Aligning quantitative vegetation classification and landscape scale mapping: updating the classification approach of the Regional Ecosystem classification system used in Queensland
Eda Addicott A B C D , Victor John Neldner
A B C D , Victor John Neldner  A and Timothy Ryan A
A and Timothy Ryan A
A Queensland Herbarium, Mt Coot-tha Road, Toowong, Department of Environment & Science, Queensland Government, Qld 4066, Australia.
B Australian Tropical Herbarium, James Cook University, Cairns, Qld 4870, Australia.
C Centre for Tropical Environmental and Sustainability Science (TESS) and College of Science & Engineering, James Cook University, PO Box 6811, Cairns, Qld 4870, Australia.
D Corresponding author. Email: eda.addicott@des.qld.gov.au
Australian Journal of Botany 69(7) 400-413 https://doi.org/10.1071/BT20108
Submitted: 22 August 2020 Accepted: 15 February 2021 Published: 11 May 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Vegetation classification systems form a base for conservation management and the ecological exploration of the patterns and drivers of species’ distributions. A standardised system crossing administrative and geographical boundaries is widely recognised as most useful for broad-scale management. The Queensland Government, recognising this, uses the Regional Ecosystem (RE) classification system and accompanying mapping as a state-wide standardised vegetation classification system. This system informs legislation and policy at local, state and national levels, underpinning decisions that have wide-ranging implications for biodiversity and people’s livelihoods. It therefore needs to be robust from a scientific and legal perspective. The current approach in the RE system for identifying vegetation communities relies on expert-based class definition procedures. This is in contrast to best practice, which is based on quantitative procedures. This paper discusses the RE system in a global context and outlines the updated approach that incorporates quantitative class definition procedures, synthesises the research behind the updated approach and discusses its implications and implementation.
Keywords: Queensland, Qld, mapping, class definition procedures, vegetation communities, plant association, conservation management, classification system.
Introduction
Vegetation classification systems form a base for conservation management and provide for the ecological exploration of the natural patterns and drivers of species’ distributions (Kent 2012). Applying a vegetation classification system for management purposes through a map showing geographical areas of similarity within the jurisdiction is common with maps being a frequently associated component. Although vegetation classification systems are based on plot-based data, and maps on remotely sensed data, they both require a simplification of the complexity of the natural world. A classification system may describe detailed floristic composition of areas, however a map describing this detail quickly becomes too complicated for practical use. Contrastingly, a map that does not describe the complexity sufficiently is inadequate for land management (Kuchler 1951). Hence, a vegetation classification system and an accompanying map are interdependent and need to relate to each other, despite their different primary data sources.
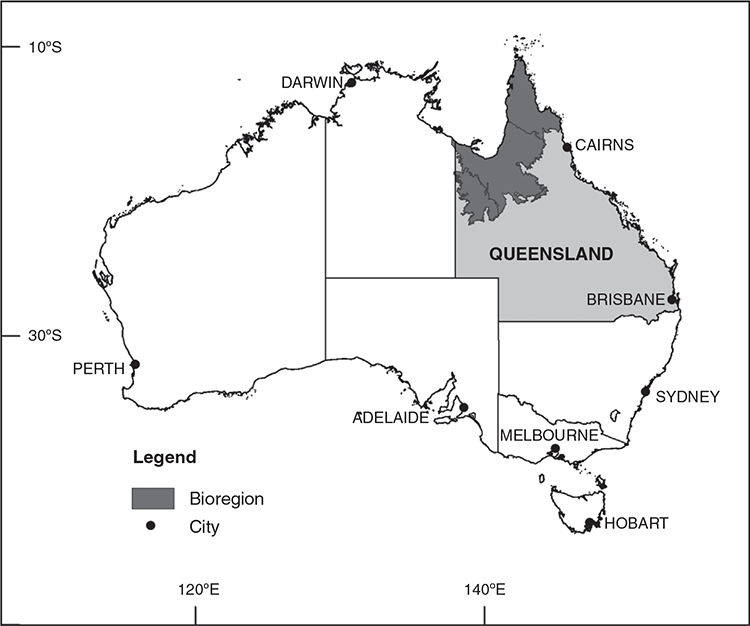
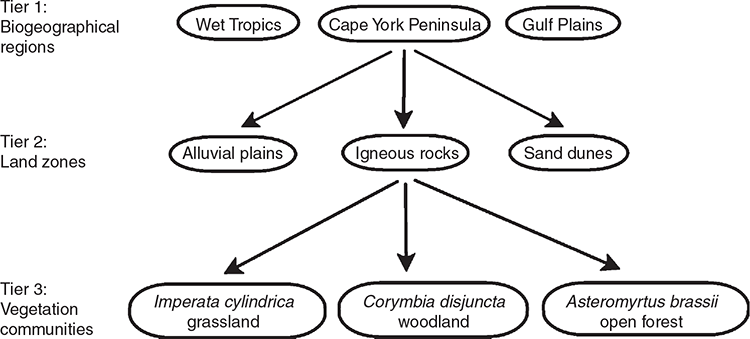
The demand for vegetation classification systems and maps is steadily increasing because of their direct applicability across a broad range of issues (Chytrý et al. 2011; Wesche and von Wehrden 2011). The globalisation of planning and management issues has created an increasing need to manage landscapes across geographical and administrative boundaries (Peet and Roberts 2013) thus a consistent vegetation classification system crossing these boundaries is desirable (De Cáceres et al. 2015; Franklin 2015). Recognising this, the government of the state of Queensland in north-eastern Australia (Fig. 1), adopted a state-wide, landscape-scale classification system for planning for conservation and biodiversity in 1999 (Sattler and Williams 1999). Queensland covers an area of 1.7 × 106 km2 and has a sparse population of 5.1 million people. Approximately 80% of its area is natural ‘remnant’ (Neldner et al. 2020) vegetation, of which 61.6% is sclerophyll forest, woodland and shrublands, 17.7% is grasslands and 1.2% is rainforest (Accad et al. 2019). The Queensland Government adopted the Regional Ecosystem (RE) classification system, which is three-tiered (Fig. 2) and incorporates geodiversity and floristic diversity. The first tier recognises geological and biological diversity at a continental scale by using a biogeographical regionalisation (Stanton and Morgan 1977) aligned with the Interim Biogeographical Regions of Australia (Thackway and Cresswell 1995). The second tier, termed ‘land zones’ and recognising diversity at a regional level, is based on broad geological divisions of the landscape that take account of geomorphological processes and soils (Wilson and Taylor 2012). Land zone concepts are groups such as ‘tidal flats and beaches’, ‘Quaternary inland dunefields’ or ‘basalt plains and hills’. The third tier is plant communities identified at the plant association level and termed ‘vegetation communities’. A RE is therefore defined as ‘a vegetation community, or communities, in a bioregion that are consistently associated with a particular combination of geology, landform and soil’ (Neldner et al. 2020, p. 116) noting that a RE may contain more than one vegetation community, but a vegetation community cannot occur in more than one RE. The classification approach used to identify vegetation communities specifies that communities be identified at the plant association level using plot-based records and characteristics of the pre-dominant layer (defined as that layer contributing most to the above-ground biomass; Neldner et al. 2020). Communities are recognised using the height, cover and dominant species in this pre-dominant layer, with secondary consideration given to associated species in other layers (see ‘Guidelines for defining a new regional ecosystem or vegetation community’ of Neldner et al. 2020, pp. 116–118). Vegetation communities are defined as plant associations where the predominant layer has a uniform floristic composition and exhibits a uniform structure (Neldner et al. 2020) aligning with both the Beadle (1981) definition of a plant association and a necessary emphasis on canopy species used for vegetation mapping.
|
|
Recognising the importance of maps in the role of vegetation management, a government-funded state-wide RE mapping program commenced in 1999 with the introduction of the RE system. REs are mappable entities with a distinctive signature recognisable from remotely sensed imagery at the landscape scale of 1 : 100 000 (Neldner et al. 2017). REs form the basis for mapping and survey projects at all scales across the state and are embedded in both national and Queensland Government legislation (Vegetation Management Act 1999 (Qld), see https://www.legislation.qld.gov.au/view/html/inforce/current/act-1999-090; Environment Protection and Biodiversity Conservation Act 1999, see https://www.environment.gov.au/epbc). Local governments within Queensland frequently use REs and RE mapping for planning at a local scale. They have become the fundamental baseline dataset for biodiversity information across Queensland.
The identification of vegetation communities within the RE system has predominantly used expert-based plot-grouping techniques. These are most often used in remote areas with limited researchers, such as in Queensland (Peet and Roberts 2013); however, they have acknowledged problems including their lack of transparency, repeatability and consistency between researchers (Mucina 1997; Kent 2012; Oliver et al. 2013). The outcomes are heavily dependent on a researcher’s knowledge of the vegetation of the area, their skills in image interpretation, and are biased by a researcher’s assumptions of the ecological and biophysical processes important to landscape function and biodiversity (Kent 2012). Best practice recommends using quantitative analysis procedures to identify communities. To this end, the classification approach of the RE system has been updated to incorporate quantitative procedures for identifying vegetation communities. The aim of this paper is to outline the updated approach and synthesise the background research underpinning it. We establish the need for an updated approach by comparing and contrasting the RE classification system with some of those used internationally. Finally, we discuss the implications and implementation of the updated approach.
Rainforests are included in the RE system, but the current classification approach for these communities specifies concepts based on structure with criteria based on dominance (Neldner et al. 2020). Thus, there is a mis-match between the concepts and criteria of the classification approach for identifying rainforest communities in Queensland. Therefore, this paper specifically outlines an updated classification approach for non-rainforest vegetation communities. The need to update the classification approach for identifying rainforest vegetation communities is an important next step in aligning the RE system with current best practice.
Global context
Classifying vegetation patterns into vegetation types has a long history (Goodall 2014) with a consequent evolution of ideas, concepts and methods (Peet and Roberts 2013) designed to meet specific end-uses. However, with the need to address global environmental issues has come the need to relate systems developed in isolation to each other (De Cáceres et al. 2015). To this end a framework and terminology for comparing plot-based vegetation classification systems, and the processes used to develop them, have been outlined (De Cáceres et al. 2015, 2018). In this, plot-based classification of vegetation is broken into two distinct sections: the structural elements and the procedural elements. The structural elements include the vegetation plot data, the vegetation type identified by the classification exercise and the classification system itself (made up of vegetation types). The primary procedural element is the classification approach. This includes the concepts and the classification protocols used to define vegetation types. The classification protocols, in turn, include the criteria and the class-definition procedures used to identify vegetation types. These procedures include such elements as the data collection methods, taxonomic resolution, the primary vegetation attributes, and the plot-grouping techniques. Primary vegetation attributes are those attributes of plants specifically used to consistently group plots into vegetation types (e.g. species, abundance or physiognomy). Associated environmental attributes used to help to align plots to vegetation types are considered as secondary attributes.
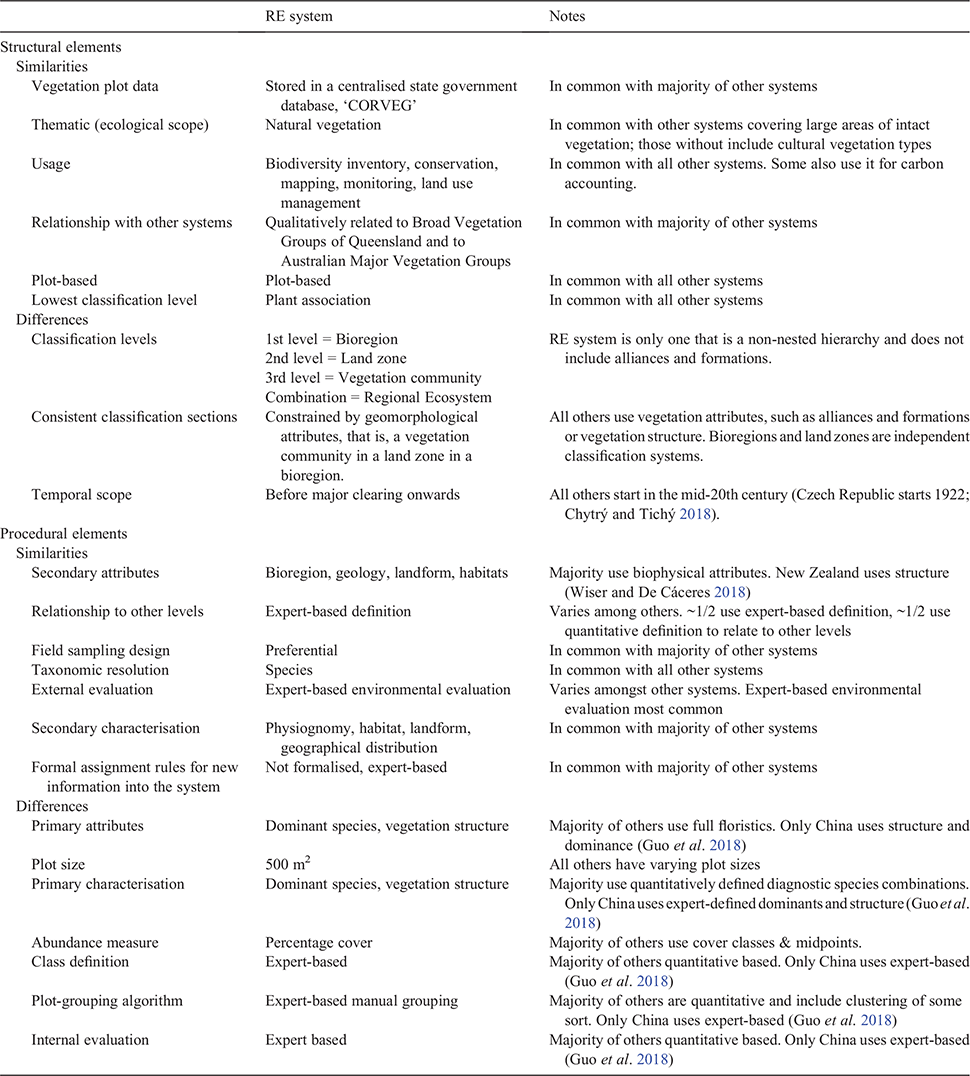
The structural and procedural elements of the RE system are outlined in documentation specifying standardised survey and mapping methods and classification criteria (Neldner et al. 2020). To compare and contrast the RE system with others used around the world we cast the RE system into the framework developed by De Cáceres et al. (2015) (Table 1). Using this common framework, we discuss the structural and procedural elements of the RE system compared with the classification systems included in a special issue of the journal Phytocoenologia (Vol. 48, issue 2, 2018). This highlights the similarities and differences of the RE system with those used elsewhere (Table 1).
|
In terms of structural elements, the RE system, along with all the systems included in the special issue, has the plant association as the lowest classification level (a reflection of scale and not importance). However, most systems place the plant association within a hierarchy of classification levels based on plant alliances and formations. Contrastingly, the RE system is one of the few systems that formally includes the environmental variables of biogeographical and geological divisions of the landscape as mandatory. However, the vegetation communities making up REs have also been used in a more conventional hierarchy to form the Broad Vegetation Groups of Queensland, which more closely align with the concepts of alliance and formation (Neldner et al. 2019). The only other classification system reviewed that used a low classification level to form another conceptual hierarchy was the Biogeoclimatic Ecosystem Classification used in Canada (MacKenzie and Meidinger 2018). In line with other systems in countries where managing existing natural vegetation types is the primary purpose of the system, the ecological scope of the RE system is confined to natural ‘remnant’ vegetation, (Brown and Bredenkamp 2018; MacKenzie and Meidinger 2018; Walker et al. 2018; Wiser and De Cáceres 2018). In countries where highly modified landscapes predominate, semi-natural and cultural vegetation types are included in their classification system (Gillet and Julve 2018; Guarino et al. 2018; Rodwell 2018; Federal Geographic Data Vegetation Subcommittee, see http://usnvc.org/revisions/)
Major differences become apparent when the procedural elements are compared (Table 1). A major difference is that the RE system uses dominant species to identify plant communities. A practice more common in the past (Whittaker 1973), it is usually used today in landscapes of low species richness (Wesche and von Wehrden 2011; Faber-Langendoen et al. 2014; Landucci et al. 2015). Using the full species composition of vascular plants is now more common (De Cáceres et al. 2018), but may not be possible where many species are absent because of seasonal conditions, for example drought. The RE system recommends consistent sampling methods and a standard plot size of 500 m2, shown to adequately capture the α diversity of plots in all vegetation types (apart from rainforest vegetation) in Queensland (Neldner and Butler 2008). This also contrasts with all other systems reviewed, which have variable plot sizes, as do the plot-grouping techniques used to identify communities in the RE system that are predominantly determined by expert-based definition. All others either already incorporate, or are working to incorporate, quantitative plot-grouping techniques to identify plant associations (Faber-Langendoen et al. 2014; De Cáceres et al. 2018). Consequently, unlike most of the systems reviewed, within the RE system there is no evaluation of the effectiveness of communities using characteristics of the communities themselves (internal evaluation). There is only external evaluation through peer-review and stake-holder feedback. REs are therefore currently both identified and evaluated using expert knowledge.
Adequacy of the survey design to capture data underpinning the RE system
To understand and apply a classification system, it is important to understand the efficacy of the data informing it and one of the common questions asked by users of the RE system is ‘How good is the sampling?’ (H. Dillewaard, pers. comm.). The sampling design used by the Queensland Government within the RE system is described by Neldner et al. (2020), and can be summarised as directed preferential with study-wide recognition of unique landscape situations derived from imagery. Large numbers of observational records are collected across these landscape situations and these are used to position detailed vegetation data plots at sites that are representative of the surrounding community. In a vegetation survey that aims to identify and characterise the range of communities in an extensive study area some level of preferential placement of plots is necessary (Peet and Roberts 2013) and preferential sampling designs cover a greater range of environmental extremes than other designs (Roleček et al. 2007). Greater environmental variability is associated with greater heterogeneity of community distribution (β-diversity) (Lepš 2008; Kent 2012) and to maximise the capture of β-diversity and species richness it is therefore necessary to have adequate spatial distribution of plots across major environmental gradients (Kent 2012). With 288 detailed plots and 6177 observation records from a surveyed area of ~54 000 km2, the two-tiered preferential sampling design used in the RE program captured the environmental variability (represented by 200-m pixels) of >96% of the area (Addicott et al. 2018a). The β-diversity, measured by the average Bray–Curtis dissimilarity between detailed plots, was within the 95% confidence intervals (Addicott 2020). However, this sampling design did not adequately capture the species richness in the landscape (Addicott 2020). This supports a finding in a Queensland wide study, which found that even though the RE program, using this sampling design, contributed over 90 000 specimens to the Queensland Herbarium collection (28% of the total over a 43-year period) only 79% of the total native and 73% of the naturalised vascular flora of the State were captured (Neldner 2014). The RE system is therefore likely to identify most of the vegetation communities in a region but not adequately describe the floristic diversity of those communities. Although a low density of detailed plots (288 across ~54 000 km2) may not be regarded as suitable for a quantitative classification exercise (Gellie et al. 2018), this two-tiered approach appears to achieve comprehensive sampling with low density (Addicott 2020). To describe the full diversity of species, whose distribution may be driven by factors operating at finer scales than those driving plant community patterns, is beyond the scope of the RE system, a landscape-scale classification system whose primary function is as a surrogate for biodiversity.
The two-tiered sampling design for collecting vegetation data has been designed to combine the process of mapping and species survey across extensive areas and is equally dependent on both rapid observational records and quantitative detailed vegetation plots. A potential drawback of this design is that collecting detailed vegetation plots is time-consuming (Neldner et al. 2020) and may be regarded as not cost effective. Mapping can be done using observational records with expert-based allocation of records to communities. However, if collecting observational data is prioritised over detailed plots, then the adequacy of sampling within the RE system, and hence the quality of the mapping, will be reduced. It is therefore important that detailed vegetation plots are collected as well as observation records. Ensuring enough detailed plots are collected can be done by comparing the sampling adequacy across environmental variables of the observational records versus detailed plots. This will help reduce tendencies to collect observational records at the expense of time-consuming detailed data plots.
Gellie et al. (2018) argue for a move away from vegetation mapping as a basis for vegetation classification systems. However, in contrast to this, in broad, often inaccessible parts of the country this two-tiered survey design shows how vegetation mapping and classification systems developed in conjunction can provide comprehensive capturing of environmental variability and β-diversity at a landscape scale (Addicott et al. 2018a; Addicott 2020). When a vegetation classification system, underpinned by a quantitatively based classification approach, is combined with mapping their application becomes powerful in allowing possibilities of ecological comparisons on a national or global scale (Addicott et al. 2020).
Appropriate class definition procedures for the RE system
Choosing appropriate quantitatively based class definition procedures to incorporate into a classification approach involves three critical components; (1) the primary vegetation attributes with which to recognise communities, (2) the techniques used to identify groups of co-occurring plant species forming communities, and (3) the techniques to validate them as fit-for-purpose. There is no general agreement on those most suitable and the number of techniques available complicates the choice and application (Kočí et al. 2003; Wesche and von Wehrden 2011; De Cáceres et al. 2015). However, those used need to be consistent with the concepts and criteria of the classification system (De Cáceres et al. 2015).
Primary vegetation attributes
Choosing appropriate primary vegetation attributes to align with the concepts and criteria of the RE system (see ‘Guidelines for defining a new regional ecosystem or vegetation community’ of Neldner et al. 2020, pp. 116–118) requires consideration of four factors. These are: the abundance measure to use; the species used to recognise communities; the vegetation layers to include; and how to include vegetation structure (Kent 2012).
Species abundance measure
Species abundance can be measured in several ways useful for classifying vegetation communities (Kent 2012) and in Queensland the recommended data collection methodology focuses on three; basal area, stem counts and percentage cover (Neldner et al. 2020). All of these can be used to estimate relative dominance and abundance (for example (Lehmann et al. 2014; Memiaghe et al. 2016; Cavada et al. 2017; Eldridge et al. 2018); however, the arguments supporting the use of percentage cover as the abundance measure in quantitative class definition procedures for the RE system are compelling. The most common abundance measure used globally in vegetation classification systems (De Cáceres et al. 2018) is percentage cover and within Australia it is used to determine national vegetation types (Executive Steering Committee for Australian Vegetation Information and Department of the Environment and Heritage 2003; Hnatiuk et al. 2009). Using percentage cover has been shown to reflect geological and slope gradients better than classification exercises using frequency or biomass, and tended to identify the same groups as a pre-existing supervised classification system (Smartt et al. 1974, 1976). This is important as both geology and landform are structural and procedural elements of the RE system. At a plot level, woody vegetation species are measured in three ways, and the ground layer species are measured as percentage cover. The criteria in the RE system use percentage cover to identify communities (see ‘Guidelines for defining a new regional ecosystem or vegetation community’ of Neldner et al. 2020, pp. 116–118) and it is the abundance measure referred to in existing end-uses of the RE system with, for example, legislation that currently uses the RE system using cover as a determinant of legal outcomes (Vegetation Management Act 1999 (Qld). Finally the RE mapping program distinguishes communities based on cover; a structural attribute extracted from imagery and that forms the key data source for mapping.
Species used to recognise communities
The identification of communities within the RE system is based on plot-data which collects the percentage cover of as many species as possible (Neldner et al. 2020). However, with REs being defined at a landscape-scale, and integrated into a mapping program, the classification criteria necessarily specify that communities are recognised by dominant species in the pre-dominant layer. Thus it becomes important to understand the levels of percentage cover of species that represent dominance at scales commensurate with the RE system and mapping. Species that do not fit the criteria of dominant contribute to ‘noise’ in the dataset, and may mask the relationships of interest between vegetation plots at landscape levels (Kent 2012; Pos et al. 2014). Additionally communities that are recognised using non-dominant species may be characterised by species responding to habitat changes below the scale of the classification system and mapping, thus reducing the confidence of end-users and the applicability of both the mapping and the classification system. Removing sparse species based on low frequency of occurrence (McCune and Grace 2002; Kent 2012) or low contribution to cumulative abundance (Field et al. 1982; Grime 1998; Mariotte 2014) is recommended. Using low frequency of occurrence to remove species from datasets is problematic in the RE system because of the preferential sampling design, which may lead to spatially restricted communities being represented by single plots. Removing infrequent species may consequently remove species dominating unique communities identified by these single plots, risking misclassification of plots.
Removing species from a classification exercise based on contribution to percentage cover rather than frequency is therefore necessary within the RE system. Testing the levels of contribution to total foliage cover that represented dominance in grassland, shrubland and woodland formations in north-eastern Queensland, Addicott et al. (2018b) found the levels of contribution to percentage total foliage cover that influenced classification outcomes relevant to broad-scale map communities differed between the three formations. In grasslands a contribution of 8% to total foliage cover in any plot was the optimal threshold for removing species, in shrublands a contribution of 1% was optimal and in woodlands a contribution of 10% was optimal. However the 1% threshold was adequate (if not optimal) for all vegetation formations tested. This 1% threshold removed up to 46% of the total species from analysis when applied to two savanna landscapes in north-eastern Queensland (Addicott et al. 2018a).
Although concepts of dominance differed between vegetation formations, a standard threshold of 1% has been adopted within the RE system across all non-rainforest formations. Although this extra species information may introduce ‘noise’, this threshold has been used to recognise appropriate vegetation communities across three bioregions of northern Queensland (Addicott et al. 2018a; Queensland Herbarium 2019) (Fig. 1). A consistent threshold across non-rainforest formations provides a standardised approach and the extra information may contribute to other projects such as documenting the floristic diversity of the landscape. Importantly, having a threshold of dominance allows a regional species list for recognising vegetation communities to be defined for end-users of the RE system. This may help direct survey time and effort (Marignani et al. 2008), indicate when seasonally dependent annual species may be important, and indicate new vegetation communities where plots are dominated by species not on this list (Addicott et al. 2018b).
Incorporating vegetation layers and structure
Incorporating vegetation structure in identifying communities is useful at landscape and regional scales (Beard 1973) and has a long history in Australia (Beard 1973; Specht 1981; Executive Steering Committee for Australian Vegetation Information and Department of the Environment and Heritage 2003). Plot data collected within the RE system includes percentage cover in all woody vegetation layers and the ground layer (Neldner et al. 2020). The considerations about including these layers in the primary vegetation attributes centre on mapping and scale issues. REs are mapped from remotely sensed imagery using techniques dependent on recognising changes in patterns of cover and dominant species, which in forested areas is the woody vegetation layers (Neldner et al. 2020). This method is supported by research that shows communities identified by woody vegetation are more stable for mapping (Hüttich et al. 2011), more recognisable at a landscape scale and by experts (Neldner and Howitt 1991; Mucina and Tichý 2018), and as informative about the distribution of all species across a landscape as communities identified using all vegetation layers (Bedward et al. 1992). Additionally, at landscape scales the ground layer composition has a low correlation with the distribution of woody species across a variety of biomes (Neldner and Howitt 1991; Neldner et al. 2004; Nezerkova-Hejcmanova et al. 2005; Lewis 2012) due to differences in species composition resulting from disturbance, seasonal changes and microclimate (Mucina and Daniel 2013; Mucina and Tichý 2018). Therefore, within the RE system in plots dominated by woody vegetation the ground layer is not included in the information used by the plot-grouping techniques.
The RE system recognises the importance of life-forms and species heights in ecosystem function (Küchler and Zonneveld 1988; Sattler and Williams 1999; De Cáceres et al. 2013), and in differentiating vegetation communities at landscape scales, by weighting vegetation layers in the classification criteria (see ‘Guidelines for defining a new regional ecosystem or vegetation community’ of Neldner et al. 2020, pp. 116–118). In quantifying these criteria Addicott et al. (2018b) found weighting species by actual layer height identified communities by canopy then subcanopy species and was substantially better at predicting species foliage cover within the dataset than alternative weightings. The approach adopted for incorporating vegetation structure to identify communities in the RE system is therefore to multiply species cover by the height of layer and sum across layers, giving a total volumetric abundance for each species at each plot Addicott et al. (2018b). Although total volumetric abundance to ensure clustering was driven by the pre-dominant layer was found to be appropriate for grasslands, shrublands and woodlands this may not be applicable for identifying vegetation communities in more structurally and floristically complex formations (Mucina and Tichý 2018). De Cáceres et al. (2013) successfully developed an approach for incorporating vegetation layers that is instead based on a cumulative volumetric abundance. This should be tested for applicability in complex vegetation formations such as rainforests, which are included in the RE system. These are currently classified using expert-based assessment of vegetation structure but with criteria of dominance dictating their characterisation.
Recognising communities
Plot grouping techniques
Identifying plant communities using plot-based data was historically carried out using expert knowledge to allocate plots into groups of similar vegetation (Whittaker 1973). With the advent of computers, statistical and mathematical models have become established techniques for grouping plots with high similarity to form clusters representing vegetation types (Kent 2012; Goodall 2014). There has been a multiplicity of these techniques developed (Peet and Roberts 2013; Goodall 2014; Chytrý et al. 2019) and the choice can influence the vegetation types identified (Wiser and De Cáceres 2013; De Cáceres et al. 2018). The techniques most commonly used are either agglomerative hierarchical clustering (AHC) or non-hierarchical partitioning (Kent 2012; De Cáceres et al. 2018; Addicott 2020). Clusters in both techniques are taken to represent vegetation types. AHC techniques assess the similarity of individual plots and produce a dendrogram representing a hierarchy of the dissimilarity between plots. Cutting the dendrogram at a given level of similarity forms clusters (Goodall 1973; Kent 2012). Non-hierarchical partitioning assesses plots sitting close to each other in multi-dimensional space and partitions it so that plots close to each other are considered a cluster (Kent 2012).
Although non-hierarchical partitioning techniques have been successfully used for determining vegetation types (Feoli and Zuccarello 1991; Moraczewski 1993; De Cáceres et al. 2010; Wiser and De Cáceres 2013; Tichý et al. 2014), AHC is most commonly used (Addicott 2020) and has been adopted as the standard plot-grouping technique for recognising the vegetation communities within the RE system because of its widespread use and proven robustness. Preparing datasets by square-root transforming data, calculating similarities between plots using the Bray–Curtis coefficient and using unweighted pair group mean average as the sorting technique for clustering, is the recommended combination as it has shown to be the optimal statistical approach for species datasets (Belbin and McDonald 1993; Clarke and Gorley 2006; Kent 2012; Tichý et al. 2020) and is the most commonly used combination in AHC (Addicott 2020).
There are advantages and disadvantages associated with all plot-grouping techniques (Kent 2012; De Cáceres et al. 2018). One drawback of using AHC to identify communities is the difficulty of incorporating new plot data into the system without re-running the whole classification exercise (De Cáceres and Wiser 2012) and, as has happened in the United Kingdom, this may result in a static classification system that is hard to update (Rodwell 2018). New plot data will continue to be acquired as the RE mapping program is a continuing Queensland Government project. The RE system needs to be stable from a social and political perspective and developing an approach for incorporating new information into the system that maximises the stability of the existing vegetation communities, and identifies new ones, is a priority. Various techniques exist (for example Oliver et al. 2013; Tichý et al. 2014) and these will need to be tested for suitability.
Quantitative evaluation techniques
Evaluating the identified vegetation communities as fit-for-purpose is an essential part of the class definition procedures and there are two types of evaluators; internal and external (Gauch and Whittaker 1981). Internal evaluators rely on criteria that assess clusters using the primary vegetation attributes and the cluster’s compositional characteristics and are most commonly used to decide the level of cluster division that form communities (Gauch and Whittaker 1981; Peet and Roberts 2013). External evaluators are factors outside the clustering analysis, for example secondary attributes such as environmental gradients, and are more often used to validate the final clusters as communities (Gauch and Whittaker 1981; De Cáceres et al. 2015).
Internal evaluation techniques
Internal evaluators are either geometric or non-geometric (Aho et al. 2008). Geometric evaluators are based on comparing the similarity of plots within and between clusters and non-geometric evaluators are based on the strength of species’ association with clusters (Aho et al. 2008). There is no general agreement on preferable internal evaluators and clusters chosen by one or other method may be contrastingly different (Aho et al. 2008). Comparing the classification outcomes produced by both types is therefore recommended (Aho et al. 2008; Lötter et al. 2013; Peet et al. 2018). Although there are a multiplicity of geometric evaluators available (e.g. Aho et al. 2008; Clarke et al. 2008; Lyons et al. 2016) the SIMPROF (similarity profile analysis) algorithm (Clarke et al. 2008) uses permutation tests to give a statistical measure of the similarity of plots within versus between clusters. It is provided in the PRIMER-e software package (Clarke and Gorley 2015) and so is readily accessible and well supported. This routine is well tested in the marine science literature using cover as the abundance measure (Clarke et al. 2014) and increasingly used in vegetation science (Oliver et al. 2013; Stromberg and Merritt 2016; Addicott et al. 2018a; Hunter and Lechner 2018). Again, several non-geometric evaluators are available (for example Dufrêne and Legendre (1997), Roberts (2015), Tichý et al. (2010) and of these indicator species analysis (Dufrêne and Legendre 1997) best suits AHC (Aho et al. 2008). It gives information on the distribution of species across the clusters as well as being useful in choosing the final cluster division, which is that with the highest number of significant indicator species (McCune and Grace 2002). As an alternative evaluator Lyons et al. (2016) developed a modelling approach that quantifies how well different classification outcomes predict the distribution of species occurrence and abundance across the dataset. These three individual evaluators have been used in identifying vegetation communities across three bioregions of northern Queensland (Fig. 1), with each providing different, but important, information about clusters as proposed vegetation types (Addicott et al. 2018a, 2018b; Queensland Herbarium 2019). Individually they do not form a final answer but are used in the body of work considered during the external evaluation process (Addicott et al. 2018a).
External evaluation process
The two components of external evaluation of communities resulting from a classification exercise are expert-based peer review and quantitative analysis. Whilst expert recognition of communities as ecologically interpretable and fit-for-purpose is one of the most important validation criteria (Goodall 1973; Whittaker 1973; Kent 2012; Lötter et al. 2013), expert-based review necessarily involves assumptions about the ecological drivers of patterns and what constitutes a community (Kent 2012). Therefore external evaluation using quantitative techniques is an important component in validating communities, feeding back in to the expert-based review. Of the quantitative techniques available, complimentary analysis comparing outcomes across different techniques are most common (Addicott 2020). Where vegetation mapping is the primary aim of the project, assessment of the recognition of cluster groups on remotely sensed imagery is used (Neldner and Howitt 1991; Bedward et al. 1992; Neldner et al. 1997; Penn et al. 2004; Lewis 2012).
Expert-based review is currently incorporated into the RE system, but the process has only been partially formalised. For each bioregion, a bioregional coordinator is appointed whose primary role is to facilitate consistency of mapping and RE concepts across the bioregion (Neldner et al. 2017) and a technical review panel (which includes the bioregional coordinator) is formed consisting of recognised experts in the flora and vegetation ecology of the area from both academia and industry. These panels are responsible for validating communities and assigning them into the RE system (Addicott et al. 2018a) and perform similar functions to those of international and other Australian jurisdictions overseeing the maintenance of vegetation classification systems and implementation of changes (e.g. European Vegetation Survey Working Group 2017; Office of Environment & Heritage and NSW Office of Environment and Heritage 2018; Federal Geographic Data Vegetation Subcommittee, see http://usnvc.org/revisions/).
The first step in the expert review process in the RE system is the assessment of communities from the classification exercise by the bioregional coordinator. To formalise this process, it is now done using quantitative analysis and a suite of environmental variables where communities identified are unexpected. Although available environmental variables differ between bioregions, landform, geology, geomorphology, soils, geographic distribution and modelled datasets such as radiometrics form a standard set of variables. It is important that specific questions testing the assumptions about the drivers of expected differences are formulated. These may be, for example, expectations of floristic difference between landforms, substrate or in ground layer composition. Standard variables and techniques used to test differences are suggested in Table 2 but others may be appropriate in some bioregions. In the second step of the expert-review process, the panel reviews the outcomes of this assessment, and in an iterative process, requests more quantitative analysis of communities when there are concerns. It is important that these requests are also based on questions that specifically address assumptions regarding the drivers of expected difference (Addicott et al. 2018a).

|
The RE classification system will remain one that retains an expert-based process because, in common with all classification systems reviewed, it reflects the reality that a classification system must be interpretable by end-users (De Cáceres et al. 2018; Addicott 2020). Secondly, because of the sparse population and low numbers of experts working in the vegetation science field in Queensland (Gellie et al. 2018), there will always be areas of the landscape where the full variability is not sampled by detailed plots (Addicott 2020), but this variability may be captured from remote sensing imagery and observational records (Addicott et al. 2018a). Consequently, there will always be communities in the RE system identified using expert-based techniques and observational records rather than quantitative analysis. In saying this, it is important to recognise that expert-recognised communities will reflect some of the biases of the experts and their assumptions regarding the drivers of ecological patterns. When the updated class definition procedures were applied to savanna communities of two landscapes in north-eastern Queensland, a 49% reduction in the number of communities compared to those identified using expert-based techniques resulted (Addicott et al. 2018a) and these were more recognisable and useful for conservation planning (Addicott and Laurance 2019). In total, 96% of the suggested changes were accepted during the review process, despite the extensive modifications to the existing expert-based communities. Requiring experts to explicitly define and quantitatively test their assumptions about ecological drivers ensures that communities identified a posteriori to the plot-grouping techniques are evidence-based and scientifically defensible (Addicott et al. 2018a). This, plus having class definition procedures specifically chosen to allow experts to compare like with like, may help reduce reluctance to adopt new classification outcomes as found in other jurisdictions (Wiser and De Cáceres 2018).
Final class definition procedures
The final suite of techniques making up the class definition procedures of the RE system incorporate the various considerations in identifying appropriate communities (Table 2). These include considering the adequacy of the available dataset to sample the β diversity and the environmental variability, the un-supervised plot-grouping techniques, the internal evaluation techniques and external evaluation process.
Conclusions
Queensland is a large state with a sparse population and a limited number of ecologists working in the field of vegetation classification. For example, across Queensland there are 5–12 ecologists involved in this work compared with European countries or the United States of America where there are many more ecologists working in smaller areas (Mucina et al. 2016; Gellie et al. 2018). This has led to the RE system being inextricably linked to vegetation mapping to cover the vast areas involved, and resulted in a system developed for a specific end use. Despite this, the structural and procedural elements of the RE classification system are similar to many others used elsewhere around the globe. The major differences are first, the mandatory inclusion of environmental variables as a structural element in the classification hierarchy. In most other systems environmental variation is highlighted as result of the classification exercise, for example higher levels of plant groupings in the hierarchy may indicate bioregional differences or climatic gradients (Faber-Langendoen et al. 2014; De Cáceres et al. 2018). The second major difference between the RE system and the majority of classification systems internationally is the use of the expert-based identification of plant associations. Updating the procedural elements to include quantitative techniques for identifying and evaluating plant associations brings the RE system further in to line with global best practice.
Also, in contrast to Europe and other parts of the world, the expertise in the RE classification system lies with the Queensland Government rather than academic or non-government institutions. Whilst this facilitates implementing a standardised approach there are some possible resulting limitations. One, as previously mentioned, is the perceived cost versus benefit of collecting time-consuming detailed vegetation plot data. Another is a greater difficulty in acquiring the skill set required to apply quantitative class definition procedures as government is necessarily oriented towards provision of services rather than research. Queensland is dominated by savanna and rangeland systems with broad environmental gradients and the fact that ecologists may perceive differences that do not represent vegetation communities at the appropriate scale in these situations (Addicott and Laurance 2019) means it is important the updated classification approach is applied in a standardised manner across the state. In order to avoid an idiosyncratic application and embed it into corporate knowledge, investment in training in the quantitative class definition procedures is highly desirable.
With the greatest proportion of the natural vegetation in Queensland being non-rainforest communities (98.5%; Accad et al. 2019) the updated classification approach has wide applicability. It has been implemented across three bioregions of Queensland (930 000 km2) (Fig. 1) thus establishing its functionality (Addicott et al. 2018a; Queensland Herbarium 2019). With the improvement of consistency and repeatability comes the potential for statistically robust communities to be compared across administrative and regional boundaries (Goodall 1973). As well as the current comparisons of spatial and temporal change of REs (Accad et al. 2019) statistical comparisons between vegetation communities at regional scales will become possible (Goodall 1973; Addicott et al. 2018a). Importantly, it will also form statistically supported base-line data against which to measure the effects of future changes, such as climate and land use. Having vegetation communities, the base-line level of the RE hierarchy, underpinned by quantitative analyses will ensure REs are more readily defensible and robust, helping to instil greater confidence in the classification system in both legislators and end users.
Conflicts of interest
Eda Addicott is a Guest Editor for the ‘ESA Conference’ special issue for Australian Journal of Botany. Despite this relationship, she did not at any stage have Editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Journal of Botany encourages its editors to publish in the journal and they are kept totally separate from the decision-making process for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
This work was supported by the Queensland Herbarium, Department of Environment and Science. We thank Peter Bannink for Fig. 1 and Andrew Franks for comment on the manuscript.
References
Accad A, Neldner VJ, Kelley JAR, Li J, Richter D (2019) Remnant regional ecosystem vegetation in Queensland. Analysis 1997–2017. (Queensland Department of Environment and Science: Brisbane, Qld, Australia) Available at https://www.qld.gov.au/environment/plants-animals/plants/ecosystems/remnant-vegetation [Verified 18 March 2021]Addicott E (2020) A new classification approach: Improving the Regional Ecosystem Classification in Queensland, Australia. PhD thesis. James Cook University, Cairns, Qld, Australia.
Addicott E, Laurance SGW (2019) Supervised versus un-supervised classification: a quantitative comparison of plant communities in savanna vegetation. Applied Vegetation Science 22, 373–382.
| Supervised versus un-supervised classification: a quantitative comparison of plant communities in savanna vegetation.Crossref | GoogleScholarGoogle Scholar |
Addicott E, Newton M, Laurance S, Neldner J, Laidlaw M, Butler D (2018a) A new classification of savanna plant communities on the igneous rock lowlands and Tertiary sandy plain landscapes of Cape York Peninsula bioregion. Cunninghamia 18, 29–72.
Addicott E, Laurance S, Lyons M, Butler D, Neldner J (2018b) When rare species are not important: Linking plot-based vegetation classifications and landscape-scale mapping in Australian savanna vegetation. Community Ecology 19, 67–76.
| When rare species are not important: Linking plot-based vegetation classifications and landscape-scale mapping in Australian savanna vegetation.Crossref | GoogleScholarGoogle Scholar |
Addicott E, Laurance SGW, Bannink P, Thompson S (2020) The intertidal plant communities in north-eastern Australia, their carbon stores and vulnerability to extreme climate events. Aquatic Conservation 30, 2298–2312.
| The intertidal plant communities in north-eastern Australia, their carbon stores and vulnerability to extreme climate events.Crossref | GoogleScholarGoogle Scholar |
Aho K, Roberts DW, Weaver T (2008) Using geometric and non-geometric internal evaluators to compare eight vegetation classification methods. Journal of Vegetation Science 19, 549–562.
| Using geometric and non-geometric internal evaluators to compare eight vegetation classification methods.Crossref | GoogleScholarGoogle Scholar |
Beadle NCW (1981) ‘The vegetation of Australia.’ (Cambridge University Press: New York, NY, USA)
Beard JS (1973) The Physiognomic Approach. In ‘Classification of Plant Communities. Handbook of Vegetation Science’. (Ed. RH Whittaker) pp. 33–64. (Junk: The Hague, Netherlands).
Bedward M, Keith DA, Pressey RL (1992) Homogeneity analysis: assessing the utility of classifications and maps of natural resources. Australian Journal of Ecology 17, 133–139.
| Homogeneity analysis: assessing the utility of classifications and maps of natural resources.Crossref | GoogleScholarGoogle Scholar |
Belbin L, McDonald C (1993) Comparing three classification strategies for use in ecology. Journal of Vegetation Science 4, 341–348.
| Comparing three classification strategies for use in ecology.Crossref | GoogleScholarGoogle Scholar |
Brown LR, Bredenkamp GJ (2018) An overview of the vegetation classification approach in South Africa. Phytocoenologia 48, 163–170.
| An overview of the vegetation classification approach in South Africa.Crossref | GoogleScholarGoogle Scholar |
Carpenter G, Gillison AN, Winter J (1993) DOMAIN: a flexible modelling procedure for mapping potential distributions of plants and animals. Biodiversity and Conservation 2, 667–680.
| DOMAIN: a flexible modelling procedure for mapping potential distributions of plants and animals.Crossref | GoogleScholarGoogle Scholar |
Cavada N, Ciolli M, Rocchini D, Barelli C, Marshall AR, Rovero F (2017) Integrating field and satellite data for spatially explicit inference on the density of threatened arboreal primates. Ecological Applications 27, 235–243.
| Integrating field and satellite data for spatially explicit inference on the density of threatened arboreal primates.Crossref | GoogleScholarGoogle Scholar | 28052505PubMed |
Chytrý M, Tichý L (2018) National vegetation classification of the Czech Republic: a summary of the approach. Phytocoenologia 48, 121–131.
| National vegetation classification of the Czech Republic: a summary of the approach.Crossref | GoogleScholarGoogle Scholar |
Chytrý M, Schaminee JHJ, Schwabe A (2011) Vegetation survey: a new focus for applied vegetation science. Applied Vegetation Science 14, 435–439.
| Vegetation survey: a new focus for applied vegetation science.Crossref | GoogleScholarGoogle Scholar |
Chytrý M, Chiarucci A, Pärtel M, Pillar VD, Bakker JP, Mucina L, Peet RK, White PS (2019) Progress in vegetation science: trends over the past three decades and new horizons. Journal of Vegetation Science 30, 1–4.
| Progress in vegetation science: trends over the past three decades and new horizons.Crossref | GoogleScholarGoogle Scholar |
Clarke KR, Gorley RN (2006) ‘PRIMER v6: User Manual and Tutorial.’ (PRIMER-E Ltd: Plymouth, UK)
Clarke KR, Gorley RN (2015) ‘PRIMER v7: User Manual and Tutorial.’ (PRIMER-E Ltd: Plymouth, UK)
Clarke KR, Somerfield PJ, Gorley RN (2008) Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. Journal of Experimental Marine Biology and Ecology 366, 56–69.
| Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage.Crossref | GoogleScholarGoogle Scholar |
Clarke KR, Gorley RN, Somerfield PJ, Warwick RM (2014) ‘Change in Marine Communities. An Approach to Statistical Analysis and Interpretation.’ (PRIMER-E: Plymouth, UK)
De Cáceres M, Wiser SK (2012) Towards consistency in vegetation classification. Journal of Vegetation Science 23, 387–393.
| Towards consistency in vegetation classification.Crossref | GoogleScholarGoogle Scholar |
De Cáceres M, Font X, Oliva F (2010) The management of vegetation classifications with fuzzy clustering. Journal of Vegetation Science 21, 1138–1151.
| The management of vegetation classifications with fuzzy clustering.Crossref | GoogleScholarGoogle Scholar |
De Cáceres M, Legendre P, He F, Faith D (2013) Dissimilarity measurements and the size structure of ecological communities. Methods in Ecology and Evolution 4, 1167–1177.
| Dissimilarity measurements and the size structure of ecological communities.Crossref | GoogleScholarGoogle Scholar |
De Cáceres M, Chytrý M, Agrillo E, Attorre F, Botta-Dukát Z, Capelo J, Czúcz B, Dengler J, Ewald J, Faber-Langendoen D, Feoli E, Franklin SB, Gavilán R, Gillet F, Jansen F, Jiménez-Alfaro B, Krestov P, Landucci F, Lengyel A, Loidi J, Mucina L, Peet RK, Roberts DW, Roleček J, Schaminée JHJ, Schmidtlein S, Theurillat JP, Tichý L, Walker DA, Wildi O, Willner W, Wiser SK (2015) A comparative framework for broad-scale plot-based vegetation classification. Applied Vegetation Science 18, 543–560.
| A comparative framework for broad-scale plot-based vegetation classification.Crossref | GoogleScholarGoogle Scholar |
De Cáceres M, Franklin SB, Hunter JT, Landucci F, Dengler J, Roberts DW (2018) Global overview of plot-based vegetation classification approaches. Phytocoenologia 48, 101–112.
| Global overview of plot-based vegetation classification approaches.Crossref | GoogleScholarGoogle Scholar |
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67, 345–366.
| Species assemblages and indicator species: the need for a flexible asymmetrical approach.Crossref | GoogleScholarGoogle Scholar |
Eldridge DJ, Delgado-Baquerizo M, Travers SK, Val J, Oliver I (2018) Livestock grazing and forest structure regulate the assembly of ecological clusters within plant networks in eastern Australia. Journal of Vegetation Science 29, 788–797.
| Livestock grazing and forest structure regulate the assembly of ecological clusters within plant networks in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
European Vegetation Survey Working Group (2017) Procedures for updating the standard European vegetation classification (draft). Available at http://euroveg.org/news#70 [Verified 9 August 2017].
Executive Steering Committee for Australian Vegetation Information, Department of the Environment and Heritage (2003) Australian Vegetation Attribute Manual: National Vegetation Information System, Version 6.0. (Executive Steering Committee for Australian Vegetation Information: Canberra, ACT, Australia) Available at https://environment.gov.au/system/files/pages/06613354-b8a0-4a0e-801e-65b118a89a2f/files/vegetation-attribute-manual-6.pdf [Verified 15 August 2020]
Faber-Langendoen D, Keeler-Wolf T, Meidinger D, Tart D, Hoagland B, Josse C, Navarro G, Ponomarenko S, Saucier J-P, Weakley A, Comer P (2014) EcoVeg: a new approach to vegetation description and classification. Ecological Monographs 84, 533–561.
| EcoVeg: a new approach to vegetation description and classification.Crossref | GoogleScholarGoogle Scholar |
Feoli E, Zuccarello V (1991) Syntaxonomy: a source of useful fuzzy sets for environmental analysis? In ‘Computer Assisted Vegetation Analysis’. (Eds E Feoli, L Orloci) pp. 265–271. (Kluwer: Dordrecht, Netherlands).
Field JG, Clarke KR, Warwick RM (1982) A practical strategy for analysing multispecies distribution patterns. Marine Ecology Progress Series 8, 37–52.
| A practical strategy for analysing multispecies distribution patterns.Crossref | GoogleScholarGoogle Scholar |
Franklin S (2015) How a national vegetation classification can help ecological research and management. Frontiers in Ecology and the Environment 13, 185–186.
| How a national vegetation classification can help ecological research and management.Crossref | GoogleScholarGoogle Scholar |
Gauch HG, Whittaker RH (1981) Hierarchical classification of community data. Journal of Ecology 69, 537–557.
| Hierarchical classification of community data.Crossref | GoogleScholarGoogle Scholar |
Gellie NJH, Hunter JT, Benson JS, Kirkpatrick JB, Cheal DC, McCreery K, Brocklehurst P (2018) Overview of plot-based vegetation classification approaches within Australia. Phytocoenologia 48, 251–272.
| Overview of plot-based vegetation classification approaches within Australia.Crossref | GoogleScholarGoogle Scholar |
Gillet F, Julve P (2018) The integrated synusial approach to vegetation classification and analysis. Phytocoenologia 48, 141–152.
| The integrated synusial approach to vegetation classification and analysis.Crossref | GoogleScholarGoogle Scholar |
Goodall DW (1973) Numerical Classification. In ‘Ordination and Classification of Communities. Handbook of Vegetation Science’. (Ed. RH Whittaker) pp. 575–615. (Junk: The Hague, Netherlands).
Goodall DW (2014) A century of vegetation science. Journal of Vegetation Science 25, 913–916.
| A century of vegetation science.Crossref | GoogleScholarGoogle Scholar |
Grime JP (1998) Benefits of Plant Diversity to Ecosystems: immediate, filter and founder effects. Journal of Ecology 86, 902–910.
| Benefits of Plant Diversity to Ecosystems: immediate, filter and founder effects.Crossref | GoogleScholarGoogle Scholar |
Guarino R, Willner W, Pignatti S, Attorre F, Loidi JJ (2018) Spatio-temporal variations in the application of the Braun-Blanquet approach in Europe. Phytocoenologia 48, 239–250.
| Spatio-temporal variations in the application of the Braun-Blanquet approach in Europe.Crossref | GoogleScholarGoogle Scholar |
Guo K, Liu C-C, Xie Z-Q, Li FY, Franklin SB, Lu Z-J, Ma K-P (2018) China Vegetation Classification: concept, approach and applications. Phytocoenologia 48, 113–120.
| China Vegetation Classification: concept, approach and applications.Crossref | GoogleScholarGoogle Scholar |
Hnatiuk RJ, Thackway R, Walker J (2009) Vegetation. In ‘Australian Soil and Land Survey Field Handbook’. (Ed. National Committee on Soil and Terrain) pp. 73–125. (CSIRO Publishing: Melbourne, Vic., Australia)
Hunter JT, Lechner AM (2018) A multiscale, hierarchical, ecoregional and floristic classification of arid and semi-arid ephemeral wetlands in New South Wales, Australia. Marine and Freshwater Research 69, 418–431.
| A multiscale, hierarchical, ecoregional and floristic classification of arid and semi-arid ephemeral wetlands in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Hüttich C, Herold M, Wegmann M, Cord A, Strohbach B, Schmullius C, Dech S (2011) Assessing effects of temporal compositing and varying observation periods for large-area land-cover mapping in semi-arid ecosystems: Implications for global monitoring. Remote Sensing of Environment 115, 2445–2459.
| Assessing effects of temporal compositing and varying observation periods for large-area land-cover mapping in semi-arid ecosystems: Implications for global monitoring.Crossref | GoogleScholarGoogle Scholar |
Kent M (2012) ‘Vegetation Description and Data Analysis: a Practical Approach.’ (Wiley-Blackwell: Oxford, UK)
Kočí M, Chytrý M, Tichý L (2003) Formalized reproduction of an expert-based phytosociological classification: a case study of subalpine tall-forb vegetation. Journal of Vegetation Science 14, 601–610.
| Formalized reproduction of an expert-based phytosociological classification: a case study of subalpine tall-forb vegetation.Crossref | GoogleScholarGoogle Scholar |
Kuchler AW (1951) The relation between classifying and mapping vegetation. Ecology 32, 275–283.
| The relation between classifying and mapping vegetation.Crossref | GoogleScholarGoogle Scholar |
Küchler AW, Zonneveld IS (1988) ‘Vegetation Mapping.’ (Kluwer: Dordrecht, Netherlands)
Landucci F, Tichý L, Šumberová K, Chytrý M (2015) Formalized classification of species-poor vegetation: a proposal of a consistent protocol for aquatic vegetation. Journal of Vegetation Science 26, 791–803.
| Formalized classification of species-poor vegetation: a proposal of a consistent protocol for aquatic vegetation.Crossref | GoogleScholarGoogle Scholar |
Lehmann CER, Felfili J, Hutley LB, Ratnam J, San Jose J, Montes R, Franklin D, Russell-Smith J, Ryan CM, Durigan G, Hiernaux P, Anderson TM, Haidar R, Bowman DMJS, Bond WJ, Sankaran M, Higgins SI, Archibald S, Hoffmann WA, Hanan NP, Williams RJ, Fensham RJ (2014) Savanna vegetation-fire-climate relationships differ among continents. Science 343, 548–552.
| Savanna vegetation-fire-climate relationships differ among continents.Crossref | GoogleScholarGoogle Scholar |
Lepš J (2008) Diversity and ecosystem function. In ‘Vegetation Ecology’. (Ed. E van der Maarel) pp. 199–237. (Wiley-Blackwell: Oxford, UK)
Lewis DL (2012) An evaluation of image and field data for vegetation community mapping in tropical savannas. PhD thesis. University of Queensland, Brisbane, Qld, Australia. Available at https://espace.library.uq.edu.au/view/UQ:301982.
Lötter MC, Mucina L, Witkowski ETF (2013) The classification conundrum: species fidelity as leading criterion in search of a rigorous method to classify a complex forest data set. Community Ecology 14, 121–132.
| The classification conundrum: species fidelity as leading criterion in search of a rigorous method to classify a complex forest data set.Crossref | GoogleScholarGoogle Scholar |
Lyons M, Keith DA, Warton DI, Somerville M, Kingsford RT (2016) Model-based assessment of ecological community classifications. Journal of Vegetation Science 27, 704–715.
| Model-based assessment of ecological community classifications.Crossref | GoogleScholarGoogle Scholar |
MacKenzie WH, Meidinger DV (2018) The Biogeoclimatic Ecosystem classification approach: an ecological framework for vegetation classification. Phytocoenologia 48, 203–213.
| The Biogeoclimatic Ecosystem classification approach: an ecological framework for vegetation classification.Crossref | GoogleScholarGoogle Scholar |
Marignani M, Del Vico E, Maccherini S (2008) Performance of indicators and the effect of grain size in the discrimination of plant communities for restoration purposes. Community Ecology 9, 201–206.
| Performance of indicators and the effect of grain size in the discrimination of plant communities for restoration purposes.Crossref | GoogleScholarGoogle Scholar |
Mariotte P (2014) Do subordinate species punch above their weight? Evidence from above‐ and below‐ground. New Phytologist 203, 16–21.
| Do subordinate species punch above their weight? Evidence from above‐ and below‐ground.Crossref | GoogleScholarGoogle Scholar |
McCune B, Grace JB (2002) ‘Analysis of Ecological Communities.’ (MjM Software Design)
Memiaghe HR, Lutz JA, Korte L, Alonso A, Kenfack D (2016) Ecological importance of small-diameter trees to the structure, diversity and biomass of a tropical evergreen forest at Rabi, Gabon. PLoS One 11, e0154988
| Ecological importance of small-diameter trees to the structure, diversity and biomass of a tropical evergreen forest at Rabi, Gabon.Crossref | GoogleScholarGoogle Scholar | 27186658PubMed |
Moraczewski IR (1993) Fuzzy logic for phytosociology – 1. Syntaxa as vague concepts. Vegetatio 106, 1–11.
| Fuzzy logic for phytosociology – 1. Syntaxa as vague concepts.Crossref | GoogleScholarGoogle Scholar |
Mucina L (1997) Classification of vegetation: past, present and future. Journal of Vegetation Science 8, 751–760.
| Classification of vegetation: past, present and future.Crossref | GoogleScholarGoogle Scholar |
Mucina L, Daniel G (2013) ‘Vegetation Mapping in the Northern Kimberley, Western Australia.’ (Ed. L Mucina) (Curtin University: Perth, WA)
Mucina L, Tichý L (2018) Forest classification: data-analytical experiments on vertical forest layering and flattened data. In ‘Vegetation Survey and Classification of Subtropical Forests of Southern Africa’. (Ed. L Mucina) pp. 47–57. (Springer).
Mucina L, Bültmann H, Dierssen K, Theurillat J-P, Raus T, Carni A, Šumberová K, Willner W, Dengler J, Schaminee JHJ, Hennekens SM (2016) Vegetation of Europe: hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Applied Vegetation Science 19, 3–264.
| Vegetation of Europe: hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities.Crossref | GoogleScholarGoogle Scholar |
Neldner V (2014) The contribution of vegetation survey and mapping to Herbarium collections and botanical knowledge: a case study from Queensland. Cunninghamia 14, 77–87.
| The contribution of vegetation survey and mapping to Herbarium collections and botanical knowledge: a case study from Queensland.Crossref | GoogleScholarGoogle Scholar |
Neldner VJ, Butler DW (2008) Is 500 m2 an effective plot size to sample florisitic diversity for Queensland’s vegetation? Cunninghamia 10, 513–519.
Neldner VJ, Howitt CJ (1991) Comparision of an intuitive mapping classification and numerical classifications of vegetation in south-east Queensland, Australia. Vegetatio 94, 141–152.
| Comparision of an intuitive mapping classification and numerical classifications of vegetation in south-east Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Neldner VJ, Fensham RJ, Clarkson JR, Stanton JP (1997) The natural grasslands of Cape York Peninsula, Australia. Description, distribution and conservation status. Biological Conservation 81, 121–136.
| The natural grasslands of Cape York Peninsula, Australia. Description, distribution and conservation status.Crossref | GoogleScholarGoogle Scholar |
Neldner VJ, Kirkwood AB, Collyer BS (2004) Optimum time for sampling floristic diversity in tropical eucalypt woodlands of northern Queensland. The Rangeland Journal 26, 190–203.
| Optimum time for sampling floristic diversity in tropical eucalypt woodlands of northern Queensland.Crossref | GoogleScholarGoogle Scholar |
Neldner VJ, Butler DW, Guymer GP (2017) Queensland’s regional ecosystems: Building and maintaining a biodiversity inventory, planning framework and information system for Queensland. Ver. 2.0. (Queensland Herbarium, Department of Environment and Science: Brisbane, Australia) Available at https://www.qld.gov.au/environment/plants-animals/plants/herbarium/publications [Verified 15 August 2020]
Neldner VJ, Niehus RE, Wilson BA, McDonald WJF, Ford AJ, Accad AN (2019) The vegetation of Queensland: descriptions of broad vegetation groups. Ver. 4.0. (Queensland Herbarium Department of Environment and Science) Available at https://www.publications.qld.gov.au/dataset/redd/resource/78209e74-c7f2-4589-90c1-c33188359086 [Verified 18 March 2021]
Neldner VJ, Wilson BA, Dillewaard HA, Ryan TS, Butler DW, McDonald WJF, Addicott E, Appelman CN (2020) Methodology for survey and mapping of vegetation communities and regional ecosystems in Queensland. Ver. 5.1. Available at https://www.publications.qld.gov.au/dataset/redd/resource/6dee78ab-c12c-4692-9842-b7257c2511e4 [Verified 18 March 2021]
Nezerkova-Hejcmanova P, Hejcman M, Camara AA, Antonínová M, Pavlů V, Černý T, Bâ AT (2005) Analysis of the herbaceous undergrowth of the woody savanna in the Fathala Reserve, Delta du Saloum National Park (Senegal). Belgian Journal of Botany 138, 119–128.
Office of Environment & Heritage, NSW Office of Environment and Heritage (2018) NSW plant community type – change control. Available at http://www.environment.nsw.gov.au/research/PCTchangecontrol.htm [Verified 15 August 2020]
Oliver I, Broese EA, Dillon ML, Sivertsen D, McNellie MJ (2013) Semi-automated assignment of vegetation survey plots within an a priori classification of vegetation types. Methods in Ecology and Evolution 4, 73–81.
| Semi-automated assignment of vegetation survey plots within an a priori classification of vegetation types.Crossref | GoogleScholarGoogle Scholar |
Peet RK, Roberts DW (2013) Classification of natural and semi-natural vegetation. In ‘Vegetation Ecology’. (Eds E van der Maarel, J Franklin) pp. 28–70. (Wiley: Chichester, UK)
Peet RK, Palmquist KA, Wentworth TR, Schafale MP, Weakley AS, Lee MT (2018) Carolina vegetation survey: an initiative to improve regional implementation of the US National Vegetation Classification. Phytocoenologia 48, 171–179.
| Carolina vegetation survey: an initiative to improve regional implementation of the US National Vegetation Classification.Crossref | GoogleScholarGoogle Scholar |
Penn MG, Sutton DA, Monro A (2004) Vegetation of the Greater Maya Mountains, Belize. Systematics and Biodiversity 2, 21–44.
| Vegetation of the Greater Maya Mountains, Belize.Crossref | GoogleScholarGoogle Scholar |
Pos E, Andino JEG, Sabatier D, Molino JF, Pitman N, Mogollon H, Neill D, Ceron C, Rivas G, Di Fiore A, Thomas R, Tirado M, Young KR, Wang O, Sierra R, Garcia-Villacorta R, Zagt R, Palacios W, Aulestia M, ter Steege H (2014) Are all species necessary to reveal ecologically important patterns? Ecology and Evolution 4, 4626–4636.
| Are all species necessary to reveal ecologically important patterns?Crossref | GoogleScholarGoogle Scholar | 25558357PubMed |
Queensland Herbarium (2019) Regional Ecosystem Description Database (REDD). Ver. 11.1. Available at https://apps.des.qld.gov.au/regional-ecosystems/ [Verified 15 August 2020]
Roberts DW (2015) Vegetation classification by two new iterative reallocation optimization algorithms. Plant Ecology 216, 741–758.
| Vegetation classification by two new iterative reallocation optimization algorithms.Crossref | GoogleScholarGoogle Scholar |
Rodwell JS (2018) The UK national vegetation classification. Phytocoenologia 48, 133–140.
| The UK national vegetation classification.Crossref | GoogleScholarGoogle Scholar |
Roleček J, Chytrý M, Hájek M, Lvončik S, Tichý L (2007) Sampling design in large-scale vegetation studies: do not sacrifice ecological thinking to statistical purism. Folia Geobotanica 42, 199–208.
| Sampling design in large-scale vegetation studies: do not sacrifice ecological thinking to statistical purism.Crossref | GoogleScholarGoogle Scholar |
Sattler PS, Williams RD (1999) ‘The Conservation Status of Queensland’s Bioregional Ecosystems.’ (Environmental Protection Agency: Brisbane, Qld, Australia)
Smartt PFM, Meacock SE, Lambert JM (1974) Investigations into the properties of quantitative vegetational data: I. Pilot study. Journal of Ecology 62, 735–759.
| Investigations into the properties of quantitative vegetational data: I. Pilot study.Crossref | GoogleScholarGoogle Scholar |
Smartt PFM, Meacock SE, Lambert JM (1976) Investigations into the properties of quantitative vegetational data: II. Further data type comparisons. Journal of Ecology 64, 41–78.
| Investigations into the properties of quantitative vegetational data: II. Further data type comparisons.Crossref | GoogleScholarGoogle Scholar |
Specht RL (1981) Foliage projective cover and standing biomass. In ‘Vegetation Classification in Australia’. (Eds AN Gillison, DJ Anderson) pp. 10–21. (CSIRO in association with Australian National University Press: Canberra)
Stanton JP, Morgan MG (1977) Project RAKES – The rapid selection and appraisal of key and endangered sites: the Queensland case study. PR 4. (Department of Environment, Housing and Community Development, University of New England: Armidale, NSW, Australia)
Stromberg JC, Merritt DM (2016) Riparian plant guilds of ephemeral, intermittent and perennial rivers. Freshwater Biology 61, 1259–1275.
| Riparian plant guilds of ephemeral, intermittent and perennial rivers.Crossref | GoogleScholarGoogle Scholar |
Thackway R, Cresswell ID (1995) ‘An interim Biogeographic Regionalisation for Australia: a Framework for Establishing the National System of Reserves. Ver. 4.0.’ (Australian Nature Conservation Agency: Canberra)
Tichý L, Chytrý M, Hajek M, Talbot SS, Botta-Dukát Z (2010) OptimClass: using species-to-cluster fidelity to determine the optimal partition in classification of ecological communities. Journal of Vegetation Science 21, 287–299.
| OptimClass: using species-to-cluster fidelity to determine the optimal partition in classification of ecological communities.Crossref | GoogleScholarGoogle Scholar |
Tichý L, Chytrý M, Botta-Dukát Z (2014) Semi-supervised classification of vegetation: preserving the good old units and searching for new ones. Journal of Vegetation Science 25, 1504–1512.
| Semi-supervised classification of vegetation: preserving the good old units and searching for new ones.Crossref | GoogleScholarGoogle Scholar |
Tichý L, Hennekens SM, Novák P, Rodwell JS, Schaminée JHJ, Chytrý M (2020) Optimal transformation of species cover for vegetation classification. Applied Vegetation Science 23, 710–717.
| Optimal transformation of species cover for vegetation classification.Crossref | GoogleScholarGoogle Scholar |
Walker DA, Daniels FJA, Matveyeva NV, Sibik J, Walker MD, Breen AL, Druckenmiller LA, Raynolds MK, Bultmann H, Hennekens S, Buchhorn M, Epstein HE, Ermokhina K, Fosaa AM, Heidmarsson S, Heim B, Jonsdottir IS, Koroleva N, Levesque E, MacKenzie WH, Henry GHR, Nilsen L, Peet R, Razzhivin V, Talbot SS, Telyatnikov M, Thannheiser D, Webber PJ, Wirth LM (2018) Circumpolar Arctic vegetation classification. Phytocoenologia 48, 181–201.
| Circumpolar Arctic vegetation classification.Crossref | GoogleScholarGoogle Scholar |
Wesche K, von Wehrden H (2011) Surveying southern Mongolia: application of multivariate classification methods in drylands with low diversity and long floristic gradients. Applied Vegetation Science 14, 561–570.
| Surveying southern Mongolia: application of multivariate classification methods in drylands with low diversity and long floristic gradients.Crossref | GoogleScholarGoogle Scholar |
Whittaker RH (1973) Approaches to classifying vegetation. In ‘Ordination and Classification of Communities’. (Ed. RH Whittaker) pp. 323–354. (Junk: The Hague, Netherlands).
Wilson PR, Taylor PM (2012) Land zones of Queensland. Available at http://www.ehp.qld.gov.au/assets/documents/plants-animals/ecosystems/land-zones-queensland.pdf. [Verified 15 August 2020]
Wiser SK, De Cáceres M (2013) Updating vegetation classifications: an example with New Zealand’s woody vegetation. Journal of Vegetation Science 24, 80–93.
| Updating vegetation classifications: an example with New Zealand’s woody vegetation.Crossref | GoogleScholarGoogle Scholar |
Wiser SK, De Cáceres M (2018) New Zealand’s plot-based classification of vegetation. Phytocoenologia 48, 153–161.
| New Zealand’s plot-based classification of vegetation.Crossref | GoogleScholarGoogle Scholar |


