Controls on myxomycete species and species assemblages
Peter Wellman
17 Warragamba Avenue, Duffy, ACT 2611, Australia. Email: wellmanp@iinet.net.au
Australian Journal of Botany - https://doi.org/10.1071/BT20118
Submitted: 11 September 2020 Accepted: 30 November 2020 Published online: 22 January 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
This paper uses data from previous worldwide myxomycete surveys to determine the controls on the occurrence of myxomycete species, and on species assemblages. The main findings are as follows. The effect of substrate pH can be modelled, in that each species has a preferred pH value relative to the mean of a survey; errors from the model are 0.2 pH units. The substrate physical properties, evaluated by subjective hardness, showed no correlation with pH measurements. Hence, myxomycete species seem to have distinct ecological niches in substrate, with preferred pH and preferred physical properties. Comparison of the species found from the liana stem substrate shows that the species association does not change within angiosperm forests. Further, the species association is the same as that found in other angiosperm litter substrates: twigs on trees or on the ground, and leaves. This and a previous finding are consistent with similar ecological environments around the world having the same myxomycete species association within sampling error. In mixed angiosperm forests around the world the pH of un-decayed wood is ~4.9, and for decayed wood and tree litter is ~6.5 in tropical latitudes, and ~5.5 at 35° latitude, so on decaying the change in pH varies with latitude.
Keywords: acidity, latitude, myxomycete, pH, plasmodial slime mould, species assemblages.
Introduction
Myxomycetes (plasmodial slime moulds) are single celled organisms in the phylum Amoebozoa. Their life cycle has two feeding stages; a relatively small uninucleate phase, and a larger multinucleate plasmodium. The life cycle ends with a fruiting body which contains spores. When there is moisture the myxomycetes feed mainly on bacteria, but otherwise they survive as dormant stages (microcysts, sclerotia, and spores). There are ~1000 species (Stephenson and Rojas 2017), with almost all species identified from their fruiting bodies in terms of a morphological species concept. A summary of our present knowledge of myxomycetes is provided by Stephenson and Rojas (2017).
Myxomycetes can be obtained in the fruiting stage either by collecting naturally occurring fruiting bodies in the field, or by collecting substrates with resting phases and cultivating the substrates. It is not practical to address most of the subjects discussed in this paper by using the fruiting bodies collected in the field, because where myxomycete fruiting bodies are found is not necessarily where they have fed. The moist culture method of cultivation is relatively easy: it can be applied to any organic matter; a sample can be collected over a large part of the year; the relation between the number of fruit and the feeding source is known; and the number of common species on a single type of substrate is ~30, so a survey to determine the relative abundance of the common species of a type of substrate needs only ~30 diverse samples if five Petri dishes are grown per sample (data of Wellman 2019; this paper).
A large number of myxomycete moist-culture surveys have been carried out. In a great majority of the surveys the objective was to obtain a listing of the species on one or more substrates, particularly the rare species. In many cases the surveys included an analysis of the completeness of the species list. Some of the major surveys have also recorded environmental parameters and analysed the correlation within the survey area between the myxomycete species and the various environmental niches (e.g. Schnittler 2001). There have been many comparisons of species assemblages using measures of diversity. Previous papers by Wellman (2016, 2019) have tackled some general topics using Australian data from the bark substrate: the number of species associations over Australia; the nature of the species association boundaries; the effects of pH on the productivity; the variation over Australia of productivity and family dominance; and the typical growth and decay over four weeks of a large mixed myxomycete ‘population’. This paper uses both Australian and overseas data to investigate other general myxomycete ecological topics which affect myxomycete species. These topics include: the extent of species associations around the world, the effects of pH and substrate physical properties on a species, and the average change of substrate acidity with latitude.
The study of myxomycete growth controls is important both to understanding myxomycetes (plasmodial slime moulds), and, because the cultivation of myxomycetes is so easy, to providing important pointers to growth controls in other simple organisms which are more difficult to study. The related cellular slime moulds (Distyostelia) are the model organism for investigating the workings of a single cell.
Materials and methods
The studies in this paper were initiated by a desire to compare the myxomycete species assemblage on Australian temperate liana substrates with the assemblage on tropical liana substrate. A temperate liana substrate survey was carried out as follows. The samples were collected near the coast in eastern New South Wales (NSW) at different months during the years 2014–2018. They were selected from five areas over seven degrees of latitude to provide variety in location. They were taken from 14 liana species and a wide range of families to provide variety in substrate pH and bark texture. In all species a section of the woody stem showed numerous wide water conduits. The liana samples were collected during a long period of dry weather, so the myxomycete material should be in a resting phase when collected. The material cultivated for myxomycetes was liana stem sections ~20–45 mm long (bark and wood). Most of the stem material was of small diameter so the stem was cultivated whole, but a few stems, with a diameter >8 mm, were cut lengthwise, and the half sections were cultivated with the bark uppermost. Myxomycete fruit were obtained by the moist culture method (Stephenson and Stempen 1994). For each sample five Petri dishes were cultivated, each dish 90 mm in diameter and 10 mm high. Filter paper was placed at the base of each Petri dish, and sections of the stem were placed on this with the stem sides touching, to fill the Petri dish. In order to obtain as many myxomycete species as possible in each Petri dish, the pieces of stem used sampled the range of stem diameter, degree of decay and bark texture. For each selected stem a portion was placed in each Petri dish. The Petri dishes were half filled with ‘distilled’ water, left for 24 h and then the water was drained. The Petri dishes were then maintained at ~22°C in diffuse natural light. Every week the dishes were inspected, mature fruiting bodies removed for drying and study, and Petri-dish moistness was maintained by adding drops of water. Any pieces of substrate with significant fungus was discarded. The duration of cultivation was 6–8 weeks. This procedure aimed at getting a large diverse species assemblage and a large number of fruiting bodies so the relative productivity of species could be better determined. The myxomycete fruiting bodies were identified mainly using Ing (1999), Poulain et al. (2011) and Discover Life (see http://discoverlife.org). Appendix 1 lists for each sample the sample number, location, liana species, mean stem diameter and, when known, the pH of the stems. Appendix 2 lists the myxomycete species found, and for each species lists the records – sample number and the number of fruiting bodies.
The new pH measurements reported in Appendix 3 were made on four types of myxomycete substrates: liana stems; partly decayed twigs on trees; decayed wood and non-decayed wood. The samples were collected from indigenous and exotic trees growing in the city of Canberra (35°S, south-east Australia). The exotic trees originated in other temperate climates. Small pieces of the samples materials were placed in small plastic cups, 20 mm of de-ionised water was added, the cups were left for 24 h, and then the pH of the water was determined by a pH meter that had been calibrated by standard solutions.
Results
Geographic extent of litter-substrate species assemblages
The aim of this study was to obtain the myxomycete species assemblage for temperate rainforest liana stems, so the species found could be compared with nine assemblages previously reported on liana in the tropics. The results of the 10 available surveys are listed in Table 1 as columns 1–10, with the temperate survey as column 3. The main difference in species association between the temperate survey and elsewhere is that in NSW Physarum oblatum is common, and the Stemonitis fusca is subspecies fusca not nigrescens. The NSW data does not record Comatricha tenerrima (which apparently does not occur in Australia), or Perichaena dictyonema and Physarum didermoides. These differences are minor. Column 3 was from the temperate forest reported above, column 1 was sampled well above the ground at the very top of the rainforest, column 9 samples a cloud forest at 1300–2700 m altitude with liana stems having a thick epiphyte covering, and in other surveys the samples were collected close to the ground near sea level. After allowing for the effect of sampling errors, there appears to be no difference between the species assemblages of these 10 surveys; that is, the surveys are consistent with a liana myxomycetes species association being uniform across angiosperm closed rainforest. If we take the species which occur on three or more surveys as forming the liana species assemblage, then this assemblage consists of 22 species, and they occur with an abundance of 6–160 parts per thousand (‰) This species assembly is listed in Table 1, column 19.
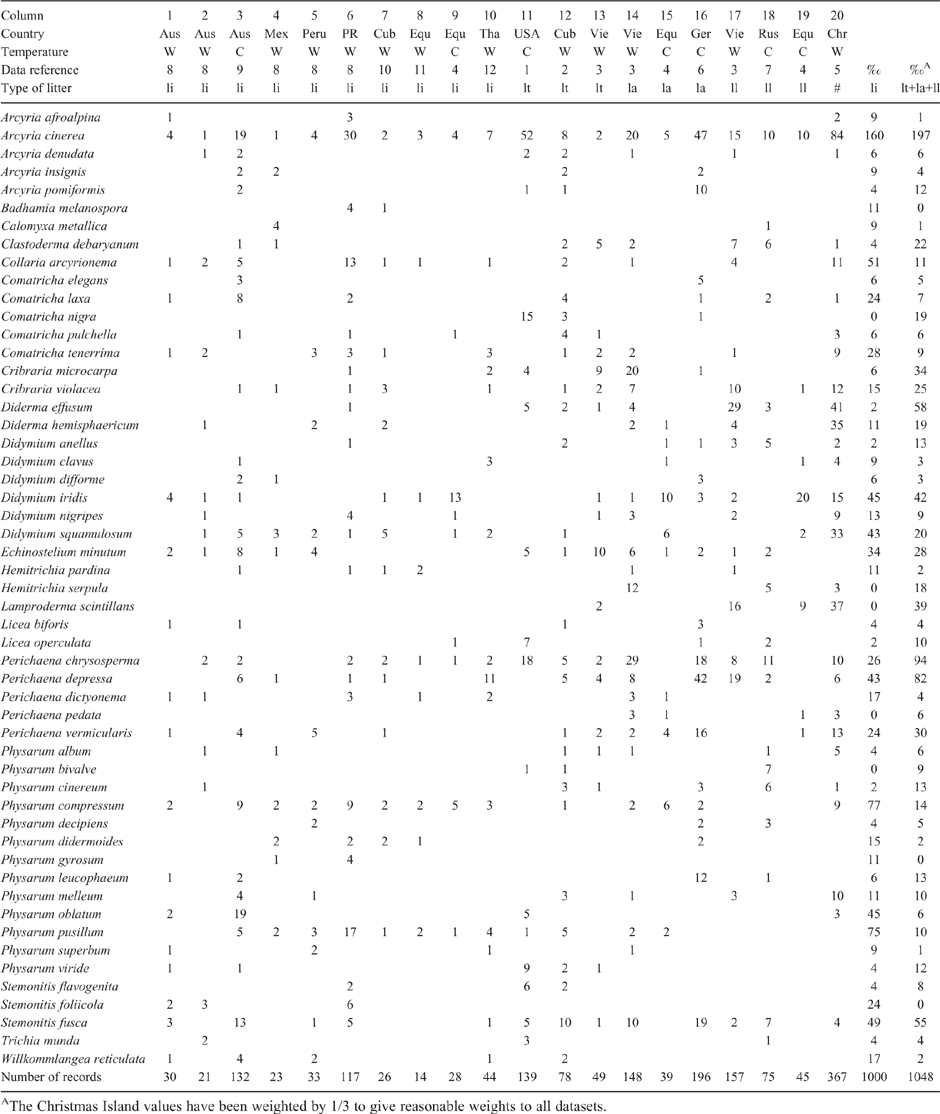
|
Table 1, columns 1–20 list the published myxomycete species assemblages for four types of litter substrates in angiosperm forests: dead liana stems on the vine; dead twigs on the ground; dead twigs still on the tree or shrub, and mainly dead leaves on the ground. If there are any differences between the species assemblages of the litter types, or at any particular location, then these differences should show on the table. The table does not list species occurring in total fewer than three times because these species are too rare to help in any comparison. Column 20 of the table shows the mean species assemblage for the additional three litter types combined. The dataset is not perfect, and interpretations of a species assemblage have to take into account of a species having no records, or a very large number of records due to the following factors. For some surveys the number of records is small relative to the number of species being considered, so strictly the survey is not large enough for the purpose. Some continents do not contain some of the species on the list, explaining a survey’s absence of a record. The sample area may be in a ‘swarm’ of one species, so the numbers of one species may be very large. In terms of a random sample some surveys err in collecting substrates of different tree species only in a small area, whereas other surveys err in collecting from many areas, but from too few substrate tree species.
In general, there is an excellent consistency between all surveys and the four types of litter, with most of the species listed in Table 1 being part of an angiosperm litter species association. Species that are part of this species association generally occur reasonably randomly across the table, and their abundance is reasonably consistent. This angiosperm litter species association incorporates the previously published assessments for liana stems (Wrigley de Basanta et al. 2008), and twigs (Cedeño et al. 2014). Hence it seems likely that there is only one myxomycete species association on angiosperm forest tree litter – that defined by Table 1. Complementing this is the conclusion of Wellman (2017) that for temperate angiosperm forests the myxomycete species associations on dead wood were similar for similar climate areas – for Australia, New Zealand, Patagonia, Britain and Spain.
There is an apparent inconsistency between the conclusion that worldwide the angiosperm closed-forest litter types have a single species association, and the conclusion of Walker (2016) that the litter types within one myxo survey have different species assemblages. Walker cultivated 288 samples of both leaf litter and small woody debris. The 10 most abundant species for each substrate had six species in common between the substrates, and four species each not in common between the substrates, so she concluded that there was a difference in species between the two substrates. However almost all these 15 selected species are members of the litter species association of Table 1, so they are found in both leaf litter and woody debris worldwide. Everywhere the separation of species into substrate type is likely to be influenced by the local pH and substrate physical effects discussed later in the paper.
This conflict can be summarised as follows. It is generally accepted that in a survey the different substrates give different species assemblages. Presumably one can get a better mean of the substrate’s species association by adding the species occurrences in many surveys. However, when we do this the mean assemblages of the various substrates are the same within experimental error. Something is wrong. The error is thought to be in assuming that for surveys in two places, with different trees, there is the same division of species between substrates.
Myxomycete species and substrate acidity
For a long time it has been known that substrate acidity (pH) is an important control on which myxomycete species is present in moist culture studies (e.g. Härkönen 1977). However, the details of the pH effect have not been studied. This is in part because the form of the relationship has not been known, and in part because previously there was not enough survey information available to form the basis for a pH study. To study the effect of pH we need a range of surveys, large and small, from wet to arid climate, the surveys having at least some species in common. To be useful, the surveys should have at least six species with six or more records, so that for these species the mean pH is known with reasonable accuracy. Within each survey there can be a mixture of substrates (bark, litter), the pH distribution should approximate a normal curve, and if the survey is over a large distance it will only be useful if there is little change in the mean pH with distance. All large surveys were considered for inclusion in this analysis but many could not be used because the pH values were not reported in a useful form (pH range is not useable).
The surveys used in this analysis are as follows.
-
A survey of myxomycetes found on bark on several traverses across Australia (Wellman 2019). This survey is of much wider extent than other surveys, consequently the mean pH of sections of the traverses changes along the traverses. This effect has been corrected by adjusting the pH within some parts of the traverses. Records in the Tropical Species Association have been adjusted by 1.0 pH units, and records in the Tropical/Northern Arid Species Association transition have been adjusted by 0.54 pH units.
-
A survey in the winter cold desert of western Kazakhstan (Schnittler 2001).
-
A survey in the arid climate Volga River Basin in Russia (Novozhilov et al. 2006). The reported mode pH of each species was used, not a mean.
-
A survey in the monsoon lowland tropical rainforest in southern Vietnam (Novozhilov et al. 2017a).
-
A survey in the temperate deciduous forest in Germany (Schnittler et al. 2006).
-
A survey in the tropical forests in eastern Mexico (Lado et al. 2003).
-
A survey of liana stems from tropical rain forests in a variety of countries (Wrigley de Basanta et al. 2008). The mean pH of all liana substrates was similar in the various areas.
-
A survey in the tropical forests of Christmas Island in the north-east Indian Ocean (Stephenson and Stephenson 2019). The analysis does not use substrates where the description mentions palms, Pandanus, or seed pods as these have very different pH from the pH range of other substrates.
-
Surveys in Twin Creeks and Baskin localities within the southern Appalachian Mountains, USA (Stephenson SL, unpubl. data).
-
A survey in Norfolk Island, in the western Pacific Ocean between New Zealand and New Caledonia (Stephenson and Stephenson 2020).
Surveys 2–7 were analysed using information within each published paper, whereas surveys 1 and 8–11 were analysed using unpublished spreadsheets of the survey records. The surveys range from large to small in number of samples and area, and range from rain-forests within the tropics to mid-latitude, wet forests and arid regions at mid latitudes where the dominant plant is grass or scrub. The surveys used one or more of a wide range of substrate types. A paper by Lado et al. (2011) with mainly cacti and succulent substrates was not used in this study because of suspected change in the mean substrate pH over the survey, due to both the large range in altitude of the substrates, and to some collections being from internal drainage areas with alkaline soils.
The surveys were analysed as follows. A spreadsheet was made with one column giving the myxomycete species, and two columns for each survey. The two columns give, for each species found in that survey with more than 6 records, the number of records and the mean pH of the record’s substrate. Only species which have a mean pH available for two or more surveys are listed in this table. The eleven surveys had a mean pH of all records varying from 5.2 to 7.7. First, for each survey a constant (survey pH6.0) was added to the species’ mean pH values to adjust the mean survey pH value to 6.0. Then a mean pH for each species was calculated (species pH6.0). These preliminary estimates of survey pH6.0 and species pH6.0 were then adjusted alternately to minimise the residuals. Table 2 gives the final model: survey pH6.0 corrections, the species pH6.0 values, and the residuals of the model. The residuals on the spreadsheet have a standard deviation of 0.2 pH unit. The strength of the above analysis depends greatly on a few species that are relatively common. Species that were observed on four or five surveys are Arcyria cinerea, Echinostelium minutum, Macbridela oblonga, Perichaena corticalis, Perichaena depressa, Perichaena vermicularis, and Stemonitis fusca. Table 2 shows that the survey pH values standard deviation (s.d.) is relatively large in surveys 1 and 5 (s.d. 0.86, 0.84) and is relatively small in survey 10 (s.d. 0.51), but there is no change in the spread of species pH6.0 values between Arcyria cinerea and Perichaena vermicularis. Hence Table 2 is consistent with species pH6.0 being constant within sampling error with varying standard deviation in survey pH values.
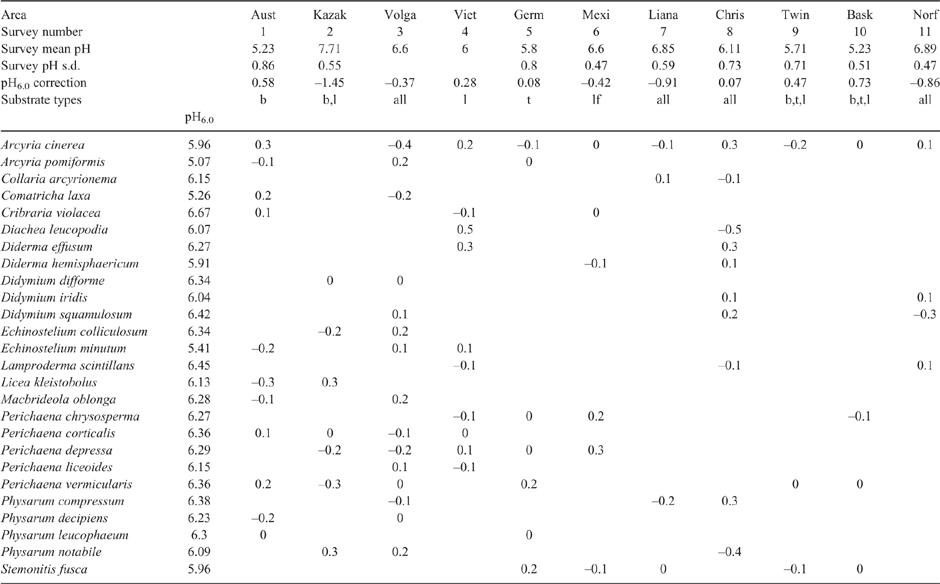
|
Table 3 is a longer species list that includes species with a less accurate species pH6.0 estimate. This list has been derived from a spreadsheet of the 11 surveys with all species with five or more records and their pH6.0 values. The species mean pH6.0 values listed are of four accuracies; the most accurate values are derived from two or more surveys each with 6 or more records (Table 2), less accurate values are from one survey with 10 or more records, still less accurate values from one survey with 6 or more records, and the least accurate from one survey with only 5 records. The species pH6.0 values are most accurate if there is more than one survey used in the calculation. If there is only one survey used then the accuracy decreases fast with fewer records, by 1/√n, where n in the number of pH records. In order to understand the significance of the species pH6.0 values the species have been listed in the systematic order of Leontyev et al. (2019), using subclass, order and family.
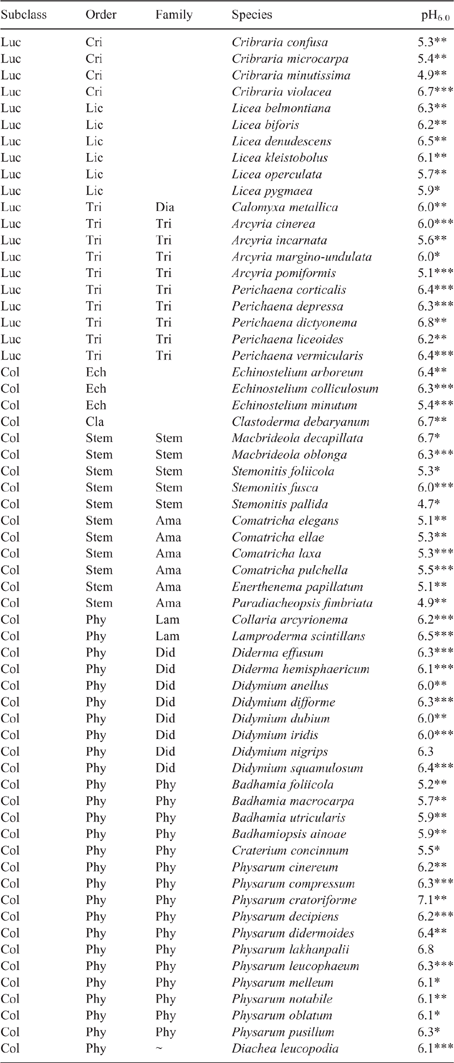
|
From the published literature one would anticipate that species having lime in the fruiting body (most of the Order Physarales) would prefer more alkaline environments than species without lime in the fruiting body. However what is found in Table 3 is a different pattern. Species with lime in the fruiting body have a pH6.0 that is slightly below 6.0 (Badhamia) or above 6.0 (Didymium, Physarum), and species without lime in the fruiting body also have a preferred pH6.0 both above and below 6.0. The grouping of similar pH6.0 seems to be more a characteristic of genera, with many genera having a narrow range in pH6.0; with Perichaena having values of 6.2–6.8, Didymium 6.0–6.4, Badhamia 5.2–5.9, Physarum 6.1–7.1 and Comatricha 5.1–5.3. Genera listed with a reasonable number of species but with a greater range of pH6.0 above and below 6.0 are Cribraria, Licea and Arcyria. It seems unlikely that the species pH6.0 numbers in Table 3 are random, and the model that best fits the information is that species in the same genus tend to have a similar pH6.0 value. This is likely to be because of inheritance; that is because they have inherited the same gene.
The major control on the mean survey pH of Table 2 is not clear. It could in part be soil pH, because the two highly alkaline mean surveys pH are in cold deserts (surveys 2 and 3 of Table 2). However the other values of mean survey pH show little correlation with the map of alkaline and acid soils derived from soil aridity (Slessarev et al. 2016; Wikimedia 2020).
A budget of pH variance
Another method of expressing the control of substrate pH on the distribution of myxomycetes is to subdivide the pH effects into a pH budget - that is quantifying the various pH effects. The estimates of the effects have been derived from data given in published papers, and spreadsheets of records as follows. The pH mean scatter of one substrate at one site has been calculated using the pH measurements in three Petri dishes from the triplicate site samples in Stephenson and Stephenson (2019) (and also in Baskins survey and Gaudineer survey, Stephenson SL, unpubl. data) using species record spreadsheets. This gives a s.d. of generally 0.2–0.35 pH units, where the lower values are probably in cases where collected substrate material is from few tree species, and higher values from many tree species. Within a survey the variation of pH within one species has (for surveys 1 and 8 using spreadsheets) a mean s.d. of 0.60 pH units. The differences between the mean pH6.0 of the myxomycete species is calculated from Table 2 as a s.d. of 0.37 pH units. The effects of physical (and other chemical) factors of the substrate is given by the residuals of the mean pH in the model of Table 2, with a s.d. of 0.20 pH units. The total variation in pH measurements within a large survey is between 0.5 to 0.8 pH units (surveys 1, 8, 10–12 using spreadsheets). The variation in mean pH of surveys (from Table 2) has a s.d. of 0.76 pH units, but the true World value is likely to be higher as not all localities and substrates in the World were sampled.
Table 4 lists the pH budget. The effects are also expressed as variances, because the variances can be added to get the combined effect. The variance is the square of the s.d. Within a survey the total variance calculated from the components approximately equals the total variance calculated directly from all the survey pH values treated as one population. Within a survey, the main variance is from the pH variation within a species. The control on the species is only understood if there are sufficiently records for each species such that the mean pH can be better defined. A species pH measurements have a s.d. of 0.60, so if there are six records for a species then the species pH mean value has a s.d. of 0.60/√6 = 0.25. Then, considering the species means (not measured values), the differences between the species mean pH (with a s.d. of ~0.37) are the dominant control on the pH pattern observed.
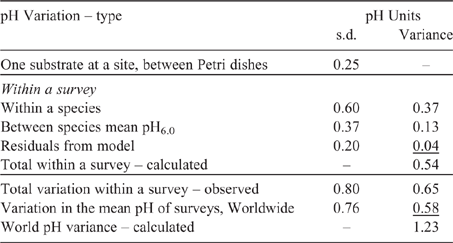
|
Not all myxomycete surveys will give results consistent with the above budget.
-
The surveys selected must have pH values with a reasonable spread (over 2 pH units). If substrates of only a few tree genera are sampled then the preferred mean pH of the myxomycetes species will be constrained to be less than the spread of the tree’s pH.
-
In many surveys the distribution of substrate pH is a broad single peak, approximating a normal distribution. Substrates forming a secondary peak in the pH distribution (such as palms or Pandanus) have to be excluded from an analysis if you are going to assume a normal distribution.
-
The mean pH of the survey cannot vary significantly across the survey, or must be corrected for. In this respect the Australian survey used in this analysis has not really been adequately adjusted to one mean pH value, but the adjustment was the best available with sample spacing of 30–50 km across a continent.
Substrate frequency against rank
Information about myxomycete species apparent substrate preference can be summarised in tables showing a list of myxomycetes species, and for each species a list of the substrate tree genera and the number of records for each host genus ordered by rank. The potential information can be divided into the numeric information of such a table (the shape of the relationship of substrate frequency against rank ignoring the tree names), and the tree name information with or without substrate physical properties. This section discusses the numeric information. This numeric information can be derived from the results of any myxomycete survey, the important question is whether the information is informative, or only reflects random processes.
A common myxomycete species always occurs most commonly on several tree species substrates and less commonly on many other substrates. What is given here is a model quantifying the effect of chance on substrate selection. The data used here is from a uniform, large dataset: the raw data used to prepare table 2 in Wellman (2019) for myxomycetes on bark collected from trees growing across Australia. Plotted on the y-axis of Fig. 1 is the log of the number of records of a tree genus substrate, and plotted on the x-axis is the log of the rank of the tree genus. Tree genera are ranked with the genus with the maximum number of records having a rank of one, and the genera with only one record having the highest rank. As the data is noisy the myxomycetes species that have the same number of tree genera as substrate have had their data averaged, so lines are shown for the average relationship for 3, 4, 5, 8, 9 and 12 tree genera, with the number of myxomycete species data averaged being 5, 6, 4, 4, 2 and 3 respectively. When allowance is made for experimental error, the lines are approximately straight and parallel, with mean slope of ~1.1. Importantly, there is no evidence for the lines having a change in slope part way across the figure. This is consistent with a single power law relationship between the number of records of a host tree genus and the rank of this number. In particular, there is no obvious surplus or deficit of tree genera with single records. Hence, the data are consistent for each myxomycete species, with the number of records for each host being determined by one sampling relationship – a random sampling of substrates In particular there is no evidence for myxomycetes growing poorly on less common substrates. Although Fig. 1 is consistent with a single model of abundance against rank, it does not prove that there is a single straight-line relationship. A model with two straight lines of slightly different slope needs a very large amount of data to be evident, and for each myxomycete species we only have a little data. Table 2 is consistent with there being a lot of chance in where myxomycete fruit are found.
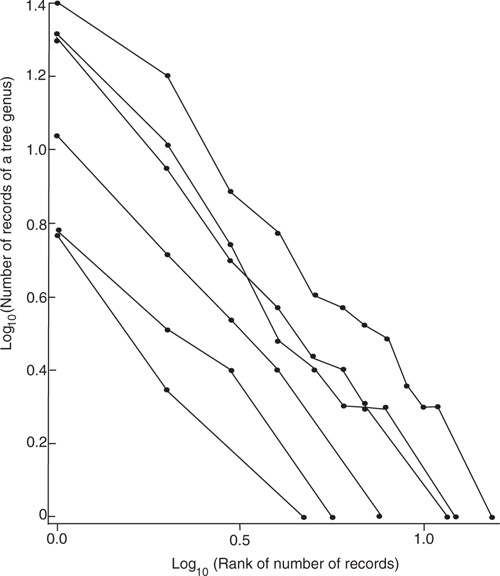
|
The sampling of the bark samples is really not random, in that the sampled substrates do not have an equal chance of being selected. Some substrates are preferentially sampled because they give many myxomycete species or are common; other substrates are rarely sampled because they are marginally suitable or uncommon. The statistical relationship of Fig. 1 seems to be robust even with non-random sampling.
A possible difference between common and less-common substrates can be investigated another way. If we take the data for 57 sites of Arcyria cinerea in the Wellman (2019) record list then the most common substrate for Arcyria cinerea is on Eucalyptus of ‘coolabah’ type bark (37% of sites). Eucalyptus with stringy bark and Corymbia are next most common, and the remainder of the less common substrates with 3, 2 and 1 sites per tree genera form 37% of the sites. When the most common substrate is compared with the less common substrates they have the same average number of fruiting bodies (21), and nearly the same average ratio of ‘A. cinerea fruit volume /total sample myxomycete fruit volume’ of 0.27 compared with 0.25. Hence this small sample is consistent with A. cinerea being equally productive on the substrate of trees on which it is common, as those on which it is rarely found.
A related question is whether a tree that is exotic to the area can get suitable myxomycete spores from elsewhere and have normal myxomycete productivity. Wellman (2019) mentioned that very isolated exotic trees in the arid inland of Australia had small myxomycete productivity. Samples of seven isolated trees exotic to the area gave (for five Petri dishes) a spore harvest with a mean of 1.8 mm3, and number of species with a mean of 3.4 species. This is much smaller than the mean harvest for arid Australia: a spore harvest of ~5 mm3, and number of species of 6. However the number of exotic trees sampled is small, so the lower productivity may be due to chance. A confirmation of this lower productivity of myxomycetes on locally exotic trees in arid areas is required. The colonisation of isolated exotic trees is in part addressed by the habitat colonisation model of Schnittler and Tesmer (2008), looking at the ‘island’ effect on myxomycete species numbers.
Physical properties of substrates
The dominant myxomycete species growth requirements in terms of physical properties of the substrate can be determined if we can obtain numerous large surveys with the critical physical properties of the substrates. Unfortunately, this information is not available at present. However, some of the issues are discussed below by looking at a single survey, with the substrate tree names and a limited amount of information about the physical properties of the substrate.
The dataset used is again the Wellman (2019) survey of myxomycetes on bark in Australia. The samples are from a very large area of arid land and the dryer parts of the non-arid areas. Only thick, old and weathered bark was sampled. For this survey the collected substrates fall into three major groups: 1. Acacia has hard and massive bark. 2. The eucalypts (Eucalyptus, Corymbia and Angophora) have variable bark but those collected were mainly soft, but with some of medium density. 3. The numerous remaining tree species mainly had medium density and medium porosity bark, but a few are hard or soft. There are 298 records of Acacia, 544 records of eucalypts, and 512 records of other tree species. Table 5 (from table 2 of Wellman 2019) gives a list of myxomycete species, and for each species the total number of records, and the substrate tree genera listed in order of the number of records. One tree genus, Eucalyptus, has been subdivided into 4 bark types. This procedure is very useful for future Australian surveys, as it directly relates myxomycete species to known Australian trees. However to people on other continents it gives no quantitative information on bark properties, and because the number of samples in the various genera or species groups is very uneven, the inferred myxomycete species groups of Table 5 are dominated by the common genera, hence any information analysis is biased.
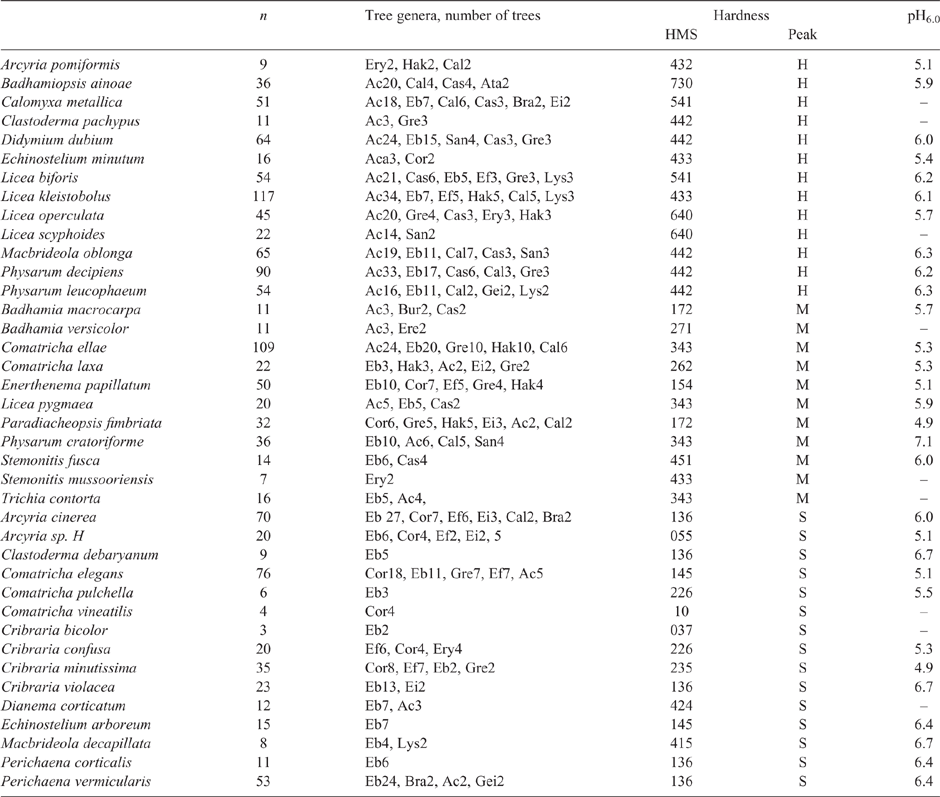
|
However, there is a way to use subjective information on the myxomycetes’ preference for substrates of different hardness. The relative hardness of the various barks has been subjectively estimated during a survey: in collecting the bark samples, in the cutting of the bark slivers for the Petri dishes, in the cutting out of myxomycete fruit, and through poking the bark by a sharp spike after cultivation. Note that most of these procedures indicate the hardness of dry bark. If the barks genera/species can be divided into hard, medium and soft, then we get a measure of the myxo species preference for the types of bark. This is given in the last column of Table 5 as a three-figure number. For Calomyxa metallica the number 541 means that 50% of the records were hard, 40% were medium and 10% were soft. The three digits give both a measure of the mean hardness observed in the records, and the observed spread in the hardness values. In theory, these proportions should be corrected to allow for the different numbers of hard, medium and soft substrates; however, the number of records for the various degrees of hardness was similar (hard 413 records, medium 503 records and soft 403 records), so no correction was made. In Table 5 these proportions have been used to subdivide the species into three groups based on whether the maximum number of records was in the hard, medium, or easy group. The two problems with the hardness reported in Table 5, is that the hardness used is subjective, and the mode hardness used has low reliability for those species with few records. Any future use of hardness should be quantitative, and use wet bark. Table 5 gives for the myxo species average hardness (peak of the subjective hardness) and the pH6.0 value. Importantly there seems to be no correlation between the hardness values and the pH values. This is consistent with myxomycete species (or groups of species) having distinct ecological niches, differing in both preferred pH6.0 and in preferred hardness.
It is quite possible that, other than pH, the substrate property that is most important to myxomycete growth is water holding capacity. There are two reasons for this: water holding capacity is a measure of the proportion of the internal substrate that is accessible by interconnecting voids which controls its access to food, and it is a measure of the length of time after rain that the myxomycetes can eat before the water evaporates and they transform to a resting phase. Fortunately, water holding capacity is relatively easily measured by weighing a substrate before and after soaking. Hardness or density are both techniques difficult to measure accurately for normal samples, particularly twigs and leaves. They are not good measures of suitability for myxomycete feeding because they are a measure of the proportion of air in a dry sample, not the proportion of the substrate that is accessible. It seems likely that there will be no major progress in further defining myxomycete species microhabitat until future major surveys are carried out with both chemical (pH) and physical properties (water holding capacity) of the substrates.
Two myxomycete species where substrate choice, other than pH, is more, or less, important can be identified by having raw pH values with a high standard deviation about their mean of 0.7 to 1.0 pH units. Acyria cinerea prefers the softer barks of Eucalyptus of the ‘coolabah’ type or Corymbia, even though neither of these substrates has a relative pH close to what it prefers elsewhere in the World. For A. cinerea soft bark is so important that in the driest part of Australia where there are no soft barks the species does not occur on bark. Licea kleistobolus in unusual in having no particular preference of substrate.
Myxomycete total spore productivity of different substrates
A measure of the myxomycete productivity in the New South Wales liana survey is the total spore volume harvested for the five Petri dishes of each sample. The average volume of the fruiting body of each species has been estimated, mainly using fruiting body dimensions of Poulain et al. (2011). The harvest for each sample is calculated by multiplying the number of fruiting bodies by this fruiting body volume, and the summing the volumes for all the species in the sample. Fig. 2b gives a histogram of the logarithms of these volumes. The curve is rather noisy because of the small number of samples. The majority of the volumes form a histogram peak with a high ~9 mm3, with a rather fat tail to 47 mm3, and a very long tail to small volumes. The curve is consistent with most samples giving a harvest forming a broad peak with a mode at about a 0.9 mm3, with about one-quarter of the samples giving low harvests due, it is thought, to the stems being unsuitable for bacterial growth. The histogram is very similar to that of bark samples from Western Australia (Wellman 2019) shown in Fig. 2a. As the shape of the curves are similar, it is likely that similar processes are controlling myxomycete productivity on liana twigs and dead bark on a living tree. The average mass of plant material in the Petri dishes differs between the two surveys, with a greater mass of bark material, whereas the surface area cultivated is very similar. Most pieces of bark have a thin, weak, grey outer layer, and a stronger darker inner part. It seems likely that the inner part of the bark samples is not sufficiently decomposed for bacteria, and that the similarity in productivity of the two materials is due to the similar surface area of the cultivated material.
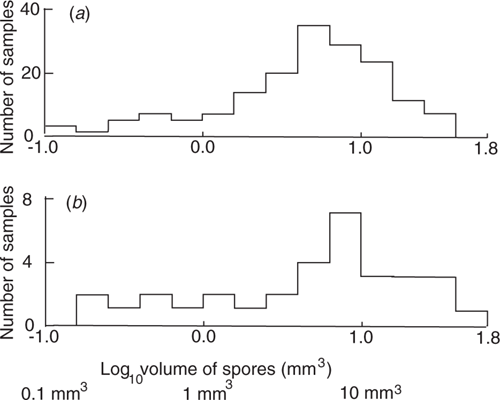
|
While the two histograms of Fig. 2 have a similar shape, the origin of the highest volumes is different. Wellman (2019) showed for the bark survey the samples with the highest productivity have an abnormally big difference between the total spore volumes of the species with the highest volume and the next highest volume. This is thought to be due to carry over of mass between feeding events, leading to a large ‘swarm’ event giving the highest volume. In contrast, in the liana survey the samples with the highest productivity have a normal difference between the total spore volumes of the species with the highest volume and the next highest volume, so their harvest is thought to be due solely to the last growing event.
Change in mean substrate acidity with latitude
Myxomycete (and fungal) species assemblages would be affected by systematic differences in mean acidity (pH) of the decayed plant material substrates, either a difference in the mean acidity of different substrates at one latitude, or a systematic variation of acidity with latitude. To study this there are a large number of measurements of acidity reported by myxomycete researchers, and measurements of timber pH. Table 6 lists the acidity measurements relevant to the present paper, both from previous publications and some new measurements. Within a myxomycete survey the pH measurements generally have good internal consistency, with a standard deviation of 0.4–0.6 pH units. The latitude attributed to the pH mean is the mean of the samples latitudes and 10° for tropical samples. A set of measurements in Finland (Härkönen 1977) was not used because of possible effects of acid rain. The accuracy of the mean pH values in Table 6 depends on both the number of measurements made (‘N’ in Table 6) and the standard deviation of the samples, and on the form of the real population – in part whether the samples are a random sample of the real acidity population, and in part whether some samples are less or more decayed.
|
Fig. 3 shows the data from all decayed substrates plotted together. Different substrates have different symbols. There is considerable scatter in the mean values derived from single surveys but the scatter of mean values forms a pattern. In the relation of mean pH with latitude there appear to be no systematic differences between the decayed substrate types (aerial liana, aerial twigs, bark, ground twigs, ground leaf litter, or ground logs). In this figure the average pH for all decayed substrates is 6.5 at tropical latitudes (23°N–23°S), and is 5.6 at ~35° latitude (6.52, with 2 × sdm = 0.18; 5.59, 2 × sdm = 0.29; sdm being standard deviation of the mean). The form of the mean variation of pH with latitude is not defined by the data points, but the model shown as a dotted line on the figure is possible - a constant value of 6.5 in the tropics, with a linear decrease with higher latitude. The measured pH values have a variance about their mean of 0.49, and a variance about the model line on Fig. 3 of 0.24, so the model line explains about one-half the scatter of the observed pH measurements.
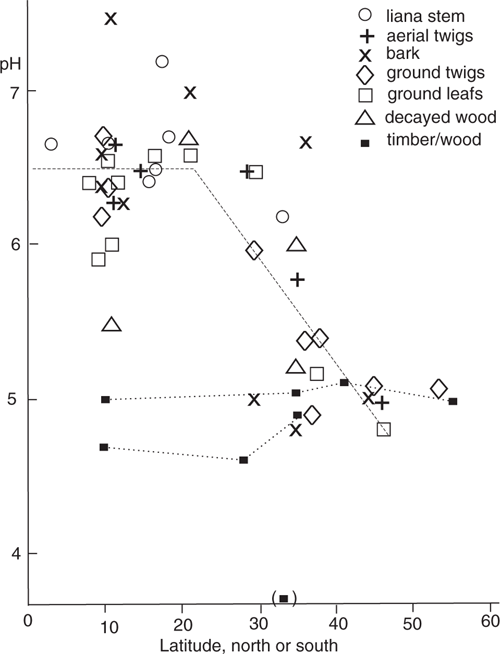
|
It is interesting that the bark substrate gives a similar pH to the other substrates. Bark samples in Australia collected for myxomycete substrates are of two types. For a minority of trees (those with bark similar to Eucalyptus coolabah) the bark is uniformly decomposed for the first few centimetres, as judged by uniform colour and texture. These are the ones that can be invaded by very fast-growing fungi when water is added. Most suitable samples of dead bark for myxomycete cultivation the bark samples have a thin (1–2 mm thick), lighter, outer layer of much decomposed bark, on a thicker layer that is darker and appears to be little decomposed, although dead. From appearance one would think that this thicker layer was less decomposed than aerial and ground litter. However, the mean-pH data points on Fig. 3 for bark have the same pattern as those for aerial and ground litter, and are broadly consistent with a mean pH of 6.5 within the tropics, and a mean pH of 5.6 near 35° latitude.
To discuss the origin of the variation in pH of decayed tree litter a set of measurements on non-decayed angiosperm timber/wood is used. Timber industry measurements for Australia (in the southern hemisphere) are reported by Bootle (1983) and measurements for the northern hemisphere collated by Tetréault (1999). These reports show that the pH of most sampled trees is in the range of 3 to 7. Lachowicz et al. (2019) shows that the variation in pH within and between adjacent forest stands of one species is less than 0.5 pH units, and Hernandez (2013) shows that the wood within one tree has a variation of ~1 pH unit. The Bootle (1983) and Tetréault (1999) report a range of pH for some species, the mode of these ranges is 0.8 pH units for both datasets. These four papers are consistent with the pH of one tree species generally having a range of less than ~1.8 pH units. A dataset of angiosperm timber pH has been constructed as follows. The Bootle (1983) data has been divided into three datasets: tropical imported timber, Australian rainforest timber from southern Queensland and northern New South Wales, and Australian Eucalyptus timber south of ~30°S. The Tetréault (1999) data has also been divided into three datasets: tropical trees, trees south of 50°N and trees north of 50°N. The two papers include some of the same Tropical timber species. The latitude attributed to the samples is the mean of the species distribution area for non-tropical timber species, and has been put as 10° for tropical timber species. The Canberra dataset of Appendix 3 consists of one collection of local indigenous trees, and one of temperate exotic (northern hemisphere) trees. All these estimates of the mean pH for timber/wood are listed in Table 6 and plotted in Fig. 3. A sample with only one genera (Eucalyptus) has a mean of 3.7; this low value is presumably characteristic of the genus, and this data point is not discussed further. The majority of the timber/wood mean values listed are for mixed genera, and these have a pH range of only 4.6–5.1, and a mean of ~4.9. The samples range in latitude from the equator to a latitude of 55°, so there is no correlation of pH and latitude found for timber/wood for mixed species samples. The simplest explanation for the correlations between pH and latitude differing between un-decayed materials (timber/wood) and decayed materials (litter, bark and rotten wood), is that the decay of the organic material resulted in a change in pH, with the amount of change varying with latitude.
Conclusions
For surveys where the substrate is liana stems in closed forests, there were no significant differences in myxo species assemblages on substrates in forests with different altitudes above sea level, tropical or temperate climates, or collections from near ground to tree top level. Further, there were no significant differences between the species assemblages from all the major types of litter substrate in closed angiosperm forests. Similarly, Wellman (2017) found that the myxomycete species associations collected by field surveys on wood in closed temperate forests in the southern hemisphere and in the UK and Spain had the same species in the same relative abundance. These observations are consistent with a model of similar ecological environments around the world having the same myxomycete species association within sampling error.
The pH data from numerous published myxomycete surveys can be modelled, such that the mean pH of a species in a survey gives a residual from the model of 0.2 pH unit. As a consequence of this model the various myxo species preferred pH relative to a standard survey mean of pH of 6.0 (pH6.0) can be determined. The preferred pH of the myxomycete species does not correlate with the presence of lime in the fruiting bodies, but is generally constant within genus.
Using subjective measures of hardness the Australian barks can be divided into hard, medium and soft, and for each myxomycete species the average hardness of its records can be determined. There is no apparent correlation of pH6.0 and average hardness values. This is consistent with the myxomycete species differentiating in ecological niche both by pH6.0 value, and preferred hardness. A fuller understanding of the controls on myxomycete species occurrence would appear to come not from the substrates name or frequency but from the measurement for each sample of the chemical (pH) and physical properties, where the preferred physical property is water holding capacity.
In mixed angiosperm forests the (not-decayed) timber has a mean pH of ~4.9 at latitudes of 0–55°. The mean pH values for decayed timber, bark and various types of litter all have a large scatter. The average pH values of the decayed substrates do not differ significantly from one another, and they have a mean value of 6.5 in tropical latitudes and 5.6 at 35° latitude. The data is consistent with the angiosperm timber increasing in pH on decay, with the amount of increase being greater at low latitude.
Conflicts of interest
The author declares that he has no conflicts of interest.
Declaration of funding
The research did not receive any specific funding.
Acknowledgements
I thank the Australian National Herbarium for the use of laboratory facilities, and Christine Cargill and various librarians of the Australian National Botanic Garden for support. The pH studies were much facilitated by Steve Stephenson giving permission for me to use the record spreadsheets for published and unpublished surveys. I am grateful for the two reviews.
References
Black DR, Stephenson SL, Pearce CA (2004) Myxomycetes associated with the aerial litter microhabitat in tropical forests of northern Queensland, Australia. Systematics and Geography of Plants 74, 129–132.Bootle KR (1983) ‘Wood in Australia, properties and uses.’ (McCraw Hill Book Co: Sydney, NSW)
Camino M, Stephenson SL, Krivomaz T, Wrigley de Basanta D, Lado C, Estrada-Torres A (2008) Biodiversity survey for myxomycetes in the mountains of central Cuba. Revista Mexicana de Micología 27, 39–51.
Cedeño M, Clayton M, Stephenson SL (2014) Woody twigs as a microhabitat for myxomycetes in the upland forests of southwestern Virginia. Mycosphere: Journal of Fungal Biology 5, 673–680.
| Woody twigs as a microhabitat for myxomycetes in the upland forests of southwestern Virginia.Crossref | GoogleScholarGoogle Scholar |
Davison EM, Davison PJN, Brims MH (2008) Moist chamber and field collections of myxomycetes from the northern Simpson Desert, Australia. Australasian Mycologist 27, 129–135.
Härkönen M (1977) Coricolous myxomycetes in three different habitats in southern Finland. Karstenia 17, 19–32.
| Coricolous myxomycetes in three different habitats in southern Finland.Crossref | GoogleScholarGoogle Scholar |
Hernandez V (2013) Radiata pine pH and buffering capacity: effect of age and location in the stem. Maderas. Ciencia y Tecnología 15, 73–78.
| Radiata pine pH and buffering capacity: effect of age and location in the stem.Crossref | GoogleScholarGoogle Scholar |
Ing B (1999) ‘The myxomycetes of Britain and Ireland.’ (The Richmond Publishing Co. Ltd: Slough, UK)
Ko Ko TW, Stephenson SL, Hyde KD, Rojas C, Lumyong S (2010) Patterns of occurrence of myxomycetes on lianas. Fungal Ecology 3, 302–310.
| Patterns of occurrence of myxomycetes on lianas.Crossref | GoogleScholarGoogle Scholar |
Lachowicz H, Wróblewska H, Sajdak M, Komorowicz H, Wojtaan R (2019) The chemical composition of silver birch (Betula pendula Roth.) wood in Poland depending on forest stand location and forest habitat type. Cellulose 26, 3047–3067.
| The chemical composition of silver birch (Betula pendula Roth.) wood in Poland depending on forest stand location and forest habitat type.Crossref | GoogleScholarGoogle Scholar |
Lado C, Estrada-Torres A, Stephenson SL, Wrigley de Basanta D, Schnittler M (2003) Biodiversity assessment of myxomycetes from two tropical forest reserves in Mexico. Fungal Diversity 12, 67–110.
Lado C, Wrigley de Basanta D, Estrada-Torres A (2011) Biodiversity of myxomycetes from the Monte Desert of Argentina. Anales del Jardin Botanico de Madrid 68, 61–95.
| Biodiversity of myxomycetes from the Monte Desert of Argentina.Crossref | GoogleScholarGoogle Scholar |
Leontyev DV, Schnittler M, Stephenson SL, Novozhilov YK, Shehepin ON (2019) Towards a phylogenetic classification of the Myxomycetes. Phytotaxa 399, 209–238.
| Towards a phylogenetic classification of the Myxomycetes.Crossref | GoogleScholarGoogle Scholar |
Massingill JM, Stephenson SL (2013) Myxomycetes appearing in moist chamber cultures on samples of bark and wood collected from coarse woody debris. Mycosphere: Journal of Fungal Biology 4, 627–633.
| Myxomycetes appearing in moist chamber cultures on samples of bark and wood collected from coarse woody debris.Crossref | GoogleScholarGoogle Scholar |
McHugh R (2005) Moist chamber and field collections of Ecuador myxomycetes. Mycotaxon 92, 107–118.
Novozhilov YK, Zemlianskaia IV, Schnittler M, Stephenson S (2006) Myxomycete diversity and ecology in the arid regions of the Lower Volga River Basin (Russia). Fungal Diversity 23, 193–241.
Novozhilov YK, Erastova DA, Shchepin ON, Schnittler M, Alexandrova AV, Popov ES, Kuznetsov AN (2017a) Myxomycetes associated with monsoon lowland tropical forests in southern Vietnam. Nova Hedwigia 104, 143–182.
| Myxomycetes associated with monsoon lowland tropical forests in southern Vietnam.Crossref | GoogleScholarGoogle Scholar |
Novozhilov YK, Schnittler M, Erastova DA, Shchepin ON (2017b) Myxomycetes of the Sikhote-Alin State Nature Biosphere Reserve (Far East, Russia). Nova Hedwigia 104, 183–209.
| Myxomycetes of the Sikhote-Alin State Nature Biosphere Reserve (Far East, Russia).Crossref | GoogleScholarGoogle Scholar |
Poulain M, Meyer M, Bozonnet J (2011) ‘Les myxomycÈtes.’ (Fédération mycologique et botanique Dauphiné-Savoie: Sevrier, 2 volumes)
Rosing WC, Mitchell DW, Stephenson SL (2007) Corticolous myxomycetes from Victoria. Australasian Mycologist 26, 9–15.
Schnittler M (2001) Ecology of myxomycetes of a winter-cold desert in western Kazakhstan. Mycologia 93, 653–669.
| Ecology of myxomycetes of a winter-cold desert in western Kazakhstan.Crossref | GoogleScholarGoogle Scholar |
Schnittler M, Stevenson SL (2000) Myxomycete diversity in four different forest types of Costa Rica. Mycologia 92, 626–637.
| Myxomycete diversity in four different forest types of Costa Rica.Crossref | GoogleScholarGoogle Scholar |
Schnittler M, Tesmer J (2008) A habitat colonisation model for spore-dispersed organisms - Does it work with eumycetozoans? Mycological Research 112, 697–707.
| A habitat colonisation model for spore-dispersed organisms - Does it work with eumycetozoans?Crossref | GoogleScholarGoogle Scholar | 18495452PubMed |
Schnittler M, Lado C, Stephenson SL (2002) Rapid biodiversity assessment of a tropical myxomycete assemblage - Maquipucuna Cloud Forest Reserve, Ecuador. Fungal Diversity 9, 135–167.
Schnittler M, Unterseher M, Tesmer J (2006) Species richness and ecological characterization of myxomycetes and myxomycete-like organisms in the canopy of a temperate deciduous forest. Mycologia 98, 223–232.
| Species richness and ecological characterization of myxomycetes and myxomycete-like organisms in the canopy of a temperate deciduous forest.Crossref | GoogleScholarGoogle Scholar | 16894967PubMed |
Slessarev EW, Lin Y, Bingham NL, Johnson JE, Dai Y, Chadwick OA (2016) Water balance creates a threshold in soil pH at the global scale. Nature 540, 567–569.
| Water balance creates a threshold in soil pH at the global scale.Crossref | GoogleScholarGoogle Scholar | 27871089PubMed |
Stephenson SL, Rojas C (2017) ‘Myxomycetes: biology, systematics, biogeography and ecology.’ (Academic Press: London)
Stephenson SL, Stempen H (1994) ‘Myxomycetes, a handbook of slime moulds.’ (Timber Press: Portland, OR, USA)
Stephenson SL, Stephenson BC (2019) Distribution and ecology of myxomycetes on Christmas Island, Indian Ocean. Phytotaxa 416, 138–148.
| Distribution and ecology of myxomycetes on Christmas Island, Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Stephenson SL, Stephenson BC (2020) Biodiversity of myxomycetes on Norfolk Island, South Pacific Ocean. Cunninghamia 20, 115–121.
Stephenson SL, Urban LA, Rojas C, McDonald MS (2008) Myxomycetes associated with woody twigs. Revista Mexicana de Micología 27, 21–28.
Tetréault J (1999) Coatings for storage and display in museums. Canadian Conservation Institute Technical Bulletin 21, Department of Canadian Heritage, Ottawa, Canada.
Walker LM (2016) Shifts in myxomycete community structure in selected microhabitats across nutrient treatments in lowland tropical forest of Panama. University of Arkansas theses and dissertations, 1697. Available at http://scholarworks.uark.edu/etd/1697
Wellman P (2016) Distinctive corticolous myxomycete assemblages of tropical, temperate and arid regions of Australia. Cunninghamia 16, 101–114.
Wellman P (2017) Temperate-climate myxomycetes in Australia and the Southern Hemisphere. Cunninghamia 17, 1–13.
Wellman P (2019) Australian corticolous myxomycetes: models of distribution and development. Australian Journal of Botany 67, 617–629.
| Australian corticolous myxomycetes: models of distribution and development.Crossref | GoogleScholarGoogle Scholar |
Wikimedia (2020) World soil pH. Available at https://commons.wikimedia.org/wiki/File:World_Soil_pH.svg
Wrigley de Basanta D, Stephenson SL, Lado C, Estrada-Torres A, Nieves-Rivera AM (2008) Lianas as a microhabitat for myxomycetes in tropical forests. Fungal Diversity 28, 109–125.
Wrigley de Basanta D, Lado C, Estrada-Torres A, Stephenson SL (2010) Biodiversity of myxomycetes in subantarctic forests of Patagonia and Tierra del Fuego, Argentina. Nova Hedwigia 90, 45–79.
| Biodiversity of myxomycetes in subantarctic forests of Patagonia and Tierra del Fuego, Argentina.Crossref | GoogleScholarGoogle Scholar |
Appendix 1. List of sample details
A list the sample numbers, name of liana, latitude, longitude, altitude, diameter of liana stems, and if known the pH of the stems
900A, Cissus antarctica, 35.603°S, 150.324°E, 52 m, d = 9 mm. 900B, unknown, 35.603°S, 150.324°E, 52 m, d = 10 mm. 900C, unknown, 35.603°S, 150.324°E, 52 m, d = 5 mm. 900D, Palmeria scandens, 35.603°S, 150.324°E, 52 m, d = 5 mm. 900E, unknown, 35.603°S, 150.324°E, 52 m, d = 10 mm. 900F, Eustrephus latifolius, 35.603°S, 150.324°E, 52 m, d = 1 mm. 900G, Smilax australis, 35.603°S, 150.324°E, 52 m, d = 7 mm. 900H, Cissus antarctica, 35.603°S, 150.324°E, 52 m, d = 4 mm. 900J, Cissus hypoglauca, 35.603°S, 150.324°E, 52 m, d = 3 mm. 900K, Smilax australis, 35.603°S, 150.324°E, 52 m, d = 5 mm. 933, Marsdenia flavescens, 36.615°S, 150.029°E, 6 m, d = 9 mm. 934, Kennedia rubicunda, 36.615°S, 150.029°E, 6 m, d = 2 mm. 936, Aphanopetalum resinosum, 36.605°S, 150.033°E, 8 m, d = 5 mm. 937, Marsdenia rostrata, 36.605°S, 150.033°E, 8 m, d = 5 mm. 938, Sarcopetalum harveyanum, 36.605°S, 150.033°E, 8 m, d = 4 mm. 939, Eustrephus latifolius, 36.605°S, 150.033°E, 8 m, d = 4 mm. 1115, Melodinus australis, 35.504°S, 150.308°E, 127 m, d = 4 mm. 1117, Parsonsia straminea, 35.635°S, 150.281°E, 32 m, d = 5 mm. 1118, Morinda jasminoides, 36.617°S, 150.272°E, 39 m, d = 5 mm. 1120, unknown, 36.617°S, 150.272°E, 39 m, d = 6.5 mm. 1121, Parsonsia straminea + Cissus hypoglauca, 36.617°S, 150.272°E, 39 m, d = 7 mm. 1123, Cissus hypoglauca, 36.617°S, 150.272°E, 39 m, d = 6 mm. 1124, Smilax australis, 37.413°S, 149.812°E, 222 m, d = 6 mm. 1125, Cissus hypoglauca, 37.413°S, 149.812°E, 222 m, d = 5 mm. 1126, Cissus hypoglauca, 37.413°S, 149.812°E, 222 m, d = 12 mm. 1127, Marsdenia rostrata, 37.415°S, 149.815°E, 202 m, d = 4 mm. 1128, Parsonia straminea, 37.415°S, 149.815°E, 202 m, d = 4 mm. 1431, Cissus antarctica, 32.233°S, 152.554°E, 31 m, d = 5 mm, pH = 5.9. 1432, Parsonsia straminea, 32.233°S, 152.554°E, 31 m, d = 4, pH = 6.2. 1433, unknown, 32.233°S, 152.554°E, 31 m, d = 5 mm, pH = 6.0. 1440, Parsonsia straminea, 30.368°S, 152.795°E, 716 m, d = 4 mm, pH = 5.5. 1441, Parsonsia straminea, 30.361°S, 152.798°E, 753 m, d = 6 mm. 1442, Stephania japonica, 30.41°S, 153.075°E, 5 m, d = 2 mm, pH = 6.6.
Appendix 2. List of myxomcete records
This lists the myxomycete species recorded, the localities where they were found, and the number of fruiting bodies of each record.
Arcyria cinerea (Bull.) Pers.; 900A, 55; 900D, 4; 900E, 230; 900F, 7; 900G, 4; 900H, 9; 900K, 42. Arcyria denudata Fr.; 900C, 91; 900E, 32; 900G, 10; 936, 2; 937, 8; 938, 6; 1118, 3; 1120, 8; 1124, 4; 1125, 3; 1126, 22; 1128, 6; 1433, 29; 1440, 2; 1442, 79. Arcyria insignis Kalchbr. and Cooke; 900H, 11;1117, 6; Arcyria pomiformis (Leers) Rostaf.; 900K, 13. Badhamia nitens Berk.; 900D, 8; 1431, 98. Clastoderma debaryanum A. Blytt; 900G, 1. Comatricha elegans (Racib.) G. Lister; 900A, 8; 1124, 5; 1440, 8. Comatricha ellae Härk.; 937, 3; Comatricha laxa Rostaf.; 900A, 2; 900F, 2; 900G cf, 87; 936, 1; 937, 9; 1118, 1; 1123, 3; 1433, 2. Comatricha pulchella (C. Bab.) Rostaf.; 900K, 10; 1121, 1. Comatricha sp; 1440, 4. Cribraria violacea Rex; 900H, 8. Didymium clavus (Alb. and Schwein.) Rabenh.; 1117 cf, 15. Didymium difforme (Pers.) Gray; 900F cf, 4; 934, 2. Didymium iridis (Ditmar) Fr.; 1432 cf, 33. Didymium squamulosum (Alb. and Schwein) Fr.; 900F, 68; 934, 19; 1118, 2; 1127, 72; 1442, 27. Didymium sp.; 1125, 24. Echinostelium minutum de Bary; 934, 30, 936, 200; 1118, 10; 1123, 2; 1126, 10; 1431, 10; 1440, 12; 1441, 200. Hemitrichia pardina (Minakata) Ing; 1118, 4. Lamproderma arcyrionema Rostaf.; 900A, 5; 900D, 20; 900E, 12; 900F, 1; 900H, 2. Licea biforis Morgan; 900F, 12. Licea kleistobolus G. W. Martin; 900H, 10. Macbrideola declinata T. E. Brooks and H. W Keller; 1123, 8. Macbrideola sp.; 900G, 2; 900H, 1. Perichaena chrysosperma (Curr.) Lister; 900H, 18; 1118, 19. Perichaena depressa Lib.; 900A, 127; 900B, 12; 900H, 25; 1117 cf; 1; 1118, 31; 1433, 145. Perichaena sp.; 1120, 2. Perichaena vermicularis (Schwein) Rostaf. 934, 5; 1128, 16; 1432, 2; 1442, 16. Physarum compressum Alb. and Schwein.; 900A, 216; 900B, 195; 900F, 10; 933, 425; 934, 50; 937, 50; 1117, 16; 1125, 76; 1127, 50. Physarum hongkongense Chao H. Chung; 900E, 5. Physarum leucophaeum Fr.; 1118, 4; 1442, 22. Physarum melleum (Berk. and Broome) Massee; 900D, 105; 900H, 121; 900J, 50; 1118, 16. Physarum oblatum T. Macbr.; 900A, 327; 900D, 9; 900F, 81; 900H, 12; 900J, 24; 900K, 70; 933, 1; 937, 170; 1115, 4; 1117, 103; 1118, 12; 1120, 19; 1121, 5; 1124, 11; 1125, 7; 1126, 1; 1127, 6; 1128, 1; 1442, 350. Physarum pusillum (Berk. and M. A. Curtis) G. Lister; 900E, 23; 900B cf, 3; 934, 3; 1432, 5; 1433, 43. Physarum sp.; 936, 3; 1118, 1; 1123, 5; 1125, 2; 1126, 2; 1433, 5. Physarum viride (Bull.) Pers.; 900K cf, 2. Stemonitis fusca Roth; 900A, 34; 900C, 37; 900D, 3; 900E, 5; 900G, 17; 900H, 7; 900K, 1; 936, 12; 937, 7; 1118, 11; 1126, 13; 1440, 51; 1441, 29. Stemonitis mussooriensis G. W. Martin, K. S. Thind and Sohi; 900F, 24; 939, 13; 1125, 500; 1127, 70; 1432, 43; 1433, 15. Willkommlangea reticulata (Alb. and Schwein.) Kuntze; 900A, 45; 900D, 93; 900G, 4; 1431, 2.
Appendix 3. Additional pH measurements
Asterisks (*) indicate trees exotic to Australia
Wood and timber
Grown in Canberra, 35°S. Acacia dealbata, 4.7; Alnus glutinosa*, 5.4; Carya sp.*, 4.8; Casuarina cunninghamiana, 6.1; Eucalyptus globulus bicostata, 4.9; Eucalyptus delegatensis, 3.9; Eucalyptus marginata, 4.1; Eucalyptus polyanthemos, 5.2; Eucalyptus sideroxylon, 4.7; Exocarpus cupressiformis, 5.6; Gleditsia triconthos*, 5.2; Nothofagus cunninghamii, 3.9; Pinus radiata*, 4.6; Platanux acerifolia*, 5.7; Populus canescens*, 3.7; Populus lasiocarpa*, 4.9; Populus nigra ‘Italica’*, 6.7; Quercus × hispanica*, 5.6; Quercus robur*, 4.8; Salix fragilis*, 5.3; Ulmus procera*, 5.2.
Decayed liana stem
Grown in Canberra, 35°S. Actinidia chinensis*, 6.1; Akebia quinata*, 6.8; Hardenbergia violacea, 5.8; Jasminum nudiflorum*, 6.7; Lonicera hildebrandiana*, 6.3; Pandorea jasminoides, 5.3; Pandorea pandorana, 5.6; Trachelospermum jasminoides*, 6.7; Vitus vinifera*, 6.7.
NE New South Wales 32°S. 1434, unknown, 7.0; 1436, Cissus antarctica, 6.1; 1437, Marsdenia rostrata, 6.2.
Decayed branch wood
Grown in Canberra 35°S. Acacia sp., 4.9; Brachychiton populneus, 6.4; Casuarina sp., 4.7; Eucalyptus globulus, 5.0; Eucalyptus sp. 4.4, 5.3, 5.4; Exocarpus compressiformis, 5.1; Gleditsia triconthos*, 4.9; Quercus suber*, 5.0; Salix sp.*, 5.5; Tilia sp.*5.3; Ulmus sp.*, 5.3
Aerial litter – twigs
Grown Canberra 35°S. Brachychiton populneus, 6.6; Castanea sativa*, 5.7; Casuarina cunninghamiana, 5.1; Eriobotrya japonica*, 6.7; Eucalyptus cinomonum, 5.2; Eucalyptus mannifera, 5.0; Juglands nigra*, 5.6; Prunus sp.*, 5.8; Quercus suber*, 5.4; Tilia sp.*, 7.2; Ulmus procera*, 6.3; Ulmus sp.*, 5.6


