Comparing three filter-bag types for accuracy and precision of in sacco undigestible neutral detergent fibre determination of various dicotyledon forages
A. Jonker A * and M. M. Della Rosa
A * and M. M. Della Rosa  A
A
A
Abstract
Internationally, undigestible neutral detergent fibre (uNDF) has become a standard feed analysis to quantify the potential digestible NDF fraction and to be an internal marker to estimate diet total-tract digestibility in animal studies. However, this analysis is labour-intensive/expensive and not commercially available in New Zealand and many other countries.
To compare the uNDF after rumen incubation determined for various forages by using filter bags that can be used sequentially for NDF and uNDF analysis with the standard method using Saatifil bags.
Freeze-dried material of 15 forage samples (mainly dicotyledons) was weighed into three types of bags (Saatifil, 12 μm; F57, 25 μm; F58, 6–9 μm) in sextuplicate and then incubated across two runs for 12 days in the rumen of a fistulated non-lactating pasture-fed dairy cow. After incubation, the NDF of the residue was determined.
Overall, the average (±within forage sample CV%) uNDF was 5.4% (9.8), 6.7% (15.2) and 6.3% (16.0) of DM for Saatifil, F57 and F58, respectively, and the mean bias (±95% confidence interval, CI) was 1.3 (0.3–2.3) and 1.2 (−0.1–2.5) for F57 and F58 versus Saatifil respectively. The 95% CI indicated that the intercept and slope for the orthogonal regression of F57 and F58 versus Saatifil were not different from zero and one, respectively, and the correlation for F57 with Saatifil was stronger than for F58 (r = 0.65 and 0.40 respectively).
Both F57 and F58 bags resulted in similar uNDF values as with the Saatifil bag; however, numerically uNDF values were greater and with a large within-sample CV.
The results of the current study suggest that the F57 bag is suitable for uNDF determination, but that some further modifications to the protocol need to be made to improve the accuracy and precision of the uNDF determination.
Keywords: alternative forage, digestibility, fodder crops, in situ, indigestible fibre, marker, proxy, pulverized sample.
Introduction
Undigestible neutral detergent fibre (uNDF) has become an analytical standard for several feed-evaluation systems to quantify the potential digestible NDF fraction of a feed (Sniffen et al. 1992; Volden 2011). Unlike, neutral detergent fibre (NDF), uNDF is a feed fraction with a digestibility of zero. Subtracting uNDF from NDF improves the predictability of the dry-matter digestibility (DMD) of a feed (Mertens 2015). Furthermore, performing total faecal collection for determination of DMD is not practical in most animal studies, and therefore dietary internal markers have been used to estimate apparent degradation after passing through the digestive tract, i.e. from faecal spot samples (Tamminga et al. 1989; Huhtanen et al. 1994). Undigestible NDF has been found to be a suitable internal maker for this purpose in several studies (Morris et al. 2018; Beck et al. 2023).
However, uNDF analysis is not commercially available in New Zealand and the in sacco nylon-bag incubation methods or in vitro methods are labour-intensive/expensive (Lund et al. 2004; Foster et al. 2023). The ANKOM F57 bags (ANKOM Technology, Macedon, NY, USA) are cheaper than nylon bags and can be used sequentially for both NDF analysis and rumen incubations and therefore reduce labour requirements and costs compared with using nylon bags or in vitro methods. Furthermore, the F57 bags can be used for boiling samples in neutral detergent solution in a cooking pot, which allows performing NDF analysis on a large number of samples at once (McRoberts and Cherney 2014), reducing the labour requirements for NDF analysis and, consequently, for uNDF analysis. However, the initial analysis with ANKOM F57 bags in our laboratory showed some challenges for uNDF determination, especially with forage rape that had a low fibre content (Della Rosa et al. 2022a), which might result in an overestimation of the DMD. (To note, acid detergent lignin was also below the detection limit when the same forage sample was analysed using the ANKOM F57 method by a commercial laboratory). Dicotyledon forages such as forage rape (and other forage brassicas), lucerne and white clover have different and less tensile fibre than do monocotyledons and typically are pulverised during grinding and turn into a dusty bulky fine powder, independent of grinding screen size. The relatively large pore size of 25 μm of the F57 bag might have allowed undigested small particles to escape from the bag (Damiran et al. 2008) and therefore reduce the uNDF value. ANKOM has released a new bag (F58) with a pore size of 6–9 μm for fibre analysis of samples that have had significant pulverization during grinding. Unlike the F57 bag, the F58 bag is not specifically designed for in vitro or in sacco rumen incubations, but the pore size of F58 bag is similar to that of the 12 μm of the Saatifil bag (Saati S.p.A., Veniano, Italy) that is recommended for uNDF analysis for the NorFor feed-evaluation system (Volden 2011). The objective of the current study was to compare the uNDF for various forages after 12 days of in sacco rumen incubation by using ANKOM F57 and F58 filter bags and Saatifil bags. Second, the effect of sequentially determining NDF and uNDF and bag sealing direction were tested for the two ANKOM bags. The hypothesis was that uNDF determined with the F58 would be similar to uNDF determined with the Saatifil bag, whereas uNDF determined with the F57 would be underestimated.
Materials and methods
Two in sacco rumen incubation experiments with a fistulated non-lactating non-pregnant Jersey dairy cow was conducted. The rumen incubations adhered to the guidelines of the 1999 New Zealand Animal Welfare Act and the AgResearch code of ethical conduct, and animal manipulations were approved by the AgResearch Animal Ethics Committee (#1933). The cow was grazing ryegrass-based pasture during the in sacco rumen incubations.
In Experiment 1, samples from different forage species (Table 1; some of which have known high (e.g. plantain) or low (e.g. forage rape) uNDF contents were compared with ryegrass (Della Rosa et al. 2022a; 2022b)) were weighed in sextuplicate into three filter-bag types, namely, Saatifil (12 μm pore size, 10 × 10 cm, fabric code PES 12/6), and ANKOM F57 and F58 bags (25 μm 6–9 μm pore size, respectively, dimensions of the trapezoid shaped bags were approximately 5 cm at the top, 3 cm wide at the bottom and 5 cm high). The bags were incubated for 12 days in the rumen of a fistulated non-lactating dairy cow grazing ryegrass-based pasture. Two other procedures also were tested in Experiment 1. A second set of F57 and F58 bags containing substrates was analysed for NDF followed by the determination of uNDF, and a third set of F57 and F58 bags containing substrates was heat-sealed with the sides of the bags pushed to each other (instead of the flat side of the bag) to maintain an gas pocket inside the bag (Fig. 1).
| Forage species and cultivar | Growth stage | Harvest season | NDF (%DM) | |
|---|---|---|---|---|
| Experiment 1 | ||||
| Brassica napus cv. Titan | Vegetative | Summer | 34.0 | |
| Brassica oleracea cv. Regal | Vegetative | Winter | 30.7 | |
| Plantago laceolata cv. AgriTonic | Vegetative | Spring | 44.5 | |
| Lolium perenne cv. Ceres 150 | Veg/rep | Summer | 60.4 | |
| Trifolium repens cv. Brace | Flowering | Summer | 45.5 | |
| Experiment 2 | ||||
| Cichorium intybus cv. Puna | Vegetative | Autumn | 39.4 | |
| Brassica oleracea cv. Coleor | Vegetative | Autumn | 29.1 | |
| Brassica oleracea cv. Kestrel | Vegetative | Autumn | 33.8 | |
| Trifolium pratense cv. Blamash | Vegetative | Autumn | 46.4 | |
| Trifolium repens cv. Brace | Vegetative | Autumn | 45.7 | |
| Trifolium repens cv. Legacy | Vegetative | Autumn | 41.4 | |
| Trifolium repens cv. Attribute | Vegetative | Autumn | 48.7 | |
| Trifolium repens cv. Tribute | Vegetative | Autumn | 45.1 | |
| Trifolium repens cv. Barblanka | Vegetative | Autumn | 44.5 | |
| Trifolium repens cv. Quartz | Vegetative | Autumn | 45.8 | |
The Saatifil bag (left) and ANKOM F57 and F58 bags sealed on the flat sides (top middle and right) and side-by-side (bottom middle and right) to create a gas pocket.

In Experiment 2, 10 samples from dicotyledon forages (Table 1), which are known to result in fine powdery material after grinding, were used, including samples from six white clover cultivars to enable the determination of within-species variance. The forage samples were each weighed into sextuplicate bags for each bag type (Saatifil, F57 and F58) and incubated for 12 days in the rumen of one fistulated cow grazing ryegrass based pasture.
Forage samples used for in sacco incubations
The forage samples used for Experiments 1 and 2 are described in Table 1. The brassica forages were collected in the field by cutting the stems at 15–20 cm above ground level and other forages were collected by cutting the foliage at ~5 cm above ground level by using battery-powered sheep-shearing clippers. The forage harvested was frozen at −20°C and later freeze-dried and ground to pass through either a 1.5 or a 1.0 mm screen by using a Wiley Mill, Mod. 4 (Arthur Thomas Company, Philadelphia, USA). The residual moisture of the freeze-dried samples was determined following Method 934.01 (AOAC 1990).
Samples ground to pass through a 1.0 mm screen were used to quantify NDF. The NDF content of the samples was quantified in triplicate by boiling ~0.5 g of sample in F57 ANKOM bags in a neutral detergent solution (NDS) for 1 h (Van Soest et al. 1991). The bags with samples were boiled in NDS at a ratio of 150 mL NDS/g DM of sample in an kitchen pot similar to that described by (McRoberts and Cherney 2014). After NDS boiling, the bags were rinsed three times with boiling water and then soaked in acetone for 3–5 min. After the acetone had evaporated, the bags were oven-dried at 65°C for 48 h and weighed after the bags were cooled to room temperature in a desiccator.
In sacco rumen incubations for undigestible NDF quantification
The quantity of forage sample weight in each bag type was adjusted according to specific recommendations for Saatifil bags (Volden 2011) and NDF quantification method for ANKOM bags (Method 13, ANKOM 2017). The actual amounts incubated were 1.93 ± 0.06 g dry matter (DM) for Saatifil bags, 0.49 ± 0.02 DM for F57 bags and 0.48 ± 0.02 DM for F58 bags, resulting in sample dry weight to filter-bag surface area ratios of approximately 12.5 mg/cm2 (approximate surface area of 20 cm2 × two sides for ANKOM bags; Adesogan 2005). The forage samples ground through a 1.5 mm screen were used for the Saatifil bags as recommended in the NorFor feed-evaluation system (Volden 2011) and forages samples ground through a 1 mm screen were used for the F57 and F58 ANKOM bags, as recommended in the guidelines of ANKOM (2017).
All bags were heat sealed using a hand-operated, 5-mm impulse heat sealer (MeC 2010 HC). Saatifil and F57 ANKOM bags were sealed using temperature level eight, whereas F58 bags were sealed with temperature level 4.5. The bags containing substrates were contained in three small laundry bags (24 × 34 cm polyester bags with 4 mm pore size), with bags of each treatment in each laundry bag. The small laundry bags were then contained in a 60 × 60 cm laundry bag (4 mm pore size) with a 130 g weight before being placed in the rumen of a fistulated cow. The uNDF was determined on the basis of 288-h ruminal in sacco incubations (Volden 2011) in a fistulated non-lactating non-pregnant dairy cow. In Experiment 1, the bags were rinsed under tap water by hand until the water became clear. In Experiment 2, the bags were contained in a laundry bag (as describe above) and were rinsed with water in a washing machine for ~50 min (‘Delicate’ wash cycle, Mod WF16T9500GV, Samsung, Suwon-si, South Korea).
Rinsed bags were oven dried at 65°C for 48 h and then placed in a desiccator prior to weighing. The residue present following the initial wash was considered undigestible DM (uDM). The dried bags were boiled for 1 h in NDS as described for NDF quantification. Bags were oven dried for 48 h at 65°C and then placed in a desiccator prior to weighing; the residues were considered the uNDF. The bags were processed in six batches per experiment. Each batch included one of the replicates of each treatment. Undigestible NDF was expressed inclusive of residual ash, as follows:
where W1 is the initial bag weight (g, as is), W2 is the initial sample weight (g), W3 is the weight of the dried bag and sample residue following the NDS wash (g), C1 is the blank correction factor following the NDS wash (average weight of dry bag following NDS wash divided by the initial dry weight of the bag). The blank correction factors for uNDF of Experiment 1 were 1.010, 1.075 and 1.059 for Saatifil, F57 and F58 bags respectively, and for Experiment 2 were 0.999, 1.001 and 1.003 for Saatifil, F57 and F58 bags respectively.
Statistical analyses
For each forage sample by bag type (and other procedures tested in Experiment 1), the mean uNDF and within-forage sample coefficient of variations (CV%, based on sexduplicate bags per forage sample) were calculated. Then the mean uNDF and pooled within forage sample CV% (i.e. weighted average of the within sample CV% for the forage samples (five in Experiment 1 and 10 in Experiment 2) within bag type in an experiment) were calculated. The mean bias (i.e. mean difference in uNDF between Saatifil and alternative method) and 95% confidence interval (CI) of the mean bias were calculated for each experiment (Giavarina 2015). The Pearson correlation of uNDF between Saatifil and alternative methods was determined in each experiment.
The orthogonal regression (total least-squares regression) and 95% CI of the intercept and slope for uNDF data across the two experiments between Saatifil and F57 or F58 bags were determined using Minitab (ver. 18; Minitab LCC, State College, Pennsylvania, USA). Bland–Alman plots showing the mean bias and limits of agreement were also generated (Giavarina 2015).
Results
Results of rumen Experiment 1
The mean (±pooled within sample CV%) uNDF was 7.2% (17.9), 9.2% (20.3) and 5.5% (18.6) of DM for Saatifil, F57 and F58 bags, respectively, and the mean bias (±95% CI) was 2.0 (−0.4–4.4) and −1.7 (−3.2 – −0.3) for F57 and F58 versus Saatifil, respectively (Table 2; note, for F57 and F58 these are the averages across the three procedures within bag type). Sealing the F57 and F58 bags from side-to-side instead of on the flat sides or performing sequential NDF analysis before uNDF determination had minor effects on the uNDF determination in terms of mean bias and within-sample pooled CV. The main differences observed in uNDF were due to bag type compared with the Saatifil uNDF determination.
| Item | uNDF (CV%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Saatifil | F57 | F58 | ||||||
| Flat | By side | Flat | Flat | By side | Flat | Flat | ||
| No | No | No | Yes | No | No | Yes | ||
| Forage species | ||||||||
| Forage rape | 5.9 (44.3) | 12.4 (28.0) | 10.3 (32.7) | 4.5 (22.8) | 2.8 (19.7) | 2.8 (45.2) | 2.4 (48.7) | |
| Kale | 2.7 (13.0) | 4.1 (29.9) | 5.2 (17.1) | 3.7 (27.5) | 2.1 (44.8) | 2.8 (4.2) | 1.6 (33.7) | |
| Plantain | 13.9 (9.9) | 21.3 (26.9) | 15.6 (3.8) | 11.8 (13.1) | 12.3 (15.7) | 10.9 (14.8) | 11.5 (10.0) | |
| Ryegrass | 8.3 (13.6) | 6.6 (29.1) | 6.4 (18.2) | 8.7 (10.4) | 6.6 (7.4) | 5.5 (19.8) | 6.9 (16.5) | |
| White clover | 5.4 (18.6) | 5.7 (23.5) | 10.3 (22.7) | 12.1 (13.2) | 4.5 (13.6) | 4.9 (25.8) | 5.2 (29.7) | |
| Mean uNDF | 7.2 (19.9) | 10.2 (27.5) | 9.3 (18.9) | 8.7 (17.4) | 6.0 (20.2) | 6.1 (22.0) | 6.2 (27.7) | |
| Mean diff. from Saatifil | – | 2.8 | 2.3 | 0.9 | −1.6 | −1.9 | −1.7 | |
| 95% confidence interval | – | (−2.1–7.7) | (−1.0–5.7) | (−3.4–5.2) | (−2.7– −0.4) | (−3.8–0.1) | (−3.3– −0.2) | |
| Corr. with Saatifil | – | 0.87 | 0.79 | 0.65 | 0.97 | 0.95 | 0.96 | |
Results of rumen Experiment 2
The average (±pooled within sample CV%) uNDF was 4.5% (4.8), 5.3% (13.4) and 6.9% (12.7) of DM for Saatifil, F57 and F58, respectively (Table 3). The mean bias for uNDF (±95% CI) relative to Saatifil was 1.0 (0.6–1.4) for F57 and 2.5 (2.1–2.9) for F58.
| Item | uNDF (CV%) | |||
|---|---|---|---|---|
| Saatifil | F57 | F58 | ||
| Forage species/cultivar | ||||
| Chicory (cv. Puna) | 4.6 (8.9) | 4.9 (23.7) | 7.5 (10.4) | |
| Kale (cv. Coleor violet) | 2.0 (4.8) | 2.5 (34.6) | 3.9 (20.7) | |
| Kale (Kestrel white) | 2.2 (5.7) | 2.3 (24.1) | 3.8 (16.7) | |
| Red clover (cv. Blamash) | 5.5 (5.9) | 6.6 (2.4) | 8.1 (9.9) | |
| White clover (cv. Brace) | 4.8 (4.3) | 6.0 (8.7) | 7.6 (19.3) | |
| White clover (cv. Legacy) | 5.2 (4.7) | 6.2 (9.3) | 7.7 (9.9) | |
| White clover (cv. Attribute) | 5.8 (2.5) | 7.2 (4.3) | 8.8 (10.2) | |
| White clover (cv. Tribute) | 5.1 (3.0) | 7.0 (10.3) | – | |
| White clover (cv. Barblanka) | 4.7 (1.7) | 5.0 (7.5) | 6.9 (8.5) | |
| White clover (cv. Quartz) | 5.0 (6.0) | 5.6 (8.7) | 7.5 (8.9) | |
| Mean uNDF | 4.5 (4.8) | 5.3 (13.4) | 6.9 (12.7) | |
| Mean diff. from Saatifil | 1.0 | 2.5 | ||
| 95% confidence interval | (0.6–1.4) | (2.1–2.9) | ||
| Corr. with Saatifil | 0.96 | 0.99 | ||
Combined results of Experiments 1 and 2
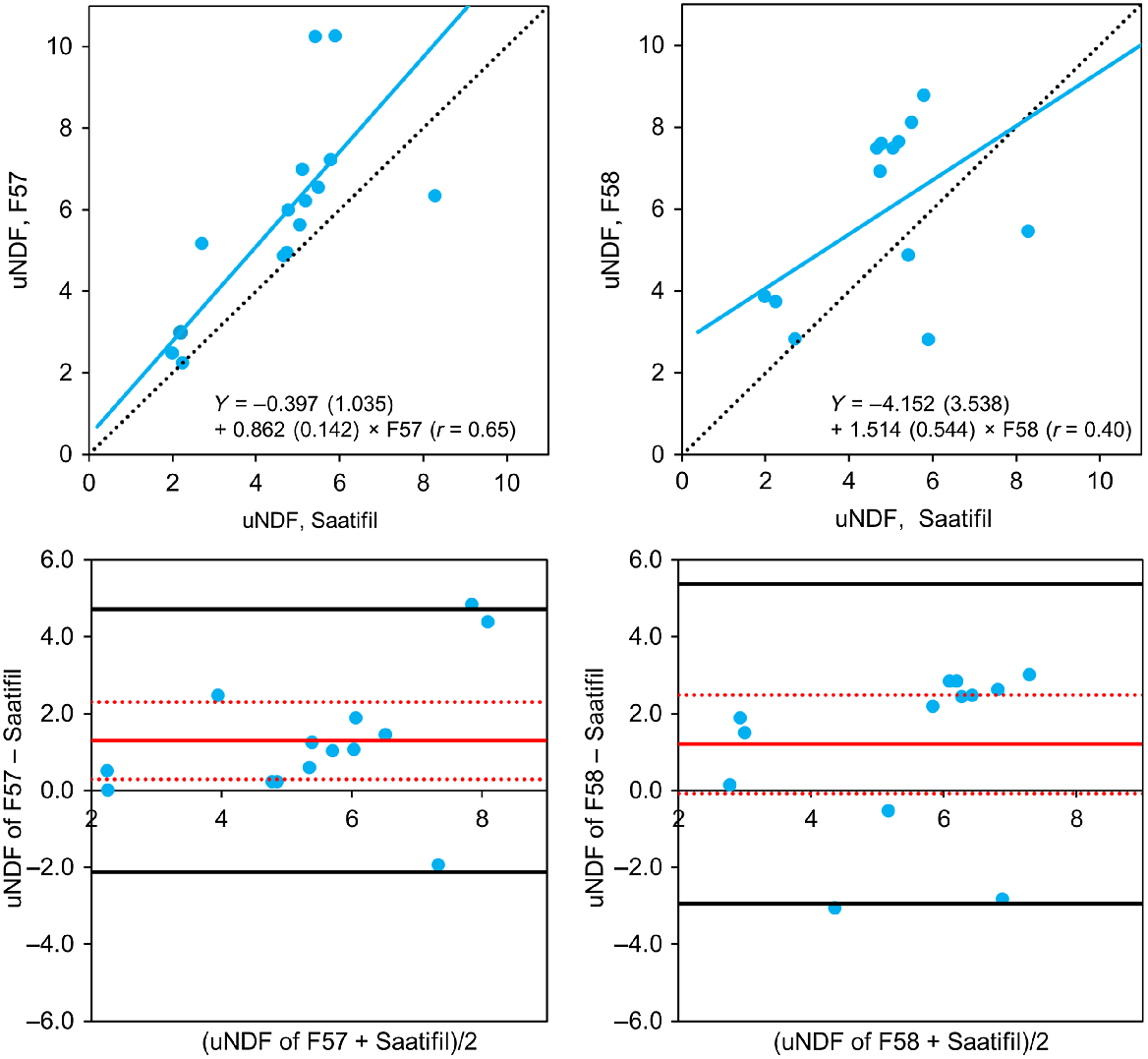
The average uNDF (±pooled within sample CV%) for all forage samples across the two experiments was 5.4% (9.8), 6.7% (15.2) and 6.3% (16.0) of DM for Saatifil, F57 and F58, respectively. The mean bias for uNDF (±95% CI) relative to Saatifil was 1.3 (0.3–2.3) for F57 and 1.2 (−0.1–2.5) for F58 (Fig. 2). The mean squared prediction error (MSPE, % of DM) relative to Saatifil was 2.1% of DM for F57 and 2.7% of DM for F58. The 95% CI indicated that the intercept and slope for the orthogonal regression of Saatifil vs F57 and F58 were not different from zero and one, respectively, suggesting that the uNDF values of F57 and F58 were similar to Saatifil. The correlation of Saatifil with F57 was stronger than for F58 (r = 0.65 and 0.40, respectively).
Orthogonal regression (top graphs) and Bland–Altman plots (bottom grahps) of undigestible neutral detergent fibre (uNDF, % of DM) determined using the F57 or F58 bags compared with the Saatifil bag (left-hand and right-hand graphs, respectively) of various forages incubated in the rumen for 12 days across Experiments 1 and 2 (see Table 1). The Bland–Altman plot shows the mean bias (solid red line), 95% confidence interval for the mean bias (red dotted lines) and limits of agreement (solid black lines). In the orthogonal regressions, the 95% confidence interval for F57 versus Saatifil was −2.423–1.628 for the intercept and 0.585–1.139 for the slope and F58 versus Saatifil was −11.097–2.773 for the intercept and 0.448–2.581 for the slope.
Discussion
The hypothesis that uNDF determined with the F58 would be similar to the uNDF determined with the Saatifil bag and that uNDF determined with the F57 would be underestimated was not supported by findings of the current study. Instead, the F58 bag did not appear to result in improved uNDF determination compared with using the F57 bag, and both resulted in similar values as did the Saatifil bag; however, numerically, uNDF values were greater and with a larger within-forage CV for the two ANKOM bag types. Furthermore, sealing the ANKOM bags from side-to-side to create a air space in the bag or sequentially analysing uNDF after NDF analysis did not result in great changes in the uNDF determination with the F57 and F58 bags, compared with sealing the bags on the flat sides and without pre-NDF determination.
Although the F57 has a larger pore size (25 μm) than recommended (~12 μm), on the basis of findings with nylon bags (Volden 2011; Krizsan et al. 2015), results of the current study and by Norris et al. (2019) did not indicate that the F57 loses more undigested particles than does the F58 bag, which has a smaller pore size of 6–9 μm. This was similar to the findings of Norris et al. (2019), but different from the findings of Adams et al. (2020) who observed that uNDF was approximately 20% greater in F58 than in F57 bags. Casali et al. (2009) tested and observed no undigested particle losses from F57 bags with different types of feed, but they found particle losses from nylon bags with 50 μm pore size. Using electron microscopy, they observed that the fabric of the nylon bag had a geometric arrangement, whereas the fabric of the F57 bag had an irregular mesh arrangement, which might affect the optimum pore size for uNDF analysis. It was observed that the external layer of most of the F58 bags was peeled and rolled, retaining some particles after washing (M. M. Della Rosa, pers. obs.). This could have contributed to the greater within-sample CV observed for the F58 bags. Similar observations for F58 bags were described by Adams et al. (2020).
The uNDF values of clover and ryegrass with the three bag types fell largely within the range of 2.5–11.1% of DM across grass/clover samples from Denmark analysed according to Volden (2011) and reported in Krämer et al. (2012) and the NorFor feed tables (https://feedstuffs.norfor.info/), suggesting that values observed in this study were within the normal range. However, numerically the uNDF values were, on average, greater with the F57 bag during both experiments and F58 bag in Experiment 2 than with the Saatifil bag, suggesting that the true uNDF might not yet have been reached.
A factor found to affect uNDF was the sample size in the bag, with uNDF being ~10% less when the sample size was reduced from 40 to 20 mg/cm2 (Norris et al. 2019). The sample size in the bag in the current study was approximately 12.5 mg/cm2 for the two ANKOM bags and slightly less at 9.65 mg/cm2 for the Saatifil bag. Fluffy, bulky samples can make the ANKOM bag appear very full and become compacted when the bag is sealed. Therefore, we tested whether sealing the ANKOM bags from side-to-side, which created an air space in the bag, would improve the uNDF determination. Especially in the F57 bag, it appeared to make the uNDF determination worse, both in terms of a numerically larger mean bias than in the Saatifil bag and a greater within-sample CV. Coblentz et al. (2019) compared incubating samples in F57 bags at 12.5 mg/cm2 or 6.25 mg/cm2 in DaisyII in vitro system and found that results with the smaller sample size were in closer agreement with results using the in vitro method, based on Goering and Van Soest (1970), which was based on incubating each sample in buffered rumen fluid in Erlenmeyer flasks, followed by NDS washing after incubation and filtering through crucibles. Therefore, reducing the sample size in the bag might further improve the accuracy of the uNDF determination.
Sequentially determining NDF and uNDF versus direct determination of uNDF had little effect on the accuracy and precision of the uNDF determination. In contrast to these findings, Battelli et al. (2020) found that DMD estimation in pigs was more accurate when using uNDF determination after NDF analysis than using uNDF directly. These suggest that sequential determination of NDF and uNDF can be employed.
Feed uNDF was found to be 10–29% less when incubating in the rumen for 576 h than when incubating 288 h (in F57 and F58 bags) (Norris et al. 2019; Adams et al. 2020). The faecal recovery of NDF was over 100% for both 288 and 576 h of incubation, as well as for the F57 and F58 bas in the study of Adams et al. (2020), which indicates that the true uNDF was still not reached, even after 576 h of incubation. This was mainly due to the uNDF of the feed, which reduced to a greater extent between 288 and 576 h of incubation than did the uNDF of faeces (Norris et al. 2019; Adams et al. 2020). In contrast, Beck et al. (2024) observed that the recovery of uNDF was near 100% when incubating F57 bags in the rumen for 288 h and this was shortened to 120 h if the feed was predigested with pepsin and HCl before rumen incubations. These studies did not compare the ANKOM bags with nylon-type filter bags with a small pore size. It is therefore not clear whether uNDF in these bags would further decrease when incubated for more than 288 h in the rumen of fistulated cows.
The within-sample CV (precision) was similar for the three bag types tested in the first experiment, but the pooled CV was less for the Saatifil bag in the second experiment than for the F57 and F58 bags. The similar pooled CV for the F57 and F58 bags in the current study was different from the findings of Adams et al. (2020) who observed a greater CV for F58 than for F57 bags. The within-sample CV of Experiment 1 was similar to that observed in Norris et al. (2019), but this was larger than that observed in Experiment 2 and that observed by Adams et al. (2020). This might be partly due to the feeds incubated and, in the current study, the bags were hand-washed after Experiment 1 while a washing machine was used after Experiment 2. However, Cherney et al. (1990) observed similar variance for filter bags washed by hand or with a washing machine, whereas residual DM was greater in hand-washed than machine-washed bags.
The clover samples in the F57 bags in Experiment 2 had a smaller within-sample CV than did the chicory and kale samples in the F57 bags in Experiment 2, suggesting that there might be a difference in the precision of the uNDF determination for different forages, as was also observed by Norris et al. (2019). The samples of chicory, forage rape and kale contained less NDF and less uNDF than did the clover samples and this results in a lower signal-to-noise ratio, which might increase the error (imprecision) of the uNDF determination. The signal-to-noise ratio can be improved by incubating a greater amount of sample, but the F57 bag comes only in one size, and, as indicated above, incubating a greater sample in the F57 is not advisable (Norris et al. 2019).
The within-sample CV for the NDF analysis of the feed samples was 6.0%, which also contributed to the error of the uNDF analysis. Norris et al. (2019) modelled that the number of bags incubated per sample needs to be increased by three- to four-fold to improve the precision to enable detection of differences between feeds or diets. Although there are many recommendations in the literature to improve the accuracy of the uNDF determination, there are very limited recommendations for improving the precision of the uNDF determination (Foster et al. 2023).
Implications
The F58 bag did not appear to result in improved uNDF determination compared with using the F57 bag, and both resulted in similar values as with the Saatifil bag; however, numerically uNDF values were greater and with a large within-sample CV for the two ANKOM bag types. The results of the current study suggest that some further modifications to the protocol need to be made to improve the accuracy and precision of the uNDF determination using the F57 bag. The F57 bag can be used sequentially (i.e. weigh samples only once) to determine the different fibre fractions (NDF and uNDF) and using the simple kitchen pot method for NDF determination, making it a more practical alternative than uNDF determination with the Saatifil bag.
Conflicts of interest
A. Jonker is a Guest Editor on the Australasian Dairy Science Symposium 2024 collection in Animal Production Science. To mitigate this potential conflict of interest he had no editor-level access to this manuscript during peer review. The other author has no other conflicts of interest to declare.
Declaration of funding
This project was financially supported by the method development fund of the AgResearch Strategic Science Investment Fund (Ministry of Business, Innovation and Employment, Wellington, New Zealand).
References
Adams JM, Norris AB, Dias Batista LF, Rivera ME, Tedeschi LO (2020) Comparison of in situ techniques to evaluate the recovery of indigestible components and the accuracy of digestibility estimates. Journal of Animal Science 98, skaa296.
| Crossref | Google Scholar |
Adesogan AT (2005) Effect of bag type on the apparent digestibility of feeds in ANKOM DaisyII incubators. Animal Feed Science and Technology 119(3–4), 333-344.
| Crossref | Google Scholar |
ANKOM (2017) Neutral detergent fiber in feeds: filter bag technique (for A2000 and A2000I). Available at https://www.ankom.com/sites/default/files/document-files/Method_13_NDF_A2000.pdf [accessed 19 August 2024]
Battelli M, Rapetti L, Rota Graziosi A, Colombini S, Crovetto GM, Galassi G (2020) Use of undigested NDF for estimation of diet digestibility in growing pigs. Animals 10(11), 2007.
| Crossref | Google Scholar | PubMed |
Beck MR, Proctor JA, Smith JK, Gouvêa VN, Kasuske Z, Foote AP, Gunter SA, Beck PA (2023) Assessing different sampling regimens for estimating dietary characteristics using internal markers. Applied Animal Science 39(6), 411-422.
| Crossref | Google Scholar |
Beck MR, Griffin ML, Proctor JA, Foster R, Long NS, Smith JK, Gouvêa VN (2024) Optimization of indigestible neutral and acid detergent fiber measurement protocols. Applied Animal Science 40(2), 124-131.
| Crossref | Google Scholar |
Casali AO, Detmann E, Filho SdCV, Pereira JC, Cunha Md, Detmann KdSC, Paulino MF (2009) Estimation of fibrous compounds contents in ruminant feeds with bags made from different textiles. Revista Brasileira de Zootecnia 38, 130-138.
| Crossref | Google Scholar |
Cherney DJR, Patterson JA, Lemenager RP (1990) Influence of in situ bag rinsing technique on determination of dry matter disappearance. Journal of Dairy Science 73(2), 391-397.
| Crossref | Google Scholar |
Coblentz WK, Akins MS, Ogden RK, Bauman LM, Stammer AJ (2019) Effects of sample size on neutral detergent fiber digestibility of triticale forages using the Ankom DaisyII incubator system. Journal of Dairy Science 102(8), 6987-6999.
| Crossref | Google Scholar | PubMed |
Damiran D, DelCurto T, Bohnert DW, Findholt SL (2008) Comparison of techniques and grinding size to estimate digestibility of forage based ruminant diets. Animal Feed Science and Technology 141(1–2), 15-35.
| Crossref | Google Scholar |
Della Rosa MM, Sandoval E, Reid P, Luo D, Pacheco D, Janssen PH, Jonker A (2022a) Substituting ryegrass-based pasture with graded levels of forage rape in the diet of lambs decreases methane emissions and increases propionate, succinate, and primary alcohols in the rumen. Journal of Animal Science 100(9), skac223.
| Crossref | Google Scholar |
Della Rosa MM, Sandoval E, Luo D, Pacheco D, Jonker A (2022b) Effect of feeding fresh forage plantain (Plantago lanceolata) or ryegrass-based pasture on methane emissions, total-tract digestibility, and rumen fermentation of nonlactating dairy cows. Journal of Dairy Science 105(8), 6628-6638.
| Crossref | Google Scholar | PubMed |
Foster JL, Smith WB, Rouquette FM, Tedeschi LO (2023) Forages and pastures symposium: an update on in vitro and in situ experimental techniques for approximation of ruminal fiber degradation. Journal of Animal Science 101, skad097.
| Crossref | Google Scholar |
Giavarina D (2015) Understanding Bland Altman analysis. Biochemia Medica 25(2), 141-151.
| Crossref | Google Scholar | PubMed |
Huhtanen P, Kaustell K, Jaakkola S (1994) The use of internal markers to predict total digestibility and duodenal flow of nutrients in cattle given six different diets. Animal Feed Science and Technology 48(3–4), 211-227.
| Crossref | Google Scholar |
Krämer M, Weisbjerg MR, Lund P, Jensen CS, Pedersen MG (2012) Estimation of indigestible NDF in forages and concentrates from cell wall composition. Animal Feed Science and Technology 177(1–2), 40-51.
| Crossref | Google Scholar |
Krizsan SJ, Rinne M, Nyholm L, Huhtanen P (2015) New recommendations for the ruminal in situ determination of indigestible neutral detergent fibre. Animal Feed Science and Technology 205, 31-41.
| Crossref | Google Scholar |
Lund P, Weisbjerg MR, Ahvenjärvi S, Huhtanen P, Udén P, Olafsson B, Volden H (2004) Nordic ringtest on INDF content and NDF degradation characteristics in three feeds. Journal of Animal and Feed Sciences 13, 139-142.
| Crossref | Google Scholar |
McRoberts KC, Cherney DJR (2014) Low-infrastructure filter bag technique for neutral detergent fiber analysis of forages. Animal Feed Science and Technology 187, 77-85.
| Crossref | Google Scholar |
Morris DL, Rebelo LR, Dieter PA, Lee C (2018) Validating intrinsic markers and optimizing spot sampling frequency to estimate fecal outputs. Journal of Dairy Science 101(9), 7980-7989.
| Crossref | Google Scholar | PubMed |
Norris AB, Tedeschi LO, Muir JP (2019) Assessment of in situ techniques to determine indigestible components in the feed and feces of cattle receiving supplemental condensed tannins. Journal of Animal Science 97(12), 5016-5026.
| Crossref | Google Scholar | PubMed |
Sniffen CJ, O’Connor JD, Van Soest PJ, Fox DG, Russell JB (1992) A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. Journal of Animal Science 70(11), 3562-3577.
| Crossref | Google Scholar | PubMed |
Tamminga S, Robinson PH, Meijs S, Boer H (1989) Feed components as internal markers in digestion studies with dairy cows. Animal Feed Science and Technology 27(1–2), 49-57.
| Crossref | Google Scholar |
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74, 3583-3597.
| Crossref | Google Scholar | PubMed |


