Broad near-infrared spectroscopy calibrations can predict the nutritional value of >100 forage species within the Australian feedbase
Hayley C. Norman A E , Elizabeth Hulm A , Alan W. Humphries B , Steve J. Hughes C and Philip E. Vercoe DA CSIRO Agriculture and Food, Private Bag 5, Wembley, WA 6913, Australia.
B SARDI Livestock Feed & Forage, GPO Box 397, Adelaide, SA 5001, Australia.
C SARDI, Australian Pastures Genebank, GPO Box 397, Adelaide, SA 5001, Australia.
D School of Animal Biology, University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
E Corresponding author. Email: hayley.norman@csiro.au
Animal Production Science 60(8) 1111-1122 https://doi.org/10.1071/AN19310
Submitted: 28 May 2019 Accepted: 23 October 2019 Published: 7 April 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Context: Near-infrared reflectance spectroscopy (NIRS) is a tool that permits rapid and inexpensive prediction of the nutritional characteristics of forages consumed by ruminants.
Aim: Our aim was to investigate the feasibility of developing a NIRS calibration to predict the nutritional value of the majority of grasses, legumes and forbs that are utilised for sheep and cattle production in southern Australia.
Methods: More than 100 annual and perennial forage species were grown in replicated plots at two locations over a period of 3 years. Biomass was sampled every 3–6 weeks, dried, ground and scanned with a desktop NIRS machine (n = 4385). One-quarter of these samples were subjected to laboratory analysis for calibration development or validation.
Key results: Despite the large variation in the taxonomy and maturity of the plants when sampled, we successfully developed broad calibrations that predicted key nutritional traits. We achieved excellent predictions for crude protein, with a ratio of standard error of performance : standard deviation (RPD) of 5.3, and standard error of cross validation (SECV) of 1.06%. Predictions of neutral detergent fibre were also excellent (RPD 4.3, SECV. 3.5%). For pepsin–cellulase DM digestibility and acid detergent fibre, predictions were very good (RPD 3.7, SECV 2.6% and RPD 3.9, SECV 2.1%). Predictions for organic matter were less reliable (RPD 2.2). We achieved very promising predictions of methane production during batch culture fermentation (RPD 3.1, SECV 3.5 mL/gDM). Predictions of ammonia and total volatile fatty acid concentrations in the post-fermentation substrate were poor.
Conclusions: We found that the broad calibrations predicted the nutritional traits of annual grasses, annual legumes and forb species with greater accuracy than perennial grasses or legumes. This could be associated with the accuracy of the wet chemistry methods. As a general rule, separating taxonomically similar species into groups before the development of calibrations, did not lead to more accurate predictions.
Implications: If more spatial and temporal diversity can be built in without a large reduction in accuracy, these broad NIRS calibrations represent a valuable tool for Australian researchers, feed testing agents and livestock producers, as they encompass nearly all of the species that appear in monocultures or mixed swards.
Additional keywords: feeding value, feed testing, fibre, pasture quality, proximate analysis, ruminant.
Introduction
Near-infrared reflectance spectroscopy (NIRS) is used to predict nutritional characteristics that contribute to the intake and utilisation of forages by ruminants. The method relies on the development of mathematical relationships between measured traits and light absorption properties within the near-infrared region (wavelength range 700–2500 nm). Once calibration equations are developed, predictions of nutritional traits using NIRS are faster and less expensive than chemical analyses (Deaville and Flinn 2000). Therefore, NIRS is a powerful tool within forage improvement programs, as a greater number of samples can be assessed for nutritional value before narrowing down the pool of candidate genotypes for selection.
There are many examples of NIRS calibrations to predict the nutritional value of forages, such as whole cereal plants (Deaville et al. 2009; Stubbs et al. 2010), lucerne (alfalfa, Medicago sativa) (Halgerson et al. 2004; Brogna et al. 2009), perennial grasses (Myer et al. 2011; Burns et al. 2013), forage maize (Zea mays; Hetta et al. 2017) and even woody forage shrubs, such as tagasaste (Cytisus proliferus; Flinn et al. 1996) and sagebrush (Artemisia tridentata; Olsoy et al. 2016). These examples are all characterised by narrow taxonomic diversity with only one or two plant species within the calibration set. It has been suggested that across NIRS predictions of forage quality, species-specific calibrations are more accurate than broad, taxonomically diverse calibrations (Dryden 2003; Landau et al. 2006). Accurate, species-specific calibrations are useful for single-species forage improvement programs and assessment of widely sown species, such as oaten or lucerne hays. These calibrations are not feasible for forage testing laboratories and researchers who work with a wide range of species, mixed swards or have samples submitted with uncertain identification.
There have been several studies exploring how much diversity is required to develop robust multispecies NIRS calibrations. Shenk and Westerhaus (1993) concluded that if enough samples are utilised, broad multiforage species calibrations can be nearly as accurate as those for single species. Andueza et al. (2011) explored the development of calibrations for single forage species and compared them with mixed grass (comprising five species), mixed legume (comprising three species) and a broad, global calibration encompassing all eight species of grasses and legumes. For predictions of crude protein, (CP), the ratio of standard error of performance : standard deviation (RPD) value was higher for the calibration developed for the most taxonomically diverse data. Standard error of prediction values were similar for the broad and grass-only calibrations (1.1%). For individual species, some calibrations had lower errors of prediction than others, with standard error of prediction values from 0.9 to 1.7%. There are several other examples of mixed calibrations; however, the taxonomic diversity rarely exceeds 15 species. In southern Chile, a calibration was successfully developed for mixed swards, comprising eight perennial grass and legume species by using nearly 300 spectra/chemistry pairs (Lobos et al. 2013). In Italy, calibrations have been developed for 13 species that are endemic to native grasslands, including grasses and legumes (Parrini et al. 2018). In southern Australia, calibrations were successfully developed for eight woody shrub species (Norman and Masters 2010). Furthermore, a team in Uganda developed calibrations for 11 diverse species of herbs and trees that were eaten by mountain gorillas (Rothman et al. 2009).
Extensive grazing systems in southern Australia are based on a diverse range of forage species, dominated by annual and perennial grasses, legumes, and forbs. The aim of this project was to investigate the feasibility of developing broad NIRS calibrations to predict the nutritional value of the majority of annual and perennial forage species in the feedbase of southern Australia. We tested the hypothesis that it would be possible to develop a global calibration that provides accurate predictions across a diverse range of forage species for total nitrogen (N), pepsin–cellulase dry matter digestibility (DMD), fibre fractions (neutral detergent fibre (NDF) and acid detergent fibre (ADF)), organic matter (OM), methane produced during 24-h batch fermentation and subsequent fermentation products (ammonia and volatile fatty acids). We also hypothesised that predictions from the global calibration would be equally, or more, accurate than those from calibrations derived from groups of species from similar taxonomic groups.
Materials and methods
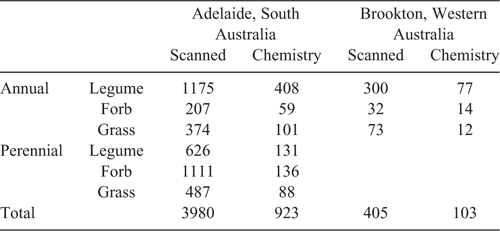
To test our hypotheses, we utilised 4385 plant samples originating from 102 forage species (representing 150 accessions or cultivars). The experiment was designed for a project to investigate the feeding value and antimethanogenic potential of the Australian feedbase. The diversity of the sample base included commercialised and experimental accessions, comprising 50 species of annual legumes (60 accessions), 20 species of perennial legumes (30 accessions), nine species of annual grasses (18 accessions), 13 species of perennial grasses (25 accessions), seven species of annual forbs (11 accessions) and three species of perennial forbs (6 accessions; Table 1).

|
Plot management
The primary field site was located in Adelaide, at the Australian Pastures Genebank field nursery, at the Waite Institute in South Australia. The soil at the site consists of fine, red–brown sandy loam with a pH (in CaCl2) of 6.2. The site was rainfed with additional subsurface drip irrigation in the first 12 months to match average monthly rainfall. The long-term average rainfall in Adelaide is 528 mm. The experimental site was split into five experimental units within the same paddock for ease of management, namely: (1) annual legumes, (2) annual grasses and forbs, (3) annual grasses, (4) perennial grasses and forbs, and (5) chicory. The annual legumes, grass and forbs were sown on 11 June 2012, and the perennials were sown on 11 August 2012. Plots were 1 × 8 m in size, and forage yield was assessed every 3–6 weeks after an initial establishment phase of 77 days. Each of the 150 accessions within the experimental cohort was replicated across three plots, and material from each plot was analysed separately. Basal fertilizer was applied (at recommended rates for each cohort of plants within the five experimental units) and the legumes were inoculated on the day before sowing with the recommended class of rhizobia for the species. Plants were sampled using quadrat cuts across all growth stages (approximately every 3–6 weeks). Annual legumes were allowed to set seed and regenerate in 2013, both perennials and the regenerating annuals were sampled over two seasons. When sampling, each quadrat cut was taken from a new part of the plot, so regrowth after cutting was not sampled.
The field site in Western Australia was located on a rainfed commercial farm near Brookton (mean annual rainfall 430 mm). At this site, a subset of 16 annual legumes, forbs and grasses were grown in two consecutive seasons (Table 1). Each year, the plants were established from seed in adjacent paddocks on 14 June 2013 and 28 May 2014. The light brown sandy loam had a pH (in CaCl2) of 4.6. Basal fertiliser was applied across all plots and the legumes were inoculated with appropriate rhizobia before sowing. Plots were sampled with quadrats every 3 weeks through the growing season, and for 2 months after senescence of all species.
Sample processing
The 4385 samples were either immediately frozen and eventually freeze-dried (for the Adelaide site in the first season) or placed in a paper bag then oven-dried for 48 h at 60°C (for the Adelaide site in the second season and the Brookton site in both seasons). Samples were ground to pass through a 1-mm screen using either a Cyclotech (FOSS, Hillerød, North Zealand, Denmark) or Cyclone Mill Twister (RETSCH, Haan, North Rhine-Westphalia, Germany) grinder. A preliminary study was conducted with unground samples that were divided and subsequently ground in each of the grinders to establish whether the type of grinder created any spectral bias. There was no spectral bias associated with these grinders detected. Across the 3-year project, a total of 1086 of the 4385 samples were subjected to the full range of wet chemical analyses in the laboratory (Table 2).
|
NIRS scanning, mathematical treatments and validation
Spectra were collected using a Unity Spectrastar 2500X rotating top window system (Unity Scientific, Milford, MA, USA). The spectrum file data from the NIRS machine were converted to a multifile, and the chemometric software package Ucal (Unity Scientific) was used to generate predictions using partial least-squares regression methods. We tested a range of pretreatment options including standard normal variate detrending, scatter correction, and derivatisation with different derivative gap and smoothing. From this the best performing equations were selected. No wave specification trims were utilised, the entire available spectra from 680 nm to 2500 nm was employed. Critical levels to remove outliers were left at default settings with the T limit equalling 2.5. The GD limit was 3.0, and the neighbourhood size was set to 0.20
In 2012, an initial cohort of 113 samples from the SA site was subjected to wet chemistry. A total of 100 samples were used to develop the first iteration of the global calibration, and 13 were set aside for immediate validation. A further 44 samples were selected (based on high standardised distance from the mean, as indicated by global H and neighbourhood H values) and subjected to wet chemistry, and added to the independent validation set. Cross-validation was used to calculate the standard error of cross-validation (SECV). This “preliminary global” calibration was expanded over the following 2 years.
During the 2013 and 2014 seasons, the preliminary global calibration, based on samples from the first year from one site, was used to predict incoming samples. Samples that had either high global H or high neighbourhood H values were prioritised for chemistry. At the end of the project, approximately half the dataset was used to develop the mature “global” calibration, and the remaining spectra were used for independent validation (Table 2).
During the project, a subset of 187 samples were subjected to 24-h in vitro batch fermentation with sheep rumen fluid (Durmic et al. 2010). A calibration was attempted for total methane produced during 24-h fermentation, and both ammonia and total volatile fatty acid concentrations in the fermentation liquor. A total of 17 samples were randomly selected and kept aside for an independent validation set.
To investigate the value of lumping similar samples into a dedicated calibration, we sorted the data into four groups: (1) annual grasses, (2) annual legumes, (3) mixed perennial grasses and legumes, and (4) forbs. The perennial grasses and legumes were combined to allow for sufficient sample numbers. For each of these groups, ~70% of the chemistry/spectra pairs were used for calibration, and the rest were kept for independent validation. The “group” calibrations were developed according to the method described previously.
Assessing the predictive ability of equations
The performance of the preliminary global, global and group calibration equations was assessed using several criteria, including the coefficient of determination for a linear model (R2 value), 1-VR (1 minus variance ratio) and SECV. To aid interpretation of the data and to allow simple comparison with other studies, we calculated RPD from R2 values using the following equation:
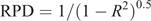
We adopted the guide of Williams (2014), who suggested RPD values of 0–1.9 are very poor and not recommended for forage testing; RPD values of 2.0–2.4 are poor and only of use for rough screening; RPD values of 2.5–2.9 offer a fair screening potential; RPD values of 3.0–3.4 are good (quality control); RPD values of 3.5–4.0 are very good (suited to process control); and RPD values >4.1 are deemed excellent. All RPD values that are discussed in this paper are calculated from independent validation statistics.
Wet chemistry
In vitro DMD was determined in duplicate using a modified pepsin–cellulase technique described by Clarke et al. (1982). Modifications include the use of ANKOM Technology F57 filter bags, plastic boxes as incubation vessels and the use of an orbital mixer incubator (set at 48°C with dial set to 2RPM). Duplicate samples of eight Australian Fodder Industry Association standards (AFIA 2007), with known in vivo DMD, were included in each batch to allow raw laboratory values to be adjusted to predict in vivo digestibility using linear regression. The AFIA forage samples had in vivo DMD values ranging from 48 to 69%. The R2 value of a regression between mean in vivo and mean in vitro data was 0.986, and the average standard error of the measured values of standards across the batches was 0.261%.
Concentrations of NDF and ADF (on a DM basis, without heat stable α amylase for NDF) were measured sequentially, according to operating instructions, using an ANKOM 200/220 Fibre Analyser (ANKOM Technology, Fairport, NY, USA). Duplicate samples were analysed, and an oaten hay quality control sample (NDF of 30.19 ± 0.1137% DM and ADF of 19.71 ± 0.0665% DM) was included in each of the 103 fibre analyses during the project. Total N was determined by combustion using a Leco CN628 N Analyser (Leco, St. Joseph, MI, USA) (Sweeney and Rexroad 1987). CP was calculated using total N × 6.25. OM was measured by ashing duplicate samples according to the methods of Faichney and White (1983).
Samples (n = 187) were analysed for in vitro fermentability and methanogenic potential using an in vitro batch fermentation system (Durmic et al. 2010). In each batch fermentation, five controls, including a negative batch control (rumen fluid only), positive batch control (oaten chaff + rumen fluid) and three AFIA pasture plant standards were included in each run to correct for differences between rumen fluid batch and run. The samples were fermented for 24 h before gas pressure, methane, ammonia and VFA were measured according to the methods described by Durmic et al. (2010).
Results
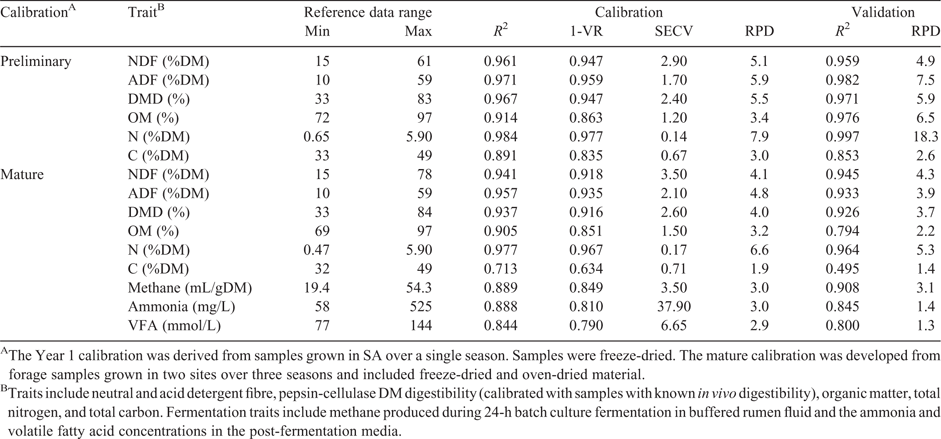
The preliminary global calibration, developed from a broad range of species at a single site in a single season, was very successful (Table 3), as evidenced by coefficient of determination or RPD values from independent validation (samples not used to generate the model) and SECV values. Total N was predicted with an RPD of 18.3, falling into the “excellent” category of Williams (2014). Predictions of DMD, OM, NDF and ADF were also “excellent”, with RPD values (validation) of 5.9, 6.5, 4.9 and 7.5 respectively. Total carbon was predicted less successfully with a RPD value (validation) of 2.6 (“fair screening potential” according to Williams (2014)). The SECV values for total N, DMD, OM, NDF and ADF were 0.14%, 2.4%, 1.2%, 2.9% and 1.7% respectively.
The mature global calibration, based on samples across seasons and sites, did not perform as well as the preliminary calibration, as evidenced by the validation statistics (Table 3). For validation samples, total N was predicted with an RPD of 5.3, thus remaining in the “excellent”’ category of Williams (2014). The SECV was 0.17% (equating to ~1.06% CP). Predictions of NDF were also “excellent”, with an RPD of 4.3 and a SECV of 3.5%. The ADF predictions were “very good”, with an RPD of 3.9 and an SECV of 2.1%. DMD predictions were “very good”, with an RPD of 3.7 and an SECV of 2.6%. The ability to predict OM seemed to decline markedly after the first year, with an RPD of 2.2 and SECV of 0.85%.
Performance of the mature global calibration across different taxonomic groups and within individual species
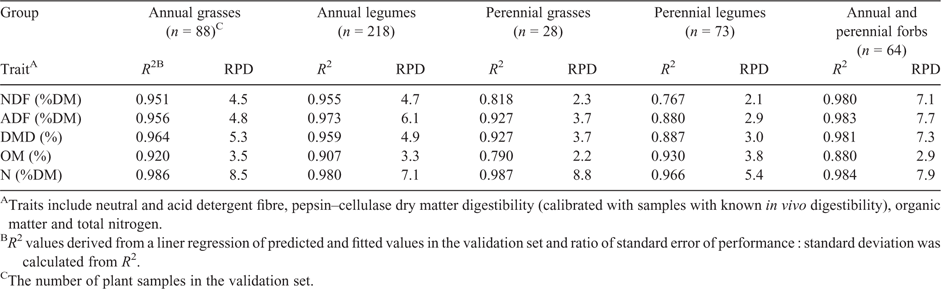
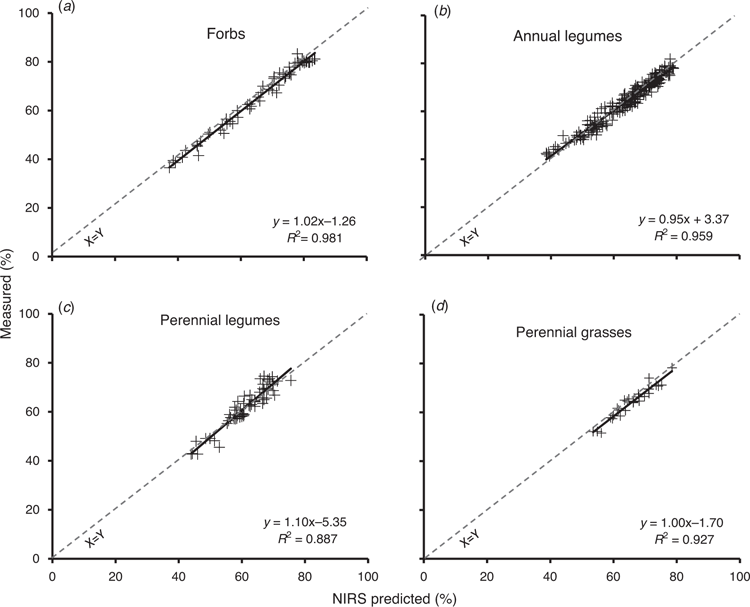
Using the validation dataset (n = ~500), we investigated errors of prediction for the following groups: annual grasses, annual legumes, perennial grasses, perennial legumes and forbs (annuals and perennials combined). The R2 values calculated from a linear regression of measured (laboratory) against values that were predicted using the final global calibrations are presented in Table 4. Graphs of DMD and NDF for four of the taxonomic groups are shown in Figs 1 and 2 respectively. As a rule, the broad calibration gave more accurate predictions for the forbs, annual grasses and annual legumes than for the perennial grasses and perennial legumes.
Across all taxonomic groups, predictions of total N were “excellent”, with RPD values of 8.5, 7.1, 8.8, 5.5 and 7.9 for annual grasses, annual legumes, perennial grasses, perennial legumes and mixed annuals, and perennial forbs respectively (Table 4). Predictions of DMD from the global calibration were “excellent’” for forbs, annual grasses and annual legumes, and “very good” for perennial grasses, but not as accurate, with just a “good” rating for perennial legumes (RPD values of 7.3, 5.3, 4.9, 3.7 and 3.0 respectively; Table 4, Fig. 1). Predictions of NDF that were derived from the global calibration were “excellent” for forbs, annual grasses and annual legumes, and “poor or rough screening potential” for perennial grasses and perennial legumes (RPD values of 7.1, 4.5, 4.7, 2.3 and 2.1 respectively; Table 4, Fig. 2). For ADF, predictions from the global calibration were “excellent” for forbs, annual grasses and annual legumes, and “very good” for perennial grasses and “fair screening potential” for perennial legumes (RPD values of 7.7, 4.8, 6.1, 3.7 and 2.9 respectively; Table 4). Predictions of OM for the perennial legumes, annual grasses and annual legumes were “very good” or “good” (RPD values of 3.8, 3.5 and 3.3 respectively). OM predictions for forbs and perennial grasses were “fair” to “poor” (RPD values of 2.9 and 2.2).
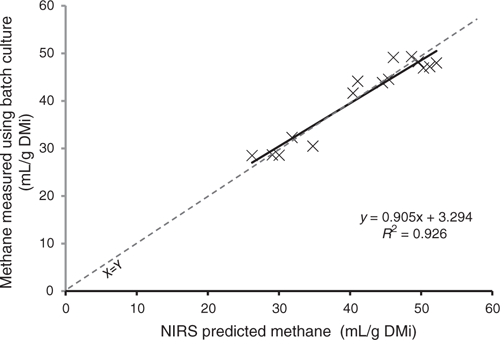
We developed a very promising global calibration that predicted total methane produced during 24-h fermentation in rumen fluid (RPD 3.1, SECV 3.5 mL/gDM; Fig. 3). Calibrations to predict ammonia (RPD 1.4) or total VFA (RPD 1.3) concentrations in the fluid after fermentation were less successful (Table 3).
|
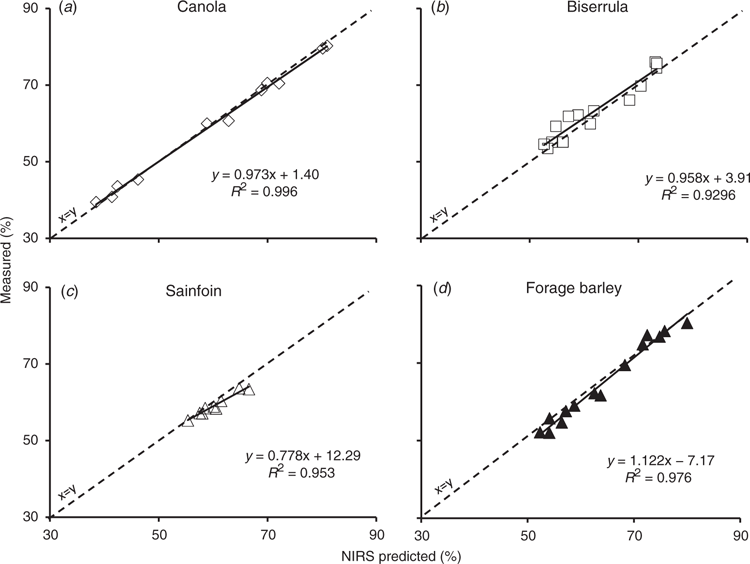
Fig. 4 presents measured versus predicted DMD values (using the global calibration) for four species where we had the greatest numbers of samples represented in the validation set. They include canola (Brassica napus; an annual forb), biserrula (Biserrula pelecinus; an annual legume), forage barley (Hordeum vulgare; an annual grass) and sainfoin (Onobrichis viciifolia; a perennial legume). For canola, sainfoin and barley, the RPD values placed them in the “excellent” predictive category (RPD of 15.8, 4.6 and 6.4). The R2 value for biserrula was 0.93, placing it in the “very good” categories of Williams (2014).
Calibrations generated with species that have been split and grouped by taxonomic and life history traits
The performance statistics of four NIRS calibrations that were generated using data for species that had been arbitrarily grouped by taxonomy and life cycle before calibration development are presented in Table 5. Perennial legumes and perennial grasses were grouped to ensure enough samples. For predictions of total N, the RPD values indicated stronger predictions were generated using the mature global calibration rather than the group calibrations. Only annual legumes were predicted with a higher RPD using an annual legume-only calibration (RPD 8.8), compared with the global calibration (RPD 7.1). SECV values indicate that the mature global calibration tended to give lower errors of prediction (SECV of 0.17% DM for the mature global calibration compared with 0.15–0.33% DM for the group calibrations).
For NDF, the mature global calibration resulted in similar or higher RPD values compared with RPD values from the group calibrations for annual grasses, annual legumes and forbs. Restricting a calibration set to just perennial legumes and grasses led to an improvement in RPD values (RPD for the group calibration was 4.15, compared with calculated RPD values of 2.3 and 2.1 for perennial grasses and perennial legumes that were predicted with the global calibration). The SECV value for the mature global calibration (calculated across groups) was generally lower than SECV values for the four group calibrations.
The mature global calibration generally gave ADF predictions with similar or higher RPD values than the group calibrations. The SECV value of the mature global calibration (2.1% DM) was higher than the SECV generated for a group calibration for annual grasses, and perennial grasses and legumes (2.0% DM and 1.7% DM respectively), but equal to or lower than the SECV values from group calibrations for annual legumes and forbs (2.1% DM and 3.3% DM respectively).
DMD predictions for annual legumes had higher RPD values when they were generated from the mature global calibration (RPD of 4.9) than the group calibrations (RPD of 4.1). For the forbs, the global and group calibration had similar RPD values. For annual grasses, and mixed perennial grasses and legumes, the group calibrations yielded predictions with higher RPD values than the global calibration (6.3 compared with 5.3 for annual grasses, and 4.2 compared with 3.7/3.0 for perennial grasses and legumes). Grouping annual legumes before development of the calibration tended to give a lower SECV value (2.0% DM) than the SECV value for the mature calibration (2.6% DM). For all other groups, the SECV value generated from a global calibration was lower than those generated from group calibrations.
Across all groups, the global calibration gave predictions of OMD with equal or higher RPD values.
Discussion
The data presented in this paper suggest that a large, multispecies NIRS calibration to predict the nutritional value of forage species within the southern feedbase of Australia is feasible, supporting our hypothesis. With the inclusion of 102 annual and perennial species across several plant families, the taxonomic diversity in this data is considerably larger than the diversity reported in other studies that we identified in the literature. After comparing performance statistics for the global and group calibrations, we found that there was rarely any value in splitting the samples into groups, based on taxonomic and/or life cycle traits, before calibration development. For total N, OM and ADF, the mature global calibration consistently outperformed calibrations that were developed for groups of plants with similar taxonomy or maturity. Restricting the dataset to just perennial legumes and grasses before calibration yielded an improvement in the RPD values for this group of plants for both NDF and DMD.
Throughout the project, total N was the trait that was predicted with the highest RPD values and lowest errors. With a RPD value for validation of 5.3, our prediction of CP (total N × 6.25) using the global calibration was comparable to RPD values reported in the literature. Studies with narrow taxonomic diversity report RPD values for CP of 1.8 and 2.2 for cereals (Deaville et al. 2009; Stubbs et al. 2010), 3.5 for sagebrush (Olsoy et al. 2016), 5.0 for barley hay (Durmic et al. 2010), and 7.1 for lucerne (Hsu et al. 2000). In studies where there were more than five species from at least two plant families, the RPD values for CP include 4.5 (Rothman et al. 2009), 6.6 (Andueza et al. 2011) and 10.3 (Lobos et al. 2013). Our results were consistent with those of Andueza et al. (2011), who also demonstrated that increasing diversity in the reference samples led to improved predictive capacity for CP.
For in vitro DMD, the broad calibration gave a RPD and SECV values (3.7 and 2.6%), suggesting better predictive ability than many others have reported in the literature. For mixed swards comprising eight species, Lobos et al. (2013) reported RPD and SECV values of 3.0 and 3.1%. Norman and Masters (2010) achieved RPD and SECV values of 3.5 and 2.5% for eight woody shrub species. It appears from the literature that calibrations based on a narrow range of species tend to have lower RPD values. Examples include 1.7 for grass silages (De Boever et al. 1996), 1.8 for sagebrush and 2.3 for forage maize (Hetta et al. 2017). In the present study, the RPD values for in vitro DMD of annual grasses, and mixed perennial grasses and legumes could be further improved with grouping before calibration development.
Although it would be better to develop calibrations with samples of known in vivo digestibility, these samples are expensive to generate. It is also difficult to produce samples at the extreme ends of the spectrum due to welfare concerns with ruminants offered very poor or extremely fermentable diets. We feel that our approach, by using a broad range of samples with known in vivo digestibility to calibrate our laboratory in vitro enzymatic digestibility, is a good compromise.
The broad calibration offered comparable RPD validation values for fibre fractions as other studies reported in the literature. For ADF, our RPD value of 3.9 was similar to numbers reported in the literature. For NDF, the broad calibration gave RPD and SECV values of 4.3 and 3.5%. This RPD value is higher than some (e.g. 3.5 and 3.4; Hsu et al. 2000; Stubbs et al. 2010) and lower than others (e.g. 4.5; Rothman et al. 2009; Parrini et al. 2018). For perennial legumes and grasses, we were able to achieve higher RPD values for NDF after restricting the calibration set to just perennial legumes and grasses. Abrams et al. (1987) also suggested that for NDF, species-specific models may improve the prediction of samples. Our inability to develop good predictions for NDF in perennial legumes is not surprising. Perennial legume samples consistently have much greater variances between replicates in the laboratory than annual legume or grass samples. This variance is associated with the ANKOM method, and is not discernible after the subsequent ADF phase. Others have reported that the ANKOM NDF method is problematic for samples with high starch, protein or other mucilaginous materials (Goering and Van Soest 1997; McRoberts and Cherney 2014). Addressing this laboratory analysis issue is critical if we desire better calibrations for NDF in perennial legumes.
This study provides greater confidence in the ability to predict methane production during fermentation of forage using rumen fluid. The majority of studies to date have involved methane produced by grass samples fermented in bioreactors with a manure-based inoculum, rather with rumen fluid. RPD values from these studies include 1.75 and 2.49 (Raju et al. 2011; Triolo et al. 2014). In this study, we achieved an RPD value of 3.1, indicating that NIRS does have significant potential as a screening tool for methanogenic potential of forages. Unfortunately, despite 170 samples, we could not develop calibrations to predict ammonia or volatile fatty acid content of the fermentation liquor.
The accuracy of predictions from the calibrations declined after the first year, as we increased the temporal diversity of the sample range with a second season in South Australia and the spatial diversity with a new site in Western Australia. The preliminary global calibration was generated with just 100 samples with matched NIR spectra and chemistry. The RPD values declined when new samples were added, even though the new samples were from the same species that featured in the preliminary calibration and the reference data range was not extended markedly. This highlights the need to include spatial, temporal and management diversity within the dataset if calibrations are to be used beyond the reference sample collection sites. This would be especially important for feed testing laboratories where the diversity of growing sites and seasons for forages – and forage management regimes – would be very high. This outcome was expected, as several authors have stated that calibration populations must encompass all sources of variation likely to be found in future unknown samples of similar material (Windham et al. 1989; Deaville and Flinn 2000).
A critical factor leading to the success of this work has been the quality of the laboratory data behind the calibration. Not all differences between NIRS predictions and reference values can be ascribed to NIRS prediction error (Coates 2002), as the error sources of the reference method are incorporated into the model (Murray 1993). By using a single, highly trained laboratory operator and adoption of a broad range of quality control samples, we kept laboratory errors to a minimum (in vitro pepsin–cellulase DMD 0.23%, NDF 0.11% and ADF 0.7%).
The current dataset with >1000 samples of a very diverse range of species, with matching scans and chemistry, provides an excellent platform for future refinement or generation of calibrations for new traits. If more spatial and temporal diversity can be built in without a large reduction in accuracy, these broad NIRS calibrations represent a useful tool for researchers, feed testing agents and livestock producers in Australia, as they encompass nearly all of the species that appear in monocultures or mixed swards. Inexpensive and rapid prediction of the nutritional value of forages assists producers to optimise livestock management and productivity. This may lead to increased profitability and reduced methane emissions intensity if maternal stock have higher reproductive rates and young stock reach slaughter weight faster, with fewer feed inputs. Development of accurate calibrations can also be very useful in plant breeding and selection programs where large numbers of plants require assessment of their nutritional value. The NIRS database also provides an opportunity for producers to measure improvements in the feedbase (or estimate total methane outputs from the feedbase) for future carbon reduction schemes.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was a component of “Efficient Livestock and Low Emissions (ELLE) from southern grazing systems” (project number: 01200.042; B.CCH.6540), a collaborative project involving the University of Western Australia (UWA), South Australian Research and Development Institute (SARDI), and the Commonwealth Scientific and Industrial Research Organisation (CSIRO). The work was funded through the Australian Department of Agriculture and Water Resources “Filling the Research Gap” program, and managed as part of the National Livestock Methane Program by Meat and Livestock Australia. The authors thank and acknowledge Robert Law and Trevor Rowe (SARDI) for the mammoth task of sample collection and processing in SA, and Matt Wilmot, Joshua Hendry and Miranda Macintyre (CSIRO) for sample collection and processing in WA. Paul Young (CSIRO) performed nitrogen analyses, and batch culture analyses were undertaken by Zoey Durmic, Joy Vadhanabhuti, Bidhyut Banik and Marga Joy (UWA). We are grateful to Anna and Colin Butcher (Brookton) for hosting a research site. Brad Nutt, Ronald Yates (Murdoch University) and Angelo Loi (DPIRD) kindly sowed and managed the Brookton site. Seed of all accessions used in this study are maintained and available for further R&D at the Australian Pastures Genebank https://apg.pir.sa.gov.au/gringlobal. Thank you to the National Livestock Methane Program team, especially Dr John Black, for advice throughout the project. Finally, we appreciated the considered advice from the anonymous reviewers.
References
Abrams SM, Shenk JS, Westerhaus MO, Barton FE (1987) Determination of forage quality by nearinfrared reflectance spectroscopy: Efficiency of broad based calibration equations. Journal of Dairy Science 70, 806–803.AFIA (2007) ‘Laboratory methods manual. Publication no 63/001.’ (Australian Fodder Industry Association: Melbourne)
Andueza D, Picard F, Jestin M, Andrieu J, Baumont R (2011) NIRS prediction of the feed value of temperate forages: efficacy of four calibration strategies. Animal 5, 1002–1013.
| NIRS prediction of the feed value of temperate forages: efficacy of four calibration strategies.Crossref | GoogleScholarGoogle Scholar | 22440096PubMed |
Brogna N, Pacchioli MT, Immovilli A, Ruozzi F, Ward R, Formigoni A (2009) The use of near-infrared reflectance spectroscopy (NIRS) in the prediction of chemical composition and in vitro neutral detergent fiber (NDF) digestibility of Italian alfalfa hay. Italian Journal of Animal Science 8, 271–273.
| The use of near-infrared reflectance spectroscopy (NIRS) in the prediction of chemical composition and in vitro neutral detergent fiber (NDF) digestibility of Italian alfalfa hay.Crossref | GoogleScholarGoogle Scholar |
Burns GA, Gilliland TJ, Grogan D, Watson S, O’Kiely P (2013) Assessment of herbage yield and quality traits of perennial ryegrasses from a national variety evaluation scheme. The Journal of Agricultural Science 151, 331–346.
| Assessment of herbage yield and quality traits of perennial ryegrasses from a national variety evaluation scheme.Crossref | GoogleScholarGoogle Scholar |
Clarke PC, Flinn P, McGowan AA (1982) Low-cost pepsin-cellulase assays for prediction of digestibility of herbage. Grass and Forage Science 37, 147–150.
| Low-cost pepsin-cellulase assays for prediction of digestibility of herbage.Crossref | GoogleScholarGoogle Scholar |
Coates DB (2002) An empirical approach to the question ‘Is NIRS only as good as the laboratory reference values?’. Spectroscopy Europe 14, 24–26.
De Boever JG, Cottyn BL, De Brabander DM, Vanacker JV, Boucque CH (1996) Prediction of the feeding value of grass silages by chemical parameters, in vitro digestibility and near-infrared reflectance spectroscopy. Animal Feed Science and Technology 60, 103–115.
| Prediction of the feeding value of grass silages by chemical parameters, in vitro digestibility and near-infrared reflectance spectroscopy.Crossref | GoogleScholarGoogle Scholar |
Deaville ER, Flinn PC (2000) Near-infrared (NIR) spectroscopy: an alternative approach for the estimation of forage quality and voluntary intake. In ‘Forage evaluation in ruminant nutrition’. (Eds DI Givens, E Owen, RFE Axford, HM Ohmed) pp. 301–320. (CABI Publishing: Wallingford, UK)
Deaville ER, Humphries DJ, Givens DI (2009) Whole crop cereals 2. Prediction of apparent digestibility and energy values from in vitro digestion techniques and near infrared reflectance spectroscopy and chemical composition by near infrared reflectance spectroscopy. Animal Feed Science and Technology 149, 114–124.
| Whole crop cereals 2. Prediction of apparent digestibility and energy values from in vitro digestion techniques and near infrared reflectance spectroscopy and chemical composition by near infrared reflectance spectroscopy.Crossref | GoogleScholarGoogle Scholar |
Dryden GM (2003) Near infrared spectroscopy: applications in deer nutrition. Report: Rural Industries Research and Development Corporation. Available at: www.rirdc.gov.au/reports/DEE/w03-007.pdf [Verified July 2003]
Durmic Z, Hutton P, Revell DK, Emms J, Hughes S, Vercoe PE (2010) In vitro fermentative traits of Australia woody perennial plant species that may be considered as potential sources of feed for grazing ruminants. Animal Feed Science and Technology 160, 98–109.
| In vitro fermentative traits of Australia woody perennial plant species that may be considered as potential sources of feed for grazing ruminants.Crossref | GoogleScholarGoogle Scholar |
Faichney GJ, White GA (1983) ‘Methods for the analysis of feeds eaten by ruminants.’ (CSIRO: Melbourne)
Flinn PC, Edwards NJ, Oldham CM, McNeill DM (1996) Near infrared analysis of the fodder shrub tagasaste (Chamaecytisus proliferus) for nutritive value and anti‐nutritive factors. In AMC Davies, P Williams (Eds.), ‘Near Infrared Spectroscopy: The Future Waves’. pp. 576–580. (NIR Publications: Chichester, UK)
Goering HK, Van Soest PJ (1997) ‘Forage fiber analyses (apparatus, reagents, procedures, and some Applications).’ Agriculture Handbook No. 379. (USDA-ARS: Washington, DC)
Halgerson JL, Sheaffer GC, Martin NP, Peterson PR, Wes SJ (2004) Near-infrared reflectance spectroscopy prediction of leaf and mineral concentrations in alfalfa. Agronomy Journal 96, 344–351.
| Near-infrared reflectance spectroscopy prediction of leaf and mineral concentrations in alfalfa.Crossref | GoogleScholarGoogle Scholar |
Hetta M, Mussadiq Z, Wallsten J, Halling M, Swensson C, Geladhi P (2017) Prediction of nutritive values, morphology and agronomic characteristics in forage maize using two applications of NIRS spectrometry. Soil and Plant Science 67, 326–333.
Hsu H, Mathison GW, McNeil A, Tsai C, Recinos-Diaz G, Okine E, McKenzie RH, Wright S (2000) Determination of feed quality for barley hay and silage by near infrared spectroscopy. In ‘Near Infrared spectroscopy: Proceedings 9th International Conference’ (Eds AMC Davis, R Giangiacomo) pp. 643–649. (NIR Publications: Chichester, UK)
Landau S, Glasser T, Dvash L (2006) Monitoring nutrition in small ruminants with the aid of near infrared reflectance spectroscopy (NIRS) technology: a review. Small Ruminant Research 61, 1–11.
| Monitoring nutrition in small ruminants with the aid of near infrared reflectance spectroscopy (NIRS) technology: a review.Crossref | GoogleScholarGoogle Scholar |
Lobos I, Gou P, Saldana R, Alfaro M (2013) Evaluation of potential NIRS to predict pastures nutritive value. Journal of Soil Science and Plant Nutrition 13, 463–468.
McRoberts KC, Cherney DJR (2014) Low-infrastructure filter bag technique for neutral detergent fiber analysis of forages. Animal Feed Science and Technology 187, 77–85.
| Low-infrastructure filter bag technique for neutral detergent fiber analysis of forages.Crossref | GoogleScholarGoogle Scholar |
Murray I (1993) Forage analysis by near-infrared reflectance spectroscopy. In ‘Sward management handbook’. (Eds A Davies, RD Baker, SA Grant, AS Laidlaw) pp. 285–312. (British Grassland Society: Reading, UK)
Myer R, Blount A, Coleman S, Carter J (2011) Forage nutritional quality evaluation of bahiagrass selections during autumn in Florida. Communications in Soil Science and Plant Analysis 42, 167–172.
| Forage nutritional quality evaluation of bahiagrass selections during autumn in Florida.Crossref | GoogleScholarGoogle Scholar |
Norman HC, Masters DG (2010) Predicting the nutritive value of saltbushes (Atriplex spp) with near infrared reflectance spectroscopy. In ‘Proceedings of the International Conference on Management of Soil and Groundwater Salinization in Arid Regions’, 11–14 January 2010, Muscat, Oman. (Eds M Ahmed, SAl-Rawahy) pp. 51–57. (SQU Press: Muscat, Oman)
Olsoy PJGriggs TCUlappa ACGehlken KShipley LAShewmaker GEForbey JS (2016 ) Nutritional analysis of sagebush by near-infrared reflectance spectroscopy.Journal of Arid Environments 134 125131
Parrini S, Acciaioli A, Crovetti A, Bozzi R (2018) Use of FT-NIRS for the determination of chemical components and nutritional value of natural pasture. Italian Journal of Animal Science 17, 87–91.
| Use of FT-NIRS for the determination of chemical components and nutritional value of natural pasture.Crossref | GoogleScholarGoogle Scholar |
Raju CS, Ward AJ, Nielsn L, Moller HB (2011) Comparison of near infra-red spectroscopy, neutral detergent fibre assay and in-vitro organic matter digestibility assay for rapid determination of biochemical methane potential of meadow grasses. Bioresource Technology 102, 7835–7839.
| Comparison of near infra-red spectroscopy, neutral detergent fibre assay and in-vitro organic matter digestibility assay for rapid determination of biochemical methane potential of meadow grasses.Crossref | GoogleScholarGoogle Scholar | 21708461PubMed |
Rothman JM, Chapman CA, Hansen JL, Cherney DJR, Pell AN (2009) Rapid assessment of the nutritional value of foods eaten by mountain gorillas: applying near-infrared reflectance spectroscopy to primatology. International Journal of Primatology 30, 729–742.
| Rapid assessment of the nutritional value of foods eaten by mountain gorillas: applying near-infrared reflectance spectroscopy to primatology.Crossref | GoogleScholarGoogle Scholar |
Shenk JS, Westerhaus MO (1993) Near infrared reflectance analysis with single and multiproduct calibrations. Crop Science 33, 582–584.
| Near infrared reflectance analysis with single and multiproduct calibrations.Crossref | GoogleScholarGoogle Scholar |
Stubbs TL, Kennedy AC, Fortuna AM (2010) Using NIRS to predict fiber and nutrient content of dryland cereal cultivars. Journal of Agricultural and Food Chemistry 58, 398–403.
| Using NIRS to predict fiber and nutrient content of dryland cereal cultivars.Crossref | GoogleScholarGoogle Scholar | 19961223PubMed |
Sweeney RA, Rexroad PR (1987) Comparison of LECO FP-228 “nitrogen determinator” with AOAC copper catalyst Kjeldahl method for crude protein. Journal - Association of Official Analytical Chemists 70, 1028–1030.
Triolo JM, Ward AJ, Pederson L, Lokke MM, Qu H, Sommer SG (2014) Near infrared reflectance spectroscopy (NIRS) for rapid determination of biochemical methane potential of plant biomass. Applied Energy 116, 52–57.
| Near infrared reflectance spectroscopy (NIRS) for rapid determination of biochemical methane potential of plant biomass.Crossref | GoogleScholarGoogle Scholar |
Williams P (2014) Tutorial: the RPD statistic: a tutorial note. NIR News 25, 22–26.
| Tutorial: the RPD statistic: a tutorial note.Crossref | GoogleScholarGoogle Scholar |
Windham WR, Mertens DR, Barton FE (1989) Supplement 1. Protocol for NIRS calibration: sample selection and equation development and validation. In ‘Near Infrared Reflectance Spectroscopy (NIRS): analysis of forage quality’. (Eds GC Marten, SJ Shenk, FE Barton) pp. 96–103. USDA Agriculture Handbook No 643 (U.S. Gov. Print. Office, Washington DC, WA).