Feeding twin-bearing Merino ewes above the metabolisable energy requirements for maintenance during late gestation increases the duration of parturition of the first-born lamb
Amy L. Munn A * , William H. E. J. van Wettere A , Alyce M. F. Swinbourne B and Alice C. Weaver B
A * , William H. E. J. van Wettere A , Alyce M. F. Swinbourne B and Alice C. Weaver B
A
B
Abstract
In Australia, approximately 53% of lamb deaths are caused by dystocia. One of the main welfare concerns in the sheep industry is under- and overfeeding ewes, which may be contributing to cases of dystocia.
This pilot study aimed to investigate how increasing energy intake affects the duration of parturition and predictors of lamb survival.
On Day 100 of gestation (dG), 20 twin-bearing and 10 singleton Merino ewes were selected and allocated to three treatment groups; (1) singleton ewes fed at 1.0× maintenance levels (n = 10); (2) twin-bearing ewes fed at 1.0× maintenance levels (n = 10) or (3) twin-bearing ewes fed at 1.25× maintenance (n = 10). Ewes were housed indoors in individual pens. Urine and blood were sampled from ewes on dG 130, 140, then daily from dG 145 through to parturition, and blood was sampled at the onset of parturition and 30 min post-partum. Urine was analysed for pH and blood was analysed for metabolic parameters, mineral concentration and acid–base balance. Predictors of lamb survival from birth to 24 h consisted of weight, rectal temperature, blood glucose and lactate, and body morphology.
Serum calcium in late gestation and blood base excess pre-parturition were higher in Singletons 1M compared with Twins 1M (P < 0.05). The Twins 1.25M group took longer to give birth to the first-born lamb (104.4 ± 21.1 min) compared with the Twins 1M group (44.1 ± 6.6 min; P = 0.015). There were no differences in the predictors of lamb survival measures between the twin-bearing groups (P > 0.05).
Ewes from the Twins 1.25M group took significantly longer to give birth to the first-born lamb. There were no other significant findings between the twin-bearing groups, including lamb liveweight, however, lambs born to ewes from the Twins 1.25 group were numerically heavier, which may explain the increase in parturition length.
Feeding ewes above maintenance did not provide any production benefits to metabolic health or any other physiological parameters. Producers should avoid overfeeding ewes during late gestation.
Keywords: calcium, glucose, ketone bodies, maternal supplementation, Merino, nutrition, parturition, sheep.
Introduction
In Australia, the average lamb mortality rate is between 20 and 30% (Hinch and Brien 2014), and approximately 53% of lamb mortality and approximately 35% of ewe mortality can be associated with prolonged and/or assisted parturitions (dystocia) (Bruce et al. 2021). Ewe and lamb deaths associated with dystocia costs the industry approximately A$780 million annually (Bruce et al. 2021), making dystocia a significant economical and welfare concern in Australia.
There are many factors that cause dystocia, and these can vary between breeds. These factors include genetics, ewe weight and parity, lamb birthweight, litter size and nutrition (Dwyer and Lawrence 2005; Dwyer and Bünger 2012; Refshauge et al. 2016; Horton et al. 2017; Jacobson et al. 2020). Nutrition, in particular the role of energy, has been linked to incidences of dystocia; however, there is limited information on how ewe metabolic state can affect the parturition process (Jacobson et al. 2020).
A recent report assessing and addressing sheep welfare in Australia found that under- and overfeeding ewes was one of the main welfare concerns found on farms (Doyle 2018). Furthermore, based on a producer survey (n = 2003), only 29% of producers in Australia manage singleton and twin-bearing ewes separately, and may be under- or overfeeding their flocks as a result due to the different energy requirements of multiple-bearing ewes (Sheep Sustainability Framework 2022). Under- and overfeeding is a risk factor for pregnancy toxaemia (Crilly et al. 2021), which is a disease that can increase the risk of dystocia in sheep (Barbagianni et al. 2015). In contrast, increasing energy intake can improve ewe condition and in turn improve milk production, as well as lamb survival due to an increase in fetal growth and milk intake (Thompson et al. 2011; McGovern et al. 2015a, 2015b), however, the incidence of dystocia is unknown.
Although a significant proportion of ewes may be overfed, the current understanding of how increased energy intake impacts the duration of parturition and subsequent predictors of lamb survival is limited. Therefore, this project investigated (1) how energy intake of ewes during late pregnancy impacts the duration of parturition and predictors of lamb survival in twin-bearing ewes, and (2) whether there are differences in metabolic state, mineral status and acid–base balance between singleton and twin-bearing ewes fed at maintenance levels. It was hypothesised that (1) ewes with higher energy intake would have an increased duration of parturition as a result of heavier lambs, and (2) ewes bearing singleton lambs would have improved metabolic state, mineral status and acid–base balance during the transition period compared with twin-bearing ewes.
Materials and methods
This pilot experiment was approved by the Department of Primary Industries and Regions’ (South Australia) Animal Ethics Committee (#06/21) and conducted in accordance with the ‘Australian code for the care and use of animals for scientific purposes 8th edition’ (National Health and Medical Research Council: Canberra, 2013). All animal work was completed during August/September 2021 at the South Australian Research and Development Institute’s Turretfield Research Centre, Rosedale, South Australia.
Animal selection and dietary groups
On Day 100 of gestation (dG), 20 twin-bearing and 10 singleton Merino ewes that were artificially inseminated (Merino sire) were selected based on liveweight (LW), body condition score (BCS) and age (ranging from 3 to 4 years-old). Ewes were group housed and managed in small outdoor pens (10 m2; n = 5 ewes per pen; twin-bearing and singleton ewes were housed separately) to acclimatise ewes to human interaction. Ewe LW and BCS were measured weekly to ensure healthy weights were maintained during gestation. At dG 130, ewes were moved indoors into individual pens (2.4 m2) and allocated to three treatment groups; (1) singleton ewes receiving a basal diet at 1.0 times maintenance level (Singletons 1M; n = 10); (2) twin-bearing ewes receiving a basal diet at 1.0 times maintenance level (Twins 1M; n = 10); (3) twin-bearing ewes receiving a basal diet at 1.25 times maintenance (Twins 1.25M; n = 10).
Diets consisted of a commercial pellet (metabolisable energy (ME): 12.9 MJ/kg dry matter (DM), crude protein (CP): 18.7% DM;Ewe and Lamb Pellet, Laucke Feed Mills, Daveyston, SA, Australia) containing a mineral and vitamin pre-mix, barley grain (ME: 13.3 MJ/kg DM, CP: 12.3% DM), peas (ME: 13.3 DM, CP: 25.8% DM) and oaten hay (ME: 8.1 MJ/kg DM, CP: 5.9% DM). Ewes were fed 200 g of the commercial pellet and 200 g of oaten hay. The amount of barley grain and peas were adjusted based on the Agricultural and Food Research Council recommendations (Alderman and Cottrill 1996) to meet the metabolisable energy requirements of 80 kg singleton and twin-bearing maintenance levels at each stage of gestation. To reach 1.25 times maintenance requirements, the metabolisable energy requirements of twin-bearing ewes were increased by 25%. Each ingredient was individually weighed to meet the experimental diet requirements of 1.0× or 1.25× maintenance feed. Feed residuals were recorded on a daily basis.
Ewe pre-partum measures
On dG 130, 140 and then daily from dG 145 until parturition, a 10 mL blood and approximately 30 mL urine sample were sampled from each ewe. Blood was collected via jugular venepuncture from alternate sides of the neck using a 10 mL syringe and 18-gauge (G) 1.5″ needle, and then dispensed evenly across a 9 mL Lithium Heparin and 9 mL clot activator vacutainer tube (BD Vacutainer, BD, Belliver Industrial Estate, Plymouth, UK) and stored on ice upon collection. The remaining 1 mL of whole blood was used to measure ketone and glucose levels (Abbots Freestyle Optium Neo, Melbourne, Vic, Australia). Samples in Lithium Heparin tubes were centrifuged at 3024 g for 15 min within 1 h of collection. Samples in clot activator tubes were stored at 4°C for 24 h to allow the sample to clot before being centrifuged. Plasma and serum supernatant were divided into three aliquots and stored at −80°C. Urine samples were collected via transient apnoea as previously described in Benech et al. (2015) into a 70 mL plastic container (Sardsted, AG & Co. Kg, Germany). Urine pH was immediately analysed using a benchtop pH meter (CyberScan pH 510, Eutech Instruments, Thermo Fisher Scientific Inc).
Parturition measures
From dG 147, ewes were monitored 24 h per day for the onset of parturition through both direct and video surveillance observation using an infrared video surveillance system previously described by Murdock et al. (2021). Straw bedding was provided to ewes when signs of parturition were shown (vaginal discharge, contractions, visible amniotic sac). At the onset of parturition, as determined by the observance of visible contractions or expulsion of an amniotic sac (whichever was observed first), a 10 mL blood sample was collected from the ewe, and again 30 min after the delivery of the first lamb for singleton ewes or second-born lamb for twin-bearing ewes. At birth of the lamb, a 1 mL blood sample was collected via jugular venepuncture using a 2 mL syringe and 21 G 1″ needle. Both the ewe and lamb blood samples were immediately measured for blood gas, chemistry and metabolites using a blood gas analyser (EPOC, Alere, Waltham, MA, USA). The ewe blood samples were processed and stored as previously described in the ewe pre-partum measures section.
When a lamb was born, the time of birth, birth type (singleton; twin), parturition difficulty score (1 = no assistance, 2 = lamb position corrected, and ewe left to lamb naturally, 3 = manually delivered) (Dwyer 2003), lamb birth order (if twins) and meconium score (1 = no staining, 2 = mild staining, 3 = moderate staining and 4 = severe staining) (Brougham et al. 2020) were recorded.
Post-partum measures
At 4 h post-parturition, measures were recorded/collected from each lamb consisting of LW, sex, body morphology (crown–rump and forelimb length, thoracic and abdominal circumference and crown width) and rectal temperature, and lambs were tagged with an individual identification ear tag. A 3 mL blood sample was collected from the lamb using a 3 mL syringe and 21 G 1″ needle via jugular venepuncture, whole blood glucose was measured and the sample dispensed into a 5 mL clot activator vacutainer tube (BD Vacutainer). The sample was processed and stored in duplicate as previously described in the ewe pre-partum measures section. At 24 h post-parturition, the ewe and lamb/s were weighed, and ewe BCS was recorded upon exiting the lambing shed. Lamb ponderal and body mass index were calculated using a previously described method Baxter et al. (2008). Seven days after birth of the last lamb, all animals were returned to a paddock and monitored daily until lambs were marked (tails removed, vaccinated, and males castrated) at approximately 40 days of age, at which point the lambs were weighed again.
Retrospective sample analysis and behavioural observation
Ewe serum was analysed for calcium, cholesterol, phosphate (reagent; Thermo Fisher), beta-hydroxybutyrate (BHB), non-esterified fatty acids (NEFA) and magnesium (reagent; Randox) via Konelab20Xti (Thermo Scientific, Finland) by the University of Sydney, Veterinary Pathology Diagnostic Services, NSW, Sydney.
Footage of parturition (infrared video surveillance) was backed up daily onto external hard drives and retrospectively analysed with the start of parturition considered to be during the second stage of labour when the ewe was actively pushing/contracting and the end of parturition being the expulsion of the fetus.
Sample size estimations
Duration of parturition was the outcome of interest in this study and the ewe was the experimental unit used to determine sample size. The software G*Power (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany, ver. 3.1.9.7) was used to estimate sample size. A standard deviation of 0.6 (10 min) was determined from previous studies using the same facilities with duration of parturition one of the outcomes of interest. The inputs provided into the software consisted of an effect size = 0.5, α = 0.05 and power = 0.8. Based on the output from the software, the total number of ewes per treatment was estimated to be 10.
Statistical analyses
SPSS (IBM) ver. 29 was used for statistical analysis and P value of <0.05 was considered significant and <0.1 was considered a trend. A general linear model was used to analyse scale variables, a multinominal logistic regression was used to analyse ordinal variables (meconium stain score), a chi-squared analysis was used to analyse categorical variables (assistance at parturition). A repeated measure mixed model analysis was used to assess the effect of the dietary treatment over time on mineral and metabolite concentrations during gestation (dG 130, 140, Day −2 and −1 relative to parturition). A Kaplan–Meier parametric time failure model was used to analyse time events (duration of parturition) on the basis of satisfying the model’s assumptions. The experimental unit used was the ewe, due to each animal receiving the experimental diet in individual pens. When analysing ewe data, the dependent variables consisted of blood parameters, duration of parturition and assistance rate, urine pH, LW and BCS. The independent variables include dietary group, ewe age and birth type (singleton versus twin-bearing). When analysing lamb data, dependent variables consisted of blood parameters, LW, meconium score, body morphology and rectal temperature. The independent variables included dietary group, ewe age, sex and birth type. A linear regression was performed with parturition length as the dependent variable and metabolic, mineral and acid–base balance parameters for the independent variables for all twin-bearing ewes in the study. One ewe from each treatment group was removed from the project due to either metabolic disease or because the assumed fetal number was found to be incorrect when the ewe gave birth, resulting in nine ewes per treatment for the statistical analysis.
Results
Metabolic state, mineral status and acid–base balance in singleton vs twin-bearing ewes fed at maintenance levels and in twin-bearing ewes fed at different maintenance levels
The metabolic parameters blood glucose, ketones, and serum NEFAs, cholesterol and BHB (P > 0.05) were similar for singleton and twin-bearing ewes fed at 1.0 times maintenance levels during late gestation (Table 1). Metabolic state parameters were also were similar (P > 0.05) for both the Twins 1M group and Twins 1.25 group during late gestation (Table 1).
| Singletons 1M | Twins 1M | Twins 1.25M | P-value | |||
|---|---|---|---|---|---|---|
| 1M groups | Twin groups | |||||
| Metabolites | ||||||
| n | 9 | 9 | 9 | |||
| Blood glucose (mmol/L) | 3.2 ± 0.2 | 2.7 ± 0.2 | 2.7 ± 0.2 | 0.106 | 0.855 | |
| Blood ketones (mmol/L) | 0.7 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.2 | 0.433 | 0.627 | |
| Serum cholesterol (mmol/L) | 2.0 ± 0.1 | 2.0 ± 0.1 | 1.9 ± 0.1 | 0.736 | 0.544 | |
| Serum NEFA (mmol/L) | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.150 | 0.833 | |
| Serum BHB (mmol/L) | 0.6 ± 0.3 | 1.1 ± 0.3 | 0.8 ± 0.3 | 0.191 | 0.617 | |
| Minerals | ||||||
| n | 9 | 9 | 9 | |||
| Serum calcium (mmol/L) | 2.3 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 | 0.024 | 0.931 | |
| Serum magnesium (mmol/L) | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 0.835 | 0.114 | |
| Serum phosphate (mmol/L) | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.8 ± 0.1 | 0.766 | 0.614 | |
| Urine pH | ||||||
| n | 9 | 9 | 9 | |||
| Urine pH | 7.5 ± 0.7 | 6.3 ± 0.5 | 7.4 ± 0.4 | 0.251 | 0.103 | |
The 1M groups consist of the singleton and twin-bearing groups fed at maintenance and the Twin groups consists of the twin-bearing ewes fed at either 1.0× or 1.25× maintenance levels. Data presented as mean ± s.e.m.
dG, day of gestation; NEFA, non-esterified fatty acids; BHB, beta-hydroxybutyrate.
All data in parenthesises throughout the text are displayed as mean ± s.e.m. Ewes from the Singletons 1M group had higher serum calcium levels during late gestation (2.3 ± 0.1 mmol/L) compared with ewes in the Twins 1M group (2.1 ± 0.1 mmol/L; P = 0.024). No differences were seen in serum magnesium, phosphate or urine pH between singletons and twin-bearing ewes (P > 0.05; Table 1). There were no differences in serum calcium, magnesium and phosphate or urine pH between Twins 1M and Twins 1.25M (P > 0.05; Table 1).
There were no differences in the blood metabolites (measured by EPOC) glucose, lactate, blood urea nitrogen (BUN) or creatinine pre- and post-partum (P > 0.05), except singleton ewes tended to have higher creatinine levels post-partum (1.5 ± 0.1 mg/dL) compared with twin-bearing ewes fed at maintenance levels (1.2 ± 0.1 mg/dL; P = 0.070), and, the Twins 1M group tended to have higher glucose levels post-partum (11.7 ± 0.9 mmol/L) compared with the ewes from the Twins 1.25M group (9.2 ± 0.9 mmol/L; P = 0.097).
There were no differences in whole blood glucose, ketones, serum cholesterol, NEFA, BHB, calcium, magnesium, phosphate or urine pH pre- and post-partum between singleton and twin-bearing ewes fed at maintenance levels (P > 0.05; Table 2). Ewes from the Twins 1M group tended to have higher whole blood glucose (glucometer) pre- (7.8 ± 0.8 mmol/L; P = 0.081) and post-parturition (11.0 ± 0.8 mmol/L; P = 0.079) compared with the Twins 1.25 group (5.4 ± 0.7 and 8.5 ± 0.8 mmol/L respectively; Table 2).
| Singletons 1M | Twins 1M | Twins 1.25M | P-value | |||
|---|---|---|---|---|---|---|
| 1M groups | Twin groups | |||||
| Pre-partum | ||||||
| n | 8 | 7 | 9 | |||
| Blood glucose (mmol/L) | 7.0 ± 0.7 | 7.8 ± 0.8 | 5.4 ± 0.7 | 0.500 | 0.081 | |
| Blood ketones (mmol/L) | 0.7 ± 0.4 | 1.9 ± 0.5 | 1.1 ± 0.4 | 0.141 | 0.300 | |
| Serum cholesterol (mmol/L) | 1.8 ± 0.1 | 1.9 ± 0.1 | 1.7 ± 0.1 | 0.696 | 0.228 | |
| Serum NEFA (mmol/L) | 1.3 ± 0.2 | 1.5 ± 0.3 | 1.6 ± 0.2 | 0.534 | 0.899 | |
| Serum BHB (mmol/L) | 0.6 ± 0.3 | 1.5 ± 0.4 | 1.0 ± 0.3 | 0.134 | 0.448 | |
| Serum calcium (mmol/L) | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 0.358 | 0.558 | |
| Serum magnesium (mmol/L) | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.884 | 0.741 | |
| Serum phosphate (mmol/L) | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 0.651 | 0.956 | |
| Post-partum | ||||||
| n | 9 | 9 | 9 | |||
| Blood glucose (mmol/L) | 12.0 ± 0.8 | 11.0 ± 0.8 | 8.5 ± 0.8 | 0.422 | 0.079 | |
| Blood ketones (mmol/L) | 0.5 ± 0.3 | 1.0 ± 0.3 | 0.5 ± 0.3 | 0.270 | 0.267 | |
| Serum cholesterol | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.6 ± 0.1 | 0.676 | 0.150 | |
| Serum NEFA (mmol/L) | 0.9 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.457 | 0.828 | |
| Serum BHB (mmol/L) | 0.4 ± 0.2 | 0.9 ± 0.2 | 0.5 ± 0.2 | 0.213 | 0.291 | |
| Serum calcium (mmol/L) | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 0.164 | 0.646 | |
| Serum magnesium (mmol/L) | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.729 | 0.626 | |
| Serum phosphate (mmol/L) | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 0.510 | 0.956 | |
The 1M groups consists of the singleton and twin-bearing groups fed at maintenance and the Twin groups consists of the twin-bearing ewes fed at either 1.0× or 1.25× maintenance levels. Data presented as mean ± s.e.m.
NEFA, non-esterified fatty acids; BHB, beta-hydroxybutyrate.
Base excess in extracellular fluid (ecf) was higher in singletons (2.1 ± 1.0 mmol/L) compared with twin-bearing ewes fed at maintenance levels (−1.4 ± 1.1 mmol/L) pre-partum (P = 0.46; Table 3). In the Singletons 1M group, the partial pressure of oxygen (pO2) tended to be lower (P = 0.073) and bicarbonate levels (cHCO3) tended to be higher (P = 0.055) compared with the Twins 1M group pre-partum (31.6 ± 1.8 vs 36.2 ± 2.0 mmHg and 26.1 ± 0.9 vs 23.2 ± 0.9 mmol/L respectively; Table 3). There were no differences in blood pH, partial pressure of carbon dioxide (pCO2) and oxygen saturation (cSO2) between singletons and twin-bearing ewes pre- or post-parturition (P > 0.05; Table 3). In the twins only groups, for the acid–base balance parameters, blood pO2 tended to be higher (P = 0.082) pre-partum in the Twins 1M group (36.2 ± 2.0 mmHg) compared with the Twins 1.25M group (30.4 ± 1.8 mmHg) but not post-partum (P > 0.05; Table 3).
| Singletons 1M | Twins 1M | Twins 1.25M | P-value | |||
|---|---|---|---|---|---|---|
| 1M groups | Twin groups | |||||
| Pre-partum | ||||||
| n | 8 | 7 | 8 | |||
| pH | 7.4 ± 0.03 | 7.5 ± 0.03 | 7.4 ± 0.03 | 0.935 | 0.577 | |
| pCO2 (mmHg) | 37.7 ± 1.9 | 36.8 ± 2.0 | 37.1 ± 1.9 | 0.765 | 0.896 | |
| pO2 (mmHg) | 31.6 ± 1.8 | 36.2 ± 2.0 | 30.4 ± 1.8 | 0.073 | 0.082 | |
| cHCO3 (mmol/L) | 26.1 ± 0.9 | 23.2 ± 0.9 | 24.2 ± 0.9 | 0.055 | 0.435 | |
| BE (ecf) (mmol/L) | 2.1 ± 1.0 | −1.4 ± 1.1 | −0.2 ± 1.0 | 0.046 | 0.425 | |
| cSO2 (%) | 63.6 ± 4.3 | 68.9 ± 4.6 | 58.5 ± 4.3 | 0.311 | 0.163 | |
| Post-partum | ||||||
| n | 9 | 9 | 9 | |||
| pH | 7.4 ± 0.02 | 7.4 ± 0.02 | 7.5 ± 0.02 | 0.617 | 0.129 | |
| pCO2 (mmHg) | 36.0 ± 1.5 | 35.4 ± 1.5 | 34.0 ± 1.5 | 0.810 | 0.331 | |
| pO2 (mmHg) | 35.0 ± 2.4 | 33.4 ± 2.4 | 33.1 ± 2.4 | 0.677 | 0.889 | |
| cHCO3 (mmol/L) | 23.9 ± 0.9 | 23.3 ± 0.9 | 23.8 ± 0.9 | 0.574 | 0.707 | |
| BE (ecf) (mmol/L) | −2.4 ± 1.7 | −1.1 ± 1.7 | −0.1 ± 1.7 | 0.631 | 0.548 | |
| cSO2 (%) | 65.9 ± 4.1 | 65.2 ± 4.1 | 66.4 ± 4.1 | 0.915 | 0.781 | |
The 1M groups consists of the singleton and twin-bearing groups fed at maintenance and the Twin groups consists of the twin-bearing ewes fed at either 1.0× or 1.25× maintenance levels. Data presented as mean ± s.e.m.
pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; cHCO3, bicarbonate; BE (ecf), base excess in extracellular fluid; cSO2, oxygen saturation.
Twin-bearing ewes were heavier at dG 142 (83.8 ± 2.1 kg) compared with singleton ewes (76.6 ± 2.1 kg; P = 0.027); however, LW and BCS were similar (P > 0.05) at all other time points (Table 4). Ewe weight and BCS was similar for Twins 1M and Twins 1.25M at all timepoints (P > 0.05; Table 4).
| Singletons 1M | Twins 1M | Twins 1.25M | P-value | |||
|---|---|---|---|---|---|---|
| 1M groups | Twin groups | |||||
| LW (kg) | ||||||
| n | 9 | 9 | 9 | |||
| dG 142 | 76.6 ± 2.1 | 83.8 ± 2.1 | 85.1 ± 2.1 | 0.027 | 0.824 | |
| 24 h post-partum | 69.7 ± 2.5 | 71.1 ± 2.5 | 71.2 ± 2.5 | 0.732 | 0.718 | |
| Day 40 post-partum | 67.1 ± 3.0 | 68.3 ± 3.0 | 66.1 ± 3.2 | 0.773 | 0.947 | |
| BCS | ||||||
| n | 9 | 9 | 9 | |||
| dG 142 | 3.3 ± 0.1 | 3.4 ± 0.1 | 3.5 ± 0.1 | 0.375 | 0.188 | |
| 24 h post-partum | 3.0 ± 0.1 | 3.1 ± 0.1 | 3.4 ± 0.1 | 0.495 | 0.663 | |
| Day 40 post-partum | 3.2 ± 0.1 | 3.2 ± 0.1 | 3.3 ± 0.1 | 1.000 | 0.871 | |
The 1M groups consists of the singleton and twin-bearing groups fed at maintenance and the Twin groups consists of the twin-bearing ewes fed at either 1.0× or 1.25× maintenance levels. Data presented as mean ± s.e.m.
Duration of parturition and predictors of lamb survival in twin-bearing ewes fed at different maintenance levels
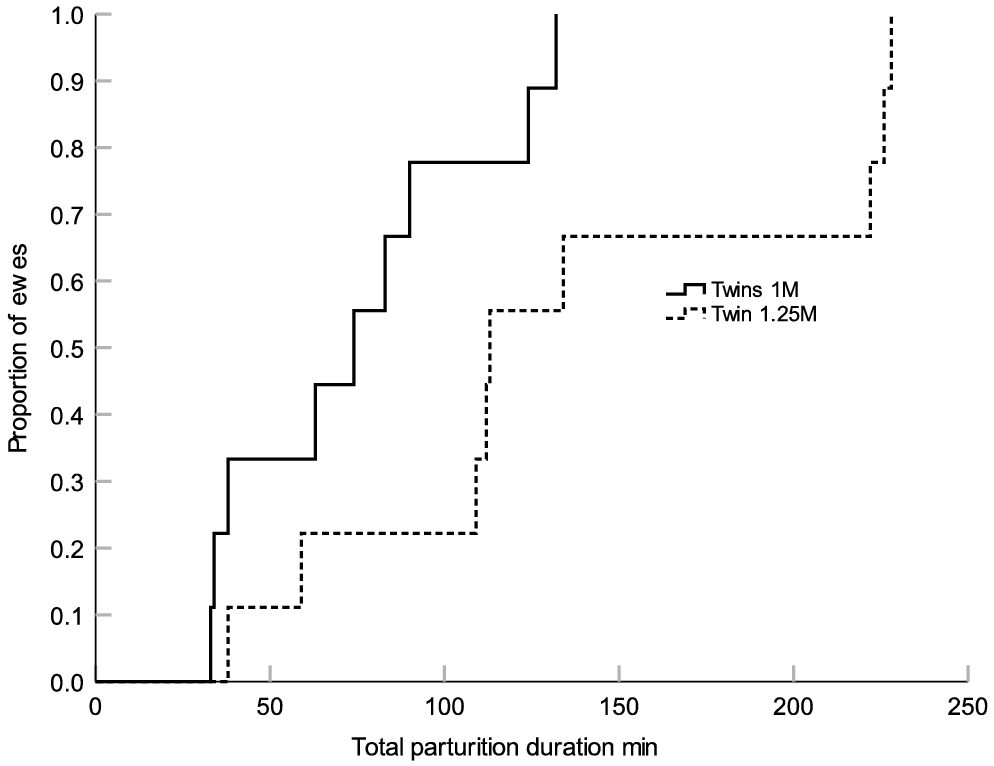
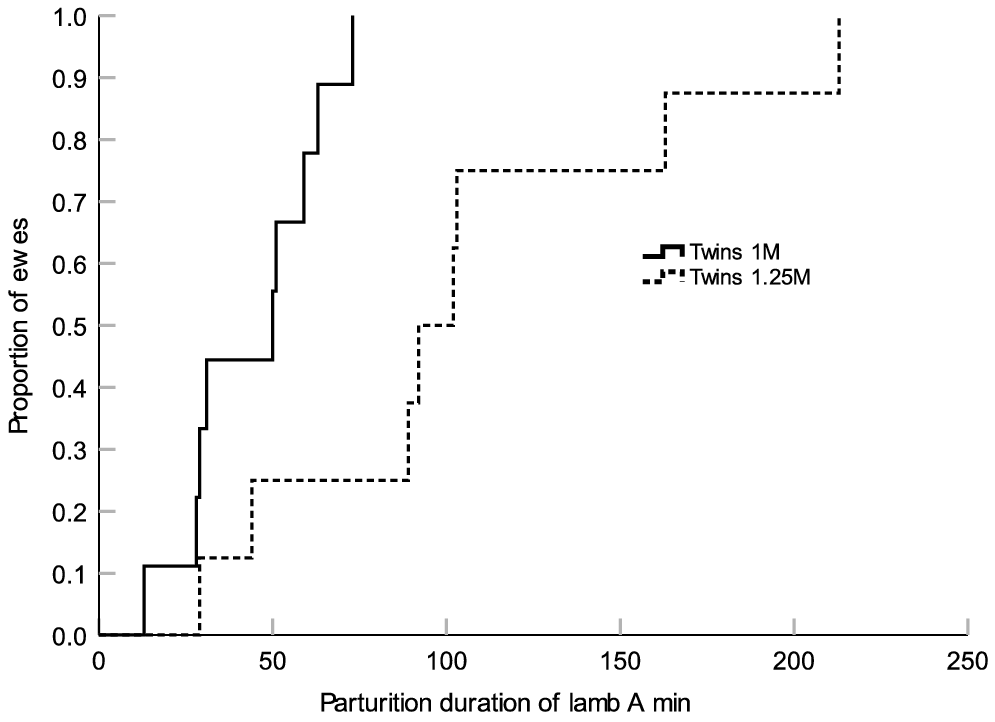
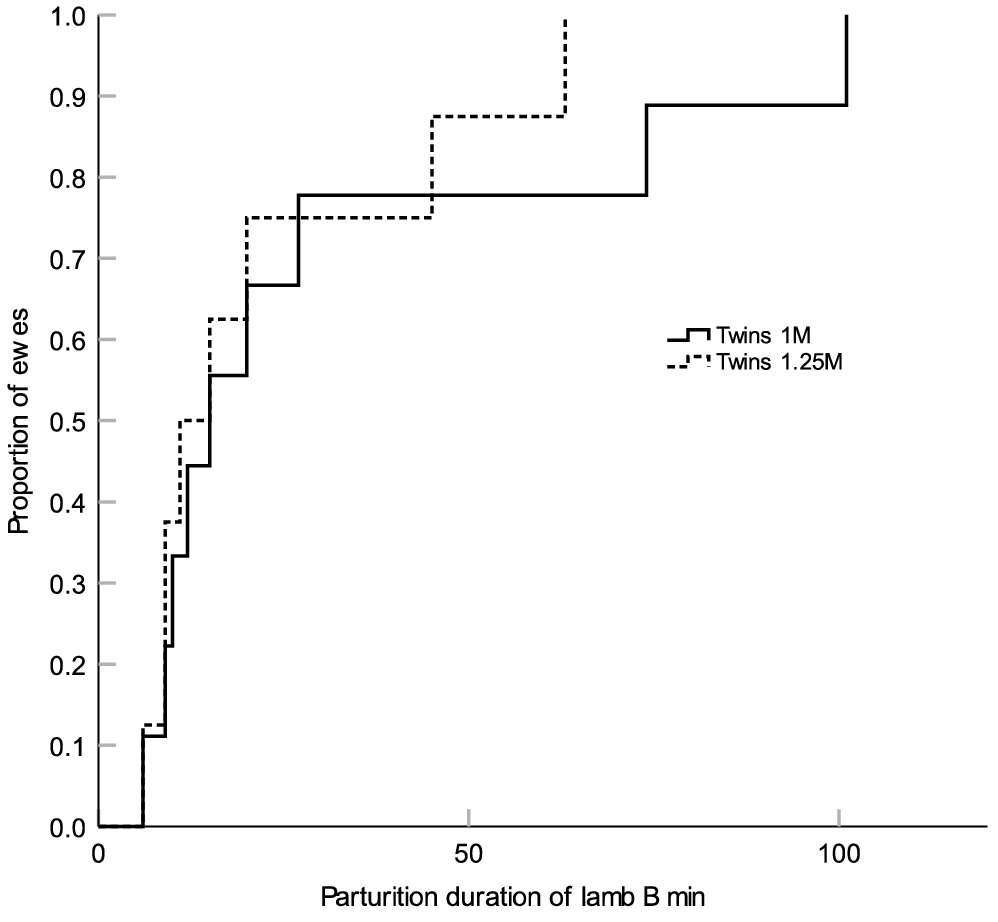
Ewes in the Twins 1M group had a shorter total duration of parturition (P = 0.047; Fig. 1) and a shorter duration of parturition for the first-born lamb (P = 0.015; Fig. 2) compared with the ewes from the Twins 1.25M group. There were no treatment differences in the duration of parturition for the second-born lamb (P > 0.05; Fig. 3). There were also no treatment differences in the percent of ewes that required assistance at delivery (Twins 1M = 11.1% (n = 1/9) and Twins 1.25M = 33.3% (n = 3/9)) or in gestation length (Twins 1M = 149.3 ± 0.6 days (n = 9) and Twins 1.25M = 149.7 ± 0.6 (n = 9) (P > 0.05). Irrespective of treatment, there were no significant linear relationships between metabolic state or mineral status on the duration of parturition (P > 0.05).
Kaplan–Meier time failure graph for the total parturition length of the ewes from groups Twins 1M (n = 9) and Twins 1.25M (n = 9). The mean total duration of parturition was 74.6 ± 12.3 (s.e.m.) min for the ewes from Twins 1M and 137.9 ± 24.0 min for the ewes from Twins 1.25M respectively.
Kaplan–Meier time failure graph for the duration of parturition of Lamb A (first-born lamb) from groups Twins 1M (n = 9) and Twins 1.25M (n = 9). The mean duration of parturition of Lamb A was 44.1 ± 6.6 (s.e.m.) min for the ewes from Twins 1M and 104.4 ± 21.1 min for the ewes from Twins 1.25M respectively.
Kaplan–Meier time failure graph for the duration of parturition of Lamb B (second-born lamb) from groups Twins 1M (n = 9) and Twins 1.25M (n = 9). The mean duration of parturition of Lamb B was 30.4 ± 11.2 (s.e.m.) min for the ewes from Twins 1M and 22.3.4 ± 7.3 min for the ewes from Twins 1.25M respectively.
Predictors of lamb survival were similar for lambs born to ewes from the Twins 1M group and the Twins 1.25M group (P > 0.05; Table 5), as were the measures of body morphology (body mass index and ponderal index) for lambs born to ewes from the Twins 1M group and Twins 1.25M group (P > 0.05; Table 5).
| Twins 1M | Twins 1.25M | P-value | ||
|---|---|---|---|---|
| n | 18 | 17 | ||
| Meconium staining (n) | 2.1 ± 0.3 | 2.9 ± 0.3 | 0.378 | |
| 0 h blood lactate (mmol/L) | 8.4 ± 0.9 | 10.2 ± 0.9 | 0.192 | |
| 4 h blood glucose (mmol/L) | 4.4 ± 0.5 | 4.8 ± 0.5 | 0.571 | |
| 4 h rectal temp (°C) | 38.8 ± 0.1 | 38.8 ± 0.1 | 0.968 | |
| Ponderal index (n) | 30.1 ± 1.8 | 32.8 ± 1.9 | 0.333 | |
| Body mass index (n) | 15.5 ± 0.7 | 16.8 ± 0.7 | 0.217 | |
| 4 h LW (kg) | 4.2 ± 0.1 | 4.5 ± 0.1 | 0.168 | |
| 24 h LW (kg) | 4.2 ± 0.1 | 4.5 ± 0.1 | 0.132 | |
| n | 16 | 15 | ||
| Day 40 LW (kg) | 12.2 ± 0.4 | 12.9 ± 0.4 | 0.280 |
Discussion
Ewes in the Twins 1.25M group took significantly longer to give birth to the first-born lamb compared with ewes from the Twins 1M group, supporting our hypothesis. Although ewes that were fed above maintenance requirements took longer to give birth, there were no significant differences in metabolic state, mineral status and acid–base balance between twin-bearing ewes fed at 1.0. There were also no differences in ewe LW, BCS and predictors of lamb survival. The other objective of this study was to determine whether singleton-bearing Merino ewes had a healthier metabolic state compared with twin-bearing Merino ewes during late gestation. In this study, twin-bearing ewes showed no differences in metabolic state, mineral status or acid–base balance except twin-bearing ewes had lower serum calcium during gestation and lower base excess (ecf) pre-partum compared with singleton ewes. Limitations of the study include relatively low numbers of ewes to evaluate these outcomes as the sample size estimation was designed to detect differences in the duration of parturition induced by diet. Further, blood sampling close to parturition may have disrupted normal parturition behaviour.
The transition period is considered the most challenging period for the pregnant ewe, with the ewe required to provide energy for the rapidly growing fetus/es, the initiation, production and secretion of colostrum, the birthing process and milk production (Fthenakis et al. 2012; El-Sayed et al. 2020). If the energy demands are not met, multiple-bearing ewes are more susceptible to hypoglycaemic stress and enter into a negative energy balance as they must accommodate for more than one fetus (Schlumbohm and Harmeyer 2008). For example, singleton ewes had lower blood NEFA, BHB, BUN and cholesterol compared with twin-bearing ewes (Balıkcı et al. 2007; Moallem et al. 2012; Raoofi et al. 2013, 2015; González-García et al. 2015). Singleton ewes have also been reported to have higher blood glucose levels compared with multiple-bearing ewes (Kleemann et al. 1988; Firat and Özpinar 1996; Balıkcı et al. 2007; Moallem et al. 2012; Raoofi et al. 2013, 2015). In contrast to other studies, where singleton ewes had a healthier metabolic state compared with twin-bearing ewes, the current study reported no difference in metabolic state between singleton and twin-bearing ewes. In this study, the diets were formulated specifically to meet the fetal requirements of either singleton or twin-bearing ewes, which differs to the previous studies in which singleton and twin-bearing ewes were offered the same amount of feed irrespective of their fetal number (Balıkcı et al. 2007; Moallem et al. 2012; Raoofi et al. 2013, 2015; González-García et al. 2015) which makes the results difficult to compare with literature. Despite this, the results suggest that as long as singleton and twin-bearing ewes are fed according to their gestational requirements there are likely to be no differences in metabolic state. Lastly, twin-bearing ewes were heavier on dG 142 compared with singleton ewes; however, this difference was no longer present post-partum, which was expected as twin-bearing ewes carry a higher fetal and placental weight during gestation compared with singleton-bearing ewes (Hocking Edwards et al. 2011).
Improving the plane of nutrition can improve ewe condition, colostrum yield and lamb growth rates in both twin and singleton lambs (Behrendt et al. 2011; Thompson et al. 2011; McGovern et al. 2015a, 2015b). In contrast, pregnancy toxaemia, which can occur in overfed ewes, increases the incidence of dystocia, lamb mortality and reduces colostrum production (Louca et al. 1974; Russel et al. 1977; Jordan and Mayer 1989; Gardner et al. 2007; Tygesen et al. 2008; Barbagianni et al. 2015). In the current study, no differences were observed between twin-bearing ewes fed at 1.0× or 1.25× maintenance levels on ewe LW and BCS and lamb LW. This finding agrees with other studies that fed ewes a higher plane of nutrition during late gestation (Kleemann et al. 1993; Kenyon et al. 2011). Additionally, there were no differences in other predictors of lamb survival such as meconium staining and lactate at birth, 4 h rectal temperature and body morphology and/or 4 h blood glucose. The low numbers in this study (n = 9/treatment) when evaluating parameters other than the duration of parturition, may have resulted in non-significant results, especially lamb liveweights. Although lamb birthweight differences were not significant in the current study, lambs born to ewes that were fed 25% above maintenance were numerically heavier. Heavier lambs may provide an explanation as to why these ewes took significantly longer to give birth to the first-born lamb, as heavy lambs are a risk factor for dystocia (Horton et al. 2017), however, the difference in birthweight was not large (300 g). McGovern et al. (2015a) reported that increasing ewe metabolisable energy intake by 20% during the final four weeks of gestation did not alter ewe metabolic state compared with maintenance fed ewes post-partum. In addition, Kenyon et al. (2011) also reported no differences in ewe metabolic state measures when feeding ewes either maintenance levels or ad libitum. Together, these findings support those of the current study, which suggests that feeding at or above maintenance requirements does not improve measures of ewe metabolic state. Regardless of treatment group, all ewes had blood glucose levels above baseline values (1.4–2.8 mmol/L) for sheep (Reid 1950) during parturition and shortly post-partum. This may be due to the physiological response to stress and pain during parturition where the activation of the sympathetic nervous system causes hyperglycaemia (Hiew et al. 2020). However, blood metabolites used to measure pain/stress, such as cortisol, was not measured in the current study.
Similar to metabolic state, mineral status, particularly calcium, can also be influenced by fetal number during late gestation. In ewes, the highest demand for calcium typically occurs 2–6 weeks prior to parturition and is due to mineralisation of the fetal skeleton in conjunction with the diversion of calcium to milk production (Freer et al. 2007; Brozos et al. 2011; Povey et al. 2016). Therefore, ewes carrying more than one fetus have a greater risk of experiencing calcium deficiency compared with singleton ewes. In addition to calcium, magnesium and phosphorus are important minerals during late gestation, mainly due to their role in the regulation of calcium (Treacher and Caja 2002). In the current study, magnesium and phosphorous concentrations were similar between singleton and twin-bearing ewes. In singleton ewes, serum calcium levels were higher during late gestation. This is supported by previous evidence of higher calcium levels in singleton compared with multiple-bearing ewes (Moallem et al. 2012; Raoofi et al. 2015). There were no differences in serum calcium levels immediately pre- and post-partum, suggesting the presence of multiple fetuses did not affect calcium during the parturition process. Ataollahi et al. (2021) demonstrated that improving the calcium and magnesium status in ewes tended to decrease the duration of parturition in the second-born lamb. In the current study, regardless of energy group, there were no linear relationships between serum calcium, magnesium or phosphate on the duration of parturition. However, it is important to note that all ewes were within the normal calcium reference range for sheep (2.12–2.87 mmol/L (Paynter 1996)) and all ewes received a commercial pellet that contained a suitable vitamin and mineral pre-mix. Testing a diet sub-clinically deficient in calcium on the effect of parturition length/difficulty score would be more suited to explore the relationship between ewe mineral status and the duration of parturition. The number of fetuses may also influence the acid–base balance of the ewe. As previously mentioned, twin-bearing ewes require more energy during late gestation, which increases the use of ketone bodies as an energy source compared to singleton ewes. This in turn can shift the acid–base balance of the blood to become more acidic (González et al. 2012; Lima et al. 2012); however, there is little literature available comparing the acid–base balance of singleton and twin-bearing ewes. In this study, there were no differences in the acid–base balance (pH, pCO2, pO2, cHCO3, and cSO2) between singleton and twin pregnancies, except that twin-bearing ewes had a lower base excess pre-partum compared with singleton ewes. This suggests that twin-bearing ewes may use more ketone bodies as an energy source during parturition, causing a base deficit (Santarosa et al. 2019). However, this refutes the findings of Santarosa et al. (2019) who reported that singleton ewes had a lower base excess compared with twin-bearing ewes. This difference may be a result of breed differences as the Santarosa et al. (2019) study was conducted using Dorper ewes, which is a meat breed that typically produces heavier lambs. This may have resulted in greater energy expenditure during parturition of the singleton ewes to expel the high birthweight lamb, resulting in a lower base excess (Fogarty and Thompson 1974; George 1976; Hall et al. 1994). There were no differences in the acid–base balance parameters pre- and post-partum in both twin groups, and these were within normal healthy ranges (Hajikolaei et al. 2006; Redfearn et al. 2022), which indicates that increasing the energy of the diet does not affect the acid–base balance of the ewe during parturition.
Conclusions
Feeding twin-bearing ewes 25% above maintenance requirements increased the duration of parturition of the first-born lamb. There were no significant changes in physiological parameters between the two twin-bearing groups, however, lambs born to ewes from the Twins 1.25 group were numerically heavier, which may explain the increase in parturition length. Only minor differences in metabolic state was found when feeding singleton and twin-bearing ewes according to their gestational maintenance requirements. This indicates that producers that have issues with metabolic disease in their flock might benefit from separating singleton and twin-bearing ewes to ensure that the gestational energy requirements are being met, and that overfeeding ewes in the transition period provides no production benefits.
Author contributions
Conceptualisation: WHvW, AMS and ACW. Methodology: ALM, WHvW, AMS and ACW. Formal analysis: ALM, AMS and ACW. Data curation: ALM, AMS and ACW. Writing – original draft: ALM. Writing – review and editing: WHvW, AMS and ACW. Supervision: WHvW, AMS and ACW. Funding acquisition: ACW.
Acknowledgements
The authors gratefully acknowledge the assistance given during the research project by volunteers and South Australian Research and Development Institute staff members at Turretfield Research Centre.
References
Alderman G, Cottrill B (1996) Feed evaluation and diet formulation energy and protein requirements of ruminants. In ‘Energy and protein requirements of ruminants’. (Eds G Alderman, B Cottrill) pp. 41–56. (An Advisory Manual Prepared by the AFRC Technical Committee on Responses to Nutrients: Wallingford, Oxon, UK)
Balıkcı E, Yıldız A, Gürdoğan F (2007) Blood metabolite concentrations during pregnancy and postpartum in Akkaraman ewes. Small Ruminant Research 67, 247-251.
| Crossref | Google Scholar |
Barbagianni MS, Spanos SA, Ioannidi KS, Vasileiou NGC, Katsafadou AI, Valasi I, Gouletsou PG, Fthenakis GC (2015) Increased incidence of peri-parturient problems in ewes with pregnancy toxaemia. Small Ruminant Research 132, 111-114.
| Crossref | Google Scholar |
Baxter EM, Jarvis S, D’eath RB, Ross DW, Robson SK, Farish M, Nevison IM, Lawrence AB, Edwards SA (2008) Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology 69, 773-783.
| Crossref | Google Scholar |
Behrendt R, van Burgel AJ, Bailey A, Barber P, Curnow M, Gordon DJ, Edwards JEH, Oldham CM, Thompson AN (2011) On-farm paddock-scale comparisons across southern Australia confirm that increasing the nutrition of Merino ewes improves their production and the lifetime performance of their progeny. Animal Production Science 51, 805-812.
| Crossref | Google Scholar |
Benech A, Cal-Pereyra L, Da Silva S, Acosta-Dibarrat J, González-Montaña JR (2015) Transient apnoea in sheep: an alternative method for serial urine sample collection. Veterinarski Arhiv 85, 293-307.
| Google Scholar |
Brougham B-J, Weaver AC, Swinbourne AMF, Lewis Baida BE, Kelly JM, Walker SK, Kleemann DO, van Wettere WHEJ (2020) Maternal supplementation with dietary betaine during gestation to improve twin lamb survival. Animals 10, 1749.
| Crossref | Google Scholar | PubMed |
Brozos C, Mavrogianni VS, Fthenakis GC (2011) Treatment and control of peri-parturient metabolic diseases: pregnancy toxemia, hypocalcemia, hypomagnesemia. Veterinary Clinics of North America: Food Animal Practice 27, 105-113.
| Crossref | Google Scholar | PubMed |
Bruce M, Young JM, Masters DG, Refshauge G, Thompson AN, Kenyon PR, Behrendt R, Lockwood A, Miller DW, Jacobson C (2021) The impact of lamb and ewe mortality associated with dystocia on Australian and New Zealand sheep farms: a systematic review, meta-analysis and bio-economic model. Preventive Veterinary Medicine 196, 105478.
| Crossref | Google Scholar | PubMed |
Crilly JP, Phythian C, Evans M (2021) Advances in managing pregnancy toxaemia in sheep. In Practice 43, 79-94.
| Crossref | Google Scholar |
Dwyer CM (2003) Behavioural development in the neonatal lamb: effect of maternal and birth-related factors. Theriogenology 59, 1027-1050.
| Crossref | Google Scholar | PubMed |
Dwyer CM, Bünger L (2012) Factors affecting dystocia and offspring vigour in different sheep genotypes. Preventive Veterinary Medicine 103, 257-264.
| Crossref | Google Scholar | PubMed |
Dwyer CM, Lawrence AB (2005) A review of the behavioural and physiological adaptations of hill and lowland breeds of sheep that favour lamb survival. Applied Animal Behaviour Science 92, 235-260.
| Crossref | Google Scholar |
El-Sayed A, El-Ashker M, Ibrahim H, Shoieb S, Ibrahim F, Youssef M, El-Khodery S (2020) Blood metabolic profile in barki ewes during transition period. Journal of the Hellenic Veterinary Medical Society 71, 2261-2266.
| Crossref | Google Scholar |
Firat A, Özpinar A (1996) The study of changes in some blood parameters (glucose, urea, bilirubin, AST) during and after pregnancy in association with nutritional conditions and litter size in ewes. Turkish Journal of Veterinary & Animal Sciences 20, 387-393.
| Crossref | Google Scholar |
Fogarty NM, Thompson JM (1974) Relationship between pelvic dimensions, other body measurements and dystocia in Dorset Horn ewes. Australian Veterinary Journal 50, 502-506.
| Crossref | Google Scholar | PubMed |
Freer M, Dove H, Nolan J (2007) ‘Nutrient requirements of domesticated ruminants’. (CSIRO Publishing: Melbourne, Australia) doi:10.1071/9780643095106
Fthenakis GC, Arsenos G, Brozos C, Fragkou IA, Giadinis ND, Giannenas I, Mavrogianni VS, Papadopoulos E, Valasi I (2012) Health management of ewes during pregnancy. Animal Reproduction Science 130, 198-212.
| Crossref | Google Scholar |
Gardner DS, Buttery PJ, Daniel Z, Symonds ME (2007) Factors affecting birth weight in sheep: maternal environment. Reproduction 133, 297-307.
| Crossref | Google Scholar | PubMed |
George JM (1976) The incidence of dystocia in Dorset Horn ewes. Australian Veterinary Journal 52, 519-523.
| Crossref | Google Scholar | PubMed |
González FHD, Hernández F, Madrid J, Martínez-Subiela S, Cerón JJ, Tecles F (2012) Acid–base and electrolyte status during early induced pregnancy toxaemia in goats. The Veterinary Journal 193, 598-599.
| Crossref | Google Scholar | PubMed |
González-García E, Tesnière A, Camous S, Bocquier F, Barillet F, Hassoun P (2015) The effects of parity, litter size, physiological state, and milking frequency on the metabolic profile of Lacaune dairy ewes. Domestic Animal Endocrinology 50, 32-44.
| Crossref | Google Scholar | PubMed |
Hajikolaei MRH, Nouri M, Afshar FS, Dehkordi AJ (2006) Effects of experimentally induced ruminal lactic acidosis on blood pH, bicarbonate and pCO2 in the sheep. Pakistan Journal of Biological Sciences 9, 2003-2005.
| Crossref | Google Scholar |
Hall DG, Gilmour AR, Fogarty NM (1994) Variation in reproduction and production of Poll Dorset ewes. Australian Journal of Agricultural Research 45, 415-425.
| Crossref | Google Scholar |
Hiew MWH, Megahed AA, Horstman LA, Constable PD (2020) Clinical utility of plasma progesterone and blood and plasma glucose concentrations in predicting parturition in Holstein cows. Journal of Dairy Science 103, 5575-5590.
| Crossref | Google Scholar | PubMed |
Hinch GN, Brien F (2014) Lamb survival in Australian flocks: a review. Animal Production Science 54, 656-666.
| Crossref | Google Scholar |
Hocking Edwards JE, Copping KJ, Thompson AN (2011) Managing the nutrition of twin-bearing ewes during pregnancy using Lifetimewool recommendations increases production of twin lambs. Animal Production Science 51, 813-820.
| Crossref | Google Scholar |
Horton BJ, Corkrey R, Hinch GN (2017) Estimation of risk factors associated with difficult birth in ewes. Animal Production Science 58, 1125-1132.
| Crossref | Google Scholar |
Jacobson C, Bruce M, Kenyon PR, Lockwood A, Miller D, Refshauge G, Masters DG (2020) A review of dystocia in sheep. Small Ruminant Research 192, 106209.
| Crossref | Google Scholar |
Jordan DJ, Mayer DG (1989) Effects of udder damage and nutritional plane on milk yield, lamb survival and lamb growth of Merinos. Australian Journal of Experimental Agriculture 29, 315-320.
| Crossref | Google Scholar |
Kenyon PR, Pain SJ, Hutton PG, Jenkinson CMC, Morris ST, Peterson SW, Blair HT (2011) Effects of twin-bearing ewe nutritional treatments on ewe and lamb performance to weaning. Animal Production Science 51, 406-415.
| Crossref | Google Scholar |
Kleemann DO, Smith DH, Walker SK, Walkley JRW (1988) Plasma glucose levels in South Australian Merino ewes. Australian Veterinary Journal 65, 99-100.
| Crossref | Google Scholar | PubMed |
Kleemann DO, Walker SK, Walkley JRW, Ponzoni RW, Smith DH, Grimson RJ, Seamark RF (1993) Effect of nutrition during pregnancy on birth weight and lamb survival in FecB Booroola× South Australian Merino ewes. Animal Reproduction Science 31, 213-224.
| Crossref | Google Scholar |
Lima MS, Pascoal RA, Stilwell GT (2012) Glycaemia as a sign of the viability of the foetuses in the last days of gestation in dairy goats with pregnancy toxaemia. Irish Veterinary Journal 65, 1.
| Crossref | Google Scholar | PubMed |
Louca A, Mavrogenis A, Lawlor MJ (1974) Effects of plane of nutrition in late pregnancy on lamb birth weight and milk yield in early lactation of Chios and Awassi sheep. Animal Science 19, 341-349.
| Crossref | Google Scholar |
McGovern FM, Campion FP, Lott S, Boland TM (2015a) Altering ewe nutrition in late gestation: I. The impact on pre-and postpartum ewe performance. Journal of Animal Science 93, 4860-4872.
| Crossref | Google Scholar | PubMed |
McGovern FM, Campion FP, Sweeney T, Fair S, Lott S, Boland TM (2015b) Altering ewe nutrition in late gestation: II. The impact on fetal development and offspring performance. Journal of Animal Science 93, 4873-4882.
| Crossref | Google Scholar | PubMed |
Moallem U, Rozov A, Gootwine E, Honig H (2012) Plasma concentrations of key metabolites and insulin in late-pregnant ewes carrying 1 to 5 fetuses. Journal of Animal Science 90, 318-324.
| Crossref | Google Scholar | PubMed |
Murdock NJ, Weaver AC, Kelly JM, Kleemann DO, van Wettere WHEJ, Swinbourne AM (2021) Supplementing pregnant Merino ewes with caffeine to improve neonatal lamb thermoregulation and viability. Animal Reproduction Science 226, 106715.
| Crossref | Google Scholar | PubMed |
Povey G, Stubbings L, Phillips K (2016) Feeding the ewe: a literature review. Coventry, England. Available at https://ahdb.org.uk/update-feeding-the-ewe [accessed 22 April 2021]
Raoofi A, Jafarian M, Safi S, Vatankhah M (2013) Fluctuations in energy-related metabolites during the peri-parturition period in Lori-Bakhtiari ewes. Small Ruminant Research 109, 64-68.
| Crossref | Google Scholar |
Raoofi A, Jafarian M, Safi S, Vatankhah M (2015) Comparison of energy related metabolites during peri-parturition period in single and twin-bearing Lori-Bakhtiari ewes. Iranian Journal of Veterinary Medicine 9, 149-154.
| Crossref | Google Scholar |
Redfearn A, McNally J, Brewer H, Doyle E, Schmoelzl S (2022) Using pen-side measurable blood parameters to predict or identify dystocic lambing events. Biology 11, 206.
| Crossref | Google Scholar | PubMed |
Refshauge G, Brien FD, Hinch GN, van de Ven R (2016) Neonatal lamb mortality: factors associated with the death of Australian lambs. Animal Production Science 56, 726-735.
| Crossref | Google Scholar |
Reid RL (1950) Studies on the carbohydrate metabolism of sheep. I. The range of blood-sugar values under several conditions. Australian Journal of Agricultural Research 1, 182-199.
| Crossref | Google Scholar |
Russel AJF, Maxwell TJ, Sibbald AR, McDonald D (1977) Relationships between energy intake, nutritional state and lamb birth weight in Greyface ewes. The Journal of Agricultural Science 89, 667-673.
| Crossref | Google Scholar |
Santarosa BP, Dantas GN, Ferreira DOL, Carvalho MG, Rodrigues M, Pereira PFV, Silva AA, Gonçalves RC (2019) Comparison of electrolyte and acid-base balances of Dorper breed ewes between single and twin pregnancies. Pesquisa Veterinaria Brasileira 39, 789-795.
| Crossref | Google Scholar |
Schlumbohm C, Harmeyer J (2008) Twin-pregnancy increases susceptibility of ewes to hypoglycaemic stress and pregnancy toxaemia. Research in Veterinary Science 84, 286-299.
| Crossref | Google Scholar | PubMed |
Thompson AN, Ferguson MB, Campbell AJD, Gordon DJ, Kearney GA, Oldham CM, Paganoni BL (2011) Improving the nutrition of Merino ewes during pregnancy and lactation increases weaning weight and survival of progeny but does not affect their mature size. Animal Production Science 51, 784-793.
| Crossref | Google Scholar |
Tygesen MP, Nielsen MO, Nørgaard P, Ranvig H, Harrison AP, Tauson A-H (2008) Late gestational nutrient restriction: Effects on ewes’ metabolic and homeorhetic adaptation, consequences for lamb birth weight and lactation performance. Archives of Animal Nutrition 62, 44-59.
| Crossref | Google Scholar | PubMed |


