Aquaculture nutrition in Australia: challenges and trends
H. H. Truong A * , B. M. Hines B , A. N. Rombenso A and C. J. Simon B
A * , B. M. Hines B , A. N. Rombenso A and C. J. Simon B
A CSIRO, Livestock and Aquaculture Program, Bribie Island Research Centre, Woorim, Qld 4507, Australia.
B CSIRO, Livestock and Aquaculture Program, Queensland Bioscience Precinct, St. Lucia, Qld 4067, Australia.
Abstract
This review provides an outline of some of the challenges facing nutritionists in the Australian aquaculture industry. It commences with a brief description of how aquaculture nutrition differs from that for terrestrial livestock – the challenges of providing nutrition in aqueous environments, the diversity of species and the high trophic level of most. Specific challenges of aquaculture nutrition are then discussed in further detail, including the difficulty of measuring feed intake and digestibility, the limited ability to use carbohydrates for carnivores, and the specific requirements of species for lipids, including cholesterol, phospholipids, long-chain polyunsaturated fatty acids and carotenoids. The review then examines how environmental, social and governance concerns are leading to new trends in nutrition for the Australian industry. This includes topics such as the replacement of wild-caught fish meal and fish oil, in terms of both sources of omega-3 lipids and protein. For the former, possible solutions include greater use of seafood trimming, algal oil, and GMO-derived products. For the latter, solutions can include use of livestock render, plant protein meals, fermented products, and insects. Nutrient discharge is also a concern for the industry and nutritionist can assist by improving digestibility and nutrient retention from feeds. Finally, the carbon footprint of aquaculture is leading to new directions for industry and, in turn, for the field of aquaculture nutrition.
Keywords: alternative ingredients, diet formulations, digestibility, fatty acids, feed intake, nutrient requirements, protein, sustainable aquaculture.
Introduction
The Australian aquaculture industry supplies to premium markets by leveraging on a ‘clean and green’ reputation of producing products that are safe to consume, are highly regarded for their health benefits and taste and have a low environmental impact (Department of Agriculture and Water Resources 2017). The value of the industry is a commendable AUD1.6 billion (Steven et al. 2021), despite its small size of just 100 000 t per annum. Furthermore, the industry is growing and is expected to produce 150 000 t a year by 2030 (Food and Agriculture Organization of the United Nations 2020). Diversification is expected to broaden the range of aquaculture products on offer and supplement the current important species for Australian aquaculture, namely, Atlantic salmon (Salmo salar), barramundi (Lates calcarifer) and black tiger prawn (Penaeus monodon), which together represent over 70% of the total farmed value for fed aquaculture (Steven et al. 2021).
This review provides an outline of some of the challenges faced by aquaculture nutritionists and by the Australian aquaculture industry. It comprises the following three sections: (1) an initial overview of how aquaculture broadly differs from that of terrestrial livestock; (2) an overview of specific challenges of nutrition-based research that remain unresolved; and (3) an examination of how environmental, social and governance (ESG) concerns are leading the aquaculture industry in new directions and how this will influence nutrition research.
Overview of differences between nutrition for aquaculture and terrestrial livestock
Aquaculture nutrition presents a unique set of challenges compared with nutrition of terrestrial livestock. The water in which animals are reared makes measurement of feed intake and observation of feeding behaviours difficult. Water also affects how feeds are manufactured, because all require a degree of stability in this medium. Unstable feeds disintegrate and leach, which reduces the effectiveness of feeding and causes greater nutrification of the environment (Obaldo et al. 2002; Sørensen 2012). In addition, water contains many dissolved minerals and harbours micro-organisms that might vary throughout the seasons and provide additional sources of nutrition (Moriarty 1997; Moss et al. 2006; Boyd 2020). Measuring the nutritive contribution of each of these is difficult.
The diversity of species cultured in aquaculture far exceeds that of terrestrial livestock. Terrestrial livestock is dominated by a handful of species, while aquaculture farms are dominated by hundreds of species, which has resulted in a limited knowledge and research base specific to each species. Species-specific nutritional requirements are very hierarchical, with the most commercially important species receiving the most research (Rombenso et al. 2021). Knowledge is significantly reduced for less commonly cultured species including invertebrates such as crabs, lobsters, and shrimp. These species present challenges for researchers due to their divergence from vertebrates, small size, complex larval cycles, unique digestive physiology, feeding habits, and diversity.
Some of Australia’s most important species have more mature industries overseas and so the local industry can leverage off an international body of research and commercial practice. This is particularly true for Atlantic salmon, but also applies to a lesser extent to other species such as cobia (Rachycentron canadum), Queensland grouper (Epinephelus lanceolatus), yellowtail kingfish (Seriola lalandi), barramundi (Lates calcarifer) and black tiger prawn (Penaeus monodon). In contrast, species that are reared only in Australia are often poorly known. This includes species such as Murray cod (Maccullochella peelii), barcoo grunter (Scortum barcoo) and redclaw (Cherax quadricarinatus), as well as many other less-cultured species. These species are further neglected as the economics of producing bespoke feeds is usually prohibitive, because it is costly for feed companies to change feed formulations, manufacture reduced volumes, and fund research into these species. Consequently, growers of minor species are often reliant on adopting feeds tailored to another animal.
Most aquaculture species cultured in Australia are carnivorous and require high protein diets that vary markedly in formulation from those for terrestrial livestock. Formulations are rich in energy, protein, essential amino acids, lipids and long-chain polyunsaturated fatty acids (LC-PUFA; National Research Council 2011). Aquatic species often have a very limited ability to utilise carbohydrates (Tacon 1987; Wilson 1994). In addition, there are often species-specific requirements for nutrients such as cholesterol and phospholipids for crustaceans and LC-PUFA and taurine for some fish species (Abramo 1989; Trushenski and Rombenso 2020; Hardy and Kaushik 2021). These formulation requirements strongly distinguish aquaculture from traditional livestock production.
One illustration of how aquaculture varies from terrestrial livestock is the area of mineral nutrition. Mineral nutrition in aquaculture is strongly affected by the mineral profile of the water in which the animals are cultured, rather than from dietary sources. Seawater contains many minerals, such that most elements do not need to be supplemented (Truong et al. 2022a). In freshwater systems, supplementation may be required, but often both indirectly by adjusting or maintaining water chemistry as well as directly via dietary supplementation.
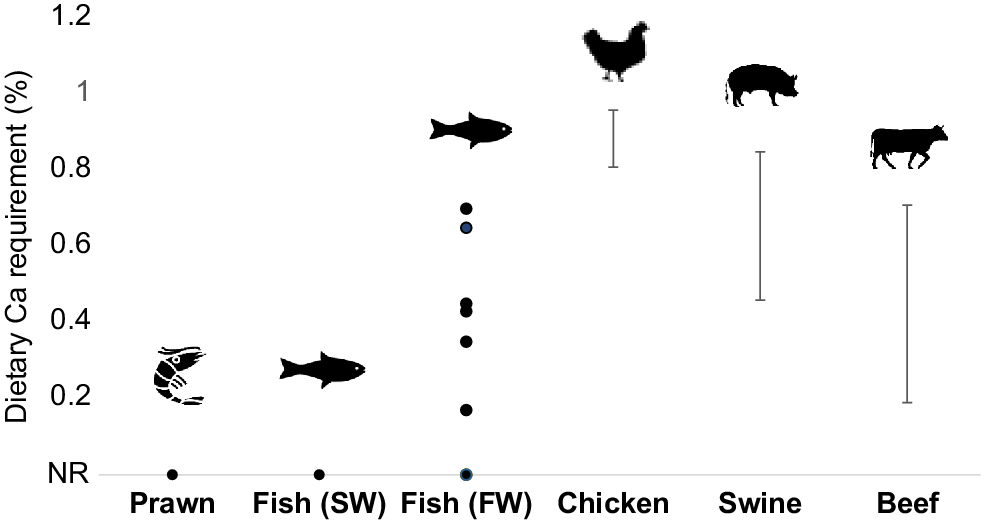
Calcium (Ca) is a good example of how aquaculture and terrestrial livestock nutrition differ. This element is essential and is described as a macromineral, due to its importance to an animal. Despite its importance, Ca supplementation for animals grown in seawater is usually not required so long as concentrations of available Ca in the water are maintained (Fig. 1, derived from Truong et al. 2022a).
Calcium (Ca) requirements of different species. Points indicate the value(s) that was recommended. Whiskers indicate the range that was recommended. Values on the x-axis (x = 0) are not required (NR). Requirement values were obtained for shrimp species (Penaeus monodon and vannamei), saltwater (SW) and freshwater (FW) fish (via Truong et al. 2022b), chicken (Aviagen 2022), swine (Southern et al. 2012) and beef (National Academies of Sciences 2016).
Specific challenges in aquaculture nutrition
Measuring feed intake
Feed intake is a key parameter to measure both palatability of feeds and feed conversion efficiency. By knowing feed intake, nutritionists can make informed decisions about the ingredients and feed additives (attractants, digestibility enhancers, binders) used in formulations (Glencross et al. 2007).
In aquaculture, feed intake is usually measured for a group of animals rather than for individuals (Helland et al. 1996; Folorunso et al. 2017; Chen et al. 2020; Truong et al. 2021; Yuan et al. 2021). There are various reasons why groups of animals are used as compared with individuals. First, there is the cost. Housing fish or other animals in individual tanks across treatments soon becomes logistically prohibitive. There are also behavioural problems whereby species that school become stressed when housed alone. Finally, there is a sampling issue of trying to measure what was and was not eaten for animals that can be quite small, such as prawns. For these reasons, group scale measures of feed intake have prevailed.
Technologies have been developed to assist in the measurement of feed intake. In a terrestrial system, accurate feed-intake measurements are possible with the controlled delivery of feed (e.g. automatic/electronic feeding) and identification of individuals (e.g. radio-frequency identification tags, GPS collars, individual feeders; Andonovic et al. 2018; Muir et al. 2020; Vargovic et al. 2020; Moss et al. 2021). However, these technologies are rarely translatable to the aqueous environment. Some efforts have been made using markers and dyes, X-rays (Jobling et al. 2001) and video (Zhou et al. 2018a). However, each of these has limitations and broadscale adoption of any of these technologies has yet to occur.
The difficulty in measuring feed intake has broad implications for aquaculture. In other animal production areas such as poultry, great gains have been achieved by selecting lines of animals that are most efficient in converting feed into product. However, for aquaculture, measuring feed intake is difficult and so selective breeding of feed-efficient lines rarely occurs, if ever (de Verdal et al. 2018). Instead, breeding programs select broodstock principally on growth. This discrepancy can lead to the breeding of animals that grow quickly through dominance, whereby dominant individuals are able to consume more feed than their peers, as evident in terrestrial animal examples (Jensen 2006; Canario et al. 2012; Adamczyk et al. 2013). If feed intake is not considered, the industry could coincidentally develop lines that are less feed efficient, not more.
Measuring digestibility
Accurate measurement of apparent digestibility (AD) is essential to characterise the nutritive value of ingredients. Apparent digestibility of an ingredient or feed is species-specific and techniques to measure apparent digestibility are reflective of the animal’s digestive system. In terrestrial animals, total tract digestibility is determined from excreted faeces (McGhee and Stein 2020; Adekoya et al. 2021; Wang et al. 2022). This is challenging in aquaculture, because nutrients leach from any excreted faeces, making accurate estimation of apparent digestibility difficult. To prevent leaching, nutritionists prefer to obtain faecal samples before they are excreted, either by applying pressure to the abdominal cavity (‘stripping’) or by dissection (Storebakken et al. 1998; Glencross et al. 2007). However, the collection of faeces of sufficient quantity by either of these methods is not always possible, particularly for small animals.
In crustaceans, stripping is not possible as the hard exoskeletons prevent the application of enough pressure to move digesta along the gastro-intestinal tract (Glencross et al. 2007). For large crustaceans such as lobster, faecal-collection devices have been developed (Irvin and Tabrett 2005; Simon 2009). However, for small crustaceans such as prawns, these techniques are not suitable. Furthermore, dissection of prawns has not historically provided enough sample for analysis. This has meant that digestibility estimates have been obtained by collecting excreted faeces from the bottom of tanks that have been exposed to the effects of leaching. While important information on feedstuff digestibility in shrimp has been acquired over the years by using indirect-marker methods on recovered faecal strands, there are certain aspects of prawn digestive physiology that need to be better understood to satisfy certain digestibility marker assumptions (Wade et al. 2018) and leaching losses have hindered understanding intricate differences in digestive capacity for certain ingredients.
Techniques for measuring apparent digestibility in prawns were recently compared, whereby apparent digestibility values obtained using techniques of collecting excreted faeces that have been traditionally relied on in prawn nutrition where compared with values obtained by dissecting digesta (Truong et al. 2022b). These methods showed no difference when determining the apparent digestibility of starch and lipid. However, significant differences were observed for apparent digestibility of crude protein and amino acids, which are highly water-soluble. Traditional faecal-collection methods that rely on recovering faeces excreted into water consistently over-estimated the digestibility of protein and amino acids compared with the dissection method, which produced more conservative values. For example, protein digestibility of fishmeal was 82% when using a faeces-settlement-in-water method and was 49% when using the dissection method (Truong et al. 2022b). The apparent digestibility values obtained by dissection are likely closer to reality because they agree with the poor protein retention efficiency commonly observed in prawns.
Near-infrared (NIR) spectroscopy has been used as a tool to estimate apparent digestibility (Glencross et al. 2015; Glencross et al. 2017; Simon et al. 2022). These authors developed NIR models to predict the nutrient content of diets and faeces from barramundi (Lates calcarifer), rainbow trout (Oncorhynchus mykiss) and yellowtail kingfish (Seriola lalandi). For example, in kingfish, the NIR model in combination with a digestibility marker was able to successfully predict, by using a small amount of faeces (<1 g dry weight), the nutrient AD of dry matter, protein, and gross energy with a degree of error of ±1–3% and lipid with a degree of error of ±6–8%. These results indicate that such models can play an important role in the industry, by providing a rapid and cost-effective method to assess apparent digestibility.
Carbohydrates
A conundrum exists in nutrition of carnivorous fish with the use of carbohydrates. Use of carbohydrates is required for the structural formation of the feed. However, overuse of the carbohydrates can cause health issues for these animals. Carbohydrates are required to make the desired physical properties of the pellets of feed. These properties include high stability in water, texture, buoyancy, and porosity. For example, porosity is required to achieve high-lipid diets (e.g. higher than 30% dietary lipid content for salmon) where extruded pellets are produced to contain air-pockets in which oils can be vacuumed-sealed within. In this regard, carbohydrates contribute important functional roles to achieve specific pellet characteristics. However, while carbohydrates are important in feeds for structural purposes, they can cause problems if they are fed to carnivorous fish.
Carnivorous fish such as salmon and barramundi have a very limited ability to digest carbohydrates, which contrasts strongly with livestock such as poultry (Truong et al. 2016), ruminants (National Academies of Sciences 2016) and swine (Southern et al. 2012), whereby this food class is the major source of energy for production. For carnivorous fish, catabolism is inefficient in regard to protein and lipid (Azevedo et al. 2005; Sá et al. 2007; Glencross et al. 2017). Health issues arise because these animals have no adaptation to digest carbohydrates. ‘Fatty liver disease’ in the Australian grouper (Epinephelus sp.) industry is an example, whereby overfeeding of carbohydrates is thought to be linked to pathology whereby animals accumulate too much lipid and glycogen in the liver (Nankervis et al. 2022).
Requirement for specific lipids and pigments
Lipids serve several roles in nutrition as an energy source, source of essential fatty acids and facilitate absorption of fat-soluble vitamins. For terrestrial animals, many ingredients can contribute to meeting an animal’s lipid requirement and the addition of fat to diets is largely driven by economics (cost per unit of energy; Southern et al. 2012). Concentrated lipid sources are restricted in feeds for ruminants because they can have an antimicrobial effect, be hydrolysed by rumen bacteria and provide little energy for microbial fermentation (Bauman et al. 2003). Lipid requirements for aquaculture species are comparatively more significant, encompassing a higher gross requirement for energy as well as more specific requirements for functional lipid classes such as cholesterol, intact phospholipid, and essential fatty acids (National Research Council 2011). These requirements vary between species and are briefly discussed below.
For fish, cholesterol and phospholipids can each be produced in vivo; however, it is a metabolically expensive process and providing dietary sources assists in promoting growth (Hardy and Kaushik 2021). Prawns are not able to synthesise cholesterol and so it needs to be provided in feeds (Teshima and Kanazawa 1971). Prawns also benefit from dietary inclusion of phospholipids, despite having the capability to synthesise them (Coutteau et al. 1997). Live feed and ingredients derived from marine sources such as pelagic fish, krill, and microalgae are rich sources of cholesterol and phospholipids. Formulated feeds for fish and prawns are often supplemented with phospholipids (soy lecithin) and cholesterol (krill meal, fish oil, poultry oil) to ensure that requirements are met (National Research Council 2011).
Long-chain poly unsaturated fatty acids (LC-PUFA) are of particular interest in fish nutrition, with some (i.e. docosahexaenoic acid, DHA, and arachidonic acid, ARA) being more physiologically relevant than others (i.e. eicosapentaenoic acid, EPA) in respect of growth, survival and stress resilience (Trushenski and Rombenso 2020). Dietary ARA was reported to be strategic to fish cultured at suboptimal temperatures (warmer and colder; Norambuena et al. 2016; Araújo et al. 2019; Araújo et al. 2021). This is of particular interest not only to the Tasmanian salmon industry faced with high summer seawater temperatures, but also to the broad Australian aquaculture industry as it faces the consequences of climate change. Regardless the fish species’ capacity to produce or not in vivo, dietary supplementation of these key LC-PUFA can benefit the production of higher trophic-level (more carnivorous) species (Trushenski and Rombenso 2020).
Omega-3 fatty acids are important for the aquaculture industry as there are consumer expectations that many seafood products, such as salmon, contain high concentrations of omega-3 for human health benefits. Strategies to maintain or enhance omega-3 content in fish fillets via dietary manipulations are well developed for mature industries such as salmon (Rombenso et al. 2022). These include the use of finishing diets with high fish oil concentrations, supplementation of omega-3 fatty acids and formulation strategies to promote an omega-3 LC-PUFA sparing effect. Industry adoption of these dietary strategies is predominantly driven by consumer expectations, and regulations and guidelines that may be associated with this, rather than as a dietary requirement of the fish.
Carotenoid pigments such as astaxanthin are added to diets for both salmon and prawns to provide colour to flesh, skin, shells, and fins at levels to meet customer demands and preferences. In addition, carotenoids have been shown to be beneficial as antioxidants and immunomodulators (de Carvalho and Caramujo 2017). Sources of lipid influenced the absorption of algal carotenoids in salmon where canola oil facilitates a higher apparent digestibility of carotenoids than does tallow (Courtot et al. 2022). Furthermore, algal carotenoids had significantly higher apparent digestibility than did synthetic astaxanthin. While this finding would align with an increasing consumer preference for the use of naturally derived feed additives in animal feeds, the cost of such naturally derived astaxanthin is substantially higher than that of the synthetic forms currently used by industry.
ESG concerns
It is forecasted that there will be a reduced exportation of Australian-produced seafood due to the ongoing effects of the COVID-19 pandemic, rising production and freight costs and reduced export-market profile related to changing consumer behaviour and international relationship tensions (Curtotti et al. 2023). Thus, it is necessary to increase the domestic consumption of Australian seafood products to maintain a viable Australian aquaculture industry. Cost of seafood undoubtedly plays a role in consumer preferences. However, there is also a need to improve the public perception of Australian-produced seafood where there is an increasing spotlight on ESG challenges faced by the industry. We discuss the foremost challenges and possible new directions to safeguard the future of Australian aquaculture in the domestic market.
Replacement of wild-caught fishmeal and fish oil
Fishmeal and fish oil (FMFO) are used extensively in aquaculture feed. They are often considered gold-standard ingredients as they meet the demand for many macronutrients and even unknown growth factors (Turchini et al. 2019). However, much of the FMFO has been sourced from wild-caught fisheries. Although being well managed (Food and Agriculture Organization of the United Nations 2020), there is ongoing environmental and social pressure to divert use of wild-caught FMFO away from aquaculture as it is considered unsustainable, by some, to use wild-caught fish to produce ‘farmed fish’, measured as fish-in-fish-out ratios. Coupled with this has been an increase in cost of FMFO as demand has grown concurrent with the industry, while supply has reached a plateau. Together, these two factors are a strong driver for the industry to judiciously use FMFO and find alternative and complementary ingredients to satisfy the industry and social demands.
Australia’s aquaculture industry is responding to these concerns. For example, feeds that are free of wild-caught FMFO are commercially available for prawns. For salmon, one of the main producers in Australia, Huon Aquaculture, has reportedly reduced the inclusion of FMFO by 20% to current levels of between 15% and 18% (Huon Aquaculture 2021). However, complete replacement of wild-caught FMFO is not yet commercially feasible for carnivorous species such as salmon, as the cost of many alternatives with the required nutrient content remains prohibitive. Nonetheless, reliance on this resource is likely to reduce through time as social pressure on the industry increases, the resource itself becomes more expensive, and alternatives become cheaper. Some of these alternatives are discussed below.
Alternatives to wild-caught FMFO
Both fishmeal and fish oil can be generated from seafood processing waste, collectively known as trimmings. Trimmings can be ground and pressed/extruded to produce fishmeal and fish oil. Lesser-quality wastes may be rendered to produce hydrolysates to be used as feed attractants and as a source of amino acids (Howieson and Choo 2017; Siddik et al. 2021). The reuse of offal that would have otherwise been disposed as waste presents major circularity benefits for the aquaculture industry. However, greater opportunity is possible when trimmings from the fishing industry are also considered (FAIRR 2022). Large volumes of waste are generated in fishing trawlers and are usually pitched overboard. Trimmings (mainly heads and guts) can account for 37–50% of the weight of caught fish such as cod, haddock and tuna. Iceland, as an example, has improved their proportion of fish utilised post-catch by 30-fold since the 1990s by generating co-products such as omega-3 oil, roe and fish leather (Archer and Jacklin 2022). However, in Australia, limitations to accessing this source relate to its stabilisation and storage on trawlers and then logistical challenges to transport this material for further processing (FAIRR 2022).
The most desired component of fish oil is the omega-3 content, particularly DHA and EPA. Fish are not efficient at producing omega-3 fatty acids. Instead, they bioaccumulate by consuming either microalgae or prey on fish that have in turn accumulated omega-3 fatty acids.
Microalgae are receiving much attention as it is these organisms that are the original source of omega-3 oils (Tocher 2015). However, producing microalgae at scale is expensive and this has limited their use in the aquaculture industry. Furthermore, processing to extract the oil from the organism may be required, due to the poor digestibility of the rigid cell wall (Nagappan et al. 2021). Thraustochytrids are one organism grown to produce algal oil at commercial scale. BioMar has used thraustochytrid-sourced oil to produce over one million tonnes of aquaculture feeds globally since 2016 (The Fish Site 2021) and Skretting is also using this product to replace fish oil in a FMFO-free feed range (Skretting 2023). However, while overseas companies have progressed with the use of algal oil, there has been little use in Australian manufactured fish feeds.
Genetic engineering represents another possible solution to the need to produce omega-3 fatty acids more sustainably. Nuseed’s Omega-3 canola is the world’s first plant-based source of DHA (Nuseed 2022) and was developed in collaboration with CSIRO and the Grains Research and Development Corporation (GRDC). Derived from its oil are Aquaterra® for aquaculture feeds and Nutriterra® for human nutrition. Use of Aquaterra® Omega-3 oil in aquaculture feeds has been shown to maintain growth in salmon, while also delivering favourable omega-3 concentrations in fish fillets at harvest (Nuseed 2022). However, to our knowledge the use of this product is not occurring in Australia, where its lack of use may relate to Tasmania, which is the main state for salmon production, having a GMO-free moratorium that precludes the use of GMO supplements in fish feed (Department of Primary Industries Parks Water and Environment 2019).
Alternative sources of protein
Animal by-products such as meat, bone and blood meal, and poultry by-products are major constituents of many aquaculture feeds (Colombo et al. 2022). Ingredients derived from animal carcasses have desirable nutrient profiles, because their content and bioavailability are comparable to fishmeal (Galkanda-Arachchige et al. 2020; Jia et al. 2022). Using these products can reduce reliance on wild-caught marine resources as well as reducing waste and improving circularity from the meat-processing industries.
There are some risks associated with the use of animal by-products in aquaculture feeds. One risk is the transmission of disease. This risk has prevented the use of animal by-products in the EU until recently (Jędrejek et al. 2016). However, the risk of disease transfer is generally considered low (Klinger and Naylor 2012). Another risk is that measures of sustainability of aquaculture feeds may be affected, as rendering of meat is energy intensive and so the carbon footprint of these products is typically higher than that of wild-caught seafood (Ramirez 2012; Ghamkhar and Hicks 2020). Finally, industries using animal by-products can become associated with consumer concerns relating to animal welfare. These issues may prevent greater adoption of the use of animal by-products in aquaculture feeds.
In Australia, some community groups maintain a social argument against the use of animal by-products in aquaculture diets (Percival 2021). Some companies have responded to this by reducing the reliance of animal by-products. For examples, Huon Aquaculture has reduced the use of animal by-product meal in the diets for salmon from 40% in 2015 to 18% in 2020 (Huon Aquaculture 2021). Despite the social pressures, animal by-product meals remain readily available locally, are high-quality ingredients and the arguments for their use in terms of circular use of resources remains strong.
Ingredients that are rich in vegetable protein are attractive to the aquaculture nutritionist due to their low cost and high availability. Soybean meal has been most researched and utilised for aquaculture diets. It is a well known protein source for animal feeding, with a desirable nutritional profile (up to 48% crude protein and ~2.5% fat after lipid extraction; National Research Council 2011). Typical digestibility of soybean meal (solvent extracted) averages 76% and 68% for crude protein and gross energy respectively, in salmon (Hajen et al. 1993), and 80% and 46% crude protein and gross energy respectively, in prawns (Glencross et al. 2018), which is comparable to animal-derived protein sources. Although soy meals and concentrates are useful ingredients to reduce the reliance on fishmeal, there are also caveats. Like most plant-based protein ingredients, these ingredients can contain anti-nutritional substances that can effect animal health and digestibility (Zhou et al. 2018b). In addition, soybean products can be expensive as a source of plant protein due to competing demand from livestock industries. There are also community concerns regarding the global impact of soybean farming on deforestation and carbon emissions (McFarlane and O’Connor 2014).
Australia has a wealth of other plant protein crops that may be increasingly used in the aquaculture industry. For example, Australia is the largest producer of lupins, accounting for 85% of the world’s production. Lupins have high contents of protein and can be grown in nutrient deficient soils. Aquaculture species fed feeds based on lupins can grow as well and, at times, better, than when fed fish meal-based diets (Glencross 2001). Similarly, canola is the main oilseed grown in Australia and its high protein by-product, canola meal, possesses an amino acid profile comparable to soybean meal (Enami 2011). Other protein meals produced in Australia include sunflower, safflower, hemp and chickpea. Currently, these are all underutilised by aquaculture.
Plant protein crops are being increasingly improved to better meet the needs of animal and aquafeed industries. For example, Australian canola breeding programs have successfully reduced glucosinolate content to trace amounts, with current research aiming to reduce fibre and increase protein content (Mailer et al. 2008).
Yeast, bacteria and microalgae have been used for fermentation to achieve high biomass yields (Glencross et al. 2020; Sharif et al. 2021). Fermentation technology is particularly attractive for aquaculture because high-carbohydrate substrates can be converted into high-protein microbial cells that contain a more desirable nutrient profile for feeding. Fermented ingredients are also associated with immune-modulatory properties where micro-organisms constituents (e.g. β-glucans, nucleotides) and their co-products (e.g. organic acids) can improve immune resistance and digestive tract function of aquatic animals (Glencross et al. 2020; Woolley et al. 2023).
Fermented ingredients, including single-cell proteins and fermented feedstuff, have potential as quality protein ingredients for aquaculture feeds (Glencross et al. 2020; Jones et al. 2020). Commercially available fermented ingredients can be incorporated into diets from 15% to 40% inclusion without any adverse effect on growth parameters for salmon and prawns (Aas et al. 2006; Øverland et al. 2013; Guo et al. 2019; Jintasataporn et al. 2022). The impact of fermented ingredients of carbon and environment is dependent on the product. Fermentation can be used to upcycle food and agriculture waste so that nutrients can be rescued from landfill (Dou et al. 2018).
Insect protein has attracted significant investment as it promises to be a sustainable (low carbon-emitting and land-use) protein source for humans and animals. However, as a replacement to fishmeal, insect meal is not necessarily more sustainable. Production of insect meal can have greater carbon emissions, electricity use and nitrogen waste than have other protein sources, including fishmeal and single-cell protein (Quang Tran et al. 2022). However, it is likely that the industry will evolve to address these concerns. For example, one company, Ÿnsect, claims that cultivating mealworms in vertical farm uses 98% less land while having a lower carbon and biodiversity footprint than for traditional animal protein meals (Hogan 2021). These trends towards more sustainable insect rearing are likely to continue and increase the viability of these products for aquaculture nutrition applications.
Insects are a natural diet for many aquaculture species, which gives insect meal a nutritive advantage over many other terrestrial-derived meals. A caveat is that insect meals containing high levels of chitin can reduce digestibility. However, the literature assessing insect meals in aquaculture feeds continues to expand, showing benefits in growth and immunity for many aquaculture species, with some insects such as black soldier fly larvae exhibiting bioactive properties (Freccia et al. 2020; Rombenso et al. 2023). This growing literature is vital to building industry confidence on the reliability of commercial insect meals for aquaculture.
The main hurdles for greater adoption of insect meal in the aquaculture industry is the limited volumes currently produced, inconsistencies in composition and high prices (higher than for fishmeal). To enable greater expansion of the sector, insect production companies are seeking links with aquaculture feed producers to fuel the growth plans of the sector (Fletcher 2021).
Nutrient discharge
Nutrient discharge from aquaculture production poses a significant ESG risk for the acceptance of aquaculture in Australia (Verdegem 2013). Nutritionist can play a role in reducing nutrient discharge by ensuring that feeds are well formulated to reduce leaching, to have high digestibility, and to ensure that feeding regimes meet the metabolic and growth requirements of the animal.
Leaching of diets can account for a significant loss of nutrients, particularly highly soluble amino acids. For example, prawn diets have been shown to lose 20% of crystalline methionine after 10 min and 50% loss after 2 h immersion in water (Simon et al. 2021). Diets can be manufactured to improve stability in water. The use of functional ingredients such as diet binders, and starches that gelatinise and entrap water-soluble ingredients, can improve diet stability (Dominy et al. 2004; Paolucci et al. 2012). However, overuse of these ingredients may reduce the attractiveness of the diet to the animal, palatability, and digestibility. Thus, a compromise between diet stability and nutrient bioavailability needs to be achieved.
Improving the digestibility of diets can reduce nutrient discharge by reducing both the amount of feed needed to produce a harvest and the amount of nutrients lost through faeces. Digestibility can be improved by judicious selection of ingredients that match the biology of the species in culture. In addition, additives can be incorporated into diets to facilitate gut function and digestibility. Some examples include amino acids, fatty acids, algae extracts, bacteria mixes and organic acids (Lara-Flores et al. 2010; Magouz et al. 2020; Tharaka et al. 2020; Li et al. 2021; Khorshidi et al. 2022).
Improving nutrient retention can also assist to reduce nutrition discharge from aquaculture. Nutrient retention efficiency is an indicator of the proportion of nutrients retained by an animal in anabolic processes (growth) versus how much is lost in faeces (not digested) and urea (catabolised). Protein retention efficiency of shrimp is ~10–25% (Truong et al. 2022b), while fish can achieve two to three times this, such as, for example, 36% in barramundi (Simon et al. 2019). However, these protein retention efficiencies are low compared with those for poultry, which range from ~25% up to 80% (Gous et al. 2018). Nutrient retention-efficiency gains achieved in poultry are predominately attributed to genetic improvement (McKay 2009). Unfortunately, this strategy is not easily achievable in aquaculture, due to the challenges in measuring individual feed intake. As such, dietary approaches remain the most likely means of improving nutrient retention efficiency in the short term. This has been observed in P. monodon prawns through the addition of microbial biomass, which acts to improve the absorption of amino acids into the haemolymph (Truong et al. 2021) and more than doubles the protein retention efficiency compared with a control diet (10.9 vs 22.6%; Simon et al. 2020).
Precision feeding can be used to ensure that the provision of feed is adequate to sustain growth but is not wasteful. Technologies such as acoustic feeders, which can detect when prawns are feeding, can better align feed delivery with prawn appetite (Ullman et al. 2019). In Australia, this technology has resulted in up to 20% improvement in feeding efficiency (M. Goodey, Tassal, Australia, pers. comm., 27 June 2022). Video technologies to observe how fish are consuming feed are also used for this purpose in the salmon industry (Ang and Petrell 1997).
Carbon footprint
The carbon footprint of producing aquaculture feeds is high and accounts for much of the carbon footprint to produce farmed seafood. For example, a recent report indicated that for salmon farming, feed accounts for 80% of all greenhouse-gas emissions from farming when Scope 3 emissions (emissions in which activities are indirectly responsible, up and down its value chain) are considered (FAIRR 2022). This includes feed production, ingredient, and feed transport to and from feed mills, and downstream transport of finished products. This scenario would also hold for most of the fed aquaculture businesses in Australia, including barramundi and prawns.
Producing aquaculture feeds with a lower carbon footprint will be a challenge for industry. It will involve sourcing ingredients from suppliers with lower carbon footprints, minimising emissions in feed production, and decreasing the distance between feed mills and farmers. Overseas companies are leading the way, with the development of carbon-neutral or low-carbon aquaculture feeds. Major feed producers are utilising low carbon-producing ingredients, maximising productivity (so that less feed is required to rear fish) and carbon offsetting to produce a low-carbon feed range (The Fish Site 2020; Hogan 2021).
Aquaculture will need to adopt a whole-of-system approach to reduce carbon emissions. Carbon footprint calculators are being used to assist with the adoption of practices with reduced carbon emissions, including transport. For example, Danish Salmon producer Hiddenfjord has eliminated all air freight by switching to sea freight. This has reduced transport CO2 emissions by 94% (Hogan 2021).
Conclusions
Australian aquaculture nutrition is facing new challenges not imagined by previous generations. Not only do formulations have to meet the rigid nutrient requirements of the high-trophic species cultured, but they will also have to adhere to increasing expectations of seafood quality, animal health and welfare, and environmental sustainability. The shift away from fishmeal and fish oil has resulted in more complex formulations utilising a greater array of ingredients and feed additives. Companies producing alternative ingredients are aligning with major feed producers to acquire the necessary industry conviction to increase production volumes and be more cost competitive. In turn, feed producers are acquiring ingredients from trusted suppliers who can provide full traceability of their products. These actions are all in the efforts to gain social licences to operate and meet the increasing consumer expectation for responsible and sustainable farming.
The Australian aquaculture industry is facing increasing pressure to ensure that these products have ESG acceptance. Whether driven by government regulations or consumer expectations, the onus of environmentally conscious production of food has fallen on the producers themselves. Criticism of any animal production industry will taint the reputation of all animal production industries and so, we are seeing an increasingly consolidated effort to adopt sustainable practices throughout the production value chain. Aquaculture nutrition will play a key role in delivering ESG goals for the Australian industry.
Acknowledgements
The authors thank Dr Sarah Berry (CSIRO) and the review panel of Animal Production Science for their careful consideration and constructive comments on previous versions of the paper. We also acknowledge Martyn Goodey (Tassal, Australia) who provided his observations of prawn production improvements using acoustic feeders.
References
Aas TS, Grisdale-Helland B, Terjesen BF, Helland SJ (2006) Improved growth and nutrient utilisation in Atlantic salmon (Salmo salar) fed diets containing a bacterial protein meal. Aquaculture 259, 365-376.
| Crossref | Google Scholar |
Abramo D (1989) Lipid requirements of shrimp. Actes de colloques Ifremer, Tahiti, French Polynesia, 20 Feb–4 Mar 1989, no. 9, chap. 28, pp. 271–285. Available at https://archimer.ifremer.fr/doc/00000/1470/
Adamczyk K, Pokorska J, Makulska J, Earley B, Mazurek M (2013) Genetic analysis and evaluation of behavioural traits in cattle. Livestock Science 154, 1-12.
| Crossref | Google Scholar |
Adekoya A, Park CS, Adeola O (2021) Energy and phosphorus evaluation of poultry meal fed to broiler chickens using a regression method. Poultry Science 100, 101195.
| Crossref | Google Scholar |
Ang KP, Petrell RJ (1997) Control of feed dispensation in seacages using underwater video monitoring: effects on growth and food conversion. Aquacultural Engineering 16, 45-62.
| Crossref | Google Scholar |
Araújo BC, Honji RM, Rombenso AN, Souza GBd, Mello PHd, Hilsdorf AWS, Moreira RG (2019) Influences of different arachidonic acid levels and temperature on the growth performance, fatty acid profile, liver morphology and expression of lipid genes in cobia (Rachycentron canadum) juveniles. Aquaculture 511, 734245.
| Crossref | Google Scholar |
Araújo BC, Rodriguez M, Honji RM, Rombenso AN, del Rio-Zaragoza OB, Cano A, Tinajero A, Mata-Sotres JA, Viana MT (2021) Arachidonic acid modulated lipid metabolism and improved productive performance of striped bass (Morone saxatilis) juvenile under sub- to optimal temperatures. Aquaculture 530, 735939.
| Crossref | Google Scholar |
Archer M, Jacklin M (2022) Global at-sea fish processing: a review of current practice, and estimates of the potential volume of by-products and their nutritional contribution from at-sea processing operations, 2022. Friends of Ocean Action. Available at https://www3.weforum.org/docs/WEF_FOA_SFLW_Global_at_sea_fish_processing_report_2022.pdf [Accessed 3 February 2023]
Aviagen (2022) Broiler ROSS nutrient specifications 2022. Available at http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerNutritionSpecifications2022-EN.pdf [Accessed 5 June 2023]
Azevedo P, Van Milgen J, Leeson S, Bureau D (2005) Comparing efficiency of metabolizable energy utilization by rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar) using factorial and multivariate approaches. Journal of Animal Science 83, 842-851.
| Crossref | Google Scholar |
Boyd CE (2020) Typical chemical characteristics of full-strength seawater. Available at https://www.globalseafood.org/advocate/typical-chemical-characteristics-of-full-strength-seawater/ [Accessed 20 April 2023]
Canario L, Turner SP, Roehe R, Lundeheim N, D’Eath RB, Lawrence AB, Knol E, Bergsma R, Rydhmer L (2012) Genetic associations between behavioral traits and direct-social effects of growth rate in pigs1. Journal of Animal Science 90, 4706-4715.
| Crossref | Google Scholar |
Chen L, Yang X, Sun C, Wang Y, Xu D, Zhou C (2020) Feed intake prediction model for group fish using the MEA-BP neural network in intensive aquaculture. Information Processing in Agriculture 7, 261-271.
| Crossref | Google Scholar |
Colombo SM, Roy K, Mraz J, Wan AHL, Davies SJ, Tibbetts SM, Øverland M, Francis DS, Rocker MM, Gasco L, Spencer E, Metian M, Trushenski JT, Turchini GM (2022) Towards achieving circularity and sustainability in feeds for farmed blue foods. Reviews in Aquaculture 15(3), 1115-1141.
| Crossref | Google Scholar |
Courtot E, Musson D, Stratford C, Blyth D, Bourne NA, Rombenso AN, Simon CJ, Wu X, Wade N (2022) Dietary fatty acid composition affects the apparent digestibility of algal carotenoids in diets for Atlantic salmon, Salmo salar. Aquaculture Research 53, 2343-2353.
| Crossref | Google Scholar |
Coutteau P, Geurden I, Camara MR, Bergot P, Sorgeloos P (1997) Review on the dietary effects of phospholipids in fish and crustacean larviculture. Aquaculture 155, 149-164.
| Crossref | Google Scholar |
Curtotti R, Dylewski CA, Tuynman H (2023) Australian fisheries and aquaculture outlook to 2027−28, ABARES research report. Canberra. Available at https://daff.ent.sirsidynix.net.au/client/en_AU/search/asset/1034561/0 [Accessed 12 April 2023]
de Carvalho CCCR, Caramujo MJ (2017) Carotenoids in aquatic ecosystems and aquaculture: a colorful business with implications for human health. Frontiers in Marine Science 4, 93.
| Crossref | Google Scholar |
de Verdal H, Komen H, Quillet E, Chatain B, Allal F, Benzie JAH, Vandeputte M (2018) Improving feed efficiency in fish using selective breeding: a review. Reviews in Aquaculture 10, 833-851.
| Crossref | Google Scholar |
Department of Agriculture and Water Resources (2017) National aquaculture strategy, Canberra, August. CC BY 4.0. Available at http://agriculture.gov.au/fisheries/aquaculture/national-aquaculture-strategy
Department of Primary Industries Parks Water and Environment (2019) Review of Tasmania’s genetically modified organisms (GMO) moratorium: final report. (Tasmanian Government) Available at https://nre.tas.gov.au/Documents/GMO%20Final%20Report.pdf [Accessed 3 February 2023]
Dominy WG, Cody JJ, Terpstra JH, Obaldo LG, Chai MK, Takamori TI, Larsen B, Forster IP (2004) A comparative study of the physical and biological properties of commercially-available binders for shrimp feeds. Journal of Applied Aquaculture 14, 81-99.
| Crossref | Google Scholar |
Dou Z, Toth JD, Westendorf ML (2018) Food waste for livestock feeding: feasibility, safety, and sustainability implications. Global Food Security 17, 154-161.
| Crossref | Google Scholar |
Enami HR (2011) A review of using canola/rapeseed meal in aquaculture feeding. Journal of Fisheries and Aquatic Science 6, 22-36.
| Crossref | Google Scholar |
FAIRR (2022) Oceans and biodiveristy impact report: phase 2 sustainable aquafeed engagement update and FAIRR’s action on fisheries. Coller FAIRR Protein Producer Index 2021/22, London, UK. Available at https://www.fairr.org/article/index-chapter-3-aquaculture/ [Accessed 3 February 2022]
Fletcher R (2021) Why insect production may have minimal impact on aquaculture sustainability. The Fish Site. Available at https://thefishsite.com/articles/why-insect-production-may-have-minimal-impact-on-aquaculture-sustainability [Accessed 6 February 2023]
Folorunso L, Emikpe B, Falaye E, Dauda AB, Ajani EK (2017) Evaluating feed intake of fishes in aquaculture nutrition experiments with due consideration of mortality and fish survival. Journal of Northeast Agricultural University (English Edition) 24, 45-50.
| Google Scholar |
Food and Agriculture Organization of the United Nations (2020) ‘The state of world fisheries and aquaculture 2020 – sustainability in action.’ (FAO: Rome, Italy) doi:10.4060/cc0461en
Galkanda-Arachchige HSC, Wilson AE, Davis DA (2020) Success of fishmeal replacement through poultry by-product meal in aquaculture feed formulations: a meta-analysis. Reviews in Aquaculture 12, 1624-1636.
| Crossref | Google Scholar |
Ghamkhar R, Hicks A (2020) Comparative environmental impact assessment of aquafeed production: sustainability implications of forage fish meal and oil free diets. Resources, Conservation and Recycling 161, 104849.
| Crossref | Google Scholar |
Glencross BD, Booth M, Allan GL (2007) A feed is only as good as its ingredients – a review of ingredient evaluation strategies for aquaculture feeds. Aquaculture Nutrition 13, 17-34.
| Crossref | Google Scholar |
Glencross B, Bourne N, Hawkins W, Karopoulos M, Evans D, Rutherford N, McCafferty P, Dods K, Burridge P, Veitch C, Sipsas S, Buirchell B, Sweetingham M (2015) Using Near Infrared Reflectance Spectroscopy (NIRS) to predict the protein and energy digestibility of lupin kernel meals when fed to rainbow trout, Oncorhynchus mykiss. Aquaculture Nutrition 21, 54-62.
| Crossref | Google Scholar |
Glencross B, Bourne N, Irvin S, Blyth D (2017) Using near-infrared reflectance spectroscopy to predict the digestible protein and digestible energy values of diets when fed to barramundi, Lates calcarifer. Aquaculture Nutrition 23, 397-405.
| Crossref | Google Scholar |
Glencross B, Blyth D, Wade N, Arnold S (2018) Critical variability exists in the digestible value of raw materials fed to black tiger shrimp, Penaeus monodon: the characterisation and digestibility assessment of a series of research and commercial raw materials. Aquaculture 495, 214-221.
| Crossref | Google Scholar |
Glencross BD, Huyben D, Schrama JW (2020) The application of single-cell ingredients in aquaculture feeds – a review. Fishes 5, 22.
| Crossref | Google Scholar |
Gous RM, Faulkner AS, Swatson HK (2018) The effect of dietary energy: protein ratio, protein quality and food allocation on the efficiency of utilisation of protein by broiler chickens. British Poultry Science 59, 100-109.
| Crossref | Google Scholar |
Guo J, Reis J, Salze G, Rhodes M, Tilton S, Davis DA (2019) Using high protein distiller’s dried grain product to replace corn protein concentrate and fishmeal in practical diets for the Pacific white shrimp Litopenaeus vannamei. Journal of the World Aquaculture Society 50, 983-992.
| Crossref | Google Scholar |
Hajen WE, Higgs DA, Beames RM, Dosanjh BS (1993) Digestibility of various feedstuffs by post-juvenile chinook salmon (Oncorhynchus tshawytscha) in sea water. 2. Measurement of digestibility. Aquaculture 112, 333-348.
| Crossref | Google Scholar |
Helland SJ, Grisdale-Helland B, Nerland S (1996) A simple method for the measurement of daily feed intake of groups of fish in tanks. Aquaculture 139, 157-163.
| Crossref | Google Scholar |
Hogan H (2021) Aquaculture, feed companies embark on a carbon-cutting journey. Global Seafood Alliance. Available at https://www.globalseafood.org/advocate/aquaculture-feed-companies-embark-on-a-carbon-cutting-journey/ [Accessed 6 February 2023]
Huon Aquaculture (2021) Feed ingredients fact sheet. Available at https://www.huonaqua.com.au/wp-content/uploads/2021/12/Feed-Ingredients-Fact-Sheet-FINAL.pdf
Irvin SJ, Tabrett SJ (2005) A novel method of collecting fecal samples from spiny lobsters. Aquaculture 243, 269-272.
| Crossref | Google Scholar |
Jensen P (2006) Domestication – from behaviour to genes and back again. Applied Animal Behaviour Science 97, 3-15.
| Crossref | Google Scholar |
Jintasataporn O, Chumkam S, Triwutanon S, LeBlanc A, Sawanboonchun J (2022) Effects of a single cell protein (Methylococcus capsulatus, Bath) in Pacific White Shrimp (Penaeus vannamei) diet on growth performance, survival rate and resistance to Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease. Frontiers in Marine Science 8, 764042.
| Crossref | Google Scholar |
Jones SW, Karpol A, Friedman S, Maru BT, Tracy BP (2020) Recent advances in single cell protein use as a feed ingredient in aquaculture. Current Opinion in Biotechnology 61, 189-197.
| Crossref | Google Scholar |
Jędrejek D, Levic J, Wallace J, Oleszek W (2016) Animal by-products for feed: characteristics, European regulatory framework, and potential impacts on human and animal health and the environment. Journal of Animal and Feed Sciences 25, 189-202.
| Crossref | Google Scholar |
Khorshidi Z, Paknejad H, Sodagar M, Hajimoradloo A, Shekarabi SPH (2022) Effect of a commercial multi-effect additive (Biotronic® Top3) on growth performance, digestive enzymes, and intestinal barrier gene expression in common carp (Cyprinus carpio). Aquaculture 560, 738588.
| Crossref | Google Scholar |
Klinger D, Naylor R (2012) Searching for solutions in aquaculture: charting a sustainable course. Annual Review of Environment and Resources 37, 247-276.
| Crossref | Google Scholar |
Lara-Flores M, Olivera-Castillo L, Olvera-Novoa MA (2010) Effect of the inclusion of a bacterial mix (Streptococcus faecium and Lactobacillus acidophilus), and the yeast (Saccharomyces cerevisiae) on growth, feed utilization and intestinal enzymatic activity of Nile tilapia (Oreochromis niloticus). International Journal of Fisheries and Aquaculture 2, 93-101.
| Google Scholar |
Li Y, Yang Y, Song L, Wang J, Hu Y, Yang Q, Cheng P, Li J (2021) Effects of dietary supplementation of Lactobacillus plantarum and Bacillus subtilis on growth performance, survival, immune response, antioxidant capacity and digestive enzyme activity in olive flounder (Paralichthys olivaceus). Aquaculture and Fisheries 6, 283-288.
| Crossref | Google Scholar |
Magouz FI, Dawood MAO, Salem MFI, El-Ghandour M, Van Doan H, Mohamed AAI (2020) The role of a digestive enhancer in improving the growth performance, digestive enzymes activity, and health condition of Nile tilapia (Oreochromis niloticus) reared under suboptimal temperature. Aquaculture 526, 735388.
| Crossref | Google Scholar |
Mailer RJ, McFadden A, Ayton J, Redden B (2008) Anti-nutritional components, fibre, sinapine and glucosinolate content, in Australian Canola (Brassica napus L.) Meal. Journal of the American Oil Chemists’ Society 85, 937-944.
| Crossref | Google Scholar |
McFarlane I, O’Connor EA (2014) World soybean trade: growth and sustainability. Modern Economy 5, 580-588.
| Crossref | Google Scholar |
McGhee ML, Stein HH (2020) The apparent ileal digestibility and the apparent total tract digestibility of carbohydrates and energy in hybrid rye are different from some other cereal grains when fed to growing pigs. Journal of Animal Science 98, skaa218.
| Crossref | Google Scholar |
Moriarty DJW (1997) The role of microorganisms in aquaculture ponds. Aquaculture 151, 333-349.
| Crossref | Google Scholar |
Moss SM, Forster IP, Tacon AGJ (2006) Sparing effect of pond water on vitamins in shrimp diets. Aquaculture 258, 388-395.
| Crossref | Google Scholar |
Moss AF, Chrystal PV, Cadogan DJ, Wilkinson SJ, Crowley TM, Choct M (2021) Precision feeding and precision nutrition: a paradigm shift in broiler feed formulation? Animal Bioscience 34, 354-362.
| Crossref | Google Scholar |
Muir SK, Linden NP, Kennedy A, Calder G, Kearney G, Roberts R, Knight MI, Behrendt R (2020) Technical note: validation of an automated feeding system for measuring individual animal feed intake in sheep housed in groups. Translational Animal Science 4, 1006-1016.
| Crossref | Google Scholar |
Nagappan S, Das P, AbdulQuadir M, Thaher M, Khan S, Mahata C, Al-Jabri H, Vatland AK, Kumar G (2021) Potential of microalgae as a sustainable feed ingredient for aquaculture. Journal of Biotechnology 341, 1-20.
| Crossref | Google Scholar |
Nankervis L, Cobcroft JM, Nguyen NV, Rimmer MA (2022) Advances in practical feed formulation and adoption for hybrid grouper (Epinephelus fuscoguttatus♀× E. lanceolatus♂) aquaculture. Reviews in Aquaculture 14, 288-307.
| Crossref | Google Scholar |
Norambuena F, Rombenso A, Turchini GM (2016) Towards the optimization of performance of Atlantic salmon reared at different water temperatures via the manipulation of dietary ARA/EPA ratio. Aquaculture 450, 48-57.
| Crossref | Google Scholar |
Nuseed (2022) Aquaterra®, a novel source of omega-3, delivers aquaculture improved production with unique fatty acid profile. Available at https://aquaterraomega3.com/wp-content/themes/aquaterra/images/whitepaper.pdf [Accessed 3 February 2023]
Obaldo LG, Divakaran S, Tacon AG (2002) Method for determining the physical stability of shrimp feeds in water. Aquaculture Research 33, 369-377.
| Crossref | Google Scholar |
Øverland M, Krogdahl Å, Shurson G, Skrede A, Denstadli V (2013) Evaluation of distiller’s dried grains with solubles (DDGS) and high protein distiller’s dried grains (HPDDG) in diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 416–417, 201-208.
| Crossref | Google Scholar |
Percival S (2021) The importance of balanced information. Available at https://www.huonaqua.com.au/the-importance-of-balanced-information/ [Accessed 15 May 2023]
Quang Tran H, Van Doan H, Stejskal V (2022) Environmental consequences of using insect meal as an ingredient in aquafeeds: a systematic view. Reviews in Aquaculture 14, 237-251.
| Crossref | Google Scholar |
Rombenso A, Esmaeili M, Araujo B, Emerenciano MG, Truong HH, Viana M, Li E, Simon C (2021) Macronutrient research in aquaculture nutrition. Global Seafood Alliance. Available at https://www.globalseafood.org/advocate/macronutrient-research-in-aquaculture-nutrition/ [Accessed 3 February 2023]
Rombenso AN, Turchini GM, Trushenski JT (2022) The omega-3 sparing effect of saturated fatty acids: a reason to reconsider common knowledge of fish oil replacement. Reviews in Aquaculture 14, 213-217.
| Crossref | Google Scholar |
Rombenso A, Rusu A, Porter A, Bourne N, Truong HH, Simon CJ, Osborne SA (2023) Bioactivity of black soldier fly larvae meal. Global Seafood Alliance. Available at https://www.globalseafood.org/advocate/bioactivity-of-black-soldier-fly-larvae-meal/ [Accessed 9 February 2023]
Sá R, Pousão-Ferreira P, Oliva-Teles A (2007) Growth performance and metabolic utilization of diets with different protein: carbohydrate ratios by white sea bream (Diplodus sargus, L.) juveniles. Aquaculture Research 38, 100-105.
| Crossref | Google Scholar |
Sharif M, Zafar MH, Aqib AI, Saeed M, Farag MR, Alagawany M (2021) Single cell protein: sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 531, 735885.
| Crossref | Google Scholar |
Siddik MAB, Howieson J, Fotedar R, Partridge GJ (2021) Enzymatic fish protein hydrolysates in finfish aquaculture: a review. Reviews in Aquaculture 13, 406-430.
| Crossref | Google Scholar |
Simon CJ (2009) The effect of carbohydrate source, inclusion level of gelatinised starch, feed binder and fishmeal particle size on the apparent digestibility of formulated diets for spiny lobster juveniles, Jasus edwardsii. Aquaculture 296, 329-336.
| Crossref | Google Scholar |
Simon C, Salini M, Irvin S, Blyth D, Bourne N, Smullen R (2019) The effect of poultry protein concentrate and phosphorus supplementation on growth, digestibility and nutrient retention efficiency in barramundi Lates calcarifer. Aquaculture 498, 305-314.
| Crossref | Google Scholar |
Simon CJ, Truong HH, Noble TH, Osborne SA, Wynne JW, Wade NM (2020) Microbial biomass, marine invertebrate meals and feed restriction influence the biological and gut microbiota response of shrimp Penaeus monodon. Aquaculture 520, 734679.
| Crossref | Google Scholar |
Simon CJ, Truong H, Habilay N, Hines B (2021) Feeding behaviour and bioavailability of essential amino acids in shrimp Penaeus monodon fed fresh and leached fishmeal and fishmeal-free diets. Animals 11, 847.
| Crossref | Google Scholar |
Simon CJ, Bourne N, Hines BM, Pirozzi I, Booth M (2022) Estimation of apparent dietary nutrient digestibility in Yellowtail Kingfish Seriola lalandi by Near-Infrared Spectroscopy (NIRS). Aquaculture 548, 737624.
| Crossref | Google Scholar |
Skretting (2023) Omega-3 from marine algae – an innovative solution for increased protein production. Available at https://www.skretting.com/en-au/innovation/our-innovations/ingredient-innovation/omega-3-from-marine-algae--an-innovative-solution-for-increased-protein-production/ [Accessed 10 February 2023]
Steven A, Dylewski M, Curtotti R (2021) ‘Australian fisheries and aquaculture statistics 2020.’ Fisheries Research and Development Corporation. ABARES, Canberra, ACT, Australia. August. CC BY 4.0. https://doi.org/10.25814/0wzy-re76
Storebakken T, Kvien IS, Shearer KD, Grisdale-Helland B, Helland SJ, Berge GM (1998) The apparent digestibility of diets containing fish meal, soybean meal or bacterial meal fed to Atlantic salmon (Salmo salar): evaluation of different faecal collection methods. Aquaculture 169, 195-210.
| Crossref | Google Scholar |
Sørensen M (2012) A review of the effects of ingredient composition and processing conditions on the physical qualities of extruded high-energy fish feed as measured by prevailing methods. Aquaculture Nutrition 18, 233-248.
| Crossref | Google Scholar |
Tacon AGJ (1987) The nutrition and feeding of farmed fish and shrimp; a training manual. 1: The essential nutrients. p. 126. Food and Agriculture Organization of the United Nations (FAO). Available at https://www.fao.org/3/AB470E/AB470E00.htm#TOC
Teshima S-I, Kanazawa A (1971) Biosynthesis of sterols in the lobster, Panulirus japonica, the prawn, Penaeus japonicus, and the crab, Portunus trituberculatus. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 38, 597-602.
| Crossref | Google Scholar |
Tharaka K, Gunathilaka BE, Veille A, Kim M-G, Shin J, Lim H, Jeong J-B, Meallet V, Lee K-J (2020) Algae-clay powder (sea lettuce, Ulva lactuca and red algae, Solieria chordalis in exfoliated micronized montmorillonite) supplementation in a fish meal-reduced diet for olive flounder (Paralichthys olivaceus). Aquaculture Reports 18, 100498.
| Crossref | Google Scholar |
The Fish Site (2020) Aquafeed company launches ‘carbon neutral’ aquaculture initiative. The Fish Site website. Available at https://thefishsite.com/articles/aquafeed-company-launches-carbon-neutral-fish-farm-initiative [Accessed 6 February 2023]
The Fish Site (2021) Aquafeed giant reports microalgal milestone. Available at https://thefishsite.com/articles/aquafeed-giant-reports-microalgal-milestone [Accessed 3 February 2023]
Tocher DR (2015) Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449, 94-107.
| Crossref | Google Scholar |
Truong HH, Liu SY, Selle PH (2016) Starch utilisation in chicken-meat production: the foremost influential factors. Animal Production Science 56, 797-814.
| Crossref | Google Scholar |
Truong HH, Hines BM, Rombenso AN, Simon CJ (2021) Feed intake, gastro-intestinal transit and haemolymph free amino acids in the shrimp Penaeus monodon are influenced by marine meal supplementation. Aquaculture 533, 736171.
| Crossref | Google Scholar |
Truong HH, Hines BM, Emerenciano MG, Blyth D, Berry S, Noble TH, Bourne NA, Wade N, Rombenso AN, Simon CJ (2022a) Mineral nutrition in penaeid shrimp. Reviews in Aquaculture
| Crossref | Google Scholar |
Truong H, Blyth D, Habilay N, Bourne N, Wade N, Hines B, Rombenso A, Simon C (2022b) Faecal collection methods result in different estimates of nutrient apparent digestibility in Penaeus monodon. Aquaculture 551, 737957.
| Crossref | Google Scholar |
Trushenski JT, Rombenso AN (2020) Trophic levels predict the nutritional essentiality of polyunsaturated fatty acids in fish – introduction to a special section and a brief synthesis. North American Journal of Aquaculture 82, 241-250.
| Crossref | Google Scholar |
Turchini GM, Trushenski JT, Glencross BD (2019) Thoughts for the future of aquaculture nutrition: realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. North American Journal of Aquaculture 81, 13-39.
| Crossref | Google Scholar |
Ullman C, Rhodes MA, Allen Davis D (2019) Feed management and the use of automatic feeders in the pond production of Pacific white shrimp Litopenaeus vannamei. Aquaculture 498, 44-49.
| Crossref | Google Scholar |
Vargovic L, Hermesch S, Athorn RZ, Bunter KL (2020) Feed intake and feeding behavior traits for gestating sows recorded using electronic sow feeders. Journal of Animal Science 99, skaa395.
| Crossref | Google Scholar |
Verdegem MCJ (2013) Nutrient discharge from aquaculture operations in function of system design and production environment. Reviews in Aquaculture 5, 158-171.
| Crossref | Google Scholar |
Wade NM, Bourne N, Simon CJ (2018) Influence of marker particle size on nutrient digestibility measurements and particle movement through the digestive system of shrimp. Aquaculture 491, 273-280.
| Crossref | Google Scholar |
Wang WJ, Larsen M, Weisbjerg MR, Johansen M, Hellwing ALF, Lund P (2022) Effects of particle size and toasting of fava beans and forage source on nutrient digestibility, ruminal fermentation, and metabolizable protein supply in dairy cows. Journal of Dairy Science 105, 8806-8823.
| Crossref | Google Scholar |
Wilson RP (1994) Utilization of dietary carbohydrate by fish. Aquaculture 124, 67-80.
| Crossref | Google Scholar |
Woolley L, Chaklader MR, Pilmer L, Stephens F, Wingate C, Salini M, Partridge G (2023) Gas to protein: microbial single cell protein is an alternative to fishmeal in aquaculture. Science of The Total Environment 859, 160141.
| Crossref | Google Scholar |
Yuan Y, Lawrence AL, Chehade SB, Jensen KE, Barry RJ, Fowler LA, Makowsky R, Powell ML, Watts SA (2021) Feed intake as an estimation of attractability in Pacific white shrimp Litopenaeus vannamei. Aquaculture 532, 736041.
| Crossref | Google Scholar |
Zhou C, Xu D, Lin K, Sun C, Yang X (2018a) Intelligent feeding control methods in aquaculture with an emphasis on fish: a review. Reviews in Aquaculture 10, 975-993.
| Crossref | Google Scholar |
Zhou Z, Ringø E, Olsen RE, Song SK (2018b) Dietary effects of soybean products on gut microbiota and immunity of aquatic animals: a review. Aquaculture Nutrition 24, 644-665.
| Crossref | Google Scholar |


