Pre-rigor carcass stretching counteracts the negative effects of high rigor temperature on tenderness and water-holding capacity – using lamb muscles as a model
R. D. Warner A B E , M. Kerr B , Y. H. B. Kim C and G. Geesink DA Present address: CSIRO Animal, Food and Health Sciences, 671 Sneydes Road, Werribee, Vic. 3030, Australia.
B Department of Primary Industries, 600 Sneydes Road, Werribee, Vic. 3030, Australia.
C AgResearch Ltd, Ruakura Research Centre, Hamilton, New Zealand.
D University of New England, Armidale, NSW 2351, Australia.
E Corresponding author. Email: robyn.warner@csiro.au
Animal Production Science 54(4) 494-503 https://doi.org/10.1071/AN13062
Submitted: 14 February 2013 Accepted: 23 January 2014 Published: 27 February 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
High pre-rigor muscle temperature has negative consequences on quality and has been predominantly studied in the excised longissimus muscle of beef and lamb carcasses. There is little data on other muscles, the application in whole carcasses or potential amelioration techniques such as stretching. This study evaluated the effects of electrical stimulation, high pre-rigor temperature and stretching of lamb sides on quality traits and protein denaturation in four leg muscles [gluteus medius (GM), rectus femoris (RF), semimembranosus (SM) and semitendinosus (ST)]. Twenty lamb carcasses were used with two electrical stimulation treatments (stimulated or non-stimulated, +/−) and two pre-rigor temperature treatments (chilled at 2°C directly after slaughter, or held in 37°C water for 4.5 h before transfer to a 2°C chiller) applied. One side of each carcass was suspended from the Achilles tendon, whereas the other side was stretched by allowing the leg to drop and tying it to the ribs. Electrical stimulation did not influence the different traits except for pH fall post slaughter and myosin denaturation. Stretching resulted in greater muscle and sarcomere lengths for the GM, SM and ST, but a decrease in sarcomere length for the RF. For the non-stretched GM and SM, the 37°C treatment resulted in tougher meat at 1 and 8 days post mortem compared with the 2°C treatment. In contrast, the stretched 37°C treatment resulted in more tender meat for the GM, SM and ST at 1 day post mortem compared with the 2°C treatment. For all muscles, the 37°C treatment resulted in a decrease in the water-holding capacity (increased purge, surface exudate and cooking losses). The magnitude of this effect was generally diminished by stretching for the GM, SM and ST, but for the RF, (which was not stretched by the treatment) this effect was reversed. The 37°C treatment resulted in paler meat (increased L*-values) for the GM, SM and ST relative to the 2°C treatment. The observed effects of the 37°C treatment on water-holding capacity and colour could be explained by the effects of this treatment on indicators of protein denaturation (sarcoplasmic protein solubility and myofibrillar ATPase activity), which were decreased for the GM, SM and ST. The response to both temperature and stretching varied between the muscles, due to different anatomical location and also due to postulated differences in the fibre types. In conclusion, pre-rigor stretching of lamb sides can counteract the negative effects of high early post-mortem temperature on tenderness and water-holding capacity for those muscles that are stretched as a result of this hanging method.
Introduction
A high muscle temperature pre-rigor in meat carcasses causes quality defects including pale colour, moisture exudates from the meat surface, water loss during storage and a failure to tenderise during aging (Warner et al. 2014b). The condition has previously been known as ‘heat-toughening’ or ‘heat-shortening’ [see Jacob and Hopkins (2014) for discussion] although neither toughening nor shortening is necessarily involved with the defect. High rigor temperature in beef and lamb muscles is a similar phenomenon to PSE (pale, soft, exudative) in pig meat [see Barbut et al. (2008) for a review], although less severe. The main cause of the quality defect is denaturation of sarcoplasmic proteins and myosin, leading to a decrease in water-binding capacity of the proteins and, as a result, a decrease in the water-holding capacity of the meat (Barbut et al. 2008). In addition, denaturation of µ-calpain, the principal enzyme involved in post-mortem proteolysis of myofibrillar proteins, appears to lead to a reduction in the improvement of tenderness during aging (Dransfield 1994).
Although not as extensively investigated as the PSE problem in pork, the high rigor temperature problem in beef and lamb has been the topic of several studies with sometimes, conflicting results. Devine et al. (1999) attempted to quantify the toughening effect of high rigor temperature per se and the unfavourable effects of high temperature/low pH conditions on aging by comparing the tenderness of excised beef longissimus muscle samples, which entered rigor at different temperatures (15–35°C) while being restrained, or free to contract. The results of this study indicated that the high rigor temperature toughening was due to excessive muscle contraction at rigor and a diminished tenderisation response during aging. Kim et al. (2010) compared protein denaturation and degradation of myofibrillar proteins in deep and superficial portions of beef semimembranosus muscle, which correspond to relatively fast and slowly chilled parts of this muscle. Their results indicated that low pH and high temperature conditions caused protein denaturation, including denaturation of µ-calpain, and as a consequence, limited post-mortem proteolysis and pale colour of the meat. Similarly, Kim et al. (2012) reported increased protein denaturation, drip loss and shear force and less degradation of desmin during aging in beef longissimus muscles that entered rigor at 38°C compared with samples that entered rigor at 15°C.
Given the size of lamb carcasses, the risk of cold-shortening is considered to be greater than the risk of heat-toughening. Yet Pearce et al. (2010) and Thompson et al. (2005) in Australia and Matthews (2011) in the UK, have quite clearly shown that lamb carcasses can have a high temperature at rigor (temperature at pH 6 >35°C). This is particularly the case as abattoirs use stimulations systems to prevent cold-shortening. The effects of high temperature and low pH conditions on meat quality in excised longissimus muscle have been investigated in lamb in several studies. Geesink et al. (2000) reported on meat quality characteristics of excised lamb longissimus muscle, which entered rigor at different temperatures (5–35°C) and were then aged for a further 2 weeks at 2°C. Muscle contraction was most severe when muscles entered rigor at 35°C while tenderness and water-holding capacity were negatively affected when muscles entered rigor at temperatures of 25°C or higher. Devine et al. (2002) reported similar negative effects on tenderness after aging and on colour characteristics of lamb longissimus muscles that entered rigor mortis at 35°C compared with 18°C. Although most papers refer to the ‘temperature at rigor’ it is actually temperature before rigor, and duration at that temperature, which is probably most important, as discussed by Offer (1991). According to Offer (1991), myosin bound to actin is less susceptible to denaturation than unbound myosin. Thus, high pre-rigor temperature per se may protect against myosin denaturation and lessen the impact of high temperature and low pH conditions on water-holding capacity of meat. Stretching of muscles or muscle strips limits the overlap between actin and myosin. This may limit the detrimental effects of heat-shortening on meat tenderness, but at the same time may increase myosin denaturation and, consequently, decrease water-holding capacity. Furthermore, it is most likely the duration at a high temperature (and low pH) that is important and that there is a critical temperature/duration when denaturation starts to increase significantly. This paper and also the paper by Kim et al. (2014) attempt to address this deficiency in our knowledge.
Most of the studies on beef and lamb discussed above relate to experiments in which the muscles were excised before rigor, and therefore, free to contract and are limited to the longissimus muscle. Although the high rigor temperature conditions used in this experiment are extreme for lamb muscle, it was proposed to use the results to understand the occurrence of high rigor temperature in beef muscle. Thus the aim of this study was to determine the effect of high rigor temperature conditions in four different hind leg muscles in intact lamb carcasses and whether muscle stretching promotes or lessens the impact of high rigor temperature conditions on protein denaturation and water-holding capacity.
Material and methods
Animals, experimental design and treatments
Twenty 8-month-old Poll Dorset × Border Leicester–Merino lambs [average hot carcass weight = 21.3 kg ± 2.3 and the GR (total tissue thickness at the 12th rib, 110 mm from the midline) fat depth = 12.4 mm ± 3.6] were used in an experiment with two post-slaughter electrical stimulation treatments (ES; stimulated or non-stimulated, +/−), two pre-rigor temperature treatments (37°C for 4.5 h v. 2°C) applied to the carcass and two stretch treatments (stretched or non-stretched, +/−) applied to sides of the carcass. The experiment was conducted using five slaughter times involving four lambs per slaughter. The carcasses were randomly allocated to ES and temperature treatments within slaughter days and the sides were randomly allocated to +/− stretch treatment within a carcass.
Lambs were slaughtered by head-only electrical stunning, while restrained in a V-Restrainer, followed by exsanguination and, depending on the treatment group, ES was applied as described below. Low voltage ES was applied at 1 min post slaughter using a rectal probe and electrode clip applied to the stick wound. A square bipolar wave form was applied, providing 157-mA peak-to-peak (28–33V) with a frequency of 14 Hz for a duration of 60 s. The carcass was then suspended from the tail bone. Each carcass had one side stretched (leg pulled down tightly and tied to ribs) and the other non-stretched (hung from the Achilles tendon) (see Fig. 1). The temperature treatments were (i) high temperature (37°C) where the carcass was placed in a 37°C vat of water for 4.5 h (see Fig. 2) and then placed in a chiller at 2°C, (ii) low temperature (2°C) where the carcass was placed directly into a chiller at 2°C.

|

|
Carcass measurements
As an indication of the effectiveness of the temperature and ES treatments, the pH and temperature of the longissimus thoracis muscle over the 13th rib, at a depth of ~2–3 cm, was measured at 30 min post slaughter, then hourly for 4 h post slaughter. A pH meter with temperature compensation (WP-80, TPS Pty Ltd, Brisbane, Qld, Australia) and a polypropylene spear-type gel electrode (Ionode IJ 44) were used and calibration occurred at ambient temperature.
Sample collection and meat quality determination
At 24 h post slaughter, the muscles gluteus medius (GM), rectus femoris (RF), semimembranosus (SM) and semitendinosus (ST) were removed from both sides of each carcass. The length of each muscle was measured and recorded. The GM and SM were cut in half and each piece was randomly allocated to 0 or 7 days of storage. Samples destined for 7 days’ storage were weighed, vacuum packed and stored at 2°C for the duration of the storage period.
The following measurements were conducted on all muscles after 0 days’ storage; colour of the muscle surface after 30 min bloom at 4°C, ultimate pH, surface exudate 10 min after exposing the surface (Kauffman et al. 1986), Warner–Bratzler peak shear force and cooking loss. Also at 0 days of storage, samples were removed for sarcoplasmic protein solubility, sarcomere length and myofibrillar ATPase activity. As muscles can vary in the fibre type and quality in both a longitudinal and transverse direction (in some cases), every attempt was made to remove samples for each assay or measurement from a similar location, in both stretched and non-stretched muscles. After 7 days of storage, the GM and SM samples were removed from the vacuum bags, the amount of purge was measured, and samples were removed for determination of sarcoplasmic protein solubility, cooking loss and Warner–Bratzler peak shear force.
Surface colour was measured using a Minolta chromameter (Model CR-200b, Minolta Pty Ltd, Japan), 2° standard observer, D65 lighting and 8-mm aperture in the measuring head. For sarcoplasmic protein solubility, duplicate 1-g samples were removed and immediately homogenised in 10 mL of ice cold 0.025 M potassium phosphate, pH 7.2 and processed as described by Warner et al. (1997). For sarcomere length, triplicate 2-cm-long by 1-cm2 samples were removed from each muscle and frozen at −20°C for subsequent measurement. Sarcomere length was measured using a helium-neon laser (Ruddick and Richards 1975) and averaged across 20 measurements for each sample. Myofibrillar ATPase activity was used as a measure of myosin denaturation. A 2-g sample was removed, immediately subjected to myofibril purification (Warner et al. 1997) before freezing at −80°C in a glycerol-rigor buffer mixture. The method for measurement of myofibrillar ATPase activity is described in Warner et al. (1997).
Aged samples were weighed before vacuum packing and then removed from the vacuum pack after the storage period, patted dry and weighed to determine purge. Shear force measurements were undertaken using the method of Hopkins and Thompson (2001). All samples (Days 1 and 8) were trimmed and prepared into 65-g blocks and cooked on the day of collection. These blocks were cooked in a water bath in plastic bags at 70°C for 30 min. The shear force samples were prepared to give five replicate samples of 1-cm2 cross-sectional area and the force required to shear these was measured perpendicular to the fibre orientation using a Lloyd texture analyser (Model LRX, Lloyd Instruments, Hampshire, UK) fitted with a V-shaped cutting blade that sheared down through the samples. The cross head speed of the analyser was 300 mm/min. Cooking losses were calculated as the difference in weight before, and subsequent to cooking, and expressed as a percent of initial weight before cooking.
Statistics
Data was analysed using the ANOVA function in Genstat (Genstat Committee 2008) with ES, temperature, stretch treatments and interactions fitted as main effects (2 × 2 × 2) and slaughter day, carcass and side fitted as block effects, with nesting (Slaughter day/Carcass/Side). The statistical method of restricted maximum likelihood was used to model the pH over time, using a selected variance-covariance structure. The selection was via a sequence of likelihood ratio tests on several nested models. A power model in which the correlation between observations from the same carcass decays as the time delay between the observations increases was selected as the most appropriate.
Temperature at pH 6 (temp@pH6) was calculated as an indication of rigor temperature, as described by Warner et al. (2014a, 2014b). Temp@pH6 was included as a covariate in the ANOVA of shear force for each muscle.
Results
Effect of electrical stimulation
Electrical stimulation did not generally influence the meat quality traits measured as a main effect (P > 0.05) except for pH fall post slaughter, and for several other exceptions. Thus main effects of ES are only presented in the pH section and also under shear force below. ST shear force and myofibrillar ATPase activity were affected by the interaction of ES and temperature and this is reported in relevant sections below.
Temperature and pH decline rates
At 0.5 h post slaughter, the temperature (°C) of the loin muscle was similar between the temperature treatments (mean = 34.5, s.e.d. = 0.74; P > 0.05). At 0.5, 1.0, 2.0, 3.0, 4.0 and 5.0 h post slaughter, the temperature of the loins treated at 2°C were 34.0, 27.1, 19.4, 14.5, 11.3 and 9.3°C, respectively, and were lower (P < 0.001) at each time point than those treated at 37°C (34.9, 33.7, 34.7, 34.3, 34.2 and 34.6°C, respectively).
There was an effect of temperature, time and ES (P < 0.001 for all) on the LT pH post slaughter and all two- and three-way interactions were also significant (P < 0.05 for all). The differences between treatments at each time point are shown in Fig. 3. The electrically stimulated carcasses had a much lower pH at 0.5 and 1.0 h post slaughter (~0.5–0.6 units) compared with the non-stimulated carcasses (Fig. 3; P < 0.001). Thereafter, for the stimulated carcasses, the pH was similar between the 2 and 37°C treatments (P > 0.05). For the non-stimulated carcasses, the pH was 0.4–0.6 units lower for the 37°C treatment compared with those held at 2°C [Temperature × ES interaction at 2–5 h post slaughter (P < 0.10 for all)]. Thus the non-stimulated carcasses held at 2°C had a much slower rate of pH fall than the other three treatment groups.

|
The GM muscle held initially at 37°C had a higher ultimate pH than that held at 2°C (5.74 v. 5.66, respectively; s.e.d. = 0.026, P < 0.01). All other muscles had a similar ultimate pH between treatments (mean pH ± s.e.d. for RF, SM and ST was 5.82 ± 0.048, 5.75 ± 0.045 and 5.78 ± 0.053, respectively; P > 0.05).
The temp@pH6 (rigor temperature) was influenced by temperature, ES and by the interaction between temperature and ES (P < 0.001 for all). For the non-ES carcasses, the temp@pH6 was much lower for the 2°C treatment than for the 37°C (9.2 v. 25.7°C, respectively; s.e.d. = 2.27) whereas for the ES carcasses, the temp@pH6 was similar for the 2 and 37°C treatments (34.4 v. 32.5°C, respectively; s.e.d. = 2.27).
Sarcomere and muscle length
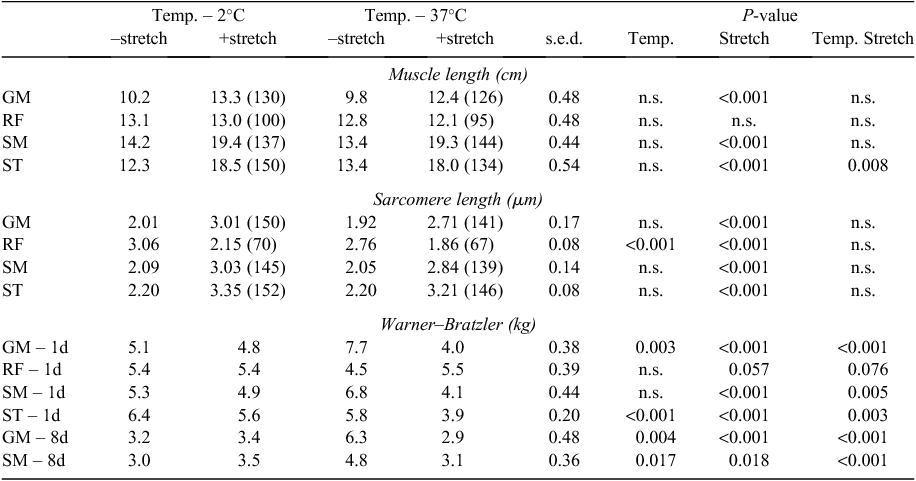
The +stretch treatment (see Fig. 4) resulted in longer muscle length in the GM (~3 cm), SM (~4 cm) and ST (~6 cm) and also longer sarcomeres (~0.9–1.0 μm) in all three muscles compared with the –stretch treatment (Table 1; P < 0.001 for all). The GM, SM and ST lengthened by 130–150% with the stretch treatment and the sarcomeres were 140–150% longer, relative to the non-stretched treatment (see Table 1). The non-stretched sides appeared to have slightly longer sarcomeres in the GM, SM and ST, compared with normal (Bouton et al. 1971) and thus the Achilles suspension used may have resulted in a slight stretch. Temperature appeared to have little effect on the muscle length or sarcomere length of the SM, ST or GM with one exception. For the ST, there was a bigger difference in sarcomere length between – and +stretch for the 2°C temperature treatment than for the 37°C treatment (interaction, P = 0.008). The +stretch treatment had no effect on the muscle length of the RF muscle (P > 0.05) but the sarcomere length of +stretch RF muscles was ~0.9 μm shorter than –stretch muscles (P < 0.05), which was about a 30% shortening. This indicates that the RF muscle is normally stretched by Achilles suspension and was free to shorten in the stretched side. In addition, the RF muscles from the carcasses chilled at 2°C were 0.3 μm longer than those held at 37°C. This indicates that the RF muscle underwent shortening as a result of the 37°C treatment, relative to the 2°C treatment.

|
Shear force
The shear force values showed a significant interaction between stretch and temperature treatments at both 1 and 8 days post slaughter for all muscles (Table 1). For the –stretch treatment, the 37°C treatment produced significantly tougher meat in the GM and SM at 1 and 8 days post slaughter compared with the 2°C. In contrast, for the muscles GM, SM and ST from the stretched sides, the 37°C treatment produced significantly more tender meat in all three muscles at 1 day post slaughter compared with the 2°C treatment. However, stretching did not affect the shear force of the RF at 2°C, whereas an adverse effect of stretching was found on the shear force values of the RF at 37°C (increasing shear force over 1 kg).
The shear force of the GM was lower with ES compared with no ES (5.17 v. 5.73 kg, respectively, s.e.d. = 0.235; P = 0.053). The shear force values also showed an interaction between electrical ES and temperature (P = 0.002) for the ST muscle only. For the ST muscles from the carcasses kept at 37°C, the shear force values were similar for plus and minus ES (4.93 v. 4.84 kg, respectively, s.e.d. = 0.179) but for the muscles held at 2°C, the shear force values were lower for plus ES compared with minus ES (5.51 v. 6.53 kg, respectively, s.e.d. = 0.179).
The inclusion of temp@pH6 as a covariate was not significant in any of the statistical models for shear force (P > 0.05).
Water-holding capacity
For all muscles at 1 day post slaughter, the muscles at 37°C had a higher surface exudate (~20 mg higher), a higher cooking loss (~6% higher) and a higher purge (~2% higher) than the muscles at 2°C (Table 2; P < 0.001 for all). At 8 days post slaughter, the GM and SM muscles from the carcasses held at 37°C had a similar cooking loss to the muscles from the carcasses chilled at 2°C (P > 0.05). Thus the higher temperature treatment clearly reduced the water-holding capacity of the muscle, but in the case of cooking loss, this effect appears to be removed by storing the muscle.
All of the stretched muscles except RF had a lower surface exudate (P < 0.05 for all), lower purge (P < 0.01) and a lower cooking loss at both 1 and 8 days post slaughter (P < 0.05). This indicates positive impacts of stretching carcasses on water-holding capacity of the muscles. Conversely, stretching of the sides resulted in more surface exudates from the RF regardless of holding temperatures, confirming that it was not stretched but actually shortened.
There was an interaction, or a trend for an interaction, between stretch and temperature treatment for the SM and ST surface exudate and the SM purge (P = 0.044, 0.055, 0.056, respectively). For the muscles from the carcasses held at 2°C, there was no difference in purge or exudate between the stretch treatments (P > 0.05), whereas for the muscles from the carcasses held at 37°C, the +stretch muscles had lower exudate and purge (P < 0.05).
Colour
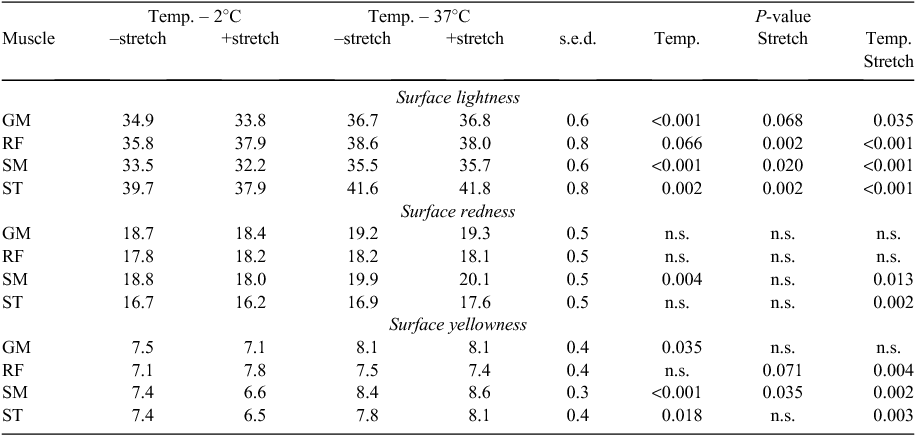
High pre-rigor temperature resulted in a significant increase in the lightness (L*) of the muscles regardless of the stretching treatment in general (Table 3). The redness (a*) values of the GM and RF were not affected by any of the treatments, but the redness of SM was increased by the high pre-rigor temperature condition (37°C).
For the SM and ST muscles, the –stretch muscles had a similar a* value between the two temperature treatments (P > 0.05) whereas the +stretched muscles were redder for the 37°C treatment than for the 2°C muscles (temperature × stretch interaction, P < 0.05 for both). The surface yellowness (b*) of the all muscles (except RF) was increased by the high pre-rigor temperature.
Protein denaturation
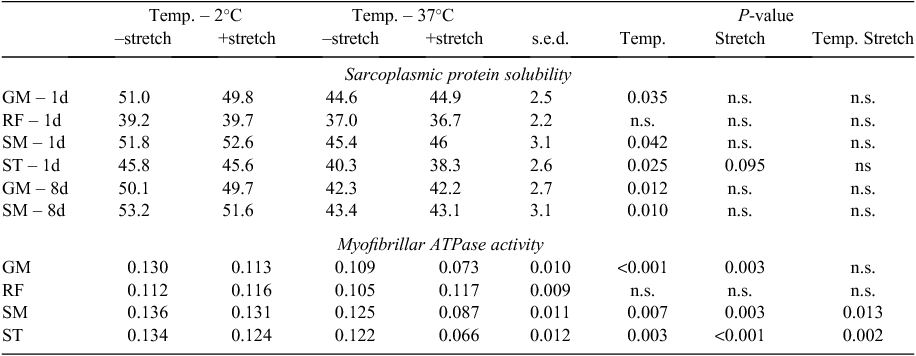
For all muscles except for the RF, the 37°C treatment reduced sarcoplasmic protein solubility compared with the 2°C treatment at both 1 and 8 days post slaughter (Table 4; P < 0.05 for all). For the ST muscle, 2°C-treated muscles did not show any effect of stretch but for the 37°C treatment, the +stretch muscles had lower sarcoplasmic protein solubility than the –stretch muscles (temperature × stretch interaction, P = 0.095). This interaction was also evident in the myofibrillar ATPase activity for several muscles. For the SM and ST muscles, 2°C-treated muscles did not show any effect of stretch but for the 37°C treatment, the +stretch had lower myofibrillar ATPase activity than the –stretch muscles (temperature × stretch interaction, P < 0.01 for all). For the GM muscle, 37°C muscles had lower myofibrillar ATPase activity than the 2°C muscles (P < 0.001) and the +stretch muscles had lower myofibrillar ATPase activity than the –stretch muscles (P = 0.003). In the GM, muscles from carcasses subjected to ES had lower myofibrillar ATPase activity than the non-ES muscles (0.087 v. 0.113 µmol/mg protein.min, respectively, s.e.d. = 0.0109; P = 0.032). Furthermore, for the ST, muscles from sides undergoing ES had lower myofibrillar ATPase activity with stretching, although this was not the case if the carcasses were not electrically stimulated (ES × stretch interaction, P = 0.056; see Table 5).

|
Thus the high temperature treatment clearly resulted in increased myofibrillar and sarcoplasmic muscle protein denaturation. In addition, the stretching of the muscles generally resulted in denaturation of the myosin head, as indicated by reduced myofibrillar ATPase activity, and particularly when combined with the high temperature treatment, or for the ST, when combined with ES.
Discussion
Unstretched GM, SM and RF muscles held at 37°C for 4.5 h clearly demonstrated reduced initial tenderness and a reduced rate of aging, whereas the ST showed increased initial tenderness. All of the hind leg muscles held at 37°C for 4.5 h also showed substantially reduced water-holding capacity, regardless of stretch treatment. Although it is unlikely that muscles in a lamb carcass would be >35°C for 5 h, (Jacob et al. 2014) clearly showed that the interface between the SM and ST muscles in a beef carcass can be >35°C for 5 h. More importantly, Jacob et al. (2014) showed that at a depth of 10 cm into the SM/ST interface, the temperature can be in the range of 40−42°C for the first 2 h post slaughter, which are known to be extremely damaging denaturing conditions for muscle proteins. There is a high incidence (75%) of high rigor temperature in the longissimus muscles of beef carcasses in Australia (Warner et al. 2014a) and the muscles in the hind leg of a beef carcass are much slower to cool than the longissimus muscle (Jacob et al. 2014). Thus the implications of this study are that beef carcasses classified as high rigor temperature in the longissimus muscle are likely to have reduced tenderness and aging in some leg muscles as well as high water loss and pale colour. Tenderstretch can be used to reliably produce tender meat and both Thompson et al. (2006) and Warner et al. (2014b) have shown that tenderstretching can provide insurance for palatability against processing carcasses at hot rigor temperatures.
The influence of high pre-rigor temperature on meat quality attributes such as tenderness, water-holding capacity and colour has been studied in excised muscle, and almost exclusively on the longissimus muscle. It has been generally agreed that keeping excised pre-rigor longissimus muscles at a high temperature (normally ≥35°C) results in lower tenderness (often called heat-induced toughening), more fluid loss from meat and paler colour (Jaime et al. 1992; Hertzman et al. 1993; Devine et al. 1999, 2002; Geesink et al. 2000; Rosenvold et al. 2008). Warner et al. (2014b) and others have shown the detrimental effects of high rigor temperature on the tenderness, water-holding capacity and colour of beef longissimus and GM muscles from a carcass (rather than excised). But there have been no studies on muscles from a lamb carcass, or on the muscles SM, ST and RF in either lamb or beef carcasses. Positive impacts of high pre-rigor temperature on longissimus muscle tenderness have been also reported (Locker and Daines 1975; Hwang et al. 2004; Bekhit et al. 2007), which we also found for the ST muscle.
It is of interest to note that in the present study, both the positive and negative impacts of high pre-rigor temperature on meat tenderness (based on Warner–Bratzler shear force values) were observed in the different hind leg muscles. The non-stretched GM and SM (–stretch) were influenced by heat-induced toughening as higher shear force values of the muscles from 37°C at 1 day and 8 days post slaughter were observed compared with the muscles held at 2°C. On the other hand, the shear force values of non-stretched RF and ST (–stretch) were actually decreased at 1 day post slaughter by placing carcasses at high pre-rigor temperature. This suggests that different muscles within a carcass may respond differently to high pre-rigor temperature in regards to tenderness. The predominant fibre type in a muscle is thought to determine the ultimate quality of the meat. This is because variations in the cellular enzymes and proteins between fibre types determine the variations in post-mortem biochemistry that can determine the final quality. It is quite difficult to generalise regarding the predominant fibre type in specific muscles because this varies with breed, age, species and the technique used to identify the fibre types. But in general terms, in the sheep carcass, the ST is composed of predominantly white glycolytic fibres Type IIb, and the SM, GM and RF are a mixture of oxidative-glycolytic Type IIa and glycolytic type IIb (White et al. 1978; Suzuki and Tamate 1988; Greenwood et al. 2006), with the predominant percentage varying with location in the muscle (Suzuki and Tamate 1988). It has been reported that the aging rate is faster in glycolytic fibres than in oxidative red fibres (Ouali 1990), which could suggest the importance of the initial aging condition for the glycolytic fibres. This is supported by our data as we showed that the glycolytic ST held at 37°C was more tender than the oxidative-glycolytic SM and GM held at the same temperature. Although, glycolytic fibres are likely to be more susceptible to the protein denaturing condition (such as high temperature and rapid pH decline) (Klont et al. 1998), the SM and GM muscles are also larger and less superficial muscles and thus would chill more slowly than the ST. Therefore, it would be reasonable to speculate that the high pre-rigor temperature might induce more negative effects on the muscles which chill more slowly, exhibiting reduced aging potential by adversely affecting proteolytic enzyme activities.
Moreover, the high pre-rigor temperature condition had no impact on either muscle length or sarcomere length (except RF), while the shear force values of the muscles were either increased (GM and SM) or decreased (RF and ST) by the high pre-rigor temperature condition. Furthermore, in contrast to the different shear force values of the muscles responding differently to the high temperature condition, all the muscles showed decreased water-holding capacity and more protein denaturation under the high temperature condition. This observation suggests that no cold-shortening or heat-shortening occurred in the muscles under the two different pre-rigor temperature conditions (2 and 37°C), and increased shear force values of the GM and SM was neither attributed to heat-shortening nor increased water loss. Kim et al. (2012) also found no influence of high pre-rigor temperature (38°C) on sarcomere length of beef loin (P > 0.05), but did find significantly increased shear force values of the loins. They also reported that the beef loins placed at 38°C had greater protein denaturation, more purge and drip loss, and less myofibrillar protein degradation due to lower µ-calpain activity (based on less autolysis) compared with the loins placed at 15°C. Thus, they concluded that high pre-rigor temperature induces heat-toughening primarily due to protein denaturation with subsequent limitation of proteolysis by µ-calpain. In the present study, we also observed that the muscles from pre-rigor carcasses held at 37°C appeared to have more protein denaturation (indicated by lower sarcoplasmic protein solubility and lower myofibrillar ATPase activity) and more fluid loss (purge and surface exudate).
However, it would appear that the stretch treatment applied overcame the heat-toughening caused by the 37°C treatment that was evident in the non-stretched muscles (GM and SM) as well as the non-heat toughened muscle (ST). This concurs with the findings of Thompson et al. (2005) in lamb and Warner et al. (2014b) in beef that stretching (tenderstretch in each case) overcomes the negative effects of high rigor temperature on tenderness in longissmus muscles. This is highly likely due to the fact that the stretch treatment resulted in substantial increase in sarcomere length of the muscles (except RF) regardless of the pre-rigor temperature conditions (~49% increase at 2°C and 42% increase at 37°C). It is also important to note that the improvement in tenderness during aging was not negatively affected when the stretched muscles were kept at 37°C before rigor. Numerous studies have reported limited aging potential of muscles that were exposed to high pre-rigor temperature (Lee and Ashmore 1985; Hertzman et al. 1993; Dransfield 1994; Devine et al. 1999, 2002; Geesink et al. 2000; Hwang and Thompson 2001; Thomson et al. 2008; Kim et al. 2010, 2012; Rosenvold and Wiklund 2011). Further, some studies have reported a transient effect of stretching muscles – improved tenderness during the early post-mortem period, but no further benefit with aging (Taylor et al. 2012; Toohey et al. 2012). Stretching, particularly super stretching (stretching with force applied, similar to our technique), has also been postulated to cause disassembly of the sarcomeric structure, which negates autolysis of enzymes like calpain (Hopkins et al. 2000). Taken together, it seems that applying stretching to carcasses placed at high temperature can improve meat tenderness by overcoming heat-toughening caused by protein denaturation and limited aging potential, and also possibly by disassembly of the sarcomeric structure. Further, the stretch treatment also resulted in less surface exudate and purge loss of the muscles (GM, SM and ST) compared with the muscles from the non-stretched carcasses placed at 37°C, although the water loss was still greater than that of the muscles at 2°C. The opposite was achieved by the stretch treatment in the RF muscle likely due to its anatomical location in the carcass.
Reduced sarcoplasmic protein solubility and myofibrillar ATPase activity have previously been associated with PSE meat in pork carcasses, which is a phenomenon of high rigor temperature at low pH and is similar to high rigor temperature (Warner et al. 1997). PSE muscles exhibit a pale colour and reduced water-holding capacity, similar to that seen with our 37°C treatment. Our data shows that in stretched muscles, where the actin-myosin overlap would have been minimal, the myosin denaturation was more extensive, as measured by the myofibrillar ATPase activity. This agrees with the theory of Offer (1991) that exposed myosin is more susceptible to denaturation than myosin bound to actin. Thus stretching induced myosin denaturation but still resulted in beneficial impacts on water-holding capacity and shear force.
It is interesting that there were few effects of ES on quality traits, particularly interesting was the lack of effect on shear force. There was no doubt that the ES treatment was effective, as indicated by the accelerated pH decline in the longissimus muscle and the higher temp@pH6 with ES. Jacob et al. (2008) also reported a lack of effect on the shear force of longissimus lamb muscles in response to ES. Many studies report positive effects of ES on the shear force of the longissimus muscle, through the prevention of cold-shortening. In our study, the longissimus muscles of unstimulated carcasses chilled at 2°C were in the cold-shortening region but it is unlikely the GM, SM, ST and RF were in the cold-shortening region as these are larger muscles which chill more slowly.
Conclusion
In conclusion, the stretch treatment was successful in achieving a significant lengthening of the muscle and sarcomeres in the GM, SM and ST muscles, and subsequently resulted in substantial increase in tenderness at 1 day post mortem and throughout the whole storage period. Muscles exposed to high temperature conditions in the sheep carcass demonstrated excessive water loss, high levels of muscle protein denaturation and variable effects on tenderisation in different muscles in the hind leg. The implications of this study are that beef carcasses classified as high rigor temperature in the longissimus are likely to have variable tenderness and aging in hind leg muscles as well as high water loss and pale colour. The negative effects of high rigor temperature can be alleviated by stretching.
Acknowledgements
The funding provided by Meat and Livestock Australia and the Department of Primary Industries Victoria is gratefully acknowledged. We are also grateful for the assistance with experimental conduct and laboratory assays provided by Melissa Rees.
References
Barbut S, Sosnicki AA, Lonergan SM, Knapp T, Ciobanu DC, Gatcliffe LJ, Huff-Lonergan E, Wilson EW (2008) Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Science 79, 46–63.| Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitlGmsLo%3D&md5=271fb5f91ea9de2c0e92c86a970471c1CAS | 22062597PubMed |
Bekhit AED, Farouk MM, Cassidy L, Gilbert KV (2007) Effects of rigor temperature and electrical stimulation on venison quality. Meat Science 75, 564–574.
| Effects of rigor temperature and electrical stimulation on venison quality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbns1amtg%3D%3D&md5=1c089d2e2c53750300287af5f86e53f0CAS |
Bouton PE, Harris PV, Shorthose R (1971) Effect of ultimate pH upon the water-holding capacity and tenderness of mutton. Journal of Food Science 36, 435–439.
| Effect of ultimate pH upon the water-holding capacity and tenderness of mutton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3MXhtl2ltr0%3D&md5=ce77347a08f7de8998a7f6fcc61ee76eCAS |
Devine CE, Wahlgren NM, Tornberg E (1999) Effect of rigor temperature on muscle shortening and tenderisation of restrained and unrestrained beef M. longissimus thoracicus et lumborum. Meat Science 51, 61–72.
| Effect of rigor temperature on muscle shortening and tenderisation of restrained and unrestrained beef M. longissimus thoracicus et lumborum.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVWqtA%3D%3D&md5=0fa670a2c0be3f3e48dbef00926751beCAS | 22061537PubMed |
Devine CE, Payne SR, Peachey BM, Lowe TE, Ingram JR, Cook CJ (2002) High and low rigor temperature effects on sheep meat tenderness and ageing. Meat Science 60, 141–146.
| High and low rigor temperature effects on sheep meat tenderness and ageing.Crossref | GoogleScholarGoogle Scholar | 22063237PubMed |
Dransfield E (1994) Modelling post-mortem tenderisation – V: inactivation of calpains. Meat Science 37, 391–409.
| Modelling post-mortem tenderisation – V: inactivation of calpains.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmt12htrg%3D&md5=0f7a088f9aab1db34f9490fe94eb77d9CAS |
Geesink GH, Bekhit AD, Bickerstaffe R (2000) Rigor temperature and meat quality characteristics of lamb longissimus muscle. Journal of Animal Science 78, 2842–2848.
Genstat Committee (2008) ‘Genstat for Windows.’ (VSN International Ltd: Hertfordshire, UK)
Greenwood PL, Gardner GE, Hegarty RS (2006) Lamb myofibre characteristics are influenced by sire estimated breeding values and pastoral nutritional system. Australian Journal of Agricultural Research 57, 627–639.
| Lamb myofibre characteristics are influenced by sire estimated breeding values and pastoral nutritional system.Crossref | GoogleScholarGoogle Scholar |
Hertzman C, Olsson U, Tornberg E (1993) The influence of high temperature, type of muscle and electrical stimulation on the course of rigor, ageing and tenderness of beef muscles. Meat Science 35, 119–141.
| The influence of high temperature, type of muscle and electrical stimulation on the course of rigor, ageing and tenderness of beef muscles.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbntlKjsQ%3D%3D&md5=6f3ca62e08b17e48f991ff29bf19a844CAS | 22060841PubMed |
Hopkins DL, Thompson JM (2001) The relationship between tenderness, proteolysis, muscle contraction and dissociation of actomyosin. Meat Science 57, 1–12.
| The relationship between tenderness, proteolysis, muscle contraction and dissociation of actomyosin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmslKltrg%3D&md5=44ece769c509c2ec60743751ec9ea15dCAS | 22061160PubMed |
Hopkins DL, Garlick PR, Thompson JM (2000) The effect on the sarcomere structure of super tenderstretching. Asian-Australasian Journal of Animal Sciences 13, 233
Hwang IH, Thompson JM (2001) The interaction between pH and temperature decline early postmortem on the calpain system and objective tenderness in electrically stimulated beef longissimus dorsi muscle. Meat Science 58, 167–174.
| The interaction between pH and temperature decline early postmortem on the calpain system and objective tenderness in electrically stimulated beef longissimus dorsi muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1Wgurc%3D&md5=40939faba202cd6c32db4d2d60e5d1ecCAS | 22062112PubMed |
Hwang IH, Park BY, Cho SH, Lee JM (2004) Effects of muscle shortening and proteolysis on Warner-Bratzler shear force in beef longissimus and semitendinosus. Meat Science 68, 497–505.
| Effects of muscle shortening and proteolysis on Warner-Bratzler shear force in beef longissimus and semitendinosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsVSmu7o%3D&md5=47c3afc94e61d28200f7ed6c2a3e3db9CAS | 22062419PubMed |
Jacob RH, Hopkins DL (2014) Techniques to reduce the temperature of beef muscle early in the post mortem period – a review. Animal Production Science 54, 482–493.
| Techniques to reduce the temperature of beef muscle early in the post mortem period – a review.Crossref | GoogleScholarGoogle Scholar |
Jacob RH, Pearce KL, Smith N (2008) Validating the benefits of a medium voltage electrical stimulation unit at an abattoir in Western Australia. Australian Journal of Experimental Agriculture 48, 898–903.
| Validating the benefits of a medium voltage electrical stimulation unit at an abattoir in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Jacob RH, Beatty DT, Warner RD (2014) A preliminary study into the use of ‘heat pipes’ to prevent high rigor temperature in beef carcasses by increasing cooling rate. Animal Production Science 54, 504–509.
| A preliminary study into the use of ‘heat pipes’ to prevent high rigor temperature in beef carcasses by increasing cooling rate.Crossref | GoogleScholarGoogle Scholar |
Jaime I, Beltrán JA, Ceña P, López-Lorenzo P, Roncalés P (1992) Tenderisation of lamb meat: effect of rapid postmortem temperature drop on muscle conditioning and aging. Meat Science 32, 357–366.
| Tenderisation of lamb meat: effect of rapid postmortem temperature drop on muscle conditioning and aging.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbntlSqtQ%3D%3D&md5=89c3c4e2c797083706a0d3bba594d11dCAS | 22059887PubMed |
Kauffman RG, Eikelenboom G, Van der Wal PG (1986) The use of filter paper to estimate drip loss of porcine musculature. Meat Science 18, 191–200.
| The use of filter paper to estimate drip loss of porcine musculature.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFahsw%3D%3D&md5=f5476b35b84a1c8a6fa9cf4c97c3c4c4CAS | 22055647PubMed |
Kim YH, Lonergan SM, Huff-Lonergan E (2010) Protein denaturing conditions in beef deep semimembranosus muscle results in limited µ-calpain activation and protein degradation. Meat Science 86, 883–887.
| Protein denaturing conditions in beef deep semimembranosus muscle results in limited µ-calpain activation and protein degradation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFCrurnE&md5=4becf077926d7fe23677f72d367918a4CAS | 20674187PubMed |
Kim YHB, Stuart A, Nygaard G, Rosenvold K (2012) High pre rigor temperature limits the ageing potential of beef that is not completely overcome by electrical stimulation and muscle restraining. Meat Science 91, 62–68.
| High pre rigor temperature limits the ageing potential of beef that is not completely overcome by electrical stimulation and muscle restraining.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtVCmsLg%3D&md5=1f902cbe6afe74ff48f6d5b2ac116177CAS |
Kim YHB, Kerr M, Geesink G, Warner RD (2014) Impacts of hanging method and high pre-rigor temperature and duration on quality attributes of ovine muscles. Animal Production Science 54, 414–421.
| Impacts of hanging method and high pre-rigor temperature and duration on quality attributes of ovine muscles.Crossref | GoogleScholarGoogle Scholar |
Klont RE, Brocks L, Eikelenboom G (1998) Muscle fibre type and meat quality. Meat Science 49, S219–S229.
| Muscle fibre type and meat quality.Crossref | GoogleScholarGoogle Scholar |
Lee YB, Ashmore CR (1985) Effect of early postmortem temperature on beef tenderness. Journal of Animal Science 60, 1588–1596.
Locker RH, Daines GJ (1975) Rigor mortis in beef sternomandibularis muscle at 37°C. Journal of the Science of Food and Agriculture 26, 1721–1733.
| Rigor mortis in beef sternomandibularis muscle at 37°C.Crossref | GoogleScholarGoogle Scholar |
Matthews K (2011) Evaluation of the pH fall in English lamb abattoirs against the Meat Standards Australia pH/temperature window, Report to EBLEX. Available at http://www.eblex.org.uk/wp/wp-content/uploads/2013/04/evaluationofthephfallinenglishlambabattoirs_271011-final-report.pdf [Verified 8 February 2014]
Offer G (1991) Modelling of the formation of Pale, Soft and Exudative meat effects of chilling regime and rate and extent of glycolysis. Meat Science 30, 157–184.
| Modelling of the formation of Pale, Soft and Exudative meat effects of chilling regime and rate and extent of glycolysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmslygu7Y%3D&md5=ab78c2132e3b69fa280d2bb8d1e81744CAS | 22061833PubMed |
Ouali A (1990) Meat tenderization: possible causes and mechanisms. A review. Journal of Muscle Foods 1, 129–165.
| Meat tenderization: possible causes and mechanisms. A review.Crossref | GoogleScholarGoogle Scholar |
Pearce KL, Van De Ven R, Mudford CR, Warner RD, Hocking-Edwards JE, Pethick DW, Hopkins DL (2010) Case studies demonstrating the optimisation of medium voltage electrical stimulation of lamb carcases. Animal Production Science 50, 1107–1114.
| Case studies demonstrating the optimisation of medium voltage electrical stimulation of lamb carcases.Crossref | GoogleScholarGoogle Scholar |
Rosenvold K, Wiklund E (2011) Retail colour display life of chilled lamb as affected by processing conditions and storage temperature. Meat Science 88, 354–360.
| Retail colour display life of chilled lamb as affected by processing conditions and storage temperature.Crossref | GoogleScholarGoogle Scholar | 21316158PubMed |
Rosenvold K, North M, Devine C, Micklander E, Hansen P, Dobbie P, Wells R (2008) The protective effect of electrical stimulation and wrapping on beef tenderness at high pre rigor temperatures. Meat Science 79, 299–306.
| The protective effect of electrical stimulation and wrapping on beef tenderness at high pre rigor temperatures.Crossref | GoogleScholarGoogle Scholar | 22062758PubMed |
Ruddick JE, Richards JF (1975) Comparison of sarcomere length measurement of cooked chicken pectoralis muscle by laser diffraction and oil immersion microscopy. Journal of Food Science 40, 500–501.
| Comparison of sarcomere length measurement of cooked chicken pectoralis muscle by laser diffraction and oil immersion microscopy.Crossref | GoogleScholarGoogle Scholar |
Suzuki A, Tamate H (1988) Distribution of myofiber types in the hip and thigh musculature of sheep. The Anatomical Record 221, 494–502.
| Distribution of myofiber types in the hip and thigh musculature of sheep.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c3mslGjtw%3D%3D&md5=8652f7183c47fb841df8e1d719c133ccCAS | 2968770PubMed |
Taylor J, Toohey ES, van de Ven R, Hopkins DL (2012) SmartStretch technology. IV. The impact on the meat quality of hot-boned beef rostbiff (m. gluteus medius). Meat Science 91, 527–532.
| SmartStretch technology. IV. The impact on the meat quality of hot-boned beef rostbiff (m. gluteus medius).Crossref | GoogleScholarGoogle Scholar | 22503482PubMed |
Thompson JM, Hopkins DL, D’Souza DN, Walker PJ, Baud SR, Pethick DW (2005) The impact of processing on sensory and objective measurements of sheep meat eating quality. Australian Journal of Experimental Agriculture 45, 561–573.
| The impact of processing on sensory and objective measurements of sheep meat eating quality.Crossref | GoogleScholarGoogle Scholar |
Thompson JM, Perry D, Daly B, Gardner GE, Johnston DJ, Pethick DW (2006) Genetic and environmental effects on the muscle structure response post-mortem. Meat Science 74, 59–65.
| Genetic and environmental effects on the muscle structure response post-mortem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xms1GrsbY%3D&md5=3d30dde2c61a1d825d8a38f9663b2007CAS | 22062716PubMed |
Thomson KL, Gardner GE, Simmons N, Thompson JM (2008) Length of exposure to high post-rigor temperatures affects the tenderisation of the beef M. longissmus dorsi. Australian Journal of Experimental Agriculture 48, 1442–1450.
| Length of exposure to high post-rigor temperatures affects the tenderisation of the beef M. longissmus dorsi.Crossref | GoogleScholarGoogle Scholar |
Toohey ES, de Ven RV, Thompson JM, Geesink GH, Hopkins DL (2012) SmartStretch Technology: V. The impact of SmartStretch technology on beef topsides (m. semimembranosus) meat quality traits under commercial processing conditions. Meat Science 92, 24–29.
| SmartStretch Technology: V. The impact of SmartStretch technology on beef topsides (m. semimembranosus) meat quality traits under commercial processing conditions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38rotl2lug%3D%3D&md5=dac20dadf8c226c686c4b62c6f4de867CAS | 22537469PubMed |
Warner RD, Kauffman RG, Greaser ML (1997) Muscle protein changes post mortem in relation to pork quality traits. Meat Science 45, 339–352.
| Muscle protein changes post mortem in relation to pork quality traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXisFyrtrY%3D&md5=7a2e794968870801cd62ddf945b9145dCAS | 22061472PubMed |
Warner RD, Dunshea FR, Gutzke D, Lau J, Kearney G (2014a) Factors influencing the incidence of high rigor temperature in beef carcasses in Australia. Animal Production Science 54, 363–374.
| Factors influencing the incidence of high rigor temperature in beef carcasses in Australia.Crossref | GoogleScholarGoogle Scholar |
Warner RD, Thompson JM, Polkinghorne R, Gutzke D, Kearney GA (2014b) A consumer sensory study of the influence of rigor temperature on eating quality and ageing potential of beef striploin and rump. Animal Production Science 54, 396–406.
| A consumer sensory study of the influence of rigor temperature on eating quality and ageing potential of beef striploin and rump.Crossref | GoogleScholarGoogle Scholar |
White NA, McGavin MD, Smith JE (1978) Age-related changes in percentage of fiber types and mean fiber diameters of the ovine quadriceps muscle. American Journal of Veterinary Research 39, 1297–1302.