Dietary betaine supplementation has energy-sparing effects in feedlot cattle during summer, particularly in those without access to shade
K. DiGiacomo A D , R. D. Warner B , B. J. Leury A , J. B. Gaughan C and F. R. Dunshea AA Melbourne School of Land and Environment, The University Of Melbourne, Parkville, Vic. 3010, Australia.
B CSIRO Animal, Food and Health Sciences, 671 Sneydes Road, Werribee, Vic. 3030, Australia.
C School of Agriculture and Food Sciences, The University of Queensland, Gatton, Qld 4343, Australia.
D Corresponding author. Email: kristyd@unimelb.edu.au
Animal Production Science 54(4) 450-458 https://doi.org/10.1071/AN13418
Submitted: 9 October 2013 Accepted: 12 February 2014 Published: 11 March 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Dietary betaine supplementation improves water retention in steers and may influence lean-tissue deposition, while also acting as an osmolyte to help regulate cellular osmotic balance. This study investigated the interactions between shade and dietary betaine on carcass characteristics, tissue enzyme activity and gene expression in 48 feedlot steers during summer. Steers were randomly allocated to a 4 × 2 factorial design with the factors being dietary betaine (0, 10, 20 or 40 g) and shade (with and without shade) for 120 days. Tissue samples were obtained at slaughter and analysed for gene expression of heat shock proteins 70 and 90 (HSP70/90) and expression of heat shock factor 1 (HSF1), and enzyme activity of fatty acid synthase (FAS) and glycerol-6-phosphate dehydrogenase (G6PDH). Carcasses were evaluated for quality. Carcass weight at slaughter was not altered by shade (P = 0.18) but tended to be increased by dietary betaine (306 v. 314 kg, P = 0.09). The P8 backfat was not altered by shade (P = 0.43) or dietary betaine (P = 0.32), although there was a within dietary betaine effect whereby P8 backfat tended to be greater in steers fed 10 g compared with 40 g betaine/day (17.4 v. 14.5 mm, P = 0.06). Muscle pH at 1 h (5.97 v. 6.03, P = 0.01) and 2 h (5.73 v. 5.80, P = 0.04) post-slaughter was higher in shaded steers, and muscle pH at 1 h post-slaughter was higher in steers fed 10 or 20 g than those fed 40 g betaine/day (6.03 v. 6.03 v. 5.95, P = 0.005). Gene expression was not altered by betaine, while adipose tissues expressed more of each gene than muscle (P < 0.001). The mRNA expression of HSF1 and HSP90 was influenced by a shade × betaine interaction, although the direction of this interaction was irregular (P = 0.03 and 0.03, respectively). Adipose tissue FAS and G6PDH enzyme activity was unaffected by shade and betaine. The results of this study indicate that betaine supplementation may be a successful carcass modifier in growing feedlot steers during summer. Provision of shade during summer may reduce the rate of pH decline in the first 2 h after slaughter and reduce the risk of high rigor temperature.
Introduction
Animals in intensive feedlot systems often experience high heat loads that can lead to heat stress. Heat stress can be a major cause of illness and production losses resulting in economic losses and welfare issues for the beef industry (Sackett et al. 2006), and managing cattle to reduce the impact of heat stress remains a challenge (Hahn 1999; Mader 2003). Solar radiation is often a major contributor to heat stress, particularly in feedlot cattle, which are more susceptible to heat stress as they are more confined than if at pasture and they often consume a high-energy diet (Blackshaw and Blackshaw 1994). Thus, the provision of shade is an important factor that can reduce the radiant heat load on the animal and decrease heat loads (Ittner et al. 1951; Blackshaw and Blackshaw 1994). However, the provision of shade is costly and accounts for nearly AU$10 million of the estimated annual losses of approximately $16.5 million in feedlot systems in northern Australia alone (Sackett et al. 2006). Furthermore, there are inconsistencies between studies (Clarke and Kelly 1996; Gaughan et al. 2004; Brown-Brandl et al. 2005) in the effectiveness of shade for improving cattle performance, and therefore the efficiency of this management practice remains contentious (Sullivan et al. 2011). As the number of cattle being raised in feedlots increases and the effects of climate variability are set to increase environmental temperatures, the need to ameliorate the effects of heat stress in cattle becomes increasingly important to maintain animal welfare and production levels.
The provision of nutritional supplements to ameliorate the effects of heat stress in production animals is an attractive method and is potentially the fastest and easiest option for producers, particularly for animals in feedlot situations. One such supplement that has recently been utilised to prevent thermal stress in cattle, poultry and pigs is the organic amino acid derivative, dietary betaine. Physiologically, mammals utilise betaine as a methyl donor able to participate in protein and lipid metabolism, or when not catabolised, betaine can be used as an organic cellular osmoprotectant (Fernández et al. 1998; Huang et al. 2007). In steers, acute (7 days) dietary betaine supplementation did not alter bodyweight, backfat, hot carcass weight or marbling score (Bock et al. 2002), while longer term dietary betaine supplementation in feedlot steers improved final fat thickness, hot carcass weight and backfat thickness and decreased longissimus muscle area (Loest et al. 2002). In pigs, dietary betaine supplementation reduced the mRNA expression and activity of fatty acid synthase (FAS) (Huang et al. 2008), a key lipogenic enzyme; however, in cattle the effects of betaine supplementation on enzymatic gene expression and activities has not been explored. This is important as an animal with a reduced ability to produce fatty acids may have an increased capacity for protein deposition. However, as dietary betaine supplementation increases fat thickness in feedlot steers, it is expected that the expression of lipogenic enzyme mRNA and their activity will increase in supplemented animals.
Cattle that are grain-fed have a higher core body temperature (Jacob et al. 2014) and are more susceptible to exhibiting the quality condition called ‘high rigor temperature’ or ‘heat-toughening’, which is caused by rapid pH fall at high muscle temperatures, and the meat exhibits failure to tenderise, softness and excessive weep (Warner et al. 2014). Thus, any remedies for reducing the core body temperature of grain-fed cattle will potentially reduce the levels of heat-toughening and improve product quality.
Heat shock proteins (HSPs) are a set of protective proteins involved in cellular stress responses, of which the HSP70 family is the most readily induced by stress and is the most abundantly studied. This family is expressed under most physiological conditions, although expression is tightly regulated by heat shock factors (HSF) (Pockley 2003). Heat shock proteins are present in the circulation of rats (Fleshner et al. 2004), humans (Campisi et al. 2003; Lancaster and Febbraio 2005; Pshennikova et al. 2006; Ruell et al. 2007) and cattle (Kristensen et al. 2004). The presence of HSPs in circulation is yet to be fully understood, but it appears they play a role in the innate immune system and inflammation (Terry et al. 2006) and are released intra- and extra-cellularly in response to stress (Hightower and Guidon 1989). Thus, the aim of the present experiment was to explore the effects of long-term dietary betaine supplementation with and without shade on carcass yield and quality, HSP (HSF1, HSP70 and HSP90) gene expression, and enzyme (FAS and G6PDH) activity in feedlot steers exposed to heat. It was hypothesised that long-term supplementation of dietary betaine with and without shade would increase fat depth in steers exposed to a high heat load.
Materials and methods
Experimental design—animals and housing
All procedures involving animals were approved by the University of Queensland Animal Ethics Committee. The study was undertaken from November to March during the Australian summer. The summer climate in this region is characterised by high temperature, a high solar load and high humidity. An on-site automated weather station (as described by Gaughan et al. 2010) located 15 m behind the feedlot collected climatic data every 30 min, which was used to calculate the heat load index (HLI). This experiment was part of a larger trial of 164 Black Angus steers as described by Gaughan et al. (2010). The experiment involved 48 Black Angus steers 12–15 months old that were randomly allocated to a 4 × 2 factorial design with the respective factors being dietary betaine (96% betaine; Feedworks, Romsey, Vic.) supplement (0, 10, 20 or 40 g head/day) and shade (with and without shade). These doses were chosen to explore any possible dose responses present based on the results of previous studies (Bock et al. 2002, 2004; Loest et al. 2002) and equated to ~0.1, 0.2 and 0.4% of the diet. Steers were housed in treatment groups within feedlot pens at a stocking rate of one steer per 18–19 m2 (8–9 steers per pen) in the feedlot at The Queensland Department of Primary Industries and Fisheries Brigalow Research Station, Theodore, Queensland, Australia, as previously described in Gaughan et al. (2010). Shade was provided by shade cloth (80% solar block) with ~3.2 m2 of shade available per steer. Supplemented and control steers were fed the same basal diet that included a mineral supplement formulated by Integrated Animal Production (Toowoomba, Qld) (Table 1). Starter diets were fed for 4 days, followed by the intermediate diet 1 for 7 days and intermediate diet 2 for 6 days. Steers were then fed the finisher diet for the rest of the experiment (120 days). Dietary betaine supplements were incorporated into daily rations. Water was available ad libitum in each pen.

|
Sample collection and analysis
After 120 days of the finisher diet treatment, the steers were transported to the abattoir and remained in covered lairage overnight before being slaughtered at random over 2 h in two groups at a commercial abattoir. After placement in the chiller, pH/temperature and time measurements were recorded in the m. longissimus lumborum in the region of the second to fifth lumbar vertebrae on individual carcasses at 1, 2 and 3 h post-slaughter. At grading (approximately 24 h post-slaughter), the pH and temperature were measured in the exposed surface of the longissimus thoracis (LT) at the 12th/13th rib. For each measurement, care was taken to place the pH meter into a fresh incision rather than using the site where the previous measurement had been recorded. A pH meter (Jenco 6009; Jenco Instruments Inc., San Diego, CA, USA) with a polypropylene spear-type gel electrode (IJ44; Ionode Pty Ltd, Brisbane, Qld) and temperature probe allowing temperature compensation was used to measure pH and temperature. The pH meter and electrode were calibrated at ambient temperature using buffers of 4.00 and 7.00.
Within 5–10 min after slaughter, samples (20–30 g) were taken from the trapezius muscle and adipose tissue was collected from the subcutaneous (above the trapezius muscle) and omental regions. On removal, the tissue samples were trimmed and immediately frozen in liquid nitrogen and stored at −80°C before analysis. AUS-MEAT (Authority for Uniform Specifications for Meat and Livestock) carcass measurements, including hot standard carcass weight (HSCW) and P8 fat depth, were also recorded. After splitting, the sides were identified and chilled overnight. At about 22 h post-mortem, after quartering of the carcass between the 12th and 13th rib, MSA (Meat Standards Australia) graders made the following measurements: AUS-MEAT texture score (1, fine; 7, coarse) and AUS-MEAT firmness score (1, firm; 7, soft) (Aus-Meat 1996). Muscle colour was measured using a HunterLab Miniscan (Model 45/0-L; aperture size 25 mm, illuminant D65, standard observer 10, calibrated with black and white tiles) (HunterLab Inc., Reston, VA, USA), after a 30-min bloom, to measure the colour parameters Hunter L, which is the muscle lightness value (0 is black and 100 is white), Hunter a, which is a measure of muscle redness–greenness (higher values are more red, lower values are less red), and Hunter b, which is a measure of muscle yellowness–blueness (higher values are more yellow, lower values less yellow).
Tissue RNA was extracted from samples from the control and treatments of 40 g betaine/day with or without shade, using an Invitrogen PureLink™ RNA micro to midi kit and TriZol reagent (Invitrogen, Mulgrave, Vic.) as per the manufacturer’s instructions. Isolated RNA was evaluated for quality using the Bio-Rad Experion automated electrophoresis station (Bio-Rad Laboratories Inc., Hercules, CA, USA) to examine the ratio of 28S to 18S, and samples with two clear peaks and a ratio close to 1.7 were accepted. Tissue RNA was converted to cDNA using the SuperScriptIII first-strand synthesis system for RT–PCR (Invitrogen) as per the manufacturer’s instructions. The reverse transcription PCR was performed in triplicate in a 25-µL volume using SYBR green (Bio-Rad) as fluorescein and an iQ5 system (Bio-Rad). The gene expression of HSP70 (forward 5′AAC ATG AAG AGC GCC GTG GAGG, reverse 5′GTT ACA CAC CTG CTC CAG CTCC), HSP90 (forward 5′GAC TCC CAG GCA TAC TGC TC, reverse 5′GGC GCT GAT ATC TCC ATG AT), HSF1 (forward 5′ACC TCT GGA GGC AGA GAA AAG, reverse 5′TCT CCC TCG GAG AAG TAG GAG) and β-actin (forward 5′CAT CGA GCA CGG CAT CGT CA, reverse 5′TAG CAC AGC CTG GAT AGC AAC) was examined. For each assay, 40 PCR cycles were run and a dissociation curve was produced. The RT–PCR data were analysed as the threshold cycle (Ct) relative to that of β-actin. A ΔCt of –1.0 is associated with a doubling (200%) and +1.0 a halving (50%) of expression.
Adipose tissue samples were analysed for the activity of FAS and G6PDH using a Thermo Multiskan Spectrum Microplate Spectrophotometer (accuracy ± 0.005) (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 37°C and 340 nm wavelength. The concentration of protein in each homogenate was measured using a Bradford assay and all assays were conducted using BD Falcon clear 69-well flat bottom micro-well plates (BD, Franklin Lakes, NJ, USA). Each method was optimised to find the correct concentration of sample required to achieve a change of absorbance of 0.1 absorbance unit every 3 min. All co-factors were obtained from Sigma-Aldrich (Castle Hill, NSW). The FAS (E.C. 2.3.1.85) activity was measured using the method of Martin et al. (1961), with modifications by Ingle et al. (1972). The average enzymatic rate was calculated by determining the linear decrease in absorbance over a minimum 3-min period, and FAS activity was expressed as nmol NADP reduced/min.mg cytosolic protein. The G6PDH (E.C. 1.1.1.49) activity was measured according to the adapted methods of Deutsch (1983), which uses the increase of absorbance at 340 nm due to NADPH to determine the rate of increase. The average enzymatic rate was calculated by determining the linear increase in absorbance over a minimum 3-min period. Total enzyme activity was expressed as nmol NADP reduced/min.mg cytosolic protein.
Statistical analyses
All analyses were undertaken using residual maximum likelihood (REML) in Genstat (11th edition, VSN International, Hemel Hempstead, UK). Analysis included the responses of betaine dose, shade and, where applicable, tissue. In addition, comparisons were made between diets containing no betaine or added betaine (i.e. regardless of betaine dose), in which case the model included betaine, shade and, where appropriate, tissue. Gene expression was analysed Ct relative to that of β-actin (ΔCt). All analyses were undertaken using individual animal as the random effect.
Results
The climatic conditions experienced in the study have been described previously (Gaughan et al. 2010). Briefly, the average maximum ambient temperature for the duration of the study was 36.3°C and the temperature humidity index ranged from 57.7 to 84.5 units over the duration of the study. There was a 21-day period of increased heat load from day 71 to day 91, during which cattle were exposed to >30.0°C temperatures for 8–10 h/day.
Carcass and meat quality responses measured are presented in Table 2. Carcass weight at slaughter was not significantly altered by shade (309 v. 315 kg, P = 0.18) but tended towards being increased by dietary betaine (306 v. 314 kg, P = 0.09). Backfat depth at the P8 site was not altered by shade (15.1 v. 15.8 mm, P = 0.43) or dietary betaine (14.7 v. 15.7 mm, P = 0.32). However, there was a within dietary betaine effect such that P8 backfat tended to be greater in steers fed 10 g betaine/day compared with 40 g betaine/day (17.4 v. 14.5 mm, P = 0.06). There were no main effects of shade or dietary betaine on temperature at 1 h after slaughter. However, there was a shade × within betaine interaction (P = 0.06) such that muscle temperature increased with increasing dose of betaine in steers maintained in shade but was highest in steers supplemented 10 g betaine/day and kept out of shade (Table 2). Muscle pH at 1 h (5.97 v. 6.03, P = 0.014) and 2 h (5.97 v. 6.03, P = 0.014) post-slaughter was lower in steers that had been maintained in shade (5.73 v. 5.80, P = 0.043). While there was no main effect of betaine on muscle pH at 1 h (5.99 v. 6.00, P = 0.74), there was a within dietary betaine effect such that muscle pH was higher in steers fed 10 or 20 g betaine/day than those fed 40 g betaine/day (6.03 v. 6.03 v. 5.95, P = 0.005) at 1 h. There was no effect of dietary betaine on pH at 2 h or 22 h, but muscle pH at 3 h was lower in steers fed dietary betaine (5.63 v. 5.57, P = 0.026). Firmness, texture and drip loss were not altered by betaine or shade.
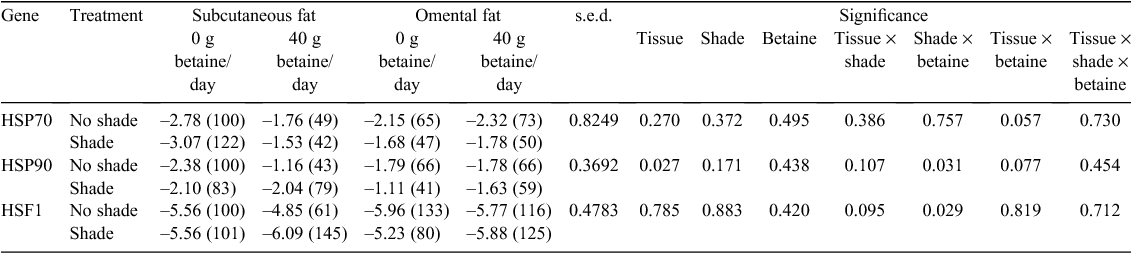
The expression of HSP70, HSP90 and HSF1 was significantly different between tissue types (P < 0.001) such that the expression in muscle tissue was much lower than in adipose tissues (Fig. 1). The expression of HSP70, HSP90 and HSF1 mRNA in omental and subcutaneous adipose tissue are presented in Table 3. For clarity, data are also presented as a percentage of expression at 0 g betaine/day from unshaded steers in subcutaneous tissue. When analysing adipose tissue responses, HSF1 was unaffected by dietary betaine, shade or tissue type, whereas there was a dietary betaine × shade interaction such that expression was slightly greater in unshaded tissue without betaine supplementation but greater in shaded animals supplemented with 40 g betaine/day: –5.76 (no shade, no betaine), –5.31 (no shade, betaine) and –5.40 (shade, no betaine), –5.98 (shade, betaine); s.e.d. 0.293, P = 0.03. The expression of HSP70 tended to be greater in subcutaneous adipose tissue from unsupplemented animals but greater in the omental adipose tissue from those supplemented with 40 g betaine/day: –2.93 (subcutaneous, no betaine), –1.65 (subcutaneous, betaine) and –1.92 (omental, no betaine), –2.05 (omental, betaine); s.e.d. 0.557, P = 0.06.

|
The expression of HSP90 was greater in subcutaneous than omental adipose tissue (–1.92 v. –1.58 for subcutaneous and omental adipose tissue, respectively; s.e.d. 0.207, P = 0.03). There was a dietary betaine × shade interaction such that HSP90 expression in adipose tissue was greater in unshaded, unsupplemented animals compared with those with access to shade and supplemented with 40 g betaine/day: –2.08 (no shade, no betaine), –1.47 (no shade, betaine) and –1.60 (shade, no betaine), –1.83 (shade, betaine); s.e.d. 0.218, P = 0.03. In skeletal muscle tissue, the expression of HSF1 was not affected by dietary betaine or shade. The expression of HSP70 in muscle was greater in unshaded than shaded animals (0.43 v. 1.11 for unshaded and shaded animals, respectively; s.e.d. 0.297, P = 0.04). The expression of HSP90 in muscle tended to be greater in unshaded than shaded animals (0.53 v. 1.14 for unshaded and shaded animals, respectively; s.e.d. 0.321, P = 0.09).
When analysed incorporating all three tissue types into the model, dietary betaine supplementation did not alter the expression of HSP70 (–1.3 v. –1.0; s.e.d. 0.372, P = 0.70), HSP90 (–0.89 v. –0.87; s.e.d. 0.169, P = 0.75) or HSF1 (–4.4 v. –4.5; s.e.d. 0.224, P = 0.29) for control and 40 g betaine/day, respectively. The expression of HSP90 mRNA was decreased by shade (–1.0 v. –0.77 for unshaded and shaded respectively; s.e.d. 0.169, P = 0.03). The HSF1 mRNA expression was not altered by shade. There was a shade × tissue interaction such that omental adipose tissue from steers kept out of shade expressed more HSF1 than omental adipose tissue from shaded steers, while the converse was true for subcutaneous adipose tissue. The expression of HSF1 in muscle was not affected by dietary betaine or shade. The expression of HSP70 in muscle was greater in unshaded than shaded animals (0.43 v. 1.11 for unshaded and shaded animals, respectively; s.e.d. 0.297, P = 0.04). The expression of HSP90 in muscle tended to be greater in unshaded than shaded animals (0.53 v. 1.14 for unshaded and shaded animals, respectively; s.e.d. 0.321, P = 0.09). All genes were expressed more in adipose than muscle tissue.
The enzyme activity of FAS and G6PDH are presented in Table 4. The activity of FAS was greater in subcutaneous than in omental adipose tissue (7.8 v. 5.8 nmol NADPH/min.mg cytosolic protein; P = 0.009). Shade and betaine treatments did not alter FAS or G6PDH activity. There was a shade × betaine × tissue type interaction on FAS activity (P = 0.05) such that subcutaneous adipose tissue from unshaded steers supplemented with 40 g betaine/day had the greatest FAS activity compared with all other treatments, while omental adipose tissue from shaded control steers had the least activity.
Discussion
Shade can provide immediate relief from the effects of solar radiation, which is a large contributor to heat stress in cattle housed outdoors. This project aimed to explore what effects, if any, the provision of shade had on some physiological and carcass characteristics in feedlot steers housed in central Queensland. This location was selected based on climate, which is sufficient to induce a heat-stress response during summer. As discussed by Gaughan et al. (2010) the weather conditions were milder than expected but sufficient to observe heat-stress responses (increased respiration rate) in the cattle. In the results presented from this aspect of the larger study, the provision of shade did not have any conclusive effects on the cattle, although there were some interactions of shade and dietary betaine.
The data from the present experiment indicate some interesting carcass and meat quality responses to dietary betaine and shade, some of which were moderated by the dose of dietary betaine. The P8 backfat increased with the 10 g dietary betaine dose, which supports our initial hypothesis that betaine supplementation would increase fat depth in feedlot steers. Furthermore, carcass weight tended to be increased by dietary betaine, although this occurred in response to overall betaine and there were no within betaine dose responses (Table 2). This supports the findings of Bock et al. (2004), who demonstrated that 40 g head/day of betaine supplemented to beef steers consuming pasture for 7 days pre-slaughter improved carcass weight, although this was not the case for steers supplemented with dietary betaine while in the feedlot. Betaine can reduce maintenance energy requirements (Schrama et al. 2003) by reducing ion pumping associated with maintaining cellular osmolarity (Nakanishi et al. 1990; Lohr et al. 1991; Peterson et al. 1992), and in cattle betaine is lipotropic and may increase fat thickness (Bock et al. 2004; Loest et al. 2002), as somewhat supported by the results of the present experiment in the P8 backfat responses to the 10 g dose. Thus, the trend for carcass weight to be increased in steers supplemented with betaine may be the result of increased fat thickness. There is no evidence to support betaine directly influencing lipid metabolism; instead, betaine is thought to reduce maintenance energy requirements by maintaining cellular osmolarity without requiring ion pumps in cells exposed to hyperosmotic conditions (Moeckel et al. 2002), which allows more energy to be directed towards fat accretion (Campbell et al. 1995; Suster et al. 2004). In chickens, betaine is able to maintain gut cellular osmolarity and improve and stabilise the structure of gut mucosa (Kettunen et al. 2001), although in meat lambs, betaine supplementation did not alter the hydration status of the animals at slaughter (Pearce et al. 2008).
It is also suggested that betaine is able to increase cell proliferation within gut tissues, which increases the available surface for nutrient absorption and, hence, can improve the efficiency of feed utilisation (Eklund et al. 2005). While we observed some effects of betaine on carcass quality, these responses were not entirely clear. Furthermore, measurements of gut mucosa and overall gut weights were not made; thus, inferences about potential differences in gut physiology and size due to betaine and/or shade treatments are not possible in the present study. While most studies examining betaine supplementation have been with poultry and pigs, its effects are equivocal in other species where the responses are less obvious (Bock et al. 2002; Fernández et al. 1998; Loest et al. 2002). In pigs, the varied responses to betaine are thought to be due to the protein and energy content of the diet (Matthews et al. 1998) and this may also be the case in cattle. There is little uniformity between studies, deeming the interpretation of pooled responses to betaine in cattle impractical; however, the effect of betaine on lipid metabolism is a recurring theme.
Pre-slaughter environmental conditions, alongside animal factors such as stress and physiology, affect meat quality. Post-mortem, muscle ATP ceases to be produced via mitochondria due to lack of oxygen, and thus muscle metabolism is driven by anaerobic glycolysis, which leads to lactic acid production. The rate of pH decline is influenced by the rate of glycolysis, the rate of temperature decline post-slaughter and the temperature at which the pH is obtained, all of which can be influenced by fat depth (Tornberg et al. 2000). In the present experiment there appeared to be a small effect of shade and betaine dose on the muscle temperature at 1 and 3 h post-slaughter, although there was no clear pattern to these responses. Although ultimate pH was not influenced by betaine, the first pH measurement was increased in steers supplemented with 10 and 20 g betaine, whereas 40 g betaine decreased the first pH measure. The temperature and pH of the carcass at slaughter is important for lasting effects on meat quality, and increased temperatures pre-slaughter combined with a rapid temperature decline post-slaughter can lead to heat-toughening. Warmer post-mortem temperatures will result in a more rapid pH decline and high pre-slaughter glycogen levels, which are increased by pre-slaughter stress responses. Heat-toughening leads to paler meat (Hughes et al. 2014), increased drip loss, and tough, less juicy meat (Warner et al. 2014), although in the present experiment drip loss and colour were not altered by shade or betaine. A pH between 5.9 and 6.2 at 1.5 h post-slaughter is suggested as optimum for tenderness (Hwang and Thompson 2001), and our data show that these animals were in this range at 1–2 h post-slaughter. However, it is also suggested that a pH <5.6 at 3 h post-mortem results in significantly tougher meat (Pike et al. 1993), and carcasses with a pH ≤6.25 at 1 h post-slaughter are said to have ‘faster rigor’ onset, resulting in less tender meat (Khan and Ballantyne 1973). Under the conditions in the present study, the meat from these steers is likely to be tough regardless of shade or dietary treatments, as all treatments had a temperature >35°C at pH 6, which is classed as high rigor temperature (as discussed by Warner et al. 2014). Shaded animals also exhibited increased muscle temperature when supplemented with 10 g betaine, whereas unshaded animals demonstrated the opposite effect. Thus, betaine was not effective in reducing the occurrence of heat-toughening post-slaughter in grain-fed beef carcasses. Feeding betaine for a shorter time might have more effect in reducing the core body temperature of the cattle and thus post-slaughter temperature and metabolism.
The effects of betaine supplementation on lipid metabolism were further examined through enzyme activity measures. Fatty acid synthase, which catalyses the final step in the lipogenic (fatty acid synthesis) pathway and is hence a key determinant of the maximal capacity of a tissue to synthesise fatty acids, was altered by adipose tissue type. Furthermore, there was a shade × tissue × betaine treatment interaction for FAS activity. Subcutaneous adipose tissue had improved FAS activity over that of omental, indicating that this type of fat was better able to synthesise fatty acids. In addition, subcutaneous adipose tissue from unshaded animals supplemented with 40 g betaine displayed more FAS activity than control animals, whereas all other betaine doses had decreased subcutaneous FAS activity. Huang et al. (2006) demonstrated reduced FAS mRNA in subcutaneous adipose tissue from pigs fed 0.125% betaine compared with control animals, whereas the increased FAS activity noted here is novel. Contrasting with these results, P8 backfat, which is a measure of subcutaneous fat depth, was increased the most by the lower (10 g) dose of betaine. However, the enzyme expression analysis was undertaken on adipose tissue sampled at a different site from the P8 depth measure, and this may explain the discordance between actual fat depth and enzyme activity. On the other hand, the activity of G6PDH was not affected by either shade or betaine, although there tended to be a shade × tissue × betaine supplementation interaction. Unlike FAS activity, there tended to be more G6PDH activity in omental compared with subcutaneous adipose tissues. G6PDH is required for fatty acid synthesis as it is the main enzyme available to supply NADPH, the coenzyme required to synthesise fatty acids (Huang et al. 2006).
In addition to the effects of shade and dietary betaine supplementation on carcass measures, there were some interesting HSP gene and protein expression responses. HSP/HSF mRNA was present in both types of adipose as well as muscle tissue, supporting the findings of Chung et al. (2008), who demonstrated HSP70 expression in adipose tissue and skeletal muscle of mice. However, there were no effects of the highest dose of betaine (40 g) on the expression of HSPs or HSF in fat or muscle tissues. It was initially hypothesised that the highest dose of betaine would elicit the greatest effects on the animals, which is why this treatment was examined; however, the 10 g dose appears to have the greatest influence carcass composition, which may have also been reflected by gene expression. Perhaps supplementation with the 10 g dose of betaine was the ideal dose rate, with the 20 and 40 g doses ineffective for reasons that cannot be deduced from the present experiment.
Tissue type significantly altered the expression of HSPs and HSF1, with adipose tissues expressing more than muscle tissue. Furthermore, there appeared to be differences between the two types of adipose measured, although neither type was consistently more expressive than the other. Metabolically, subcutaneous fat is less lipolytic than omental fat due to the presence of fewer insulin and catecholamine receptors, but this was not reflected in the enzyme activities measured here (Pérez-Pérez et al. 2009). Perhaps, then, the metabolic activity of the tissue is not responsible for the induction of HSPs, and it is the location of the subcutaneous fat (close to the skin surface) that induces more HSP70, as the tissue may be under more thermal stress due to solar radiation. However, there were no apparent differences in expression of HSPs between shaded and unshaded animals, although the unshaded animals would be expected to be under a greater heat load. As the tissue used in this experiment was collected at slaughter after all steers were at lairage under shade for >8 h, any effects of the shade treatment may have been eliminated by then, which is supported by the measurement of body temperature obtained at lairage showing no differences between shaded and unshaded steers (P = 0.7; J. B. Gaughan, unpubl. data). Nevertheless, the effectiveness of shade in reducing the thermal load and improving production responses in feedlot cattle is contentious, as discussed by Blackshaw and Blackshaw (1994) and Sullivan et al. (2011). Steers (n = 164) involved in the larger overall study of which this experiment was a part, as presented by Gaughan et al. (2010), demonstrated that steers with access to shade had higher HSCW, average daily gain and grain : feed compared with unshaded steers. The unshaded steers also had a greater mean panting score over the duration of the study than shaded steers, although this did not translate into differences in body temperature (Gaughan et al. 2010).
Expression of HSF1 tended to be the greatest of the three measured genes. There was a tissue × shade interaction whereby subcutaneous adipose tissue had increased expression in control steers, and omental fat from steers supplemented with 40 g betaine had decreased HSF1 expression in shaded animals. In the shaded animals, this interaction appears to follow results seen for HSP90, whereby greater expression was seen in subcutaneous adipose tissue, and as HSF1 regulates HSP expression, this makes sense, although this is not supported by HSP70 responses. The reason for this interaction is not immediately clear and it is even counter-intuitive, as we would predict that shaded animals would be under a lesser thermal load. In vitro studies using canine kidney cells show that betaine can inhibit HSP70 mRNA at high temperatures, likely by stabilising cellular proteins and protecting them from heat (stress) induced denaturisation (Sheikh-Hamad et al. 1994). Thus, the lack of tissue HSP70 expression in the present study may be, at least in part, due to supplementary betaine stabilising cellular proteins. Tissue variations in HSF1 expression have been noted in pigs (Yue et al. 2010), although muscle and fat tissue was not measured, and as demonstrated here it appears that HSF gene expression is tissue-specific. Finally, it is possible that the stress gene expression and interactions presented were caused by the transportation and slaughter processes that the animals experienced before the sample collection; however, they could not be avoided in this experiment.
There were no differences in mean body temperature when the steers were in lairage pre-slaughter, although the shaded steers had significantly (P < 0.05) lower temperatures from approximately 12 : 00 to 17 : 00, during their final 24 h at the feedlot (J. B. Gaughan, unpubl. data.). Because this study was undertaken over the summer, and both animals were under the same thermal environment (shaded) for ~8 h at lairage, the presence of HSP mRNA in the tissue of both shaded and unshaded animals is logical. This is particularly true for HSP90, which is able to form long-term associations with cellular proteins. Furthermore, a constant concentration of HSPs under ‘normal’, non-stress situations is thought to exist due to their roles in immune and chaperoning processes. The expression profiles of HSP have been shown elsewhere to return to basal levels within hours of a stress event (Collier et al. 2006), although acclimation would deem that these animals, after 120 days in the feedlot, had acclimatised to their thermal environment. In rats however, acclimated animals had an increased concentration of HSP70 mRNA compared with non-acclimated animals (Maloyan et al. 1999). This was further demonstrated by Horowitz (2002), who showed that long-term acclimated rats have drastically increased HSP70 mRNA, although this is in the heart and brain tissue. On the other hand, HSP protein in the quadriceps muscle in rats is not influenced by short-term increases in environmental temperature (Flanagan et al. 1995). Unfortunately, baseline (pre thermal stress) samples were not obtained, and it would have been interesting to compare the tissue HSP expression pre- and post-heat, as this may provide a link to animals that are more sensitive to elevated temperatures and are thus more likely to exhibit high rigor temperature and other meat quality defects, and vice versa. This has been demonstrated in pigs, in which polymorphisms in the HSP70 gene have been shown to alter meat quality measures such as backfat thickness (Huang et al. 2004). Together, the findings from this experiment and other literature indicate that there are differences in tissue, species and individual animal HSP gene expression for reasons that are not clear. This may be explained by different tissues having different baseline temperatures and different thermogenic capacity, which would therefore influence their heat shock responses and hence HSP expression.
Conclusion
Dietary betaine tended to increase HSCW and there was a within betaine dose response such that P8 backfat depth was greater in steers supplemented with 10 compared with 40 g betaine/day, supporting our hypothesis that dietary betaine supplementation can increase fat depth in feedlot steers. Muscle pH at 1 and 2 h post-slaughter was lower in shaded compared with unshaded steers and pH tended to be higher in steers fed 10 or 20 g betaine/day compared with those fed 40 g betaine/day. Provision of shade during summer may therefore reduce the rate of pH decline in the first 2 h after slaughter and reduce the risk of high rigor temperature. Furthermore, in feedlot steers, dietary betaine may be eliciting some energy-sparing responses that are manifested in carcass fat depth and measures of pH, and betaine supplementation may be a useful carcass modifier in growing feedlot steers during summer. The mRNA of HSP70/90 and HSF1 was expressed in muscle, omental and subcutaneous adipose tissues, which is a novel finding. Dietary betaine supplementation did not directly alter the expression of HSP/HSF mRNA in either muscle or adipose tissue, although there were some interactions between betaine and presence of shade. All genes were expressed more in adipose than muscle tissues. The reasons for the differences in tissue HSP/HSF mRNA expression are unknown but may reflect the different thermogenic capacities of the tissue types.
Acknowledgements
The technical assistance throughout this investigation from Fahri Fahri and Athula Naththarampatha are gratefully acknowledged. The funding provided by Meat and Livestock Australia and Department of Primary Industries Victoria is also gratefully acknowledged.
References
Aus-Meat (1996) Beef and veal chiller assessment language. In ‘Australian beef carcase evaluation’. (Meat Standards Australia)Blackshaw JK, Blackshaw AW (1994) Heat stress in cattle and the effect of shade on production and behaviour: A review. Australian Journal of Experimental Agriculture 34, 285–295.
| Heat stress in cattle and the effect of shade on production and behaviour: A review.Crossref | GoogleScholarGoogle Scholar |
Bock BJ, Brethour JR, Goodall SR (2002) Effect of 7-day pre-harvest 40g betaine regimen on dressing percent of feedlot steers. In ‘Proceedings of the Western Section American Society of Animal Science’. pp. 1–3. (American Society of Animal Science: Fort Collins, CO)
Bock BJ, Brethour JR, Harmoney KR, Goodall SR (2004) Influence of betaine on pasture, finishing, and carcass performance in steers. The Professional Animal Scientist 20, 53–57.
Brown-Brandl TM, Eigenberg RA, Nienaber JA, Hahn GL (2005) Dynamic response indicators of heat stress in shaded and non-shaded feedlot cattle, part 1: Analyses of indicators. Biosystems Engineering 90, 451–462.
| Dynamic response indicators of heat stress in shaded and non-shaded feedlot cattle, part 1: Analyses of indicators.Crossref | GoogleScholarGoogle Scholar |
Campbell RG, Cadogan DJ, Morley WC, Uusitalo R, Virtanen E (1995) Interrelationships between dietary methionine and betaine on growth performance in pigs 65 to 100kg. Journal of Animal Science 73, 82
Campisi J, Leem TH, Fleshner M (2003) Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress & Chaperones 8, 272–286.
| Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtVWnsb%2FN&md5=6887bb720d670a33687378678107dcc7CAS |
Chung J, Nguyen A-K, Henstridge DC, Holmes AG, Stanley Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA (2008) Hsp72 protects against obesity-induced insulin resistance. Proceedings of the National Academy of Sciences of the United States of America 105, 1739–1744.
| Hsp72 protects against obesity-induced insulin resistance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhvFalsbs%3D&md5=cfd7a9651935744fdf7680ac2c704468CAS | 18223156PubMed |
Clarke MR, Kelly AM (1996) Some effects of shade on Hereford steers in a feedlot. Proceedings of the Australian Society of Animal Production 21, 235–239.
Collier RJ, Dahl GE, VanBaale MJ (2006) Major advances associated with environmental effects on dairy cattle. Journal of Dairy Science 89, 1244–1253.
| Major advances associated with environmental effects on dairy cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjt1SjsLk%3D&md5=2f5bbe9b1bfbc89034cd50d0670ae57bCAS | 16537957PubMed |
Deutsch J (1983) Glucose-6-phosphate dehydrogenase. In ‘Methods of enzymatic analysis’. (Eds H Bergmeyer, J Bergmeyer) pp. 190–197. (Verlag Chemie: Weinheim, Germany)
Eklund M, Bauer E, Wamatu J, Mosenthin R (2005) Potential nutritional and physiological functions of betaine in livestock. Nutrition Research Reviews 18, 31–48.
| Potential nutritional and physiological functions of betaine in livestock.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXntFSktLk%3D&md5=f946c9aecbbd77322ee21b2444cb50e1CAS | 19079893PubMed |
Fernández C, Gallego L, Lopez-Bote CJ (1998) Effect of betaine on fat content in growing lambs. Animal Feed Science and Technology 73, 329–338.
| Effect of betaine on fat content in growing lambs.Crossref | GoogleScholarGoogle Scholar |
Flanagan SW, Ryan AJ, Gisolfi CV, Moseley PL (1995) Tissue specific HSP70 response in animals undergoing heat stress. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 268, R28–R32.
Fleshner M, Campisi J, Amiri L, Diamond DM (2004) Cat exposure induces both intra- and extracellular Hsp72: The role of adrenal hormones. Psychoneuroendocrinology 29, 1142–1152.
| Cat exposure induces both intra- and extracellular Hsp72: The role of adrenal hormones.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlt1Wjtrs%3D&md5=62fa145d248161ce1b66ba89a3c493e9CAS | 15219638PubMed |
Gaughan JB, Tait LA, Eigenberg RA, Bryden WL (2004) Effect of shade on respiration rate and rectal temperature of angus heifers. Animal Production in Australia 25, 69–72.
Gaughan JB, Bonner S, Loxton I, Mader TL, Lisle A, Lawrence R (2010) Effect of shade on body temperature and performance of feedlot steers. Journal of Animal Science 88, 4056–4067.
| Effect of shade on body temperature and performance of feedlot steers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFGnsrjE&md5=dc150240e651f372d6f572ab8fa8cf8bCAS | 20709874PubMed |
Hahn GL (1999) Dynamic responses of cattle to thermal heat loads. Journal of Animal Science 77, 10–20.
Hightower LE, Guidon PT (1989) Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. Journal of Cellular Physiology 138, 257–266.
| Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXhtlaltbg%3D&md5=1d333e465950b0f2ff76c5c4dc77ffc4CAS | 2918030PubMed |
Horowitz M (2002) From molecular and cellular to integrative heat defense during exposure to chronic heat. Comparative Biochemistry and Physiology. A. Comparative Physiology 131, 475–483.
| From molecular and cellular to integrative heat defense during exposure to chronic heat.Crossref | GoogleScholarGoogle Scholar |
Huang SY, Lee WC, Chen MY, Wang SC, Huang CH, Tsou HL, Lin EC (2004) Genotypes of 5′-flanking region in porcine heat-shock protein 70.2 gene affect backfat thickness and growth performance in duroc boars. Livestock Production Science 85, 181–187.
| Genotypes of 5′-flanking region in porcine heat-shock protein 70.2 gene affect backfat thickness and growth performance in duroc boars.Crossref | GoogleScholarGoogle Scholar |
Huang QC, Xu ZR, Han XY, Li WF (2006) Changes in hormones, growth factor and lipid metabolism in finishing pigs fed betaine. Livestock Science 105, 78–85.
| Changes in hormones, growth factor and lipid metabolism in finishing pigs fed betaine.Crossref | GoogleScholarGoogle Scholar |
Huang QC, Xu ZR, Han XY, Li WF (2007) Effect of betaine on growth hormone pulsatile secretion and serum metabolites in finishing pigs. Journal of Animal Physiology and Animal Nutrition 91, 85–90.
| Effect of betaine on growth hormone pulsatile secretion and serum metabolites in finishing pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksFWqs78%3D&md5=843ed5645cb597fa8fb6f16150c075ffCAS | 17355337PubMed |
Huang QC, Xu ZR, Han XY, Li WF (2008) Effect of dietary betaine supplementation on lipogenic enzyme activities and fatty acid synthase mRNA expression in finishing pigs. Animal Feed Science and Technology 140, 365–375.
| Effect of dietary betaine supplementation on lipogenic enzyme activities and fatty acid synthase mRNA expression in finishing pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlvV2ltg%3D%3D&md5=93bf7878e7ad9cbf3129a9a5dc63ad9fCAS |
Hughes JM, Kearney G, Warner RD (2014) Improving beef meat colour scores at carcass grading. Animal Production Science 54, 422–429.
| Improving beef meat colour scores at carcass grading.Crossref | GoogleScholarGoogle Scholar |
Hwang IH, Thompson JM (2001) The interaction between ph and temperature decline early postmortem on the calpain system and objective tenderness in electrically stimulated beef longissimus dorsi muscle. Meat Science 58, 167–174.
| The interaction between ph and temperature decline early postmortem on the calpain system and objective tenderness in electrically stimulated beef longissimus dorsi muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1Wgurc%3D&md5=40939faba202cd6c32db4d2d60e5d1ecCAS | 22062112PubMed |
Ingle DL, Bauman DE, Garrigus US (1972) Lipogenesis in the ruminant: in vitro study of tissue sites, carbon source and reducing equivalent generation for fatty acid synthesis. The Journal of Nutrition 102, 609–616.
Ittner NR, Kelly CF, Guilbert HR (1951) Water consumption of Hereford and Brahman cattle and the effect of cooled drinking water in a hot climate. Journal of Animal Science 10, 742–751.
Jacob RH, Surridge VSM, Beatty DT, Gardner GE, Warner RD (2014) Grain feeding increases core body temperature of beef cattle. Animal Production Science 54, 444–449.
| Grain feeding increases core body temperature of beef cattle.Crossref | GoogleScholarGoogle Scholar |
Kettunen H, Peuranen S, Tiihonen K (2001) Betaine aids in the osmoregulation of duodenal epithelium of broiler chicks, and affects the movement of water across the small intestinal epithelium in vitro. Comparative Biochemistry and Physiology. A. Comparative Physiology 129, 595–603.
| Betaine aids in the osmoregulation of duodenal epithelium of broiler chicks, and affects the movement of water across the small intestinal epithelium in vitro.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MzmtF2hsw%3D%3D&md5=e0f809f93fe7fccc40961fbf689f96f0CAS |
Khan AW, Ballantyne WW (1973) Post-slaughter ph variation in beef. Journal of Food Science 38, 710–711.
| Post-slaughter ph variation in beef.Crossref | GoogleScholarGoogle Scholar |
Kristensen TN, Lovendahal P, Berg P, Loeschcke V (2004) Hsp72 is present in plasma from Holstein-Friesian dairy cattle, and the concentration level is repeatable across days and age classes. Cell Stress & Chaperones 9, 143–149.
| Hsp72 is present in plasma from Holstein-Friesian dairy cattle, and the concentration level is repeatable across days and age classes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntVGqtrY%3D&md5=dcb100f2f4b9e29a741ef9a01d93e5c2CAS |
Lancaster GI, Febbraio MA (2005) Exosome-dependent trafficking of HSP70. The Journal of Biological Chemistry 280, 23349–23355.
| Exosome-dependent trafficking of HSP70.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltVygsrc%3D&md5=457995c73f275021e38ea5188f79367eCAS | 15826944PubMed |
Loest CA, Titgemeyer EC, Drouillard JS, Coetzer CM, Hunter RD, Bindel DJ, Lambert BD (2002) Supplemental betaine and peroxide-treated feather meal for finishing cattle. Journal of Animal Science 80, 2234–2240.
Lohr JW, Pochal MA, Acara M (1991) Osmoregulatory betaine uptake by rat renal medullary slices. Journal of the American Society of Nephrology 2, 879–884.
Mader TL (2003) Environmental stress in confined beef cattle. Journal of Animal Science 81, E110–E119.
Maloyan A, Palmon A, Horowitz M (1999) Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 276, R1506–R1515.
Martin DB, Horning MG, Vagelos PR (1961) Fatty acid synthesis in adipose tissue: I. Purification and properties of a long chain fatty acid-synthesizing system. The Journal of Biological Chemistry 236, 663–668.
Matthews JO, Southern LL, Pontif JE, Higbie AD, Bidner TD (1998) Interactive effects of betaine. Crude protein, and net energy in finishing pigs. Journal of Animal Science 76, 2444–2455.
Moeckel GW, Shadman R, Fogel JM, Sadrzadeh SMH (2002) Organic osmolytes betaine, sorbitol and inositol are potent inhibitors of erythrocyte membrane ATPases. Life Sciences 71, 2413–2424.
| Organic osmolytes betaine, sorbitol and inositol are potent inhibitors of erythrocyte membrane ATPases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmvVansLo%3D&md5=2968b1822f5f8c8a26de524018566720CAS | 12231402PubMed |
Nakanishi T, Turner RJ, Burg MB (1990) Osmoregulation of betaine transport in mammalian renal medullary cells. The American Journal of Physiology 258, F1061–F1067.
Pearce KL, Masters DG, Jacob RH, Hopkins DL, Pethick DW (2008) Effects of sodium chloride and betaine on hydration status of lambs at slaughter. Australian Journal of Experimental Agriculture 48, 1194–1200.
| Effects of sodium chloride and betaine on hydration status of lambs at slaughter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXptlKlu7g%3D&md5=b9cc1d406eec2f985d6f06136848e487CAS |
Pérez-Pérez R, Ortega-Delgado FJ, Garcia-Santos E, Lopez JA, Camafeita E, Ricart W, Fernandez-Real J-M, Peral B (2009) Differential proteomics of omental and subcutaneous adipose tissue reflects their unalike biochemical and metabolic properties. Journal of Proteome Research 8, 1682–1693.
| Differential proteomics of omental and subcutaneous adipose tissue reflects their unalike biochemical and metabolic properties.Crossref | GoogleScholarGoogle Scholar | 19714809PubMed |
Peterson DP, Murphy KM, Ursino R, Streeter K, Yancey PH (1992) Effects of dietary protein and salt on renal osmolytes: Covariation in urea and GPC contents. The American Journal of Physiology 263, F594–F600.
Pike MM, Ringkob TP, Beekman DD, Koh YO, Gerthoffer WT (1993) Quadratic relationship between early-post-mortem glycolytic rate and beef tenderness. Meat Science 34, 13–26.
| Quadratic relationship between early-post-mortem glycolytic rate and beef tenderness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXisFeltrk%3D&md5=4e8f2f89c9df9b4b2b21d0dec19eddf1CAS | 22060264PubMed |
Pockley GA (2003) Heat shock proteins as regulators of the immune response. Lancet 362, 469–476.
| Heat shock proteins as regulators of the immune response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmt1ajsrk%3D&md5=21bfe0ed39aba8af3170897bac8adf01CAS |
Pshennikova MG, Zelenina OM, Kruglov SV, Pokidyshev DA, Shimkovich MV, Malyshev IY (2006) Synthesis of heat shock proteins (HSP70) in blood leukocytes as a criterion of the resistance to stress injury. Bulletin of Experimental Biology and Medicine: General Pathology and Pathophysiology 142, 660–660.
| Synthesis of heat shock proteins (HSP70) in blood leukocytes as a criterion of the resistance to stress injury.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjvFaht7c%3D&md5=942b4352ff47f655b8c47ebc7c96a7aeCAS |
Ruell PA, Thompson MW, Hoffman KM, Brotherhood JR, Richards DAB (2007) Lymphocyte HSP72 following exercise in hyperthermic runners: The effect of temperature. Journal of Thermal Biology 32, 406–412.
| Lymphocyte HSP72 following exercise in hyperthermic runners: The effect of temperature.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1amtrbM&md5=f5b21af315b12b3b7cbbb1d8d050ba44CAS |
Sackett D, Holmes P, Abbott K, Jephcott S, Barber M (2006) ‘Assessing the economic cost of endemic disease on the profitability of Australian beef cattle and sheep producers.’ (Meat & Livestock Australia Ltd: North Sydney)
Schrama JW, Heetkamp MJW, Simmins PH, Gerrits WJJ (2003) Dietary betaine supplementation affects energy metabolism of pigs. Journal of Animal Science 81, 1202–1209.
Sheikh-Hamad D, Garcia-Perez A, Ferraris JD, Peters EM, Burg MB (1994) Induction of gene expression by heat shock versus osmotic stress. American Journal of Physiology. Renal Physiology 267, F28–F34.
Sullivan ML, Cadwell-Smith AJ, Mader T, Gaughan J (2011) Effect of shade area on performance and welfare of short-fed cattle. Journal of Animal Science 89, 2911–2925.
| Effect of shade area on performance and welfare of short-fed cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFWqtrbI&md5=9bcf3c2ccab2c778922cc1028f8611a9CAS | 21478450PubMed |
Suster D, Leury BJ, King RH, Mottram M, Dunshea FR (2004) Interrelationships between porcine somatotropin (pST), betaine, and energy level on body composition and tissue distribution of finisher boars. Australian Journal of Agricultural Research 55, 983–990.
| Interrelationships between porcine somatotropin (pST), betaine, and energy level on body composition and tissue distribution of finisher boars.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnvVKiurk%3D&md5=547e84d4cbe64a23b882b2e9501680e4CAS |
Terry DF, Wyszynski DF, Nolan VG, Atzmon G, Schoenhofen EA, Pennington JY, Andersen SL, Wilcox MA, Farrer LA, Barzilai N, Baldwin CT, Asea A (2006) Serum heat shock protein 70 level as a biomarker of exceptional longevity. Mechanisms of Ageing and Development 127, 862–868.
| Serum heat shock protein 70 level as a biomarker of exceptional longevity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFektLjO&md5=7da49067371c7893f973aa484798db53CAS | 17027907PubMed |
Tornberg E, Wahlgren M, Brøndum J, Engelsen SB (2000) Pre-rigor conditions in beef under varying temperature- and ph-falls studied with rigometer, NMR and NIR. Food Chemistry 69, 407–418.
| Pre-rigor conditions in beef under varying temperature- and ph-falls studied with rigometer, NMR and NIR.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXis12ktL0%3D&md5=2b3896818a0f466fea001ff4ee46eb00CAS |
Warner RD, Dunshea FR, Gutzke D, Lau J, Kearney G (2014) Factors influencing the incidence of high rigor temperature in beef carcasses in Australia. Animal Production Science 54, 363–374.
| Factors influencing the incidence of high rigor temperature in beef carcasses in Australia.Crossref | GoogleScholarGoogle Scholar |
Yue Z, Hao Q, Tang S, Bao E, Hartung J (2010) Variation in Hsp90, HSF-1, and hsp90 mRNA expression in tissues of pigs exposed to different durations of transport. Livestock Science 129, 141–145.
| Variation in Hsp90, HSF-1, and hsp90 mRNA expression in tissues of pigs exposed to different durations of transport.Crossref | GoogleScholarGoogle Scholar |