Multivariate drivers of diversity in temperate Australian native grasslands
Jodi N. Price A G , Megan K. Good B , Nick L. Schultz C , Lydia K. Guja D E and John W. Morgan FA Institute of Land, Water and Society, Charles Sturt University, Albury, NSW 2640, Australia.
B School of BioSciences, The University of Melbourne, Vic. 3010, Australia.
C School of Health and Life Sciences, Federation University Australia, Ballarat, Vic. 3350, Australia.
D National Seed Bank, Biodiversity Science Section, Australian National Botanic Gardens, Canberra, ACT 2601, Australia.
E Centre for Australian National Biodiversity Research, CSIRO, Canberra, ACT 2601, Australia.
F Department of Ecology, Environment and Evolution, La Trobe University, Bundoora, Vic. 3083, Australia.
G Corresponding author. Email: joprice@csu.edu.au
Australian Journal of Botany 67(5) 367-380 https://doi.org/10.1071/BT18190
Submitted: 4 October 2018 Accepted: 9 August 2019 Published: 18 September 2019
Abstract
Disturbance has been considered essential for maintaining biodiversity in temperate grassy ecosystems in Australia. This has been particularly well demonstrated for inter-tussock plant species in C4 Themeda-dominated grasslands in mesic environments. Disturbance is also thought crucial to maintain the structure of preferred habitat for some animals. Relationships between disturbance and diversity may be contingent on ecosystem productivity, but little is known about the generality of the disturbance-promoting-diversity paradigm across the range of temperate grasslands. To date, the disturbance-promoting-diversity paradigm has taken a univariate approach to the drivers of biodiversity; rainfall is seen as a key driver of productivity, which then drives diversity, mediated by disturbance. We argue that this framework is too simplistic as biodiversity drivers are multivariate. We suggest that the accumulation of phytomass (live and dead plant material) is an important determinant of diversity in grassy ecosystems and that phytomass accumulation is governed by multiple drivers (of which disturbance is just one). For fauna, it is structure – not biomass – that determines habitat suitability, and this can be moderated by both abiotic and biotic drivers. The assumption that there is a consistent effect of disturbance on diversity through the range of temperate grassland settings in southern Australia ignores the likelihood that biodiversity also responds to other factors such as spatial heterogeneity in the environment, resource availability and climatic variation. We developed a conceptual model of the multivariate drivers of grassland diversity that explores mechanisms underpinning patterns of species richness. Despite four decades of research, it is clear that our understanding of the multivariate drivers of diversity across the range of temperate grasslands in Australia is still incomplete. Further research into the conditions under which disturbance is required to maintain biodiversity in grasslands is integral to conservation planning in these endangered systems.
Additional keywords: beta diversity, biomass, disturbance, fire, grazing, regeneration, resources, spatial scale.
Introduction
Disturbance theory has served as the primary framework for understanding species coexistence in temperate grasslands in Australia (Lunt and Morgan 2002; Prober et al. 2013) and elsewhere (e.g. Grime 1973; Huston 1979; Collins and Steinauer 1998; Fynn et al. 2004). In highly productive environments, large dominant species (usually grasses) can monopolise resources (such as light) and competitively exclude other species (Grime 1973; Grace 1999; Harpole et al. 2016), as well as regulate many ecosystem functions including nutrient cycling, population dynamics and animal habitat (Knapp and Seastedt 1986; Lunt 1997a; Morgan and Lunt 1999). Disturbances such as fire can promote species diversity by constraining biomass and hence, the competitive effects of dominant species. In unproductive environments, plants tend to recover more slowly after disturbance, leading to slower rates of competitive exclusion. Hence, disturbance-promoting-diversity is thought to be contingent on productivity. Rates of biomass accumulation are dictated by the rates of primary productivity and decomposition, both of which are regulated by climate (Knapp and Smith 2001; O’Halloran et al. 2013) and, at smaller scales, by soil fertility (Harpole et al. 2016). Hence, productivity gradients controlled by climate and edaphic factors are likely to regulate the degree to which disturbance is necessary in grasslands to maintain diversity and ecosystem function.
The temperate grasslands of southern Australia are among the nation’s most well studied ecosystems (see synthesis by Williams et al. 2015) and biomass accumulation is known to be rapid in some parts of the range (McDougall 1989; Morgan and Lunt 1999; Prober et al. 2007; Schultz et al. 2011). As a consequence, moderate to high-frequency fire regimes typically promote plant species coexistence in productive grasslands (Morgan 1999), whereas infrequently-burned grasslands have lower plant species density (Stuwe and Parsons 1977). Such findings underpin management recommendations that have promoted frequent burning as necessary to maintain plant biodiversity, particularly in those sites that have a long-history of fire. Indeed, Williams et al. (2006) found that local extinction of plants was highest in native grasslands where burning regimes were relaxed, an outcome also observed in tallgrass prairie (Leach and Givnish 1996).
Prior to European colonisation, Australian temperate grasslands were burnt by Indigenous peoples for hunting and to promote food plants (Gott 2005). The details of the pre-European fire regime (i.e. frequency, intensity) are largely unknown in southern Australia due to the history of colonial invasion which meant Indigenous people had few opportunities to remain on their country and maintain traditional practices (Neale et al. 2019). It is generally thought that lowland grassland distributions are mostly driven by bottom-up processes (resource-constrained) rather than top-down (disturbance-driven) (Morgan et al. 2017). However, it is likely fire played a role in promoting plant diversity in these ecosystems. The disturbance created from Indigenous people collecting roots of food plants is likely to have also influenced the dynamics of grasslands (Gott 2005). Changes in disturbance regimes following European colonisation had dramatic and rapid effects – the removal of fire and introduction of livestock grazing resulted in the local extinction of grazing-sensitive and fire-dependent species. Additionally, grasslands have been heavily cleared and degraded by agricultural and urban development (McDougall and Kirkpatrick 1993), and all temperate grasslands in southern Australia are now endangered or critically endangered (Morgan et al. 2017).
A common assumption is that native grasslands require frequent disturbance to remove accumulated biomass (i.e. live + dead plant material = phytomass) in order to maximise local species diversity. In the absence of disturbance, perennial grass phytomass accumulates as annual leaf production by the dominant grass adds to the previous year’s litter which is slow to decompose. The diverse array of species occupying the inter-tussock spaces decline because of the direct relationship between phytomass, light availability and plant competitive interactions (Grace 1999). In temperate grasslands in south-eastern Australia, kangaroo grass (Themeda triandra) tussocks create a thatch of dead leaves over the soil surface that decomposes slowly (Morgan and Lunt 1999), shading out inter-tussock species (Morgan 1997, 1998b), smothering seedlings (although quantitative data are lacking) and contributing to the decline of plant diversity (Williams et al. 2006). However, the applicability of this model to other ecosystems is less well documented.
For fauna, disturbance has also been thought crucial to maintain habitat suitability of grassland species. The plains-wanderer (Pedionomus torquatus), for example, is sensitive to grassland biomass – as it affects structure – which can either be too dense or too open (Baker-Gabb et al. 2016), whereas the striped legless lizard (Delma impar) is thought to be suited to a complex grassland structure that develops with increasing time since disturbance (Dorrough and Ash 1999; Howland et al. 2016). Howland et al. (2014) showed that reptile richness and abundance are positively related to grass cover. Although disturbance-diversity relationships are often invoked to explain faunal habitat suitability, the mechanisms underpinning such relationships are rarely explored, nor are how these relationships change over a species’ range.
A simplified model summarising over 40 years of research on disturbance-diversity relationships in lowland temperate Australian grasslands is presented in Fig. 1. The approach to understanding drivers of biodiversity has been univariate, largely focussed on rainfall as the key driver of biomass, and disturbance as the single factor that modifies biomass, which then governs small-scale richness. A more nuanced model incorporating some of the contingencies in this relationship (e.g. species identity, resource availability) has not been incorporated into the disturbance-promoting-diversity paradigm (although see Clarke 2003; Prober et al. 2013 for notable exceptions). Such nuances are necessary when making predictions about the need for, and type of, management interventions to maintain biodiversity.
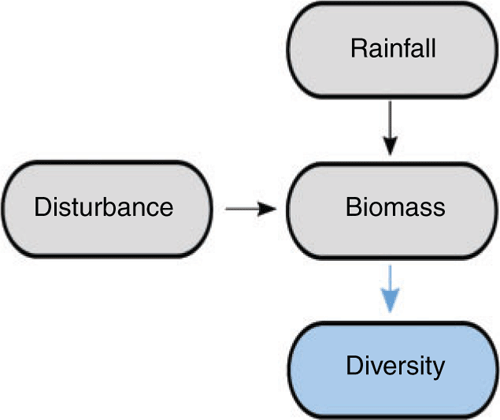
|
We developed a conceptual model of the multivariate drivers of grassland diversity that explores mechanisms underpinning patterns of species richness. Overwhelmingly the grassland literature in temperate Australia has come from one type of grassland (Themeda-dominated), at one spatial scale (small), in one bioregion (Victorian Volcanic Plains). Hence, these findings are unlikely to be generalisable to other grasslands, particularly more xeric ecosystems. Despite this, disturbances (fire, grazing or slashing) are being employed as a biomass reduction management tool in many temperate grasslands, with little understanding of the effects on biodiversity (especially at large spatial scales) and often without a clear theoretical underpinning for doing so.
We review the literature from lowland temperate grasslands in southern Australia and use examples from other grasslands to understand the mechanisms governing diversity and how they may vary in space and time. In particular, we explore the limitations of focusing on small-spatial scales and α diversity for understanding the relationship between disturbance and diversity (see section ‘The scales at which grasslands have been studied limit generality about landscape patterns’), the multivariate drivers of phytomass accumulation (see section ‘Phytomass accumulation is governed by multiple drivers; disturbance is just one of them’), the importance of grassland structure for plants and animals (see section ‘Grassland structure – more than just biomass’), multivariate drivers of grassland function (see section ‘The need to account for multivariate drivers of grassland function’), and if disturbances are substitutable (see section ‘Disturbances are not substitutable: on the need to understand why disturbance affects function differently’). We then identify key research questions that must be addressed to advance the conservation management of these endangered ecosystems (see section ‘Future research directions’).
The scales at which grasslands have been studied limit generality about landscape patterns
Most ecological research in Australian temperate grasslands has been conducted at small spatial scales, limiting our ability to understand relationships that occur at larger scales. As a result, many management interventions are focussed on maximising plant and animal diversity at the plot or (less typically) the site scale. Yet, we know that average plant size increases with biomass (Oksanen 1996), and fewer individuals can occur in small plots at high biomass, even if there are no ecological interactions. As a result, species density (the number of species in an area of fixed size) inevitably will decline with increasing time-since-disturbance, even if species richness does not. The focus on maximising small-scale plant richness (α diversity), typically by promoting forb species over grass cover, has come at the expense of understanding the complex, interacting drivers of diversity (such as climate, species identity, disturbance, regeneration) of this ecosystem across its range. Of the 21 published studies reporting fire effects on temperate grassy ecosystems in Australia between 1977 and 2017, all use plots ≤20 m2 to describe compositional, structural or functional response to fire (see Supplementary Material table S1 available at the journal’s website). Hence, an important and as yet unanswered question is: do small plots adequately describe the effects of long-term fire regimes on species persistence in temperate native grasslands in southern Australia, or are these regime effects scale-dependent?
Similarly, the effects of grazing on grassland diversity have predominantly been observed at small spatial scales (0.01–400 m2), with the common conclusion being that moderate grazing can promote species diversity at local scales by negating competitive exclusion (Tremont 1994; Fensham 1998; McIntyre and Martin 2001). However, grazing can reduce plant species diversity at the landscape or regional scale by removing grazing-sensitive species from the species pool, or by favouring grazing-tolerant species (Lunt 1997a; Landsberg et al. 2002; Dorrough et al. 2007; Schultz et al. 2016). Grassland management often aims to create a structure that accommodates the highest diversity of species, but the landscape configuration that most effectively conserves the highest proportion of the local species pool may include a variety of grassland structures, some of which may be relatively species poor (Schultz et al. 2016; Shahan et al. 2017; Abraham and Morgan 2018).
At larger spatial scales, species richness in less-frequently disturbed temperate grasslands may be comparable to frequently-disturbed grasslands due to within-community habitat heterogeneity which can promote species coexistence (Harper et al. 1965; Grace 1999). Environmental heterogeneity occurs due to spatial variation in soil properties (Harrison et al. 2003; Lundholm and Larson 2003; Price et al. 2017), animal disturbance (Hobbs and Mooney 1985; Martin 2003), topographic and edaphic factors (Fuhlendorf and Engle 2001) and variable competitive interactions among plant species (Aarssen 2001). Hence, frequent disturbance is perhaps only necessary to maintain small-scale (e.g. 1–100 m2) richness in productive grasslands, by allowing mechanisms such as species packing to operate (Oksanen 1996). In tallgrass prairie, Collins (1992) found that heterogeneity was significantly negatively related to burning frequency, perhaps because nitrogen-limitation is higher in frequently burned areas (Seastedt et al. 1991; Collins and Steinauer 1998). Collins (1992) also found a significant positive relationship between heterogeneity and species diversity and total species richness. Hence, if unburned grasslands develop patchiness based on topography and edaphic factors, high diversity might be permitted at larger scales despite dominance by tussock grasses that constrain small-scale diversity. In Australia, moderate to high disturbance is not consistently beneficial for plant diversity; rather, diversity can depend on management history and spatial heterogeneity (Prober et al. 2013).
Fauna-habitat relationships are also likely to be influenced by spatial scale, but for most fauna that occur in grasslands, there is little information on their habitat requirements. Globally, studies of fauna–habitat relationships have also suffered from a lack of acknowledgement of the effects of spatial scale on the observed patterns (Levin 1992; Shahan et al. 2017).
Phytomass accumulation is governed by multiple drivers; disturbance is just one of them
We argue that it is the accumulation of phytomass and the arrangement of biomass (see section ‘Grassland structure – more than just biomass’), not productivity per se, that indirectly affects grassland diversity (Fig. 2). Data on phytomass accumulation rates in temperate grasslands are sparse (Morgan et al. 2017); this is perplexing given biomass is thought to be a key driver of local species richness. Phytomass in mesic Themeda-dominated grassland increases after fire (Groves 1965; McDougall 1989); increases are linear because annual leaf production of Themeda subsequently contributes to accumulation of dead leaves that fail to decompose quickly (see fig. 1 in Morgan and Lunt 1999). Phytomass of up to 4.6 t ha−1 has been recorded within 2 years of burning a Themeda grassland in southern Australia (Morgan and Lunt 1999), and 2.5 t ha−1 has been recorded within 2.5 years in subtropical Themeda grasslands in north-western Australia (Bennett et al. 2003). At regional scales, Schultz et al. (2011) found phytomass accumulation in ungrazed grasslands varied from 28 to 944 g m–2, increasing with mean annual rainfall (independent of time-since-grazing exclusion). In low rainfall grasslands (<500 mm annual rainfall), rates of biomass accumulation were slow (Schultz et al. 2011).

|
Most grass leaves have short lifespans, so accumulated phytomass is mostly comprised of dead leaf material produced in earlier years. Undisturbed Themeda grasslands in mesic regions, for example, accumulated large quantities of dead grass; 88–93% of phytomass in long unburnt (11–14 years) Themeda and Themeda–Poa grasslands was dead material (Morgan and Lunt 1999; Prober et al. 2007). Environmental controls on litter decomposition are typically related to differences in litter quality (e.g. N, C : N, lignin content) and local climate (precipitation) (Brandt et al. 2010; Gaxiola and Armesto 2015). These factors modulate the activity of microbes and other decomposers. The effects of photodegradation (UV radiation during rainless periods) also contributes to differences in decomposition rates (Austin and Vivanco 2006). Photodegradation is greater in arid than in mesic ecosystems, and in litter that is more recalcitrant to microbial decomposition (with high lignin concentrations) (Brandt et al. 2010).
In subtropical and semiarid ecosystems, low biomass accumulation might reflect high decomposition rates (Fensham et al. 2017) or erratic production (e.g. tied to above-average rainfall; Austin and Williams 1989). Importantly, phytomass accumulation does not follow a linear function of time-since-disturbance, i.e. it is decoupled from disturbance (Fensham et al. 2017). Lewis et al. (2008, 2010) found in arid (Astrebla) grasslands, with a highly variable climate, that recent rainfall events were far more important for sward density than disturbance. In drier grasslands, litter accumulation is also negligible over long periods; either it decays quickly or gets blown away (Lewis et al. 2008; Morgan and Williams 2015). For example, Conway (2000) found that litter cover declined from over 50 to <10% in a single year during drought conditions in northern Victoria. Thus, low levels of accumulated biomass in semiarid grasslands probably reflect high decomposition rates as well as low productivity.
Given that phytomass accumulation varies in different grasslands, disturbance may only promote small-scale richness in areas where phytomass accumulation is substantial. Indeed, Schultz et al. (2011) found diversity in Victorian grasslands declined when biomass was >500 g m–2. Disturbances are thought to maintain richness in these high productivity sites because the removal of the phytomass produced by the dominant grass species frees up resources (light, space, nutrients and moisture) for subordinate species to germinate, grow or persist in the ecosystem (Fig. 2). Lunt and Morgan (2002) found light availability was reduced to less than 10% beneath Themeda tussocks when phytomass exceeded 4 t ha–1. This level of phytomass can accumulate within just 2–4 years after burning (Morgan and Lunt 1999). Phytomass accumulation is also influenced by disturbance history and species identity (Fig. 2). For example, Prober et al. (2013) found declines in richness with increasing time-since-fire in sites with a recent history of tree clearing and burning and with high biomass (>600 g m–2), a response that was not reported in uncleared sites without a recent history of fire and with low biomass (314 g m–2). These sites have similar rainfall to mesic Themeda-dominated grasslands in Victoria, which highlights the importance of considering multiple drivers of phytomass accumulation (Fig. 2). Although Themeda recovers rapidly after fire, other dominant grasses require more time to return biomass to pre-disturbance levels (Prober et al. 2007).
Given that we identify phytomass accumulation (and decomposition) as a key indirect driver of species diversity in grasslands (Fig. 2), it is crucial that data be obtained to test this relationship. Additionally, we need studies from xeric sites dominated by other species, as most studies in south-eastern Australia are primarily from Themeda-dominated grasslands (Stuwe and Parsons 1977; Lunt and Morgan 2002; Lunt et al. 2012), in which fast accumulation of phytomass smothers inter-tussock species and reduces light availability. In particular, uncoupling the effects of species identity and climate on phytomass accumulation should be a priority. This can be done experimentally (e.g. standardised decomposition rates using teabag protocols, Didion et al. 2016) and by field studies coupled with models (Grace 1999).
Grassland structure – more than just biomass
The horizontal and vertical arrangement of biomass – not just the quantity of biomass – has profound effects on ecological processes in grasslands, particularly as it affects light interception, microclimate, plant–soil interactions, canopy gap availability and animal behaviour (Baker-Gabb 1988; Lunt 1997b; Zehm et al. 2003; Baker-Gabb et al. 2016). Biomass per se is a poor measure to capture the spatial (i.e. horizontal and vertical arrangement), temporal and functional aspects of grassland swards. Light penetration may vary according to a combination of factors including phytomass, but also plant architecture (Fig. 3). High biomass grasslands can provide abundant gaps if the sward is open (Fig. 3b), whereas a closed sward with high biomass is unlikely to provide safe sites for recruitment (Fig. 3a). Conversely, low biomass (when comprising a closed sward) may provide limited safe sites for establishment (Fig. 3c). Currently, few examples exist that document the variation in temperate grassland structure as a function of climate variation (i.e. temporal rhythmicity) and disturbance processes (but see Lunt 1997b for an exception).
Measures of grassland structure that include multiple variables, such as grass height and cover (or grass volume, Howland et al. 2014; Schultz et al. 2017), likely provide a better estimate of the vegetation profile than biomass, and might capture attributes of grass swards that plants and animals respond to (e.g. habitat complexity, light availability). There are other approaches for measuring grassland structure and heterogeneity, including variance in biomass (Dorrough and Ash 1999), point quadrats to capture height and canopy distribution (Lunt 1997b), the ‘golf ball method’ to provide an integrative measure of vegetation density, height and heterogeneity (Schultz et al. 2017), and horizontal photography to measure vertical arrangement of vegetation (Zehm et al. 2003). Measuring the amount of bare ground, cryptogamic crust, and soil cracks is also important as these attributes affect seed germination of native (Briggs and Morgan 2011) and exotic species (Morgan 2006), provide important refuges for fauna (Hadden 1995; Bourke et al. 2017), and are responsive to management interventions (Morgan 2004; Wong and Morgan 2012). Indeed, it is likely that structure (and its complexity and heterogeneity) may be a more important driver of small-scale richness than biomass per se through impacts on regeneration (Fig. 2).
We poorly understand the habitat preferences of most grassland fauna, but structure (not biomass per se) is typically invoked as a key attribute of grasslands that needs to be manipulated to maximise selected faunal taxa (Supplementary Material table S2). Habitat suitability for grassland birds is directly related to the structural environment provided by the dominant tussock grasses, with some species preferring short and sparse grassland (Baker-Gabb et al. 2016) while others utilise denser, closed swards (Antos and Williams 2015). Clearly, some species are more abundant in closed than open swards e.g. stubble quail (Coturnix pectoralis) and Richard’s pipit (Anthus richardi) (Neave and Tanton 1989) – hinting that a ‘one size fits all’ approach to biodiversity conservation has limited application. At landscape-scales, a range of different sward structures is required to maintain habitat for a range of different species (McIntyre 2005). Reptiles typically require a grass canopy to avoid heat stress and predation (Howland et al. 2014), but also need a degree of canopy openness to permit sufficient sunlight for metabolic function. Some reptiles, however, are found across a range of grassland structures and respond to other habitat elements. The abundance of the hooded-scaly foot (Pygopus schraderi), for example, is determined by the cover of cryptogams and the incidence of spider holes and soil cracks (Brown and Scroggie 2012), whereas the earless dragon (Tympanocryptis pinguicolla) requires access to holes excavated by arthropods (Stevens et al. 2010).
For many faunal species, the effects of grassland structure may be mediated by indirect factors such as food resource availability (i.e. invertebrates, seeds). King and Hutchinson (1983) showed that the biomass and diversity of invertebrates declined with increasing intensity of sheep grazing in the New England Tablelands, and similar trends have been demonstrated in grasslands elsewhere (Dennis et al. 1998; McCracken and Tallowin 2004). Typically, there are fewer decomposers in a grassland system when a greater proportion of the biomass is removed (King and Hutchinson 1976), and the life cycle stages of invertebrates are disrupted by biomass removal (McQuillan 1999). Ant composition, however, shows a different trend, and is mostly determined by grassland structural complexity. High ant diversity has been demonstrated across a range of grassland habitats and conditions (New 2000), although ant abundance is generally higher in heavily grazed, low-biomass sites (Hutchinson and King 1980). Phytophagous species such as the golden sun moth (Synemon plana) and morabine grasshopper (Keyacris scurra) appear to respond more to the presence of particular food plant species than to grassland structure (O’Dwyer and Attiwill 1999; Griffith and Nano 2011; Richter et al. 2013). While the abundance and diversity of invertebrates in grasslands is assumed to influence grassland food webs, we do not have a good understanding of how invertebrates influence grassland ecosystem function (Abraham and Morgan 2018).
The need to account for multivariate drivers of grassland function
Univariate drivers of diversity, such as those presented in the simplified model (Fig. 1), underplay the complex processes that affect biodiversity in grasslands (Grace 1999; Harpole et al. 2017). Biodiversity is invariably influenced by, and interacts with, multiple drivers through both direct and indirect pathways. To account for this complexity, models of ecological dynamics need to be developed that incorporate these multivariate drivers of diversity. We introduce a conceptual model of temperate grassland dynamics that proposes the key multivariate drivers that underpin diversity in grasslands (Fig. 2). Here, accumulated biomass governs the structure of grasslands and this, in turn, affects diversity through its effects on resource availability. Regeneration opportunities are influenced by species-specific responses to disturbance regimes, resources and structure (Fig. 2). We outline the key direct drivers of diversity in temperate grasslands – disturbance, resources and regeneration – in an effort to better understand how grasslands vary ecologically across their range, and why structure, function and diversity are more than outcomes of the amount of biomass singularly.
Species identity
Despite four decades of research, we actually know very little about most grassland species. Hence, our understanding of grassland dynamics comes from a small subset of the species pool. Grassland management has mostly focussed on the ecology of the dominant grass (Themeda triandra), a limited number of forbs from (mostly) the Asteraceae, and a suite of highly threatened species, while the literature on land-use intensification has identified those species sensitive to nutrient addition and grazing (Vesk and Westoby 2001; Clarke 2003; Dorrough et al. 2004). Plant functional traits have been used to generalise responses to disturbance (e.g. McIntyre and Lavorel 1994) and management (Williams et al. 2006). For example, Zeeman and Morgan (2018) found that grassland habitat suitability varies with plant species and can be predicted by traits affecting competition such as height. Native species that increase in little disturbed grasslands (after being absent from frequently disturbed grasslands) typically comprise tall, wind-dispersed species, while those native species that decline are often short, rosette forming species. Studies of faunal responses are generally limited to charismatic threatened species such as plains wanderer (Pedionomus torquatus), striped legless lizard (Delma impar) and golden sun-moth (Synemon plana). Similarly, little is known about the role of species identity in ecosystem function (Abraham and Morgan 2018) and how the loss of rare species affects ecosystem function (Mokany et al. 2008).
What is clear is that species respond differently to disturbance, a point poorly articulated in the simplified model (Fig. 1). Stuwe and Parsons (1977) showed that native grasslands in grazed areas support a different suite of species, not just a subset of those in ungrazed and burnt grasslands. For example, Glycine latrobeana (Fabaceae) and Comesperma polygaloides (Polygalaceae) are disadvantaged by frequent fire regimes, their occurrence being favoured in unburnt and/or grazed areas (Scarlett and Parsons 1982). There are also many examples of grassland species that do well under anthropogenic disturbance regimes (Kirkpatrick 2007). Lunt (1997a) coined this process ‘habitat segregation’ and found evidence for different species complements in ungrazed, burnt railway verges compared with grazed, unburnt forests. Hence, species responses to disturbance are more nuanced than is depicted in the current simplified model of grassland function. As a case in point, Sinclair et al. (2014) showed that long-unburnt C3 grasses such as Austrostipa (spear grasses) did not recover by vegetative resprouting after a summer fire, despite the general temperate grassland literature suggesting that grasses are resistant (via resprouting) to fire. Moore et al. (2019) showed that temperate C4 grasses had higher probabilities of surviving fire relative to C3 grasses, and that drought plays a role in the ability of native grasses to vegetatively recover after fire. This has implications for understanding the persistence of species in landscapes where fire management is practiced. Hence, models need to specify which species are advantaged or disadvantaged by a given disturbance regime, and identify the mechanisms that underpin such responses.
Regeneration opportunities
It is commonly thought that fire promotes regeneration indirectly – by removing biomass and providing gaps for recruitment – rather than directly stimulating germination. As such, much of the grassland literature has focussed on the importance of biomass removal in promoting diversity (Fig. 1). However, studies have mostly examined particular plant groups (mostly Poaceae and Asteraceae, e.g. Morgan 1998a; Clarke and French 2005) and few studies have directly explored the effect of fire-cues on germination. Disturbances such as fire, grazing and bioturbation can cause a myriad of changes in abiotic and biotic conditions that can affect seedling recruitment. We expect germination cues to be related to gap formation and resource availability, or to fire-related cues such as heat or smoke (Fig. 2). Hence, we require an understanding of the factors that directly affect germination as a result of gap formation e.g. nutrient changes, light intensity changes, temperature changes, temperature during fire (dormancy removal), soil changes (disturbance/compaction) and cryptogram cover, and how the effects of these factors may also vary with species identity.
Germination of some grassland forbs are promoted by fire-cues (Vening et al. 2017; Hodges et al. 2019) and restoration practitioners have also identified many difficult-to-germinate species from grassy ecosystems, indicating seed dormancy may be more prevalent than previously recognised. As such, fire may directly promote germination or remove dormancy for grassland species, as found in other fire-prone ecosystems (Bell 1999). Many herbaceous species have types of dormancy (physiological or morphological) that often require a combination of cues to remove dormancy and enable germination (Merritt et al. 2007; Baskin and Baskin 2014). Some species may only become responsive to fire-cues once dormancy has been removed and may therefore require a combination of dormancy removal and fire-cues to enable germination (Ooi et al. 2006; Thompson and Ooi 2010; Long et al. 2011). For example, Dianella revoluta had highest germination when subject to warm stratification (dormancy removal treatment) and smoke (fire-cues) in combination (Hodges et al. 2019), and further investigations of such treatments are required to determine if other grassland species have a similar strategy. Determining the processes involved in gap-driven germination is important for understanding the effect of disturbance type and timing on seedling recruitment. For example, if fire directly promotes seed germination, then fire may promote diversity regardless of biomass accumulation and this has implications for considering if disturbances are substitutable (see section ‘Disturbances are not substitutable: on the need to understand why disturbance affects function differently’). Resource variation and the environmental conditions during seedling emergence and establishment are also critical for recruitment success.
Below ground, whether species are able to form persistent soil seed banks that survive the disturbance interval is relatively unstudied, but vital for understanding recruitment potential after different disturbance types. Seed persistence or longevity across the diversity of species in grasslands is likely to have a much greater range than the transient soil seed banks known for a select few daisies and forbs (Lunt 1995), and requires further investigation.
Resource variation
Temperate grasslands exist over a range of climates as a consequence of their wide distribution across latitude, distance to coast and altitude (Fig. 4). Importantly, grasslands also differ in their seasonality of precipitation (uniform to distinctly winter-dominated), frequency of drought (return interval), and the severity of drought (duration) throughout the range of climates that support temperate grasslands. As a result, gradients in rainfall quantity and seasonality, as well as temperature, drive turnover in plant species composition (McDougall and Kirkpatrick 1993), and long-term ecological dynamics as driven by exposure to different disturbance regimes (Morgan et al. 2017).
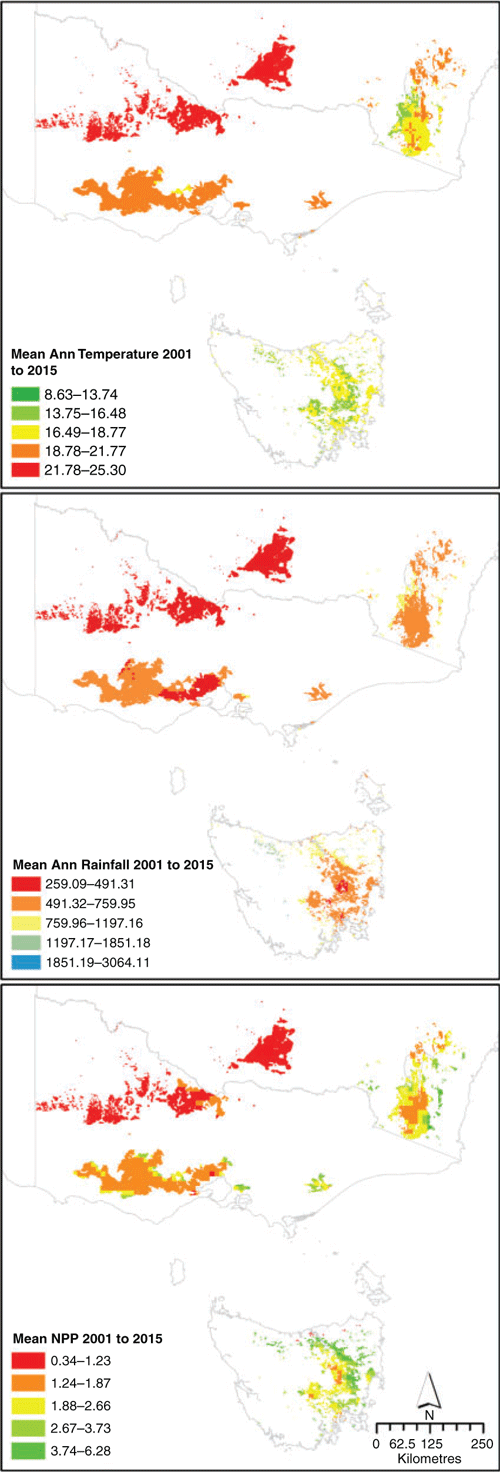
|
Availability and use of water by plants is one of the dominant forces influencing the phenology and productivity of ecosystems, and deserves greater recognition in temperate grasslands. Interannual variation in precipitation, for instance, has been shown to be a strong determinant of yearly primary productivity in Australian temperate grasslands (e.g. Groves 1965; McDougall 1989; Morgan et al. 2017) and elsewhere (Knapp and Smith 2001). Year-dependent effects on primary production are particularly important in Australian grasslands because the El Niño–Southern Oscillation cycles cause large inter-annual variation in precipitation (Vines et al. 2004). This introduces the potential for significant variation in the rates of canopy recovery after disturbance, seedling recruitment opportunities, as well as species coexistence processes. Elsewhere, temporal variation in water availability influences community composition in grasslands (Pitt and Heady 1978; Hamilton et al. 1999; Corbin et al. 2005) and herb-rich savanna (Myers and Harms 2011).
Grassland productivity is also influenced by soil nutrients, either singly or via co-limitation (Elser and Bracken 2007; Harpole and Ngai 2011; Fay et al. 2015). Temperate grasslands generally occur on fine-textured soils (Fensham et al. 2015), but soil nutrients vary substantially as a function of parent material and age. Temperate grasslands are mostly associated with cracking clay vertisols derived from recent deposits of basalt and alluvium, although in some regions, such as the Monaro Tablelands, grasslands also occur on clays derived from a greater range of parent rocks including limestone, fine-grained sedimentary rocks and even granite (Benson 1994). Such differences in soils likely play a role in the rate at which competitive exclusion occurs. Multiple nutrient limitations have also been shown to structure plant diversity and composition in grasslands, independently of changes in light and biomass (Harpole et al. 2017). Indeed, in the global NutNet experiment examining drivers of plant diversity in grasslands (which includes temperate grasslands from south-east Australia; Morgan et al. 2016), many sites showed no significant increase in biomass with nutrient addition, yet lost diversity when nutrients were added (Harpole et al. 2017). Hence, although light limitation can be an important contributor to diversity loss in grasslands, it is not always a causal mechanism. Additionally, the magnitude of the nutrient addition effect in grasslands increases with increasing plant available water, i.e. nutrient addition interacts positively with growing season precipitation (Prober and Wiehl 2012; Dwyer et al. 2015). A robust assessment of the extent to which water and nutrient availability limit productivity in Australian temperate grasslands under present climatic conditions is needed. Variation in rainfall and soil nutrients in time and space, likely modifies species’ responses to disturbance and competitive exclusion and it can be predicted that patterns of diversity will respond in non-linear ways to these key drivers; hence the need for their inclusion in updated models of grassland function (Fig. 2).
Disturbances are not substitutable: on the need to understand why disturbance affects function differently
The ‘state’ of a grassland (e.g. species composition, degree of invasion by exotic species, life form representation) has typically been regarded as a function of its long-term management history, i.e. its disturbance regime (Stuwe and Parsons 1977; Lunt 1997a; McIntyre and Lavorel 2007). Based on this, literature often recommends maintaining the status quo in relation to disturbance regimes to manage grasslands (Fig. 1; Ross 1999; Wong and Morgan 2007). This is based on the idea that maintenance of the processes that gave rise to the patterns of diversity is viewed as the best way of maintaining those patterns. For example, cessation of fire results in local extinction of native forbs from temperate grasslands that previously had a history of frequent fire (Williams et al. 2006; Zeeman et al. 2017). Maintaining the status quo, however, may no longer be an option with global climate change (Harris et al. 2015).
Little is known about whether different disturbance types are substitutable, i.e. moving beyond the status quo. Disturbance, as defined by Grime (1977), is any event that removes biomass, but disturbance type can have fundamentally different outcomes on vegetation; grazing is selective, fire consumes all biomass, and slashing generally leaves litter on the ground. This will have impacts on the post-disturbance ecosystem that is created, with consequent effects on the biota that utilise grassland habitat. Such changes are already occurring as land management practices change with rural population decline (Williams 2007), urbanisation (Williams et al. 2006; Zeeman et al. 2017) and conservation covenanting (Wong and Morgan 2012). Changing long-term regimes might lead to instability in the system (in the short term), but to improved conservation outcomes because it ultimately favours more species (at landscape scales). Legacy effects will influence the response of grasslands to new disturbances. Species identity is likely to be an important determinant of how grasslands respond to different types of disturbances (Kirkpatrick et al. 2005). For example, high mortality has been observed in C3 tussock grasses when reinstating fire into a long unburnt site (Sinclair et al. 2014), and C4 grasses are more resilient to burning than C3 grasses (McDougall 1989; Moore et al. 2019). Additionally, two types of disturbances applied concurrently can have different impacts than a single disturbance type. For example, fire and grazing can benefit the striped legless lizard but concurrent application of both disturbances may trigger population collapse (Scroggie et al. 2019).
Fire is a ‘pulse’ disturbance (Bender et al. 1984), typically destroying all plant material in a relatively homogenous way, but it can vary in intensity both spatially and temporally (Morgan 1999). The effects of fire and fire regimes on grasslands are generally well described, particularly for plants (Lunt and Morgan 2002; Morgan et al. 2017). For invertebrate species, abundance and richness are reduced after fire, but can recover relatively quickly to pre-fire levels (Greenslade 1997; Abraham and Morgan 2018). Among vertebrates, reptiles can suffer elevated mortality from fire, with mortality influenced by the season of burning (Griffiths and Christian 1996) and burrow use (Fenner and Bull 2007). Marsupials like fat-tailed dunnarts aim to evade fire; they can arouse from torpor when they detect smoke, increasing their likelihood of evading an approaching fire (Stawski et al. 2015; Nimmo et al. 2019), suggesting that the presence of unburnt refuges, and the speed of an approaching fire front may influence mortality. For soil fungal communities, fungal diversity and composition were influenced by fire regime (long-term fire frequency), but not time-since-fire (Egidi et al. 2016). It is clear that removing fire has negative effects for many species (Williams et al. 2006; Zeeman et al. 2017), but there has been little quantification of (1) the time that grasslands can remain unburned before irreversible declines in diversity occur, and (2) how substituting other disturbances in place of fire may mitigate such declines.
Livestock grazing can reduce vegetation cover, with substantial reductions persistent over time in many areas (i.e. a ‘press’ disturbance sensu Bender et al. 1984). Grazing is used as a management tool because it can maintain species richness (at high numbers in small quadrats)—based on the idea that competitive exclusion reduces small scale diversity (Fig. 1). Although α diversity is high, β diversity across the landscape may be low when grazed (Schultz et al. 2016) and fire-cued recruitment opportunities will be lost. Conservation managers are loathe to remove stock from native grasslands because they are acutely aware that destocking might lead to declines in α diversity. This fails to recognise that destocking might lead to increases in β diversity if it promotes heterogeneity via microsite differentiation. So far, the evidence for effects of changing disturbance – such as moving from a grazing to fire regime, abandonment, or altering the status quo grazing regime – is mixed, with positive, neutral and negative impacts on plant, invertebrate and bird diversity observed (Hadden 1995; Kirkpatrick et al. 2005; Zimmer et al. 2010; Wong and Morgan 2012). If a grassland has a long history of grazing, it is likely that grazing-sensitive plant species have been lost from the system (Price et al. 2010). Hence, reinstating fire or grazing removal is unlikely to recover those species without further management interventions such as seed addition and could lead to loss of species that require some disturbance (Kirkpatrick et al. 2005; Johnson et al. 2018; Zamin et al. 2018). Little is known about the effects of native marsupial grazing on Australian temperate grasslands; a few studies have explored impacts of over abundant native animals, and high selectivity has been observed (Leonard et al. 2010).
In general, mowing promotes greater native richness than grazing (Verrier and Kirkpatrick 2005), but reduced richness compared with fire (Prober et al. 2013). Slashing is comparatively a fairly homogenous disturbance, particularly if applied frequently, as it generally encourages low-biomass, closed grassland structure (Prober et al. 2008; Fig. 3c). Mowing can result in aggregated litter which can have negative consequences unless slash is removed (Verrier and Kirkpatrick 2005; Morgan 2015). Slashing is generally viewed as unfavourable in temperate grasslands (Kirkpatrick 1986), particularly for taller species, but Smith et al. (2018) show that annual mowing can have positive influences on plant diversity, and may promote structural heterogeneity when applied patchily, and can also favour some rare species (Gilfedder and Kirkpatrick 1997). Mowing increased cover of native species (including some rare or threatened species) and reduced exotic grass cover compared with grazing in Tasmania (Verrier and Kirkpatrick 2005). In degraded grasslands in the ACT, tussock thinning and litter removal favoured native forbs, but only when seeds were added (Johnson et al. 2018). Positive effects of slashing at small spatial scales (α diversity), might not translate to larger scales (β diversity) (Smith et al. 2018).
Bioturbation is another disturbance type that has been understudied, particularly in the context of Australian temperate grasslands. Burrowing mammals are recognised globally as ecosystem engineers in grasslands, increasing biodiversity and habitat heterogeneity (Davidson et al. 2012). In Australian ecosystems, the ecological roles of digging animals are best understood in semiarid and arid ecosystems, where digging by burrowing bettongs (Bettongia lesueur) can suppress shrubs and increase the cover and diversity of perennial grasses by altering soil characteristics, trapping litter and water, and enhancing seed germination (Noble et al. 2007; Eldridge and James 2009). In Australia’s temperate grasslands, bioturbation by digging animals such as eastern-barred bandicoots (Perameles gunnii) could perform similar or other ecological roles, but such animals have been lost from temperate grasslands (Dufty 1994; Reading et al. 1996). A recent study highlights the potential for bettongs (Bettongia gaimardi) to enhance soil processes in degraded temperate woodlands (Munro et al. 2019). We predict that digging might provide a relatively low-intensity disturbance that could have ecological benefits in the more xeric temperate grasslands that do not require frequent disturbance.
Future research directions
Despite four decades of research in temperate grasslands, much remains unknown about the multivariate drivers of animal and plant diversity as outlined in Fig. 2. Using this model as a starting point for future research, we believe there are five key questions that need to be urgently addressed.
-
1. What are the rates, and drivers, of phytomass accumulation in different grasslands?
Rates of phytomass accumulation across the range of temperate grasslands are likely to be influenced by climate, dominant grass species identity, and decomposition. Understanding how these processes vary in space and time would allow us to predict phytomass accumulation in a given location enabling better prediction of the disturbance requirements for promoting diversity, and hence more tailored management decisions.
-
2. Under what conditions is disturbance required for recruitment?
More research is required on seed persistence, dormancy and germination cues for many grassland plant species. From a management perspective, identifying responses to fire-cues for many component species is required to determine if fire per se is required to promote diversity, or simply the removal of biomass. We also need to explore the ability of species to form persistent soil seed banks and the effect of other disturbance types on seedling regeneration, e.g. soil disturbance, animal diggings.
-
3. What are the impacts of changing from long implemented management regimes (status quo) to alternative disturbance regimes?
Little is known about whether a switch from one long-term management regime (the status quo) to another management regime (e.g. fire to grazing and vice versa) has positive, negative or neutral outcomes for diversity. Responses of plants and animals will depend on land-use legacies and if sites are stable under the current management regime.
-
4. Do small plots adequately describe the effects of long-term disturbance regimes on species persistence?
We require a better understanding of the drivers of β and gamma diversity in grasslands, as well as the drivers of spatial and temporal variation in composition. There is almost no data to address these questions, both of which logically flow to landscape-scale conservation planning.
-
5. How does disturbance influence faunal assemblages and habitat?
More research is needed on the effect of disturbance on habitat provision and food resources for most grassland fauna. In particular, we need to understand the relationship between faunal diversity and grassland structure, and how habitat diversity and disturbances might affect different faunal food webs.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Bob Parsons and two anonymous reviewers for comments on an early version of the manuscript that helped improve its clarity. We thank Zac Walker for drawing Figs 1 and 2. We thank Simon McDonald (Spatial Data Analysis Network) for producing Fig. 4. The concepts developed here owe much to the long-term contributions of grassland ecologists Bob Parsons, Ian Lunt, Suzanne Prober, Josh Dorrough and Paul Foreman. This research did not receive any specific funding.
References
Aarssen LW (2001) On correlations and causations between productivity and species richness in vegetation: predictions from habitat attributes. Basic and Applied Ecology 2, 105–114.| On correlations and causations between productivity and species richness in vegetation: predictions from habitat attributes.Crossref | GoogleScholarGoogle Scholar |
Abraham J, Morgan JW (2018) Effect of time-since-fire on the invertebrate communities of kangaroo grass Themeda triandra-dominated grasslands in Melbourne, Victoria. Victorian Naturalist 135, 36–46.
Antos M, Williams NSG (2015) The wildlife of our grassy landscapes. In ‘Land of sweeping plains: managing and restoring the native grasslands of south-eastern Australia’. (Eds NSG Williams, A Marshall, JW Morgan) pp. 87–114. (CSIRO Publishing: Melbourne, Vic.)
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442, 555–558.
| Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation.Crossref | GoogleScholarGoogle Scholar | 16885982PubMed |
Austin MP, Williams OB (1989) Influence of climate and community composition on the population demography of pasture species in semi-arid Australia. In ‘Temporal and spatial patterns of vegetation dynamics’. (Eds J Miles, W Schmidt, E van der Maarel) pp. 43–49. (Springer: Dordrecht, The Netherlands)
Baker-Gabb DJ (1988) The diet and foraging behaviour of the plains-wanderer Pedionomus torquatus. Emu - Austral Ornithology 88, 115–118.
| The diet and foraging behaviour of the plains-wanderer Pedionomus torquatus.Crossref | GoogleScholarGoogle Scholar |
Baker-Gabb D, Antos M, Brown G (2016) Recent decline of the critically endangered Plains-wanderer (Pedionomus torquatus), and the application of a simple method for assessing its cause: major changes in grassland structure. Ecological Management & Restoration 17, 235–242.
| Recent decline of the critically endangered Plains-wanderer (Pedionomus torquatus), and the application of a simple method for assessing its cause: major changes in grassland structure.Crossref | GoogleScholarGoogle Scholar |
Baskin CC, Baskin JM (2014) ‘Seeds: ecology, biogeography and evolution of dormancy and germination.’ 2nd edn. (Elsevier: Oxford, UK)
Bell DT (1999) Turner Review No. 1 – The process of germination in Australian species. Australian Journal of Botany 47, 475–517.
| Turner Review No. 1 – The process of germination in Australian species.Crossref | GoogleScholarGoogle Scholar |
Bender EA, Case TJ, Gilpin ME (1984) Perturbation experiments in community ecology: theory and practice. Ecology 65, 1–13.
| Perturbation experiments in community ecology: theory and practice.Crossref | GoogleScholarGoogle Scholar |
Bennett LT, Judd TS, Adams MA (2003) Growth and nutrient content of perennial grasslands following burning in semi-arid, sub-tropical Australia. Plant Ecology 164, 185–199.
| Growth and nutrient content of perennial grasslands following burning in semi-arid, sub-tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Benson JS (1994) The native grasslands of the Monaro region: southern tablelands of NSW. Cunninghamia 3, 609–650.
Bourke G, Matthews A, Michael DR (2017) Can protective attributes of artificial refuges offset predation risk in lizards? Austral Ecology 42, 497–507.
| Can protective attributes of artificial refuges offset predation risk in lizards?Crossref | GoogleScholarGoogle Scholar |
Brandt LA, King JY, Hobbie SE, Milchunas DG, Sinsabaugh RL (2010) The role of photodegradation in surface litter decomposition across a grassland ecosystem precipitation gradient. Ecosystems 13, 765–781.
| The role of photodegradation in surface litter decomposition across a grassland ecosystem precipitation gradient.Crossref | GoogleScholarGoogle Scholar |
Briggs AL, Morgan JW (2011) Seed characteristics and soil surface patch type interact to affect germination of semi-arid woodland species. Plant Ecology 212, 91–103.
| Seed characteristics and soil surface patch type interact to affect germination of semi-arid woodland species.Crossref | GoogleScholarGoogle Scholar |
Brown G, Scroggie M (2012) Monitoring of the threatened hooded scaly-foot Pygopus schraderi in north-central Victoria: program establishment and initial results. A Report to the Department of Sustainability and Environment, Parks Victoria and Trust for Nature. Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, Heidelberg, Vic.
Clarke PJ (2003) Composition of grazed and cleared temperate grassy woodlands in eastern Australia: patterns in space and inferences in time. Journal of Vegetation Science 14, 5–14.
| Composition of grazed and cleared temperate grassy woodlands in eastern Australia: patterns in space and inferences in time.Crossref | GoogleScholarGoogle Scholar |
Clarke S, French K (2005) Germination response to heat and smoke of 22 Poaceae species from grassy woodlands. Australian Journal of Botany 53, 445–454.
| Germination response to heat and smoke of 22 Poaceae species from grassy woodlands.Crossref | GoogleScholarGoogle Scholar |
Collins SL (1992) Fire frequency and community heterogeneity in tallgrass prairie vegetation. Ecology 73, 2001–2006.
| Fire frequency and community heterogeneity in tallgrass prairie vegetation.Crossref | GoogleScholarGoogle Scholar |
Collins SL, Steinauer EM (1998) Disturbance, diversity and species interactions in tallgrass prairie. In ‘Grassland dynamics: long-term ecological research in tallgrass prairie’. (Eds AK Knapp, JM Briggs, DC Hartnett, SL Collins) pp. 140–156. (Oxford University Press: New York)
Conway MG (2000) The effects of grazing exclusion on a long-grazed species-rich Riverina grassland. Honours thesis, Charles Sturt University, Wagga Wagga, NSW, Australia.
Corbin JD, Thomsen MA, Dawson TE, D’Antonio CM (2005) Summer water use by California coastal prairie grasses: fog, drought, and community composition. Oecologia 145, 511–521.
| Summer water use by California coastal prairie grasses: fog, drought, and community composition.Crossref | GoogleScholarGoogle Scholar | 16001220PubMed |
Davidson AD, Detling JK, Brown JH (2012) Ecological roles and conservation challenges of social, burrowing, herbivorous mammals in the world’s grasslands. Frontiers in Ecology and the Environment 10, 477–486.
| Ecological roles and conservation challenges of social, burrowing, herbivorous mammals in the world’s grasslands.Crossref | GoogleScholarGoogle Scholar |
Dennis P, Young MR, Gordon IJ (1998) Distribution and abundance of small insects and arachnids in relation to structural heterogeneity of grazed, indigenous grasslands. Ecological Entomology 23, 253–264.
| Distribution and abundance of small insects and arachnids in relation to structural heterogeneity of grazed, indigenous grasslands.Crossref | GoogleScholarGoogle Scholar |
Didion M, Repo A, Liski J, Forsius M, Bierbaumer M, Djukic I (2016) Towards harmonizing leaf litter decomposition studies using standard tea bags – a field study and model application. Forests 7, 167
| Towards harmonizing leaf litter decomposition studies using standard tea bags – a field study and model application.Crossref | GoogleScholarGoogle Scholar |
Dorrough J, Ash JE (1999) Using past and present habitat to predict the current distribution and abundance of a rare cryptic lizard, Delma impar (Pygopodidae). Australian Journal of Ecology 24, 614–624.
| Using past and present habitat to predict the current distribution and abundance of a rare cryptic lizard, Delma impar (Pygopodidae).Crossref | GoogleScholarGoogle Scholar |
Dorrough J, Yen A, Turner V, Clark SG, Crosthwaite J, Hirth JR (2004) Livestock grazing management and biodiversity conservation in Australian temperate grassy landscapes. Australian Journal of Agricultural Research 55, 279–295.
| Livestock grazing management and biodiversity conservation in Australian temperate grassy landscapes.Crossref | GoogleScholarGoogle Scholar |
Dorrough JW, Ash JE, Bruce S, McIntyre S (2007) From plant neighbourhood to landscape scales: how grazing modifies native and exotic plant species richness in grassland. Plant Ecology 191, 185–198.
| From plant neighbourhood to landscape scales: how grazing modifies native and exotic plant species richness in grassland.Crossref | GoogleScholarGoogle Scholar |
Dufty A (1994) Habitat and spatial requirements of the eastern barred bandicoot (Perameles gunnii) at Hamilton, Victoria. Wildlife Research 21, 459–471.
| Habitat and spatial requirements of the eastern barred bandicoot (Perameles gunnii) at Hamilton, Victoria.Crossref | GoogleScholarGoogle Scholar |
Dwyer JM, Hobbs RJ, Wainwright CE, Mayfield MM (2015) Climate moderates release from nutrient limitation in natural annual plant communities. Global Ecology and Biogeography 24, 549–561.
| Climate moderates release from nutrient limitation in natural annual plant communities.Crossref | GoogleScholarGoogle Scholar |
Egidi E, McMullan-Fisher S, Morgan JW, May T, Zeeman B, Franks AE (2016) Fire regime, not time-since-fire, affects soil fungal community diversity and composition in temperate grasslands. FEMS Microbiology Letters 363, fnw196
| Fire regime, not time-since-fire, affects soil fungal community diversity and composition in temperate grasslands.Crossref | GoogleScholarGoogle Scholar | 27528692PubMed |
Eldridge DJ, James AI (2009) Soil‐disturbance by native animals plays a critical role in maintaining healthy Australian landscapes. Ecological Management & Restoration 10, S27–S34.
| Soil‐disturbance by native animals plays a critical role in maintaining healthy Australian landscapes.Crossref | GoogleScholarGoogle Scholar |
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10, 1135–1142.
| Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems.Crossref | GoogleScholarGoogle Scholar | 17922835PubMed |
Fay PA, Prober SM, Harpole WS, Knops JMH, Bakker JD, Borer ET, Lind EM, MacDougall AS, Seabloom EW, Wragg PD, et al (2015) Grassland productivity limited by multiple nutrients. Nature Plants 1, 15080
| Grassland productivity limited by multiple nutrients.Crossref | GoogleScholarGoogle Scholar | 27250253PubMed |
Fenner AL, Bull CM (2007) Short-term impact of grassland fire on the endangered pygmy bluetongue lizard. Journal of Zoology 272, 444–450.
| Short-term impact of grassland fire on the endangered pygmy bluetongue lizard.Crossref | GoogleScholarGoogle Scholar |
Fensham RJ (1998) The grassy vegetation of the Darling Downs, south-eastern Queensland, Australia. Floristics and grazing effects. Biological Conservation 84, 301–310.
| The grassy vegetation of the Darling Downs, south-eastern Queensland, Australia. Floristics and grazing effects.Crossref | GoogleScholarGoogle Scholar |
Fensham RJ, Butler DW, Foley J (2015) How does clay constrain woody biomass in drylands? Global Ecology and Biogeography 24, 950–958.
| How does clay constrain woody biomass in drylands?Crossref | GoogleScholarGoogle Scholar |
Fensham RJ, Butler DW, Laffineur B, MacDermott HJ, Morgan JW, Silcock JL (2017) Subtropical native grasslands may not require fire, mowing or grazing to maintain native-plant diversity. Australian Journal of Botany 65, 95–102.
| Subtropical native grasslands may not require fire, mowing or grazing to maintain native-plant diversity.Crossref | GoogleScholarGoogle Scholar |
Fuhlendorf SD, Engle DM (2001) Restoring heterogeneity on rangelands: ecosystem management based on evolutionary grazing patterns: we propose a paradigm that enhances heterogeneity instead of homogeneity to promote biological diversity and wildlife habitat on rangelands grazed by livestock. Bioscience 51, 625–632.
| Restoring heterogeneity on rangelands: ecosystem management based on evolutionary grazing patterns: we propose a paradigm that enhances heterogeneity instead of homogeneity to promote biological diversity and wildlife habitat on rangelands grazed by livestock.Crossref | GoogleScholarGoogle Scholar |
Fynn RWS, Morris CD, Edwards TJ (2004) Effect of burning and mowing on grass and forb diversity in a long-term grassland experiment. Applied Vegetation Science 7, 1–10.
| Effect of burning and mowing on grass and forb diversity in a long-term grassland experiment.Crossref | GoogleScholarGoogle Scholar |
Gaxiola A, Armesto JJ (2015) Understanding litter decomposition in semiarid ecosystems: linking leaf traits, UV exposure and rainfall variability. Frontiers of Plant Science 6, 140
| Understanding litter decomposition in semiarid ecosystems: linking leaf traits, UV exposure and rainfall variability.Crossref | GoogleScholarGoogle Scholar |
Gilfedder L, Kirkpatrick JB (1997) Aspects of the distribution, phytosociology, ecology and management of Danthonia popinensis D.I. Morris, an endangered wallaby grass from Tasmania Papers and Proceedings of the Royal Society of Tasmania 131, 31–35.
| Aspects of the distribution, phytosociology, ecology and management of Danthonia popinensis D.I. Morris, an endangered wallaby grass from TasmaniaCrossref | GoogleScholarGoogle Scholar |
Gott B (2005) Aboriginal fire management in south-eastern Australia: aims and frequency. Journal of Biogeography 32, 1203–1208.
| Aboriginal fire management in south-eastern Australia: aims and frequency.Crossref | GoogleScholarGoogle Scholar |
Grace JB (1999) The factors controlling species density in herbaceous plant communities: an assessment. Perspectives in Plant Ecology, Evolution and Systematics 2, 1–28.
| The factors controlling species density in herbaceous plant communities: an assessment.Crossref | GoogleScholarGoogle Scholar |
Greenslade P (1997) Short-term effects of a prescribed burn on invertebrates in grassy woodland in south-eastern Australia. Memoirs of the Museum of Victoria 56, 305–312.
| Short-term effects of a prescribed burn on invertebrates in grassy woodland in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Griffith C, Nano A (2011) Moths in the sun: community monitoring for the golden sun moth at Derrimut Grassland Reserve, Victoria 2008–2010. NatureWatch Monitoring Report No. 1. Victorian National Parks Association, Melbourne, Vic.
Griffiths AD, Christian KA (1996) The effects of fire on the frillneck lizard (Chlamydosaurus kingii) in northern Australia. Australian Journal of Ecology 21, 386–398.
| The effects of fire on the frillneck lizard (Chlamydosaurus kingii) in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Grime JP (1973) Competitive exclusion in herbaceous vegetation. Nature 242, 344–347.
| Competitive exclusion in herbaceous vegetation.Crossref | GoogleScholarGoogle Scholar |
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist 111, 1169–1194.
| Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory.Crossref | GoogleScholarGoogle Scholar |
Groves R (1965) Growth of Themeda australis tussock grassland at St Albans, Victoria. Australian Journal of Botany 13, 291–302.
| Growth of Themeda australis tussock grassland at St Albans, Victoria.Crossref | GoogleScholarGoogle Scholar |
Hadden S (1995) Distribution, status and habitat requirements of the striped legless lizard Delma impar (Fischer). Australian Nature Conservation Agency, Melbourne, Vic.
Hamilton JG, Holzapfel C, Mahall BE (1999) Coexistence and interference between a native perennial grass and non-native annual grasses in California. Oecologia 121, 518–526.
| Coexistence and interference between a native perennial grass and non-native annual grasses in California.Crossref | GoogleScholarGoogle Scholar | 28308361PubMed |
Harper JL, Williams JT, Sagar GR (1965) The behaviour of seeds in soil: I. The heterogeneity of soil surfaces and its role in determining the establishment of plants from seed. Journal of Ecology 53, 273–286.
| The behaviour of seeds in soil: I. The heterogeneity of soil surfaces and its role in determining the establishment of plants from seed.Crossref | GoogleScholarGoogle Scholar |
Harpole WS, Ngai JT, Cleland EE, Seabloom EW, Borer ET, Bracken MES, Elser JJ, Gruner DS, Hillebrand H, Shurin JB, Smith JE (2011) Nutrient co-limitation of primary producer communities. Ecology Letters 14, 852–862.
| Nutrient co-limitation of primary producer communities.Crossref | GoogleScholarGoogle Scholar | 21749598PubMed |
Harpole WS, Sullivan LL, Lind EM, Firn J, Adler PB, Borer ET, Chase J, Fay PA, Hautier Y, Hillebrand H, MacDougall AS, et al (2016) Addition of multiple limiting resources reduces grassland diversity. Nature 537, 93
| Addition of multiple limiting resources reduces grassland diversity.Crossref | GoogleScholarGoogle Scholar | 27556951PubMed |
Harpole WS, Sullivan LL, Lind EM, Firn J, Adler PB, Borer ET, Chase J, Fay PA, Hautier Y, Hillebrand H, MacDougall AS, et al (2017) Out of the shadows: multiple nutrient limitations drive relationships among biomass, light and plant diversity. Functional Ecology 31, 1839–1846.
| Out of the shadows: multiple nutrient limitations drive relationships among biomass, light and plant diversity.Crossref | GoogleScholarGoogle Scholar |
Harris RMB, Carter O, Gilfedder L, Porfirio LL, Lee G, Bindoff NL (2015) Noah’s Ark conservation will not preserve threatened ecological communities under climate change. PLoS ONE 10, e0124014
| Noah’s Ark conservation will not preserve threatened ecological communities under climate change.Crossref | GoogleScholarGoogle Scholar |
Harrison S, Inouye BD, Safford HD (2003) Ecological heterogeneity in the effects of grazing and fire on grassland diversity. Conservation Biology 17, 837–845.
| Ecological heterogeneity in the effects of grazing and fire on grassland diversity.Crossref | GoogleScholarGoogle Scholar |
Hobbs RJ, Mooney HA (1985) Community and population dynamics of serpentine grassland annuals in relation to gopher disturbance. Oecologia 67, 342–351.
| Community and population dynamics of serpentine grassland annuals in relation to gopher disturbance.Crossref | GoogleScholarGoogle Scholar | 28311567PubMed |
Hodges JA, Price JN, Nimmo DG, Guja LK (2019) Evidence for direct effects of fire-cues on germination of some perennial forbs common in grassy ecosystems. Austral Ecology
| Evidence for direct effects of fire-cues on germination of some perennial forbs common in grassy ecosystems.Crossref | GoogleScholarGoogle Scholar | ,
Howland B, Stojanovic D, Gordon IJ, Manning AD, Fletcher D, Lindenmayer DB (2014) Eaten out of house and home: impacts of grazing on ground-dwelling reptiles in Australian grasslands and grassy woodlands. PLoS One 9, e105966
| Eaten out of house and home: impacts of grazing on ground-dwelling reptiles in Australian grasslands and grassy woodlands.Crossref | GoogleScholarGoogle Scholar | 25501680PubMed |
Howland BWA, Stojanovic D, Gordon IJ, Fletcher D, Snape M, Stirnemann IA, Lindenmayer DB (2016) Habitat preference of the striped legless lizard: implications of grazing by native herbivores and livestock for conservation of grassland biota. Austral Ecology 41, 455–464.
| Habitat preference of the striped legless lizard: implications of grazing by native herbivores and livestock for conservation of grassland biota.Crossref | GoogleScholarGoogle Scholar |
Huston M (1979) A general hypothesis of species diversity. American Naturalist 113, 81–101.
| A general hypothesis of species diversity.Crossref | GoogleScholarGoogle Scholar |
Hutchinson KJ, King KL (1980) The effects of sheep stocking level on invertebrate abundance, biomass and energy utilization in a temperate, sown grassland. Journal of Applied Ecology 17, 369–387.
| The effects of sheep stocking level on invertebrate abundance, biomass and energy utilization in a temperate, sown grassland.Crossref | GoogleScholarGoogle Scholar |
Johnson DP, Catford JA, Driscoll DA, Gibbons P (2018) Seed addition and biomass removal key to restoring native forbs in degraded temperate grassland. Applied Vegetation Science 21, 219–228.
| Seed addition and biomass removal key to restoring native forbs in degraded temperate grassland.Crossref | GoogleScholarGoogle Scholar |
King KL, Hutchinson KJ (1976) The effects of sheep stocking intensity on the abundance and distribution of mesofauna in pastures. Journal of Applied Ecology 13, 41–55.
| The effects of sheep stocking intensity on the abundance and distribution of mesofauna in pastures.Crossref | GoogleScholarGoogle Scholar |
King KL, Hutchinson KJ (1983) The effects of sheep grazing on invertebrate numbers and biomass in unfertilized natural pastures of the New England Tablelands (NSW). Australian Journal of Ecology 8, 245–255.
| The effects of sheep grazing on invertebrate numbers and biomass in unfertilized natural pastures of the New England Tablelands (NSW).Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick J (1986) The viability of bush in cities – ten years of change in an urban grassy woodland. Australian Journal of Botany 34, 691–708.
| The viability of bush in cities – ten years of change in an urban grassy woodland.Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick JB (2007) Collateral benefit: unconscious conservation of threatened plant species. Australian Journal of Botany 55, 221–224.
| Collateral benefit: unconscious conservation of threatened plant species.Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick JB, Gilfedder L, Bridle K, Zacharek A (2005) The positive and negative conservation impacts of sheep grazing and other disturbances on the vascular plant species and vegetation of lowland subhumid Tasmania. Ecological Management & Restoration 6, 51–60.
| The positive and negative conservation impacts of sheep grazing and other disturbances on the vascular plant species and vegetation of lowland subhumid Tasmania.Crossref | GoogleScholarGoogle Scholar |
Knapp AK, Seastedt TR (1986) Detritus accumulation limits productivity of tallgrass prairie. Bioscience 36, 662–668.
| Detritus accumulation limits productivity of tallgrass prairie.Crossref | GoogleScholarGoogle Scholar |
Knapp AK, Smith MD (2001) Variation among biomes in temporal dynamics of aboveground primary production. Science 291, 481–484.
| Variation among biomes in temporal dynamics of aboveground primary production.Crossref | GoogleScholarGoogle Scholar | 11161201PubMed |
Landsberg J, James CD, Maconochie J, Nicholls AO, Stol J, Tynan R (2002) Scale-related effects of grazing on native plant communities in an arid rangeland region of South Australia. Journal of Applied Ecology 39, 427–444.
| Scale-related effects of grazing on native plant communities in an arid rangeland region of South Australia.Crossref | GoogleScholarGoogle Scholar |
Leach MK, Givnish TJ (1996) Ecological determinants of species loss in remnant prairies. Science 273, 1555–1558.
| Ecological determinants of species loss in remnant prairies.Crossref | GoogleScholarGoogle Scholar |
Leonard S, Kirkpatrick J, Marsden-Smedley J (2010) Variation in the effects of vertebrate grazing on fire potential between grassland structural types. Journal of Applied Ecology 47, 876–883.
| Variation in the effects of vertebrate grazing on fire potential between grassland structural types.Crossref | GoogleScholarGoogle Scholar |
Levin SA (1992) The problem of pattern and scale in ecology: the Robert H MacArthur award lecture. Ecology 73, 1943–1967.
| The problem of pattern and scale in ecology: the Robert H MacArthur award lecture.Crossref | GoogleScholarGoogle Scholar |
Lewis T, Clarke PJ, Reid N, Whalley RDB (2008) Perennial grassland dynamics on fertile plains: is coexistence mediated by disturbance? Austral Ecology 33, 128–139.
| Perennial grassland dynamics on fertile plains: is coexistence mediated by disturbance?Crossref | GoogleScholarGoogle Scholar |
Lewis T, Reid N, Clarke PJ, Whalley RDB (2010) Resilience of a high-conservation-value, semi-arid grassland on fertile clay soils to burning, mowing and ploughing. Austral Ecology 35, 464–481.
| Resilience of a high-conservation-value, semi-arid grassland on fertile clay soils to burning, mowing and ploughing.Crossref | GoogleScholarGoogle Scholar |
Long RL, Stevens JC, Griffiths EM, Adamek M, Powles SB, Merritt DJ (2011) Detecting karrikinolide responses in seeds of the Poaceae. Australian Journal of Botany 59, 610–619.
| Detecting karrikinolide responses in seeds of the Poaceae.Crossref | GoogleScholarGoogle Scholar |
Lundholm JT, Larson DW (2003) Relationships between spatial environmental heterogeneity and plant species diversity on a limestone pavement. Ecography 26, 715–722.
| Relationships between spatial environmental heterogeneity and plant species diversity on a limestone pavement.Crossref | GoogleScholarGoogle Scholar |
Lunt I (1995) Seed longevity of six native forbs in a closed Themeda triandra grassland. Australian Journal of Botany 43, 439–449.
| Seed longevity of six native forbs in a closed Themeda triandra grassland.Crossref | GoogleScholarGoogle Scholar |
Lunt ID (1997a) Effects of long-term vegetation management on remnant grassy forests and anthropogenic native grasslands in south-eastern Australia. Biological Conservation 81, 287–297.
| Effects of long-term vegetation management on remnant grassy forests and anthropogenic native grasslands in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Lunt ID (1997b) A multivariate growth-form analysis of grassland and forest forbs in south-eastern Australia. Australian Journal of Botany 45, 691–705.
| A multivariate growth-form analysis of grassland and forest forbs in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Lunt ID, Morgan JW (2002) Grasslands of southern Australia. In ‘Flammable Australia: the fire regimes and biodiversity of a continent’. (Eds RJ Williams, AM Gill, RA Bradstock) pp. 177–196. (CSIRO Publishing: Melbourne, Vic.)
Lunt ID, Prober SM, Morgan JW (2012) How do fire regimes affect ecosystem structure, function and diversity in grasslands and grassy woodlands of southern Australia. In ‘Flammable Australia: fire regimes, biodiversity and ecosystems in a changing world’. (Eds RJ Williams, AM Gill, RA Bradstock) pp. 253–270. (CSIRO Publishing: Melbourne, Vic.)
Martin G (2003) The role of small ground-foraging mammals in topsoil health and biodiversity: Implications to management and restoration. Ecological Management & Restoration 4, 114–119.
| The role of small ground-foraging mammals in topsoil health and biodiversity: Implications to management and restoration.Crossref | GoogleScholarGoogle Scholar |
McCracken DI, Tallowin JR (2004) Swards and structure: the interactions between farming practices and bird food resources in lowland grasslands. The Ibis 146, 108–114.
| Swards and structure: the interactions between farming practices and bird food resources in lowland grasslands.Crossref | GoogleScholarGoogle Scholar |
McDougall KL (1989) The re-establishment of Themeda triandra (kangaroo grass): implications for the restoration of grasslands. Technical Report Series No. 52, Arthur Rylah Institute for Environmental Research, Melbourne, Vic.
McDougall KL, Kirkpatrick JB (1993) ‘Conservation of lowland native grasslands in south-eastern Australia.’ (Worldwide Fund for Nature: Sydney, NSW)
McIntyre S (2005) Biodiversity attributes of different sward structures in grazed grassland. Ecological Management & Restoration 6, 71–73.
| Biodiversity attributes of different sward structures in grazed grassland.Crossref | GoogleScholarGoogle Scholar |
McIntyre S, Lavorel S (1994) How environmental and disturbance factors influence species composition in temperate Australian grasslands. Journal of Vegetation Science 5, 373–384.
| How environmental and disturbance factors influence species composition in temperate Australian grasslands.Crossref | GoogleScholarGoogle Scholar |
McIntyre S, Lavorel S (2007) A conceptual model of land use effects on the structure and function of herbaceous vegetation. Agriculture, Ecosystems & Environment 119, 11–21.
| A conceptual model of land use effects on the structure and function of herbaceous vegetation.Crossref | GoogleScholarGoogle Scholar |
McIntyre S, Martin TG (2001) Biophysical and human influences on plant species richness in grasslands: comparing variegated landscapes in subtropical and temperate regions. Austral Ecology 26, 233–245.
| Biophysical and human influences on plant species richness in grasslands: comparing variegated landscapes in subtropical and temperate regions.Crossref | GoogleScholarGoogle Scholar |
McQuillan PB (1999) The effect of changes in Tasmanian grasslands on the geometrid moth Tribe Xanthorhoini (Geometridae: Larentiinae). In ‘The other 99% the conservation and biodiversity of invertebrates’. (Eds W Ponder, D Lunney) pp. 121–129. (Surrey Beatty & Sons: Sydney, NSW)
Merritt DJ, Turner SR, Clarke S, Dixon KW (2007) Seed dormancy and germination stimulation syndromes for Australian temperate species. Australian Journal of Botany 55, 336–344.
| Seed dormancy and germination stimulation syndromes for Australian temperate species.Crossref | GoogleScholarGoogle Scholar |
Mokany K, Ash J, Roxburgh S (2008) Functional identity is more important than diversity in influencing ecosystem processes in a temperate native grassland. Journal of Ecology 96, 884–893.
| Functional identity is more important than diversity in influencing ecosystem processes in a temperate native grassland.Crossref | GoogleScholarGoogle Scholar |
Moore NA, Camac JS, Morgan JW (2019) Effects of drought and fire on resprouting capacity of 52 temperate Australian perennial native grasses. New Phytologist 221, 1424–1433.
| Effects of drought and fire on resprouting capacity of 52 temperate Australian perennial native grasses.Crossref | GoogleScholarGoogle Scholar | 30216446PubMed |
Morgan JW (1997) The effect of grassland gap size on establishment, growth and flowering of the endangered Rutidosis leptorrhynchoides (Asteraceae). Journal of Applied Ecology 34, 566–576.
| The effect of grassland gap size on establishment, growth and flowering of the endangered Rutidosis leptorrhynchoides (Asteraceae).Crossref | GoogleScholarGoogle Scholar |
Morgan JW (1998a) Comparative germination responses of 28 temperate grassland species. Australian Journal of Botany 46, 209–219.
| Comparative germination responses of 28 temperate grassland species.Crossref | GoogleScholarGoogle Scholar |
Morgan JW (1998b) Importance of canopy gaps for recruitment of some forbs in Themeda triandra-dominated grasslands in south-eastern Australia. Australian Journal of Botany 46, 609–627.
| Importance of canopy gaps for recruitment of some forbs in Themeda triandra-dominated grasslands in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Morgan JW (1999) Defining grassland fire events and the response of perennial plants to annual fire in temperate grasslands of south-eastern Australia. Plant Ecology 144, 127–144.
| Defining grassland fire events and the response of perennial plants to annual fire in temperate grasslands of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Morgan JW (2004) Bryophyte composition in a native grassland community subjected to different long-term fire regimes. Cunninghamia 8, 485–489.
Morgan JW (2006) Bryophyte mats inhibit germination of non-native species in burnt temperate native grassland remnants. Biological Invasions 8, 159–168.
| Bryophyte mats inhibit germination of non-native species in burnt temperate native grassland remnants.Crossref | GoogleScholarGoogle Scholar |
Morgan JW (2015) Biomass management in native grasslands. In ‘Land of sweeping plains: managing and restoring the native grasslands of south-eastern Australia’. (Eds NSG Williams, JW Morgan) pp. 201–222. (CSIRO Publishing: Melbourne, Vic.)
Morgan JW, Lunt ID (1999) Effects of time-since-fire on the tussock dynamics of a dominant grass (Themeda triandra) in a temperate Australian grassland. Biological Conservation 88, 379–386.
| Effects of time-since-fire on the tussock dynamics of a dominant grass (Themeda triandra) in a temperate Australian grassland.Crossref | GoogleScholarGoogle Scholar |
Morgan JW, Williams NSG (2015) The ecology and dynamics of temperate native grasslands in south-eastern Australia. In ‘Land of sweeping plains’. (Eds NSG Williams, A Marshall, JW Morgan) pp. 61–85. (CSIRO Publishing: Melbourne, Vic.)
Morgan JW, Dwyer JM, Price JN, Prober SM, Power SA, Firn J, Moore JL, Wardle GM, Seabloom EW, Borer ET, Camac JS, et al (2016) Species origin affects the rate of response to inter-annual growing season precipitation and nutrient addition in four Australian native grasslands. Journal of Vegetation Science 27, 1164–1176.
| Species origin affects the rate of response to inter-annual growing season precipitation and nutrient addition in four Australian native grasslands.Crossref | GoogleScholarGoogle Scholar |
Morgan JW, Fensham RJ, Godfree R, Foreman PW (2017) Australian tussock grasslands. In ‘Australian vegetation’. 3rd edn. (Ed. DA Keith) pp. 438–460. (Cambridge University Press: Cambridge, UK)
Munro NT, McIntyre S, Macdonald B, Cunningham SA, Gordon IJ, Cunningham RB, Manning AD (2019) Returning a lost process by reintroducing a locally extinct digging marsupial. PeerJ 7, e6622
| Returning a lost process by reintroducing a locally extinct digging marsupial.Crossref | GoogleScholarGoogle Scholar | 31497393PubMed |
Myers JA, Harms KE (2011) Seed arrival and ecological filters interact to assemble high-diversity plant communities. Ecology 92, 676–686.
| Seed arrival and ecological filters interact to assemble high-diversity plant communities.Crossref | GoogleScholarGoogle Scholar | 21608476PubMed |
Neale T, Carter R, Nelson T, Bourke M (2019) Walking together: a decolonising experiment in bushfire management on Dja Dja Wurrung country. Cultural Geographies 26, 341–359.
| Walking together: a decolonising experiment in bushfire management on Dja Dja Wurrung country.Crossref | GoogleScholarGoogle Scholar |
Neave H, Tanton M (1989) The effects of grazing by kangaroos and rabbits on the vegetation and the habitat of other fauna in the Tidbinbilla Nature Reserve, Australian Capital Territory. Wildlife Research 16, 337–351.
| The effects of grazing by kangaroos and rabbits on the vegetation and the habitat of other fauna in the Tidbinbilla Nature Reserve, Australian Capital Territory.Crossref | GoogleScholarGoogle Scholar |
New TR (2000) How useful are ant assemblages for monitoring habitat disturbance on grasslands in south eastern Australia? Journal of Insect Conservation 4, 153–159.
| How useful are ant assemblages for monitoring habitat disturbance on grasslands in south eastern Australia?Crossref | GoogleScholarGoogle Scholar |
Nimmo DG, Avitabile S, Banks SC, Bliege Bird R, Callister K, Clarke MF, Dickman CR, Doherty TS, Driscoll DA, Greenville AC, Haslem A, et al (2019) Animal movements in fire-prone landscapes. Biological Reviews of the Cambridge Philosophical Society 94, 981–998.
| Animal movements in fire-prone landscapes.Crossref | GoogleScholarGoogle Scholar | 30565370PubMed |
Noble JC, Mueller WJ, Detling JK, Pfitzner GH (2007) Landscape ecology of the burrowing bettong: warren distribution and patch dynamics in semiarid eastern Australia. Austral Ecology 32, 326–337.
| Landscape ecology of the burrowing bettong: warren distribution and patch dynamics in semiarid eastern Australia.Crossref | GoogleScholarGoogle Scholar |
O’Dwyer C, Attiwill PM (1999) A comparative study of habitats of the golden sun moth Synemon plana Walker (Lepidoptera: Castniidae): implications for restoration. Biological Conservation 89, 131–141.
| A comparative study of habitats of the golden sun moth Synemon plana Walker (Lepidoptera: Castniidae): implications for restoration.Crossref | GoogleScholarGoogle Scholar |
O’Halloran LR, Borer ET, Seabloom EW, MacDougall AS, Cleland EE, McCulley RL, Hobbie S, Harpole WS, DeCrappeo NM, Chu C, Bakker JD, et al (2013) Regional contingencies in the relationship between aboveground biomass and litter in the world’s grasslands. PLoS One 8, e54988
| Regional contingencies in the relationship between aboveground biomass and litter in the world’s grasslands.Crossref | GoogleScholarGoogle Scholar | 23405103PubMed |
Oksanen J (1996) Is the humped relationship between species richness and biomass an artefact due to plot size? Journal of Ecology 84, 293–295.
| Is the humped relationship between species richness and biomass an artefact due to plot size?Crossref | GoogleScholarGoogle Scholar |
Ooi M, Auld TD, Whelan RJ (2006) Dormancy and the fire-centric focus: germination of three Leucopogon species (Ericaceae) from south-eastern Australia. Annals of Botany 98, 421–430.
| Dormancy and the fire-centric focus: germination of three Leucopogon species (Ericaceae) from south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 16735409PubMed |
Pitt MD, Heady HF (1978) Responses of annual vegetation to temperature and rainfall patterns in northern California. Ecology 59, 336–350.
| Responses of annual vegetation to temperature and rainfall patterns in northern California.Crossref | GoogleScholarGoogle Scholar |
Price JN, Wong NK, Morgan JW (2010) Recovery of understorey vegetation after release from a long history of sheep grazing in a herb-rich woodland. Austral Ecology 35, 505–514.
| Recovery of understorey vegetation after release from a long history of sheep grazing in a herb-rich woodland.Crossref | GoogleScholarGoogle Scholar |
Price J, Tamme R, Gazol A, de Bello F, Takkis K, Uria-Diez J, Kasari L, Pärtel M (2017) Within-community environmental variability drives trait variability in species-rich grasslands. Journal of Vegetation Science 28, 303–312.
| Within-community environmental variability drives trait variability in species-rich grasslands.Crossref | GoogleScholarGoogle Scholar |
Prober SM, Wiehl G (2012) Relationships among soil fertility, native plant diversity and exotic plant abundance inform restoration of forb-rich eucalypt woodlands. Diversity & Distributions 18, 795–807.
| Relationships among soil fertility, native plant diversity and exotic plant abundance inform restoration of forb-rich eucalypt woodlands.Crossref | GoogleScholarGoogle Scholar |
Prober SM, Thiele KR, Lunt ID (2007) Fire frequency regulates tussock grass composition, structure and resilience in endangered temperate woodlands. Austral Ecology 32, 808–824.
| Fire frequency regulates tussock grass composition, structure and resilience in endangered temperate woodlands.Crossref | GoogleScholarGoogle Scholar |
Prober SM, Lunt ID, Thiele KR (2008) Effects of fire frequency and mowing on a temperate, derived grassland soil in south-eastern Australia. International Journal of Wildland Fire 17, 586–594.
| Effects of fire frequency and mowing on a temperate, derived grassland soil in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Prober SM, Thiele KR, Speijers J (2013) Management legacies shape decadal-scale responses of plant diversity to experimental disturbance regimes in fragmented grassy woodlands. Journal of Applied Ecology 50, 376–386.
| Management legacies shape decadal-scale responses of plant diversity to experimental disturbance regimes in fragmented grassy woodlands.Crossref | GoogleScholarGoogle Scholar |
Reading RP, Clark TW, Seebeck JH, Pearce J (1996) Habitat suitability index model for the eastern barred bandicoot, Perameles gunnii. Wildlife Research 23, 221–235.
| Habitat suitability index model for the eastern barred bandicoot, Perameles gunnii.Crossref | GoogleScholarGoogle Scholar |
Richter A, Osborne W, Hnatiuk S, Rowell A (2013) Moths in fragments: insights into the biology and ecology of the Australian endangered golden sun moth Synemon plana (Lepidoptera: Castniidae) in natural temperate and exotic grassland remnants. Journal of Insect Conservation 17, 1093–1104.
| Moths in fragments: insights into the biology and ecology of the Australian endangered golden sun moth Synemon plana (Lepidoptera: Castniidae) in natural temperate and exotic grassland remnants.Crossref | GoogleScholarGoogle Scholar |
Ross J (1999) ‘Guide to best practice conservation of temperate native grasslands.’ (WWF Australia: Sydney, NSW)
Scarlett NH, Parsons RF (1982) Rare plants of the Victorian plains. In ‘Species at risk: research in Australia’. (Eds R Groves, WDL Ride) pp. 89–105. (Australian Academy of Science: Canberra, ACT)
Schultz NL, Morgan JW, Lunt ID (2011) Effects of grazing exclusion on plant species richness and phytomass accumulation vary across a regional productivity gradient. Journal of Vegetation Science 22, 130–142.
| Effects of grazing exclusion on plant species richness and phytomass accumulation vary across a regional productivity gradient.Crossref | GoogleScholarGoogle Scholar |
Schultz NL, Reid N, Lodge G, Hunter JT (2016) Habitat specificity analyses reveal the importance of grazing refugia for plant diversity conservation in a warm-temperate grassy ecosystem. Applied Vegetation Science 19, 578–588.
| Habitat specificity analyses reveal the importance of grazing refugia for plant diversity conservation in a warm-temperate grassy ecosystem.Crossref | GoogleScholarGoogle Scholar |
Schultz N, Keatley M, Antos M, Wong N, Moxham C, Farmilo B, Morgan JW (2017) The golf ball method for rapid assessment of grassland structure. Ecological Management & Restoration 18, 134–140.
| The golf ball method for rapid assessment of grassland structure.Crossref | GoogleScholarGoogle Scholar |
Scroggie MP, Peterson GNL, Rohr DH, Nicholson E, Heard GW (2019) Disturbance has benefits as well as costs for fragmented populations of a cryptic grassland reptile. Landscape Ecology 34, 1949–1965.
Seastedt TR, Briggs JM, Gibson DJ (1991) Controls of nitrogen limitation in tallgrass prairie. Oecologia 87, 72–79.
| Controls of nitrogen limitation in tallgrass prairie.Crossref | GoogleScholarGoogle Scholar | 28313354PubMed |
Shahan JL, Goodwin BJ, Rundquist BC (2017) Grassland songbird occurrence on remnant prairie patches is primarily determined by landscape characteristics. Landscape Ecology 32, 971–988.
| Grassland songbird occurrence on remnant prairie patches is primarily determined by landscape characteristics.Crossref | GoogleScholarGoogle Scholar |
Sinclair SJ, Duncan DH, Bruce MJ (2014) Mortality of native grasses after a summer fire in natural temperate grassland suggests ecosystem instability. Ecological Management & Restoration 15, 91–94.
| Mortality of native grasses after a summer fire in natural temperate grassland suggests ecosystem instability.Crossref | GoogleScholarGoogle Scholar |
Smith AL, Barrett RL, Milner RNC (2018) Annual mowing maintains plant diversity in threatened temperate grasslands. Applied Vegetation Science 21, 207–218.
| Annual mowing maintains plant diversity in threatened temperate grasslands.Crossref | GoogleScholarGoogle Scholar |
Stawski C, Matthews JK, Körtner G, Geiser F (2015) Physiological and behavioural responses of a small heterothermic mammal to fire stimuli. Physiology & Behavior 151, 617–622.
| Physiological and behavioural responses of a small heterothermic mammal to fire stimuli.Crossref | GoogleScholarGoogle Scholar |
Stevens TA, Evans MC, Osborne WS, Sarre SD (2010) Home ranges of, and habitat use by, the grassland earless dragon (Tympanocryptis pinguicolla) in remnant native grasslands near Canberra. Australian Journal of Zoology 58, 76–84.
| Home ranges of, and habitat use by, the grassland earless dragon (Tympanocryptis pinguicolla) in remnant native grasslands near Canberra.Crossref | GoogleScholarGoogle Scholar |
Stuwe J, Parsons RF (1977) Themeda australis grasslands on the Basalt Plains, Victoria: floristics and management effects. Australian Journal of Ecology 2, 467–476.
| Themeda australis grasslands on the Basalt Plains, Victoria: floristics and management effects.Crossref | GoogleScholarGoogle Scholar |
Thompson K, Ooi MKJ (2010) To germinate or not to germinate: more than just a question of dormancy. Seed Science Research 20, 209–211.
| To germinate or not to germinate: more than just a question of dormancy.Crossref | GoogleScholarGoogle Scholar |
Tremont R (1994) Life-history attributes of plants in grazed and ungrazed grasslands on the northern tablelands of New South Wales. Australian Journal of Botany 42, 511–530.
| Life-history attributes of plants in grazed and ungrazed grasslands on the northern tablelands of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Vening GS, Guja LK, Spooner PG, Price JN (2017) Seed dormancy and germination of three grassy woodland forbs required for diverse restoration. Australian Journal of Botany 65, 625–637.
| Seed dormancy and germination of three grassy woodland forbs required for diverse restoration.Crossref | GoogleScholarGoogle Scholar |
Verrier FJ, Kirkpatrick JB (2005) Frequent mowing is better than grazing for the conservation value of lowland tussock grasssland at Pontville, Tasmania. Austral Ecology 30, 74–78.
| Frequent mowing is better than grazing for the conservation value of lowland tussock grasssland at Pontville, Tasmania.Crossref | GoogleScholarGoogle Scholar |
Vesk PA, Westoby M (2001) Predicting plant species’ responses to grazing. Journal of Applied Ecology 38, 897–909.
| Predicting plant species’ responses to grazing.Crossref | GoogleScholarGoogle Scholar |
Vines GR, Noble JC, Marsden SG (2004) Australian rainfall patterns and the southern oscillation. 1. A continental perspective. Pacific Conservation Biology 10, 28–48.
| Australian rainfall patterns and the southern oscillation. 1. A continental perspective.Crossref | GoogleScholarGoogle Scholar |
Williams NSG (2007) Environmental, landscape and social predictors of native grassland loss in western Victoria, Australia. Biological Conservation 137, 308–318.
| Environmental, landscape and social predictors of native grassland loss in western Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Williams NSG, Morgan JW, McCarthy MA, McDonnell MJ (2006) Local extinction of grassland plants: the landscape matrix is more important than patch attributes. Ecology 87, 3000–3006.
| Local extinction of grassland plants: the landscape matrix is more important than patch attributes.Crossref | GoogleScholarGoogle Scholar |
Williams NSG, Marshall A, Morgan JW (2015) ‘Land of sweeping plains.’ (CSIRO Publishing: Melbourne, Vic.)
Wong N, Morgan JW (2007) Review of grassland management in south eastern Australia. Parks Victoria Technical Report No. 39. Parks Victoria, Melbourne Vic.
Wong NK, Morgan JW (2012) Experimental changes in disturbance type do not induce short-term shifts in plant community structure in three semi-arid grasslands of the Victorian Riverine Plain managed for nature conservation. Ecological Management & Restoration 13, 175–182.
| Experimental changes in disturbance type do not induce short-term shifts in plant community structure in three semi-arid grasslands of the Victorian Riverine Plain managed for nature conservation.Crossref | GoogleScholarGoogle Scholar |
Zamin TJ, Jolly A, Sinclair S, Morgan JW, Moore JL (2018) Enhancing plant diversity in a novel grassland using seed addition. Journal of Applied Ecology 55, 215–224.
| Enhancing plant diversity in a novel grassland using seed addition.Crossref | GoogleScholarGoogle Scholar |
Zeeman BJ, Morgan JW (2018) Increasing and declining native species in urban remnant grasslands respond differently to nitrogen addition and disturbance. Annals of Botany 121, 691–697.
| Increasing and declining native species in urban remnant grasslands respond differently to nitrogen addition and disturbance.Crossref | GoogleScholarGoogle Scholar | 29324992PubMed |
Zeeman BJ, McDonnell MJ, Kendal D, Morgan JW (2017) Biotic homogenization in an increasingly urbanized temperate grassland ecosystem. Journal of Vegetation Science 28, 550–561.
| Biotic homogenization in an increasingly urbanized temperate grassland ecosystem.Crossref | GoogleScholarGoogle Scholar |
Zehm A, Nobis M, Schwabe A (2003) Multiparameter analysis of vertical vegetation structure based on digital image processing. Flora - Morphology, Distribution, Functional Ecology of Plants 198, 142–160.
| Multiparameter analysis of vertical vegetation structure based on digital image processing.Crossref | GoogleScholarGoogle Scholar |
Zimmer HC, Turner VB, Mavromihalis J, Dorrough J, Moxham C (2010) Forb responses to grazing and rest management in a critically endangered Australian native grassland ecosystem. The Rangeland Journal 32, 187–195.
| Forb responses to grazing and rest management in a critically endangered Australian native grassland ecosystem.Crossref | GoogleScholarGoogle Scholar |



