Unassisted invasions: understanding and responding to Australia’s high-impact environmental grass weeds
Rieks D. van Klinken A C and Margaret H. Friedel BA CSIRO, EcoSciences Precinct, PO Box 2583, Brisbane, Qld 4001, Australia.
B CSIRO, PO Box 2114, Alice Springs, NT 0871, Australia.
C Corresponding author. Email: rieks.vanklinken@csiro.au
Australian Journal of Botany 65(8) 678-690 https://doi.org/10.1071/BT17152
Submitted: 24 August 2017 Accepted: 5 December 2017 Published: 11 January 2018
Journal compilation © CSIRO 2017 Open Access CC BY-NC-ND
Abstract
Alien grass species have been intentionally introduced into Australia since European settlement over 200 years ago, with many subsequently becoming weeds of natural environments. We have identified the subset of these weeds that have invaded and become dominant in environmentally important areas in the absence of modern anthropogenic disturbance, calling them ‘high-impact species’. We also examined why these high-impact species were successful, and what that might mean for management. Seventeen high-impact species were identified through literature review and expert advice; all had arrived by 1945, and all except one were imported intentionally, 16 of the 17 were perennial and four of the 17 were aquatic. They had become dominant in diverse habitats and climates, although some environments still remain largely uninvaded despite apparently ample opportunities. Why these species succeeded remains largely untested, but evidence suggests a combination of ecological novelty (both intended at time of introduction and unanticipated), propagule pressure (through high reproductive rate and dominance in nearby anthropogenically-disturbed habitats) and an ability to respond to, and even alter, natural disturbance regimes (especially fire and inundation). Serious knowledge gaps remain for these species, but indications are that resources could be better focused on understanding and managing this limited group of high-impact species. They require new management approaches, especially to counteract the advantages of ecological novelty, reduce propagule pressure and better direct the large-scale disturbance regimes that continue to shape plant communities across Australia.
Additional keywords: disturbance regime, ecological novelty, invasive plants, pasture grass, Poaceae, propagule pressure.
Introduction
Poaceae is one of the largest, most cosmopolitan and ecologically important of the flowering plant families globally with ~10 000 species (McCusker 2002; Clayton et al. 2006). Due to their value for crops, pasture, horticultural amenity and land rehabilitation, grasses have been deliberately redistributed globally since at least the mid-19th century (Russell 2001; Lazarides 2002; Cook and Dias 2006; Martins et al. 2009). Many successful introductions have subsequently led to alien grasses becoming dominant in diverse and often non-target ecosystems, causing profound ecosystem impacts and important conservation challenges (Mack 1989; D’Antonio and Vitousek 1992; Knapp 1996). In Australia the introduction history of invasive grasses is relatively well documented (Thorp and Lynch 2000; Groves et al. 2005; Cook and Dias 2006; Setterfield et al. 2010; Friedel et al. 2011; van Klinken et al. 2013), and so we have an opportunity to ask a central question in invasion biology: what is it that allows species to invade and dominate environmentally important areas, even without the assistance of anthropogenic disturbance? We use ‘anthropogenic disturbance’ to describe post-European settlement practices such as livestock grazing, vegetation clearance, burning and roadside slashing, in contrast to ‘natural disturbance’, which includes pre-European indigenous fire regimes, floods and windstorms.
Current literature offers numerous hypotheses and predictors of invasion success, especially concerning the demographic consequences of species traits, environment and their interactions (Colautti et al. 2006; Richardson and Pyšek 2006; Catford et al. 2009; van Kleunen et al. 2010; Gurevitch et al. 2011). However, invasiveness is generally not a good predictor of impact (Ricciardi and Cohen 2007; van Klinken et al. 2013): ruderal species, for example, can be highly invasive yet may cause little environmental impact (van Klinken et al. 2013). By contrast, there has been relatively little attention paid to predictors of alien species that cause serious impact. A recent analysis of invasive alien tropical grasses in Australia identified some limited predictors for grass species causing serious environmental impacts. The analysis concluded that improved prediction would require a deeper understanding of the circumstances in which impact occurs – whether in environmental, pastoral or agricultural sectors (van Klinken et al. 2013).
In the present work, we identified those ‘high impact’ alien grass species that have invaded and become dominant in Australia without the assistance of anthropogenic disturbance and asked why these have been so successful, with the intention of improving management focus and outcomes. Since the availability of data on impacts is limited, we took a qualitative approach, through surveying experts, literature review and analysis. We did so by:
-
extending the list of tropical grass species that currently cause serious environmental impact in Australia (see van Klinken et al. 2013) to include temperate species;
-
providing a national overview of the habitats that have, and have not, become dominated by invasive alien grasses; and
-
identifying and assessing hypotheses as to why some invasive alien grasses have had a high environmental impact in Australia.
Results are discussed from the perspective of how to better anticipate and manage such species into the future.
Identifying alien grass species in Australia with high environmental impact
Species with high environmental impact were defined following van Klinken et al. (2013) as those species that have become dominant (that is, the species with the highest percent herbaceous cover) on land managed for environmental values as the result of natural spread (implying an ability to invade), and not dependent on human-related disturbance (e.g. excludes roadsides that are regularly slashed, high-use areas such as campgrounds, and land that has historically had heavy, prolonged grazing). Dominance was used as a surrogate for impact because environmental impact of most alien grass species has not been quantified. Specific examples where all criteria were met were required for a species to be considered as high impact. Details of the methodology are provided in Table S1, available as Supplementary Material to this paper.
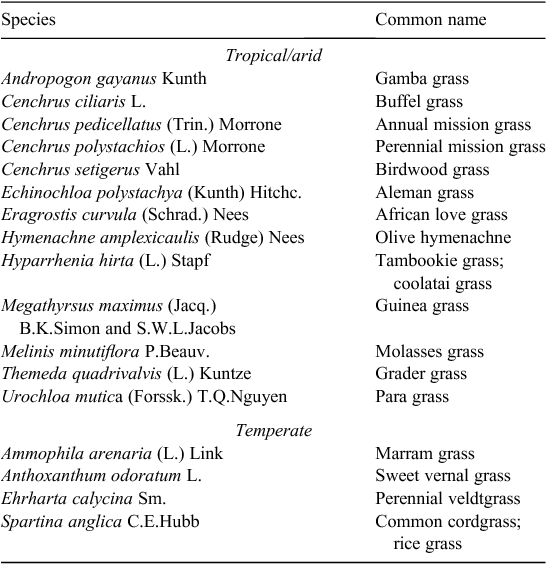
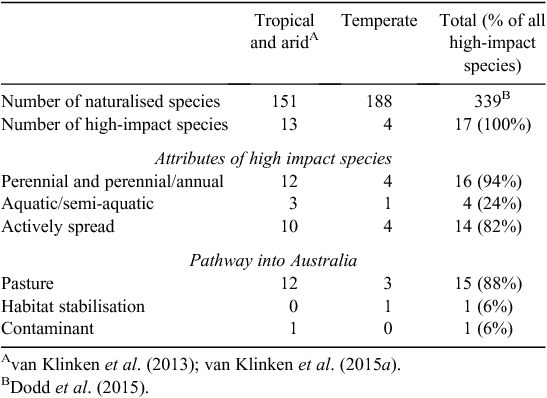
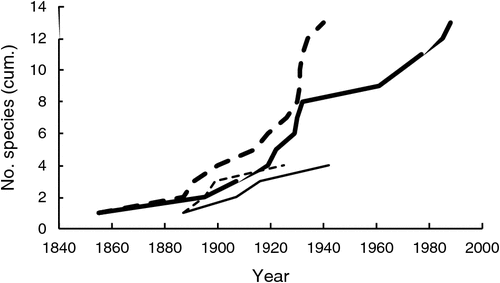
A total of 17 species met the criteria for being high impact (Table 1), or 5.0% of all naturalised species (Table 2). There were three times as many high-impact tropical species as temperate species, despite the number of naturalised species being similar in both regions. Most high-impact species were perennial, having been introduced for use in pastures and subsequently actively spread due to their perceived benefits. The timing of first records and naturalisation for the first six tropical species were quite similar to that of temperate species which had all naturalised by 1945 (Fig. 1). However, the next seven tropical species were rapidly introduced during the late 1920s to 1940, but it took until 1988 for all to be considered naturalised. This activity was the direct result of an active pasture improvement program conducted in northern Australia throughout much of the twentieth century (Cook and Dias 2006; van Klinken et al. 2013).
|
|
|
Finding evidence of species meeting our criteria for high impact was difficult, even for many of the species on the final list (Table 1). Published observations were rare, normally focused on ‘threat’ rather than impact, and they generally provided few insights into the disturbance regimes under which invasion occurred. All high-impact species were widely distributed but some, such as African love grass (Eragrostis curvula (Schrad.) Nees) and grader grass (Themeda quadrivalvis (L.) Kuntze), met our criteria only in quite localised circumstances (Table S1). Many species listed as high-impact environmental weeds by other authors (Thorp and Lynch 2000; Groves 2003) did not meet our stricter and more explicit criteria. For example, the relatively well studied Chilean needle grass (Nassella neesiana (Trin. & Rupr.) Barkworth) is ranked as one of the 20 ‘Weeds of National Significance’ (Thorp and Lynch 2000) for its pastoral and environmental impact, but was not included due its apparent requirement for high levels of anthropogenic disturbance to facilitate invasion (Faithful 2012).
Invasion outcomes: which ecosystems have been invaded?
A wide range of tropical, subtropical, temperate, arid and semiarid ecosystems have been transformed by high-impact grasses (Fig. 2).
Under temperate climates only one high-impact grass, rice grass (Spartina anglica C.E.Hubb.), occurred in aquatic systems, in estuarine mudflats (Hindell and Warry 2010). Demonstration of invasion under natural disturbance regimes was difficult in temperate grasslands and woodlands as these habitats were typically fragmented and subjected to significant anthropogenic disturbance (Faithful 2012). The presence of invasive grasses was often part of a ‘syndrome’ of problems (Mack 1989; Faithful 2012). Examples of high-impact grasses were therefore limited to restricted, but often environmentally significant, habitats. These grasses include marram grass (Ammophila arenaria (L.) Link) on beaches, sweet vernal grass (Anthoxanthum odoratum L.) in alpine habitats and perennial veldtgrass (Ehrharta calycina Sm.) in Banksia woodlands.
The greatest diversity of high-impact grasses is present in the woodlands with grass understoreys that occur across about a third of tropical and subtropical Australia. Of these the most spectacular is gamba grass (Andropogon gayanus Kunth), which grows to 4 m in height and becomes dominant through altering fire regimes (Setterfield et al. 2010). It has the capacity to invade all but the wetter savanna habitats (Flores et al. 2005). Perennial mission grass (Cenchrus polystachios (L.) Morrone) can grow in the same areas as gamba grass, while annual mission grass (Cenchrus pedicellatus (Trin.) Morrone) readily invades dry savanna habitats (Australian Government: Department of the Environment and Energy 2012).
All of these high-impact grasses also commonly dominate habitats such as roadsides, forest margins, clearings and riparian zones with a history of severe anthropogenic disturbance. Finding evidence of invasion and dominance without the assistance of anthropogenic disturbance was difficult for some, such as grader grass, guinea grass (Megathyrsus maximus (Jacq.) B.K.Simon & S.W.L.Jacobs) and molasses grass (Melinis minutiflora P.Beauv.). Most records for these species are from highly disturbed areas and records rarely provide information on disturbance history (see Table S1).
Subtropical and tropical wetlands are frequently heavily invaded by high-impact grasses, with extensive monocultures occurring even under limited anthropogenic disturbance. Olive hymenachne (Hymenachne amplexicaulis (Rudge) Nees) and Aleman grass (Echinochloa polystachya (Kunth) Hitchc.) can grow in water to a depth of 2.5 m or more, the latter preferring seasonal flooding, whereas para grass (Urochloa mutica (Forssk.) T.Q.Nguyen) grows on seasonally flooded soils, in shallower water (Hannon-Jones and Weber 2008; Wearne et al. 2010; Bayliss et al. 2012; Queensland Department of Agriculture and Fisheries 2012, 2016).
Buffel grass (Cenchrus ciliaris L.) and several other Cenchrus species are causing serious impacts in arid Australia. The extent of invasion by buffel grass and the diversity of habitats it invades means it ranks as among the most serious environmental weeds in Australia (Australian Government: Department of Environment 2015; Martin et al. 2015).
The environmental impacts of high-impact grass species are expected to continue to increase, with most still having relatively restricted distributions compared with their potential distribution. Buffel grass is the main threat: modelling suggests that ~68% of mainland Australia is vulnerable to invasion (Lawson et al. 2004), across a broad range of habitats (Clarke et al. 2005; Martin et al. 2015). Gamba grass is expected to have the potential to invade 70% of Australian upland savanna communities (Petty et al. 2012). However, disturbance requirements are generally not considered in species distribution modelling which can therefore result in weed potential being seriously overestimated (Murray et al. 2012): some habitats may be susceptible to invasion in the absence of anthropogenic disturbance, whereas others may require disturbance before invasion can occur.
Invasion outcomes: limits to grass invasion success
Some environments remain largely uninvaded. Understanding why is important for conservation: will they inevitably succumb to invasion as well, or is that an unlikely outcome or one that can be prevented?
Failure to invade and become dominant under relatively natural disturbance regimes can simply be due to weeds having insufficient time to do so. The lack of propagule pressure (Colautti et al. 2006) certainly explains some apparent invasion failures, especially when combined with infrequent invasion opportunities such as rare high rainfall years in arid regions. In fact, there are several examples where such lag phases have led to spurious conclusions regarding either the limits of tolerance of the invading species or the resistance of communities to invasion. For example, gamba grass was not expected by pasture scientists to readily invade intact savannas (Cameron and Lemcke 2006) but this has proved to be incorrect (Brooks et al. 2010). Likewise, buffel grass was initially thought to be limited to mesic parts of arid environments, but has since established on spinifex sandplains (hummock grasslands) and rocky terrain (e.g. Dixon et al. 2002; Binks et al. 2005; Puckey et al. 2007; Murray et al. 2012).
Nonetheless, some environments do remain largely uninvaded despite apparently ample opportunity. Notable examples include native perennial grasslands covering black soil plains across vast areas of northern Australia (Fensham et al. 2014; R Silcock pers. comm.), much of the hummock or spinifex grasslands on arid sandplains, dunefields and mountain ranges that are typically dominated by native Triodia species (Griffin 1984), and many intact forests, including those with native grass understoreys (often dominated by kangaroo grass (Themeda triandra Forssk.)) in south-eastern Queensland (Fig. 3). Generally speaking, disturbance regimes in native habitats that maintain dense natural ground or tree cover are least likely to be invaded by alien grasses (McIvor 2003; Eyre et al. 2009; Robbins 2009). Modelling showed that there was a critical threshold of ~30% retained native woodland vegetation in semiarid woodlands of eastern Australia, above which buffel grass was not likely to occur in woodland fragments (Eyre et al. 2009). The inability of buffel grass to invade above this threshold was attributed to insufficient propagule pressure from the grass, suppression of seedling emergence by native plant litter, competition from established plants and limited bare ground in the absence of disturbance.
What makes high-impact species special?
There are many hypotheses regarding how species become invasive, and various syntheses of those. However, little specific attention has been given to hypotheses as to why some species go on to become ‘high impact’. A recent quantitative analysis of high-impact grass species in northern Australia found no strong predictors, and concluded that future analyses needed to take account of the individual circumstances under which some invasive plants cause serious impact (van Klinken et al. 2013). Standard correlative approaches were hampered by lack of data, even for relatively well studied species, and by the small number of species that actually become high impact.
We identified three inter-related hypotheses as being especially important in allowing some species to become dominant without the assistance of anthropogenic disturbance: (i) ecological novelty, (ii) colonisation and propagule pressure, and (iii) favourable natural disturbance regimes. A broad review of the history of introduction, combined with a literature analysis conducted on each of the high impact species (Web of Science, using species names, main synonyms and widely used common names; broader web-based searches of grey literature, and grey literature sourced from the Australian weed research community) were used to assess evidence for and against these main hypotheses. We hope that this case-study approach will ultimately lead to more systematic testing of hypotheses relating to why some species can invade and become dominant in natural environments.
Ecological novelty
From the perspective of species invasions, we define ecological novelty as species characteristics that confer particular advantages in invaded environments over existing flora (‘limiting similarity’, Catford et al. 2009). Ecological novelty has been the driving motivation for importing new species into Australia. Thus species were actively sought to fill what were viewed as empty or depauperate niches (Hindell and Warry 2010), to address environmental issues such as erosion that resulted from intensifying land use practices (Lonsdale 1994; Cook and Dias 2006; Dear and Ewing 2008), and to meet ongoing demand for aesthetic novelty in ornamental plants (Groves et al. 2005). All but one of the 17 high-impact species were introduced intentionally (Table 2), in each case to address a perceived need that was not being met by the existing flora. The potential of native species to meet these needs was not investigated in any depth until the 1970s, after more than a century of plant importation and selection (Cook and Dias 2006). In most cases the primary focus of grass introductions was pasture improvement; other goals included addressing salinity issues, sand dune stabilisation, mud-flat reclamation (and its conversion to pasture) and erosion control (Cook and Dias 2006).
Australian native grasses generally evolved under relatively low grazing pressures exerted by marsupials, they developed early flowering to ensure seed set in response to unpredictable climates, thereby limiting their nutritive value to animals later in the season, and they are often slow growing (Cook and Clem 2000; Sinclair 2002; Orians and Milewski 2007). It was commonly assumed that native grasses were inadequate as pasture (Lonsdale 1994; Cook and Dias 2006). As a result, pasture research in Australia was broadly aimed at finding high production perennial pasture species that were tolerant of heavy, prolonged grazing, would prevent erosion, and could grow in diverse climates and soil types (Cook et al. 2005; Cook and Dias 2006). In temperate regions this also included addressing salinity challenges (Dear and Ewing 2008). African grasses for example were sought that were adapted to heavy grazing by large wild herds and their associated disturbances, and under less variable moisture conditions (Cook and Clem 2000; Foxcroft et al. 2010). Some, such as buffel grass, are now viewed as critical to pasture production in parts of northern Australia (Friedel et al. 2011) and several underpin grazing in southern Australia (Mack 1989; Dear and Ewing 2008). These same adaptations have helped some of these alien species to become dominant in natural habitats, even under limited anthropogenic disturbance.
An important subset of introduced pasture grasses was the so-called ponded pasture species (e.g. Aleman grass, para grass and olive hymenachne), which were semi-aquatic and selected to combine with ‘water catching infrastructure’ in northern Australia (Wildin 1991). Several species were successfully imported and commercialised, and were considered far superior to corresponding native species (Wildin and Chapman 1987; Wildin 1991). For example the introduced ponded pasture species, olive hymenachne, was found to be a much better performer than the native congener and ecological analogue, Hymenachne acutigluma (Steud.) Gilliland, which had a lower photosynthetic rate at cooler temperatures and reduced photosynthetic leaf area when flooded (Kibbler and Bahnisch 1999).
Naturalised species have also often demonstrated unanticipated ecological novelty. Many of the pasture grass introductions have different fire responses to native species (see below regarding disturbance regimes). For example, the native savanna biota evolved with frequent but relatively low intensity fire, and is poorly adapted to the hot canopy fires generated by gamba grass (Setterfield et al. 2010). In a comparative study perennial veldtgrass shared several important traits with other native fire ephemerals and ‘fire weeds’ in Western Australia that allowed perennial veldt grass to utilise the increased availability of nutrients following fire (Fisher et al. 2006). Other ecological novelties have also been identified retrospectively among the high-impact species. These include an ability to perform well under high-light conditions (Robbins 2009), rapid post-rain germination (Keir and Vogler 2006; Vogler and Owen 2008), broad germination requirements that include the ability to germinate in drier soils than native species (Chejara et al. 2008) and the ability to rapidly exploit phosphorus through fast-growing root systems (Christie 1975; Christie and Moorby 1975). However, the potential of such ecological novelties to enable species to successfully invade and become dominant without the assistance of anthropogenic disturbance still needs to be properly tested.
Colonisation and propagule pressure
The more grass species that are introduced (colonisation pressure) and the greater the number and size of plantings for each species (propagule pressure), the greater the number of new species that will overcome stochastic events that could limit successful establishment and spread (Lockwood et al. 2009).
Colonisation pressure for grasses entering Australia has been very high since European settlement, perhaps greater than for any other plant family. No other taxon, with the possible exception of legumes, has been so targeted for importation and, for a subset of species, establishment across vast areas of Australia (Winter et al. 1985; Lonsdale 1994; Cook and Dias 2006). Approximately 22% of the global grass flora (2250 species) have been recorded as being intentionally imported into Australia since formal records began in 1929 (Cook and Dias 2006), including all the high-impact species with the exception of rice grass. The fates of many of the introduced accessions are not known, although many were planted out in a network of experimental field stations across Australia (Winter et al. 1985; Lonsdale 1994; Cook and Dias 2006). High colonisation pressure can help explain why so many high-impact species established widely in Australia, especially the last several tropical species to become naturalised (Fig. 1), but not why they became high impact.
High propagule pressure can account for invasion of areas of low anthropogenic disturbance (Panetta and Hopkins 1991; Robbins 2009; Fensham et al. 2013), although its role in those species subsequently becoming dominant is less clear. Many of the high-impact species produce large numbers of seeds (Smith et al. 1999; Keir and Vogler 2006; Martins et al. 2009). In contrast, the widespread native kangaroo grass, which is often displaced by alien grasses, produces relatively few seeds and is a poor invader and disperser (Everson et al. 2009). Most high-impact species have also been actively spread and planted (Table 2) and may be dominant in habitats adjacent to environmental reserves, and on linear features such as transport corridors and disturbed riparian strips, as has been observed with buffel grass in arid Australia (Griffin 1993; van Vreeswyk et al. 2004). However, very high propagule pressure on its own is insufficient to facilitate invasion into adjacent environmental reserves for many of these species, such as grader grass, which generally also requires bare ground to invade under relatively natural disturbance regimes (Table S1; van Klinken et al. 2013).
Ability to invade under natural disturbance regimes
The ability to invade and become dominant under natural disturbance regimes is a pre-requisite for high-impact species as we define them here. Disturbance regimes are often critical in shaping plant communities, including providing opportunities for aliens to invade, dominate and persist. Altered or newly imposed disturbance regimes can be an important mechanism for plant invasion (Hobbs and Humphries 1995; Moles et al. 2012), by increasing resource availability or resetting succession (Colautti et al. 2006; Catford et al. 2009). However, most literature addresses situations where human disturbance is high, pervasive and long term. In fact, a common theme is the resilience of natural vegetation in Australia to invasion by most grass (and other plant) species in the absence of anthropogenic disturbance (McIvor 2003; Loo et al. 2009; Catford et al. 2011).
Teasing apart causes of successful invasions remains challenging (e.g. see Eyre et al. 2009), and we found few studies that specifically examined the relative role of natural versus anthropogenic disturbance in aiding successful establishment of alien grasses. One exception is Chilean needle grass, which is recognised as one of the worst environmental weeds in Australia, dominating grasslands, many of which are endangered native grasslands (McLaren et al. 2004). However, invasion by this species has generally been facilitated by anthropogenic disturbance (Faithful 2012) such as slashing activities, although once dominant it can remain so even with the subsequent cessation of anthropogenic disturbance (van Klinken et al. 2015b). In many cases multiple causes of disturbance have been implicated. The widespread adoption of buffel grass as a pasture species in north-western Western Australia a century ago coincided with loss of native flora due to overgrazing (van Vreeswyk et al. 2004). The subsequent invasion of the Pilbara Region (Western Australia) was assisted by massive flood events (Mitchell and Leighton 1998), drought and continued overgrazing. In fact, widespread grass introductions were often intended to address the negative impacts of imposed disturbance as well as provide improved pasture production (Payne et al. 2004; Cook and Dias 2006).
Some natural disturbances can provide opportunities for initial invasion whereas others can enhance invasion once species are present as propagules or established plants. Drought and flooding create opportunities for invasion, whereas fire commonly enhances invasions already underway (D’Antonio and Vitousek 1992). Other more localised disturbances, such as shifting dunes, can also assist invasion (e.g. marram grass). In most cases evidence suggests that invasion or dominance followed changes in disturbance regimes, albeit natural in the case of drought and flood. This supports a recent meta-analysis that found changing disturbance regimes was a better predictor of invasive species richness and relative cover than was disturbance per se (Moles et al. 2012).
Drought
Drought appears to be an important agent in initial invasion by high-impact species, at least in arid and semiarid Australia. In examples we located, grazing was not currently occurring although it had at some time in the past. In a central Australian National Park, buffel grass became dominant in the 1990s when drought in the mid-1980s reduced native grass cover (Clarke et al. 2005). Buffel grass also expanded after a return to above average rainfall following a drought period in semiarid Queensland in the period 2005–2011 (Fensham et al. 2013) and, in the semiarid tropics, it increased rapidly after a severe drought followed by above average rainfall in the period 1992–2001 (Ash et al. 2011). Invasion by buffel grass after high rainfall reported by Griffin (1993) for the 1970s was also preceded by drought conditions (Griffin and Friedel 1985). Climate and the build-up of inorganic nitrogen following loss of native species during drought could be triggers for invasion (Ash et al. 2011).
Inundation
Four of the high-impact species were aquatic, including more tropical species than expected from overall naturalisations (van Klinken et al. 2013). In some cases they were invading relatively empty niches such as mudflats (see above); in others they appeared better adapted to inundation regimes than native species, thereby becoming dominant (Ferdinands et al. 2005).
Major floods can open up opportunities for invasion, but we found no evidence that natural flooding alone was sufficient for environmental weeds to invade and become dominant. Riparian zones are often highly invaded (Lawes and Grice 2010), but we found little support for widespread invasion in the absence of post-European anthropogenic disturbance. Riparian zones are frequently highly modified by human activity, including through altered flow and fire regimes, grazing, nutrient addition and clearing of vegetation to the edge of the riparian corridor (Richardson et al. 2007; Loo et al. 2009). This confluence of disturbance regimes can favour alien grasses. For example, guinea grass and signal grass (Urochloa decumbens (Stapf) R.D.Webster) can dominate in eastern Australia, especially where clearing has provided a high-light understorey and large invasion front (Robbins 2009).
Fire
Evidence of fire-assisted invasion of land not subject to anthropogenic disturbance was elusive. For example, no evidence was found that fire enhanced the invasion of a central Queensland woodland by buffel grass (Fensham et al. 2013). A study of buffel grass invasion in a Sonoran desert ecosystem in the USA reached the same conclusion (Olsson et al. 2012). However there is widespread evidence that, once established, invading grasses can alter the existing fire regime to their own advantage (grass-fire positive feedback cycle; D’Antonio and Vitousek 1992; Brooks et al. 2010; Miller et al. 2010). Some invasions result in fires becoming more intense and more frequent (e.g. gamba grass (Rossiter et al. 2003); perennial veldtgrass (Baird 1977); buffel grass (Butler and Fairfax 2003; Miller et al. 2010). The altered fire regimes in savannas and open woodlands can increase shrub and tree mortality and prevent recruitment, so that the vegetation structure shifts towards open grasslands dominated by alien species (Rossiter et al. 2003; Miller et al. 2010). The fire-generated transition from savanna woodland to gamba grassland can occur within a decade (Brooks et al. 2010). This transition can be assisted by increased nitrogen losses during fires (Rossiter-Rachor et al. 2009) and other invasion-related changes in soil nutrients and water required by native species (Rossiter-Rachor et al. 2009), resulting in the crossing of an ‘abiotic threshold’ beyond which ecosystem function is very different (Brooks et al. 2010).
Managing the conservation challenge
As a group, alien grasses have clearly been outstandingly successful invaders in Australia. Environmental impacts are already substantial and will increase further. This situation is by no means unique to Australia, given temperate grass invaders in North and South America (Mack 1989), and buffel grass in southern USA and northern Mexico (Arriaga et al. 2004). However, significant environmental impacts are not inevitable. For example, few grasses have been identified as important environmental invaders in analogous environments in Africa (Rahlao et al. 2009; Foxcroft et al. 2010), although they are becoming increasingly prevalent (Milton 2004). Here, we identify broad management responses that are required to mitigate the current and future environmental impacts of invasive grasses in Australia, for conservation outcomes and more generally. These derive from our three main hypotheses for invasion success: ecological novelty, colonisation and propagule pressure, and changes in natural disturbance regimes.
Sources of ecological novelty
High-impact alien species have many ecological novelties when compared with native flora, both intentionally sought and unintended ones. However, more empirical work is needed to determine to what extent such novelty is responsible for those species invading and becoming dominant without the assistance of anthropogenic disturbance. In some cases this may help identify management solutions, such as maintaining forest cover to counter the advantage many high-impact species have under high light (Amor and Stevens 1976; Robbins 2009).
It appears that the ecological novelties enabling grass species to invade in the absence of anthropogenic disturbance are rare among the entire global pool of grass species. Of the relatively few species (17) that met our criteria for high impact in Australia, a number could only do so in relatively limited situations. Also, many environments such as low-light habitats, heavy clay soils and nutrient-poor soils remain relatively uninvaded, despite often significant efforts to source species worldwide that could invade and dominate there (Cook and Dias 2006).
One potentially important source for ecological novelty is new cultivars of existing species, or the hybridisation of cultivars that are already present in Australia (Friedel et al. 2006). Australian legislation does not prevent importation of cultivars of species already in Australia (Spafford-Jacob et al. 2004), and this loophole needs to be closed. For example, introduction of ‘Frio’, a frost-tolerant buffel grass cultivar bred in the United States (Hussey and Burson 2005), could expose new areas within Australia to unacceptable risk of invasion. Furthermore, future potential high-impact species may already be naturalised in Australia, for instance ornamentals imported by nursery and garden industries, but have so far lacked the opportunity to invade suitable areas, or else have long residence times. Continued vigilance is required to recognise such species in time for preventative actions to be taken.
Colonisation and propagule pressure
The rates of naturalisations are not increasing in Australia for the flora overall (Dodd et al. 2015), and both importation (‘colonisation pressure’) and naturalisation rates are approaching historical lows at least for tropical grasses (van Klinken et al. 2015a). It therefore appears that existing quarantine restrictions are effective, provided no new entry pathways arise, and existing regulations and efforts are not weakened. Even in the absence of strongly supported predictors of high-impact grass species (van Klinken et al. 2013), existing pre-border weed risk assessment (e.g. Pheloung et al. 1999; Auld 2012) are providing a strong barrier to introduction of any new species without detailed examination.
High propagule pressure is an important means by which some high-impact species invade and dominate environmental reserves. This pressure can potentially be reduced through carefully-timed herbicide application, fire and/or grazing management practices (Friedel et al. 2011) but, inevitably, these will also introduce new disturbance regimes and may only be applicable to relatively small areas. Biological control offers the prospect of reducing propagule pressure over large areas without altering disturbance regimes. In South Africa biological control has been used successfully to reduce seed production without affecting the value of highly invasive but beneficial woody weeds (Van Wilgen et al. 2001). No releases of bio-control agents have been made against the current high-impact grasses in Australia (Julien and Griffiths 1998).
Disturbance regimes
Our review shows that very few grass species have the ability to invade and dominate under natural disturbance regimes in Australia. Managing disturbance therefore becomes the critical tool for managing invasions. Once alien grasses become dominant, the process will often be difficult to reverse. Monitoring for early signs of invasion, and interpreting them in the context of current and historical disturbance regimes, may give time to alter disturbance regimes in conservation lands before this happens.
Disturbance regimes have changed dramatically across most of Australia since European settlement, and continue to do so (Moore 1970; Mack 1989; White 1997; Dyer et al. 2001; Groves and Whalley 2002; Russell-Smith et al. 2003; Clarke et al. 2005; Diamond 2005; Lindsay and Cunningham 2011), but we still have only a rudimentary understanding as to how disturbance regimes in the most susceptible habitats can be managed to minimise or reduce invasion and dominance by alien grasses. Large-scale disturbance regimes offer both opportunities and threats for managing invasive grasses for conservation outcomes. Paradoxically, imposing anthropogenic disturbance regimes, such as strategic grazing, within conservation reserves may help suppress high impact environmental alien grasses (Popay and Field 1996; Friedel et al. 2011). Prescriptions for optimal fire regimes continue to be refined in different parts of Australia in response to competing land uses (Russell-Smith et al. 2009; Friedel et al. 2014) and major ongoing changes in water management and flow regimes are expected in Australia’s largest catchment, the Murray-Darling Basin (Pittock and Connell 2010) and elsewhere. However, implications for grass invasions need to be better integrated into land management recommendations and prescriptions.
The challenge remains to identify ways that natural resource management regimes can be shaped to minimise the success and impact of invasive grasses. A distinction can be made between structural and functional responses, that is, the mechanical manipulation of ecosystem structure v. the manipulation of interactions and dynamics of ecosystems. Structural intervention is less likely than functional intervention to be sustainable (e.g. removing and replacing undesirable species v. altering the disturbance regime to favour desirable species) (King and Hobbs 2006). Similarly a distinction can be made between biotic and abiotic thresholds of degradation which present barriers to ecosystem recovery, abiotic thresholds (e.g. soil erosion) potentially posing the greater obstacle. This framework was applied to tropical savannas invaded by gamba grass and perennial mission grass (Brooks et al. 2010). It was concluded that biotic changes had occurred, both structural (biomass increase) and functional (fire-mediated nitrogen loss), but that abiotic changes (soil erosion and sealing) generally had not. Removal of the alien grasses from less-impacted areas might therefore initiate recovery, and manipulation of fire regimes might slow the degradation process. Hawaiian pili grasslands (Heteropogon contortus (L.) P.Beauv. ex Roem. and Schult.) were successfully returned to pili grass dominance after invasion by buffel grass in an experiment that combined removal of buffel grass with seeding of H. contortus, watering and fire (Daehler and Goergen 2005). In Australia perennial veldtgrass abundance decreased following fire exclusion (Baird 1977).
Conclusions
Globally, the management of existing high-impact alien grass species for conservation outcomes presents major challenges because available management options are not always effective or feasible at the scales required (Chambers and Wisdom 2009; Brooks et al. 2010; Grice et al. 2012). Our investigation of alien grasses in Australia suggests that despite over a century of systematic introduction and redistribution of alien grass species, and naturalisation of 339 of them, very few (we have identified 17) are capable of invading and becoming dominant under natural disturbance regimes and of those most can only do so under a limited set of circumstances. Designing and maintaining appropriate disturbance regimes, through ongoing study, monitoring and adaptive management (Foxcroft and McGeoch 2011) will be critical for these species.
Wide-scale management of most high-impact species has remained intractable and new tools and approaches are required. Management options include the related objectives of counteracting the ecological novelties that confer advantage on alien species (for example, through increasing forest cover levels), reducing propagule pressure (for example, through timing of fire management activities) and creating disturbance regimes that favour the native community or disadvantage alien grasses. To date most attention has been on restoration (Daehler and Goergen 2005; Tjelmeland et al. 2008; Brooks et al. 2010); however, beyond localised asset protection, it remains unrealistic for extensive and remote conservation lands, and may also not in itself lead to the stable recovery of ecosystems (Reid et al. 2009). In these cases, disturbance management, bio-control and possibly the application of new genetic approaches such as CRISPR-based gene drive technology (Webber et al. 2015) offer the greatest hope. There will also be circumstances where the economic, social or environmental cost of interventions is so great as to be impractical or undesirable, in which case the invaded environment becomes the ‘new normal’. The decision to intervene will depend on the values held for the invaded environment, and the benefits and costs of intervention.
Where high-impact invasive grasses are of commercial value, the social and economic dimensions of proposed management cannot be ignored (Marshall et al. 2011). For example broad scale solutions like bio-control could impact livelihoods of individuals and communities, even if conservation outcomes are desirable. In the case of buffel grass, an Australian study has shown that diverse stakeholders were able to agree broadly on positive and negative impacts, and objectives and tools for management, for production and conservation lands (Friedel et al. 2011), leaving room for optimism.
There are opportunities to devise policies that support management objectives for invasive alien grasses (Grice et al. 2012). Any policy development would require consultation with all stakeholders. Goals would need to apply to a range of spatial scales, and to take into account regional differences in biophysical and socioeconomic attributes and the benefits and costs to all stakeholders. Although some policy might be legislated, other measures could include codes of practice, insurance mechanisms and certification. Where multiple varieties of a species occur, new varieties should not be developed or distributed and, if some varieties are more problematic than others, control should focus on the most detrimental (Grice et al. 2012).
High-impact weed species are ‘low probability but high consequence’ and as such are difficult to study as a group, and may defy generalisation. A more explicit focus on what allows ‘high-impact species’ to cause unaided harm to our most important natural environments is needed. Causes of impact remains relatively unexplored within the broader discipline of invasion biology, but are where new management approaches are likely to be found.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgements
We thank Richard Groves and Helen Murphy for providing comments on an earlier draft, the Global Invasions Network for helping to motivate this work, and the many people who generously provided advice on the status of candidate species (see Table S1, available as Supplementary Material to this paper).
References
Amor R, Stevens P (1976) Spread of weeds from a roadside into sclerophyll forests at Dartmouth, Australia. Weed Research 16, 111–118.| Spread of weeds from a roadside into sclerophyll forests at Dartmouth, Australia.Crossref | GoogleScholarGoogle Scholar |
Arriaga L, Castellanos AE, Moreno E, Alarcón J (2004) Potential ecological distribution of alien invasive species and risk assessment: a case study of buffel grass in arid regions of Mexico. Conservation Biology 18, 1504–1514.
| Potential ecological distribution of alien invasive species and risk assessment: a case study of buffel grass in arid regions of Mexico.Crossref | GoogleScholarGoogle Scholar |
Ash AJ, Corfield JP, McIvor JG, Ksiksi TS (2011) Grazing management in tropical savannas: utilization and rest strategies to manipulate rangeland condition. Rangeland Ecology and Management 64, 223–239.
| Grazing management in tropical savannas: utilization and rest strategies to manipulate rangeland condition.Crossref | GoogleScholarGoogle Scholar |
Auld B (2012) An overview of pre-border weed risk assessment and post-border weed risk management protocols. Plant Protection Quarterly 27, 105–111.
Australian Government: Department of Environment (2015) ‘Threat abatement advice for ecosystem degradation, habitat loss and species decline in arid and semi-arid Australia due to the invasion of buffel grass (Cenchrus ciliaris and C. pennisetiformis).’ Available at http://www.environment.gov.au/biodiversity/threatened/threat-abatement-advices/buffel-grass-introduction [Verified 8 December 2017].
Australian Government: Department of the Environment and Energy (2012) ‘Background: threat abatement plan to reduce the impacts on northern Australia’s biodiversity by the five listed grasses.’ Available at http://www.environment.gov.au/biodiversity/threatened/publications/threat-abatement-plan-reduce-impacts-northern-australias-biodiversity-five-listed-grasses [Verified 8 December 2017].
Baird A (1977) Regeneration after fire in King’s Park, Perth, Western Australia. Journal of the Royal Society of Western Australia 60, 1–22.
Bayliss P, van Dam RA, Bartolo RE (2012) Quantitative ecological risk assessment of the Magela Creek floodplain in Kakadu National Park, Australia: comparing point source risks from the Ranger Uranium Mine to diffuse landscape-scale risks. Human and Ecological Risk Assessment 18, 115–151.
| Quantitative ecological risk assessment of the Magela Creek floodplain in Kakadu National Park, Australia: comparing point source risks from the Ranger Uranium Mine to diffuse landscape-scale risks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XovFGmtw%3D%3D&md5=ee7e9e0833baf3a08dc1ad264e341082CAS |
Binks R, Cann A, Perks S, Silla A, Young M (2005) The effect of introduced buffel grass (Cenchrus ciliaris L.) on terrestrial invertebrate communities in the Pilbara region, Western Australia. Honours thesis. School of Animal Biology, University of Western Australia, Perth, WA.
Brooks KJ, Setterfield SA, Douglas MM (2010) Exotic grass invasions: applying a conceptual framework to the dynamics of degradation and restoration in Australia’s tropical savannas. Restoration Ecology 18, 188–197.
| Exotic grass invasions: applying a conceptual framework to the dynamics of degradation and restoration in Australia’s tropical savannas.Crossref | GoogleScholarGoogle Scholar |
Butler BDW, Fairfax RJ (2003) Buffel grass and fire in a gidgee and brigalow woodland: a case study from central Queensland. Ecological Management & Restoration 4, 120–125.
| Buffel grass and fire in a gidgee and brigalow woodland: a case study from central Queensland.Crossref | GoogleScholarGoogle Scholar |
Cameron A, Lemcke B (2006) ‘Kent gamba grass.’ Northern Territory Department of Primary Industry, Fisheries and Mines. Agnote E8. Available at https://dpir.nt.gov.au/__data/assets/pdf_file/0017/233522/413.pdf [Verified 8 December 2017].
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Diversity & Distributions 15, 22–40.
| Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework.Crossref | GoogleScholarGoogle Scholar |
Catford JA, Vesk PA, White MD, Wintle BA (2011) Hotspots of plant invasion predicted by propagule pressure and ecosystem characteristics. Diversity & Distributions 17, 1099–1110.
| Hotspots of plant invasion predicted by propagule pressure and ecosystem characteristics.Crossref | GoogleScholarGoogle Scholar |
Chambers JC, Wisdom MJ (2009) Priority research and management issues for the imperiled Great Basin of the western United States. Restoration Ecology 17, 707–714.
| Priority research and management issues for the imperiled Great Basin of the western United States.Crossref | GoogleScholarGoogle Scholar |
Chejara VK, Kristiansen P, Whalley RDB, Sindel BM, Nadolny C (2008) Factors affecting germination of Coolatai grass (Hyparrhenia hirta). Weed Science 56, 543–548.
| Factors affecting germination of Coolatai grass (Hyparrhenia hirta).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXotlKitLw%3D&md5=7ed59d0f575fc19f67dcd8b81863989cCAS |
Christie E (1975) A note on the significance of Eucalyptus populnea for buffel grass production in infertile semi-arid rangelands. Tropical Grasslands 9, 243–246.
Christie E, Moorby J (1975) Physiological responses of semiarid grasses. I. The influence of phosphorus supply on growth and phosphorus absorption. Crop and Pasture Science 26, 423–436.
| Physiological responses of semiarid grasses. I. The influence of phosphorus supply on growth and phosphorus absorption.Crossref | GoogleScholarGoogle Scholar |
Clarke PJ, Latz PK, Albrecht DE (2005) Long-term changes in semi-arid vegetation: Invasion of an exotic perennial grass has larger effects than rainfall variability. Journal of Vegetation Science 16, 237–248.
| Long-term changes in semi-arid vegetation: Invasion of an exotic perennial grass has larger effects than rainfall variability.Crossref | GoogleScholarGoogle Scholar |
Clayton W, Vorontsova M, Harman K, Williamson H (2006) ‘GrassBase – The online world grass flora.’ Available at http://www.kew.org/data/grasses-db.html [Verified 8 December 2017].
Colautti RI, Grigorovich IA, MacIsaac HJ (2006) Propagule pressure: a null model for biological invasions. Biological Invasions 8, 1023–1037.
| Propagule pressure: a null model for biological invasions.Crossref | GoogleScholarGoogle Scholar |
Cook B, Clem R (2000) Which grass for where? Tropical Grasslands 34, 156–161.
Cook GD, Dias L (2006) It was no accident: deliberate plant introductions by Australian government agencies during the 20th century. Australian Journal of Botany 54, 601–625.
| It was no accident: deliberate plant introductions by Australian government agencies during the 20th century.Crossref | GoogleScholarGoogle Scholar |
Cook BG, Pengelly BC, Brown SD, Donnelly JL, Eagles DA, Franco MA, Hanson J, Mullen BF, Partridge IJ, Peters M, Schultze-Kraft R (2005) ‘Tropical forages: an interactive selection tool.’ Available at http://www.tropicalforages.info/ [Verified 8 December 2017].
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annual Review of Ecology and Systematics 23, 63–87.
| Biological invasions by exotic grasses, the grass/fire cycle, and global change.Crossref | GoogleScholarGoogle Scholar |
Daehler CC, Goergen EM (2005) Experimental restoration of an indigenous Hawaiian grassland after invasion by buffel grass (Cenchrus ciliaris). Restoration Ecology 13, 380–389.
| Experimental restoration of an indigenous Hawaiian grassland after invasion by buffel grass (Cenchrus ciliaris).Crossref | GoogleScholarGoogle Scholar |
Dear B, Ewing M (2008) The search for new pasture plants to achieve more sustainable production systems in southern Australia. Animal Production Science 48, 387–396.
| The search for new pasture plants to achieve more sustainable production systems in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Diamond J (2005) ‘Collapse: how societies choose to fail or succeed.’ (Penguin: New York)
Dixon I, Dixon K, Barrett M (2002) Eradication of buffel grass (Cenchrus ciliaris) on Airlie Island, Pilbara Coast, Western Australia. In ‘Turning the tide: eradication of invasive species’. (Eds CR Veitch, MN Clout) pp. 92–101. (IUCN SSC Invasive Species Specialist Group: Gland, Switzerland)
Dodd AJ, Burgman MA, McCarthy MA, Ainsworth N (2015) The changing patterns of plant naturalization in Australia. Diversity & Distributions 21, 1038–1050.
| The changing patterns of plant naturalization in Australia.Crossref | GoogleScholarGoogle Scholar |
Dyer R, Jacklyn P, Partridge I, Russell-Smith J, Williams D (2001) ‘Savanna burning: understanding and using fire in northern Australia.’ (Tropical Savannas Co-operative Research Centre: Darwin, NT)
Everson T, Yeaton R, Everson C (2009) Seed dynamics of Themeda triandra in the montane grasslands of South Africa. African Journal of Range & Forage Science 26, 19–26.
| Seed dynamics of Themeda triandra in the montane grasslands of South Africa.Crossref | GoogleScholarGoogle Scholar |
Eyre TJ, Wang J, Venz MF, Chilcott C, Whish G (2009) Buffel grass in Queensland’s semi-arid woodlands: response to local and landscape scale variables, and relationship with grass, forb and reptile species. The Rangeland Journal 31, 293–305.
| Buffel grass in Queensland’s semi-arid woodlands: response to local and landscape scale variables, and relationship with grass, forb and reptile species.Crossref | GoogleScholarGoogle Scholar |
Faithful IG (2012) Biodiversity impacts of Chilean needle grass Nassella neeisiana on Australia’s indigenous grasslands. PhD thesis. School of Engineering and Science, Victoria University, Melbourne, Vic.
Fensham RJ, Donald S, Dwyer JM (2013) Propagule pressure, not fire or cattle grazing, promotes invasion of buffel grass Cenchrus ciliaris. Journal of Applied Ecology 50, 138–146.
| Propagule pressure, not fire or cattle grazing, promotes invasion of buffel grass Cenchrus ciliaris.Crossref | GoogleScholarGoogle Scholar |
Fensham RJ, Silcock JL, Firn J (2014) Managed livestock grazing is compatible with the maintenance of plant diversity in semidesert grasslands. Ecological Applications 24, 503–517.
| Managed livestock grazing is compatible with the maintenance of plant diversity in semidesert grasslands.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2cjjvVCgtw%3D%3D&md5=4c213433c701279388abcd2a6112b184CAS |
Ferdinands K, Beggs K, Whitehead P (2005) Biodiversity and invasive grass species: multiple-use or monoculture? Wildlife Research 32, 447–457.
| Biodiversity and invasive grass species: multiple-use or monoculture?Crossref | GoogleScholarGoogle Scholar |
Fisher JL, Veneklaas EJ, Lambers H, Loneragan WA (2006) Enhanced soil and leaf nutrient status of a Western Australian Banksia woodland community invaded by Ehrharta calycina and Pelargonium capitatum. Plant and Soil 284, 253–264.
| Enhanced soil and leaf nutrient status of a Western Australian Banksia woodland community invaded by Ehrharta calycina and Pelargonium capitatum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntF2isrY%3D&md5=065c1233be5774cd4d0e88f3f949b0b4CAS |
Flores TA, Setterfield SA, Douglas MM (2005) Seedling recruitment of the exotic grass Andropogon gayanus (Poaceae) in northern Australia. Australian Journal of Botany 53, 243–249.
| Seedling recruitment of the exotic grass Andropogon gayanus (Poaceae) in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Foxcroft LC, McGeoch M (2011) Implementing invasive species management in an adaptive management framework. Koedoe 53, 105–115.
| Implementing invasive species management in an adaptive management framework.Crossref | GoogleScholarGoogle Scholar |
Foxcroft LC, Richardson DM, Rejmánek M, Pyšek P (2010) Alien plant invasions in tropical and sub-tropical savannas: patterns, processes and prospects. Biological Invasions 12, 3913–3933.
| Alien plant invasions in tropical and sub-tropical savannas: patterns, processes and prospects.Crossref | GoogleScholarGoogle Scholar |
Friedel M, Puckey H, O’Malley C, Waycott M, Smyth A, Miller G (2006) ‘Buffel grass: both friend and foe. An evaluation of the advantages and disadvantages of buffel grass use and recommendations for future research.’ (Desert Knowledge Co-operative Research Centre: Alice Springs, NT)
Friedel MH, Grice AC, Marshall NA, van Klinken RD (2011) Reducing contention amongst organisations dealing with commercially valuable but invasive plants: the case of buffel grass. Environmental Science & Policy 14, 1205–1218.
| Reducing contention amongst organisations dealing with commercially valuable but invasive plants: the case of buffel grass.Crossref | GoogleScholarGoogle Scholar |
Friedel M, Allan GE, Duguid A (2014) Do we know enough about vegetation dynamics to manage fire regimes in central Australia? Ecological Management & Restoration 15, 128–132.
| Do we know enough about vegetation dynamics to manage fire regimes in central Australia?Crossref | GoogleScholarGoogle Scholar |
Grice AC, Friedel MH, Marshall NA, Van Klinken RD (2012) Tackling contentious invasive plant species: a case study of buffel grass in Australia. Environmental Management 49, 285–294.
| Tackling contentious invasive plant species: a case study of buffel grass in Australia.Crossref | GoogleScholarGoogle Scholar |
Griffin GF (1984) Hummock grasslands. In ‘Management of Australia’s rangelands’. (Eds GN Harrington, AD Wilson, MD Young) pp. 271–284. (CSIRO Publishing: Melbourne, Vic.)
Griffin G (1993) The spread of buffel grass in inland Australia: land use conflicts. In ‘Proceedings I: 10th Australian Weeds Conference and 14th Asian Pacific Weed Science Society Conference’. pp. 501–504. (Weed Society of Queensland: Brisbane, Qld.)
Griffin G, Friedel M (1985) Discontinuous change in central Australia: some implications of major ecological events for land management. Journal of Arid Environments 9, 63–80.
Groves RH (2003) ‘Weed categories for natural and agricultural ecosystem management.’ (Bureau of Rural Sciences: Canberra, ACT)
Groves R, Whalley R (2002) Grass and grassland ecology in Australia. Flora of Australia 43, 157–182.
Groves RH, Boden R, Lonsdale WM (2005) ‘Jumping the garden fence: invasive garden plants in Australia and their environmental and agricultural impacts.’ WWF-Australia: Ultimo, NSW, Australia. Available at http://awsassets.wwf.org.au/downloads/sp090_jumping_the_garden_fence_invasive_1feb05.pdf [Verified 8 December 2017].
Gurevitch J, Fox GA, Wardle GM, Inderjit , Taub D (2011) Emergent insights from the synthesis of conceptual frameworks for biological invasions. Ecology Letters 14, 407–418.
| Emergent insights from the synthesis of conceptual frameworks for biological invasions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mvls1Sgug%3D%3D&md5=40c3070d0c774e10edbfe2bf10c66e6cCAS |
Hannon-Jones M, Weber J (2008) ‘Pest plant risk assessment: Aleman grass (Echinochloa polystachya).’ (Department of Primary Industries and Fisheries: Brisbane, Qld)
Hindell J, Warry F (2010) Nutritional support of estuary perch (Macquaria colonorum) in a temperate Australian inlet: evaluating the relative importance of invasive Spartina. Estuarine, Coastal and Shelf Science 90, 159–167.
| Nutritional support of estuary perch (Macquaria colonorum) in a temperate Australian inlet: evaluating the relative importance of invasive Spartina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlehtLjL&md5=ccef2c16097083847608c407959c4d90CAS |
Hobbs RJ, Humphries SE (1995) An integrated approach to the ecology and management of plant invasions. Conservation Biology 9, 761–770.
| An integrated approach to the ecology and management of plant invasions.Crossref | GoogleScholarGoogle Scholar |
Hussey M, Burson B (2005) Registration of ‘Frio’ buffelgrass. Crop Science 45, 411–412.
| Registration of ‘Frio’ buffelgrass.Crossref | GoogleScholarGoogle Scholar |
Julien M, Griffiths M (1998) ‘Biological control of weeds: a world catalogue of agents and their target weeds.’ (CABI Publishing: New York)
Keir AF, Vogler WD (2006) A review of current knowledge of the weedy species Themeda quadrivalvis (grader grass). Tropical Grasslands 40, 193–201.
Kibbler H, Bahnisch L (1999) Distribution of Hymenachne acutigluma (Steudel) Guilliland in ponded pasture is limited by photosynthetic response to temperature. Australian Journal of Experimental Agriculture 39, 437–443.
| Distribution of Hymenachne acutigluma (Steudel) Guilliland in ponded pasture is limited by photosynthetic response to temperature.Crossref | GoogleScholarGoogle Scholar |
King EG, Hobbs RJ (2006) Identifying linkages among conceptual models of ecosystem degradation and restoration: towards an integrative framework. Restoration Ecology 14, 369–378.
| Identifying linkages among conceptual models of ecosystem degradation and restoration: towards an integrative framework.Crossref | GoogleScholarGoogle Scholar |
Knapp PA (1996) Cheatgrass (Bromus tectorum L.) dominance in the Great Basin Desert: history, persistence, and influences to human activities. Global Environmental Change 6, 37–52.
| Cheatgrass (Bromus tectorum L.) dominance in the Great Basin Desert: history, persistence, and influences to human activities.Crossref | GoogleScholarGoogle Scholar |
Lawes R, Grice A (2010) War of the weeds: competition hierarchies in invasive species. Austral Ecology 35, 871–878.
| War of the weeds: competition hierarchies in invasive species.Crossref | GoogleScholarGoogle Scholar |
Lawson B, Bryant M, Franks A (2004) Assessing the potential distribution of buffel grass (Cenchrus ciliaris L.) in Australia using a climate-soil model. Plant Protection Quarterly 19, 155–163.
Lazarides M (2002) Economic attributes of Australian grasses. Flora of Australia 43, 213–244.
Lindsay EA, Cunningham SA (2011) Native grass establishment in grassy woodlands with nutrient enriched soil and exotic grass invasion. Restoration Ecology 19, 131–140.
| Native grass establishment in grassy woodlands with nutrient enriched soil and exotic grass invasion.Crossref | GoogleScholarGoogle Scholar |
Lockwood JL, Cassey P, Blackburn TM (2009) The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Diversity & Distributions 15, 904–910.
| The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology.Crossref | GoogleScholarGoogle Scholar |
Lonsdale WM (1994) Inviting trouble – introduced pasture species in northern Australia. Australian Journal of Ecology 19, 345–354.
| Inviting trouble – introduced pasture species in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Loo SE, Mac Nally R, O’Dowd DJ, Thomson JR, Lake P (2009) Multiple scale analysis of factors influencing the distribution of an invasive aquatic grass. Biological Invasions 11, 1903–1912.
| Multiple scale analysis of factors influencing the distribution of an invasive aquatic grass.Crossref | GoogleScholarGoogle Scholar |
Mack RN (1989) Temperate grasses vulnerable to plant invasions: characteristics and consequences. In ‘Biological invasions: a global perspective. 1989 SCOPE edn’. (Wiley: Chichester, UK)
Marshall N, Friedel M, Van Klinken R, Grice A (2011) Considering the social dimension of invasive species: the case of buffel grass. Environmental Science & Policy 14, 327–338.
| Considering the social dimension of invasive species: the case of buffel grass.Crossref | GoogleScholarGoogle Scholar |
Martin TG, Murphy H, Liedloff A, Thomas C, Chadès I, Cook G, Fensham R, McIvor J, van Klinken RD (2015) Buffel grass and climate change: a framework for projecting invasive species distributions when data are scarce. Biological Invasions 17, 3197–3210.
| Buffel grass and climate change: a framework for projecting invasive species distributions when data are scarce.Crossref | GoogleScholarGoogle Scholar |
Martins CR, Hay JD, Carmona R (2009) Invasion potential of two cultivars of Melinis minutiflora in the Brazilian Cerrado – seed characteristics and seedling establishment. Revista Árvore 33, 713–722.
| Invasion potential of two cultivars of Melinis minutiflora in the Brazilian Cerrado – seed characteristics and seedling establishment.Crossref | GoogleScholarGoogle Scholar |
McCusker A (2002) Poaceae: family description. Flora of Australia 43, 1–3.
McIvor JG (2003) Competition affects survival and growth of buffel grass seedlings – is buffel grass a coloniser or an invader? Tropical Grasslands 37, 176–181.
McLaren D, Stajsic V, Iaconis L (2004) The distribution, impacts and identification of exotic stipoid grasses in Australia. Plant Protection Quarterly 19, 59–66.
Miller G, Friedel M, Adam P, Chewings V (2010) Ecological impacts of buffel grass (Cenchrus ciliaris L.) invasion in central Australia – does field evidence support a fire-invasion feedback? The Rangeland Journal 32, 353–365.
| Ecological impacts of buffel grass (Cenchrus ciliaris L.) invasion in central Australia – does field evidence support a fire-invasion feedback?Crossref | GoogleScholarGoogle Scholar |
Milton SJ (2004) Grasses as invasive alien plants in South Africa. South African Journal of Science 100, 69–75.
Mitchell A, Leighton K (1998) ‘Report on damage caused by the flood of the Ashburton River in February 1997.’ (Agriculture Western Australia: Perth, WA)
Moles AT, Flores-Moreno H, Bonser SP, Warton DI, Helm A, Warman L, Eldridge DJ, Jurado E, Hemmings FA, Reich PB, Cavender-Bares J, Seabloom EW, Mayfield MM, Sheil D, Djietror JC, Peri PL, Enrico L, Cabido MR, Setterfield SA, Lehmann CER, Thomson FJ (2012) Invasions: the trail behind, the path ahead, and a test of a disturbing idea. Journal of Ecology 100, 116–127.
| Invasions: the trail behind, the path ahead, and a test of a disturbing idea.Crossref | GoogleScholarGoogle Scholar |
Moore RM (1970) Australian grasslands. In ‘Australian grasslands.’ (Ed. RM Moore) pp. 87–100. (Australian National University Press: Canberra, ACT)
Murray JV, Stokes KE, Klinken RD (2012) Predicting the potential distribution of a riparian invasive plant: the effects of changing climate, flood regimes and land‐use patterns. Global Change Biology 18, 1738–1753.
| Predicting the potential distribution of a riparian invasive plant: the effects of changing climate, flood regimes and land‐use patterns.Crossref | GoogleScholarGoogle Scholar |
Olsson AD, Betancourt J, McClaran MP, Marsh SE (2012) Sonoran Desert ecosystem transformation by a C4 grass without the grass/fire cycle. Diversity & Distributions 18, 10–21.
| Sonoran Desert ecosystem transformation by a C4 grass without the grass/fire cycle.Crossref | GoogleScholarGoogle Scholar |
Orians GH, Milewski AV (2007) Ecology of Australia: the effects of nutrient‐poor soils and intense fires. Biological Reviews of the Cambridge Philosophical Society 82, 393–423.
| Ecology of Australia: the effects of nutrient‐poor soils and intense fires.Crossref | GoogleScholarGoogle Scholar |
Panetta F, Hopkins A (1991) Weeds in corridors: invasion and management. Nature and Conservation 2, 341–351.
Payne A, Watson I, Novelly P (2004) Spectacular recovery in the Ord River catchment. Miscellaneous Publication 17/2004, Department of Agriculture, Perth, WA.
Petty AM, Setterfield SA, Ferdinands KB, Barrow P (2012) Inferring habitat suitability and spread patterns from large-scale distributions of an exotic invasive pasture grass in north Australia. Journal of Applied Ecology 49, 742–752.
Pheloung P, Williams P, Halloy S (1999) A weed risk assessment model for use as a biosecurity tool evaluating plant introductions. Journal of Environmental Management 57, 239–251.
| A weed risk assessment model for use as a biosecurity tool evaluating plant introductions.Crossref | GoogleScholarGoogle Scholar |
Pittock J, Connell D (2010) Australia demonstrates the planet’s future: water and climate in the Murray-Darling Basin. Water: Research and Development 26, 561–578.
Popay I, Field R (1996) Grazing animals as weed control agents. Weed Technology 10, 217–231.
Puckey H, Brock C, Yates C (2007) Improving the landscape scale management of buffel grass Cenchrus ciliaris using aerial survey, predictive modelling, and a geographic information system. Pacific Conservation Biology 13, 264–273.
| Improving the landscape scale management of buffel grass Cenchrus ciliaris using aerial survey, predictive modelling, and a geographic information system.Crossref | GoogleScholarGoogle Scholar |
Queensland Department of Agriculture and Fisheries (2012) ‘Invasive species risk assessment: Para grass Brachiaria mutica.’ Available at https://www.daf.qld.gov.au/__data/assets/pdf_file/0004/65254/IPA-Para-Grass-Risk- Assessment.pdf [Verified 8 December 2017].
Queensland Department of Agriculture and Fisheries (2016) ‘Ponded pastures.’ Available at https://www.daf.qld.gov.au/__data/assets/pdf_file/0007/77092/IPA-Hymenachne-PP54.pdf [Verified 8 December 2017].
Rahlao S, Milton S, Esler K, Van Wilgen B, Barnard P (2009) Effects of invasion of fire‐free arid shrublands by a fire‐promoting invasive alien grass (Pennisetum setaceum) in South Africa. Austral Ecology 34, 920–928.
| Effects of invasion of fire‐free arid shrublands by a fire‐promoting invasive alien grass (Pennisetum setaceum) in South Africa.Crossref | GoogleScholarGoogle Scholar |
Reid AM, Morin L, Downey PO, French K, Virtue JG (2009) Does invasive plant management aid the restoration of natural ecosystems? Biological Conservation 142, 2342–2349.
| Does invasive plant management aid the restoration of natural ecosystems?Crossref | GoogleScholarGoogle Scholar |
Ricciardi A, Cohen J (2007) The invasiveness of an introduced species does not predict its impact. Biological Invasions 9, 309–315.
| The invasiveness of an introduced species does not predict its impact.Crossref | GoogleScholarGoogle Scholar |
Richardson DM, Holmes PM, Esler KJ, Galatowitsch SM, Stromberg JC, Kirkman SP, Pyšek P, Hobbs RJ (2007) Riparian vegetation: degradation, alien plant invasions, and restoration prospects. Diversity & Distributions 13, 126–139.
| Riparian vegetation: degradation, alien plant invasions, and restoration prospects.Crossref | GoogleScholarGoogle Scholar |
Richardson DM, Pyšek P (2006) Plant invasions: merging the concepts of species invasiveness and community invasibility. Progress in Physical Geography 30, 409–431.
| Plant invasions: merging the concepts of species invasiveness and community invasibility.Crossref | GoogleScholarGoogle Scholar |
Robbins S (2009) The role of disturbance in exotic grass invasions in open Eucalyptus woodlands. Honours thesis. School of Integrative Biology, University of Queensland, Brisbane, Qld.
Rossiter-Rachor NA, Setterfield SA, Douglas MM, Hutley LB, Cook GD, Schmidt S (2009) Invasive Andropogon gayanus (gamba grass) is an ecosystem transformer of nitrogen relations in Australian savanna. Ecological Applications 19, 1546–1560.
| Invasive Andropogon gayanus (gamba grass) is an ecosystem transformer of nitrogen relations in Australian savanna.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MnjvVSgsA%3D%3D&md5=ccfe352f112a9d55d0b443c41346c088CAS |
Rossiter NA, Setterfield SA, Douglas MM, Hutley LB (2003) Testing the grass-fire cycle: alien grass invasion in the tropical savannas of northern Australia. Diversity & Distributions 9, 169–176.
| Testing the grass-fire cycle: alien grass invasion in the tropical savannas of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith J, Yates C, Edwards A, Allan GE, Cook GD, Cooke P, Craig R, Heath B, Smith R (2003) Contemporary fire regimes of northern Australia, 1997–2001: change since Aboriginal occupancy, challenges for sustainable management. International Journal of Wildland Fire 12, 283–297.
| Contemporary fire regimes of northern Australia, 1997–2001: change since Aboriginal occupancy, challenges for sustainable management.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith J, Murphy BP, Meyer CM, Cook GD, Maier S, Edwards AC, Schatz J, Brocklehurst P (2009) Improving estimates of savanna burning emissions for greenhouse accounting in northern Australia: limitations, challenges, applications. International Journal of Wildland Fire 18, 1–18.
| Improving estimates of savanna burning emissions for greenhouse accounting in northern Australia: limitations, challenges, applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhvFaqs74%3D&md5=75941b3ebfdb5c582da458e04022182aCAS |
Russell M (2001) ‘Grasses and humans: the story of human dependence on grasses and the science of grass use.’ (Russell and Daughters: North Tamborine, Qld)
Setterfield SA, Rossiter-Rachor NA, Hutley LB, Douglas MM, Williams RJ (2010) Turning up the heat: the impacts of Andropogon gayanus (gamba grass) invasion on fire behaviour in northern Australian savannas. Diversity & Distributions 16, 854–861.
| Turning up the heat: the impacts of Andropogon gayanus (gamba grass) invasion on fire behaviour in northern Australian savannas.Crossref | GoogleScholarGoogle Scholar |
Sinclair R (2002) Ecophysiology of grasses. Flora of Australia 43, 133–156.
Smith MA, Bell DT, Loneragan WA (1999) Comparative seed germination ecology of Austrostipa compressa and Ehrharta calycina (Poaceae) in a Western Australian banksia woodland. Australian Journal of Ecology 24, 35–42.
| Comparative seed germination ecology of Austrostipa compressa and Ehrharta calycina (Poaceae) in a Western Australian banksia woodland.Crossref | GoogleScholarGoogle Scholar |
Spafford-Jacob H, Randall RP, Lloyd SG, King C (2004) Quarantine law loophole: an examination of the known weed species permitted for import without weed risk assessment. In ‘Weed management: balancing people, planet, profit: 14th Australian Weeds Conference’. (Eds B Sindel, S Johnson) pp. 684–689. (Weed Society of New South Wales: Wagga Wagga, NSW)
Thorp JR, Lynch R (2000) ‘The determination of weeds of national significance.’ (National Weeds Strategy Executive Committee: Launceston, Tas.)
Tjelmeland AD, Fulbright TE, Lloyd-Reilley J (2008) Evaluation of herbicides for restoring native grasses in buffelgrass-dominated grasslands. Restoration Ecology 16, 263–269.
| Evaluation of herbicides for restoring native grasses in buffelgrass-dominated grasslands.Crossref | GoogleScholarGoogle Scholar |
van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecology Letters 13, 235–245.
| A meta-analysis of trait differences between invasive and non-invasive plant species.Crossref | GoogleScholarGoogle Scholar |
van Klinken RD, Panetta FD, Coutts SR (2013) Are high-impact species predictable? An analysis of naturalised grasses in northern Australia. PLoS One 8, e68678
| Are high-impact species predictable? An analysis of naturalised grasses in northern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFyis7nE&md5=e0550e553bbdf6859734ddda0c8673a4CAS |
van Klinken RD, Panetta FD, Coutts S, Simon BK (2015a) Learning from the past to predict the future: an historical analysis of grass invasions in northern Australia. Biological Invasions 17, 565–579.
| Learning from the past to predict the future: an historical analysis of grass invasions in northern Australia.Crossref | GoogleScholarGoogle Scholar |
van Klinken R, Murray JV, Smith C, Venette R (2015b) Process-based pest risk mapping using Bayesian networks and GIS. In ‘Pest risk modelling and mapping for invasive alien species’. (Ed. RC Venette) pp. 171–188. (CAB International: Wallingford, Oxfordshire, UK)
van Vreeswyk A, Leighton K, Payne A, Hennig P (2004) ‘An inventory and condition survey of the Pilbara region, Western Australia.’ (Department of Agriculture: Perth, WA)
Van Wilgen B, Richardson D, Le Maitre D, Marais C, Magadlela D (2001) The economic consequences of alien plant invasions: examples of impacts and approaches to sustainable management in South Africa. Environment, Development and Sustainability 3, 145–168.
| The economic consequences of alien plant invasions: examples of impacts and approaches to sustainable management in South Africa.Crossref | GoogleScholarGoogle Scholar |
Vogler WD, Owen NA (2008) Grader grass (Themeda quadrivalvis): changing savannah ecosystems. In ‘Hot topics in the tropics: 16th Australian Weeds Conference’. (Eds RD van Klinken, VA Osten, FD Panetta, JC Scanlan) pp. 213–214. (Queensland Weeds Society: Coomera, Qld)
Wearne LJ, Clarkson J, Grice AC, van Klinken RD, Vitelli JS (2010) The biology of Australian weeds. 56. Hymenachne amplexicaulis (Rudge) Nees. Plant Protection Quarterly 25, 146–161.
Webber BL, Raghu S, Edwards OR (2015) Opinion: is CRISPR-based gene drive a bio-control silver bullet or global conservation threat? Proceedings of the National Academy of Sciences of the United States of America 112, 10565–10567.
| Opinion: is CRISPR-based gene drive a bio-control silver bullet or global conservation threat?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsVWgtr3L&md5=c4a6be6d1a8b2a965e0693ea006f8a20CAS |
White ME (1997) ‘Listen, our land is crying: Australia’s environment: problems and solutions.’ (Rosenberg Publishing: Kenthurst, NSW)
Wildin J (1991) Aleman grass, Hymenachne and other forage species for ponded pasture systems. In ‘Proceedings of probing ponded pastures workshop’. (University of Central Queensland: Rockhampton, Qld)
Wildin JH, Chapman DG (1987) ‘Ponded pasture systems – capitalising on available water.’ (Queensland Department of Primary Industries: Brisbane, Qld)
Winter W, Cameron A, Reid R, Stockwell T, Page M (1985) Improved pasture plants. In ‘Agro-research for the semiarid tropics: north-west Australia’. (Ed. RC Muchow) pp. 227–269. (University of Queensland Press: Brisbane, Qld)




