A handbook for the standardised sampling of plant functional traits in disturbance-prone ecosystems, with a focus on open ecosystems
B. J. Wigley A B X , T. Charles-Dominique C , G. P. Hempson D , N. Stevens E F , M. TeBeest G H I , S. Archibald D , W. J. Bond J , K. Bunney K , C. Coetsee L B , J. Donaldson M , A. Fidelis N , X. Gao O , J. Gignoux C , C. Lehmann P Q , T. J. Massad R , J. J. Midgley J , M. Millan D F , D. Schwilk O , F. Siebert S , C. Solofondranohatra T U , A. C. Staver V , Y. Zhou V and L. M. Kruger W J
A B X , T. Charles-Dominique C , G. P. Hempson D , N. Stevens E F , M. TeBeest G H I , S. Archibald D , W. J. Bond J , K. Bunney K , C. Coetsee L B , J. Donaldson M , A. Fidelis N , X. Gao O , J. Gignoux C , C. Lehmann P Q , T. J. Massad R , J. J. Midgley J , M. Millan D F , D. Schwilk O , F. Siebert S , C. Solofondranohatra T U , A. C. Staver V , Y. Zhou V and L. M. Kruger W J
A Department of Environmental Biology, Sapienza University of Rome, Piazzale Aldo Moro 5, I-00185 Rome, Italy.
B School of Natural Resource Management, Nelson Mandela University, George, 6529, South Africa.
C CNRS UMR7618, Institute of Ecology and Environmental Sciences, Paris, Sorbonne University, 4 Place Jussieu, F-75252 Paris, France.
D Centre for African Ecology, School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, 2050, South Africa.
E Environmental Change Institute, School of Geography and the Environment, University of Oxford, Oxford, OX1 3QY, UK.
F Department of Botany and Zoology, Stellenbosch University, Private Bag X1, Matieland 7602, South Africa.
G Copernicus Institute of Sustainable Development, Utrecht University, PO Box 80115, NL-3508 TC Utrecht, Netherlands.
H Centre for African Conservation Ecology, Nelson Mandela University, PO Box 77000, Port Elizabeth, 6031, South Africa.
I South African Environmental Observation Network (SAEON), Grasslands, Forests, Wetlands Node, Montrose, 3201, South Africa.
J Department of Biological Sciences, University of Cape Town, Rondebosch, 7701 South Africa.
K Department of Zoology and Entomology, University of Pretoria, Hatfield, South Africa.
L Scientific Services, Kruger National Park, Private Bag X402, Skukuza, 1350, South Africa.
M Department of Biology, Wake Forest University, 049 Winston-Salem, NC 27109, USA.
N Universidade Estadual Paulista (UNESP), Instituto de Biociências, Lab of Vegetation Ecology, Avenida 24-A 1515, 13506-900, Rio Claro, Brazil.
O Department of Biological Sciences, Texas Tech University, Lubbock, TX 79409, USA.
P Tropical Diversity, Royal Botanic Garden Edinburgh, Edinburgh, EH35NZ, UK.
Q School of GeoSciences, University of Edinburgh, Edinburgh, EH9 3FF, UK.
R Department of Scientific Services, Gorongosa National Park, Sofala, Mozambique.
S Unit for Environmental Sciences and Management, North-West University, Private Bag X6001, Potchefstroom 2520, South Africa.
T Laboratoire de Botanique, Département de Biologie et Ecologie Végétales, Faculté des Sciences, Université d’Antananarivo BP 906, Antananarivo 101, Madagascar.
U Kew Madagascar Conservation Centre, Lot II J 131 B, Ambodivoanjo-Ivandry, Antananarivo 101, Madagascar.
V Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06511, USA.
W Organization for Tropical Studies, PO Box 33, Skukuza, 1350, South Africa.
X Corresponding author. Email: benwigley@gmail.com
Australian Journal of Botany 68(8) 473-531 https://doi.org/10.1071/BT20048
Submitted: 6 May 2020 Accepted: 8 October 2020 Published: 30 November 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Plant functional traits provide a valuable tool to improve our understanding of ecological processes at a range of scales. Previous handbooks on plant functional traits have highlighted the importance of standardising measurements of traits to improve our understanding of ecological and evolutionary processes. In open ecosystems (i.e. grasslands, savannas, open woodlands and shrublands), traits related to disturbance (e.g. herbivory, drought, and fire) play a central role in explaining species performance and distributions and are the focus of this handbook. We provide brief descriptions of 34 traits and list important environmental filters and their relevance, provide detailed sampling methodologies and outline potential pitfalls for each trait. We have grouped traits according to plant functional type (grasses, forbs and woody plants) and, because demographic stages may experience different selective pressures, we have separated traits according to the different plant life stages (seedlings saplings and adults). We have attempted to not include traits that have been covered in previous handbooks except for where updates or additional information was considered beneficial.
Keywords: demographic bottlenecks, disturbance, drought, fire, forbs, grasses, herbivory, plant functional traits, saplings, seedlings, woody plants.
Introduction and discussion
The standardisation of methodologies used for measuring plant functional traits at the global scale allows us to (1) better understand the selective forces shaping the functioning of plant communities, (2) better describe ecosystems in terms of a limited number of ecological component types, and (3) better predict species-response rules to ecosystem perturbations (Cornelissen et al. 2003; Pérez-Harguindeguy et al. 2013; Díaz et al. 2016). The standardised measurement of traits also allows for drawing comparisons among different regions of the globe (Rusch et al. 2003). While previous trait handbooks by Cornelissen et al. (2003) and Pérez-Harguindeguy et al. (2013) have immense pertinence to open ecosystems (i.e. areas with climates suitable for forest, but dominated, instead, by grasslands, savannas, open woodlands or shurblands; Bond 2019), they do not capture the full set of traits that are relevant to the functioning of these widespread ecosystems. The selection pressures faced by plants in open ecosystems are largely driven by disturbance, often in the form of fire and herbivory (but also seasonal flooding or drought). The plants found in these open ecosystems have, therefore, had to evolve a diverse suite of plant traits, many of which are related to resisting or tolerating the dominant disturbance agent(s) (Bond and Midgley 2001; Bond 2005).
Open ecosystems: savannas, grasslands and shrublands
Open ecosystems, largely dominated by shade-intolerant plants, occur in consumer-controlled (i.e. disturbance-prone) environments that often have the potential to support closed canopy vegetation if not for the ecological processes that remove trees (Pausas and Bond 2020). The savanna biome, one of the largest terrestrial biomes (Lehmann et al. 2014), supports significant animal and plant biodiversity (Bond and Parr 2010; Murphy et al. 2016) and provides ecosystem services that sustain a large proportion of the world’s human population (Scholes and Archer 1997). Savannas are defined as having a discontinuous woody overstorey with a continuous, predominantly C4 grassy understorey (Ratnam et al. 2011). Tree densities vary widely, from treeless grasslands to savannas with near-continuous tree cover (respectively classified as campo sujo and cerrado sensu stricto in Brazilian savannas). The most important criterion to distinguish savannas from closed systems (e.g. thicket or forest) is the presence of shade-intolerant and typically C4 grasses. This definition allowed for the reclassification of many systems that were previously thought of as degraded ecosystems, as ecosystems with shared ecological functioning on different continents (Bond et al. 2008; Ratnam et al. 2011; Ratnam et al. 2016), and opened opportunities for global comparisons. Although trees and grasses are typically used to identify savannas, forbs should not be overlooked because they often contribute the highest levels of diversity in old-growth savannas (Zaloumis and Bond 2011). Throughout this handbook, we regard the functioning of grasslands, shrublands and savannas to be similar, because of the strong parallels in their drivers. Although much of the work included in this trait handbook has focused on African, South American and Asian savannas and grasslands, we consider these effective exemplars of disturbance-driven ecosystems and we anticipate that the traits covered here can be applied to other open ecosystems.
Demographic bottlenecks
The strong effect of disturbances on savanna structure and composition is shown both by a demographic bottleneck specific to savannas, characterised by a high mortality of woody saplings within the flame and herbivore zones (Higgins et al. 2000; Sankaran et al. 2005; Wakeling et al. 2011; Hoffmann et al. 2012), and by the distinct vertical distribution of vegetation when fire or megaherbivores such as elephant are present (Higgins et al. 2007; Asner et al. 2009; Smit et al. 2010; Midgley et al. 2011; Asner and Levick 2012; Bond 2019). In mesic savannas, which burn frequently, tree saplings must grow above the flame zone of grass-fuelled surface fires to escape top-kill and reach adult sizes (Trollope 1984). The fire trap, a concept first made explicit by Bell (1984), refers to the 0–3-m zone that experiences repeated fires, resulting in the top-kill or aboveground mortality of plants that are not adequately protected from the effects of fires (Fig. 1). In herbivore-driven savannas, elephants (see Kerley et al. 2008 for review) and other mega-herbivore species, e.g. giraffes, have been shown to regulate tree allometries (Moncrieff et al. 2011) and height (Fornara and du Toit 2007). Staver and Bond (2014) provided experimental evidence for the effects of meso-herbivores (e.g. impala, nyala and kudu) in regulating tree growth and recruitment, resulting in a browse-trap (Fig. 1).
In frequently disturbed savanna systems, the main constraints on plant fitness include resource competition (for growth and reproduction) and resource storage (for either surviving or escaping disturbances, or both). Escape opportunities are rare in savannas, but many savanna trees, such as Acacia karroo (Schutz et al. 2009; Wigley et al. 2009a), can resprout repeatedly from belowground root stores. Plants may remain in the flame zone for decades, until a sufficient interval between fires occurs or until growth rates are such that recruitment into larger size classes occurs between fires (Bond and van Wilgen 1996; Gignoux et al. 1997; Schutz et al. 2009; Wigley et al. 2009a). Furthermore, intraspecific growth rates of savanna woody saplings have been found to be highly variable; for example, Wakeling et al. (2011) found that only 5% of saplings had growth rates that were fast enough to escape being top-killed under the dominant fire regime at their mesic, grass-dominated study site.
Consumption patterns
Chronic disturbances, such as fire and vertebrate herbivory, can remove significant plant biomass in savannas. While fire and herbivores both consume vegetation, the manner in which they do so, and thus what plants need to respond to, differs substantially (Archibald and Hempson 2016). Fires are typically widespread episodic events (that generate heat damage and combustion), whereas herbivory tends to be a spatially patchy but more continuous occurrence (that causes breaking, stripping, biting and trampling). Plants may, thus, be able to gradually develop their fire responses to meet a requisite effectiveness threshold (such as a bark thickness threshold to survive fire), while responses to herbivory may need to be more immediate (e.g. grow longer spines). Plant strategies that ensure survival in frequently disturbed systems can be classified into the following four main strategies: (1) escaping the negative effects of consumers by either spatially or temporally evading the disturbance, such as growing out of the reach of flames or herbivores; (2) resisting the disturbance, i.e. remaining exposed to the disturbance but minimising its effect; (3) tolerating disturbance, i.e. being affected by disturbance but having a strategy to replace damaged or removed parts, the tolerance strategy can operate at the individual, population or landscape level (Archibald et al. 2019); and (4) promoting the disturbance, i.e. by providing the right conditions for the disturbance to occur (e.g. by having high flammability or high palatability).
Fire characteristics
Fire effects depend on the size, intensity, season and return interval of any given fire (Martin and Sapsis 1992); all these properties vary in a predictable way (to some extent) along a rainfall gradient. The occurrence of fire is determined by the availability of dry-season grass fuel (Bond, 1997), and production of fuel is linearly dependent on moisture availability (O’Connor and Bredenkamp 1997; Scholes and Walker 2004). High-rainfall savannas (values differ among continents; Lehmann et al. 2014) have rapid accumulation of grass biomass, and thus burn at frequent intervals (1–2 years). Because of high humidity, they burn mostly during the late dry season. By contrast, drier savannas burn less frequently (3–10 years), but with hot fires burning throughout the longer dry season (Scholes 1997; Williams et al. 1998).
Herbivore characteristics
Herbivore pressure depends on the density of herbivores (total biomass) at a site and the functional composition of herbivore communities. Herbivores fall on a spectrum, from being exclusive grazers to specialist browsers (some herbivores tend to be mixed-feeders and consume a mix of grass and browse), with specific dietary adaptations (e.g. mouth dimensions and teeth, neck size and trunk) that influence the quality of the consumed forage (e.g. tree leaves, long grass and short grass). Larger and smaller herbivore species have different diets and feeding modes, with larger animals needing to eat more each day, taking bigger bites but accepting a lower forage quality. Gut types also influence herbivore diets, with ruminants (slower retention times) typically being able to accept a lower-quality diet (Illius and Gordon 1992).
In African savannas, herbivore pressure correlates with rainfall and soil nutrient gradients (Hempson et al. 2015). Mesic savannas (>800-mm mean annual precipitation) tend to have a large quantity of low-quality forage and browse and host communities dominated by large-bodied ‘bulk feeders’, such as elephants. African semi-arid savannas (400–800 mm) usually have high-quality forage and browse and typically host-abundant and diverse herbivore communities with a wide range of body sizes and feeding guilds. Whether other continents show similar turnover in the composition of herbivore communities, and at what thresholds such transitions occur (e.g. along rainfall gradients), remain to be tested (Lehmann et al. 2014). The role of invertebrate herbivory has often been neglected in many open ecosystems but has begun to receive more attention. Termites have been shown to have a profound effect on savanna vegetation, especially during the wet season (Davies et al. 2016), and have been found to remove as much grass biomass as mammalian herbivores do in broadleaf savannas (Scholes and Walker 2004).
Co-variation, feedbacks and effect traits
Many of the core drivers of savanna functioning tend to co-vary and interact with each other. This should be kept in mind when making predictions related to environmental filtering in savannas. In African savannas, for example, herbivores preferentially consume high-quality plant material with low C : N ratios and high moisture content, whereas fires require dry fuels and readily consume grasses with high C : N ratios. Higher quality ‘forage’ resources are typically more abundant in drier regions because: (1) soils are more nutrient-poor in wet areas due to increased leaching, and (2) fast growing grasses that develop in wetter regions allocate more to structural support tissues with a high C : N ratio. Consequently, in African savannas, herbivory plays a greater role in filtering plant communities in drier systems, whereas fire becomes more important in wetter systems (Archibald and Hempson 2016). In other systems, for example, Brazilian cerrado, Alvarado et al. (2017) found that fires are weakly associated with annual rainfall; however, spatial and temporal variation in fires was determined by drought during the ignition season. Although the determinants of consumer patterns may differ across continents, such covariation among different controls in savannas will necessarily produce non-causal correlations between bottom-up drivers and traits related to disturbance, and vice versa.
Positive feedback loops occur in savannas when keystone species help create the conditions required for the maintenance of the system. Fires and herbivory typically involve positive feedback loops (Sankaran et al. 2008, 2013; Staver et al. 2009, 2011; Hoffmann et al. 2012). In the case of fire, it is well established that as C4 grasses evolved to become more flammable, they created the conditions necessary to open forested environments and, thereby, facilitated their own spread (Beerling and Osborne 2006). In the absence of fire, shade-intolerant C4 grasses are progressively filtered out by shade as both tree canopy cover increases and moribund grass material accumulates (Bond et al. 2005; Staver et al. 2011; Hoffmann et al. 2012). This feedback, therefore, not only determines the presence of flammable grasses, but also affects the performance of other functional types; many savanna tree and forb species are well adapted to fire but are frequently poor competitors for light, and often tend to be shade intolerant. Grazing lawns provide another example of a positive feedback. In this case, highly palatable grass species on grazing lawns are adapted to survive high grazing pressure and provide highly palatable forage to grazers, which further increase the productivity of the system through the deposition of dung and urine (e.g. see McNaughton 1984; Hempson et al. 2015, 2019).
As savanna functioning is often dependent on positive feedback loops, it is particularly important to understand how plants contribute to the modification of their own environment (as opposed to responding to climatic variables only). This emphasises the importance of traits that directly modify the environment of a species, i.e. effect traits (Violle et al. 2007). Some of the effect traits responsible for the stability of savannas (or for transitions to alternative biome states; Higgins et al. 2012; Charles-Dominique et al. 2015a; Kruger et al. 2017) have been well investigated (such as flammability of the grass layer or the palatability of the grass layer; Hempson et al. 2019), whereas others are still under active scrutiny (e.g. shading abilities by trees; Charles-Dominique et al. 2018).
Trait descriptions
Here we include a total of 34 traits, 28 of which have been previously published, and six that have not been previously described. Several of the traits in this handbook overlap with those in previously published handbooks (i.e. Cornelissen et al. 2003; Pérez-Harguindeguy et al. 2013), but we have refined these to include additional information relevant to open ecosystems. Previously undescribed traits are included to fill gaps identified in the previous handbooks. Some traits have been published in dedicated articles, whereas some have been validated only by the respective authors (Box 1) at their respective field sites. For the new traits outlined here, we hope that the standardisation of the methods outlined in this handbook will provide useful guidance about how to apply them in the field and facilitate collaborations among researchers.
| Box 1. Life forms, environmental filters and the difficulty level for quantification of each of the traits described in this protocol |
| The corresponding authors (and email addresses) for each trait description are as follows: {GH}, Gareth Hempson (gareth.hempson@wits.ac.za); {SA}, Sally Archibald (sally.archibald@wits.ac.za); {CS}, Cedrique Solofondranohatra (lovacedrique@gmail.com); {JD}, Jason Donaldson (jubatusdnl@gmail.com); {DS}, Dylan Schwilk (Dylan.Schwilk@ttu.edu); {XG}, Xiulin Gao (xiulin.gao@ttu.edu); {FS}, Frances Siebert (Frances.Siebert@nwu.ac.za); {AF}, Alessandra Fidelis (alessandra.fidelis@unesp.br); {KB}, Katherine Bunney (katherine.bunney@gmail.com); {NS}, Nicola Stevens (nicolastvns@gmail.com); {JG}, Jacques Gignoux (jacques.gignoux@upmc.fr); {TCD}, Tristan Charles-Dominique (tristan.charles-dominique@sorbonne-universite.fr); {JM}, Jeremy Midgley (Jeremy.Midgley@uct.ac.za); {LK}, Laurence Kruger (laurence.kruger@gmail.com); {BW}, Benjamin Wigley (benwigley@gmail.com); {CC}, Corli Coetsee (Corli.Coetsee@sanparks.org); {TM}, Tara Massad (tmassad77@gmail.com); {YZ}, Yong Zhou (yong.zhou@aggienetwork.com); {ACS}, Ann Carla Staver (carla.staver@yale.edu}. |
 |
For each trait described in this handbook, we provide a brief trait description, an outline of the relevant environmental filters, detailed sampling methodologies and, where possible or necessary, we have included extra notes, special cases, and warnings or common pitfalls in sampling. In contrast to previous handbooks (Cornelissen et al. 2003; Pérez-Harguindeguy et al. 2013), we treat grasses, forbs and woody plants separately, as each functional type faces a distinct set of constraints and plays a different role in ecosystem functioning. Although not quantified for all the listed traits, we have provided an indication of whether traits have low or high levels of intraspecific variability. For the traits for which we have information on the levels of intraspecific variability, we attempted to provide recommendations with respect to sampling replication. However, sampling replication ultimately depends on the question being asked, the study design and the available resources. We have ordered the trait descriptions to begin with traits that are relevant to seedlings and adults of all plant functional types, followed by traits relevant to one functional type only, namely, grasses, forbs and woody plants.
Seed and seedling traits relevant to forbs, grasses and woody plants
How to define a seedling?
There are many possible ways to define a seedling, which vary according to definitions based on physiology, life history, plant architecture, time or functional type. For the purposes of this handbook, a woody seedling is defined as ‘the plantlet resulting from germination that is produced during its first growing season’. This will differ for forbs and grasses, which are often able to grow into adults within a few weeks or months. According to the above definition, a woody seedling cannot be more than 1 year old. An undisturbed woody seedling becomes a sapling, juvenile or subadult in its second year.
Dispersal mode (DM)
Trait description
Here, we adapt the definition of dispersal mode from Pérez-Harguindeguy et al. (2013) for savanna ecosystems. Dispersal mode (DM) is a categorical trait. The assignment of a propagule to a particular dispersal mode is based on either the morphological features of the fruit or seed (Table 1, Fig. 2) or observation of the seed-dispersal vector. The DM of a propagule determines the distance it can travel from the parent plant. Dispersal mode can be used as a proxy for the likelihood of escaping from both ‘density-dependent’ predation (e.g. host-specific herbivores and pathogens: Janzen 1970; Connell 1978) and from physical damage (e.g. fire or desiccation) on the soil surface. Dispersal mode applies to woody plants, forbs and grasses in their reproductive stages.

|

|
Relevant environmental filters
In areas with high densities of megaherbivores (large vertebrates with a body mass typically exceeding 1000 kg; Owen-Smith 1988), one might predict the presence of large specialised fruit (megafaunal fruit). Large seed size confers faster initial seedling growth, i.e. better performance in a competitive context where a large seedling size prevents overtopping by others, but generally comes at the cost of reduced dispersal ability (Ezoe 1998). Large vertebrates circumvent the dispersal–survivability trade-off in seed size because they can transport seeds over long distances (Dudley 2000; Guimarães et al. 2008; Bunney et al. 2017). Large fruits (megafaunal fruits) have evolved alongside large vertebrates to take advantage of these remarkable seed vectors. In addition, trees that retain indehiscent pods for consumption by large vertebrates escape insect damage (e.g. Acacia seeds and bruchid beetle damage) and, subsequently, have significantly higher rates of germination (Or and Ward 2003). The historical distribution of megaherbivores does not guarantee the occurrence of megafaunal fruit (Bunney et al. 2019). Megafaunal fruit are a tropical phenomenon, namely, they have largely evolved in the Paleotropics. Abiotic factors, such as precipitation and temperature, that underpinned their evolution now limit their distribution.
In areas with high rates of herbivory, the foliage-as-fruit dispersal mechanism is also likely to be selected for. In this scenario, the foliage of small-seeded plants may function ecologically as fruit (Janzen 1984), attracting large herbivores in much the same way as berries and arils attract and reward birds. Large ungulates ingest minute seeds and fruiting stalks that are veiled in leaf tissue. These seeds would likely be tough, digestion-resistant seeds, so as to increase survival past the massive grinding molars and through the lengthy gut (Dinerstein 1989). These seeds would frequently be defecated in nutrient-rich hotspots (dung piles) and in favourable germination sites, such as the edges of game trails, within bush clumps, wallow edges and along riverbanks. In addition, African savannas with their high diversity and biomass of ungulates are likely to have selected for higher than expected rates of attachment (epizoochory). This is particularly true of the African achyranthoids (Amaranthaceae), where attachment (epizoochory) is the primary dispersal mode in 80% of the species. African achyranthoids largely occur in Acacia–Commiphora savannas, dry evergreen afromontane forests and grasslands and are, thus, suitable for long-distance dispersal via attachment. The diaspores of Pupalia have been described as possessing ‘the most persistently adhesive burrs known’ (Ridley 1930).
Vertebrate dispersal increases the probability that a dispersed seed will find itself near fire-protecting trees (sufficiently large to have competitively displaced grass and, thus, reduced the fuel load) because tree clumps are foci for animal activity (Dean et al. 1999). We predict that in savannas with a high fire frequency, woody plants that present their propagules above the height of the fire trap (flame zone) improve their chances of survival. Presenting propagules at a height where wind, large vertebrates and birds are the only possible agents of dispersal, is likely to confer an advantage to savanna tree species. Wind dispersal may be enhanced in the immediate post-fire period because fire clears the canopy and ground of many obstacles to the passage of wind and seeds. For example, Bond (1988) showed that the fruits of some Protea and Leucadendron species may be tumbled over 50 m by wind in post-fire environments.
Method
The propagules of the plant species under investigation are assigned to one of the dispersal modes in Table 1 on the basis of either the morphological features of the fruit or seed or of observations of the seed-dispersal vector.
Special cases
Assigning a single dispersal mode to a species, on the basis of the morphological features of the fruit or seeds, implies an exclusive plant–disperser interaction. However, it is common for a plant to be dispersed by several dispersal agents (Herrera 1985; Blüthgen et al. 2007). The dispersal mode should suggest the agent for which the plant has been primarily adapted. That is, the agent that is likely to provide the most effective dispersal service. Observational studies of seed dispersal are necessary to appreciate the suite of agents that each plant species engages.
Seed-burying mode (SBM)
Current knowledge does not yet allow for a full understanding of the relationship between burial depth and seed morphology. For this reason, this trait is a first attempt to list known burial mechanisms. The burying modalities listed here are most likely not exhaustive, and we encourage readers to complement this trait with all contextual and specific modalities promoting seed burial found in the area or in the group of species under study.
Trait description
The probability of successful germination depends on the depth at which a seed is buried in the soil. Seeds tend to have lower rates of successful germination or to die when they are too close to the surface or if they are buried too deep (Collins and Wein 1997; Garnier and Dajoz 2001; Benvenuti 2016). Seed dispersal usually results in the seed lying on the ground surface. The seed may then get buried below the soil surface. The following three main strategies are used by plants to bury their seeds: (1) attract animals that will place the seed underground (e.g. ants or squirrels), (2) develop morphological structures that move according to humidity, such as coil or bend as they dry (Abraham and Elbaum 2013), thereby pushing the seed into the soil, (3) develop a particular germination sequence that will push the embryo deep into the soil, while the seed stays on the ground surface (e.g. many Arecaceae: Tomlinson and Jeffrey 1990).
The attraction of burying insects (mainly ants) usually relies on the presence of an aril or elaiosome (cf. dispersal mode). The attraction of squirrels, or more generally, seed-caching rodents, is a matter of seed size and chance (success depends on the death or poor memory of the seed owner). The burial depth in these strategies will depend on the behaviour of the particular animal species, so that the most important feature to record is the animal species.
Self-burial depth depends on many factors, including seed size (Bond et al. 1999, Benvenuti 2007), soil texture (Benvenuti 2007; Molano-Flores 2012), presence of cracks in the soil (Stamp 1984), and morphological features enabling self-burial. There is considerable variation in these features. For example, seed awns, which have been proved to be efficient burial mechanisms (Murbach 1900; Stamp 1984; Garnier and Dajoz 2001; Elbaum et al. 2007) have been found to be highly variable. Cavanagh et al. (2019) analysed 1000 native Australian grass species and identified 20 different morphological types of awn. Biomechanical studies show that seed self-burial using hygroscopic movements is often associated with (1) oblique bristles, hairs or hooklets located on the seed or the base of the awn, that act as ratchets that prevent the seed from exiting the soil once it has started to enter it (Elbaum et al. 2007; Kulić et al. 2009; Grohmann et al. 2019), (2) a tapered or twisted seed tip (Stamp 1984) similar to a screw tip, and (3) a long awn with one or more bends that will act as a lever to move the seed on the soil surface until it reaches an obstacle (crack, pebble, other plant), then orientate the seed vertically and push it into the soil by its rotating movements induced by its shape and variations of humidity (Stamp 1984; Garnier and Dajoz 2001; Evangelista et al. 2011; Molano-Flores 2012).
Arecaceae (palm trees) have developed specific germination modes where their typically large seeds lay on the ground but send the apical meristem below ground where growth and survival conditions are better for the seedling (Tomlinson and Jeffrey 1990).
Relevant environmental filters
In undisturbed environments, there is an optimal burial depth for germination, depending on soil texture and seed size (Molano-Flores 2012). Poor germination close to the surface may result from the quick alternance of dry and wet conditions; deeper layers are more predictably humid. Poor germination deep in the soil may result from the heterotrophic phase of germination not permitting the first leaves to reach the surface, where autotrophic growth can start. Accordingly, small seeds cannot germinate as deep as can large seeds (Benvenuti 2016). Note that this pattern can also be explained by predation and pathogens; animal predators will find the seeds very easily when on or close to the soil surface, whereas fungi in the deeper, wetter soil layers are more likely to attack germinating seedlings.
In fire-prone environments such as humid savannas, fire adds a further constraint on seed germination. The deeper a seed is buried, the better its chances of surviving fire (up to a threshold, which we suggest to be 5 cm), as temperature quickly decreases with depth, with no measurable increase in temperature below 5 cm during most savanna fires (Abbadie et al. 2006). Under natural conditions, all these constraints interact (e.g. Garnier and Dajoz 2001), resulting in the existence of an optimal depth for germination that depends on seed size (Bond et al. 1999). Larger seeds typically need more rain to bury (Benvenuti 2007). Small seeds can self-bury deeper than can large seeds (Benvenuti 2007).
Method
Seed-burying mode of the plant species under investigation is assigned to one of the modes listed in Table 2. The grouping of the 20 awn types of Cavanagh et al. (2019) into five classes is based on the expected different mechanical behaviour of single straight, bent or curved awns v. multiple awns (Elbaum et al. 2007), which suggests that the number of functional classes is smaller than the number of morphological classes. Classes and subclasses are exclusive, so that a given species should be attributed to exactly one of the nine possible SBM values.

|
Related measures and traits are as follows:
-
For Class A, it may be useful to record as a companion variable the animal disperser species (SBM-A:ADS).
-
For Class S, presence of ratchet-like structures (SBM-S:RS) and a tapered seed tip (SBM-S:TT) should be recorded, along with awn anatomy, because experimental studies have suggested that an awn would be useless without a ratchet (little is known about the role of seed tip, but it might be as important).
-
Because burying is dependent on seed mass (SM), it should also be recorded as a related trait (cf. measurement of seed mass in Cornelissen et al. 2003).
-
Because the strength of seed movements, and, as a consequence, the burial depth, depend on awn length (Garnier and Dajoz 2001; Kulić et al. 2009) on one hand, and on seed size on the other hand (Benvenuti 2007), it may be useful to measure the ratio of average awn length to average seed mass (SBM-S:ALM) as a measure of the effort of a species to develop self-burying (a large ALM means a higher investment in awn length relative to overall seed size).
-
After a germination event in the field (e.g. a heavy-rainfall event at the onset of the rainy season), a careful measurement of the depth at which the seed of an emerging seedling (SBD) is found could be used as a direct measure of this trait. Auld and Denham (2005) used the length of the hypocotyl, measured from its colour change when reaching the soil surface to the attachment of the first radicle, noticeable by a sharp decrease in diameter, to measure burial depth. In the case of hypogeal germination, the decrease in diameter corresponds with the attachment of the (buried) cotyledons, an even clearer indication of where the seed lay before germination.
Seed dormancy (SD)
Trait description
Seed dormancy (SD) is a mechanism to prevent germination during unsuitable ecological conditions (Baskin and Baskin 1998; Poschlod et al. 2013). The presence of dormancy and the cues to break it are an important first step in the successful establishment of a plant (Donohue et al. 2010). Within a savanna context, the specific cues required to break SD can act as filters of regional species pools into local plant communities (Jiménez-Alfaro et al. 2016), and identifying the mechanisms required to break dormancy indicate when species filtering may occur through the absence of suitable germination cues.
Relevant environmental filters
Seeds germinating in arid savannas typically face the problem of small and infrequent rainfall events that create a recruitment bottleneck through limiting successful seedling establishment in the first month following germination (Higgins et al. 2000; Botha et al. 2007; Stevens et al. 2014). We predict that in arid savanna environments, plants will adopt an arid germination strategy, with a plant community characterised by no coat-imposed dormancy and rapid seed germination when exposed to sufficient water. Seeds are likely to have high germination rates, and an increased tolerance to moderate water stress (Choinski and Tuohy 1991; Baskin and Baskin 1998; Stevens et al. 2014). When the initial germination event is not followed by suitable environmental conditions, such as rainfall (Jurado and Westoby 1992), germination failure can result and cause a rapid depletion of the seed bank, thus creating a potential additional limitation to seedling establishment.
As rainfall increases across the savanna moisture gradient, we propose that there is a trade-off between high germinability and dormancy (Stevens et al. 2014). In semi-arid regions with a higher mean annual rainfall characterised by frequent small rainfall events, more unsuccessful germination events can increase the likelihood of seed-bank depletion if seed coat-imposed dormancy is not present (Botha et al. 2007; Stevens et al. 2014). We propose that there will be an increase in the frequency of coat-imposed dormancy as rainfall becomes more predictable. This pattern has been recorded in grasses (Anderson et al. 2012) but has not been recorded at the global scale in woody species and remains to be tested for savanna woody species (Jurado and Flores 2005). As herbivores are dominant at the lower end of the savanna rainfall gradient, we predict that dormancy-breaking mechanisms associated with herbivores (e.g. seed scarification and acid-breaking dormancy processes) will be most common. How successful these (mega)-herbivore-adapted species are in the absence of megaherbivores remains to be conclusively tested for savannas.
Method
The most common way to record dormancy is to measure it through the absence of germination (Finch-Savage and Leubner-Metzger 2006). Whereas a full range of dormancy-breaking protocols utilised by the Millenium seedbank are listed and described in Liu et al. (2020), for open ecosystems, we propose that treatments associated with drivers of these ecosystems will show how strongly these species can be filtered from the community. Germination trails can be conducted using 25–50 seeds replicated five times, using seeds that were produced within the same growing season (less than 6 months old). Seeds should be sown onto 1% aqueous agar in plastic Petri dishes and incubated at a warm temperature of ~25°C (savanna seed thermal niches commonly vary between 20 and 35°C) and exposed to a 12 h light–12 h dark photoperiod (Choinski and Tuohy 1991; Baskin and Baskin 1998; Stevens et al. 2014; Liu et al. 2020). To test whether dormancy is present, compare the germination rates of untreated to treated seeds germinating in agar. We suggest applying dormancy-breaking treatments that replicate ecosystem processes that represent potential ecosystem filters. Herbivory as a dormancy-breaking mechanism can be represented using either mechanical or acid scarification, or both. Mechanical scarification involves the physical abrasion of the seed coat by using clippers or sandpaper and acidic scarification involves exposing seeds to 93% sulphuric acid for 1–5 min (Baskin and Baskin 1998). In mesic savannas where fire is the dominant process, heat treatment is effective for breaking the dormancy of seeds (Baskin and Baskin 1998; Light et al. 2004). Although smoke may stimulate germination, post-dormancy breaking it is not widely used as a dormancy-breaking mechanism for savanna species (Dayamba et al. 2010; Fichino et al. 2016). Thermal scarification is undertaken by either applying a dry-heat treatment when seeds are placed in an oven at 80–100°C for 2–4 min, or a wet-heat treatment when seeds are immersed and soaked in boiled water (85–100°C), which is left to cool with seeds remaining immersed in the water for 12 h. The treated seeds are then placed on agar (as described above) and left to germinate, while monitoring the germination process for 30 days.
Radicle extension rate (RER)
Trait description
Radicle extension rate (RER) quantifies the extension rate of a radicle in the first month following germination. Seedling survival depends on water availability in the upper layers of the soil and the capacity of the seedling to extend its radicle into the moister layers below the evaporation zone (Knoop and Walker 1985; Scholes and Walker 2004; February and Higgins 2010). Measuring the seedling RER in water-limited systems allows us to estimate how long a seedling will take to extend its radicle out of the evaporation zone of the soil into deeper layers and is a critical bottleneck in seedling establishment (Stevens et al. 2014).
Relevant environmental filters
Rapid RER is an important drought-survival mechanism to ensure establishment by allowing the radicle to reach the moister soil layers out of the wetting or drying surfaces of the soil evaporation zone (Choinski and Tuohy 1991; Johnson et al. 1996). We predict that seedlings with a rapid RER will have higher survival in arid and semi-arid savannas. This strategy allows plants to escape the impacts of extended dry periods or droughts during the growing season or to reach moister soil layers below the wetting or drying surface layers.
Method
This method is best performed in the laboratory under standard-temperature (20–35°C) and soil-moisture-stress (0–2 MPa) ranges, because both of these influence radicle-extension rates. Radicle extension can be tracked directly, and newly germinated seeds can be grown in agar on Petri dishes or in growth boxes (Stevens et al. 2014). Osmotic stress can be imposed using polyethylene glycol 6000 (using PEG), where PEG solution is added to water in the growth medium (Michel and Kaufmann 1973; Michel 1983). Radicle length should be measured every third day for the first 30 days after germination. Alternatively, for larger species, 200 newly germinated seeds can be planted in a 1 : 1 vermiculite : sand mix, after which 10 seedlings are harvested every 3 days for the duration of 1 month. On harvesting, radicle length should be recorded for each seedling.
Seedling root : shoot ratio (SRS)
Trait description
For simplicity, we denote ‘roots’ here as any belowground storage structure, and ‘shoots’ as all of the aboveground parts of the seedling. The trait considered here is the seedling root : shoot ratio, computed as the ratio of aboveground dry plant biomass to belowground dry plant biomass at the end of the first growing season (SRS). SRS measures how much a plant invests in belowground reserves at the seedling stage before its first dry season, when fire may occur. For a young seedling, the first dry season and the first fire it encounters are potentially deadly obstacles to its survival following the first wet season. At the onset of the dry season, in the absence of fire, a deciduous species seedling will lose its leaves, which must be set from reserves that may be stored aboveground or belowground. An evergreen species has no such constraint. In the presence of fire, all seedlings (deciduous or evergreen) will lose their leaves and their stems through top-kill, so that only belowground reserves may be used for regrowth at the onset of the next growing season. We, therefore, expect the root : shoot ratio to be critically important to all species in the presence of fire, important for deciduous species in the absence of fire, and unimportant for evergreen species in the absence of fire.
Gignoux et al. (2009) found that surviving the first fire was a sine qua non condition for a species to be present in a fire-prone environment. In their study, forest species were excluded from the savanna environment at the seedling stage because their investments in roots were insufficient (Gignoux et al. 2016). Seedlings of fire-resistant species tend to invest a large proportion of their early growth in roots, resulting in higher survival. The root : shoot ratio quantifies this investment. The importance of this trait has been demonstrated for savanna tree seedlings, but all plants growing in ecosystems that are subject to frequent fires face the same problem, namely, they must grow a fire-resistant structure quickly enough to resist their first fire. We, thus, expect this trait to be applicable to many other species and ecosystems.
Relevant environmental filters
We expect SRS to be directly correlated with the likelihood of surviving the first fire when fire frequency is very high. Fire is not the only possible reason why plants may seek a refuge below the soil surface. Other environmental and disturbance factors, such as frost, drought, herbivory, or any other feature relating to a ‘stressful period’, may also select for high and quick allocation to roots after germination (e.g. biennial plants with belowground storage organs). However, fire is the most severe control, because it is highly unlikely for the aerial parts of any plant species to survive a fire after just one growing season; i.e. for a plant to survive a fire when small, it must rely on belowground storage. Fire should, therefore, be the most effective selective pressure for a quick and high investment into roots.
Method
Measuring the growth of seedlings parts is challenging, because it is subject to a lot of individual and environmental variation. There is a trade-off here. If seedlings growing under natural conditions are sampled, they will most probably be subject to strong competition with grass (e.g. the removal of grass resulted in a 10-fold increase in tree seedling growth rates; J. Gignoux, unpubl. data; see also Cramer et al. 2012), and individual variation in their local environment will be high. If seedlings are grown in a greenhouse or laboratory, better control of individual variation can be achieved, but it will be difficult to reproduce the competitive conditions present in the field. If these traits are required to be reproducible and comparable among species, standardised greenhouse conditions should be used; however, the values obtained will probably be misleading as absolute values. If trait values that reflect growth and allocation in the natural environment of the species are required, seedlings should be sampled in the field, with the risk of low precision in trait values. This choice will have to be clearly stated in any work using this trait. Semi-natural experiments, where seedlings are grown in the greenhouse and then transplanted into a natural environment at the beginning of the rainy season, may constitute a reasonable compromise. Sampling should take place as late as possible in the wet season, but before the seedling has shed its leaves, so as to avoid underestimation of the aboveground mass. Seedlings must be carefully dug out from the soil, so as to lose as few roots as possible. After washing the soil, the aboveground and belowground parts should be separated, oven-dried to a constant weight (usually 48 h at 60°C is sufficient) and weighed. It is good practice to precisely record the method used to wash out the roots from the soil (e.g. mesh size or time spent sorting roots) because final root masses are significantly affected by the method (Gignoux et al. 2006). Root : shoot ratios are then measured as the ratio of belowground biomass to aboveground biomass. Because sampling is destructive, the average root : shoot ratios of several seedlings must be used, or, better, the slope of the principal component regression of seedling root mass over seedling shoot mass (assuming the intercept is not different from zero, the slope is equal to the root : shoot ratio).
Shade tolerance (STol)
Trait description
This trait has been adapted from Gignoux et al. (2016). Shade tolerance (STol) can be measured using a gas-exchange meter on plants in the field. It is not possible for a plant to survive in dense shade and to grow fast. This is a limitation of photosynthesis (Taiz and Zeiger 2006), visible on assimilation-response curves to light irradiance (Fig. 3). The compensation point cp determines when the plant will die because of a negative carbon budget (assimilation = photosynthesis – respiration < 0). To survive under dense shade requires a very low compensation point; however, this is possible only at the cost of a lower maximal assimilation rate (Fig. 3). A species adapted to low light (typically, less than 1% of full light irradiance, e.g. as low as 10–15 µmol photons m–2 s–1, as found in rainforests) will, in a typical savanna environment (irradiance I = 500 in tree clumps to 1200 µmol photons m–2 s–1 in the open, from Simioni et al. 2004), not show any response to variation in light irradiance, whereas a species adapted to full light will respond to a change from 500 to 1200 µmol photons m–2 s–1.

|
Leaf assimilation at various light levels, for example, 500 and 1200 µmol photons m–2 s–1, as shown here, is easy to measure with a gas-exchange meter device, and much faster than constructing an entire assimilation curve to work out the compensation point. The A500 and A1200 values can then be combined in a shade-tolerance trait; for example, the ratio: STol = A500 : A1200 constitutes an easy trait relating to the ability of the plant to survive in dense shade or to grow fast under high light. A species with STol close to 1 is shade-tolerant (no response to high light levels), whereas a species with STol close to 0.5 is light-demanding. Measurements obtained in rainforests (Zotz and Winter 1994; Tinoco-Ojanguren and Pearcy 1995; Nogueira et al. 2004) and savannas (Ronquim et al. 2003; Gignoux et al. 2016) have confirmed this trend.
Relevant environmental filters
Shade tolerance as proposed here measures whether a plant can survive in dense shade and trades-off with its ability for fast growth in open environments. It separates forest tree species adapted to dense shade from pioneer forest species very well, but also the latter from savanna tree species (Gignoux et al. 2016). The low irradiance level used here, 500 µmol photons m–2 s–1, is typically found under tree clumps in savannas, i.e. ‘shady’ conditions (Mordelet and Roux 1993), and also in large treefall gaps in rainforests, i.e. ‘high-light’ conditions (Tinoco-Ojanguren and Pearcy 1995).
Method
Leaf assimilation at I = 500 and 1200 µmol photons m–2 s–1 should be measured with a leaf gas-exchange device such as the LICOR-6400 (LI-COR, Inc., Lincoln, NE, USA) or equivalent. These devices enable the measurement of leaf gas-exchange parameters from leaves directly in the field, while precisely controlling the leaf irradiance and gas composition in the measurement chamber. The measurements should be taken on unstressed plants (during the wet season), on fully developed leaves that grow in full light, and should be repeated on a few individuals (5–10). Standardisation is important for future comparisons of a species; so, whereas irradiance levels other than those proposed here can be used, A500 and A1200 should always be measured. Carbon dioxide (CO2) concentrations in the chamber should be the same as those in the ambient air.
Special cases
Leaves and their photosynthetic capacities are known to have high levels of plasticity. A major source of variation in leaf-photosynthesis response curves is the light environment in which the leaves grew. The variation in maximal assimilation and compensation point can be as large between a sun and a shade leaf of a single individual plant as between species (e.g. Kubiske and Pregitzer 1996; Walters and Reich 2000; Lusk and Del Pozo 2002). Because the light compensation point depends on respiration, it also varies significantly with temperature (Kollmann and Reiner 1996). For these reasons, one should be careful to (1) use this trait only for comparisons within the same study site, not for regional or global comparisons, unless controlling for temperature and light environment, (2) always work on leaves grown in similar environments, for example, in controlled experiments, and (3) carefully check that the light levels proposed here are suited to the local environment, and if not, adapt them.
Seedling leaf : shoot ratio (SLS)
Trait description
The seedling leaf to shoot biomass ratio (SLS) is a measure of a plant’s investment into quick production v. occupation of the vertical space. The extreme disturbance regime of fire- and herbivore-driven savanna ecosystems forces plants, especially small ones, to react quickly to biomass losses. This requires specific adaptations, including very efficient production. Efficient production can be reached through physiological adaptations (e.g. C4 photosynthesis) or through an appropriate allocation strategy, partly measured by this trait. It has been found that savanna tree seedlings invest much more into leaves than do forest tree seedlings, which favour stems (Gignoux et al. 2016).
Relevant environmental filters
In fire-prone savannas, tree seedlings must quickly build up belowground reserves to survive their first fire (Gignoux et al. 2009). Under such constraints, high SLS values are expected, because quick production is more important than exploring the aboveground space (light is easier to access in a savanna than in a forest environment). Hence, savanna tree seedlings, which must quickly build a root system with stored reserves, are expected to have higher investments in leaves than are forest species, which do not have to deal with fire (Gignoux et al. 2016). In herbivore-driven savannas, seedlings may suffer heavy defoliation. When a seedling loses aboveground biomass to herbivory, it needs to be able to recover this quickly to keep growing and resist further herbivory, but it also needs to start building a ‘cage’ or structural defences to resist herbivores. Species growing in areas with a high herbivore pressure are expected to have intermediate to low SLS because they need to produce a lot of stems. In forest, shade is usually the dominant constraint. Species growing in such environments are expected to invest in an erect stem (low SLS), because shading out their neighbours is the only way to win the competition for light.
Method
It is preferable to sample seedlings growing under natural conditions, we also recommend that the same individuals are used for measurements of all allocation traits. Sampling should take place only once the growing season has started, when leaves are fully developed. Seedling leaf : shoot ratio is measured by clipping the aboveground parts then separating all leaf and stem material. If a seedling starts developing belowground stems as storage organs at an early stage, these should not be sampled; only the aerial stems are important because they are likely to be lost in the first fire. Leaves and stems are oven-dried to a constant weight (usually 48 h at 60°C is sufficient) then weighed. Seedling leaf : shoot ratio is then computed as the ratio of leaf biomass to stem biomass. For species with long, unwinged petioles (>5% leaf weight), it makes more sense to group the petioles with the stems because they constitute a structural rather than assimilation organ.
Traits relevant to forbs, grasses and woody plants
Non-structural carbon (NSC)
Trait description
Non-structural carbohydrates (NSCs) play a central role in plant metabolism (Sala et al. 2012). As the primary products of photosynthesis, NSCs carry both energy and carbon for plant biosynthesis, and are involved in almost all critical plant physiological processes (Landhäusser et al. 2018). Hoch et al. (2003), suggested that non-structural carbohydrates are quantitatively the most important carbon compounds for storage, and recent work on the importance of NSCs in plant responses to environmental change (e.g. climate change and drought) has created renewed interest in their storage role. Total NSCs are a good proxy for quantifying the reserves that can be remobilised to fuel growth during the next growing season or after an injury. The non-structural carbon pool (see Fig. 4) comprises several compound classes (Chapin et al. 1990); these include low-molecular-weight sugars (e.g. glucose, fructose and sucrose), starch, oligosaccharides of the raffinose series, and fructans (Fischer and Höll 1991, 1992; Eissenstat and Duncan 1992; Hoch et al. 2003). Although other organic compounds (e.g. other sugars, alcohols and lipids) are important storage compounds in some species (Hoch and Körner 2003; Hoch et al. 2003; Martínez-Vilalta et al. 2016), we refer specifically to NSCs as the sum of starch, fructans (when present), and soluble sugars, because these are the most routinely measured ones.

|
Relevant environmental filters
Non-structural carbohydrates are associated with resprouting, allowing plants to survive when the aboveground part of the plant is repeatedly ‘top-killed’ by aboveground disturbances such as fire (Wigley et al. 2009b; Dietze et al. 2014). As a result, belowground NSC storage is predicted to increase in systems with high levels of aboveground disturbances (Schutz et al. 2009, 2011; Wigley et al. 2009b; Martínez-Vilalta et al. 2016). Palacio et al. (2008) and several references therein have reported a decrease in starch pools after browsing or defoliation in deciduous and evergreen species. Martínez -Vilalta et al. (2016) suggested that high NSC concentrations in tropical species may indicate an adaptation to high levels of disturbance from herbivory or shade. Myers and Kitajima (2007) concluded that carbohydrate storage (stems and roots) in neotropical seedlings enhances long-term survival in shade. Tropical tree species with higher foliar and whole-plant NSC concentrations display higher survival levels during drought (O’Brien et al. 2014) and previous work has shown a carbon-metabolism role in drought-induced mortality (Adams et al. 2013; Wiley et al. 2016). For instance, Bréda et al. (2006) showed that low starch concentrations of stem tissues in European trees correlate with large twig and branch declines during drought.
Method
See reviews by Quentin et al. (2015) and Landhäusser et al. (2018) for comprehensive comparisons and discussions of the main problems involved with the different methods commonly used to estimate NSC concentrations. A standard protocol is proposed in the supplementary material of Landhäusser et al. (2018). On the basis of all previous evidence, we recommend that samples are collected and stored according to the following guidelines.
Samples should be collected at similar phenological stages; Martínez-Vilalta et al. (2016) found that NSC concentrations in most organs and functional types showed maxima before, or at the onset of, the growing season. Concentrations of NSCs in leaves should be at a maximum when growing in sun and follow a diurnal cycle, so should be collected at the same time of day (Landhäusser 2011). Concentrations of NSCs also vary depending on which part(s) of the plant is measured, and the plant organ to be sampled will depend on the question being addressed (e.g. belowground organs should be sampled to determine resprouting potential after top-kill). Concentrations in the global synthesis of Martínez-Vilalta et al. (2016) were highest in leaves and belowground reserve organs, and lowest in stems, with values being intermediate in roots. Starch concentrations were as high in roots as in leaves, and highest in belowground reserve organs such as bulbs and lignotubers. Where possible, keep samples cold and process samples within 8 h; microwaving may contribute to sample integrity because enzyme activity is halted. For sugars, ethanol extraction rather than water extraction is recommended. Choose a laboratory that can analyse your samples using one of the three recommended quantification methods (i.e. ion chromatographic method, enzyme method, or acid method) and make sure the standardised methods have been followed as suggested in the supplementary material of Landhäusser et al. (2018).
Martínez-Vilalta et al. (2016) suggested that concentrations and not pools should be measured. Although absolute pool sizes are desirable in some contexts (Martínez-Vilalta 2014), they must be weighted by the biomass of the organ or plant to give an idea of the availability of NSCs per unit tissue. Because carbon allocation to tissues and organs with different NSC concentrations may differ across species and sites, pools weighted by biomass may provide a better measure to compare with overall sources and sinks. However, because seasonal changes in biomass partitioning are likely to be smaller than changes in concentrations (particularly in woody tissues), changes in concentrations are likely to reflect changes in pools. In general, a detailed description of the plant, location and phenological stage and type of material analysed will facilitate future comparison across studies.
Notes
-
It is extremely challenging (especially in large woody plants) to measure the weight of different plant organs to calculate NSC pools. We, therefore, recommend that if it is not possible to weigh the whole root system or stem, it would be helpful to at least estimate the volumes of these organs (e.g. see Wigley et al. 2019a) and measure the wet and dry weight of a subsample of each sampled organ type (i.e. leaf, stem or root).
-
If only interested in NSC concentration, the concentrations of NSCs at a depth of 15 cm were found to be very similar to concentrations at a 30-cm depth in a set of ~70 species (B. J. Wigley, unpubl. data). We, therefore, suggest that sampling of roots at shallower depths (10–15 cm) should usually suffice.
Plant chemical defence (PCD)
Trait description
Plant chemical defences (PCD) can be described both qualitatively and quantitatively. The detection and measurement of secondary metabolites may involve sophisticated laboratory techniques or simple bioassays, depending on the questions under investigation and means available. Savanna plants have evolved a diverse suite of defence strategies, including physical (e.g. tough leaves, spines and thorns), biotic (e.g. mutualisms with ants) and chemical defences. Multiple lines of defence are often employed by a single plant species. Here, we focus on chemical defences in woody, long-lived plants, examining what is known about their detection and quantification in savanna systems. Most previous work has focused on mature, reproductive plants; however, future work would do well to focus on the more vulnerable seedling and sapling stages, which may be more strongly defended because growth and survival early in ontogeny are often more limited by herbivory. Most of literature on savanna plant defences has examined carbon-based (C-based) polyphenolics, with condensed tannins figuring prominently among them.
All tannins function to precipitate proteins (Robbins 1994), reducing digestion and nutrient acquisition and limiting microbial growth (Zucker 1983). Condensed tannins especially deter feeding by ruminants (Cooper and Owen-Smith 1985), whereas hydrolysable tannins are thought to be more effective against insect herbivores (Zucker 1983; Cooper and Owen-Smith 1985; Furstenburg and van Hoven 1994). Many studies of tannins in savanna plants have not actually examined the link between metabolites and their possible function(s). Future studies should, therefore, attempt to better quantify the role of these metabolites in limiting both mammalian and insect herbivory.
Here, we summarise the most up-to-date methods for polyphenol quantification and suggest other chemical defences that are likely to be important in woody savanna plants.
Relevant environmental filters
The phenolics most often studied in savanna plants are often expressed at higher concentrations in resource-poor environments, defined by either water or nutrient limitations (Wigley et al. 2018), supporting predictions of the carbon–nutrient balance hypothesis (Bryant et al. 1983), the resource-availability hypothesis (Coley et al. 1985), and the growth-differentiation balance hypothesis (Herms and Mattson 1992). Studies have shown that C-based defences in savannas may decrease in the presence of herbivores, which is likely because of resource stress (Scogings et al. 2013, 2014). However, in some species phenolic concentrations increase with herbivory (Scogings et al. 2011) or increase post-simulated herbivory (Kohi et al. 2010; Hean and Ward 2012). Species-specific responses are, therefore, important to consider before attempting to arrive at generalisations regarding defence expression, as phylogenetic and environmental constraints may both influence the production of chemical defences.
Methods
Phenolic chemistry in savanna plants varies within individuals. The expression of phenolics changes across ontogeny (Gowda and Palo 2003; Rooke et al. 2004), with phenology (Furstenburg and van Hoven 1994), by season (Scogings et al. 2004), in response to damage (Stock et al. 1993; Furstenburg and van Hoven 1994; Scogings et al. 2013), with light (Furstenburg and van Hoven 1994) and water (Scogings and Mopipi 2008), with interactions between herbivory and water availability (Scogings et al. 2017), and with soil nutrients (Wigley et al. 2018). Large sample sizes and careful control of sources of the abovementioned variation are critical when sampling.
Typical extraction methods include drying leaf material at ambient temperatures, grinding the samples, and extracting 1 g or less of dry leaf material in an organic solvent. Extractions should be performed at low temperatures, and sonication can improve yields. Repeated extractions are also often employed. The ratio of wet to dry weight will vary according to species, but may be expected to be ~3 : 1. If one were to evaluate a plant species for all the methods listed below, it would be necessary to collect ~12 g of fresh leaf material.
Total phenolics. Total phenolics are often determined colorimetrically by reacting phenolics with phosphomolybdic–phosphotungstic acid complexes and measuring absorbance (the Folin–Ciocalteu assay). Improved methods of this technique are presented by Ainsworth and Gillespie (2007). The Price–Butler or Prussian blue method is also often used for colorimetric analysis (Price and Butler 1977). Gallic acid is typically used to create a calibration curve (D. Hattas, pers. comm.).
Tannins. Condensed tannins (proanthocyanidins) are typically measured colorimetrically after reaction with vanillin–HCl (Price et al. 1978) or butanol–HCl and ferric ammonium sulfate (Porter et al. 1985), with the latter being more reliable than the former (Hagerman 1987). Tannins are extracted as per measurements of total phenolics, and resulting proanthocyanidins are oxidatively cleaved in acid butanol to yield anthocyanidin pigments. The pigments are reacted with FeNH4(SO4)2 in HCl and absorbance of the product is read at 550 nm.
Condensed and hydrolysable tannins are sometimes quantified via their protein-precipitation activity measured with the radial diffusion assay (Hagerman 1987). Hydrolysable tannins can also be measured colorimetrically; the preferred method involves reacting extracts with potassium iodate (Hartzfeld et al. 2002). The most accurate quantification of tannins relies on high-performance liquid chromatography combined with mass spectrometry (HPLC–MS). More improved methods for phenolic extraction and quantification have been described by Khoddami et al. (2013).
The quantification of condensed and hydrolysable tannins can be difficult owing to challenges in completely extracting these phenolics from plant material and selecting appropriate standards. Ideally, standards should come from tannins purified from the study species itself, but in the absence of species-specific standards, it has been found that sorghum tannin underestimates condensed tannin concentrations in African savanna trees by 0.26–0.79 times. As a result, it is preferable to the widely used quebracho tannin standard, which can overestimate tannins by as much as eight times (Hattas and Julkunen-Tiitto 2012).
Terpenoids. Terpenoids are diverse (Gershenzon and Dudareva 2007; Pichersky and Raguso 2018), but little work has been conducted on the ecological effects of terpenoids in savannas, although their bioactivity is recognised (Rahman et al. 2008; Su et al. 2009). Both volatile and non-volatile terpenes can be quantified with gas chromatography. Flame-ionisation detection and gas chromatography–mass spectrometry (GC–MS) are preferred detection methods (Merfort 2002). HPLC can be used in the quantification of non-volatile terpenoids; optimal detection methods will depend on the class of terpenoids under study (Wu et al. 2013).
Triterpenoid saponins. Triterpenoid saponins are important anti-feeding compounds that inhibit mammalian and insect herbivores and have antifungal properties (Oleszek et al. 1999; Agrell et al. 2004). They are common in members of the Fabaceae (Cooper and Owen-Smith 1985) and can be detected and measured via basic laboratory methods (the foam test; Massad et al. 2012) or more sophisticated HPLC techniques (Kursar et al. 2009; Massad et al. 2012).
Cyanogenic glycosides and alkaloids. Cyanogenic glycosides are present across plant families; they interrupt respiration and are, therefore, toxic to vertebrate and invertebrate herbivores (Zagrobelny et al. 2004). Their presence is easily detectable with the picric acid assay (Francisco and Pinotti 2000; Vetter 2000). Alkaloids are also toxic to vertebrate and invertebrate herbivores; these compounds can be detected with GC–MS, HPLC–MS and thin layer chromatography (TLC; Röder 1999; Wu et al. 2013).
Metabolomics and bioassays. The field of chemical ecology is moving in an exciting direction with the advent of metabolomics (Dyer et al. 2018). Secondary chemistry metabolomics is demonstrating that phytochemical diversity in and of itself is an important defence property (Richards et al. 2015; Salazar et al. 2016; Massad et al. 2017). Most of this work has been conducted on tropical forest plants; however, applying these techniques to savanna plants may provide insight into herbivore choice and plant–herbivore interactions. The ability of compounds to actually limit herbivory can be tested with bioassays. Targeted or holistic extractions can be produced, diluted in sucrose solution, and applied to leaves of herbivores’ preferred food plants. The adjusted consumption index can be calculated to determine the deterrent properties of the extract (Dyer et al. 2003). This is an attainable and effective way to determine the defensive properties of secondary metabolites for insects, which are largely overlooked when studying herbivory in savannas (Davies et al. 2016), and could also be attempted with mammalian herbivores.
Special cases
There are generally high levels of intraspecific variation in chemical defence expression. Care should be taken to minimise unwanted variation by careful standardisation of sample collection (e.g. by ensuring that fully developed sun-exposed leaves are always sampled in the same season). Although chemical analyses are labour- and time-intensive, we recommend as large a sample size as possible to ensure accurate results. Many plant defences operate in synergy to limit herbivory. More holistic analyses of plant defences combined with bioassays should, therefore, be favoured over the isolation and quantification of single compounds to provide a mechanistic understanding of plant defence.
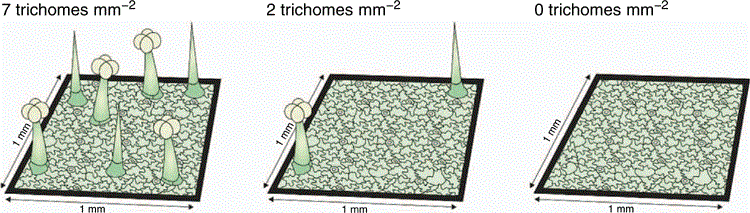
Trichome density (TD)
Trait description
Trichome density (TD) is a measure of leaf pubescence or hairs. Trichome density is calculated by dividing the total number of trichomes on a leaf by the leaf area (trichomes mm–2). Werker (2000, p. 3) defined trichomes as ‘unicellular or multicellular appendages which originate from the epidermal cells only and develop outwards on the surface of various plant organs’ (i.e. not connected to the vascular system). Trichomes are further classified as glandular and non-glandular; glandular trichomes secrete a variety of unmodified (e.g. salt) or synthesised (secondary) compounds (Werker 2000).
Relevant environmental filters
Trichomes have a diverse set of functions that are most commonly linked to an increased tolerance of xeric environments and resistance to herbivory (both invertebrate and vertebrate). Trichomes increase a plant’s capacity to tolerate dry and sunny environments by protecting against excessive light, absorbing (and shedding) water, preventing desiccation and reducing transpiration and secreting excess salts (Jeffree 1986; Werker 2000). Additionally, TD is correlated with increased resistance to invertebrate herbivory (Mauricio 1998; Agrawal 1999; Valverde et al. 2001; Kaplan et al. 2009; Pott et al. 2012) and decreased insect oviposition and egg numbers (Handley et al. 2005). Trichomes also increase resistance against vertebrate herbivory (Levin 1973; Stuart-Hill and Mentis 1982; Pullin and Gilbert 1989; Hanley et al. 2007). Trichomes are often induced by herbivory (Pullin and Gilbert 1989; Agrawal 1999; Dalin and Björkman 2003), depending on plant-species identity, intensity of herbivory (Sletvold et al. 2010) and interactions with other environmental conditions (Gonzáles et al. 2008). This trait is expected to relate to herbivore pressure, and we expect increased trichome densities with intense herbivory. Trichomes constitute part of a plant’s structural or architectural defence strategy (as opposed to chemical defences, e.g. see Wigley et al. 2018) and, as such, may form part of a structural defence syndrome (e.g. edible milkweeds employ high trichome densities paired with high latex as a defence syndrome, Agrawal and Fishbein 2006).
Method
The classification of trichomes according to shape is complicated as trichomes differ characteristically among species (Payne 1978). Rather than classifying trichomes according to shape, they are more regularly classified as glandular or non-glandular (Gonzáles et al. 2008). However, there are no straightforward patterns enabling the easy identification of glandular v. non-glandular trichomes. For instance, both glandular and non-glandular trichomes may be branched or unbranched (Werker 2000), and both glandular and non-glandular trichomes can occur simultaneously on the same plant and there may also be more than one type of each on a plant (Werker 2000). For simplicity, we propose that all trichomes are counted and presented as total trichome density, unless there is a specific interest in glandular v. non-glandular trichomes. Trichome density is a trait most commonly used for interspecific comparisons but can also be used to compare different treatments (e.g. herbivore intensities) within a species. We recommend that 5–10 replicates are sampled per species for inter-specific comparisons. The methods provided to describe TD are not restricted to one ontological stage; therefore, the age of leaves to be sampled will depend on the research question. The following steps are described in detail below: (1) choosing an appropriate leaf surface, (2) measuring leaf area, (3) counting trichomes, (4) dividing the trichome counts by the leaf area.
-
Trichome density is usually measured on the surface(s) at which a herbivore(s) of interest feeds or where trichomes occur. It should be determined and noted which leaf surface(s) have trichomes.
-
Leaf area is most easily measured (in the absence of a leaf area meter) by laying out the leaves on a white surface with a scale (typically a bar with 1-mm increments) and taking a photo with a digital camera. The area of the leaves can then be computed with appropriate software, such as Image J (Rueden et al. 2017).
-
Trichomes can be counted by using a stereomicroscope or binocular microscope linked to a digital camera. If leaves are small, trichomes can be counted on the entire leaf surface. For larger leaves, a disc of a known area is removed by using a cork borer or punch (e.g. Sütterlin and van Lenteren 1997; Mauricio 1998; Agrawal 1999; Kaplan et al. 2009). Cheng et al. (2014) suggested that the Cell Counter plugin (http://rsbweb.nih.gov/ij/plugins/cell-counter.html, accessed 20 Octobre 2020) in Image J can be used to facilitate trichome counting.
-
Trichome density is calculated by dividing the number of trichomes by the leaf area (trichomes mm–2 see Fig. 5).
Grass traits
General introduction to grasses
Characteristics of the grass layer underpin the flammability and grazer productivity of savannas and grasslands (Archibald et al. 2019). Determining how grass functional traits relate to fire and grazer prevalence is central to understanding the ecology and evolutionary history of grasslands and savannas. From a management perspective, the potential for rapid shifts in grass community composition makes monitoring of community shifts in traits a valuable tool for land management and conservation interventions. Although we focus on Poaceae (particularly in tropical environments), many of the traits covered in this handbook are likely to be applicable to graminoids more generally (e.g. Cyperaceae, Juncaceae, Restionaceae and Xyridaceae) because of their generally similar growth form.
The traits presented here are useful for quantifying different components of grass life histories, providing the foundation for quantifying how grass species avoid, resist or tolerate either fire or grazing, or both. Flammability and palatability, and thus the likelihood of a grass being burned or grazed, are broadly captured by three axes, namely, the quality, quantity and spatial arrangement of plant material. Fire and grazing ‘select’ for opposing qualities in grasses, with grasses with high C : N ratios, low bulk densities, low moisture content and high concentrations of tannins and volatile oils enhancing flammability, and vice versa for palatability (e.g. Archibald et al. 2019; Hempson et al. 2019; Solofondranohatra et al. 2020). However, structural constraints mean that maximum quantities of high-quality fire fuel or grazer forage are achieved via different plant architectures. Tall grasses require higher relative structural support and, hence, have higher C : N ratios, with vertical accumulation of biomass tending to enhance grass flammability. By contrast, the intake rates of grazers are highest on densely packed forage that maximises the amount of food per bite, with a dense leafy sward with little stem material simultaneously optimising both quality and quantity (Murray and Illius 2000). Therefore, grass communities associated with high herbivore densities tend to be composed of short and often laterally spreading grasses (McNaughton 1984). By contrast, flammable grass communities comprise tall grasses with higher relative C : N ratios with well aerated canopies. At an individual level, grasses can minimise the likelihood of being either burned or grazed by having low biomass and sparse architectures, making them both poor fuel for fires and of little grazing value.
Resistance and tolerance in grasses imply surviving fire or grazing. A central tenet is to determine which plant parts are protected during a ‘consumption’ event, and thereby shape a plant’s competitive ability post-fire or post-grazing (Coughenour 1985). Grasses that resist defoliation minimise material consumed by fire or grazing, for example, by remaining green and moist late into the dry season, thereby reducing flammability (McGranahan et al. 2018), or via tough leaves and being strongly rooted, thus minimising plant material lost when grazed. Alternately, grazing lawn species are short and expose only their leaf blades to grazers, with intercalary and basal meristems being protected below grazing depth (McNaughton 1984). At an individual level, tolerance strategies in grasses typically entail a combination of resprouting from belowground-stored reserves and high photosynthetic rates, which enable individuals to be strong competitors for space and light post-disturbance (Ripley et al. 2015). A trait such as crown depth (i.e. the depth of the grass crown and basal buds below the soil surface) can be effective against both fire and grazing, serving both to insulate basal meristematic tissue from intense heat when burned, and providing rooting strength when grazed (Lemon 1949). Crown and basal buds can be protected from fire by high biomass ratios, which elevate the combustible material and, thus, vent the heat of the fire away from the soil surface (Gao and Schwilk 2018). Alternatively, dense packing of leaf sheaths around culm bases provides insulation against the heat of the fire (Lemon 1949). The risk of being uprooted when grazed can also be mitigated by having culms that separate easily from the crown when pulled. These and several other traits that are detailed below provide an opportunity for describing the variety of grass life histories that have evolved across a wide range of environmental conditions, fire regimes and diverse grazer communities. Whereas the focus of this section is on traits linked to fire and grazing, other key drivers of grass traits and growth forms are competition and drought. Situations where fire and grazing traits are potentially associated with or influenced by adaptations related to competition and drought have been noted in the text.
Biomass ratio (BR)
Trait description
Biomass ratio (BR) is the ratio of canopy biomass above 10-cm height to canopy biomass below 10-cm height (i.e. from the soil surface to 10 cm; Gao and Schwilk 2018). This trait quantifies how canopy architecture influences temperature at the soil surface when a plant burns. Plants with higher biomass ratios (i.e. relatively more biomass above 10 cm) vent heat upwards, which results in lower soil-surface temperatures when burned once total aboveground biomass is accounted for (Gao and Schwilk 2018). Lower soil-surface temperatures during fire increases the likelihood of basal meristems and other crown tissues surviving the fire, allowing the plant to survive and resprout after fires (Choczynska and Johnson 2009). Although developed to quantify canopy architecture effects on soil-surface (and basal-bud) temperature when burned, this trait is also useful for separating broad architectural differences among grasses, with prostrate growth forms having very low biomass ratio values. This functional trait can be measured on all mature grasses.
Relevant environmental filters
Biomass ratio should increase with productivity (i.e. rainfall, soil nutrients) in grassy ecosystems, and decrease with grazer density. Two other grass traits, namely, crown depth and leaf-sheath packing, function similarly to insulate basal meristems and crown tissues from the heat of fires and may obscure biomass ratio v. productivity relationships. By describing canopy architecture, this trait provides an index of the degree to which flammable biomass is held above the soil surface in a way that can help carry a fire. Hence, values of the BR trait may be maximised in fire-grass communities where positive feedbacks promote frequent, hot fires (Hempson et al. 2019). Frequent grazing reduces fuel loads for fires, and BR should decrease in tufted, upright grasses as grazer densities increase; further increases in grazer densities can produce a shift to prostrate growth forms, such as grazing lawn species, with biomass ratios ultimately declining to zero once no canopy material occurs above 10 cm.
Method
Biomass ratio is quantified by first clipping and collecting all biomass (i.e. leaves, culms and inflorescences) of an individual at 10 cm above the soil surface, and then clipping the remaining aboveground biomass at the soil surface. This allows aboveground biomass to be separated into plant material held above 10 cm, and plant material occurring between the soil surface and 10 cm. These samples then need to be dried to a constant mass and weighed, with biomass ratio calculated as the ratio of mass for material above 10 cm to the mass of material below 10 cm. The trait is fairly stable within a species (Gao and Schwilk 2018) and measuring five individuals per species at a location should be adequate. Biomass ratios should ideally be measured on individuals in the first-year post-fire, once peak flowering has been reached, so as to standardise for the effect of biomass accumulation over time.
Notes
High BRs have been found to reduce soil-surface temperatures in fully dry grass fuels; however, when grasses are actively growing, high fuel moisture can lead to inefficient combustion and smouldering, which can override the effect of high biomass ratios on reducing soil-surface temperatures (Gao and Schwilk 2018). For laterally spreading species, such as mat-forming grazing lawn grasses, where individuals are difficult to isolate, a 20- × 20-cm section of the grazing lawn can be clipped as a representative sample. Very large tufted, upright grasses can also be subsampled in a similar manner. However, care should be taken that the sample remains representative of the overall canopy architecture, which may be more spreading on the margins and more densely packed in the middle of the tuft.
Crown depth (CD)
Trait description
Crown depth (CD) is how deep the crown is positioned below the soil surface. The crown is defined here as the point at the base of the leafy stems where adventitious roots are produced and where reserve buds (developing new shoots) are located. The trait is a simple measure of the distance between the soil surface and the crown (Fig. 6b). Crown depth provides a measure of how well insulated the crown and associated meristematic tissues are from the heat of fires (Choczynska and Johnson 2009) and may also be related to how easily a grass can be uprooted by grazers. This functional trait can be measured on all mature grasses.
Relevant environmental filters
Crown depth is predicted to have the highest values in areas experiencing frequent fires, i.e. highly productive systems. This trait allows critical tissues to resist being damaged during a fire and forms a crucial component of the tolerance strategy of perennial grasses by enabling them to resprout after fires. Lemon (1949) recognised the importance of the insulation effect provided by positioning leaf meristems below the soil surface, suggesting that a depth of 38 mm (i.e. 1.5 inches) provided adequate protection in his study site in Alapaha, Georgia, USA. This has been reiterated in Daubenmire (1968) and Bond and van Wilgen (1996). On the basis of models of soil heat transfer, Choczynska and Johnson (2009) suggested that temperatures lethal to buds are likely to be restricted to the top 20 mm of the soil profile. Where CD functions to reduce the likelihood of a grass being uprooted by grazers, it is likely to be greater in sandier soils. With regard to uprooting, this acts as a resistance trait preventing total plant death and limiting the material lost during a grazing event to aboveground parts only.
Method
Crown depth should be measured on five mature individuals (minimum three) per locality. A mature individual should be excavated, noting the soil-surface level on the culms, and then measuring from this point to the crown (Fig. 6c). In situations where this is variable, three measurements on the individual are taken and averaged. In some species or localities, the crown may be positioned above the soil surface, either on a tufted pedestal or, owing to soil erosion, around the tuft base. In these instances, CD should be recorded as a negative value.
Culm diameter (CUD)
Trait description
Culm diameter (CUD) is the maximum diameter of the internode of the culm (Fig. 6c). Culm diameter is positively related to the tensile strength of the culm and negatively related to its digestibility, and, hence, how likely it is to be grazed (Benvenutti et al. 2009). With regard to fire, CUD is probably also negatively related to the ignitability of the culm. Large CUDs are a requirement for structural stability of tall-statured grasses. This functional trait can be measured on all mature grasses.
Relevant environmental filters
Culm diameter increases with plant height and is, thus, broadly correlated with productivity. However, where high CUDs constitute a specific adaption to reduce grazer preference or surviving cool fires, high CUD values will be observed at lower productivities, or in shorter grasses. High CUD values may allow grasses to avoid being grazed, particularly by selective grazers (Benvenutti et al. 2009). Culms typically have higher C : N ratios than does leaf material, owing to their primary function of providing structural support. Culms are, thus, lower-quality forage for grazers, the more so as their diameter increases (Benvenutti et al. 2006). When grazed, CUD provides a measure of resistance, by influencing how much of the plant is consumed (Drescher et al. 2006, see ‘stemminess’ trait). Similarly, thick culms may not ignite during cool fires (particularly if moisture levels are high; Cardoso et al. 2018), and, thus, allow parts of the plant to resist being burned. Culm diameter should be considered concurrently with stemminess because the ratio of leaf to stem material is more informative when forage value of those culms is understood (i.e. using CUD as proxy for physical strength and digestibility; Benvenutti et al. 2009). Flammability traits that influence the intensity at which a plant burns will also influence whether culms of a particular diameter are likely to burn or not. Note that the benefits of the fire resistance of culms conferred by high diameters are likely to be restricted to enhancing post-fire grazing avoidance and resistance, by increasing the stemminess of the resprouting individual. This is because epicormic resprouting on culms that persist through fires is unlikely, unless combustion is almost entirely incomplete.
Method
Culm diameter should be measured on five mature individuals (minimum three) per locality. Culm diameter should be measured using digital callipers at the thickest point of the second internode on the tallest culm of an individual (Fig. 6c). Care should be taken to avoid the swelling associated with the node. The leaf sheath may be included in the measurement (see notes).
Notes
One or more leaf sheaths will often be wrapped around the culm at its thickest point on the second internode. We suggest including these in the CUD measurement, because they (1) increase the thickness through which a grazer needs to bite and are often lower-quality forage material than is the leaf blade, and (2) provide insulation that relates to fire resistance aspects of CUD. In some species, the culm itself may be much thinner than it appears, with the bulk of the structural support and apparent CUD constituted by multiple layers of rigid leaf sheaths (e.g. Aristida stenostachya). For these reasons, we suggest that including leaf sheaths in the CUD measurement provides a more ecologically meaningful measure than does removing them.
Foliar sodium (FS)
Trait description
Foliar sodium (FS) is a measure of the sodium concentration in the leaves of a plant. Foliar sodium can be measured using a variety of standard laboratory procedures. Sodium is an essential element for all animals, and plants with a high sodium concentration are actively sought out by herbivores (McNaughton et al. 1997; Veldhuis et al. 2014). This functional trait can be measured on all mature grasses.
Relevant environmental filters
Foliar sodium is expected to be highest in soils with high sodium concentrations, but also in areas experiencing frequent drought (Veldhuis et al. 2014). Foliar sodium is most relevant as a grazer-attraction trait (Borer et al. 2019). All animals require sodium (Robbins 1994), but it is not considered an essential nutrient for plants and can be toxic at high concentrations (Kronzucker et al. 2013). Because of their sodium requirements, grazers seek out plants with high sodium concentrations (Griffith et al. 2017), and, consequently, by concentrating sodium in their leaves, grasses cannot only get rid of excess sodium, but promote regular grazing to maintain high light conditions close to the soil level (i.e. high sodium concentrations can facilitate the persistence of short-statured grasses in communities that might otherwise grow tall and shade them out). Grasses with high FS concentrations should, thus, also have traits conferring high resistance or tolerance to grazing (Griffith et al. 2017).
Method
Foliar sodium should be measured on healthy leaves collected from five mature individuals (minimum three) per locality. Mature, healthy leaf material should be collected, dried and ground for standard laboratory-based nutrient analysis (e.g. mass spectrometry, flame-emission spectroscopy or inductively coupled plasma–atomic emission spectroscopy). The amount of material required will depend on the analytical method, but 1 g of wet material would typically be adequate (0.1 g required for atomic-absorption spectroscopy; Ford and Wilson 1981).
Notes
Despite not being considered essential for plants, sodium can replace potassium (K) in various functions, including osmotic adjustments (Wakeel et al. 2011), and is required in small amounts by C4 plants during the Calvin cycle (Brownell and Crossland 1972). In communities that experience frequent droughts, high FS concentrations may primarily form as a response to maintain osmotic potential under water-stressed conditions (Ford and Wilson 1981). Attractiveness to herbivores may, thus, be a by-product of a drought response in some species or environments.
Grass bulk density (GBD)
Trait description
Grass bulk density (GBD) is the ratio between grass biomass and the volume it occupies. It is calculated by dividing the total aboveground biomass by an estimate of the aboveground grass-canopy volume. Grass bulk density should ideally be collected on mature grasses that do not retain senesced growth from previous years. Grass bulk density is associated with both flammability and palatability (Solofondranohatra et al. 2020). With respect to grazing, GBD provides a measure of the bite size and, hence, intake rate that grazers can achieve (Benvenutti et al. 2006; Drescher et al. 2006). Higher GBDs allow for higher intake rates, but the quality of the material (e.g. leaf C : N and stemminess) also needs to be considered to determine the likelihood of the plant being grazed (along with grazer body size). With respect to fire, GBD describes fuel aeration through its influences on the adequacy of fuel to enable fire spread and the air flow properties that replenish oxygen concentrations to sustain combustion (Grootemaat et al. 2017). Grass bulk density can be particularly meaningful when interpreted at a community level, where dominance by high bulk-density grazing lawn species, or by low–moderate bulk-density fire grasses, can have positive feedbacks to grazer and fire prevalence in the community (Hempson et al. 2019; Solofondranohatra et al. 2020).
Notes
Fire ecologists often use the packing ratio (defined as the volume of fuel : volume of fuelbed; Grootemaat et al. 2017) instead of bulk density, and herbivore ecologists sometimes use the term ‘biomass concentration’ instead of ‘bulk density’ (McNaughton 1984). Simpson et al. (2016) used ‘biomass density’ (slope of the biomass accumulation curve with height) in a similar context. All of these measures or terms describe the same functional trait.
Relevant environmental filters
-
Avoidance strategies. Grasses with very low GBDs (i.e. very sparse architectures) have low grazing value (very low intake rates) and are poor fuel for fires (low probability of fire spread, little material to combust to sustain the fire; Archibald et al. 2019). Thus, these grasses largely avoid being grazed or burned. However, the sparse biomass of these grasses makes them poor competitors in communities with species with higher GBDs.
-
Grazer attraction. Grazing lawn species promote grazing by having high GBDs of high-quality leaf material. These species are short and resist grazers by keeping most stems and buds below grazing depth (but with high bulk-density leaf material being accessible) and rely on grazers to maintain a high light environment by preventing taller species from invading (Hempson et al. 2019). Because of the short sward height, bite size is maximised in grazers with wide mouths.
-
Fire attraction. Grasses that promote frequent hot fires have intermediate GBDs (Simpson et al. 2016), which provide adequate fuel to sustain the fire but also allow for sufficient air flow to provide the oxygen necessary for combustion. These species often have associated traits that allow them to survive fires and to regrow rapidly after fires to regain competitive dominance for light (Ripley et al. 2015; Hempson et al. 2019).
-
Fire resistance. Grasses with a high GBD and with low curing rates and ignition point are able to resist fire by limiting air flow through the sward and having reduced ignitability (Archibald et al. 2019). This is particularly relevant at the base of the plant, where tightly packed stems can protect basal meristems from burning.
Method
Grass bulk density should be measured on at least five mature individuals (minimum three) per locality. Canopy volume of a tufted grass is calculated using measures of the tuft basal diameter (DB), leaf-table height (HLT) and leaf-table diameter (DLT; Fig. 7). Leaf-table height is defined as the height visually estimated to correspond to the ~80th quantile of leaf biomass (the height below which the main bulk of the leaf canopy occurs). For plants with base and leaf tables that are not circular, diameters are estimated as the average of the longest axis and the corresponding perpendicular axis. Volume (V) is then calculated using the formula for a truncated cone, as follows (Fig. 7):
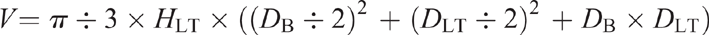
Canopy volume of mat-forming grasses or very large tufts can be obtained by calculating the volume of a subsample of the individual, using the same basal diameter, leaf-table height and leaf-table diameter measures as described above. For mat-forming grasses, it is often convenient to mark out a square or rectangular section of the individual(s) using a spade, and to calculate the volume as (Fig. 7):

Aboveground biomass can be determined by clipping, drying and weighing the parts of the individual for which the volume estimate was made. Bulk density is then quantified as aboveground biomass divided by canopy volume.
Notes
Grass bulk density considers the total plant biomass but can also be calculated for leaf biomass only. This can be performed by separating the leaf and culm material and weighing these separately, or by subtracting the culm proportion of the total biomass using the stemminess -trait estimate. The potential subjectivity associated with visually estimating the 80th quantile of leaf biomass can be minimised by either keeping the observer constant or comparing estimates from different observers. Techniques such as placing a polystyrene board on the canopy are subject to interference by culms, such that stemminess or culm diameter may unduly influence leaf table-height estimates.
Integrated flammability protocol (IFP)
Trait description
Grasses become flammable once they are dry enough to ignite, with their subsequent combustion characteristics reflecting their overall architecture as well as leaf chemical properties. Flammability is divided into three separate properties, namely, ignitability, heat release and fire spread rate (Pausas et al. 2017), all of which are usually measured on dry (cured) plant material, preferably at a whole-plant level to accommodate architecture (Schwilk 2015). Because the moisture content of grass fuels is such a strong driver of seasonal patterns of fire, we suggest that when assessing flammability, one should include information on the curing rates of different grass species (the rate at which different grass species lose moisture when under water stress), as well as the moisture content at which they are able to ignite (ignition point). Here, we provide an integrated flammability protocol (IFP) to optimise the efficiency of collecting these labour-intensive plant traits.
Notes
The flammability traits described here represent the emergent properties of several key plant traits such as leaf C : N ratio, leaf thickness, biomass, bulk density, biomass allocation and physiological drought response, each of which has been shown to influence different aspects of flammability. See details in the particular trait descriptions above. Note that although several other flammability protocols have been developed and used (e.g. see White and Zipperer 2010), these are either for testing the flammability of the leaf material independent of plant architecture (Simpson et al. 2016), or more appropriate for shrubby or woody fire regimes where pre-heating is important (Jaureguiberry et al. 2011). Therefore, here we present a consolidated method that we consider most appropriate for assessing whole plant species-specific differences in grass flammability.
Relevant environmental filters
Biomass is a major driver of flammability and, therefore, we expect more flammable species in systems that can support highly productive (high biomass = high heat release and spread rate) and tall grasses with aerated canopies (low bulk density = high ignitability). Thus, high rainfall ecosystems with tall tussock grasses are often considered more flammable. Leaf chemistry with a high carbon, low N and P content is more flammable, and decomposes more slowly; so, nutrient-poor systems generally have more flammable grasses (higher ignitability and sustainability; Scarff and Westoby 2008). Curing occurs in all systems with seasonal rainfall, but curing rate is linked to a plant’s water-use strategy; so, we expect curing rates to vary with gradients of water availability. Systems dominated by annual grasses will have very fast curing rates and, consequently, longer fire seasons (e.g. Northern Territory in Australia).
Grass-flammability traits all have important effects on the emergent fire regime, in particular, the rate of spread, fire severity (completeness of combustion) and fire-return time. Curing rates of grass species in more mesic savanna climates are an important determinant of the fire-season length and the patchiness of the burn (i.e. variability in curing rates among species produces a patchier fuel load), as well as affecting fire emissions (smouldering moist fuels emit more greenhouse gases). Therefore, combined, these traits have an influence on the effect that fires have on atmospheric properties, vegetation structure and the forest–savanna boundary (Biddulph and Kellman 1998; Cardoso et al. 2018).
Method
Curing rate, ignition point and flammability (ignitability, sustainability, and combustibility) can be measured in one integrated protocol (Fig. 8), starting with fully hydrated plants clipped in the field or grown in pots, with canopy architecture preserved as far as possible. If curing rate and ignition point are not going to be measured, this protocol can be followed by skipping the first few steps and using fully cured plant material instead. The protocol requires a large number of samples per species (Table 3). Five samples should be used for determining curing rates; these five specimens are weighed each day, and once fully cured, need to be oven-dried to determine dry mass. Ignitability tests should be performed on three new samples each day. Combustion properties should be measured on five fully cured samples.

|
Measurement protocols
Part 1: curing rate. Here we define an experimental method for estimating curing rate. The assumption is that this correlates with differences in observed curing rates of species rooted in the soil as the soil profile dries; however, this should be tested to demonstrate validity.
Curing-rate measurements should be initiated on mature plants that are not water-stressed. This can be ensured by commencing measurements after substantive rainfall events or by watering plants for a few days before starting the study. Samples are collected by clipping the grass at the base. The sample should include the entire tuft up to a maximum of 10-cm diameter. Samples should be weighed immediately to obtain fresh mass (MT0). Samples are then placed in wire-mesh tubes that preserve their original canopy architecture (wire-mesh tubes may thus differ among samples). These samples should be kept in a room with constant conditions of ~25°C and 60% humidity and weighed every 24 h, recording the mass and time since drying commenced (h). Once constant mass is attained (i.e. fully cured), samples are dried in an oven for 48 h at 70°C and reweighed to determine dry mass (MDry).
Moisture content is calculated for each weighing point (MTi) as follows:
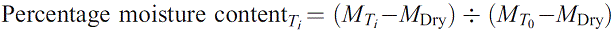
Finally, curing rate is quantified as the slope of the linear regression of log(Moisture content) v. time (h).
Notes. We also recommend that measurements of stomatal control, or ‘physiological drought tolerance’ following the methods of Craine et al. (2013) and Tucker et al. (2011) be performed because this associated physiological trait probably underlies differences in observed curing rates. Note that grass plants will also cure when exposed to frost, and as part of phenological processes as plants age and allocate resources to flowering instead of maintenance of leaves.
Part 2: ignition point. Samples for ignition-point tests are collected and kept in the same manner as curing-rate samples. Ignition tests are performed on three samples each day, at the same time as when curing-rate samples are weighed. Ignition tests are performed by holding a lit butane lighter to the base of the sample for 10 s. Whether the sample ignites, and whether it combusts completely or partially (estimated percentage) is recorded. Ignition point is the percentage moisture content at which >50% combustion of the specimen occurs. The percentage moisture content is determined from the associated curing-rate samples. An alternative quantification of ignition point involves using an epiradiator to apply a constant temperature to a sample of known mass and measuring the time to ignition. However, these two methods are not directly comparable.
Part 3: combustion properties. Samples for combustion properties tests are collected and kept in the same manner as curing-rate samples and should be weighed daily until constant mass is attained. Combustion measurements are made by placing these fully cured specimens on a scale attached to a flame-proof bed and attempting to ignite them. A continuous record of mass change and time is required, either by connecting the scale to a computer, or by video recording the scale display and logging mass and time. Ignition attempts are made by holding a butane lighter to the base of the upright orientated sample for 10 s. The following metrics are then derived from these measures:
-
Time to ignition (s): the amount of time between introducing the flame and the sample catching alight
-
Flaming time (s): the amount of time between a sample ignition and flame extinction
Maximum combustion rate (g s–1) is the slope of the linear regression through the inflection point of the mass v. time curve during combustion, assessed as follows:
-
Constructing a curve of sample mass at 0.2-s intervals through the combustion period.
-
Isolating data from 3 s either side of the inflection point of this sigmoidal curve.
-
Fitting a linear regression through these data points.
Heat release during combustion can be measured by placing a black aluminium disc at 50-cm height and at the plant base, and measuring the temperature of these discs using an infrared thermometer gun before ignition, after flame extinction, and when no embers remain (X. Gao and D. W. Schwilk, unpubl. data).
Notes. Most studies do not measure ‘ignitability’ at a whole-plant level (e.g. Simpson et al. 2016); so, using time to ignition in this way has not yet been verified as an effective method.
An alternative ignition method is to ignite a cotton ball soaked in 10 mL of pure ethanol and place it at the base of the sample (X. Gao and D. W. Schwilk, unpubl. data).
Leaf-sheath packing (LSP)
Trait description
Leaf-sheath packing (LSP) is the ratio of the number of basal leaf sheaths in the short internode zone to the length of the short internode zone. It is calculated by counting the number of basal leaf sheaths in the short internode zone and dividing this by the length of the short internode zone (Fig. 6d). Leaf-sheath packing provides a measure of how well insulated the crown and associated meristematic tissues are from the heat of fires (Lemon 1949). This functional trait can be measured on all mature grasses and is somewhat analogous to bark thickness in woody plants.
Relevant environmental filters
Leaf-sheath packing is predicted to have highest values in areas experiencing frequent fires, i.e. highly productive systems. This trait allows critical tissues to resist fire-induced damage and forms a crucial component of the tolerance strategy of perennial grasses, by enabling them to resprout after fires. We are not aware of this trait previously being described or quantified. However, Lemon (1949) appears to be the first to recognise the insulating effect of closely packed basal leaf sheaths, and the potential for these to limit air flow (i.e. oxygen) necessary for combustion. This has been reiterated in Daubenmire (1968) and Bond and van Wilgen (1996). The insulation provided by LSP is likely to also provide protection against cold temperatures and may prevent water infiltration that could hasten tuft decomposition.
Method
Leaf-sheath packing should be measured on five mature individuals (minimum three) per locality. A mature flowering culm is excavated, and the leaf sheaths in the short internode zone at the base of the culm are sequentially removed and counted. Once the entire short internode zone has been exposed, the distance between the first and last leaf sheath to be removed is measured. The number of leaf sheaths is then divided by the length measurement (Fig. 6d). The short internode zone can be distinguished from the long internode zone from a marked shift in the length of internode sections (see Perreta et al. 2011 for further detail). However, in some species or individuals, this transition is gradual or otherwise unclear, with delimitation of the short internode zone thus being somewhat arbitrary (Perreta et al. 2011); a more conservative assessment is recommended, with the crucial point being that the leaf-sheath count is restricted to the section considered to be the short internode zone.
Notes
Difficulties may arise where the extent of the short internode zone is unclear, either being long and without a clear transition into the long internode zone, or effectively being absent as in the case of some annual species. Some species may not retain any basal leaf sheaths (i.e. they decompose), although the scars may still be evident. In this case, a value of zero is simply recorded for the number of leaf sheaths; we suggest that the length of the short internode zone is still measured, because it can provide insights into the architectural flexibility of the species (Perreta et al. 2011). Considering this trait alongside information on tuft basal diameter and number of culms may provide a more complete assessment of the degree to which buds are protected. This trait remains to be tested experimentally. Note that this method does not account for varying thickness of leaf sheaths, and an alternate approach to quantifying leaf sheath insulation properties may be to measure the thickness of the leaf sheaf layer at a standardised position, such as at ground-level or 10 mm above the crown.
Physiological drought tolerance (PDT)
Trait description
Physiological drought tolerance (PDT) is measured as the critical leaf water potential (Ψcrit) at which a plant closes its stomata and stops transpiring, identified at the point where its stomatal conductance is 5% of maximum (Tucker et al. 2011). Plants vary in the degree to which they reduce photosynthetic rates (slow water loss) in response to increasing water stress. In woody plants with persistent aboveground biomass, this trait relates to a trade-off between safety (preventing cavitation) v. efficiency (maximising photosynthesis); it can describe how susceptible plants are to water stress and predict drought mortality (Choat et al. 2012). Tropical grasses generally abandon their aboveground biomass annually during an ~6-month dry season, so the trait is less obviously linked to drought survival (S. Archibald, unpubl. data), but represents a useful measure of how quickly grasses cure at the end of the dry season, as well as how they are likely to respond to mid-season droughts (periods of low water availability during the growing season).
Notes
Physiological drought tolerance is expected to be strongly correlated with curing rate (see integrated flammability protocol); however, this remains to be tested experimentally.
Relevant environmental filters
Although large variation in this trait has been observed globally in grasses (Craine et al. 2013), its environmental correlates are still not obvious. In fact, there is disagreement in the literature about whether grasses with high v. low Ψcrit would be considered the most drought-tolerant (S. Archibald, unpubl. data). Within a landscape, plants with a high Ψcrit (sensitive stomatal control) dominate in shallow soils and long-unburned sites (Tucker et al. 2011). We expect systems that experience frequent, temporary water stress during the growing season to be dominated by species with high stomatal control (high Ψcrit), whereas places with predictably high water availability during the growing season would continue to transpire and photosynthesise to the point of senescence.
As an effect trait, plants with a low Ψcrit are likely to have to discard some living aboveground leaf material during a drought because they are functioning closer to their safety limit and will, therefore, accumulate more dead biomass by the end of the growing season. They will also cure faster than those with a high Ψcrit at the end of the dry season. Systems dominated by plants with a low Ψcrit are, therefore, likely to have longer dry seasons and be more flammable (see discussion on curing rates in the ‘Integrated flammability protocol (IFP)’ section, ‘Measurement protocols’ subsection).
Method
Methods described here largely follow Tucker et al. (2011). The physiological drought index should be measured on at least 8–10 individuals; so, it is necessary to germinate ~100 individuals from seed (or from individual culms; du Toit 2009) in standard conditions in a greenhouse or, preferably, a controlled growth chamber. Vapour pressure deficit should be as constant as possible among days, so that the plants are mostly responding to the changes in soil water content. Once they have reached sufficient size (8–12 weeks old), a drought is applied by withholding water. The soil moisture and stomatal conductance are measured daily on three non-senesced leaves of at least eight individual plants of each species, by using a porometer. Once the plant has reached 5% of maximum stomatal conductance (our definition of stomatal closure), the same three leaves are harvested and the leaf water potential (Ψ) measured by using a Scholander pressure device. The leaf water potential corresponding to stomatal closure is the ‘physiological drought tolerance’ of that species.
The measurements should always be taken at the same time of day to control for diurnal patterns of water stress. We recommend sampling in the morning before 1100 hours in the tropics. Other studies (e.g. Ocheltree et al. 2016) have sampled from 1100 to 1400 hours to get midday water potential.
Notes
Ideally, the drought should follow the dry-down curve observed in the field, which would mean adding water in the pots daily to achieve an idealised soil moisture curve. However, in our experience this is not necessary. Likewise, it would be ideal to harvest one leaf from each individual daily to calculate the relative leaf water content (according to standard methods described in Pérez-Harguindeguy et al. 2013).
Regrowth in the dark (RITD)
Trait description
Regrowth in the dark (RITD) is the amount of aboveground biomass produced in the absence of any light following the initial removal of all aboveground biomass by clipping, burning or grazing, relative to the initial size of the plant. This trait estimates the capacity of grasses to resprout using remobilised resources stored belowground (Ripley et al. 2015). This functional trait can be measured on all mature grasses.
Relevant environmental filters
Regrowth in the dark provides a measure of a plant’s stored carbohydrates, which represents the ability to maintain high growth rates even with low photosynthetically active leaf area following complete defoliation. Resprouting from stored reserves is a tolerance strategy that allows plants to persist under frequent defoliation by grazing (Qian et al. 2017). An effective strategy appears to be to rapidly regain aboveground biomass following defoliation, allowing individuals to quickly become effective competitors for light (Archibald et al. 2019). Large below-ground reserves could also be effective for plants growing in regions where droughts are frequent, but this has not been tested for grasses as far as we are aware.
Notes
Large underground stored reserves may also provide an advantage to grasses in areas with frequent fires, by allowing them to resprout rapidly after being burned and, thus, re-establish their aboveground photosynthetic material. However, because even annual fires are infrequent within a typical grass growth cycle, it is possible that the benefits of high photosynthetic rates outweigh those of large stored reserves, so long as there are adequate reserves to initiate regrowth in the post-fire environment (Ripley et al. 2015). In areas where fire is infrequent and litter accumulates, underground reserve can initiate growth through the litter layer.
Method
Regrowth in the dark should be measured on a minimum of 10 individuals per species per site at each sampling interval after defoliation by clipping, burning or grazing. We suggest that 2-week (14-day) intervals and two harvests should be sufficient. For greenhouse experiments, mature plants grown in pots can either be burned or clipped to remove all aboveground biomass, after which an inverted plant pot should be placed over each individual. If plants are of a known or similar age and size, then the replicates could be reduced, but in the field, it is necessary to account for initial size. Black or dark green pots are recommended, because these block out more light than do white pots. Ripley et al. (2015) recommended that the inverted pots have ventilation holes covered with black cloth and are painted silver to avoid overheating, which also allows for better air circulation and minimises effects on humidity and fungal development. Because of reduced transpiration and evaporation, watering should be reduced such that the soil is kept slightly moist, to avoid over-watering. It is necessary to account for the effect of the initial size of a plant on the amount of stored reserves. In clipping experiments, the initially clipped biomass should be dried and weighed. For field-based studies involving burning or grazing, species-level allometric relationships should be derived to estimate initial aboveground biomass from basal area, canopy area and plant height (Oliveras et al. 2014). Alternately, tuft diameter can be used as a crude proxy for initial biomass within a species. At each sampling interval after initial defoliation, the new growth of each of at least five plants should be harvested, oven-dried, and the dry weights should be determined. The slope of the regrowth as a function of the initial biomass is the trait to be recorded. Slope estimates will be most robust where a large range of plant sizes are included. Where there is a low variation in plant size, forcing the regression line through the origin should provide a more reliable slope estimate. However, this comes at the cost of assuming that there are no consequential changes in regrowth rates, such as an initial lag or surge in the regrowth rate.
Notes
This trait can also be measured on plants growing under natural conditions in the field, by placing an inverted pot over a plant before it regrows after fire or grazing. To avoid potentially measuring regrowth fuelled by clonal growth, a metal plate should be used to sever roots around the pot edge. Differences in the age and micro-environment of different individuals may also influence results. The proportion of total regrowth attributable to the use of remobilised stored reserves can be estimated by measuring regrowth rates in the dark in association with regrowth rates in the light (Ripley et al. 2015).
Shade biomass ratio (SBR)
Trait description
Shade biomass ratio (SBR) is the ratio of biomass produced under 50% shading to the biomass produced under full sunlight and is calculated separately for aboveground v. belowground biomass (Solofondranohatra et al., in press; X. Gao and D. W. Schwilk, unpubl. data). This trait quantifies how shade tolerant a species or population is, by contrasting plant growth achieved under shaded conditions with that achieved under full-light conditions, while keeping other environmental conditions constant. Species or populations with high aboveground and belowground shade biomass ratios are considered more shade tolerant because shading has less effect on biomass production than for species or populations with low shade biomass ratios. This functional trait is measured on grasses grown in a greenhouse under controlled conditions.
Relevant environmental filters
The proportion of understorey plants in a community that show some level of shade tolerance is anticipated to increase with tree cover, and, hence, broadly with rainfall. Species with high levels of shade tolerance are nonetheless expected to occur below tree canopies in most savanna ecosystems, although other aspects of the understorey microhabitat (e.g. soil moisture and nutrients) will also likely contribute to the commonly observed differences in understorey plant communities below and beyond tree canopies (Ludwig et al. 2001). There is also evidence that grass flammability is negatively related to aboveground shade biomass ratios in grassy ecosystems in the south-western United States, which suggests that species with a high shade tolerance may be able to facilitate the persistence of trees in savannas, by reducing the temperatures that they are exposed to during fires (X. Gao and D. W. Schwilk, unpubl. data). By contrast, Solofondranohatra et al. (in press) observed no response in aboveground shade biomass ratios in Madagascan grasses, but strong effects of shading on belowground biomass, which is likely to reduce the resprouting ability of these grasses after defoliation.
Method
Shade biomass ratio can be determined by growing plants in a greenhouse, with half of the individuals grown in full light, and the other half in 50% shade. After germination, 10 potted seedlings should be randomly allocated to the 0% and 50% shade treatments and grown for 4 months while maintaining equivalent water and nutrient-supply conditions in each treatment. Pots should be randomly relocated within each treatment each month, to minimise any effect of variation in light intensity within treatments. After 4 months, each plant should be carefully uprooted, and any attached soil gently washed off the roots to minimise loss of fine roots. Each plant should then be split into aboveground and belowground biomass, and then dried and weighed. The aboveground and belowground shade biomass ratios are respectively calculated by dividing the total aboveground or belowground biomass of plants grown under 50% shade by the aboveground or belowground biomass of plants grown under 0% shade. Gao and Schwilk (unpubl. data) made use of a split-block design, where five blocks in a greenhouse were each evenly split into 0% and 50% shade treatments. They constructed a 1.2 m-high PVC pipe frame over each block, with polypropylene shade cloth used to cast 50% shade over half of each block. For each species in their study, one pot was allocated to each treatment in each block, and shade biomass ratio was calculated at the block level, resulting in one biomass ratio estimate per species per block.
Notes
Fifty per cent shading is suggested as a general point of comparison across studies, which reflects a common shade level underneath trees in savanna ecosystems (Ludwig et al. 2001). However, a range of other shade levels can also be used to better characterise the SBR response curves where this is of interest (e.g. Solofondranohatra et al., in press). Further measurements of potential interest include (1) the rate of aboveground biomass gain in shade v. full sunlight, because this influences the potential for a plant to effectively compete for light in a community, and (2) both the rate and amount of regrowth following single or repeat defoliation events, which would provide insight into the longer-term effects of shading on plant competitive abilities.
Stemminess (SS)
Trait description
Stemminess (SS) is the ratio of stem to leaf material in the aboveground biomass. It is calculated by dividing the culm biomass by the leaf biomass. Stemminess provides an indication of how attractive an individual grass is to grazers by (1) describing the proportion of lower-quality culm material to higher-quality leaf material in a non-selective bite, and (2) providing a proxy for how difficult it is to select leaf material from the sward, and hence the forage-intake rate (Benvenutti et al. 2006, 2009; Drescher et al. 2006). This functional trait can be measured on all mature grasses.
Relevant environmental filters
In general, SS is predicted to increase as a simple function of plant height, which is broadly correlated with productivity. However, where this trait constitutes a specific adaptation to reduce grazer preference, high SS values will be observed at lower productivities. High SS values will allow grasses to largely avoid being grazed (O’Reagain and Mentis 1989), particularly by selective grazers. When grazed, SS provides a measure of resistance, by decreasing intake rates (slower rates for more selective grazers) and, thus, the amount of leaf material likely to be consumed (O’Reagain and Mentis 1989; Benvenutti et al. 2006, 2009). Stem material has a higher C : N ratio than does the leaf material, owing to its primary function of providing structural support. This requirement increases when plants become taller at higher productivities. Grazer body size is positively related to the ability to digest forage with higher C : N ratios, and partly as a consequence of this, larger grazers tend to be less selective than are smaller grazers. Stemminess should be considered concurrently with culm diameter, because very thin culms will not deter grazers (Benvenutti et al. 2009). Note that SS can be increased in the following two ways: (1) by increasing culm diameter and culm density, which will often be a by-product of becoming taller, and (2) by having many culms with few leaves per culm, which may represent a specific adaptation to grazer avoidance in less productive ecosystems.
Method
Stemminess should be measured on five mature individuals per locality near the peak of the growing season. A representative sample (e.g. a ‘pie slice’ that includes material from the centre to the edge of the tuft) of aboveground material from a mature (flowering) individual can be clipped and separated into leaf and culm, which is then dried and weighed to calculate the proportion of culm material (culm mass ÷ total mass OR 1 – leaf mass ÷ total mass). An alternative, less precise but quicker, method to estimate total culm mass is to count the number of culms and multiply this by the mass of a single culm.
Notes
The trait is mostly intended to provide broad interspecific comparisons; SS values tend to show fairly high variability within a species at a locality and will also change as a plant matures. Stemminess can be reduced by intense fires, whereas cool fires will often leave thick stems unburned. When interpreting SS trait values for different species, it is thus useful to have recorded whether stems represent new growth after fire, or whether they have been retained through one or more fires. O’Reagain and Mentis (1989) used a visual estimate of SS, subjectively assessing the proportion of stems present on a scale of 0–5, where 0 = no stems and 5 = many stems.
Forb traits
Defining forbs
A common definition of a forb is a non-grassy herbaceous plant, although definitions vary greatly. The total woody tissue in an adult plant remains debatable when it comes to defining a forb. For the purpose of this study, we will use a broad definition of forbs, which represents an adapted version of the US Department of Agriculture PLANTS Database definition (USDA, Natural Resources Conservation Service, see https://plants.sc.egov.usda.gov/java/, accessed 4 November 2020), as follows: all non-graminoid vascular plants with a limited degree of aboveground woody tissue and with buds at or below the ground surface.
Forbs are widely acknowledged for their significant contribution to overall species richness of savanna ecosystems, and yet their value in addressing ecological questions remains poorly studied. Various strategies to avoid or tolerate disturbances and climatic stress are displayed by a wide array of plant families within the savanna forb flora (Bond et al. 2008). For instance, forbs often dominate savanna herbaceous communities after a drought event through drought-avoidance strategies (see regenerative traits), drought-tolerance traits (e.g. underground storage organs) and resprouting capacity (Siebert et al. 2020). Similarly, when exposed to heavy grazing, herbaceous communities may be transformed in favour of grazing-resistant or -tolerant forbs (Siebert and Dreber 2019).
Grassland and savanna forbs are particularly adapted to fire through various traits and strategies, from underground storage organs, resprouting capacity, and bud protection to post-fire flowering, fire-dependent seed germination and dispersal mechanisms (Fidelis and Blanco 2014). Despite intraspecific variations, forbs are also generally well adapted to changes in rising temperature, rainfall variability and elevated atmospheric CO2 concentrations because of their diverse gene pool, high phylogenetic diversity and greater physiological plasticity through representing all three photosynthetic pathways (C3, C4 and CAM; Varanasi et al. 2016). Photosynthetic pathway is well described for trees (e.g. Sage and Kubien 2003) and grasses (e.g. Taylor et al. 2010), but less known for forbs. Because forbs may exhibit differential responses to higher CO2 concentrations, drought and rising temperatures, describing their photosynthetic pathway is required for an improved understanding of grass–forb–tree coexistence in savanna ecosystems. Further examples of the relevance of describing forb traits in open ecosystems include their significance in the maintenance of ecosystem resilience (e.g. Bond and Parr 2010; Buisson et al. 2019) and supporting key questions related to the antiquity of grassy biomes (Zaloumis and Bond 2016; Buisson et al. 2019).
The integration of forb traits in cross-continental savanna studies that attempt to address complex global-change effects are therefore much needed. Many of the traits that are important to define the success of forbs in open ecosystems are universal and have already been described in previous handbooks (Cornelissen et al. 2003; Pérez-Harguindeguy et al. 2013; Table 4). Similarly, several of the traits described for grasses and trees in this handbook, have relevance to forbs as well. In Table 4, we have listed these traits, as well as their main environmental filters in savannas, notably herbivory, fire, and frequent drought.

|
Underground storage organs (USOs)
Trait description
Underground storage organs (USOs) describe the presence or absence and type of belowground organs. The type of USOs is described by root morphology and anatomy (see Fig. 9). Underground storage organs describe the capacity of plants to regenerate after disturbance. The presence of USOs guarantees the ability to resprout due to the allocation from belowground parts to the formation of new shoots. Since USO stores not only include buds and nutrients, but also water and carbon (e.g. starch or fructans, see Pausas et al. 2018), they can be used to assess carbon reserves (using dry biomass) and water reserves (using difference between fresh and dry biomass). This functional trait can be described for perennial grasses, forbs, shrubs and trees and it is better described for adult plants, although most species will have their USOs formed in the early stages of development.

|
Relevant environmental filters
The protection of buds in USOs is known to occur in disturbed systems (see Clarke et al. 2013; Pausas et al. 2018). Certain types of USOs are related to specific disturbances, such as the relationship between xylopodia and fire (Fidelis et al. 2014). Some USOs are related to clonality traits (see Pérez-Harguindeguy et al. 2013) such as rhizomes, whereas others function only as bud-bearing organs, such as xylopodia (Appezzato-da-Glória et al. 2008; Fidelis et al. 2014; Pausas et al. 2018). Underground storage organs is a trait related to regeneration strategy (persistence), and the diversity of USOs in an ecosystem can help describe or predict responses to disturbances such as fire at the community level (see Fidelis et al. 2014). Underground storage organs are also related to the resprouting ability and clonality of plants. The presence of USOs increases plants’ resilience to disturbance, because they store buds, carbohydrates, water and nutrients for plants to resprout after disturbance (Clarke et al. 2013; Pausas et al. 2018).
Method
This trait is stable at the intraspecific level (i.e. low levels of plasticity within species) and is, therefore, most useful when analysed at the interspecific or community level. The best classification for USOs is provided by Pausas et al. (2018) who provided a key (see Fig. 9) to identify the most important types of USOs found in tropical savannas, namely, xylopodium, lignotuber, root crown, woody rhizome, bud-bearing roots, taproot tuber, bulb, corm, stem tuber, non-woody rhizome, rhizophore and belowground caudex. Belowground roots and shoots can be distinguished by their primary anatomy, the presence or absence of leaves or leaf scales, the regularity of insertion of organs (generally more regular on stem). Woody roots are lignified, whereas non-woody roots are not. Note that some USOs include organs used for clonal propagation (e.g. rhizomes) and others do not (e.g. xylopodium). Most USOs have some kind of storage function (e.g. starch), whereas certain types may function only as bud-bearing organs that sometimes also store water (e.g. xylopodium; Appezzato-da-Glória et al. 2008; Pausas et al. 2018). At least three individuals per species should be dug up during the peak of the growing season and the USOs should be placed in plastic bags. In the laboratory, they should be washed to remove excess soil, weighed and stored in 70% alcohol or placed in a freezer until ready for identification. To identify the different types of USOs, the identification key of Pausas et al. (2018) should be used (Fig. 9). For further analyses, USOs can be oven-dried at 85°C for 48 h (or until constant weight is achieved for large samples) to assess carbon (dry mass) or water content (fresh minus dry mass).
Post-fire flowering (PFFLO)
Trait description
Post-fire flowering (PFFLO) refers to the ability of a plant to flower after a fire (but could also be applied to other major disturbances, e.g. herbivory or drought) and whether this response is fire-dependent or not. This trait is described by evaluating flowering responses in post-fire environments by counting the number of individuals flowering in a population. The ratio of reproductive to vegetative shoots, as well as the aboveground allocation to reproduction (ratio of reproductive to vegetative parts of the plant) can also be used to quantify a plant’s allocation to post-disturbance reproduction. This functional trait applies to forbs, grasses and woodies in the adult life stage.
Relevant environmental filters
Most handbooks do not consider reproductive phenology in their list of traits. In fire-prone ecosystems, PFFLO is a well described event, and fire-stimulated flowering (disturbance-dependent) can be classified as protanthy (flower then foliate), seranthy (foliate then flower) and synathy (foliate and flower at the same time; see Lamont and Downes 2011). Some species may not be affected by disturbance (disturbance-independent) or even be negatively affected by disturbance (disturbance-sensitive). Reproductive phenology of some groups (e.g. forbs) responds more to disturbance than others. Post-fire flowering typically occurs in the first year post-fire (usually within a few weeks or months after a fire) when flowering peaks (Lamont and Downes 2011; Fidelis and Blanco 2014). According to Lamont and Downes (2011), the majority of species that exhibit PFFLO are monocots that often also have USOs (e.g. orchids). This functional trait describes the ability of adult plants to allocate resources to reproduction v. vegetative regrowth (i.e. production of shoots) after disturbance. Plants that are able to rapidly allocate resources to reproduction should have an advantage in post-fire environments, because they are able to attract pollinators, replenish the seed bank while more soil is exposed, and disperse seeds longer distances. Finally, plants that can rapidly flower after fire must first be able to resprout (Lamont and Downes 2011) and, therefore, the presence of USOs is typically expected to accompany PFFLO.
Method
A minimum of 10 individuals should be sampled in the post-disturbance population of each species. This number can be increased if the species that are studied are abundant. There are the following two methods that can be followed: (1) individuals are marked and their phenology followed for the first 3 months following fire, or until the start of the next rainy season (although note that fire-induced flowering has been recorded up to 1 year after fire, depending on the species and growth form), or (2) individuals are sampled when they are flowering for biomass allocation. In both cases, one should monitor the plants regularly (at least monthly) so as to sample plants during the flowering period. Plants in non-disturbed neighbouring areas should also be marked to ensure that flowering was triggered by the disturbance.
Method 1. If PFFLO is to be followed (with no destructive sampling), plants should be marked, and their vegetative and reproductive shoots should be counted when flowers are fully open. If plants flower within 3 months (or before the next rainy season) in burned areas, but not in non-burnt areas, plants should be classified as having fire-dependent flowering. If plant flowering increases by more than 50% (i.e. higher biomass or reproductive shoots) or earlier in burnt areas, they should be classified as having fire-stimulated flowering. If flowering happens equally at both sites (burnt and unburnt), plants should be classified as having fire-independent flowering. Finally, if flowering does not happen in the burnt area, only in the unburnt areas, plants should be classified as having fire-sensitive flowering. This classification can be performed by counting only the proportion of individuals that are flowering.
Method 2. If PFFLO is to be evaluated by sampling flowering individuals, only individuals with at least 50% of fully open flowers should be sampled. Individuals should be cut at the soil level and should be at least 5 m distant from each other (owing to the presence of clonal plants). Reproductive parts (including flowers and flower stalks) should be separated from vegetative parts and put in paper bags. In the laboratory, they should be dried (65°C for 48 h) and weighed separately. The ratio of reproductive to vegetative biomass can then be calculated. This ratio can be used to determine whether a plant has high investment in flowering in relation to vegetative resprouting.
Bud bank (BB)
Trait description
Bud bank (BB) describes the number of viable buds (axillary and adventitious buds) and their position in relation to the soil surface (see Fig. 10). Bud bank (position and density) describes the potential of a plant to regenerate after disturbance (fire, herbivory) or to produce new shoots outside of the normal (wet) growing season. This functional trait can be measured on perennial forbs, grasses and shrubs in all life stages, but should preferentially be measured on adult individuals. Bud bank can be observed by external morphological observations (base of leaf sheaths, surface of underground organs), but it is sometimes necessary to cut these structures to ensure that what is being observed is a bud and not a root apex.
Relevant environmental filters
This trait is related to the ability of a plant to resprout after fire, herbivory or drought. If plants have a high investment in BBs, it suggests that they have the capacity to respond to disturbance by producing numerous new shoots or stems. A belowground BB would be advantageous where fires are intense (depending on the depth of burial), whereas an aboveground BB would be linked to bud protection (see bud-protection trait). Bud bank is a trait related to the persistence strategy because it allows for the rapid recovery of damaged vegetative structures after a major disturbance (e.g. fire, herbivory or drought). Species adapted to high fire frequencies should have a higher BB density. Post-disturbance BB density is expected to decrease over time because of bud mortality. The durability of the buds should match or be slightly longer than the average return interval of the local disturbance regime (see Benson et al. 2004; Dalgleish and Hartnett 2006; Fidelis et al. 2014).
Method
This trait is highly variable within a species, depending on the age of the plant and the local disturbance regime. Because it is difficult to define the age of plants in savanna systems (Wigley et al. 2019a), Klimešová and de Bello (2009) recommended that the BB distribution (vertical distribution of buds) be evaluated in five layers in relation to the soil surface. The seasonality, type of perennation of BB organs and number of buds per shoot should also be evaluated according to the layers. However, because the size of a plant usually does not correspond to its age in savanna systems, it is difficult to use the number of buds per shoot, or even just to count buds or bud-bearing organs, because it might fluctuate according to plant age. In cases where the age of a plant is known (e.g. seedlings), the method proposed by Klimešová and de Bello (2009) could be used. However, we propose that only three layers are used for forbs and grasses, namely, belowground, soil surface (0–5 cm above the soil surface) and aboveground (i.e. >5 cm from the soil surface). For woody plants, see the Presence of accessory buds (ACCB) trait in the woody plant section. If the age of the plant is known, we recommend sampling 10 individuals of the same age. These should then be carefully dug out. It is usually not necessary to dig deeper than 10 cm as most of the belowground buds are located in the first 10 cm of the belowground structures. Plants should be placed in plastic bags in the field and taken to the laboratory. Plants should be washed to remove excess soil and fixed in 70% alcohol or placed in the freezer until ready for bud counting. Each individual should have their buds located (belowground, surface and aboveground) and counted according to the position (see Fig. 10). Axillary and adventitious buds can be counted using a binocular microscope. If there is any doubt about the origin of the structure (bud or root apex), anatomical cuts should be performed to assure that leaf primordia are observed. Usually one can observe these structures according to their morphology. For the aboveground parts, axillary and accessory buds should be counted (see ACCB trait).
If the age of the plant is not known (which will usually be the case), plots will need to be established within populations of the target species. The plots can vary according to the size of the plants under investigation (0.5 × 0.5 to 1 × 1 m). All individuals of the target species should be sampled, and buds counted (as described above). For grasses with large tussocks, one can establish a smaller sample within the tussock (such as 0.1 × 0.1 m) and sample the same size in different tussocks. For forbs, individuals should be sampled, and bud counting should be separated according to the three layers. Bud density should be expressed as buds per square metre, whereas bud position is a categorical trait. If the seasonal dynamics of the BB are to be assessed, the same procedures should be used during the wet and dry seasons, at the same sites. The best time to sample the BB is during the wet season, at the peak of growth. Bud bank should be assessed in conjunction with USO type (see USO trait). For sites that have been excluded from disturbance, it might be difficult to find forbs with aboveground biomass, because they may be dormant in the belowground parts.
Woody plant traits: saplings and adults
For woody plants, each life-stage (i.e. seedlings, saplings and adults) faces a particular set of challenges (e.g. within or out of shading, flame or herbivore zones). Furthermore, as a consequence of the vertical structure of savannas (browse height, fire escape height, see Fig. 1), plant demography is a useful framework for understanding bottlenecks specific to each life stage, and, hence, traits that plants might rely on to survive, grow or recruit (Midgley and Bond 2001). Bottom-up and top-down controls have contrasting influences at different life stages. For example, bottom-up controls (e.g. nutrients, water availability and climate) affect germination and establishment, growth rates, hydraulic limitation to height and senescence, whereas top-down controls (e.g. herbivory, fire) influence establishment, recruitment into larger size classes, and mortality across all size classes. Here we have grouped the traits that are relevant to both woody sapling and adult life stages.
Bark traits
We use the term bark to describe all tissues external to the wood, following Pérez-Harguindeguy et al. (2013). For all bark traits, we recommend that both inner- and outer-bark thickness is measured and recorded insofar as possible. Four main approaches have been adopted to describe how bark can protect a plant from fire. Each of these methods provides slightly different information on the protective role of bark. The most common approach is to measure bark thickness (described in detail in Pérez-Harguindeguy et al. 2013). This first approach measures the combined outcome of bark production and bark shedding (i.e. losses due to active shedding by the plant, weathering, fire, abrasion from animals). Bark thickness is expected to be strongly related to the survival of aboveground parts in systems where moderate to intense fires (fuel mostly composed of dead branches and woody plants themselves) are common (e.g. Quercus suber in Mediterranean systems). The interval between fires tends to be long (>10 years) in these systems, allowing trees enough time to grow and develop very thick bark. However, when fire intensity is high, even trees with thick bark do not survive and basal resprouters or trees protecting their seeds are favoured. The second approach used by Staver et al. (2012) and Dantas and Pausas (2013) is an adaptation of the above method. In this second approach, the bark thickness is recorded on saplings of different stem diameter-size classes. The measurement of bark thickness for given diameter-size classes provides meaningful information when analysing size-dependent stem mortality and has the advantage of being relevant to the life stages that are the most sensitive to fire. A third method (bark growth rate) has been developed to predict the performance of species in relation to fire frequencies. The fire regime typically found in savannas is characterised by frequent fires of low intensity (as the fuel is mainly composed of dry grasses). In savanna systems, it is, therefore, important to develop enough bark in the period between germination or resprouting and the next fire event. This trait is calculated as bark thickness (for a portion of stem that has not started bark shedding) divided by the age of the structure. It allows for a yearly rate of bark production to be calculated at the species level. The diameter is not equivalent to an age when comparing multiple species because wood annual increment varies tremendously among species. The fourth approach usually described as relative bark thickness or bark investment (see Pérez-Harguindeguy et al. 2013) is measured by dividing bark thickness by stem diameter. Bark relative thickness, therefore, describes a plant’s respective investment in bark v. wood and provides meaningful information about allocation trade-offs. Finally, bark damage is often a major constraint on woody plants growing in savanna systems, especially when mega-herbivores (e.g. elephant) and fires are common. We have, therefore, also included methods that can be used to determine a plant’s ability to resist bark damage and recover from bark damage (defence against bark stripping).
Bark growth rate (BGR)
Trait description
Bark growth rate (BGR) is the annual rate of bark production measured on the main stem or trunk. It is calculated by dividing the total bark thickness of the oldest portion of trunk without bark shedding by the age of the stem at the level where bark thickness was measured (Fig. 11). Bark growth rate describes the rate of bark accumulation and, therefore, predicts how quickly a species will be protected against fire after germination or resprouting. This functional trait applies only to woody species with a phellogen (excludes most forbs, grasses, palms, cycads). This trait requires prior basic training in plant architecture to identify growth units (sensu Barthélémy and Caraglio 2007).
Relevant environmental filters
A high BGR is linked to higher survival of species (Charles-Dominique et al. 2015a, 2017a) growing in areas that are frequently burnt at low fire intensities. Bark growth rate best describes the likelihood of survival for the life stages (i.e. saplings) within the flame zone that are the most sensitive to fire. Bark growth rate describes the rate of accumulation of bark on the trunk (and, thus, does not describe the total thickness of bark found on a trunk that is a function of this rate, the number of years the bark accumulated and the amount of bark that was shed). This trait was defined to predict how fast a species can develop enough bark to face the first fire in environments with frequent fires of low intensity. This trait is expected to be strongly related to fire frequency and, to a lesser extent, to fire intensity (Charles-Dominique et al. 2015a, 2017a). Bark growth rate should be low in tropical environments where surface fires are rare and where grass biomass is low (e.g. arid savanna, forest, thicket).
Method
This trait is suitable for interspecific comparisons. Because BGR is expected to be stable at the intraspecific level, we suggest that sampling can be limited to three to five replicates per species where destructive methods are problematic, or to 5–10 individuals per species where they are not. Ideally 2–3-m-tall saplings growing within the flame height should be selected: then, (1) a location on the trunk that has not started shedding bark is identified (Fig. 11a), (2) a section of the trunk is cut and bark thickness measured (Fig. 11b), (3) the age of the section that was cut is estimated and (4) bark thickness is divided by the age to obtain BGR in millimetres per year.
-
Measuring bark on young trees reduces the problem of confounding bark gains from growth with losses from bark shedding. To ensure that no bark has been shed, a dead epidermis must be visible at the location on the trunk where bark is to be measured. The epidermic layer can easily be identified by looking at young stems (at the crown level) and comparing this with older and older stems (as you move towards the base of the trunk), until signs of shedding are visible on the outermost layer (Fig. 11a). If the epidermis is still visible at the base of the trunk, measurement is made at ~20–30-cm height (and not lower), so as to describe the area exposed to high temperatures and without the particular anatomy of collar, lignotuber, or basal burl tissues.
-
Many methods exist to measure bark thickness (see Pérez-Harguindeguy et al. 2013). The same cutting can be used to measure bark thickness and to estimate the age. After taking a photograph orthogonally to the cutting (under a binocular if needed; Fig. 11b) with a scale in place, bark thickness is measured in four directions on the cutting (top, bottom, left and right), while avoiding fissures and protuberances. The average per sample is taken.
-
The age of the section (where bark thickness was measured) can be evaluated through morphological and anatomical methods (ideally both to get higher confidence), but not by stem diameter (because wood production is not equivalent across species). Morphological method counts the number of growth units from the top of the plant to the cutting position using the following morphological markers: shortening of internodes, presence of bud scale leaves and events of branching for species with a 1-year-delayed development of new shoots (Barthélémy and Caraglio 2007). The relationship between number of growth units and age (it is important to make sure species are not polycyclic, i.e. with more than one growth cycle per year) should be checked at sites where the age of plants is known (by using a site where the date of the last fire event is known and measuring growth units on the oldest individuals growing after fire). Anatomical method counts the number of wood rings on stem cross-sections (Fig. 11c; also requires checking for false rings, especially for polycyclic species, ideally using individuals growing at a site that was disturbed at a known date). The mean of the age estimated by the two methods is used (if they differ and neither appears to be more accurate). In cases where one of the two methods led to unclear estimations (absence of morphological markers or undistinguishable wood rings), only one method is used.
-
Bark growth rate is calculated as mean bark thickness divided by mean stem age.
Special cases
-
Multi-stemmed species. For multi-stemmed species, one of the main or largest stems should be sampled. Lateral horizontal branches and twigs with a lower diameter should be avoided because they frequently have a lower BGR than do the main stems.
-
Root suckering species. The BGRs of species with the ability to spread vegetatively using underground specialised organs, either by underground modified stems or by true root-suckering (both referred to here as ‘root-suckers’), are frequently not related to fire frequency (Charles-Dominique et al. 2017a). It is, therefore, recommended to record this information together with BGR.
-
Species with highly developed inner bark. Bark growth rate is not expected to be a good predictor of how well species with extremely high levels of inner bark (ratio inner bark thickness : outer bark thickness of >5) are protected against fire because (1) the protective role of inner bark is still under evaluation (Rosell 2016; Pausas 2017), and (2) inner bark is composed of primary tissues (produced during the first year) and secondary tissues (with an annual increment; Evert 2006) in proportions that are difficult to evaluate.
Relative bark thickness (RBT)
Trait description
Fires damage trees in proportion to their absolute bark thickness (ABT), which is simply a measurement of how thick the bark is. For example, 0.6-cm thickness of bark provides an ~50% chance for stems to survive top-kill in South American savannas (Hoffmann et al. 2009). Absolute bark thickness is not a useful species-specific trait because it depends very strongly on plant size; larger stems have thicker bark. Relative bark thickness (RBT) is the ratio of bark thickness divided by the bole diameter (BD = stem diameter – 2 × bark thickness; Midgley and Lawes 2016). Relative bark thickness is a species-specific trait and linked to plant life histories and plant biogeography (Pellegrini et al. 2017). For example, epicormic resprouters tend to have a high RBT and epicormic resprouters are most common where ground fires, rather than canopy fires, take place (Pausas and Keeley 2017). Relative bark thickness is strongly dominated by two factors; primarily, by rates of bark retention and, secondarily, by bark growth rates. For example, a bark thickness of 0.6 cm may be achieved in 3 years, by growth of 2 mm year–1, if all bark is retained, but at faster growth rates if bark is shed. At this stage, not enough information is available on the variability of bark-retention rates and, thus, bark-thickness growth rates should be measured on young shoots and branches where shedding is limited. Australian eucalypts are notorious bark-shedders (e.g. analysis of Eucalyptus obliqua in Midgley and Lawes 2016) and, in this instance, their bark thickness is more strongly determined by growth rates, than by retention rates.
Special cases
A subtlety in the trait of bark thickness is when bark thickness varies abruptly with stem height. These are the ‘half-butts’ very common in Australian eucalypts (e.g. Eucalyptus miniata), which maintain a 1-2 m-high sleeve of thick bark at the plant base and above this bark is rapidly shed. Stems above the butt are typically green and photosynthetic.
Relevant environmental filters
High RBT in adult woody plants is indicative of greater allocation to defence and confers resistance to fire, pathogens and insect attack (Rosell 2016). Trees growing in fire-prone environments are likely to have a high RBT, so as to cope with an increased frequency and intensity of fires (Pellegrini et al. 2017). A low RBT cannot protect the xylem (primarily) and cambium from heat transfer and renders the adult woody plant vulnerable to xylem cavitation, top-kill and death. There are trade-offs between RBT and other life-history traits, such as growth rates, plant architecture and resprouting ability.
Method
Relative bark thickness should be sampled from a minimum of 10 individuals, and of stems no thicker than 100-mm bole diameter.
Bark can be sampled using a variety of tools, such as bark punch (or narrow-gauge leather punch) or sharp knife; bark thickness (BT) and stem diameter (SD), which includes bole, inner and outer bark, are measured using a pair of Vernier callipers; inner and outer bark (Rosell 2016) are included in the measurement of bark thickness.
A minimum of 50 measures are to be taken from the thinnest mature twig to a maximum of 100 mm BD, from a range of individuals, so as to obtain a reasonable regression relationship between BD and BT.
RBT can be calculated as
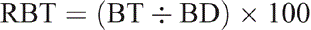
or by ordinary least-square (OLS) regression analysis of the data, with the line forced through zero, whereby the slope of the line provides the metric for the trait.
Defence against bark stripping (DABS)
Trait description
Global patterns of mortality in woody plants in non-savanna biomes are primarily driven by stress (in particular, drought and frost), pests (McDowell et al. 2018), fire, windthrow (Mitchell 2013) and anthropomorphic drivers (Lindenmayer et al. 2012). Herbivory and fires, and their interactions (e.g. elephant feeding and fire), are an important determinant of top-kill and adult mortality in savanna systems (especially African and Asian savannas). Although elephants are the primary agent of bark removal, humans (Williams et al. 2007), porcupines (Yeaton 1988), browsing antelope and buffalo (through the rubbing of the horns) can also damage the bark of woody plants and expose the xylem. Elephants are mixed feeders that predominantly graze in the wet growing season, but switch to browse during the dry season. During the dry season when many trees have lost their leaves, elephants also utilise the bark and roots of woody plants to access digestible proteins, stored carbohydrates and water-rich phloem and cambium (O’Connor et al. 2007). If entirely ringbarked or girdled (i.e. all bark, cambium and phloem is removed from the complete circumference of the tree), mortality follows as a consequence of the interruption of sap and nutrient flow, root starvation and canopy death. However, especially in more fire-prone savannas, after bark (rhytidome and living inner bark (phloem and cambium)) is removed, the xylem is exposed to pathogens, wood-boring insects (Rosell 2019) and fire (N’Dri et al. 2014). Following stripping of bark, successive fires may result in cambial death or embolisms in the xylem and, ultimately, top-kill or mortality (see Midgley et al. 2010 for review). For example, the proportion of bark stripped below 3 m has been found to be the best predictor of Acacia nigrescens mortality (Moncrieff et al. 2008). The manner in which an elephant uses a plant, and the plant’s growth response to resist or compensate for utilisation, should determine whether it survives (O’Connor et al. 2007). A suite of stem traits plays an important, yet poorly studied, role in limiting top-kill and mortality in savanna systems. Woody species vary considerably in the degree to which their bark may be damaged by herbivores. This is typically not determined by one single trait, but rather through an interaction of stem traits. Bark damage can be limited by avoidance, resistance (limiting stripping) or tolerance (recovery) to stripping. In general, the survival of savanna trees is strongly linked to limiting or recovering from bark stripping, i.e. fluted bark, less fibrous bark and the ability to regrow bark following stripping. The key traits associated with each of these strategies include the following:
(1) Avoidance of stripping
Multi-stemmedness. Allows avoidance of bark stripping of inward-facing sides of the outer stems as well as total protection of inner stems.
(2) Resistance to stripping
Flutedness of the trunk. Trunk flutes, i.e. convolutions or folds in the trunk (see Fig. 12), reduce the potential for bark removal and can protect up to ~70% of the surface area of the trunk in Balanites maughamii (Williams et al. 2007).
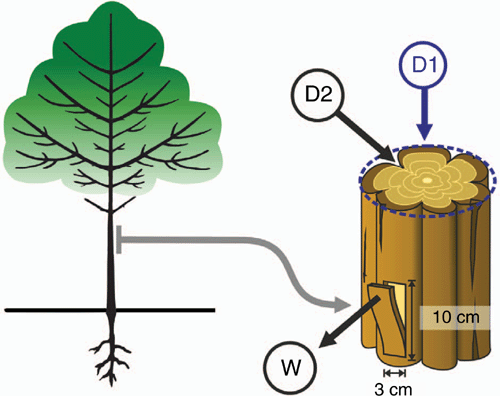
|
(3) Bark fibrousness and stem spines
Both of these play a role in determining how easily and how much bark can be removed from a stem, thereby exposing the xylem to fire, insects and other pathogens (O’Connor et. al. 2007). Fibrous bark, when stripped, results in the exposure of long portions for the stem. Less fibrous, ‘blocky’ bark typically shears sooner, exposing less xylem, cambium or phloem. Less fibrous ‘blocky’ bark increases the effort and time required to remove bark, limiting herbivore feeding efficiency. Stem spines are purported to limit bark stripping by increasing resistance to stripping. Vascularised knobs or hooks, as opposed to stem emergences (Bell 2008), provide greater resistance to stripping, reducing the surface area removed.
(4) Recovery from stripping
Bark regrowth is a compensatory mechanism that allows woody plants to recover their resistance to fire and pathogens. Bark regrowth may be influenced by age or size and total area removed (Vermeulen et al. 2012), and differs across species (Wigley et al. 2019b).
Relevant environmental filters
(1) Resistance to stripping
In disturbance-prone environments, greater trunk flutedness could be linked to a reduction in bark loss, particularly in adult savanna trees, and, hence, a reduction in top-kill or mortality. Low flutedness exposes more of the trunk, rendering the tree vulnerable to either ringbarking or significant bark removal, resulting in top-kill or mortality. We predict that greater bark fibrousness (‘stringy bark’) indicates a greater vulnerability to being stripped by megaherbivores and exposure to fire and insect pathogens. Low fibrousness is linked to limited bark loss and increased stem survival. Increased density of vascularised spines or knobs is associated with reducing the surface area of stripped bark, in turn reducing stem mortality. The presence of knobs may confer some advantage by limiting stripping of the stems.
(2) Bark regrowth
Greater bark regrowth increases the probability of survival, whereas limited bark regrowth exposes the stem to pathogens, insect feeding and fires (Moncrieff et al. 2008; Wigley et al. 2019b). Limited unpublished data suggest that resistance to bark stripping and increased bark recovery is highest in fire-driven systems, i.e. mesic savannas, where the increased intensity and frequency would select for these traits.
Method
(1) Resistance to stripping
Flutedness of trunk. The ratio of the circumference at breast height (CBH; or at the base if branched close to the ground) relative to the actual circumference is a useful measure of flutedness (see Fig. 12). Actual stem circumference can be measured with a flexible tape measure. Flutedness should be measured on at least 10 individuals per species.
Bark-fibrousness index. The bark-fibrousness index should be measured on at least 10 individuals per species. At a height of 1.5–2 m on the trunk, a strip that is 3 cm wide and 10 cm long (avoiding lateral stems and spines) is cut, but the bottom edge is left attached (Fig. 12). The top edge of the bark is separated from the stem and a (self-locking) heavy duty pulling clamp (or any similar clamp) is attached, connected to a Pesola (www.Pesola.com) scale (50 kg capacity) that measures the force required to tear the bark from the stem. Pulling is then required until the portion of the bark is removed entirely from the stem, i.e. bark becomes entirely separated from the stem. The maximum weight is read off the Pesola scale and converted into Newtons (N) by multiplying by 9.80665. This will provide the maximum force required to tear the bark from the stem. The area of the removed bark section is then calculated by either photographing the area of removed bark on the stem or the actual piece of removed bark can be laid on a flat surface and photographed. For both of the above options, a 30-cm ruler needs to be included for scale. Software such as ImageJ can then be used to calculate the area of the removed bark section. The bark-fibrousness index (cm2 N–1) can then be calculated by dividing the total area of bark removed (cm2) by the force (N) required to remove the bark section. The removal of a strip of bark usually represents little threat to the tree’s survival.
Bark regrowth. Bark recovery following stripping is recorded as the proportion of bark recovered following a stripping event after a full growing season. However, it is worth noting whether the tree can continue to recover its bark during subsequent growing seasons. Bark regrowth can be empirically measured by removing a circular section of bark of 5-cm diameter (~20 cm2) with a mallet and a sharpened corer before the growing season. Bark regrowth focuses on the recovery of the living inner bark. The thin layer of outer bark (i.e. periderm and cork) is removed using a wood chisel and then the entire layer of inner bark (i.e. secondary phloem) is removed, without damaging the underlying wood (Wigley et al. 2019b). Regrowth can be calculated by measuring the regrowth on two perpendicular axes (see Fig. 13) on a regular basis (3–4 months), but should be enumerated after a full growing season. Regrowth can also be measured by taking a photograph with a scale bar included, and calculating percentage recovery by using ImageJ or similar software. The removal of a small disc of bark usually poses little risk to the tree.
Bud traits
Bud protection (BP)
Trait description
Bud protection (BP) describes the location of buds sensu lato (including epicormic strands without scale leaves) within the bark layer. It is described using macro-anatomical cutting and external morphological observations at the base of the trunk. Bud protection describes how well buds are protected against fire and relates to a plant’s ability to resprout after fire. This functional trait applies only to woody species with an active phellogen (excluding most forbs, grasses, palms and cycads).
Relevant environmental filters
A high level of BP is linked to a greater ability to resprout after fire and higher survival rates for species growing in frequently burned areas with low to moderate fire intensities (Burrows 2013; Charles-Dominique et al. 2015b). Bud protection describes a tolerance strategy of woody life stages (saplings to mature trees) exposed to fire (within the dominant flame height). Bud protection should be high for most species in areas with frequent fires (with the exception of root suckers sensu lato, Burrows et al. 2010; Charles-Dominique et al. 2015b). In systems with infrequent but very intense fire (e.g. Mediterranean), the whole aboveground structure of saplings is often burned, with no obvious advantage for high levels of BP. Bud protection is expected to be high in all systems with high (>2 Mg ha–1) grass biomass (fuel loads). Bud protection has been shown to differ between biomes with contrasting fire regimes (Burrows et al. 2010; Charles-Dominique et al. 2015a). Because this is a very stable trait at the species level (low intraspecific variability), it can be used as a specific attribute on phylogenies, such as to understand the rise of fire-dominated systems during evolutionary times (Crisp et al. 2011).
Method
This trait is suitable for interspecific comparisons. It is a very stable trait requiring three destructively sampled replicates per species. The methods are easiest to perform on 2–3-m tall saplings growing within the flame height (the trunks of bigger trees can prove challenging to cut accurately). The detailed steps to determine BP for each species under investigation are as follows:
-
Ideally, BP should be assessed on a trunk that has not started bark shedding (Fig. 14a). Potential location of preventitious buds (buds that are found in leaf axils) is determined using the external morphology of the trunk.
-
A bud is located using scars, phyllotaxy and angles visible on the stem (Fig. 14b). Ideally, the bud should be in the middle portion of a growth unit (see Barthélémy and Caraglio 2007). For species producing extensive bark, we recommend preliminary comparative observations with younger structures (at the crown level) to clarify which scars correspond to bud and leaf scars (and which ones correspond to branch scars) and where the bud should be located. Cuttings should not be performed through branch scars, because buds would have already been used to produce branches. Branch scars can be easily identified because they leave a big circular scar with radial symmetry. Leaf scars with an intact bud generally produce bilateral symmetry. When scars are not visible anymore (owing to intensive bark production), the angles observed on the stem inform as to the presence of a node (leaf + bud) and can help locate the bud.
-
A 20-cm-long stem section, including the observed bud, is cut with a handsaw or secateurs. The section is cut length-wise with a razor or chisel, so as to reveal the trunk and bud pith (Fig. 14c). Some adjustments may be necessary after the initial cut. We advise practising the method before describing the samples.
-
Bud protection is a semiquantitative variable with the following five categories: (1) not protected (bud entirely outside the bark surface); (2) low protection (bud emerging from the bark surface, but bud apical meristem below the surface); (3) intermediate protection (bud at the base of a deep narrow depression in the bark); (4) high bud protection (bud totally covered by bark); and (5) extreme protection (bud embedded within the wood layer; see Fig. 14d). Score BP for each longitudinal section according to the five categories listed above and shown in Fig. 14d. Take the average score of the three sections. External morphological observations can complement macro-anatomical cuttings.

|
Special cases
-
Multi-trunk and -stemmed species. For multi-stemmed species, one of the main stems should be sampled (frequently vertical stems with larger diameters).
-
Type of stem to sample. Trunks or main stems. Lateral branches and twigs should be avoided because they frequently have a lower BP than do the main stems.
-
Root-suckering species. For root-suckering species sensu lato (underground modified stems or true root-suckering, see the ‘Resprouting as a trait syndrome (RAATS)’ section), the BP is poorly informative of species survival in fire-dominated systems. It is suggested to record this information together with BP.
-
Limitation of the method. This method applies only to preventitious buds (located at the axil of a leaf (or leaf scar)). Some species can additionally develop adventitious buds (out of leaf axils) that reinforce species ability to survive fire (for more information, see Meier et al. 2012), but these will not be disclosed by the described method.
-
Tips. When performing the cutting, one may find only empty axils or buds already developed (for example, into floral structures). In these cases, target smaller buds (frequently located at the extremity of the growth unit) that are usually more likely to persist as epicormics buds (Meier et al. 2012).
Presence of accessory buds (ACCB)
Trait description
The presence of accessory buds (ACCB, more than one bud at the leaf axil; see Bell 2008) can be assessed from macro-anatomical longitudinal cutting, external morphological observations or both. This trait provides information on how effectively a species can resprout following disturbance. All groups (trees, grasses, forbs) described in this handbook can develop accessory buds but with some variation in their expression. Here, we describe the criteria needed to determine whether woody species develop accessory buds and provide some information about their development in monocots. This trait requires prior basic training in macro-anatomy to identify macro-anatomical structures.
Relevant environmental filters
Species with accessory buds have enhanced resprouting abilities (tolerance strategy) and are favoured in frequently disturbed environments, because the number of available buds increases without involving a greater cost of bark production (Burrows et al. 2008; Charles-Dominique et al. 2015b). This trait seems to confer an advantage to plants both in fire-dominated and in herbivore-dominated systems and has been found to be high in species in disturbance-driven savannas and intermediate in systems rich in browsing mammalian herbivores (Charles-Dominique et al. 2015a)
Method
This trait is suitable for interspecific comparisons. It is a stable trait requiring approximately three to five replicates per species. It is usually a destructive sampling method. One positive observation is sufficient to confirm the presence of accessory buds, but several observations should be performed to conclude that they are absent. The presence of ACCB can sometimes be inferred form morphological observations. However, accessory buds frequently remain dormant and can be very small, in which case they cannot be observed from morphological observations only. If external morphological observations (Fig. 15a) fail to show the presence of accessory buds, macro-anatomical cuttings (Fig. 15b) have to be performed to conclude that the species does not have accessory buds.
External morphological observations
In dicotyledons, accessory buds are typically found either above or below the main bud on the bearing stem (see Fig. 15a). They are usually aggregated with the main bud, but can be spaced regularly as shown in Fig. 16c. The following observations can be made to conclude that a woody species has accessory buds (Fig. 15a): only one leaf (or leaf scar) with several aligned buds at its axil. Special attention needs to be paid to the location of the axillary leaf (Fig. 15a) and to the first leaves of the bud (prophylls α and β); although these two leaves can be extremely reduced (cataphylls), in dicotyledons, they are always located in a lateral position at the base of any new stem (or buds). Locating α and β leaves helps understand the arrangement and timing of bud development. Branches developed from accessory buds produce a distinctive branching pattern (Fig. 16b, 15a) where two (or more) branches are emitted, clustered on the main stem and in the alignment of the main stem axis.

|
Macro-anatomical observations
The presence of accessory buds can be recorded together with bud protection (cf. bud-protection trait), in which case samples should be prepared for assessing bud protection. If the presence of accessory buds is assessed independently, i.e. a young, vertical stem with long internodes and relatively large diameter (i.e. not on short shoots or twigs) is chosen.
A bud is located using scars, phyllotaxy and angles visible on the stem (Fig. 15a). Ideally, the bud should be in the middle portion of a growth unit (see Barthélémy and Caraglio (2007). Steps 2 and 3 in the methods for bud protection (Fig. 14, Steps 2, 3) are followed. Accessory buds (if any) should be visible in the same cutting plane with the traces of the main bud and leaf (Fig. 15b, Step 3, 16d). Presence or absence is scored.
Special cases
When performing the cutting, one may find only empty axils or buds that have already developed (e.g. into floral structures). In these cases, smaller buds (frequently located at the extremity of growth unit) are targeted, that usually live longer than do larger buds (Meier et al. 2012).
Monocotyledons develop collateral accessory buds (Bell 2008). The accessory buds can, therefore, be accessed only by cutting in a different direction (Fig. 15a, morphological observations).
Bite-size index (BSI)
Trait description
The effectiveness of structural defences can be determined by measuring the amount of material a feeding vertebrate herbivore can remove with each bite. Several studies have shown that the bite-size index (BSI) is an effective proxy for measuring the effectiveness of a plant’s structural defences against browsing by medium-sized ungulates (Wigley et al. 2014, 2018; Charles-Dominique et al. 2015c). This method uses human bites to simulate the bite size of meso-herbivores and has been found to be correlated with goat bite size (Charles-Dominique et al. 2015c). Bite-size index is expected to decline with ramification and physical defences, and increase with leaf size. Plants with physical defences (spines, thorns and prickles or being highly branched, or both) should be harder to browse, decreasing BSI values.
Relevant environmental filters
A low BSI is indicative of high levels of structural defences in plants (e.g. cage-like architectures, spines) and is expected in areas where impact by medium-sized ungulates is high. A high BSI is indicative of high availability of browse for herbivores. Low levels of structural defences and large leaves are expected where herbivore pressure is low or for plants with effective chemical defences. The BSI is strongly related to the survival and growth of coppice and sapling life stages for trees and shrubs that are within the feeding height of small and medium-sized mammal herbivores.
Method
Bite-size index should be measured during the growing season when leaves are fully expanded and still green. Bite-size index should be measured on at least five different individuals per species. Even though BSI is a stable trait allowing for inter-specific comparisons, it has been shown to vary as a function of browsing intensity and can be used to assess intraspecific responses to browsing pressure (e.g. see Wigley et al. 2015, 2019c). Bite-size index should, therefore, be sampled at different herbivore intensities, depending on the question. The BSI of a plant is the total dry weight (g) of the leaf and stem material removed by 10 human bites (without using hands to manipulate the stems or branches). All bites should be taken while standing on the ground and can be taken anywhere within reach of the mouth (see Fig. 17). The recorder should avoid taking all measurements at the same level and attempt to take bites across the range of the plant canopy that is within reach. Leaves and stems from each bite are weighed together. For each bite, the person measuring the BSI should attempt to remove the maximum amount of leaf material possible. When comparing multiple species in a study, we recommend that BSI is measured by the same person, so as to control for potential differences among individual recorders. However, Charles-Dominique et al. (2015c) found that the BSI differed only for one of nine recorders in a controlled experiment. This trait should only be measured on species that are known to be non-toxic (see Box 2).

|
| Box 2. Special cases and caveats to be considered when measuring bite-size index (BSI) |
 |
 |
Height of the fifth fork (H5)
Trait description
The height of the fifth fork (H5) describes how woody species explore vertical space and is independent from the age of the plant as long as it has developed five forks. For most species, after the fifth fork is established, the plant has already performed a large part of its vertical exploration, and subsequent branching contributes mostly to the lateral expansion of the crown and multiplication of number of apices. Height of the fifth fork describes a species organisation (independent from growth rates) and determines the height at which plants can establish their crown. Foresters frequently use the height of the first fork to evaluate whether a stand will produce straight boles (which is directly related to the height of the crown). We instead chose the fifth fork as a compromise because the height of the first fork is highly variable, and higher-level forks are more difficult to locate within the crown. This trait applies to all species that develop forks (excluding species with unbranched main stem and species not producing forks with only one main stem and lateral subordinate branches). This trait potentially applies to woody and non woody species, but its ecological significance has been investigated only for woody plants (Charles-Dominique et al. 2015a).
Relevant environmental filters
Height of the fifth fork can be used to predict the ability of a species to grow in shaded environments. Many woody species in closed environments have a high H5, allowing them to develop their crown at the canopy level, whereas plants more adapted to open environments frequently develop their canopy at lower heights (Charles-Dominique et al. 2015a). In more open environments, this trait can also allow a species to set up its canopy out of the range of major disturbances, such as out of the flame zone or above browse height (Wakeling et al. 2011; Staver et al. 2012). This trait is interpreted as part of an escape strategy because it allows a tree species to set up its crown above light-limited environments and major disturbances.
Method
Height of the fifth fork can be used both for intraspecific and interspecific comparisons. Height of the fifth fork has been found to be variable within species according to the light environment (Charles-Dominique et al. 2012) and can be used to analyse strategies of vertical exploration under different levels of canopy cover. For interspecific comparisons, this can be used as a categorical trait, with height categories being adapted to the local regime of disturbance (average flame height and herbivore range) and dominant canopy height in the co-occurring vegetation types (Charles-Dominique et al. 2015a). For interspecific comparisons, we recommend sampling a minimum of 5–10 individuals per species that have not been previously top-killed or had the main stem broken or toppled. Measurement of this trait is non-destructive and can easily be undertaken on a large number of individuals. For intraspecific comparison, 5–10 individuals are described for all levels of the environmental gradient to be analysed. To record the height of the fifth fork, all forks from the ground level to the fifth fork are counted and the height of the fifth fork is recorded (Fig. 18). (Hint: imagine the tree being gradually submerged by rising water; the fifth branch is the fifth to be submerged, anywhere in the canopy, as the water level rises.) A height pole can be used for more accurate measurements (e.g. for intraspecific comparisons), whereas height classes are usually sufficient for interspecific comparisons. When recording the H5 on plants with multiple basal trunks or stems, choose one of the main stems to record the trait (see example in Fig. 18c). The basal branching (within 30 cm from the ground level) around the collar region (such as from basal burl, lignotuber and rootstock) does not provide information about the vertical exploration strategy and should not be recorded as a fork. Once a stem has been chosen, the first fork is identified. On each branch of this fork, subsequent forks are identified. The observations are repeated until reaching the fifth-lowest fork in the structure (Fig. 18c). The height of the fifth fork is recorded. To properly identify a fork, the two branches of the fork should be (1) of a similar diameter, (2) generally have a similar growth direction (Fig. 18a), (3) and give rise to branched systems that are equivalent. It is useful to make these observations from a distance to have an overview of the full plant structure, especially when diameters are not strikingly different (50% differences and less) and doubts arise about whether the two branches are equivalent or not. For more information about the developmental processes generating these forks and additional criteria, see information about total sequential reiterates in Barthélémy and Caraglio (2007). Check that the two branches of the fork have been developed simultaneously (or with a delay of only one growing season). Otherwise, what is interpreted as a fork could simply result from the late development of a new trunk (that usually does not affect the vertical exploration strategy). Advanced methods can be applied to check this point (ageing the branches morphologically or using scar morphology), but a coarse assessment can be performed by checking that bark and epidermis structure and colours are similar on both branches of the fork (Fig. 18b).

|
Special cases
For species with a single stem (such as many palms) or without forks (such as some conifers), a well developed individual should be chosen and two-thirds of the species maximal height should be used.
Index of cage architecture (ICA)
Two methods have been proposed to describe branching patterns in woody plants, i.e. levels of branching that can offer protection against feeding herbivores. The first, called ‘branching architecture’, is a highly variable trait that can capture intraspecific variation resulting from genetic differences, local growing conditions and induced responses after herbivory (Staver et al. 2012) and is described in Pérez-Harguindeguy et al. (2013). The second, called ‘index of cage architecture’ (ICA), is a measure of investments in structural defences (Charles-Dominique et al. 2017b). This is a trait that is stable at the species level and useful for interspecific comparisons, but requires prior basic training in plant architecture to identify axis categories (sensu Barthélémy and Caraglio 2007).
Trait description
The ICA is calculated from the number of different types of stems encountered in the architecture of a tree, woodiness and the level of spinescence. When a highly branched structure is composed of stems that are either woody or spiny, it impedes the movement of mammalian browsers attempting to access the soft and nutritive edible parts of a plant (leaf, buds, fruits) and, therefore, reduces their biting rate. This trait applies to woody plants only (trees and shrubs) and should be described for life stages within reach of the dominant meso-browsers (antelopes, cervids) at a site, usually below ~2–3 m. Cage architecture may be less effective in reducing feeding rates of animals that use their forelimbs for plucking leaves (e.g. primates, Australian macropods).
Relevant environmental filters
Species with a higher ICA maintain a higher proportion of leaves inside the cage in herbivore-rich natural systems. High herbivore densities are expected to result in plant communities with high ICAs. Woody species growing in herbivore-dominated savannas typically have higher values of ICA than do species from co-occurring biomes (e.g. thicket and forest, Kruger et al. 2017). A high ICA forms part of a resistance strategy that minimises the negative effects of herbivores. An experimental study using goats found that a high ICA resulted in a slower biting rate inside the cage (Charles-Dominique et al. 2017b). The low feeding rates achieved by herbivores attempting to feed inside the cage of plant species with high ICAs would not fulfil their daily nutritional requirements. High ICAs are expected to have large impacts on herbivore feeding preferences, including selection of less defended individuals or species (Charles-Dominique et al. 2017b).
Method
This trait is suitable for interspecific comparisons. It is stable at the species level and, therefore, requires observations only on a limited number of individuals (description of one individual followed by visual confirmation on several individuals in varied ecological situations). The method to describe ICA applies to saplings within the feeding height of meso-herbivores. It consists of the following four parts, which are described in detail below: (1) identifying axis categories on the basis of their morphological attributes, (2) assessing the conicity of these categories, (3) establishing the location of spines, if any, and (4) calculating the index on the basis of these observations.
(1) Identifying axis categories
The method for identifying axis categories was introduced in Barthélémy and Caraglio (2007). It consists of grouping all stems (i.e. trunk, branches, twigs and short shoots) in the plant body into axes with similar morphological properties. This process highlights duplicated structures (reiteration) within the plant body (see Fig. 19a, 20). Morphological descriptors prescribed for describing plant axis categories (sensu Barthélémy 1991) include growth direction (plagiotropy, orthotropy or mixed), stem shape, length of internodes (portion of stem between two consecutive leaves; can be scored as categorical, see Charles-Dominique et al. 2017b), phyllotaxy and mode of branching (subapical, acrotonic, mesotonic or basitonic). These descriptors can be complemented by observing symmetry and other morphological descriptors such as ability to produce flowers (for a more complete list, see Barthélémy and Caraglio 2007). Fig. 19b illustrates three theoretical architectural units consisting of one, two and three axis categories, with the first axis category (C1) being orthotropic, spiny and conical, C2 is plagiotropic, spiny and cylindrical and C3 is ageotropic, cylindrical and spiny (Fig. 20). It is important to note that axis categories are not equivalent to branching order. Short shoots can, for example, be borne on both trunk (Order 2) and main branches (Order 3).

|
(2) Conicity of axis categories
A stem with a difference between basal (i.e. near the ground (for trunk) or insertion point (for other stems)) and distal (i.e. near the stem apex) diameters of more than 20% is considered conic, otherwise cylindrical. Conicity provides information on the wood production of axis categories (i.e. it results from differences in secondary growth between the base and the newly formed apex) and can determine whether the resulting structure can impede the movement of herbivores (with the assumption that thin stems have less effect on the movement of mammals within the canopy).
(3) Spinescence
For each axis category, the ability to bear a lateral spiny organ with a sharp tip (thorn, spine, hook) is recorded. This ability does not mean that spinescence has to be systematically expressed on all stems for this category (frequently, the ability of axis categories to produce spines is not expressed higher in the canopy).
(4) Calculating the index
The index is calculated according to the following formula:
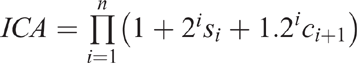
where i is the rank of the axis categories ordered from the inside of the species’ architectural unit to the periphery, si is the presence of spines borne by an axis category i (1 = presence, 0 = absence) and ci describes the conicity of the axis category i (1 = conic, 0 = cylindrical). Any axis category that does not bear a conical axis category, or that is not spiny, does not contribute to a plant’s investments in defence.
Special cases
Situations where cylindrical stems are woody and potentially impede the movements of herbivores, or where the stems are conic but do not create a barrier to movement, might exist but have not been encountered. In these situations, this criterion might have to be adjusted, keeping in mind its aim.
Resprouting as a trait syndrome (RAATS)
Trait description
The ability to resprout is widespread in woody taxa, and virtually all angiosperms, and some gymnosperm plants, are able to do so (Keeley and Zedler 1998; Del Tredici 2001; Bond and Midgley 2003; Vesk and Westoby 2004b). Although the manner in which plants recover their lost aboveground biomass after severe disturbance varies quantitatively and qualitatively, the ability to resprout is often treated as binary (reseeders and resprouters). However, resprouting ability varies among and within species (Kruger et al. 1997; Bond and Midgley 2001; Clarke et al. 2013), in type (Del Tredici 2001; Clarke et al. 2013), life-history stages (Peterson and Carson 1996; Del Tredici 2001; Bond and Midgley 2003; Vesk 2006), life form (Vesk 2006) and disturbance severity (Pausas 1999; Bellingham and Sparrow 2000). We define resprouting in a broader sense than previously defined e.g. Pérez-Harguindeguy et al. (2013). Here, we define resprouting as the production of secondary shoots or root suckers as an induced response to either (1) injury or (2) a dramatic change in surrounding environmental conditions. Although ubiquitous across all biomes, resprouting plays a disproportionately important role in savanna systems, given the frequent and often intense disturbances in the form of fire and herbivory.
Within the context of broader regeneration strategies, plants may be categorised into obligate reseeders, obligate resprouters and facultative reseeders or resprouters (Clarke et al. 2015); the latter is a group of species that have the ability to resprout but rely on the production of viable seeds as a means of reproduction. Resprouting syndromes can then be divided into aerial (primarily epicormic), basal and belowground resprouting. Resprouting ability can vary with phylogeny (Burrows 2013), growth form (Clarke et al. 2015), plant size (Vesk 2006) and can vary intraspecifically with changes in ecosystem drivers, such as soils, fire, herbivory and competition (Bond and Midgley 2003; Moreira et al. 2012; Clarke et al. 2015). These factors determine sprouting vigour, survival, persistence and reproduction. There are allocational trade-off costs associated with resprouting, specifically the production and maintenance of buds, storage organs and stored NSCs (Bond and Midgley 2003). Resprouting ability, therefore, comes at the cost of a range of other plant traits including, but not limited to, reduced reproductive allocation or output, slower maturation rates and plant height (Midgley 1996). Resprouting can have profound effects on ecosystem dynamics. Given that resprouting enhances individual persistence and, hence, individual turnover, it influences plant demography (Kruger et al. 1997) and community dynamics (Kruger and Midgley 2001).
Clarke et al. (2013) provided the most comprehensive functional classification for resprouting types on the basis of the fundamental traits required to resprout, namely, bud availability, bud location and protection and funding the bud bank (by NSCs). The bud–protection–resource (BPR) schema treats resprouting as a continuum from apical to root suckering. Depending on the intensity of disturbance (Kruger et al. 1997; Bellingham and Sparrow 2000) and, secondarily, resource availability, sprouting may occur using axillary buds (axillary resprouting), epicormic buds in branch and stem epicormic sprouting and, ultimately, basal resprouting from adventitious buds at the base of the stem. More specifically, resprouting can be broadly categorised according to Fig. 21 (after Clarke et al. 2013). We have defined resprouting as a syndrome because resprouting cannot be defined by any single trait, but rather is a combination of several traits.
Note
Sprouting differs from clonal growth in that it does not imply the potential for vegetative spread (Del Tredici 2001). Many more species are sprouters than clonal, although virtually all clonal species are sprouters (Bond and Midgley 2001).
Relevant environmental filters
The ability to resprout is ubiquitous across all woody plants in savannas, particularly as seedlings or saplings, but less so as adults (e.g. Kruger et al. 2017). However, resprouting syndromes are likely to vary with changing disturbance regimes (Kruger et al. 1997, 2017; Bellingham and Sparrow 2000; Clarke et al. 2013; Pausas and Keeley 2017), site productivity (Pausas and Keeley 2017) and also with changes in environmental conditions, such as light regimes. The conceptual framework of Bellingham and Sparrow (2000), refined by Clarke et al. (2013) and Pausas and Keeley (2017), provides the simplest and easiest model for understanding aboveground resprouting in relation to disturbance. Basal resprouting is likely to be most prevalent following frequent or high-intensity disturbance, such as intense fires, windthrow and herbivory, whereas epicormic resprouting is a response to lower intensity of disturbance regimes. Root suckering is a special case because it is both a response to disturbance and a reproductive strategy and is relatively ubiquitous across disturbance regimes. Some forms, for example, geoxyles or underground trees, are more common in fire-dominated savannas. Since sprouting can be limited by stored NSC and, hence, resource availability, resprouting can be less common in resource-limited savannas (Clarke et al. 2013). Resprouting can also be a response to changing local environmental conditions; for example, a change in light regimes results in resprouting in forest trees, leading to ‘opportunistic branching’ Hallé et al. (1978). Root suckering and epicormic resprouting may also occur without any disturbance.
Method
The traits that enable plants to resprout have been described above. These include the presence of accessory buds (ACCB), bud protection (BP) and NSCs. This section outlines the identification of resprouting in the field. Generally, multi-stemmedness (multiple stems emerging from the base of a tree) and the presence of swollen underground storage organs are a good indication of resprouting in adult woody plants; however, the absence of these traits does not imply the inability to resprout. Without assessing ACCB, BP and NSCs (which identify the potential to resprout), resprouting ability will typically be shown only after a disturbance event. The various types of resprouting can be assessed according to the methods outlined below. Plants are defined as branch or stem epicormic, basal resprouting or root suckering.
Branch or stem epicormic resprouting
Sprouts are often most prevalent but are not limited to the site of the damage. At the site of the injury, resprouts are enumerated, and identified as shoots smaller in diameter than the original branch or stem. Sprouting vigour can be assessed by counting and measuring the length of sprouts. It is best to wait for 6 months (a growing season) after the injury to assess resprouting efficacy, i.e. assess whether resprouts survive, resulting in plant persistence.
Basal resprouting
Excavation is undertaken around the base of the stem to assess whether the plant has a swollen root stock or an underground storage organ (e.g. lignotuber or rhizome). The swollen portion will be significantly greater in diameter than the aboveground stem or trunk. All the stems emerging from the ground are counted as a measure of multi-stemmedness and, hence, basal resprouting. Identifying what constitutes an individual can be difficult without excavating the entire plant, so an objective means to quantify this is to count the number of stems emerging from the soil in a radius of 1/10th of the canopy height. This standardises enumeration to account for plant size (Kruger et al. 1997).
Root suckering
Superficially, root-suckering species can be identified by clumped or symmetrically arranged individuals in a local population in close proximity of an adult tree. For example, root suckers of Balanites maughamii are often found in a straight line from the adult tree. Excavating the roots is the only certain, non-destructive way to identify root-suckering species and the magnitude of the belowground root suckering. Population-genetic techniques, such as whole-genotype SNP or micro-satellite techniques (see e.g. Saari et al. 2005; Morin et al. 2009; Javed Muhammad et al. 2017), may also be used to identify genets of an individual. Some roots connecting genets may suberise and abort, in which case the links among individuals are no longer evident. In this case, a ‘t-junction’ of a smaller vertical root with a larger horizonal root is often an indication of a connection between a genet and parent plant.
Special cases
Because age and size can have a profound influence on sprouting ability, a further measure might include assessing the point at which a plant loses its ability to resprout. Aside from field surveys, experimental methods include cutting the main stem or branches of the tree, so as to simulate top-kill or branch removal, across a range of size classes to assess the threshold of resprouting ability. Alternatively, as a laboratory proxy, plants can be germinated in nurseries and the threshold size can be determined. However, this will allow assessment of only smaller individuals. Resprouting can be assessed 6–12 months after the removal after the growth season.
Tree and shrub rooting depth (RD)
Trait description
Rooting depth (RD) describes either the maximum depth to which a plant’s roots can reach in the soil profile (‘absolute rooting depth’) or some biomass-weighted measure of the depth of root allocation by a plant. This trait has important implications for ecosystem functioning, including hydrological balance and carbon and nutrient cycling (Canadell et al. 1996; Schenk and Jackson 2002). In arid ecosystems, the survival of some species has been shown to be highly dependent on their RDs (Padilla and Pugnaire 2007; Fensham et al. 2009; Zhou et al. 2020). Rooting depth has been shown to be an important trait in savanna ecosystems, because it determines the competitive outcome between trees and grasses for water and nutrient resources (e.g. niche-partitioning theory for tree–grass coexistence), thereby affecting the establishment and persistence of savanna trees (Walker et al. 1981; Holdo 2013; Kulmatiski and Beard 2013).
Relevant environmental filters
Rooting depth in savanna trees appears to be phylogenetically constrained, with some tree species or families able to send their roots deeper into the soil profile than others (Canadell et al. 1996). However, environmental variables also explain some variation in RD, where tree species that occur on sandy, nutrient-poor soils tend to have deeper roots relative to those on clayey, nutrient-rich ones (Zhou et al. 2020). Several studies have shown that RD is not strongly related to mean annual rainfall for savanna trees (Schenk and Jackson 2002; Bhattachan et al. 2012; Zhou et al. 2020), but RD may increase with the length of the dry season. Fire and herbivory have been reported to increase biomass allocation to belowground root systems (Wigley et al. 2019a), but their effects on the rooting depth of savanna trees remain unclear.
Method
The typical way to estimate RD is through the full excavation of the entire root zone (e.g. O’Donnell et al. 2015). However, full root-system excavations are laborious and expensive and may still be insufficient to trace the deepest root of individual trees. The absolute rooting depth is a desirable metric, but not practical to collect, given that roots of many savanna trees can reach >5 m (Schenk and Jackson 2002). In many cases, functional traits are used for comparative purposes (e.g. Franco et al. 2005; Staver et al. 2012); thus, an alternative proxy of RD that represents some biomass-weighted measure of RD and generally varies predictably with respect to the maximum RD might be sufficient for facilitating inter-specific comparisons.
Because taproots can potentially better characterise the maximum rooting depth of individual trees, we introduce the ratio of taproot diameter at deep (30 cm) to shallow (10 cm) depths (hereafter DS ratio) as an easily measurable proxy for RD. This alternative proxy has been validated by the excavation of taproots to 50–100-cm depth for several dominant savanna trees in Kruger National Park, South Africa (see Zhou et al. 2020); however, there is still a need for further work to validate this proxy (i.e. the maximum RD based on full excavations of the entire rooting zone still needs to be determined for more savanna tree species).
To facilitate comparisons among species, trees of a similar size (e.g. basal diameter or diameter at breast height) are recommended. To standardise across individuals, trees with a single stem, with no sign of post-fire resprouting at the base, and of sufficient height (e.g. >3 m) should be selected. Before excavating the tree, the ground level is marked on the stem, (if necessary, the ground is levelled, especially if soil is piled up around the stem base, so as to standardise the location where the stem ends and the root system begins). All the soil within a cylindrical pit with a 30-cm radius and 35-cm depth around the stem should then be excavated using a trowel, spade and pick. If present, any lateral roots spreading out from the taproot at depths of 10- and 30-cm depth should be removed, then the taproot diameters at these two depths (i.e. D10 and D30) can be measured (Fig. 22). The DS ratio (i.e. D30 : D10) is computed accordingly. Larger DS ratios indicate that trees are rooting relatively deeper into the soil profile (Fig. 22). Five or more replicates per species are recommended to achieve a reasonable estimate of DS ratios. The DS ratio can be obtained by excavating to slightly beyond 30 cm along the taproot with minimal effects on the survival of sampled trees.

|
Special cases
This proxy is not suitable for multi-stemmed trees or trees without taproots (e.g. with a lignotuber, see Wigley et al. 2009b; Pausas et al. 2018).
Conclusions
The need for a ‘periodic table for ecology’ was recognised almost 40 years ago (Barh 1982). More than a decade later, plant functional types (PFTs) were proposed as the ecological equivalent of chemical elements, which could be classified by the manner in which they function within an ecosystem (Steffen 1996). Since then, we have witnessed a steady and continued increase in the development and use of functional trait-based approaches in ecological research (Lavorel and Garnier 2002; McGill et al. 2006; de Bello et al. 2015). However, one of the biggest challenges in this endeavour is to find convergence in multiple traits and, ultimately, define ecological strategy schemes or trait syndromes (e.g. Westoby et al. 2002; McGill et al. 2006; Moles et al. 2013). Prior efforts to effectively define plant functional traits and the standardisation of trait-measurement techniques have been phenomenally successful. Previously published plant functional-trait handbooks (i.e. Cornelissen et al. 2003; Pérez-Harguindeguy et al. 2013) are testament to this and have witnessed a steady increase in usage over the past two decades (>5400 citations for these two publications; Google Scholar).
Although many of the traits and methods described in previous handbooks have been applicable to and widely used in savanna, grassland and shrubland ecosystems across the globe, an important suite of traits, some previously described and some still undescribed, remains missing for open ecosystems. One of the main reasons for this is that many of the traditionally used plant functional traits are typically related to resource use, and often do not discriminate between species from open v. closed vegetation types (Charles-Dominique et al. 2015a). Instead, quite different traits have recently emerged as indicators of open v. closed states and of the consumers (mostly fire and herbivory) that maintain ecosystems in an open state (Bond 2019). This handbook is an attempt at updating the growing list of plant functional traits that are relevant to open ecosystems. The traits and sampling protocols presented here have been informed by years of collective experience gained from measuring plant traits in the field. Because of the constantly evolving nature of ecological thinking and, by proxy, our understanding of plant functional traits, we have attempted to make this handbook a living document as far as possible and we encourage readers to contact, question, interact or collaborate with the contributors to this handbook (details provided in Box 1).
Ultimately, this handbook will help tackle some of the important issues that will be necessary to better understand, forecast and manage open ecosystems in a changing world (Osborne et al. 2018; Bond 2019). The handbook is intended to promote better integration of knowledge and research from scientists that are scattered around the globe. This will help identify which open systems are functionally analogous among continents (and better disclose regional specificities), while increasing our understanding of functional biogeography and the sharing of management solutions. The traits outlined in this handbook should also allow for a better understanding of the functional changes that have resulted from past (and will continue to result from future) human disturbances. Finally, we hope that the handbook will help set clear conservation goals based on ecological functioning, which can be used to complement current efforts that are often solely based on measures of species diversity.
Author contributions
The idea for this handbook was conceived during a savanna plant functional trait workshop held in Skukuza, Kruger National Park, South Africa, in 2016, hosted and funded by the Skukuza Science Leadership Initiative (SSLI), a partnership between South African National Parks, Nsasani Trust and OTS, as part of the Bond Review Ecological Workshop Series (BREWS). B. J. Wigley, T. Charles-Dominique and L. M. Kruger conceptualised and steered the process from beginning to end. G. P. Hempson coordinated the working group responsible for the writing-up of the grass traits. The grass-trait group also included S. Archibald, J. Donaldson, C. Lehmann, C. Solofondranohatra, D. Schwilk and X. Gao. M. TeBeest coordinated the working group responsible for the writing-up of the forb traits. The forb-trait group also included A. Fidelis and F. Siebert. N. Stevens coordinated the working group responsible for the writing-up of the seedling traits. The seedling-trait group also included K. Bunney and J. Gignoux. B. J. Wigley and L. M. Kruger coordinated the working group responsible for the writing-up of the woody-plant sapling and adult traits. The woody plant-trait group also included T. Charles-Dominique, C. Coetsee, A. C. Staver, J. J. Midgley, T. J. Massad and Y. Zhou. All authors contributed to the writing of this manuscript and commented on earlier drafts.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
We are grateful for funding and support from School of Natural Resource Management, Nelson Mandela University; Nsasani Trust; Organisation for Tropical Studies (OTS), South Africa.
References
Abbadie L, Gignoux J, Lepage M, Roux X (2006) Environmental constraints on living organisms. Ecological Studies 179, 46–61.| Environmental constraints on living organisms.Crossref | GoogleScholarGoogle Scholar |
Abraham Y, Elbaum R (2013) Hygroscopic movements in Geraniaceae: the structural variations that are responsible for coiling or bending. New Phytologist 199, 584–594.
| Hygroscopic movements in Geraniaceae: the structural variations that are responsible for coiling or bending.Crossref | GoogleScholarGoogle Scholar | 23574364PubMed |
Adams HD, Germino MJ, Breshears DD, Barron-Gafford GA, Guardiola-Claramonte M, Zou CB, Huxman TE (2013) Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytologist 197, 1142–1151.
| Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism.Crossref | GoogleScholarGoogle Scholar | 23311898PubMed |
Agrawal AA (1999) Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness. Ecology 80, 1713–1723.
| Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness.Crossref | GoogleScholarGoogle Scholar |
Agrawal AA, Fishbein M (2006) Plant defence syndromes. Ecology 87, S132–S149.
| Plant defence syndromes.Crossref | GoogleScholarGoogle Scholar | 16922309PubMed |
Agrell J, Anderson P, Oleszek W, Stochmal A, Agrell C (2004) Combined effects of elevated CO2 and herbivore damage on alfalfa and cotton. Journal of Chemical Ecology 30, 2309–2324.
| Combined effects of elevated CO2 and herbivore damage on alfalfa and cotton.Crossref | GoogleScholarGoogle Scholar | 15672673PubMed |
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nature Protocols 2, 875–877.
| Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent.Crossref | GoogleScholarGoogle Scholar | 17446889PubMed |
Alvarado ST, Fornazari T, Cóstola A, Morellato LPC, Silva TSF (2017) Drivers of fire occurrence in a mountainous Brazilian Cerrado savanna: tracking long-term fire regimes using remote sensing. Ecological Indicators 78, 270–281.
| Drivers of fire occurrence in a mountainous Brazilian Cerrado savanna: tracking long-term fire regimes using remote sensing.Crossref | GoogleScholarGoogle Scholar |
Anderson TM, Schütz M, Risch AC (2012) Seed germination cues and the importance of the soil seed bank across an environmental gradient in the Serengeti. Oikos 121, 306–312.
| Seed germination cues and the importance of the soil seed bank across an environmental gradient in the Serengeti.Crossref | GoogleScholarGoogle Scholar |
Appezzato-da-Glória B, Cury G, Kasue Misaki Soares M, Rocha R, Hissae Hayashi A (2008) Underground systems of Asteraceae species from the Brazilian Cerrado. The Journal of the Torrey Botanical Society 135, 103–113.
| Underground systems of Asteraceae species from the Brazilian Cerrado.Crossref | GoogleScholarGoogle Scholar |
Archibald S, Hempson GP (2016) Competing consumers: contrasting the patterns and impacts of fire and mammalian herbivory in Africa. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 371, 20150309
| Competing consumers: contrasting the patterns and impacts of fire and mammalian herbivory in Africa.Crossref | GoogleScholarGoogle Scholar | 27502384PubMed |
Archibald S, Hempson GP, Lehmann C (2019) A unified framework for plant life-history strategies shaped by fire and herbivory. New Phytologist 224, 1490–1503.
| A unified framework for plant life-history strategies shaped by fire and herbivory.Crossref | GoogleScholarGoogle Scholar | 31177547PubMed |
Asner GP, Levick SR (2012) Landscape-scale effects of herbivores on treefall in African savannas. Ecology Letters 15, 1211–1217.
| Landscape-scale effects of herbivores on treefall in African savannas.Crossref | GoogleScholarGoogle Scholar | 22863324PubMed |
Asner GP, Levick SR, Kennedy-Bowdoin T, Knapp DE, Emerson R, Jacobson J, Colgan MS, Martin RE (2009) Large-scale impacts of herbivores on the structural diversity of African savannas. Proceedings of the National Academy of Sciences of the United States of America 106, 4947–4952.
| Large-scale impacts of herbivores on the structural diversity of African savannas.Crossref | GoogleScholarGoogle Scholar | 19258457PubMed |
Auld TD, Denham AJ (2005) A technique to estimate the pre-fire depth of burial of Grevillea seeds by using seedlings after fire. Australian Journal of Botany 53, 401–405.
| A technique to estimate the pre-fire depth of burial of Grevillea seeds by using seedlings after fire.Crossref | GoogleScholarGoogle Scholar |
Bahr LM Jr (1982) Functional taxonomy: an immodest proposal. Ecological Modelling 15, 211–233.
Barthélémy D (1991) Levels of organization and repetition phenomena in seed plants. Acta Biotheoretica 39, 309–323.
| Levels of organization and repetition phenomena in seed plants.Crossref | GoogleScholarGoogle Scholar |
Barthélémy D, Caraglio Y (2007) Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of Botany 99, 375–407.
| Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny.Crossref | GoogleScholarGoogle Scholar | 17218346PubMed |
Baskin CC, Baskin JM (1998) ‘Seeds – Ecology, Biogeography, and Evolution of Dormancy and Germination.’ (Academic Press: San Diego, CA, USA)
Beerling DJ, Osborne CP (2006) The origin of the savanna biome. Global Change Biology 12, 2023–2031.
| The origin of the savanna biome.Crossref | GoogleScholarGoogle Scholar |
Bell RHV (1984) ‘Notes on Elephant–Woodland Interactions. Status and Conservation of Africa’s Elephants and Rhinos.’ (IUCN: Gland, Switzerland)
Bell AD (2008) ‘Plant Form: An Illustrated Guide to Flowering Plant Morphology.’ (Wiley: Hoboken, NJ, USA)
Bellingham PJ, Sparrow AD (2000) Resprouting as a life history strategy in woody plant communities. Oikos 89, 409–416.
| Resprouting as a life history strategy in woody plant communities.Crossref | GoogleScholarGoogle Scholar |
Benson EJ, Hartnett DC, Mann KH (2004) Belowground bud banks and meristem limitation in tallgrass prairie plant populations. American Journal of Botany 91, 416–421.
| Belowground bud banks and meristem limitation in tallgrass prairie plant populations.Crossref | GoogleScholarGoogle Scholar | 21653397PubMed |
Benvenuti S (2007) Natural weed seed burial: effect of soil texture, rain and seed characteristics. Seed Science Research 17, 211–219.
| Natural weed seed burial: effect of soil texture, rain and seed characteristics.Crossref | GoogleScholarGoogle Scholar |
Benvenuti S (2016) Seed ecology of Mediterranean hind dune wildflowers. Ecological Engineering 91, 282–293.
| Seed ecology of Mediterranean hind dune wildflowers.Crossref | GoogleScholarGoogle Scholar |
Benvenutti MA, Gordon IJ, Poppi DP (2006) The effect of the density and physical properties of grass stems on the foraging behaviour and instantaneous intake rate by cattle grazing an artificial reproductive tropical sward. Grass and Forage Science 61, 272–281.
| The effect of the density and physical properties of grass stems on the foraging behaviour and instantaneous intake rate by cattle grazing an artificial reproductive tropical sward.Crossref | GoogleScholarGoogle Scholar |
Benvenutti MA, Gordon IJ, Poppi DP, Crowther R, Spinks W, Moreno FC (2009) The horizontal barrier effect of stems on the foraging behaviour of cattle grazing five tropical grasses. Livestock Science 126, 229–238.
| The horizontal barrier effect of stems on the foraging behaviour of cattle grazing five tropical grasses.Crossref | GoogleScholarGoogle Scholar |
Bhattachan A, Tatlhego M, Dintwe K, O’Donnell F, Caylor KK, Okin GS, Perrot DO, Ringrose S, D’Odorico P (2012) Evaluating ecohydrological theories of woody root distribution in the Kalahari. PLoS One 7, e33996
| Evaluating ecohydrological theories of woody root distribution in the Kalahari.Crossref | GoogleScholarGoogle Scholar | 22470506PubMed |
Biddulph J, Kellman M (1998) Fuels and fire at savanna–gallery forest boundaries in southeastern Venezuela. Journal of Tropical Ecology 14, 445–461.
| Fuels and fire at savanna–gallery forest boundaries in southeastern Venezuela.Crossref | GoogleScholarGoogle Scholar |
Blüthgen N, Menzel F, Hovestadt T, Fiala B, Blüthgen N (2007) Specialization, constraints and conflicting interests in mutualistic networks. Current Biology 17, 341–346.
| Specialization, constraints and conflicting interests in mutualistic networks.Crossref | GoogleScholarGoogle Scholar | 17275300PubMed |
Bond WJ (1988) Proteas as ‘tumbleseeds’: wind dispersal through the air and over soil. South African Journal of Botany 54, 455–460.
| Proteas as ‘tumbleseeds’: wind dispersal through the air and over soil.Crossref | GoogleScholarGoogle Scholar |
Bond WJ (1997) Fire. In ‘Vegetation of Southern Africa’. (Eds RM Cowling, DM Richardson, SM Pierce) pp. 421–446. (Cambridge University Press: Cambridge, UK)
Bond WJ (2005) Large parts of the world are brown or black: a different view on the ‘Green World’ hypothesis. Journal of Vegetation Science 16, 261–266.
| Large parts of the world are brown or black: a different view on the ‘Green World’ hypothesis.Crossref | GoogleScholarGoogle Scholar |
Bond WJ (2019) ‘Open Ecosystems: Ecology and Evolution beyond the Forest Edge.’ (Oxford University Press: Oxford, UK)
Bond WJ, Midgley JJ (2001) Ecology of sprouting in woody plants: the persistence niche. Trends in Ecology & Evolution 16, 45–51.
| Ecology of sprouting in woody plants: the persistence niche.Crossref | GoogleScholarGoogle Scholar |
Bond WJ, Midgley JJ (2003) The evolutionary ecology of sprouting in woody plants. International Journal of Plant Sciences 164, S103–S114.
| The evolutionary ecology of sprouting in woody plants.Crossref | GoogleScholarGoogle Scholar |
Bond WJ, Parr CL (2010) Beyond the forest edge: ecology, diversity and conservation of the grassy biomes. Biological Conservation 143, 2395–2404.
| Beyond the forest edge: ecology, diversity and conservation of the grassy biomes.Crossref | GoogleScholarGoogle Scholar |
Bond WJ, van Wilgen BW (1996) ‘Fire and Plants.’ (Chapman: New York, NY, USA)
Bond WJ, Honig M, Maze KE (1999) Seed size and seedling emergence: an allometric relationship and some ecological implications. Oecologia 120, 132–136.
| Seed size and seedling emergence: an allometric relationship and some ecological implications.Crossref | GoogleScholarGoogle Scholar | 28308044PubMed |
Bond WJ, Woodward FI, Midgley GF (2005) The global distribution of ecosystems in a world without fire. New Phytologist 165, 525–538.
| The global distribution of ecosystems in a world without fire.Crossref | GoogleScholarGoogle Scholar | 15720663PubMed |
Bond WJ, Silander JA, Ranaivonasy J, Ratsirarson J (2008) The antiquity of Madagascar’s grasslands and the rise of C4 grassy biomes. Journal of Biogeography 35, 1743–1758.
| The antiquity of Madagascar’s grasslands and the rise of C4 grassy biomes.Crossref | GoogleScholarGoogle Scholar |
Borer ET, Lind EM, Firn J, Seabloom EW, Anderson TM, Bakker ES, Biederman L, La Pierre KJ, MacDougall AS, Moore JL, Risch AC, Schutz M, Stevens CJ (2019) More salt, please: global patterns, responses and impacts of foliar sodium in grasslands. Ecology Letters 22, 1136–1144.
| More salt, please: global patterns, responses and impacts of foliar sodium in grasslands.Crossref | GoogleScholarGoogle Scholar | 31074933PubMed |
Botha MS, Bond WJ, February E (2007) How to handle rainfall variability when you can’t phone the weatherman: the challenge of a savanna tree seedling. South African Journal of Botany 73, 282
| How to handle rainfall variability when you can’t phone the weatherman: the challenge of a savanna tree seedling.Crossref | GoogleScholarGoogle Scholar |
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Annals of Forest Science 63, 625–644.
| Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences.Crossref | GoogleScholarGoogle Scholar |
Brownell PF, Crossland CJ (1972) The requirement for sodium as a micronutrient by species having the C4 dicarboxylic photosynthetic pathway. Plant Physiology 49, 794–797.
| The requirement for sodium as a micronutrient by species having the C4 dicarboxylic photosynthetic pathway.Crossref | GoogleScholarGoogle Scholar | 16658050PubMed |
Bryant JP, Chapin FS, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40, 357
| Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory.Crossref | GoogleScholarGoogle Scholar |
Buisson E, Le Stradic S, Silveira FA, Durigan G, Overbeck GE, Fidelis A, Fernandes GW, Bond WJ, Hermann JM, Mahy G, Alvarado ST (2019) Resilience and restoration of tropical and subtropical grasslands, savannas, and grassy woodlands. Biological Reviews of the Cambridge Philosophical Society 94, 590–609.
| Resilience and restoration of tropical and subtropical grasslands, savannas, and grassy woodlands.Crossref | GoogleScholarGoogle Scholar | 30251329PubMed |
Bunney K, Bond WJ, Henley M (2017) Seed dispersal kernel of the largest surviving megaherbivore: the African savanna elephant. Biotropica 49, 395–401.
| Seed dispersal kernel of the largest surviving megaherbivore: the African savanna elephant.Crossref | GoogleScholarGoogle Scholar |
Bunney K, Robertson M, Bond W (2019) The historical distribution of megaherbivores does not determine the distribution of megafaunal fruit in southern Africa. Biological Journal of the Linnean Society. Linnean Society of London 128, 1039–1051.
| The historical distribution of megaherbivores does not determine the distribution of megafaunal fruit in southern Africa.Crossref | GoogleScholarGoogle Scholar |
Burrows GE (2013) Buds, bushfires and resprouting in the eucalypts. Australian Journal of Botany 61, 331
| Buds, bushfires and resprouting in the eucalypts.Crossref | GoogleScholarGoogle Scholar |
Burrows GE, Hornby SK, Waters DA, Bellairs SM, Prior LD, Bowman DMJS (2008) Leaf axil anatomy and bud reserves in 21 Myrtaceae species from northern Australia. International Journal of Plant Sciences 169, 1174–1186.
| Leaf axil anatomy and bud reserves in 21 Myrtaceae species from northern Australia.Crossref | GoogleScholarGoogle Scholar |
Burrows GE, Hornby SK, Waters DA, Bellairs SM, Prior LD, Bowman DMJS (2010) A wide diversity of epicormic structures is present in Myrtaceae species in the northern Australian savanna biome: implications for adaptation to fire. Australian Journal of Botany 58, 493
| A wide diversity of epicormic structures is present in Myrtaceae species in the northern Australian savanna biome: implications for adaptation to fire.Crossref | GoogleScholarGoogle Scholar |
Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) Maximum rooting depth of vegetation types at the global scale. Oecologia 108, 583–595.
| Maximum rooting depth of vegetation types at the global scale.Crossref | GoogleScholarGoogle Scholar | 28307789PubMed |
Cardoso AW, Oliveras I, Abernethy KA, Jeffery KJ, Lehmann D, Edzang Ndong J, McGregor I, Belcher CM, Bond WJ, Malhi YS (2018) Grass species flammability, not biomass, drives changes in fire behavior at tropical forest–savanna transitions. Frontiers in Forests and Global Change 1, 6
| Grass species flammability, not biomass, drives changes in fire behavior at tropical forest–savanna transitions.Crossref | GoogleScholarGoogle Scholar |
Cavanagh AM, Godfree RC, Morgan JW (2019) An awn typology for Australian native grasses (Poaceae). Australian Journal of Botany 67, 309–334.
| An awn typology for Australian native grasses (Poaceae).Crossref | GoogleScholarGoogle Scholar |
Chapin FS, Schulze E, Mooney HA (1990) The ecology and economics of storage in plants. Annual Review of Ecology and Systematics 21, 423–447.
| The ecology and economics of storage in plants.Crossref | GoogleScholarGoogle Scholar |
Charles-Dominique T, Edelin C, Brisson J, Bouchard A (2012) Architectural strategies of Rhamnus cathartica (Rhamnaceae) in relation to canopy openness. Botany 90, 976–989.
| Architectural strategies of Rhamnus cathartica (Rhamnaceae) in relation to canopy openness.Crossref | GoogleScholarGoogle Scholar |
Charles-Dominique T, Staver AC, Midgley GF, Bond WJ (2015a) Functional differentiation of biomes in an African savanna/forest mosaic. South African Journal of Botany 101, 82–90.
| Functional differentiation of biomes in an African savanna/forest mosaic.Crossref | GoogleScholarGoogle Scholar |
Charles-Dominique T, Beckett H, Midgley GF, Bond WJ (2015b) Bud protection: a key trait for species sorting in a forest–savanna mosaic. New Phytologist 207, 1052–1060.
| Bud protection: a key trait for species sorting in a forest–savanna mosaic.Crossref | GoogleScholarGoogle Scholar | 25856385PubMed |
Charles-Dominique T, Midgley GF, Bond WJ (2015c) An index for assessing effectiveness of plant structural defences against mammal browsing. Plant Ecology 216, 1433–1440.
| An index for assessing effectiveness of plant structural defences against mammal browsing.Crossref | GoogleScholarGoogle Scholar |
Charles-Dominique T, Midgley GF, Bond WJ (2017a) Fire frequency filters species by bark traits in a savanna–forest mosaic. Journal of Vegetation Science 28, 728–735.
| Fire frequency filters species by bark traits in a savanna–forest mosaic.Crossref | GoogleScholarGoogle Scholar |
Charles-Dominique T, Barczi J-F, Le Roux E, Chamaillé-Jammes S (2017b) The architectural design of trees protects them against large herbivores. Functional Ecology 31, 1710–1717.
| The architectural design of trees protects them against large herbivores.Crossref | GoogleScholarGoogle Scholar |
Charles-Dominique T, Midgley GF, Tomlinson KW, Bond WJ (2018) Steal the light: shade vs fire adapted vegetation in forest–savanna mosaics. New Phytologist 218, 1419–1429.
| Steal the light: shade vs fire adapted vegetation in forest–savanna mosaics.Crossref | GoogleScholarGoogle Scholar | 29604213PubMed |
Cheng Y, Cao L, Wang S, Li Y, Wang H, Zhou Y (2014) Analyses of plant leaf cell size, density and number, as well as trichome number using cell counter plugin. Bio-Protocol 4, e1165
| Analyses of plant leaf cell size, density and number, as well as trichome number using cell counter plugin.Crossref | GoogleScholarGoogle Scholar |
Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, Jacobsen AL (2012) Global convergence in the vulnerability of forests to drought. Nature 491, 752–755.
| Global convergence in the vulnerability of forests to drought.Crossref | GoogleScholarGoogle Scholar | 23172141PubMed |
Choczynska J, Johnson EA (2009) A soil heat and water transfer model to predict belowground grass rhizome bud death in a grass fire. Journal of Vegetation Science 20, 277–287.
| A soil heat and water transfer model to predict belowground grass rhizome bud death in a grass fire.Crossref | GoogleScholarGoogle Scholar |
Choinski JS, Tuohy JM (1991) Effect of water potential and temperature on the germination of four species of African savanna trees. Annals of Botany 68, 227–233.
| Effect of water potential and temperature on the germination of four species of African savanna trees.Crossref | GoogleScholarGoogle Scholar |
Clarke PJ, Lawes MJ, Midgley JJ, Lamont BB, Ojeda F, Burrows GE, Enright NJ, Knox KJE (2013) Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire. New Phytologist 197, 19–35.
| Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire.Crossref | GoogleScholarGoogle Scholar | 23110592PubMed |
Clarke PJ, Lawes MJ, Murphy BP, Russell-Smith J, Nano CE, Bradstock R, Enright NJ, Fontaine JB, Gosper CR, Radford I, Midgley JJ (2015) A synthesis of postfire recovery traits of woody plants in Australian ecosystems. The Science of the Total Environment 534, 31–42.
| A synthesis of postfire recovery traits of woody plants in Australian ecosystems.Crossref | GoogleScholarGoogle Scholar | 25887372PubMed |
Coates-Palgrave KC (2015) ‘Palgrave’s Trees of Southern Africa.’ (Penguin Random House: Cape Town, South Africa)
Coley PD, Bryant JP, Chapin FS (1985) Resource availability and plant antiherbivore defense. Science 230, 895–899.
| Resource availability and plant antiherbivore defense.Crossref | GoogleScholarGoogle Scholar | 17739203PubMed |
Collins BS, Wein GR (1997) Mass allocation and self-burial of Aristida tuberculosa florets. The Journal of the Torrey Botanical Society 124, 306–311.
| Mass allocation and self-burial of Aristida tuberculosa florets.Crossref | GoogleScholarGoogle Scholar |
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199, 1302–1310.
| Diversity in tropical rain forests and coral reefs.Crossref | GoogleScholarGoogle Scholar | 17840770PubMed |
Cooper SM, Owen-Smith N (1985) Condensed tannins deter feeding by browsing ruminants in a South African savanna. Oecologia 67, 142–146.
| Condensed tannins deter feeding by browsing ruminants in a South African savanna.Crossref | GoogleScholarGoogle Scholar | 28309860PubMed |
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51, 335–380.
| A handbook of protocols for standardised and easy measurement of plant functional traits worldwide.Crossref | GoogleScholarGoogle Scholar |
Coughenour MB (1985) Graminoid responses to grazing by large herbivores: adaptations, exaptations, and interacting processes. Annals of the Missouri Botanical Garden 72, 852–863.
| Graminoid responses to grazing by large herbivores: adaptations, exaptations, and interacting processes.Crossref | GoogleScholarGoogle Scholar |
Craine JM, Ocheltree TW, Nippert JB, Towne EG, Skibbe AM, Kembel SW, Fargione JE (2013) Global diversity of drought tolerance and grassland climate-change resilience. Nature Climate Change 3, 63–67.
| Global diversity of drought tolerance and grassland climate-change resilience.Crossref | GoogleScholarGoogle Scholar |
Cramer MD, Wakeling JL, Bond WJ (2012) Belowground competitive suppression of seedling growth by grass in an African savanna. Plant Ecology 213, 1655–1666.
| Belowground competitive suppression of seedling growth by grass in an African savanna.Crossref | GoogleScholarGoogle Scholar |
Crisp MD, Burrows GE, Cook LG, Thornhill AH, Bowman DMJS (2011) Flammable biomes dominated by eucalypts originated at the Cretaceous–Palaeogene boundary. Nature Communications 2, 193
| Flammable biomes dominated by eucalypts originated at the Cretaceous–Palaeogene boundary.Crossref | GoogleScholarGoogle Scholar | 21326225PubMed |
Dalgleish HJ, Hartnett DC (2006) Below-ground bud banks increase along a precipitation gradient of the North American Great Plains: a test of the meristem limitation hypothesis. New Phytologist 171, 81–89.
| Below-ground bud banks increase along a precipitation gradient of the North American Great Plains: a test of the meristem limitation hypothesis.Crossref | GoogleScholarGoogle Scholar | 16771984PubMed |
Dalin P, Björkman C (2003) Adult beetle grazing induces willow trichome defence against subsequent larval feeding. Oecologia 134, 112–118.
| Adult beetle grazing induces willow trichome defence against subsequent larval feeding.Crossref | GoogleScholarGoogle Scholar | 12647188PubMed |
Dantas VL, Pausas JG (2013) The lanky and the corky: fire-escape strategies in savanna woody species. Journal of Ecology 101, 1265–1272.
| The lanky and the corky: fire-escape strategies in savanna woody species.Crossref | GoogleScholarGoogle Scholar |
Daubenmire R (1968) Ecology of fire in grasslands. In ‘Advances in Ecological Research’. (Ed. JM Cragg) Vol. 5, pp. 209–266. (Academic Press)
Davies AB, van Rensburg BJ, Robertson MP, Levick SR, Asner GP, Parr CL (2016) Seasonal variation in the relative dominance of herbivore guilds in an African savanna. Ecology 97, 1618–1624.
| Seasonal variation in the relative dominance of herbivore guilds in an African savanna.Crossref | GoogleScholarGoogle Scholar | 27459791PubMed |
Dayamba SD, Sawadogo L, Tigabu M, Savadogo P, Zida D, Tiveau D, Oden PC (2010) Effects of aqueous smoke solutions and heat on seed germination of herbaceous species of the Sudanian savanna–woodland in Burkina Faso. Flora – Morphology, Distribution, Functional Ecology of Plants 205, 319–325.
| Effects of aqueous smoke solutions and heat on seed germination of herbaceous species of the Sudanian savanna–woodland in Burkina Faso.Crossref | GoogleScholarGoogle Scholar |
de Bello F, Berg MP, Dias AT, Diniz-Filho JAF, Götzenberger L, Hortal J, Ladle RJ, Lepš J (2015) On the need for phylogenetic ‘corrections’ in functional trait-based approaches. Folia Geobotanica 50, 349–357.
| On the need for phylogenetic ‘corrections’ in functional trait-based approaches.Crossref | GoogleScholarGoogle Scholar |
Dean WRJ, Milton SJ, Jeltsch F (1999) Large trees, fertile islands, and birds in arid savanna. Journal of Arid Environments 41, 61–78.
| Large trees, fertile islands, and birds in arid savanna.Crossref | GoogleScholarGoogle Scholar |
Del Tredici P (2001) Sprouting in temperate trees: a morphological and ecological review. Botanical Review 67, 121–140.
| Sprouting in temperate trees: a morphological and ecological review.Crossref | GoogleScholarGoogle Scholar |
Díaz S, Kattge J, Cornelissen JH, Wright IJ, Lavorel S, Dray S, Reu B, Kleyer M, Wirth C, Prentice I, Garnier E, Bönisch G, Westoby M, Poorter H, Reich P, Moles A, Dickie J, Gillison A, Zanne A, Gorné L (2016) The global spectrum of plant form and function. Nature 529, 167–171.
| The global spectrum of plant form and function.Crossref | GoogleScholarGoogle Scholar | 26700811PubMed |
Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plants. Annual Review of Plant Biology 65, 667–687.
| Nonstructural carbon in woody plants.Crossref | GoogleScholarGoogle Scholar | 24274032PubMed |
Dinerstein E (1989) The foliage-as-fruit hypothesis and the feeding behavior of South Asian ungulates. Biotropica 21, 214
| The foliage-as-fruit hypothesis and the feeding behavior of South Asian ungulates.Crossref | GoogleScholarGoogle Scholar |
Donohue K, Rubio de Casas R, Burghardt L, Kovach K, Willis CG (2010) Germination, postgermination adaptation, and species ecological ranges. Annual Review of Ecology, Evolution, and Systematics 41, 293–319.
| Germination, postgermination adaptation, and species ecological ranges.Crossref | GoogleScholarGoogle Scholar |
Drescher M, Heitkönig IMA, Raats JG, Prins HHT (2006) The role of grass stems as structural foraging deterrents and their effects on the foraging behaviour of cattle. Applied Animal Behaviour Science 101, 10–26.
| The role of grass stems as structural foraging deterrents and their effects on the foraging behaviour of cattle.Crossref | GoogleScholarGoogle Scholar |
du Toit JC (2009) Early survival and growth of vegetatively propagated indigenous grasses in a clear-felled timber plantation in KwaZulu–Natal, South Africa. African Journal of Range & Forage Science 26, 97–101.
| Early survival and growth of vegetatively propagated indigenous grasses in a clear-felled timber plantation in KwaZulu–Natal, South Africa.Crossref | GoogleScholarGoogle Scholar |
Dudley JP (2000) Seed dispersal by elephants in semiarid woodland habitats of Hwange National Park, Zimbabwe. Biotropica 32, 556–561.
| Seed dispersal by elephants in semiarid woodland habitats of Hwange National Park, Zimbabwe.Crossref | GoogleScholarGoogle Scholar |
Dyer LA, Dodson CD, Gentry G (2003) A bioassay for insect deterrent compounds found in plant and animal tissues. Phytochemical Analysis 14, 381–388.
| A bioassay for insect deterrent compounds found in plant and animal tissues.Crossref | GoogleScholarGoogle Scholar | 14667066PubMed |
Dyer LA, Philbin CS, Ochsenrider KM, Richards LA, Massad TJ, Smilanich AM, Forister ML, Parchman TL, Gallard LK, Hurtado PJ, Espeset AE, Glassmire AE, Harrison JG, Mo C, Yoon S, Pardikes NA, Muchoney ND, Jahner JP, Slinn HL, Shelef O, Dodson CD, Kato MJ, Yamaguchi LF, Jeffrey CS (2018) Modern approaches to study plant-insect interactions in chemical ecology. Nature Reviews. Chemistry 2, 50–64.
| Modern approaches to study plant-insect interactions in chemical ecology.Crossref | GoogleScholarGoogle Scholar |
Eissenstat DM, Duncan LW (1992) Root growth and carbohydrate responses in bearing citrus trees following partial canopy removal. Tree Physiology 10, 245–257.
| Root growth and carbohydrate responses in bearing citrus trees following partial canopy removal.Crossref | GoogleScholarGoogle Scholar | 14969982PubMed |
Elbaum R, Zaltzman L, Burgert I, Fratzl P (2007) The role of wheat awns in the seed dispersal unit. Science 316, 884–886.
| The role of wheat awns in the seed dispersal unit.Crossref | GoogleScholarGoogle Scholar | 17495170PubMed |
Evangelista D, Hotton S, Dumais J (2011) The mechanics of explosive dispersal and self-burial in the seeds of the filaree, Erodium cicutarium (Geraniaceae). The Journal of Experimental Biology 214, 521–529.
| The mechanics of explosive dispersal and self-burial in the seeds of the filaree, Erodium cicutarium (Geraniaceae).Crossref | GoogleScholarGoogle Scholar | 21270299PubMed |
Evert RF (2006) ‘Esau’s Plant Anatomy’ (Wiley: Hoboken, NJ, USA)
Ezoe H (1998) Optimal dispersal range and seed size in a stable environment. Journal of Theoretical Biology 190, 287–293.
| Optimal dispersal range and seed size in a stable environment.Crossref | GoogleScholarGoogle Scholar | 9514653PubMed |
February EC, Higgins SI (2010) The distribution of tree and grass roots in savannas in relation to soil nitrogen and water. South African Journal of Botany 76, 517–523.
| The distribution of tree and grass roots in savannas in relation to soil nitrogen and water.Crossref | GoogleScholarGoogle Scholar |
Fensham RJ, Fairfax RJ, Ward DP (2009) Drought-induced tree death in savanna. Global Change Biology 15, 380–387.
| Drought-induced tree death in savanna.Crossref | GoogleScholarGoogle Scholar |
Fichino BS, Dombroski JR, Pivello VR, Fidelis A (2016) Does fire trigger seed germination in the Neotropical savannas? Experimental tests with six Cerrado species. Biotropica 48, 181–187.
| Does fire trigger seed germination in the Neotropical savannas? Experimental tests with six Cerrado species.Crossref | GoogleScholarGoogle Scholar |
Fidelis A, Blanco C (2014) Does fire induce flowering in Brazilian subtropical grasslands? Applied Vegetation Science 17, 690–699.
| Does fire induce flowering in Brazilian subtropical grasslands?Crossref | GoogleScholarGoogle Scholar |
Fidelis A, Appezzato-da-Glória B, Pillar VD, Pfadenhauer J (2014) Does disturbance affect bud bank size and belowground structures diversity in Brazilian subtropical grasslands? Flora – Morphology, Distribution, Functional Ecology of Plants 209, 110–116.
| Does disturbance affect bud bank size and belowground structures diversity in Brazilian subtropical grasslands?Crossref | GoogleScholarGoogle Scholar |
Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytologist 171, 501–523.
| Seed dormancy and the control of germination.Crossref | GoogleScholarGoogle Scholar | 16866955PubMed |
Fischer C, Höll W (1991) Food reserves of scots pine (Pinus sylvestris L.) I. Seasonal changes in the carbohydrate and fat reserves of pine needles. Trees 5, 187–195.
| Food reserves of scots pine (Pinus sylvestris L.) I. Seasonal changes in the carbohydrate and fat reserves of pine needles.Crossref | GoogleScholarGoogle Scholar |
Fischer C, Höll W (1992) Food reserves of scots pine (Pinus sylvestris L.) II. Seasonal changes and radial distribution of carbohydrate and fat reserves in pine wood. Trees 6, 147–155.
| Food reserves of scots pine (Pinus sylvestris L.) II. Seasonal changes and radial distribution of carbohydrate and fat reserves in pine wood.Crossref | GoogleScholarGoogle Scholar |
Ford CW, Wilson JR (1981) Changes in levels of solutes during osmotic adjustment to water stress in leaves of four tropical pasture species. Functional Plant Biology 8, 77
| Changes in levels of solutes during osmotic adjustment to water stress in leaves of four tropical pasture species.Crossref | GoogleScholarGoogle Scholar |
Fornara DA, du Toit JT (2007) Browsing lawns? Responses of Acacia nigrescens to ungulate browsing in an African savanna. Ecology 88, 200–209.
| Browsing lawns? Responses of Acacia nigrescens to ungulate browsing in an African savanna.Crossref | GoogleScholarGoogle Scholar | 17489468PubMed |
Francisco IA, Pinotti MHP (2000) Cyanogenic glycosides in plants. Brazilian Archives of Biology and Technology 43, 487–492.
| Cyanogenic glycosides in plants.Crossref | GoogleScholarGoogle Scholar |
Franco AC, Bustamante M, Caldas LS, Goldstein G, Meinzer FC, Kozovits AR, Rundel P, Coradin VTR (2005) Leaf functional traits of Neotropical savanna trees in relation to seasonal water deficit. Trees 19, 326–335.
| Leaf functional traits of Neotropical savanna trees in relation to seasonal water deficit.Crossref | GoogleScholarGoogle Scholar |
Furstenburg D, van Hoven W (1994) Condensed tannin as anti-defoliate agent against browsing by giraffe (Giraffa camelopardalis) in the Kruger National Park. Comparative Biochemistry and Physiology – A. Physiology 107, 425–431.
| Condensed tannin as anti-defoliate agent against browsing by giraffe (Giraffa camelopardalis) in the Kruger National Park.Crossref | GoogleScholarGoogle Scholar |
Gao X, Schwilk D (2018) Grass canopy architecture influences temperature exposure at soil surface. Fire 1, 35
| Grass canopy architecture influences temperature exposure at soil surface.Crossref | GoogleScholarGoogle Scholar |
Garnier LKM, Dajoz I (2001) Evolutionary significance of awn length variation in a clonal grass of fire-prone savannas. Ecology 82, 1720–1733.
| Evolutionary significance of awn length variation in a clonal grass of fire-prone savannas.Crossref | GoogleScholarGoogle Scholar |
Gershenzon J, Dudareva N (2007) The function of terpene natural products in the natural world. Nature Chemical Biology 3, 408–414.
| The function of terpene natural products in the natural world.Crossref | GoogleScholarGoogle Scholar | 17576428PubMed |
Gignoux J, Clobert J, Menaut J-C (1997) Alternative fire resistance strategies in savanna trees. Oecologia 110, 576–583.
| Alternative fire resistance strategies in savanna trees.Crossref | GoogleScholarGoogle Scholar | 28307253PubMed |
Gignoux J, Mordelet P, Menaut JC (2006) Biomass cycle and primary production. In ‘Lamto: Structure, Functioning and Dynamics of a Savanna Ecosystem’. (Eds L Abbadie, J Gignoux, X Le Roux, M Lepage) pp. 115–137. (Springer-Verlag: New York, NY, USA)
Gignoux J, Lahoreau G, Julliard R, Barot S (2009) Establishment and early persistence of tree seedlings in an annually burned savanna. Journal of Ecology 97, 484–495.
| Establishment and early persistence of tree seedlings in an annually burned savanna.Crossref | GoogleScholarGoogle Scholar |
Gignoux J, Konaté S, Lahoreau G, Le Roux X, Simioni G (2016) Allocation strategies of savanna and forest tree seedlings in response to fire and shading: outcomes of a field experiment. Scientific Reports 6, 38 838
| Allocation strategies of savanna and forest tree seedlings in response to fire and shading: outcomes of a field experiment.Crossref | GoogleScholarGoogle Scholar | 28000732PubMed |
Gonzáles WL, Negritto MA, Suarez LH, Gianoli E (2008) Induction of glandular and non-glandular trichomes by damage in leaves of Madia sativa under contrasting water regimes. Acta Oecologica 33, 128–132.
| Induction of glandular and non-glandular trichomes by damage in leaves of Madia sativa under contrasting water regimes.Crossref | GoogleScholarGoogle Scholar |
Gowda JH, Palo RT (2003) Age-related changes in defensive traits of Acacia tortilis Hayne. African Journal of Ecology 41, 218–223.
| Age-related changes in defensive traits of Acacia tortilis Hayne.Crossref | GoogleScholarGoogle Scholar |
Griffith DM, Anderson TM, Hamilton EW (2017) Ungulate grazing drives higher ramet turnover in sodium-adapted Serengeti grasses. Journal of Vegetation Science 28, 815–823.
| Ungulate grazing drives higher ramet turnover in sodium-adapted Serengeti grasses.Crossref | GoogleScholarGoogle Scholar |
Grohmann C, Hartmann JN, Kovalev A, Gorb SN (2019) Dandelion diaspore dispersal: frictional anisotropy of cypselae of Taraxacum officinale enhances their interlocking with the soil. Plant and Soil 440, 399–408.
| Dandelion diaspore dispersal: frictional anisotropy of cypselae of Taraxacum officinale enhances their interlocking with the soil.Crossref | GoogleScholarGoogle Scholar |
Grootemaat S, Wright IJ, van Bodegom PM, Cornelissen JHC (2017) Scaling up flammability from individual leaves to fuel beds. Oikos 126, 1428–1438.
| Scaling up flammability from individual leaves to fuel beds.Crossref | GoogleScholarGoogle Scholar |
Guimarães PR, Galetti M, Jordano P (2008) Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS One 3, e1745
| Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate.Crossref | GoogleScholarGoogle Scholar | 18320062PubMed |
Hagerman AE (1987) Radial diffusion method for determining tannin in plant extracts. Journal of Chemical Ecology 13, 437–449.
| Radial diffusion method for determining tannin in plant extracts.Crossref | GoogleScholarGoogle Scholar | 24301886PubMed |
Hallé F, Oldeman RAA, Tomlinson PB (1978) ‘Tropical Trees and Forests’. (Springer: Berlin, Germany)
Handley R, Ekbom B, Ågren J (2005) Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana. Ecological Entomology 30, 284–292.
| Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM (2007) Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematics 8, 157–178.
| Plant structural traits and their role in anti-herbivore defence.Crossref | GoogleScholarGoogle Scholar |
Hartzfeld PW, Forkner R, Hunter MD, Hagerman AE (2002) Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. Journal of Agricultural and Food Chemistry 50, 1785–1790.
| Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate.Crossref | GoogleScholarGoogle Scholar | 11902913PubMed |
Hattas D, Julkunen-Tiitto R (2012) The quantification of condensed tannins in African savanna tree species. Phytochemistry Letters 5, 329–334.
| The quantification of condensed tannins in African savanna tree species.Crossref | GoogleScholarGoogle Scholar |
Hean JW, Ward D (2012) Fire and herbivory are not substitutable: evidence from regrowth patterns and changes in physical and chemical defences in Acacia seedlings. Journal of Vegetation Science 23, 13–23.
| Fire and herbivory are not substitutable: evidence from regrowth patterns and changes in physical and chemical defences in Acacia seedlings.Crossref | GoogleScholarGoogle Scholar |
Hempson GP, Archibald S, Bond WJ (2015) A continent-wide assessment of the form and intensity of large mammal herbivory in Africa. Science 350, 1056–1061.
| A continent-wide assessment of the form and intensity of large mammal herbivory in Africa.Crossref | GoogleScholarGoogle Scholar | 26612946PubMed |
Hempson GP, Archibald S, Donaldson JE, Lehmann CER (2019) Alternate grassy ecosystem states are determined by palatability–flammability trade-offs. Trends in Ecology & Evolution 34, 286–290.
| Alternate grassy ecosystem states are determined by palatability–flammability trade-offs.Crossref | GoogleScholarGoogle Scholar |
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. The Quarterly Review of Biology 67, 283–335.
| The dilemma of plants: to grow or defend.Crossref | GoogleScholarGoogle Scholar |
Herrera CM (1985) Determinants of plant–animal coevolution: the case of mutualistic dispersal of seeds by vertebrates. Oikos 44, 132–141.
| Determinants of plant–animal coevolution: the case of mutualistic dispersal of seeds by vertebrates.Crossref | GoogleScholarGoogle Scholar |
Higgins SI, Bond WJ, Trollope WSW (2000) Fire, resprouting and variability: a recipe for grass–tree coexistence in savanna. Journal of Ecology 88, 213–229.
| Fire, resprouting and variability: a recipe for grass–tree coexistence in savanna.Crossref | GoogleScholarGoogle Scholar |
Higgins SI, Bond WJ, February EC, Bronn A, Euston-Brown DIW, Enslin B, Govender N, Rademan L, O’Regan S, Potgieter ALF, Scheiter S, Sowry R, Trollope L, Trollope WSW (2007) Effects of four decades of fire manipulation on woody vegetation structure in savanna. Ecology 88, 1119–1125.
| Effects of four decades of fire manipulation on woody vegetation structure in savanna.Crossref | GoogleScholarGoogle Scholar | 17536398PubMed |
Higgins SI, Bond WJ, Combrink H, Craine JM, February EC, Govender N, Lannas K, Moncrieff G, Trollope WS (2012) Which traits determine shifts in the abundance of tree species in a fire-prone savanna? Journal of Ecology 100, 1400–1410.
| Which traits determine shifts in the abundance of tree species in a fire-prone savanna?Crossref | GoogleScholarGoogle Scholar |
Hoch G, Körner C (2003) The carbon charging of pines at the climatic treeline: a global comparison. Oecologia 135, 10–21.
| The carbon charging of pines at the climatic treeline: a global comparison.Crossref | GoogleScholarGoogle Scholar | 12647099PubMed |
Hoch G, Richter A, Körner C (2003) Non-structural carbon compounds in temperate forest trees. Plant, Cell & Environment 26, 1067–1081.
| Non-structural carbon compounds in temperate forest trees.Crossref | GoogleScholarGoogle Scholar |
Hoffmann WA, Adasme R, Haridasan MT, de Carvalho M, Geiger EL, Pereira MAB, Gotsch SG, Franco AC (2009) Tree topkill, not mortality, governs the dynamics of savanna-forest boundaries under frequent fire in central Brazil. Ecology 90, 1326–1337.
| Tree topkill, not mortality, governs the dynamics of savanna-forest boundaries under frequent fire in central Brazil.Crossref | GoogleScholarGoogle Scholar | 19537552PubMed |
Hoffmann WA, Geiger EL, Gotsch SG, Rossatto DR, Silva LCR, Lau OL, Haridasan M, Franco AC (2012) Ecological thresholds at the savanna-forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecology Letters 15, 759–768.
| Ecological thresholds at the savanna-forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes.Crossref | GoogleScholarGoogle Scholar | 22554474PubMed |
Holdo RM (2013) Revisiting the two-layer hypothesis: coexistence of alternative functional rooting strategies in savannas. PLoS One 8, e69625
| Revisiting the two-layer hypothesis: coexistence of alternative functional rooting strategies in savannas.Crossref | GoogleScholarGoogle Scholar | 23950900PubMed |
Illius AW, Gordon IJ (1992) Modelling the nutritional ecology of ungulate herbivores: evolution of body size and competitive interactions. Oecologia 89, 428–434.
| Modelling the nutritional ecology of ungulate herbivores: evolution of body size and competitive interactions.Crossref | GoogleScholarGoogle Scholar | 28313093PubMed |
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. American Naturalist 104, 501–528.
| Herbivores and the number of tree species in tropical forests.Crossref | GoogleScholarGoogle Scholar |
Janzen DH (1984) Dispersal of small seeds by big herbivores: foliage is the fruit. American Naturalist 123, 338–353.
| Dispersal of small seeds by big herbivores: foliage is the fruit.Crossref | GoogleScholarGoogle Scholar |
Jaureguiberry P, Bertone G, Díaz S (2011) Device for the standard measurement of shoot flammability in the field. Austral Ecology 36, 821–829.
| Device for the standard measurement of shoot flammability in the field.Crossref | GoogleScholarGoogle Scholar |
Javed Muhammad A, Abdullah MZ, Muhammad N, Ratnam W (2017) Detecting mislabeling and identifying unique progeny in Acacia mapping population using SNP markers. Journal of Forestry Research 28, 1119–1127.
| Detecting mislabeling and identifying unique progeny in Acacia mapping population using SNP markers.Crossref | GoogleScholarGoogle Scholar |
Jeffree CE (1986) The cuticle, epicuticular waxes and trichomes of plants, with reference to their structure, functions and evolution. In ‘Insects and the Plant Surface’. (Eds BE Juniper, TRE Southwood) pp. 23–64. (Edward Arnold Publishers: London, UK)
Jiménez-Alfaro B, Silveira FAO, Fidelis A, Poschlod P, Commander LE (2016) Seed germination traits can contribute better to plant community ecology. Journal of Vegetation Science 27, 637–645.
| Seed germination traits can contribute better to plant community ecology.Crossref | GoogleScholarGoogle Scholar |
Johnson JM, Pritchard J, Gorham J, Tomos AD (1996) Growth, water relations and solute accumulation in osmotically stressed seedlings of the tropical tree Colophospermum mopane. Tree Physiology 16, 713–718.
| Growth, water relations and solute accumulation in osmotically stressed seedlings of the tropical tree Colophospermum mopane.Crossref | GoogleScholarGoogle Scholar | 14871694PubMed |
Jurado E, Flores J (2005) Is seed dormancy under environmental control or bound to plant traits? Journal of Vegetation Science 16, 559–564.
| Is seed dormancy under environmental control or bound to plant traits?Crossref | GoogleScholarGoogle Scholar |
Jurado E, Westoby M (1992) Seedling growth in relation to seed size among species of arid Australia. Journal of Ecology 80, 407–416.
| Seedling growth in relation to seed size among species of arid Australia.Crossref | GoogleScholarGoogle Scholar |
Kaplan I, Dively GP, Denno RF (2009) The costs of anti-herbivore defense traits in agricultural crop plants: a case study involving leafhoppers and trichomes. Ecological Applications 19, 864–872.
| The costs of anti-herbivore defense traits in agricultural crop plants: a case study involving leafhoppers and trichomes.Crossref | GoogleScholarGoogle Scholar | 19544730PubMed |
Keeley JE, Zedler PH (1998) Evolution of life histories in Pinus. In ‘Ecology and Biogeography of Pinus’. pp. 219–250. (Cambridge University Press: Cambridge, UK)
Kerley GIH, Landman M, Kruger L, Owen-Smith N, Balfour D, de Boer WF, Gaylard A, Lindsay K, Slotow R (2008) Effects of elephants on ecosystems and biodiversity. In ‘The 2007 Scientific Assessment of Elephant Management in South Africa’. (Eds K Mennell, R Scholes) pp. 101–147. (Wits University Press: Johannesburg, South Africa)
Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18, 2328–2375.
| Techniques for analysis of plant phenolic compounds.Crossref | GoogleScholarGoogle Scholar | 23429347PubMed |
Kleyer M, Bekker R, Knevel I, Bakker J, Thompson K, Sonnenschein M, Poschlod P, Van Groenendael J, Klimeš L, Klimešová J, Klotz S, Rusch G, Hermy M, Adriaens D, Boedeltje G, Bossuyt B, Dannemann A, Endels P, Götzenberger L, Hodgson J, Jackel A-K, K� Kunzmann D, Ozinga W, Römermann C, Stadler M, Schlegelmilch J, Steendam H, Tackenberg O, Wilmann B, Cornelissen J, Eriksson O, Garnier E, Peco B (2008) The LEDA Traitbase: a database of life history traits of the Northwest European flora. Journal of Ecology 96, 1266–1274
Klimešová J, de Bello F (2009) CLO-PLA: the database of clonal and bud bank traits of Central European flora. Journal of Vegetation Science 20, 511–516.
| CLO-PLA: the database of clonal and bud bank traits of Central European flora.Crossref | GoogleScholarGoogle Scholar |
Knoop WT, Walker BH (1985) Interactions of woody and herbaceous vegetation in a southern African savanna. Journal of Ecology 73, 235
| Interactions of woody and herbaceous vegetation in a southern African savanna.Crossref | GoogleScholarGoogle Scholar |
Kohi EM, De Boer WF, Slot M, Van Wieren SE, Ferwerda JG, Grant RC, Heitkönig IMA, De Knegt HJ, Knox N, Van Langevelde F, Peel M, Slotow R, Van Der Waal C, Prins HHT 2010 Effects of simulated browsing on growth and leaf chemical properties in Colophospermum mopanesaplings.African Journal of Ecology48 190196
Kollmann J, Reiner SA (1996) Light demands of shrub seedlings and their establishment within scrublands. Flora 191, 191–200.
| Light demands of shrub seedlings and their establishment within scrublands.Crossref | GoogleScholarGoogle Scholar |
Kronzucker HJ, Coskun D, Schulze LM, Wong JR, Britto DT (2013) Sodium as nutrient and toxicant. Plant and Soil 369, 1–23.
| Sodium as nutrient and toxicant.Crossref | GoogleScholarGoogle Scholar |
Kruger LM, Midgley JJ (2001) The influence of resprouting forest canopy species on richness in Southern Cape forests, South Africa. Global Ecology and Biogeography 10, 567–572.
| The influence of resprouting forest canopy species on richness in Southern Cape forests, South Africa.Crossref | GoogleScholarGoogle Scholar |
Kruger LM, Midgley JJ, Cowling RM (1997) Resprouters vs reseeders in South African forest trees; a model based on forest canopy height. Functional Ecology 11, 101–105.
| Resprouters vs reseeders in South African forest trees; a model based on forest canopy height.Crossref | GoogleScholarGoogle Scholar |
Kruger LM, Charles-Dominique T, Bond WJ, Midgley JJ, Balfour DA, Mkhwanazi A (2017) Woody plant traits and life-history strategies across disturbance gradients and biome boundaries in the Hluhluwe–iMfolozi park. In ‘Conserving Africa’s Mega-Diversity in the Anthropocene’. pp. 189–210. (Cambridge University Press: Cambridge, UK)
Kubiske ME, Pregitzer KS (1996) Effects of elevated CO2 and light availability on the photosynthetic light response of trees of contrasting shade tolerance. Tree Physiology 16, 351–358.
| Effects of elevated CO2 and light availability on the photosynthetic light response of trees of contrasting shade tolerance.Crossref | GoogleScholarGoogle Scholar | 14871736PubMed |
Kulić IM, Mani M, Mohrbach H, Thaokar R, Mahadevan L (2009) Botanical ratchets. Proceedings of the Royal Society of London – B. Biological Sciences 276, 2243–2247.
| Botanical ratchets.Crossref | GoogleScholarGoogle Scholar |
Kulmatiski A, Beard KH (2013) Root niche partitioning among grasses, saplings, and trees measured using a tracer technique. Oecologia 171, 25–37.
| Root niche partitioning among grasses, saplings, and trees measured using a tracer technique.Crossref | GoogleScholarGoogle Scholar | 22752210PubMed |
Kursar TA, Dexter KG, Lokvam J, Pennington RT, Richardson JE, Weber MG, Murakami ET, Drake C, McGregor R, Coley PD (2009) The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proceedings of the National Academy of Sciences of the United States of America 106, 18 073–18 078.
| The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga.Crossref | GoogleScholarGoogle Scholar |
Lamont BB, Downes KS (2011) Fire-stimulated flowering among resprouters and geophytes in Australia and South Africa. Plant Ecology 212, 2111–2125.
| Fire-stimulated flowering among resprouters and geophytes in Australia and South Africa.Crossref | GoogleScholarGoogle Scholar |
Landhäusser SM (2011) Aspen shoots are carbon autonomous during bud break. Trees 25, 531–536.
| Aspen shoots are carbon autonomous during bud break.Crossref | GoogleScholarGoogle Scholar |
Landhäusser SM, Chow PS, Dickman LT, Furze ME, Kuhlman I, Schmid S, Wiesenbauer J, Wild B, Gleixner G, Hartmann H, Hoch G, McDowell NG, Richardson AD, Richter A, Adams HD (2018) Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiology 38, 1764–1778.
| Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates.Crossref | GoogleScholarGoogle Scholar | 30376128PubMed |
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology 16, 545–556.
| Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail.Crossref | GoogleScholarGoogle Scholar |
Lehmann CER, Anderson TM, Sankaran M, Higgins SI, Archibald S, Hoffmann WA, Hanan NP, Williams RJ, Fensham RJ, Felfili J, Hutley LB, Ratnam J, San Jose J, Montes R, Franklin D, Russell-Smith J, Ryan CM, Durigan G, Hiernaux P, Haidar R, Bowman DMJS, Bond WJ (2014) Savanna vegetation–fire–climate relationships differ among continents. Science 343, 548–552.
| Savanna vegetation–fire–climate relationships differ among continents.Crossref | GoogleScholarGoogle Scholar |
Lemon PC (1949) Successional responses of herbs in the longleaf– slash pine forest after fire. Ecology 30, 135–145.
| Successional responses of herbs in the longleaf– slash pine forest after fire.Crossref | GoogleScholarGoogle Scholar |
Levin DA (1973) The role of trichomes in plant defense. The Quarterly Review of Biology 48, 3–15.
| The role of trichomes in plant defense.Crossref | GoogleScholarGoogle Scholar |
Light ME, Van Staden J, Bornman CH (2004) The potential of smoke in seed technology. South African Journal of Botany 70, 97–101.
| The potential of smoke in seed technology.Crossref | GoogleScholarGoogle Scholar |
Lindenmayer DB, Laurance WF, Franklin JF (2012) Global decline in large old trees. Science 338, 1305–1306.
| Global decline in large old trees.Crossref | GoogleScholarGoogle Scholar | 23224548PubMed |
Liu C, He N, Zhang J, Li Y, Wang Q, Sack L, Yu G (2018) Variation of stomatal traits from cold temperate to tropical forests and association with water use efficiency. Functional Ecology 32, 20–28.
| Variation of stomatal traits from cold temperate to tropical forests and association with water use efficiency.Crossref | GoogleScholarGoogle Scholar |
Liu U, Cossu TA, Davies RM, Forest F, Dickie JB, Breman E (2020) Conserving orthodox seeds of globally threatened plants ex situ in the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK: the status of seed collections. Biodiversity and Conservation 29, 2901–2949.
| Conserving orthodox seeds of globally threatened plants ex situ in the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK: the status of seed collections.Crossref | GoogleScholarGoogle Scholar |
Ludwig F, Kroon H, Prins HHT, Berendse F (2001) Effects of nutrients and shade on tree–grass interactions in an East African savanna. Journal of Vegetation Science 12, 579–588.
| Effects of nutrients and shade on tree–grass interactions in an East African savanna.Crossref | GoogleScholarGoogle Scholar |
Lusk CH, Del Pozo A (2002) Survival and growth of seedlings of 12 Chilean rainforest trees in two light environments: gas exchange and biomass distribution correlates. Austral Ecology 27, 173–182.
| Survival and growth of seedlings of 12 Chilean rainforest trees in two light environments: gas exchange and biomass distribution correlates.Crossref | GoogleScholarGoogle Scholar |
Martin RE, Sapsis DB (1992) Fires as agents of biodiversity: pyrodiversity promotes biodiversity. In ‘Proceedings of the Conference on Biodiversity of Northwest California Ecosystems’, 28–30 October 1991, Santa Rosa, CA, USA. (Eds RR Harris, DC Erman, HM Kerner) pp. 150–157. (Cooperative Extension, University of California: Berkeley, CA, USA)
Martínez-Vilalta J (2014) Carbon storage in trees: pathogens have their say. Tree Physiology 34, 215–217.
| Carbon storage in trees: pathogens have their say.Crossref | GoogleScholarGoogle Scholar | 24619516PubMed |
Martínez-Vilalta J, Sala A, Asensio D, Galiano L, Hoch G, Palacio S, Piper FI, Lloret F (2016) Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Ecological Monographs 86, 495–516.
| Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis.Crossref | GoogleScholarGoogle Scholar |
Massad TJ, Dyer LA, Vega CG (2012) Costs of defense and a test of the carbon-nutrient balance and growth-differentiation balance hypotheses for two co-occurring classes of plant defense. PLoS One 7, e47554
| Costs of defense and a test of the carbon-nutrient balance and growth-differentiation balance hypotheses for two co-occurring classes of plant defense.Crossref | GoogleScholarGoogle Scholar | 23115654PubMed |
Massad TJ, Martins de Moraes M, Philbin C, Oliveira C Jr, Cebrian Torrejon G, Fumiko Yamaguchi L, Jeffrey CS, Dyer LA, Richards LA, Kato MJ 2017 Similarity in volatile communities leads to increased herbivory and greater tropical forest diversity.Ecology98 17501756
Mauricio R (1998) Costs of resistance to natural enemies in field populations of the annual plant Arabidopsis thaliana. American Naturalist 151, 20–28.
| Costs of resistance to natural enemies in field populations of the annual plant Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 18811421PubMed |
McDowell N, Allen CD, Anderson-Teixeira K, Brando P, Brienen R, Chambers J, Christoffersen B, Davies S, Doughty C, Duque A, Espirito-Santo F (2018) Drivers and mechanisms of tree mortality in moist tropical forests. New Phytologist 219, 851–869.
| Drivers and mechanisms of tree mortality in moist tropical forests.Crossref | GoogleScholarGoogle Scholar | 29451313PubMed |
McGill BJ, Enquist BJ, Weiher E, Westoby M (2006) Rebuilding community ecology from functional traits. Trends in Ecology & Evolution 21, 178–185.
| Rebuilding community ecology from functional traits.Crossref | GoogleScholarGoogle Scholar |
McGranahan DA, Archibald S, Kirkman KP, O’Connor TG (2018) A native C3 grass alters fuels and fire spread in montane grassland of South Africa. Plant Ecology 219, 621–632.
| A native C3 grass alters fuels and fire spread in montane grassland of South Africa.Crossref | GoogleScholarGoogle Scholar |
McNaughton SJ (1984) Grazing lawns: animals in herds, plant form, and coevolution. American Naturalist 124, 863–886.
| Grazing lawns: animals in herds, plant form, and coevolution.Crossref | GoogleScholarGoogle Scholar |
McNaughton SJ, Banyikwa FF, McNaughton MM (1997) Promotion of the cycling of diet-enhancing nutrients by African grazers. Science 278, 1798–1800.
| Promotion of the cycling of diet-enhancing nutrients by African grazers.Crossref | GoogleScholarGoogle Scholar | 9388182PubMed |
Meier AR, Saunders MR, Michler CH (2012) Epicormic buds in trees: a review of bud establishment, development and dormancy release. Tree Physiology 32, 565–584.
| Epicormic buds in trees: a review of bud establishment, development and dormancy release.Crossref | GoogleScholarGoogle Scholar | 22555307PubMed |
Merfort I (2002) Review of the analytical techniques for sesquiterpenes and sesquiterpene lactones. Journal of Chromatography. A 967, 115–130.
| Review of the analytical techniques for sesquiterpenes and sesquiterpene lactones.Crossref | GoogleScholarGoogle Scholar | 12219925PubMed |
Michel BE (1983) Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiology 72, 66–70.
| Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes.Crossref | GoogleScholarGoogle Scholar | 16662983PubMed |
Michel BE, Kaufmann MR (1973) The osmotic potential of polyethylene glycol 6000. Plant Physiology 51, 914–916.
| The osmotic potential of polyethylene glycol 6000.Crossref | GoogleScholarGoogle Scholar | 16658439PubMed |
Midgley JJ (1996) Why the world’s vegetation is not totally dominated by resprouting plants; because resprouters are shorter than reseeders. Ecography 19, 92–94.
| Why the world’s vegetation is not totally dominated by resprouting plants; because resprouters are shorter than reseeders.Crossref | GoogleScholarGoogle Scholar |
Midgley JJ, Bond WJ (2001) A synthesis of the demography of African acacias. Journal of Tropical Ecology 17, 871–886.
| A synthesis of the demography of African acacias.Crossref | GoogleScholarGoogle Scholar |
Midgley JJ, Lawes MJ (2016) Relative bark thickness: towards standardised measurement and analysis. Plant Ecology 217, 677–681.
| Relative bark thickness: towards standardised measurement and analysis.Crossref | GoogleScholarGoogle Scholar |
Midgley JJ, Lawes MJ, Chamaillé-Jammes S (2010) Savanna woody plant dynamics: the role of fire and herbivory, separately and synergistically. Australian Journal of Botany 58, 1
| Savanna woody plant dynamics: the role of fire and herbivory, separately and synergistically.Crossref | GoogleScholarGoogle Scholar |
Midgley JJ, Kruger LM, Skelton R (2011) How do fires kill plants? The hydraulic death hypothesis and Cape Proteaceae ‘fire-resisters’. South African Journal of Botany 77, 381–386.
| How do fires kill plants? The hydraulic death hypothesis and Cape Proteaceae ‘fire-resisters’.Crossref | GoogleScholarGoogle Scholar |
Mitchell SJ (2013) Wind as a natural disturbance agent in forests: a synthesis. Forestry 86, 147–157.
| Wind as a natural disturbance agent in forests: a synthesis.Crossref | GoogleScholarGoogle Scholar |
Molano-Flores B (2012) Diaspore morphometrics and self-burial in Hesperostipa spartea from loam and sandy soils. The Journal of the Torrey Botanical Society 139, 56–62.
| Diaspore morphometrics and self-burial in Hesperostipa spartea from loam and sandy soils.Crossref | GoogleScholarGoogle Scholar |
Moles AT, Peco B, Wallis IR, Foley WJ, Poore AG, Seabloom EW, Vesk PA, Bisigato AJ, Cella-Pizarro L, Clark CJ, Cohen PS (2013) Correlations between physical and chemical defences in plants: tradeoffs, syndromes, or just many different ways to skin a herbivorous cat? New Phytologist 198, 252–263.
| Correlations between physical and chemical defences in plants: tradeoffs, syndromes, or just many different ways to skin a herbivorous cat?Crossref | GoogleScholarGoogle Scholar | 23316750PubMed |
Moncrieff GR, Kruger LM, Midgley JJ (2008) Stem mortality of Acacia nigrescens induced by the synergistic effects of elephants and fire in Kruger National Park, South Africa. Journal of Tropical Ecology 24, 655–662.
| Stem mortality of Acacia nigrescens induced by the synergistic effects of elephants and fire in Kruger National Park, South Africa.Crossref | GoogleScholarGoogle Scholar |
Moncrieff GR, Chamaillé-Jammes S, Higgins SI, O’Hara RB, Bond WJ (2011) Tree allometries reflect a lifetime of herbivory in an African savanna. Ecology 92, 2310–2315.
| Tree allometries reflect a lifetime of herbivory in an African savanna.Crossref | GoogleScholarGoogle Scholar | 22352170PubMed |
Mordelet P, Roux X (1993) Tree/grass interactions. In ‘Ecological Studies’. pp. 139–161. (Springer: New York, NY, USA)
Moreira B, Tormo J, Pausas JG (2012) To resprout or not to resprout: factors driving intraspecific variability in resprouting. Oikos 121, 1577–1584.
| To resprout or not to resprout: factors driving intraspecific variability in resprouting.Crossref | GoogleScholarGoogle Scholar |
Morin PA, Martien KK, Taylor BL (2009) Assessing statistical power of SNPs for population structure and conservation studies. Molecular Ecology Resources 9, 66–73.
| Assessing statistical power of SNPs for population structure and conservation studies.Crossref | GoogleScholarGoogle Scholar | 21564568PubMed |
Murbach L (1900) Note on the mechanics of the seed-burying awns of Stipa avenacea. Botanical Gazette 30, 113–117.
| Note on the mechanics of the seed-burying awns of Stipa avenacea.Crossref | GoogleScholarGoogle Scholar |
Murphy BP, Andersen AN, Parr CL (2016) The underestimated biodiversity of tropical grassy biomes. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 371, 20150319
| The underestimated biodiversity of tropical grassy biomes.Crossref | GoogleScholarGoogle Scholar | 27502382PubMed |
Murray MG, Illius AW (2000) Vegetation modification and resource competition in grazing ungulates. Oikos 89, 501–508.
| Vegetation modification and resource competition in grazing ungulates.Crossref | GoogleScholarGoogle Scholar |
Myers JA, Kitajima K (2007) Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. Journal of Ecology 95, 383–395.
| Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest.Crossref | GoogleScholarGoogle Scholar |
N’Dri AB, Gignoux J, Barot S, Konaté S, Dembélé A, Werner PA (2014) The dynamics of hollowing in annually burnt savanna trees and its effect on adult tree mortality. Plant Ecology 215, 27–37.
| The dynamics of hollowing in annually burnt savanna trees and its effect on adult tree mortality.Crossref | GoogleScholarGoogle Scholar |
Niklas K (1995) Plant height and the properties of some herbaceous stems. Annals of Botany 75, 133–142.
| Plant height and the properties of some herbaceous stems.Crossref | GoogleScholarGoogle Scholar |
Nogueira A, Martinez CA, Ferreira LL, Prado CHBA (2004) Photosynthesis and water use efficiency in twenty tropical tree species of differing succession status in a Brazilian reforestation. Photosynthetica 42, 351–356.
| Photosynthesis and water use efficiency in twenty tropical tree species of differing succession status in a Brazilian reforestation.Crossref | GoogleScholarGoogle Scholar |
O’Brien MJ, Leuzinger S, Philipson CD, Tay J, Hector A (2014) Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nature Climate Change 4, 710–714.
| Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels.Crossref | GoogleScholarGoogle Scholar |
O’Connor TG, Bredenkamp GJ (1997) Grassland. In ‘Vegetation in South Africa’. (Eds RM Cowling, DM Richardson, SM Pierce) pp. 215–257. (Cambridge University Press: Cambridge, UK)
O’Connor TG, Goodman PS, Clegg B (2007) A functional hypothesis of the threat of local extirpation of woody plant species by elephant in Africa. Biological Conservation 136, 329–345.
| A functional hypothesis of the threat of local extirpation of woody plant species by elephant in Africa.Crossref | GoogleScholarGoogle Scholar |
O’Donnell FC, Caylor KK, Bhattachan A, Dintwe K, D’Odorico P, Okin GS (2015) A quantitative description of the interspecies diversity of belowground structure in savanna woody plants. Ecosphere 6, art154
| A quantitative description of the interspecies diversity of belowground structure in savanna woody plants.Crossref | GoogleScholarGoogle Scholar |
O’Reagain PJ, Mentis MT (1989) The effect of plant structure on the acceptability of different grass species to cattle. Journal of the Grassland Society of Southern Africa 6, 163–170.
| The effect of plant structure on the acceptability of different grass species to cattle.Crossref | GoogleScholarGoogle Scholar |
Ocheltree TW, Nippert JB, Prasad PVV (2016) A safety vs efficiency trade-off identified in the hydraulic pathway of grass leaves is decoupled from photosynthesis, stomatal conductance and precipitation. New Phytologist 210, 97–107.
| A safety vs efficiency trade-off identified in the hydraulic pathway of grass leaves is decoupled from photosynthesis, stomatal conductance and precipitation.Crossref | GoogleScholarGoogle Scholar | 26680276PubMed |
Oleszek WA, Hoagland RE, Zablotowicz RM (1999) Ecological significance of plant saponins. In ‘Principles and Practices in Plant Ecology: Allelochemical Interactions’. pp. 451–65. (CRC Press: New York, NY, USA)
Oliveras I, van der Eynden M, Malhi Y, Cahuana N, Menor C, Zamora F, Haugaasen T (2014) Grass allometry and estimation of above-ground biomass in tropical alpine tussock grasslands. Austral Ecology 39, 408–415.
| Grass allometry and estimation of above-ground biomass in tropical alpine tussock grasslands.Crossref | GoogleScholarGoogle Scholar |
Or K, Ward D (2003) Three-way interactions between Acacia, large mammalian herbivores and bruchid beetles: a review. African Journal of Ecology 41, 257–265.
| Three-way interactions between Acacia, large mammalian herbivores and bruchid beetles: a review.Crossref | GoogleScholarGoogle Scholar | 33237498PubMed |
Osborne CP, Charles-Dominique T, Stevens N, Bond WJ, Midgley G, Lehmann CE (2018) Human impacts in African savannas are mediated by plant functional traits. New Phytologist 220, 10–24.
| Human impacts in African savannas are mediated by plant functional traits.Crossref | GoogleScholarGoogle Scholar | 29806964PubMed |
Owen-Smith RN (1988) ‘Megaherbivores. The Influence of Very Large Body Size on Ecology.’ (Cambridge University Press: Cambridge, UK)
Padilla FM, Pugnaire FI (2007) Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Functional Ecology 21, 489–495.
| Rooting depth and soil moisture control Mediterranean woody seedling survival during drought.Crossref | GoogleScholarGoogle Scholar |
Palacio S, Hester AJ, Maestro M, Millard P (2008) Browsed Betula pubescens trees are not carbon-limited. Functional Ecology 22, 808–815.
| Browsed Betula pubescens trees are not carbon-limited.Crossref | GoogleScholarGoogle Scholar |
Pausas JG (1999) Response of functional types to changes in the fire regime in Mediterranean ecosystems: a simulation approach. Journal of Vegetation Science 10, 717–722.
| Response of functional types to changes in the fire regime in Mediterranean ecosystems: a simulation approach.Crossref | GoogleScholarGoogle Scholar |
Pausas JG (2017) Bark thickness and fire regime: another twist. New Phytologist 213, 13–15.
| Bark thickness and fire regime: another twist.Crossref | GoogleScholarGoogle Scholar | 27891644PubMed |
Pausas JG, Bond WJ (2020) Alternative biome states in terrestrial ecosystems. Trends in Plant Science 25, 250
| Alternative biome states in terrestrial ecosystems.Crossref | GoogleScholarGoogle Scholar | 31917105PubMed |
Pausas JG, Keeley JE (2017) Epicormic resprouting in fire-prone ecosystems. Trends in Plant Science 22, 1008–1015.
| Epicormic resprouting in fire-prone ecosystems.Crossref | GoogleScholarGoogle Scholar | 28927652PubMed |
Pausas JG, Keeley JE, Schwilk DW (2017) Flammability as an ecological and evolutionary driver. Journal of Ecology 105, 289–297.
| Flammability as an ecological and evolutionary driver.Crossref | GoogleScholarGoogle Scholar |
Pausas JG, Lamont BB, Paula S, Appezzato-da-Glória B, Fidelis A (2018) Unearthing belowground bud banks in fire-prone ecosystems. New Phytologist 217, 1435–1448.
| Unearthing belowground bud banks in fire-prone ecosystems.Crossref | GoogleScholarGoogle Scholar | 29334401PubMed |
Payne WW (1978) A glossary of plant hair terminology. Brittonia 30, 239
| A glossary of plant hair terminology.Crossref | GoogleScholarGoogle Scholar |
Pellegrini AFA, Anderegg WRL, Paine CET, Hoffmann WA, Kartzinel T, Rabin SS, Sheil D, Franco AC, Pacala SW (2017) Convergence of bark investment according to fire and climate structures ecosystem vulnerability to future change. Ecology Letters 20, 307–316.
| Convergence of bark investment according to fire and climate structures ecosystem vulnerability to future change.Crossref | GoogleScholarGoogle Scholar |
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, van der Heijden MGA, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61, 167–23.
| New handbook for standardised measurement of plant functional traits worldwide.Crossref | GoogleScholarGoogle Scholar |
Perreta M, Ramos J, Tivano JC, Vegetti A (2011) Descriptive characters of growth form in Poaceae: an overview. Flora – Morphology, Distribution, Functional Ecology of Plants 206, 283–293.
| Descriptive characters of growth form in Poaceae: an overview.Crossref | GoogleScholarGoogle Scholar |
Peterson CJ, Carson WP (1996) Generalizing forest regeneration models: the dependence of propagule availability on disturbance history and stand size. Canadian Journal of Forest Research 26, 45–52.
| Generalizing forest regeneration models: the dependence of propagule availability on disturbance history and stand size.Crossref | GoogleScholarGoogle Scholar |
Pichersky E, Raguso RA (2018) Why do plants produce so many terpenoid compounds? New Phytologist 220, 692–702.
| Why do plants produce so many terpenoid compounds?Crossref | GoogleScholarGoogle Scholar | 27604856PubMed |
Porter LJ, Hrstich LN, Chan BG (1985) The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25, 223–230.
| The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin.Crossref | GoogleScholarGoogle Scholar |
Poschlod P, Abedi M, Bartelheimer M, Drobnik J, Rosbakh S, Saat-kamp A (2013) Seed ecology and assembly rules in plant communities. In ‘Vegetation Ecology’, 2nd edn. (Eds van der Maarel E, J Franklin) pp. 164–202. (Wiley-Blackwell: Chichester, UK)
Pott C, McLoughlin S, Wu S, Friis EM (2012) Trichomes on the leaves of Anomozamites villosus sp. nov. (Bennettitales) from the Daohugou beds (Middle Jurassic), inner Mongolia, China: mechanical defence against herbivorous arthropods. Review of Palaeobotany and Palynology 169, 48–60.
| Trichomes on the leaves of Anomozamites villosus sp. nov. (Bennettitales) from the Daohugou beds (Middle Jurassic), inner Mongolia, China: mechanical defence against herbivorous arthropods.Crossref | GoogleScholarGoogle Scholar |
Price ML, Butler LG (1977) Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. Journal of Agricultural and Food Chemistry 25, 1268–1273.
| Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain.Crossref | GoogleScholarGoogle Scholar |
Price ML, Van Scoyoc S, Butler LG (1978) A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. Journal of Agricultural and Food Chemistry 26, 1214–1218.
| A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain.Crossref | GoogleScholarGoogle Scholar |
Pullin AS, Gilbert JE (1989) The stinging nettle, Urtica dioica, increases trichome density after herbivore and mechanical damage. Oikos 54, 275
| The stinging nettle, Urtica dioica, increases trichome density after herbivore and mechanical damage.Crossref | GoogleScholarGoogle Scholar |
Qian J, Wang Z, Liu Z, Busso CA (2017) Belowground bud bank responses to grazing intensity in the inner-Mongolia steppe, China. Land Degradation & Development 28, 822–832.
| Belowground bud bank responses to grazing intensity in the inner-Mongolia steppe, China.Crossref | GoogleScholarGoogle Scholar |
Quentin AG, Pinkard EA, Ryan MG, Tissue DT, Baggett LS, Adams HD, Maillard P, Marchand J, Landhäusser SM, Lacointe A, Gibon Y 2015 Non-structural carbohydrates in woody plants compared among laboratories.Tree Physiology35 11461165
Quinn JA, Mowrey DP, Emanuele SM, Whalley RDB (1994) The ‘foliage is the fruit’ hypothesis: Buchloe dactyloides (Poaceae) and the shortgrass prairie of North America. American Journal of Botany 81, 1545–1554.
| The ‘foliage is the fruit’ hypothesis: Buchloe dactyloides (Poaceae) and the shortgrass prairie of North America.Crossref | GoogleScholarGoogle Scholar |
Rahman MM, Garvey M, Piddock LJV, Gibbons S (2008) Antibacterial terpenes from the oleo-resin of Commiphora molmol (Engl.). Phytotherapy Research 22, 1356–1360.
| Antibacterial terpenes from the oleo-resin of Commiphora molmol (Engl.).Crossref | GoogleScholarGoogle Scholar | 18570217PubMed |
Ratnam J, Bond WJ, Fensham RJ, Hoffmann WA, Archibald S, Lehmann CER, Anderson MT, Higgins SI, Sankaran M (2011) When is a ‘forest’ a savanna, and why does it matter? Global Ecology and Biogeography 20, 653–660.
| When is a ‘forest’ a savanna, and why does it matter?Crossref | GoogleScholarGoogle Scholar |
Ratnam J, Tomlinson KW, Rasquinha DN, Sankaran M (2016) Savannahs of Asia: antiquity, biogeography, and an uncertain future. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 371, 20150305
| Savannahs of Asia: antiquity, biogeography, and an uncertain future.Crossref | GoogleScholarGoogle Scholar | 27502371PubMed |
Richards LA, Dyer LA, Forister ML, Smilanich AM, Dodson CD, Leonard MD, Jeffrey CS (2015) Phytochemical diversity drives plant–insect community diversity. Proceedings of the National Academy of Sciences of the United States of America 112, 10973–10978.
| Phytochemical diversity drives plant–insect community diversity.Crossref | GoogleScholarGoogle Scholar | 26283384PubMed |
Ridley HN (1930). ‘The Dispersal of Plants throughout the World.’ (L. Reeve & Company, Limited)
Ripley B, Visser V, Christin P-A, Archibald S, Martin T, Osborne C (2015) Fire ecology of C3 and C4 grasses depends on evolutionary history and frequency of burning but not photosynthetic type. Ecology 96, 2679–2691.
| Fire ecology of C3 and C4 grasses depends on evolutionary history and frequency of burning but not photosynthetic type.Crossref | GoogleScholarGoogle Scholar | 26649389PubMed |
Robbins C (1994) ‘Wildlife Feeding and Nutrition.’ (Elsevier: Cambridge, MA, USA)
Röder E (1999) Analysis of pyrrolizidine alkaloids. Current Organic Chemistry 3, 32
Ronquim CC, Prado CHBA, Paula NF (2003) Growth and photosynthetic capacity in two woody species of Cerrado vegetation under different radiation availability. Brazilian Archives of Biology and Technology 46, 243–252.
| Growth and photosynthetic capacity in two woody species of Cerrado vegetation under different radiation availability.Crossref | GoogleScholarGoogle Scholar |
Rooke T, Danell K, Bergström R, Skarpe C, Hjältén J (2004) Defensive traits of savanna trees: the role of shoot exposure to browsers. Oikos 107, 161–171.
| Defensive traits of savanna trees: the role of shoot exposure to browsers.Crossref | GoogleScholarGoogle Scholar |
Rosell JA (2016) Bark thickness across the angiosperms: more than just fire. New Phytologist 211, 90–102.
| Bark thickness across the angiosperms: more than just fire.Crossref | GoogleScholarGoogle Scholar | 26890029PubMed |
Rosell JA (2019) Bark in woody plants: understanding the diversity of a multifunctional structure. Integrative and Comparative Biology 59, 535–547.
| Bark in woody plants: understanding the diversity of a multifunctional structure.Crossref | GoogleScholarGoogle Scholar | 31120526PubMed |
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 529
| ImageJ2: ImageJ for the next generation of scientific image data.Crossref | GoogleScholarGoogle Scholar | 29187165PubMed |
Rusch GM, Pausas JG, Lepš J (2003) Plant functional types in relation to disturbance and land use: an introduction. Journal of Vegetation Science 14, 307–310.
| Plant functional types in relation to disturbance and land use: an introduction.Crossref | GoogleScholarGoogle Scholar |
Saari SK, Campbell CD, Russell J, Alexander IJ, Anderson IC (2005) Pine microsatellite markers allow roots and ectomycorrhizas to be linked to individual trees. New Phytologist 165, 295–304.
| Pine microsatellite markers allow roots and ectomycorrhizas to be linked to individual trees.Crossref | GoogleScholarGoogle Scholar | 15720641PubMed |
Sage RF, Kubien DS (2003) Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants. Photosynthesis Research 77, 209–225.
| Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants.Crossref | GoogleScholarGoogle Scholar | 16228377PubMed |
Sala A, Woodruff DR, Meinzer FC (2012) Carbon dynamics in trees: feast or famine? Tree Physiology 32, 764–775.
| Carbon dynamics in trees: feast or famine?Crossref | GoogleScholarGoogle Scholar | 22302370PubMed |
Salazar D, Jaramillo A, Marquis RJ (2016) The impact of plant chemical diversity on plant–herbivore interactions at the community level. Oecologia 181, 1199–1208.
| The impact of plant chemical diversity on plant–herbivore interactions at the community level.Crossref | GoogleScholarGoogle Scholar | 27129320PubMed |
Sankaran M, Hanan NP, Scholes RJ, Ratnam J, Augustine DJ, Cade BS, Gignoux J, Higgins SI, Le Roux X, Ludwig F, Ardo J 2005 Determinants of woody cover in African savannas.Nature438 846849
Sankaran M, Ratnam J, Hanan N (2008) Woody cover in African savannas: the role of resources, fire and herbivory. Global Ecology and Biogeography 17, 236–245.
| Woody cover in African savannas: the role of resources, fire and herbivory.Crossref | GoogleScholarGoogle Scholar |
Sankaran M, Augustine DJ, Ratnam J (2013) Native ungulates of diverse body sizes collectively regulate long-term woody plant demography and structure of a semi-arid savanna. Journal of Ecology 101, 1389–1399.
| Native ungulates of diverse body sizes collectively regulate long-term woody plant demography and structure of a semi-arid savanna.Crossref | GoogleScholarGoogle Scholar |
Scarff FR, Westoby M (2008) The influence of tissue phosphate on plant flammability: a kinetic study. Polymer Degradation & Stability 93, 1930–1934.
| The influence of tissue phosphate on plant flammability: a kinetic study.Crossref | GoogleScholarGoogle Scholar |
Schenk HJ, Jackson RB (2002) Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. Journal of Ecology 90, 480–494.
| Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems.Crossref | GoogleScholarGoogle Scholar |
Scholes RJ (1997) ‘Savannas in Vegetation of Southern Africa.’ (Eds RM Cowling, DM Richardson, SM Pierce) pp. 258–277. (Cambridge University Press: Cambridge, UK)
Scholes RJ, Archer SR (1997) Tree–grass interactions in savannas. Annual Review of Ecology and Systematics 28, 517–544.
| Tree–grass interactions in savannas.Crossref | GoogleScholarGoogle Scholar |
Scholes RJ, Walker BH (2004) ‘An African Savanna.’ (Cambridge University Press: Cambridge, UK)
Schutz AEN, Bond WJ, Cramer MD (2009) Juggling carbon: allocation patterns of a dominant tree in a fire-prone savanna. Oecologia 160, 235–246.
| Juggling carbon: allocation patterns of a dominant tree in a fire-prone savanna.Crossref | GoogleScholarGoogle Scholar |
Schutz AEN, Bond WJ, Cramer MD (2011) Defoliation depletes the carbohydrate reserves of resprouting Acacia saplings in an African savanna. Plant Ecology 212, 2047–2055.
| Defoliation depletes the carbohydrate reserves of resprouting Acacia saplings in an African savanna.Crossref | GoogleScholarGoogle Scholar |
Schwilk DW (2015) Dimensions of plant flammability. New Phytologist 206, 486–488.
| Dimensions of plant flammability.Crossref | GoogleScholarGoogle Scholar | 25800615PubMed |
Scogings PF, Mopipi K (2008) Effects of water, grass and N on responses of Acacia karroo seedlings to early wet season simulated browsing: aboveground growth and biomass allocation. Journal of Arid Environments 72, 509–522.
| Effects of water, grass and N on responses of Acacia karroo seedlings to early wet season simulated browsing: aboveground growth and biomass allocation.Crossref | GoogleScholarGoogle Scholar |
Scogings PF, Dziba LE, Gordon IJ (2004) Leaf chemistry of woody plants in relation to season, canopy retention and goat browsing in a semiarid subtropical savanna. Austral Ecology 29, 278–286.
| Leaf chemistry of woody plants in relation to season, canopy retention and goat browsing in a semiarid subtropical savanna.Crossref | GoogleScholarGoogle Scholar |
Scogings PF, Hjältén J, Skarpe C (2011) Secondary metabolites and nutrients of woody plants in relation to browsing intensity in African savannas. Oecologia 167, 1063–1073.
| Secondary metabolites and nutrients of woody plants in relation to browsing intensity in African savannas.Crossref | GoogleScholarGoogle Scholar | 21660581PubMed |
Scogings PF, Hjältén J, Skarpe C (2013) Does large herbivore removal affect secondary metabolites, nutrients and shoot length in woody species in semi-arid savannas? Journal of Arid Environments 88, 4–8.
| Does large herbivore removal affect secondary metabolites, nutrients and shoot length in woody species in semi-arid savannas?Crossref | GoogleScholarGoogle Scholar |
Scogings PF, Hjältén J, Skarpe C, Hattas D, Zobolo A, Dziba L, Rooke T (2014) Nutrient and secondary metabolite concentrations in a savanna are independently affected by large herbivores and shoot growth rate. Plant Ecology 215, 73–82.
| Nutrient and secondary metabolite concentrations in a savanna are independently affected by large herbivores and shoot growth rate.Crossref | GoogleScholarGoogle Scholar |
Scogings PF, Mamashela TC, Zobolo AM (2017) Growth, nitrogen and tannins of semi-arid savanna saplings in response to herbivory and water. Journal of Arid Environments 137, 21–29.
| Growth, nitrogen and tannins of semi-arid savanna saplings in response to herbivory and water.Crossref | GoogleScholarGoogle Scholar |
Siebert F, Dreber N (2019) Forb ecology research in dry African savannas: knowledge, gaps, and future perspectives. Ecology and Evolution 9, 7875–7891.
| Forb ecology research in dry African savannas: knowledge, gaps, and future perspectives.Crossref | GoogleScholarGoogle Scholar | 31346447PubMed |
Siebert F, Klem J, Van Coller H (2020) Forb community responses to an extensive drought in two contrasting land-use types of a semi-arid Lowveld savanna. African Journal of Range & Forage Science 37, 53–64.
| Forb community responses to an extensive drought in two contrasting land-use types of a semi-arid Lowveld savanna.Crossref | GoogleScholarGoogle Scholar |
Simioni G, Le Roux X, Gignoux J, Walcroft AS (2004) Leaf gas exchange characteristics and water- and nitrogen-use efficiencies of dominant grass and tree species in a West African savanna. Plant Ecology 173, 233–246.
| Leaf gas exchange characteristics and water- and nitrogen-use efficiencies of dominant grass and tree species in a West African savanna.Crossref | GoogleScholarGoogle Scholar |
Simpson KJ, Ripley BS, Christin P-A, Belcher CM, Lehmann CER, Thomas GH, Osborne CP (2016) Determinants of flammability in savanna grass species. Journal of Ecology 104, 138–148.
| Determinants of flammability in savanna grass species.Crossref | GoogleScholarGoogle Scholar | 26877549PubMed |
Sletvold N, Huttunen P, Handley R, Kärkkäinen K, Ågren J (2010) Cost of trichome production and resistance to a specialist insect herbivore in Arabidopsis lyrata. Evolutionary Ecology 24, 1307–1319.
| Cost of trichome production and resistance to a specialist insect herbivore in Arabidopsis lyrata.Crossref | GoogleScholarGoogle Scholar |
Smit IPJ, Asner GP, Govender N, Kennedy-Bowdoin T, Knapp DE, Jacobson J (2010) Effects of fire on woody vegetation structure in African savanna. Ecological Applications 20, 1865–1875.
| Effects of fire on woody vegetation structure in African savanna.Crossref | GoogleScholarGoogle Scholar |
Solofondranohatra CL, Vorontsova MS, Hempson GP, Hackel J, Cable S, Vololoniaina J, Lehmann CER (2020) Fire and grazing determined grasslands of central Madagascar represent ancient assemblages. Proceedings of the Royal Society of London – B., Biological Sciences 287, 20200598
| Fire and grazing determined grasslands of central Madagascar represent ancient assemblages.Crossref | GoogleScholarGoogle Scholar |
Solofondranohatra CL, Vorontsova MS, Dewhirst RA, Belcher CM, Cable S, Jeannoda V, Lehmann CER Shade alters grass growth and architecture by reducing root biomass. Biotropica.
Stamp NE (1984) Self-burial behaviour of erodium cicutarium seeds. Journal of Ecology 72, 611–620.
| Self-burial behaviour of erodium cicutarium seeds.Crossref | GoogleScholarGoogle Scholar |
Staver AC, Bond WJ (2014) Is there a ‘browse trap’? Dynamics of herbivore impacts on trees and grasses in an African savanna. Journal of Ecology 102, 595–602.
| Is there a ‘browse trap’? Dynamics of herbivore impacts on trees and grasses in an African savanna.Crossref | GoogleScholarGoogle Scholar |
Staver AC, Bond WJ, Stock WD, van Rensburg SJ, Waldram MS (2009) Browsing and fire interact to suppress tree density in an African savanna. Ecological Applications 19, 1909–1919.
| Browsing and fire interact to suppress tree density in an African savanna.Crossref | GoogleScholarGoogle Scholar | 19831079PubMed |
Staver AC, Archibald S, Levin SA (2011) The global extent and determinants of savanna and forest as alternative biome states. Science 334, 230–232.
| The global extent and determinants of savanna and forest as alternative biome states.Crossref | GoogleScholarGoogle Scholar | 21998389PubMed |
Staver AC, Bond WJ, Cramer MD, Wakeling JL (2012) Top-down determinants of niche structure and adaptation among African Acacias. Ecology Letters 15, 673–679.
| Top-down determinants of niche structure and adaptation among African Acacias.Crossref | GoogleScholarGoogle Scholar | 22507561PubMed |
Steffen WL (1996) A periodic table for ecology? A chemist’s view of plant functional types. Journal of Vegetation Science 7, 425–430.
| A periodic table for ecology? A chemist’s view of plant functional types.Crossref | GoogleScholarGoogle Scholar |
Stevens N, Seal CE, Archibald S, Bond W (2014) Increasing temperatures can improve seedling establishment in arid-adapted savanna trees. Oecologia 175, 1029–1040.
| Increasing temperatures can improve seedling establishment in arid-adapted savanna trees.Crossref | GoogleScholarGoogle Scholar | 24805202PubMed |
Stock WD, Le Roux D, Van der Heyden F (1993) Regrowth and tannin production in woody and succulent karoo shrubs in response to simulated browsing. Oecologia 96, 562–568.
| Regrowth and tannin production in woody and succulent karoo shrubs in response to simulated browsing.Crossref | GoogleScholarGoogle Scholar | 28312463PubMed |
Stuart-Hill GC, Mentis MT (1982) Coevolution of African grasses and large herbivores. Proceedings of the Annual Congresses of the Grassland Society of Southern Africa 17, 122–128.
| Coevolution of African grasses and large herbivores.Crossref | GoogleScholarGoogle Scholar |
Su S-L, Duan J-A, Tang Y-P, Zhang X, Yu L, Jiang F-R, Zhou W, Luo D, Ding A-W (2009) Isolation and biological activities of neomyrrhaol and other terpenes from the resin of Commiphora myrrha. Planta Medica 75, 351–355.
| Isolation and biological activities of neomyrrhaol and other terpenes from the resin of Commiphora myrrha.Crossref | GoogleScholarGoogle Scholar | 19101885PubMed |
Sütterlin S, van Lenteren JC (1997) Influence of hairiness of Gerbera jamesonii leaves on the searching efficiency of the parasitoid Encarsia formosa. Biological Control 9, 157–165.
| Influence of hairiness of Gerbera jamesonii leaves on the searching efficiency of the parasitoid Encarsia formosa.Crossref | GoogleScholarGoogle Scholar |
Taiz L, Zeiger E (2006) ‘Plant Physiology.’ (Sinauer: Sunderland, MA, USA)
Taylor SH, Hulme SP, Rees M, Ripley BS, Ian Woodward F, Osborne CP (2010) Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment. New Phytologist 185, 780–791.
| Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment.Crossref | GoogleScholarGoogle Scholar | 20002318PubMed |
Tinoco-Ojanguren C, Pearcy RW (1995) A comparison of light quality and quantity effects on the growth and steady-state and dynamic photosynthetic characteristics of three tropical tree species. Functional Ecology 9, 222
| A comparison of light quality and quantity effects on the growth and steady-state and dynamic photosynthetic characteristics of three tropical tree species.Crossref | GoogleScholarGoogle Scholar |
Tomlinson PB, Jeffrey EC (1990) ‘The Structural Biology of Palms.’ (Clarendon Press: Oxford, UK)
Trollope WSW (1984) Fire in savanna. In ‘Ecological Studies’. (Eds PV Booysen, NM Tainton) pp. 149–175. (Springer: Berlin, Germany)
Tucker SS, Craine JM, Nippert JB (2011) Physiological drought tolerance and the structuring of tallgrass prairie assemblages. Ecosphere 2, art48
| Physiological drought tolerance and the structuring of tallgrass prairie assemblages.Crossref | GoogleScholarGoogle Scholar |
Valladares F, Niinemets à (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology, Evolution, and Systematics 39, 237–257.
| Shade tolerance, a key plant feature of complex nature and consequences.Crossref | GoogleScholarGoogle Scholar |
Valverde PL, Fornoni J, Nunez-Farfan J (2001) Defensive role of leaf trichomes in resistance to herbivorous insects in Datura stramonium. Journal of Evolutionary Biology 14, 424–432.
| Defensive role of leaf trichomes in resistance to herbivorous insects in Datura stramonium.Crossref | GoogleScholarGoogle Scholar |
van der Pijl L (1982) ‘Principles of Dispersal in Higher Plants.’ (Springer-Verlag: Berlin, Germany)
Varanasi A, Prasad PVV, Jugulam M (2016) Impact of climate change factors on weeds and herbicide efficacy. In ‘Advances in Agronomy’. pp. 107–146. (Elsevier)
Veldhuis MP, Howison RA, Fokkema RW, Tielens E, Olff H (2014) A novel mechanism for grazing lawn formation: large herbivore-induced modification of the plant-soil water balance. Journal of Ecology 102, 1506–1517.
| A novel mechanism for grazing lawn formation: large herbivore-induced modification of the plant-soil water balance.Crossref | GoogleScholarGoogle Scholar |
Vermeulen WJ, Geldenhuys CJ, Esler KJ (2012) Response of Ocotea bullata, Curtisia dentata and Rapanea melanophloeos to medicinal bark stripping in the southern Cape, South Africa: implications for sustainable use. Southern Forests 74, 183–193.
| Response of Ocotea bullata, Curtisia dentata and Rapanea melanophloeos to medicinal bark stripping in the southern Cape, South Africa: implications for sustainable use.Crossref | GoogleScholarGoogle Scholar |
Vesk PA (2006) Plant size and resprouting ability: trading tolerance and avoidance of damage? Journal of Ecology 94, 1027–1034.
| Plant size and resprouting ability: trading tolerance and avoidance of damage?Crossref | GoogleScholarGoogle Scholar |
Vesk PA, Westoby M (2004b) Sprouting ability across diverse disturbances and vegetation types worldwide. Journal of Ecology 92, 310–320.
| Sprouting ability across diverse disturbances and vegetation types worldwide.Crossref | GoogleScholarGoogle Scholar |
Vetter J (2000) Plant cyanogenic glycosides. Toxicon 38, 11–36.
| Plant cyanogenic glycosides.Crossref | GoogleScholarGoogle Scholar | 10669009PubMed |
Violle C, Navas M-L, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116, 882–892.
| Let the concept of trait be functional!Crossref | GoogleScholarGoogle Scholar |
Wakeel A, Farooq M, Qadir M, Schubert S (2011) Potassium substitution by sodium in plants. Critical Reviews in Plant Sciences 30, 401–413.
| Potassium substitution by sodium in plants.Crossref | GoogleScholarGoogle Scholar |
Wakeling JL, Staver AC, Bond WJ (2011) Simply the best: the transition of savanna saplings to trees. Oikos 120, 1448–1451.
| Simply the best: the transition of savanna saplings to trees.Crossref | GoogleScholarGoogle Scholar |
Walker BH, Ludwig D, Holling CS, Peterman RM (1981) Stability of semi-arid savanna grazing systems. Journal of Ecology 69, 473–498.
| Stability of semi-arid savanna grazing systems.Crossref | GoogleScholarGoogle Scholar |
Walters MB, Reich PB (2000) Trade-offs in low-light CO2 exchange: a component of variation in shade tolerance among cold temperate tree seedlings. Functional Ecology 14, 155–165.
| Trade-offs in low-light CO2 exchange: a component of variation in shade tolerance among cold temperate tree seedlings.Crossref | GoogleScholarGoogle Scholar |
Wang R, Yu G, He N, Wang Q, Xia F, Zhao N, Xu Z, Ge J (2014) Elevation-related variation in leaf stomatal traits as a function of plant functional type: evidence from Changbai Mountain, China. PLoS One 9, e115395
| Elevation-related variation in leaf stomatal traits as a function of plant functional type: evidence from Changbai Mountain, China.Crossref | GoogleScholarGoogle Scholar | 25531884PubMed |
Wang R, Yu G, He N, Wang Q, Zhao N, Xu Z, Ge J (2015) Latitudinal variation of leaf stomatal traits from species to community level in forests: linkage with ecosystem productivity. Scientific Reports 5, 14454
| Latitudinal variation of leaf stomatal traits from species to community level in forests: linkage with ecosystem productivity.Crossref | GoogleScholarGoogle Scholar | 26403303PubMed |
Werker E (2000) Trichome diversity and development. In ‘Plant Trichomes’. (Eds DL Hallahan, JC Gray) pp. 1–35. (Academic Press: New York, NY, USA)
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33, 125–159.
| Plant ecological strategies: some leading dimensions of variation between species.Crossref | GoogleScholarGoogle Scholar |
White RH, Zipperer WC (2010) Testing and classification of individual plants for fire behaviour: plant selection for the wildland–urban interface. International Journal of Wildland Fire 19, 213–227.
| Testing and classification of individual plants for fire behaviour: plant selection for the wildland–urban interface.Crossref | GoogleScholarGoogle Scholar |
Wigley BJ, Bond WJ, Hoffman MT (2009a) Bush encroachment under three contrasting land-use practices in a mesic South African savanna. African Journal of Ecology 47, 62–70.
| Bush encroachment under three contrasting land-use practices in a mesic South African savanna.Crossref | GoogleScholarGoogle Scholar |
Wigley BJ, Cramer MD, Bond WJ (2009b) Sapling survival in a frequently burnt savanna: mobilisation of carbon reserves in Acacia karroo. Plant Ecology 203, 1–11.
| Sapling survival in a frequently burnt savanna: mobilisation of carbon reserves in Acacia karroo.Crossref | GoogleScholarGoogle Scholar |
Wigley BJ, Fritz H, Coetsee C, Bond WJ (2014) Herbivores shape woody plant communities in the Kruger National Park: lessons from three long-term exclosures. Koedoe 56, a1165
| Herbivores shape woody plant communities in the Kruger National Park: lessons from three long-term exclosures.Crossref | GoogleScholarGoogle Scholar |
Wigley BJ, Bond WJ, Fritz H, Coetsee C (2015) Mammal browsers and rainfall affect Acacia leaf nutrient content, defense, and growth in South African savannas. Biotropica 47, 190–200.
| Mammal browsers and rainfall affect Acacia leaf nutrient content, defense, and growth in South African savannas.Crossref | GoogleScholarGoogle Scholar |
Wigley BJ, Fritz H, Coetsee C (2018) Defence strategies in African savanna trees. Oecologia 187, 797–809.
| Defence strategies in African savanna trees.Crossref | GoogleScholarGoogle Scholar | 29754291PubMed |
Wigley BJ, Staver AC, Zytkowiak R, Jagodzinski AM, Wigley-Coetsee C (2019a) Root trait variation in African savannas. Plant and Soil 441, 555–565.
| Root trait variation in African savannas.Crossref | GoogleScholarGoogle Scholar |
Wigley BJ, Coetsee C, Kruger LM, Ratnam J, Sankaran M (2019b) Ants, fire, and bark traits affect how African savanna trees recover following damage. Biotropica 51, 682–691.
| Ants, fire, and bark traits affect how African savanna trees recover following damage.Crossref | GoogleScholarGoogle Scholar |
Wigley BJ, Coetsee C, Augustine DJ, Ratnam J, Hattas D, Sankaran M (2019c) A thorny issue: woody plant defence and growth in an East African savanna. Journal of Ecology 107, 1839–1851.
| A thorny issue: woody plant defence and growth in an East African savanna.Crossref | GoogleScholarGoogle Scholar |
Wiley E, Rogers BJ, Hodgkinson R, Landhausser SM (2016) Nonstructural carbohydrate dynamics of lodgepole pine dying from mountain pine beetle attack. New Phytologist 209, 550–562.
| Nonstructural carbohydrate dynamics of lodgepole pine dying from mountain pine beetle attack.Crossref | GoogleScholarGoogle Scholar | 26256444PubMed |
Williams RJ, Gill AM, Moore PHR (1998) Seasonal changes in fire behaviour in a tropical savanna in northern Australia International Journal of Wildland Fire 8, 227–239.
| Seasonal changes in fire behaviour in a tropical savanna in northern AustraliaCrossref | GoogleScholarGoogle Scholar |
Williams VL, Balkwill K, Witkowski ETF (2007) Stem diameter and bark surface area of the fluted trunk of Balanites maughamii (Balanitaceae). Bothalia 37, 211–214.
| Stem diameter and bark surface area of the fluted trunk of Balanites maughamii (Balanitaceae).Crossref | GoogleScholarGoogle Scholar |
Willson MF, Irvine AK, Walsh NG (1989) Vertebrate dispersal syndromes in some Australian and New Zealand plant communities, with geographic comparisons. Biotropica 21, 133
| Vertebrate dispersal syndromes in some Australian and New Zealand plant communities, with geographic comparisons.Crossref | GoogleScholarGoogle Scholar |
Willson MF, Rice BL, Westoby M (1990) Seed dispersal spectra: a comparison of temperate plant communities. Journal of Vegetation Science 1, 547–562.
| Seed dispersal spectra: a comparison of temperate plant communities.Crossref | GoogleScholarGoogle Scholar |
Wu H, Guo J, Chen S, Liu X, Zhou Y, Zhang X, Xu X (2013) Recent developments in qualitative and quantitative analysis of phytochemical constituents and their metabolites using liquid chromatography–mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis 72, 267–291.
| Recent developments in qualitative and quantitative analysis of phytochemical constituents and their metabolites using liquid chromatography–mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 23031576PubMed |
Yeaton RI (1988) Porcupines, fires and the dynamics of the tree layer of the Burkea Africana savanna. Journal of Ecology 76, 1017
| Porcupines, fires and the dynamics of the tree layer of the Burkea Africana savanna.Crossref | GoogleScholarGoogle Scholar |
Zagrobelny M, Bak S, Rasmussen AV, Jørgensen B, Naumann CM, Møller BL (2004) Cyanogenic glucosides and plant–insect interactions. Phytochemistry 65, 293–306.
| Cyanogenic glucosides and plant–insect interactions.Crossref | GoogleScholarGoogle Scholar | 14751300PubMed |
Zaloumis NP, Bond WJ (2011) Grassland restoration after afforestation: no direction home? Austral Ecology 36, 357–366.
| Grassland restoration after afforestation: no direction home?Crossref | GoogleScholarGoogle Scholar |
Zaloumis NP, Bond WJ (2016) Reforestation or conservation? The attributes of old growth grasslands in South Africa. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 371, 20150310
| Reforestation or conservation? The attributes of old growth grasslands in South Africa.Crossref | GoogleScholarGoogle Scholar | 27502375PubMed |
Zhou Y, Wigley BJ, Case MF, Coetsee C, Staver AC (2020) Rooting depth as a key woody functional trait in savannas. New Phytologist 227, 1350–1361.
| Rooting depth as a key woody functional trait in savannas.Crossref | GoogleScholarGoogle Scholar | 32306404PubMed |
Zotz G, Winter K (1994) Photosynthesis of a tropical canopy tree, Ceiba pentandra, in a lowland forest in Panama. Tree Physiology 14, 1291–1301.
| Photosynthesis of a tropical canopy tree, Ceiba pentandra, in a lowland forest in Panama.Crossref | GoogleScholarGoogle Scholar | 14967618PubMed |
Zucker WV (1983) Tannins: does structure determine function? An ecological perspective. American Naturalist 121, 335–365.
| Tannins: does structure determine function? An ecological perspective.Crossref | GoogleScholarGoogle Scholar |