Length of exposure to high post-rigor temperatures affects the tenderisation of the beef M. longissmus dorsi
K. L. Thomson A B E G , G. E. Gardner A C , N. Simmons D F and J. M. Thompson A BA Australian Sheep Industry Cooperative Research Centre, University of New England, Armidale, NSW 2351, Australia.
B School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia.
C School of Veterinary and Biomedical Sciences, Murdoch University, Murdoch, WA 6150, Australia.
D AgResearch, MIRINZ Centre, Hamilton, New Zealand.
E Department of Primary Industries, Mount Napier Road, Hamilton, Vic. 3300, Australia.
F Carne Technologies Ltd, PO Box 740, Cambridge, New Zealand.
G Corresponding author. Email: kthomson@dpi.vic.gov.au
Australian Journal of Experimental Agriculture 48(11) 1442-1450 https://doi.org/10.1071/EA07132
Submitted: 8 May 2007 Accepted: 20 June 2008 Published: 16 October 2008
Abstract
This experiment examined the effect of length of exposure of bovine M. longissmus dorsi to high temperatures (37°C) on proteolytic activity during post-mortem aging and subsequent meat tenderness. To avoid confounding between pH decline and incubation treatments, the experiment was conducted on post-rigor meat (pH < 5.6), which had entered rigor at 15°C. This meat was first incubated at 15°C until rigor (pH < 5.6), whereupon samples were then reheated and maintained at 37°C for 0, 1 or 3 h. Following incubation, samples were aged at 1°C for 1–21 days. Control groups were incubated at either 15 or 37°C until pH < 5.6, followed by aging at 1°C. High rigor temperatures accelerated post-mortem proteolysis early in the post-mortem period, as indicated by a rapid decline in shear force (P < 0.01), but post-mortem aging ceased at about day 3 post-mortem and the high rigor temperature treatment was ultimately 30% tougher at day 21 post-mortem (P < 0.01). The process of reheating samples from 15 to 37°C had minimal effect on tenderness levels, but was associated with a slight increase in proteolysis, identified by myofibril length, and was associated with an increase in cook loss percentage (P < 0.05). Shear force in the length of exposure experiment was affected by an incubation time × post-mortem aging interaction (P < 0.01). This indicated an initial acceleration of post-mortem aging with increased length of exposure, but also a reduction in the ultimate tenderness of product after extended post-mortem aging. This was presumably due to the loss of proteolytic enzyme activity caused by the instability of proteolytic enzymes at high ionic strength conditions such as those present in post-mortem muscle.
Introduction
The Meat Standards Australia (MSA) grading scheme uses critical control points (CCPs) to predict the palatability of individual cuts in the beef carcass (Thompson 2002; Polkinghorne et al. 2008). These CCPs cover the entire beef production chain and include genetics, production, lairage, processing and chilling through to value adding and the final process, cooking. Glycolytic rate and chilling rate have been identified as CCPs and the MSA pH/temperature window was established to ensure that pH and temperature relationships were kept within the prescribed window to achieve optimal palatability (Thompson 2002). The MSA pH/temperature window stipulates that pH should be greater than 6.0 at temperatures above 35°C and below pH 6.0 before the temperature falls below 12°C (Thompson 2002). Product that falls outside the upper limits of the MSA pH/temperature window is termed ‘heat-toughened product’, where the combination of high muscle temperatures and low pH caused a reduction in palatability and an increase in drip loss, due largely to the early exhaustion of proteolytic activity (Dransfield 1993; Simmons et al. 1997) and the denaturation of proteins (Hwang et al. 2004).
As reviewed by Hopkins and Taylor (2004) and Koohmaraie and Geesink (2006), the calpain system appears to be largely responsible for tenderisation during aging, although the impact of post-mortem conditions (e.g. variation in pH, ionic strength and temperature) on the rate of proteolysis and subsequent tenderness is still not well understood. This is due in part to the difficulty of studying the activity of these enzymes in vitro. Previous studies have shown that exposure of pre-rigor meat to temperatures above 15°C caused increased activation and autolysis of µ-calpain resulting in early proteolytic activity (Simmons et al. 1996; Geesink et al. 2000; Hwang and Thompson 2001; Hwang et al. 2004). However, while increased activation led to rapid tenderisation early post-mortem, the increased autolysis of µ-calpain limited subsequent tenderisation during aging (Dransfield 1993; Taylor et al. 1995; Simmons et al. 1996; Geesink et al. 2000).
The activity of proteolytic enzymes and their inhibitors after slaughter is of particular interest in high value muscles such as the M. longissimus dorsi, where ultimate tenderness is a function of proteolytic activity during post-mortem storage (Tornberg 1996). Although it was known that exposure of meat to increased rigor temperatures affected the rate and extent of tenderisation during post-mortem storage, it was not clear how length of exposure to these temperatures impacted on aging rate and ultimate tenderness. Geesink et al. (2000) suggested that the effect of temperature between 5 and 35°C on µ-calpain and calpastatin activity may not be linear as tenderness and levels of post-mortem proteolysis were negatively affected by incubation at temperatures above 25°C.
This study examined the relationship between length of exposure of post-rigor muscle to high temperatures and meat quality attributes to better understand the effect of exposure to heat toughening conditions on the subsequent rate of proteolysis during post-mortem aging and tenderness of the M. longissimus dorsi. To avoid the confounding interaction between temperature and pH conditions, incubation treatments were applied once pH < 5.6 was achieved. Due to the difficulty of measuring the specific activities of proteolytic enzymes, the breakdown of the myofibrillar proteins, desmin (Lametsch et al. 2004) and troponin-T (Penny and Dransfield 1979), which are known to be degraded by these enzymes, were used as indicators of proteolytic activity.
Materials and methods
Animals and slaughter procedures
A total of 15 dairy cross cattle (13 heifers and two steers) with a mean liveweight of 219 ± 15 kg were used in this experiment. Following captive bolt stunning and dressing, the striploins (M. longissimus dorsi, from the 10th rib to the lumbar sacral joint) from both sides of the carcass were removed from the hot carcass within 30 min of slaughter. Striploins were denuded of subcutaneous fat and epimysium before being weighed and divided into anterior and posterior portions. The four striploin portions from each animal were then twice wrapped tightly in thick plastic to minimise muscle fibre shortening (Devine et al. 1999).
Experimental design
The four striploin portions from each animal were allocated to four of the five treatments listed in Table 1. In allocating the striploin portions to treatments, the first treatment was incremented by 1 for each animal, with the 15 animals providing 12 replicates per treatment. The wrapped striploin portions were placed in a water bath at 15°C, or a wind tunnel at 37°C, according to their treatment allocation.

|
Changes in muscle pH were monitored in all striploin portions using a MP125 pH meter (Mettler-Toledo, Columbus, OH). When the pH of individual portions fell below 5.6, each portion was further subdivided into 6 × 35-mm-thick steaks. It had been previously determined that it took 25 min to raise the internal temperature of 35-mm-thick steaks from 15 to 36°C (data not shown). The six steaks from each striploin portion were put in individual plastic bags, placed in a weighted plastic bag (ensuring steaks did not overlap), and suspended in a 37°C water bath for either 25 min (reheating), 85 min (reheating + 1 h incubation), or 205 min (reheating + 3 h incubation) according to their allocated treatment. Steaks were then cooled in an ice slurry and vacuum packed for storage at 1°C. The first steak from each striploin portion was used for measurement of sarcomere length (day 1 only), myofibril length, and proteolysis using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)/western blotting techniques at each of the five aging periods. The five remaining steaks were used as cook blocks and were randomly allocated across the post-mortem aging periods (at 1, 3, 7, 12 and 21 days post-rigor).
Meat quality measurements
Shear force
Shear force was determined using the Meat Industry Research Institute of New Zealand tenderometer (Graafhuis et al. 1991). At the pre-allocated aging times, chilled steaks were cooked in polyethylene bags to an internal temperature of 75°C using a 99°C water bath, then cooled in an ice slurry before storing overnight at 1°C. From each steak, 8–10 samples were prepared for shearing perpendicular to the myofibril direction. Shear force was measured on samples at 1, 3, 7, 12 and 21 days post-rigor. Cooking loss was calculated as the proportion of weight lost from raw to cooked weight, after chilling overnight at 1°C.
Myofibril length
The technique used was similar to that described by Devine et al. (1999). Briefly, 1 g of frozen meat at rigor, after incubation, days 1, 3 and 7 (free from visible fat or connective tissue) was weighed and placed in a test tube on ice with 10 mL of chilled standard salt solution (100 mmol/L KCL, 20 mmol/L K-phosphate, 1 mmol/L EDTA, 1 mmol/L NaN3, pH 7.0). The tube was homogenised (Ultra-Turrax T25, IKA, Wilmington, NC) for 60 s at 11 000 rpm then centrifuged for 15 min at 1000g at 4°C. The supernatant was discarded and 5 mL of standard salt solution added to the pellet for resuspension. Of this suspension, 200 µL was added to 5 mL of standard salt solution and vortexed before 5–8 µL of sample was placed on a microscope slide, covered with a glass slip and viewed under the microscope at 40× magnification. For each slide, five images were captured and myofibril length measured using the ScionImage Program 2000 (Scion Corporation, Frederick, MA). Three repeats were recorded per image, before averaging the 15 measurements per sample.
Sarcomere length
Using the myofibril length slides from day 1 samples, myofibril strands were viewed at 100× magnification and sarcomere length was measured on individual myofibril strands using the ScionImage Program 2000. The average number of sarcomere length measurements per sample was 44.
Proteolysis (SDS-PAGE and western blots)
SDS-PAGE (12% acrylamide) gels were used to separate myofibrillar proteins based on the methodology of Laemmli (1970). Samples taken at each aging period (at rigor, after incubation, and at days 1, 3, 7, 12 and 21) were homogenised (homogenising MOPS/sucrose, pH 7.0, 4°C), and denatured samples with standard protein concentration of 5 mg/mL were prepared. After running SDS-PAGE, proteins were transferred from gels onto nitrocellulose membranes overnight at 30V. Immunodetection of proteins proceeded after rinsing and washing the nitrocellulose membranes with blocking buffer [dH20, 1 × Tris-buffered saline, 0.05% Tween 20, 5% blotting-grade blocker – non-fat dry milk (Bio-Rad, Gladesville, NSW)]. Primary antibodies JLT-12 (dilution 1 : 5000, Sigma, St Louis, MO) and DE-U-10 (dilution 1 : 2500, Sigma) were used to identify troponin-T and desmin, respectively, followed by a secondary antibody (total mouse IgG, dilution 1 : 10 000, Sigma). An alkaline phosphatase reaction (alkaline phosphatase conjugate substrate kit, Bio-Rad) was used to identify protein bands. The reaction proceeded with constant rocking until the signal/background ratio was sufficient in conjunction with the pre-stained molecular weight marker (Bio-Rad). Care was taken to prevent blots from becoming saturated so that signal intensities would remain within the linear range. Figure 1 shows the relationship between signal intensity and amount of muscle protein loaded for desmin. The relative intensity of individual bands on the western blots, measured as Gaussian volume, was determined using Phoretix 1D software (Nonlinear Dynamics, Durham, NC). The bands investigated were the 48-kDa band for desmin and <31-kDa bands for troponin-T degradation products.

|
Calculation of glycolytic rate
To calculate glycolytic rate, the exponential rate of pH decline (k) was estimated using the non-linear procedure (PROC NLIN, SAS 1996) where the following function was fitted to the pHt and time data:
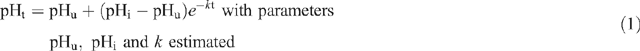
where pHt is pH at time t, pHu is estimated ultimate pH, pHi is estimated initial pH and k is the exponential decay constant.
Statistical analyses
Data analyses were conducted in three parts. Part 1 tested the effects of rigor temperature (treatments 1 and 2) on meat quality and proteolysis traits. Part 2 tested the effect of the reheating procedure (treatments 2 and 3), whereas part 3 tested the effect of length of exposure to high temperatures on the same traits (treatments 3, 4 and 5).
For part 1, the initial models for shear force and cook loss percentage contained fixed effects for rigor temperature (treatments 1 and 2, which were 37 and 15°C rigor temperature, respectively), post-mortem aging (day 1, 3, 7, 12 and 21), striploin portion (left anterior, left posterior, right anterior, right posterior), and steak location (positions 2–6) within striploin portion. Pre-cook weight and sarcomere length (both linear and curvilinear terms) were included as covariates and animal as a random term (PROC MIXED, SAS 1996). For myofibre length, native desmin and troponin-T, samples were analysed from steak position 1 and so steak location was not appropriate. To account for potential differences in SDS-PAGE gel conditions, the analysis of muscle proteins were restricted to within gel, i.e. using gel nested within animal as a random term.
For part 2, the initial models were similar to those used in the first analysis with post-mortem aging, treatment (treatments 2 and 3, which were held at 15°C, or reheated in a 37°C water bath for 25 min, respectively) and striploin portion included as fixed effects. For the shear force and cook loss percentage analyses, steak location within sample was included as a fixed effect and pre-cook weight and average sarcomere length (both linear and curvilinear terms) included as covariates. For the desmin and troponin-T analysis post-mortem aging was limited to days 1, 3, 7, 12 and 21 (rigor samples were not processed for treatment 3). Animal was used as the random term, except for the muscle protein analysis where the random term was gel within animal.
Part 3 examined length of incubation at elevated temperatures (treatments 3, 4 and 5, which were 0, 1 and 3 h incubation, respectively) and post-mortem aging effects. For the shear force and cook loss percentage analyses, steak location within sample was included as a fixed effect and pre-cook weight and sarcomere length (both linear and curvilinear terms) as covariates. For the desmin and troponin-T analyses post-mortem aging was limited to days 1, 3, 7, 12 and 21 (rigor samples were not processed for treatments 3, 4 or 5). Animal was used as the random term, except for the muscle protein analyses where the random term was gel nested within animal.
For analyses within parts 1, 2 and 3, appropriate first order interactions were tested and non-significant terms (P > 0.05) excluded from the analyses using stepwise regression.
Results
Effect of rigor temperature on meat quality and proteolytic rate
Table 2 showed an increase in glycolytic rate in the 37°C compared with the 15°C rigor temperature treatment, as indicated by the 2-fold increase in pH(k) and almost 3-fold decrease in the time to reach pH 5.6. Although incubation temperature affected the rate of pH decline, it had little effect on ultimate pH or sarcomere length (Table 2).

|
At day 1, shear force values for the 37°C treatment were 13% lower (P < 0.01) than the 15°C treatment, indicating that a greater degree of tenderisation had occurred by then (Table 3; Fig. 2). However, from day 3 onwards there was little further decline in shear force in the 37°C treatment, whereas in the 15°C treatment shear force continued to decline up to day 12, where it was ~20% more tender than the 37°C treatment (P < 0.01).

|
Shear force values were affected by the rigor temperature × striploin portion interaction (P < 0.05), such that in the 15°C rigor temperature treatment, samples from the left anterior portion had higher shear force values than samples from the left posterior and right anterior positions (P < 0.05). There was no difference in shear force between striploin portions from the 37°C rigor temperature (P > 0.05). There was a curvilinear relationship between sarcomere length and shear force (P < 0.05) with a point of inflexion (corresponding to the lowest shear force value) occurring at 1.72 µm.
For cook loss percentage there was a significant (P < 0.05) rigor temperature × post-mortem aging interaction (Table 3). Table 4 showed that with the exception of day 1, there was a trend for the low rigor treatment to have a higher cook loss percentage, which achieved significance at 3 and 7 days aging (P < 0.05). Cook loss percentage was affected by steak pre-cook weight (Table 3, P < 0.05) such that cook loss percentage decreased by –0.054 ± 0.018% per gram increase in pre-cook weight. Similarly, cook loss percentage was affected by sarcomere length (Table 3, P < 0.05) with a decrease in cook loss percentage of –3.66 ± 1.64 per micron increase in sarcomere.

|
For myofibril length there was a significant (P < 0.05) aging × incubation temperature interaction (Table 3). Figure 3 showed that while there was no treatment effect on myofibril length at rigor, by day 7 post-mortem, the 15°C rigor treatment had significantly (P < 0.05) shorter myofibrils, which indicated greater proteolysis had occurred.

|
The level of native desmin declined (P < 0.01) by ~80% from rigor to day 21, and this decline did not interact with rigor temperature (Table 3). As indicated by a slower accumulation of degradation products (<31 kDa), the 37°C rigor temperature treatment decreased troponin-T degradation, which was significant from day 7 onwards (see Fig. 4).

|
Effect of reheating samples from 15 to 37°C on meat quality traits
Table 5 shows that the pH values of the striploin portions that were reheated were similar to the ultimate pH readings obtained from the day 1 aged samples, which indicated that little change in pH in samples were reheated. This indicated that all samples had achieved ultimate pH when the treatments were imposed.

|
The analysis tested the effect of the sample reheating procedure, from 15 to >35°C (25 min incubation in the 37°C water bath) on objective meat quality measures and proteolysis (Table 6). There was a significant striploin portion × treatment interaction (P < 0.01) on shear force, such that in the 15°C to rigor treatment samples from the left anterior and right posterior positions had higher shear force values than those in the left posterior and right anterior striploin portions. Shear force values for the left posterior and right anterior portions in the 15°C were similar to those attained in the 37°C rigor temperature. Likewise, no striploin position effect was identified in the 37°C rigor temperature (P > 0.05). In the presence of the striploin portion treatment interaction, the reheating procedure lowered shear force values by ~20 N, indicating an acceleration of tenderisation (P < 0.01).
There was an aging effect (P < 0.01) on shear force, with a decline as post-mortem aging increased (Table 6). There was also a significant (P < 0.05) steak position within striploin portion effect on shear force, which highlighted the importance of randomising the allocation of aging periods across the individual steak positions within the striploin portions.
Reheated samples had a lower cook loss percentage (26.9 ± 0.5% and 24.9 ± 0.4% for the 15°C rigor treatment and reheating treatments, respectively). Post-mortem aging also affected cook loss percentage (P < 0.01, Table 6) with lower cook loss percentage in samples aged for 12 days (P < 0.05).
Average myofibril length declined with post-mortem aging (P < 0.01) and the reheating treatment (P < 0.05, Table 6). At rigor the average myofibril length was 33.9 ± 1.1 µm, and this declined to 21.4 ± 1.1 µm at day 7 post-rigor. The reheating treatment accelerated myofibrillar degradation (P < 0.05), but did not interact with post-mortem aging (P > 0.05). The average myofibril length of the reheated samples was ~8% shorter than samples held at 15°C and not reheated (P < 0.05). Native desmin declined by ~90% from day 1 to day 21 post-mortem (P < 0.01) and was not affected by the reheating procedure (P > 0.05, Table 6). Likewise, troponin-T degradation products increased (P < 0.01) with post-mortem aging but were not affected by the reheating procedure (P > 0.05, Table 6).
Effect of length of exposure of post-rigor meat to temperatures >35°C on meat quality traits
For shear force there was a significant (P < 0.01) incubation time × aging interaction (Table 7), which showed that at day 1 post-mortem shear force value for the 3-h incubation was ~19 N lower than the 0-min incubation (P < 0.01, Fig. 5), whereas at the completion of aging, the 3-h incubation treatment was 13.4 ± 5.3 N and 17.4 ± 5.1 N higher than the 1- and 0-h incubation treatments (P < 0.05). Figure 6 shows the trend that an increasing length of exposure initially accelerated post-mortem proteolysis at day 1, but also limited the extent of tenderisation as the length of exposure increased. This suggested that the effect of increasing the length of exposure to high temperature was linear for both the improvement of initial tenderness and also the subsequent inhibition of further tenderisation. It was of interest that at day 21 post-rigor, the 3-h incubation was 17.4 N tougher than the 0-h treatment as no difference between these treatment was observed at days 7 and 12 (P > 0.05). This suggested that the initial acceleration of post-mortem proteolysis early post-mortem was not overcome until the meat was aged out beyond 12 days post-mortem.

|

|
For shear force there was a significant incubation time × striploin portion interaction (Table 7, P < 0.05). This again highlighted the importance of randomising experimental treatments across the sampling positions. Shear force was also affected by sarcomere length (P < 0.05), with a 0.1-µm decrease in sarcomere length increasing shear force by ~5 N.
Cook loss percentage declined with post-mortem aging (P < 0.01) from 27.8 ± 0.6% at day 1 post mortem to 24.9 ± 0.6% at day 21. Table 7 showed that cook loss percentage was also affected by sample pre-cook weight (P < 0.01), with a lower cook loss percentage in heavier samples. There was an effect of striploin portion on cook loss percentage with a greater cook loss percentage in the anterior striploin portions than the posterior striploin portions (P < 0.05). Myofibril length declined by ~35% between rigor and day 7 post-mortem (P < 0.01), with predicted means (±s.e.) of 33.4 ± 0.9 µm and 22.2 ± 0.9 µm for rigor and day 7.
The rate of desmin degradation during early post-mortem aging was affected by the length of incubation at high temperatures (P < 0.01). At the conclusion of the incubation treatments, the 3-h treatment showed more desmin degradation; however, from day 1 onwards there were no differences in the levels of native desmin between incubation treatments (Fig. 7). Likewise, there was a trend (P < 0.07) for the rate of troponin-T degradation to be affected by the length of exposure to high temperatures. Figure 8 showed that while early post-mortem there were no differences in the level of troponin-T degradation products (<31 kDa), at day 21 aging the 3-h incubation treatment had less troponin-T degradation than both the 0- and 1-h incubations (P < 0.05).

|

|
Discussion
Effect of rigor temperature on meat quality and proteolysis
High rigor temperatures initially resulted in more tender meat, but this was reversed with aging and meat from the high rigor temperature treatment was ultimately 30% tougher. Presumably, this interaction occurred because of the instability of the autolysed enzyme at elevated temperatures (Geesink and Koohmaraie 2000). Simmons et al. (1996) also reported a similar crossover in shear force in un-aged and aged samples subjected to a range of rigor temperatures. In addition, a similar trend for titin degradation was shown by Geesink et al. (2000) in ovine M. longissimus thoracis et lumborum. Simmons et al. (1996) suggested that calpain activity declined more rapidly when rigor was achieved at higher muscle temperatures, with an increased rate of calpain autolysis relative to calpain-mediated proteolysis, leading to tougher meat during post-mortem storage. Similarly, Geesink et al. (2000) and Hwang et al. (2004) also showed that proteolysis of myofibrillar proteins and µ-calpain autolysis was increased by incubation at high temperatures.
In some studies the effect of temperature on proteolysis has been studied by holding pre-rigor meat at different temperatures for the same length of time (Geesink et al. 2000; Hwang et al. 2004). In these studies, it was likely that higher temperatures increased glycolytic rate such that rigor mortis occurred earlier in the incubation period, which would effectively result in the early activation of µ-calpain and potentially a longer period of µ-calpain activity.
Although the high incubation temperature accelerated the rate of glycolysis, ultimate pH was not affected. Similar findings were identified by Hertzman et al. (1993) and Simmons et al. (1996). However, Geesink et al. (2000) and Devine et al. (2002) found that higher ultimate pH values were associated with increased rigor temperatures. These findings were in ovine M. longissimus thoracis et lumborum, and no suggestions regarding the cause of these results were provided.
While several studies report that high rigor temperatures were associated with increased cooking losses (Geesink et al. 2000; Devine et al. 2002; Hwang et al. 2004), this was not apparent in the present results. Hwang et al. (2004) proposed that increased cooking loss may result from the denaturation of proteins at high rigor temperatures, but also suggested that muscle shortening could also potentially contribute to this effect. In the present experiment, a muscle wrapping technique was used to limit sarcomere shortening and proved to be quite effective since both treatment groups had relatively long sarcomere lengths, which was unusual for hot-boned products. Given that muscle shortening may in part be responsible for the cooking loss effect (Hwang et al. 2004), this may explain the absence of a cook loss effect in our experiment. In addition, rigor temperature treatments in our study were only imposed until rigor was achieved, which differed from Geesink et al. (2000) and Hwang et al. (2004) where incubation times were applied for either 16 or 24 h. The increased length of incubation at high temperatures in these experiments may have also contributed to the magnitude of the cook loss effect in their studies. It may also be possible that changes in the weep or purge process during aging may have impacted on the subsequent cook loss measures in these studies.
Troponin-T is generally used as an indicator of proteolysis rather than as a predictor of tenderness per se (Hopkins and Taylor 2004). Our results showed that at the conclusion of aging, the low rigor temperature treatment had 35% more troponin-T degradation products. Desmin is known to be degraded by µ-calpain under post-mortem conditions (Lametsch et al. 2004) and has also been used to investigate the rate of proteolysis. In the present experiment, levels of native desmin declined during aging, but no effect of rigor temperature was identified. The presence of large standard errors for desmin suggested that the western blotting methodology used in this experiment may not have been sensitive enough to detect the treatment differences observed by shear force assessment or the degradation of troponin-T. The use of an internal standard in each gel would have enabled individual gels to be directly compared, which may have increased the ability to detect treatment effects.
This experiment showed that extreme rigor temperatures of greater than 35°C produced tougher meat after post-mortem aging at 1°C. This was similar to Devine et al. (2002), where differences in tenderness did not result as a consequence of muscle shortening. Rather, they were caused by the 15°C rigor treatment exhibiting a faster rate of tenderisation and tenderising to a greater extent than the 35°C rigor treatment. This provided further confirmation of the detrimental effects of the combination of high rigor temperatures and low muscle pH (Thompson et al. 2006). Our results demonstrated that exposure to extreme heat toughening conditions contributed to toughening of the M. longissimus dorsi and supported the heat toughening region on the MSA pH/temperature window (Thompson 2002).
Effect of reheating samples from 15 to 37°C on meat quality and tenderisation during aging
The reheating procedure appeared to have minimal effect on tenderness levels although a slight acceleration in post-mortem proteolysis was identified by myofibril length, and higher cook loss percentage were associated with the reheated procedure. Given the treatment × striploin portion interaction it was difficult to quantify the effect of the reheating treatment on shear force, although the absence of any treatment × aging interaction indicated that the reheating process did not affect the rate of tenderisation. No reheating effects were observed in the degradation patterns of desmin or troponin-T, but given the insensitivity of the SDS-PAGE/western blot methodology this result was perhaps not unexpected.
Effect of length of exposure of post-rigor meat to temperatures >35°C
Increased length of exposure to high temperatures (>35–37°C) in post-rigor meat accelerated proteolysis early in the post-mortem aging period such that ultimate tenderness was achieved more rapidly. However, the trade-off with early exhaustion of protease activity meant that the meat was tougher at 21 days compared with product not exposed to high temperatures. While the effect of length of exposure was difficult to investigate in pre-rigor carcasses due to the changes in pH (or changes in ionic strength), results from this experiment suggested that increasing the length of exposure activated greater levels of proteolytic enzymes, which either contributed to the proteolysis of myofibrillar proteins, or underwent further autolysis. Using myofibril length and levels of native desmin and troponin-T as indicators of proteolysis failed to show any effect of incubation time on post-mortem proteolysis.
It was interesting that while the degradation pattern for desmin did identify the early post-mortem effect of extended exposure to high rigor temperatures, in all three incubation treatments, no native desmin was detected at day 21 post-mortem. Lametsch et al. (2004) previously reported that desmin was completely degraded when incubated with µ-calpain. It would, therefore, appear that neither muscle proteins investigated in the present experiment provided a sensitive enough measure to identify similar treatment differences observed by shear force.
Subjecting post-rigor meat samples to various periods of high temperature treatment impacted on both initial tenderness and ultimate shear force. While there was a linear improvement in initial shear force from exposure to high temperatures, there was also a linear increase in shear force after extended aging. This suggested that while exposure to high temperatures accelerated myofibrillar proteolysis early post-mortem, it was also related to the loss of µ-calpain activity, presumably due to the instability of the autolysed calpains due to high ionic strength conditions (Geesink and Koohmaraie 2000). It was, therefore, likely that this was responsible for the subsequent failure to tenderise in the long incubation treatments. It was interesting that exposure of post-rigor meat to high temperatures only resulted in a 17.4-N difference after extended incubation (3 h). Considering that conditions post-rigor displayed the extremes of low pH and high ionic strength (Geesink and Koohmaraie 2000) it could have been expected that under the conditions imposed in this study, significant inhibition of myofibrillar proteolysis would result.
Conclusion
In this experiment, the rigor temperature treatments applied were more extreme than would normally be encountered commercially. However, they clearly demonstrated that high rigor temperatures produced tougher meat after post-mortem aging, by affecting both the rate and extent of tenderisation. This confirmed the need for carcasses to avoid the heat shortening region of the MSA pH/temperature window.
Increasing the length of time meat was exposed to heat toughening conditions reduced tenderness levels after extended post-mortem aging. However, if the meat was not aged for extended periods then exposure to heat toughening conditions (as long as product safely is not jeopardised) should not pose significant problems, and may in fact promote increased tenderness. Future work to clarify the commercially acceptable levels of pre-rigor/post-rigor exposure to high temperatures is required to enable further refinement of the MSA pH/temperature window.
Acknowledgements
This experiment was conducted at the AgResearch MIRNZ Ruakura Research Centre, Hamilton, New Zealand. The authors also wish to thank Peter Dobbie and Tracey Cumming from AgResearch, New Zealand and Dr Pierre Baudoux from Meat Science, University of New England, Australia for their skilled technical expertise, assistance and support during this experiment.
Devine CE,
Wahlgren NM, Tornberg E
(1999) Effect of rigor temperature on muscle shortening and tender of restrained and unrestrained beef M. longissimus thoracic lumborum.
Meat Science 51, 61–72.
| Crossref | GoogleScholarGoogle Scholar |

Devine CE,
Payne SR,
Peachey BM,
Lowe TE,
Ingram JR, Cook CJ
(2002) High and low rigor temperature effects on sheep meat tenderness and ageing. Meat Science 60, 141–146.
| Crossref | GoogleScholarGoogle Scholar |

Dransfield E
(1993) Modelling post-mortem tenderisation. IV. Role of calpains and calpastatin in conditioning. Meat Science 34, 217–234.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Geesink GH, Koohmaraie M
(2000) Ionic strength-induced inactivation of μ-calpain in postmortem muscle. Journal of Animal Science 78, 2336–2343.
|
CAS |
PubMed |

Geesink GH,
Bekhit AD, Bickerstaffe R
(2000) Rigor temperature and meat quality characteristics of lamb longissimus muscle. Journal of Animal Science 78, 2842–2848.
|
CAS |
PubMed |

Hertzman C,
Olsson U, Tornberg E
(1993) The influence of high temperature, type of muscle and electrical stimulation on the course of rigor, ageing and tenderness of beef muscles. Meat Science 35, 119–141.
| Crossref | GoogleScholarGoogle Scholar |

Hwang IH, Thompson JM
(2001) The interaction between pH and temperature decline early postmortem on the calpain system and objective tenderness in electrically stimulated beef longissimus dorsi muscle. Meat Science 58, 167–174.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Hwang IH,
Park BY,
Cho SH, Lee JM
(2004) Effects of muscle shortening and proteolysis on Warner-Bratzler shear force in beef longissimus and semitendinosus. Meat Science 68, 497–505.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Koohmaraie M, Geesink GH
(2006) Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Science 74, 34–43.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Laemmli UK
(1970) Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 227, 680–685.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Lametsch R,
Roepstorff P,
Moller HS, Bendixen E
(2004) Indentification of myofibrillar substrates of µ-calpain. Meat Science 68, 515–521.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Penny IF, Dransfield E
(1979) Relationship between toughness and troponin-T in conditioned beef. Meat Science 3, 135–141.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Polkinghorne R,
Thompson JM,
Watson R,
Gee A, Porter M
(2008) Evolution of the Meat Standards Australia (MSA) beef grading system. Australian Journal of Experimental Agriculture 48, 1351–1359.

Taylor RG,
Geesink GH,
Thompson VE,
Koohmaraie M, Goll DE
(1995) Is Z-disk degradation responsible for postmortem tenderization? Journal of Animal Science 73, 1351–1367.
|
CAS |
PubMed |

Thompson J
(2002) Managing meat tenderness. Meat Science 62, 295–308.
| Crossref | GoogleScholarGoogle Scholar |

Thompson JM,
Perry D,
Daly B,
Gardner GE,
Johnston DJ, Pethick DW
(2006) Genetic and environmental effects on the muscle structure response post-mortem. Meat Science 74, 59–65.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Tornberg E
(1996) Biophysical aspects of meat tenderness. Meat Science 43, S175–S191.
| Crossref | GoogleScholarGoogle Scholar |






