Increased light scattering in electrically stimulated beef longissimus muscle fibres contributes to the observed meat colour at grading
J. Hughes A , N. McPhail A , P. Watkins B , J. Stark A and R. D. Warner
B , J. Stark A and R. D. Warner  C *
C *
A CSIRO Agriculture and Food, 39 Kessels Road, Coopers Plains, Qld 4108, Australia.
B CSIRO Agriculture and Food, Sneydes Road, Werribee, Vic. 3030, Australia.
C Faculty of Veterinary and Agricultural Science, The University of Melbourne, Parkville, Vic. 3010, Australia.
Animal Production Science 63(7) 673-680 https://doi.org/10.1071/AN22390
Submitted: 17 October 2022 Accepted: 23 January 2023 Published: 20 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Electrical stimulation is often used by meat processors to promote fast muscle pH decline and optimise meat quality. Meat colour can be made more acceptable by this process, but how this relates to the microstructure and light-scattering properties of muscle is still unknown.
Aims: To investigate the effect of electrical stimulation of beef carcasses on the meat colour at grading and the role of the muscle fibre microstructure and light scattering in determining colour differences.
Methods: Electrical stimulation inputs (electrical stimulation inputs (ES), n = 8; no electrical stimulation inputs (NS), n = 8) were applied to beef carcasses from female cattle of approximately 18–24 months of age. ES comprised electrical immobilisation, bleed rail electric simulation and hide puller rigidity probe, which have all been shown to increase pH fall post-mortem in beef carcasses. pH fall was monitored, the longissimus thoracis was graded at 20–22 h postmortem and measurements were made of colour, muscle-fibre structure and light scattering.
Key results: The decline of pH was increased in ES relative to NS, as indicated by lower pH at 2 h postmortem (5.83 vs 6.86 respectively, s.e. = 0.068; P < 0.05) as well as changes in both chromatic colour a* b* and achromatic (no colour) lightness in the muscle. Chromatic changes were evident as higher grader colour scores, increased redness (a*) and yellowness (b*) with higher levels of oxymyoglobin and lower levels of deoxymyoglobin. Achromatic changes were evident as increased lightness (L*) and surface reflectance (%R) at the meat surface, and increased global brightness within the muscle fibres.
Conclusions: Increased lightness and brightness in electrically stimulated muscles were likely to be due to formation of contraction nodes and distortion of muscle fibres, which changed the microstructure of muscle in ways that increased its light-scattering properties.
Implications: Consideration of the role of light scattering in determining beef colour at grading will advance understanding of how to improve this important quality trait.
Keywords: beef, confocal microscopy, contraction nodes, grading, light scattering, meat colour, myoglobin, sarcomere, skeletal muscle structure.
Introduction
Meat is often described as being translucent (MacDougall and Jones 1981; Swatland 2012; Jacques 2013), meaning that light is permitted to pass through, but objects on the opposite side are not clearly visible. Opacity is the opposite of translucence and is defined as the condition of lacking transparency or translucence. These terms have been used to describe extremes of meat colour from dark to pale, and to explain the nature by which the structural properties of meat can influence the scattering of light within the muscle structure (MacDougall 1982). The extent of this transition from translucent to opaque depends on the time postmortem and has been reported to occur at ~pH 5.9 (MacDougall and Jones 1981). Dark, high ultimate-pH (pHu) meat is normally associated with an increased translucency (MacDougall and Jones 1981), whereas lower pHu meat is associated with opacity. Therefore, this transition is considered a critical factor in colour development early postmortem and also for meat colour when grading occurs. It would be useful to understand how electrical stimulation of carcasses affects this transition from translucent to opaque and the development of light scattering during accelerated pH decline postmortem and what, if any, structural changes occur in the muscle that may contribute. This information could be used to advise meat processors on developing strategies to improve meat colour at grading.
In the early 1980s, meat scientists demonstrated that electrical stimulation can be used to increase the lightness and redness of beef longissimus muscles (Savell et al. 1978; Sleper et al. 1983; Eikelenboom et al. 1985), with a dualistic mechanism being proposed of myoglobin oxygenation and light scattering. Some authors have observed higher reflectance (%R630–%R580) at the surface of electrically stimulated muscles and hypothesised that the ‘looser muscle structure permits deeper oxygen penetration and a thicker oxymyoglobin layer’ in conjunction with ‘structural damage, which causes more surface reflectance’ (Sleper et al. 1983). In addition, substantial evidence exists of structural alterations (formation of contraction bands, I-band fractures, Z-line disruption and sarcoplasmic protein denaturation and deposition on myofibrillar surfaces), which occur after electrical stimulation, but these mechanisms are often discussed in context of tenderisation or water-holding capacity (Savell et al. 1978; George et al. 1980; Ho et al. 1996; Hwang et al. 2003). To our knowledge, although hypothesised, there are no studies that quantitate the link between electrically stimulated induced structural changes in the muscle and modifications in light-scattering properties. The rate of the early postmortem pH–temperature decline is important for many meat-quality parameters, including colour. In Australia, Meat Standards Australia (MSA) provides recommendations for an optimal pH–temperature ‘window’ when the muscle passes through pH 6 (Temp@pH6) and recommended it should be within the range 15°C to 35°C (Thompson 2002). In terms of meat colour, higher temperatures pre-rigour have been shown to produce carcasses of paler colour scores (higher lightness and redness scores), especially with a rigour temperature of >35°C. This has been described as heat-induced toughening and has been previously reviewed (Kim et al. 2014).
In the context of this optimal ‘pH–temperature window’, beef sternomandibularis (a low-value muscle) passing through a high rigour temperature (35°C) is known to exhibit increased light scattering compared with a lower rigour temperature (15°C; Jeacocke 1984; Hughes et al. 2018) but the effect on higher-value muscles is still unknown. In addition, it is well known that pre-rigour muscles exposed to a higher than normal temperature accompanied by a rapid pH decline result in a paler colour (see review by Kim et al. 2014). Thus, the aim of this study was to determine the effect of electrical stimulation (which induces a fast pH decline) on the light-scattering properties of beef longissimus thoracis muscle fibres post-rigour and to quantify the changes in microstructure. The hypothesis was that electrical stimulation will promote structural modifications to the microstructure, consequently increasing light scattering, induced either through muscle fibre shrinkage, or distortion of the sarcomeres. Often there is a misconception by meat processors that meat colour is ‘set’ on the basis of glycogen concentrations and conditions of the animal before slaughter. Consequently, by performing this quantitative analysis linking structural light-scattering properties to postmortem electrical stimulation, it would provide evidence to meat processors that colour development during the early postmortem period is a dynamic process that, to some extent, can be under their control.
Materials and methods
Collection of muscles
Sixteen beef carcasses were acquired from the same group of carcasses, which were all MSA graded. All carcasses were from female animals, approximately 18–24 months of age, and none were treated with hormonal growth promotants. Animals were stunned using a mushroom head non-penetrating stunner followed by halal slaughter. The treatments were; no electrical inputs on the slaughter floor for the first eight carcasses versus full electrical inputs applied to the next eight carcasses. The electrical inputs comprised electrical immobilisation, bleed rail electric simulation and hide puller rigidity probe, which have all been shown to increase pH fall postmortem in beef carcasses (Warner et al. 2014). For carcasses with electrical inputs, immediately after stunning, carcasses were placed on an Applied Sorting Technologies CPMS Electronic immobiliser at 1250 mA (frequency: 2000 Hz; pulse width: 100 μs; period: 500 ms) for 4–14 s, electrical stimulation was applied using an Applied Sorting Technologies CPMS bleed rail stimulator at 300 V (frequency: 15 Hz; pulse width: 500 μs; period: 68 ms) with contact of the carcass on the stimulation bar being 30–64 s and an Applied Sorting Technologies CPMS back stiffener rigidity probe was also applied at 300 amps for 3–6 s for hide removal. All Applied Sorting Technology equipment was sourced from Stim Tech, Queensland, Australia. In this paper, the carcasses with electrical inputs are labelled ‘electrically stimulated’. After evisceration, carcasses were weighed and the hot carcass weight was recorded (175–230 kg). Carcasses were then subjected to a step-wise chilling regime for 16 h (0°C for 8 h with spray chilling for 2 mins every 30 min, followed by 4°C for 8 h), followed by chiller temperature of 6°C.
At 20–21 h postmortem, carcasses were quartered between the 12th and 13th vertebrae and after about 30–60 min, the exposed longissimus thoracis (LT) was graded by a qualified AUS-MEAT grader for meat colour (AMC; 1 = very pale to 7 = very dark purple), ossification, eye-muscle area (cm2), rib fat (mm) and marbling by using methods described in AUS-MEAT (2005).
Temperature/pH decline and muscle collection
At 1 h postmortem, and hourly for a further 8 h, the pH and temperature was measured in the longissimus thoracis muscle, at a depth of 3–4 cm, using a fresh incision for each measurement. A TPS WP-80 pH meter with a polypropylene spear-type gel electrode (Ionode IJ 44) and temperature probe (all from TPS, Springwood, Qld, Australia) were used. Calibration was performed at ~10°C, using pH 4.00 and 7.00 buffers (TPS, Product numbers 121 382 and 121 388 respectively). At 22 h postmortem, pH was again measured. Carcasses were moved to the boning room (~10°C) and approximately 40 mm of LT above the 12th rib was excised from one side of each carcass at 22–23 h postmortem and transported on ice, in insulated containers, to the laboratory. A final pHu measurement was also conducted in triplicate in the LT at the laboratory at ~26 h postmortem.
Colour measurements
Objective colour measurements were taken on the exposed loin after blooming at 0–4°C, for 105–120 min at 22–23 h postmortem, prior to relocation to the boning room. This blooming time was selected on the basis of the stability of colour co-ordinates L*, a* and b* after blooming for >78 min, as reported previously (Wulf and Wise 1999). A Hunterlab Miniscan EZ 45/0 LAV (light source A, observer angle 10°, aperture diameter size 5 cm) was used to measure lightness, redness and yellowness attributes in triplicate (L*, a*, b* values respectively). The instrument was calibrated at ~4°C, using white and black calibration tiles supplied with the instrument (Novasys Group, Melbourne, Vic., Australia). Colour parameters were calculated as follows: hue (degrees) = [arctangent (b*/a*) × 180/π] and chroma = (a*2 + b*2)1/2. A spectral reflectance scan from 400 to 700 nm, in 10 nm intervals, was also completed and used to calculate three forms of myoglobin, namely, deoxymyoglobin (DMb), oxymyoglobin (OMb) and metmyoglobin (MMb), by using the concept of reflectance attenuance (Krzywicki 1979; Hunt 2012).
Fluid collection
Muscle fluid was collected at 3–4 days postmortem by placing 10–20 g of LT muscle in a 50 mL plastic tube and holding for 24 h at 4°C. An aliquot (100 μL) of collected fluid was pipetted into a 96-well plate (Sarstedt product no. 82.1581) and the absorbance was measured between 400 and 700 nm at 10 nm intervals by using an Infinite M200 microplate reader (Tecan Australia, Clontarf, Qld, Australia).
Protein extraction and concentration determination
Fresh muscle-fibre fragments were extracted according to the method of Warner et al. (1997), with modifications. Samples (1.00 ± 0.05 g) were homogenised on ice (Ultraturrax, 4000g, 3 × 4 s pulses, 0–1°C) in mannitol (10 mL, 380 mM) and potassium acetate buffer (50 mM, pH 5.6), to minimise osmotic modification of fibre fragments (Winger and Pope 1981). Homogenates were centrifuged (4000g, 10 min, 4°C) and the supernatant was retained (sarcoplasmic fraction). Pellets were resuspended in homogenising buffer (5 mL), stirred rigorously and were filtered through flyscreen (1 × 2 mm dimensions) to remove the connective tissue. The residue was washed using homogenising buffer (5 mL × 2) and resuspended in 5 mL of homogenising buffer. From the final suspension, a dilution (1 in 10) was used for determination of the myofibrillar protein solubility in the specified buffer, using the Biuret method with bovine serum albumin as a standard (Gornall et al. 1949). The same dilution was performed on the sarcoplasmic fraction for determination of the protein concentration. This was conducted using 40 μL of the sample and 160 μL of Biuret reagent measured using a spectrophotometer at 550 nm, by using a 96-well plate and plate reader as described previously. These fractions are defined as myofibrillar and sarcoplasmic protein solubility similar to Warner et al. (1997), except the buffers used for extraction were different, and were expressed in mg/g meat.
Reflection confocal scanning laser microscopy (RCLSM)
An Olympus Fluoview™ 1000 confocal laser scanning microscope at Griffith University (Eskitis Centre), was used in the reflectance mode (473 nm blue diode laser applied at 15%, bandwidth of 470–545 nm). The microscope had an inverted objective lens and a numerical aperture (NA) 1.35; ×60 magnification and aperture of 105 μm were used. Signal amplification was achieved using photomultiplier tube at 400 V (8% offset) and 2× gain. For image acquisition, high pixel resolution at 1024 × 1024 and a slow speed (20 μs/pixel) was used. Muscles (four images per sample) were viewed longitudinally (20 μm depth) and global brightness (mean greyscale pixel intensity, as an indicator of light scattering) and fibre fragment widths (μm) were determined by using Image J software (Rasband 2014), as previously described (Hughes et al. 2017).
Sarcomere length
Prior to analysis (approximately 30–34 h postmortem), a small longitudinal section of muscle (20 × 30 × 5 mm) was removed, placed flat in a plastic bag and frozen at −20°C. A razor blade was used to shave sections (10 × 5 × 1 mm: length × width × depth), which were then mounted on glass microscope slides and sarcomere lengths were measured using the laser diffraction procedure (Koolmees et al. 1986).
Statistical analysis
Data analysis was completed using Genstat 20th edition (GenStat 2019). One-way ANOVA was used for comparison among treatments. The pH and temperature declines were modelled as a function of time using an exponential function of the form: y = A + Be−kt, where y is the pH or temperature, A and B are calculated parameters, k is the related decay constant and t is the time of measurement postmortem. The parameters were calculated using non-linear regression by using R (R Core Team 2020), using a self-starting function (SSasymp) that guesses its own start parameters, rather than relying on initial estimates. A two-step approach was used to calculate the temperature at pH 6.0. The first step was to determine the time needed to reach pH 6.0 using the ‘optimise’ command in R. The time (tp) was determined using as an optimisation calculation which minimised the function (6 – (A + Be−ktp))2. In the second step, this value was used to determine the temperature at this time point using the model equation relating temperature with time. The calculations were performed individually for each carcass.
Results and discussion
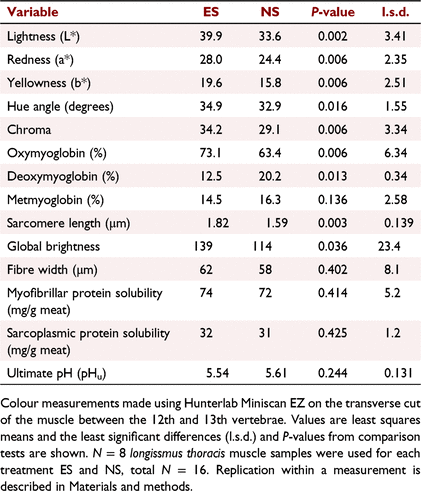
The carcass attributes are summarised in Table 1. The carcass ossification scores between 130 and 200 were indicative of cattle approximately 15–30 months old in an optimum condition carcass, as described by Romans et al. (1994). No difference was observed in hot carcass weights among treatment groups (P > 0.05). Both MSA marbling and rib fat scores were low, being indicative of the leanness of the carcasses, with some rib fat values just falling above the MSA compliance value of ≥3 mm (Meat and Livestock Australia 2019). Meat colour (AMC) scores, as assessed by the grader, showed a higher incidence of dark meat in the non-stimulated carcasses, with seven of eight having a AMC > 4, whereas stimulated carcasses had only one carcass with AMC > 4 and six of eight having a paler AMC of 1C or 2.

|
The pH decline for stimulated carcasses was much faster than that for non-stimulated carcasses, being indicative of a faster glycolytic rate early postmortem, as shown in Fig. 1 and observed in previous studies as reviewed by Hwang et al. (2003) and Adeyemi and Sazili (2014). The temperature–pH profiles during the postmortem period are shown in Fig. 1 and the Temp@pH6 was calculated to be 25.5°C reached at 2 h 20 min postmortem, and 3.7°C reached at 13 h 25 min, for electrically and non-electrically stimulated muscles respectively. As the stimulated carcasses passed through pH 6 at 25.5°C, they would pass through the ‘pH–temperature window’ recommended by MSA (Thompson 2002), whereas non-stimulated carcasses passed through pH 6 at <10°C and were considered to be cold-shortened, which was confirmed by the shorter sarcomere lengths (P < 0.05; Table 2). The cold-shortening effect was not ideal, but most likely the result of the standard meat processors chilling regime used on the carcasses and was necessary to ensure consistency in all other treatment parameters used in the experiment apart from that being tested, i.e. electrical stimulation. It was evident that the pH–temperature decline of stimulated carcasses was non-linear, with a steep decline occurring <2 h postmortem, being indicative of the faster rate of glycolysis, whereas in non-stimulated carcasses the decline was more linear with a progressively slower rate of glycolysis. However, no differences (P > 0.05) were observed in the pHu after 26 h postmortem (Table 2), with 5.61 and 5.54 being observed for non-stimulated and stimulated muscles respectively. These are well below the MSA non-compliance value of pH ≤ 5.70 and are similar to previous findings (Hopkins et al. 2014). Thus, electrically stimulated meat had a faster pH decline than non-stimulated controls, which were cold-shortened.
|
The colour differences observed by the meat grader between the two sets of carcasses were confirmed by colour measurements, as shown in Table 2. Electrically stimulated longissimus muscles were lighter, redder, yellower and, consequently, had higher hue and chroma values (P < 0.05 for all). The electrically stimulated muscles had higher concentrations of red OMb and lower concentrations of purple DMb (P < 0.05), while the brown MMb concentrations were similar. Data for DMb were heteroscedastic and a natural-log transformation was used for comparison, with back-transformed means being reported. Fig. 2a shows the reflectance scans, highlighting that electrically stimulated muscles had higher reflectance values, especially in the amber/red part (>600 nm) of the visible spectrum, which is consistent with increased reflectance values observed at 24 h postmortem by Sleper et al. (1983). Increases in these colour attributes with electrical stimulation have been previously reported (Tang and Henrickson 1980; Li et al. 2011; Biraima et al. 2019), but are not always consistent (Ledward et al. 1986; Hope-Jones et al. 2012), depending on many other attributes, including time postmortem (Sleper et al. 1983; Adeyemi and Sazili (2014). Electrical stimulation generates a brighter colour, especially early postmortem (12–20 h), but these differences can be less obvious after longer periods (48–72 h) (Orcutt et al. 1984; Adeyemi and Sazili 2014). In this study, although colour was measured at 23–24 h postmortem, colour differences between stimulation treatments were still apparent (Orcutt et al. 1984). The increase in chromatic attributes (redness, yellowness and OMb) was likely a result of the treatment promoting oxygenation of myoglobin, an increase in mitochondrial treason, changes in inner mitochondrial membrane permeability and/or or opening of the mitochondrial permeability transition pores, induced by the influx of calcium as described previously (Tang and Henrickson 1980; Sleper et al. 1983; Hudson 2012; England et al. 2018). These changes are known to alter oxygen consumption of mitochondria, and as mitochondria are known to compete with myoglobin for oxygen, it is likely that the lower pH, faster pH decline and higher rigour temperature in the electrically stimulated muscles had a role in this chromatic development (Jacob 2020; Ramanathan et al. 2021). In addition, mitochondrial swelling has been observed in previous work and was likely to be involved, causing colour variations among different muscle types (Devine et al. 1984), but this study focused on overall muscle fibre dimensions and characteristics. Electrical stimulation increased both the chromatic parameters, with higher concentrations of OMb being observed, and the achromatic parameters, with higher lightness values being observed than in non-stimulated carcasses.
The increase in achromatic attributes post-treatment was further substantiated with increased global brightness (an indicator of light scattering) in muscle fibres by using reflectance confocal microscopy, confirming our hypothesis that microstructural changes occurred as a result of electrical stimulation. Figure 3 shows RCLSM images from non-stimulated and stimulated muscles, with the latter having higher (P < 0.05) pixel intensity, expressed as global brightness values (Table 2). Contrary to our hypothesis, no differences were observed in the muscle fibre width, indicating for the first time that muscle fibre shrinkage was not a key driver of the structural changes involved in the development of light scattering in electrically stimulated meat. For non-stimulated muscles, the sarcomere lengths were shorter, indicating that cold-shortening had occurred which may have contributed to the lower global brightness values. For stimulated muscles, there was clear evidence of formation of contraction nodes in the muscle fibres (Fig. 3d–f), resulting in distortion and bending of muscle fibres from their usual configuration, which has been described previously (Ho et al. 1996; Hwang et al. 2003). These contraction nodes were typically 10–25 μm in length, and have been defined previously as 2–10 highly contracted sarcomeres per myofibril (Ho et al. 1996). They are formed due to the localised excessive calcium ion release from the sarcoplasmic reticulum and irreversible tetanic contracture as a result of the frequency of electrical stimulation, as explained by Hwang et al. (2003). This was not evident in all micrographs/samples, and may have been due to the mixture of fibre types present in the longissimus thoracis, where red oxidative fibre types have been reported to be more susceptible to super-contracture than the white fibre types (Devine et al. 1984). The contraction nodes evident in electrically stimulated muscles with subsequent reconfiguration of the muscle fibre packing density are likely to be the main components responsible for changes in the muscle’s achromatic properties. Thus, this study has highlighted that the main component for achromatic light-scattering development in electrically stimulated meat is not from muscle fibre shrinkage, but rather from the development of contraction nodes in individual muscle fibres, which, consequently, change the deflection of light between myofibrils, increasing global brightness of each individual muscle fibre. The distortion of individual muscle fibres would also affect scattering between individual muscle fibres.
Additionally, there are likely to be other components of the muscle that affect the scattering to a smaller extent, such as, for example, sarcoplasmic proteins binding to myofilaments, integrity of cytoskeleton, and myosin denaturation, which have been discussed in a more general fashion in relation to light-scattering development (Hughes et al. 2019; Purslow et al. 2020). It is evident that the exact mechanisms are still unknown and involve numerous structural proteins, as outlined by Hwang et al. (2003). In this study, measurements of the sarcoplasmic and myofibrillar protein solubility and the absorbance of muscle fluid were not indicative of any role for denaturation of these proteins in the observed light scattering. First, the extraction of both sarcoplasmic and myofibrillar muscle proteins did not reveal any alterations in protein solubility under these extraction conditions (Table 2, note our values for myofibrillar and sarcoplasmic protein solubility were lower than would be expected according to other data such as in Feng et al. (2020)) and, second, no differences were observed in the muscle fluid scan profiles shown in Fig. 2b.
Conclusions
Electrical stimulation promotes changes in colour of the longissimus thoracis muscle that are both chromatic colour and achromatic (no colour) lightness. These changes were evident chromatically as increased redness, yellowness and higher concentrations of OMb and lower concentrations of DMb in the muscle. For the first time, this study also demonstrated that colour changes with electrical stimulation are achromatic, and associated with light scattering and higher global brightness values in the muscle fibre. In addition, this study has highlighted that the achromatic changes are not caused by muscle fibre transverse shrinkage, but are likely to arise from changes in the muscle fibres and myofibril packing density. These achromatic changes appear to occur due to formation of contraction nodes and distortion of muscle fibres, which increase the light scattering properties of the individual muscle fibres and the overall lightness of the muscle. It is evident that muscle is in a structurally dynamic state postmortem and the slaughter-floor methods applied to pre-rigour muscle can have large effects on the colour properties of the muscle. Meat processors should ensure that electrical stimulation units are operational to prevent the negative colour effects of slow pH fall and cold-shortening, especially in smaller, pasture-fed animals.
Data availability
The data that support this study cannot be publicly shared due to ethical or privacy reasons and may be shared upon reasonable request to the corresponding author if appropriate.
Conflicts of interest
Robyn Warner is an Associate Editor for Animal Production Science but was blinded from the peer-review process for this paper. The other authors declare no other conflicts of interest.
Declaration of funding
Funding was provided by Australian Meat Processor Corporation (AMPC).
Acknowledgements
The authors acknowledge funding provided by Australian Meat Processor Corporation (AMPC) and matching funds provided from the Australian Government, via Meat and Livestock Australia (MLA), to support the research and development detailed in this publication. The support of Imaging and Image Analysis Facility at Griffith University is also gratefully acknowledged.
References
Adeyemi KD, Sazili AQ (2014) Efficacy of carcass electrical stimulation in meat quality enhancement: a review. Asian–Australasian Journal of Animal Science 27, 447–456.| Efficacy of carcass electrical stimulation in meat quality enhancement: a review.Crossref | GoogleScholarGoogle Scholar |
AUS-MEAT (2005) Handbook of Australian meat. In ‘International red meat manual’. (Ed. I King) pp. 8–11. (AUS-MEAT: Brisbane, Qld, Australia)
Biraima ADA, Mohammed AM, Webb EC (2019) Effects of electrical stimulation and age at slaughter on carcass and meat quality of two Sudanese Baggara beef types. South African Journal of Animal Science 49, 904–913.
| Effects of electrical stimulation and age at slaughter on carcass and meat quality of two Sudanese Baggara beef types.Crossref | GoogleScholarGoogle Scholar |
Devine CE, Ellery S, Averill S (1984) Responses of different types of ox muscle to electrical stimulation. Meat Science 10, 35–51.
| Responses of different types of ox muscle to electrical stimulation.Crossref | GoogleScholarGoogle Scholar |
Eikelenboom G, Smulders FJM, Ruderus H (1985) The effect of high and low voltage electrical stimulation on beef quality. Meat Science 15, 247–254.
| The effect of high and low voltage electrical stimulation on beef quality.Crossref | GoogleScholarGoogle Scholar |
England EM, Matarneh SK, Mitacek RM, Abraham A, Ramanathan R, Wicks JC, Shi H, Scheffler TL, Oliver EM, Helm ET, Gerrard DE (2018) Presence of oxygen and mitochondria in skeletal muscle early postmortem. Meat Science 139, 97–106.
| Presence of oxygen and mitochondria in skeletal muscle early postmortem.Crossref | GoogleScholarGoogle Scholar |
Feng Y-H, Zhang S-S, Sun B-Z, Xie P, Wen K-X, Xu C-C (2020) Changes in physical meat traits, protein solubility, and the microstructure of different beef muscles during post-mortem aging. Foods 9, 806
| Changes in physical meat traits, protein solubility, and the microstructure of different beef muscles during post-mortem aging.Crossref | GoogleScholarGoogle Scholar |
GenStat (2019) ‘GenStat.’ (VSN International: Hempstead, UK)
George AR, Bendall JR, Jones RCD (1980) The tenderising effect of electrical stimulation of beef carcasses. Meat Science 4, 51–68.
| The tenderising effect of electrical stimulation of beef carcasses.Crossref | GoogleScholarGoogle Scholar |
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. Journal of Biological Chemistry 177, 751–766.
| Determination of serum proteins by means of the biuret reaction.Crossref | GoogleScholarGoogle Scholar |
Ho CY, Stromer MH, Robson RM (1996) Effect of electrical stimulation on postmortem titin, nebulin, desmin, and troponin-T degradation and ultrastructural changes in bovine longissimus muscle. Journal of Animal Science 74, 1563–75.
| Effect of electrical stimulation on postmortem titin, nebulin, desmin, and troponin-T degradation and ultrastructural changes in bovine longissimus muscle.Crossref | GoogleScholarGoogle Scholar |
Hope-Jones M, Strydom PE, Frylinck L, Webb EC (2012) Effect of dietary beta-agonist treatment, vitamin D3 supplementation and electrical stimulation of carcasses on colour and drip loss of steaks from feedlot steers. Meat Science 90, 607–612.
| Effect of dietary beta-agonist treatment, vitamin D3 supplementation and electrical stimulation of carcasses on colour and drip loss of steaks from feedlot steers.Crossref | GoogleScholarGoogle Scholar |
Hopkins DL, Ponnampalam EN, van de Ven RJ, Warner RD (2014) The effect of pH decline rate on the meat and eating quality of beef carcasses. Animal Production Science 54, 407–413.
| The effect of pH decline rate on the meat and eating quality of beef carcasses.Crossref | GoogleScholarGoogle Scholar |
Hudson NJ (2012) Mitochondrial treason: a driver of pH decline rate in post-mortem muscle? Animal Production Science 52, 1107–1110.
| Mitochondrial treason: a driver of pH decline rate in post-mortem muscle?Crossref | GoogleScholarGoogle Scholar |
Hughes J, Clarke F, Purslow P, Warner R (2017) High pH in beef longissimus thoracis reduces muscle fibre transverse shrinkage and light scattering which contributes to the dark colour. Food Research International 101, 228–238.
| High pH in beef longissimus thoracis reduces muscle fibre transverse shrinkage and light scattering which contributes to the dark colour.Crossref | GoogleScholarGoogle Scholar |
Hughes J, Clarke F, Purslow P, Warner R (2018) A high rigor temperature, not sarcomere length, determines light scattering properties and muscle colour in beef M. sternomandibularis meat and muscle fibres. Meat Science 145, 1–8.
| A high rigor temperature, not sarcomere length, determines light scattering properties and muscle colour in beef M. sternomandibularis meat and muscle fibres.Crossref | GoogleScholarGoogle Scholar |
Hughes JM, Clarke FM, Purslow PP, Warner RD (2019) Meat color is determined not only by chromatic heme pigments but also by the physical structure and achromatic light scattering properties of the muscle. Comprehensive Reviews in Food Science and Food Safety 19, 44–63.
| Meat color is determined not only by chromatic heme pigments but also by the physical structure and achromatic light scattering properties of the muscle.Crossref | GoogleScholarGoogle Scholar |
Hunt M (2012) ‘AMSA meat color measurement guidelines.’ (American Meat Science Association: IL, USA)
Hwang IH, Devine CE, Hopkins DL (2003) The biochemical and physical effects of electrical stimulation on beef and sheep meat tenderness. Meat Science 65, 677–691.
| The biochemical and physical effects of electrical stimulation on beef and sheep meat tenderness.Crossref | GoogleScholarGoogle Scholar |
Jacob R (2020) Implications of the variation in bloom properties of red meat: a review. Meat Science 162, 108040
| Implications of the variation in bloom properties of red meat: a review.Crossref | GoogleScholarGoogle Scholar |
Jacques SL (2013) Optical properties of biological tissues: a review. Physics in Medicine and Biology 58, R37–R61.
| Optical properties of biological tissues: a review.Crossref | GoogleScholarGoogle Scholar |
Jeacocke RE (1984) Light scattering from muscle during the onset of rigor mortis. In ‘Proceedings of 30th European meeting of meat research workers’, Bristol, UK. pp. 104–105. https://digicomst.ie/1984/1984_03_06/
Kim YHB, Warner RD, Rosenvold K (2014) Influence of high pre-rigor temperature and fast pH fall on muscle proteins and meat quality: a review. Animal Production Science 54, 375–395.
| Influence of high pre-rigor temperature and fast pH fall on muscle proteins and meat quality: a review.Crossref | GoogleScholarGoogle Scholar |
Koolmees PA, Korteknie F, Smulders FJM (1986) Accuracy and utility of sarcomere length assessment by laser diffraction. Food Microstructure 5, 71–76.
Krzywicki K (1979) Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Science 3, 1–10.
| Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef.Crossref | GoogleScholarGoogle Scholar |
Ledward DA, Dickinson RF, Powell VH, Shorthose WR (1986) The colour and colour stability of beef Longissimus dorsi and Semimembranosus muscles after effective electrical stimulation. Meat Science 16, 245–265.
| The colour and colour stability of beef Longissimus dorsi and Semimembranosus muscles after effective electrical stimulation.Crossref | GoogleScholarGoogle Scholar |
Li C, Li J, Li X, Hviid M, Lundström K (2011) Effect of low-voltage electrical stimulation after dressing on color stability and water holding capacity of bovine longissimus muscle. Meat Science 88, 559–565.
| Effect of low-voltage electrical stimulation after dressing on color stability and water holding capacity of bovine longissimus muscle.Crossref | GoogleScholarGoogle Scholar |
MacDougall DB (1982) Changes in the colour and opacity of meat. Food Chemistry 9 75–88.
| Changes in the colour and opacity of meat.Crossref | GoogleScholarGoogle Scholar |
MacDougall DB, Jones SJ (1981) Translucency and colour defects of dark-cutting meat and their detection. The problem of dark-cutting in beef. Current Topics in Veterinary Medicine and Animal Science 10, 328–43.
Meat and Livestock Australia (2019) 2019 Australian beef eating quality insights. Meat and Livestock Australia, Qld, Australia. Available at https://www.mla.com.au/globalassets/mla-corporate/marketing-beef-and-lamb/documents/meat-standards-australia/abeqi-2019-interactive.pdf. [Accessed 1 March 2022]
Orcutt MW, Dutson TR, Cornforth DP, Smith GC (1984) Factors affecting the formation of a dark, coarse band (‘heat-ring’) in bovine longissimus muscle. Journal of Animal Science 58, 1366–1375.
| Factors affecting the formation of a dark, coarse band (‘heat-ring’) in bovine longissimus muscle.Crossref | GoogleScholarGoogle Scholar |
Purslow PP, Warner RD, Clarke FM, Hughes JM (2020) Variations in meat colour due to factors other than myoglobin chemistry; a synthesis of recent findings (invited review). Meat Science 159, 107941
| Variations in meat colour due to factors other than myoglobin chemistry; a synthesis of recent findings (invited review).Crossref | GoogleScholarGoogle Scholar |
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available at https://www.r-project.org/
Ramanathan R, Hunt MC, Price T, Mafi GG (2021) Strategies to limit meat wastage: focus on meat discoloration. Advances in Food and Nutrition Research 95, 183–205.
| Strategies to limit meat wastage: focus on meat discoloration.Crossref | GoogleScholarGoogle Scholar |
Rasband W (2014) ImageJ.Ink. Available at http://imagej.nih.gov/ij/index.html [Accessed 6 February 2020]
Romans JR, Costello WJ, Carlson CW, Greaser ML, Jones KW (1994) ‘The meat we eat.’ (Interstate Publishers, Inc.: Danville, IL, USA)
Savell JW, Dutson TR, Smith GC, Carpenter ZL (1978) Structural changes in electrically stimulated beef muscle. Journal of Food Science 43, 1606–1607.
| Structural changes in electrically stimulated beef muscle.Crossref | GoogleScholarGoogle Scholar |
Sleper PS, Hunt MC, Kropf DH, Kastner CL, Diekman ME (1983) Electrical stimulation effects on myoglobin properties of bovine longissimus muscle. Journal of Food Science 48, 479–483.
| Electrical stimulation effects on myoglobin properties of bovine longissimus muscle.Crossref | GoogleScholarGoogle Scholar |
Swatland HJ (2012) Optical properties of meat. In ‘65th annual reciprocal meat conference’. (Ed. American Meat Science Association), pp. 1–7 (American Meat Science Association): Chicago, Illinois, USA.
Tang BH, Henrickson RL (1980) Effect of postmortem electrical stimulation on bovine myoglobin and its derivatives. Journal of Food Science 45, 1139–1141.
| Effect of postmortem electrical stimulation on bovine myoglobin and its derivatives.Crossref | GoogleScholarGoogle Scholar |
Thompson J (2002) Managing meat tenderness. Meat Science 62, 295–308.
| Managing meat tenderness.Crossref | GoogleScholarGoogle Scholar |
Warner RD, Kauffman RG, Greaser ML (1997) Muscle protein changes post mortem in relation to pork quality traits. Meat Science 45, 339–352.
| Muscle protein changes post mortem in relation to pork quality traits.Crossref | GoogleScholarGoogle Scholar |
Warner RD, Dunshea FR, Gutzke D, Lau J, Kearney G (2014) Factors influencing the incidence of high rigor temperature in beef carcasses in Australia. Animal Production Science 54, 363–374.
| Factors influencing the incidence of high rigor temperature in beef carcasses in Australia.Crossref | GoogleScholarGoogle Scholar |
Winger RJ, Pope CG (1981) Osmotic properties of post-rigor beef muscle. Meat Science 5, 355–369.
| Osmotic properties of post-rigor beef muscle.Crossref | GoogleScholarGoogle Scholar |
Wulf DM, Wise JW (1999) Measuring muscle color on beef carcasses using the L*a*b* color space. Journal of Animal Science 77, 2418–2427.
| Measuring muscle color on beef carcasses using the L*a*b* color space.Crossref | GoogleScholarGoogle Scholar |


