Improving floral nectar storage on filter paper for sugar recovery
Bianca Amato A , Sophie Petit A B * and Russell Schumann B
A B * and Russell Schumann B
A UniSA STEM, University of South Australia, Mawson Lakes, SA 5095, Australia.
B Kangaroo Island Research Station, Dudley West, SA 5222, Australia.
Australian Journal of Botany 69(8) 585-595 https://doi.org/10.1071/BT21006
Submitted: 18 January 2021 Accepted: 21 July 2021 Published: 29 October 2021
© 2021 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC)
Abstract
Nectar analysis has been used to understand pollination systems, but nectar storage methods have rarely been considered as potential sources of inaccuracy in the recovery of data. Prompt nectar sugar analysis is not always possible and storage methods can affect results. We aimed to develop an effective method to store nectar on filter paper. Nectars from two subspecies of Eremophila maculata (Scrophulariaceae) and Strelitzia reginae (Strelitziaceae) were spotted on filter papers. Nectars were redissolved and assayed by high-performance liquid chromatography to determine the masses of sugars recovered from the papers from Day 0 to Day 30. We evaluated the effects of the method of elution, paper type and size, and storage treatments on sugar recovery. Liquid nectars were also stored in the refrigerator. Sugars were best eluted from filter papers in 15 mL of water and agitated for 1 min. Nectar sugars stored on small papers tended to be recovered more successfully than those stored on larger papers (significantly for glucose). Paper performed better than nylon for glucose. Desiccant had a marginal positive effect on nectar sugar recovery, and filter paper performed better than did refrigeration of liquid nectar for storage. If highly accurate measurements are needed, nectars should be eluted with large volumes of water from small filter papers stored with desiccant within a few days of collection.
Keywords: nectar elution, nectar storage methods, nectar storage on filter paper, nectar sugar analysis, zeolite.
Introduction
Nectar is the main reward for flower visitors (Simpson and Neff 1981), and its most commonly measured sugars are sucrose, glucose, and fructose (Baker and Baker 1983b). Other sugars are sometimes present in small traces (e.g. mannose, arabinose, xylose, maltose, melibiose, raffinose, melezitose, stachyose; Baker and Baker 1983b), as well as amino acids, enzymes, lipids, phenolics, salts, and alkaloids (Baker and Baker 1973; Gottsberger et al. 1984; Galletto and Bernardello 2005; Nicolson and Thornburg 2005). Floral nectar sugar characteristics have been the focus of many investigations, including how viscosity, sugar composition and concentration, and energetic reward are shaped by microclimate (Corbet 1979), nectar-dwelling yeasts and microorganisms (Herrera et al. 2009), and flower age and structure (Percival 1961; Nicolson and Van Wyk 1998). The evolutionary and behavioural consequences of nectar characteristics also represent key aspects of the pollination literature (e.g. pollinator feeding rates, preferences, distributions, and plant fitness; Kingsolver and Daniel 1995; Brown et al. 2010; Amorim et al. 2013; Staab et al. 2017).
The accuracy of ecological studies involving nectar sugar measurements depends on the method of collection, laboratory analysis, and storage. Different methods are likely to produce inconsistent results. Nectar can be sampled using microcapillary pipettes, syringes, and graduated pipettes (Kearns and Inouye 1993; Corbet 2003), by using paper wicks (McKenna and Thomson 1988; Dungan et al. 2004; Dósa 2008), by washing cut flowers (Morrant et al. 2009), and by rinsing uncut flowers (Mallick 2000). Morrant et al. (2009) and Petit et al. (2011) demonstrated that sampling techniques affected results, with washing and rinsing yielding the greatest amount of sugar among the methods they compared.
When determining nectar sugar concentrations in the field, a hand-held refractometer is the most common instrument used. However, a refractometer is unsuitable if nectar volumes are too small (e.g. <0.5 μL; Kearns and Inouye 1993; Corbet 2003), or if a detailed sugar analysis is required (Aronne and Malara 2019). In this case, chromatography in the laboratory is required. Historically, paper chromatography (PC; Baker and Baker 1983a; Kearns and Inouye 1993), thin-layer chromatography (TLC; Lewis and Smith 1969; Baker 1977), and gas chromatography (GC; Baskin and Bliss 1969) were chromatographic procedures used to identify nectar sugars. However, high-performance liquid chromatography (HPLC) is now the preferred chromatographic method for nectar sugar analyses. It permits the identification of different sugars and quantifies their masses, whereas GC requires complex methods to generate volatile derivatives of nectar before analysis, and TLC and PC are slow to run, and their results are qualitative (Gilbert 1987). HPLC results are based on precise area counts of peaks that are measured with software, rather than visual comparisons or spot intensity matching techniques, as with TLC and PC (Coskun 2016). HPLC also offers different detector, software, stationary phase, and column options (Dhandhukia and Thakker 2011; Coskun 2016) to improve separation and provide the required sensitivity.
Between collection and laboratory analysis, nectar is often refrigerated, frozen, or preserved with an antimicrobial agent to prevent changes in sugar composition. However, these treatments may result in poor sugar concentration stability over time (Morrant et al. 2009). Nectar should not be stored for extended periods because sucrose is hydrolysed to glucose and fructose (Nicolson and Thornburg 2005). Enzymes such as invertase that are found in flowers during pre-secretion can alter sugar composition (Nichol and Hall 1988; Pate et al. 1998; Nicolson and Thornburg 2005; Ruhlmann et al. 2010). Invertase activity has been reported for several species (Baker and Baker 1983a; Pate et al. 1985; Heil et al. 2005; Shenoy et al. 2012), and could be a significant contributor to altered sugar characteristics during storage. Microbial growth can also affect nectar. For example, yeasts and bacteria affected sucrose, glucose, and fructose concentrations in studies by Herrera et al. (2009) and de Vega and Herrera (2012).
Considering the lack of nectar stability, storage conditions have the potential to affect the results of a nectar study greatly. We aimed to develop an effective method to store nectar on filter paper in the field. Filter paper is a common storage medium for nectar (Galletto and Bernardello 2005). Once the nectar has air-dried on the filter paper, it can be eluted by washing with a solvent before analysis. Elution methods are often complex, non-standardised, and mostly unreported in published work; storage methods are, likewise, rarely reported (Amato and Petit 2017).
Preliminary work on nectar that was stored on filter paper and conducted over 30 days showed that sugar recovery was not consistent for sucrose in Eremophila maculata nectar (Amato 2015). We hypothesised that elution volume, size and type of filter paper, and desiccating regime could have affected the results. Desiccation inhibits enzymes and microbial metabolism in the nectar and on the filter papers and prevents microbial growth.
Consequently, we designed this study to address the following objectives: (1) to test the effect of water volume and agitation on sugar recovery on the day of collection; (2) to determine whether filter paper size and type influence sugar recovery of stored nectar; and (3) to compare drying and storage methods for nectars stored on Whatman number 1 filter paper. The results will be useful to researchers investigating sugar contents of nectar when immediate analysis is not possible and the storage of samples is required.
Materials and methods
Plant species and nectar collection
Eremophila maculata ssp. brevifolia (Benth.) Chinnock (Scrophulariaceae) was used to address Objective 1 (to test the effect of water volume and agitation on sugar recovery on the day of collection). Eremophila maculata ssp. maculata (Ker Gawl.) F.Muell. was used to address Objectives 2 (to determine the influence of filter paper size and type on sugar recovery) and 3 (to compare drying and storage methods). We used the bird of paradise Strelitzia reginae Aiton (Strelitziaceae) as well for Objective 3. The experiments required large volumes of nectar, which all three species had. The experiments conducted are presented in a chronological order. Eremophila m. ssp. maculata nectar was used because E. m. ssp. brevifolia had stopped flowering after the first objective. We used Strelitzia reginae nectar because we wanted to determine whether different nectar characteristics influenced storage. Eremophila m. maculata nectar is hexose dominant, whereas S. reginae nectar is balanced for the three sugars. With a 100-μL pipette, we drew up the nectar of six individual E. m. maculata, eight E. m. brevifolia, and 12 S. reginae plants throughout Adelaide, South Australia (AGD94 –34.919822, 138.612568). We sampled all the flowers from each plant, combining nectar from over 500 Eremophila flowers and 50 S. reginae flowers for their respective experiments. All nectar collection occurred between 08:00 and 09:30. Nectar was pooled into 50-μL Eppendorf tubes and stored on ice during transportation to the laboratory. We sampled unbagged E. m. maculata flowers of all ages from May to September 2015 and S. reginae flowers in July 2016.
High-performance liquid chromatography analysis of sugars
We used HPLC to measure sugar concentrations in nectars and in water eluted from filter paper on which nectar had been stored. All solutions were filtered using a 0.45-nm nylon membrane (Celltreat Scientific Products, Shirley, MA, USA) into a 7 × 40-mm, 800-μL crimp-top vial, and sealed with 8-mm ‘silver’ seals with PTFE/silicone liners (ThermoFisher Scientific, Waltham, MA, USA). We injected 100 μL of each sample by using a LS-3200 auto-sampler (SGE, Melbourne, Vic., Australia) and chromatographed with a carbohydrate, Pb2+ 300 × 7.8-mm column (Benson Polymeric, Inc., Reno, NV, USA) heated to 85°C with a column-heating module. Water as solvent was pumped using a Waters 600E solvent delivery system at a flow rate of 0.8 mL min−1. Detection was achieved with an evaporative light-scattering detector (Alltech Model 3300 ELSD, Flemington, NJ, USA) heated to 45°C with a gain set at 8 or a differential refractive index (RI) detector (Waters Model 431), heated to 35°C. Data acquisition and handling were performed using Baseline 815 software from Waters (Dynamic Solutions of Millipore, Ventura, CA, USA). Calibration curves between 2 and 80 mg L−1 were made from stock solutions of sucrose, glucose and fructose (Sigma−Aldrich, Saint Louis, MO, USA). Fresh calibration standards were made during every objective, and we recalibrated the HPLC frequently. The detector response was non-linear over this range, and so calibration curves were constructed using a second-order polynomial fit for both detectors.
Nectar and synthetic nectar
The freshly collected nectar was pooled in a 10-mL vial and magnetically stirred for 5 min. A volume of 10 mg of nectar was diluted with 15 mL of Milli-Q water (EMD Millipore, Darmstadt, Germany), and assayed using HPLC to determine the sugar masses and concentrations. The synthetic nectar (control) was prepared using sugar concentrations similar to those of the floral nectars collected from E. m. maculata, E. m. brevifolia, and S. reginae, by weighing high-purity sucrose, glucose, and fructose in 10-mL volumetric flasks and dissolving with milli-Q water. The homogenised nectar and synthetic nectar were used for each experiment.
Sugar recovery
Recovery is the ratio of the observed mass to known (true) mass. Recovery calculations assess the accuracy of the experiment and allow the determination of whether low recoveries are the result of the analytical procedure or other external factors, such as losses during the sample storage. To evaluate the instrument’s accuracy, we assayed quality-control (QC) samples at five-sample intervals. QC samples of low (∼25 mg L−1), high (∼70 mg L−1), and similar nectar concentrations were prepared from determined volumes of stock solutions. The instrument accuracy was calculated as the percentage deviation from the known concentration of the QC or percentage recovery value:

QC recoveries of 100 ± 2% meant that any low sugar recoveries were unlikely to have resulted from the analytical procedure. Sugar recovery calculations were also used to determine the difference between the sugar masses recovered from the filter paper and calculated known sugar masses (determined from the liquid nectar at Day 0); they were expressed as sugar percentage recovery. We chose to express the spread of the data with two standard errors to represent 95% confidence that the sample mean was within two standard errors of the population mean.
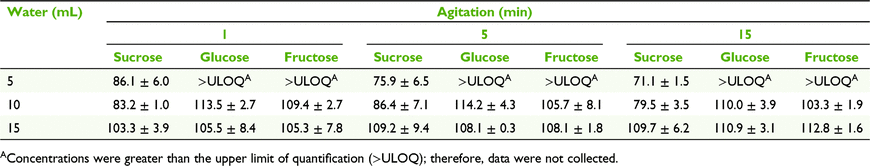
Effect of water volume and agitation on sugar recovery
To evaluate the effects of water volume and agitation time on extraction of sugars from the filter paper, nectar was loaded onto the filter paper, air-dried and extracted immediately to minimise any chance of sugar decomposition. We cut Whatman number 1 filter paper (GE Healthcare, Chicago, IL, USA) into squares of 1.5 × 1.5 cm, wearing gloves to prevent contamination, and spotted 10 μL of the homogenised nectar on each filter-paper square. The spotted filter paper was immediately weighed on a microbalance to determine nectar mass, and air-dried for 1 min. Spotted papers were stored in labelled 15- × 10-cm plastic Clickzip plastic sandwich bags (Hercules, THA) at room temperature until analysis.
We placed each spotted paper in a 70-mL stoppered container and subjected it to one of nine treatments to redissolve the nectar (n = 3 for each treatment). Treatments consisted of three volumes of milli-Q water (5, 10, and 15 mL) added to the stoppered vial × agitation with an auto-shaker for three durations (1, 5, and 15 min). The solution was assayed using HPLC to determine the mass of sucrose, glucose, and fructose removed from the paper.
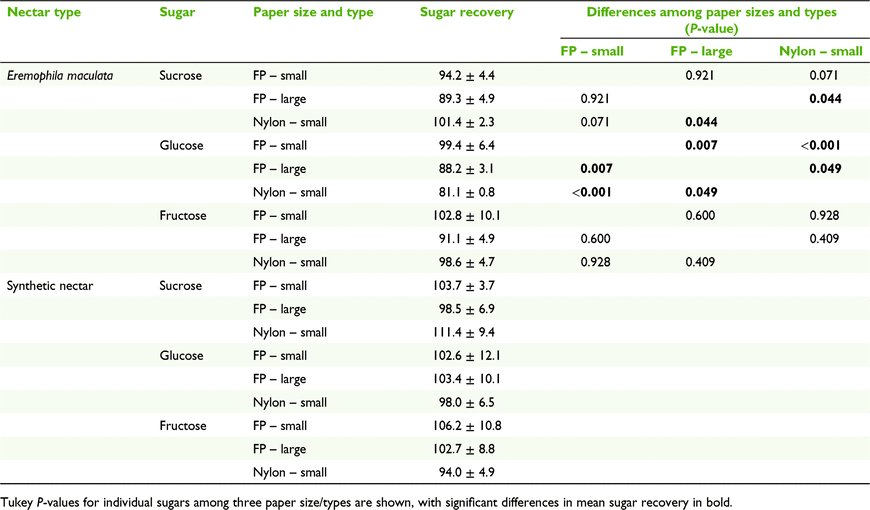
Effect of filter paper size and type on sugar recovery
Whatman number 1 filter paper was cut into 3.0- × 3.0-cm and 1.5- × 1.5-cm squares, and Whatman 7404-004 nylon membrane filter (GE Healthcare, Chicago, IL, USA) into 1.5- × 1.5-cm squares and spotted with homogenised natural and synthetic nectars to compare the effects of different sizes and types of paper on nectar sugar recovery. Spotted squares were immediately weighed on a microbalance to determine nectar mass, then air dried. They were stored in labelled Clickzip sandwich bags until analysis. Each spotted paper was placed in a 70-mL stoppered container with 15 mL of milli-Q water and agitated for 1 min before analysis.
Effect of drying treatments on sugar recovery
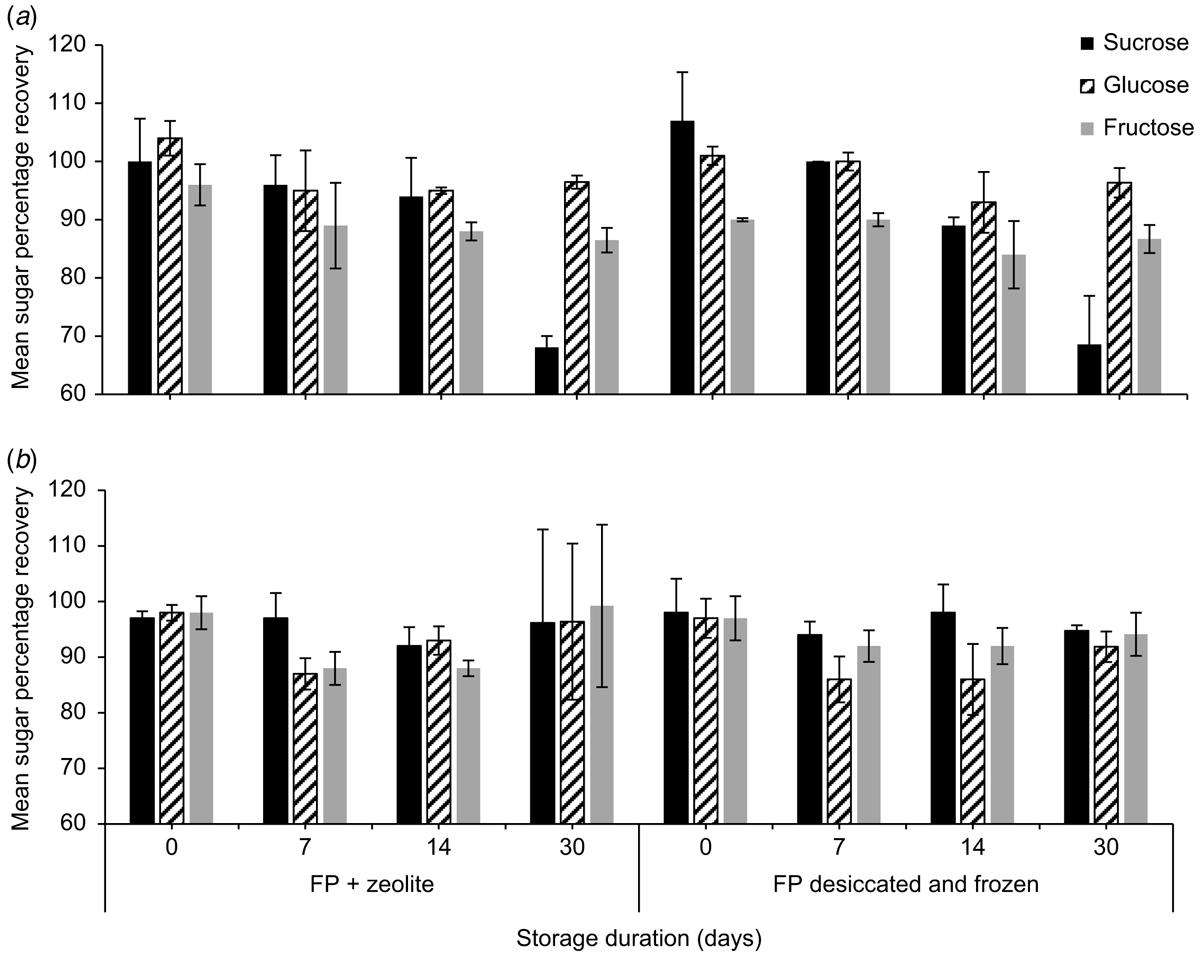
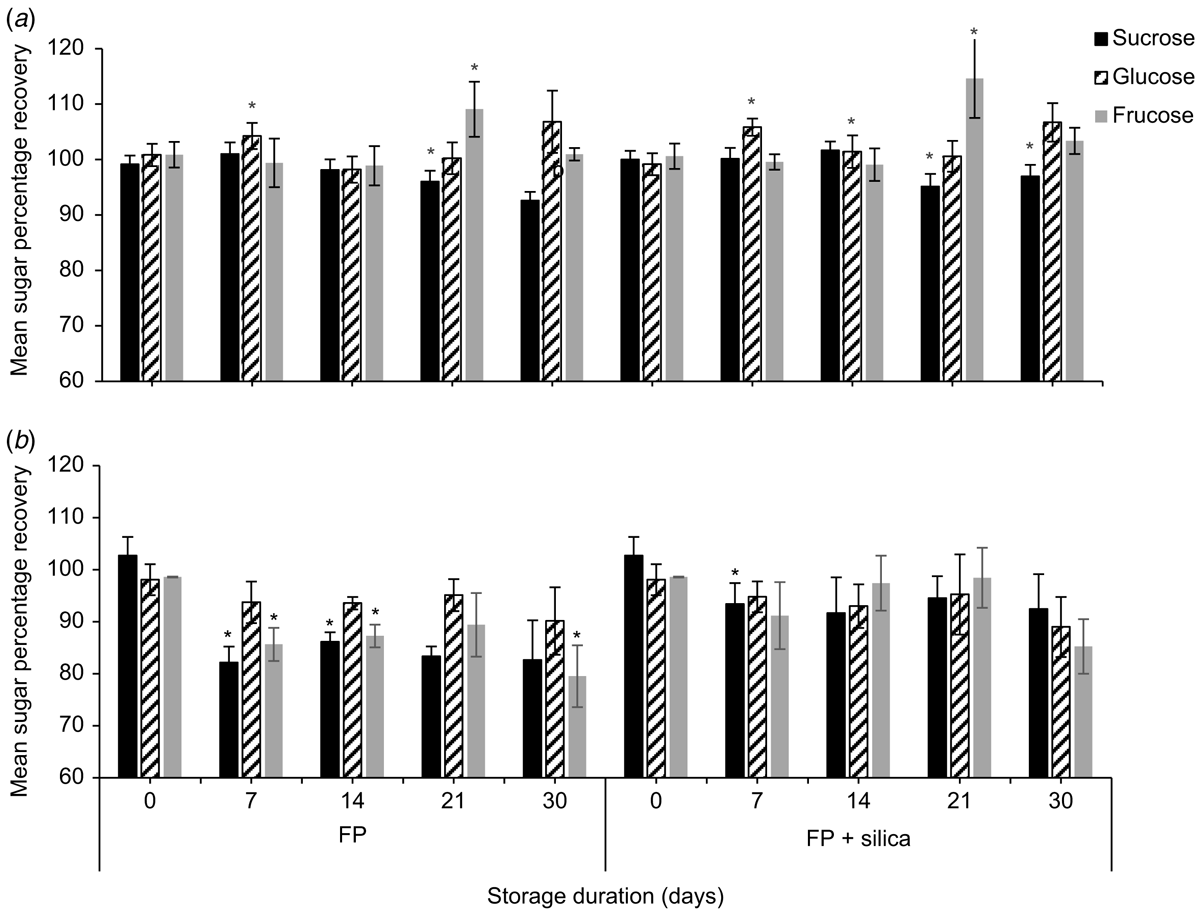
To evaluate the effects of different drying treatments on nectar sugar recovery, Whatman filter paper number 1 was cut into 1.5- × 1.5-cm pieces. Homogenised nectar from E. m. maculata was stored, using the following treatments: (a) spotted on filter papers that had been desiccated overnight in a forced-draught oven at 50°C, re-dried in the oven overnight, placed in 15- × 10-cm plastic Clickzip plastic bags, and frozen (n = 2); and (b) spotted on filter paper, placed in Clickzip sandwich bags with eight pieces of ∼3- × 7-mm natural zeolite (Zeolite Australia Pty Ltd, Werris Creek, NSW, Australia) and stored at room temperature (n = 2). During subsequent nectar storage experiments, E. m. maculata was no longer flowering, so we used a different species for further testing. Homogenised S. reginae nectar was stored on (c) filter papers and placed in Clickzip sandwich bags, and stored at room temperature (n = 5), and (d) filter papers placed in Clickzip sandwich bags with silica gel (0.5-g Tyvek packets, DuPont, MI, USA) and stored at room temperature (n = 5). The homogenised liquid nectars of E. m maculata and S. reginae that were left over from the filter paper treatments were transferred to 7-mL bijou vials and stored in the refrigerator. As before, papers from all four treatments were placed in a 70-mL stoppered container with 15 mL of milli-Q water and agitated for 1 min. The extracts were assayed using HPLC to determine the masses of sugars recovered from the treatments at Days 0, 7, 14, 21 and 30. Concentrations of sugars in the homogenised liquid nectar samples stored refrigerated were also determined at each of the above time intervals by dissolving 10 mg of the refrigerated nectar in 15 mL of water and assaying the solution by using HPLC.
Data analysis
We used the statistical package SPSS (IBM Corp., Armonk, NY, USA) to conduct analyses (α = 0.05). For Objective 1, we used a multivariate analysis of variance (MANOVA) to compare the effect of different volumes and agitation times on sugar recovery. Univariate analysis of variance (ANOVA) identified effects on individual sugars (Field 2009), and we also analysed sucrose separately. For Objective 2, sugar recoveries from the collection day were compared among the three treatments with a MANOVA. For Objective 3, sugar recoveries were compared separately among the different treatments using a repeated-measure one-way ANOVA. Repeated contrasts were used to compare each storage day against the previous one. If sphericity was not met, a Greenhouse–Geisser correction was applied to all tests. Data were tested for normality using a Kolmogorov–Smirnov goodness-of-fit test. We conducted statistical analyses only for treatments with three or more replicates.
Results
Sugar recovery using three water volumes and agitation periods
Mean (±2 s.e., n = 3) sucrose, glucose and fructose concentrations measured on the day of collection for E. m. brevifolia nectar were 0.8 ± 0.2% (w/w), 6.2 ± 0.2% (w/w) and 6.9 ± 0.2% (w/w) respectively. The MANOVA testing the effects of elution volume and agitation time indicated a significant effect of volume (F3,10 = 47.72, P < 0.001), highlighting sucrose (univariate ANOVA F1,12 = 115.28, P < 0.001) and glucose (F1,12 = 6.13, P = 0.029). We had no data for glucose and fructose at 5 mL; because of the low dilution factor, their concentrations were not within the calibration range of the HPLC (2–80 mg L−1). Therefore, we performed a separate univariate ANOVA with sucrose and found that volume (F2,18 = 122.98, P < 0.001) and volume + agitation (F4,18 = 4.12, P = 0.015) were significant, with the main effect for volume, indicating that large volume and small agitation time should be preferrable (Table 1).
Sugar recovery from filter papers of different sizes and types
We found a significant effect of different paper sizes and types on nectar sugar recovery (F6,10 = 3.749, P = 0.032). Separate univariate ANOVA on the sugar recoveries indicated that sucrose and glucose were significantly affected by the different paper sizes and types (F2,6 = 6.00, P = 0.037; F2,6 = 31.33, P < 0.001 respectively; Table 2). Sucrose recovery was better on the 1.5- × 1.5-cm nylon paper than the 3.0- × 3.0-cm filter paper (P = 0.044), but the opposite was true for glucose recovery (P = 0.049). Glucose recovery was better on the 1.5- × 1.5-cm filter paper than on the 3.0- × 3.0-cm filter paper (P = 0.007). The MANOVA did not highlight a significant effect of different paper size and type on nectar sugar recovery for synthetic nectar.
Sucrose in E. m. maculata nectar accounted for 2–6% of the total sugar mass. When sucrose, glucose, and fructose masses were combined and the total sugar recovery was calculated, the low recovery of sucrose had little effect on the total sugar mass and recovery. The differences in the total sugar mass recovery among the three treatments were small (107.1 ± 9.3% for 1.5- × 1.5-cm filter paper and 93.08 ± 3.8% for 3.0- × 3.0-cm filter paper, and 96.7 ± 1.9% for the nylon paper).
Sugar recovery after different drying regimes
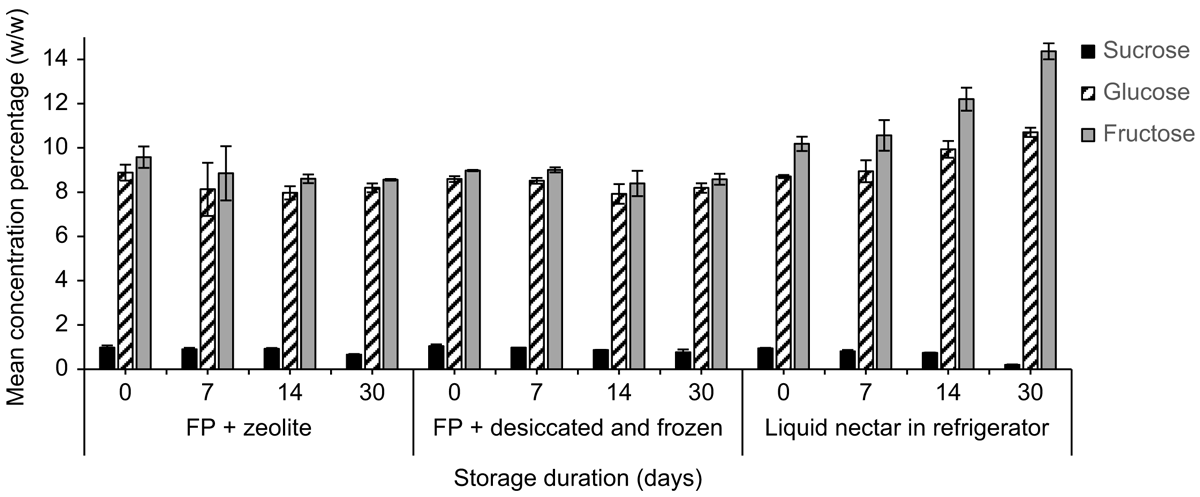
Mean (±2 s.e., n = 3) sucrose, glucose, and fructose concentrations (%, w/w) of E. m. maculata on the day of collection were 0.9 ± 0.1%, 10.5 ± 2.9% and 11.2 ± 1.6% respectively. Both storage treatments (desiccated and frozen, and zeolite at room temperature) of E. m. maculata nectar, which is hexose-rich, produced sucrose and glucose recoveries >100% on the day of collection (Fig. 1; n = 2).
By Day 30, mean (±2 s.e., n = 2) sucrose recovery decreased to 68.1 ± 8.3% from the desiccated and frozen treatment and 68.6 ± 2.0% from paper stored with zeolite. Fructose on the desiccated and frozen papers had a poorer initial recovery on Day 0 (90.0 ± 8.3%) than when stored with zeolite (96.0 ± 3.5%). Synthetic nectar sugar recoveries were ∼100% and were relatively consistent from Day 0 to Day 30 (Fig. 1).
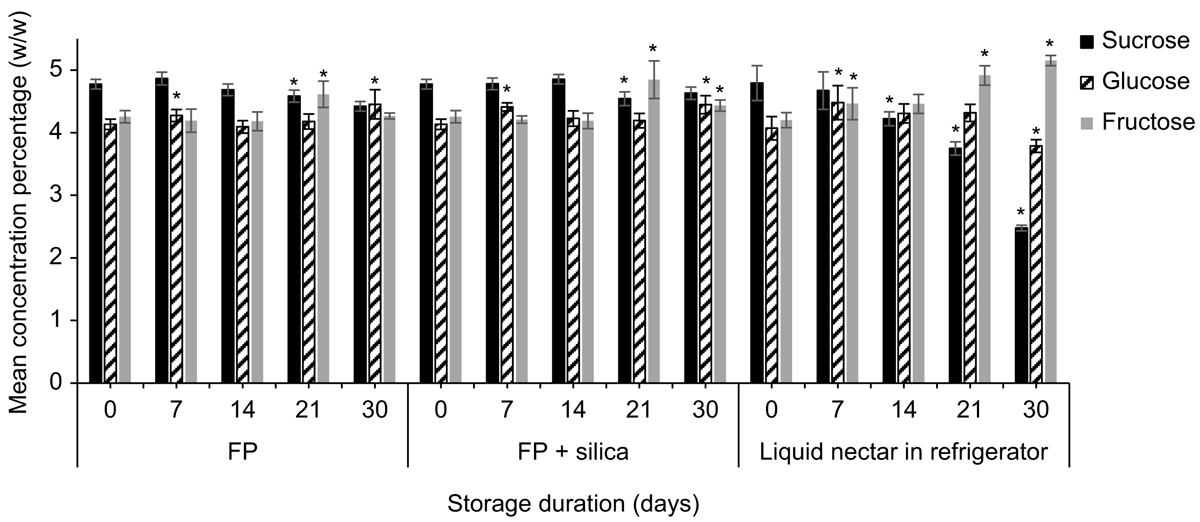
Strelitzia reginae nectar was richer in sucrose than was E. m. maculata nectar. Mean (±2 s.e., n = 5) sucrose, glucose, and fructose concentrations (%, w/w) of S. reginae on the day of collection were 4.79 ± 0.28%, 4.08 ± 0.19%, and 4.20 ±0.12% respectively. Time had a significant effect on mean nectar sucrose (F4,32 = 15.88, P < 0.001), glucose (F4,32 = 11.99, P < 0.001), and fructose (F2.31,18.49 = 19.73, P < 0.001) recoveries for both filter paper and filter paper + silica gel treatments (Fig. 2; n = 5). Contrasts between storage days showed significant differences in sugar recovery from the previous test day at Day 21 for sucrose (F1,4 = 13.13, P = 0.022) and for fructose (F1,4 = 28.55, P = 0.022), and at Day 7 for glucose (F1,4 = 8.32, P = 0.045) when nectar was stored on filter paper with silica gel. Similarly, sucrose (F1,4 = 38.53, P = 0.003) and fructose (F1,4 = 21.97, P = 0.001) were significantly different from the previous test day at Day 21, and glucose (F1,4 = 27.52, P = 0.006) at Day 7 when stored on filter paper without silica gel. Glucose recovery had surpassed Day 0 recovery by 3.5 ± 2.8% when stored with silica gel and by 6.8 ± 3.0% when stored without silica gel by Day 7.
We also found a significant difference in mean sucrose recovery of S. reginae nectar when stored on filter paper with vs without silica gel (F1,8 = 24.96, P = 0.034). On Day 30, mean (±2 s.e., n = 5) sucrose recovery from the filter papers without silica gel was 92.6 ± 1.6%. The addition of silica gel showed a small improvement in sucrose recovery (97.0 ± 2.4%).
Sugar recoveries of the synthetic control nectar stored on filter paper with and without silica gel were ∼100% at the day of collection. Time had a significant effect on the mean sucrose (F2.98,23.84 = 13.92, P < 0.001), glucose (F2.55,20.42 = 3.69, P = 0.014), and fructose (F2.310,18.49 = 14.44, P < 0.001) recoveries for both treatments for synthetic nectar stored on filter paper with and without silica gel (Fig. 2; n = 5). Contrasts between storage days showed a significant difference from the previous test day at Day 7 for sucrose (F1,4 = 10.33, P = 0.032) when the paper was stored with silica gel. When the synthetic nectar was stored on filter paper without silica gel, Day 7 was significantly different from the previous test day for sucrose (F1,4 = 174.99, P < 0.001) and fructose (F1,4 = 65.47, P < 0.001; Fig. 2). By Day 7, the mean (±2 s.e., n = 5) sucrose and fructose recoveries for the filter papers without silica gel were 82.1 ± 3.1% and 85.7 ± 3.2% respectively. We found a significant difference in mean sucrose (F1,8 = 63.66, P < 0.001) and fructose (F1,8 = 25.57, P < 0.001) recovery for synthetic nectar between filter paper with silica gel, and filter paper without.
Concentrations of glucose and fructose measured in nectar from E. m. maculata stored on filter papers with zeolite over a 30-day period were relatively stable, whereas sucrose concentrations decreased by Day 30 for both treatments (Fig. 3). For nectar stored in the refrigerator, at Day 30, sucrose was not detected, glucose was similar, and fructose concentration had a 67.3% increase compared with the day of collection.
Time had a significant effect on the mean sucrose (F1.60,6.38 = 30.68, P < 0.001), glucose (F1.60,6.41 = 12.64, P = 0.017), and fructose (F2.19,8.75 = 28.67, P < 0.001) concentrations of S. reginae liquid nectar stored in the refrigerator. Sucrose concentration had changed significantly from the previous test day by Day 14 (F1,4 = 49.39, P = 0.002), glucose (F1,4 = 6.82, P = 0.050) and fructose (F1,4 = 7.89, P = 0.050) by Day 7 when liquid nectar was stored in the refrigerator (Fig. 4). When stored on filter paper, mean sucrose (F1,4 = 19.245, P = 0.012) and fructose (F1,4 = 29.51, P = 0.006) concentrations had changed by Day 21, and glucose (F1,4 = 8.30, P = 0.045) by Day 7. For nectar stored in the refrigerator, at Day 30, sucrose and glucose concentrations had decreased by 48.2% and 7.1% respectively, whereas fructose had increased by 22.7% compared with Day 0.
Discussion
In the field, biologists rarely know what the sugar constituents, concentrations, or proportions are for the nectars they collect; they rely on different methods of storage and analysis to measure sugars. We used several test plant species to demonstrate variations in the effectiveness of storing nectar and eluting it. The results showed that (a) only 1 min of agitation was required for nectar elution and a large volume of water was more effective, (b) small filter paper generally performed better for sugar recovery than did large filter paper, (c) for hexose-dominant E. m. maculata nectar, sugar concentrations were relatively stable and changed by Day 14 when stored on filter paper, compared with Day 7 when stored in its liquid form in the refrigerator. The addition of zeolite made a small improvement to sugar measurements at Day 14. (d) For balanced S. reginae nectar, significant differences in sugar recovery were found at Day 7 for glucose, and at Day 21 for sucrose and fructose with and without silica gel. The addition of silica gel made an overall improvement to sucrose recovery. Refrigeration of S. reginae liquid nectar led to a change in sucrose by Day 14 and glucose and fructose by Day 7. We caution that analyses were conducted on three to five replicates and may suffer from Type II errors; it is likely that a greater number of significant results would be generated from larger sample sizes.
Elution and paper size techniques
Water volume had a significant effect on sugar recovery; the greatest water volume had the best result and an agitation time of 1 min was as effective as was 15 min. We did not expect that agitation time would affect sugar recovery because the movement of sugar from filter paper to water is likely to be very fast. We recommend eluting with a large volume of water and a short agitation time. The amount of water will vary depending on the detection limits of the instruments being used.
Our preliminary work (Amato 2015) showed that nectar sugar recoveries were not consistent over time for E. m. maculata, and sucrose recoveries from the filter paper were often less than 65% on the day of collection. Amato (2015) had not measured the size of the filter papers on which the nectar was stored. We hypothesised that the size of the filter paper affected the recovery of sucrose, with the filter paper structure detaining sucrose molecules, preventing complete dissolution into the water. Consequently, the larger the filter paper, the more opportunity existed for sugar to remain on the filter paper. Comparatively large volumes of water would dissolve the sugar to a greater extent and reduce the driving force (concentration gradient) for re-adsorption. Our results support the hypothesis that we generated after our preliminary work because small (1.5- × 1.5 cm) pieces of paper led to better sugar recovery than did larger (3.0- × 3.0 cm) ones for natural nectar, significantly so for glucose. Sucrose and glucose appeared to behave slightly differently on filter paper and nylon paper (Table 2), and paper type should be considered in the context of the experiment. For example, nylon may be preferable for high-sucrose nectars, but the unlikely mean sucrose recovery of 111% for synthetic nectar (Table 2) indicates that more work is needed to explore the functionality of nylon paper. We recommend using pieces of paper that are as small as possible, with no ‘blank space’ around the nectar spot, and a generous amount of water that still allows the accurate detection of sugars.
Nectar storage on filter paper
Sucrose, glucose, and fructose recoveries of E. m. maculata nectar stored on filter paper with and without zeolite experienced changes within 7 days. Zeolite had a marginally positive effect compared with desiccated and frozen nectar, but we had only two replicates. Eremophila m. maculata liquid nectar that was stored in the refrigerator experienced only small changes to its sugar concentrations for up to 7 days. By Day 30, no sucrose was detected in E. m. maculata, and mould was growing in E. m. maculata and S. reginae liquid nectars that were stored in the refrigerator. Statistical analysis of sucrose, glucose, and fructose recoveries of S. reginae nectar showed that glucose was not stable, and its recovery was greater than on the day of collection by Day 7. Sucrose and fructose were stable for 14 days. The addition of silica gel made a minor improvement to the overall mean sucrose recovery. Glucose and fructose concentrations from S. reginae liquid nectar stored in the refrigerator were stable for less than a week. The benefit of storing nectar in the refrigerator is that there is no need for complex washing techniques to prepare the samples. However, we would recommend storing nectar in the refrigerator for fewer than 7 days.
The decrease in sucrose in nectar from E. m. maculata over time did not result in a great change in the nectar sugar content because this plant’s nectar is very low in sucrose. By Day 30, at least one-third of the sucrose was lost in some treatments, but this amount represented less than 0.3% of the total nectar sugar mass in E. m. maculata. By contrast, by Day 30, we reported only a 3–7% loss of sucrose from the equally proportioned sugars in the nectar of S. reginae in all filter paper treatments. The increase in glucose recovery by Day 7 for S. reginae also made a relatively small difference to the total sugar mass.
Storage in an air-conditioned laboratory contrasts with storage in many field situations, such as the wet tropics. Zeolite and silica gel in those conditions may have a greater impact, which is why we recommend their use. As opposed to desiccation and freezing, storage with zeolite or silica gel can take place immediately after collection in the field and does not require specialised equipment.
Considering the microorganisms and other components present in nectar, we predicted that the synthetic nectars would have similar or better recoveries than their natural nectar counterparts. However, the synthetic nectar made to represent S. reginae sugar concentrations had the lowest recovery of all the treatments, even if silica gel improved recovery. Because this experiment was not conducted under sterile conditions, it is possible that microorganisms in the synthetic nectar used sugar carbon for growth, thus reducing sugar recovery. No further decreases in sugar recoveries after Day 7 suggest that microbial growth was inhibited after this time, perhaps as a result of depletion of other essential nutrients (e.g. phosphorus and nitrogen). Some nectars have stabilising compounds such as proteins and enzymes that protect the nectar from microbial infestation (Carter and Thornburg 2000; Nicolson and Thornburg 2005). Nectarin enzymes found in the floral nectar of Nicotiana produce hydrogen peroxide to create a microbe-free environment in the nectar (Carter and Thornburg 2004). Jaroenkit et al. (2008) reported that in cut flowers of S. reginae, mould growth was present on the stamens and stigma, but not in the nectar. These types of compounds may reduce microbial growth in S. reginae nectar. It is wise to use sterile methods and equipment, including filter paper, in methodological experiments involving nectar.
HPLC and sugar analysis limitations and recommendations
Sugar recoveries were often greater than 100%. Sucrose hydrolyses into glucose and fructose, thus generating a greater mass of the hexose sugars. Because of the low mass of sucrose present in our nectar, it is unlikely that the high recoveries of hexose sugars were the result of sucrose hydrolysis. We checked that sugar contamination of the syringe (used to inject samples into the HPLC vials) from previous samples was not the cause of high sugar recovery (not presented in Results). Washing the syringe between each sample with distilled water eliminated sugar from previous samples and did not influence sugar recovery. We washed and assayed blank filter papers, and found no positive interference, so recoveries above 100% could not be accounted for by contamination from the filter papers. We suggest that recoveries of over 100% are the result of interference from impurities within the nectars, water evaporation from the nectar when it was weighed and spotted on the filter paper, or both factors together. Biological samples with low concentrations often show positive interference from background impurities (chemical noise), which can overshadow the peak of the analyte (sucrose, glucose, and fructose) on the chromatogram and lead to inaccurate measurements (Guo et al. 2006). Nectar was weighed with a precision of 0.1 mg when spotting it onto the filter paper. Water may have evaporated from the nectar while the microbalance was stabilising, thus recording a smaller total mass than was actually added, a higher apparent sugar concentration, and resulting in initial sugar recoveries greater than 100%. Evaporation rates of nectar droplets would vary among samples because evaporation rates are governed by the size, shape, surface area and constituents of the droplet, as well as environmental factors such as humidity, vapour concentration, and temperature (Shahidzadeh-Bonn et al. 2006; Girard et al. 2008). In the field, nectar is often collected and volumetrically measured with microcapillary pipettes (Corbet 2003), whereas in the laboratory we measured it gravimetrically on a microbalance. A closed vessel such as microcapillary pipette may reduce evaporation and the lack of precision from gravimetric measurements before analysis, although this collection method is associated with other problems (Petit et al. 2011).
During the experiment, we had to change detectors from an ELSD to RI. RI is less sensitive than is ELSD (Clement et al. 1992). Although we found no notable difference between QC recoveries using the ELSD and RI detectors for all three sugars, RI can be more susceptible to interference from impurities, which may have influenced the results for our nectar samples. The accuracy and reliability of the data can be a direct result of the type of HPLC detector (or chromatographic procedure) chosen. Errors can be large (Karkacier et al 2003). We recommend that researchers quantify the sources of error when assaying nectar sugars, including those from the instruments.
Sampling from sucrose-poor nectars requires a low volume of water to ensure that the concentration can be measured within the range of detection of the instrument. Increasing the volume of water to rinse the samples can dilute sucrose concentrations to the point where they fall under the limit of detection of HPLC. Pulsed-amperometric detection (PAD) might overcome problems of diluting nectar with low sucrose concentrations. PAD is highly sensitive and accurate, offering a low limit of detection for simple carbohydrates (Bailey et al. 2014).
Conclusions
We have shown several sources of uncertainty and error during the process, from collection, storage, and elution, to HPLC analysis. Variances for replicates were sometimes large. Biologists need a level of certainty from their results, especially when most ecological investigations assay only one replicate per nectar sample. For fieldwork, we recommend the use of small pieces of filter paper with a desiccant, and later elution with as large a volume of water as permitted to achieve the desired sensitivity for analysis. Analysis should be performed within a few days if high accuracy is needed; storage as liquid in the refrigerator is not recommended. However, these recommendations do not apply to the recovery of amino acids (Power et al. 2017), EFNs, and one method does not fit all purposes. Detailed storage and laboratory methods, including elution techniques, QC procedures, and detailed analytical procedures, need to be reported in published works. We suggest that other factors that may influence sugar recoveries should be investigated, such as the mass of nectar spotted on the filter paper, different nectar concentrations and proportions, different amino acids compositions, and microorganisms.
Data availability
The data that support this study are available in the article and accompanying supplementary material online.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This work was supported by a Lirabenda Endowment Fund Research Grant (Field Naturalists Society of South Australia); Nature Foundation SA Honours research grant; two Playford Memorial Trust Science and Engineering Scholarships; UniSA Rural and Isolated Scholarship; Australian Postgraduate Award to B. Amato.
Acknowledgements
We thank Tim Quinn, Joan Gibbs, Irina Ametov, Sayaka Hidaki, Robert Aebi, and George Levay for assistance. Zeolite Australia Pty Ltd donated the zeolite.
References
Amato B (2015) Evaluating field methods for the storage of floral nectar. BSc(Hons) thesis, University of South Australia, Mawson Lakes, SA, Australia.Amato B, Petit S, (2017) A review of the methods for storing floral nectars in the field. Plant Biology 19, 497–503.
| A review of the methods for storing floral nectars in the field.Crossref | GoogleScholarGoogle Scholar | 28303638PubMed |
Amorim FW, Galetto L, Sazima M (2013) Beyond the pollination syndrome: nectar ecology and the role of diurnal and nocturnal pollinators in the reproductive success of Inga sessilis (Fabaceae). Plant Biology 15, 317–327.
| Beyond the pollination syndrome: nectar ecology and the role of diurnal and nocturnal pollinators in the reproductive success of Inga sessilis (Fabaceae).Crossref | GoogleScholarGoogle Scholar | 22823072PubMed |
Aronne G, Malara P (2019) Fiber‐optic refractometer for in vivo sugar concentration measurements of low‐nectar‐producing flowers. New Phytologist 224, 987–993.
| Fiber‐optic refractometer for in vivo sugar concentration measurements of low‐nectar‐producing flowers.Crossref | GoogleScholarGoogle Scholar |
Bailey B, Ullucci P, Bauder R, Plante M, Crafts C, Acworth I (2014) ‘Carbohydrate analysis using HPLC with PAD, FLD, charged aerosol detection, and MS detectors.’ (ThermoFisher Scientific: Boston, MA, USA)
Baker HG (1977) Non-sugar chemical constituents of nectar. Apidologie 8, 349–356.
| Non-sugar chemical constituents of nectar.Crossref | GoogleScholarGoogle Scholar |
Baker HG, Baker I (1973) Amino-acids in nectar and their evolutionary significance. Nature 241, 543–545.
| Amino-acids in nectar and their evolutionary significance.Crossref | GoogleScholarGoogle Scholar |
Baker HG, Baker I (1983a) Floral nectar sugar constituents in relation to pollinator type. In ‘Handbook of experimental pollination biology’. (Eds C Jones, R Little) pp. 117–141. (van Nostrand Reinhold Co Inc.: New York, NY, USA)
Baker HG, Baker I (1983b) A brief historical review of the chemistry of floral nectar. In ‘The biology of nectaries’. (Eds B Bentley, T Elias) pp. 126–152. (Columbia University Press: New York, NY, USA)
Baskin SI, Bliss CA (1969) Sugar occurring in the extrafloral exudates of the Orchidaceae. Phytochemistry 8, 1139–1145.
| Sugar occurring in the extrafloral exudates of the Orchidaceae.Crossref | GoogleScholarGoogle Scholar |
Brown M, Downs CT, Johnson SD (2010) Sugar preferences of a generalist nonpasserine flower visitor, the African speckled mousebird (Colius striatus. Auk 127, 781–786.
| Sugar preferences of a generalist nonpasserine flower visitor, the African speckled mousebird (Colius striatus.Crossref | GoogleScholarGoogle Scholar |
Carter C, Thornburg RW (2000) Tobacco nectarin I purification and characterisation as a germin-like, manganese superoxide dismutase implicated in the defense of floral reproductive tissues. Journal of Biological Chemistry 275, 36726–36733.
| Tobacco nectarin I purification and characterisation as a germin-like, manganese superoxide dismutase implicated in the defense of floral reproductive tissues.Crossref | GoogleScholarGoogle Scholar |
Carter C, Thornburg RW (2004) Is the nectar redox cycle a floral defense against microbial attack? Trends in Plant Science 9, 320–324.
| Is the nectar redox cycle a floral defense against microbial attack?Crossref | GoogleScholarGoogle Scholar | 15231276PubMed |
Clement A, Yong D, Brechet C (1992) Simultaneous identification of sugars by HPLC using evaporative light scattering detection (ELSD) and refractive index detection (RI). Application to plant tissues. Journal of Liquid Chromatography 15, 805–817.
| Simultaneous identification of sugars by HPLC using evaporative light scattering detection (ELSD) and refractive index detection (RI). Application to plant tissues.Crossref | GoogleScholarGoogle Scholar |
Corbet SA (2003) Nectar sugar content: estimating standing crop and secretion rate in the field. Apidologie 34, 1–10.
| Nectar sugar content: estimating standing crop and secretion rate in the field.Crossref | GoogleScholarGoogle Scholar |
Corbet S, Unwin DM, Prys-Jones O (1979) Humidity, nectar and insect visits to flowers, with special reference to CrataegusTilia and Echium. Ecological Entomology 4, 9–22.
| Humidity, nectar and insect visits to flowers, with special reference to CrataegusTilia and Echium.Crossref | GoogleScholarGoogle Scholar |
Coskun O (2016) Separation techniques: chromatography. Northern Clinics of Istanbul 3, 156–160.
| Separation techniques: chromatography.Crossref | GoogleScholarGoogle Scholar | 28058406PubMed |
de Vega C, Herrera CM (2012) Relationships among nectar-dwelling yeasts, flowers and ants: patterns and incidence on nectar traits. Oikos 121, 1878–1888.
| Relationships among nectar-dwelling yeasts, flowers and ants: patterns and incidence on nectar traits.Crossref | GoogleScholarGoogle Scholar |
Dhandhukia P, Thakker J (2011) Quantitative analysis and validation of method using HPTLC. In ‘High-performance thin-layer chromatography (HPTLC)’. (Ed. M Srivastava) pp. 203–221. (Springer: Berlin, Germany)
Dósa G (2008) Nectar collection and analysis with wick-sampling method. Acta Botanica Hungarica 50, 93–96.
| Nectar collection and analysis with wick-sampling method.Crossref | GoogleScholarGoogle Scholar |
Dungan RJ, Beggs JR, Wardle DA (2004) A simple gravimetric technique for estimating honeydew or nectar production. New Zealand Journal of Ecology 28, 283–288.
Field A (2009) ‘Discovering statistics using SPSS’, 3rd edn. (Sage Publication Ltd: London, UK)
Galletto L, Bernardello G (2005) Nectar. In ‘Practical pollination biology’. (Eds A Dafni, PG Kevan, BC Husband) pp. 156–216. (Enviroquest: Cambridge, ON, Canada)
Gilbert MT (1987) ‘High performance liquid chromatography.’ (IOP Publishing: Bristol, UK)
Girard F, Antoni M, Sefiane K (2008) On the effect of Marangoni flow on evaporation rates of heated water drops. Langmuir 24, 9207–9210.
| On the effect of Marangoni flow on evaporation rates of heated water drops.Crossref | GoogleScholarGoogle Scholar | 18671417PubMed |
Gottsberger G, Schrauwen J, Linskens HF (1984) Amino acids and sugars in nectar, and their putative evolutionary significance. Plant Systematics and Evolution 145, 55–77.
| Amino acids and sugars in nectar, and their putative evolutionary significance.Crossref | GoogleScholarGoogle Scholar |
Guo X, Bruins AP, Covey TR (2006) Characterisation of typical chemical background interferences in atmospheric pressure ionisation liquid chromatography–mass spectrometry. Rapid Communications in Mass Spectrometry 20, 3145–3150.
| Characterisation of typical chemical background interferences in atmospheric pressure ionisation liquid chromatography–mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 16998786PubMed |
Heil M, Rattke J, Boland WJS (2005) Postsecretory hydrolysis of nectar sucrose and specialisation in ant/plant mutualism. Science 308, 560–563.
| Postsecretory hydrolysis of nectar sucrose and specialisation in ant/plant mutualism.Crossref | GoogleScholarGoogle Scholar | 15845855PubMed |
Herrera CM, de Vega C, Canto A, Pozo MI (2009) Yeasts in floral nectar: a quantitative survey. Annals of Botany 103, 1415–1423.
| Yeasts in floral nectar: a quantitative survey.Crossref | GoogleScholarGoogle Scholar | 19208669PubMed |
Jaroenkit T, Chen NJ, Paull RE (2008) Nectar secretion, mucilage production and mold growth on bird-of-paradise inflorescences. Postharvest Biology and Technology 49, 431–435.
| Nectar secretion, mucilage production and mold growth on bird-of-paradise inflorescences.Crossref | GoogleScholarGoogle Scholar |
Karkacier M, Erbas M, Uslu MK, Aksu M (2003) Comparison of different extraction and detection methods for sugars using amino-bonded phase HPLC. Journal of Chromatographic Science 41, 331–333.
| Comparison of different extraction and detection methods for sugars using amino-bonded phase HPLC.Crossref | GoogleScholarGoogle Scholar | 12935307PubMed |
Kearns CA, Inouye DW (1993) ‘Techniques for pollination biologists.’ (University Press of Colorado: Boulder, CO, USA)
Kingsolver J, Daniel T (1995) Mechanics of food handling by fluid-feeding insects. In ‘Regulatory mechanisms in insect feeding’. (Eds R Chapman, G de Boer) pp. 32–73. (Springer: Boston, MA, USA)
Lewis B, Smith F (1969) Sugars and derivatives. ‘Thin-layer chromatography’. (Ed. E Stahl) pp. 807–837. (Springer: Berlin, Germany)
Mallick SA (2000) Technique for washing nectar from the flowers of Tasmanian leatherwood (Eucryphia lucida Eucryphiaceae). Austral Ecology 25, 210–212.
| Technique for washing nectar from the flowers of Tasmanian leatherwood (Eucryphia lucida Eucryphiaceae).Crossref | GoogleScholarGoogle Scholar |
McKenna MA, Thomson JD (1988) A technique for sampling and measuring small amounts of floral nectar. Ecology 69, 1306–1307.
| A technique for sampling and measuring small amounts of floral nectar.Crossref | GoogleScholarGoogle Scholar |
Morrant DS, Schumann R, Petit S (2009) Field methods for sampling and storing nectar from flowers with low nectar volumes. Annals of Botany 103, 533–542.
| Field methods for sampling and storing nectar from flowers with low nectar volumes.Crossref | GoogleScholarGoogle Scholar | 19074446PubMed |
Nichol P, Hall JL (1988) Characteristics of nectar secretion by the extrafloral nectaries of Ricinus communis. Journal of Experimental Botany 39, 573–586.
| Characteristics of nectar secretion by the extrafloral nectaries of Ricinus communis.Crossref | GoogleScholarGoogle Scholar |
Nicolson SW, Thornburg RW (2005) Nectar chemistry. In ‘Nectaries and nectar’. (Eds SW Nicolson, M Nepi, E Pacini) pp. 215–263. (Springer: Dordrecht, Netherlands)
Nicolson SW, Van Wyk B-E (1998) Nectar sugars in Proteaceae: patterns and processes. Australian Journal of Botany 46, 489–504.
| Nectar sugars in Proteaceae: patterns and processes.Crossref | GoogleScholarGoogle Scholar |
Pate JS, Peoples MB, Storer PJ, Atkins CA (1985) The extrafloral nectaries of cowpea (Vigna unguiculata (L.) Walp.). II. Nectar composition, origin of nectar solutes, and nectary functioning. Planta 166, 28–38.
| The extrafloral nectaries of cowpea (Vigna unguiculata (L.) Walp.). II. Nectar composition, origin of nectar solutes, and nectary functioning.Crossref | GoogleScholarGoogle Scholar | 24241308PubMed |
Pate J, Shedley E, Arthur D, Adams M (1998) Spatial and temporal variations in phloem sap composition of plantation-grown Eucalyptus globulus. Oecologia 117, 312–322.
| Spatial and temporal variations in phloem sap composition of plantation-grown Eucalyptus globulus.Crossref | GoogleScholarGoogle Scholar | 28307910PubMed |
Percival MS (1961) Types of nectar in angiosperms. New Phytologist 60, 235–281.
| Types of nectar in angiosperms.Crossref | GoogleScholarGoogle Scholar |
Petit S, Rubbo N, Schumann R (2011) Nectar collected with microcapillary tubes is less concentrated than total nectar in flowers with small nectar volumes. Australian Journal of Botany 59, 593–599.
| Nectar collected with microcapillary tubes is less concentrated than total nectar in flowers with small nectar volumes.Crossref | GoogleScholarGoogle Scholar |
Power EF, Stabler D, Borland AM, Barnes J, Wright GA, Durand P (2017) Analysis of nectar from low-volume flowers: a comparison of collection methods for free amino acids. Methods in Ecology and Evolution 9, 734–743.
| Analysis of nectar from low-volume flowers: a comparison of collection methods for free amino acids.Crossref | GoogleScholarGoogle Scholar | 29938013PubMed |
Ruhlmann JM, Kram BW, Carter CJ (2010) CELL WALL INVERTASE 4 is required for nectar production in Arabidopsis. Journal of Experimental Botany 61, 395–404.
| CELL WALL INVERTASE 4 is required for nectar production in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 19861655PubMed |
Shahidzadeh-Bonn N, Rafai S, Azouni A, Bonn D (2006) Evaporating droplets. Journal of Fluid Mechanics 549, 307–313.
| Evaporating droplets.Crossref | GoogleScholarGoogle Scholar |
Shenoy M, Radhika V, Satish S, Borges RM (2012) Composition of extrafloral nectar influences interactions between the myrmecophyte Humboldtia brunonis and its ant associates. Journal of Chemical Ecology 38, 88–99.
| Composition of extrafloral nectar influences interactions between the myrmecophyte Humboldtia brunonis and its ant associates.Crossref | GoogleScholarGoogle Scholar | 22234428PubMed |
Simpson BB, Neff JL (1981) Floral rewards: alternatives to pollen and nectar. Annals of the Missouri Botanical Garden 68, 301–322.
| Floral rewards: alternatives to pollen and nectar.Crossref | GoogleScholarGoogle Scholar |
Staab M, Methorst J, Peters J, Blüthgen N, Klein AM (2017) Tree diversity and nectar composition affect arthropod visitors on extrafloral nectaries in a diversity experiment. Journal of Plant Ecology 10, 201–212.