Effects of Tremella fuciformis extract on growth performance, biochemical and immunological parameters of weaned piglets challenged with lipopolysaccharide
Linlin Qin A , Guoqi Su B , Cheng Wu A , Qiang Zhou A , Xie Peng A , Liang Hu A , Yang Liu A , Ru Wang A , Qin Xu A , Zhengfeng Fang A , Yan Lin A , Shengyu Xu A , Bin Feng A , Jian Li A , De Wu A and Lianqiang Che
B , Cheng Wu A , Qiang Zhou A , Xie Peng A , Liang Hu A , Yang Liu A , Ru Wang A , Qin Xu A , Zhengfeng Fang A , Yan Lin A , Shengyu Xu A , Bin Feng A , Jian Li A , De Wu A and Lianqiang Che  A *
A *
A Key Laboratory for Animal Disease Resistance Nutrition of the Ministry of Education, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu 611130, China.
B Chongqing Academy of Animal Science, Rongchang, Chongqing, China.
Animal Production Science 62(5) 462-469 https://doi.org/10.1071/AN20425
Submitted: 6 August 2020 Accepted: 29 November 2021 Published: 1 February 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: In-feed antibiotics are commonly used to improve growth and gut health of weaning pigs. Due to anti-microbial resistance by extensively using antibiotics, however, in-feed antibiotics have been banned in Europe and China. Tremella fuciformis is a traditional edible fungus in China. Recent studies have found that Tremella fuciformis extract (TFE) has anti-inflammatory, anti-cancer and immune-modulatory functions. Therefore, there is the potential to develop Tremella fuciformis as an alternative to antibiotics.
Aims: The study was performed to explore the effects of TFE on growth performance, and biochemical and immunological parameters of weaned piglets under lipopolysaccharide (LPS) challenge.
Methods: Forty-eight weaned piglets were assigned into two groups with six pens (four piglets per-pen), receiving a control diet or a control diet with 400 mg/kg TFE (TFE), respectively. After 28 days of the trial, two piglets per pen were selected to be injected with LPS (50 μg/kg of BW) or an equivalent amount of sterile saline. Blood samples were collected at 0 and 3 h after LPS challenge.
Key results: The results showed that TFE supplementation significantly increased the average daily gain (P < 0.05) and decreased the faecal score (P < 0.05) during the first week, improved the feed conversion ratio (P < 0.05) and BWt gain (P < 0.05) during the whole period. Piglets fed the TFE diet had higher plasma levels of white blood cells (P < 0.05) than that of piglets fed the control diet diet before the LPS challenge. Regardless of the dietary treatment, the LPS challenge significantly decreased the level of white blood cells, and increased the levels of red blood cells, haemoglobin, haematocrit, total protein, interleukin-1β and tumour necrosis factor-α (all P < 0.05). Regardless of the LPS challenge, however, the concentrations of total protein, interleukin-1β and tumour necrosis factor-α were decreased (all P < 0.05) in the plasma of piglets fed the TFE diet compared with the control diet diet.
Conclusions: In summary, the supplementation of TFE in the weaning diet could improve the growth performance and immunity of piglets.
Implication: TFE could be used as a bioactive substance for improving growth and immune response in pig production.
Keywords: growth, immune, inflammation, lipopolysaccharide, pigs, polysaccharides, Tremella fuciformis extract, weaning.
Introduction
Early weaning is one of the most stressful events that induces intestinal and immunological dysfunction, which impairs growth and immune responses (Pluske et al. 1997; Smith et al. 2010). It has been reported that polysaccharides from Chinese medicinal herbs could improve the growth performance and health status of piglets (Yuan et al. 2006; Kang et al. 2010).
Tremella fuciformis, classified as the order of the Tremellales and the family of the Tremellaceae, has been appreciated as an edible mushroom in Asia (Cho et al. 2006). The polysaccharides from T. fuciformis (TPS) have been regarded as the primary active component. For example, TPS could inhibit cyclophosphamide-induced leucopenia in mice, and leucocytes increased in a dose-dependent fashion (Jiang et al. 2012). Nitric oxide synthase (iNOS), interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α were also upregulated by Tremella fuciformis extract (TFE) when cultured in RAW264.7 (Han et al. 2015). Meanwhile, TFE suppressed lipopolysaccharide (LPS)-induced production of iNOS and COX-2 in RAW264.7, and oral administration of TFE significantly inhibited LPS-induced production of IL-1β, IL-6 and TNF-α, and expressions of iNOS and COX-2 (Lee et al. 2016).
It has been found that the Tremella fuciformis polysaccharides was composed of α-d-mannose in the main chain, and β-d-xylose, β-d-gluconic acid and β-d-xylobiose linked to the C-2 of the main chain mannose (Yui et al. 1995). Another analysis on Tremella fuciformis polysaccharides, named TL04, was composed of (1→2)-and (1→4)-linked-mannose, and (1→3)-linked-glucans (Jin et al. 2016). d-Mannose has been proven to have beneficial effects on the immune system and against metabolic syndrome (Hu et al. 2016); in particular, d-Mannose could strongly inhibit the attachment of ST-10 Salmonella typhimurium to intestinal cells and offer a competitive binding site for this class of bacteria (Hu et al. 2016). Although the sugar composition and relative molecular ratios of T. fuciformis polysaccharides varied among studies, the bioactive effects are similar, such as anti-inflammatory and immunomodulatory effects (Wu et al. 2019). However, data are limited about the effects of T. fuciformis extract (TFE) on growth performance, immune response and blood biochemistry of weaned piglets.
Therefore, this study was performed to determine the effects of Tremella fuciformis extract on growth performance, and biochemical and immunological parameters of weaned piglets under LPS challenge.
Materials and methods
Preparation of TFE
The TFE was a mixture of T. fuciformis spore liquid fermentation broth and carrier wheat bran, which was produced by our laboratory. The preserved strains were streamed and inoculated on potato dextrose agar solid medium in a thermostatic chamber at 25°C for 4 days. A single colony was picked and inoculated in a 500-mL culture flask containing 200 mL of potato dextrose agar medium with an inoculation ring, and cultured in an incubator shaker at 25°C/220 rpm for 4 days to obtain the seed fungus liquid. The seed fungus liquid was inoculated in sterilised potato dextrose agar liquid fermentation medium with 10% inoculation amount and cultured at 25°C/220 rpm for 7 days until the fermentation broth contained up to 5 × 108 T. fuciformis spore per mL. The T. fuciformis spore liquid fermentation broth was mixed with wheat bran (SICHUAN Giastar Group) in a ratio of 5:1, dried at 60°C in a drying oven, then grounded into powder and screened through a 0.178-mm mesh screen.
Experimental design and animal management
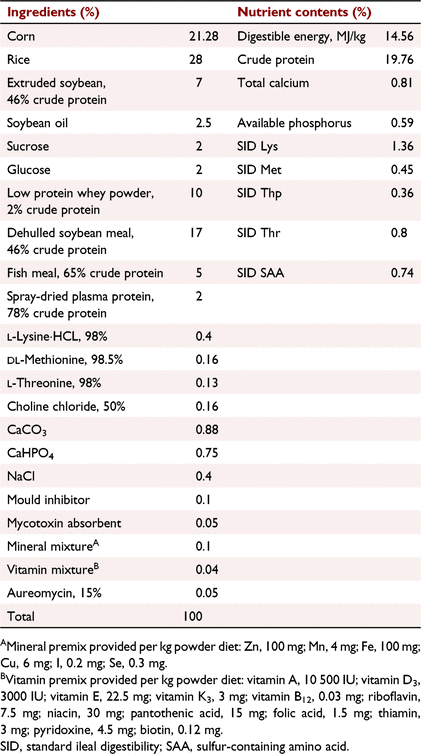
As shown in Fig. 1, a total of 48 piglets (Duroc × Landrace × Yorkshire) weaned at 21 days (BWt 6.40 ± 0.17 kg) were randomly assigned into two groups, with six pens and four piglets per pen at 21 days, receiving a control diet (CON diet) or control diet with 400 mg/kg TFE (TFE diet) for a period of 28 days. Diets were formulated according to the nutrient requirements of swine recommended by the American National Research Council. The feed ingredients (SICHUAN Giastar Group) and nutrient composition of the experimental diets are shown in Table 1. Diets were fed in mash form throughout the experiment. On Day 29, after an overnight fast, two piglets (one challenged with LPS, the other treated with sterile saline) were selected according to their BWs near the average BW of each pen. No diarrhoea or other disease were observed. The selected piglets were intramuscularly injected with LPS (Escherichia coli serotype 055: B5, Sigma Chemical) at 50 μg/kg BW, and another piglet was injected with the same amount of sterile saline. The temperature of the feeding room was maintained between 26 and 30°C, and the humidity was maintained between 50 and 60%. The 12 h of light and 12 h of dark were provided in the stall. Piglets had free access to water and feed, and feed intake was recorded daily.
|
|
|
The experimental protocol was approved by the Ethics Committee of Feed Research Institute, Sichuan Agricultural University, China.
Rectum digesta
On Day 28, fresh faeces were collected from two piglets without disease and diarrhoea in each pen. Faecal samples were collected in duplicate into sterile tubes and then stored at −80°C for analysis of volatile fatty acids (VFA).
Blood sample
On Day 29, approximately 10 mL of blood samples were collected via the anterior vena cava puncture at 0 and 3 h after challenge, respectively, then injected into EDTA-Na2 and sodium heparin tubes, respectively. The vacuum tubes of EDTA-Na2 were immediately placed on ice for analysis of blood biochemistry. Blood samples with sodium heparin were centrifuged (3000g, 15 min, 4°C) to obtain plasma samples, and stored at −80°C for further analysis (Su et al. 2020).
Growth performance
Individual piglet BW was measured, then average daily feed intake, average daily gain (ADG) and feed:gain ratio (F:G) were calculated.
Faecal score
Piglets were observed for clinical signs of diarrhoea from Day 1 to Day 28 of the experiment, and a scoring system was applied to indicate the diarrhoea severity. The following scoring system was used: 0, normal; 1, pasty; 2, semi-liquid; and 3, watery. Piglets with a faecal score of ≤1 were considered to not have diarrhoea (Bhandari et al. 2008). Scores were evaluated daily for individual pens, and the average faecal score per piglet was calculated.
Haematological and biochemical parameters
Haematological parameters, including white blood cells (WBC), red blood cells (RBC), haemoglobin, haematocrit and platelets (PLT), were analysed by an automatic biochemical analyser (model BC-2800vet, Mindray, Yaan, China). The serum concentrations of complement C3, complement C4, immunoglobulin G (IgG), immunoglobulin M (IgM), C-reactive protein (CRP), total protein (TP), albumin (ALB), alanine aminotransferase (ALT) and aspartate amino transferase (AST) were detected using a Hitachi 7020 Automatic Analyser (Tokyo, Japan) with the assay kits (Sichuan Maker Biotechnology Co. Ltd, Chengdu, China), according to the method of Su et al.(2018). Moreover, TNF-α and IL-1β were analysed by a commercially available swine ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer’s instructions.
VFA
The VFA concentrations of rectum digesta were measured with a gas chromatographic method according to our previous study (Pena et al. 2013). The thawed digesta samples (1 g) were suspended in 2 mL of distilled water in a screw-capped tube, then vortexed and centrifuged at 12 000g for 10 min at 4°C. Then, 1 mL of supernatant was transferred to 1.5-mL PE centrifuge tubes and mixed with 0.2 mL of metaphosphoric acid, and incubated at 4°C for 30 min, then the mixture was centrifuged at 12 000g for 10 min at 4°C; 1 μL of supernatant was used to analyse VFA by Varian CP-3800 gas chromatograph (Agilent Technologies). A flame ionisation detector was used at an oven temperature of 100–150°C. The polyethylene glycol column was operated with highly purified N2, as the carrier gas, at 1.8 mL/min. The detectable limit for all VFA was 0.1 mmol/L.
Statistical analyses
The pen was considered as the experimental unit for data about growth performance and VFA. The data about growth performance and VFA was performed using the t-test procedure (SAS 9.0). The data were expressed as means ± standard deviations. The data of haematological parameters were analysed using the general linear model procedures of SAS appropriate for a 2 × 2 factorial design (SAS 9.0). The statistical model included the effects of challenge (saline or LPS), diet (CON or TFE) and their interactions. The variability of all the data were expressed as the pooled standard error of the mean. Differences were considered as significant when P < 0.05, and a tendency was recognised when P < 0.10.
Results
Components of TFE
The TFE contained ∼200 mg polysaccharides per gram detected using anthrone colourimetry, as before (Wu et al. 2007). The monosaccharide composition of polysaccharides was measured by gas chromatography–mass spectrometry (model 7890a-5975c, Agilent), as detailed in a previous study (Wahjudi et al. 2010), which was composed of 0.56% arabinose, 0.86% fucose, 1.31% xylose, 1.36% galactose, 10.10% mannose and 85.50% glucose.
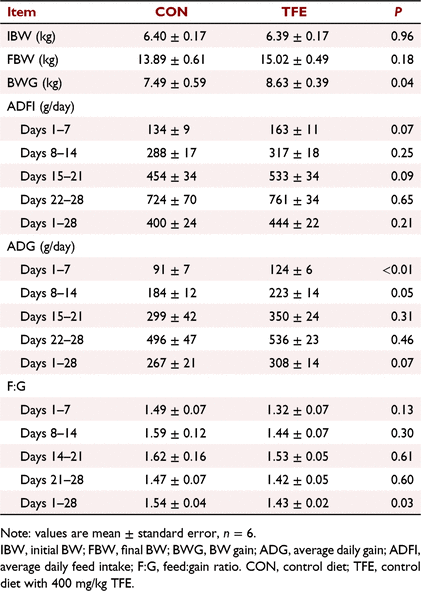
Effect of TFE on growth performance
The effects of TFE on growth performance of weaned piglets are shown in Table 2. TFE supplementation significantly increased ADG (P = 0.006) and BW gain (P = 0.04) from Day 1 to Day 7, and decreased F:G (P = 0.03) during the whole experimental period compared with CON. The ADG of piglets fed the TFE diet tended to increase during Day 8 to Day 14 and the whole experimental period (P = 0.05–0.07) compared with piglets fed the CON diet. Piglets fed the TFE diet tended to have higher average daily feed intake than piglets fed the CON diet during Day 1 to Day 7 and Day 15 to Day 21 (P = 0.07–0.09).
|
Effect of TFE on faecal score
In Fig. 2, the faecal score of piglets fed the TFE diet was significantly decreased during Day 1–7 (P = 0.02) compared with piglets fed the CON diet, and tended to be decreased during the whole experimental period (P = 0.08).
Effects of TFE on VFA
In Fig. 3, there was no significant difference on acetic acid (P = 0.55), propionic acid (P = 0.93) and butyric acid (P = 0.88) in the rectal digesta between piglets fed the TFE and CON diet.
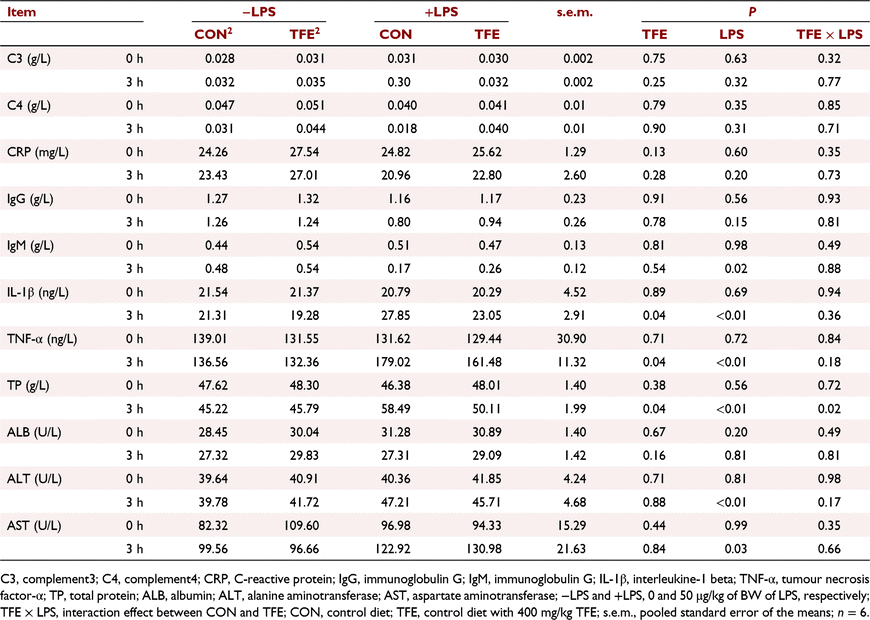
Effects of TFE on serum biochemistry
As shown in Table 3, LPS challenge significantly increased the concentrations of IL-1β and TNF-α, and decreased the concentration of immunoglobulin M in plasma (all P < 0.05) at 3 h post-challenge. However, piglets fed the TFE diet showed significantly decreased the concentrations of IL-1β (P = 0.04) and TNF-α (P = 0.04), compared with piglets fed the CON diet. The LPS challenge markedly increased concentrations of TP (P = 0.04), ALT (P = 0.04) and AST (P = 0.04) in the plasma of piglets, compared with piglets without LPS challenge. However, piglets fed the TFE diet had markedly lower concentrations of TP (P = 0.04) compared with piglets fed CON diet at 3 h post-challenge. Moreover, LPS challenge × diet interaction for TP was observed (P = 0.02).
|
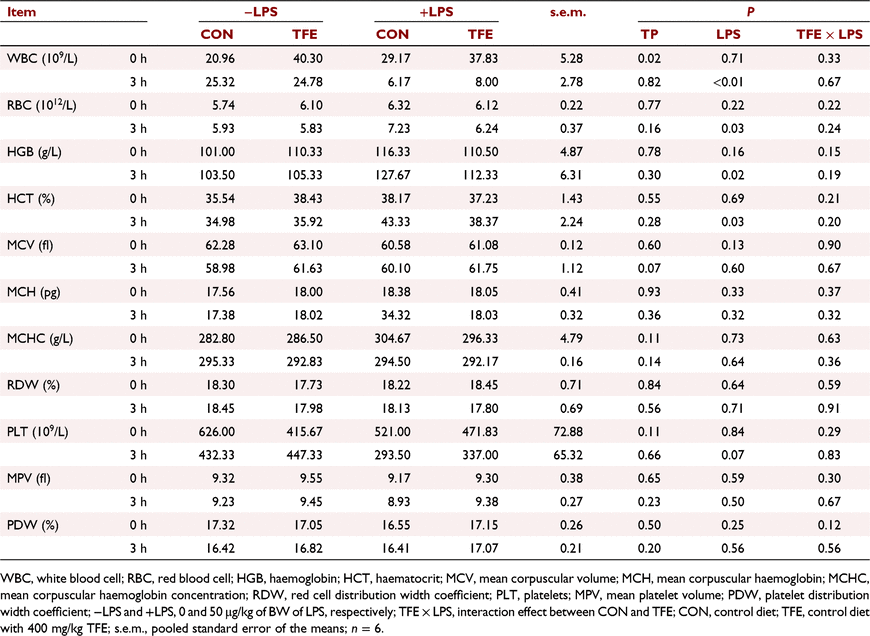
Effects of TFE on haematological parameters
The effects of TFE on haematological parameters are presented in Table 4. The level of WBC was significantly increased in piglets fed the TFE diet compared with piglets fed the CON diet (P = 0.02). At 3 h post-challenge, the level of WBC in piglets challenged with LPS was notably decreased (P < 0.05), whereas the levels of RBC (P = 0.03), haemoglobin (P = 0.02) and haematocrit (P = 0.03) were observably increased compared with piglets without LPS challenge. The level of platelets (P = 0.07) in piglets challenged with LPS tended to increase compared with piglets without LPS.
|
Discussion
In the present study, the effects of TFE supplementation on growth performance of weaned piglets were explored. The results showed that TFE supplementation increased ADG, BW gain and the feed conversion ratio of weaned piglets. The polysaccharide or oligosaccharide by TFE, composed of fucose, xylose, galactose and mannose, could be the main bioactive components to enhance the growth performance of piglets. It has been widely reported that mannan oligosaccharides have beneficial effects on growth performance and nutrient digestibility in weanling pigs (LeMieux et al. 2003; Zhao et al. 2012). A previous study also demonstrated that TFE improved ADG and average daily feed intake in finishing pigs (Ding et al. 2012). In addition, birds fed TFE had better growth performance than the non-supplemented birds, but were not significantly different from those fed virginiamycin (Guo et al. 2004). As an edible mushroom, the supplementation of Pleurotus ostreatus mushroom in the diet of piglets increased feed consumption, gut microbial composition and diversity, as well as short-chain fatty acids synthesis, consequently prevented the occurrence of diarrhoea and increased the growth of piglets (Adams et al. 2019). Various bioactive effects had been observed by the polysaccharides in TFE (Kakuta et al. 1979), the better growth performance of piglets fed TFE may also be related to the beneficial effect of TFE on alleviating weaning stress.
Weaning stress, particularly occurring in the first week post-weaning, leads to excessive inflammation, in which immune activation could distribute the nutrients from muscular growth into the immune system. Futhermore, weaning stress leads to intestinal damage and being more susceptible to pathogen infection (Hu et al. 2015; Wan et al. 2016; Che et al. 2017). TFE contains polysaccharides that are responsible for anti-inflammation and immunomodulation (Jiang et al. 2012; Hu et al. 2016). In agreement with a previous study (Lee et al. 2016), we found T. fuciformis extract could suppress the inflammatory response via reducing the levels of LPS-induced IL-1β, IL-6 and TNF-α. Similarly, Ruan et al. (2018) reported that RAW264.7 cells pre-treated with TFE profoundly inhibited the activation of protein kinase B, p38 mitogen-activated protein kinases and nuclear factor-κB, and attenuated the expression of MCP-1 in macrophages. Meanwhile, TFE also decreased cytokine and reactive oxygen species levels, and attenuated cell inflammation after treatment with LPS. In addition, the lower faecal score of TFE-supplemented piglets in the study further suggested TFE could alleviate the intestinal dysfunction induced by weaning stress.
Haematological parameters are usually common indicators of physical condition in humans and animals (Savran et al. 2020). It has been reported that liver injury was associated with the increased concentration of plasma TP (Wang et al. 2015). In addition, damage to the hepatic function by LPS can be reflected in the increased concentrations of AST and ALT (Hanley et al. 2004; Pan et al. 2015). In our study, LPS challenge increased the levels of ALT, AST and TP in serum, but TFE decreased the level of TP when piglets were challenged with LPS, which indicated that TFE might protect hepatic function from LPS challenge. WBC are essential immune cells associated with the induction of inflammation (Li et al. 2018). Previous studies reported that the level of WBC was decreased, whereas the levels of RBC and HGB were increased after immune challenge (Reiner et al. 2007; Che et al. 2011). Consistently, LPS challenge decreased the level of WBC, and increased the levels of RBC and HGB in this study, which could be partly recovered by supplementing TFE. In addition, the current results showed that the level of WBC was markedly increased by the TFE diet, which is similar to previous studies showing that T. fuciformis polysaccharides enhanced immunity (Wang et al. 1983; Cheung 1996; Reshetnikov et al. 2000). The activation of immune cells may be a positive response to inflammation by protecting the host from pathogenic insult (Kauppinen et al. 2013).
Conclusions
In conclusion, TFE supplementation in the weaning piglet diet exhibited positive effects on ADG, F:G and BW gain, as well as the potential capacity to enhance immune response.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Research funding support is acknowledged from the National Key Research and Development Program of China (2016YFD0501204), and Sichuan provincial project on S&T application and demonstration (2016CC0070).
Acknowledgements
The authors acknowledge Mr Fangqi Wu for providing the strain of T. fuciformis spore.
References
Adams S, Che D, Hailong J, Zhao B, Rui H, Danquah K, Qin G (2019) Effects of pulverized oyster mushroom (Pleurotus ostreatus) on diarrhea incidence, growth performance, immunity, and microbial composition in piglets. Journal of the Science of Food and Agriculture 99, 3616–3627.| Effects of pulverized oyster mushroom (Pleurotus ostreatus) on diarrhea incidence, growth performance, immunity, and microbial composition in piglets.Crossref | GoogleScholarGoogle Scholar | 30628086PubMed |
Bhandari SK, Xu B, Nyachoti CM, Giesting DW, Krause DO (2008) Evaluation of alternatives to antibiotics using an Escherichia coli K88+ model of piglet diarrhea: effects on gut microbial ecology. Journal of Animal Science 86, 836–847.
| Evaluation of alternatives to antibiotics using an Escherichia coli K88+ model of piglet diarrhea: effects on gut microbial ecology.Crossref | GoogleScholarGoogle Scholar | 18192551PubMed |
Che TM, Johnson RW, Kelley KW, Van Alstine WG, Dawson KA, Moran CA, Pettigrew JE (2011) Mannan oligosaccharide improves immune responses and growth efficiency of nursery pigs experimentally infected with porcine reproductive and respiratory syndrome virus. Journal of Animal Science 89, 2592–2602.
| Mannan oligosaccharide improves immune responses and growth efficiency of nursery pigs experimentally infected with porcine reproductive and respiratory syndrome virus.Crossref | GoogleScholarGoogle Scholar | 21454863PubMed |
Che L, Xu Q, Wu C, Luo Y, Huang X, Zhang B, Auclair E, Kiros T, Fang Z, Lin Y, Xu S, Feng B, Li J, Wu D (2017) Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88. British Journal of Nutrition 118, 949–958.
| Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88.Crossref | GoogleScholarGoogle Scholar |
Cheung PCK (1996) The hypocholesterolemic effect of two edible mushrooms: Auricularia auricula (tree-ear) and Tremella fuciformis (white jelly-leaf) in hypercholesterolemic rats. Nutrition Research 16, 1721–1725.
| The hypocholesterolemic effect of two edible mushrooms: Auricularia auricula (tree-ear) and Tremella fuciformis (white jelly-leaf) in hypercholesterolemic rats.Crossref | GoogleScholarGoogle Scholar |
Cho EJ, Oh JY, Chang HY, Yun JW (2006) Production of exopolysaccharides by submerged mycelial culture of a mushroom Tremella fuciformis. Journal of Biotechnology 127, 129–140.
| Production of exopolysaccharides by submerged mycelial culture of a mushroom Tremella fuciformis.Crossref | GoogleScholarGoogle Scholar | 16872706PubMed |
Ding WQ, Jia G, Wang KN (2012) Effects of Tremella fuciformis Berk spore fermentation and probiotic on growth performance and meat quality of growing-finishing pigs. Chinese Journal of Animal Nutrition 24, 1912–1919.
Guo FC, Kwakkel RP, Williams BA, Li WK, Li HS, Luo JY, Li XP, Wei YX, Yan ZT, Verstegen MWA (2004) Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on growth performance of broilers. British Poultry Science 45, 684–694.
| Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on growth performance of broilers.Crossref | GoogleScholarGoogle Scholar | 15623224PubMed |
Han C-K, Chiang H-C, Lin C-Y, Tang C-H, Lee H, Huang D-D, Zeng Y-R, Chuang T-N, Huang Y-L (2015) Comparison of immunomodulatory and anticancer activities in different strains of Tremella fuciformis Berk. The American Journal of Chinese Medicine 43, 1637–1655.
| Comparison of immunomodulatory and anticancer activities in different strains of Tremella fuciformis Berk.Crossref | GoogleScholarGoogle Scholar | 26621447PubMed |
Hanley AJG, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Kempf J, Zinman B, Haffner SM (2004) Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 53, 2623–2632.
| Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study.Crossref | GoogleScholarGoogle Scholar |
Hu L, Liu Y, Yan C, Peng X, Xu Q, Xuan Y, Han F, Tian G, Fang Z, Lin Y, Xu S, Zhang K, Chen D, Wu D, Che L (2015) Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction. British Journal of Nutrition 114, 53–62.
| Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction.Crossref | GoogleScholarGoogle Scholar |
Hu X, Shi Y, Zhang P, Miao M, Zhang T, Jiang B (2016) d-Mannose: properties, production, and applications: an overview. Comprehensive Reviews in Food Science and Food Safety 15, 773–785.
| d-Mannose: properties, production, and applications: an overview.Crossref | GoogleScholarGoogle Scholar | 33401842PubMed |
Jiang R-Z, Wang Y, Luo H-M, Cheng Y-Q, Chen Y-H, Gao Y, Gao Q-P (2012) Effect of the Molecular Mass of Tremella Polysaccharides on Accelerated Recovery from Cyclophosphamide-Induced Leucopenia in Rats. Molecules 17, 3609–3617.
| Effect of the Molecular Mass of Tremella Polysaccharides on Accelerated Recovery from Cyclophosphamide-Induced Leucopenia in Rats.Crossref | GoogleScholarGoogle Scholar | 22447024PubMed |
Jin Y, Hu X, Zhang Y, Liu T (2016) Studies on the purification of polysaccharides separated from Tremella fuciformis and their neuroprotective effect. Molecular Medicine Reports 13, 3985–3992.
| Studies on the purification of polysaccharides separated from Tremella fuciformis and their neuroprotective effect.Crossref | GoogleScholarGoogle Scholar | 27035561PubMed |
Kakuta M, Sone Y, Umeda T, Misaki A (1979) Comparative structural studies on acidic heteropolysaccharides isolated from “shirokikurage,” fruit body of Tremella fuciformis Berk and the growing culture of its yeast-like cells. Agricultural and Biological Chemistry 43, 1659–1668.
| Comparative structural studies on acidic heteropolysaccharides isolated from “shirokikurage,” fruit body of Tremella fuciformis Berk and the growing culture of its yeast-like cells.Crossref | GoogleScholarGoogle Scholar |
Kang P, Xiao HL, Hou YQ, Ding BY, Liu YL, Zhu HL, Hu QZ, Hu Y, Yin YL (2010) Effects of astragalus polysaccharides, Achyranthes bidentata polysaccharides, and Acantbepanax senticosus saponin in the performance and immunity in weaned pigs. Asian-Australasian Journal of Animal Sciences 23, 750–756.
| Effects of astragalus polysaccharides, Achyranthes bidentata polysaccharides, and Acantbepanax senticosus saponin in the performance and immunity in weaned pigs.Crossref | GoogleScholarGoogle Scholar |
Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A (2013) Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cellular Signalling 25, 1939–1948.
| Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.Crossref | GoogleScholarGoogle Scholar | 23770291PubMed |
Lee J, Ha SJ, Lee HJ, Kim MJ, Kim JH, Kim YT, Song K-M, Kim Y-J, Kim HK, Jung SK (2016) Protective effect of Tremella fuciformis Berk extract on LPS-induced acute inflammation via inhibition of the NF-κB and MAPK pathways. Food & Function 7, 3263–3272.
| Protective effect of Tremella fuciformis Berk extract on LPS-induced acute inflammation via inhibition of the NF-κB and MAPK pathways.Crossref | GoogleScholarGoogle Scholar |
LeMieux FM, Southern LL, Bidner TD (2003) Effect of mannan oligosaccharides on growth performance of weanling pigs. Journal of Animal Science 81, 2482–2487.
| Effect of mannan oligosaccharides on growth performance of weanling pigs.Crossref | GoogleScholarGoogle Scholar | 14552375PubMed |
Li Y, Kakkar R, Wang J (2018) In vivo and in vitro approach to anti-arthritic and anti-inflammatory effect of crocetin by alteration of nuclear factor-E2-related factor 2/hem oxygenase (HO)-1 and NF-κB expression. Frontiers in Pharmacology 9, 1341
| In vivo and in vitro approach to anti-arthritic and anti-inflammatory effect of crocetin by alteration of nuclear factor-E2-related factor 2/hem oxygenase (HO)-1 and NF-κB expression.Crossref | GoogleScholarGoogle Scholar | 30618728PubMed |
Pan C-W, Zhou G-Y, Chen W-L, Zhuge L, Jin L-X, Zheng Y, Lin W, Pan Z-Z (2015) Protective effect of forsythiaside A on lipopolysaccharide/d-galactosamine-induced liver injury. International Immunopharmacology 26, 80–85.
| Protective effect of forsythiaside A on lipopolysaccharide/d-galactosamine-induced liver injury.Crossref | GoogleScholarGoogle Scholar | 25797347PubMed |
Pena OM, Afacan N, Pistolic J, Chen C, Madera L, Falsafi R, Fjell CD, Hancock REW (2013) Synthetic cationic peptide IDR-1018 modulates human macrophage differentiation. PLoS ONE 8, e52449
| Synthetic cationic peptide IDR-1018 modulates human macrophage differentiation.Crossref | GoogleScholarGoogle Scholar | 23308112PubMed |
Pluske JR, Hampson DJ, Williams IH (1997) Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livestock Production Science 51, 215–236.
| Factors influencing the structure and function of the small intestine in the weaned pig: a review.Crossref | GoogleScholarGoogle Scholar |
Reiner G, Fischer R, Hepp S, Berge T, Köhler F, Willems H (2007) Quantitative trait loci for red blood cell traits in swine. Animal Genetics 38, 447–452.
| Quantitative trait loci for red blood cell traits in swine.Crossref | GoogleScholarGoogle Scholar | 17627803PubMed |
Reshetnikov SV, Wasser SP, Nevo E, Duckman I, Tsukor K (2000) Medicinal value of the genus Tremella Pers. (Heterobasidiomycetes). International Journal of Medicinal Mushrooms 2, 26
| Medicinal value of the genus Tremella Pers. (Heterobasidiomycetes).Crossref | GoogleScholarGoogle Scholar |
Ruan Y, Li H, Pu L, Shen T, Jin Z (2018) Tremella fuciformis polysaccharides attenuate oxidative stress and inflammation in macrophages through miR-155. Analytical Cellular Pathology 2018, 5762371
| Tremella fuciformis polysaccharides attenuate oxidative stress and inflammation in macrophages through miR-155.Crossref | GoogleScholarGoogle Scholar |
Savran M, Aslankoc R, Ozmen O, Erzurumlu Y, Savas HB, Temel EN, Kosar PA, Boztepe S (2020) Agomelatine could prevent brain and cerebellum injury against LPS-induced neuroinflammation in rats. Cytokine 127, 154957
| Agomelatine could prevent brain and cerebellum injury against LPS-induced neuroinflammation in rats.Crossref | GoogleScholarGoogle Scholar | 31869757PubMed |
Smith F, Clark JE, Overman BL, Tozel CC, Huang JH, Rivier JEF, Blikslager AT, Moeser AJ (2010) Early weaning stress impairs development of mucosal barrier function in the porcine intestine. American Journal of Physiology Gastrointestinal and Liver Physiology 298, G352–G363.
| Early weaning stress impairs development of mucosal barrier function in the porcine intestine.Crossref | GoogleScholarGoogle Scholar | 19926814PubMed |
Su G, Zhou X, Wang Y, Chen D, Chen G, Yan L (2018) Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs. Lipids in Health and Disease 17, 139
| Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs.Crossref | GoogleScholarGoogle Scholar | 29903022PubMed |
Su G, Zhou X, Wang Y, Chen D, Chen G, Li Y, He J (2020) Dietary supplementation of plant essential oil improves growth performance, intestinal morphology and health in weaned pigs. Journal of Animal Physiology and Animal Nutrition 104, 579–589.
| Dietary supplementation of plant essential oil improves growth performance, intestinal morphology and health in weaned pigs.Crossref | GoogleScholarGoogle Scholar | 31854008PubMed |
Wahjudi PN, Patterson ME, Lim S, Yee JK, Mao CS, Lee W-NP (2010) Measurement of glucose and fructose in clinical samples using gas chromatography/mass spectrometry. Clinical Biochemistry 43, 198–207.
| Measurement of glucose and fructose in clinical samples using gas chromatography/mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 19747474PubMed |
Wan H, Zhu J, Su G, Liu Y, Hua L, Hu L, Wu C, Zhang R, Zhou P, Shen Y, Lin Y, Xu S, Fang Z, Che L, Feng B, Wu D (2016) Dietary supplementation with β-hydroxy-β-methylbutyrate calcium during the early postnatal period accelerates skeletal muscle fibre growth and maturity in intra-uterine growth-retarded and normal-birth-weight piglets. British Journal of Nutrition 115, 1360–1369.
| Dietary supplementation with β-hydroxy-β-methylbutyrate calcium during the early postnatal period accelerates skeletal muscle fibre growth and maturity in intra-uterine growth-retarded and normal-birth-weight piglets.Crossref | GoogleScholarGoogle Scholar |
Wang ZC, Yang S, Li LX, Zhou FM, Wang RD (1983) Studies on the effects of Tremella fuciformis Berk preparation on immunity and blood formation in rhesus monkeys. Journal of Traditional Chinese Medicine 3, 13–16.
Wang L, Hou Y, Yi D, Li Y, Ding B, Zhu H, Liu J, Xiao H, Wu G (2015) Dietary supplementation with glutamate precursor α-ketoglutarate attenuates lipopolysaccharide-induced liver injury in young pigs. Amino Acids 47, 1309–1318.
| Dietary supplementation with glutamate precursor α-ketoglutarate attenuates lipopolysaccharide-induced liver injury in young pigs.Crossref | GoogleScholarGoogle Scholar | 25795418PubMed |
Wu K, Dai ZY, Ma B (2007) An improved anthrone colorimetry for determination of polysaccharide concentration in streptococcus pneumoniae capsular polysaccharide conjugate. Chinese Journal of Biologicals 20, 536–538+549.
Wu Y-J, Wei Z-X, Zhang F-M, Linhardt RJ, Sun P-L, Zhang A-Q (2019) Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: a review. International Journal of Biological Macromolecules 121, 1005–1010.
| Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: a review.Crossref | GoogleScholarGoogle Scholar | 30342120PubMed |
Yuan SL, Piao XS, Li DF, Kim SW, Guo PF (2006) Effects of dietary Astragalus polysaccharide on growth performance and immune function in weaned pigs. Animal Science 82, 501–507.
| Effects of dietary Astragalus polysaccharide on growth performance and immune function in weaned pigs.Crossref | GoogleScholarGoogle Scholar |
Yui T, Ogawa K, Kakuta M, Misaki A (1995) Chain conformation of a glucurono-xylo-mannan isolated from fruit body of Tremella fuciformis Berk. Journal of Carbohydrate Chemistry 14, 255–263.
| Chain conformation of a glucurono-xylo-mannan isolated from fruit body of Tremella fuciformis Berk.Crossref | GoogleScholarGoogle Scholar |
Zhao PY, Jung JH, Kim IH (2012) Effect of mannan oligosaccharides and fructan on growth performance, nutrient digestibility, blood profile, and diarrhea score in weanling pigs. Journal of Animal Science 90, 833–839.
| Effect of mannan oligosaccharides and fructan on growth performance, nutrient digestibility, blood profile, and diarrhea score in weanling pigs.Crossref | GoogleScholarGoogle Scholar | 21984718PubMed |


