Changes in blood parameters and electroencephalogram of cattle as affected by different stunning and slaughter methods in cattle
I. Zulkifli A B E , Y. M. Goh B , B. Norbaiyah A , A. Q. Sazili A , M. Lotfi C , A. F. Soleimani B and A. H. Small DA Institute of Halal Products Research, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia.
B Institute of Tropical Agriculture, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia.
C AHFS Australian Halal Food Services, Highpoint Business Centre – Unit 26, PO Box 775, Springwood, Qld 4127, Australia.
D CSIRO FD McMaster Research Laboratory, Chiswick, New England Highway, Armidale, NSW 2350, Australia.
E Corresponding author. Email: zulkifli@agri.upm.edu.my
Animal Production Science 54(2) 187-193 https://doi.org/10.1071/AN12128
Submitted: 12 April 2012 Accepted: 24 January 2013 Published: 17 April 2013
Abstract
The present study aimed to provide a comparative analysis of the effects of penetrative stunning, non-penetrative stunning and post-slaughter stunning on biochemical parameters and electroencephalogram (EEG) associated with stress in heifers and steers. Ten animals were assigned to each of the following four treatment groups: (1) animals were subjected to conventional halal slaughter (a clean incision through the structures on the ventral neck at the approximate level of vertebrae C2–C3 – the trachea, oesophagus, carotid arteries and jugular veins) and post-cut penetrating mechanical stun within 10–20 s of the halal cut (U); (2) high-power non-penetrating mechanical stunning using a mushroom-headed humane killer, followed by conventional halal slaughter (HPNP); (3) low-power non-penetrating mechanical percussive stunning using a mushroom-headed humane killer, followed by conventional halal slaughter (LPNP); and (4) penetrative stunning using a captive-bolt pistol humane killer, followed by conventional halal slaughter (P). For each animal, blood samples and electroencephalogram recordings were taken before stunning, post-stunning (if applicable) and post-slaughter, and plasma concentrations of cortisol, adrenocorticotrophic hormone (ACTH), adrenaline, noradrenaline and β-endorphin were determined. Irrespective of the stunning method, except for percentage change in plasma concentrations of noradrenaline, the values of blood parameters attained before and after stunning were not significantly different. The plasma noradrenaline concentration of the HPNP animals was significantly elevated following stunning. Following slaughter, the percentage change of plasma ACTH concentration in the P animals was significantly elevated. Neither stunning method nor sampling time had a significant effect on plasma β-endorphin concentration. On the basis of the EEG results, penetrative stunning seemed to be better in maximising the possibility of post-stunning insensibility, whereas U animals appeared to demonstrate an evident increase in EEG activity which is consistent with the presence of post-slaughter noxious stimuli associated with tissue cut and injury. The U animals had consistently higher, if not the highest, RMS values than did other stunned animals. This indicates a degree of EEG changes associated with stress and pain. On the basis of EEG data, our results suggested that penetrative stunning would be the most reliable method of ensuring insensibility and minimising pain. However, at slaughter, the P animals showed a dramatic elevation in the percentage change of circulating ACTH, suggesting physiological stress response. On a cautionary note, the results are not unequivocal, and it may be that the range of analyses available to researchers at this point of time are not sufficiently specific to allow definitive conclusions to be drawn.
Introduction
The manner in which livestock are immobilised, slaughtered and exsanguinated can affect their welfare and final meat quality. Legal, moral and ethical requirements dictate that animals should be insensible to noxious, potentially painful, stimuli during slaughter. According to Gregory and Shaw (2000), when stunning is performed correctly, the animal feels no pain and becomes instantly unconscious. In Australia, cattle are stunned before slaughter using penetrative captive bolt, non-penetrative (percussive) captive bolt, or electrical methods. Electrical stunning in cattle, however, has been associated with blood speckle and blood splash in the carcass (Gregory 2007). With mechanical stunning, the intent is to cause concussion with or without penetration.
Non-penetrating captive-bolt stunners may or may not fracture the skull. According to Grandin (2009), non-penetrating captive bolt that does not fracture the skull could be less effective than a stunner that does fracture the skull, and effectiveness increases as the degree of skull fracturing increases. Head injuries caused by non-penetrating stunning that fractures the skull can be severe. The impact of the heavy mushroom head against the relatively thin frontal bone, which forms the roof of the cranium in cattle, can result in a severe, well circumscribed, depressed fracture of the skull, with a subarachnoid haemorrhage in the subadjacent brain (Finnie 1995). In some countries, non-penetrative percussive stunning is disallowed because of a risk that insufficient power could result in an ineffective stun and, hence, compromise animal welfare (Grandin and Smith 2004). The prevalence of error in performing non-penetrative percussive stunning is a major welfare concern; however, when carried out correctly, and with appropriate power for the class of animal, non-penetrative stunning can produce an insensible animal.
Pre-slaughter stunning is practised to render animals insensible to potential pain and distress of slaughter. Cutting of the throat stimulates nociceotors located in the neck tissue that cause a barrage of impulses to travel to the brain (Mellor et al. 2009). Previous work reported by Gibson et al. (2009a, 2009b, 2009c, 2009d) clearly illustrated the benefits of stunning in ameliorating the noxious stimuli associated with throat cut, on the basis of electroencephalogram (EEG) measurements in calves. However, interpretation of EEG changes for pain and stress evaluation should not be carried out without inferring to other concurrent measurements. Blackmore and Newhook (1982) proposed a window of sensibility, indicated by EEG recordings between 10 and 35 µV. However, this may not be applicable under all stunning conditions, as shown by Devine et al. (1986). Furthermore, newer EEG analytical techniques suggest that the determination of the window of sensibility in cattle may be more complex than anticipated. For instance, periods following non-penetrative stunning often result in a rapid and spiking barrage of transitional EEG, which is not necessarily an indicator of pain perception in animals (Gibson et al. 2009c). It was, therefore, the aim of the current work to corroborate and to relate changes between EEG and plasma hormones to arrive at a more objective assessment of pain and stress in animals subjected to stunning and slaughter.
The concentrations of cortisol, adrenocorticotrophic hormone (ACTH), adrenaline, noradrenaline and β-endorphin in plasma are commonly used to measure the stress response to pre-slaughter and slaughter treatments in animals (Warriss 2010). Although the main purpose of stunning is to eliminate animal suffering during slaughter, increases in plasma cortisol, cathecolamines and β-endorphin concentrations have been reported in horses subjected to captive-bolt stunning (Micera et al. 2010a). These authors, however, concluded that the increase in circulating hormones could be attributed to pre-slaughter handling. In another study, Micera et al. (2010b) reported that captive-bolt stunning did not influence concentrations of adrenaline, noradrenaline, cortisol and β-endorphin in plasma in Limousine steers. The aim of the present study was to compare changes in blood parameters and EEG in cattle subjected to different mechanical stunning methods, including high-power non-penetrating mechanical stun (HPNP), low-power non-penetrating stun (LPNP), penetrative mechanical stunning (P) and unstunned slaughter, followed by penetrative mechanical stun (U) in cattle.
Materials and methods
Animals
The work was conducted during the period of July and August 2009 in Queensland (outdoor temperature 15–20°C). The cattle processed were heifers and steers, of liveweights between 268 and 635 kg (mean = 446 kg, s.d. = 67.33), resulting in hot carcass weights of 138–326 kg (mean = 233 kg, s.d. = 35.19). They were sourced from one of two feedlots, either 50 km or 160 km from the abattoir, and had been held in feedlot pens at the abattoir for up to 12–36 h before slaughter. They were Bos taurus × Bos indicus crossbreds, and were representative of the normal class of animals slaughtered at the abattoir for the Halal market.
The animals were handled using the emergency slaughter area at the abattoir for the following two main reasons: (1) it was not feasible to collect blood samples from animals in the stun box used regularly by the abattoir, because it is a fully enclosed box, with no access to personnel; (2) the regular stun box, being fully enclosed, does not allow access to the neck, so as to carry out unstunned slaughter (U). The lairage design was such that animals taken from the holding pen could either be placed in the crowd pen, and then enter the single file race to the regular stun box, or be placed into a crowd pen, leading to a short race into the emergency slaughter crush. The crush was fitted with a neck yoke and head restraint that lifted the head extending the neck for exsanguination. Thus, the degree of handling experienced by the trial animals would not have been greatly different from that experienced by animals slaughtered under normal conditions.
Experimental procedure
In total, 40 steers were randomly assigned to one of the following four treatments:
-
conventional Halal slaughter (a clean incision through the structures at the front of the neck – the trachea, oesophagus, carotid arteries and jugular veins) and post-cut penetrating mechanical stun within 10–20 s of the Halal cut (after the post-sticking blood sample had been taken). The post-cut stun was applied to satisfy the requirements of the Animal Ethics Approval obtained (CSIRO A7/08) (U);
-
high-power non-penetrating mechanical stunning using a mushroom-headed humane killer (Cash Magnum Knocker Concussion Stunner, 0.25-calibre, 4-grain cartridge; Accles and Shelvoke, Sutton Coldfield, UK), followed by a conventional Halal slaughter (HPNP);
-
low-power non-penetrating mechanical percussive stunning using a mushroom-headed humane killer (Cash Magnum Knocker Concussion Stunner, 0.25-calibre, 3-grain cartridge), followed by a conventional Halal slaughter (LPNP); and
-
penetrative stunning using a captive-bolt pistol humane killer (Cash 8000 Model Stunner, 0.22-calibre, 4.5-grain cartridge), followed by a conventional Halal slaughter (P).
The experiment was carried out over three slaughter dates. On the first two slaughter dates, three animals of each treatment were processed during a period of 90 min; on the third date, four animals of each treatment were processed during a period of 2 h. The treatment order was randomly drawn on the day before processing, and the animals were allowed to enter the crush as they came up.
Blood parameters
All animals were transported for 5 min by a truck from the holding yard to the lairage. The animals arrived at the lairage between 2 and 4 h before blood sampling. The range in lairage time for a single group of animals was artificially extended in this trial, because animals were processed sequentially and time was taken between each animal so as to ensure that all measurements were taken before the carcass was delivered to the processing line. Blood collection was carried out in the stunning box and the distance between the box and lairage was ~50 m. For each animal, before slaughter, a baseline blood sample (10 mL) (T1) was taken from the coccygeal vein with an 18-G needle and EDTA tubes (Vacutainer Brand, Becton Dickinson, Sydney, NSW, Australia). During the slaughter process, further blood samples were taken immediately following each action carried out, including stunning (from coccygeal vein) (T2), and the transverse section of the neck (from blood flow) (T3). Thus, from the U animals, only two blood samples were collected, namely, baseline (T1) and post-transverse section of the neck (T3); from the HPNP, LPNP and P animals, three blood samples were collected, including baseline (T1), post-stun (T2) and post-transverse section of the neck (T3). Once blood was collected, sample tubes were kept at 4°C and centrifuged at 1260g for 15 min within the first hour after sampling. The recovered plasma fraction was divided into aliquots and stored at −80°C until analysis. Concentrations of ACTH, cortisol, adrenaline and noradrenaline in plasma were determined using the appropriate EIA kits supplied by IBL Hamburg, Germany. The concentration of β-endorphin in plasma was determined using an EIA kit supplied by Phoenix Pharmaceuticals Inc., Burlingam, CA, USA. All measurements were carried out in duplicate.
Electroencephalogram recordings
Electroencephalogram activity at baseline (T1), immediately post-stunning (T2) and 30 s post-slaughter (T3) was recorded telemetrically with PowerLab Biopotential Recordings systems (ADInstruments, Bella Vista, NSW, Australia). On entry to the crush, the animal was allowed to relax for a few seconds, the baseline blood sample was taken and then low-impedance surface electrodes (<5 kOhm; Red Dot, 3M 2248-50; Neuss, Germany) were placed 6–8 cm distally from the poll, at an equal distance from anterior orbital prominences of both the left and right eyes, and on the left base of the poll. The animal was allowed to stand for up to 30 s while a clear telemetry signal was verified, before any further procedure was carried out. The EEG recordings were acquired within a band-pass signal range between 0.1 to 200 Hz, at the sampling rate of 1 kHz. These signals were then analysed offline by using the Chart 5.0 software (ADInstruments). Video recordings with a time stamp synchronised to the EEG recording were also made for each experimental animal, so as to enable movement and other artefacts to be ruled out from EEG traces (Devine et al. 1986). Prior to EEG analysis, the raw EEG recordings were resampled at 1024 Hz and only frequencies between 0.1 to 30 Hz were obtained to minimise the presence of artefacts. Possible interferences from concurrent electrocardiography signals were digitally removed from the raw EEG recordings by using the Chart 5.0 software (ADInstruments) before analysis. Signals were then processed in blocks of 1-s epochs, yielding 60 epochs per minute. The signal was then filtered into band-pass filters to yield delta (0.1–4 Hz), theta (4.1–8 Hz), alpha (8.1–12 Hz) and beta (12.1–20 Hz) waves. The Chart Spectral Analysis Function (Chart 5.0 software, ADInstruments) was used to analyse each frequency component. Briefly, the signals were subjected to fast fourier transformation (FFT), and power-density curves for each frequency band were derived on the basis of cosine bell distribution. The median frequency (F50), or the frequency below which 50% of the total EEG power lies, was derived; F50 has been previously associated with arousal and nociception in horses, dogs and pigs, as reviewed by Murrell and Johnson (2006). The root mean square (RMS) for each wave form at T1, T2 and T3 was calculated. An average of 10 serial epochs with minimal interference was sampled to arrive at the mean values for T1, T2 and T3. The RMS values for the lowest or terminal values of alpha, beta, delta and theta waves were also determined. This point corresponded with removal of the electrodes at the absence of corneal reflex. The time taken from the point of slaughter to the point of attainment of the terminal RMS value was recorded in seconds. These datasets were then compared across the treatment groups and across T1, T2 and T3, to determine the effects of low-power, high-power and penetrative stunning versus the unstunned animals.
Statistical analysis
All hormone and β-endorphin data are expressed as a percentage of T1 values (Lacroix and Hontela 2006). All analyses were conducted using the general linear models procedure of SAS (SAS Institute 2003). When significant effects were found, comparison among multiple means was made by Duncan’s multiple-range test. Data were analysed, with sampling time and stunning method as main effects. Statistical significance is considered as P < 0.05 throughout the paper.
Results
Results of blood parameters are presented in Table 1 as percentages of T1. Among the groups subjected to stunning (HPNP, LPNP and P), stunning method had no significant effect on the percentage change in plasma concentrations of cortisol, ACTH, adrenaline, noradrenaline and β-endorphin. Irrespective of the stunning method, except for mean percentage changes in plasma noradrenaline concentration, the values of blood parameters attained at T1 and T2 were not significantly different. Within the HPNP animals, the percentage change in the plasma concentration of noradrenaline was significantly higher at T2. Comparison among HPNP, LPNP, P and U at T3 showed that stunning method had no significant effect on any of the blood parameters. However, the percentage changes in plasma ACTH concentration of P animals were significantly higher at T3 than at T1 and T2.
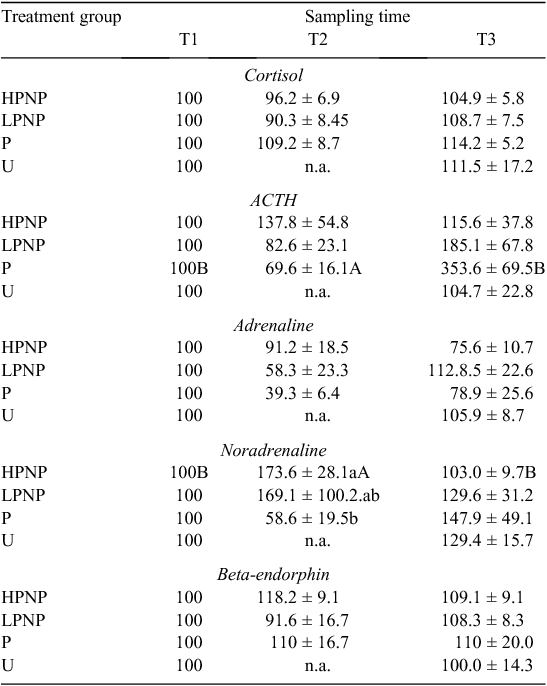
|
Electroencephalogram activities of different treatment groups are presented in Tables 2 and 3. The U animals showed a greater increase in the levels of alpha- and beta-wave activities post-slaughter at T3, than they did at T1. The RMS for alpha waves among U animals increased by almost three-fold, from 11.77 ± 2.70 µV to 31.44 ± 8.94 µV, within 30 s post-slaughter. Whereas the RMS value for beta waves doubled within 30 s after the initial throat cut. The EEG median frequency for the animals in U group also showed a significant (P < 0.05) post-slaughter increase. In contrast, none of the stunned animals (P, LPNP and HPNP) experienced a significant elevation in brain electrical activity. Animals that were stunned using the penetrative method consistently showed lower alpha-, beta- and theta-wave activities. In fact, the values of EEG median frequency were significantly lower in all stunned animals, than they were in U animals at T2 and T3. As for the P animals, the beta wave form post-stunning (T2) and post-slaughter (T3) were suppressed greatly when compared to the other groups.
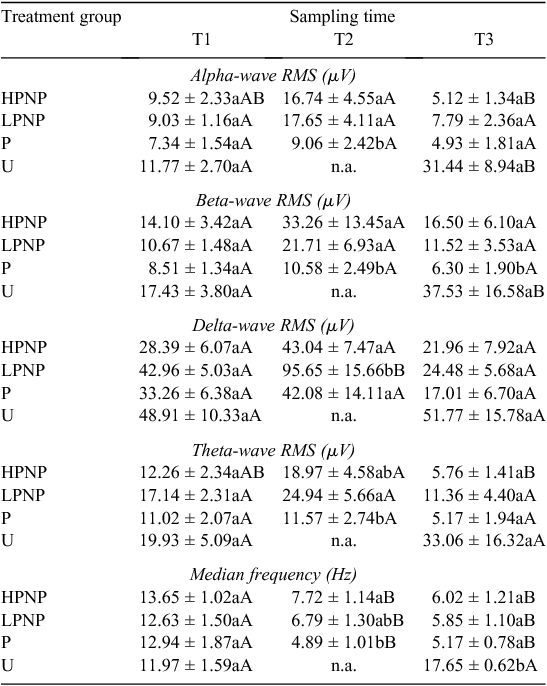
|
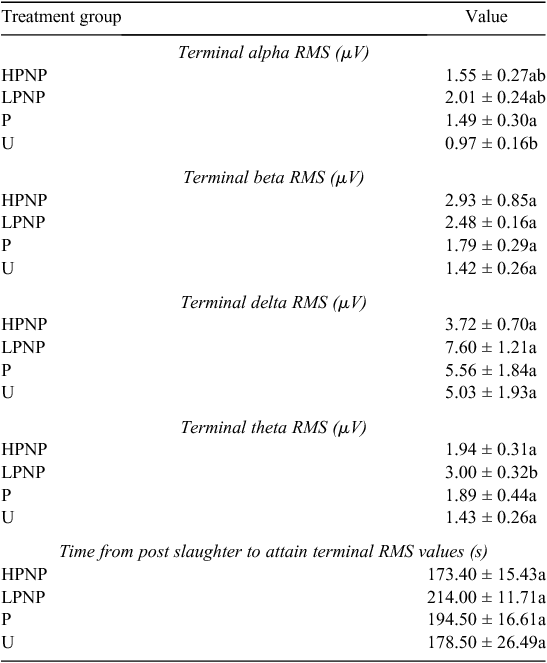
|
The time from post-slaughter to the attainment of terminal RMS values, or terminal time, for all wave forms was used in conjunction with the absence of vital signs such as corneal reflex, to determine the point of cessation of brain electrical activity. The results revealed that the terminal time was not significantly different across treatment groups (Table 3), indicating that stunning method was not a significant contributor to shortening terminal time, or cessation of all visible vital signs and reflexes.
Discussion
When discussing the results of the present experiment, it should be remembered that different methods of stunning, species and the time frame of action may have different physiological effects that could complicate the assessment of stress-related hormones. One of the main objectives of the present study was to compare the effects of various stunning methods as indicated by both hormonal assay and EEG recording. There are no previous studies in cattle that have compared hormonal changes following non-penetrative and penetrative mechanical stunning. Pain caused by neck or throat cut has been the subject of debate. It has been suggested that the use of a very sharp knife produces little behavioural reaction in non-stunned cattle and, hence, such a neck cut is not perceived by the animal as painful (Grandin and Smith 2004). On the basis of hormonal data, there is little indication that U treatment was more stressful than LPNP or HPNP treatments at T3 (post-neck cut). Stunning within 5 s after throat cut would be expected to render the animals insensible to further pain and distress (Gibson et al. 2009d).
Shaw and Tume (1992) indicated that mechanical stunning elevates concentrations of circulating cathecolamines in cattle. In the present study, the percentage change in the concentration of plasma noradrenaline, but not adrenaline, in the HPNP animals was higher at T2 than at T1. Adrenaline is known to be more affected by psychological stress, whereas noradrenaline is commonly associated with physical disturbances (Broom and Johnson 1993). The noted increase in the concentration of plasma noradrenaline may suggest that the mechanical stunning procedure itself could be physiologically stressful to animals (Micera et al. 2010a). However, changes in the concentrations of hormones may also be attributed to pre-slaughter stress, rather than to the method of stunning and/or slaughter.
The concentrations of circulating ACTH and cortisol were not significantly different between T1 and T2. There is a possibility that maximum secretions of ACTH and cortisol were reached before stunning in the present study. Elevation in the concentrations of circulating ACTH and cortisol before stunning could be attributed to distractions that impede forward movement of animals from lairage to stun box, noise, prolonged head restraint or the animals not being accustomed to contact with humans (Grandin 1996).
On the basis of the noradrenaline data at T1 and T2, it appears that HPNP treatment was more stressful to cattle than were P and LPNP treatments. Increase in cathecolamines indicated stimulation of the adrenal medulla and suggested that animals were experiencing some emotional or physical distress and presumably damage to tissues (Mellor et al. 2002; Nowak et al. 2007). Working with lambs, Finnie et al. (2000) demonstrated that, generally, both penetrative and non-penetrative percussive stunning can result in similar structural brain damage. However, these authors reported that while focal injury was more severe in penetrative percussive stunning, non-penetrative percussive stunning caused a more widely distributed damage. Grandin and Smith (2004) suggested that penetrative captive-bolt stunning was more effective and the likelihood of error was lower than for the non-penetrative method. The percentage change in circulating noradrenaline at T1 and T2 also suggested that the power of the non-penetrative mechanical stunning is crucial in determining the magnitude of physiological stress experienced following stunning.
Wide variation in individual noradrenaline concentrations in LPNP animals at T2 suggested that there is considerable variation either in the level of stress imposed on individual animals or in their response to stressors, or both. Variation in response to stressors could be associated with genetics, social status and prior experience (Sapolsky 1992; Zulkifli and Siegel 1995).
The concentration of plasma β-endorphin has been used as an index of physiological stress associated with pre-slaughter and slaughter of livestock (Anil et al. 1990; Shaw and Tume 1992; Micera et al. 2010a). Beta-endorphin is an endogenous opioid released primarily from the adenohypophysis. Beta-endorphin is involved in modulation of pain (Shaw and Tume 1992). In the present study, although HPNP treatment, as measured by cathecolamines, was physiologically stressful to the animals, neither sampling time nor stunning method had a significant effect on the concentration of plasma β-endorphin. Similarly, Micera et al. (2010a) noted no significant increase in the concentration of β-endorphin following captive-bolt stunning in horses. In contrast, Anil et al. (1990) reported a two-fold increase in β-endorphin concentration following electrical stunning in sheep. These authors, however, suggested that the release of β-endorphin could be a direct result of the electrical current.
Pre-slaughter stunning is carried out to rapidly render the animals insensible to the pain of slaughter. Examination of changes in the EEG as well as the hormonal response may provide important clues into the extent of pain and stress (Gibson et al. 2009b) experienced by the animals. The present findings suggested that alpha and beta waves spiked rapidly post-stunning, but declined gradually to their respective terminal values among the HPNP and LPNP animals. The present results are consistent with the concentrations of circulating noradrenaline, especially among the HPNP animals. The appearance of high-amplitude, slow-frequency waves in theta- and delta-frequency bands was noted in all stunned animals post-stunning. This led to the conclusion that stunning rendered the animal unconscious, and therefore less likely to perceive noxious stimuli than were the animals in U treatment group. The appearance of slow-frequency waves was similar to that reported by Lambooy and Spanjaard (1981), and consistent with trauma-induced unconsciousness of the brain, as described by (Shaw 2002) in human patients. However, on the basis of the EEG results, penetrative stunning seemed to be better in maximising the possibility of post-stunning insensibility, while U animals appeared to demonstrate evident increase in EEG activities that are consistent with the presence of post-slaughter noxious stimuli associated with tissue cut and injury, as proposed by Gibson et al. (2009b). On the basis of the window-of-sensibility concept put forth earlier by Blackmore and Newhook (1982), it is clear that all terminal RMS values of alpha, beta, delta and theta wave forms were below 10 µV. This indicates deep unconsciousness, or even death itself in the case of the current study. At this stage, most of the terminal RMS values are associated with isoelectric EEG traces. It should be noted that stunning resulted in slightly higher terminal RMS values than those in U animals. However, some of these values should be inferred with caution because their coefficient of variation normally ranges between 30% and >60%. The results suggested that while effective stunning may result in a period of insensibility, stunning is generally not associated with the onset of death. All animals started with statistically similar values of median frequency, alpha, beta, delta and theta RMS at baseline. Thus, the increase in the brain electrical activity in U animals could be attributed to conscious pain even at 30-s post-slaughter. It was postulated that a longer duration between completing the cut and the onset of unconsciousness greatly increases the risk of pain and distress in cattle (Gregory et al. 2010). The HPNP, LPNP and P groups demonstrated the immediate prominence of slow-frequency delta and theta waves post-stunning, although this was not reflected in the RMS values. The appearance of slow-frequency delta and theta waves suggested the possible loss of consciousness. These wave forms were also accompanied by a significant increase in alpha- and beta-wave RMS values at T2 in the LPNP and HPNP animals. When interpreted alongside their respective median frequencies, these values are suggestive of post-stunning noxious stimuli (Murrell and Johnson 2006). On the contrary, the alpha and beta RMS values were significantly lower immediately after stunning at T2 in the P animals. These showed that the P animals were less sensible to pain following the penetrative stunning. In their counter argument to the use of sticking during slaughter, Gregory and Shaw (2000) pointed out that a good stunning technique is more important in improving animal welfare because other procedures such as sticking serve only at promoting rapid exsanguination in a state where the animal may already be insensible. This is congruent to our results where U animals had consistently higher, if not the highest, RMS values than did other stunned animals. The current work also demonstrated the close similarities between the results obtained for our non-stunned animals and those for the minimally anaesthetised calves by Gibson et al. (2009a). There was a notable increase in the median frequency by almost 50% in the U animals post-slaughter, which was consistent with findings by Gibson et al. (2009a). This was accompanied by significant increases in both beta- and alpha-wave power densities among U animals following slaughter, at 170% and 110% of their T1 values, respectively. Collectively, these results showed that noxious stimuli are present from the point of neck cut to the loss of consciousness in unstunned and non-anaesthetised animals, as pointed earlier by Johnson et al. (2012) via their minimally anaesthetised-cattle model.
The present EEG results clearly showed that the P treatment was effective in rendering animals insensible. However, a dramatic increase in the percentage change of plasma ACTH concentration from T2 to T3 in the P animals was noted. It appears that the present EEG results do not correlate well with the percentage change in the plasma concentration of ACTH. There is no clear explanation for the phenomenon. However, previous studies in domestic animals have suggested that EEG variables correlated well with the cognitive perception of pain (Ong et al. 1997; Hemsworth et al. 2009).
Conclusions
‘Humane slaughter’ in many countries requires that an animal becomes unconscious and does not regain consciousness until death. Percussive stunning using a penetrating or non-penetrating captive-bolt gun is a common method used to render animals unconscious rapidly and effectively. One of the concerns in industry is that LPNP, as used to ensure that skulls are not cracked by stunning, is likely to result in a greater number of animals that are not effectively stunned and therefore likely to suffer pain at sticking. In the present study, although a lower grain cartridge was used to deliver the low-power percussive stun than that used for the-high power percussive stun, no attempt was made to assess the skulls for damage; so, it is unknown as to whether the power used would comply with these requirements. On the basis of percentage change in plasma noradrenaline concentration following stunning, it is evident that the HPNP treatment could be more physiologically stressful to the animals than is the LPNP treatment. Animals subjected to P appeared to experience a greater magnitude of physiological stress at slaughter, as indicated by a significant increase in the percentage change of circulating ACTH. However, the results are not unequivocal, and it may be that the range of analyses available to researchers at this point in time are not sufficiently specific to allow definitive conclusions to be drawn from a small study such as this. The concentrations of the hormones measured are influenced by a large number of physiological conditions, not only pain and distress, and the large variation in the results gathered is likely to be a result of many factors such as pre-slaughter handling stress, hydration state or psychological stress, none of which was quantified in the present study. It appears that penetrative stunning induces a period of insensibility as suggested by EEG evidence. When stunning was performed correctly, it did not cause or hasten death in the present study because the cessation of vital life functions such as heart beat, and subsequent brain death, are attributable to the act of throat cut and exsanguination.
Acknowledgements
The authors thank Stephen Humphries, Janet Stark, Joanne Mountford, Gabriele Netzel, Omar M. Lotfi, Terry Nolan and AHFS for technical support and assistance during this project. This project was funded by Meat and Livestock Australia and matching funds were provided by the Australian Government and Universiti Putra Malaysia.
References
Anil MH, Fordham DP, Rodway RG (1990) Plasma β-endorphin increase following electrical stunning in sheep. The British Veterinary Journal 146, 476–477.| Plasma β-endorphin increase following electrical stunning in sheep.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M%2FivVKrsw%3D%3D&md5=eb9c939338aa49cae7895028d28e63c4CAS | 2224492PubMed |
Blackmore DK, Newhook JC (1982) Electroencephalographic studies of stunning and slaughter of sheep and calves – Part 3: the duration of insensibility induced by electrical stunning in sheep and calves. Meat Science 7, 19–28.
| Electroencephalographic studies of stunning and slaughter of sheep and calves – Part 3: the duration of insensibility induced by electrical stunning in sheep and calves.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvVyltA%3D%3D&md5=f9d0b2df7e33856265f08845a75b499bCAS | 22055065PubMed |
Broom DM, Johnson KG (1993) ‘Stress and animal welfare.’ (Chapman and Hall: London)
Devine CE, Gilbert KV, Graafhuis AE, Tavener A, Reed H, Leigh P (1986) The effect of electrical stunning and slaughter on the electroencephalogram of sheep and calves. Meat Science 17, 267–281.
| The effect of electrical stunning and slaughter on the electroencephalogram of sheep and calves.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvFSntQ%3D%3D&md5=18ebea66285985cd1843b1b66b0ff29dCAS | 22055358PubMed |
Finnie JW (1995) Neuropathological changes produced by non-penetrating percussive captive bolt stunning of cattle. New Zealand Veterinary Journal 43, 183–185.
| Neuropathological changes produced by non-penetrating percussive captive bolt stunning of cattle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2MzntlOltg%3D%3D&md5=ab6d0a7e758b2a8c31fedd8ff1411bfeCAS | 16031846PubMed |
Finnie JW, Blumberg PC, Manavis J, Summersides GE, Davies RA (2000) Evaluation of brain damage resulting from penetrating and non-penetrating captive bolt stunning using lambs. Australian Veterinary Journal 78, 775–778.
| Evaluation of brain damage resulting from penetrating and non-penetrating captive bolt stunning using lambs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M7ks1OgsQ%3D%3D&md5=bcaf5cdb6af9f055871aeeec4d65d918CAS | 11194725PubMed |
Gibson TJ, Johnson CB, Murrell JC, Hulls CM, Mitchinson SL, Stafford KJ, Johnstone AC, Mellor DJ (2009a) Electroencephalographic responses of halothane-anaesthetised calves to slaughter by ventral-neck incision without prior stunning. New Zealand Veterinary Journal 57, 77–83.
| Electroencephalographic responses of halothane-anaesthetised calves to slaughter by ventral-neck incision without prior stunning.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MzntlOmsA%3D%3D&md5=6a00162d1462412355c92681213f0177CAS | 19471325PubMed |
Gibson TJ, Johnson CB, Murrell JC, Stafford KJ, Chambers PJ, Mellor DJ (2009b) Components of electroencephalographic responses to slaughter in halothane-anaesthetised calves: effects of cutting neck tissues compared with major blood vessels. New Zealand Veterinary Journal 57, 84–89.
| Components of electroencephalographic responses to slaughter in halothane-anaesthetised calves: effects of cutting neck tissues compared with major blood vessels.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MzntlOmsQ%3D%3D&md5=599e8408867a091b439df8350b457877CAS | 19471326PubMed |
Gibson TJ, Johnson CB, Murrell JC, Mitchinson SL, Stafford KJ, Mellor DJ (2009c) Electroencephalographic response to concussive non-penetrating captive-bolt stunning in halothane-anaesthetised calves. New Zealand Veterinary Journal 57, 90–95.
| Electroencephalographic response to concussive non-penetrating captive-bolt stunning in halothane-anaesthetised calves.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MzntlOmtg%3D%3D&md5=a981e0bd2ea6fa2b88d3b42cfce7ccf4CAS | 19471327PubMed |
Gibson TJ, Johnson CB, Murrell JC, Mitchinson SL, Stafford KJ, Mellor DJ (2009d) Amelioration of electroencephalographic responses to slaughter by non-penetrative captive-bolt stunning after ventral neck incision in halothane-anaesthetised calves. New Zealand Veterinary Journal 57, 96–101.
| Amelioration of electroencephalographic responses to slaughter by non-penetrative captive-bolt stunning after ventral neck incision in halothane-anaesthetised calves.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MzntlOmtw%3D%3D&md5=c07639db980adaaac52d9bdee1ecf028CAS | 19471328PubMed |
Grandin T (1996) Factors that impede animal movement at slaughter plants. Journal of the American Veterinary Medical Association 209, 757–759.
Grandin T (2009) ‘Recommended captive bolt stunning techniques for cattle.’ Available at http://www.grandin.com/humane/cap.bolt.tips.html [verified 26 March 2013].
Grandin T, Smith GC (2004) ‘Animal welfare and humane slaughter.’ (Colorado State University: Fort Collins, CO). Available at http://www.grandin.com/references/humane.slaughter.html [verified 26 March 2013].
Gregory NG (2007) ‘Animal welfare and meat production.’ 2nd edn. (CABI Publishing: Wallingford, UK)
Gregory NG, Shaw F (2000) Penetrating captive bolt stunning and exsanguinations of cattle in abattoirs. Journal of Applied Animal Welfare Science 3, 215–230.
| Penetrating captive bolt stunning and exsanguinations of cattle in abattoirs.Crossref | GoogleScholarGoogle Scholar |
Gregory NG, Fielding HR, Von Wenzlawowicz M, Von Holleben K (2010) Time to collapse following slaughter without stunning in cattle. Meat Science 85, 66–69.
| Time to collapse following slaughter without stunning in cattle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3ks1agtA%3D%3D&md5=d09aad38f6cdba912e4468c0b7dbf100CAS | 20374866PubMed |
Hemsworth PH, Mellor DJ, Johnson CB (2009) A scientific comment on the welfare of sheep slaughtered without stunning. Available at http://www.daff.gov.au/animal-plant-health/welfare/aaws/a_scientific_comment_on_the_welfare_of_sheep_slaughtered_without_stunning [verified 26 March 2013].
Johnson CB, Gibson TJ, Stafford KJ, Mellor DJ (2012) Pain perception at slaughter. Animal Welfare 21, 113–122.
| Pain perception at slaughter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XotVymtLk%3D&md5=c17106c6f85f97021adf4682b504c860CAS |
Lacroix A, Hontela A (2006) Role of calcium channels in cadmium-induced disruption of cortisol synthesis in rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology 144, 141–147.
| Role of calcium channels in cadmium-induced disruption of cortisol synthesis in rainbow trout (Oncorhynchus mykiss).Crossref | GoogleScholarGoogle Scholar | 16959544PubMed |
Lambooy E, Spanjaard W (1981) Effect of the shooting position on the stunning of calves by captive bolt. Veterinary Record 109, 359–361.
| Effect of the shooting position on the stunning of calves by captive bolt.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL387gvFSmsg%3D%3D&md5=41c88177e80846c2aaf4ae500829e1c7CAS | 7324352PubMed |
Mellor DJ, Stafford KJ, Todd SE, Lowe TE, Gregory NG, Bruce RA, Ward M (2002) A comparison of catecholamine and cortisol responses of young lambs and calves to painful husbandry procedures. Australian Veterinary Journal 80, 228–233.
| A comparison of catecholamine and cortisol responses of young lambs and calves to painful husbandry procedures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltlOrsbc%3D&md5=2741927414af683256f1994bb2db1247CAS | 12054287PubMed |
Mellor DJ, Gibson TJ, Johnson CB (2009) A re-evaluation of the need to stun calves prior to slaughter by ventral-neck incision: an introductory review. New Zealand Veterinary Journal 57, 74–76.
| A re-evaluation of the need to stun calves prior to slaughter by ventral-neck incision: an introductory review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1Mznt1Srsw%3D%3D&md5=b6af6e825cf6e8b84ab4c4d2e9611891CAS | 19471324PubMed |
Micera E, Albrizio M, Surdo NC, Moramarco AM, Zarrilli A (2010a) Stress-related hormones in horses before and after stunning by captive bolt gun. Meat Science 84, 634–637.
| Stress-related hormones in horses before and after stunning by captive bolt gun.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXit1ajsLo%3D&md5=93c01a7f215dd2187e36477ea2fbca2fCAS | 20374835PubMed |
Micera E, Dimatteo S, Grimaldi M, Marsico G, Zarrilli A (2010b) Stress indicators in steers at slaughtering. Italian Journal of Animal Science 6, 457–459.
| Stress indicators in steers at slaughtering.Crossref | GoogleScholarGoogle Scholar |
Murrell JC, Johnson CB (2006) Neurophysiological techniques to assess pain in animals. Journal of Veterinary Pharmacology and Therapeutics 29, 325–335.
| Neurophysiological techniques to assess pain in animals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28rjsFCjtw%3D%3D&md5=d91c042127bde8a1370279167f28dd62CAS | 16958776PubMed |
Nowak B, Mueffling TV, Hartung J (2007) Effect of different carbon dioxide concentrations and exposure times in stunning of slaughter pigs: impact on animal welfare and meat quality. Meat Science 75, 290–298.
| Effect of different carbon dioxide concentrations and exposure times in stunning of slaughter pigs: impact on animal welfare and meat quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFerurbF&md5=16b8e84a3e4703915d58a203e1f5dc8aCAS | 22063661PubMed |
Ong RM, Morris JP, O’Dwyer JK, Barnett JL, Hemsworth PH, Clarke IJ (1997) Behavioural and EEG changes in sheep in response to painful acute electrical stimuli. Australian Veterinary Journal 75, 189–193.
| Behavioural and EEG changes in sheep in response to painful acute electrical stimuli.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s3ktlKluw%3D%3D&md5=35d5289f22342b890f935af4d0247b38CAS | 9088510PubMed |
Sapolsky RM (1992) ‘Stress, the aging brain, and the mechanisms of neuron death.’ (The MIT Press: Cambridge, MA)
SAS Institute (2003) ‘SAS/STAT user’s guide. Release 9.1.’ (SAS Institute: Cary, NC)
Shaw NA (2002) The neurophysiology of concussion. Progress in Neurobiology 67, 281–344.
| The neurophysiology of concussion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xms1KisbY%3D&md5=c5db6735dd8e33e35c97e69890a5c995CAS | 12207973PubMed |
Shaw FD, Tume RK (1992) The assessment of pre-slaughter and slaughter treatments of livestock by measurement of plasma constituents – A review of recent work. Meat Science 32, 311–329.
| The assessment of pre-slaughter and slaughter treatments of livestock by measurement of plasma constituents – A review of recent work.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XltVylsrk%3D&md5=ca52108db38a24330fe902b7f1e29750CAS | 22059818PubMed |
Warriss P (2010) ‘Meat science.’ 2nd edn. (CABI Publishing: Wallingford, UK)
Zulkifli I, Siegel PB (1995) Is there a positive a positive side to stress? World’s Poultry Science Journal 51, 63–76.
| Is there a positive a positive side to stress?Crossref | GoogleScholarGoogle Scholar |


