Assessment of loliolide extracted from Biserula pelecinus, present during in vitro oocyte maturation, on fertilisation and embryo development in sheep
A. A. Amir A # , A. A. Algreiby B # , J. M. Kelly C , D. O. Kleemann C , Z. Durmic
C , Z. Durmic  A , G. R. Flematti D , D. Blache A and G. B. Martin
A , G. R. Flematti D , D. Blache A and G. B. Martin  A *
A *
A
B
C
D
# These authors contributed equally to this paper
Handling Editor: Joanna Souza-Fabjan
Abstract
As a ‘duty of care’, it is important to test whether new forage plants for ruminants contain secondary compounds (PSCs) that affect reproductive performance. We have previously observed, a posteriori, that the presence of a methanolic extract of Biserrula pelecinus during maturation of sheep oocytes increased fertilisation rate and blastocyst development. This result needed to be verified a priori and, if the outcome was repeated, we needed to identify the plant secondary metabolite responsible.
To test whether PSCs from B. pelecinus, when added to the oocyte maturation medium, improve fertilisation rate and blastocyst development; to test whether loliolide is the active molecule produced by B. pelecinus.
Methanol–chloroform extracts of B. pelecinus were fractionated using rapid silica filtration and solvents of increasing polarity. Fractions at final concentrations of 0, 100 or 200 μg mL−1 were added to the medium used to mature sheep cumulus–oocyte complexes (COCs) and effects were determined for maturation, subsequent cleavage rate, blastocyst rate, hatching rate, blastocyst efficiency and total blastocyst cell number (TCN).
Fraction BP-6 at 100 μg mL−1 reduced blastocyst rate (P < 0.05), but had no effect when the dose was doubled to 200 μg mL−1. Further fractionation using semi-preparative high-performance liquid chromatography showed loliolide as the most abundant compound in BP-6. Supplementation of the in vitro maturation medium with loliolide (0, 2.5, 5, 10 and 25 μg mL−1) did not affect any measure of embryo development. All COCs treated with B. pelecinus fractions reached the final stage of embryo development, blastocyst hatching. Total blastocyst cell number was not affected.
The presence of fractions of B. pelecinus extract during in vitro oocyte maturation can reduce embryo development.
In vitro techniques can detect potential effects of forages on reproduction. Some fractions from an extract of B. pelecinus when present during oocyte maturation can reduce embryo development. The abundant PSC, loliolide, was not responsible. There was no indication that a PSC in B. pelecinus improves outcomes.
Keywords: Biserrula pelecinus, blastocyst, fertilisation, IVEP, IVMFC, loliolide, oocyte, sheep.
Introduction
Dietary factors that affect the reproductive performance of livestock, such as timely supplements or leguminous forages, are important tools for the management of grazing sheep and goats (review: Martin 2022). There is a continual search for alternative forage species. However, the introduction of new species carries the risk of detrimental effects of novel plant secondary compounds (PSCs) on health and productivity (Revell and Revell 2007; Durmic and Blache 2012). Perhaps the clearest example of this potential problem is the ‘clover disease’ that disrupted fertility in sheep that were grazing cultivars of Trifolium subterraneum containing a high concentration of phytoestrogens (Adams and Martin 1983; Adams 1995). Consequently, there is considerable interest in testing for the potential effects of PSCs on reproductive performance in ruminants as a ‘duty of care’ that must be integrated into programs of forage development (Revell and Revell 2007).
A new leguminous species, Biserrula pelecinus, has been shown to grow in infertile, sandy, acidic soils, and to adapt readily to Australian conditions where it is becoming an increasingly important forage for grazing sheep (Loi et al. 2015). Recent studies on the effects of PSCs from B. pelecinus in sheep have focused on aversion behaviour, photosensitisation, and the potential for reducing methane production (Banik et al. 2013; Kessell et al. 2015; Thomas et al. 2015).
In ruminants, several PSCs can affect reproductive capacity by reducing fertilisation, embryo survival and fetal development (McEvoy et al. 2001; Amir et al. 2018). The measurement of these effects is difficult in grazing animals; so, we have utilised in vitro embryo production (IVEP) technology because it offers rapid, cost-effective screening for effects of compounds of interest on oocytes, fertilisation and embryo development (Leite et al. 2004; Wang et al. 2005, 2007; Spinaci et al. 2008; Rajabi-Toustani et al. 2013; Santos et al. 2014). In a previous study, we used IVEP to demonstrate the disruptive effects of the isoflavones responsible for ‘clover disease’ (Amir et al. 2018). In addition, we tested methanolic extracts of several novel Australian forages, including B. pelecinus (Amir et al. 2019). Surprisingly, the presence of the methanolic extract of B. pelecinus during oocyte maturation increased fertilisation rate and blastocyst development (Amir et al. 2019).
The present study aimed to identify the compound(s) responsible for any effects of B. pelecinus on early reproductive events. The compound with the greatest effect was identified as loliolide, so it was studied further, using IVEP. We therefore tested the hypothesis that loliolide is the PSC in B. pelecinus that is responsible for enhancing oocyte maturation and subsequent fertilisation and early embryo development of sheep oocytes in vitro.
Materials and methods
Experimental design
In Experiment 1, methanol–chloroform extracts of B. pelecinus were fractionated using rapid silica filtration (RSF) and solvents of increasing polarity. Each final RSF fraction (about 10%) was tested in the IVEP-system, with each of the seven fractions compared at three concentrations (0, 100 and 200 μg mL−1). Fractions were added to the medium (final concentrations: 0, 100 or 200 μg mL−1) used for in vitro maturation (IVM) of abattoir-derived ovine cumulus–oocyte complexes (COC). After IVM (24 h), mature oocytes underwent in vitro fertilisation (IVF) and subsequent IVC procedures. Cleavage rate, blastocyst rate, hatching rate, blastocyst efficiency and total blastocyst cell number (TCN) were assessed. The most active fraction was selected for Experiment 2.
In Experiment 2, the fraction with the greatest effect was further fractionated using semi-preparative high-performance liquid chromatography (HPLC). The most abundant compound was identified then added as a supplement to IVM medium at different concentrations (0, 2.5, 5, 10 and 25 μg mL−1) to test for effects on cleavage rate, blastocyst rate, hatching rate, blastocyst efficiency and TCN.
In both experiments, dimethyl sulfoxide (DMSO) was used to dilute (1 in 500) the fractions/compound, and was therefore used as the control treatment (+DMSO). A negative control was also included (–DMSO).
Collection of ovaries and oocytes
Ovaries from adult ewes were collected from an abattoir and transported to the laboratory in warm (33°C) phosphate-buffered saline (PBS). COCs were aspirated from follicles ≥2 mm in diameter by using an 18 g needle and a vacuum pump (Cook Australia, Queensland, Australia) with a pressure equivalent to 25 mm Hg. The COCs were collected into 2 mL aspiration medium that comprised Hepes-buffered TCM199 supplemented with 2% (v/v) heat-inactivated ovine oestrous serum (SS), 100 IU mL−1 heparin (Pharmacia and Upjohn; Bentley, Western Australia, Australia), 100 μg mL−1 streptomycin sulfate (CSL, Parkville, Victoria, Australia), and 100 IU mL−1 penicillin G (CSL). Unexpanded COCs were recovered using a stereo-microscope (×40 magnification) (Olympus, Tokyo, Japan). Oocytes that were atretic or showed signs of an expanded cumulus were discarded.
In vitro maturation (IVM)
The procedures used were similar to those outlined by Walker et al. (1996) and Kelly et al. (2007). In brief, COCs were rinsed in IVM maturation medium containing sodium bicarbonate-buffered TCM199 supplemented with 20% (v/v) SS, 5 μg/mL follicle-stimulting hormone (Folltropin, Bioniche, Inc.), 0.1 IU mL−1 hCG (Chorulon, Intervet, USA) and 1 μg mL−1 oestradiol-17β. COCs were matured in four-well culture dishes (Nunc Inc., Naperville, IL, USA) containing 500 μL maturation medium covered with 300 μL mineral oil for approximately 24 h at 38.8°C in a humidified atmosphere of 5% CO2 in air. The COCs were allocated equally among treatments.
Sperm preparation and in vitro fertilisation (IVF)
After maturation, COCs were gently stripped of excess cumulus cells in 400 IU mL−1 hyaluronidase by using a fine-bore pipette, followed by three washes in IVF medium containing synthetic oviduct fluid (SOF) supplemented with 2% (v/v) SS. Up to 25 COCs were placed into four-well culture dishes containing 450 μL IVF medium overlaid with 300 μL mineral oil. Motile sperm were obtained using the ‘swim-up’ procedure in which 200 μL of frozen-thawed semen, pooled from two rams of proven fertility, were layered for 30 min under 1 mL of IVF medium in a 14 mL tube (Falcon, Becton Dickinson, Melbourne, Victoria, Australia). Approximately 0.5 × 106 sperm were placed in each well and co-incubated with the COCs for 24 h at 38.8°C in a humidified atmosphere of 5% CO2 in air.
In vitro culture (IVC)
After approximately 24 h, remnant cumulus cells were removed by gentle pipetting with a fine bore pipette and the presumptive zygotes were washed three times in IVC medium, which included SOF containing 8 mg mL−1 bovine serum albumin (Fraction V; Invitrogen Corporation, Auckland, New Zealand) and amino acids at concentrations typical of oviduct fluid [22–23]. Presumptive zygotes (n = 20–25 per well) were cultured in 600 μL IVC medium under 300 μL of mineral oil in a humidified atmosphere of 5% CO2:5% O2:90% N2 at 38.8°C. Oocytes that failed to divide (‘single cell’) were removed after 24 h, allowing the cleavage (fertilisation) rate to be determined.
Embryo assessment
Embryo development was assessed on Day 7 (Day 0 = day of IVF). Blastocysts, expanded blastocysts, hatching blastocysts and totally hatched blastocysts were collected, and stained so that the cells could be counted. Embryos were mounted on a microscope slide in a drop of glycerol containing Hoechst 33342 (1 mg mL−1) and covered with a coverslip. The total cell count was recorded using a fluorescent microscope (Olympus Optical Co., Tokyo, Japan). Blastocyst rate was defined as the total number of blastocysts expressed as a percentage of the number of cleaved oocytes; hatching rate was defined as the total number of hatching and totally hatched blastocysts as a percentage of the total number of blastocysts; blastocyst efficiency was defined as the total number of blastocysts produced, expressed as a percentage of the original number of COCs.
Extraction and fractionation of B. pelecinus
For HPLC, we used an Agilent 1200 system equipped with a photodiode array detector (PDA) and fraction collector. Analytical separations were conducted using an Apollo C18 column (250 mm × 4.6 mm i.d., 5 μm, Grace-Davison, Deerfield, USA). Absorbance of UV was routinely measured at wavelengths of 220, 254 and 280 nm, and full photodiode spectra were collected between 200 and 600 nm. Semi-preparative HPLC was conducted using an Apollo C18 column (250 mm × 10.0 mm i.d., 5 μm, Grace-Davison). For high-resolution mass spectrometry (HR-MS), we used a Waters Alliance e2695 HPLC interfaced to a Waters LCT Premier XE time-of-flight (TOF) mass spectrometer fitted with an atmospheric pressure chemical-ionisation source (APCI) scanning at a mass range of 80–1000 amu. The mobile phase was 50% water (0.1% formic acid) and 50% acetonitrile at a flow rate of 0.3 mL min−1 operating at ambient temperature. Nitrogen (20 psi) was used as the nebulising gas, vaporiser temperature was 350°C, drying gas flow was 10 mL min−1, and capillary voltage was set to 2000 V. To obtain nuclear magnetic resonance (NMR) spectra, we used a Bruker Avance 600, with the 1 H NMR spectra being recorded at 600 MHz. Chemical shifts were expressed as δ values in parts per million (ppm) and coupling constants (J) in Hertz (Hz). For gas chromatography-mass spectrometry (GC-MS), we used a Hewlett Packard 6890 gas chromatograph connected to a HP 5973 mass spectral detector. The GC was equipped with a BPX5 column [(5% phenyl polysilphenylene-siloxane), 30 m × 0.25 mm × 0.25 μm film thickness, SGE Australia], by using helium as a carrier gas. All solvents used were of technical grade and distilled before use. All solvents used for HPLC were of HPLC grade.
Experiment 1
Samples of B. pelecinus in the vegetative stage were collected from UWA Farm Ridgefield, Western Australia (32°29′S, 116°58′E). The plant material (15.1 g dry weight) was extracted by stirring overnight with 1:1 (v/v) methanol:chloroform (MeOH:CHCl3, 2 × 150 mL). The solvent phase was filtered (Whatman No. 1, 32.0 cm) and evaporated to dryness to provide 1.82 g of crude extract. Analysis of the crude extract by HPLC showed that a complex mixture of compounds was present (Fig. 1a), indicating the need for further purification steps.
(a) HPLC chromatogram of a crude methanolic extract of B. pelecinus and (b) a fraction of BP-6, showing the presence of only two major compounds. Loliolide accounted for more than 80% of the sample.
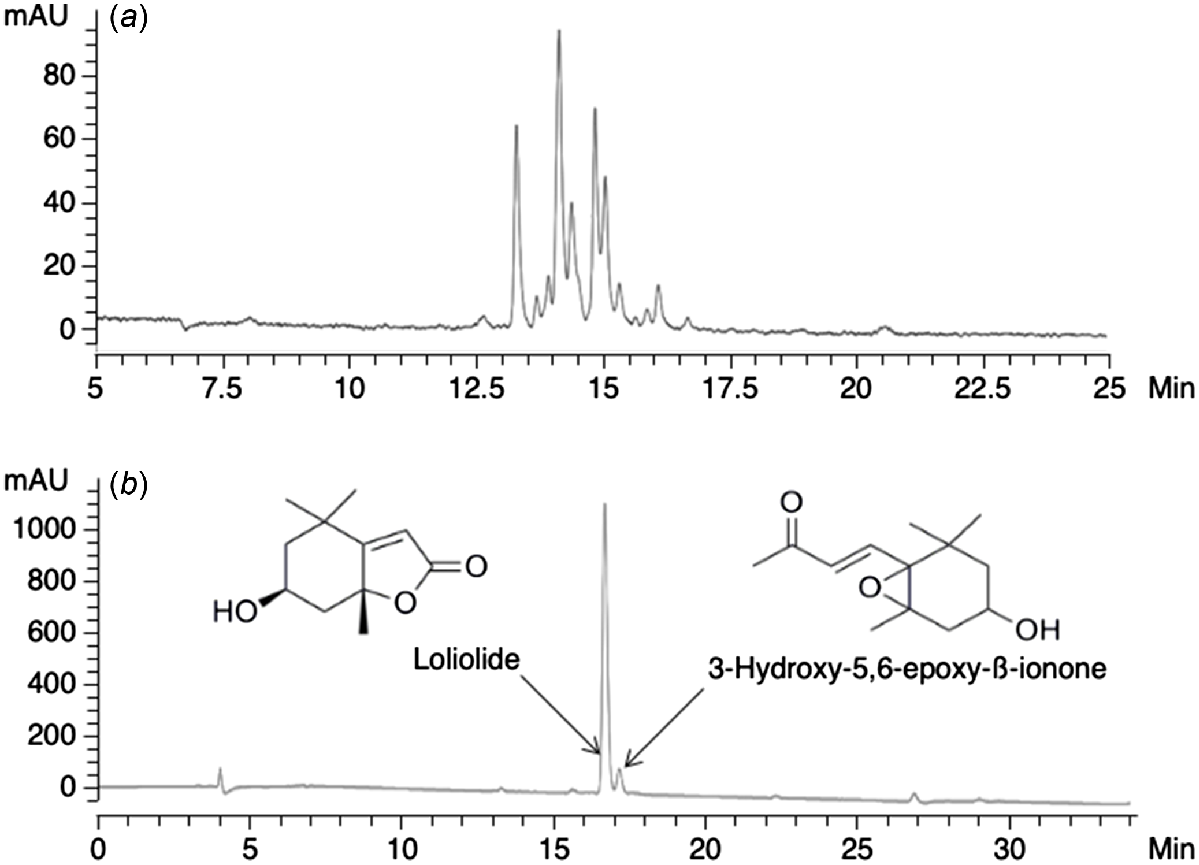
To separate the crude extract into fractions, it was dissolved in a minimal volume of MeOH/hexanes and added to a RSF column (4.5 cm × 12 cm) containing silica gel (10 g, Grace-Davisil, 40–63 μm) equilibrated with hexanes. The column was first eluted with hexanes (200 mL), followed by different solvent mixtures of increasing polarity from 20% to 100% (v/v) ethyl acetate (EtOAc) in hexanes and finally mixtures of 20–100% MeOH in EtOAc (all 200 mL volumes). A sample of each of the 10 fractions (Supplementary Table S1) was analysed by HPLC. Fractions with similar chromatograms (BP.1 and BP.2; BP.6 and BP.7; BP.9 and BP.10) were pooled, giving a total of seven fractions to be tested during IVM (Table S1). The fractions were concentrated under reduced pressure and dried under a gentle flow of nitrogen gas at room temperature.
Each final RSF fraction (about 10%) was tested in the IVEP system, with each of seven fractions compared at three concentrations of 0, 100 and 200 μg mL−1. DMSO was used to dilute (1 in 500) the fractions and was therefore used as one of the control treatments. The most active fraction was selected for Experiment 2.
Experiment 2
Fraction BP-6 was selected for Experiment 2 because it decreased blastocyst rate when tested at 100 μg mL−1. Fraction BP-6 from the RSF separation (16.1 mg) was subjected to semi-preparative HPLC. The sample was dissolved in a minimal volume of methanol and a volume of 500 μL was injected. The column was eluted with a gradient mobile phase, beginning with 1% acetonitrile in water (+0.1% trifluoroacetic acid) and increasing to 100% acetonitrile over 35 min at a flow rate of 4 mL min−1. Fractions were collected every 1 min. Allowing for solvent delay and column cleaning, 20 fractions were collected and, on the basis of similarities in the HPLC chromatogram, they were combined to give a total of six fractions for testing.
One of the subfractions of BP-6 was shown to be pure compared to the unfractionated extract and contained two compounds, one of which accounted for >80% of the fraction (Fig. 1b). High-resolution mass spectrometry (HR-MS) of the main compound gave a protonated molecular ion [M + H] + at m/z 197.1186, corresponding to a molecular formula of C11H16O3 (C11H17O3 requires 197.1178). Careful analysis and comparison of the HR-MS data and NMR spectroscopic properties (Supplementary Fig. S1, Table S2) with a commercial sample confirmed the isolated compound to be the monoterpene, (–)-loliolide.
Loliolide was purchased from Toronto Research Chemicals (Canada). The effect of pure loliolide was assessed by adding it to the IVM medium at five concentrations (0, 2.5, 5, 10 and 25 μg mL−1). We used DMSO (1 in 200) to dilute the loliolide; so, DMSO was used as one of the control treatments (0 μg mL−1) and we compared the two controls. The experiment was replicated four times.
For the minor compound in fraction BP-6, along with NMR, GC-MS and accurate mass measurements were also conducted. HR-MS returned a protonated molecular ion [M + H] + of m/z 225.1498 corresponding to a molecular formula of C13H20O3 (C13H21O3 requires 225.1491). Comparison of the NMR spectroscopic data and electron ionization mass spectrum with literature values (Table S3) confirmed the compound to be 3-hydroxy-5,6-epoxy-β-ionone. A commercial sample of this compound was not available for testing.
Statistical analyses
For both experiments, treatment effects were initially analysed by comparing the two controls (‒DMSO, +DMSO). The controls did not differ significantly for any of the variables; so, the +DMSO control was used as the zero concentration for both experiments. The seven fractions of B. pelecinus (Experiment 1), tested at three concentrations (0, 100 and 200 μg mL−1) and replicated five times, were compared within fraction. Loliolide (Experiment 2) was tested at five concentrations (0, 2.5, 5, 10 and 25 ng mL−1) with three replications. Data for embryonic development (cleavage rate, blastocyst rate, hatching rate, blastocyst efficiency) were analysed using the procedure CATMOD in SAS (Statistical Analysis System, Cary, NC, USA). Concentration (fraction or loliolide) and replicate were included in the model. Total cell numbers (Experiment 2) were analysed using the GLM procedure in SAS, with concentration, embryo stage (early blastocyst, blastocyst, hatched) and replicate being included in the model. Probability values of <0.05 were considered significant.
Results
Experiment 1
There were no significant differences between the two controls (+DMSO and –DMSO) for any of the measures of oocyte and embryo development (Table 1). For fractions BP-1, BP-4, and BP-5, there were no consistent effects on any measure of embryo development. Compared with the DMSO control, no fraction affected cleavage rate.
| Item | Control (–DMSO) | Control (+DMSO) | BP-1 | BP-3 | BP-4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Dose (μg mL−1) | 0 | 0 | 100 | 200 | 100 | 200 | 100 | 200 | |
| Oocytes tested | 120 | 127 | 132 | 133 | 137 | 133 | 134 | 131 | |
| Cleavage rate (%) | 90.0 (108)a | 85.8 (109)a | 81.1 (107)a | 83.5 (111)a | 79.6 (109)a | 83.5 (111)a | 82.1 (110)a | 73.3 (96)a | |
| Blastocyst rate (%) | 64.8 (70)a | 70.6 (77)a | 60.7 (65)a | 64.0 (71)a | 65.1 (71)a | 56.8 (63)b | 65.5 (72)a | 71.9 (69)a | |
| Hatching rate (%) | 24.3 (17)a | 15.6 (12)a | 18.5 (12)a | 28.2 (20)a | 19.7 (14)a | 25.4 (16)a | 23.6 (17)a | 26.1 (18)a | |
| Blastocyst efficiency (%) | 58.3 (70)a | 60.6 (77)a | 49.2 (65)a | 53.4 (71)a | 51.8 (71)a | 47.4 (63)b | 53.7 (72)a | 52.7 (69)a | |
| Item | BP-5 | BP-6 | BP-8 | BP-9 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Dose (μg mL−1) | 100 | 200 | 100 | 200 | 100 | 200 | 100 | 200 | |
| Oocytes tested | 134 | 131 | 132 | 128 | 130 | 137 | 129 | 130 | |
| Cleavage rate (%) | 78.4 (105)a | 83.2 (109)a | 79.5 (105)a | 79.7 (102)a | 76.2 (99)a | 86.9 (119)a | 76.7 (99)a | 76.9 (100)a | |
| Blastocyst rate (%) | 70.5 (74)a | 67.0 (73)a | 48.6 (51)b | 61.8 (63)a | 64.6 (64)a | 60.5 (72)a | 52.5 (52)b | 54.0 (54)b | |
| Hatching rate (%) | 28.4 (21)a | 19.2 (14)a | 17.6 (9)a | 36.5 (23)a | 15.6 (10)a | 19.4 (14)a | 26.9 (14)a | 24.1 (13)a | |
| Blastocyst efficiency (%) | 55.2 (74)a | 55.7 (73)a | 38.6 (51)b | 49.2 (63)b | 49.2 (64)b | 52.6 (72)a | 40.3 (52)b | 41.5 (54)b | |
Numbers of oocytes or embryos used to calculate percentages are given in parentheses.
Cleavage rate, oocytes cleaved/total oocytes; blastocyst rate, blastocysts/oocytes cleaved; hatching rate, blastocysts hatched/total blastocysts; blastocyst efficiency, total blastocysts/total oocytes.
Values within a row sharing the same letter as the control (+DMSO) are not significantly different from the control (at P = 0.05).
At 100 μg mL−1, blastocyst development rate was about 22% lower for BP-6, and about 18% lower for BP-9, than for the DMSO control (Table 1). At 200 μg mL−1, blastocyst rate was about 14% lower for BP-3 and 17% lower for BP-9 than for the DMSO control.
For BP-9, the effect of lower blastocyst development rate at 100 and 200 μg mL−1 was reflected in lower blastocyst efficiency for both concentrations. BP-6 blastocyst development rate was significantly lower at 100 but not 200 μg mL−1, and the lower blastocyst rate at 100 μg mL−1 was reflected in a lower blastocyst efficiency (Table 1). For BP-6 and BP-8 at 200 μg mL−1, blastocyst efficiency was not affected. Although hatching rate for BP-6 was not significantly affected, more blastocysts were produced, leading to a higher hatching rate than the control values (Table 1).
Experiment 2
As expected, TCN increased as the embryo developed. Least-square means (±s.e.m.) for TCN were 66.1 ± 3.2 at the blastocyst stage, 109.1 ± 2.4 at the expanded blastocyst stage, and 168.9 ± 3.3 at the hatching stage.
There were no significant differences between the two controls (+DMSO, –DMSO) for any of the measures of oocyte and embryo performance, including TCN (Table 2). No effects of loliolide were observed on any of the measures of embryonic development, over the range of loliolide concentrations tested (Table 2). For TCN, there were no significant interactions between loliolide concentration and stage of embryo development.
| Item | Control | Loliolide (μg mL−1) | |||||
|---|---|---|---|---|---|---|---|
| –DMSO | +DMSO | 2.5 | 5 | 10 | 25 | ||
| Oocytes tested | 142 | 161 | 146 | 151 | 132 | 142 | |
| Cleavage rate (%) | 82.4 (117)a | 76.4 (123)a | 81.5 (119)a | 83.4 (126)a | 82.6 (109)a | 78.9 (112)a | |
| Blastocyst rate (%) | 54.7 (64)a | 56.1 (69)a | 62.2 (74)a | 60.3 (76)a | 63.3 (69)a | 56.3 (63)a | |
| Hatching rate (%) | 32.8 (21)a | 30.4 (21)a | 21.6 (16)a | 19.7 (15)a | 18.8 (13)a | 33.3 (21)a | |
| Blastocyst efficiency (%) | 45.1 (64)a | 42.9 (69)a | 50.7 (74)a | 50.3 (76)a | 52.3 (69)a | 44.4 (63)a | |
| Total blastocyst cell number (TCN) | 117.7 ± 4.2a | 109.8 ± 3.7a | 115.8 ± 4.1a | 114.2 ± 4.4a | 118.3 ± 4.4a | 112.4 ± 4.0a | |
Numbers of oocytes or embryos used to calculate percentages are given in parentheses.
Cleavage rate, oocytes cleaved/total oocytes; blastocyst rate, blastocysts/oocytes cleaved; hatching rate, blastocysts hatched/total blastocysts; blastocyst efficiency, total blastocysts/total oocytes; total blastocyst cell number (TCN), total cell number/blastocyst (TCN values are least-square means ± s.e.m.).
No values differ significantly (at P = 0.05) from the control (+DMSO), as indicated by values sharing the same lower-case letter.
Discussion
From our previous study with a crude methanolic extract of B. pelecinus (Amir et al. 2019), we reported an enhancement of fertilisation and early embryo development, but those effects were observed a posteriori; so, in the present study, that outcome was re-tested as an a priori hypothesis. The hypothesis was rejected. On the contrary, fraction BP-6 at 100 μg mL−1 reduced blastocyst rate and blastocyst efficiency. At 200 μg mL−1, blastocyst rate was not significantly affected, but blastocyst efficiency was reduced. In addition, other fractions of B. pelecinus extract had negative effects on blastocyst rate. The most likely PSC in fraction BP-6, loliolide, did not affect any measure of fertilisation and early embryo development; so, it seems unlikely that it would be responsible for this biological activity of B. pelecinus.
The concentrations of extract chosen for testing were based on our previous experience (Amir et al. 2019) where we used the range of 0–100 μg mL−1 and found that all embryos developed safely. We increased the upper limit to 200 μg mL−1 because it seemed likely that some biologically active material would have been lost through the fractionation process. Similar decision processes have been used in reproductive toxicology studies using bovine and porcine IVM/IVF models, and Rajabi-Toustani et al. (2013) used the range 0–200 μg mL−1 to study the effects of an extract of Papaver rhoeas in in vitro maturation of sheep oocytes.
With respect to loliolide, it was not known that B. pelecinus produced this molecule until the present study, and we have been unable to find any data for loliolide concentrations in sheep blood or follicular fluid. Considering that we were working with a defined molecule, rather than a mixture, we chose the range of 2.5–25 mg mL−1 on the basis of our previous study with defined phytoestrogenic isoflavones (Amir et al. 2018). The upper value may exceed those expected in vivo; for example, circulating isoflavone concentrations can reach 7 μg mL−1 in sheep consuming oestrogenic clover (Shutt et al. 1967) and this single value is a third of the concentration that affects oocyte maturation in vitro (Amir et al. 2018). There are few data on the concentration of phytoestrogens in follicular fluid of sheep consuming oestrogenic clovers; however, in humans, there is a strong correlation between the blood and follicular fluid concentrations of endocrine disruptors (Petro et al. 2012). These issues are complex because, even for a dietary phytoestrogen, the dose will vary with plant genotype, season and fertiliser practices (Adams 1995).
Clearly, any reproductive outcomes from feeding B. pelecinus under field conditions could reflect a combination of the stimulatory and inhibitory effects. Importantly, in the present study, the fractions and loliolide were present only during oocyte maturation; so, effects on fertilisation, blastocyst development and TCN are consequences of the actions on the oocyte before fertilisation. Oocyte nuclear and cytoplasmic maturation, in vitro and in vivo, involves cellular processes that control meiosis, germinal vesicle breakdown, and the extrusion of the first polar body, any of which could be influenced by any of the PSCs in B. pelecinus, with consequences for oocyte quality that flow-on to the pre-implantation embryo or hatching blastocyst.
It is also important to note that the solvent polarity determines the types of molecules that are extracted from plant material. In our previous study of B. pelecinus (Amir et al. 2019), a relatively polar solvent, methanol, was used to extract samples and provide treatments for the in vitro maturation medium. In the present study, the crude methanolic extract was fractionated by increasing the solvent polarity, beginning with 100% hexanes and then increasing the concentration of ethyl acetate (EtOAc) until it reached 100%, after which the proportion of MeOH in EtOAc was gradually increased to 100% MeOH. The final outcome was seven fractions for in vitro screening. Loliolide was found in the 100% EtOAc (original BP-6) and 20:80 MeOH:EtOAc fractions (original BP-7). We were further encouraged to focus on loliolide, a widely occurring lactone in insects and plants (Grabarczyk et al. 2015), because it has a long history in traditional folk medicine, and a variety of benefits have been reported, including anti-inflammatory, anti-tumor, antioxidant, antiviral, antifungal, and antibacterial activities (Cheng et al. 2010; Yang et al. 2011; Grabarczyk et al. 2015). To the best of our knowledge, the present study is the first report of the isolation of loliolide from B. pelecinus, but the process we used is not exhaustive and other, as yet undiscovered, bioactive compounds in B. pelecinus may be awaiting discovery.
Conclusions
Treatment of ovine oocytes, during in vitro maturation, with a fraction of B. pelecinus, exerted some inhibitory effects on subsequent embryo development; however, the lactone identified in that fraction, loliolide, had no effects. We conclude that the stimulatory effects we reported after our previous study were probably chance observations. The positive outcome is that B. pelecinus seems to present, at most, a small risk to early reproductive events in sheep, although it would be wise to be vigilant with sheep grazing pastures dominated by this new legume where there might be effects on fertilisation and early embryo development in the oviduct. Finally, the present study has again demonstrated the value of in vitro reproductive technology as a tool for detecting plant secondary compounds that might affect reproduction in animals grazing novel forages, for assessing risks and benefits, and for exploring mechanisms of action of specific compounds.
Data availability
None of the data were deposited in an official repository. Data are available from authors upon request.
Conflicts of interest
All authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Declaration of funding
Laboratory costs were covered by the UWA School of Animal Biology. A. A. Amir was financially supported by a scholarship from Universiti Putra Malaysia. The interstate collaboration was supported by UWA through a Mike Carroll Travelling Scholarship and a Convocation Travel Award. A. A. Algreiby was financially supported by Qassim University, represented by the Deanship of Graduate Studies and Scientific Research.
Author contributions
Anna AA conceived the study, extracted the plant, carried out all embryo work, and wrote the first draft of the paper. Azizah AA and GRF performed the extract fractionation and compound identification. JMK and DOK resourced and supervised the in vitro work and helped analyse the data. ZD and DB were co-supervisors and edited the paper. GBM was the principal supervisor and organised the funding and paper editing. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2024-9/1). This project was supported by the UWA School of Animal Biology, a Mike Carroll Travelling Fellowship, and a UWA Convocation Travel Award. This paper forms part of the PhD thesis of Anna Aryani Amir.
References
Adams NR (1995) Detection of the effects of phytoestrogens on sheep and cattle. Journal of Animal Science 73(5), 1509-1515.
| Crossref | Google Scholar | PubMed |
Adams NR, Martin GB (1983) Effects of oestradiol on plasma concentrations of luteinizing hormone in ovariectomized ewes with clover disease. Australian Journal of Biological Sciences 36(3), 295-303.
| Crossref | Google Scholar | PubMed |
Amir AA, Kelly JM, Kleemann DO, Durmic Z, Blache D, Martin GB (2018) Phyto-oestrogens affect fertilisation and embryo development in vitro in sheep. Reproduction, Fertility and Development 30(8), 1109-1115.
| Crossref | Google Scholar | PubMed |
Amir AA, Kelly JM, Kleemann DO, Durmic Z, Blache D, Martin GB (2019) Extracts of forage plants affect the developmental competence of ovine oocytes in vitro. Animal Production Science 59(10), 1814-1819.
| Crossref | Google Scholar |
Banik BK, Durmic Z, Erskine W, Ghamkhar K, Revell C (2013) In vitro ruminal fermentation characteristics and methane production differ in selected key pasture species in Australia. Crop & Pasture Science 64(9), 935-942.
| Crossref | Google Scholar |
Cheng S-Y, Chuang C-T, Wang S-K, Wen Z-H, Chiou S-F, Hsu C-H, Dai C-F, Duh C-Y (2010) Antiviral and anti-inflammatory diterpenoids from the soft coral Sinularia gyrosa. Journal of Natural Products 73(6), 1184-1187.
| Crossref | Google Scholar | PubMed |
Durmic Z, Blache D (2012) Bioactive plants and plant products: effects on animal function, health and welfare. Animal Feed Science and Technology 176(1–4), 150-162.
| Crossref | Google Scholar |
Grabarczyk M, Wińska K, Mączka W, Pontaniec B, Anioł M (2015) Loliolide – the most ubiquitous lactone. Folia Biologica Et Oecologica 11, 1-8.
| Crossref | Google Scholar |
Kelly JM, Kleemann DO, Rudiger SR, Walker SK (2007) Effects of grade of oocyte–cumulus complex and the interactions between grades on the production of blastocysts in the cow, ewe and lamb. Reproduction in Domestic Animals 42(6), 577-582.
| Crossref | Google Scholar | PubMed |
Kessell AE, Ladmore GE, Quinn JC (2015) An outbreak of primary photosensitisation in lambs secondary to consumption of Biserrula pelecinus (biserrula). Australian Veterinary Journal 93, 174-178.
| Crossref | Google Scholar | PubMed |
Leite SP, de Medeiros PL, da Silva EC, de Souza Maia MB, de Menezes Lima VL, Saul DE (2004) Embryotoxicity in vitro with extract of Indigofera suffruticosa leaves. Reproductive Toxicology 18(5), 701-705.
| Crossref | Google Scholar | PubMed |
Loi A, Franca A, Nutt BJ, Yates RJ, D’Antuono MF, Howieson JG (2015) Important ecological traits for selecting Biserrula pelecinus L. (biserrula) genotypes for their potential introduction into agricultural systems. Grass and Forage Science 70(3), 519-529.
| Crossref | Google Scholar |
Martin GB (2022) Frontiers in sheep reproduction – making use of natural responses to environmental challenges to manage productivity. Animal Reproduction 19(4), e20220088.
| Crossref | Google Scholar | PubMed |
McEvoy TG, Robinson JJ, Ashworth CJ, Rooke JA, Sinclair KD (2001) Feed and forage toxicants affecting embryo survival and fetal development. Theriogenology 55(1), 113-129.
| Crossref | Google Scholar | PubMed |
Petro EML, Leroy JLMR, Covaci A, Fransen E, De Neubourg D, Dirtu AC, De Pauw I, Bols PEJ (2012) Endocrine-disrupting chemicals in human follicular fluid impair in vitro oocyte developmental competence. Human Reproduction 27(4), 1025-1033.
| Crossref | Google Scholar | PubMed |
Rajabi-Toustani R, Motamedi-Mojdehi R, Roostaei-Ali Mehr M, Motamedi-Mojdehi R (2013) Effect of Papaver rhoeas L. extract on in vitro maturation of sheep oocytes. Small Ruminant Research 114(1), 146-151.
| Crossref | Google Scholar |
Revell C, Revell D (2007) Meeting ‘duty of care’ obligations when developing new pasture species. Field Crops Research 104(1–3), 95-102.
| Crossref | Google Scholar |
Santos RR, Schoevers EJ, Roelen BAJ (2014) Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reproductive Biology and Endocrinology 12, 117.
| Crossref | Google Scholar | PubMed |
Shutt DA, Axelsen A, Lindner HR (1967) Free and conjugated isoflavones in the plasma of sheep following ingestion of oestrogenic clover. Australian Journal of Agricultural Research 18(4), 647-655.
| Crossref | Google Scholar |
Spinaci M, Volpe S, De Ambrogi M, Tamanini C, Galeati G (2008) Effects of epigallocatechin-3-gallate (EGCG) on in vitro maturation and fertilization of porcine oocytes. Theriogenology 69(7), 877-885.
| Crossref | Google Scholar | PubMed |
Thomas DT, Milton JTB, Revell CK, Ewing MA, Lindsay DR (2015) Individual and socially learned preferences for biserrula (Biserrula pelecinus L.) in sheep. Grass and Forage Science 70(2), 374-380.
| Crossref | Google Scholar |
Walker SK, Hill JL, Kleemann DO, Nancarrow CD (1996) Development of ovine embryos in synthetic oviductal fluid containing amino acids at oviductal fluid concentrations. Biology of Reproduction 55(3), 703-708.
| Crossref | Google Scholar | PubMed |
Wang S, Panter KE, Gaffield W, Evans RC, Bunch TD (2005) Effects of steroidal glycoalkaloids from potatoes (Solanum tuberosum) on in vitro bovine embryo development. Animal Reproduction Science 85(3–4), 243-250.
| Crossref | Google Scholar | PubMed |
Wang Z-G, Yu S-D, Xu Z-R (2007) Improvement in bovine embryo production in vitro by treatment with green tea polyphenols during in vitro maturation of oocytes. Animal Reproduction Science 100(1–2), 22-31.
| Crossref | Google Scholar | PubMed |
Yang X, Kang M-C, Lee K-W, Kang S-M, Lee W-W, Jeon Y-J (2011) Antioxidant activity and cell protective effect of loliolide isolated from Sargassum ringgoldianum subsp. coreanum. Algae 26(2), 201-208.
| Crossref | Google Scholar |


