Impact of parasites on Australian laying hen welfare
Peter J. Groves
Faculty of Science, Sydney School of Veterinary Science, The University of Sydney, NSW 2006, Australia. Email: peter.groves@sydney.edu.au
Animal Production Science 61(10) 1031-1036 https://doi.org/10.1071/AN19693
Submitted: 2 December 2019 Accepted: 15 September 2020 Published: 24 May 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Cage housing systems separate the hen from her excreta and, thus, break the transmission cycle for most internal parasites. However, production systems where the birds are on litter or have access to the outdoors allow parasite life-cycle completion and, hence, these systems have seen a resurgence of intestinal parasites (worms, coccidia and histomonads). Effective registered anthelminthics are few in Australia and there are no registered products available to treat tapeworms in hens that are laying eggs for human consumption. Hence, internal parasites represent a challenge to the welfare of free-range and cage-free flocks. This is even more problematic in ‘organic’ production systems, as none of the effective treatments can be used. This is a considerable welfare issue for the organic system of production where the only measure available is lengthy range-area resting. External parasites can infest birds in any production system, although these too are regarded as more likely in extensive systems. Parasites are increasing in the layer industry and this is compounded by the parasites’ ability to infest a variety of bird species, making introduction from wild birds a significant source. New antiparasitic treatments that can be used during egg production for human consumption will be required in future.
Keywords: external parasites, protozoa, roundworms, tapeworms.
Introduction
Internal and external parasites represent a considerable health hazard to Australian egg-layer flocks. While housing birds in cages almost completely negates the faecal–oral infection route, the increase in cage-free systems puts birds at a considerable risk of internal parasitism. External parasites remain a threat in all housing systems, although some sources suggest that poultry red mite is reported to be becoming more prevalent in cage-free housing (DEFRA 2011; Hartcher and Jones 2017).
The most notable internal parasites of concern in Australia are nematodes (roundworms) and cestodes (tapeworms), while red mite (Dermanyssus gallinae) and poultry lice are the most commonly encountered external parasite types. The fowl tick (Argas persicus) and the stickfast flea (Echidnophaga gallinacea) are rarely encountered (possibly never commercially), although they may be found uncommonly in backyard flocks, as can the scaly leg mite (Knemdiocoptes mutans).
Internal parasites
Intestinal worm infestations are commonly found and vary in severity, mainly due to the numbers of parasites present (Sharma et al. 2019; P. J. Groves, pers. obs.).
Nematodes
The most common roundworms found in commercial poultry are Ascaridia galli (the large roundworm) and Heterakis gallinarum (the caecal worm). Capillaria species (hairworms, crop worms, gizzard worms) have been reported historically as not uncommonly causing heavy infestations (Seddon and Albiston 1967a), but do not seem to be a major concern in commercial flocks, only being seen occasionally (P. J. Groves, pers. obs.). Capillaria may cause enteritis and anaemia in heavy infestations.
Infection with poultry nematodes is usually direct; the parasite passes eggs that become embryonated in the environment and these infective eggs are ingested by the chicken, hatching within the intestines and proceeding with their life cycle. However, some of the Capillaria species have indirect cycles (i.e. involving some development of eggs or larvae in an intermediate host, usually earthworms; Small 1996; McDougald 2020a).
Ascaridia galli is the most common intestinal worm observed in poultry in Australia (Seddon and Albiston 1967b). It is also thought to parasitise turkeys, ducks, geese and doves, although these historic reports may not be fully reliable (McDougald 2020a). The adult stage is a large worm (50–76 mm long; McDougald 2020a). Once the eggs hatch in the proventriculus or upper intestine, the young larvae are released and will burrow into the intestinal lining for a few days. (McDougald 2020a). When the larvae mature, they emerge into the intestinal lumen and may begin to produce more eggs at 28–30 days of age (McDougald 2020a). These eggs pass into the environment where they may take 10–12 days to become infective. The mature worms are free inside the intestine and can migrate to places such as the oesophagus and crop or the gizzard (McDougald 2020a). Worms may be passed in excreta and can be observed in their droppings (Westwood 2013). Occasionally they can migrate into the oviduct, where they may become enclosed in a developing hen egg and create quite a surprise for an unsuspecting consumer (Business Queensland 2017a; McDougald 2020a). Pathogenicity can vary depending on the number of worms present in a hen, the immune status of the bird and the bird’s nutritional status (McDougald 2020a). Ascarid infections were previously documented to be able to retard growth rate, reduce appetite, decrease egg production and cause diarrhoea that may be blood-stained and cause weight loss, leading to emaciation (Small 1996), but more recent consideration suggests that A. galli pathogenicity is low and may cause only a decrease in bodyweight (McDougald 2020a). Affected birds may appear to be droopy, have ruffled feathers and have pale or droopy combs (Small 1996; Westwood 2013; Business Queensland 2017a). Heavy infestations can cause anaemia or intestinal obstruction (Small 1996; Trees 2008; Westwood 2013; McDougald 2020a). Infestation is generally higher in birds younger than 6 months of age, but will persist at a lower prevalence in birds over 3 years of age (Seddon and Albiston 1967b). Adult worms may survive in the host for up to 6 months; hence, burdens seen in older birds represent ongoing infestation being obtained by the birds. Other intestinal disease or peritonitis can occur as a sequel to ascarid infection (Westwood 2013). Apparently, birds fed diets with protein sourced predominantly from animal products are more resistant to ascarid infections, where chicks receiving feed lower in lysine had significantly higher worm burdens (Cuca et al. 1968). Also, heavier layer breeds, such as Rhode Island breeds, are also more resilient to ascarids (McDougald 2020a). Older birds can mount an immune response to ascarids that results in their larvae being arrested in developmental stages (McDougald 2020a). Ascarid eggs can remain infective in soil for long periods (at least for 4 months; Small 1996; maximally perhaps up to 2 years; Thapa et al. 2018); hence, infection can be facilitated with the introduction of fresh birds onto a site that has been considerably spelled. In Australia, piperazine and levamisole are registered anthelminthics for poultry with zero days egg withdrawal and both should be effective against ascarids. Alternative treatments suggested include apple cider vinegar (which is ineffective in the author’s experience) and diatomaceous earth, neither of which appears to have scientific evidence of support (Murray undated; The Raw Food Company 2017).
Heterakis gallinarum, the caecal worm, can infect most gallinaceous birds, ducks and geese. These are very small worms between 7 and 13 cm long (McDougald 2020a). The life cycle is direct, worm eggs passed in excreta undergo embryonation over ~2 weeks and can then establish an infection if swallowed by a suitable host. Earthworms can ingest the eggs and harbour the worms until eaten by a bird. The larvae hatch in the intestinal tract and travel to the caeca, mostly in their distal ends. There is no tissue phase. It is not regarded as being pathogenic in its own right (Trees 2008), although McDougald (2020a) reports marked inflammatory effects. The major concern with caecal worms is their ability to carry the causative organism of blackhead, the protozoan Histomonas meleagridis (Trees 2008; McDougald 2020b).
Examination of excreta for presence of ascarid eggs is useful for diagnosis. An ELISA test, which can be used on serum or egg yolk, has been shown to be able to detect infection with both A. galli and H. gallinarum (Daş et al. 2017; Sharma et al. 2019). However, commercially diagnosis is most readily and quickly confirmed by postmortem examination of dead or culled birds.
Neither infection with A. galli nor H. gallinarum has been shown to affect internal egg quality or eggshell quality (Sharma et al. 2019).
Capillaria species are small worms (hair worms) that can be highly pathogenic (Trees 2008). Different species infect different parts of the gastrointestinal tract, mostly the upper intestine and caeca, although some species are known to parasitise the oesophagus, crop or gizzard (McDougald 2020a). Diagnosis of capillariasis is not easy as the worms are microscopic and burrow into the intestinal mucosa and, hence, are not readily seen. In the author’s experience, heavily infested chickens have thickened, rubbery intestinal walls. Diagnosis requires finding capillarid eggs in excreta or finding worms from mucosal scrapings microscopically. Signs of infection include poor thrift, malnutrition, droopiness, emaciation, diarrhoea (sometimes with blood) and anaemia (McDougald 2020a). Deaths have been reported (McDougald 2020a).
Cestodes
The prevalence of cestode (tapeworm) infestations has been recently documented in Australia at 39.9% in free-range layers (Sibanda et al. 2020) and it is recognised by Australian poultry veterinarians that this has become prominent in cage-free systems, particularly where the hens have access outdoors (free-range conditions). Cestodes have indirect life cycles involving intermediate hosts (McDougald 2020a) that are much more likely encountered by the hens with access to the outdoor environment. Intermediate hosts include various species of earthworms, beetles, ants, houseflies, snails and slugs. There is a large number of tapeworm species identified to parasitise chickens. Genera described in chickens are Amoebotaenia, Choanotaenia, Davainea, Hymenolepsis and Raillietina. Species within these genera vary considerably in their reported pathogenicity, from harmless to severe (McDougald 2020a). Tapeworms are rarely examined to determine genus or species by Australian and international veterinarians or poultry producers. Simply noting a presence of ‘tapeworms’ in a flock does not assist in assessing the potential damage the parasite found may be contributing. An Agnote from the Northern Territory Department of Primary Industry (Small 1996) reported Raillietina sp, Choanotaenia sp. and Hymenolepsis sp. as being present in that jurisdiction. Historically, most species have been frequently reported, with more being noted in Queensland than in other states (Seddon and Albiston 1967c). Genus and species are difficult to determine without detailed laboratory examination. The author has submitted several specimens to NSW DPI for species identification in recent years and the results are reported in Table 1 (this represents a limited sample base only). Even so, precise species identification is difficult. Relative pathogenicity of the various species was described by McDougald (2020a) and this is outlined in Table 1 for local species identified.
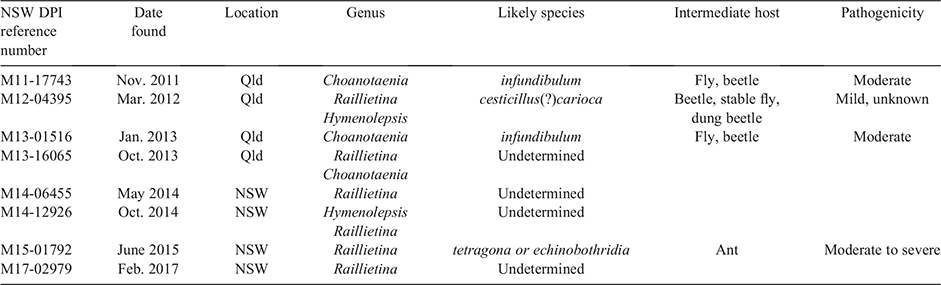
|
Hence, various genera and species of poultry tapeworms are present in Australia, some of which can have moderate to severe pathogenic effects in chickens. House flies and ants rank as important intermediate hosts, and efforts to control these need to be addressed (Seddon and Albiston 1967c).
Worms: general comments
Many other species of roundworms and tapeworms are rare or not generally present in commercial flocks but may occur in backyard and small-holding situations (McDougald 2020a). The long-term use of all-in all-out confined rearing of pullets and cage housing has limited the existence of these other species in commercial flocks (McDougald 2020a). The substantial move to large-scale extensive free-range production systems could lead to the re-establishment of some of these rarer parasites in future commercial situations. These may have already seen some emergence in Europe (McDougald 2020a). We may see the return of genera such as Syngamus, Gonglyonema, Subulura, Strongyloides, Heterakis isolonche and Oxyspirura.
Control and treatment of internal parasites
Worm infestations can cause variable problems, depending on the worm species and their number. The potential for their eggs to accumulate and remain viable in the environment is considerable and the involvement of intermediate hosts adds to their prolonged presence.
In Australia, piperazine and levamisole are registered for use in poultry, with no requirement to withdraw eggs during or after treatment. Piperazine has a label claim only for Ascaridia galli, while levamisole has label indications including Heterakis and Capillaria (MIMS 2017). Some levamisole preparations claim effectiveness against Raillietina species, but this is only registered for use in ornamental birds. There have not been registered products for control of tapeworms in commercial chickens in Australia for many years. Benzimadazoles (mebendazole, fenbendazole) are used off-label under veterinary authorisation for Heterakis, Capillaria and tapeworm control in flocks before lay, but no maximum residue level (MRL) has been established for eggs with these products in Australia; hence, use of these during lay would necessitate a lengthy withdrawal of eggs for human consumption to ensure no residues would occur. This egg withdrawal essentially precludes their use during lay. Several products claiming tapeworm-control capabilities are available, but these are registered only for ornamental birds (e.g. ivermectin, moxidectin andpraziquantel; Bird Vet Melbourne 2019), not commercial egg producers. Flubendazole at the time of writing is about to become available in Australia (Hack 2020). Flubendazole is used as a feed additive for a 1-week treatment and is active against all likely intestinal worms of poultry. It can be used with no need to withdraw eggs for human consumption (Hack 2020).
A recent study explored the value of a nematophagus fungus in reducing ascarid egg contamination of soil and, while this was able to decrease viable ascarid egg numbers, the reduced hen exposure increased the mature worm proportion within the hens, which lead to higher worm-egg excretion (Thapa et al. 2018).
Alternative treatments suggested in the popular literature include apple cider vinegar, diatomaceous earth, wormwood and garlic (Westwood 2013). Although strongly purported by internet websites, there is no scientific support for the effectiveness of these products. In fact, the likely effect of apple cider vinegar on internal parasites is strongly questioned (Murray undated) and this agrees with the author’s field experience. Diatomaceous earth has been shown to be ineffective against worms in ruminants (Fernandez et al. 1998; Barnard et al. 2009) and its lack of effect against internal parasites is explainable, even though it has insecticidal activity against insects in a dry environment (The Raw Food Company 2017). The lack of effective alternatives to anthelminthics leaves the organic egg layer-production systems basically unable to treat worm infestations and with no ammunition to prevent continual build-up of parasite exposure over time.
Spelling of range areas is recommended for reduction of infective stages for internal parasites in free-range layer flocks (Westwood 2013). This requires mobile facilities or being able to leave stationary facilities empty for prolonged periods. This does have limitations, given the long survival times of A. galli and Heterakis eggs (1–2 years, Thapa et al. 2018) and the survival of parasitic forms in robust intermediate hosts, and the eventual build-up of infective forms in the environment will be unavoidable. Good drainage of range areas and the avoidance of puddles and wet areas are important, as these enable embryonation of the parasite eggs.
Protozoa
Histomoniasis (‘blackhead’)
Blackhead (Histomonas meleagridis) is a major disease of turkeys but can cause variable problems to chicken flocks (from inapparent to severe; McDougald 2020b). This parasite is a protozoan organism carried within the egg of the caecal worm, Heterakis gallinarum (Trees 2008). Earthworms and some insects that may ingest caecal worm eggs carrying the histomonads may also harbour them and be a source of infection to the birds (Trees 2008; McDougald 2020b). The variety of birds that can carry caecal worms makes the risk of this disease higher in free-ranging flocks (Trees 2008). Once some birds are affected, bird to bird direct transmission is possible and morbidity can become extensive (McDougald 2020b). H. meleagridis invades via the caecal wall and is carried by the blood to the liver where it enters liver cells and multiplies, leading to the creation of typical blackhead liver lesions (Trees 2008). There are no longer any medications available for the specific control of blackhead (dimetridazole was removed from sale in most countries in the late 1990s). The only current control mechanism for blackhead revolves around the effective control of the caecal worm. In Australia, this is attempted mainly by application of effective anthelminthics (often mebendazole or flubendazole by off-label authorisation by veterinarians) late in the rearing period, sufficiently before egg production starts, so as to allow for a substantial withdrawal period.
Coccidiosis
Coccidia of chickens (Eimeria species) can be a serious problem in intensively reared flocks (Trees 2008). Each of the pathogenic species requires exposure to the chicken to establish immunity (Trees 2008). Flocks reared on the floor may be treated with an in-feed anticoccidial product for the first part of rearing, in the hope that all species will have exposure to stimulate immunity before the drug is withdrawn. The use of a precocious (Trees 2008) live multi-species coccidiosis vaccine (EimeriavaxTM or ParacoxTM) is becoming popular and is usually administered by spray in the hatchery. The success of these vaccines requires the vaccine coccidia to cycle through the flock to induce immunity. This factor limits the ability to use vaccine when birds are cage reared. Cages generally interfere with the transmission and build-up of coccidia and, hence, treatment is unnecessary for this system. However, a problem can occur when birds are cage reared (virtually with no exposure to coccidia) and then released into a floor system (barn or free range). In this case, fully susceptible birds can become exposed to coccidial oocysts and an outbreak may occur. Avoidance of this latter situation may require therapeutic anticoccidials (via drinking water or feed) and many producers use this routinely after the birds are placed on litter. Coccidiosis is generally well controlled in Australia at present, but outbreaks in lay can cause substantial performance problems and sometimes mortality.
External parasites
Poultry lice (many species, >40 have been described), but commonly the chicken body louse, Menacanthus stramineus, the wing louse, Lipeurus caponis, and the shaft louse, Menopon gallinae, are found on chicken flocks, although the author is unaware of any documented occurrences or species identification in Australia. State government departments mention only that many species have been seen (Graham 1996; Business Queensland 2017b; Atlas of Living Australia undated). Lice spend their entire life on the birds but are quite mobile and transfer among birds readily. Lice are grazing parasites, feeding on shed skin cells and feather debris (Hinkle and Corrigan 2020). Graham (1996) noted that the body louse is the most important, as it does pierce the skin at the base of the feather shaft, but the shaft louse is more common. Lice cause general irritation to the bird but only heavy infestations would cause production declines (Business Queensland 2017b). Lice tend to parasitise specific host species; however, they may transfer to other species transiently (Hinkle and Corrigan 2020). Lice can be well controlled with insecticidal dusts and sprays (Arends 2003). Malathion and a combined product containing sulfur and rotenone (PesteneTM) are the only insecticides registered for use on birds in Australia (MIMS 2017).
Both the red poultry mite (Dermanyssus gallinae) and the northern fowl mite (Ornithonyssus sylviarum) have been described as being present in Australia (Watson 2003). O. sylviarum is regarded as the most important mite in caged layers in North America (Hinkle and Corrigan 2020). O. sylviarum has been found associated with wild birds (starlings, pigeons, mynahs) in Australia (Doggett 2003), but it has seldom been reported in commercial poultry here, if at all. In contrst, D. gallinae is commonly found in commercial chicken flocks in Australia (Graham 1996; Business Queensland 2017b). The red mite has an aversion to light and spends much of the daylight hours hiding in crevices and under structures like perches and emerge to parasitise the birds at night, completing much of its life-cycle stages in these sites. Red mites are blood-sucking pests and severe infestations can cause severe anaemia in flocks (Fossum et al. 2008). They can be introduced into a flock by rodents or wild birds (Hinkle and Corrigan 2020). The life cycle is short (Arends 2003) and, hence, populations can increase rapidly under conducive conditions (warmer weather). Infestations have been known to cause restlessness at night, drops in egg production and pale combs and even death in heavy infestations (Business Queensland 2017b). Red mites and lice can infest any layer production system, but are significantly recognised to be more of a problem in flocks on litter, especially with outdoor access, in Australia (Hartcher and Jones 2017) and overseas (Fossum et al. 2008). Treatment of red mite is more difficult as the parasites extend throughout the facility and comprehensive insecticidal or acaricidal treatment is necessary to control the infestation.
The wide host range of external parasites makes the likelihood of introduction to a commercial poultry flock of a higher risk in birds with outdoor access.
Products available in Australia for external parasite control in commercial poultry with nil egg-withdrawal requirements include malathion for lice and a combination of sulfur and rotenone (PesteneTM) for mites (MIMS 2017).
Welfare implications of poultry parasites
Cage production systems have effectively reduced the opportunities for internal parasites in chickens by separating the bird from the source(s) of infection. The increase in floor-based systems, free-range layers and, particularly, organic production systems allow for the exposure of birds to internal-parasite infestations and an increased exposure to external parasite sources, which are difficult to remove. In Australia, we have evidence that some of the cestode species commonly seen can have moderate to severe pathogenicity. Fortunately, an effective cestode treatment is about to become available in Australia (Hack 2020). Other treatments that are not registered in commercial poultry in Australia should not be used without consideration of withdrawal of eggs for human consumption, if used during lay. Range-area resting appears the only viable option to prevent parasite build-up in the birds’ environment, but this will not eliminate the infective stages completely. There is a long-recognised need to establish effective anthelminthics that do not require egg withdrawal, for use in Australia. The size of the Australian poultry anthelminthic market is an obstacle to this.
The welfare effects of external and internal parasitism in commercial layer flocks vary depending on the parasite involved. Lice infestations cause considerable irritation and may increase feather pecking and may lead to scab development (Hinkle and Corrigan 2020). Red mite infestations can become serious if left unchecked, leading to anaemia and possibly death of the bird (Hinkle and Corrigan 2020). While the keeping of birds in cages had previously provided a disadvantage to the red mite, due to its life history of transiting between the birds and environmental sites, the resurgence in alternative systems has given this parasite an ecological advantage again (Hinkle and Corrigan 2020).
The effects of the commonly encountered intestinal parasites in commercial layer flocks are generally of low numbers and the effects are predominantly mild (McDougald 2020a). However, the potential risk of the occurrence of histomoniasis through Heterakis infection remains a considerable source of anxiety without an effective treatment available. Producers need to remain vigilant in the control of intestinal worms as continued build-up of infective stages in the environment may lead to subsequent heavy infections, which may cause poor egg production, bodyweight loss and deteriorations in flock uniformity. Under organic production conditions, where there are no proven means of parasite treatment, the only option remains frequent rotation of paddocks. Given the long survival of some parasite eggs (Thapa et al. 2018) and of the intermediate host for cestodes, this represents a considerable challenge to long-term production.
In general, external and internal parasites that are increasing in presence are mainly those that have been commonly found in commercial situations. As some of these are capable of infestation of multiple bird host species, the chances of introduction of some of the more obscure parasite species finding their way into commercial size poultry operations will increase in line with the increasing potential contact with wild birds and rodents (McDougald 2020a).
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
This work was funded by Australian Eggs Limited.
References
Arends JJ (2003) External parasites and poultry pests. In ‘Diseases of poultry’. 11th edn. (Ed. YM Saif) pp. 905–927. (Iowa State Press: Ames, IA, USA)Atlas of Living Australia (undated) Lipeurus caponis. Available at https://bie.ala.org.au/species/urn:lsid:biodiversity.org.au:afd.taxon:e6ba28f1-6530-42d9-a0c1-9a1c96589554#names [Verified 5 December 2018]
Barnard G, Worku M, Ahmedna M (2009) The effects of Diatomaceous earth on parasite infected goats. Bulletin of the Georgia National Academy of Sciences 3, 129–135.
Bird Vet Melbourne (2019). ‘Worms in birds.’ (Burwood Bird and Animal Hospital: Melbourne, Vic., Australia) Available at https://birdvetmelbourne.com/worms-in-birds/ [Verified 24 July 2020]
Business Queensland (2017a) ‘Worm parasites in poultry.’ Available at https://www.business.qld.gov.au/industries/farms-fishing-forestry/agriculture/livestock/animal-welfare/pest-diseases-disorders/worm-parasites [Verified 6 October 2018]
Business Queensland (2017b) ‘External parasites in poultry.’ Available at https://www.business.qld.gov.au/industries/farms-fishing-forestry/agriculture/livestock/animal-welfare/pests-diseases-disorders/external-parasites-poultry [Verified 5 December 2018]
Cuca M, Todd AC, Sunde (1968) Effects of levels of calcium and lysine upon the growth of Ascaridia galli in chicks. The Journal of Nutrition 94, 83–88.
| Effects of levels of calcium and lysine upon the growth of Ascaridia galli in chicks.Crossref | GoogleScholarGoogle Scholar | 5638642PubMed |
Daş G, Hennies M, Sohnrey B, Rahimian S, Wongrak K, Stehr M, Gauly M (2017) A comprehensive evaluation of an ELISA for the diagnosis of the two most common ascarids in chickens using plasma or egg yolks. Parasites and Vectors 10, 187
| A comprehensive evaluation of an ELISA for the diagnosis of the two most common ascarids in chickens using plasma or egg yolks.Crossref | GoogleScholarGoogle Scholar | 28420423PubMed |
DEFRA (2011) Pest control. In ‘The welfare of hens in free range systems’. pp. 12–13. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/69366/pb6490-hens-020130.pdf [Verified 26 November 2018]
Doggett S (2003) Letter to the editor: human infestation with bird mites in Wollongong. Communicable Diseases Intelligence 27, 394–395.
Fernandez MI, Woodward BW, Stromberg BE (1998) Effect of diatomaceous earth as an anthelmintic treatment on internal parasites and feedlot performance of beef steers. Animal Science 66, 635–641.
| Effect of diatomaceous earth as an anthelmintic treatment on internal parasites and feedlot performance of beef steers.Crossref | GoogleScholarGoogle Scholar |
Fossum O, Jansson DS, Etterlin PE, Vågsholm I (2009) Causes of mortality in laying hens in different housing systems in 2001 to 2004. Acta Veterinaria Scandinavica 51, 3
| Causes of mortality in laying hens in different housing systems in 2001 to 2004.Crossref | GoogleScholarGoogle Scholar | 19146656PubMed |
Graham P (1996) ‘External parasites of poultry. Agnote 364 no. K3.’ (Northern Territory Government) Available at www.primaryindustry.nt.gov.au [Verified 5 December 2018]
Hack R (2020) Flubendazole for intestinal worm control. In ‘Proceedings of the Australasian Veterinary Poultry Association’, 20 May 2020 (online conference).
Hartcher KM, Jones B (2017) The welfare of layer hens in cage and cage-free housing systems. World’s Poultry Science Journal 73, 767–782.
| The welfare of layer hens in cage and cage-free housing systems.Crossref | GoogleScholarGoogle Scholar |
Hinkle NC, Corrigan RM (2020) Chapter 26. External parasites and poultry pests. In ‘Diseases of poultry’. 14th edn. (Ed. DE Swayne) pp. 1157–1191. (John Wiley & Sons: Hoboken, NJ, USA)
McDougald LR (2020a) Section V. Parasitic diseases. In ‘Diseases of poultry’. 14th edn. (Ed. DE Swayne) pp. 1185–1191. (John Wiley & Sons: Hoboken, NJ, USA)
McDougald LR (2020b) Protozoal infections. In ‘Diseases of poultry’. 14th edn. (Ed. DE Swayne) pp. 1192–1294. (John Wiley & Sons: Hoboken, NJ, USA)
MIMS (2017) ‘MIMS IVS version 2.00.0010.’ (MIMS Australia Pty Ltd: Sydney, NSW, Australia) Available at www.mims.com.au
Murray A (undated) ‘Can apple cider vinegar kill parasites?’ Available at https://www.hunker.com/13418902/can-apple-cider-vinegar-kill-parasites [Verified 5 December 2018]
Seddon HR, Albiston HE (1967a) Capillaria infestation of poultry. In ‘Diseases of domestic animals in Australia. Part 1’. 2nd edn. (Ed. HE Albiston) p. 127. Service Publication Number 5. (Department of Health, Commonwealth of Australia: Canberra, ACT, Australia)
Seddon HR, Albiston HE (1967b) Tapeworm infestations of poultry. In ‘Diseases of domestic animals in Australia. Part 1’. 2nd edn. (Ed. HE Albiston) p. 58–60. Service Publication Number 5. (Department of Health, Commonwealth of Australia: Canberra, ACT, Australia)
Seddon HR, Albiston HE (1967c) Ascarosis of poultry. In ‘Diseases of domestic animals in Australia. Part 1’. 2nd edn. (Ed. HE Albiston) pp. 160–164. Service Publication Number 5. (Department of Health, Commonwealth of Australia: Canberra, ACT, Australia)
Sharma N, Hunt PW, Hine BC, Runke I (2019) The impacts of Ascaridia galli on performance, health, and immune responses of laying hens: new insights into an old problem. Poultry Science 98, 6517–6526.
| The impacts of Ascaridia galli on performance, health, and immune responses of laying hens: new insights into an old problem.Crossref | GoogleScholarGoogle Scholar | 31504894PubMed |
Sibanda TZ, Kolakshyapati M, Walkden-Browb SW, de Souza Vilela J, Courtice J, Ruhnke I (2020) Body weight sub-populations are associated with significant different welfare, health and egg production status in Australian commercial free-range laying hens in an aviary system. Archiv für Geflügelkunde 84,
| Body weight sub-populations are associated with significant different welfare, health and egg production status in Australian commercial free-range laying hens in an aviary system.Crossref | GoogleScholarGoogle Scholar |
Small L (1996) ‘Internal parasites (worms) of poultry. Agnote 669, K4.’ (Northern Territory Government: Darwin, NT, Australia) Available at www.primaryindustry.nt.gov.au [Verified 27 November 2018]
Thapa S, Thamsborg SM, Wang R, Meyling NV, Dalgaard TS, Petersen HH, Mejer H (2018) Effect of the nematophagus fungus Pochonia chlamydosporia on soil content of ascarid eggs and infection levels in exposed hens. Parasites & Vectors 11, 319–330.
| Effect of the nematophagus fungus Pochonia chlamydosporia on soil content of ascarid eggs and infection levels in exposed hens.Crossref | GoogleScholarGoogle Scholar |
The Raw Food Company (2017) ‘DE is not an intestinal parasite treatment!’ Available at https://therawfeedingcommunity.com/2017/05/30/de-is-not-an-intestinal-parasite-treatment/ [Verified 3 December 2018]
Trees AJ (2008) Parasitic diseases. In ‘Poultry diseases’. 6th edn. (Eds M. Pattison, PF McMullin, JM Bradbury, DJ Alexander) pp. 444–467. (Elsevier: Edinburgh, UK)
Watson CR (2003) Human infestation with bird mites in Wollongong. Communicable Diseases Intelligence 27, 259–261.
Westwood P (2013) ‘Worming free range hens.’ Available at https://freerangeeggs.blogspot.com/2013/03/worming-free-range-hens.html [Verified 6 October 2018]


