Genetics of heifer performance in ‘wet’ and ‘dry’ seasons and their relationships with steer performance in two tropical beef genotypes
S. A. Barwick A B G , D. J. Johnston A B , H. M. Burrow A C , R. G. Holroyd A D , G. Fordyce A E , M. L. Wolcott A B , W. D. Sim A C and M. T. Sullivan A FA Cooperative Research Centre for Beef Genetic Technologies, Armidale, NSW 2351, Australia.
B Animal Genetics and Breeding Unit 1 , University of New England, Armidale, NSW 2351, Australia.
C CSIRO Livestock Industries, Rockhampton, Qld 4702, Australia.
D Queensland Department of Primary Industries and Fisheries, Rockhampton, Qld 4702, Australia.
E Queensland Department of Primary Industries and Fisheries, Charters Towers, Qld 4820, Australia.
F Queensland Department of Primary Industries and Fisheries, PO Box 1333, Mount Isa, Qld 4825, Australia.
G Corresponding author. Email: steve.barwick@dpi.nsw.gov.au
Animal Production Science 49(6) 367-382 https://doi.org/10.1071/EA08273
Submitted: 11 November 2008 Accepted: 16 February 2009 Published: 13 May 2009
Abstract
The genetics of heifer performance in tropical ‘wet’ and ‘dry’ seasons, and relationships with steer performance, were studied in Brahman (BRAH) and Tropical Composite (TCOMP) (50% Bos indicus, African Sanga or other tropically adapted Bos taurus; 50% non-tropically adapted Bos taurus) cattle of northern Australia. Data were from 2159 heifers (1027 BRAH, 1132 TCOMP), representing 54 BRAH and 51 TCOMP sires. Heifers were assessed after post-weaning ‘wet’ (ENDWET) and ‘dry’ (ENDDRY) seasons. Steers were assessed post-weaning, at feedlot entry, over a 70-day feed test, and after ∼120-day finishing. Measures studied in both heifers and steers were liveweight (LWT), scanned rump fat, rib fat and M. longissimus area (SEMA), body condition score (CS), hip height (HH), serum insulin-like growth factor-I concentration (IGF-I), and average daily gains (ADG). Additional steer measures were scanned intra-muscular fat %, flight time, and daily (DFI) and residual feed intake (RFI). Uni- and bivariate analyses were conducted for combined genotypes and for individual genotypes. Genotype means were predicted for a subset of data involving 34 BRAH and 26 TCOMP sires. A meta-analysis of genetic correlation estimates examined how these were related to the difference between measurement environments for specific traits.
There were genotype differences at the level of means, variances and genetic correlations. BRAH heifers were significantly (P < 0.05) faster-growing in the ‘wet’ season, slower-growing in the ‘dry’ season, lighter at ENDDRY, and taller and fatter with greater CS and IGF-I at both ENDWET and ENDDRY. Heritabilities were generally in the 20 to 60% range for both genotypes. Phenotypic and genetic variances, and genetic correlations, were commonly lower for BRAH. Differences were often explained by the long period of tropical adaptation of B. indicus. Genetic correlations were high between corresponding measures at ENDWET and ENDDRY, positive between fat and muscle measures in TCOMP but negative in BRAH (mean of 13 estimates 0.50 and –0.19, respectively), and approximately zero between steer feedlot ADG and heifer ADG in BRAH. Numerous genetic correlations between heifers and steers differed substantially from unity, especially in BRAH, suggesting there may be scope to select differently in the sexes where that would aid the differing roles of heifers and steers in production. Genetic correlations declined as measurement environments became more different, the rates of decline (environment sensitivity) sometimes differing with genotype. Similar measures (LWT, HH and ADG; IGF-I at ENDWET in TCOMP) were genetically correlated with steer DFI in heifers as in steers. Heifer SEMA was genetically correlated with steer feedlot RFI in BRAH (0.75 ± 0.27 at ENDWET, 0.66 ± 0.24 at ENDDRY). Selection to reduce steer RFI would reduce SEMA in BRAH heifers but otherwise have only small effects on heifers before their first joining.
Additional keywords: adaptation, Bos indicus, genetic correlation, genotype by environment, residual feed intake, sexual dimorphism.
Introduction
Greater knowledge is needed of the potential for trade-offs between breeding female and slaughter steer performance in tropical beef cattle breeding, where it is critical that production gains made do not compromise survival and reproduction (Burrow et al. 2003; Chase et al. 2005). In tropical environments, cattle experience extremes of heat and humidity, cattle tick (Boophilus microplus) and other parasites, marked annual ‘wet’ and ‘dry’ seasons, and feed deficiencies (Syrstad 1989; Burrow 2001). The environment for steers may be less severe than for heifers and breeding cows; steers bred in tropical and subtropical northern Australia, for example, are often feedlot-finished. Knowledge of genetic relationships between similar, early-in-life traits of heifers and steers could help anticipate later trade-offs between breeding female and slaughter steer performance, as the early-in-life relationships will usually be components of later trade-offs. Genetic correlations between similar traits of heifers and steers are expected to reflect both the differing environments of heifers and steers and any sexual dimorphism existing for the traits (Eisen and Legates 1966).
A study encompassing both young animal and cow performance was initiated in Brahman (BRAH) and Tropical Composite (TCOMP) cattle in northern Australia to assess genetic relationships among a range of traits. The TCOMP is ~50% derived from tropically adapted and 50% from non-tropically adapted breeds. Barwick et al. (2009) presented results from this study for daily (DFI) and residual feed intake (RFI), body composition, growth and other post-weaning measures of feedlot-finished steers. This paper reports on the genetics of heifer post-weaning performance in ‘wet’ (between December and May) and ‘dry’ (between June and November) seasons; and on relationships between this and the performance of their steer half-sibs. As well as examining whether similar relationships occur within steers and heifers in their differing environments, the study examines genetic parameter and mean differences between the genotypes, and how selection for steer traits, including for lower RFI of steers in the feedlot, is expected to impact on heifer performance.
Materials and methods
Animals
Heifers in the study were born in 1999–2000 through 2002–2003 using AI and natural service on seven cooperating properties (4 BRAH and 3 TCOMP) and the ‘Belmont’ research station (both BRAH and TCOMP). Calvings commenced in the ‘dry’ season and continued into the ‘wet’ season of the new calendar year. Data were available for 2159 heifers (1027 BRAH, 1132 TCOMP), representing 54 BRAH and 51 TCOMP sires. Heifers born and managed together at ‘Belmont’ represented 34 BRAH and 26 TCOMP sires. Other details of sire and dam groups in the TCOMP genotype, and of the BRAH and TCOMP sires used are given by Barwick et al. (2009). Briefly, the 50% tropically adapted component of the TCOMP is approximately one-half derived from the Bos indicus Brahman and one-half from African Sanga (Frisch et al. 1997) (24% Africander) or other adapted Bos taurus (2% N’Dama, through the Senepol). The 50% non-tropically adapted component of the TCOMP derives from non-tropically adapted B. taurus.
On each property, date of birth (sometimes accurate only to within a month), calf sex, sire, dam, dam year of birth, and sire group and dam group for TCOMP, were recorded. Sire parentage was determined by DNA fingerprinting (Vankan 2005). The use of AI generated genetic linkage across properties of origin and years, and across AI and natural service calving groups. Additional linkage was generated by re-using natural service sires across years. The distribution of heifer progeny by genotype, property of origin and post-weaning location-year of birth cohort, and the genetic linkage generated are described in Table 1.
Environments
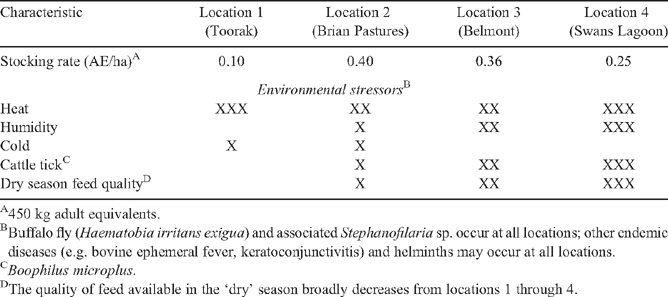
Following weaning, heifers were transferred to one of four locations that represented the range of northern Australian cow herd environments normally encountered by each genotype. Locations 1 and 2 were the Queensland Department of Primary Industries (QDPI) ‘Toorak’ and ‘Brian Pastures’ research stations; location 3 was the AgForce-owned and CSIRO-managed ‘Belmont’ research station; and location 4 was the QDPI ‘Swans Lagoon’ research station. The environmental stressors and other features typifying each location in an average year are summarised in Table 2. Other location details are given below. While all of the locations included environmental stressors, the severity of environments was broadly expected to increase from locations 1 through 4. Based on industry advice, all of the locations were judged to be suitable for BRAH, and all except location 4 were suitable for TCOMP. The combined effects of cattle tick (Boophilus microplus) and poor quality feed in the ‘dry’ season were the basis for the industry advice that location 4 was unsuitable for TCOMP. Heifers of the TCOMP genotype were assigned to locations 1, 2 and 3, and BRAH heifers to locations 1, 3 and 4. Within a property of origin and year of birth, heifers were assigned to one or more locations. Allocation to locations was on a within-sire basis to maintain genetic linkage, but otherwise, allocation was at random. Heifers of the two genotypes that were both born and retained at location 3 were fully comparable, as they were always similarly treated.
Location 1 – ‘Toorak’
‘Toorak’ is the hottest and driest of the locations, and has the highest quality ‘dry’ season feed. The research station is tick-free as a consequence of being located within a cattle tick exclusion zone. It is located 50 km south of Julia Creek (21°02′S, 141°47′E) in north-western Queensland. Average annual rainfall is 439 mm, 80% of which falls between November and March. Mean daily maximum temperatures exceed 35°C from October to March. The mean daily minimum temperature in the coldest month (July) is 8°C. The country comprises mainly treeless plains on predominantly grey and brown cracking clays. The dominant perennial and annual pasture species are Astrebla spp. (curly and bull Mitchell grasses) and Iseilema spp. (Flinders grass), respectively. Cattle are watered from artesian bores reticulated to tanks and troughs.
Location 2 – ‘Brian Pastures’
‘Brian Pastures’ is located 18 km ESE of Gayndah (25°39′S, 151°45′E) in the Burnett region of south-eastern Queensland. Average annual rainfall is 702 mm, 70% of which falls between October and March. Mean daily maximum temperatures exceed 30°C from October to March. The mean daily minimum temperature in the coldest month (July) is 7°C. The country varies from steep hills to alluvial flats. Heteropogon contortus (black spear grass) is the dominant pasture species. On higher slopes it is associated with Aristida spp. (wiregrass), and on alluvial flats with Bothriochloa bladii (forest bluegrass). Introduced species include Chloris gayana (Rhodes grass), Megathyrsus maximus var. pubiglumis (green panic), Pennisetum ciliare (buffel grass), Bothriochloa insculpta (creeping bluegrass), Stylosanthes spp. (stylo) and Leucaena leucocephala (leucaena). Cattle are watered from the Burnett River and from water reticulated to tanks and troughs.
Location 3 – ‘Belmont’
‘Belmont’ is located 24 km north-west of Rockhampton (23°13′S, 150°23′E), 40 km from the central Queensland coast and 26 km north of the Tropic of Capricorn. Average annual rainfall is 792 mm, 70% of which occurs between October and March. Mean daily maximum temperatures exceed 30°C from October to March. The mean daily minimum temperature in the coldest month (July) is 9.5°C. A large area is subject to periodic flooding. Pasture species on flooded country include Panicum spp., Chloris spp. (couch grass), Dichanthium sericeum (Queensland blue grass), Sporobolus spp. and Imperata cylindrica (blady grass). Bothriochloa spp. dominate on medium level country, and Heteropogon contortus (black spear grass) and Themeda triandra (kangaroo grass) on sandy loams and podsols. Species on heavier soils include C. gayana, P. ciliare, Aristida spp. (spear grass) and Axonopus spp. (carpet grass), and other introduced species are M. maximus var. pubiglumis, and the legumes Centrosema pubescens and Stylosanthes seca vr seca (seca stylo). Cattle are watered from artesian bores reticulated to all paddocks, as well as by access to lagoons and the Fitzroy River.
Location 4 – ‘Swans Lagoon’
‘Swans Lagoon’ is located 125 km south-west of Townsville (20°05′S, 147°13′E), 100 km inland in the Burdekin region of north coastal Queensland. Average rainfall is 860 mm, 80% or more of which falls between October and March. Maximum temperatures average 30−35°C from September to April. The mean daily minimum temperature in the coldest month (July) is 10°C. ‘Swans Lagoon’ is the most humid of the locations. The country is open eucalypt savannah woodland with most areas supporting native pasture species including Heteropogon contortus (black spear grass). Cattle are watered from lagoons, bores and dams reticulated to paddocks.
Management and treatment
Following weaning on each of the properties of origin, heifers were accumulated at ‘Toorak’ and ‘Swans Lagoon’ before allocation to post-weaning locations. Thereafter, they were managed according to the accepted practice of their region. This included monitoring for external and internal parasites and for nutritional deficiency, with intervention only on a needs basis. Heifers at each location were vaccinated against major diseases early in life, including for leptospirosis and clostridial diseases, for vibriosis and tick fever (Anaplasma spp., Babesia spp.) except at ‘Belmont’, for pestivirus usually before mating, and for bovine ephemeral fever at ‘Brian Pastures’. Heifers at ‘Brian Pastures’ were treated with a synthetic pyrethroid for cattle tick (Boophilus microplus) in 2001, and with an organophosphate spray or impregnated back rubber for buffalo fly (Haematobia irritans exigua) in 2002 and 2003.
Heifers were maintained in their year of birth groups from arrival at locations until their first joining, which was soon after the end of the second ‘dry’ season encountered post-weaning, and when they were ~24 months of age. From about the start of this second ‘dry’ season, heifers at ‘Toorak’ and ‘Swans Lagoon’ received a urea-based dry lick delivering ~75 g crude protein equivalent/day per 450 kg adult equivalent. Heifers in their first ‘dry’ season at ‘Swans Lagoon’, and heifers at ‘Brian Pastures’ received either a fortified (8% urea) molasses supplement ad libitum, or a fortified (3% urea, 6% protein meal) molasses supplement fed at between 1.5 and 4 kg/heifer.day, over much of the ‘dry’ season. Supplementation of heifers at ‘Belmont’ was via strategic access to an area of irrigated pasture.
Steers grazed on properties throughout central and south-western Queensland and northern New South Wales before entering a feedlot when the average of their cohort was ~400 kg. In the feedlot, they were fed a standard finisher ration of 12.2 MJ ME/kg DM energy density, 16.25% crude protein (w/w) and 87% DM for an average of 119 days to a finished average liveweight of 568 kg. Other details of the management of steers are given by Barwick et al. (2009).
Measurements
Measurements and scores were taken on heifers at intervals from weaning. The measurement times reported on were at times close to 1 June and 1 December, approximately at the end of the first ‘wet’ (ENDWET) and second ‘dry’ (ENDDRY) season post-weaning, and corresponding to heifer ages ~18 and 24 months, respectively. Measures were taken consistently close to these dates except in one cohort of 41 heifers, where ENDDRY measurements were taken about 1 month later. The steer measures reported were taken at ~80 days post-weaning (POSTW), at feedlot entry (ENTRY), over an average 71.6-day feed test (FEEDTEST), and at feedlot exit (EXIT), corresponding to steer ages ~10, 22, 24.5 and 26 months, respectively (Barwick et al. 2009). Fig. 1 illustrates the average age, liveweight and approximate growth rate of heifers and steers at each measurement time.

|
Measures assessed on heifers and steers were liveweight (LWT), average daily gain (ADG), ultrasonically scanned fat depth at the rump P8 (SP8) and 12/13th rib (SRIB) sites, scanned area of M. longissimus thoracis et lumborum at the 12/13th rib (SEMA), body condition score (CS), hip height (HH) and serum insulin-like growth factor-I concentration (IGF-I). Additional measures on steers included scanned intra-muscular fat% (SIMF), flight time (FT) (Burrow et al. 1988), daily feed intake (DFI), residual feed intake (RFI), metabolic mid-test weight (MWT) and feed test ADG (TADG). Average daily gains over specific intervals were derived as individual animal regressions of liveweights on days of age. For heifers, these regressions were derived at ENDWET and ENDDRY from an average of 3.8 and 5.4 liveweights recorded over the ‘wet’ and ‘dry’ season, respectively. Further details of measures are given by Barwick et al. (2009).
Statistical analyses
Fixed effect modelling
Fixed effect modelling for each trait was first carried out for BRAH and TCOMP individually, and then for the genotypes combined, to determine effects to include in subsequent ASReml (Gilmour et al. 1999) analyses. The fixed effect modelling used PROC MIXED in SAS (SAS Institute, Cary, NC, USA). Main effects of property of origin, age of dam (in years), birth month, cohort (post-weaning location-year of birth) and first order interactions were considered, with sire included as a random effect.
For TCOMP analyses, sire group (6 levels), dam group within herd of origin, and their interaction were additional effects fitted to account for average differences among combinations of sire groups and dam groups (Barwick et al. 2009). For analyses of combined genotypes, the effects considered were those identified for individual genotypes along with genotype and first-order interactions with genotype. In all analyses, non-significant terms (P > 0.05) were systematically removed to identify the final set of effects.
Mean prediction
Model predicted means for BRAH and TCOMP, for each trait, were derived from analyses of combined genotype data using the mean prediction procedure of ASReml. The predicted means were averaged over other fixed effect levels present (Gilmour et al. 2004). The genotype means predicted were for those heifers born and managed together at ‘Belmont’ that were also born over comparable months. Because there was a predominance of Belmont Red dams at ‘Belmont’, the TCOMP means that are predicted are for a sample of the genotype where the contribution of Africander to the tropically adapted component is higher (40% Africander, 1% N’Dama, 10% Brahman) than applied in the whole data.
Variance component estimation
BRAH- and TCOMP-specific estimates of variance components were derived from univariate analyses using ASReml. The statistical model included a random animal additive genetic effect, random common environmental effect of the dam, random residual environmental effect, and fixed effects. Relationships among the additive genetic effects were described by a relationship matrix (A) derived from pedigree. Other random effects were assumed to be uncorrelated. Components estimated were the additive direct genetic variance (σA 2), common dam environmental variance (σ2 C), residual environmental variance (σ2 E) and phenotypic variance (σP 2). Analyses were performed with and without the common dam environmental effect, the best fitting model being decided by likelihood ratio test. For TCOMP analyses, inclusion of sire group, dam group, and their interaction, where these were significant from the fixed effect modelling, removed the average effect of any heterosis occurring between groups. No other adjustment for heterosis was made.
Additive genetic and environmental covariances, and resulting genetic and phenotypic correlations among traits, were estimated from bivariate ASReml analyses. The models were as for the univariate analyses. The bivariate analyses were performed separately for each genotype and for the genotypes combined. Genetic correlations are presented from the analyses of combined genotypes. Genotype-specific genetic correlations are also presented where estimates for the individual genotypes differed by more than the sum of their approximate standard errors. To aid interpretation, presentation of genotype-specific results was further restricted to estimates where the approximate standard errors were no larger than 0.30 for both genotypes.
Meta-analysis of genetic correlations
To assist understanding of the genetic correlation estimates derived for each of the traits HH, LWT, ADG, SEMA, SP8, SRIB, and IGF-I, estimates between measurement times for heifers (between ENDWET and ENDDRY), for steers (between ENTRY and EXIT; Barwick et al. 2009), and for heifers and steers (between heifer measurement times and steer measurement times), were collated into a single dataset along with the standard errors of the estimates and a measure quantifying the environment difference associated with the measurement times. The data included 12 genetic correlation estimates for each trait, six for each of the two genotypes. The measure of environment difference used, GRdiff, was the difference in growth rate that was prevailing between measurement times, irrespective of whether the measurement was in heifers or steers, assessed in unadjusted data. Values of GRdiff for BRAH and TCOMP, respectively, were 0.08 and 0.02 kg/day between ENDWET and ENTRY, 0.39 and 0.30 kg/day between ENDDRY and ENTRY, 0.47 and 0.32 kg/day between ENDWET and ENDDRY, 0.60 and 0.99 kg/day between ENDWET and EXIT, 0.68 and 1.01 kg/day between ENTRY and EXIT, and 1.07 and 1.31 kg/day between ENDDRY and EXIT.
The collated data were used to examine how genetic correlation estimates varied with GRdiff. Relationships were plotted for each trait in each genotype (Fig. 2). The third and fifth data points in Fig. 2a–e , counting left to right in each genotype, correspond to correlations on the same sex, while the remaining points are for correlations on different sexes. Relationships were also examined with PROC MIXED in SAS (SAS Institute). The model describing genetic correlation estimates for each trait was:

|
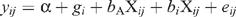
where yij is the estimate for the ith genotype and jth value of GRdiff, α is the intercept, gi is the effect of the ith genotype, X ij is the GRdiff associated with the ijth genetic correlation estimate, b A is the average regression on GRdiff, bi is the deviation from the average regression on GRdiff for the ith genotype, and eij is the residual. The model was fitted by including genotype, the regression on GRdiff, and the genotype by GRdiff interaction. The data were also analysed separately for each genotype, fitting only the regression on GRdiff. In all cases, the regressions derived were weighted regressions, weighting by the standard errors of the individual genetic correlation estimates.
Results
Data description and genotype means
Means describing the data, and model predicted means for genotypes, are in Tables 3 and 4, respectively. The model predicted means show BRAH heifers were significantly (P < 0.05) faster-growing in the ‘wet’ season, slower-growing in the ‘dry’ season, taller and fatter (for both SP8 and SRIB) with greater CS and IGF-I at both ENDWET and ENDDRY, and that BRAH heifers had greater SEMA at ENDWET than TCOMP heifers. The genotypes were not significantly different in LWT at ENDWET or ENDDRY, or in SEMA at ENDDRY (Table 4).

|

|
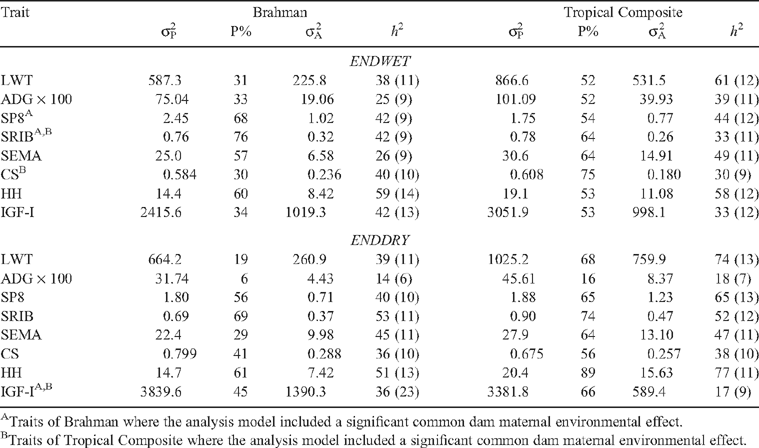
Trait variances
The percentage of observed variance represented as phenotypic variance (P%) was low for ADG at ENDDRY, and tended to be lower across traits for BRAH than for TCOMP, especially at ENDDRY (Table 5). Phenotypic variances were lower for BRAH than for TCOMP for LWT, ADG, SEMA and HH at both ENDWET and ENDDRY, and for SRIB at ENDDRY. Heritabilities for LWT, ADG at ENDWET, scanned body composition measures, CS, HH and IGF-I measures (Table 5) were generally in the 20 to 60% range for both genotypes. Heritabilities for ADG at ENDDRY (14% for BRAH, 18% for TCOMP) were lower. Resulting genetic variances were also often lower for BRAH, being lower for LWT, ADG, SEMA and HH, and for SP8 and SRIB at ENDDRY. Including a common dam environmental effect significantly improved the analysis model (as evidenced by likelihood ratio test) for some fatness measures at ENDWET, and for IGF-I at ENDDRY (Table 5).
|
Heifer measures in ‘wet’ and ‘dry’ seasons
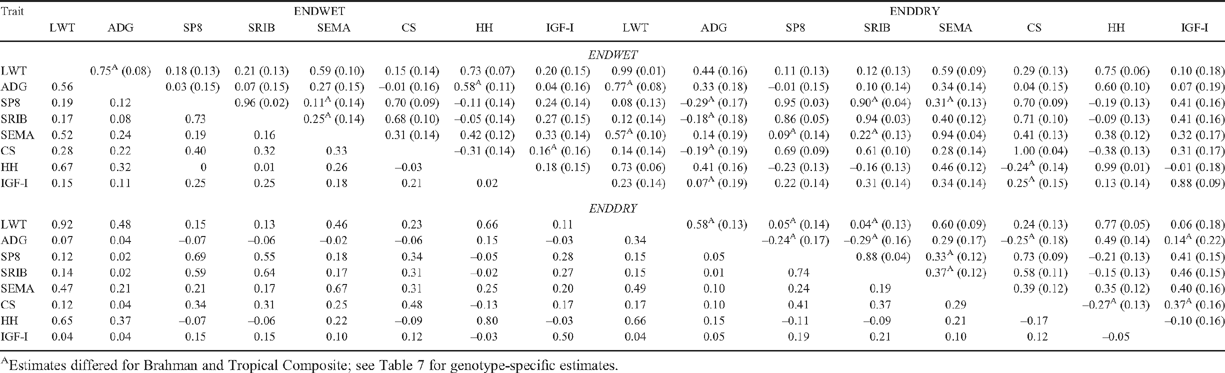
Genetic and phenotypic correlations among heifer measures at ENDWET and ENDDRY (Table 6) showed the corresponding trait measure was generally the ENDWET measure that was most genetically correlated with performance at ENDDRY, these correlations usually exceeding 0.9. An exception was the relationship between ADG at ENDWET and ENDDRY, where the genetic correlation was only 0.33. The ENDWET measure most genetically correlated with ADG at ENDDRY was LWT (0.44). Genetic and phenotypic correlations were generally symmetrical with respect to when traits were measured. For example, the genetic correlation between LWT at ENDWET and SP8 at ENDDRY (0.11) was very similar to that between SP8 at ENDWET and LWT at ENDDRY (0.08). Phenotypic correlations reflected similar trends to genetic correlations but with generally lower values (Table 6).
|
Genotype differences in the genetic correlations (Table 7) were more common among ENDDRY measures than among ENDWET measures. There were strong positive correlations between fatness measures and SEMA in TCOMP at both ENDWET and ENDDRY, whereas these correlations tended to be close to zero or negative in BRAH. Genetic correlations of fatness measures with ADG (and to a lesser extent other growth measures) at ENDDRY were strongly negative in BRAH (e.g. –0.81 with SRIB). Other results included lower genetic correlations for BRAH than for TCOMP between fat (SP8, SRIB, CS) and growth (LWT, HH) measures (e.g. –0.26 between SRIB and LWT at ENDDRY for BRAH, 0.29 for TCOMP), between some fat measures, between growth (LWT, HH) measures and ADG (e.g. 0.32 between LWT and ADG at ENDDRY for BRAH, 0.75 for TCOMP), and more negative genetic correlations for BRAH between IGF-I measures and ADG at ENDDRY. Genetic correlations between CS and IGF-I were more positive in BRAH than in TCOMP at both ENDWET and ENDDRY; and there was a more positive genetic correlation in BRAH between SEMA at ENDWET and LWT at ENDDRY (Table 7).

|
Heifer measures and steer measures pre-finishing
Genetic correlations between corresponding heifer measures at ENDWET and steer measures at POSTW and ENTRY ranged from approximately unity, for SP8 between ENDWET and ENTRY, down to 0.57 for ADG between ENDWET and ENTRY (Table 8). Those between corresponding heifer ENDDRY measures and steer measures were similarly positive but lower (range from unity to 0.41). Heavier, taller, and faster growing heifers at ENDWET were correlated with heavier and faster growing steers at ENTRY, and vice versa. There was a tendency for steer fat (SP8, SRIB) measures at ENTRY to be more negatively related to heifer growth (LWT, ADG, HH) measures at ENDWET than was so in the reverse situation; i.e. between heifer fatness (SP8, SRIB) measures at ENDWET and steer growth (LWT, ADG, HH) measures at ENTRY. Generally similar but less pronounced trends were evident between heifer measures at ENDDRY and steer measures at ENTRY (Table 8).

|
Genotype-specific genetic correlations between corresponding measures on heifers at ENDDRY and steers at ENTRY (Table 9) were effectively unity for SRIB, SEMA and IGF-I in TCOMP, but lower for BRAH (e.g. 0.73 for SEMA). Genetic correlations were also generally lower for BRAH among growth (LWT, ADG, HH, IGF-I) measures (e.g. 0.55 between HH at ENDWET and LWT at ENTRY for BRAH, 0.91 for TCOMP), among fat (SP8, SRIB, SIMF) measures (e.g. 0.69 between SP8 at ENDDRY and SRIB at ENTRY for BRAH, unity for TCOMP), and between IGF-I and growth (LWT, HH) and fat (SRIB) measures. In contrast to results for TCOMP, genetic correlations between fat (SP8, SRIB, SIMF) measures and SEMA were consistently negative for BRAH (e.g. –0.32 between SEMA at ENDWET and SRIB at ENTRY for BRAH, 0.53 for TCOMP). Genetic correlations between heifer CS measures and ENTRY IGF-I, and between some heifer growth (LWT, HH) measures and ENTRY SEMA, were higher for BRAH than for TCOMP (Table 9).

|
Heifer measures and steer measures post-finishing
Genetic correlations of heifer ENDWET and ENDDRY measures with steer measures at EXIT (Table 10) generally showed similar trends, but with lower values, to those with steer POSTW and ENTRY measures. The correlations between corresponding heifer and steer EXIT measures were close to unity for HH, but ranged down to approximately zero in BRAH for ADG at both ENDWET and ENDDRY (Tables 10 and 11). The trend for heavier and faster growing heifers to be genetically associated with heavier and faster growing steers, and vice versa, was evident for TCOMP at EXIT but hardly so for BRAH (Table 11). Heavier and faster growing steers at EXIT also tended to be genetically associated with leaner heifers, and with heifers with lower IGF-I, at ENDDRY (Table 10). Slower growing TCOMP heifers at ENDDRY were associated with leaner steers at EXIT (e.g. 0.79 with SRIB), while slower growing BRAH heifers at ENDDRY were associated with fatter steers at EXIT (e.g. –0.71 with SRIB). Other associations that were positive in TCOMP but negative in BRAH included those between heifer IGF-I and steer feedlot growth measures (MWT, LWT, TADG, ADG, HH) (Table 11).

|

|
Heifer measures and steer feed intake
Genetic correlations between heifer measures and steer FEEDTEST measures (Tables 10 and 11) showed LWT, HH and ADG, at both ENDWET and ENDDRY, and IGF-I at ENDWET in TCOMP, were the heifer measures most associated with steer DFI. Genetic correlations of heifer measures with steer RFI were low and positive for many growth and fat measures, and low and negative for HH. In BRAH, heifer SEMA was strongly positively correlated with steer RFI for SEMA measured at both ENDWET (0.75) and ENDDRY (0.66). IGF-I at ENDWET tended to be positively correlated with steer RFI (0.22), whereas IGF-I at ENDDRY and steer RFI were uncorrelated. Heifer ADG in the ‘dry’ season and steer RFI were positively genetically related in TCOMP (0.43 ± 0.25) (Table 11), negatively related in BRAH (–0.23 ± 0.34), and positively related in combined genotypes (0.32) (Table 10).
Genetic correlation meta-analysis
The trend was for genetic correlations to decline as the environment difference between measurement times (GRdiff) increased for one or both genotypes. This occurred for all traits except HH (Fig. 2). The rate of decline with increasing GRdiff was significantly greater in BRAH than in TCOMP for ADG (P < 0.05) and LWT (P < 0.10), but not significantly greater for SEMA. Genetic correlations for SP8 declined significantly with GRdiff in both TCOMP (P < 0.01) and BRAH (P < 0.05), with the rates of decline not differing (P > 0.05) between the genotypes. For SRIB and IGF-I, the rate of decline tended to be greater in TCOMP than in BRAH, but the difference was not significant (P > 0.05). In TCOMP, there was a significant decline with increasing GRdiff for SRIB (P < 0.10) and IGF-I (P < 0.05), whereas in BRAH the decline was not significant for either trait. Any heifer-steer sex difference that may exist is a possible contributor to the environment difference that was measured as GRdiff. Higher correlations might be expected between measures on the same sex than between measures on different sexes if sexual dimorphism is an important contributor to the genetic correlation differences. The third and fifth data points (correlations on the same sex) in Fig. 2a–e , counting left to right in each genotype, were consistently above the illustrated regression lines only for SEMA, IGF-I, and LWT in BRAH, suggesting that these are the traits where sexual dimorphism could have been a contributor.
Discussion
Genotype differences in mean performance
The greater fatness over the rump (SP8) for BRAH than for TCOMP heifers was similar to the trend in steers at feedlot entry (Barwick et al. 2009); and the relatively less fat over the rib (SRIB) than the rump in BRAH heifers compared with TCOMP heifers was similar to the difference seen in steers at both the start and end of finishing. Barwick et al. (2009) speculated that these fat distributional differences may partly explain the ability of B. indicus cattle to withstand tropical temperatures, as was also evidenced by significantly lower rectal temperatures for BRAH compared with TCOMP in this population (Prayaga et al. 2009). In contrast with these similar results for the sexes, in heifers, BRAH were faster growing in the ‘wet’ season and slower growing in the ‘dry’ season than TCOMP, whereas in steers, BRAH were slower growing than TCOMP throughout grow-out and finishing. BRAH heifers also had more fat over the rib and higher condition scores at ENDWET and ENDDRY than TCOMP (Table 4), whereas BRAH steers had less fat and lower condition scores at the end of feedlot finishing (Barwick et al. 2009). We are not aware of other reports of Brahman heifers being fatter than less tropically adapted heifers. Hearnshaw et al. (1994) found Brahman heifers had a higher condition score than some Hereford and crossbred heifers at weaning, but a lower condition score than Brahman × Hereford heifers at later ages. An increased ability to maintain fat in heifers through the ‘dry’ season could be an adaptation to tropical environments that has occurred in B. indicus. In Drosophila, there is evidence that genetic selection for starvation resistance increases resource storage (Chippindale et al. 1996).
The re-ranking of BRAH and TCOMP between heifers and steers for aspects of growth and fatness could represent genotype by sex interaction, or more generally, genotype by environment interaction given the confounding of sex and environment in our experiment. Growth rates and fatness were lower in heifers at ENDWET and ENDDRY than in steers at ENTRY or EXIT (Barwick et al. 2009), pointing to the heifer environments being inferior to the environments of steers. There is some evidence that the size of sex differences, and hence the potential for genotype by sex interaction (Eisen and Legates 1966), can vary between environments (Hopkins 1977; Herring et al. 2005). Also, B. indicus are widely thought to have a greater ability to utilise, partition or acquire feed on low quality or restricted diets, and a lesser ability on high energy or ad libitum diets (Moore et al. 1975; Frisch and Vercoe 1980; Turner 1980; Ferrell et al. 2005; Forbes 2005). Frisch and Vercoe (1977, 1980) suggested the B. indicus advantage in poorer environments may derive from a lower fasting heat production per unit of bodyweight. Ferrell et al. (2005) showed, for a range of genotypes, that heat production differences can usually be attributed to differences in metabolisable energy intake. Hennessy et al. (2000) reported Brahman steers had a lower intake of a low quality grass hay basal diet, and were less responsive to nitrogen and protein supplements, than Brahman × Hereford and Hereford steers. We speculate that in addition to having a generally lower feed intake (Hennessy et al. 2000; Barwick et al. 2009), the B. indicus BRAH may have evolved a capacity to better align its growth with the feed available in ‘wet’ and ‘dry’ seasons, as a part of tropical adaptation, and that this might explain the re-ranking of BRAH and TCOMP for ADG also seen between ‘wet’ and ‘dry’ seasons.
Trait variances and heritabilities
The trend for heifer trait genetic variances to be lower in BRAH than in TCOMP agrees with that seen in steers (Barwick et al. 2009), and resulted especially from BRAH heifers having lower trait phenotypic variances, despite higher observed variances, than TCOMP heifers. For ADG in the ‘dry’ season, the phenotypic variance was only a small part of the observed variance, with the large majority of the observed variance being explained by fixed effects. Heritabilities were not very different between the genotypes, nor very different from those for steers (Barwick et al. 2009). An exception was SEMA in BRAH, where heritabilities were higher for heifers (26 and 45% at ENDWET and ENDDRY) than for steers at EXIT (10%). This may have been in part due to a greater difficulty in measuring SEMA in BRAH at higher levels of fatness. The SRIB fat depth of BRAH steers averaged 7.4 mm at EXIT (Barwick et al. 2009), whereas this was 2.0 and 1.9 mm for BRAH heifers at ENDWET and ENDDRY.
Comparison of results with those for pasture-grazed steers (Barwick et al. 2009) showed no consistent direction in the difference in size of genetic variances between the sexes. Genetic variances were consistently lower in heifers than they were for steers at EXIT, except for HH in TCOMP and SEMA in BRAH. The fact that genetic variances for traits measured at pasture did not differ in a consistent direction between heifers and steers supports a similar finding by Burrow (2001) for direct additive genetic variances of weight traits in heifers and bulls. In contrast to this, the markedly lower genetic variances for heifer measures than for steer measures at EXIT emphasises the production system difference between pasture-grown heifers and feedlot-finished steers; and it suggests breeding objectives for BRAH and TCOMP in Australia will need to take account of this difference wherever possible.
Genetic correlations in heifers compared with steers
The higher genetic correlations between corresponding heifer measures at ENDWET and ENDDRY than between steer measures at ENTRY and EXIT (Barwick et al. 2009) may have been because the pasture environments of heifers were actually less different than the pasture and feedlot environments of steers (Fig. 1). The low genetic correlation between ADG at ENDWET and ENDDRY for heifers (0.33) is of similar order to that seen by Burrow (2001) for mixed sexes. The trend in steers for fat and muscle measures to be strongly positively correlated in TCOMP but negatively correlated in BRAH (Barwick et al. 2009) was also evident in the present results, being seen both within and between heifer measures at ENDWET and ENDDRY (Table 7), and between heifer measures and steer measures at ENTRY (Table 9). In fact, 13 estimates of the correlation between SEMA and SP8 or SRIB from our experiment, tabulated in either the present report or that of Barwick et al. (2009), averaged 0.50 for TCOMP and –0.19 for BRAH. These compare with average estimates of 0.01 (Koots et al. 1994) and –0.16 (Rios-Utrera 2004) that were reported in two literature reviews, where each review examined studies over a range of breeds. Koots and Gibson (1996) argue for averaging genetic correlation estimates across studies and populations because of the high sampling errors associated with individual estimates. Despite our individual genotype estimates having moderately high associated errors, the consistency of the differences here in the estimates between fat and muscle suggests averaging across individual estimates would be unwise.
Genotype differences in genetic correlations
The many lower and sometimes negative genetic correlations seen for BRAH compared with TCOMP appear consistent with theory, especially given the selection for tropical adaptation that is likely to have occurred in the BRAH, the great time period over which this could have occurred (B. indicus and B. taurus are considered to have diverged so long ago as to have been domesticated separately; MacHugh et al. 1997), and the known lesser feed intake of BRAH (Hennessy et al. 2000; Barwick et al. 2009). Selection of traits in the same direction is expected to result initially in positive genetic correlations, which then decrease over time and may become negative (Falconer 1983). Theoretical modelling also shows that when a resource is limiting, the amount of resource acquired contributes to positive covariance between traits, while any need to allocate the resource between competing functions contributes to negative trait covariance (Houle 1991). Hence, tropical adaptation over a long period and the lower feed intake of BRAH could each have led to the lower positive genetic correlations seen; and the lower feed intake and need to allocate limited feed could have contributed to the negative genetic correlations between fat and muscle, and between growth and fat. In sheep, fat and muscle levels have been shown to each vary with season (Ball et al. 1996) and apparently inversely to feed requirement (Sawalha et al. 2008), emphasising that both can be responsive to the level of feed available.
The correspondingly higher positive genetic correlations in TCOMP similarly could have resulted from the lower B. indicus content of TCOMP, or possibly from the breed crossing involved in initial composite formation. Breed crossing could have contributed by causing some amount of loss of gene combinations that had earlier been favoured under selection in TCOMP parent breeds (Kinghorn 1980). It also should be noted that genotype differences in our experiment need not have resulted only from effects deriving from B. indicus, as 100% B. indicus or 100% Sanga genotypes would each be expected to lose some adaptedness if crossed with unadapted B. taurus.
Genetic correlations in relation to the level of difference between environments
The illustrated meta-analysis results (Fig. 2) provide a different, and potentially over-arching, insight into how some key genetic correlation estimates varied over heifers and steers, genotypes, and measurement times. Correlations between corresponding traits measured in different environments generally declined as the differences between the measurement environments increased, suggesting the differences between the traits became greater. This occurred to varying extents for the traits and genotypes studied (Fig. 2), also suggesting different environment sensitivities (Falconer 1983). The greater environment sensitivity of BRAH for ADG and LWT, and lesser sensitivity for SRIB fatness, appear to reflect the earlier-mentioned lower intake and capacity of BRAH to vary growth in ‘wet’ and ‘dry’ seasons while maintaining fat. Conversely, the lesser sensitivity of TCOMP for ADG and LWT, and greater sensitivity for SRIB, seem to reflect the greater intake of TCOMP, and a lesser capacity to vary growth and to restrict gain or loss in fat. The results suggest a lesser environment sensitivity can be desirable in some traits but undesirable in others. Whether a lesser environment sensitivity is desirable will be influenced by how the selection history and early selection environment of a genotype aligns with the current objectives and environment of selection.
Genetic correlations between the performance of heifers and steers
The heifer measures with potential to be genetic indicators of steer feedlot DFI (i.e. LWT, HH, and ADG) closely resembled those found in steers (Barwick et al. 2009), while in BRAH there were high correlations, discussed further below, between heifer SEMA measures and steer feedlot RFI. The low positive genetic correlations of heifer IGF-I measures with steer RFI contrast with the consistently negative correlations that were seen in steers (Barwick et al. 2009), and re-emphasise the need to carefully define any IGF-I measures used in genetic evaluation of RFI. Of other possible indicators, ADG in the ‘dry’ season, representing the ability of heifers to grow when feed is restricted, was not consistently related to steer RFI. Frisch and Vercoe (1980) observed that the faster gaining of two genotypes on fixed intake had the lower heat production per unit of bodyweight, from which it might have been expected that ADG in the ‘dry’ season could be negatively related to RFI. We found ADG in the ‘dry’ season was negatively but lowly correlated with steer RFI in BRAH (–0.23), and positively correlated in TCOMP (0.43).
More generally, our results show that selecting for low RFI of steers would have only small effects on the heifer traits examined, except for SEMA in BRAH. Steers with lower RFI are expected to have heifer half-sibs that are slightly lighter, taller, and leaner, in addition to being less muscled. Some of these effects might be undesirable in heifers approaching their first mating in a stressful tropical environment. The strong positive correlations between SEMA and RFI in BRAH (0.75 ± 0.27 at ENDWET, 0.66 ± 0.24 at ENDDRY) could either be a sign that reducing steer RFI would markedly reduce heifer energy balance in that genotype, or it could be the source of an efficiency advantage if it is energetically cheaper for heifers to maintain fat than to maintain muscle. In steers, however, partial regressions of DFI on MWT and TADG did not differ between BRAH and TCOMP (Barwick et al. 2009). Several authors (e.g. Rauw et al. 1998; Luiting 1999; Veerkamp et al. 2003) have warned that, across species, reducing energy balance by reducing RFI could deleteriously affect fitness. Further, Johnston et al. (2009) report lower steer RFI is genetically associated with increased time to puberty in BRAH for heifers from the same experiment.
Our results for corresponding measures of heifers and steers show selection in one sex-environment will usually lead to change in the same direction in the other sex-environment, especially in TCOMP. The fact that numerous of the correlations differed substantially from unity, especially in BRAH, shows there is some scope to select differently in the sexes where it would serve the differing roles of heifers and steers in production. Greater fatness, for example, might be desired in heifers and cows going into breeding, but be not desired in finished steers. The correlation between heifer and steer fatness at these times (0.60 in combined genotypes) suggests appropriately applied selection might be effective in encouraging these differences.
Conclusions
Genotypes differed in heifer performance at the level of trait means, variances and genetic correlations, supporting separate genetic evaluation of BRAH and TCOMP. The differences often resembled those in steers (Barwick et al. 2009), but with re-ranking for aspects of growth and fatness. BRAH heifers seemed better able to align their growth with the feed available in ‘wet’ and ‘dry’ seasons, and to retain fat to the end of the ‘dry’ season. Trait phenotypic and genetic variances, and genetic correlations, were often lower for BRAH than for TCOMP. Many of the differences could be explained by the differing B. indicus content of the genotypes and the long period over which tropical adaptation of B. indicus has occurred. Trait genetic correlations between measurement environments generally decreased as the difference between the measurement environments became greater. The rate of this decrease (environment sensitivity) varied with trait and genotype. Differences in genetic correlations that could have been due specifically to a sex difference were uncommon, and existed only for some traits of BRAH.
While selection in one sex-environment generally would lead to change in the same direction in the other sex-environment, results emphasised the production system difference between heifers at pasture and feedlot-finished steers, and that there is some scope to select differently in the sexes where both are recorded. Selection to reduce steer feedlot RFI would reduce SEMA in BRAH heifers, which might have consequences for the subsequent fitness of BRAH cows. Selection to reduce steer RFI would generally otherwise have only small effects on heifers before their first joining. The performance of the present heifers as breeding cows will allow a fuller assessment of the consequences of selection for reduced feedlot RFI and other steer traits for the present genotypes.
Acknowledgements
Financial and in-kind support was provided by the Commonwealth Cooperative Research Centre program, NSW Department of Primary Industries, CSIRO, Queensland Department of Primary Industries, University of New England, Northern Pastoral Group, and Meat and Livestock Australia. We gratefully acknowledge the assistance of numerous staff with cattle management, steer feeding, data collection, laboratory analyses and data handling, including the Managers and staff of CSIRO ‘Belmont’, QDPI ‘Toorak’, ‘Brigalow’, ‘Brian Pastures’ and ‘Swans Lagoon’ Research Stations, and especially the contributions of Paul Williams, Nick Corbet, Peggy Olsson, Tracy Longhurst, Trudy Obst, Neil Cooper, Steve O’Connor and Russ Tyler.
Ball AJ,
Thompson JM, Pleasants AB
(1996) Seasonal changes in body composition of growing Merino sheep. Livestock Production Science 46, 173–180.
| Crossref | GoogleScholarGoogle Scholar |
[Verified 17 March 2009]
Chippindale AK,
Chu TJF, Rose MR
(1996) Complex trade-offs and the evolution of starvation resistance in Drosophila melanogaster. Evolution 50, 753–766.
| Crossref | GoogleScholarGoogle Scholar |
[Verified 17 March 2009]
Frisch JE, Vercoe JE
(1977) Food intake, eating rate, weight gains, metabolic rate and efficiency of food utilization in Bos taurus and Bos indicus crossbred cattle. Animal Production 25, 343–358.
[Verified 17 March 2009]
Hopkins IR
(1977) The effect of level of environment on sex differences in pre-weaning growth rate in beef cattle. Animal Production 25, 47–51.
[Verified 17 March 2009]
Sawalha RM,
Brotherstone S,
Lambe NR, Villanueva B
(2008) Association of the prion protein gene with individual tissue weights in Scottish Blackface sheep. Journal of Animal Science 86, 1737–1746.
| Crossref | GoogleScholarGoogle Scholar |
[Verified 17 March 2009].
Turner JW
(1980) Genetic and biological aspects of Zebu adaptability. Journal of Animal Science 50, 1201–1205.
[Verified 17 March 2009]
Veerkamp RF,
Beerda B, van der Lende T
(2003) Effects of genetic selection for milk yield on energy balance, levels of hormones, and metabolites in lactating cattle, and possible links to reduced fertility. Livestock Production Science 83, 257–275.
| Crossref | GoogleScholarGoogle Scholar |

1 Animal Genetics and Breeding Unit is a joint venture of New South Wales Department of Primary Industries and the University of New England.