The potential of silage lactic acid bacteria-derived nano-selenium as a dietary supplement in sheep
M. R. F. Lee A B E , H. R. Fleming A , F. Whittington B , C. Hodgson A , P. T. Suraj C and D. R. Davies A DA Rothamsted Research, North Wyke, Okehampton, Devon, EX20 2SB, UK.
B University of Bristol, Bristol Veterinary School, Langford, Somerset, BS40 5DU, UK.
C Kerala Veterinary and Animal Science University, Pookode, Wayanad, Kerala, India.
D Silage Solutions Ltd, Bwlch y Blaen, Ponthrydygroes, Ceredigion, SY25 6DP, UK.
E Corresponding author. Email: michael.lee@rothamsted.ac.uk
Animal Production Science 59(11) 1999-2009 https://doi.org/10.1071/AN19258
Submitted: 2 May 2019 Accepted: 30 May 2019 Published: 16 September 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Context: Selenium (Se) is a trace element essential for cellular function in animals as a component of the enzymes glutathione peroxidase and iodothyronine-5-deiodinase. In many parts of Europe, Se is often deficient in livestock diets due to the low Se status of soil. Supplementation of diets with selenised yeast (predominately as seleno-methionine) or inorganic sodium selenite is common practice in most livestock systems, including ruminants. Lactic acid bacteria have been shown to convert inorganic Se into predominantly elemental nano-Se, which has been used recently in human pro-biotics as a less toxic form of Se. Therefore, silage lactic acid bacteria may provide a supplementation route of bioavailable nano-Se for ruminants.
Aim: Here, we report on the effect of feeding inoculated silage enriched with a supra-nutritional level of nano-Se (Selage) versus control inoculated silage (Silage) on the Se status of finishing lambs and their products, followed by a second study where blood parameters were investigated in ewes.
Methods: In the first study, 40 Charollais × Suffolk lambs (42 ± 1.7 kg) were paired according to weight and sex, then allocated to the two treatments for 8 or 10 weeks. Uptake of Se into wool was temporally assessed, as well as excretion of Se into faeces. Selenium concentrations in blood and muscle, carcass characteristics and meat quality are reported postmortem. In the second study, individually penned Suffolk × Mule ewes (n = 12; 76 ± 4.5 kg) were offered the same diets as in the first study. Blood parameters were assessed at the start and after 6 weeks, with intake and excretion into faeces and urine assessed temporally throughout the study.
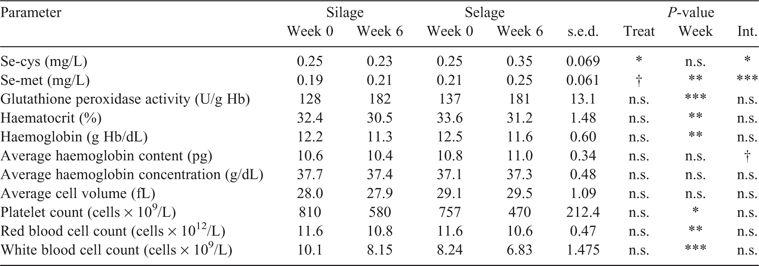
Key results: In the first study, dry-matter (DM) intake was similar in both treatment groups, at 0.8 ± 0.03 kg/day, but Se concentrations of the diets were significantly different, resulting in intakes of ~0.14 and 1.60 mg/day on the Silage and Selage diets, respectively. This was reflected in higher Se concentrations in faeces (0.4 vs 2.0 mg/kg DM; P < 0.001), wool (0.11 vs 0.25 mg/kg DM; P < 0.001), blood (0.19 vs 0.46 mg/L; P < 0.001) and muscle (0.31 vs 0.41 mg/kg: P < 0.01) on the Selage than on the Silage diet. Colour (chroma) shelf life of the meat was significantly higher on the Selage treatment (8.05 vs 9.2 days; P < 0.05). In the second trial, for ewes fed Selage, blood seleno-methionine increased from 0.21 to 0.25 mg/L and seleno-cysteine from 0.25 to 0.35 mg/L after 6 weeks on the treatment, whereas there was no change in ewes fed Silage. Glutathione peroxidase increased, whereas haematocrit, haemoglobin and platelet count were decreased across time during the study, but there was no difference between the treatments.
Conclusions: Nano-Se provided by the Selage treatment was shown to be available to sheep and improve shelf life, with no adverse haematological effects.
Implications: There is potential to use silage inoculants to provide bioavailable Se to ruminants. Further research is required to determine the most appropriate dose for animal performance and product quality.
Additional keywords: lactic acid bacteria, meat quality, nano-Se, sheep production.
Introduction
Selenium (Se) is an essential non-metallic trace element as a component of more than 20 seleno-proteins, which play critical roles in reproduction, thyroid hormone metabolism, DNA synthesis and protection from oxidative damage and infection (Sunde 2006). Typically, human diets in the UK contain roughly 50% of the recommended daily allowance of Se (Rayman 2000). Although Se-deficiency symptoms are rare in humans, subclinical incidences are increasing, with a growing reliance on dietary supplements to provide essential minerals and vitamins (Jackson et al. 2003). Animal products (fish, meat and milk) can be a reliable source of bioavailable Se and, in the classical western diet, dairy and meat are the main sources in the diet (Oster and Prellwitz 1989). Selenium has also been reported to improve sensory characteristics of meat (Joksimovic-Todorovic et al. 2012) and, along with vitamin E, could improve colour properties of the product, although, the response on shelf-life extension for Se is not as clear as with vitamin E (Ripoll et al. 2011; Libien-Jimenez et al. 2015; Rossi et al. 2015). Despite the vital role that animal products can play in Se supply to humans, concentrations of Se can be hampered by low animal uptake, as soils, and subsequently forages, in many parts of the world frequently contain inadequate concentrations of essential minerals to meet the requirements of farmed livestock (Tan et al. 2002; Lee et al. 2018).
An inadequate supply of Se to ruminant livestock can lead to increased health and welfare problems such as retained placenta, high somatic-cell count, mastitis and classic white muscle disease (Weiss et al. 1990; Lee et al. 2018). It is, therefore, common place to provide supplemental Se to reduce the risk of these deficiencies. Currently, ‘on-farm’ mineral supplementation is either by the incorporation of inorganic or organic minerals into complete rations, rumen boluses or by the provision of ad libitum mineral blocks (licks; Mehdi and Dufrasne 2016). Although supplementation with inorganic minerals such as sodium selenite should overcome deficiency, livestock, and especially ruminants, have been reported to absorb inorganic Se less efficiently than they absorb organic forms of Se (Nicholson et al. 1991; Wright and Bell 1966) and it has a high toxicity (Wang et al. 2007). Furthermore, sodium selenite has been reported to be less readily transferred into milk Se than is its organic counterpart, giving rise to additional inadequacies in the supply of Se to calves and lambs and, subsequently, into product (Juniper et al. 2006).
Currently, organic Se is predominately delivered as a selenised yeast supplement, principally in the form of seleno-methionine (Se-met). Inorganic-Se incorporation and conversion to organic forms has also been reported in mushrooms (Maseko et al. 2013), algae (Travnicek et al. 2007; Skřivan et al. 2010) and certain bacteria, including strains of lactic acid bacteria (LAB) converting sodium selenite into organic seleno-amino acids (Calomme et al. 1995; Lee et al. 2019), predominately seleno-cysteine (Se-cys), but also fully reducing it to elemental nano-Se (Eszenyi et al. 2011; Lamberti et al. 2011; Bertolini et al. 2014). This full reduction to elemental nano-Se has also been reported to occur in the rumen by B. ruminicola (Hudman and Glen 1985), although no assessment of availability in ruminants has been conducted as it has been described as inert, insoluble and biologically unavailable (Garbisu et al. 1996). However, in mice, nano-Se has been shown to be an effective antioxidant, with glutathione peroxidase activation being as high as with seleno-amino acids but without any toxicity (compared with sodium selenite; Wang et al. 2007). Nano-Se has also been developed for delivery to humans in probiotic cultures of LAB (Lamberti et al. 2011) and has been shown to be as effective in supplementing pigs as is inorganic Se, but with a lower toxicity (Svoboda et al. 2009). Recent research from our group (Lee et al. 2019) has shown that grass silage LAB inoculants that were cultured in the presence of sodium selenite converted the inorganic form of Se predominately into nano-Se. Here, we determine the potential of nano-Se produced within inoculated grass silage by LAB to act as a dietary supplement of bioavailable Se to sheep and the subsequent impact on meat-product shelf life and haematological parameters when offered at supra-nutritional concentrations because of its potential lower bioavailability.
Materials and methods
Big-bale grass silage was produced using LAB inoculants cultured in the presence or absence of sodium selenite to produce silage with elevated concentrations of nano-Se (Selage; Lee et al. 2019) and a control, respectively. These silages were then used in the following two experiments: (1) a group feeding study with lambs to determine Se uptake into biological tissues and the associated impact on meat quality (shelf life); (2) an individual-penned study with ewes to investigate intake, temporal excretion and haematological impacts of feeding nano-Se-containing silage at a supra-nutritional concentration. All animal procedures and the care for the animals were conducted under strict regulations described in the Animals (Scientific Procedures) Act 1986 issued by the Home Office of Her Majesty’s Britannic Government.
Silage production
A permanent pasture (~Lolium perenne 88%) was selected for the study at Rothamsted Research, North Wyke (UK, Devon) and fertilised with N : P : K : S (20 : 0 : 13 : 7) at a rate of 350 kg/ha on 29 June 2014. On 2 September, the field was mown, and the grass was left to wilt for 24 h. After wilting, the grass was rowed up and every other row was baled with the addition of L. plantarum SSL MC15 at a rate of 2 L/t to provide ~1012 CFU/t fresh weight (FW). The baling was performed with a New Holland BR6090 Crop Cutter that had an inoculant applicator with three spray nozzles fitted. The baler was set to chop the forage to a length of ~5 cm. Once 50% of the total grass cut had been baled (every other row; <1 h), the remaining grass was baled with the addition of L. plantarum SSL MC15 mixed with sodium selenite to provide 0.5 g Se/t FW at the same application rate. Bales were wrapped with four layers of film wrap (750 mm wide, 25 µm thick) and stored on the farm until opening for the first study on 10 November 2014 and the second study on 6 October 2015.
Group-penned study: Se uptake and meat quality
Charollais × Suffolk lambs (20 male and 20 female, mean weight 42 kg ± 1.7 kg, mean age 217 days ± 4.3 days) were paired according to weight and sex, and one of each pair was randomly allocated to one of eight pens, with each pen of five animals acting as the experimental unit. Once all animals had been allocated, the pens were randomly allocated to either control silage (Silage) or silage produced with sodium selenite included in the inoculum (Selage). All animals were housed on soft-wood shavings and had free access to fresh water. Animals were offered only ensiled forage in a manger at the front of each pen daily as either Silage or Selage ad libitum, with enough being offered to ensure 10% refusal. Forage was collected every day as fed and bulked frozen over a period of 7 days for chemical analysis. Dry-matter (DM) weight of forage offered and refused in front of each pen was calculated daily to approximate the intake of the group.
Lamb weights and body condition scores were recorded at the start of the experiment and then on a weekly basis thereafter, for the duration of the experiment. At the start of the experiment and, thereafter, weekly, wool samples were taken from each animal and bulked by pen. Samples were taken from the same area of the skin, under the neck, each week, so that the wool sampled was representative of the weekly growth. Faecal samples were taken weekly and bulked per pen. Each pen of animals was moved to a clean concrete-floored area and left for ~20 min to defaecate; clean faeces was collected from the floor; any faeces contaminated with urine was not collected.
The first slaughter group was selected as the heaviest in each pen after 8 weeks on study; 10 male and 10 female lambs from each treatment were transported to University of Bristol, Langford abattoir. After an additional 2 weeks (10 weeks in total) on the treatments, the remaining 20 lambs were slaughtered following the same procedure, to give two slaughter groups, one at 8 and one at 10 weeks. At slaughter, fresh blood samples were taken with heparin and stored frozen. Hot carcass weights were recorded, excluding kidney knob and channel fat. External fatness and conformation scores were assessed using the European Economic Community (EEC) carcass-classification scheme, as described by Kempster et al. (1986), and then the means were converted back to the EUROP classification score. After dressing, carcasses were transferred to a chiller at 2°C.
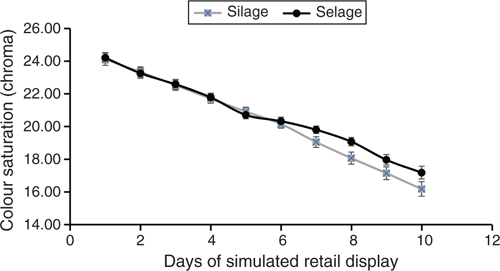
At 48 h after slaughter, carcass samples were removed for analysis. The pH was measured on the M. longissimus between the 10th and 11th rib, with a penetrating probe (Testo Type 01-06) and Testo 230 pH meter (Testo Gmbh, Lenzkirch, Germany). A 250-mm-long section of the hind loin joint containing the M. longissimus lumborum muscle was removed from the left side of the carcass, posterior to the 10th rib, and de-boned. A 20-mm-thick steak was cut, and the muscle was dissected free of subcutaneous adipose tissue, vacuum packed and frozen at –20°C for subsequent analysis of vitamin E as described by Arnold et al. (1993), using 5,7-dimethyl-tocol as the internal standard. A second 20-mm-thick steak was taken for Se analysis and stored frozen before analysis. A section of the loin was vacuum-packed and conditioned at 1°C for a further 8 days. After this, four 20-mm-thick steaks were cut and packed individually in modified-atmosphere packs (MAP, O2 : CO2, 75 : 25) and subjected to simulated retail display (4°C, 700 lx for 16 h). Colour (L*, a*, b*) coordinates (CIE 1986) were measured daily on two of the MAP steaks, on the surface of the steaks, through the film using a Minolta Chromameter CR200 (Minolta Camera Co., Milton Keynes, UK); measurements were taken for the full retail display life of the steaks. The chromameter was standardised against a white tile (L* = 97.78, a* = 0.19, b* = 1.84) covered in the MAP top web film and checked against a red plate (L* = 23.0, a* = 24.3, b* = 11.5), also covered in the MAP top web film. Colour saturation (chroma), which is a measure of the intensity of the red colour, was calculated using the formula [(a*) 2 + (b*) 2] 0.5. Loss of shelf life was determined when chroma fell below a threshold of 18. The other MAP steaks were taken at 8 days of display, trimmed of excess visible fat around the edges, homogenised and subsampled for analysis of lipid oxidation as thiobarbituric acid-reacting substances (TBARS) by the method of Tarladgis et al. (1960), modified using a Buchi 321 distillation unit.
Individual-penned study: intake, excretion and haematological parameters
Suffolk × Mule ewes (n = 12, 76 ± 4.5 kg, mean age 5 ± 1.7 years) were split into six blocks of two according to liveweight and the two animals in each block were allocated at random to the two treatments, so that the average weight of the animal in each of the two treatments was equal. Animals were penned individually, with each animal acting as the experimental unit, and treated for gastrointestinal parasites before the study was begun (Cydectin Triclamox, Fort Dodge, IA, USA). Animals were bedded on soft-wood shavings and had free access to fresh water, which was measured daily to assess water intake. In the first week, all animals were fed daily the control diet (Silage) ad libitum, with an aim of exceeding the intake by, on average, 10% DM. From the second week on, half of the animals were switched to the Selage treatment, with the intake of all animals being restricted to 90% DM intake of the first week, to ensure all feed was consumed. Animals were fed twice daily, with 50% of their feed being offered at 0900 hours and the remaining 50% at 1630 hours. Any refusals were collected and weighed at 0900 hours each morning to calculate the actual intake. Samples of forage were taken at feeding to determine oven-DM content and subsequently DM intake. Offered forage was collected and frozen at each feeding and bulked over a period of 7 days for chemical analysis and determination of Se concentration.
Ewe weights and body condition scores were recorded at the start of the study and then on a weekly basis thereafter, for the duration of the experiment. On the last day of the first week, blood, faecal and urine samples were taken (Week 0, control) and sampling was then repeated weekly for 6 weeks. For each animal, wool was clipped off from a small section of the animal’s neck to expose the jugular vein. Blood samples (2 × 10 mL) from each animal were collected into EDTA-containing vacutainers (Fisher Scientific, Loughborough, Leicestershire, UK) by a needle (20-gauge × 2.5 cm) puncture of the jugular vein. Haematological parameters were assessed from one of the tubes, with the other tube being stored frozen for Se analysis. Health parameters, i.e. haemoglobin, platelets, and red blood cell and white blood cell counts, were determined at the University of Bristol, Bristol Veterinary School, with automated electronic cell counters. A spectrophotometric method was used to measure glutathione peroxidase activity with an assay kit (ab102530, Abcam, Cambridge, UK) as described by Geraghty et al. (2013). Faecal samples were collected using the grab sampling method, by inserting a finger into the anal sphincter. Urination was induced using the method described by Hoogendoorn et al. (2010). The sheep’s vulva was wiped clean of any faecal material before its nasal and oral passages were obstructed by holding a hand tightly over the animal’s mouth and nose. A urine sample was generally produced within 5–10 s of airway obstruction. Obstruction of the airway was halted as soon as ~20 mL of mid-stream urine was collected. If a urine sample was not given within 10 s, a missing value was recorded for that animal for that week. Faecal and urine samples taken across time (temporal sampling) were stored frozen until Se analysis.
Forage-quality prediction
For the ensiled forage (Silage and Selage), a water extract was produced by mixing 50 g FW with 250 mL of deionised water, in a stomacher (Seward Limited, Worthing, West Sussex, UK) for 2 min. A glass electrode of a Jenway 3320-calibrated pH meter was used to measure the pH of the homogenised water extract (Jenway, Stone, Staffordshire, UK). Fresh samples of silage (~200 g FW) were frozen and sent on dry-ice for assessment of fermentation parameters (ammonia-N, volatile fatty acids and lactic acid) and quality predictors (digestibility (D)-value and metabolisable energy) via near-infrared spectrometry (NIRS; Sciantec Analytical Services Ltd, Selby, Yorkshire, UK). The remaining silage was freeze-dried and ground for Se analysis.
Selenium analysis
For total Se, an aliquot of the sample (0.1 g DM of forage, wool, faeces; 0.2 g of DM muscle; 0.1 mL of blood and urine) was accurately weighed into a 40-mL ultra-clean glass digestion vial along with 3 mL of concentrated nitric acid. The digestion vials were heated at 120°C for 1 h on a hot block before 1 mL of hydrogen peroxide was added and were then heated for a further 1 h. The vials were loosely capped to ensure the reflux of the acid during digestion. After the 2-h digestion period, digests were left to cool in a fume cupboard before dilution to the mark with deionised water. An aliquot of the sample digest (0.2 mL) was transferred into a clean 1-mL auto-sampler vessel and diluted to the mark with deionised water. The samples were then analysed by ultra-violet hydrogen-generation atomic fluorescence spectrometry (UV-HG-AFS) by using the Millennium Excalibur System (PS Analytical, Orpington, Kent, UK). The system used 0.7% m/v sodium hydrogen bromide in 0.1 M sodium hydroxide as the reductant and 10% v/v hydrochloric acids as the reagent blank. A pre-reductant solution (50% v/v hydrochloric acid with 5% m/v potassium bromide) was used to reduce selenate (SeVI) to selenite (SeIV) within the apparatus. Calibration standards of 0–20 ng/mL were prepared in reagent blanks.
A one-step enzymatic extraction with protease XIV (Sigma-Aldrich, Gillingham, Dorset, UK) was applied for Se speciation. An aliquot of forage and blood (0.1 g DM and 0.1 mL respectively) was accurately weighed into a 15-mL ultra-clean polypropylene centrifuge tube, followed by the addition of 20 mg of enzyme and 8 mL of phosphate buffer (60 mM, pH 7.4). The samples were then capped tightly and put on an automatic shaker for 24 h at room temperature. After proteolysis, the samples were centrifuged for 10 min (14 000g). The supernatants were filtered by 0.45-μm PTFE syringe filters (Sigma-Aldrich) and transferred into a clean vial. These solutions were then further diluted by deionised water and analysed by high-performance liquid chromatography (HPLC)–UV-HG-AFS using the Millennium Excalibur System (PS Analytical). For the forage samples, all Se that was not identified as inorganic (SeVI or SeIV) or organic (seleno-amino acids, seleno-sugars) was presumed to be nano-Se, as was previously proposed by Eszenyi et al. (2011) and Xia et al. (2007).
Statistical analyses
All forage analyses were performed using a Student’s t-test, with significance considered at P < 0.05. For the group-penned study, DM intake, body condition score, and wool and faecal Se concentrations were analysed using a repeated-measures ANOVA, with the pen mean as the experimental unit. For the post-slaughter parameters, individual animals were used as the experimental unit and blocked according to pen. A general ANOVA was used with the following treatment structure: treatment (Silage vs Selage) × sex × slaughter group (8 vs 10 weeks). Both sex and slaughter group were found not to be significant, and there was no interaction for non-carcass parameters; so, only treatment effects are reported. Shelf life across time (temporal aspect) in the group-penned study and faecal, urine and blood total Se for the individual-penned study were analysed using a repeated-measures ANOVA, with the individual animal as the experimental unit. For the haematological data, a general ANOVA was used with the following treatment structure: treatment (Silage vs Selage) × week (0 vs 6 weeks). All statistical analyses were performed using Genstat 64-bit Release 17.1 (PC/Windows 7) 11 Copyright 2014, VSN International Ltd (Hemel Hempstead, UK).
Results
Group penned study
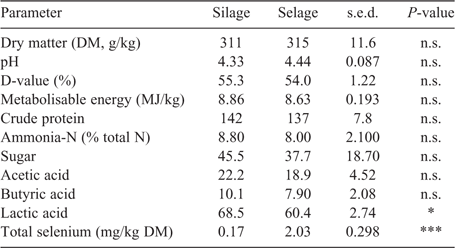
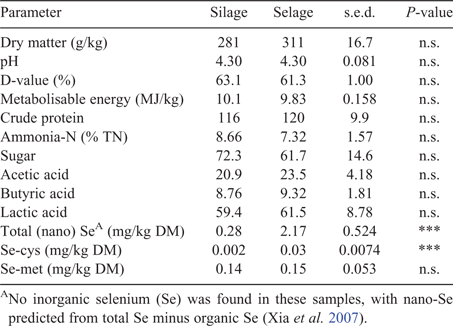
Predicted DM intakes as averaged across individual members of the pen were comparable across treatments, with no temporal difference through the study, with a mean of 0.8 ± 0.03 kg DM/head.day. Mean lamb liveweight was not different between the treatments and remained relatively constant across the study, being 41.3 ± 0.45, 40.9 ± 0.65 and 41.3 ± 1.04 kg in the Silage treatment and 41.4 ± 0.40, 41.5 ± 0.73 and 40.7 ± 0.83 kg in the Selage treatment at 0, 8 and 10 weeks, respectively. Body condition score was slightly higher (P < 0.05) in the Selage treatment at Weeks 8 and 10, being 3.30 ± 0.003, 3.50 ± 0.020 and 3.49 ± 0.043 in the Silage treatment and 3.29 ± 0.024, 3.53 ± 0.014 and 3.54 ± 0.042 in the Selage treatment at 0, 8 and 10 weeks, respectively. Forage quality, as predicted by NIRS and total Se concentration, is reported in Table 1. Both Silage and Selage forages were similar for all predictors of forage quality other than lactic acid, which was higher for Silage than for Selage. Total Se concentration was higher for Selage than Silage by approximately ×10, which would equate to a predicted intake per day for the two groups of 0.14 and 1.6 mg Se/day for Silage and Selage, respectively.
|
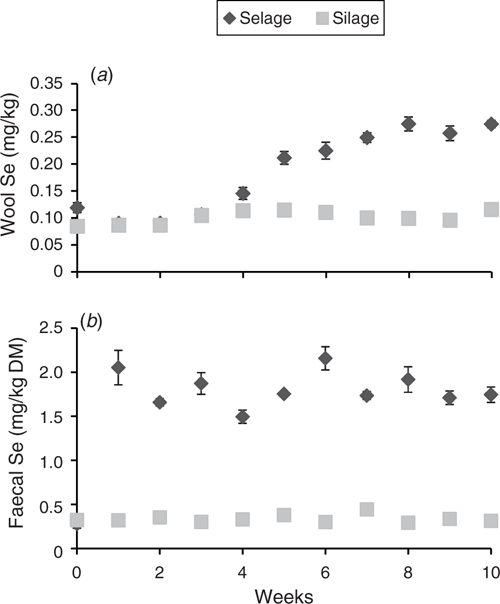
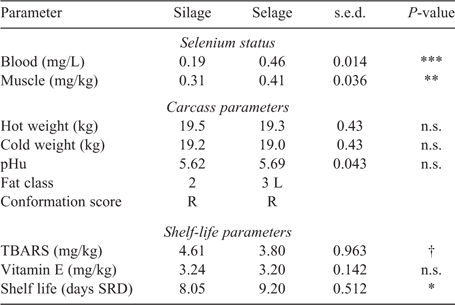
Temporal excretion of Se via faeces and deposition within wool is shown in Fig. 1. A significant rise in Se excretion was observed in the first week for the Selage-fed lambs, with the excretion then reaching a plateau at between 1.5 and 2.0 mg Se/kg DM for the rest of the study. Se excretion from the Silage-fed lambs remained constant throughout the study at ~0.4 mg Se/kg DM (Fig. 1b). Selenium deposition in wool was the same for both Silage- and Selage-fed lambs, at ~0.1 mg Se/kg DM, until Week 4, after which the Selage-fed lambs had higher concentrations in the wool, which reached a plateau at about Week 7, at 0.25 mg Se/kg DM. For Silage-fed lambs, wool Se remained constant across the study period (Fig. 1a). The average Se concentrations of the post-slaughter whole blood and muscle (Longissimus dorsi) are reported in Table 2, with the Se concentration of Selage-fed lambs being significantly higher than that of Silage-finished lambs, by 2.4 times and 1.3 times, respectively.
|
|
Carcass parameters, other than fat class, were not different between Selage- and Silage-finished lambs, with an average kill-out percentage of 47%. Conformation of all carcasses averaged at R, with Selage lambs being slightly fatter at 3 L than Silage lambs, which averaged at 2 (EEC, fat class scores; Table 2). There was a trend for TBARS, as an assessment of fatty acid oxidation of the meat, to be higher in the meat from Silage-fed lambs than in that from Selage-fed lambs, with there being no difference in vitamin E concentration between the treatments. Shelf life reported in days of simulated retail display with a chroma cut-off of 18 (Fig. 2) was significantly higher for Selage-fed lambs, by an average of 1.15 days (Table 2).
|
Individual-penned study
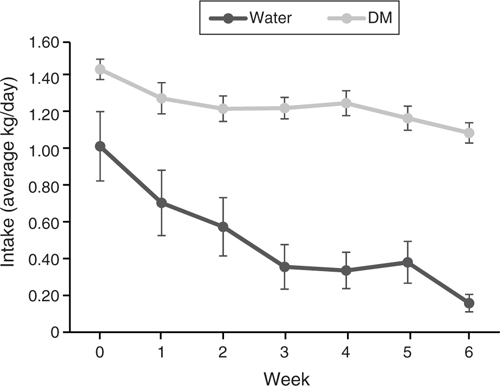
Forage quality, as predicted by NIRS, and Se concentration are reported in Table 3. Silage and Selage forages were comparable in all predictors of forage quality, with Selage providing significantly more Se-cys and total Se, predominately as nano-Se (total Se – inorganic Se – organic Se ≈ nano-Se; Xia et al. 2007), as no inorganic Se was found in any of the samples. Unlike for the group-penned study, DM intake varied across the study period. Despite reducing the offered feed to 90% of ad libitum, assessed at Week 0, ewes failed to consume their full allocation, with a non-significant decline in Week 5 and a significant decline in Week 6 for both Silage and Selage ewes (Fig. 3). This resulted in mean Se intakes of 0.35 and 2.7 mg/day from Week 1 to Week 5 and 0.30 and 2.4 mg/day for Week 6 for Silage- and Selage-fed ewes, respectively. Water intake declined throughout the study, but there was no difference between the treatments (Fig. 3). Correspondingly, there was no difference in liveweight between the treatments; however, ewes, on average, lost weight from a mean of 76.3 to a mean of 74.4 ± 0.590 during the 6-week study; however, no change was observed in the body condition score (3.07 ± 0.196) between the treatments and across the time period of the study.
|
|
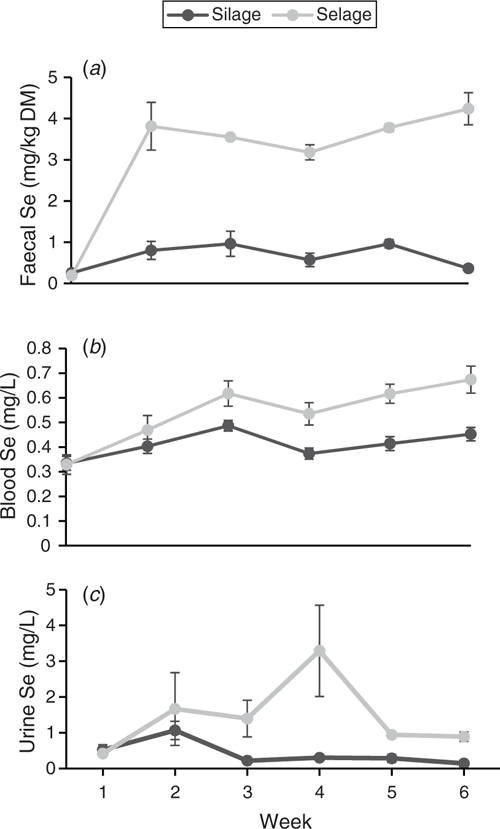
Temporal patterns of total Se in faeces, blood and urine are reported in Fig. 4. For faecal Se concentration, a pattern similar to that in the group-penned study was observed, with a rise in the Selage-fed animals in Week 1, reaching a plateau at ~4.0 mg/kg DM, declining slightly to Week 4 and then rising subsequently to Week 6. Faecal excretion of Se for the Silage-fed ewes was between 0.5 and 1.0 mg/kg DM for the duration of the study, with a drop in the concentration at Weeks 4 and 6 (Fig. 4a). Whole-blood total Se concentration showed similar patterns in both treatments, with a rise to Week 3, then reaching a plateau, although there was a non-significant drop in both treatments at Week 4. Selage-fed ewes exhibited significantly higher whole-blood total Se concentration from Week 3 than did the Silage-fed ewes (Fig. 4b). Urinary excretion of Se was more variable for Selage- than Silage-fed ewes. Both sets of ewes showed a rise to Week 2, and, after that, Silage-fed ewes showed a decline back to Week 1 concentrations from Week 3 and remained there for the rest of the study. Selage-fed ewes showed no decline from Week 2 to Week 3, and showed a sharp increase from Week 3 to Week 4, but with a high variation among animals. Urinary Se concentration then declined to Week 2 levels at Week 5, and this continued into Week 6 (Fig. 4c).
|
Blood Se-cys and Se-met were not different between the treatment groups at Week 0; however, at Week 6, Selage-fed ewes showed a significant increase in both seleno-amino acids, with no increase being observed for the corresponding values in Silage-fed ewes (Table 4). The increase in blood Se-met was smaller (×1.2) than the corresponding increase in blood Se-cys (×1.4) in the Selage-fed ewes, over the 6-week study. There was no difference in glutathione peroxidase or any of the blood-health indicators between the treatments; however, there were significant temporal effects (Week 0 vs Week 6). Glutathione peroxidase activity increased significantly between Week 0 and Week 6, whereas haematocrit, haemoglobin, platelets, red blood cells and white blood cells all significantly reduced during the 6-week study (Table 4). There was a trend for an interaction effect, with the average haemoglobin concentration in Selage-fed lambs showing an increase and in Silage-fed lambs a decrease over the 6 weeks; there was no significant difference across the whole study for either average haemoglobin concentration or average cell volume.
Discussion
Forage quality and Se content
Near-infrared spectrometry prediction for key forage-quality analyses was used in the absence of specific measured values (Beever and Mould 2000). For both studies, the nutritional differences between Silage and Selage cut from the same field at the same harvest were perceived to be small and, so, indicative quality parameters were determined. As expected, little difference was observed for most predicted quality parameters, other than a slightly lower lactic acid concentration for Silage than for Selage in the group-penned lamb study. Seppälä et al. (2014) also reported a reduced lactic acid production at the highest inclusion level of sodium selenate (500 mg/kg) within a silage acid additive treatment. This may have been due to the reduced growth rates of LAB reported in the presence of inorganic selenium (Xia et al. 2007; Lamberti et al. 2011; Lee et al. 2019). However, the same response was not observed for the conserved forages fed to the individual-penned ewes and no effect was seen in other forage-quality parameters, as was also reported by Seppälä et al. (2014). Generally, the silage quality in the two studies was poor, which was related more to the original forage harvested rather than to ensiling conditions, with DM and pH being in the range typical for big-bale silage in the UK, namely, 300–350 g/kg and 4.00–4.50, respectively (AHDB 2011). Metabolisable energy and digestibility (D-value) were low, reflecting the poor nutritional value of the forage in both studies. Total Se, predominately in the form of nano-Se (neither inorganic or organic), was higher (×10) in Selage than Silage, as predicted, with values being similar for both big-bale batches in the two studies. Selenium speciation of the forages (Table 3) showed no selenite or selenate but the seleno-amino acids were different between Silage and Selage, with Se-cys being significantly higher in the latter, whereas there was no difference in Se-met. This difference reflects the reported conversion of sodium selenite into Se-cys (up to the biological limit) and a further reduction of the remaining sodium selenite into elemental nano-Se by LAB (Lee et al. 2019; Calomme et al. 1995; Xia et al. 2007; Eszenyi et al. 2011). The Se-met concentration reflects the Se concentration of the ensiled forage, as LAB in the presence of sodium selenite have been shown to have a low Se-met concentration (Lee et al. 2019).
Intake, uptake and excretion of Se
Selenium concentrations (mg/kg DM) in ruminant (cattle, sheep and goats) rations have typically been classified as follows: deficient (<0.10); marginal (0.10–0.25); adequate (0.30–1.00); high (3.00–4.00); toxic-chronic (>5.0); and toxic-acute (>80; Puls 1988; Mehdi and Dufrasne 2016). However, the form the Se takes within in the ration is not included in such assessment or guidance, yet may influence absorption (uptake), bioavailability and toxicity of the Se supplement. Whereas most studies (Nicholson et al. 1991, dairy and beef calves; Juniper et al. 2006, dairy cattle; Ehlig et al. 1967, lambs; Qin et al. 2007, lambs; Travnicek et al. 2007, suckled lambs) have reported an increased uptake and bioavailability with organic Se (Se-met) compared with inorganic Se (selenite), others have reported little effect, or the opposite response (Koenig et al. 1997, sheep; Johansson et al. 1990, lambs). No reports are available on the uptake/bioavailability of nano-Se in livestock; however, two studies, namely Qin et al. (2007) and Svoboda et al. (2009), have reported on feeding of Se-enriched LAB to lambs and pigs, respectively. Although both studies failed to fully speciate the Se within the LAB, Qin et al. (2007) reported a greater uptake and bioavailability for Se-enriched LAB than for selenite, but lower than for Se-enriched yeast. In contrast, Svoboda et al. (2009) reported no difference between Se-enriched LAB and selenite in pigs. In terms of toxicity of the forms of Se, there is less ambiguity, with the toxicity reducing as follows in all animal species: inorganic (selenite) >> organic (Se-met) >> elemental (nano-Se; Sunde 2006; Wang et al. 2007). In the current study, we assessed the potential of nano-Se being produced through the conversion of toxic inorganic Se (selenite) by silage LAB as a dietary supplement to sheep. As the uptake/bioavailability of nano-Se has not been previously assessed and questions have been raised regarding its biological worth in ruminants due to its inert nature (Hudman and Glen 1985; Hakkarainen 1993), conserved forage was prepared (Selage), containing ~2.5 mg Se/kg DM (supra-nutritional), which is between adequate and high according to the classification (see above) to fully assess the uptake potential versus conserved forage (Silage) from UK soils, which are in the upper 90th percentile for Se (North Wyke Research Farm; 50°46′10″N, 3°54′05″W). As such, the control silage (Silage) had a high concentration of Se, namely 0.17–0.28 mg/kg DM (predominately as Se-met; Lee et al. 2018), and the Se supplemented-silage (Selage) had a Se concentration of between 2.0 and 2.2 mg/kg DM (predominately as nano-Se). These concentrations for the two studies resulted in intakes of 0.14 and 1.6 mg Se/day for the group-penned lambs and 0.30–0.35 and 2.4–2.7 mg Se/day for the individual-penned ewes, for Silage and Selage, respectively.
Uptake of Se across the study period (temporal aspect) in the group-penned lamb study was assessed in wool, which saw an increase after 4 weeks on the diet, reaching a plateau at ~0.25 mg Se/kg DM for the Selage treatment. Wool Se concentration varies based on the intake of bioavailable Se, with typical values being between 0.05 and 0.1 mg Se/kg DM on forage diets (Andrews et al. 1976), as also reported with the Silage lambs in the current study. Peak Se concentration in wool has been reported to be ~0.2 mg Se/kg DM, which aligns with our findings at plateau values, when sheep and goats were ‘Se-loaded’, with fly ash-grown sweet clover (Melilotus albus) providing ~15 mg Se/kg DM (Furr et al. 1978). This would, therefore, suggest that the supplied nano-Se within the Selage was available through absorption, counteracting the view of complete inertness previously reported (Hakkarainen 1993) and was supra-nutritional in reaching the maximum uptake into wool within the group-penned lamb study. Selenium concentrations in the muscle of the Selage-fed lambs were comparable to those reported by Qin et al. (2007) where lambs were supplemented with 0.16 mg Se/kg either as Se-enriched LAB or as Se-enriched yeast. The control group in their study, which had an intake of 0.06 mg Se/kg diet, had a significantly lower Se concentration in the muscle, at ~0.09 mg Se/kg, than did the control lambs (Silage) in the present study, at 0.31 mg Se/kg. However, the control diet in the current study had Se at concentrations similar to those of the supplemented diet in the study of Qin et al. (2007), suggesting, and being supported by the wool data, that the level supplied by the Selage was supra-nutritional and that the Silage animals were not deficient.
Total whole-blood Se in sheep has been reported to range between 0.02 and 0.36 mg/L (Ademi et al. 2017). Qin et al. (2007) and Cobanova-Boldizarova et al. (2008), when supplementing with either Se-enriched LAB or yeast at 0.3 mg Se/kg DM in the diet, recorded concentrations of ~0.3 mg/L in the whole blood. Similar whole-blood concentrations were found with the Silage diet in the present study, whereas they were higher with the Selage diet, being ~0.46 and 0.60 mg/L in the lamb and ewe studies, respectively. The high Se concentration in the whole blood was predominately accounted for, as expected, by organic Se-cys and Se-met (Table 4). The Se-met pool is unregulated as mammalian enzymes do not distinguish between methionine and Se-met. Se-met is, therefore, incorporated into any protein in place of methionine, with Se being available for seleno-protein formation only during amino acid catabolism of Se-met (Sunde 2006). Furthermore, higher animals cannot synthesise Se-met and, so, Se concentration is completely determined by intake. This explains the similar concentrations of Se-met at 0 and 6 weeks in the individual-penned ewe study and the small differential between the treatments, despite the large difference in the total Se intake, as Se-met intake was comparable between Silage and Selage. In contrast, Se-cys biological pool is highly regulated and is specifically encoded into seleno-proteins for functional metabolism (Sunde 2006). The increase in Se-cys concentration from 0 to 6 weeks in the ewes receiving Selage treatment is representative of an increased seleno-protein formation. Although glutathione peroxidase activity did not increase (discussed below), a range of seleno-proteins, such as the Se-carrier selenoprotein P, ~40% of total blood Se, may have increased due to the greater availability of Se for animals on the Selage diet, and its vital role in Se homeostasis (Burk and Hill 2005).
Excretion of Se into manure/faeces has been reported for the major livestock species, with the ratio of faecal : diet concentration being 2.8, 2.2, 2.1 and 1.4 for poultry, pigs, dairy cattle and beef cattle, respectively (Sheppard and Sanipelli 2012). This is contrary to the result of Cobanova-Boldizarova et al. (2008) who reported that excretion of Se through faeces would be higher for ruminants than for monogastrics due to the conversion of inorganic Se into unavailable elemental/nano-Se in the rumen. In the current study, the ratio of faecal : diet concentration was 2.4 and 1.0 in lambs and 2.3 and 1.8 in ewes receiving Silage and Selage treatments, respectively. This suggested that although a high volume of Se was excreted by ewes on the Selage treatment, which was clearly supra-nutritional, a higher proportion was also absorbed by ewes on this treatment than with the Silage diet, indicating an uptake of Se from LAB-produced nano-Se. However, absorption/uptake does not directly relate to the efficiency of use if excretion is via other routes. Urinary Se excretion in sheep appears to vary considerably depending of the form of Se, status of the animal and dose supplied. Koenig et al. (1997), when offering Se as either enriched yeast or selenite, reported that 7–10% was excreted in urine, whereas the control, unsupplemented animals secreted 18–24% of Se in urine. In the ‘Se-loaded’ study of Furr et al. (1978), urinary Se concentrations increased by 200 times, whereas faecal excretion increased only by 45 times. For sheep on low-Se diets, within a negative Se balance, urinary excretion can be 40–50% of the intake (Langlands et al. 1986). In the current study, the Se in urine across the time period and among animals was the most variable on the Selage diet. These variabilities in the Se excretion in urine reflect the important homeostatic control that urinary excretion has with Se (Sunde 2006). The final significant excretion route, which was not assessed in the current study but does require further investigation, is via deimethyl selenide in breath, which is also influenced by the form of Se (Davis et al. 2013).
Meat/carcass quality and haematological parameters
Seleno-proteins iodothyronine 5-deiodinase and thioredoxin reductase are involved in metabolism and growth through regulation of thyroid hormones (Mehdi and Dufrasne 2016; Sunde 2006). Subsequently, Se deficiency is associated with reduced growth rates. However, in the present study, Se was not deficient in either treatment, with there being no impact on animal performance, carcass weight or conformation, although the Selage lambs were slightly fatter at slaughter and had a greater body condition score during the study. The literature on the influence of Se on carcass quality and lipid deposition is inconclusive due to the variation in the form of Se and supplementation rate, with some studies in cattle reporting no effect (Juniper et al. 2008; Taylor et al. 2008) and others reporting a decrease in lipids (Netto et al. 2014; Mehdi et al. 2015). Reports on carcass quality in lambs supplemented with nano-Se are, to the best of the authors’ knowledge, not available; however, a study in pigs supplemented with Se-enriched LAB found no difference in carcass parameters from those supplemented with sodium selenite or Se-enriched yeast (Svoboda et al. 2009).
Ripoll et al. (2011) investigated the impact of Se and vitamin E separately and in combination on the shelf life in lambs. They found that vitamin E alone or in combination could extend the shelf life of lambs by 4 days, whereas Se alone, although increasing the lightness of the meat, had no effect on the shelf life. This contrasts with the results of the present study, which showed a longer shelf life and lower TBARS in the Selage- than the Silage-fed lambs, despite similar vitamin E concentration. It has been suggested that Se (through glutathione peroxidase) and vitamin E are inter-operable in protecting against oxidation damage through free-radical attack, as exemplified through TBARS. In the current study, vitamin E was comparable to the previously reported concentrations in forage-raised lambs, with high oxidative stability (Turner et al. 2002) in both Silage- and Selage-finished lambs. Therefore, the higher oxidative stability in Selage-fed lambs would suggest a potential additive role of Se, in contrast to the results of Ripoll et al. (2011). However, unfortunately, in the lamb group-penned study, glutathione peroxidase activity was not assessed. In the ewe study, glutathione peroxidase activity was not different between Silage and Selage treatments, with both being in the ‘adequate range’ (Counotte and Hartmans 1989). Similarly, Chauhan et al. (2015) failed to observe a response in blood glutathione peroxidase in sheep offered selenised yeast at 1.2 mg/kg DM. This may be associated with the regulated nature of seleno-protein transcription and, therefore, highlights the issues in using total blood Se concentration in determining the functional-Se status.
Due to the supra-nutritional concentrations of Se used in the present study, haematological parameters were determined to assess toxicity. For both treatments, all parameters were within a normal biological reference range for sheep, with animals showing no signs of selenosis. The temporal effects on haematocrit, haemoglobin, platelet cells, red blood cell count and white blood cell count are likely to be a response to the length of time on the poor-quality silage that may have been deficient in iron and cobalt (not assessed).
Conclusions
Nano-Se supplied through the conversion of sodium selenite by silage-LAB can be used as a source of bioavailable Se for sheep. The concentration of Se in the study (~2 mg/kg DM) was supra-nutritional, as observed with a high level of excretion in faeces and urine compared with the control treatment (~0.2 mg/kg DM). Although increases in the whole-blood Se-cys were observed, more research is required to determine the optimum level of supplementation of nano-Se and more direct comparisons at comparable intakes of organic (Se-met) and inorganic (selenite) forms. The current study showed no signs of selenosis at the levels of supplementation of nano-Se used in the study, but there was an indication of an improved shelf life.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The work was funded through a BBSRC Technology Strategy Board Project ‘Selage’ (TS/J0003069/1), with further support from a BBSRC Institute Strategic Program Grant ‘Soil to Nutrition’ (BS/E/C/000I0320). The industry consortium supporting the work consisted of Silage Solutions Ltd and Kelvin Cave Ltd. Dr P. T. Suraj was funded by a Global Innovation Initiative project (British Council) as part of the Global Farm Platform, an international initiative linking research farms around the globe to develop solutions for sustainable ruminant livestock production (www.globalfarmplatform.org).
References
Ademi A, Bernhoft A, Govasmark E, Bytyqi H, Sivertsen T, Singh BR (2017) Selenium and other mineral concentrations in feed and sheep’s blood in Kosovo. Translational Animal Science 1, 97–107.AHDB (2011) ‘Making grass silage for better return.’ Agriculture and Horticulture Development Board, Better Returns Programme. EBLEX, Huntingdon, Cambridgeshire, UK.
Andrews ED, Hogan KG, Sheppard AD (1976) Selenium in soils, pastures and animal tissues in relation to the growth of young sheep on a marginally selenium-deficient area. New Zealand Veterinary Journal 24, 111–121.
Arnold RN, Scheller KK, Arp SC, Williams SN, Schaefer D (1993) Dietary α-tocopherol acetate enhances beef quality in Holstein and beef breed steers. Journal of Food Science 58, 28–33.
| Dietary α-tocopherol acetate enhances beef quality in Holstein and beef breed steers.Crossref | GoogleScholarGoogle Scholar |
Beever DE, Mould FL (2000) Forage evaluation for efficient ruminant livestock production. In ‘Forage evaluation in ruminant nutrition’. (Ed. D Givens) pp. 15–42. (CABI Publishing: Wallingford, Oxfordshire, UK )
Bertolini C, van Aerle R, Lampis S, Moore KA, Paszkiewicz K, Butler CS, Vallini G, van der Giezen M (2014) Draft genome sequence of Stenotrophomonas maltophilia SeITE02, a gammaproteobacterium isolated from selenite-contaminated mining soil. Genome A. 2, e00331-14
| Draft genome sequence of Stenotrophomonas maltophilia SeITE02, a gammaproteobacterium isolated from selenite-contaminated mining soil.Crossref | GoogleScholarGoogle Scholar | 24812214PubMed |
Burk RF, Hill KE (2005) Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annual Review of Nutrition 25, 215–235.
| Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis.Crossref | GoogleScholarGoogle Scholar | 16011466PubMed |
Calomme M, Hu J, van den Branden K, van den Berghe DA (1995) Seleno-lactobacillus. An organic selenium source. Biological Trace Element Research 47, 379–383.
| Seleno-lactobacillus. An organic selenium source.Crossref | GoogleScholarGoogle Scholar | 7779573PubMed |
Chauhan SS, Celi P, Leury BJ, Dunshea FR (2015) High dietary selenium and vitamin E supplementation ameliorates the impacts of heat load on oxidative status and acid-base balance in sheep. Journal of Animal Science 93, 3342–3354.
| High dietary selenium and vitamin E supplementation ameliorates the impacts of heat load on oxidative status and acid-base balance in sheep.Crossref | GoogleScholarGoogle Scholar | 26440003PubMed |
CIE (1986) ‘Colorimetry.’ 2nd edn. Publication CIE 15.2. (Commision International de l’Eclairage: Vienna, Austria)
Cobanova-Boldizarova K, Gresakova L, Faix S, Petrovic V, Leng L (2008) Selenium in sheep nutrition. In ‘Current advances in selenium research and application. Part 1’. (Eds PF Surai, JA Taylor-Pickard) pp. 209–220. (Wageningen Academic Publishers: Wageningen, The Netherlands)
Counotte GHM, Hartmans J (1989) Relation between selenium content and glutathione peroxidase activity in blood of cattle. The Veterinary Quarterly 11, 155–160.
| Relation between selenium content and glutathione peroxidase activity in blood of cattle.Crossref | GoogleScholarGoogle Scholar |
Davis TZ, Stegelmeier BL, Green BT, Welch KD, Hall JO (2013) Evaluation of the respiratory elimination kinetics of selenate and Se-methylselenocysteine after oral administration in lambs. Research in Veterinary Science 95, 1163–1168.
| Evaluation of the respiratory elimination kinetics of selenate and Se-methylselenocysteine after oral administration in lambs.Crossref | GoogleScholarGoogle Scholar | 24210249PubMed |
Ehlig CF, Hogue DE, Allaway WH, Hamm DJ (1967) Fate of selenium from selenite or seleno-methionine, with or without vitamin E, in lambs. The Journal of Nutrition 92, 121–126.
| Fate of selenium from selenite or seleno-methionine, with or without vitamin E, in lambs.Crossref | GoogleScholarGoogle Scholar | 6028298PubMed |
Eszenyi P, Sztrik A, Babka B, Prokisch J (2011) Elemental, nano-sized (100–500 nm) selenium production by probiotic lactic acid bacteria. International Journal of Bioscience, Biochemistry and Bioinformatics 1, 148–152.
Furr AK, Parkinson TF, Heffron CL, Reid JT, Haschek WM, Gutenmann WH (1978) Elemental content of tissues and excreta of lambs, goats and kids fed white sweet clover growing on fly ash. Journal of Agricultural and Food Chemistry 26, 847–851.
| Elemental content of tissues and excreta of lambs, goats and kids fed white sweet clover growing on fly ash.Crossref | GoogleScholarGoogle Scholar | 670567PubMed |
Garbisu C, Ishii T, Leighton T, Buchanan BB (1996) Bacterial reduction of selenite to elemental selenium. Chemical Geology 132, 199–204.
| Bacterial reduction of selenite to elemental selenium.Crossref | GoogleScholarGoogle Scholar |
Geraghty P, Hardigan AA, Wallace AM, Mirochnitchenko O, Thankachen J, Arellanoa L, Thompson V, D’Armiento JM, Foronjy RF (2013) The glutathione peroxidase 1-protein tyrosine phosphatase 1B-protein phosphatase 2A axis. A key determinant of airway inflammation and alveolar destruction. American Journal of Respiratory Cell and Molecular Biology 49, 721–730.
| The glutathione peroxidase 1-protein tyrosine phosphatase 1B-protein phosphatase 2A axis. A key determinant of airway inflammation and alveolar destruction.Crossref | GoogleScholarGoogle Scholar | 23590304PubMed |
Hakkarainen J (1993) Bioavailability of selenium. Norwegian Journal of Agricultural Sciences 11, 21–35.
Hoogendoorn CJ, Betteridge K, Costall DA, Ledgard SF (2010) Nitrogen concentration in the urine of cattle, sheep and deer grazing a common ryegrass/cocksfoot/white clover pasture. New Zealand Journal of Agricultural Research 53, 235–243.
| Nitrogen concentration in the urine of cattle, sheep and deer grazing a common ryegrass/cocksfoot/white clover pasture.Crossref | GoogleScholarGoogle Scholar |
Hudman JF, Glen AR (1985) Selenium uptake by Butyrivibrio fibrisolvens and Bacteroides ruminicola. FEMS Microbiology Letters 27, 215–220.
| Selenium uptake by Butyrivibrio fibrisolvens and Bacteroides ruminicola.Crossref | GoogleScholarGoogle Scholar |
Jackson MJ, Broome CS, McArdle F (2003) Marginal dietary selenium intakes in the UK: are there functional consequences? The Journal of Nutrition 133, 1557S–1559S.
| Marginal dietary selenium intakes in the UK: are there functional consequences?Crossref | GoogleScholarGoogle Scholar | 12730465PubMed |
Johansson E, Jacobson SO, Luthman J, Lindh U (1990) The biological response of selenium in individual erythrocytes and GSH-Px in lambs fed sodium selenite or selenium yeast. Journal of Veterinary Medicine 37, 463–470.
| The biological response of selenium in individual erythrocytes and GSH-Px in lambs fed sodium selenite or selenium yeast.Crossref | GoogleScholarGoogle Scholar |
Joksimovic-Todorovic M, Davidovic V, Sretenovic L (2012) The effect of diet selenium supplement on meat quality. Biotechnology in Animal Husbandry 28, 553–561.
| The effect of diet selenium supplement on meat quality.Crossref | GoogleScholarGoogle Scholar |
Juniper DT, Phipps RH, Jones AK, Bertin G (2006) Selenium supplementation of lactating dairy cows: effect on selenium concentration in blood, milk, urine and feces. Journal of Dairy Science 89, 3544–3551.
| Selenium supplementation of lactating dairy cows: effect on selenium concentration in blood, milk, urine and feces.Crossref | GoogleScholarGoogle Scholar | 16899690PubMed |
Juniper DT, Phipps RH, Ramos-Morales E, Bertin G (2008) Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in beef cattle. Journal of Animal Science 86, 3100–3109.
| Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in beef cattle.Crossref | GoogleScholarGoogle Scholar | 18567732PubMed |
Kempster AJ, Cook GL, Grantley-Smith M (1986) National estimates of the body composition of British cattle, sheep and pigs with special reference to trends in fatness. A review. Meat Science 17, 107–138.
| National estimates of the body composition of British cattle, sheep and pigs with special reference to trends in fatness. A review.Crossref | GoogleScholarGoogle Scholar | 22055218PubMed |
Koenig KM, Rode LM, Cohen RDH, Buckley WT (1997) Effects of diet and chemical form of selenium on selenium metabolism in sheep. Journal of Animal Science 75, 817–827.
| Effects of diet and chemical form of selenium on selenium metabolism in sheep.Crossref | GoogleScholarGoogle Scholar | 9078502PubMed |
Lamberti C, Mangiapane E, Pessione A, Mazzoli R, Giunta C, Pessione E (2011) Proteomic characterization of a selenium-metabolizing probiotic Lactobacillus reuteri Lb2 BM for nutraceutical applications. Proteomics 11, 2212–2221.
| Proteomic characterization of a selenium-metabolizing probiotic Lactobacillus reuteri Lb2 BM for nutraceutical applications.Crossref | GoogleScholarGoogle Scholar | 21548091PubMed |
Langlands JP, Bowles JE, Donald GE, Smith AJ (1986) Selenium excretion in sheep. Australian Journal of Agricultural Research 37, 201–209.
| Selenium excretion in sheep.Crossref | GoogleScholarGoogle Scholar |
Lee MRF, Rivero MJ, Cone J (2018) The role of pasture in the diet of ruminants. In ‘Improving grassland and pasture management in agriculture’. (Eds A Marshall, R Collins) pp. 31–49. (Burleigh Dodds Science Publishing: Cambridge, UK)
Lee MRF, Fleming HR, Cogan T, Hodgson C, Davies D (2019) Assessing the ability of silage lactic acid bacteria to incorporate and transform inorganic selenium within laboratory scale silos. Animal Feed Science and Technology 253, 125–134.
| Assessing the ability of silage lactic acid bacteria to incorporate and transform inorganic selenium within laboratory scale silos.Crossref | GoogleScholarGoogle Scholar | 31293291PubMed |
Libien-Jimenez Y, Mariezcurrena-Berasain MD, de la Fuente JD, Salem AZM, Kholif AE, Vaca-Paulin R, Mariezcurrena-Berasain MA (2015) Effect of organic selenium supplementation in the diets of finishing sheep on meat color and pH during shelf life. The Indian Journal of Animal Sciences 49, 652–657.
Maseko T, Callahan DL, Dunshea FR, Doronila A, Kolev SD, Ng K (2013) Chemical characterization and speciation of organic selenium in cultivated selenium-enriched Agaricus bisporus. Food Chemistry 141, 3681–3687.
| Chemical characterization and speciation of organic selenium in cultivated selenium-enriched Agaricus bisporus.Crossref | GoogleScholarGoogle Scholar | 23993536PubMed |
Mehdi Y, Dufrasne I (2016) Selenium in cattle: a review. Molecules 21, 545
| Selenium in cattle: a review.Crossref | GoogleScholarGoogle Scholar | 27120589PubMed |
Mehdi Y, Clinquart A, Hornick J-L, Cabaraux J-F, Istasse L, Dufrasne I (2015) Meat composition and quality of young growing Belgian Blue bulls offered a fattening diet with selenium enriched cereals. Canadian Journal of Animal Science 95, 465–473.
| Meat composition and quality of young growing Belgian Blue bulls offered a fattening diet with selenium enriched cereals.Crossref | GoogleScholarGoogle Scholar |
Netto AS, Zanetti MA, Claro GR, de Melo MP, Vilala FG, Correa LB (2014) Effects of copper and selenium supplementation on performance and lipid metabolism in confined Brangus bulls. Asian-Australasian Journal of Animal Sciences 27, 488–494.
| Effects of copper and selenium supplementation on performance and lipid metabolism in confined Brangus bulls.Crossref | GoogleScholarGoogle Scholar | 25049978PubMed |
Nicholson JWG, McQueen RE, Bush RS (1991) Response of growing cattle to supplementation with organically bound or inorganic sources of selenium or yeast cultures. Canadian Journal of Animal Science 71, 803–811.
| Response of growing cattle to supplementation with organically bound or inorganic sources of selenium or yeast cultures.Crossref | GoogleScholarGoogle Scholar |
Oster O, Prellwitz W (1989) The daily dietary selenium intake of West German adults. Biological Trace Element Research 20, 1–14.
| The daily dietary selenium intake of West German adults.Crossref | GoogleScholarGoogle Scholar | 2484388PubMed |
Puls R (1988) ‘Mineral levels in animal health.’ (Sherpa International: Clearbrook, British Columbia)
Qin S, Gao J, Huang K (2007) Effects of different selenium sources on tissue selenium concentrations, blood GSH-Px activities and plasma interleukin levels in finishing lambs. Biological Trace Element Research 116, 91–102.
| Effects of different selenium sources on tissue selenium concentrations, blood GSH-Px activities and plasma interleukin levels in finishing lambs.Crossref | GoogleScholarGoogle Scholar | 17634631PubMed |
Rayman MP (2000) The importance of selenium to human health. Lancet 356, 233–241.
| The importance of selenium to human health.Crossref | GoogleScholarGoogle Scholar | 10963212PubMed |
Ripoll G, Joy M, Munoz F (2011) Use of dietary vitamin and selenium (Se) to increase the shelf life of modified atmosphere packaged light lamb meat. Meat Science 87, 88–93.
| Use of dietary vitamin and selenium (Se) to increase the shelf life of modified atmosphere packaged light lamb meat.Crossref | GoogleScholarGoogle Scholar | 20920835PubMed |
Rossi CAS, Compiani R, Baldi G, Bernardi CEM, Muraro M, Marden J-P, Dell’Orto V (2015) The effect of different selenium sources during the finishing phase on phase on beef quality Journal of Animal and Feed Sciences 24, 93–99.
| The effect of different selenium sources during the finishing phase on phase on beef qualityCrossref | GoogleScholarGoogle Scholar |
Seppälä A, Albarran YM, Miettinen H, Siguero MP, Juutinen E, Rinne M (2014) Selenium supplementation by addition of sodium selenate with silage additive. Agricultural and Food Science 23, 81–88.
| Selenium supplementation by addition of sodium selenate with silage additive.Crossref | GoogleScholarGoogle Scholar |
Sheppard SC, Sanipelli B (2012) Trace elements in feed, manure and manured soils. Journal of Environmental Quality 41, 1846–1856.
| Trace elements in feed, manure and manured soils.Crossref | GoogleScholarGoogle Scholar | 23128741PubMed |
Skřivan M, Skřivanova V, Dlouha G, Branyikova I, Zachleder V, Vitova M (2010) The use of selenium-enriched alga Scenedesmus quadricauda in a chicken diet. Czech Journal of Animal Science 55, 565–571.
| The use of selenium-enriched alga Scenedesmus quadricauda in a chicken diet.Crossref | GoogleScholarGoogle Scholar |
Sunde RA (2006) Selenium. In ‘Present knowledge in nutrition.’ 9th edn. (Eds BA Bowman, RM Russell) pp. 480–497. (ILSI Press: Washington, DC)
Svoboda M, Salakova A, Fajt Z, Ficek R, Buchtova H, Drabek J (2009) Selenium from Se-enriched lactic acid bacteria as a new Se source for growing-finishing pigs. Polish Journal of Veterinary Sciences 12, 355–361.
Tan J, Zhu W, Wang W, Li R, Hou S, Wang D, Yang L (2002) Selenium in soil and endemic disease in China. STOTEN 284, 227–235.
Tarladgis BG, Watts BM, Younathan MT, Dugan L (1960) A distillation method for the quantitative determination of malonaldehyde in rancid foods. Journal of the American Oil Chemists’ Society 37, 44–48.
| A distillation method for the quantitative determination of malonaldehyde in rancid foods.Crossref | GoogleScholarGoogle Scholar |
Taylor JB, Marchello MJ, Finley JW, Neville TL, Combs GF, Caton JS (2008) Nutritive value and display-life attributes of selenium-enriched beef-muscle foods. Journal of Food Composition and Analysis 21, 183–186.
| Nutritive value and display-life attributes of selenium-enriched beef-muscle foods.Crossref | GoogleScholarGoogle Scholar |
Travnicek J, Pisek L, Herzig I, Doucha J, Kvicala J, Kroupova V, Rodinova H (2007) Selenium content in the blood serum and urine of ewes receiving selenium-enriched unicellular alga Chlorella. Veterinarni Medicina 52, 42–48.
| Selenium content in the blood serum and urine of ewes receiving selenium-enriched unicellular alga Chlorella.Crossref | GoogleScholarGoogle Scholar |
Turner KE, McClure KE, Weiss WP, Borton RJ, Foster JG (2002) Alpha-tocopherol concentrations and case life of lamb muscle as influenced by concentrate or pasture finishing. Journal of Animal Science 80, 2513–2521.
Wang H, Zhang J, Yu H (2007) Elemental selenium at nano size possess lower toxicity without compromising the fundamental effect on selenoenzymes: comparisons with selenomethionine in mice. Free Radical Biology & Medicine 42, 1524–1533.
| Elemental selenium at nano size possess lower toxicity without compromising the fundamental effect on selenoenzymes: comparisons with selenomethionine in mice.Crossref | GoogleScholarGoogle Scholar |
Weiss WP, Todhunter DA, Hogan JS, Smith KL (1990) Effect of duration of supplementation of selenium and vitamin E on periparturient dairy cows. Journal of Dairy Science 73, 3187–3194.
| Effect of duration of supplementation of selenium and vitamin E on periparturient dairy cows.Crossref | GoogleScholarGoogle Scholar | 2273147PubMed |
Wright PL, Bell MC (1966) Comparative metabolism of selenium and tellurium in sheep and swine. The American Journal of Physiology 211, 6–10.
| Comparative metabolism of selenium and tellurium in sheep and swine.Crossref | GoogleScholarGoogle Scholar | 5911055PubMed |
Xia SK, Chen L, Liang JQ (2007) Enriched selenium and its effects on growth and biochemical composition in Lactobacillus bulgaricus. Journal of Agricultural and Food Chemistry 55, 2413–2417.
| Enriched selenium and its effects on growth and biochemical composition in Lactobacillus bulgaricus.Crossref | GoogleScholarGoogle Scholar | 17305360PubMed |