Application of gene expression studies in livestock production systems: a European perspective
I. Cassar-Malek A C , B. Picard A , C. Bernard B and J.-F. Hocquette AA INRA, UR1213, Unité de Recherches sur les Herbivores, Centre de Clermont-Ferrand/Theix, Saint-Genès-Champanelle 63122, France.
B Institut de l’Elevage, Service Aptitudes et Sélection des Races Allaitantes, 149 rue de Bercy, Paris Cedex 12, 75595, France.
C Corresponding author. Email: isabelle.cassar-malek@clermont.inra.fr
Australian Journal of Experimental Agriculture 48(7) 701-710 https://doi.org/10.1071/EA08018
Submitted: 7 January 2008 Accepted: 30 March 2008 Published: 20 June 2008
Abstract
In the context of sustainable agriculture and animal husbandry, understanding animal physiology remains a major challenge in the breeding and production of livestock, especially to develop animal farming systems that respond to the new and diversified consumer demand. Physiological processes depend on the expression of many genes acting in concert. Considerable effort has been expended in recent years on examining the mechanisms controlling gene expression and their regulation by biological and external factors (e.g. genetic determinants, nutritional factors, and animal management). Two main strategies have been developed to identify important genes. The first one has focussed on the expression of candidate genes for key physiological pathways at the level of both the transcripts and proteins. An original strategy has emerged with the advent of genomics that addresses the same issues through the examination of the molecular signatures of all genes and proteins using high-throughput techniques (e.g. transcriptomics and proteomics). In this review, the application of the gene expression studies in livestock production systems is discussed. Some practical examples of genomics applied to livestock production systems (e.g. to optimise animal nutrition, meat quality or animal management) are presented, and their outcomes are considered. In the future, integration of the knowledge gained from these studies will finally result in optimising livestock production systems through detection of desirable animals and their integration into accurate breeding programs or innovative management systems.
Introduction
In livestock production systems, an important challenge for the next century is to satisfy the predicted high demand of meat due to the increasing human population. To achieve this goal, producers will have to control animal performance more accurately by improving quantification of animal requirements and evaluating animal responses to varying nutritional inputs according to their genetic potential. At the same time, the farming and agri-food sectors in developed countries are faced with an increasing demand by consumers for safe high-quality meat and dairy products while respecting animal health and welfare and protecting the environment. The combination of these objectives has led to the concept of sustainable animal husbandry. Predicting the response of livestock animals to nutritional interventions, husbandry practices, and genetic selection will make it possible to reduce the production costs by increasing metabolic efficiency, improving growth and reproduction and preventing disease, and optimising the levels of beneficial compounds in meat and milk. Understanding animal biology still remains a major challenge in the breeding and production of livestock. Thus, there is a need for new knowledge in order to develop animal farming systems that respond to the new and diversified consumer demand.
For years, classic scientific areas have addressed studies at the DNA, RNA, protein and biological function levels by independent approaches (molecular genetics, molecular biology, biochemistry, and metabolism). Research has focussed on the mechanisms controlling gene expression and the impact of biological and external factors on gene expression (e.g. genetic determinants and nutritional factors, respectively) in tissues involved in metabolism, reproduction, growth, and production (milk and meat) traits. During the last decade, the same issues have been addressed by high-throughput genetic techniques. Nowadays, more and more examples based on genomic studies are being published in the major livestock species e.g. chicken, cattle, and swine. In this review, we will only consider gene expression approaches, especially those conducted at the level of gene transcription and protein expression; genome/genetics and metabolism/metabolomics studies are not within the scope of this paper.
Gene expression studies: strategy and techniques
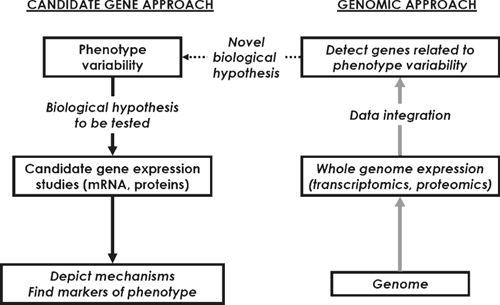
Traditional research in farm animals has focussed on requirements for specific nutrients, diet formulation, controlling performance and fuelling homeostasis in order to optimise feed and production efficiency, as well as product quality. For many years, attention was directed to specific metabolic pathways or rate-limiting enzymes in growth and metabolism. In the last 25 years, there has been a shift in focus of nutrition and growth studies towards molecular biology. Before genomics, molecular biology aimed at investigating single genes or proteins, and their structure and function, in isolation from the larger context of other genes. This is referred to as the ‘candidate gene approach’ (Fig. 1). Different methods were used to detect and quantify the expression level of individual genes (e.g. Northern blot, subtractive hybridisation, differential display, SAGE, or real-time PCR) and their products (e.g. Western blot and ELISA).
|
Gene expression depends on both genetic and environmental factors. An effective strategy when looking at gene expression is: (i) to exploit the phenotype variability among individuals within or between breeds and (ii) to compare animals with extreme characteristics (e.g. differing in their breed, nutrition, growth rate, physiological state, management system or quality of their products). Some studies have allowed a more thorough investigation of the mechanisms underlying some metabolic functions, especially in ruminants for which the major end products of digestion are qualitatively and quantitatively different than in other mammals (amino acids and volatile fatty acids in the former v. amino acids, fats and carbohydrates in the latter). For example, classic gene expression studies highly contributed to the understanding of the aetiology of fatty liver in dairy cows at calving (Gruffat et al. 1997; Bernabucci et al. 2004), increased capacity for gluconeogenesis in early lactation in cows (Greenfield et al. 2000), response of genes to nutritional or physiological status in the intestine (reviewed in Shirazi-Beechey 2004), adipose tissues (Bonnet et al. 2000) and muscle (Hocquette et al. 2001) of ruminants. Schwerin et al. (2006) identified bovine hepatic and intestinal DNA sequences (EST) expressed in a breed-specific manner that may be considered as potential candidate genes for nutrient transformation in cattle but their biological significance remains to be further studied before considering applications. Other studies have investigated the influence of breed or genetic polymorphism on gene expression. For instance, a candidate gene study was conducted in the Belgian strain of the Texel sheep harbouring a quantitative trait locus (QTL) for muscle development. The initial objective was to identify the ‘Texel’ gene and the first obvious candidate was myostatin, a negative regulator of muscle development located in the QTL. There was no differential expression of myostatin mRNA in Texel sheep. However, a mutation was identified in the 3′-untranslated region of the myostatin gene. This mutation creates a target site for microRNAs and consequently a translational inhibition of myostatin gene expression (Clop et al. 2006). As for double-muscled cattle (Grobet et al. 1997), the inactivation of the myostatin signal during development is responsible for hypermuscularity.
Actually, physiological processes are governed by several genes acting in concert rather than by only one or a few individual genes. In the last decade, the advent of genomic technologies (large-scale DNA sequencing techniques, array technology, proteomics and metabolomics) has enabled the analysis of thousands of genes or proteins or metabolites in a single experiment (genomic approach). By detecting all the transcripts or proteins in tissues, scientists hope to detect potentially interesting genes and molecular mechanisms which may be biomarkers for product quality and/or management systems. This will impact mainly on the characterisation of complex traits. Detailed reviews describing the limits and advantages of genomics have been published elsewhere (see for instance, Hocquette 2005; Mullen et al. 2006). The strategy is to identify differentially expressed genes or proteins between extreme animals without any a priori knowledge of gene functions (Fig. 1). The expected outcomes are the identification of novel key genes from a particular molecular signature, and their application to the detection of livestock animals with desirable characteristics or genetic selection. Unlike the ‘candidate gene’ approach, the genomic approach collects data without any prior hypothesis on biological pathways and is therefore generating new hypotheses.
Among the techniques available, DNA microarrays (pan genomic or tissue- or function-dedicated chips) are valuable tools to study the impact of various factors (e.g. genetics, nutrition level, type of diet, animal management) and treatments on gene expression in tissues or cells, especially for revealing novel genes that have not previously been involved in a physiological or nutritional response. However, a limit of microarrays is related to multiple gene products and the difficulty in detecting subtle transcriptional changes reflecting for example the replacement of a protein isoform by another one, such as those occurring during development (Lehnert et al. 2007).
Proteomics permits visualisation of the set of all expressed protein in tissues (proteome), combining two dimensional electrophoresis (2DE), a powerful separation technique, with highly sensitive analytical mass spectrometry. Proteomics distinguishes several isoforms of a protein with differences in molecular weight and isoelectric point values corresponding to post-translational modifications as phosphorylation, glycosylation and proteolytic cleavages. Protein isoforms can also originate from alternative splicing of mRNA as illustrated by the troponin T isoforms which are involved in muscle contraction (Bouley et al. 2005).
So far, genomic experiments were disappointing since they provided catalogues of genes or proteins regulated by various biological or external factors, but sometimes and unfortunately without any real information about gene function. Converting data into knowledge of benefit to the livestock industry is still a limitation. A better knowledge of gene function is thus required. A new challenge is also to integrate knowledge with the aim of understanding the phenotypic data based on our understanding of the parts brought by the different genomic experiments. Thus, integration of livestock genomics and physiology will be a major contribution to depict the molecular basis for physiological or nutritional responses in tissues and their regulation. This approach needs suitable databases and powerful and sometimes new statistical approaches. This is called ‘systems biology’ and it is only at its beginning in farm animals. Outcomes of systems biology could be an accurate supply of nutrients for meat or milk production or an optimisation of husbandry practices in livestock species. Another outcome of genomics is the development of diagnostic tests based on biotechnological tools, which may be useful for the livestock industry for detection of animals with desirable traits.
Genomics applied to livestock production systems
Genomic studies in livestock animals are still few despite many recent studies. However, a multitude of applications (e.g. increased livestock productivity, meat and milk quality, prevention of diseases) is driving genomic studies of farm animals. Several genomic initiatives are being conducted all over the world (e.g. AGENAE in France, FUGATO in Germany, the Biotechnology Initiative of Teagasc in Ireland, NAGRP in the USA, SheepGENOMICS and Beef CRC in Australia, see Table 1) and should contribute to making genomics fully operative in animal science in the near future. For example, the French National Institute for Agricultural Research (INRA) launched its own animal genomics program in 2000–2001, in four main species (cattle, pig, chicken, and trout). In 2002, a cooperative research program, called AGENAE, an acronym for Analyse du GENome des Animaux d’Elevage (Analysis of the genome of farm animals) was initiated (Chevalet et al. 2007). It is piloted by a consortium consisting of state-supported research organisations and private associations representing the farm animal industry. The French livestock industry, especially the beef industry, has expended great effort and resources to identify possible genomic markers that would identify animals with desirable traits. The AGENAE program covers many fields of interest: e.g. reproduction, growth and development, health, behaviour and welfare, milking ability, quality of animal products. Its main purposes are to investigate the genotype–phenotype relationships and to conduct gene expression profiling experiments (e.g. transcriptomics, proteomics). Since 2005, it has expanded to include any animal species of economic value.

|
Research was initially conducted all over the world to develop cDNA libraries and EST sequence resources as well as informatic tools and databases in the major livestock species (Fadiel et al. 2005). Tissue-specific cDNA (cDNA) libraries and microarrays have been generated in different laboratories from individual or pooled tissues, organs and cells in chicken (reviewed in Cogburn et al. 2004), cattle (reviewed in Hocquette et al. 2007) and swine (reviewed in Tuggle et al. 2007). Pan-genomic sets and microarrays have also been developed for chicken and bovine and are available in North America (http://www.fhcrc.org and http://www.pyxisgenomics.com, respectively) and in Europe (http://www.ark-genomics.org/). The sequencing of the genomes (especially for chicken and bovine, and very soon pig) and the increasing availability of microarrays are providing new opportunities to elucidate the molecular basis of physiological and productive functions in livestock species and their regulation. In particular, commercial oligonucleotide chips (http://www.affymetrix.com; http://www.agilent.com) or oligonucleotide sets (http://www.operon.com) are now available for chicken, pig and cattle. These in-laboratory printing or commercial resources have been used for gene expression studies in several areas relevant for livestock species (Table 2) and studies dealing with nutrigenomics, meat quality or characterisation of production systems are illustrated below. In the absence of full sheep genomic sequence, construction of a ‘virtual sheep genome’ is being undertaken by SheepGENOMICS, following BAC-end sequencing and alignment of sequences with the bovine genome sequence.

|
Huge progress was also recently achieved in the description of the sets of proteins expressed in key tissues of livestock (for instance, in the bovine muscle, Bouley et al. 2005) and proteomic tools were developed especially for applications in meat science (for reviews see Bendixen et al. 2005a and Hollung et al. 2007). For example, separation of proteins from the bovine Semitendinosus muscle in a pH gradient of 4–7 in the first dimension allowed the detection of roughly 500 reproducible protein spots (Bouley et al. 2004). Among them 129 were identified and cartographied. This first map was completed with the development of a separation in a basic pH gradient 7–11 allowing the mapping of 60 proteins (Chaze et al. 2006). By combining these methods, it is now possible to separate with a high resolution and reproducibility bovine proteins in a range of 4 to 11 pH units.
Nutrigenomic studies
The term ‘nutrigenomics’ refers to nutritional genomics, i.e. to the interaction between nutritional environment and gene expression, taking advantage of the genomic approach (Chadwick 2004) in human beings and, to a lesser extent, in animals. It is promising in identifying biomarkers of the nutritional status and disease, and individualised requirements of animals for nutrients. Nutrigenomics is of particular interest in the context of managing livestock animals for production. Underfeeding/refeeding protocols are generally used to identify genes responsive to nutritional manipulation, due to the close association between nutrient supply and hormonal status. Transcriptomic studies were carried out to identify genes regulated as a result of nutrient restriction in cattle and pigs. For example, the influence of prepartum nutrition on hepatic gene expression was examined in Holstein cows submitted either to moderate energy restriction or fed ad libitum (Loor et al. 2006). Energy restriction induced an upregulation of some of the genes involved in fatty acid oxidation, gluconeogenesis and cholesterol synthesis. Conversely, moderate ad libitum feeding favoured the expression of some genes associated with fat synthesis, thus predisposing cows to fatty liver. In addition, ad libitum feeding resulted in transcriptional changes, potentially compromising liver health through increased susceptibility to oxidative stress and DNA damage. These data strengthened the importance of shaping the prepartum nutrition of dairy cows and suggested that the common practice of increasing the energy density of prepartum cow diets should be rethought. Another study examined the impact of fasting on the liver transcriptome of pigs (Cheon et al. 2005). Fasting induced genes involved in mitochondrial fatty acid oxidation and ketogenesis as shown for rodents. Those genes were also induced by feeding pigs a diet supplemented with clofibric acid indicating that PPARα, encoding a transcription factor which is involved in lipid metabolism, is likely to play an active role in the metabolic adaptation to fasting in pigs.
The dairy industry is facing a problem due to the high content of saturated fatty acids in milk which are detrimental for human health. A major challenge is therefore to adapt the fatty acid profile of the milk to the dietary recommendations. In France, the influence of dietary factors on the mammary mechanisms involved in milk secretion and composition is under investigation in ruminants. An initial study examined the response to nutritional intervention in goats. Food deprivation for 48 h alters goat mammary transcriptome simultaneously with milk production and composition (Ollier et al. 2007) as shown by the identification of 161 genes responsive to food deprivation, most of which (88%) were downregulated. As expected, genes for milk components were downregulated. Expression of genes involved in the cell machinery and the cell life was also altered. These might be responsible for a decrease in cell proliferation and differentiation, and an orientation of mammary cells towards programmed cell death, which could be a signature of an early step of mammary gland involution. The analysis of the impact of dietary lipid supplementation on the mammary gene expression will undoubtedly bring additional information to the few data available in ruminants (reviewed in Bernard et al. 2008).
In all these examples, a wide and global view of metabolism and its regulation was demonstrated by genomic approaches unlike the candidate approaches described above. However, there still remains the need to convert the discovered concepts into practical considerations useful for the livestock industry. To better illustrate this global strategy, we will detail the example of studies related to beef quality.
Application to meat quality
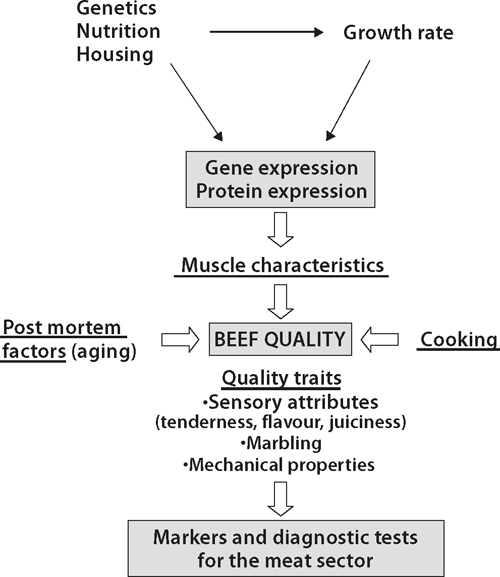
Intrinsic quality attributes of meat quality, and especially tenderness, depend on post mortem factors associated with aging, cooking, and muscle characteristics of the live animals, which depend themselves on genetic, nutritional and rearing factors (Fig. 2).
|
Today, information on meat quality is only obtainable after slaughter, which is a limitation to the delivery of a consistent quality meat. For meat producers, it is also of interest for rearing or breeding purposes to predict the ability of live animals to produce good meat in general. In the case of beef, there is specific attention towards tenderness, which is the top priority quality attribute in beef. Thus, the beef industry is looking for biological or molecular indicators that would identify live animals with desirable quality attributes, in order to orientate them towards the most appropriate production system. Genomics is thought to be helpful to achieve this general objective (reviewed by Hocquette et al. 2007).
Recently, gene expression-based research related to beef quality has focussed on identification of molecular processes involved in meat quality traits such as toughness and marbling (Lehnert et al. 2006). Other studies focussed on fetal muscle development (Sudre et al. 2003; Lehnert et al. 2007), muscle growth potential (Sudre et al. 2005; Cassar-Malek et al. 2007) and effects of nutritional changes (Byrne et al. 2005), which all influence the composition of muscle tissue. Intramuscular fat development was also examined (Wang et al. 2005; Lee et al. 2007) since it influences marbling and thus juiciness and flavour. Differential-display polymerase chain reaction analysis has allowed the detection of differential expression of the NAT1 gene (Novel APOBEC-1 target-1, a translational suppressor) between muscles differing in their intramuscular fat contents from different finishing periods on high grain feeding (Childs et al. 2002). Similarly, the transcription repressor ICER gene was found to be highly expressed in the late fattening stage of Hanwoo steers (Lee et al. 2007). Interestingly, neither NAT1 nor ICER has been previously suspected to play a role in fat accumulation. Other putative functional genes were found to be differentially expressed (e.g. adenosine triphosphate citrate lyase) or, surprisingly, not differentially expressed (e.g. PPARγ) between extreme animals (Childs et al. 2002). The A-FABP protein content (Jurie et al. 2007) and leptin and G6PDH expressions (Bonnet et al. 2007) have also been recently proposed to be relevant indicators of marbling.
In Europe (for instance France and Belgium), genetic selection in farm animals, notably in cattle, has been directed towards high muscle development at the expense of fat in order to produce leaner carcasses and increase meat production. This selection may have influenced muscle characteristics and consequently meat quality. Therefore, different studies were performed to study extreme animals with muscle hypertrophy namely Belgian Blue bulls with myostatin deletion or Texel sheep harbouring a QTL for muscle development. Using 2DE, Bouley et al. (2004) detected 17 troponin T isoforms in the bovine Semitendinosus muscle, 11 of them belonging to the fast type (fTnT) and originating from the exclusive alternative splicing of fTnT exon 16 and fTnT exon 17. Comparison of the proteomes from the Semitendinosus muscles of two groups of Belgian Blue bulls with or without myostatin deletion demonstrated that troponin T isoform patterning was altered by myostatin loss-of-function and could also be a good marker for the prediction of muscle mass (Bouley et al. 2004). The distinction of these isoforms could have important implication in beef quality since a relationship between troponin T and tenderness has been demonstrated (Tsitsilonis et al. 2002). In the same study, the proteome profiles demonstrated a shift towards a fast-twitch glycolytic muscle type in animals with a myostatin deletion. Accordingly, Hamelin et al. (2006) examined the proteome profiles in four muscles of Texel sheep harbouring a QTL for muscle development and revealed 63 differential protein spots compared with Romanov sheep without myostatin mutation. Most of them could be related to the hypertrophic status of the muscle and the associated increased levels of glycolytic enzymes and lower capillary density, respectively.
Only a few studies aimed to identify differentially expressed genes according to beef sensory quality, especially tenderness, juiciness and flavour. Tenderness is the major quality criteria of the bovine meat sector and there is still no simple, reliable and reproducible reference technique to predict it, particularly regarding carcass storage duration. In the context of the French AGENAE program and in partnership with the beef industry, Bernard et al. (2007) searched for differentially expressed genes associated with variability of beef tenderness, juiciness and flavour that may be new indicators of beef quality in Charolais steers. They found that expression of the DNAJA1 gene was strongly related to tenderness after 14 days of aging. This finding has been protected by a patent filed in Europe in September 2006 by INRA and the French Beef industry (Bernard et al. 2006). The DNAJA1 protein is a member of the heat shock 40 kDa protein family. It is a co-chaperone of the Hsc70 protein and seems to play a role in protein import into mitochondria. An emerging hypothesis is that DNAJA1 could decrease apoptosis and, therefore, meat aging and its tenderisation during days following slaughtering. Further studies are needed to characterise DNAJA1 involvement in beef tenderness and to look at the relation between DNAJA1 expression level and tenderness in other beef breeds or production systems. In addition, the regulation of DNAJA1 expression by production factors is a key issue to address for ‘paddock-to-plate’ applications. A comparative proteomic study of samples from the same program revealed that some proteins associated with tenderness variability belonged to the same family as DNAJA1, e.g. Hsp27 and α-crystallin proteins. The analysis of proteome evolution post mortem also revealed the presence of fragments of these proteins. In a regression analysis modelling sensory tenderness, Morzel et al. (2008) demonstrated that levels of the Hsp27 protein in fresh muscle and levels of Hsp27 fragments in aged meat explained up to 91% of the variation in sensory scores. In the same experiment, they showed that abundance of succinate dehydrogenase was the best common predictor of initial and overall tenderness, explaining 65.6% and 57.8% of variation of these palatability traits respectively.
A series of papers have reported the proteome changes of post mortem processes in pork, bovine and fish (reviewed in Bendixen et al. 2005a). Post mortem markers detected during the first 48 h of postslaughter storage included structural proteins (e.g. actin, myosin and troponin T) as well as metabolic enzymes (e.g. myokinase, pyruvate kinase and glycogen phosphorylase). Accumulation of these fragments was found to correlate with meat tenderness. Some papers have focussed more on proteome changes related to proteolysis during post mortem storage (reviewed in Hollung et al. 2007) or to meat quality defaults. For instance, proteomics has been applied to investigate the biochemical mechanisms behind meat colour (Sayd et al. 2006). This study showed that while the dark muscles had an increased abundance of mitochondrial proteins, indicating a more oxidative metabolism, the light muscles had an increased abundance of cytosolic proteins involved in glycolysis. Lastly, the occurrence of low-molecular weight peptides in bovine pectoralis profundus muscle during post mortem storage and cooking was analysed directly by mass spectrometry (Bauchart et al. 2006). Analysis of the peptide composition of cooked samples revealed seven peptides corresponding to five proteins. Three of them were known targets of post mortem proteolysis (troponin T, nebulin, cypher protein), while the other two were the connective tissue proteins procollagen types I and IV.
In conclusion, much progress has been made recently in our understanding of the biological processes contributing to meat quality. Gene expression profiling revealed that unsuspected genes may be potential molecular indicators of sensory attributes (tenderness, flavour, juiciness) or marbling. This should lead to the development of commercial diagnostic tests based on ‘genomic markers’ for sorting of meat quality, optimisation of husbandry, or selective breeding. However, the transition from gene expression data to practical biological assays or ideally to diagnostic tests implies many phases, especially confirmation of the association in large samples, as discussed for QTL by Barendse (2005). Moreover, the significance of these novel markers has to be tested before their commercial exploitation since recent data suggest that previous genetic markers identified in individual breeds or production systems in specific countries may not be appropriate in all production systems or in other parts of the world (Renand et al. 2007).
Characterisation of production systems
Traceability of an animal’s breed and identity, geographical origin, diet, and mode of production are increasingly important issues demanded by consumers. However, to date little data is available on tracing products back to their production source (e.g. geographical origin, intensive or extensive systems, organic systems) using gene expression studies.
One study performed at INRA examined the influence of two production systems (pasture v. maize silage indoors) on muscle gene profiles in 30-month-old Charolais using a multi-tissue bovine cDNA macroarray (Cassar-Malek et al. 2005). This strategy was designed to identify differentially expressed genes that may be potential indicators of pasture feeding systems. The muscles from Charolais grazing on pasture had more oxidative characteristics than those of steers fed maize silage indoors. An interesting finding was the decreased expression of the selenoprotein W in steers grazing on pasture. Although its metabolic function is not yet known, selenoprotein W is likely to play a role in oxidant defence (Jeong et al. 2002). Its abundance in skeletal muscles and some other tissues is regulated by dietary selenium (Yeh et al. 1997), especially in sheep, for which it is highly sensitive to selenium depletion. The differential expression of selenoprotein W in grazing beef cattle may be related to the selenium content or bioavailability in their diet (grass v. maize silage) rather than to their mobility on pasture. Thus, muscle selenoprotein W expression could be proposed as a putative indicator of a pasture-based system. Further exploration of the data thanks to the new sequencing data in cattle will undoubtedly provide additional information on novel putative biomarkers for production systems.
Another example of characterising production system is related to farm animal cloning. Somatic cloning has many applications in cattle breeding programs and promises the propagation of animals with desirable traits such as high quality food products (Heyman et al. 2007). However, the impact of cloning on animal performance and development still has to be evaluated. For this reason, INRA is conducting a genomic study within the AGENAE program in order to subtly analyse the development of the muscle tissue, from which the meat products are derived, in cloned cattle.
Ideally, this research should lead to the integration of ‘genomic tracers’ into chips to detect molecular signatures not only predicting the sensory or nutritional quality of livestock products but also ensuring traceability of production systems.
Outlook for the future
Thanks to the development of powerful technologies, genomics is reshaping biology. The expected outcomes are huge in medical science. From the economic point of view, the importance of genomic studies in agricultural practice is also likely to increase. In the near future, we will be able to detect gene polymorphisms by using high-throughput genotyping. In the more distant future, we will also be able to evaluate routinely the expression levels of genes and proteins in the tissues of interest for desirable traits or for the authentication of production systems. The genomic tools will allow establishment of interaction between the different research programs on the efficiency of production, quality of products and protection of environment for sustainable livestock. For example, with regard to cattle, reproduction and fertility of dairy or lactating females, amount and type of fat in milk or meat, sensory quality of products and excretion of nitrogen into the environment are important issues to be solved. Genomics has the potential to bring together information for all these criteria by studying the expression of all genes of any organism simultaneously.
The implementation of genomics approaches has many applications in animal science. First, they will lead to the development of diagnostic tests. Beside scientific and technical issues (the importance of the studied traits, confirmation of the gene effects on a large population, successful production of genomic tests, etc.), it is, however, crucial to determine the economic value of such diagnostic tests. For instance, it is important to know whether improvement of a specific biological trait by genomic tools will provide or not an economic return to the producer or improve competitiveness of the product compared with alternative methods. So far, the costs of DNA tests (to genotype animals) have dropped by several orders of magnitude making the livestock industry receptive to their use in a commercial way (Barendse 2005). Unfortunately, it is not yet the case for diagnostic tests based on gene expression methods. However, we must anticipate that costs will drop for array and proteomic tools as well. Second, once they have identified genes or proteins of interest for livestock production systems, researchers could modulate them. This should lead to new ways to improve health, wellbeing and performance. Lastly, new genomic knowledge will also improve disease diagnosis, detection of predisposition to disease, and enhance development of new treatments. Clearly, the sequencing of genomes in livestock will provide further impetus to research. In this respect, the bovine genomics project is a priority for public research organisations, such as INRA in partnership with the private partners of the French beef industry and many European laboratories.
Conclusion
According to Fox Keller (2002), the understanding of biology has reached a turning point and the central question has shifted from ‘who are the actors?’ [the genes and their products] to ‘what are the scripts?’ [the physiological programs]. This author said that the concept of the gene has been overused, and in future we won’t see it as being so important. We have indeed to look at things differently. There is not only one gene but rather a combination of individual genes governing biological pathways and their regulation. The level of gene expression per se, and more precisely the combination of individual expression levels, rather than the genes themselves are responsible for phenotype variability. In this respect, we will most certainly not find master genes for economically important production traits (e.g. beef tenderness or marbling) but rather a complex interplay of genes responding to intrinsic and environmental factors. Through gene expression studies and more precisely global gene expression profiling at the mRNA or protein level, we are gaining a deeper insight into the mechanisms governing the physiological functions and their regulation in livestock species. The next challenge is to integrate the knowledge gained from these studies in optimising livestock production systems through detection of desirable animals and their integration into accurate breeding programs or management systems.
Barendse W
(2005) The transistion from quantitative trait loci to diagnostic test in cattle and other livestock. Australian Journal of Experimental Agriculture 45, 831–836.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Bauchart C,
Rémond D,
Chambon C,
Patureau-Mirand M,
Savary-Auzeloux I, Reynes C
(2006) Small peptides (<5 kDa) found in ready-to- eat beef meat. Meat Science 74, 658–666.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Bendixen E,
Hedegaard J, Horn P
(2005b) Functional genomics in farm animals – microarray analysis. Meat Science 71, 128–137.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Bernabucci U,
Ronchi B,
Basirico L,
Pirazzi D,
Rueca F,
Lacetera N, Nardone A
(2004) Abundance of mRNA of apolipoprotein B100, apolipoprotein E and microsomal triglyceride transfer protein in liver from periparturient cows. Journal of Dairy Science 87, 2881–2888.
|
CAS |
PubMed |

Bernard C,
Cassar-Malek I,
Le Cunff M,
Dubroeucq H,
Renand G, Hocquette JF
(2007) New indicators of beef sensory quality revealed by expression of specific genes. Journal of Agricultural and Food Chemistry 55, 5229–5237.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bernard L,
Leroux C, Chilliard Y
(2008) Expression and nutritional regulation of lipogenic genes in the ruminant lactating mammary gland. Advances in Experimental Medicine and Biology 606, 67–108.
|
CAS |
PubMed |

Bonnet M,
Leroux C,
Faulconnier Y,
Hocquette JF,
Bocquier F,
Martin P, Chilliard Y
(2000) Lipoprotein lipase activity and mRNA are up-regulated by refeeding in adipose tissue and cardiac muscle of sheep. The Journal of Nutrition 130, 749–756.
|
CAS |
PubMed |

Bonnet M,
Faulconnier Y,
Leroux C,
Jurie C,
Cassar-Malek I,
Bauchart D,
Boulesteix P,
Pethick D,
Hocquette JF, Chilliard Y
(2007) Glucose-6-phosphate dehydrogenase and leptin are related to marbling differences among Limousin and Angus or Japanese Black × Angus steers. Journal of Animal Science 85, 2882–2894.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bouley J,
Chambon C, Picard B
(2004) Mapping of bovine skeletal muscle proteins using two-dimensional gel electrophoresis and mass spectrometry. Proteomics 4, 1811–1824.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bouley J,
Meunier B,
Chambon C,
De Smet S,
Hocquette JF, Picard B
(2005) Proteomic analysis of bovine skeletal muscle hypertrophy. Proteomics 5, 490–500.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Byrne KA,
Wang YH,
Lehnert SA,
Harper GS,
McWilliam SM,
Bruce HL, Reverter A
(2005) Gene expression profiling of muscle tissue in Brahman steers during nutritional restriction. Journal of Animal Science 83, 1–12.
|
CAS |
PubMed |

Cassar-Malek I,
Passelaigue F,
Bernard C,
Léger J, Hocquette JF
(2007) Target genes of myostatin loss-of-function in muscles of late bovine fetuses. BMC Genomics 8, 63.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chadwick R
(2004) Nutrigenomics, individualism and public health. The Proceedings of the Nutrition Society 63, 161–166.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chaze T,
Bouley J,
Chambon C,
Barboiron C, Picard B
(2006) Mapping of alkaline proteins in bovine skeletal muscle. Proteomics 6, 2571–2575.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Chaze T,
Meunier B,
Chambon C,
Jurie C, Picard B
(2008) In vivo proteome dynamics during early bovine myogenesis Proteomics in press ,

Cheon Y,
Nara TY,
Band MR,
Beever JE,
Wallig MA, Nakamura MT
(2005) Induction of overlapping genes by fasting and a peroxisome proliferator in pigs: evidence of functional PPARalpha in non proliferating species. The American Journal of Physiology 288, R1525–R1535.
|
CAS |

Childs KD,
Goad DW,
Allan MF,
Pomp D,
Krehbiel C,
Geisert RD,
Morgan JB, Malayer JR
(2002) Differential expression of NAT1 translational repressor during development of bovine intramuscular adipocytes. Physiological Genomics 10, 49–56.
|
CAS |
PubMed |

Clop A,
Marcq F,
Takeda H,
Pirottin D, Tordoir X , et al.
(2006) A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nature Genetics 38, 813–818.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Cogburn LA,
Wang X,
Carre W,
Rejto L,
Aggrey SE,
Duclos MJ,
Simon J, Porter TE
(2004) Functional genomics in chickens: development of integrated-systems microarrays for transcriptional profiling and discovery of regulatory pathways. Comparative and Functional Genomics 5, 253–261.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Fadiel A,
Anidi I, Eichenbaum KD
(2005) Farm animal genomics and informatics: an update. Nucleic Acids Research 33, 6308–6318.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Greenfield RB,
Cevana MJ, Donkin SS
(2000) Changes in mRNA expression for gluconeogenic enzymes in liver of dairy cattle during the transition to lactation. Journal of Dairy Science 83, 1228–1236.
|
CAS |
PubMed |

Grobet L,
Martin LJ,
Poncelet D,
Pirottin D, Brouwers B , et al.
(1997) A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nature Genetics 17, 71–74.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Gruffat D,
Durand D,
Chilliard Y,
Williams P, Bauchart D
(1997) Hepatic gene expression of apolipoprotein B100 during early lactation in underfed, high producing dairy cows. Journal of Dairy Science 80, 657–666.
|
CAS |
PubMed |

Hamelin M,
Sayd T,
Chambon C,
Bouix J, Bibé B , et al.
(2006) Proteomic analysis of ovine muscle hypertrophy. Journal of Animal Science 84, 3266–3276.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Heyman Y,
Chavatte-Palmer P,
Berthelot V,
Fromentin G,
Hocquette JF,
Martignat L, Renard JP
(2007) Assessing the quality of products from cloned animal: an integrative approach. Theriogenology 67, 134–141.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hocquette JF
(2005) Where are we in genomics? Journal of Physiology and Pharmacology 56, 37–70.
| PubMed |

Hocquette JF,
Graulet B,
Vermorel M, Bauchart D
(2001) Weaning affects lipoprotein lipase activity and gene expression only in adipose tissues and in masseter but not in other muscles of the calf. The British Journal of Nutrition 86, 433–441.
|
CAS |
PubMed |

Hocquette JF,
Lehnert S,
Barendse W,
Cassar-Malek I, Picard B
(2007) Recent advances in cattle functional genomics and their application to beef quality. Animal 1, 159–173.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Hollung K,
Veiseth E,
Jia X,
Moslet Faergestad E, Ivar Hildrum K
(2007) Application of proteomics to understand the molecular mechanisms behind meat quality. Meat Science 77, 97–104.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Jeong DW,
Kim TS,
Chung YW,
Lee BJ, Kim IY
(2002) Selenoprotein W is a glutathione-dependent antioxidant in vivo. FEBS Letters 517, 225–228.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Jurie C,
Cassar-Malek I,
Bonnet M,
Leroux C,
Bauchart D,
Boulesteix P,
Pethick DW, Hocquette JF
(2007) Adipocyte fatty acid-binding protein and mitochondrial enzyme activities in muscles as relevant indicators of marbling in cattle. Journal of Animal Science 85, 2660–2669.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Lee SH,
Park EW,
Cho YM,
Kim SK, Lee JH , et al.
(2007) Identification of differentially expressed genes related to intramuscular fat development in the early and late fattening stages of hanwoo steers. Journal of Biochemistry and Molecular Biology 40, 757–764.
|
CAS |
PubMed |

Lehnert SA,
Wang YH,
Tan SH, Reverter A
(2006) Gene expression-based approaches to beef quality research. Australian Journal of Experimental Agriculture 46, 165–172.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Lehnert SA,
Reverter A,
Byrne KA,
Wang Y,
Nattrass GS,
Hudson NJ, Greenwood PL
(2007) Gene expression studies of developing bovine longissimus muscle from two different beef cattle breeds. BMC Developmental Biology 7, 95.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Loor JJ,
Dann HM,
Janovick Guretzky NA,
Everts RE,
Oliveira R,
Green CA,
Litherland NB,
Rodriguez-Zas SL,
Lewin HA, Drackley JK
(2006) Plane of nutrition prepartum alters hepatic gene expression and function in dairy cows as assessed by longitudinal transcript and metabolic profiling. Physiological Genomics 27, 29–41.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Morzel M,
Terlouw C,
Chambon C,
Micol D, Picard B
(2008) Muscle proteome and meat eating qualities of Longissimus thoracis of “Blonde d’Aquitaine” young bulls: a central role of HSP27 isoforms. Meat Science 78, 297–304.
|
CAS |

Mullen AM,
Stapleton PC,
Corcoran D,
Hamill RM, White A
(2006) Understanding meat quality through the application of genomic and proteomic approaches. Meat Science 74, 3–16.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Ojha S, Kostrzynska M
(2008) Examination of animal and zoonotic pathogens using microarrays. Veterinary Research 39, 4.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ollier S,
Robert-Granié C,
Bernard L,
Chilliard Y, Leroux C
(2007) Mammary transcriptome analysis of food-deprived lactating goats highlights genes involved in milk secretion and programmed cell death. The Journal of Nutrition 137, 560–567.
|
CAS |
PubMed |

Sayd T,
Morzel M,
Chambon C,
Franck M,
Figwer P,
Larzul C,
Le Roy P,
Monin G,
Cherel P, Laville E
(2006) Proteome analysis of the sarcoplasmic fraction of pig semimembranosus muscle: implications on meat color development. Journal of Agricultural and Food Chemistry 54, 2732–2737.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Schwerin M,
Kuehn C,
Wimmers S,
Walz C, Goldammer T
(2006) Trait-associated expressed hepatic and intestine genes in cattle of different metabolic type-putative functional candidates for nutrient utilization. Journal of Animal Breeding and Genetics 123, 307–314.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Sudre K,
Leroux C,
Pietu G,
Cassar-Malek I,
Petit E,
Listrat A,
Auffray C,
Picard B,
Martin P, Hocquette JF
(2003) Transcriptome analysis of two bovine muscles during ontogenesis. Journal of Biochemistry 133, 745–756.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Sudre K,
Cassar-Malek I,
Listrat A,
Ueda Y,
Leroux C,
Jurie C,
Auffray C,
Renand G,
Martin P, Hocquette JF
(2005) Biochemical and transcriptomic analyses of two bovine skeletal muscles in Charolais bulls divergently selected for muscle growth. Meat Science 70, 267–277.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Tsitsilonis OE,
Stoeva S,
Echner H,
Balafas A,
Margomenoua L,
Katsoulasa HL,
Troy DJ,
Voelter W,
Papamichail M, Lymberi P
(2002) A skeletal muscle troponin T specific ELISA based on the use of an antibody against the soluble troponin T (16–31) fragment. Journal of Immunological Methods 268, 141–148.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Tuggle CK,
Wang Y, Couture O
(2007) Advances in swine transcriptomics. International Journal of Biological Sciences 3, 132–152.
|
CAS |
PubMed |

Wang YH,
Reverter A,
Mannen H,
Taniguchi M,
Harper GS,
Oyama K,
Byrne KA,
Oka A,
Tsuji S, Lehnert SA
(2005) Transcriptional profiling of muscle tissue in growing Japanese Black cattle to identify genes involved with the development of intramuscular fat. Australian Journal of Experimental Agriculture 45, 809–820.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Wang YH,
Reverter A,
Kemp D,
McWilliam SM,
Ingham A,
Davis CA,
Moore RJ, Lehnert SA
(2007) Gene expression profiling of Hereford Shorthorn cattle following challenge with Boophilus microplus tick larvae. Australian Journal of Experimental Agriculture 47, 1397–1407.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Yeh JY,
Gu QP,
Beilstein MA,
Forsberg NE, Whanger PD
(1997) Selenium influences tissue levels of selenoprotein W in sheep. The Journal of Nutrition 127, 394–402.
|
CAS |
PubMed |



