Advances in prebiotics for poultry: role of the caeca and oligosaccharides
Natalie K. Morgan A *
A *
A Curtin University, School of Molecular and Life Sciences, Kent Street, Bentley, WA 6102, Australia.
Animal Production Science 63(18) 1911-1925 https://doi.org/10.1071/AN23011
Submitted: 9 January 2023 Accepted: 6 March 2023 Published: 30 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Prebiotics are non-digestible carbohydrates that selectively stimulate the growth of beneficial bacteria. Prebiotic supplementation into poultry diets results in a decreased rate of pathogenic bacteria colonisation in the gastrointestinal tract. It also enhances production of volatile fatty acids and lactic acid, which provide the bird with energy. This results in improved host gastrointestinal health and productive performance. Oligosaccharides are the most notable prebiotics in poultry nutrition. Examples of prebiotic oligosaccharides include xylo-oligosaccharides, fructo-oligosaccharides, and galacto-oligosaccharides. Oligosaccharides are derived from hydrolysis of non-starch polysaccharides (NSP). They are manufactured from plant sources, synthesised by physiochemical methods or enzymatic processes. The effects of oligosaccharides occur primarily in the caeca; oligosaccharides bypass the small intestine and reach the caeca, where they are readily fermented by beneficial bacteria, such as those in family Lactobacillaceae and Bifidobacteriaceae. Caeca function is generally poorly understood, despite extensive reviews and studies in this field. A deeper understanding of the factors that influence ability of the caeca to effectively utilise oligosaccharides is warranted. This would allow new prebiotic products and NSP- degrading enzymes to be developed, targeted to specific diets and scenarios. This is required, given the lack of consistency observed in the outputs derived from different studies assessing oligosaccharide efficacy in poultry diets. A key hinderance to progression in this field is that authors rarely analyse the oligosaccharide content and composition in the test diets and products, or in the bird’s gastrointestinal tract. This review examines the mechanisms behind how oligosaccharides induce prebiotic effects in poultry, by identifying the role of the caeca in NSP digestion and identifying the impact of oligosaccharides on caeca microbiota and short-chain fatty acid composition.
Keywords: caeca, enzymes, fibre, microbiota, non-starch polysaccharides, oligosaccharides, poultry, prebiotic, short-chain fatty acids.
Introduction
The global demand for quality protein from poultry continues to increase. Meeting these demands requires development of feed technologies that beneficially affect animal performance and bird gastrointestinal health. This is particularly pertinent, given the current worldwide push to remove antibiotics from poultry feed. Application of prebiotics to poultry diets presents a promising approach. Prebiotics are non-digestible carbohydrates demonstrated to favourably manipulate the composition and fermentation patterns of gastrointestinal microbiota, selecting for beneficial species that profit the host (Ricke et al. 2020). The criterion for a qualifying prebiotic includes (a) not hydrolysed or absorbed in the upper gastrointestinal tract, (b) serve as a selective nutrient source for beneficial microbial communities in the gastrointestinal tract, and (c) induce physiological responses that benefit the host. Fermentation of prebiotics results in generation of short-chain fatty acids (SCFA), defined as the sum of volatile acids and lactic acid, which provide an important energy source for the bird and reduce gastrointestinal pH, thus inhibiting proliferation of acid-sensitive pathogenic bacteria species (Liu et al. 2021a, 2021b). The consequence is improved gastrointestinal and host health and resulting enhanced bird productive performance. Oligosaccharides are the main source of prebiotics used in poultry diets. They are carbohydrates containing 2–10 monosaccharide units, derived from hydrolysis of non-starch polysaccharides (NSP). In recent years, there has been increased interest and research into the benefits of supplementing poultry diets with different types of oligosaccharides. The caeca are the primary site of oligosaccharide fermentation. To optimise the beneficial effects of oligosaccharides and develop improved and more consistent oligosaccharide products, it is necessary to better understand how the caeca utilise oligosaccharides.
Role of the poultry caeca in carbohydrate utilisation
It is well established that the caeca play a key role in carbohydrate digestion (McNab 1973; Svihus et al. 2013; Ramírez et al. 2022). This was identified almost a century ago by Radeff (1928), who observed that digestion of crude fibre was notably greater in birds with caeca than in those that had been caecectomised; 4% and 17% more crude fibre was digested from wheat and corn respectively, in the birds with intact caeca. Extensive research in this field has been conducted since, yet uncertainty still prevails about the role of the caeca in NSP utilisation. In contrast to readily digestible starches, NSP can by-pass the small intestine and reach the caeca undigested. NSP-degrading enzymes are frequently supplemented into commercial poultry diets to facilitate degradation of NSP into carbon sources that the microbiota can utilise. The form and circumstances under which these resulting NSP fractions can enter the caeca to be converted into SCFA are poorly understood. To achieve this, it is essential to first understand how the caeca operates and recognise factors that influence its efficacy as a site for carbohydrate fermentation.
Caeca anatomy
The caeca are paired blind pouches located at the junction of the ileum and colon. They are anoxic microbial habitats, hosting the highest microbial load and diversity of species in the gastrointestinal tract (Ramírez et al. 2022). Evolutionary selection for large caeca is observed in birds fed primarily plant-based diets (namely Galliformes and Anseriformes), whereas carnivorous birds have very small or no caeca (Hunt et al. 2019). This highlights that the caeca play a key role in fibre hydrolysis. The caeca in modern commercial broilers range from approximately 13 to 22 cm in length (N.K. Morgan and A. Wallace 2022, unpubl. data; Metzler-Zebeli et al. 2018). Caeca in laying hens are slightly smaller, at approximately 9–18 cm in length (Abdallah and Beshara 2015), possibly associated with their comparatively lower feed intake. The proximal region of the caeca contains villi, lymphoid cells, and goblet cells, supporting that nutrient absorption occurs here. In contrast, the medial and distal sections of the caeca contain poorly developed villi (Ferrer et al. 1991), suggesting that nutrient absorption does not readily occur in these regions. Compared with the small intestine, the caeca contain a higher abundance of lymphoid tissue (Casteleyn et al. 2010) and lower height and density of villi (Majeed et al. 2009; Elling-Staats et al. 2022). The presence of lymphoid nodules throughout the mucus membrane of the caeca provide evidence that the caeca play a role in maintaining gut immune homeostasis (Wickramasuriya et al. 2022).
A direct relationship between caeca anatomy and dietary carbohydrate content has been observed. For example, Longstaff et al. (1988) showed that caeca were heavier and longer in birds fed a diet with 200 g/kg pentoses and uronic acid than in those fed 200 g/kg glucose. Additionally, Józefiak et al. (2006) observed a significant increase in caeca weight and contents when a barley diet, with a higher soluble-NSP content, was fed than with an oat-based diet. Also, Rehman et al. (2008) found that feeding 1% inulin increased caeca weight. This highlights that caeca functionality may be dictated by the NSP content of the diet.
Caecal movements
Digesta from the ileum enters the colon, and then a portion of this digesta is pushed into the caeca through retrograde anti-peristaltic movements. Only soluble, low molecular-weight molecules can enter the caeca. This was illustrated by Björnhag and Sperber (1977), who found that nearly half of the particles present in ileal digesta were larger than 0.2 mm in size, but only 3% of the particles in caeca digesta were this large. The materials entering the caeca are filtered by a narrow opening and muscular ring of tissue anterior to the caecal opening, coupled with a meshwork of villi and ridges (Svihus et al. 2013). These materials are pushed towards the distal end and mixed by peristaltic movements by both circular and longitudinal contractions (Sacranie et al. 2007; Janssen et al. 2009). When the caeca are full, the amplitude of circular movements increases, heightening caecal digesta mixing through moving contents from the distal and proximal ends towards each other. Viscosity of the digesta influences its ability to enter the caeca. For example, Choct et al. (1996) observed that feeding as much as 6.6% viscous arabinoxylans into the diet did not increase caecal SCFA production, indicating that these materials were not able to enter the caeca. Similarly, Langhout and Schutte (1996) found that feeding viscous pectin had no impact on fermentation, on the basis of caecal SCFA concentration; however, feeding similar quantities of less viscous pectin did significantly increase fermentation.
Digesta retention in the caeca is relatively long, compared to retention in other gastrointestinal regions, due to infrequent emptying, allowing for increased fermentation, and thus heightened SCFA manufacture. This is illustrated by Warriss et al. (2004) who observed that digesta was still present in the caeca of broilers that had been fasted for 24 h. Synchronised high-amplitude peristaltic movement of both the caeca and colon causes the caecal contents to be expelled. Antiperistaltic movements occur continuously in the colon, moving material from the anal opening into the caeca. This has been demonstrated by Sacranie et al. (2012), who injected Cr-EDTA soluble marker into the colon and observed that it was refluxed into the small intestine. This suggests that conditions in the caeca and lower digestive tract influence small-intestine microbiota composition. However, Glendinning et al. (2019) observed significant differences in microbiota composition among the duodenum, jejunum, ileum, and caeca, signifying that other factors may play a greater role in establishing ileal microbiota composition. Further research is warranted into the role that these antiperistaltic actions play in digestion of NSP.
Caeca microbiota composition
The gastrointestinal microbiome is recognised as a functional system of the bird, directly influencing animal health, productivity, and food safety. The largest concentration of microbial cells in the gastrointestinal tract are found in the caeca (Bindari and Gerber 2022). Bacteria are the primary component of the caeca ecosystem; Glendinning et al. (2020) concluded that 98.4% of the caeca microbiome originates from bacteria, 0.12% originates from Eukaryota (originated from viruses), and 0.31% originates form Archaea (single-celled prokaryotes). The most abundant phyla found within the caeca of adult birds are Firmicutes (Clavijo and Flórez 2018), with Lactobacillus, Ruminococcus, Faecalibacterium, and Clostridium being the most dominant genera within this phylum (Gong et al. 2002; Paraskeuas and Mountzouris 2019). The conformation of the caeca microbiota changes throughout the bird’s life, dictated by diet and environmental conditions.
Impact of bird age on caeca microbiota composition
It is well established that microbiota composition changes with bird age, becoming more stable as the bird gets older. However, the exact age when it stabilises has yet to be determined. The identified bird age that caeca phylogenetic diversity stabilises has varied among studies, ranging from approximately 20 to 21 days (Torok et al. 2009; Ijaz et al. 2018; Kers et al. 2020), to 28 days (Lu et al. 2003) to over 42 days (Richards et al. 2019). Nonetheless, it is well established that initial inhabitation of microbes in the gastrointestinal tract occurs within the first 72 h post-hatch (Fathima et al. 2022; Pottenger et al. 2023). In modern commercial birds, the first bacterial inoculum is derived from the environment during incubation, hatching and delivery. Variability in these early environments explains why initial colonisation differs among birds from different hatcheries. For example, Kers et al. (2020) and Pedroso et al. (2016) presented that species of Clostridiaceae were the dominant species in the caeca of newly hatched birds, whereas Ballou et al. (2016) found species of Enterobacteriaceae to be the most abundant bacteria. Birds are then exposed to more diverse microbial environments on the farm, and ingest bacteria from the feed, water, and litter.
As the bird ages, the diversity of the caeca microbiota changes (Feye et al. 2020), transitioning from species that ferment and digest simple carbohydrates towards more complex structures, easing fibre fermentation (Stanley et al. 2013; Sergeant et al. 2014). This is because bacteria populate the upper intestine and remove the readily digested fermentable oligosaccharides and soluble polysaccharides, leaving the large intestine with NSP that are more difficult to digest, such as arabinoxylans and beta-glucans. Ballou et al. (2016) and Oakley et al. (2014) found that the caecal microbiota switched from being dominated by Enterobacteriaceae to being dominated by Firmicutes, such as Clostridiales, within the first 7 days of age. Also, Gong et al. (2008) found that caecal Lactobacillus content was 100 times higher at 3 days of age than at 42 days of age. Moreover, Lu et al. (2003) found that the dominant species in the caeca were Clostridium saccharolyticum, C. oroticum, and C. orbiscindens at 7 days of age, Ruminococcus schinkii and Clostridium indolis at 14–28 days of age and Eubacterium at 49 days of age. These outputs highlight a great deal of transition in caecal microbiota composition over the lifespan of the bird, addressing the importance of successful early colonisation of the caeca; founding of an efficient microbiota in the young bird may enhance its ability to face dietary and environmental challenges when older. For example, establishing a microbiota in young birds that is efficacious at NSP hydrolysis, by increasing the abundance of NSP-degrading bacterial species, could enhance dietary NSP utilisation when the bird is older. This was illustrated by Bautil et al. (2020), who found that supplementing wheat-based diets with 0.5% arabinoxylo-oligosaccharides sped up development of a fibre-fermenting microbiome, resulting in increased arabinoxylan solubilisation and fermentation in older birds. This approach of priming the microbiota in young birds to get targeted results in adult birds could potentially increase use of more economic and environmentally friendly sources of feed ingredients that are otherwise excluded from poultry diets due to their high NSP content. For example, Duke et al. (1984) adapted turkeys to either a low- or high-NSP diet and found that cellulose degradation in the adult bird was higher in those fed the high-NSP diet. However, it is not possible to provide a target optimum microbiota composition to aim for in young birds, because this is very much dependent on the conditions the birds are to be reared in. Notably, Stanley et al. (2013) ran three successive trials in which birds were obtained from the same breeder flock, were fed the same diet, and were reared in the same facility, and saw completely different caecal microbiota compositions. This suggests that measuring absolute values of different species may be meaningless. The focus instead should be on the metabolites being produced and functionality of the microbiota. This should include measurements of oligosaccharides, focussing on both quantity and size/structure, in the diet and caeca. It may also be advantageous to measure endogenous enzyme activity, to confirm whether the oligosaccharides are stimulating NSP-degrading microbiota species.
Impact of dietary NSP on caeca microbiota composition
NSP are considered anti-nutrients because the soluble component increases intestinal viscosity, and the insoluble component acts as a nutrient diluent and physical barrier to the gastrointestinal lining, thus reducing nutrient digestion and absorption (Bedford 2018; Morgan et al. 2021). NSP composition of the diet has a notable impact on the conformation of the caeca microbiota. For example, Crisol-Martínez et al. (2017) compared wheat- and sorghum-based diets and found that Clostridium leptum predominated in the caeca of birds fed wheat, whereas strains of Lactobacillus crispatus and Lachnospiraceae were the most abundant in birds fed sorghum. Moreover, Borda-Molina et al. (2021) and Kim et al. (2022) observed that feeding broilers wheat resulted in a higher presence of Lactobacillaceae and Bifidobacteriaceae in the caeca than did feeding corn. Similarly, Rodríguez et al. (2012) observed increased caeca lactobacilli when feeding wheat and barley, compared with feeding corn. This is because grains such as wheat and barley are richer in NSP, primarily xylan and β-glucan, and products derived from hydrolysis of these NSP provide substrates for beneficial Gram-positive bacteria species. Lactobacillaceae and Bifidobacteriaceae have particularly high glycosidase activity, meaning that they can readily utilise selective oligosaccharide substrates from complex plant cell-wall substrates (Modrackova et al. 2020; Shini and Bryden 2022), converting them into SCFA. This explains why numerous studies have presented high levels of caecal Bifidobacteriaceae and Lactobacillaceae, often correlated with improved bird performance, when feeding NSP-rich diets to poultry (Apajalahti et al. 2001; Engberg et al. 2004; Józefiak et al. 2010; González-Ortiz et al. 2020). Borda-Molina et al. (2021) also observed increased levels of Bacteroides xylanisolvens in the caeca of wheat-fed birds. B. xylanisolvens is known to exert xylanolytic activity (Mirande et al. 2010), highlighting that feeding xylan results in increased proliferation of caecal bacteria that are adept at xylan degradation and endogenous xylanase manufacture. Moreover, Sergeant et al. (2014), using metagenomic analysis, identified a considerable array of genes in the caeca that encode for polysaccharide- and oligosaccharide-degradation enzymes. This highlights the capacity of the microbiota to instigate NSP hydrolysis. The resulting SCFA generated because of oligosaccharide fermentation can also directly influence microbiota composition. For example, van der Wielen et al. (2000) observed a negative correlation between caecal Enterobacteriaceae concentration and actetate, propionate and butyrate. Additionally, Liao et al. (2020) found that Escherichia–Shigella concentration in the caeca was positively correlated with isobutyrate, and caeca Salmonella was negatively correlated with isovalerate, butyrate and acetate content. These outputs reconfirmed that the microbiota can indeed be manipulated to be more efficacious at utilising dietary NSP.
Impact of NSP-degrading enzymes on caeca microbiota composition
Undigested nutrients that escape the upper digestive tract, due to the presence of NSP, become substrates for the caeca. These substrates can fuel pathogenic bacteria species (Apajalahti and Vienola 2016). NSP-degrading enzyme supplementation combats the anti-nutritional effects of NSP, facilitating removal of digestible nutrients from the diet early in the digestive tract. The consequence is restricted flow of nutrients into the caeca. This forces the caeca microbiota to adapt to use NSP as its primary substrate, instead of starch and readily fermentable carbohydrates, because NSP is the only carbohydrate source available. This highlights the importance of developing a microbiota that is efficacious at fibre-degradation as soon as possible. Clostridiales are particularly effective at degrading plant polysaccharides (Wang et al. 2021; Bedford and Apajalahti 2022), suggesting that promoting proliferation of this species should be a priority.
It is common practice to switch diets as a bird grows, so as to meet evolving energy and protein requirements. This means that birds receive different ingredient combinations as they get older, usually increased cereals and reduced protein meals. The microbiota is subsequently forced to quickly adapt to changes in nutrient availability directly following diet changes, as highlighted by Ijaz et al. (2018). The changes in NSP content and composition provided during these diet transitions are largely ignored, which is concerning given that NSP is the primary source of fuel for the caeca, as highlighted above. Pathogenic bacteria can proliferate in this unstable environment. One approach to minimise the likelihood of this is to provide an ongoing supply of fermentable substrates throughout the bird’s lifetime, so that the microbiota receives a consistent source of fuel, irrespective of diet composition (Bedford and Apajalahti 2022). Supplementing diets with NSP-degrading enzymes increases the abundance of fermentable substrates available, through hydrolysis of NSP into oligosaccharides. For example, Rodríguez et al. (2012) found that addition of xylanase and β-glucanase to wheat- and barley-based diets increased the number of bifidobacteria in the caeca. Another approach is to supplement the diets with a source of fermentable fibre, such as wheat bran or oat bran, ideally in combination with enzymes, or to directly supply oligosaccharides. For example, Morgan et al. (2022a) observed that supplementing sorghum-based diets with 2000 mg/kg xylo-oligosaccharides (XOS), xylanase and fermentable fibre (wheat bran) increased caecal SCFA concentration and xylanase and cellulase activity in 35-day-old broilers. The process of developing a microbiota that is efficacious at NSP-degradation takes time, which is why it must be established early, and why effects of dietary oligosaccharide and NSP-degrading enzyme application may only be seen in older birds.
Role of oligosaccharides as prebiotics
Oligosaccharides contain 2–10 monosaccharide units. These monosaccharides can be either linear or branched and are connected by α- or β-glycosidic linkages (Patel et al. 2014). Oligosaccharides are manufactured from plant sources, such as legumes, wholegrains and some cruciferous vegetables and fruits (Mussatto and Mancilha 2007). The NSP in these sources is hydrolysed into oligosaccharides, usually in the presence of enzymes. A review by Jahan et al. (2022) presents the sources and production techniques for common oligosaccharides used in poultry diets. Recently there has been increased interest into the benefits of supplementing poultry diets directly with oligosaccharides produced in vitro, as opposed to relying on the bird to manufacture them in situ in the digestive tract in the presence of supplemental enzymes (Morgan et al. 2019). As highlighted by Jahan et al. (2022) and in Tables 1 and 2, supplemental oligosaccharides have shown to improve bird gastrointestinal health and performance, but there is a lack of consistency among studies regarding the magnitude of their impact. This is due to variation in the size and structure of the oligosaccharides, which directly influences how and which microbiota species respond to them. The key issue with research in this field is that the majority of researchers do not measure the oligosaccharide concentration or composition (e.g. degree of polymerisation) in the diet, or in the gastrointestinal digesta. This makes it difficult to compare and interpret outputs from bird trials testing oligosaccharide efficacy. This needs to be rectified. Historically, measuring oligosaccharides has been time-consuming and expensive, requiring specialised equipment. However, there has been promising advances in this field in the past 5–10 years, particularly in analysis of XOS (Samanta et al. 2015; Ribeiro et al. 2018; Morgan et al. 2020). It is hoped this will improve the quality of oligosaccharide research in animal nutrition. Moreover, the lack of studies published in this field, particularly in laying hens, means that trends cannot yet be identified. A wider range of studies, featuring different diets, oligosaccharides derived from different substrates, and birds under varying degrees of challenge, are required. Availability of new methods for measuring oligosaccharides and increased publication of robust and repeatable studies in this field will shape development of improved and more consistent oligosaccharide products.
|
|
Properties of oligosaccharides
Diet type and enzyme treatment dictate the size and physiochemical properties of oligosaccharides. Endo-acting NSP-degrading enzymes randomly cleave the backbone of the target NSP, generating polymeric fragments. Successive hydrolytic events result in production of progressively smaller fractions (De Maesschalck et al. 2015). The aim is to manufacture oligosaccharides, as opposed to complete hydrolysis into monomers; unabsorbed pentose sugars stimulate microbial activity in the intestinal tract, causing inflow of water into the intestinal lumen via osmosis, resulting in increased moisture content in the excreta and potential litter-quality issues (Zyla et al. 1999; Mateos et al. 2012). Variability observed in response to supplemental oligosaccharides may be due to the presence of de-branching and exo-activities in the intestinal tract causing oligosaccharides to be broken down into constituent sugars, removing their prebiotic effects (Bedford and Apajalahti 2022).
Oligosaccharides generated from hydrolysis of NSP vary in degree of polymerisation (DP), monomeric units and types of linkages. An example are XOS, which are formed by xylose residues linked through β-(1→4)-linkages. The number of xylose residues in XOS vary from 2 to 10, forming xylobiose (X2; C10H18O9), xylotriose (X3; C15H26O13), xylotetraose (X4; C20H34O17) etc. (Morgan et al. 2020). Bacteria species vary significantly in their ability to use XOS of differing chain lengths (Aachary and Prapulla 2011); for example, bifidobacteria show a preference for shorter, un-substituted XOS, and struggle to use branched arabino-XOS (Okazaki et al. 1990). This was illustrated by Courtin et al. (2008), who found that adding 0.2% XOS to broiler diets had no effect on caecal enterobacteria and lactobacilli but did increase bifidobacteria concentration, after 1 week on the supplement. Dale et al. (2020) also observed that caeca bacteria prefer longer oligosaccharides, of DP 3 or larger. This knowledge can be used to develop oligosaccharide products tailored to specific scenarios, such as stimulating beneficial bacteria species that may otherwise be lacking.
The concentrations and structural features of NSP differ widely among different ingredients and batches of the same ingredient (Nguyen et al. 2021); for example, the distribution of branches along the main backbone may be more regular for some ingredients than others (Morgan et al. 2020). NSP structures also vary within different sections of the grain, such as the endosperm compared with the aleurone and bran (Burton and Fincher 2014). For example, Olukosi and Bedford (2019) found that response of wheat to xylanase varied greatly depending on the endosperm structure of the wheat. This means that it is not possible to predict in situ oligosaccharide manufacture in the gastrointestinal tract on the basis of the quantity of NSP or fibre measured in the ingredient being fed, as several factors influence response to enzyme application. Nonetheless, efficacy of NSP-degrading enzymes could be assessed by measuring the size and structure of oligosaccharides manufactured. This also reiterates that direct oligosaccharide application maybe more reliable than enzyme application alone.
Prebiotic effects of oligosaccharides
Oligosaccharides are not degraded by gastric acid or digestive enzymes and are not absorbed by intestinal mucosa (Abd El-Hack et al. 2020). This ensures that they reach the distal intestinal tract intact, where they can be fermented by probiotic bacteria in the caeca. The beneficial technological features of oligosaccharides include stability at acidic pH, heat resistance, ability to achieve significant biological effects at low daily doses, low calorie content and no toxicity (Carvalho et al. 2013).
Impact of oligosaccharides on caeca microbiota composition
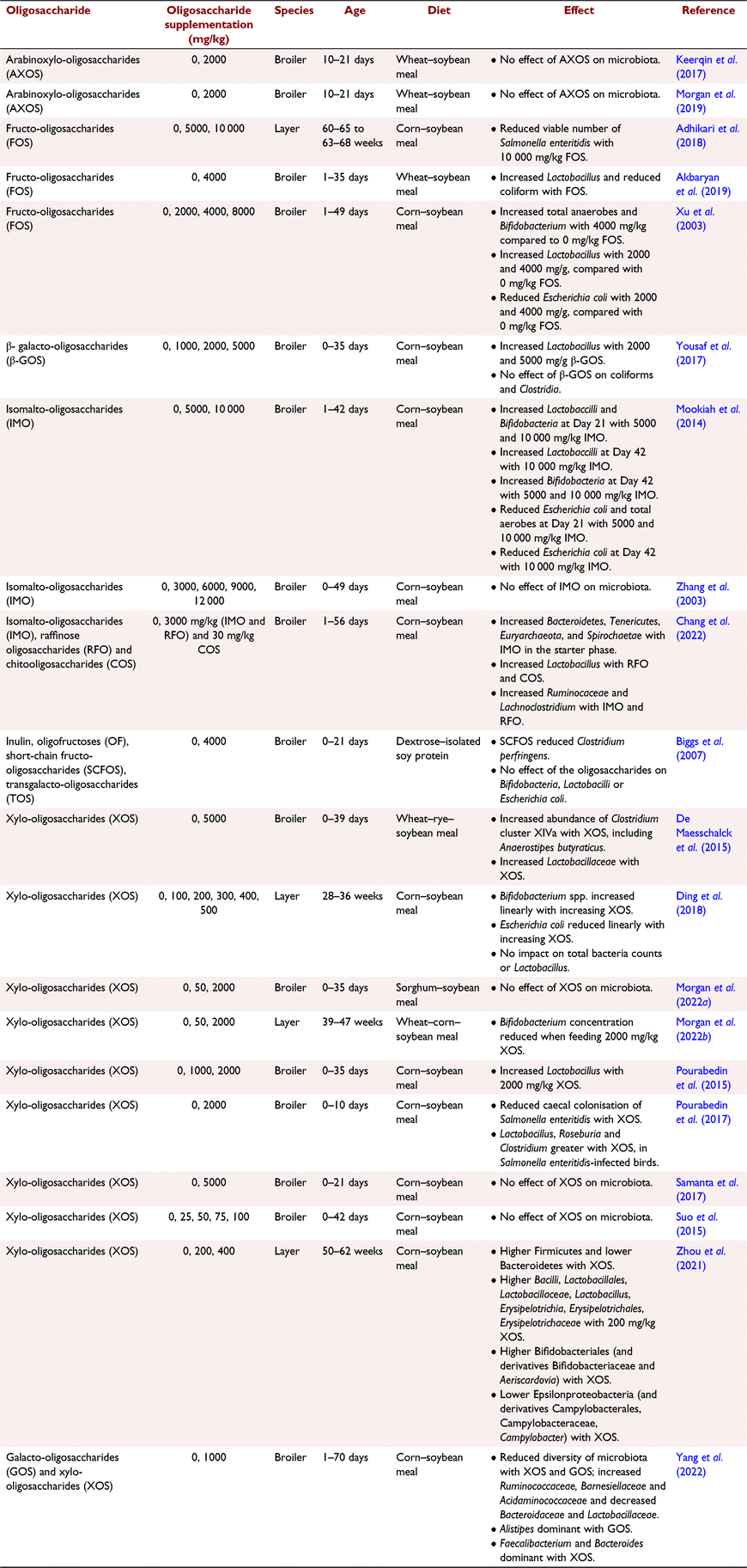
Numerous studies have presented that feeding oligosaccharides directly modifies the caeca microbiota, as highlighted in Table 1. Similar changes to caecal microbiota composition have also been observed in response to dietary NSP-degrading enzyme application (Józefiak et al. 2010; Munyaka et al. 2016; González-Ortiz et al. 2020), verifying that the positive effects of application of these enzymes can be attributed to oligosaccharide production, not just elimination of the anti-nutritional effects of NSP. Oligosaccharides have bifidogenic properties, promoting the growth of beneficial bacteria such as Bifidobacterium adolescentis, B. longum, Lactobacillus brevis and L. fermentum (Moura et al. 2007; Pourabedin et al. 2015). For example, Ding et al. (2018) observed a linear increase in bifidobacteria concentration in the caecum of White Lohmann laying hens with increasing dietary XOS supplementation, ranging from 0% to 0.05%. This confirmed that oligosaccharides cause beneficial bacteria species to be more dominant, meaning that they can competitively inhibit the growth of harmful bacteria, such as Escherichia coli. This was also illustrated by Xu et al. (2003) who observed that supplementing 4 g/kg fructo-oligosaccharides to corn–soybean-based diets increased bifidobacteria and lactobacilli and decreased E. coli concentration in the caeca of broilers. However, it should be noted that a dosage of 4 g/kg oligosaccharides is very high; as illustrated in Tables 1–3, most poultry studies test lower supplementation levels, rarely exceeding 2 g/kg. It may be disadvantageous to supplement high volumes of oligosaccharides into research diets, as these diets are likely to be unbalanced, and these high quantities would not be used in a commercial setting due to costs. Bird studies testing high supplementation levels of oligosaccharides are valuable for observing the mechanisms of their effect and how they respond in the gastrointestinal environment, but it is equally important to test levels that could be applied in commercial diets. Reduced caecal E. coli has also been observed when feeding broilers mannan-oligosaccharides (MOS; Baurhoo et al. 2007). However, care should be taken when interpreting data from research measuring bird responses to MOS, because the source of MOS in these studies is often yeast cell walls, as opposed to MOS derived from soybean meal. This is important because MOS derived from yeast have a different structure and are largely insoluble, so are polymeric, not oligomeric (Bedford and Apajalahti 2022). Researchers need to clearly define the substrate the oligosaccharides are derived from, measure the oligosaccharide concentration in the diet, and ensure test diets are balanced when designing and publishing studies in this field.
|
The preference of different bacteria species for different oligosaccharides was presented by Rycroft et al. (2001), in which in vitro fermentation of seven oligosaccharides (fructo-oligosaccharides (FOS), inulin, XOS, lactulose, isomalto-oligosaccharides, galacto-oligosaccharides (GOS) and soybean-oligosaccharides) by faecal bacteria was assessed, along with resulting SCFA production. All prebiotics were shown to increase bifidobacteria numbers, but total bacteria numbers were increased only by inulin and XOS. Additionally, it was observed that FOS produced the highest populations of lactobacilli, GOS resulted in the greatest decrease in Clostridia numbers, and caecal SCFA generation was highest with lactulose and GOS. Moreover, Yang et al. (2022) supplemented corn-based diets with either 1% GOS or 1% XOS and found that Alistipes were the dominant bacteria in the caeca of birds fed GOS, but Faecalibacterium and Bacteroides were dominant in birds fed XOS. These outputs suggest that there may be merit in using more than one oligosaccharide in a diet, to fuel a wider range of beneficial bacteria species. Further research is warranted into which oligosaccharide combinations are optimal, on the basis of the composition of the diet.
Dietary oligosaccharide application is expected to be most beneficial in broilers at an age of approximately day 10–20, given that this is when the caeca microbiota is rapidly changing, coupled with a likely change in diet from starter to grower. This is also the time when the microbiota is transitioning from dependence on starch and readily fermented carbohydrate sources to NSP, suggesting that nutrient availability may be limited, so an extra source of fuel would be beneficial. Alongside improved microbiota composition, oligosaccharides can also favourably manipulate the physical structure of the gut. For example, Ayman et al. (2022) and Li et al. (2019) found that supplementing chitosan-oligosaccharides into corn-based diets increased intestinal villus height and villus:crypt ratio. The mechanisms behind how oligosaccharides influence these parameters is not yet understood but could be associated with their ability to reduce bacteria proliferation in the lumen and produce SCFA, enhancing ability of epithelial cells to proliferate.
Impact of oligosaccharides on short-chain fatty acid production
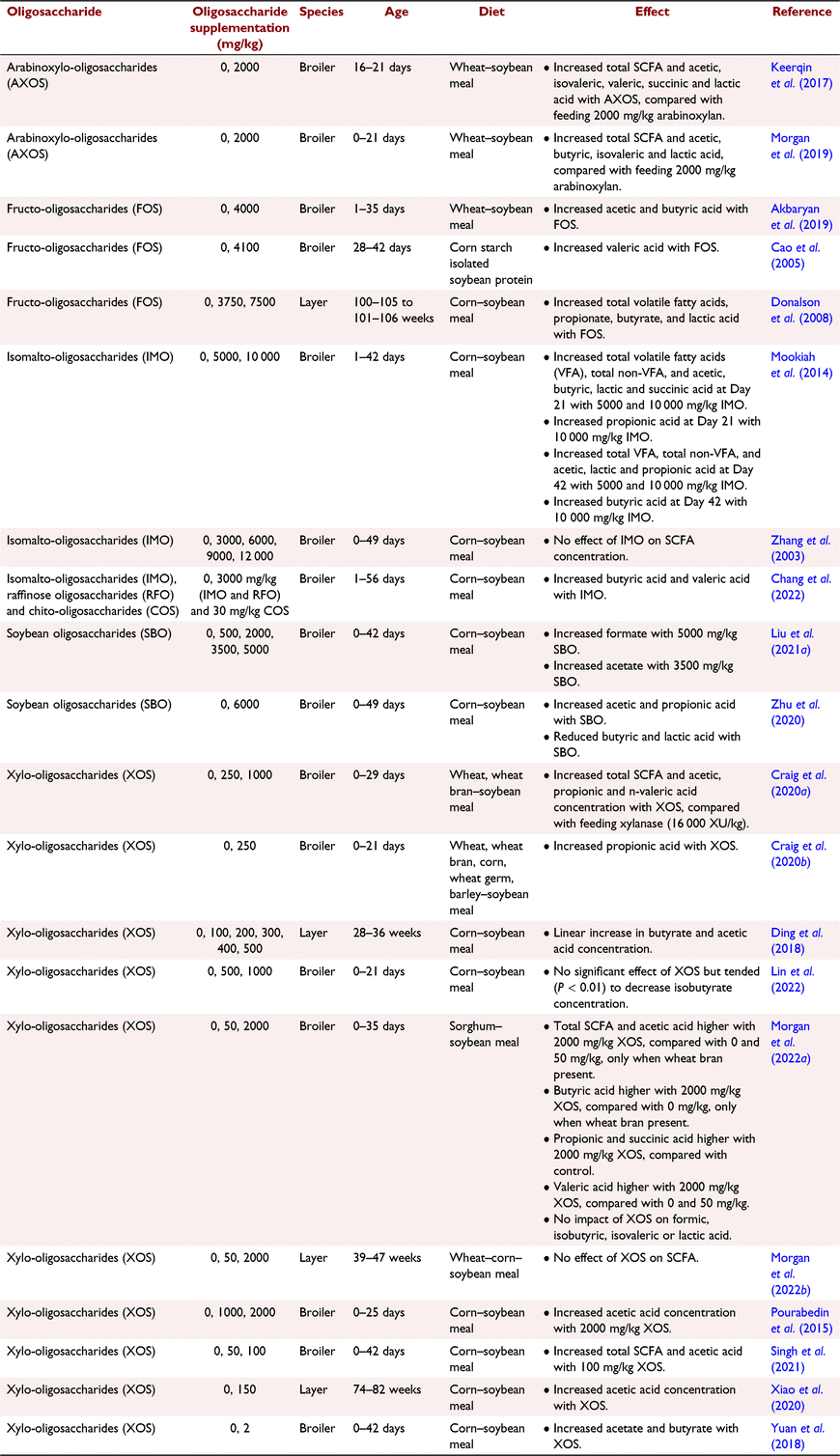
Measurement of SCFA concentration in the caeca can provide an indicator of NSP degradation. For example, Józefiak et al. (2004) observed that grain sources with varying NSP compositions responded differently to supplementation with combined xylanase, β-glucanase and protease, verified on the basis of resulting differing caecal SCFA content and concentration. Caecal fermentation of readily fermentable carbohydrates, such as starch and saccharides, typically results in production of lactate, but utilisation of more complex NSP, by families of bacteria species, such as Ruminococcaceae and Lachnospiraceae, promotes acetate and butyrate production (Mahmood and Guo 2020; Bedford and Apajalahti 2022). Lactate produced in the ileum can either be directly absorbed and used as an energy source or enters the caeca where lactate-utilising bacteria ferment it further into another SCFA, usually butyrate (Broekaert et al. 2011; De Maesschalck et al. 2015). This cross-feeding mechanism is beneficial to the bird because butyrate increases intestinal epithelial integrity by fuelling epithelial cells, displays anti-inflammatory properties, and increases the metabolic activity of the microbial ecosystem (Guilloteau et al. 2010; Canani et al. 2011). Luminal pH is also likely to be stabilised by the relationship between lactate-producing and lactate-utilising bacteria (Belenguer et al. 2006). A more acidic pH is favourable in the gastrointestinal tract because it prevents colonisation by pH-sensitive pathogenic bacteria, thus promoting growth of beneficial bacteria (Fathima et al. 2022). The high content of SCFA in the caeca means that it is usually slightly acidic, ranging from 6.3 to 6.9 in modern broilers (Asare et al. 2021).
Recent studies have presented beneficial effects of dietary supplementation of oligosaccharides on caecal SCFA concentration, as presented in Table 2. Zhu et al. (2022) fermented soybean-oligosaccharides using in vitro batch incubation inoculated with broiler caecal microbiota and observed that oligosaccharides increase SCFA production, namely propionic, butyrate and lactic acid, as well as gas production, suggesting bacterial growth-stimulating activities. This highlights the potential to use in vitro techniques, as opposed to just live-bird trials, to assess the efficacy of oligosaccharide products. Supplementing oligosaccharides into diets has been the most common route of administration, but a study by Singh et al. (2022) found that injecting 3 mg of xylotriose into broiler chicken eggs enhanced production of caecal acetate, butyrate, and total SCFA when measured in birds at 28 days of age, compared with the control. These studies highlighted the direct benefits of oligosaccharides on SCFA production and signified the need for research into new techniques and methods for assessing, developing, and administering oligosaccharides to poultry.
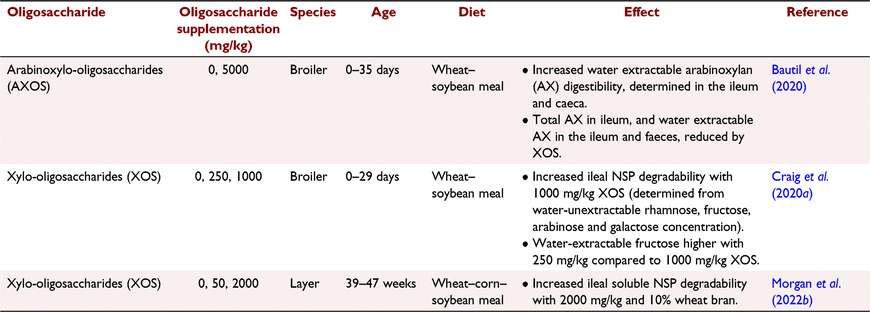
NSP-degrading enzymes stimulate caecal bacteria to produce endogenous NSP-degrading enzymes. This was shown by Bedford and Apajalahti (2018), Bautil et al. (2021) and Morgan et al. (2019) who observed that rearing birds on diets with xylanase resulted in a caecal microbiome with heightened xylanase activity, and generally increased xylan degradation. The same has also been observed with direct oligosaccharide application into diets; for example, Morgan et al. (2022a) observed increased caeca SCFA concentration and xylanase and cellulase activity from supplementing 2000 mg/kg XOS and wheat bran into sorghum-based diets fed to broilers. This suggests that the increased SCFA observed in the presence of oligosaccharides and NSP-degrading enzymes may be an indicator of the ability of these supplements to manipulate the microbiome to produce more endogenous enzymes, as opposed to just prebiotic effects. The ramifications of this on NSP degradability are still unclear, due to a lack of studies presenting this data, as highlighted in Table 3.
Gonzalez-Ortiz et al. (2019) recently proposed a new category of feed additives, stimbiotics, defined as products with the capacity to stimulate an NSP-degrading microbiome to increase NSP fermentability, at doses that are too low to contribute in a meaningful manner to an increased SCFA content. Supplemental oligosaccharides fall into this category. Bautil et al. (2019, 2020) and Morgan et al. (2022a, 2022b) presented the stimbiotic effects of XOS, with low dietary concentrations shown to stimulate production of endogenous xylanase in the caeca, resulting in enhanced degradation of xylan and increased SCFA manufacture. A stimbiotic can be the same molecule as a prebiotic, such as an oligosaccharide, but stimbiotics are fed at concentrations below that capable of directly supporting fermentation (Bedford and Apajalahti 2022). Moreover, response to stimbiotics can take multiple weeks (Morgan et al. 2021), whereas prebiotics are fermented straightaway, resulting in SCFA manufacture within hours of consumption (Pourabedin and Zhao 2015). Both stimbiotics and prebiotics will be used by similar bacteria species, but prebiotics are quantitatively fermented by the bacteria into SCFA, whereas stimbiotics stimulate growth and activity of the bacteria. Further research is warranted into the mode of action of stimbiotics, and factors that influence their efficacy, such as diet type, environmental challenges and bird age and physiological status.
Conclusions
Oligosaccharides stimulate proliferation of beneficial bacteria species in the caeca, resulting in increased production of valuable SCFA and heightened degradation of NSP. Application of enzymes to generate oligosaccharides in situ in the gastrointestinal tract is advantageous, but the effects are variable, as they are dictated by the NSP substrate in the diet. Direct application of oligosaccharides mitigates this issue. However, there is currently a deficit of data presenting the effects of oligosaccharide application in poultry. This could be attributable to how complex and time-consuming it can be to produce and measure oligosaccharides. This will improve, given the global interest in finding and testing alternative products to in-feed antibiotics, with oligosaccharides being a key contender. Presently, it is not possible to draw strong conclusions about why there is variability among studies testing the same oligosaccharide, or fully understand the mechanisms behind their effects. This is primarily because authors very rarely measure the quantity and composition of the oligosaccharides in the test diets. To rectify this, robust research trials that measure the oligosaccharides in the test diets, and preferably also in the digesta, need to be conducted. Different diets and scenarios need to be examined, as it may be that a more tailored approach to oligosaccharide application is required, primarily on the basis of the composition of the diet. This information will allow improved oligosaccharide products, with prebiotic and stimbiotic properties, to be developed for the poultry industry.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
Natalie Morgan is an Associate Editor of Animal Production Science but was blinded from the peer-review process for this paper.
Declaration of funding
This research did not receive any specific funding.
References
Aachary AA, Prapulla SG (2011) Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Comprehensive Reviews in Food Science and Food Safety 10, 2–16.| Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications.Crossref | GoogleScholarGoogle Scholar |
Abd El-Hack ME, El-Saadony MT, Shafi ME, Qattan SYA, Batiha GE, Khafaga AF, Abdel-Moneim A-ME, Alagawany M (2020) Probiotics in poultry feed: a comprehensive review. Journal of Animal Physiology and Animal Nutrition 104, 1835–1850.
| Probiotics in poultry feed: a comprehensive review.Crossref | GoogleScholarGoogle Scholar |
Abdallah AG, Beshara MM (2015) Effect of different levels and sources of dietary fibre on productive and economic performance in local laying hens during growing period and subsequent laying performance. Egyptian Poultry Science Journal 35, 367–398.
Adhikari P, Cosby DE, Cox NA, Franca MS, Williams SM, Gogal RM, Ritz CW, Kim WK (2018) Effect of dietary fructooligosaccharide supplementation on internal organs Salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with Salmonella enteritidis. Poultry Science 97, 2525–2533.
| Effect of dietary fructooligosaccharide supplementation on internal organs Salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with Salmonella enteritidis.Crossref | GoogleScholarGoogle Scholar |
Akbaryan M, Mahdavi A, Jebelli-Javan A, Staji H, Darabighane B (2019) A comparison of the effects of resistant starch, fructooligosaccharide, and zinc bacitracin on cecal short-chain fatty acids, cecal microflora, intestinal morphology, and antibody titer against Newcastle disease virus in broilers. Comparative Clinical Pathology 28, 661–667.
| A comparison of the effects of resistant starch, fructooligosaccharide, and zinc bacitracin on cecal short-chain fatty acids, cecal microflora, intestinal morphology, and antibody titer against Newcastle disease virus in broilers.Crossref | GoogleScholarGoogle Scholar |
Apajalahti J, Vienola K (2016) Interaction between chicken intestinal microbiota and protein digestion. Animal Feed Science and Technology 221, 323–330.
| Interaction between chicken intestinal microbiota and protein digestion.Crossref | GoogleScholarGoogle Scholar |
Apajalahti JHA, Kettunen A, Bedford MR, Holben WE (2001) Percent G+C profiling accurately reveals diet-related differences in the gastrointestinal microbial community of broiler chickens. Applied and Environmental Microbiology 67, 5656–5667.
| Percent G+C profiling accurately reveals diet-related differences in the gastrointestinal microbial community of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Asare PT, Greppi A, Pennacchia A, Brenig K, Geirnaert A, Schwab C, Stephan R, Lacroix C (2021) In vitro modeling of chicken cecal microbiota ecology and metabolism using the PolyFermS platform. Frontiers in Microbiology 12,
| In vitro modeling of chicken cecal microbiota ecology and metabolism using the PolyFermS platform.Crossref | GoogleScholarGoogle Scholar |
Ayman U, Akter L, Islam R, Bhakta S, Rahman MA, Islam MR, Sultana N, Sharif A, Jahan MR, Rahman MS, Haque Z (2022) Dietary chitosan oligosaccharides improves health status in broilers for safe poultry meat production. Annals of Agricultural Sciences 67, 90–98.
| Dietary chitosan oligosaccharides improves health status in broilers for safe poultry meat production.Crossref | GoogleScholarGoogle Scholar |
Ballou AL, Ali RA, Mendoza MA, Ellis JC, Hassan HM, Croom WJ, Koci MD (2016) Development of the chick microbiome: how early exposure influences future microbial diversity. Frontiers in Veterinary Science 3,
| Development of the chick microbiome: how early exposure influences future microbial diversity.Crossref | GoogleScholarGoogle Scholar |
Baurhoo B, Phillip L, Ruiz-Feria CA (2007) Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poultry Science 86, 1070–1078.
| Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Bautil A, Verspreet J, Buyse J, Goos P, Bedford MR, Courtin CM (2019) Age-related arabinoxylan hydrolysis and fermentation in the gastrointestinal tract of broilers fed wheat-based diets. Poultry Science 98, 4606–4621.
| Age-related arabinoxylan hydrolysis and fermentation in the gastrointestinal tract of broilers fed wheat-based diets.Crossref | GoogleScholarGoogle Scholar |
Bautil A, Verspreet J, Buyse J, Goos P, Bedford MR, Courtin CM (2020) Arabinoxylan-oligosaccharides kick-start arabinoxylan digestion in the aging broiler. Poultry Science 99, 2555–2565.
| Arabinoxylan-oligosaccharides kick-start arabinoxylan digestion in the aging broiler.Crossref | GoogleScholarGoogle Scholar |
Bautil A, Buyse J, Goos P, Bedford MR, Courtin CM (2021) Feed endoxylanase type and dose affect arabinoxylan hydrolysis and fermentation in ageing broilers. Animal Nutrition 7, 787–800.
| Feed endoxylanase type and dose affect arabinoxylan hydrolysis and fermentation in ageing broilers.Crossref | GoogleScholarGoogle Scholar |
Bedford MR (2018) The evolution and application of enzymes in the animal feed industry: the role of data interpretation. British Poultry Science 59, 486–493.
| The evolution and application of enzymes in the animal feed industry: the role of data interpretation.Crossref | GoogleScholarGoogle Scholar |
Bedford MR, Apajalahti J (2018) Exposure of a broiler to a xylanase for 35d increases the capacity of cecal microbiome to ferment soluble xylan. Poultry Science 97, 98–99.
Bedford MR, Apajalahti JH (2022) The role of feed enzymes in maintaining poultry intestinal health. Journal of the Science of Food and Agriculture 102, 1759–1770.
| The role of feed enzymes in maintaining poultry intestinal health.Crossref | GoogleScholarGoogle Scholar |
Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ (2006) Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Applied and Environmental Microbiology 72, 3593–3599.
| Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut.Crossref | GoogleScholarGoogle Scholar |
Biggs P, Parsons CM, Fahey GC (2007) The effects of several oligosaccharides on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poultry Science 86, 2327–2336.
| The effects of several oligosaccharides on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks.Crossref | GoogleScholarGoogle Scholar |
Bindari YR, Gerber PF (2022) Centennial Review: Factors affecting the chicken gastrointestinal microbial composition and their association with gut health and productive performance. Poultry Science 101,
| Centennial Review: Factors affecting the chicken gastrointestinal microbial composition and their association with gut health and productive performance.Crossref | GoogleScholarGoogle Scholar |
Björnhag G, Sperber I (1977) Transport of various food components through the digestive tract of turkeys, geese and guinea fowl. Swedish Journal of Agricultural Research 7, 57–66.
Borda-Molina D, Mátis G, Mackei M, Neogrády Z, Huber K, Seifert J, Camarinha-Silva A (2021) Caeca microbial variation in broiler chickens as a result of dietary combinations using two cereal types, supplementation of crude protein and sodium butyrate. Frontiers in Microbiology 11,
| Caeca microbial variation in broiler chickens as a result of dietary combinations using two cereal types, supplementation of crude protein and sodium butyrate.Crossref | GoogleScholarGoogle Scholar |
Broekaert WF, Courtin CM, Verbeke K, Van de Wiele T, Verstraete W, Delcour JA (2011) Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Critical Reviews in Food Science and Nutrition 51, 178–194.
| Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides.Crossref | GoogleScholarGoogle Scholar |
Burton RA, Fincher GB (2014) Evolution and development of cell walls in cereal grains. Frontiers in Plant Science 5,
| Evolution and development of cell walls in cereal grains.Crossref | GoogleScholarGoogle Scholar |
Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A (2011) Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World Journal of Gastroenterology 17, 1519–1528.
| Potential beneficial effects of butyrate in intestinal and extraintestinal diseases.Crossref | GoogleScholarGoogle Scholar |
Cao BH, Karasawa Y, Guo YM (2005) Effects of green tea polyphenols and fructo-oligosaccharides in semi-purified diets on broilers’ performance and caecal microflora and their metabolites. Asian-Australasian Journal of Animal Sciences 18, 85–89.
| Effects of green tea polyphenols and fructo-oligosaccharides in semi-purified diets on broilers’ performance and caecal microflora and their metabolites.Crossref | GoogleScholarGoogle Scholar |
Carvalho AFA, Neto PdO, da Silva DF, Pastore GM (2013) Xylo-oligosaccharides from lignocellulosic materials: chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Research International 51, 75–85.
| Xylo-oligosaccharides from lignocellulosic materials: chemical structure, health benefits and production by chemical and enzymatic hydrolysis.Crossref | GoogleScholarGoogle Scholar |
Casteleyn C, Doom M, Lambrechts E, Van den Broeck W, Simoens P, Cornillie P (2010) Locations of gut-associated lymphoid tissue in the 3-month-old chicken: a review. Avian Pathology 39, 143–150.
| Locations of gut-associated lymphoid tissue in the 3-month-old chicken: a review.Crossref | GoogleScholarGoogle Scholar |
Chang L, Ding Y, Wang Y, Song Z, Li F, He X, Zhang H (2022) Effects of different oligosaccharides on growth performance and intestinal function in broilers. Frontiers in Veterinary Science 9,
| Effects of different oligosaccharides on growth performance and intestinal function in broilers.Crossref | GoogleScholarGoogle Scholar |
Choct M, Hughes RJ, Wang J, Bedford MR, Morgan AJ, Annison G (1996) Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens. British Poultry Science 37, 609–621.
| Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens.Crossref | GoogleScholarGoogle Scholar |
Clavijo V, Flórez MJV (2018) The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poultry Science 97, 1006–1021.
| The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review.Crossref | GoogleScholarGoogle Scholar |
Courtin CM, Broekaert WF, Swennen K, Lescroart O, Onagbesan O, Buyse J, Decuypere E, Van de Wiele T, Marzorati M, Verstraete W, Huyghebaert G, Delcour JA (2008) Dietary inclusion of wheat bran arabinoxylooligosaccharides induces beneficial nutritional effects in chickens. Cereal Chemistry 85, 607–613.
| Dietary inclusion of wheat bran arabinoxylooligosaccharides induces beneficial nutritional effects in chickens.Crossref | GoogleScholarGoogle Scholar |
Craig AD, Khattak F, Hastie P, Bedford MR, Olukosi OA (2020a) Xylanase and xylo- oligosaccharide prebiotic improve the growth performance and concentration of potentially prebiotic oligosaccharides in the ileum of broiler chickens. British Poultry Science 61, 70–78.
| Xylanase and xylo- oligosaccharide prebiotic improve the growth performance and concentration of potentially prebiotic oligosaccharides in the ileum of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Craig AD, Khattak F, Hastie P, Bedford MR, Olukosi OA (2020b) The similarity of the effect of carbohydrase or prebiotic supplementation in broilers aged 21 days, fed mixed cereal diets and challenged with coccidiosis infection. PLoS ONE 15,
| The similarity of the effect of carbohydrase or prebiotic supplementation in broilers aged 21 days, fed mixed cereal diets and challenged with coccidiosis infection.Crossref | GoogleScholarGoogle Scholar |
Crisol-Martínez E, Stanley D, Geier MS, Hughes RJ, Moore RJ (2017) Sorghum and wheat differentially affect caecal microbiota and associated performance characteristics of meat chickens. PeerJ 5,
| Sorghum and wheat differentially affect caecal microbiota and associated performance characteristics of meat chickens.Crossref | GoogleScholarGoogle Scholar |
Dale T, Hannay I, Bedford MR, Tucker GA, Brameld JM, Parr T (2020) The effects of exogenous xylanase supplementation on the in vivo generation of xylooligosaccharides and monosaccharides in broilers fed a wheat-based diet. British Poultry Science 61, 471–481.
| The effects of exogenous xylanase supplementation on the in vivo generation of xylooligosaccharides and monosaccharides in broilers fed a wheat-based diet.Crossref | GoogleScholarGoogle Scholar |
De Maesschalck C, Eeckhaut V, Maertens L, De Lange L, Marchal L, Nezer C, De Baere S, Croubels S, Daube G, Dewulf J, Haesebrouck F, Ducatelle R, Taminau B, Van Immerseel F (2015) Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Applied and Environmental Microbiology 81, 5880–5888.
| Effects of xylo-oligosaccharides on broiler chicken performance and microbiota.Crossref | GoogleScholarGoogle Scholar |
Ding XM, Li DD, Bai SP, Wang JP, Zeng QF, Su ZW, Xuan Y, Zhang KY (2018) Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poultry Science 97, 874–881.
| Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens.Crossref | GoogleScholarGoogle Scholar |
Donalson LM, Kim WK, Chalova VI, Herrera P, McReynolds JL, Gotcheva VG, Vidanović D, Woodward CL, Kubena LF, Nisbet DJ, Ricke SC (2008) In vitro fermentation response of laying hen cecal bacteria to combinations of fructooligosaccharide prebiotics with alfalfa or a layer ration. Poultry Science 87, 1263–1275.
| In vitro fermentation response of laying hen cecal bacteria to combinations of fructooligosaccharide prebiotics with alfalfa or a layer ration.Crossref | GoogleScholarGoogle Scholar |
Duke GE, Eccleston E, Kirkwood S, Louis CF, Bedbury HP (1984) Cellulose digestion by domestic turkeys fed low or high fiber diets. The Journal of Nutrition 114, 95–102.
| Cellulose digestion by domestic turkeys fed low or high fiber diets.Crossref | GoogleScholarGoogle Scholar |
Elling-Staats ML, Gilbert MS, Smidt H, Kwakkel RP (2022) Caecal protein fermentation in broilers: a review. World’s Poultry Science Journal 78, 103–123.
| Caecal protein fermentation in broilers: a review.Crossref | GoogleScholarGoogle Scholar |
Engberg RM, Hedemann MS, Steenfeldt S, Jensen BB (2004) Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poultry Science 83, 925–938.
| Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract.Crossref | GoogleScholarGoogle Scholar |
Fathima S, Shanmugasundaram R, Adams D, Selvaraj RK (2022) Gastrointestinal microbiota and their manipulation for improved growth and performance in chickens. Foods 11,
| Gastrointestinal microbiota and their manipulation for improved growth and performance in chickens.Crossref | GoogleScholarGoogle Scholar |
Ferrer R, Planas JM, Durfort M, Moretó M (1991) Morphological study of the caecal epithelium of the chicken (Gallus Gallus Domesticus L.). British Poultry Science 32, 679–691.
| Morphological study of the caecal epithelium of the chicken (Gallus Gallus Domesticus L.).Crossref | GoogleScholarGoogle Scholar |
Feye KM, Baxter MFA, Tellez-Isaias G, Kogut MH, Ricke SC (2020) Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poultry Science 99, 653–659.
| Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes.Crossref | GoogleScholarGoogle Scholar |
Glendinning L, Watson KA, Watson M (2019) Development of the duodenal, ileal, jejunal and caecal microbiota in chickens. Animal Microbiome 1,
| Development of the duodenal, ileal, jejunal and caecal microbiota in chickens.Crossref | GoogleScholarGoogle Scholar |
Glendinning L, Stewart RD, Pallen MJ, Watson KA, Watson M (2020) Assembly of hundreds of novel bacterial genomes from the chicken caecum. Genome Biology 21,
| Assembly of hundreds of novel bacterial genomes from the chicken caecum.Crossref | GoogleScholarGoogle Scholar |
Gong J, Forster RJ, Yu H, Chambers JR, Sabour PM, Wheatcroft R, Chen S (2002) Diversity and phylogenetic analysis of bacteria in the mucosa of chicken ceca and comparison with bacteria in the cecal lumen. FEMS Microbiology Letters 208, 1–7.
| Diversity and phylogenetic analysis of bacteria in the mucosa of chicken ceca and comparison with bacteria in the cecal lumen.Crossref | GoogleScholarGoogle Scholar |
Gong J, Yu H, Liu T, Gill JJ, Chambers JR, Wheatcroft R, Sabour PM (2008) Effects of zinc bacitracin, bird age and access to range on bacterial microbiota in the ileum and caeca of broiler chickens. Journal of Applied Microbiology 104, 1372–1382.
| Effects of zinc bacitracin, bird age and access to range on bacterial microbiota in the ileum and caeca of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Gonzalez-Ortiz G, Gomes GA, dos Santos TT, Bedford MR (2019) New strategies influencing gut functionality and animal performance. In ‘The value of fibre – engaging the second brain for animal nutrition’. (Eds G González-Ortiz, MR Bedford, KE Bach Knudsen, CM Courtin, HL Classen) pp. 233–254. (Wageningen Academic Press: Netherlands)
González-Ortiz G, Olukosi OA, Jurgens G, Apajalahti J, Bedford MR (2020) Short-chain fatty acids and ceca microbiota profiles in broilers and turkeys in response to diets supplemented with phytase at varying concentrations, with or without xylanase. Poultry Science 99, 2068–2077.
| Short-chain fatty acids and ceca microbiota profiles in broilers and turkeys in response to diets supplemented with phytase at varying concentrations, with or without xylanase.Crossref | GoogleScholarGoogle Scholar |
Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F (2010) From the gut to the peripheral tissues: the multiple effects of butyrate. Nutrition Research Reviews 23, 366–384.
| From the gut to the peripheral tissues: the multiple effects of butyrate.Crossref | GoogleScholarGoogle Scholar |
Hunt A, Al-Nakkash L, Lee AH, Smith HF (2019) Phylogeny and herbivory are related to avian cecal size. Scientific Reports 9,
| Phylogeny and herbivory are related to avian cecal size.Crossref | GoogleScholarGoogle Scholar |
Ijaz UZ, Sivaloganathan L, McKenna A, Richmond A, Kelly C, Linton M, Stratakos AC, Lavery U, Elmi A, Wren BW, Dorrell N, Corcionivoschi N, Gundogdu O (2018) Comprehensive longitudinal microbiome analysis of the chicken cecum reveals a shift from competitive to environmental drivers and a window of opportunity for campylobacter. Frontiers in Microbiology 9,
| Comprehensive longitudinal microbiome analysis of the chicken cecum reveals a shift from competitive to environmental drivers and a window of opportunity for campylobacter.Crossref | GoogleScholarGoogle Scholar |
Jahan AA, González Ortiz G, Moss AF, Bhuiyan MM, Morgan NK (2022) Role of supplemental oligosaccharides in poultry diets. World’s Poultry Science Journal 78, 615–639.
| Role of supplemental oligosaccharides in poultry diets.Crossref | GoogleScholarGoogle Scholar |
Janssen PWM, Lentle RG, Hulls C, Ravindran V, Amerah AM (2009) Spatiotemporal mapping of the motility of the isolated chicken caecum. Journal of Comparative Physiology B 179, 593–604.
| Spatiotemporal mapping of the motility of the isolated chicken caecum.Crossref | GoogleScholarGoogle Scholar |
Józefiak D, Rutkowski A, Frątczak M, Boros D (2004) The effect of dietary fibre fractions from different cereals and microbial enzyme supplementation on performance, ileal viscosity and short-chain fatty acid concentrations in the caeca of broiler chickens. Journal of Animal and Feed Sciences 13, 487–496.
| The effect of dietary fibre fractions from different cereals and microbial enzyme supplementation on performance, ileal viscosity and short-chain fatty acid concentrations in the caeca of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Józefiak D, Rutkowski A, Jensen BB, Engberg RM (2006) The effect of β-glucanase supplementation of barley- and oat-based diets on growth performance and fermentation in broiler chicken gastrointestinal tract. British Poultry Science 47, 57–64.
| The effect of β-glucanase supplementation of barley- and oat-based diets on growth performance and fermentation in broiler chicken gastrointestinal tract.Crossref | GoogleScholarGoogle Scholar |
Józefiak D, Rutkowski A, Kaczmarek S, Jensen BB, Engberg RM, Højberg O (2010) Effect of β-glucanase and xylanase supplementation of barley- and rye-based diets on caecal microbiota of broiler chickens. British Poultry Science 51, 546–557.
| Effect of β-glucanase and xylanase supplementation of barley- and rye-based diets on caecal microbiota of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Keerqin C, Morgan NK, Wu SB, Swick RA, Choct M (2017) Dietary inclusion of arabinoxylo-oligosaccharides in response to broilers challenged with subclinical necrotic enteritis. British Poultry Science 58, 418–424.
| Dietary inclusion of arabinoxylo-oligosaccharides in response to broilers challenged with subclinical necrotic enteritis.Crossref | GoogleScholarGoogle Scholar |
Kers JG, Oliveira JE, Fischer EAJ, Tersteeg-Zijderveld MHG, Konstanti P, Stegeman JA(Arjan), Smidt H, Velkers FC (2020) Associations between phenotypic characteristics and clinical parameters of broilers and intestinal microbial development throughout a production cycle: a field study. MicrobiologyOpen 9,
| Associations between phenotypic characteristics and clinical parameters of broilers and intestinal microbial development throughout a production cycle: a field study.Crossref | GoogleScholarGoogle Scholar |
Kim E, Moss AF, Morgan NK, Gharib-Naseri K, Ader P, Choct M (2022) Non-starch polysaccharide-degrading enzymes may improve performance when included in wheat- but not maize-based diets fed to broiler chickens under subclinical necrotic enteritis challenge. Animal Nutrition 10, 54–67.
| Non-starch polysaccharide-degrading enzymes may improve performance when included in wheat- but not maize-based diets fed to broiler chickens under subclinical necrotic enteritis challenge.Crossref | GoogleScholarGoogle Scholar |
Langhout DJ, Schutte JB (1996) Nutritional implications of pectins in chicks in relation to esterification and origin of pectins. Poultry Science 75, 1236–1242.
| Nutritional implications of pectins in chicks in relation to esterification and origin of pectins.Crossref | GoogleScholarGoogle Scholar |
Li J, Cheng Y, Chen Y, Qu H, Zhao Y, Wen C, Zhou Y (2019) Dietary chitooligosaccharide inclusion as an alternative to antibiotics improves intestinal morphology, barrier function, antioxidant capacity, and immunity of broilers at early age. Animals 9,
| Dietary chitooligosaccharide inclusion as an alternative to antibiotics improves intestinal morphology, barrier function, antioxidant capacity, and immunity of broilers at early age.Crossref | GoogleScholarGoogle Scholar |
Liao X, Shao Y, Sun G, Yang Y, Zhang L, Guo Y, Luo X, Lu L (2020) The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers. Poultry Science 99, 5883–5895.
| The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers.Crossref | GoogleScholarGoogle Scholar |
Lin Y, Teng P-Y, Olukosi OA (2022) The effects of xylo-oligosaccharides on regulating growth performance, nutrient utilization, gene expression of tight junctions, nutrient transporters, and cecal short chain fatty acids profile in Eimeria-challenged broiler chickens. Poultry Science 101,
| The effects of xylo-oligosaccharides on regulating growth performance, nutrient utilization, gene expression of tight junctions, nutrient transporters, and cecal short chain fatty acids profile in Eimeria-challenged broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Liu HY, Li X, Zhu X, Dong WG, Yang GQ (2021a) Soybean oligosaccharides attenuate odour compounds in excreta by modulating the caecal microbiota in broilers. Animal 15,
| Soybean oligosaccharides attenuate odour compounds in excreta by modulating the caecal microbiota in broilers.Crossref | GoogleScholarGoogle Scholar |
Liu L, Li Q, Yang Y, Guo A (2021b) Biological function of short-chain fatty acids and its regulation on intestinal health of poultry. Frontiers in Veterinary Science 8,
| Biological function of short-chain fatty acids and its regulation on intestinal health of poultry.Crossref | GoogleScholarGoogle Scholar |
Longstaff MA, Knox A, McNab JM (1988) Digestibility of pentose sugars and uronic acids and their effect on chick weight gain and caecal size. British Poultry Science 29, 379–393.
| Digestibility of pentose sugars and uronic acids and their effect on chick weight gain and caecal size.Crossref | GoogleScholarGoogle Scholar |
Lu J, Idris U, Harmon B, Hofacre C, Maurer JJ, Lee MD (2003) Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Applied and Environmental Microbiology 69, 6816–6824.
| Diversity and succession of the intestinal bacterial community of the maturing broiler chicken.Crossref | GoogleScholarGoogle Scholar |
Mahmood T, Guo Y (2020) Dietary fiber and chicken microbiome interaction: where will it lead to? Animal Nutrition 6, 1–8.
| Dietary fiber and chicken microbiome interaction: where will it lead to?Crossref | GoogleScholarGoogle Scholar |
Majeed MF, Al-Asadi FS, Al-Nassir AN, Rahi EH (2009) The morphological and histological study of the caecum in broiler chicken. Basrah Journal of Veterinary Research 8, 19–25.
| The morphological and histological study of the caecum in broiler chicken.Crossref | GoogleScholarGoogle Scholar |
Mateos GG, Jiménez-Moreno E, Serrano MP, Lázaro RP (2012) Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics. Journal of Applied Poultry Research 21, 156–174.
| Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics.Crossref | GoogleScholarGoogle Scholar |
McNab JM (1973) The avian caeca: a review. World’s Poultry Science Journal 29, 251–263.
| The avian caeca: a review.Crossref | GoogleScholarGoogle Scholar |
Metzler-Zebeli BU, Magowan E, Hollmann M, Ball MEE, Molnár A, Witter K, Ertl R, Hawken RJ, Lawlor PG, O’Connell NE, Aschenbach J, Zebeli Q (2018) Differences in intestinal size, structure, and function contributing to feed efficiency in broiler chickens reared at geographically distant locations. Poultry Science 97, 578–591.
| Differences in intestinal size, structure, and function contributing to feed efficiency in broiler chickens reared at geographically distant locations.Crossref | GoogleScholarGoogle Scholar |
Mirande C, Kadlecikova E, Matulova M, Capek P, Bernalier-Donadille A, Forano E, Béra-Maillet C (2010) Dietary fibre degradation and fermentation by two xylanolytic bacteria Bacteroides xylanisolvens XB1AT and Roseburia intestinalis XB6B4 from the human intestine. Journal of Applied Microbiology 109, 451–460.
| Dietary fibre degradation and fermentation by two xylanolytic bacteria Bacteroides xylanisolvens XB1AT and Roseburia intestinalis XB6B4 from the human intestine.Crossref | GoogleScholarGoogle Scholar |
Modrackova N, Vlkova E, Tejnecky V, Schwab C, Neuzil-Bunesova V (2020) Bifidobacterium β-glucosidase activity and fermentation of dietary plant glucosides is species and strain specific. Microorganisms 8,
| Bifidobacterium β-glucosidase activity and fermentation of dietary plant glucosides is species and strain specific.Crossref | GoogleScholarGoogle Scholar |
Mookiah S, Sieo CC, Ramasamy K, Abdullah N, Ho YW (2014) Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. Journal of the Science of Food and Agriculture 94, 341–348.
| Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Morgan NK, Keerqin C, Wallace A, Wu S-B, Choct M (2019) Effect of arabinoxylo-oligosaccharides and arabinoxylans on net energy and nutrient utilization in broilers. Animal Nutrition 5, 56–62.
| Effect of arabinoxylo-oligosaccharides and arabinoxylans on net energy and nutrient utilization in broilers.Crossref | GoogleScholarGoogle Scholar |
Morgan NK, Wallace A, Bedford MR, Hawking KL, Rodrigues I, Hilliar M, Choct M (2020) In vitro versus in situ evaluation of xylan hydrolysis into xylo-oligosaccharides in broiler chicken gastrointestinal tract. Carbohydrate Polymers 230,
| In vitro versus in situ evaluation of xylan hydrolysis into xylo-oligosaccharides in broiler chicken gastrointestinal tract.Crossref | GoogleScholarGoogle Scholar |
Morgan NK, Gomes GA, Kim JC (2021) Comparing the efficacy of stimbiotic and a combination of xylanase and beta-glucanase, in broilers fed wheat-barley based diets with high or low AME. Poultry Science 100,
| Comparing the efficacy of stimbiotic and a combination of xylanase and beta-glucanase, in broilers fed wheat-barley based diets with high or low AME.Crossref | GoogleScholarGoogle Scholar |
Morgan NK, Wallace A, Bedford MR (2022a) Improving sorghum digestion in broilers by targeting fermentation of xylan. Animal Nutrition 10, 198–206.
| Improving sorghum digestion in broilers by targeting fermentation of xylan.Crossref | GoogleScholarGoogle Scholar |
Morgan NK, Wallace A, Bedford MR, González-Ortiz G (2022b) Impact of fermentable fiber, xylo-oligosaccharides and xylanase on laying hen productive performance and nutrient utilization. Poultry Science 101,
| Impact of fermentable fiber, xylo-oligosaccharides and xylanase on laying hen productive performance and nutrient utilization.Crossref | GoogleScholarGoogle Scholar |
Moura P, Barata R, Carvalheiro F, Gírio F, Loureiro-Dias MC, Esteves MP (2007) In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains. LWT - Food Science and Technology 40, 963–972.
| In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains.Crossref | GoogleScholarGoogle Scholar |
Munyaka PM, Nandha NK, Kiarie E, Nyachoti CM, Khafipour E (2016) Impact of combined β-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets. Poultry Science 95, 528–540.
| Impact of combined β-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets.Crossref | GoogleScholarGoogle Scholar |
Mussatto SI, Mancilha IM (2007) Non-digestible oligosaccharides: a review. Carbohydrate Polymers 68, 587–597.
| Non-digestible oligosaccharides: a review.Crossref | GoogleScholarGoogle Scholar |
Nguyen HT, Bedford MR, Morgan NK (2021) Importance of considering non-starch polysaccharide content of poultry diets. World’s Poultry Science Journal 77, 619–637.
| Importance of considering non-starch polysaccharide content of poultry diets.Crossref | GoogleScholarGoogle Scholar |
Oakley BB, Buhr RJ, Ritz CW, Kiepper BH, Berrang ME, Seal BS, Cox NA (2014) Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Veterinary Research 10,
| Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives.Crossref | GoogleScholarGoogle Scholar |
Okazaki M, Fujikawa S, Matsumoto N (1990) Effect of xylooligosaccharide on the growth of bifidobacteria. Bifidobacteria and Microflora 9, 77–86.
| Effect of xylooligosaccharide on the growth of bifidobacteria.Crossref | GoogleScholarGoogle Scholar |
Olukosi OA, Bedford MR (2019) Comparative effects of wheat varieties and xylanase supplementation on growth performance, nutrient utilization, net energy, and whole-body energy and nutrient partitioning in broilers at different ages. Poultry Science 98, 2179–2188.
| Comparative effects of wheat varieties and xylanase supplementation on growth performance, nutrient utilization, net energy, and whole-body energy and nutrient partitioning in broilers at different ages.Crossref | GoogleScholarGoogle Scholar |
Paraskeuas V, Mountzouris KC (2019) Broiler gut microbiota and expressions of gut barrier genes affected by cereal type and phytogenic inclusion. Animal Nutrition 5, 22–31.
| Broiler gut microbiota and expressions of gut barrier genes affected by cereal type and phytogenic inclusion.Crossref | GoogleScholarGoogle Scholar |
Patel DS, Pendrill R, Mallajosyula SS, Widmalm G, MacKerell AD (2014) Conformational properties of α- or β-(1→6)-linked oligosaccharides: Hamiltonian replica exchange MD simulations and NMR experiment. The Journal of Physical Chemistry B 118, 2851–2871.
| Conformational properties of α- or β-(1→6)-linked oligosaccharides: Hamiltonian replica exchange MD simulations and NMR experiment.Crossref | GoogleScholarGoogle Scholar |
Pedroso AA, Batal AB, Lee MD (2016) Effect of in ovo administration of an adult-derived microbiota on establishment of the intestinal microbiome in chickens. American Journal of Veterinary Research 77, 514–526.
| Effect of in ovo administration of an adult-derived microbiota on establishment of the intestinal microbiome in chickens.Crossref | GoogleScholarGoogle Scholar |
Pottenger S, Watts A, Wedley A, Jopson S, Darby AC, Wigley P (2023) Timing and delivery route effects of cecal microbiome transplants on Salmonella typhimurium infections in chickens: potential for in-hatchery delivery of microbial interventions. Animal Microbiome 5,
| Timing and delivery route effects of cecal microbiome transplants on Salmonella typhimurium infections in chickens: potential for in-hatchery delivery of microbial interventions.Crossref | GoogleScholarGoogle Scholar |
Pourabedin M, Zhao X (2015) Prebiotics and gut microbiota in chickens. FEMS Microbiology Letters 362,
| Prebiotics and gut microbiota in chickens.Crossref | GoogleScholarGoogle Scholar |
Pourabedin M, Guan L, Zhao X (2015) Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome 3,
| Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens.Crossref | GoogleScholarGoogle Scholar |
Pourabedin M, Chen Q, Yang M, Zhao X (2017) Mannan- and xylooligosaccharides modulate caecal microbiota and expression of inflammatory-related cytokines and reduce caecal salmonella enteritidis colonisation in young chickens. FEMS Microbiology Ecology 93,
| Mannan- and xylooligosaccharides modulate caecal microbiota and expression of inflammatory-related cytokines and reduce caecal salmonella enteritidis colonisation in young chickens.Crossref | GoogleScholarGoogle Scholar |
Radeff T (1928) Über die Rohfaserverdauung beim Huhn und die hierbei dem Blinddarm zukommende Bedeutung. Biocehmische Zeitschrift 193, 192–196.
Ramírez GA, Keshri J, Vahrson I, Garber AI, Berrang ME, Cox NA, González-Cerón F, Aggrey SE, Oakley BB (2022) Cecal microbial hydrogen cycling potential is linked to feed efficiency phenotypes in chickens. Frontiers in Veterinary Science 9,
| Cecal microbial hydrogen cycling potential is linked to feed efficiency phenotypes in chickens.Crossref | GoogleScholarGoogle Scholar |
Rehman H, Böhm J, Zentek J (2008) Effects of differentially fermentable carbohydrates on the microbial fermentation profile of the gastrointestinal tract of broilers. Journal of Animal Physiology and Animal Nutrition 92, 471–480.
| Effects of differentially fermentable carbohydrates on the microbial fermentation profile of the gastrointestinal tract of broilers.Crossref | GoogleScholarGoogle Scholar |
Ribeiro T, Cardoso V, Ferreira LMA, Lordelo MMS, Coelho E, Moreira ASP, Domingues MRM, Coimbra MA, Bedford MR, Fontes CMGA (2018) Xylo-oligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers. Poultry Science 97, 4330–4341.
| Xylo-oligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers.Crossref | GoogleScholarGoogle Scholar |
Richards P, Fothergill J, Bernardeau M, Wigley P (2019) Development of the caecal microbiota in three broiler breeds. Frontiers in Veterinary Science 6,
| Development of the caecal microbiota in three broiler breeds.Crossref | GoogleScholarGoogle Scholar |
Ricke SC, Lee SI, Kim SA, Park SH, Shi Z (2020) Prebiotics and the poultry gastrointestinal tract microbiome. Poultry Science 99, 670–677.
| Prebiotics and the poultry gastrointestinal tract microbiome.Crossref | GoogleScholarGoogle Scholar |
Rodríguez ML, Rebolé A, Velasco S, Ortiz LT, Treviño J, Alzueta C (2012) Wheat- and barley-based diets with or without additives influence broiler chicken performance, nutrient digestibility and intestinal microflora. Journal of the Science of Food and Agriculture 92, 184–190.
| Wheat- and barley-based diets with or without additives influence broiler chicken performance, nutrient digestibility and intestinal microflora.Crossref | GoogleScholarGoogle Scholar |
Rycroft CE, Jones MR, Gibson GR, Rastall RA (2001) A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. Journal of Applied Microbiology 91, 878–887.
| A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides.Crossref | GoogleScholarGoogle Scholar |
Sacranie A, Iji PA, Mikkelsen LL, Choct M (2007) Occurrence of reverse peristalsis in broiler chickens. In ‘Proceedings of the 19th Australian poultry science symposium, 12–14 February, Sydney, New South Wales, Australia’. pp. 161–164. (Poultry Research Foundation)
Sacranie A, Svihus B, Denstadli V, Moen B, Iji PA, Choct M (2012) The effect of insoluble fiber and intermittent feeding on gizzard development, gut motility, and performance of broiler chickens. Poultry Science 91, 693–700.
| The effect of insoluble fiber and intermittent feeding on gizzard development, gut motility, and performance of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Samanta AK, Jayapal N, Kolte AP, Senani S, Sridhar M, Dhali A, Suresh KP, Jayaram C, Prasad CS (2015) Process for enzymatic production of xylooligosaccharides from the xylan of corn cobs. Journal of Food Processing and Preservation 39, 729–736.
| Process for enzymatic production of xylooligosaccharides from the xylan of corn cobs.Crossref | GoogleScholarGoogle Scholar |
Samanta AK, Kotte AP, Elangovan AV, Dhali A, Senani S, Sridhar M, Jayapal N (2017) Effects of corn husks derived xylooligosaccharides on performance of broiler chicken. Indian Journal of Animal Science 87, 640–643.
Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Penn CW, Pallen MJ (2014) Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS ONE 9,
| Extensive microbial and functional diversity within the chicken cecal microbiome.Crossref | GoogleScholarGoogle Scholar |
Shini S, Bryden WL (2022) Probiotics and gut health: linking gut homeostasis and poultry productivity. Animal Production Science 62, 1090–1112.
| Probiotics and gut health: linking gut homeostasis and poultry productivity.Crossref | GoogleScholarGoogle Scholar |
Singh AK, Mishra B, Bedford MR, Jha R (2021) Effects of supplemental xylanase and xylooligosaccharides on production performance and gut health variables of broiler chickens. Journal of Animal Science and Biotechnology 12,
| Effects of supplemental xylanase and xylooligosaccharides on production performance and gut health variables of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Singh AK, Tiwari UP, Mishra B, Jha R (2022) Effects of in ovo delivered xylo- and mannan- oligosaccharides on growth performance, intestinal immunity, cecal short-chain fatty acids, and cecal microbiota of broilers. Journal of Animal Science and Biotechnology 13,
| Effects of in ovo delivered xylo- and mannan- oligosaccharides on growth performance, intestinal immunity, cecal short-chain fatty acids, and cecal microbiota of broilers.Crossref | GoogleScholarGoogle Scholar |
Stanley D, Geier MS, Hughes RJ, Denman SE, Moore RJ (2013) Highly variable microbiota development in the chicken gastrointestinal tract. PLoS ONE 8,
| Highly variable microbiota development in the chicken gastrointestinal tract.Crossref | GoogleScholarGoogle Scholar |
Suo H-Q, Lu L, Xu G-H, Xiao L, Chen X-G, Xia R-R, Zhang L-Y, Luo X-G (2015) Effectiveness of dietary xylo-oligosaccharides for broilers fed a conventional corn-soybean meal diet. Journal of Integrative Agriculture 14, 2050–2057.
| Effectiveness of dietary xylo-oligosaccharides for broilers fed a conventional corn-soybean meal diet.Crossref | GoogleScholarGoogle Scholar |
Svihus B, Choct M, Classen HL (2013) Function and nutritional roles of the avian caeca: a review. World’s Poultry Science Journal 69, 249–264.
| Function and nutritional roles of the avian caeca: a review.Crossref | GoogleScholarGoogle Scholar |
Torok VA, Hughes RJ, Ophel-Keller K, Ali M, MacAlpine R (2009) Influence of different litter materials on cecal microbiota colonization in broiler chickens. Poultry Science 88, 2474–2481.
| Influence of different litter materials on cecal microbiota colonization in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
van der Wielen PWJJ, Biesterveld S, Notermans S, Hofstra H, Urlings BAP, van Knapen F (2000) Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Applied and Environmental Microbiology 66, 2536–2540.
| Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth.Crossref | GoogleScholarGoogle Scholar |
Wang Y, Wang Y, Lin X, Gou Z, Fan Q, Jiang S (2021) Effects of Clostridium butyricum, sodium butyrate, and butyric acid glycerides on the reproductive performance, egg quality, intestinal health, and offspring performance of yellow-feathered breeder hens. Frontiers in Microbiology 12,
| Effects of Clostridium butyricum, sodium butyrate, and butyric acid glycerides on the reproductive performance, egg quality, intestinal health, and offspring performance of yellow-feathered breeder hens.Crossref | GoogleScholarGoogle Scholar |
Warriss PD, Wilkins LJ, Brown SN, Phillips AJ, Allen V (2004) Defaecation and weight of the gastrointestinal tract contents after feed and water withdrawal in broilers. British Poultry Science 45, 61–66.
| Defaecation and weight of the gastrointestinal tract contents after feed and water withdrawal in broilers.Crossref | GoogleScholarGoogle Scholar |
Wickramasuriya SS, Park I, Lee K, Lee Y, Kim WH, Nam H, Lillehoj HS (2022) Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry. Vaccines 10,
| Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry.Crossref | GoogleScholarGoogle Scholar |
Xiao X, Wang Y, He J, Li Y (2020) Effects of Xylooligosaccharide (XOS) and Probiotics (PRO) on production performance, egg quality and intestinal short-chain fatty acids of laying hens in late laying period. Animal Husbandry and Feed Science 12, 21–25.
Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MQ (2003) Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poultry Science 82, 1030–1036.
| Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers.Crossref | GoogleScholarGoogle Scholar |
Yang C, Qiu M, Zhang Z, Song X, Yang L, Xiong X, Hu C, Pen H, Chen J, Xia B, Du H, Li Q, Jiang X, Yu C (2022) Galacto-oligosaccharides and xylo-oligosaccharides affect meat flavor by altering the cecal microbiome, metabolome, and transcriptome of chickens. Poultry Science 101,
| Galacto-oligosaccharides and xylo-oligosaccharides affect meat flavor by altering the cecal microbiome, metabolome, and transcriptome of chickens.Crossref | GoogleScholarGoogle Scholar |
Yousaf MS, Ahmad I, Ashraf K, Rashid MA, Hafeez A, Ahmad A, Zaneb H, Naseer R, Numan M, Zentek J, Rehman H (2017) Comparative effects of different dietary concentrations of β- galacto-oligosaccharides on serum biochemical metabolites, selected caecel microbiota and immune response in broilers. The Journal of Animal and Plant Science 27, 98–105.
Yuan L, Li W, Huo Q, Du C, Wang Z, Yi B, Wang M (2018) Effects of xylo-oligosaccharide and flavomycin on the immune function of broiler chickens. PeerJ 6,
| Effects of xylo-oligosaccharide and flavomycin on the immune function of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Zhang WF, Li DF, Lu WQ, Yi GF (2003) Effects of isomalto-oligosaccharides on broiler performance and intestinal microflora. Poultry Science 82, 657–663.
| Effects of isomalto-oligosaccharides on broiler performance and intestinal microflora.Crossref | GoogleScholarGoogle Scholar |
Zhou J, Wu S, Qi G, Fu Y, Wang W, Zhang H, Wang J (2021) Dietary supplemental xylooligosaccharide modulates nutrient digestibility, intestinal morphology, and gut microbiota in laying hens. Animal Nutrition 7, 152–162.
| Dietary supplemental xylooligosaccharide modulates nutrient digestibility, intestinal morphology, and gut microbiota in laying hens.Crossref | GoogleScholarGoogle Scholar |
Zhu X, Liu J, Liu H, Yang G (2020) Soybean oligosaccharide, stachyose, and raffinose in broilers diets: effects on odor compound concentration and microbiota in cecal digesta. Poultry Science 99, 3532–3539.
| Soybean oligosaccharide, stachyose, and raffinose in broilers diets: effects on odor compound concentration and microbiota in cecal digesta.Crossref | GoogleScholarGoogle Scholar |
Zhu X, Xu M, Liu H, Yang G (2022) In vitro fermentation profiles of different soybean oligosaccharides and their effects on skatole production and cecal microbiota of broilers. Animal Bioscience 35, 1195–1204.
| In vitro fermentation profiles of different soybean oligosaccharides and their effects on skatole production and cecal microbiota of broilers.Crossref | GoogleScholarGoogle Scholar |
Zyla K, Gogol D, Koreleski J, Swiatkiewicz S, Ledoux DR (1999) Simultaneous application of phytase and xylanase to broiler feeds based on wheat: in vitro measurements of phosphorus and pentose release from wheats and wheat-based feeds. Journal of the Science of Food and Agriculture 79, 1832–1840.


