A model of flystrike pesticide resistance management on sheep: use of pesticide rotations
Pia Benedetti Vallenari A , Andrew Bailey A and Brian J. Horton A *
A *
A Tasmanian Institute of Agriculture, University of Tasmania, Private Bag 1375, Launceston, Tas. 7250, Australia.
Animal Production Science 63(8) 802-815 https://doi.org/10.1071/AN22345
Submitted: 6 September 2022 Accepted: 31 January 2023 Published: 24 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: In some regions of Australia, the Australian sheep blowfly (Lucilia cuprina) is resistant to some of the pesticides used to control flystrike in sheep. Few pesticide groups are available, so it is important to delay or prevent any increase in resistance.
Aims: This study examined some of the assumptions in a previously developed model of pesticide resistance and tested the use of pesticide rotations as a means of limiting blowfly resistance to pesticides.
Methods: A model of sheep blowfly pesticide resistance was added to a previous model of sheep blowfly strike, to allow simulation of a range of pesticide management options for control of flystrike in sheep that might avoid increasing pesticide resistance.
Key results: The model requires some assumptions of settings that are uncertain, but the effects are not sensitive to a wide range of values for these settings. Resistance may not be obvious for some years after a new product is introduced, but once it has been detected, the frequency of resistance genes will increase rapidly if use of the same pesticide continues. The use of different pesticide groups each year is preferable to continuous use of the same product, but this risks losing efficacy of multiple products rather than one product at a time. However, rotations do provide a longer period of good protection from flystrike before all products used in the rotation fail. The number of years of successful protection against flystrike is extended if there is a fitness disadvantage for resistance to the products used.
Conclusions: The model may be useful for examining interactions between genes for resistance to different pesticides and the effect of non-chemical methods of control of flystrike, to extend the useful life of the current range of pesticides.
Implications: By the time resistance is detected on a farm, the level of resistance is high and will increase rapidly if the same pesticides continue to be used. Other non-pesticide methods such as breeding sheep for resistance to flystrike may be long-term solutions where resistance has reduced pesticide protection.
Keywords: flies, flystrike, genetics, model, pesticides, resistance, sheep, sheep blowfly.
Introduction
Flystrike in Australia costs approximately AU$323 million annually in treatment, prevention and production losses but varies year to year depending on weather conditions (Shephard et al. 2022). Insecticides are a cost-effective means of flystrike control, whereby a chemical is applied to the sheep via jetting or spray-on for prevention, or a dressing is applied to an already flystruck sheep (Tellam and Bowles 1997; Heath and Levot 2015). However, over the past 60 years, the development of insecticide resistance in the Australian sheep blowfly (Lucilia cuprina) has caused some chemicals used to treat flystrike to become ineffective, resulting in reduced protection periods (Levot 2001; Levot et al. 2014) and loss of production through sheep mortality, reduced wool production and quality (Colditz et al. 2005), and lamb losses associated with severe flystrike (Horton et al. 2018).
Pesticides used to prevent flystrike usually provide protection for long periods, usually 3–5 months. This provides an ideal situation for the development of resistance to those products, because some flies will inevitably be exposed to marginal concentrations of pesticide that allow those carrying resistance genes to survive. Strategies to maintain blowfly susceptibility to currently used insecticides are critical, owing to producer reliance on chemical treatment (Levot 2001; Colvin et al. 2022).
Experimental work in which flystrike is artificially induced carries ethical concerns of sheep welfare, and anecdotal reports from producers require research for validation. In terms of studying resistance development, data need to be collected over long periods, proving difficult for experimental research and often relying on information provided by producers. A computer model was used for this research, offering a non-invasive solution to examine resistance development over periods of many years.
The focus of this research was the modelling of flystrike pesticide resistance development, using the Flystrike Resistance Decision Support System (Horton and Hogan 2010). The program used here was based on a model used previously (Lucas and Horton 2013; Percival and Horton 2014), modified to allow for resistance. In this study, the model was used to examine the effects of varying some of the settings and comparing continuous use of a product against rotations of two products.
Methods
This study used an existing weather-driven model of the risk of flystrike (Wardhaugh et al. 2007), which considers the effect of shearing, crutching and chemical treatment. The details of this model have been described previously (Lucas and Horton 2013; Percival and Horton 2014), but some changes were required so that flystrike depended on the level of resistance of the flies to the chemicals applied. A more detailed description of the model has been given by Benedetti Vallenari (2021). This study used ver. 4.51 of the Resistant Flies Model.
The flystrike risk in this study was based on weather records for Gunning (latitude −34.8, longitude 149.7), between Yass and Goulburn in New South Wales, considered to be representative of the major sheep production areas in south-eastern Australia. The program allows for a range of sheep classes so that it can be customised to individual properties. In this study, 10 000 ewes were used to simulate a large wool-producing property. The sheep were assumed to be shorn on 1 July, in order to avoid any interaction of shearing date with flystrike management in the situations tested.
Resistant flies model
The previous model estimated the risk of flystrike after treatment by using the stated length of protection for each product, with an adjustment before and after this day to smooth the transition from full protection to no protection. The current model estimates the amount (in grams) of pesticide applied to the sheep on the day of treatment and the amount expected to be left on the sheep when the listed protection period ends. The WoolRes program (Campbell and Horton 2002) was used to make these estimates for standard treatment applied 3 months after shearing. The concentration of pesticide on the last day before protection ends was considered to be the ‘lethal dose’ – the minimum concentration to kill non-resistant flies. The concentration of pesticide (g/kg wool) was estimated for each day after treatment by assuming a log–linear breakdown for pesticide on the sheep. This was converted to a multiple of the lethal dose. Flies (maggots) with a given resistance factor (see below) were assumed to survive the pesticide application if the ratio for a given day was less than their resistance factor.
On each day of the year, the model estimated the concentration of pesticide in the wool for any previously applied product. Then it considered each resistance genotype in turn, to estimate whether the maggots with that genotype could survive on that day. The proportion of all maggots surviving was multiplied by the risk of flystrike for that day, allowing for the effect of any recent shearing or crutching and the risk of strike on that day, based on normal weather conditions at the location.
Resistance factors
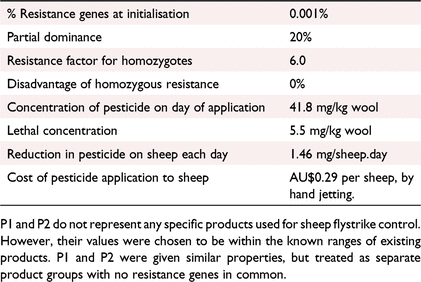
The lethal dose of a treatment is the concentration of the pesticide just before the treatment is no longer effective. Wild-type flies that are susceptible are killed by any pesticide concentration >1.0 times the lethal dose but survive at pesticide concentrations <1.0 times the lethal dose. Therefore, homozygous susceptible flies were given a resistance factor of 1.0. The resistance factor is a measure of the concentration of insecticide that a resistant fly can survive as a multiple of the lethal dose. For example, a resistance factor of 2.0 means that a resistant fly could survive up to, but not higher than, twice the lethal dose of pesticide. Initially, a resistance factor of 6 was used in the study because values of 3–8-fold resistance have been reported for other pesticides during the early stages of resistance (Levot et al. 2014). Although the homozygotes for resistance in the model have 6-fold resistance, the partial dominance of 20% allowed only 2-fold resistance in the heterozygotes. At low resistance gene frequencies, almost all resistance is due to heterozygotes.
Starting resistance level
For testing purposes, a starting resistance of 0.001%, or 1 resistant gene for every 100 000 genes, was used. The starting resistance level was the frequency of the resistance gene, not the frequency of the homozygous resistance genotype.
Partial dominance
The dominance of a gene determines the phenotype (Miko 2008). Partial dominance was used in the model to allow the genes to range from completely recessive to fully dominant. A dominance of 100% was fully dominant and meant that the heterozygotes had the same level of resistance as the homozygotes. A partial dominance of 50% meant that the resistance level of the heterozygotes was halfway between that of the wild type and that of the homozygotes, and dominance of 0% was fully recessive. In the model, an estimated partial dominance of 20% was given to genes when the true value of partial dominance was not known, as in this study.
Genetic fitness disadvantage
Genetic disadvantage determined the lethality of homozygous resistance phenotypes. For example, a 20% disadvantage meant that 20% of resistant homozygotes died, and 100% disadvantage meant that all resistant homozygotes died, with heterozygotes surviving. The default genetic disadvantage was 0%. Some cyromazine-resistant flies experience homozygous lethality (Yen et al. 1996), that is, a genetic disadvantage of 100%. However, the disadvantage, if any, of other resistance genes is not known.
Reproduction off sheep (unselected flies multiplier)
The unselected flies multiplier was a value used to adjust the number of flies not exposed to treatment and, therefore, unselected for resistance. Flies may not be exposed to treatment if they laid eggs on sheep that were not treated or came from neighbouring properties with no treatment, or if they reproduced on carcasses. An off-sheep reproduction percentage between 1.5% and 3% was judged a reasonable value (Lang et al. 2006), although there are limited studies to indicate the correct value.
Products
This study used two simulated products with similar properties but with no genetic interactions. This was done to avoid complex effects with cyromazine, where at least two different resistance loci are known (Yen et al. 1996), and interactions with dicyclanil, which is believed to have some resistance genes in common with cyromazine (Sales et al. 2020), but the genetics of resistance has not been clarified as yet.
The products used in this study are termed Product 1 (P1) and Product 2 (P2), with the properties in the model shown in Table 1.
|
The pesticide concentrations in wool and breakdown of pesticide on the wool provide a protection period of 3 months.
Optimised treatment date
The optimised treatment date was estimated as described previously (Percival and Horton 2014), using the model that does not take genetic resistance into account. This gives the same recommended treatment date as that obtained by using the FlyBoss tool (Horton and Hogan 2010).
For the products tested here for the Gunning region, on sheep shorn 1 July, the optimum treatment date for a single use of a product each year was 29 October, but if two treatments were used each year, the optimum treatment dates were 4 October and 19 January.
Results
Fig. 1 shows the expected risk of flystrike at Gunning over the period 1 July−30 June, with an expected rate of strike of 1–2.5% of sheep each week from mid-October to the end of April if no preventive treatment is applied. This is the average strike over 30 years, but in any individual year the strike is more variable from week to week. Fig. 1 also shows the expected strike if the optimised treatment is used, with P1 applied on 29 October, for both breech and body strike, providing about 3 months protection from strike. This provides protection over the highest risk period, preventing the average risk from rising above 1% per week. This is the first year of use of this treatment, so the level of resistance was low (0.001%) for this application.
|
|
Starting resistance level
The starting resistance level was the frequency of resistance genes in the starting population. Increasing the starting resistance level for each gene resulted in earlier increases in development of resistance (Fig. 2), but the resulting curves were similar, with a rapid transition from low to high gene frequency once the frequency reached 0.1%.
Fig. 3 shows the expected risk of flystrike when treatment is used in each year after beginning the use of P1 as preventive treatment. In Year 1, there was little or no effect of resistance, and Year 2 (not shown) was similar. However, by Year 3, the protective period was 12–16 days shorter; by Year 4 of regular use, some sheep could be struck within a few weeks of application of treatment; and by Year 5, there was little or no benefit of the preventive treatment. These results apply if all sheep on the property are treated with the same product at the same time each year.
|
|
Resistance factors
Increasing the resistance factor decreased the time taken for the population to be fully resistant (Fig. 4), with resistance reaching 50% within 4 years for resistance factors ≥4.0, but requiring 9 years at a resistance factor of 2.0. A resistance factor of ≤1.0 did not provide any resistance.
Partial dominance
The partial dominance of the resistance gene determined the resistance level of the heterozygotes. A partial dominance of 0% produced no resistance development (Fig. 5) over the period studied because only homozygous resistant genotypes had an advantage, and with a starting gene frequency of 0.001%, the number of homozygous resistant flies is negligible. A dominance of only 10% resulted in the development of substantial resistance within 4 years, 20% dominance required 3 years, and ≥50% dominance could produce a gene frequency >50% for resistance in only 2 years.
|
|
Fitness disadvantage
As the fitness disadvantage of homozygotes for resistance increased, the rate of resistance development decreased (Fig. 6). With a disadvantage of ≤20%, the resistance gene frequency reached almost 100% after 10 years, whereas with a disadvantage of ≥50%, a stable gene frequency was reached, with <50% resistance alleles.
Reproduction off sheep (unselected flies multiplier)
The unselected flies multiplier controlled the number of flies reproducing off sheep, therefore not being exposed to chemical treatment and consequent selection for resistance. Increasing the unselected flies multiplier, and therefore the number of flies reproducing off sheep, decreased the rate of development of resistance (Fig. 7). At zero, all flies reproduce on sheep and hence are exposed to the pesticide after treatment. This resulted in very rapid development of resistance, with >50% resistance genes after 3 years. Higher proportions of flies reproducing off sheep, and therefore not exposed to pesticide, delayed the development of resistance but did not prevent it. At a setting of 100, where the number of flies reproducing off sheep was equal to the number of flies reproducing on treated sheep, it required 21 years to reach 50% resistant genes in the population.
Rotations
In order to test rotations, a theoretical new product (P2), with the same properties as P1 but with resistance due to independent genes, was introduced (Fig. 8). Three options were tested: treatment with the same product every year for 6 years; treatment with one product until it could be determined that the product was no longer effective (3 years) then changing to another product; alternating two products each year. After 3 years of continuous P1 application, the gene frequency for resistance reached ~54%; if use of the product continued, then the gene frequency for resistance reached 98.9% after 6 years. After 3 years, if treatment switched to P2, then resistance to this product increased to reach 54% after 3 years of use, while resistance for P1 remained at 54%. Annual rotation of the products maintained a low level of resistance for at least 4 years, but after 6 years the resistance after annual rotation was 54%, the same as the gene frequency of resistance when the products were used continuously for 3 years.
Rotations with a 50% fitness disadvantage
A 50% fitness disadvantage was simulated to determine whether rotations would be of more value during periods when the product was not in use, when some reduction of the resistance gene frequency might be expected (Fig. 9). If P1 was used every year, then the resistance frequency for Gene 1 stabilised at 46.4%. If P1 and P2 were used repeatedly for 3 years, then the resistance frequency for both Gene 1 and Gene 2 fell to 6.4% when the corresponding product had not been used for 3 years, but increased to 34.6% in the year when the product was next used, rising to 46% by the third year of use. Annual rotation gave an oscillation for each gene between 18% and 40%.
Fig. 10 shows the expected percentage of sheep struck during each year for the treatment options considered in Fig. 9. If the same product was used every year, then the number of strikes rose, even though the resistance was limited by the fitness disadvantage. However, if product rotations were used then the proportion of sheep struck was only slightly elevated, with little difference between annual rotations and 3-year rotations.
|
|
Treatment twice per year
A single preventive application of P1 (or P2) each year kept the risk of strike below 1% per week, until resistance became a problem. However, this level may be too high for some sheep producers. Therefore, treatment twice each year was considered. Fig. 11 shows that treatment twice each year with the same product caused a much more rapid increase in resistance, with a gene frequency of 90% by the end of the second year, rather than after 4 years of single treatment each year. The rapid increase of resistance could be delayed by alternating P1 and P2 within each year, but this still resulted in high levels of resistance for both products after 4 years. Although P1 and P2 had identical properties in the model, P1 was applied at a time when flystrike risk is high and increasing after winter, whereas P2 was applied at a time when the fly population is stable and the risk lower than in spring. As a result, resistance to P1 increased faster than to P2, when both were used each year.
Fig. 12 shows the proportion of sheep struck for the scenarios in Fig. 11, and additional rotation options. If the same product was used twice per year, then the number of strikes increased rapidly to a level similar to that expected if no treatment was used. However, all rotation options delayed the initial increase in flystrike and resulted in a slightly lower rate of increase, although all methods were ineffective after 5 years. There was little difference among scenarios of rotations of the products during each year (P1 in spring and P2 in autumn); use of P1 twice in a year, then P2 twice in the next year; or rotations that varied the use of P1 and P2 so that each was used in spring and in autumn, but in alternate years.
Discussion
In a detailed review of sheep flystrike control, Kotze and James (2022) noted the importance of resistance management and the need for modelling of insecticide use and management strategies.
A range of assumptions must be set in the model where there is limited information; hence, the precise values are not known. However, over the range of values tested here, there were several consistent results. If the gene frequency for resistance was initially very low, then it may remain low for several years, with little or no sign of resistance. However, it will eventually increase very rapidly, with a transition from <5% to >95% over 2–3 years. A similar transition was observed for a wide range of assumptions, indicating that if the model is incorrect in some of the settings used, then this may affect the time until the resistance becomes a serious problem but not the rate of change when resistance does increase. This suggests that the model may be suitable for examining alternative management choices to delay resistance, even though it may be unable to determine the precise length of the period before the resistance is observed. Historically, chemicals have lost effectiveness in short periods once resistance has been detected (Levot 2001; Heath and Levot 2015). For example, 70% of flies were resistance to dieldrin 4 years after resistance was detected (Hughes and McKenzie 1987), confirming that the rapid changes indicated here are possible for some products if they are used on all sheep every year.
Model settings
Assigning a gene a partial dominance of 0% meant that heterozygotes had no advantage over wild-type flies in the presence of the pesticide, with only homozygous resistant flies having any advantage. At the low starting gene frequency, the number of homozygotes and resulting advantage was negligible; hence, no resistance developed over 10 years.
As partial dominance increased from 10% to 20% and 50%, the proportion of resistant flies also increased. Genes with a partial dominance <100% (but >0%) produce homozygotes with an advantage over heterozygotes in the presence of the pesticide, so the proportion of resistance genes increased more rapidly at higher dominance.
When the resistance gene has a partial dominance of 100%, the heterozygotes have the same resistance factor as homozygotes. At the low starting gene frequency, both homozygotes and heterozygotes have an initial equal advantage, but the population with resistance is almost all heterozygote. As the gene frequency for resistance increases, more homozygous resistant flies are present, which have no advantage over the heterozygous resistant flies, so the population does not approach 100% resistance as rapidly as populations with lower percentage dominance, in which homozygotes do have an advantage.
A resistance factor of 1 did not result in any resistance development because this simulated wild-type flies. Literature suggests that a resistance factor of 6–8 was reasonable for examining resistance in this model (Levot et al. 2014), although cyromazine has a resistance factor of only 3. The resistance in heterozygotes was assumed to be only 2.0.
The starting resistance level had a considerable influence on resistance development and was an important setting in the model. At higher frequency of resistance genes in the starting population, subsequent resistance development occurred sooner. A starting frequency estimation of 0.001% appears reasonable (Yen et al. 1996) for populations with limited previous exposure to the pesticide. In the case of dieldrin, 70% of flies were resistant 4 years after resistance was detected (Hughes and McKenzie 1987). This resistance development is consistent with the simulated resistance curve of starting resistance level of 0.0001%, which had ~78% resistance after 4 years. The gene frequency for resistance to some pesticides in natural populations may be much higher than 0.001% at the start, owing to frequent use of those pesticides over the last decade.
Rose Vineer (2020) examined the benefits of modelling parasite resistance and noted that a critical aspect is the size of the population in refugia, not exposed to the pesticide. For example, although a rare occurrence, Lucilia cuprina has been reported to breed off sheep and on possum carcasses (Lang et al. 2006), and the model accounts for this. Increasing the unselected flies multiplier increases the number of flies reproducing off treated sheep. As a result, increasing the unselected flies multiplier decreased resistance development, because the flies reproducing off treated sheep are not exposed to the chemical. An unselected flies multiplier of 1 indicates that 1% of the flies in the population are breeding without exposure to pesticide. The extremely low number of L. cuprina reported to emerge from goat or sheep carcasses (Cook et al. 1995) suggests that this may be the most appropriate value when all sheep on a property are treated. However, this may underestimate the proportion of flies that are able to reproduce without exposure to pesticide, and a much higher proportion of flies breeding without such exposure would slow the rate of development of resistance. There will be some flies from neighbouring properties, but if all properties in the area have a similar risk, then the incoming flies are likely to have pesticide exposure similar to the local flies. It may be necessary to use studies of gene flow in the fly population to determine the correct value.
Rotations
In the absence of assumed fitness effects, implementing a rotation using both P1 and P2 reduced the rate of development of resistance compared with continuous use of P1 alone. However, the result was the loss of both P1 and P2 in 6 years, instead of the loss of P1 in 3 years and then P2 in the following 3 years. Therefore, the final result over 6 years was similar, with resistance to both products in 6 years, whether or not a strict rotation was used. The type of rotation used did not significantly change the overall rate of resistance development.
A fitness disadvantage allowed P1 susceptibility to recover over the years P2 was in use. However, even with a fitness disadvantage, the final result was similar, whether based on continuous use or annual rotation. Nevertheless, rotation of products has the benefit of using an effective product every year in the early years, rather than waiting until a product becomes ineffective before changing to another product. This should provide a slightly better economic result because of better fly control for longer in the first half of the period considered here.
There is limited literature concerning the fitness disadvantage in fly pesticide resistance development, although (Yen et al. 1996) quantified lethality of some genes in blowfly for resistance to cyromazine. This chemical has been used for flystrike control for the past 40 years, and resistance has only recently developed of the level seen for its insecticide counterparts within a shorter time frame (Levot 2001, 2013). Tang et al. (2002) reported cyromazine resistance in houseflies, with some loss of fitness, and a loss of fitness in houseflies resistant to other pesticides has also been found (Shah et al. 2015, 2017).
Fitness disadvantage proved to be an important factor in the viability of rotations. Rotations were most beneficial when there was a fitness disadvantage, which allowed P1 susceptibility to recover when it was not in use. Regardless of whether a rotation was used, a fitness disadvantage prevented high levels of resistance developing. These results could explain why cyromazine resistance has not reached high levels, despite its being in use for >40 years (Yen et al. 1996).
Two treatments per year resulted in greater resistance development than one treatment per year. With two treatments each year, there are only short periods when flies can reproduce without exposure to concentrations of pesticide that are lethal to susceptible flies. This makes it much more difficult to find a management system that does not rapidly increase resistance, even if rotations are used within each year to limit exposure to a single product.
This study did not include genes that provide resistance to more than one product, although that is permitted by the model and known to occur for dicyclanil and cyromazine (Levot and Sales 2004), and possibly for both of those products with imidacloprid (Kotze et al. 2022). Where cross-resistance occurs, the use of rotations to delay the development of resistance will be much more difficult, owing to the limited range of products available.
The use of pesticide rotations and other methods to delay the development of resistance may only postpone for a few years the inevitable loss of most of the currently available treatments. In the long term, breeding sheep for resistance to flystrike may be the only viable option (James 2006; Greeff et al. 2014; Hatcher and Preston 2015). Pesticide resistance management may be considered an interim measure to allow preventive methods to continue to be used during years when the risk of flystrike is unusually high. Where the risk of strike is relatively low, treatment can be limited to individual struck sheep, provided that monitoring is frequent enough to allow early detection of those affected by strike (Grant et al. 2019).
Conclusions
Resistance may increase over time, without becoming obvious until it is at levels that are difficult to control. In the long term, wool producers may be unable to rely on current methods of preventive treatment every year. Non-pesticide methods must be used where possible, including breeding sheep for resistance to flystrike.
This study has shown that fitness disadvantage is an important determinant of resistance development, and further research should be conducted to determine whether other chemicals besides cyromazine have this benefit. The model could be used to examine other management changes that could reduce the rate of development of resistance of blowflies to pesticides, such as the use of shearing or crutching during the fly season to reduce the need for repeated pesticide application.
Data availability
The program is available under licence for researchers and can be used to replicate the results provided here. Applications should be made to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Funding for this work was provided by Australian Wool Innovation Ltd. AWI is grateful for its funding, which is primarily provided by Australian woolgrowers through a wool levy and by the Australian Government, which provides a matching contribution for eligible R&D activities.
References
Benedetti Vallenari P (2021) The development of a model for flystrike resistance management. Honours Thesis, University of Tasmania, Launceston, Tas., Australia.Campbell N, Horton B (2002) WoolRes: a model to assist producers to meet market requirements for low-residue wool. Wool Technology and Sheep Breeding 50, 632–637.
Colditz IG, Walkden-Brown SW, Daly BL, Crook BJ (2005) Some physiological responses associated with reduced wool growth during blowfly strike in Merino sheep. Australian Veterinary Journal 83, 695–699.
| Some physiological responses associated with reduced wool growth during blowfly strike in Merino sheep.Crossref | GoogleScholarGoogle Scholar |
Colvin AF, Reeve I, Kahn LP, Thompson LJ, Horton BJ, Walkden-Brown SW (2022) Australian surveys on incidence and control of blowfly strike in sheep between 2003 and 2019 reveal increased use of breeding for resistance, treatment with preventative chemicals and pain relief around mulesing. Veterinary Parasitology: Regional Studies and Reports 31, 100725
| Australian surveys on incidence and control of blowfly strike in sheep between 2003 and 2019 reveal increased use of breeding for resistance, treatment with preventative chemicals and pain relief around mulesing.Crossref | GoogleScholarGoogle Scholar |
Cook DF, Steiner EC, Watson I, Dadour IR (1995) Do Australian sheep blowflies, Lucilia cuprina (Diptera: Calliphoridae), breed in either goat or sheep carcasses in a semi-arid environment? The Rangeland Journal 17, 186–192.
| Do Australian sheep blowflies, Lucilia cuprina (Diptera: Calliphoridae), breed in either goat or sheep carcasses in a semi-arid environment?Crossref | GoogleScholarGoogle Scholar |
Grant EP, Wickham SL, Anderson F, Barnes AL, Fleming PA, Miller DW (2019) Remote identification of sheep with flystrike using behavioural observations. Animals 9, 368–384.
| Remote identification of sheep with flystrike using behavioural observations.Crossref | GoogleScholarGoogle Scholar |
Greeff JC, Karlsson LJE, Schlink AC (2014) Identifying indicator traits for breech strike in Merino sheep in a Mediterranean environment. Animal Production Science 54, 125–140.
| Identifying indicator traits for breech strike in Merino sheep in a Mediterranean environment.Crossref | GoogleScholarGoogle Scholar |
Hatcher S, Preston JWV (2015) Genetic parameters for breech cover, wrinkle and wool coverage scores and their implications for Merino sheep breeding programs and flock management. Small Ruminant Research 130, 36–46.
| Genetic parameters for breech cover, wrinkle and wool coverage scores and their implications for Merino sheep breeding programs and flock management.Crossref | GoogleScholarGoogle Scholar |
Heath ACG, Levot GW (2015) Parasiticide resistance in flies, lice and ticks in New Zealand and Australia: mechanisms, prevalence and prevention. New Zealand Veterinary Journal 63, 199–210.
| Parasiticide resistance in flies, lice and ticks in New Zealand and Australia: mechanisms, prevalence and prevention.Crossref | GoogleScholarGoogle Scholar |
Horton B, Hogan L (2010) FlyBoss: a web-based flystrike information and decision support system. Animal Production Science 50, 1069–1076.
| FlyBoss: a web-based flystrike information and decision support system.Crossref | GoogleScholarGoogle Scholar |
Horton BJ, Corkrey R, Doughty AK (2018) Sheep death and loss of production associated with flystrike in mature Merino and crossbred ewes. Animal Production Science 58, 1289–1296.
| Sheep death and loss of production associated with flystrike in mature Merino and crossbred ewes.Crossref | GoogleScholarGoogle Scholar |
Hughes PB, McKenzie JA (1987) Insecticide resistance in the Australian sheep blowfly, Lucilia cuprina: speculation, science and strategies. In ‘Combating resistance to xenobiotics’. (Eds MG Ford, DW Holloman, BPS Khambay, RM Sawicki) pp. 162–177. (Ellis Horwood: Chichester, UK)
James PJ (2006) Genetic alternatives to mulesing and tail docking in sheep: a review. Australian Journal of Experimental Agriculture 46, 1–18.
| Genetic alternatives to mulesing and tail docking in sheep: a review.Crossref | GoogleScholarGoogle Scholar |
Kotze AC, James PJ (2022) Control of sheep flystrike: what’s been tried in the past and where to from here. Australian Veterinary Journal 100, 1–19.
| Control of sheep flystrike: what’s been tried in the past and where to from here.Crossref | GoogleScholarGoogle Scholar |
Kotze AC, Bagnall NH, Ruffell AP, George SD, Rolls NM (2022) Resistance to dicyclanil and imidacloprid in the sheep blowfly, Lucilia cuprina, in Australia. Pest Management Science 78, 4195–4206.
| Resistance to dicyclanil and imidacloprid in the sheep blowfly, Lucilia cuprina, in Australia.Crossref | GoogleScholarGoogle Scholar |
Lang MD, Allen GR, Horton BJ (2006) Blowfly succession from possum (Trichosurus vulpecula) carrion in a sheep-farming zone. Medical and Veterinary Entomology 20, 445–452.
| Blowfly succession from possum (Trichosurus vulpecula) carrion in a sheep-farming zone.Crossref | GoogleScholarGoogle Scholar |
Levot G (2001) Implications of insecticide resistance for the control of flystrike and lice on sheep. In ‘Proceedings of the FLICS Conference, Launceston, Tasmania’. (Ed. S Champion) pp. 127–134. (Tasmanian Institute of Agricultural Research, University of Tasmania: Launceston, Tasmania)
Levot GW (2013) Response to laboratory selection with cyromazine and susceptibility to alternative insecticides in sheep blowfly larvae from the New South Wales Monaro. Australian Veterinary Journal 91, 61–64.
| Response to laboratory selection with cyromazine and susceptibility to alternative insecticides in sheep blowfly larvae from the New South Wales Monaro.Crossref | GoogleScholarGoogle Scholar |
Levot G, Sales N (2004) Insect growth regulator cross-resistance studies in field- and laboratory-selected strains of the Australian sheep blowfly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Australian Journal of Entomology 43, 374–377.
| Insect growth regulator cross-resistance studies in field- and laboratory-selected strains of the Australian sheep blowfly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae).Crossref | GoogleScholarGoogle Scholar |
Levot GW, Langfield BJ, Aiken DJ (2014) Survival advantage of cyromazine-resistant sheep blowfly larvae on dicyclanil- and cyromazine-treated Merinos. Australian Veterinary Journal 92, 421–426.
| Survival advantage of cyromazine-resistant sheep blowfly larvae on dicyclanil- and cyromazine-treated Merinos.Crossref | GoogleScholarGoogle Scholar |
Lucas P, Horton B (2013) Comparative costs, chemical treatments and flystrike rates in mulesed and unmulesed sheep flocks as predicted by a weather-driven model. Animal Production Science 53, 342–351.
| Comparative costs, chemical treatments and flystrike rates in mulesed and unmulesed sheep flocks as predicted by a weather-driven model.Crossref | GoogleScholarGoogle Scholar |
Miko I (2008) Genetic dominance: genotype–phenotype relationships. Nature Education 1, 140
Percival V, Horton B (2014) Use of a threshold of flystrike risk as a method for treatment intervention in the management of flystrike in sheep. Animal Production Science 54, 308–318.
| Use of a threshold of flystrike risk as a method for treatment intervention in the management of flystrike in sheep.Crossref | GoogleScholarGoogle Scholar |
Rose Vineer H (2020) What modeling parasites, transmission, and resistance can teach us. Veterinary Clinics of North America: Food Animal Practice 36, 145–158.
| What modeling parasites, transmission, and resistance can teach us.Crossref | GoogleScholarGoogle Scholar |
Sales N, Suann M, Koeford K (2020) Dicyclanil resistance in the Australian sheep blowfly, Lucilia cuprina, substantially reduces flystrike protection by dicyclanil and cyromazine based products. International Journal for Parasitology: Drugs and Drug Resistance 14, 118–125.
| Dicyclanil resistance in the Australian sheep blowfly, Lucilia cuprina, substantially reduces flystrike protection by dicyclanil and cyromazine based products.Crossref | GoogleScholarGoogle Scholar |
Shah RM, Shad SA, Abbas N (2015) Mechanism, stability and fitness cost of resistance to pyriproxyfen in the house fly, Musca domestica L. (Diptera: Muscidae). Pesticide Biochemistry and Physiology 119, 67–73.
| Mechanism, stability and fitness cost of resistance to pyriproxyfen in the house fly, Musca domestica L. (Diptera: Muscidae).Crossref | GoogleScholarGoogle Scholar |
Shah RM, Shad SA, Abbas N (2017) Methoxyfenozide resistance of the housefly, Musca domestica L. (Diptera: Muscidae): cross-resistance patterns, stability and associated fitness costs. Pest Management Science 73, 254–261.
| Methoxyfenozide resistance of the housefly, Musca domestica L. (Diptera: Muscidae): cross-resistance patterns, stability and associated fitness costs.Crossref | GoogleScholarGoogle Scholar |
Shephard R, Webb Ware J, Blomfield B, Niethe G (2022) Priority list of endemic diseases for the red meat industry – 2022 update. Meat & Livestock Australia, North Sydney, NSW, Australia.
Tang JD, Caprio MA, Sheppard DC, Gaydon DM (2002) Genetics and fitness costs of cyromazine resistance in the house fly (Diptera: Muscidae). Journal of Economic Entomology 95, 1251–1260.
| Genetics and fitness costs of cyromazine resistance in the house fly (Diptera: Muscidae).Crossref | GoogleScholarGoogle Scholar |
Tellam RL, Bowles VM (1997) Control of blowfly strike in sheep: current strategies and future prospects. International Journal for Parasitology 27, 261–273.
| Control of blowfly strike in sheep: current strategies and future prospects.Crossref | GoogleScholarGoogle Scholar |
Wardhaugh KG, Morton R, Bedo D, Horton BJ, Mahon RJ (2007) Estimating the incidence of fly myiases in Australian sheep flocks: development of a weather-driven regression model. Medical and Veterinary Entomology 21, 153–167.
| Estimating the incidence of fly myiases in Australian sheep flocks: development of a weather-driven regression model.Crossref | GoogleScholarGoogle Scholar |
Yen YL, Batterham P, Gelder B, McKenzie JA (1996) Predicting resistance and managing susceptibility to cyromazine in the Australian sheep blowfly Lucilia cuprina. Australian Journal of Experimental Agriculture 36, 413–420.
| Predicting resistance and managing susceptibility to cyromazine in the Australian sheep blowfly Lucilia cuprina.Crossref | GoogleScholarGoogle Scholar |


