Nitrous oxide, ammonia and methane from Australian meat chicken houses measured under commercial operating conditions and with mitigation strategies applied
S. G. Wiedemann A C , F. A. Phillips B , T. A. Naylor B , E. J. McGahan A , O. B. Keane A , B. R. Warren A and C. M. Murphy AA FSA Consulting, PO Box 2175, Toowoomba, Qld 4350, Australia.
B Centre for Atmospheric Chemistry, School of Chemistry, Faculty of Science, Medicine and Health, University of Wollongong, NSW 2522, Australia.
C Corresponding author. Email: stephen.g.wiedemann@gmail.com
Animal Production Science 56(9) 1404-1417 https://doi.org/10.1071/AN15561
Submitted: 10 September 2015 Accepted: 16 February 2016 Published: 5 May 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Greenhouse gas (GHG) and ammonia emissions are important environmental impacts from meat chicken houses. This study measured ammonia (NH3), nitrous oxide (N2O) and methane (CH4) in two trials from paired, commercial meat chicken houses using standard (control) and mitigation strategies. In Trial 1, emissions from houses with standard litter depth of 47 mm (LD47) or increased litter depth of 67 mm (LD67) were compared. When standardised to a 42-day-old bird, emissions were 11.9 g NH3/bird, 0.30 g N2O/bird and 0.16 g CH4/bird from the LD47 and 11.7 g NH3/bird, 0.69 g N2O/bird and 0.12 g CH4/bird from the LD67. Emissions per kilogram of manure N were 0.14 and 0.11 for NH3-N, 0.003 and 0.005 N2O-N and CH4 conversion factors were 0.08% and 0.05%. Total direct and indirect GHG emissions reported in carbon dioxide equivalents were found to be higher in LD67 in response to the elevated direct N2O emissions. Trial 2 compared the impact of reduced crude protein (CP19.8) and a standard diet (CP21.3) developed using least-cost ration formulation, on emissions. Emissions per bird for the CP19.8 diet were 7.7 g NH3/bird, 0.39 g N2O/bird and 0.14 g CH4/bird, while emissions from birds fed the CP21.3 diet were 10.6 g NH3/bird, 0.42 g N2O/bird and 0.19 g CH4/bird. Significant differences were observed only in the NH3 results, where emissions were reduced by 27% for the low-CP diet. Because of the low emission levels, total mitigation potential from indirect GHG emissions was relatively small in Trial 2, corresponding to 11 t carbon dioxide equivalents/year per million birds.
Additional keywords: agricultural systems, broiler, greenhouse gases, manure, nitrogen.
Introduction
Greenhouse gas (GHG) emissions have an important environmental impact globally (IPCC 2013) and emission mitigation is a priority issue in Australian agriculture. Direct emissions from meat chicken houses arise from manure management, and include nitrous oxide (N2O) and methane (CH4). Ammonia (NH3) emissions are also a regulated environmental emission in Australia (DSEWPaC 2013) and represent a health concern for poultry, together with being a precursor to indirect N2O emissions.
Gaseous emissions from meat chicken manure management are typically predicted from excreted manure properties, namely, nitrogen (N) and volatile solids (VS) when integrated into inventory methods (i.e. Dong et al. 2006; Commonwealth of Australia 2014). The Australian Emission Reduction Fund mitigation methods have also used this calculation approach in the pig industry (DIICSRTE 2013). Thus, inventory and mitigation methods are most readily applied if emissions are reported relative to excreted manure. Calculated emissions from meat chicken houses using earlier Australian inventory methods (0.02 kg N2O-N/kg N excreted, Commonwealth of Australia 2014) correspond to ~1.1 g N2O/bird for a bird marketed at 46 days. By comparison, Guiziou and Béline (2005) and Dong et al. (2011) reported negligible emission rates of N2O from meat chicken houses, while von Bobrutzki et al. (2013) reported N2O emissions of 0.34–0.44 g/bird. The more recent IPCC manual (Dong et al. 2006) reported a value of 0.001 kg N2O-N/kg N excreted, but no Australian research has been completed to verify the emissions. Similarly, reports of CH4 emissions are varied, with emissions of 6.30 ± 0.16 g/bird reported by Dong et al. (2011) while Guiziou and Béline (2005) found negligible emissions.
In contrast to N2O or CH4, NH3 emissions may be significantly higher. In their review of NH3 emissions from livestock houses in Europe, Groot Koerkamp et al. (1998) reported emissions of 9.8–22 g/bird (converted from mg/h, bird age 46 days), while Lacey et al. (2003) and Burns et al. (2007) reported emissions of 31–35 g/bird for USA production systems. Applying IPCC 2006 inventory default calculations from Dong et al. (2006) showed that indirect N2O from NH3 emissions were the largest GHG source from meat chicken housing. Thus, GHG mitigation strategies may be established that focus on reducing NH3, provided direct N2O and CH4 are equivalent or lower with mitigations applied.
While few studies have investigated mitigation of GHG for poultry, many studies have focussed on NH3 mitigation via changing litter management or feed. Reducing dietary crude protein (CP) has been effective in reducing NH3 (Elwinger and Svensson 1996; Corzo et al. 2005; Powers and Angel 2008; Liu et al. 2011). Mitigation focussed on litter management by increasing the litter depth has shown reduced NH3 emissions (Al Homidan et al. 1997). Meluzzi et al. (2008) found that increased litter and reduced stocking density corresponded to lower moisture content, N content and NH3 emissions. However, the impact of changed litter depth or dietary CP on GHGs is unknown. CH4 emissions from organic materials are promoted by high moisture leading to anaerobic conditions (Hellmann et al. 1997). Increased litter depth may reduce anaerobic conditions by reducing moisture, particularly around drinkers and around evaporative cooling pads, and may result in lower emissions from these areas. The impact on N2O emissions, which are typically low in meat chicken houses, was not known, although N2O emissions have been found to be lower in soils below field capacity (Dalal et al. 2003) and may, therefore, be reduced if moisture content decreases in response to increased litter depth. No studies, to the authors’ knowledge, have explored the impact of these strategies on GHG emissions. Other NH3 mitigation strategies have focussed on litter additives (i.e. Zhang et al. 2011; Redding 2013) but the cost effectiveness of these strategies at the commercial scale is constrained where large volumes of litter additive are required.
The aims of the present study were to determine the impact of applying two mitigation strategies, increased litter depth and reducing dietary CP, on N2O, CH4 and NH3 emissions applied at the commercial scale. The trial aimed to quantify mitigation potential for commercial meat chicken grow-out operations and to provide new baseline data for predicting emissions from Australian meat chicken production.
Materials and methods
Animal houses
The study was conducted at a commercial meat chicken farm located in south-eastern Queensland, Australia. Average annual rainfall is 770 mm, the mean maximum temperature for the region is 26.8°C, and the mean minimum temperature is 13°C (BOM 2014). Paired, tunnel-ventilated houses (120 m × 14.75 m, 1700 m2 floor area) were chosen, with the same design and using the same bird and nutrition management. The two houses (Houses A and B) were located ~10 m apart and at least 200 m from other houses, separated by a vegetative buffer. Temperature within the houses was controlled on a 3-min cycle, using temperature sensors located within the house. Ventilation fans were controlled by a cycle timer to provide minimum ventilation, with evaporative cooling used when required. Each house had 10, 48-inch-diameter, production fans. Eight of the fans were located on the end wall of the houses at a height of 1.5 m, with Fan 9 located in the gable of the house (Fig. 1). Fan 10 was located at the inlet, east-end, of the house at the brood area.
The paired houses had a stabilised clay floor with low permeability, as recommended for poultry houses in Australia (NSW DPI 2012). Prior to each trial, clean wood shavings were placed in the houses to an industry standard depth of 40–50 mm (DAFF 2012), with the exception of House B during Trial 1, where the litter depth was increased to 67 mm. Clean bedding was weighed before placement.
Mitigations
Trial 1
Trial 1 investigated the effect of litter depth on emission rates. The following two litter depths were used: standard practice for Australian houses of 47 mm (LD47) and increased litter depth to 67 mm (LD67). The trial aimed to reduce ammonia emissions and reduce or maintain GHG emissions by reducing anaerobic conditions.
Trial 2
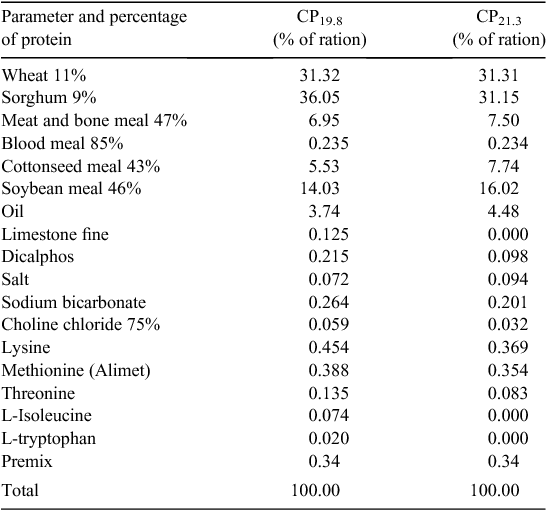
Mitigation in Trial 2 utilised a reduced CP ration formulation, aimed at reducing N excretion and N emissions after excretion. Rations were formulated on a least-cost basis at the time of the trial, by the cooperating company nutritionist. The standard diet was formulated to company specifications and resulted in a CP level of 21.3% (CP21.3) as a weighted mean of the finisher and withdrawal rations. The mitigation ration reduced dietary CP by 1.5 percentage points in the finisher and withdrawal rations, resulting in a weighted mean CP level of 19.8% (CP19.8). Mitigation was achieved by increasing the inclusion rate of primary synthetic amino acids, and introducing synthetic tryptophan and isoleucine (Table 1) in the grower, finisher and withdrawal rations. The resulting diet represented a 1.5% reduction in dietary CP for the finisher and withdrawal diets. This closely matched dietary requirements but at a higher price point.
|
Bird management and feed
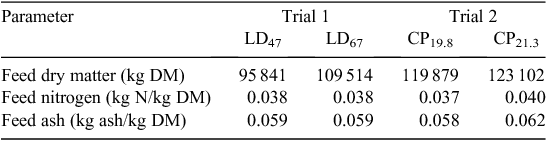
The houses were stocked according to company requirements, and stocking densities averaged 17 birds/m2. The birds were housed for a period of 7–8 weeks to a maximum bird age of 49–56 days, with four harvests beginning at ~35 days depending on market requirements. Mortalities and culls were removed from the houses daily, with numbers and mass recorded by farm staff. Feed and water were provided ad libitum. Each house was equipped with five nipple drinker lines that were adjusted to place these just above bird head height. Feed was supplied from three lines of tubed-style pan feeders that were supplied automatically from the feed silos located outside of the houses. Birds were phase-fed with commercial, sorghum-based rations developed by company nutritionists. The meat chicken performance data from each trial are given in Table 2 and the feed dry matter, N and ash concentrations are given in Table 3. As the mean age of birds at harvest differed slightly between trials, cumulative emissions per bird were reported for a standardised bird age of 42 days.

|
|
Gaseous-emission measurements
The NH3, CH4, N2O and carbon dioxide (CO2) gases emitted from the houses were measured at the ventilation-fan outlets using open-path Fourier transform infrared (OP-FTIR) spectroscopy and combined with the ventilation rate to calculate the emissions of the relevant gases from the houses.
Open-path Fourier transform infrared (OP-FTIR) spectrometer
The OP-FTIR system used here has been described elsewhere (Bai 2011; Jones et al. 2011) and only a brief description will be given here. The FTIR spectrometer (Matrix IR-Cube, Bruker Optik GmbH, Ettlingen, Germany) scans continuously to record a time-averaged (typically minutes) infrared absorption spectrum of the open atmospheric path between spectrometer and a retro-reflector, located 20–150 m from the spectrometer, to provide a path-averaged mixing-ratio (mole fraction or nmol/mol (ppbv)) of NH3, N2O, CO2, CH4, carbon monoxide (CO) and water vapour (Griffith 1996). The FTIR spectrometer is equipped with a mechanically cooled (−196°C, RicorK508) MCT detector (Infrared Associates Inc., Stuart, FL, USA, or Judson Industries, Montgomeryville, PA, USA) and coupled to a 250-mm Schmidt-Cassegrain telescope (LX 200ACF, Meade Instruments Corporation, Irvine, California, USA), modified to function as a parallel beam expander, and has a measurement precision for NH3 of 2 nmol/mol, for N2O of 0.6 nmol/mol, for CO2 of 1.5 nmol/mol and for CH4 of 6 nmol/mol (Bai 2011). The system is mounted onto a computer-controlled automated instrument mount (AIM, IAAC; Unanderra, NSW, Australia) to allow automated alignment of the beam between spectrometer and multiple retro-reflectors. The operation of the system, including orientation of spectrometer to multiple retro-reflectors, is fully computer controlled.
Instrumentation position
Two OP-FTIR spectrometers were used for emissions measurements at the site. The first was located between the paired houses with paths parallel to, and ~1 m from, the fan exhaust, with the instrument rotating between the two measurement paths every 3 min. The second instrument, located at the intake-end of the house, monitored the gas mixing-ratio at the air intake. With the nearest other animal houses ~200–300 m from the measurement site, any gas plumes from other sources were assumed to be well mixed at the measurement site.
Fan ventilation rate
The fan ventilation efficiency (fan performance curve) is required to determine the ventilation rate. The efficiency was measured using a traversing anemometer array, the fan assessment numeration system (FANS unit; Casey et al. 2008). This unit was manufactured following the design of Gates et al. (2004). The individual ventilation efficiencies of the nine fans in both houses were developed by traversing the FANS cross-arm over the fan diameter at the five static-pressure differences. For Shed A, the static-pressure difference ranged from 0 to 45 Pa, while, in Shed B, the maximum static-pressure difference achievable, with all fans operating, was 35 Pa. A single run over the fan diameter took 81–86 s with the cross-arm driven up and down twice to give a total measurement time of 324–344 s. Wind-speed data from the five propeller anemometers were recorded to a data logger (CR1000, Campbell Scientific Inc., Logan, Utah, USA) every second, together with static pressure (Setra Differential Pressure Transducer 2601-MS3-N, Setra Systems Inc, Boxborough, MA, USA). The volume of air passing through the fan was calculated and averaged for each static pressure. The resulting polynomial relationship, or fan efficiency curve was used to calculate the air-flow rate (ventilation rate) for each individual fan during emission measurements.
Auxiliary data
Auxiliary data used for the fan efficiency and emission measurements were recorded to a dedicated data logger (CR1000, Campbell Scientific Inc.). Static pressure was measured at 1 Hz and recorded as 1-min averages. Fans-open status was monitored and recorded at 1 Hz, with tilt switches mounted on each fan shutter. Anemometers located 1 m from the exhaust fans, and close to the infrared measurement path, monitored air flow from Fans 1, 3, 4, 5 and 7 of each house. Anemometers included three fan anemometers (27106T Propeller anemometers, RM Young Company, Traverse, MI, USA) measuring wind speed in one direction (horizontal wind speed from the fans) at 1 Hz, and averaged to 1 min; and two two-dimensional anemometers (WindSonic, Gill Instruments, Limited, Lymington, Hampshire, UK) provided wind speed and direction in two (north–south and east–west) directions from Fans 1 and 7. This allowed any change in fan efficiency to be monitored throughout the experiment and any cross-winds, which could result in possible cross-contamination of emissions between houses, to be identified. Temperature was measured every minute outside the house in the exhaust from Fan 6 and inside each house below Fan 9, and between Fans 4 and 5 (T107, Campbell Scientific Inc.).
A weather station located ~10 m from the houses, away from direct influence of the exhaust fans, provided data on local wind conditions. The weather station included a three-dimensional sonic anemometer that measured three-dimensional wind-speed and wind-direction data at 10-Hz resolution and averaged to 15 min (sonic anemometer, CSAT3, Campbell Scientific Inc.) and provided the wind statistical data required for the influence of emissions from nearby houses to be modelled. A wind sentry and cup anemometer (03001 RM Young Wind Sentry set, Campbell Scientific Inc.) provided additional wind direction and speed data, in conjunction with air temperature (T107, Campbell Scientific Inc.) and humidity (HMP55C, Campbell Scientific Inc.) measured each min and averaged to 5 min. All data were recorded to a data logger (CR3000, Campbell Scientific Inc.).
Emissions data analysis
The emission strength of each gas is calculated from the measured mixing ratio, above background levels, normalised to standard temperature and pressure (0°C and 101.3 kPa) and the total flow rate from all operational fans over the 3-min measurement period, using Eqn 1:

where QGas = emissions of Gas G (L/min), [G] = measured mixing ratio above background for Gas G (3-min average, nmol/mol), and AFtot = the sum of airflow from all operational fans, during the measurement of gas mixing ratio.
Emissions were converted to CO2 equivalents (CO2-e, kg) using values of IPCC AR4 global warming potential over 100 years (IPCC 2007) for measured CH4 (25) and N2O (298). These values were applied, in preference to the updated IPCC AR5 values (CH4, 28, N2O, 265), to align with Australian GHG mitigation policy instruments. Indirect N2O emissions from volatilisation and re-deposition of NH3 were determined from the mass of NH3-N volatilised, assuming a 100% deposition rate to land, and a secondary emission factor of 0.01 kg N2O-N emitted/kg of NH3 volatilised (Commonwealth of Australia 2014).
Internal tracer gas, bird-respired CO2
As CO2 from the houses is measured simultaneously with the other gases, CO2 respired by the birds can be used as an internal tracer gas in calculating the gaseous emissions (Pedersen et al. 1998; Gates et al. 2005; Casey et al. 2006; Xin et al. 2009; Calvet et al. 2011), and allows for differing losses from the houses due to different leakage rates through the roof and walls. The daily respired CO2 was predicted using a bird respiration model (Xin et al. 2009), which included bird numbers, total mass of birds, total heat production and respiratory quotient. Litter CO2 emissions were calculated on the basis of VS excretion for both trials. Carbon loss was estimated as the difference in VS excreted and VS measured at the end of the trial. After removing for CH4 losses, it was assumed that all C loss was in the form of CO2. Using this method, litter CO2 emissions were estimated to be 6–12% of total CO2 loss from the houses.
Measured CO2 emissions to model-predicted bird-respired CO2 retrieval over the trial period averaged 87.5% and 108% for House A (Trials 1 and 2 respectively) and 75.1% and 77.65% for House B (Trials 1 and 2 respectively). The measured, daily averaged emissions for each gas were normalised to 100% gas retrieval by the ratio of the measured emission estimate and the measured-to-model-predicted CO2 emission retrieval, for the relevant shed and trial.
Technique validation: controlled release of tracer gas
To validate the emission measurements, a tracer gas (N2O) was released inside the house and the retrieved emissions were compared with the known release rate of the tracer gas. Tracer gas was released in House A before the start of Trial 1, and in House B following completion of Trial 2.
The tracer gas was released from 60 aluminium gas canisters, 240 × 60-mm diameter, commonly used as ‘paint ball’ canisters fitted with a head encompassing a capillary tube to limit the flow rate of tracer gas to ~10 g/h. Each canister was filled with up to 300 g of N2O (liquid N2O, engine boost grade, product code 624, BOC Australia, Sydney, New South Wales, Australia). The canisters were distributed evenly over the floor of the house. The temperature at the canister was monitored using temperature logging buttons (Thermocron eTemperature model TCS, OnSolutions, Baulkham Hills, New South Wales, Australia) attached to 11 canisters. The average flow from the canisters was calculated from the loss of N2O and the time of release. The instantaneous flow was calculated using the temperature–pressure dependence, and the pressure dependence on the flow, as derived in the laboratory (Bai 2011). With a total flow rate of ~480 g/h, the emissions from the tracer gas dominated any residual emissions from the house. The number of fans in operation was varied over the release time and the N2O mixing ratio measured continuously at the fan exhaust.
The technique, under these operating conditions, retrieved 103.4% of the gas released from House A and 77.4% from House B. While some gas would be lost when setting up and shutting down the canister flows, these losses are expected to be minimal. The retrievals measured here were comparable to the retrieval based on the ratio of measured to model-predicted respired CO2. The sheds used in the work were older (>20 years) and the difference in retrieval between the two sheds is believed to be due to Shed B being ‘leakier’, with greater losses through gaps in the shed walls and roof. This is supported by the lower static-pressure difference that could be achieved in Shed B with all fans in operation, and from comments by the producer.
Nutrient mass balance and excretion
Nitrogen mass balance was performed to predict N excretion, using methods similar to those in Coufal et al. (2006), which are briefly explained here. Excretion of VS was determined using the approach of dry matter digestibility approximation of manure production adapted from the pig industry (Casey et al. 2000; Skerman et al. 2016). This approach utilises a digestibility model to predict total solids (TS) excreted, and an animal ash balance to determine excreted ash. VS are determined by difference.
Mass flow
The total mass of N and ash was determined from mass and concentration measurements on inputs and outputs. All mass transfers of birds, feed and litter placed in the houses, and birds (market birds and mortalities) and litter removed, were measured. Analyses confirmed that water was a negligible source of N or VS entering the system and was excluded from the balance. Replicate samples of all materials in the mass balance were taken for laboratory analysis.
Sample preparation
Whole bird carcasses were sampled from four time periods, including 1 day old, 35 days old, 42 days old and 49 days old, using carcasses supplied by the commercial operator. The carcass samples were combined for each age class and homogenised using an industrial food processor, providing a homogenised sample representative of liveweight, inclusive of feathers, bone, meat and internal organs. The material was oven-dried on aluminium trays at 60°C for 2–3 days and moisture content determined by weight loss. Dried samples were ground using a Knifetec 1095 sample mill (Foss Industries, Hillerod, Denmark), followed by a fine-grinding process using a Retsch Mortar Grinder RM 200 (Retsch, Haan, Germany) using a 1-mm steel screen.
Laboratory analysis
Following drying at 60°C during sample preparation, residual moisture in the samples was determined using a thermogravimetric analyser at 105°C and all results were corrected to dry matter basis using this residual moisture value. All samples were analysed by a commercial laboratory for N content by using the Dumas combustion method in a LECO TruSpec N analyser (Leco Corporation, Moenchengladbach, Germany). VS were determined from the difference between TS (dry weight) and ash after combustion at 550°C.
Prediction of excretion
Excretion was determined as an output of the animal mass balance, from the difference between inputs (feed and day-old birds) and outputs (market birds and mortalities). The total mass of N and ash excreted was calculated from recorded mass and characterisation data.
Statistical analyses
Gas-emission data
While the precision in measured gaseous emissions is influenced by the precision in determining the gas mixing ratio, static pressure (1.5% of full-scale reading) and ventilation rate (coefficient of determination, r2 > 0.99 for 16 fans and r2 > 0.98 for 2 fans), the accuracy of the retrieved emissions is dominated by losses through the house walls and roof and the F10-East and F9-gable fans. These losses increase as the number of fans in operation decreases.
The precision in measuring the gaseous emissions from the shed was determined as the standard deviation (s.d.) in the measured N2O emissions when N2O was released in the shed at a known, constant rate. This precision is a combination of the precision of the OP-FTIR measurement system and variations in the ventilation rates. The standard deviation in the measured emissions was less than 4% (1 s.d. n = 8, 11), except when only two fans were operating, when uncertainty increased to 9.3% (n = 10). The use of Fan 9 (gable) was limited by the producer for the trial, and generally operated in conjunction with Fans 1–8. With five fans operating, the use of the East or gable fans reduced the measured mixing ratio by 1–1.5%. From this it is estimated that the precision of the measurement technique is between 5% and 10%, depending on the number of fans in operation. A minimum number of fans (<4) were in operation in the initial weeks, when emissions were minimal, and increased to all fans in operation at the end of measurements, when emissions were greatest.
The quoted uncertainty in the CO2, CH4, N2O and NH3 emissions is 1 s.e. of the daily average of the (3-min) measured emissions, for each gas and each day (n = 92). The uncertainty in emissions was scaled to bird numbers in the same way as the emissions.
Nutrient-concentration data
A one-way ANOVA in R (R Development Core Team 2012) was used to determine significant differences in mean concentrations of N and VS in birds of different ages. Tukey’s HSD test was then used as a post hoc test. These parametric tests were deemed appropriate for use with the percentage data on the basis of previous work by (Coufal et al. 2006).
Mass-balance parameters with uncertainty
Significant differences between inputs and outputs for the N mass balance within each trial were determined by calculating confidence intervals for the inputs and outputs using a Monte Carlo simulation approach, which uses repeated random sampling of the parameters in the mass-balance model. The model was run for 5000 iterations to allow for the convergence of all output probability distributions. Parameters used in the Monte Carlo simulation are provided in Tables S1, S2, available as Supplementary material for this paper.
Results
Fan performance curves
From the fan performance curves (Fig. 2), significant differences existed in airflow efficiency among the nine fans, but there was no measurable difference in fan performance over the two trials. The total flow rate from the house with all fans operational was ~2600 m3/min. The house volume was 6500 m3, implying an exchange of the house volume in less than 3 min.
Gas emissions
Measured mixing ratios increased rapidly with the growth of the birds, with similar pattern for both houses and trials, with maximum mixing ratios of 2000 nmol/mol (NH3), 300 nmol/mol (CO2), 30 nmol/mol (N2O) and 50 nmol/mol (CH4). The exception was during the initial days of Trial 2 for CP19.8 when the N2O mixing ratio increased to ~100 nmol/mol above background levels (Fig. 3). The high mixing ratio returned to expected levels after 14 days. An increase in NH3 and N2O mixing ratio corresponding with tilling of the bedding is also noted.
Low emission rates were observed during the initial weeks for each of the trials (Trial 1, Fig. 4; Trial 2, Fig. 5) except for CP19.8 in Trial 2, where emissions were about two or three times higher (Fig. 5). This corresponded to the higher measured mixing ratios, and possibly relates to additional moisture observed on the house floor before litter placement in this house. This period of elevated emissions is equivalent to an increase in the total N2O and NH3 emissions of 5.2% and 11.3% respectively, compared with a linear interpolation of the neighbouring data. Total emissions, emission rates and emission factors were determined after replacing these anomalous data by linear interpolation. Emission estimates for all measured gases from the two houses have been normalised to the CO2 measured to model-predicted ratio.
An increase in N2O and NH3 emissions was coincident with the tillage of the litter, with the increase being more marked during Trial 2 (Fig. 5, 18 December to 19 December) and being mirrored in both houses. While the emissions increased abruptly for a short time (3–4 times above previous levels for ~10 h), emission remained at an elevated level (~2 times previous levels) following each tilling event.
For Trial 1, emissions integrated over the trial period for LD47 were 11.9 ± 0.5 g NH3/bird, 0.30 ± 0.02 g N2O/bird and 0.16 ± 0.02 g CH4/bird. Emissions for LD67 were 11.7 ± 0.7 g NH3/bird, 0.69 ± 0.05 g N2O/bird and 0.12 ± 0.02 g CH4/bird. For Trial 2, integrated emissions for CP19.8 were 7.7 ± 0.5 g NH3/bird, 0.39 ± 0.02 g N2O /bird and 0.14 ± 0.03 g CH4/bird. Emissions for CP21.3 were 10.6 ± 0.7 g NH3/bird, 0.42 ± 0.03 g N2O/bird and 0.19 ± 0.03 g CH4/bird. Total GHG emissions in CO2-e emitted, over the two trial periods, are detailed in Table 4.
Manure excretion and mass balance
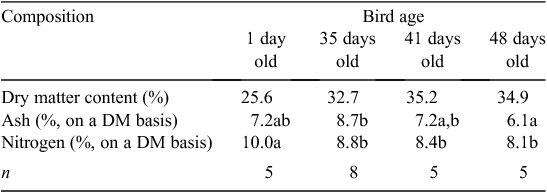
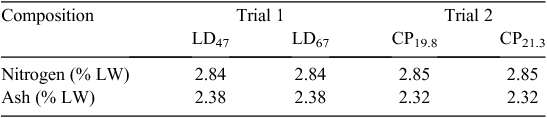
Manure nitrogen excretion relied on quantification of retention within the meat chickens. N content showed a decreasing trend between 1-day-old birds and 35-day-old birds (Table 5). Significant differences in ash content were observed only between 35- and 48-day-old birds. Weighted mean N and ash retention rates, based on the number and age of birds harvested in each trial and reported on a liveweight basis, are presented in Table 6.
|
|
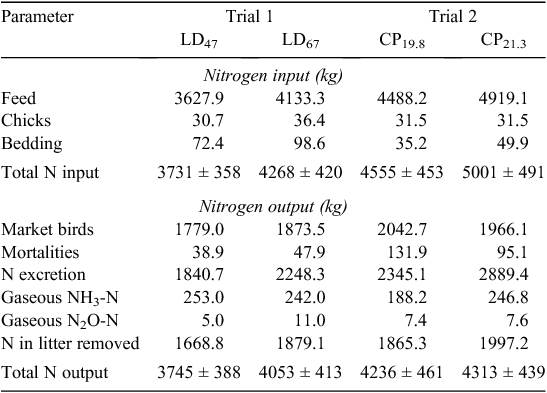
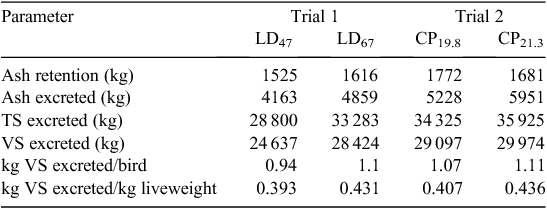
Excretion data are presented in Table 7 (N) and Table 8 (VS). N retention, as a percentage of inputs with feed and birds, was 49% and 45% for LD 47 and LD 67 in Trial 1 respectively. In Trial 2, N retention was 45% for CP19.8 and 40% for CP21.3. No significant differences were observed between inputs and outputs in the N mass balance. Manure ash, TS and VS excretion values for each trial are shown in Table 8. These values were used to predict emission factors on the basis of CH4 potential.
|
|
Emission factors
Ammonia-N emissions in Trial 1 were 0.14 and 0.11 kg/kg of excreted N for LD47 and LD67 respectively. NH3-N emissions were 0.08 and 0.09 kg/kg of excreted N for the CP19.8 and CP21.3 treatments respectively. As a fraction of N excretion, N2O-N emissions were 0.003 (LD47) and 0.005 (LD67) in Trial 1, showing proportionally higher emissions from the mitigation treatment. In Trial 2, N2O-N emissions as a fraction of N excretion were 0.003 in both treatments. CH4 conversion factors (MCFs) in Trial 1 were 0.08% LD47 and 0.05% for LD67. In Trial 2, MCFs were 0.07% for CP19.8 and 0.09% for CP21.3.
Discussion
Gaseous emissions
The NH3 emissions measured in this work, ranging from 9.8 ± 0.6 to 11.7 ± 0.5 g/bird (not standardised – CP19.8 and LD47 respectively) were similar to values for the Netherlands and Denmark reported by Groot Koerkamp et al. (1998) and at the lower end of the range reported by Guiziou and Béline (2005). Emissions were lower than the 16 g/bird reported by Harper et al. (2010), or the 31 g/bird measured by Lacey et al. (2003) and Moore et al. (2011). Each of the latter studies were conducted in the USA, with multiple batches of chickens raised on the same litter. The low emission rates in the present work may be in response to the use of fresh litter during each batch, which is a common management practice in Australia, but not in the USA, and has been found to result in lower NH3 emissions (Coufal et al. 2006). This suggests that Australian emissions may be lower than previously thought under Australian management conditions (i.e. Commonwealth of Australia 2015).
The range in N2O emissions per bird in the present study (0.30–0.69 g N2O/bird) were similar to the 0.34–0.44 g/bird reported by von Bobrutzki et al. (2013), and slightly lower than the 0.75 g N2O/bird reported by Moore et al. (2011). In contrast, Guiziou and Béline (2005) found that N2O emissions were negligible from meat chicken houses. Average flux data were 4.7–10.4 mg/m2.h in the present study, which was similar to the values in Miles et al. (2006) but at the lower end of the range reported by Miles et al. (2008). The higher values reported by Moore et al. (2011) and Miles et al. (2008) may be related to the multiple batches of chickens raised on the same litter in each of these studies. The present study found that N2O emissions were sensitive to management operations such as tillage and it is possible that disturbance between flocks, and the presence of higher levels of manure in the litter, may result in higher emissions in subsequent flocks raised on the same litter than in those raised on fresh litter. However, further research is required to understand whether this factor, or other differences between the studies, were responsible for the difference in emissions.
Methane emissions were low compared with the flux data reported by Miles et al. (2006), and closer to the negligible emissions reported by Guiziou and Béline (2005). The lower CH4 emissions in the present study than those in Miles et al. (2006) are most likely explained by differences in litter management between the two studies, with Miles et al. (2006) studying meat chickens housed on litter for multiple batches (22 consecutive batches without litter removal), which is expected to have increased the potential for anaerobic breakdown of VS in the litter, thus elevating CH4 emissions. In the present study, the use of fresh litter with each batch may have decreased anaerobic conditions suitable for CH4 generation.
Emissions of N2O and NH3 were noted to be sensitive to management. The elevated emissions in the initial weeks for CP19.8 in Trial 2 were difficult to account for as a response to the difference in treatments between the houses. For CP19.8, the litter was spread onto the floor not long after the house was washed and, from observation, with the floor still being damp from cleaning. This was the only major difference noted in the operation of the houses for the two trials. During the washing of the house, and pre- and post-emission measurements, our instruments measured high levels of NH3 and N2O (unpubl. pers. obs.). As measurements of air flow were not available, an emissions rate could not be retrieved; however, this may suggest emissions during shed washing resulted from N built-up in the clay floors. As the subsequent period of elevated emissions from the litter was early in the deployment of the chickens, the N loading from manure would have been minimal, and we postulate that the elevated emissions were associated with the wet clay floor when the litter was laid. The production of N2O is complex, with multiple pathways possible depending on the environmental conditions (Meda et al. 2011), but moisture level is known to be a driver of both N2O and NH3 emissions (Phillips et al. 2007; Meda et al. 2011; Officer et al. 2015). Meda et al. (2011) stated that higher NH3 are observed when moisture is high. However, it was reported that while N2O emissions were positively correlated with moisture at low moisture contents, at high moisture content, when conditions were more anaerobic, N2O emissions decreased with increasing moisture content. A non-significant trend towards lower recovery of outputs than inputs was observed in three of the four trials, and this may be partly explained by unaccounted losses such as those that occurred during cleaning.
Increased N2O and NH3 emissions were observed to coincide with the tillage of the litter, and remained elevated (~two times previous levels), following each tilling event. While the initial increases at tilling were anticipated, the continued elevated emissions were unexpected. There is evidence that increased aeration increases both NH3 and N2O emissions, and while much of the work on aeration was related to manure stockpiles (Jiang et al. 2011), a similar process in the house may account for the continued increased emissions observed in this current work. Tillage is practiced in Australia to maintain litter in a dry and friable condition, as specified by standards such as those set out by the RSPCA (2013). However, results from this research suggest that this practice may increase GHGs and NH3 emissions, potentially resulting in poorer conditions for animal health and the environment.
These houses were chosen as the design located most of the ventilation fans in close proximity. The emissions were monitored ~1 m from the ventilation fans and, at the velocity of the air exiting the house at the operational fans (~6 m/s), the dispersion between the fan and the measurement path is assumed to be negligible and the gases well mixed across the area of the fan. While the East and gable fans could not be monitored, the operation of these fans was minimised for the trials. Considerable difference in the venting of these two houses was apparent, based on the comparison of the external (controlled-release gas experiment, House A 103.4% and House B 77.4%) and internal (respired CO2, House A 87.5% and 108%, and House B 75.1% and 77.6%) tracer-gas retrievals, and the internal, respired CO2; tracer was used to correct for the different, not-measured, gas losses from the two houses. Also house-ventilation rates have been reported to vary by ~10% during a single batch due to aging of fan belts and accumulation of dust on the fans (Casey et al. 2008). While the precision in the measured emissions is expected to be high, based on the uncertainty in the fan-efficiency curves and the measured gas-mixing ratios, the accuracy in the measured emission rates was dominated by gas losses from the houses not easily measured. As all gases were measured simultaneously in the same air stream, by the same instrument, any losses were identical for all gases, facilitating the use of the bird-respired CO2 as an internal tracer gas. With any inaccuracies in the model-predicted respired CO2 being consistent for both houses, the difference in emissions measured from the two houses will be representative of the true difference in emissions.
Nutrient retention and excretion
Mean N retention in meat chickens across all trials and treatments was 45%, which was higher than the value (43%) applied in the Australian National Greenhouse Accounts (Commonwealth of Australia 2014), but lower than previously reported values of 55.5% (Coufal et al. 2006) and 67% (Mitran et al. 2008). As we observed higher N-retention rates with lower CP diets, the very high retention values reported by Mitran et al. (2008) may have been a response to the lower feed CP concentrations used in that study. The high retention rates observed in the literature suggest that there may be further opportunity to reduce excretion in Australian flocks beyond the extent observed in Trial 2.
Emissions factors
Emission factors for N2O in the present study ranged from 0.003 (three trial values) and 0.005 kg N2O-N/kg N excreted. These values were substantially lower than the pre-2013 Australian inventory value of 0.02 (Commonwealth of Australia 2014), although it must be noted that this value was not determined from research, but from expert opinion in the IPCC (1997) inventory methods report. Values were more comparable, although higher than the IPCC (Dong et al. 2006) recommended emission factor of 0.001 kg N2O-N/kg N excreted. MCFs were much lower than recommended by the IPCC (Dong et al. 2006), possibly in response to the use of fresh litter and dry litter conditions observed. We found no reduction in N2O emissions in response to reduced N excretion in the present study, questioning the implied association between N excretion and N2O used in most GHG inventories (i.e. Dong et al. 2006). Miles et al. (2006) found no difference in N2O fluxes between Day 1 and Day 21 of a meat chicken grow-out period on multiple-batch litter, although a later study showed that these emissions doubled over the course of a flock (Miles et al. 2008). These studies suggest that excreted N and residual N remaining in the litter do not adequately explain N2O emissions, questioning the implied association between excreted N and N2O emissions. Recently, Redding et al. (2015) observed no correlation between manure substrate N and N2O emissions from beef cattle manure pen surfaces, and similarly questioned the implied association between excreted N and N2O. Thus, further process knowledge is required to determine whether N2O emissions are similarly governed by factors other than N excretion in meat chicken houses, potentially enabling alternative inventory approaches other than predicting emissions relative to excreted N.
Mitigation of gaseous emissions
Increased litter depth was found to have no significant impact on NH3 and CH4 emissions per bird, while N2O emissions increased. Previous studies (Al Homidan et al. 1997; Meluzzi et al. 2008) have shown reduced NH3 emissions in response to increased litter depth with or without a change in stocking density, although this effect was not found by Elwinger and Svensson (1996). Considering the lack of response in ammonia emissions, and the elevated N2O in the present study, this approach was ineffective for mitigating GHG.
The results from Trial 2 showed an increase in N retention and a decrease in NH3 emissions per bird in response to reduced dietary CP. Powers and Angel (2008) reported a 15% reduction in NH3 emissions as a result of reducing dietary CP by 2 percentage points, and numerous studies support the trend towards reduced NH3 from lower CP diets (Elwinger and Svensson 1996; Corzo et al. 2005; Powers and Angel 2008; Liu et al. 2011). Reduced NH3 emissions result in lower indirect N2O emissions. In the present study, this resulted in a modest GHG mitigation effect from the reduced-CP diet. The authors found no other studies measuring the impact of reduced CP diets on CH4 or N2O.
Emission mitigation resulting from reduced dietary-N may result in broader benefits throughout the manure-management system. Litter from meat chicken houses is typically removed and used for land application for crops or pastures. Litter may be stored or composted before land application, and each of these stages result in further N2O and NH3 emissions (Dong et al. 2006). Applying the standard inventory factors, a reduction in excreted N would result in lower emissions during each further stage of the manure-management system, resulting in further mitigation effects. It is not known whether reduced emissions in response to increased litter depth will influence the magnitude of emissions at later stages in the manure-management system, and further research is required to quantify subsequent emission rates in response to this mitigation.
In the Australian Emission Reduction Fund system, viable mitigation strategies must be commercially applicable, and deliver sufficient volumes of emission abatement to make participation in the program viable. The present study identified that reduced dietary CP led to lower emissions using an approach that could be readily applied at the commercial scale. However, total abatement potential was modest because of the inherently low emission rates from the system. As an indication of total mitigation potential, a farm producing 1 million birds/year may generate 11 t CO2-e/year of abatement. This suggests that large scale aggregation would be required to deliver commercially viable levels of abatement.
Conclusions
Emissions from meat chicken houses were found to be considerably lower than predictions using the pre-2013 Australian National Greenhouse Accounts, supporting a reduction in the emission factors for the respective gases. Reduced dietary CP was effective for achieving reductions in NH3 per bird. Calculated GHG emissions per bird reported on a CO2-e basis showed a reduction in response to reduced dietary CP, in response to the 27% reduction in NH3 emissions. We note that a reduction in emissions as a result of lower excretion may flow through to later stages in the manure-management system (storage, land application), resulting in further emission reductions. The potential for the chicken meat industry to deliver significant abatement is limited by inherently low emission rates from the production system, and commercially relevant abatement quantities will require low-cost, large-scale aggregation to be viable.
Acknowledgements
This project was completed as part of the National Agricultural Manure Management Program (NAMMP) and was supported by funding from the Australian Government Department of Agriculture as part of its Carbon Farming Futures Filling the Research Gap Program, and the Rural Industry Research and Development Corporation (RIRDC) meat chicken program. We also thank the participating producer, Darwalla Milling Co Pty Ltd, for facilitating the trials, and University of Wollongong staff Graham Kettlewell, Max Desservettaz and Antony Jones for technical assistance.
References
Al Homidan A, Robertson J, Petchey A (1997) Effect of temperature, litter and light intensity on ammonia and dust production and broiler performance. British Poultry Science 38, S5–S17.Bai M (2011) Methane emissions from livestock measured by novel spectroscopic techniques. PhD Thesis, University of Wollongong, NSW.
BOM (2014) ‘Climate statistics for University of Queensland Gatton.’ Available at http://www.bom.gov.au/climate/averages/tables/cw_040082.shtml [Verified September 2014]
Burns R, Xin H, Gates R, Li H, Overhults D, Moody L, Earnest J (2007) Ammonia emissions from broiler houses in the southeastern United States. In ‘International symposium on air quality and waste management for agriculture conference proceedings’, 16–19 September 2007, Broomfield, Colorado. (Ed. L Moody) (ASABE: St Joseph, MI)
Calvet S, Estelles F, Cambra-Lopez M, Torres AG, van den Weghe HFA (2011) The influence of broiler activity, growth rate, and litter on carbon dioxide balances for the determination of ventilation flow rates in broiler production. Poultry Science 90, 2449–2458.
| The influence of broiler activity, growth rate, and litter on carbon dioxide balances for the determination of ventilation flow rates in broiler production.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbitlensg%3D%3D&md5=8594bfba1536d10ca0b573c11ab957d4CAS | 22010228PubMed |
Casey, KD, McGahan, EJ, Atzeni, MA, Gardner, EA, Frizzo, R (2000) ‘PigBal: a nutrient mass balance model for intensive piggeries.’ (Department of Primary Industries and Fisheries: Brisbane)
Casey KD, Bicudo JR, Schmidt DR, Singh A, Gay SW, Gates RS, Jacobson LD, Hoff SJ (2006) ‘Air quality and emissions from livestock and poultry production/waste management systems.’ White papers. (Animal Agriculture and the Environment, National Center for Manure and Animal Waste Management: St Joseph, MI)
Casey K, Gates R, Wheeler E, Xin H, Liang Y, Pescatore A, Ford M (2008) On-farm ventilation fan performance evaluations and implications. Journal of Applied Poultry Research 17, 283–295.
| On-farm ventilation fan performance evaluations and implications.Crossref | GoogleScholarGoogle Scholar |
Commonwealth of Australia (2014) Australian national greenhouse accounts: national inventory report 2012. Vol. 1. Department of the Environment, Canberra.
Commonwealth of Australia (2015) Australian national greenhouse accounts: national inventory report 2013. Vol. 1. Department of the Environment, Canberra.
Corzo A, Fritts C, Kidd M, Kerr B (2005) Response of broiler chicks to essential and non-essential amino acid supplementation of low crude protein diets. Animal Feed Science and Technology 118, 319–327.
| Response of broiler chicks to essential and non-essential amino acid supplementation of low crude protein diets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXitFejsg%3D%3D&md5=42625d4a533b0db107a041b21c9d5538CAS |
Coufal C, Chavez C, Niemeyer P, Carey J (2006) Nitrogen emissions measured by mass balance over eighteen consecutive flocks Japanese Poultry Science 85, 384–391.
| Nitrogen emissions measured by mass balance over eighteen consecutive flocksCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xis1aqtbk%3D&md5=20922b90ef47c74802c06fbede623eb6CAS |
DAFF (2012) ‘Queensland guidelines: meat chicken farms.’ (Department of Agriculture, Fisheries and Forestry: Brisbane)
Dalal RC, Wang WJ, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Australian Journal of Soil Research 41, 165–195.
| Nitrous oxide emission from Australian agricultural lands and mitigation options: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFKisr8%3D&md5=498c42b5dc296c33f73cd52ca7928fceCAS |
DIICSRTE (2013) ‘Carbon credits (carbon farming initiative) (destruction of methane generated from manure in piggeries: 1.1). Methodology determination 2013.’ (Department of Industry, Innovation, Climate Change, Science, Research and Tertiary Education: Canberra)
Dong H, Mangino J, McAllister TA, Hatfield JL, Johnson DE, Lassey KR, Aparecida de Lima M, Romanovskaya A, Bartram D, Gibb DJ, Martin JHJ (2006) Emissions from livestock and manure management. In ‘IPCC guidelines for national greenhouse gas inventories. Vol. 4: agriculture, forestry and other land use’. (Eds S Eggleston, L Buendia, K Miwa, T Ngara, K Tanabe) pp. 10.1–10.87. (Institute for Global Environmental Strategies: Kanagawa, Japan)
Dong H, Zhou Z, Zhu Z, Xin H, Chen Y (2011) ‘Carbon and nitrogen budget of commercial cage-grown broilers, ASABE Annual International Meeting.’ Louisville, KY.
DSEWPaC (2013) ‘National Pollutant Inventory. Emission estimation technique manual for intensive livestock: poultry raising.’ (Department of Sustainability, Environment, Water, Population and Communities, Australian Government: Canberra). Available at http://www.npi.gov.au/resource/emission-estimation-technique-manual-intensive-livestock-poultry-raising-version-30 [Verified 27 April 2015].
Elwinger K, Svensson L (1996) Effect of dietary protein content, litter and drinker type on ammonia emission from broiler houses. Journal of Agricultural Engineering Research 64, 197–208.
| Effect of dietary protein content, litter and drinker type on ammonia emission from broiler houses.Crossref | GoogleScholarGoogle Scholar |
Gates RS, Casey KD, Xin H, Wheeler EF, Simmons JD (2004) Fan assessment numeration system (FANS) design and calibration specifications. Transactions of the American Society of Agricultural Engineers 47, 1709–1715.
| Fan assessment numeration system (FANS) design and calibration specifications.Crossref | GoogleScholarGoogle Scholar |
Gates RS, Xin H, Casey KD, Liang Y, Wheeler EF (2005) Method for measuring ammonia emissions from poultry houses. Journal of Applied Poultry Research 14, 622–634.
| Method for measuring ammonia emissions from poultry houses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVGntr7F&md5=6c9dccf415e07816634f31099ecb6fc8CAS |
Griffith DWT (1996) Synthetic calibration and quantitative analysis of gas-phase FT-IR spectra. Applied Spectroscopy 50, 59–70.
| Synthetic calibration and quantitative analysis of gas-phase FT-IR spectra.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlslGrsA%3D%3D&md5=e82f41f7b9835d74f7b31c18b090d154CAS |
Groot Koerkamp P, Metz J, Uenk G, Phillips V, Holden M, Sneath R, Short J, White R, Hartung J, Seedorf J (1998) Concentrations and emissions of ammonia in livestock buildings in northern Europe. Journal of Agricultural Engineering Research 70, 79–95.
| Concentrations and emissions of ammonia in livestock buildings in northern Europe.Crossref | GoogleScholarGoogle Scholar |
Guiziou F, Béline F (2005) In situ measurement of ammonia and greenhouse gas emissions from broiler houses in France. Bioresource Technology 96, 203–207.
| In situ measurement of ammonia and greenhouse gas emissions from broiler houses in France.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXns12jur0%3D&md5=48ca28e9da66bc3cab3dfae029b00c86CAS | 15381217PubMed |
Harper LA, Flesch TK, Wilson JD (2010) Ammonia emissions from broiler production in the San Joaquin Valley. Poultry Science 89, 1802–1814.
| Ammonia emissions from broiler production in the San Joaquin Valley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFKjsbvO&md5=03bece342f54d09ac81f469f067ae5acCAS | 20709964PubMed |
Hellmann B, Zelles L, Palojarvi A, Bai Q (1997) Emission of climate-relevant trace gases and succession of microbial communities during open-windrow composting. Applied and Environmental Microbiology 63, 1011–1018.
IPCC (1997) ‘IPCC guidelines for national greenhouse gas inventories, greenhouse gas inventory reference manual. Vol. 3.’ (Meteorological Office, IPCC/OECD/IEA: Bracknell, UK)
IPCC (2007) ‘Climate change 2007: the physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change.’ (Cambridge University Press: Cambridge, UK)
IPCC (2013) ‘Technical summary. Climate change 2013: the physical science basis. Contribution of Working Group I to the fifth assessment report of the Intergovernmental Panel on Climate Change.’ (Cambridge University Press: Cambridge, UK)
Jiang T, Schuchardt F, Li G, Guo R, Zhao Y (2011) Effect of C/N ratio, aeration rate and moisture content on ammonia and greenhouse gas emission during the composting. Journal of Environmental Sciences (China) 23, 1754–1760.
| Effect of C/N ratio, aeration rate and moisture content on ammonia and greenhouse gas emission during the composting.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVCls7bL&md5=4984734ebf21ce49cba76ea407db4e56CAS |
Jones F, Phillips F, Naylor T, Mercer N (2011) Methane emissions from grazing Angus beef cows selected for divergent residual feed intake. Animal Feed Science and Technology 166-167, 302–307.
| Methane emissions from grazing Angus beef cows selected for divergent residual feed intake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsFWlsL8%3D&md5=5997878f43a92517350f947950ef070fCAS |
Lacey R, Redwine J, Parnell C (2003) Particulate matter and ammonia emission factors for tunnel-ventilated broiler production houses in the southern US. Transactions of the ASAE. American Society of Agricultural Engineers 46, 1203–1214.
| Particulate matter and ammonia emission factors for tunnel-ventilated broiler production houses in the southern US.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotVOksLg%3D&md5=7af2d077c8b585d4893ca6b69b9dfe41CAS |
Liu Z, Powers W, Karcher D, Angel R, Applegate T (2011) Effect of amino acid formulation and supplementation on nutrient mass balance in turkeys. Poultry Science 90, 1153–1161.
| Effect of amino acid formulation and supplementation on nutrient mass balance in turkeys.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXot1Ohs7g%3D&md5=9485e8897d07d81cf0c672b7d35e4716CAS | 21597053PubMed |
Meda B, Hassouna M, Aubert C, Robin P, Dourmad JY (2011) Influence of rearing conditions and manure management practices on ammonia and greenhouse gas emissions from poultry houses. World’s Poultry Science Journal 67, 441–456.
| Influence of rearing conditions and manure management practices on ammonia and greenhouse gas emissions from poultry houses.Crossref | GoogleScholarGoogle Scholar |
Meluzzi A, Fabbri C, Folegatti E, Sirri F (2008) Survey of chicken rearing conditions in Italy: effects of litter quality and stocking density on productivity, foot dermatitis and carcase injuries. British Poultry Science 49, 257–264.
| Survey of chicken rearing conditions in Italy: effects of litter quality and stocking density on productivity, foot dermatitis and carcase injuries.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cvgtV2ntg%3D%3D&md5=4da78d6dd9cb9032e5e4b8d3e46eaf4dCAS | 18568749PubMed |
Miles D, Owens P, Rowe D (2006) Spatial variability of litter gaseous flux within a commercial broiler house: Ammonia, nitrous oxide, carbon dioxide, and methane. Poultry Science 85, 167–172.
| Spatial variability of litter gaseous flux within a commercial broiler house: Ammonia, nitrous oxide, carbon dioxide, and methane.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xhs1Kltbk%3D&md5=0fd91dc8301320a7113ea986a7003622CAS | 16523609PubMed |
Miles D, Rowe D, Owens P (2008) Winter broiler litter gases and nitrogen compounds: temporal and spatial trends. Atmospheric Environment 42, 3351–3363.
| Winter broiler litter gases and nitrogen compounds: temporal and spatial trends.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkt1yiurs%3D&md5=a77b44f18081146637937d1673298252CAS |
Mitran L, Harter-Dennis J, Meisinger J (2008) Determining the nitrogen budget and total ammoniacal nitrogen emissions from commercial broilers grown in environmental chambers. Journal of Applied Poultry Research 17, 34–46.
| Determining the nitrogen budget and total ammoniacal nitrogen emissions from commercial broilers grown in environmental chambers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXos1yqurg%3D&md5=57816258b77bf59fb902181ba85441b2CAS |
Moore PA, Miles D, Burns R, Pote D, Berg K, Choi IH (2011) Ammonia emission factors from broiler litter in barns, in storage, and after land application. Journal of Environmental Quality 40, 1395–1404.
| Ammonia emission factors from broiler litter in barns, in storage, and after land application.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFGis7bN&md5=e0263c097f436f9031809b32bad3295dCAS | 21869501PubMed |
NSW DPI (2012) ‘Best practice management for meat chicken production in NSW.’ (NSW Department of Primary Industries: Orange, NSW) Available at www.dpi.nsw.gov.au [Verified 13 January 2015]
Officer SJ, Phillips FA, Kearney G, Armstrong R, Graham J, Partington D (2015) Response of soil nitrous oxide flux to nitrogen fertiliser application and legume rotation in a semi-arid climate, identified by smoothing spline models. Soil Research 53,
| Response of soil nitrous oxide flux to nitrogen fertiliser application and legume rotation in a semi-arid climate, identified by smoothing spline models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXosFGntL0%3D&md5=9df2c794794e26d4cc5b0fb3b80d03c7CAS |
Pedersen S, Takai H, Johnsen JO, Metz JHM, Groot Koerkamp PWG, Uenk GH, Phillips VR, Holden MR, Sneath RW, Short JL, White RP, Hartung J, Seedorf J, Schröder M, Linkert KH, Wathes CM (1998) A comparison of three balance methods for calculating ventilation rates in livestock buildings. Journal of Agricultural Engineering Research 70, 25–37.
| A comparison of three balance methods for calculating ventilation rates in livestock buildings.Crossref | GoogleScholarGoogle Scholar |
Phillips FA, Leuning R, Baigent R, Kelly KB, Denmead OT (2007) Nitrous oxide flux measurements from an intensively managed irrigated pasture using micrometeorological techniques. Agricultural and Forest Meteorology 143, 92–105.
| Nitrous oxide flux measurements from an intensively managed irrigated pasture using micrometeorological techniques.Crossref | GoogleScholarGoogle Scholar |
Powers W, Angel R (2008) A review of the capacity for nutritional strategies to address environmental challenges in poultry production. Poultry Science 87, 1929–1938.
| A review of the capacity for nutritional strategies to address environmental challenges in poultry production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtF2qtrbE&md5=f95891eaed3c0bdda31710a8974024d9CAS | 18809853PubMed |
R Development Core Team (2012) ‘R: a language and environment for statistical computing.’ (R Foundation for Satistical Computing: Vienna) Available at http://www.r-project.org/ [Verified 4 March 2015]
Redding MR (2013) Bentonite can decrease ammonia volatilisation losses from poultry litter: laboratory studies. Animal Production Science 53, 1115–1118.
| Bentonite can decrease ammonia volatilisation losses from poultry litter: laboratory studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVSgsb%2FM&md5=e8275d6ffac4437fb2b9be3969881e38CAS |
Redding M, Devereux J, Phillips F, Lewis R, Naylor T, Kearton T, Hill J, Wiedemann S (2015) Field measurement of beef pen manure methane and nitrous oxide reveals a surprise for inventory calculations. Journal of Environmental Quality 44, 720–728.
| Field measurement of beef pen manure methane and nitrous oxide reveals a surprise for inventory calculations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXns1Wgsbk%3D&md5=f9f44b15db27e206a93053d6e37fc965CAS | 26024253PubMed |
RSPCA (2013) ‘RSPCA approved farming scheme standards: meat chickens.’ (RSPCA Australia Inc.: Canberra). Available at http://www.rspca.org.au/sites/default/files/website/what-we-do/working-with-farming-industry/RSPCAMeatChickenStandards_May2013.pdf [Verified 19 December 2011]
Skerman AG, Willis S, McGahan EJ, Borgognone MG, Batstone DJ (2016) Validation of PigBal model predictions for pig manure production. Animal Production Science 56, 1081–1090.
| Validation of PigBal model predictions for pig manure production.Crossref | GoogleScholarGoogle Scholar |
von Bobrutzki K, Ammon C, Berg W, Fiedler M (2013) Quantification of nitrogen balance components in a commercial broiler barn. Czech Journal of Animal Science 58, 566–577.
Xin H, Li H, Gates RS, Overhults DG, Earnest JW (2009) Use of CO2 concentration difference or CO2 balance to assess ventilation rate of broiler houses. Transactions of the ASABE 52, 1353–1361.
| Use of CO2 concentration difference or CO2 balance to assess ventilation rate of broiler houses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1Wru7fN&md5=eff65084f24c5d899deefda06c99a3c7CAS |
Zhang HF, Jiao HC, Song ZG, Hai LIN (2011) Effect of alum-amended litter and stocking density on ammonia release and footpad and hock dermatitis of broilers. Agricultural Sciences in China 10, 777–785.
| Effect of alum-amended litter and stocking density on ammonia release and footpad and hock dermatitis of broilers.Crossref | GoogleScholarGoogle Scholar |








