Disease as a constraint on goat production in Lao PDR and trade to neighbouring countries: a review
P. P. Jayasekara A * , W. Theppangna B , L. Olmo
A * , W. Theppangna B , L. Olmo  A , T. Xaikhue A , C. Jenkins
A , T. Xaikhue A , C. Jenkins  C , P. F. Gerber
C , P. F. Gerber  A and S. W. Walkden-Brown
A and S. W. Walkden-Brown  A
A
A
B
C
Abstract
Goat production in Lao People’s Democratic Republic (Lao PDR) is a small but rapidly growing sector owing to strong export demand, primarily from Vietnam. Disease has been identified as one of the major constraints to goat production but there are limited data on causes and effective control strategies. The situation is exacerbated by a lack of veterinary and extension services in rural areas. Information on the major disease and clinical syndromes of goats and their causative agents is needed to develop local and national control strategies and to improve animal welfare. Zoonotic diseases involving goats are also potentially important in terms of live goat trade and public health, albeit research is lacking. This review summarises and evaluates the available published data on caprine diseases in Lao PDR and provides possible disease control strategies to improve goat production in Lao PDR. Surveys and observations suggest that lip and facial dermatitis, eye conditions and diarrhoea are the most common clinical syndromes affecting the health of Lao goats. These clinical syndromes can be considered as priorities for Lao goats. Serological surveys conducted in limited geographical areas of the country have identified moderate seroprevalence of foot and mouth disease (FMD) and low seroprevalence of bluetongue, peste des petits ruminants (PPR), brucellosis and Q fever in goats. Accordingly, the clinical signs associated with the latter diseases were not commonly reported. Trichostrongylus spp., Haemonchus contortus and coccidia are the main gastro-intestinal parasites identified among Lao goats. Despite these studies, an understanding of the causation of the most common clinical syndromes in Lao goats is still lacking, similar to the situation in many other parts of Southeast Asia. Studies to determine the causation of common clinical syndromes need to be conducted in Lao goats if progress is to be made on overcoming the disease constraint. Similarly, studies are also needed to evaluate interventions that have been introduced to limit the impact of these disease and clinical syndromes. They will likely require changes to goat management and nutrition, in addition to disease-specific interventions.
Keywords: clinical syndromes, constraints, disease diagnosis, endemic diseases, goats, Lao PDR, production, transboundary diseases, zoonoses.
Introduction
Lao People’s Democratic Republic (Lao PDR) is a landlocked country in Southeast Asia. The country borders with China to the north, Vietnam to the north-east and east, Cambodia to the south, Thailand to the west and Myanmar to the north-west. Lao PDR is a tropical country with a monsoonal climate, comprising mixed geography with woodlands, grasslands and plateaux (Osborne et al. 2021). Poverty is a constant challenge because Lao PDR is a lower middle-income country (LNCCI 2020) with a gross domestic product (GDP) of USD15.7 billion in 2022. Population density is low, with 7,529,475 civilians spread over 236,800 km2 of land in 2022 (World Bank 2023). Lao provinces are classified into three geographical regions, namely, northern, central and southern, although geographically it can be divided into lowland and upland zones (ADB 2002), with uplands being the majority (IFAD and ADB 2018). Farming is an integral part of the Lao culture and employs the majority of people (Osborne et al. 2021). Mostly, Laotians practice smallholder mixed farming with both crops and animals (Olmo et al. 2022a). Ten per cent of land in Lao PDR is used for agriculture (23,940 km2), although only 6.7% is considered arable (World Bank 2022). Livestock are mainly raised in smallholder farming systems (FAO 2020), which accounted for more than 95% of total livestock production in the country in 2002 (ADB 2002); however, more recent data on this is not readily available. Livestock farmers predominantly rear pigs, cattle, water buffaloes and poultry (Osborne et al. 2021). Statistics from 2022 showed that there were 4,408,000 pigs, 2,428,000 cattle and 1,208,634 buffaloes in Lao PDR (FAO 2024). The goat population is small but growing with the total number of goats increasing by 22% from 588,000 in 2017 to 753,860 in 2022 (FAO 2024). Goat production is a small but rapidly expanding livestock sector, driven largely by demand for live goats by neighbouring Vietnam (Walkden-Brown et al. 2023).
Disease is identified by farmers as one of the major problems they encounter (Gray et al. 2019) and the level of veterinary and extension services available to smallholders is low (Rast et al. 2014; Subharat et al. 2023). Information on the type and prevalence of diseases in goats in Lao PDR is scattered in both the formal and informal literature. The actual cause of common clinical syndromes is not known definitively. Therefore, the aim of this review is to collate the disparate data on diseases of goats in Lao PDR within the context of goat production, marketing systems and the available veterinary services in the country. This includes information on the major endemic diseases, transboundary and zoonotic diseases, and their potential to affect goat farming and trade in Lao PDR.
Goat production and marketing systems in Lao PDR
In 2011, it was estimated that 6% of all livestock farmers (43,200) reared goats (MAF and FAO 2014), but equivalent data for recent years is not available. Goats are distributed unevenly within the country, with the highest population in the central province of Savannakhet (MAF and FAO 2014). Goats are reared predominantly in smallholder systems and, during 2010–2011, the mean herd size was estimated at 4.6–6.0 in different regions, with only 11–18% of goat-rearing households having more than 10 goats (Table 1) (MAF and FAO 2014). A more recent survey from Savannakhet province in central Lao PDR identified that the goat herd size ranged from 7.3 to 9.2 goats per household (Phengvilaysouk et al. 2022). There were 93 small commercial goat farms in 2019, according to the Lao Ministry of Agriculture and Fisheries (Xayalath et al. 2021).
| Region | Farm households with goat herds of different size (%) | Mean number of goats/household | |||
|---|---|---|---|---|---|
| <5 head | 5–9 head | >10 heads | |||
| North | 66 | 23 | 11 | 4.6 | |
| Central | 50 | 32 | 18 | 6.0 | |
| South | 65 | 22 | 12 | 4.8 | |
| Total | 59 | 27 | 14 | 5.3 | |
Lao local goats, which are also known as ‘bae’ or ‘katjang’, are raised for meat production, but they are small in size. Their birth weight is usually 2 kg and their mature weight is 25–28 kg (Wilson 2007). Their coat colour is highly diverse with mixed grey/brown and black. Scimitar shaped horns, erect ears and a straight face are additional phenotypic characteristics of local goats (Keonouchanh and Xaypha 2006). There are also low numbers of imported breeds such as ‘Bac Thao’ and ‘French Alpine’ goats. The former breed is a dual-purpose breed from Vietnam, which was imported in 2002 to upgrade local goats (Wilson 2007). More recently, improved meat breeds such as the Boer can be seen in some areas and are believed to be imported from Thailand, although the origin is not well documented (Xayalath et al. 2021).
In Lao PDR, goats are utilised only for meat (Burns et al. 2018) and are mostly sold to traders from Vientiane and Vietnam (Gray 2006) rather than being kept for home consumption (Gray et al. 2019). Goat meat was allegedly not popular among locals in the past owing to its strong odour (Phengsavanh et al. 2004) and, in general, red meat is too valuable for home consumption. Importantly, goats provide manure for crops and act as vital cash reserves for farmers (Gray et al. 2019; Liehr et al. 2024). Up to 27–42% of smallholder household income is from goat sales (Phengsavanh et al. 2017). The goat industry is export-oriented and based on sale of live goats, predominantly to Vietnamese traders. There is high market demand for Lao goats in Vietnam where they may be labelled ‘Lao mountain goat’ (Gray et al. 2019). A survey from five provinces from northern and central Lao PDR showed that 90–95% of goats produced in these regions were destined for Vietnam and their price is 30% higher than that of Vietnamese crossbreds (Gray et al. 2019). The market price of Lao live goats has increased over time. Assuming 25 kg as the average weight of sale goat, the local market price of live goats in 2007 was USD45 (Wilson 2007). The price of adult goats in 2010 and 2014 was approximately USD44 and USD88 respectively (Frangi 2014). More recent data from 2017 indicated that farmers received approximately USD87 for a 20 kg male goat (Gray et al. 2019). However, the live goat export process is unofficial and poorly regulated because a formal trade agreement is lacking (Gray et al. 2019). Thus, there is a risk of transmission of transboundary as well as endemic diseases.
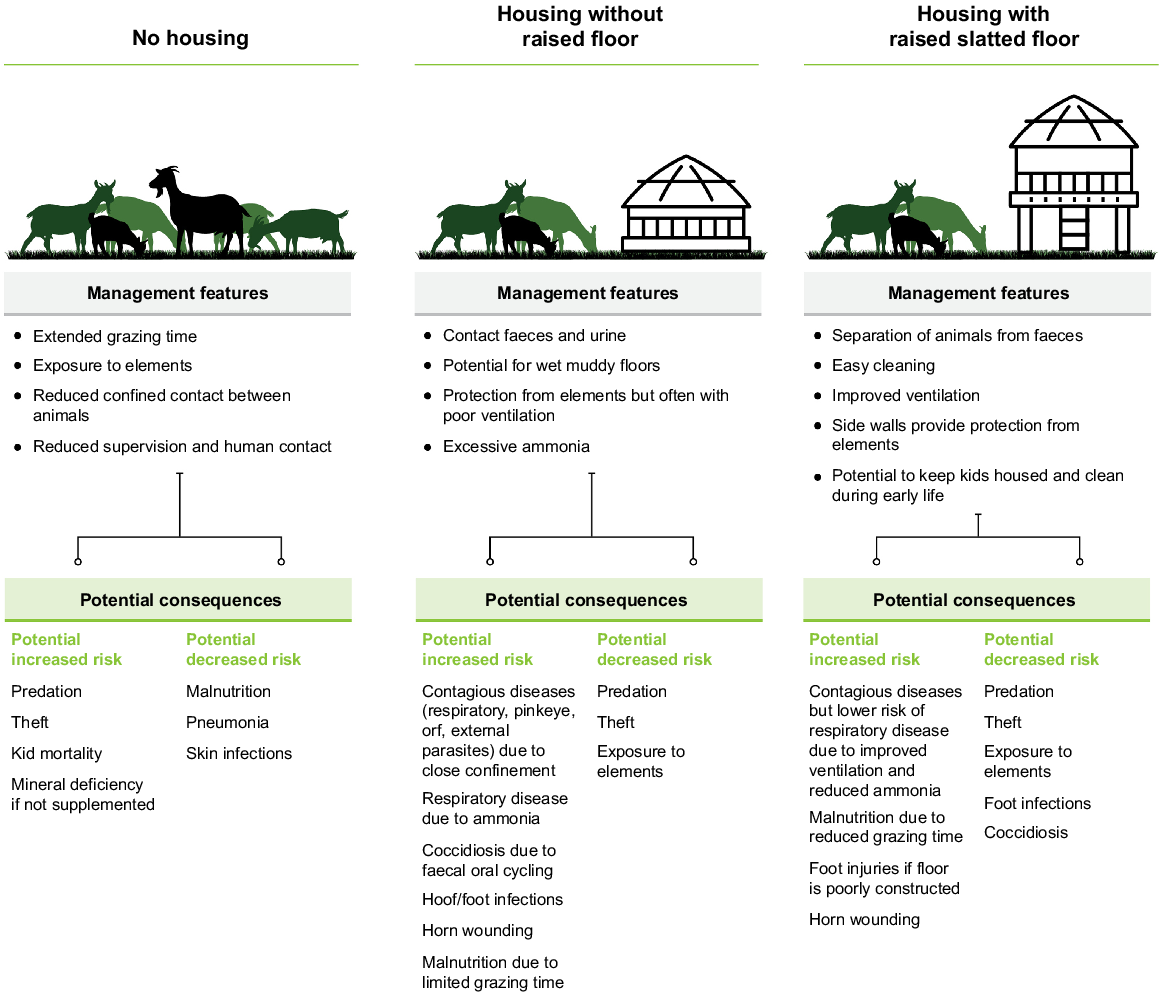
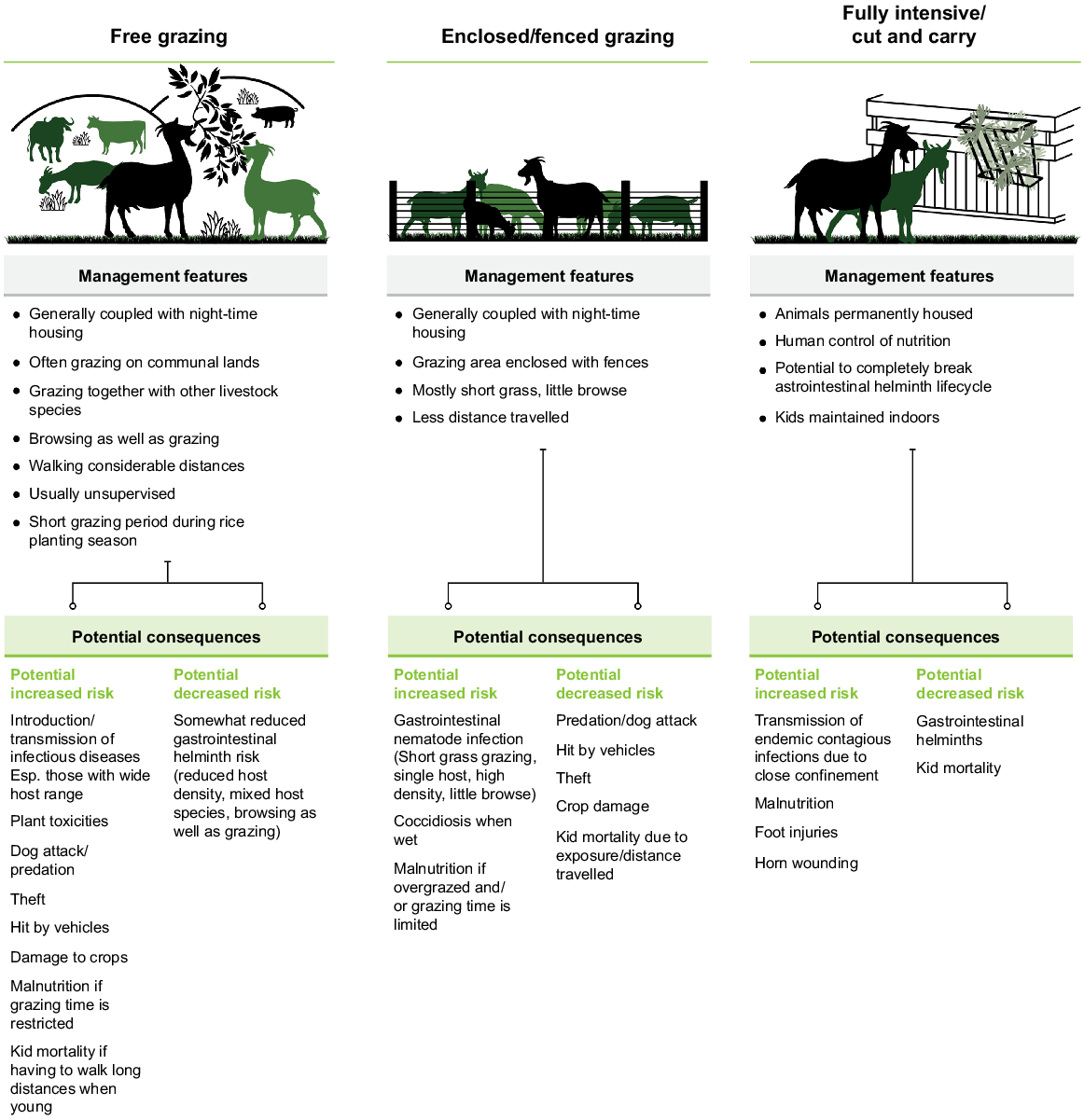
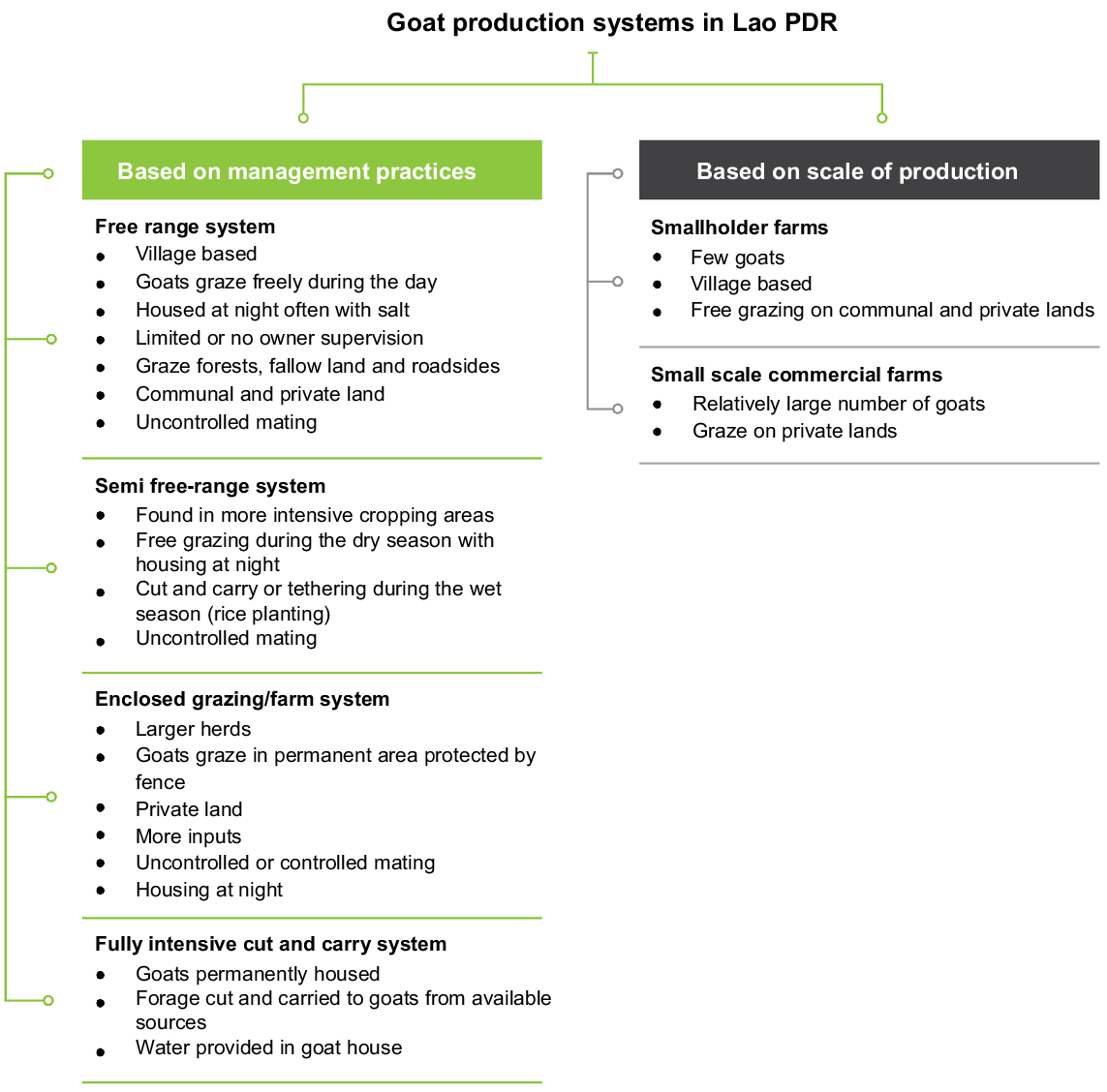
The main goat production systems in Lao PDR are free-range systems, semi free-range systems, enclosed grazing and fully intensive cut and carry systems (Fig. 1). Whereas the majority of goats are managed extensively in small herds, goats are typically enclosed in housing at night (Gray et al. 2019). The quality of goat houses varies, but they are often poorly constructed with locally available materials (Xaypha 2005; Wilson 2007) because of the limited resources available to farmers (Fig. 2). Improved housing design has been implemented by some aid and development projects.
Goat production systems in Lao PDR. Data for the management practices was retrieved from Phimpachanhvongsod, 2001 as cited by Xaypha (2005) and (Gray et al. 2019) and scale of production from Viengvilay et al. (2017).
Examples of typical goat housing and flooring in Lao PDR (Photos courtesy of P. P. Jayasekara, 2022).

As the majority of goat houses are constructed in a simple manner and are poorly ventilated or waterproofed (Gray et al. 2019), the combination of high humidity and ammonia production during the rainy season, coupled with close proximity between animals for prolonged periods of time favours the transmission of respiratory diseases. However, the majority of goat houses are raised on slatted floors (Millar et al. 2022), which prevent faecal accumulation and reduce direct contact with faeces, thereby reducing the risk of coccidiosis and transmission of worm larvae. Furthermore, elevated slatted floors keep hooves dry, reducing the risk of foot infections, although irregular spacing between slats is associated with a significant incidence of foot injury. To be effective in limiting infectious disease, goat houses need to be kept clean with the regular removal of accumulated faeces beneath the floor. The features and consequences of the different systems of housing employed by Lao goat farmers are outlined in Fig. 3.
The nutritional requirements of Lao goats are met mainly through foraging and unsupervised free grazing on private and communal lands (Sato et al. 2014; Gray et al. 2019; Olmo et al. 2022a). Consequently, there is potential for disease transmission between herds. Roadsides, grasslands, forestlands and other bare lands are used for free grazing during the dry season, while goats are tethered during the rainy season to protect cultivated crops (Xaypha 2005). Lao goats fulfil their feed requirement from local grass and fodder varieties that have seasonal low availability, low nutrient content and energy, and protein supplementation is hardly practiced (Phengvichith and Preston 2011). Some farmers practice supplementary forage feeding during the rainy season (25.9%) and virtually all provide salt in the goat house (Gray et al. 2019). Interestingly, practices such as provision of mineral blocks, concentrate feeds and water may be becoming more popular among goat farmers (Millar et al. 2022), which in turn has a positive impact on the goat health and production. However, the quality and quantity of feed material is still a significant problem (Windsor et al. 2018) affecting goat production in Lao PDR as inadequate feed availability during the late dry, early and late wet seasons has been identified from a farmer survey from central Lao PDR (Olmo et al. 2022a). The characteristics of feeding systems employed by Lao goat farmers are summarised in Fig. 4. These have particularly important consequences for diseases involving an environmental component of the lifecycle of the pathogen, such as gastro-intestinal helminth infection.
Reported mortality rates in Lao goats are highly variable and are derived from farmer survey data. Mortality of approximately 50% in kids before they reach weaning age of ~3 months has been reported (Xayalath et al. 2021), whereas a mean annual kid mortality of 26.3% has been reported from seven villages in central Savannakhet province on the basis of monthly farmer surveys on stock numbers (Colvin et al. 2022). The same study reported a total annual mortality (kid and adult combined) of 37.5%, emphasising the requirement of thorough disease investigation and control, coupled with improved management. A mean annual mortality of 47% (range 10–80%) was reported in another farmer survey from five provinces of northern (Houaphan, Oudomxay and Luang Prabang) and central (Savannakhet and Khammouane) Lao PDR (Gray et al. 2019).
Generally, natural mating with minimum human intervention occurs among Lao goats (Keonouchanh and Xaypha 2006; Gray et al. 2019). This carries the risk of spreading venereal diseases such as brucellosis which can cause abortion. The rate of abortion is not documented among Lao goats. A maximum of 3 kids/litter with a mean value of 2.1 ± 0.13 has been reported (Phengsavanh et al. 2004). Annual kidding rate of 60–88.2% has been reported from Songkhone, Sepon and Phin districts of Savannakhet province of central Lao PDR (Phengvilaysouk et al. 2022). Kid birth weight was 1.1–1.5 kg, and weaning weight was 9.7 kgXayalath et al. (2021). Lao kids take 6–8 months to reach 15 kg of bodyweight (Xayalath et al. 2021). The average liveweight of adult goats was between 25 and 35 kg (Keonouchanh and Xaypha 2006) and it took more than a year for goats to reach 20–25 kg weight (Phengsavanh et al. 2004). A farmer survey conducted in the northern Luang Prabang province reported age of first kidding at 12–18 months and a kidding interval of six months. Does were described as producing a single kid at first kidding, whereas subsequent kiddings had higher probabilities of twins (ADB 2002). The role of disease on reproductive performance of Lao goats is not documented.
Availability and structure of veterinary services to farmers in Lao PDR
Similar to other developing countries, Lao PDR has a limited capacity in the provision of animal health services (Rast 2014). Available veterinary services in Lao PDR can be broadly classified into the following three sectors: public sector, private sector and stakeholder institutions/organisations (Bastiaensen et al. 2011). Government veterinary and livestock services are organised at a national level within the Department of Livestock and Fisheries (DLF) of the Ministry of Agriculture and Forestry (MAF). Services provided at the regional and district levels are by staff of the Provincial Agriculture and Forestry Offices (PAFO) and the District Agriculture and Forestry Offices (DAFO). The National Animal Health Laboratory (NAHL) under the DLF is located in Vientiane and is responsible for disease diagnosis, containing disease outbreaks, conducting veterinary research, training students and government staff, providing technical support to provincial laboratories and collaborating with international laboratories.
Previously known as the National Animal Health Centre (NAHC), NAHL is the national technical reference centre for animal disease diagnosis and livestock product quality testing. It is the World Organization for Animal Health (WOAH, formerly OIE) focal veterinary laboratory in Lao PDR and comprises five diagnostic units, namely, virology, bacteriology, parasitology, BSL-3 (Biosafety level-3 for highly pathogenic avian influenza diagnosis) and the food safety unit together with a separate animal production centre. The animal production centre is responsible for animal vaccine production and dissemination around all provinces. They produce vaccines for pigs (classical swine fever), poultry (Newcastle disease and fowl cholera) and large ruminants (haemorrhagic septicaemia and blackleg) but not for goats or other small ruminants. The division of veterinary science under the DLF is in charge of organising animal vaccination at the government level. Other than the NAHL, there are basic provincial laboratories that conduct necropsies and sample collection to be sent to the NAHL.
A significant constraint identified is the limited number of qualified veterinarians and village veterinary workers (VVWs) in Lao PDR (Hansen 1997). Before 2009, there was no university level training of veterinarians within the country. Instead, veterinarians were sent away for training in various countries (Bastiaensen et al. 2011). In 2009, the National University of Laos (NUOL) established the Faculty of Agriculture to provide veterinary education with Bachelor and Master degrees in animal science and a Bachelor degree in animal production. The first graduates were in 2014 (WHO 2017). Because of the limited number of veterinarians, most veterinary services are provided by animal science diploma and degree holders (Subharat et al. 2023). VVWs have a legally defined role in Lao PDR and work closely with farmers to provide a basic animal health service (Bastiaensen et al. 2011). They are private entrepreneurs, selected on the basis of their willingness to work as VVWs, to conduct animal vaccination, parasite control and provide veterinary service such as treating and advising on animal diseases, although they are not always well trained, sometimes receiving training from different international projects. VVWs charge fees from farmers for the services provided (Frangi 2014).
A recent farmer survey identified that most goat farmers (85%) attain advice and veterinary treatments, predominantly traditional treatments, from neighbours. The majority purchase veterinary medicine from shops without veterinary advice (74%). Fewer purchase medicine from VVWs (18.6%) or from veterinarians (4.7%) (Phengvilaysouk et al. 2022). Vaccination is not widely practiced and the lack of vaccination and deworming (Xaypha 2005; Gray et al. 2019; Millar et al. 2022) owing to limited budget and shortage of appropriate vaccines (Frangi 2014) may contribute to high disease prevalence in Lao goats.
A considerable proportion of livestock farmers use traditional medicine to treat their goats and several sources corroborate similar methods. This includes using carambola juice, lime juice, salt, vinegar and boiled bark from specific local native trees to wash vesicular lesions of foot and mouth disease (FMD), orf-like lesions and surface lesions (Nampanya et al. 2015; Windsor et al. 2017; Phengvilaysouk et al. 2022). Similarly, carbonated drinks such as Pepsi® have been used to treat bloat (Phengvilaysouk et al. 2022). The efficacy of these remedies is unknown but the cost is presumably low.
A lack of definitive disease diagnosis is considered to be a contributor to a high disease incidence in Lao goats (Gray 2006) and there is a limited diagnostic capacity for goat diseases at the national laboratory in Vientiane between 2004 and 2014 (Frangi 2014). Even outbreaks of FMD in Lao goats have gone undiagnosed owing to limited availability of diagnostic resources (Singanallur et al. 2020). NAHL allocates its limited resources towards control of cattle and pig diseases because they are the major livestock species in Lao PDR, and transboundary diseases such as FMD because of economic losses and zoonotic diseases.
Disease surveillance systems are not well established in Lao PDR. The lack of an effective disease surveillance system has important implications for formalising trade, particularly for live animals. There are two market channels for goats in Lao PDR, namely, a domestic and an export market channel largely to Vietnam (Bui et al. 2023). The goat trade to Vietnam is based on movement of live goats across the border and three market chains have been identified, namely, north-eastern, northern and southern (Gray et al. 2019). It is estimated that monthly 2000–3000 goats are being exported to Vietnam (Windsor et al. 2018). Although there are notional inspections and certification on the Lao side of the border (Gray et al. 2019), goats largely enter Vietnam in an unregulated manner (Bui et al. 2023).
In 2018, the National Animal Disease Surveillance Network (NADSN) was established in Lao PDR as a joint venture of the Lao government (NAHL, DLF) and Mahidol Oxford Tropical Medicine Research Unit (MORU), so as to increase animal disease diagnosis, biosafety and biosecurity capacities (Siengsanan-Lamont et al. 2022). Under the NADSN, there are projects targeting animal disease surveillance in slaughter houses, laboratory capacity building and biosafety and upgrades to biosecurity at NAHL and provincial laboratories. Such surveillance programs have targeted transboundary and zoonotic diseases in cattle and pigs (FMD, brucellosis, Q fever) and pet coronavirus and other zoonoses in domestic animals. Further, the support has been given to improve the disease reporting system from district levels to the NAHL by introducing monthly Google reports and emergency reports. Currently, active surveillance is being conducted for highly pathogenic avian influenza (HPAI), antimicrobial resistance (AMR), Q fever, FMD, brucellosis and pet coronavirus. However, goats are not included in these surveys despite their growing importance (Siengsanan-Lamont and Blacksell 2021). Although the inclusion of goats in disease surveillance would be desirable, the small size and informal nature of the production and marketing systems for goats mitigate against this because goats are largely exported live without going through a slaughterhouse.
Major endemic disease and clinical syndromes and their impact
Animal disease is consistently identified as one of the major constraints to the livestock industry. In a poverty-assessment survey conducted by the Asian Development Bank (ADB), 70% of survey respondents identified animal diseases as a major problem where external intervention is required (ADB 2002). More recent surveys have ranked diseases as a major constraint on goat production specifically, and it is identified as an area where interventions are likely to be highly beneficial. In semi-structured surveys of goat farmer groups in 27 villages across 14 districts and five provinces, 93% of farmers ranked diseases as the number one constraint in goat farming (Gray et al. 2019). Other surveys in Xaythany district of Vientiane Capital, central Lao PDR in 2008 (Kongmanila et al. 2008), and Savannakhet province, central Lao PDR (Olmo et al. 2022a), also identified diseases as one of the impediments to goat production. Additionally, internal parasites were also identified as constraints to goat production by Windsor et al. (2018). Questionnaire-based surveys have also been used to identify the main health problems based on clinical signs, observed by goat farmers. Identified health problems include orf-like lip and facial dermatitis, eye conditions, diarrhoea, bloat and lameness (Gray et al. 2019; Millar et al. 2022; Olmo et al. 2022a; Phengvilaysouk et al. 2022). Some of the commonly seen clinical signs in Lao goats from Savannakhet province of central Lao PDR are indicated in Fig. 5.
Commonly seen clinical signs in Lao goats from Savannakhet province of central Lao PDR: (a) orf like lesions, (b) eye lesions, (c) diarrhoea, (d) purulent nasal discharge, (e) pale conjunctivae, and (f) skin parasites – lice (Photos courtesy of T. Xaikhue, 2022).

Lip and facial lesions are one of the commonly identified health problems in Lao goats. Orf, FMD, dermatophilosis and goat pox are the likely differential diagnosis for lip and facial lesions. In the absence of formal diagnosis, most of the lip and facial lesions were presumptively identified as orf (Gray et al. 2019; Olmo et al. 2022a). Orf is also known as scabby mouth, contagious ecthyma or pustular dermatitis. Goat farmer interviews in 2016 in the Xaythany district of Vientiane Capital of central Lao PDR disclosed that orf, FMD and sore mouth as some of the most important diseases in their herds (Windsor et al. 2017). The farmers from five provinces have also specified orf and FMD as important diseases (Gray et al. 2019). In a recent survey, lip and facial lesions that resemble orf were reported by 74% of smallholder farmers (Millar et al. 2022). Also, there is an earlier report of unconfirmed deaths of goats with symptoms of orf or goat pox (Frangi 2014). Orf was ranked as the main cause of goat mortalities by 14.4% of farmers in the survey (Gray et al. 2019). A relatively moderate herd prevalence of FMD (13%) has been reported by goat farmers from central province of Savannakhet (Olmo et al. 2022b) and FMD was identified as the main cause of goat mortalities by only 1.5% of farmers in a survey (Gray et al. 2019). The generally low seroprevalence of FMD in other studies (see below) suggest that FMD is not a major cause of lip and facial lesions and mortality in goats in Lao PDR. This is supported by the findings of Windsor et al. (2017) who identified orf as the probable cause of lip and facial lesions in an opportunistic study using 32 serum samples and eight tissue samples from goats with lip and facial lesions from central Xaythany district. The disease was diagnosed on the basis of characteristic histopathological lesions in tissue samples and animals being negative for FMD antibodies in serum samples by using an enzyme-linked immunosorbent assay (ELISA).
Eye conditions have been identified as a problem in goats in Xaythany district in Vientiane Capital of central Lao PDR (Windsor et al. 2017) and 11.9% of farmers from Phin, Sepon and Songkhone districts in Savannakhet province of central Lao PDR reported presence of sore eyes in their goats. Moreover, blindness has been reported by 10.3% of farmers in Phin district, whereas it was not reported by farmers from Sepon and Songkhone districts of Savannakhet province of central Lao PDR (Phengvilaysouk et al. 2022). Eye conditions in goats can result from pinkeye, entropion or foreign bodies. If not promptly treated, eye conditions can lead to painful ulceration and permanent blindness. Further, goats face difficulties in finding feed, resulting in a loss of body condition. A recent study from Savannakhet province of central Lao PDR has identified pinkeye as the reason for eye conditions and Mycoplasma conjunctivae as the likely causative agent (Jayasekara et al. 2023).
Clinical signs associated with the diseases in gastro-intestinal tract such as abdominal pain, bloat and diarrhoea are also commonly identified among Lao goats (Windsor et al. 2017; Gray et al. 2019; Phengvilaysouk et al. 2022). Presence of bloat was reported by 20.7% and 21.4% of farmers from Phin and Songkhone districts of Savannakhet province of central Lao PDR respectively (Phengvilaysouk et al. 2022). In a separate survey, 35.6% of respondents reported bloat as the main cause of goat mortalities (Gray et al. 2019). However, it is unclear if there is a confusion between bloat as cause of death and finding bloated goats after death, which is a natural phenomenon in the absence of gas eructation. A recent disease investigation study was unable to identify any case of pre-death bloat (ACIAR 2024). Diarrhoea has been frequently reported by over 38% of farmers as the main constraint (Millar et al. 2022; Olmo et al. 2022a; Phengvilaysouk et al. 2022). In another survey, 50.6% of farmers reported diarrhoea as the cause of mortality in Lao goats (Gray et al. 2019). Diarrhoea in goats can have several causes that are dependent on age. Bacteria and viruses are mainly responsible for neonatal diarrhoea, whereas parasites (nematodes, coccidia) and bacteria tend to cause diarrhoea in growers and adults. It is assumed that internal parasites are mainly responsible for diarrhoea in Lao goats (Xaypha 2005; Gray et al. 2019), contributing to high kid mortality leading to farmers not being able to meet market demand (Phengsavanh et al. 2004). Even though there were no epidemiological studies on nematodes in Lao goats until 2004 (Sani et al. 2004), gastro-intestinal nematodes have been suspected as the most problematic parasite group in Lao goats (Gray 2004). Further, coccidia and worms were identified as responsible for goat deaths in northern Saysathan district of Xayaboury province during the period from 2008 to 2011 by Frangi (2014), although no details are provided. A summary of the published investigations on gastro-intestinal parasites in Lao goats is available in Table 2. Trichostrongylus spp., Oesophagostomum spp. and Haemonchus spp. have been identified from coproculture and Trichostrongylus colubriformis, Oesophagostomum spp. and Haemonchus contortus were identified using DNA from adult worms recovered from a necropsy (Sato et al. 2014). Gastro-intestinal parasites reduce feed intake, thereby lowering the weight gain and also causing mortality in goats. The haematophagous parasite H. contortus causes anaemia and is a particularly important cause of mortality.
| Location and year | Methods | Results | Reference | |
|---|---|---|---|---|
| Xaythany district of Vientiane Capital and Pakseng district of Luang Prabang province (2015–2017) | Faecal samples (N = 434) – modified McMaster technique | Average EPG for, Strongylus spp. = 471 Moniezia spp. = 52 Trichuris spp. = 9 Coccidia = 1032 | Windsor et al. (2018) | |
| Songkhone district of Savannakhet province (2010) | Faecal samples (N = 14) – modified McMaster technique | Trichostrongylids (93%) with average 1728 EPG | Sato et al. (2014) | |
| Faecal samples (N = 14) – coproculture and modified Baermann technique | Haemonchus spp. (69%) Oesophagostomum spp. (15%) Trichostrongylus spp. (16%) Cooperia spp. (0%) | |||
| Necropsy (N = 1) – adult worms from GIT under light microscope | Trichuris spp. | |||
| Adult worms from GIT – PCR and sequencing | T. colubriformis H. contortus Oesophagostomum spp. | |||
| Livestock Research Centre, Vientiane Capital (2005) | Faecal samples (N = 32) – modified Wisconsin sugar flotation technique | H. contortus Strongylus spp. Coccidia oocysts | Phengvichith and Ledin (2007) |
N, sample size; EPG, eggs per gram; GIT, gastro-intestinal tract.
Pale conjunctivae and other mucous membranes are common clinical sign in Lao goats and indicate anaemia. Anaplasmosis and haemonchosis are the potential causes of anaemia in Lao goats. Clinical cases with signs of anaplasmosis have been reported in Lao PDR with laboratory confirmation based on blood samples (Frangi 2014). However, information on seroprevalence is not available. Adult worms and eggs of H. contortus have been found in parasite studies (Phengvichith and Ledin 2007; Sato et al. 2014). As indicated earlier, haemonchosis is a major cause of anaemia and mortality in small ruminants throughout the humid tropics.
Control of gastro-intestinal nematode infection is complex and an integrated parasite approach involving multiple strategies is generally advocated (Whitley et al. 2014). These involve management practices that disrupt the lifecycle of the parasites, coupled with judicious use of anthelmintic chemicals to maintain efficacy. From a gastro-intestinal nematode infection perspective, village production systems in Lao PDR benefit from uncontrolled grazing on common land together with other species, and also the housing of goats on slatted floors at nights. These have a dilution effect on the burden of infective larvae on pastures available to infect the goats (Barger 1999). Because of this, the burdens faced tend not to be overwhelming (Windsor et al. 2018). However, with intensification of production and placing of goats on fenced pastures, the parasite challenge can be expected to increase and increase the importance of appropriate and timely anthelmintic treatment in control. Fortunately, there appears to be little resistance to the currently available anthelmintics used to treat goats in Lao PDR on the basis of a faecal egg count reduction study from central Savannakhet province using 127 goats (Xaikhue et al. 2023). The efficacy of three locally available anthelmintics, namely, albendazole, ivermectin and levamisole, was evaluated against untreated controls.
Less frequent health problems have also been identified in Lao goats from farmer surveys. A study in three districts (Phin, Sepon and Songkhone) of Savannakhet province of central Lao PDR showed that 7.5%, 1.5%, 7.5% and 1.5% of goat farmers had observed skin parasites, skin problems, weak legs and coughing in their goats respectively (Phengvilaysouk et al. 2022). Further, lameness was reported (Windsor et al. 2017) owing to foot aches by 1.5% of farmers and owing to trauma from the slatted floor of goat pen by 1.5% of goat farmers (Phengvilaysouk et al. 2022). Abortions have also been reported in limited number of villages and suspected clinical cases of tetanus have been reported without laboratory confirmation (Frangi 2014). In addition, there is a record on mosquito-transmitted lumbar paralysis among exotic bucks in Lao PDR but not in native goats (Gray 2004).
Presence and importance of major transboundary and zoonotic diseases
The risk of transboundary (Windsor et al. 2017) and zoonotic diseases is also a major problem associated with livestock production in general in Lao PDR because of their economic and public health importance. It is believed that Lao PDR was free from peste des petits ruminants (PPR) and goat pox until 2004 (Gray 2004). A subsequent serological study identified PPR antibodies in a small number of goat sera by ELISA (Table 3). However, because of the apparent absence of disease manifestation of PPR in Lao PDR, these seropositive samples may indicate cross-reaction with other morbilliviruses such as measles or canine distemper, imported pre-vaccinated goats, or circulation of a less virulent virus (Burns et al. 2019). However, Lao PDR is classified as a country without an official status for PPR by the WOAH (OIE-WAHIS 2023a).
| Disease | Province | Study period | N | SP (%) | Clinical cases reported | Reference | |
|---|---|---|---|---|---|---|---|
| Bluetongue | Xayaboury | 2013–2015 | 6 | 100 | Not | Douangngeun et al. (2016) | |
| PPR | Attepeu | 2016–2017 | 11 | 9.1 | Not | Burns et al. (2019) | |
| Savannakhet | 89 | 0.6 | |||||
| Vientiane Capital | 444 | 0.3 | |||||
| Xayaboury | 273 | 2.5 | |||||
| Xiangkhouang | 255 | 3.1 | |||||
| Total | 1072 | 1.7 | |||||
| FMD | Vientiane Capital | 2016 | 32 | 0 | Suspected | Windsor et al. (2017) | |
| Borkeo | 2017–2018 | 76 | 50 | Not | Singanallur et al. (2020) | ||
| Luang Namtha | 74 | 1.4 | |||||
| Luang Prabang | 75 | 0 | |||||
| Xayaboury | 75 | 12 | |||||
| Khoummoune | 80 | 27.5 | |||||
| Savannakhet | 60 | 8.3 | |||||
| Xiangkhouang | 76 | 1.3 | |||||
| Champasak | 75 | 1.3 | |||||
| Total | 591 | 13 | |||||
| Vientiane Capital, Vientiane and Xiangkhouang | 2018 | 288 | 15 | Not mentioned | Xaydalasouk et al. (2021) | ||
| In 9 provinces | 2019 | 19 | 21.1 | Reported. Only 19 goats were included together with 602 large ruminants | MacPhillamy et al. (2022) |
N, sample size; SP, seroprevalence; PPR, peste des petits ruminants; FMD, foot and mouth disease.
Lao PDR is classified as an FMD endemic country and outbreaks of serotypes O, A and Asia 1 have been reported among cattle, buffalo and pig populations. No information was available on FMD presence in goats during 1996–2006 (Khounsy et al. 2009). Subsequent serological surveys with the use of ELISA have shown a low to moderate seroprevalence of FMD among goats with clinical evidence of infection reported in one study (Table 3). FMD outbreaks in goats have been recorded in 2013–2015 and 2017–2019 (OIE-WAHIS 2023b).
Zoonotic diseases where goats are heavily involved include brucellosis (Brucella melitensis), Q fever (Coxiella burnetii) and orf (orf virus). A small early study of only six goats in Xayaboury province of northern Lao PDR did not detect antibodies against brucellosis and Q fever by semiquantitative indirect ELISA (Douangngeun et al. 2016). A much larger subsequent serological survey with 1458 samples from five provinces from northern, central and southern Lao PDR found low overall seroprevalence of brucellosis (1.4%) and Q fever (4.1%; Table 4). The same study has also identified the risk factors of these two diseases (Table 4). Although a small proportion of Lao goats appear to have some exposure to brucellosis and Q fever pathogens, the risk of clinical disease has not yet been determined (Burns et al. 2018) and clinical diseases have not been reported (OIE-WAHIS 2023b).
| Province | N | Brucellosis | Q fever | |||
|---|---|---|---|---|---|---|
| SP (%) | Risk factors | SP (%) | Risk factors | |||
| Attepeu | 194 | 1.6 | Vientiane Capital, introduced Boer mixed breed, commercial production system, adult age, large farm size | 1.6 | Vientiane Capital, introduced Boer mixed breed, age >3 years | |
| Savannakhet | 279 | 0 | 0.4 | |||
| Vientiane Capital | 447 | 4 | 10.7 | |||
| Xayaboury | 273 | 0 | 2.9 | |||
| Xiangkhouang | 265 | 0 | 0 | |||
| Total | 1458 | 1.4 | 4.1 | |||
N, sample size; SP, seroprevalence.
Orf is another zoonotic disease that is believed to be present in Lao PDR and has been diagnosed with histology of tissue samples with clinical signs of suspected orf or FMD cases in 2016. These samples were negative for the FMD antibodies (Windsor et al. 2017). Another potential zoonotic link with goats is human infection with T. colubriformis, which has been shown in Lao PDR (Sato et al. 2011), and which shows genomic identity with T. colubriformis recovered from goats (Sato et al. 2014). Such findings further indicate the importance of continuous surveillance of zoonotic diseases in goats for timely control and prevention measures.
Melioidosis is another neglected zoonotic disease that was confirmed in Lao goats once in 2003, but for which detailed published data are not yet available (Dance et al. 2018). Anthrax was reported in Champasak province of southern Lao PDR in 2008 with the death of two domestic goats, confirmed by clinical signs and bacteriology and was successfully controlled (OIE-WAHIS 2023c). However, data on the prevalence of other important zoonotic diseases, such as leptospirosis and capripox among Lao goats, are not available.
Implications for domestic and international trade in goats
The apparent low prevalence of transboundary and zoonotic diseases in Lao goats is beneficial to the trade of Lao goats to neighbouring countries, although accurate certification of disease status in exported goats remains a challenge. However, the close proximity to China, Thailand and Vietnam where some of these diseases are endemic poses a risk to the Lao goat population, particularly because of the extent of unregulated movement of goats across country borders. Knowing the disease status of goats in neighbouring countries is valuable to Lao PDR, so as to implement specific disease control and prevention measures to promote the trade of Lao goats. Further, such disease control measures are beneficial to safeguard other livestock species in Lao PDR and to improve the value of their products. A summary of major transboundary and zoonotic diseases present in China, Thailand, Vietnam and Lao PDR goats are outlined in Table 5.
China is considered an endemic country for PPR (Liu et al. 2018), and Xinjiang and Tibet are classified as areas with higher risk (Gao et al. 2021). The disease was officially reported for the first time from Ngari region of south-western Tibet in 2007 Liu et al. (2018), followed by an outbreak in 2013 in Xinjiang. In both outbreaks, a number of goats died (Liu et al. 2018). High seroprevalence of PPR in both sheep and goats has been reported from several regions of China, including, Yannan, Jiangsu, Anhui, Hubei and Guizhou (Wu et al. 2016). The shared border with Yannan province to northern Lao PDR possesses a threat of PPR incursion. Serological studies have shown that the rate of PPR infection in Chinese goats is higher than in sheep in Tibet (Wang et al. 2009). Further, strain identification of PPR virus from goats has been performed on samples from Xinjiang (Bao et al. 2014; Li et al. 2017) and various other parts of China (Su et al. 2015; Wu et al. 2016). A national PPR eradication program has been implemented with the aim of eradicating PPR in China by 2020 (Liu et al. 2018). Currently China is reported as being in its eradication stage of PPR (Legnardi et al. 2022) and with no official reports of PPR in goat-only herds (OIE-WAHIS 2023a). However, in 2023 PPR was reported from sheep and goat mixed herds (OIE-WAHIS 2023b). Most of the available reports on Q fever in goats is in Chinese language, although 12% (176/1440) overall seropositivity has been reported for the period of 1989–2013. During this period, higher seropositivity was reported from Anhui province of central China, north-eastern provinces and inner Mongolia. Further, seroprevalence has been reported in Yannan province (El-Mahallawy et al. 2015). Furthermore, 4.8% seroprevalence has been reported in Hubei province of central China (Li et al. 2018). Of 50 B. melitensis strains isolated from Xinjiang province in a 6-year period (2010–2015), only one was from goats. Further, Brucella abortus has not been isolated from goats (Sun et al. 2016). Furthermore, only 12 Brucella strains have been isolated from goats of 600 strains collected from 29 provinces including Yannan province between 1953 and 2013. However, it was not clear whether any of the goat isolates belonged to Yannan province or not (Tian et al. 2017). Tibet, Xinjiang and Qinghai provinces in western China have been identified as animal FMD hotspots during the period from 2010 to 2016 (Ma et al. 2017). Further, south-western and central China have been identified as hotspots for FMD serotype A and serotype O respectively in all animals from 2010 to 2019 (Gao and Ma 2021). Furthermore, western, southern, including Yannan province, northern and eastern areas of China have been reported as high-risk areas of animal FMD in 2010–2020 (Haoran et al. 2021). Orf is a nationally important zoonotic disease in China and clinical cases have been reported in goats during 2009–2011 from different provinces including Yannan (Zhang et al. 2014). In 2017, clinical cases were reported in north-eastern China (Yu et al. 2020). Interestingly, orf virus has been also isolated from blood (32.4%), saliva (53%) and milk (44.7%) of asymptomatic dairy goats (Ma et al. 2022). The seroprevalence of bluetongue in goats in seven regions of China during the period from 1988 to 2019 was 28.1% (41,263/12,182) and the highest prevalence among both sheep and goats was recorded from Yannan province (35.7%) (Liu et al. 2021). Similarly, seroprevalence in goats has been reported from Hubei province (13.31%) (Luo et al. 2017) and Xinjiang province (Ma et al. 2019; Yang et al. 2021). Clinical cases of goat pox have been seen in 2009 in Chongqing municipality of China, which is closer to Hubei province (Chu et al. 2011). Goat deaths owing to melioidosis were reported in Guangxi province in 2009 Zheng et al. (2019). Several cases of caprine arthritis and encephalitis (CAE) from different provinces (Gansu, Shandong, Sichuan, Shaanxi and Guizhou), which are away from Lao PDR have been documented in Chinese language (Wang et al. 2023).
In Thailand, human brucellosis caused by B. melitensis has been reported and was primarily associated with direct contact with infected goats (Paitoonpong et al. 2006; Techapornroong and Ekgatat 2018). Low overall seroprevalence of B. melitensis (1.4%) was reported in Thai goats in 2013 (Sagarasaeranee et al. 2017). Seroprevalence of brucellosis in both sheep and goats between 2013 and 2015 was reported at 0.72% (Peck et al. 2018). Another study from Thailand reported very low seroprevalence of brucellosis, 0.6% (3/500), among goats in Chiang Mai province of northern Thailand (Kladkempetch et al. 2017). Further, a study in north-eastern provinces during 2017–2020 showed 6.8% seroprevalence; however none of these provinces share borders with Lao PDR (Rerkyusuke et al. 2022). Further, 8.3% herd-level seroprevalence has been reported from Sa Kaeo province, Thailand, sharing a border with Cambodia (Colombe et al. 2018). Moreover, Thailand is a Q fever endemic country and 3.5% seroprevalence has been reported in samples from northern Chiang Mai and north-eastern Nakornratchasima provinces (Doung-Ngern et al. 2017). Herd-level seroprevalence of Q fever has been reported as 33.3% in goats from Sa Kaeo province (Colombe et al. 2018). Higher seroprevalence of Q fever (46.61%) has been reported from north-eastern provinces, which are not sharing common borders with Lao PDR (Rerkyusuke et al. 2022). Clinical disease of FMD in goats has been reported from northern Chiang Mai province (Chomnanpood and Gleeson 1992). Further, 5.9% overall seroprevalence of CAE in three provinces of western Thailand (Lin et al. 2011) and 3.5% (68/1925) seroprevalence have been reported from five parts of Thailand including the borders with Lao PDR (Mongkonwattanaporn et al. 2021). In Thailand, 31 clinical cases of melioidosis have been reported over a 5-year period from 2006 to 2010 in areas not sharing borders with Lao PDR (Limmathurotsakul et al. 2012). In total, 65 cases of melioidosis in goats have been also reported in Thailand over a 10-year period from 2006 to 2015 and animal cases were reported from north-eastern Thailand, bordering Lao PDR (Kongkaew et al. 2017). Further, 0.33% seroprevalence has been reported during 2005–2006 among 6576 goats, covering 18 provinces including Chiang Rai Province, Thailand, which borders Lao PDR. However, the information on the presence of seroprevalent goats particularly in Chiang Rai Province is not available (Srikawkheaw and Lawhavinit 2007). Thailand was officially recognised as PPR free until 2017 (Qingdao 2017) but in February 2021 the first occurrence of PPR in domesticated goats was reported. This was the first report of PPR in Southeast Asia (OIE-WAHIS 2023d). No evidence of orf and goat pox virus infections in Thai goats are available (OIE-WAHIS 2023b).
In Vietnam, the first human brucellosis with B. melitensis was reported in 2016–2017 from four provinces towards the Cambodian border. With the history of exposure to goats in these human cases, it was assumed that the responsible pathogen is widely present among the Vietnamese goat population, particularly in the south (Campbell et al. 2017). Further, herd mortality resulting from goat pox ranging from 5.1% to 7.4% has been reported from six provinces of northern Vietnam, including Nghe An province bordering Lao PDR (Pham et al. 2020). Also, it was identified that the isolated goat pox virus is closely related to the goat pox viruses currently circulating in China, India and Pakistan (Pham et al. 2021). Even though, PPR was not reported in any animal species during the period from 2005 to 2013 (EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) 2015), a study had shown low seroprevalence of PPR (2%) among 283 goats from Ha Giang Province, which borders China, without apparent clinical signs (Maillard et al. 2008). However, Vietnam is still classified as a country without an official record for PPR (OIE-WAHIS 2023a). Being an FMD-endemic country, FMD has been reported in Vietnamese goats. Considerably low FMD prevalence (0.41%) was recorded collectively for sheep and goats from a nation-wide surveillance from 2007 to 2017 (Lee et al. 2020). However, published information on the presence of bluetongue, Q fever, orf, melioidosis and CAE in Vietnamese goats is not available (OIE-WAHIS 2023b).
Prevention and control of transboundary animal diseases requires coordination among responsible authorities within each country and also political support. Control is challenging and typically requires disease-screening centres at the most popular border-crossing sites with suitable disease diagnostic aids and competent personnel. Other measures include ensuring adequate supplies of appropriate vaccines in a vaccine bank, active and passive surveillance for the diseases of interest, control of animal movements and synchronised vaccination programs between border countries (Gongal et al. 2022).
Discussion and conclusions
This review has shown that the mortality rates in Lao goats are high on the basis of past surveys (Gray et al. 2019; Xayalath et al. 2021; Colvin et al. 2022). It has also identified that disease is a major cause of such mortalities. Poor health in Lao goats is mainly associated with several endemic clinical syndromes, while poor nutrition and poor husbandry practices also play a significant role. The most prominent endemic syndromes appear to be orf-like lip and facial lesions, eye conditions (‘pinkeye’) and diseases of the gastro-intestinal tract. Knowing the cause of some of the endemic syndromes is needed to help design appropriate treatment and control measures for a more productive goat industry in Lao PDR. This is important, given the recent increase in contribution of goats to the local and potential national income, and formed the basis for this review of the existing literature on goat diseases in Lao PDR.
High mortality and prevalence of disease and clinical syndromes in Lao goats are likely to be influenced by management systems and nutrition. The widespread use of extensive free-grazing unconstrained by fences is likely to mitigate against very heavy gastro-intestinal worm burdens and strongyle faecal egg, which generally tend to be moderate rather than very high (Windsor et al. 2018). For the smaller number of farmers who use cut and carry systems, the risk of worm infections is further reduced. In contrast, unsupervised grazing on communal lands can increase the risk of disease transmission from other goats and farm species. Further, inadequate nutrition associated with low forage availability or quality, increases host susceptibility to infections including gastro-intestinal nematode infection. The immunosuppression occurs as a result of inadequate supply of certain nutrients that are needed for optimum function of immune cells or because of increased nutritional demand as a result of environmental, physiological and management stresses (Caroprese et al. 2015) or because of changes in intestinal microbiota (Guo et al. 2022). Improving farmer knowledge on supplementary feeding, development of forage plots and extension of grazing time available for goats are likely to be advantageous in assisting disease control. Good housing with raised well-designed slatted floors, good ventilation and protection from the elements are protective for diseases transmitted by faecal–oral cycling such as coccidiosis (Smith and Sherman 2009) or respiratory disease (Constable et al. 2017), which is exacerbated by high ammonia concentrations (Smith and Sherman 2009). However, dirty, crowded goat shelters without raised floors will facilitate ammonia production and respiratory infections, especially during the rainy season under conditions of high humidity and wet floors.
The poor availability of veterinary services (Subharat et al. 2023) is a likely contributory factor to the mortality and disease problems in Lao goats. Strengthening the provision of veterinary services, which include diagnostic, extension and veterinary health workers, is needed and would benefit all livestock industries, not just goats. Strengthening of the VVW network would be beneficial because of the finite availability of qualified veterinarians in Lao PDR. While more training is needed for VVWs and village volunteers in diagnosing and treating goat diseases and ensuring that they have appropriate drugs with proper labelling for treatment is also desirable, the challenges in ensuring an effective VVW system in the field are acknowledged. Educating VVWs on the links between management practices and disease is something to consider, to introduce a role in prevention rather than only treatment of disease.
The available studies on transboundary and zoonotic diseases in Lao goats indicate low seroprevalence, except for FMD. Thus, transboundary and zoonotic diseases appear to have little impact on current goat production and trade, with a possible exception of FMD when it occurs as a result of involvement of multiple host species. However, further studies with greater geographic spread need to be conducted to confirm the low prevalence of these diseases. Such diseases represent a modest risk to the export trade because countries may close cross-border trade in live animals as a result of disease outbreaks in Lao PDR. The largely informal nature of the live-goat trade would make such closures difficult to implement. However, formalising the trade would likely require development of disease-certification procedures on the Lao side that would be difficult to implement effectively because of inadequate knowledge within Lao PDR, and insufficient human and financial resources. The informal trade, which also includes Lao PDR as a transit country in some cases, also poses a risk of disease incursions into Lao PDR, although it is not clear whether Lao goats have a superior disease status overall compared with the goats in neighbouring countries.
To conclude, goat diseases are an inadequately explored area in Lao PDR (Burns et al. 2018). Lao goats suffer from many endemic diseases and clinical syndromes and there is serological evidence that several transboundary and zoonotic diseases circulate in the country. The opportunity for Lao goat farmers to grow their household income through goat production is constrained by mortalities and endemic disease and clinical syndromes. Knowledge of disease causation, which pathogens are circulating and basic disease epidemiological data such as disease risk factors are very limited for Lao goats, despite being critical for informing appropriate disease control and treatment. Those are important areas for future research. Lack of correct disease diagnosis and using this information to design appropriate disease-control strategies have been identified as having the utmost importance for a more productive goat enterprise in Lao PDR (Gray et al. 2019). Even though some progress has been made in this area with provisional diagnosis of orf, detailed diagnosis of pinkeye and some characterisation of gastro-intestinal helminth burdens and species involved, many of the endemic disease problems of goats in Lao PDR remain poorly understood and managed. Overcoming this is a challenge for the Lao Government, non-governmental organisations (NGOs) involved in rural development and international agricultural research and development agencies. Because lip and facial dermatitis, eye conditions and diarrhoea are the most common clinical syndromes in Lao goats, these can be considered as the major priorities for determining causation. Gastro-intestinal nematode infections can also be expected to increase in importance with intensification of the production system. Information on disease causation can be used to inform appropriate control measures for the most common disease and clinical syndromes to lower the disease burden, which can exert a significant impact on Lao goat health and production. The link between management practices and disease is another recommended area of future research, with the application of good husbandry practices, nutrition and reproduction management being likely to reduce the disease burden. In addition, strengthening veterinary services in terms of surveillance systems, disease diagnostics, and field veterinary capacity, together with empowering extension workers with proper knowledge of diseases, should form part of strategies to improve disease control in Lao goats. However, the practicability, cost and durability of such interventions need to be carefully evaluated before being introduced to Lao PDR.
Data availability
Authors confirm that no data were generated in this review article. All research findings included in this review article have been cited appropriately.
Conflicts of interest
The authors declare that they have no conflicts of interest, except the author S. W. Walkden-Brown is an Associate Editor of Animal Production Science. To mitigate this potential conflict of interest they were blinded from the review process.
Declaration of funding
This review article received financial support from the Australian Centre for International Agricultural Research (Project number: LS/2017/034). Preethinie P. Jayasekara was also the recipient of a postgraduate scholarship from the University of New England, Armidale, NSW, Australia.
References
ACIAR (2024) Goat production systems and marketing in Lao PDR and Vietnam. Australian Centre for International Agricultural Research. Available at https://www.aciar.gov.au/project/ls-2017-034 [Accessed 1 February 2024]
ADB (2002) Review of the livestock sector in the Lao People’s Democratic Republic. Asian Development Bank. Available at https://cgspace.cgiar.org/bitstream/handle/10568/21136/adb_livestock_review.pdf?sequence=2 [Accessed 25 February 2022]
Bao J, Wang Q, Zhang Y, Liu C, Li L, Wang Z (2014) Complete genome sequence of a novel variant strain of Peste des Petits Ruminants virus, China/XJYL/2013. Genome Announcements 2, e00762.
| Crossref | Google Scholar | PubMed |
Barger IA (1999) The role of epidemiological knowledge and grazing management for helminth control in small ruminants. International Journal for Parasitology 29, 41-47.
| Crossref | Google Scholar | PubMed |
Bastiaensen P, Kamakawa A, Varas M (2011) PVS pathway follow-up mission report: tool for the evaluation of performance of veterinary services. OIE, Available at https://rr-asia.woah.org/wp-content/uploads/2020/05/pvs_laos_followup.pdf [Accessed 28 March 2023]
Bui TN, Nguyen HV, Nguyen XB, Le VN, Nguyen TM, Ngo CTK, Ngo QTL, Hoang N, Morales LE, Nguyen VD, Olmo L, Walken-Brown S, Le TTH (2023) An analysis of the goat value chain from Lao PDR to Vietnam and a socio-economic sustainable development perspective. Sustainability 15, 13781.
| Crossref | Google Scholar |
Burns RJL, Douangngeun B, Theppangna W, Khounsy S, Mukaka M, Selleck PW, Hansson E, Wegner MD, Windsor PA, Blacksell SD (2018) Serosurveillance of coxiellosis (Q-fever) and brucellosis in goats in selected provinces of Lao People’s Democratic Republic. PLoS Neglected Tropical Diseases 12, e0006411.
| Crossref | Google Scholar | PubMed |
Burns RJL, Douangngeun B, Theppangna W, Mukaka M, Wegner MD, Windsor PA, Blacksell SD (2019) Peste des Petits Ruminants (PPR) virus serological surveillance in goats in Lao PDR: issues for disease eradication in a low-resource disease-free setting. Transboundary and Emerging Diseases 66, 939-947.
| Crossref | Google Scholar |
Campbell JI, Lan NPH, Phuong PM, Chau LB, Trung Pham D, Guzman-Verri C, Ruiz-Villalobos N, Minh TPT, Munoz Alvaro PM, Moreno E, Thwaites GE, Rabaa MA, Chau NVV, Baker S (2017) Human Brucella melitensis infections in southern Vietnam. Clinical Microbiology and Infection 23, 788-790.
| Crossref | Google Scholar | PubMed |
Caroprese M, Giannenas I, Fthenakis GC (2015) Interactions between nutritional approaches and defences against microbial diseases in small ruminants. Veterinary Microbiology 181, 8-14.
| Crossref | Google Scholar | PubMed |
Chomnanpood P, Gleeson L (1992) Investigation into the role of goats and sheep in the epidemiology of foot-and-mouth disease in Northern Thailand. The Thai Journal of Veterinary Medicine 22, 217-231.
| Crossref | Google Scholar |
Chu Y, Yan X, Gao P, Zhao P, He Y, Liu J, Lu Z (2011) Molecular detection of a mixed infection of Goatpox virus, Orf virus, and Mycoplasma capricolum subsp. capripneumoniae in goats. Journal of Veterinary Diagnostic Investigation 23, 786-789.
| Crossref | Google Scholar | PubMed |
Colombe S, Watanapalachaigool E, Ekgatat M, Ko AI, Hinjoy S (2018) Cross-sectional study of brucellosis and Q fever in Thailand among livestock in two districts at the Thai–Cambodian border, Sa Kaeo province. One Health 6, 37-40.
| Crossref | Google Scholar | PubMed |
Colvin AF, Olmo L, Phengvilaysouk A, Millar J, Gray GD, Phengsavanh P, Walkden-Brown SW (2022) Smallholder goat production systems in Lao PDR: assessing production efficiency. In ‘34th Biennial Conference of the Australian Association of Animal Sciences (AAAS)’, Cairns, Qld, Australia, 5–7 July. (CSIRO Publishing)
Dance DAB, Luangraj M, Rattanavong S, Sithivong N, Vongnalaysane O, Vongsouvath M, Newton PN (2018) Review: melioidosis in the Lao People’s Democratic Republic. Tropical Medicine and Infectious Disease 3, 21.
| Crossref | Google Scholar |
Douangngeun B, Theppangna W, Soukvilay V, Senaphanh C, Phithacthep K, Phomhaksa S, Yingst S, Lombardini E, Hansson E, Selleck PW, Blacksell SD (2016) Seroprevalence of Q Fever, brucellosis, and bluetongue in selected provinces in Lao People’s Democratic Republic. The American Journal of Tropical Medicine and Hygiene 95, 558-561.
| Crossref | Google Scholar | PubMed |
Doung-Ngern P, Chuxnum T, Pangjai D, Opaschaitat P, Kittiwan N, Rodtian P, Buameetoop N, Kersh GJ, Padungtod P (2017) Seroprevalence of Coxiella burnetii antibodies among ruminants and occupationally exposed people in Thailand, 2012-2013. The American Journal of Tropical Medicine and Hygiene 96, 786-790 10.4269/ajtmh.16-0336.
| Google Scholar | PubMed |
EFSA AHAW Panel (European Food Safety Authority Panel on Animal Health and Welfare) (2015) Scientific opinion on peste des Petits Ruminants. European Food Safety Authority Journal 13, 3985.
| Crossref | Google Scholar |
El-Mahallawy HS, Lu G, Kelly P, Xu D, Li Y, Fan W, Wang C (2015) Q fever in China: a systematic review, 1989-2013. Epidemiology and Infection 143, 673-681.
| Crossref | Google Scholar | PubMed |
FAO (2020) Special report- 2019 FAO/WFP Crop and food security assessment mission (CFSAM) to the Lao People’s Democratic Republic. (Food and Agriculture Organization of the United Nations: Rome, Italy) Available at https://docs.wfp.org/api/documents/WFP-0000116697/download/?_ga=2.267853255.859222792.1721977833-240666296.1719539285
FAO (2024) FAOSTAT. Food and Agriculture Organization of the United Nations. Available at https://www.fao.org/faostat/en/#data/QCL [Accessed 21 March 2024]
Frangi B (2014) Factors and conditions enabling the establishment of a network of village veterinary workers in Laos: example of the village veterinary worker network in Saysathan district, Sayabouly province – practical recommendations for the replication of the network. (CARE International: Lao PDR) Available at https://data.opendevelopmentmekong.net/library_record/factors-and-conditions-enabling-the-establishment-of-a-network-of-village-veterinary-workers-in-lao [Accessed 21 February 2022]
Gao H, Ma J (2021) Spatial distribution and risk areas of foot and mouth disease in mainland China. Preventive Veterinary Medicine 189, 105311.
| Crossref | Google Scholar | PubMed |
Gao S, Xu GY, Zeng Z, Lv JN, Huang LY, Wang HN, Wang XL (2021) Transboundary spread of peste des petits ruminants virus in western China: a prediction model. PLoS ONE 16, e0257898.
| Crossref | Google Scholar | PubMed |
Gongal G, Rahman H, Thakuri KC, Vijayalakshmy K (2022) An overview of transboundary animal diseases of viral origin in South Asia: what needs to be done? Veterinary Sciences 9, 586.
| Crossref | Google Scholar | PubMed |
Gray D, Walkden-Brown S, Phengsavanh P, Patrick I, Hergenhan R, Hoang N, Phengvilaysouk A, Carnegie M, Millar J, Hữu Văn N (2019) Final report. Assessing goat production and marketing systems in Laos and market linkages into Vietnam. ACIAR Project No: LPS/2016/027. Australian Centre for International Agricultural Research, Canberra, ACT, Australia.
Guo H, Li B, Gao M, Li Q, Gao Y, Dong N, Liu G, Wang Z, Gao W, Chen Y, Yang Y (2022) Dietary nutritional level affects intestinal microbiota and health of goats. Microorganisms 10, 2322.
| Crossref | Google Scholar | PubMed |
Hansen PK (1997) Animal husbandry in shifting cultivation societies in northern Laos: technical report no.10, TR 10. Shifting Cultivation Research Sub-programme and Lao Swedish Forestry Programme, Luang Prabang, Lao PDR. Available at http://lad.nafri.org.la/fulltext/LAD010320040500.pdf [Accessed 21 February 2022]
Haoran W, Jianhua X, Maolin O, Hongyan G, Jia B, Li G, Xiang G, Hongbin W (2021) Assessment of foot-and-mouth disease risk areas in mainland China based spatial multi-criteria decision analysis. BMC Veterinary Research 17, 374.
| Crossref | Google Scholar | PubMed |
IFAD, ADB (2018) Performance evaluation report. Lao People’s Democratic Republic: Northern Region Sustainable livelihoods through livestock development project. Available at https://www.adb.org/sites/default/files/evaluation-document/235196/files/pper-lao-nrslldp.pdf [Accessed 1 November 2021]
Keonouchanh S, Xaypha S (2006) Goat production in Lao PDR. In ‘Proceedings of the APHCA-ILRI regional workshop on goat production systems and markets: Goats-Undervalued assets in Asia’, Luang Prabang, Lao PDR, 24–25 October. (Animal Production and Health Commission for Asia and the Pacific and International Livestock Research Institute: Luang Prabang, Lao PDR).
Khounsy S, Conlan JV, Gleeson LJ, Westbury HA, Colling A, Paton DJ, Ferris NP, Valarcher J-F, Wadsworth J, Knowles NJ, Blacksell SD (2009) Molecular epidemiology of foot-and-mouth disease viruses from South East Asia 1998-2006: the Lao perspective. Veterinary Microbiology 137, 178-183.
| Crossref | Google Scholar | PubMed |
Kladkempetch D, Somtua N, Maktrirat R, Punyapornwithaya V, Sathanawongs A (2017) Seroprevalence and factors affecting brucellosis in goats in Chiang Mai Province. Veterinary Integrative Sciences 15, 99-107.
| Google Scholar |
Kongkaew W, Thiptara A, Kaewkalong S, Hinjoy S (2017) Situation of melioidosis in Thailand, 2006-2015. Thai-NIAH eJournal 12, 80-102.
| Google Scholar |
Kongmanila D, Preston TR, Ledin I (2008) Survey on the utilization of local foliage species for goats in Xaythanee district, Vientiane City. Livestock Research for Rural Development 20(supplement), 6.
| Google Scholar |
Lee HS, Pham TL, Wieland B (2020) Temporal patterns and space-time cluster analysis of foot-and-mouth disease (FMD) cases from 2007 to 2017 in Vietnam. Transboundary and Emerging Diseases 67, 584-591.
| Crossref | Google Scholar | PubMed |
Legnardi M, Raizman E, Beltran-Alcrudo D, Cinardi G, Robinson T, Falzon LC, Djomgang HK, Okori E, Parida S, Njeumi F, Benfield CTO (2022) Peste des Petits Ruminants in Central and Eastern Asia/West Eurasia: epidemiological situation and status of control and eradication activities after the first phase of the PPR global eradication programme (2017-2021). Animals 12, 2030.
| Crossref | Google Scholar | PubMed |
Li J, Li L, Wu X, Liu F, Zou Y, Wang Q, Liu C, Bao J, Wang W, Ma W, Lin H, Huang J, Zheng X, Wang Z (2017) Diagnosis of peste des Petits Ruminants in wild and domestic animals in Xinjiang, China, 2013-2016. Transboundary and Emerging Diseases 64, e43-e47.
| Crossref | Google Scholar | PubMed |
Li K, Luo H, Shahzad M (2018) Epidemiology of Q-fever in goats in Hubei province of China. Tropical Animal Health and Production 50, 1395-1398.
| Crossref | Google Scholar | PubMed |
Liehr E, Millar J, Walkden-Brown S, Chittavong M, Olmo L (2024) The role of goat production in smallholder systems in Lao PDR: implications for improving productivity and scaling up production. Animal Production Science 64, AN23368.
| Crossref | Google Scholar |
Limmathurotsakul D, Thammasart S, Warrasuth N, Thapanagulsak P, Jatapai A, Pengreungrojanachai V, Anun S, Joraka W, Thongkamkoon P, Saiyen P, Wongratanacheewin S, Day NPJ, Peacock SJ (2012) Melioidosis in animals, Thailand, 2006–2010. Emerging Infectious Diseases 18, 325-327.
| Crossref | Google Scholar | PubMed |
Lin TN, Ngarmkum S, Oraveerakul K, Virakul P, Techakumphu M (2011) Seroprevalence and risk factors associated with caprine arthritis–encephalitis virus infection in goats in the western part of Thailand. The Thai Journal of Veterinary Medicine 41, 353-360.
| Crossref | Google Scholar |
Liu F, Li J, Li L, Liu Y, Wu X, Wang Z (2018) Peste des Petits Ruminants in China since its first outbreak in 2007: a 10-year review. Transboundary and Emerging Diseases 65, 638-648.
| Crossref | Google Scholar | PubMed |
Liu F, Gong Q-L, Zhang R, Chen Z-Y, Wang Q, Sun Y-H, Sheng C-Y, Ma B-Y, Li J-M, Shi K, Zong Y, Leng X, Du R (2021) Prevalence and risk factors of bluetongue virus infection in sheep and goats in China: a systematic review and meta-analysis. Microbial Pathogenesis 161, 105170.
| Crossref | Google Scholar | PubMed |
Luo HQ, Li K, Zhang H, Lan YF, Peng JP, Shahzad M, Wang JX (2017) Seroprevalence of bluetongue virus infection in goats in the central China. Tropical Biomedicine 34, 80-83.
| Google Scholar | PubMed |
Ma J, Xiao J, Gao X, Liu B, Chen H, Wang H (2017) Spatial pattern of foot-and-mouth disease in animals in China, 2010-2016. PeerJ 5, e4193.
| Crossref | Google Scholar | PubMed |
Ma J, Gao X, Liu B, Chen H, Xiao J, Wang H (2019) Epidemiology and spatial distribution of bluetongue virus in Xinjiang, China. PeerJ 7, e6514.
| Crossref | Google Scholar | PubMed |
Ma W, Pang M, Lei X, Wang Z, Feng H, Li S, Chen D (2022) Orf virus detection in the saliva and milk of dairy goats. Frontiers in Microbiology 13, 837808.
| Crossref | Google Scholar | PubMed |
MacPhillamy I, Young J, Earp F, Khounsy S, Windsor P, Toribio J-A, Bush R (2022) Foot-and-mouth disease seroprevalence and reporting behaviours in nine northern provinces in Lao PDR: the current situation and challenges for control. Transboundary and Emerging Diseases 69, 645-659.
| Crossref | Google Scholar | PubMed |
MAF, FAO (2014) Lao PDR: Lao census of agriculture 2010/11. Analysis of selected themes. (Ministry of Agriculture and Forestry: Vientiane, Lao PDR; and Food and Agriculture Organization) Available at https://www.fao.org/3/at767e/at767e.pdf [Accessed 27 February 2022]
Maillard J-C, Van KP, Nguyen T, Van TN, Berthouly C, Libeau G, Kwiatek O (2008) Examples of probable host-pathogen co-adaptation/co-evolution in isolated farmed animal populations in the mountainous regions of North Vietnam. Annals of the New York Academy of Sciences 1149, 259-262.
| Crossref | Google Scholar | PubMed |
Millar J, Colvin AF, Olmo L, Phengvilaysouk A, Phengsavanh P, Walkden-Brown SW (2022) Smallholder goat raising in Lao PDR: is there potential to improve management and productivity? The Lao Journal of Agriculture and Forestry 46, 18-34.
| Google Scholar |
Mongkonwattanaporn T, Lertwatcharasarakul P, Intaravichai P, Rukkwamsuk T (2021) Seroprevalence of small ruminant lentivirus infection in goats in Thailand. Polish Journal of Veterinary Sciences 24, 313-317.
| Crossref | Google Scholar | PubMed |
Nampanya S, Khounsy S, Phonvisay A, Young JR, Bush RD, Windsor PA (2015) Financial impact of foot and mouth disease on large ruminant smallholder farmers in the Greater Mekong Subregion. Transboundary and Emerging Diseases 62, 555-564.
| Crossref | Google Scholar | PubMed |
OIE-WAHIS (2023a) Peste des petits ruminants. World Organization for Animal Health-World Animal Health Information System. Available at https://www.woah.org/en/disease/peste-des-petits-ruminants/#ui-id-5 [Accessed 15 September 2023]
OIE-WAHIS (2023b) Disease situation. World Organization for Animal Health-World Animal Health Information System. Available at https://wahis.woah.org/#/dashboards/country-or-disease-dashboard [Accessed 19 September 2023]
OIE-WAHIS (2023c) Laos – Anthrax – immediate notification (Final). World Organization for Animal Health-World Animal Health Information System. Available at https://wahis.woah.org/#/home [Accessed 31 March 2023]
OIE-WAHIS (2023d) PPR reported for the first time in South East Asia. World Organization for Animal Health-World Animal Health Information System. Available at https://rr-asia.woah.org/en/news/ppr-in-thailand-alert-message/ [Accessed 31 March 2023]
Olmo L, Phengvilaysouk A, Colvin AF, Phengsavanh P, Millar J, Walkden-Brown SW (2022a) Improving Lao goat production when resources are limited. In ‘34th Biennial Conference of the Australian Association of Animal Sciences (AAAS)’, Cairns, Queensland, Australia, 5–7 July. (CSIRO Publishing) Available at https://doi.org/10.1071/ANv62n11abs [Verified 30 May 2024]
Olmo L, Walkden-Brown S, Phengsavanh P, Phengvilaysouk A, Van NH, Hoang N, Millar J, Cuc NTK, Werf JvD (2022b) Annual Report: goat production systems and marketing in Lao PDR and Vietnam. ACIAR Project No: LS/2017/034. Australian Centre for International Agricultural Research, Canberra, ACT, Australia.
Osborne ME, Zasloff JJ, Dommen AJ, Silverstein J, Lafont PB (2021) Laos. Online Encyclopedia. Britannica. Available at https://www.britannica.com/place/Laos [Verified 1 February 2022]
Paitoonpong L, Ekgatat M, Nunthapisud P, Tantawichien T, Suankratay C (2006) Brucellosis: the first case of King Chulalongkorn Memorial Hospital and review of the literature. Journal of the Medical Association of Thailand 89, 1313-1317.
| Google Scholar | PubMed |
Peck ME, Chanachai K, Jenpanich C, Amonsin A, Alexander BH, Bender JB (2018) Seroprevalence of brucellosis in goats and sheep in Thailand: Results from the Thai National Brucellosis Surveillance System from 2013 to 2015. Transboundary and Emerging Diseases 65, 799-805.
| Crossref | Google Scholar | PubMed |
Pham TH, Lila MAM, Rahaman NYA, Lai HLT, Nguyen LT, Do KV, Noordin MM (2020) Epidemiology and clinico-pathological characteristics of current goat pox outbreak in North Vietnam. BMC Veterinary Research 16, 128.
| Crossref | Google Scholar | PubMed |
Pham TH, Rahaman NYA, Lila MAM, Lai HLT, Nguyen LT, Van Nguyen G, Ha BX, Nguyen H, Vu HD, Noordin MM (2021) Molecular phylogenetics of a recently isolated goat pox virus from Vietnam. BMC Veterinary Research 17, 115.
| Crossref | Google Scholar | PubMed |
Phengsavanh P, Phengvilaysouk A, Viengvilai P, Gray D, Patrick I, Hergenhan R, Hoang N, Walkden-Brown S (2017) Goat production in Laos and market linkages into Vietnam. In ‘North-West Vietnam Research Symposium’, Daewoo Hotel, Hanoi, 23–24 November. (Australian Centre for International Research) Available at https://vietnam.embassy.gov.au/files/hnoi/North%20West%20Vietnam%20Research%20Symposium_%20ENG.pdf [Verified 7 March 2022]
Phengvichith V, Ledin I (2007) Effect of a diet high in energy and protein on growth, carcase characteristics and parasite resistance in goats. Tropical Animal Health and Production 39, 59-70.
| Crossref | Google Scholar | PubMed |
Phengvichith V, Preston TR (2011) Effect of feeding processed cassava foliage on growth performance and nematode parasite infestation of local goats in Laos. Livestock Research for Rural Development 23, 13.
| Google Scholar |
Phengvilaysouk A, Colvin AF, Olmo L, Phengsavanh P, Millar J, Walkden-Brown S (2022) Smallholder goat herd production characteristics and constraints in Lao PDR. The Lao Journal of Agriculture and Forestry 46, 3-19.
| Google Scholar |
Qingdao (2017) First peste des Petits Ruminants regional roadmap meeting for ASEAN countries, China, Mongolia and Timor Leste. Available at https://rr-asia.woah.org/wp-content/uploads/2020/06/200622_final-draft-communique-ppr-roadmap-meeting-qingdao.pdf [Accessed 17 September 2023]
Rast L, Toribio J-ALML, Dhand NK, Khounsy S, Windsor PA (2014) Why are simple control options for Toxocara vitulorum not being implemented by cattle and buffalo smallholder farmers in South-East Asia? Preventive Veterinary Medicine 113, 211-218.
| Crossref | Google Scholar | PubMed |
Rerkyusuke S, Lerk-u-suke S, Sirimalaisuwan A (2022) Clinical evidence and risk factors for reproductive disorders caused by bacterial infections in meat goats in northeastern Thailand. Veterinary Medicine International 2022, 1877317.
| Crossref | Google Scholar |
Sagarasaeranee O, Kaewkalong S, Sujit K, Chanachai K (2017) Seroprevalence of brucellosis in small ruminants in Thailand, 2013. Outbreak, Surveillance, Investigation & Response (OSIR) Journal 9, 7-10.
| Crossref | Google Scholar |
Sani RA, Binh DV, Ly ND, San S, Phimphachanhvongsod V (2004) Goat production, parasites and testing of control options in Lao, Cambodia and Vietnam. In ‘Worm control for small ruminants in Tropical Asia’. (Eds RA Sani, GD Gray, RL Baker) pp. 211–218. (Australian Centre for International Agricultural Research: Canberra, ACT, Australia)
Sato M, Yoonuan T, Sanguankiat S, Nuamtanong S, Pongvongsa T, Phimmayoi I, Phanhanan V, Boupha B, Moji K, Waikagul J (2011) Short report: human Trichostrongylus colubriformis infection in a rural village in Laos. The American Journal of Tropical Medicine and Hygiene 84, 52-54.
| Crossref | Google Scholar | PubMed |
Sato MO, Sato M, Chaisiri K, Maipanich W, Yoonuan T, Sanguankiat S, Pongvongsa T, Boupha B, Moji K, Waikagul J (2014) Nematode infection among ruminants in monsoon climate (Ban-Lahanam, Lao PDR) and its role as food-borne zoonosis. Brazilian Journal of Veterinary Parasitology 23, 80-84.
| Google Scholar | PubMed |
Siengsanan-Lamont J, Blacksell SD (2021) Surveillance for One Health and high consequence veterinary pathogens (brucellosis, coxiellosis and foot and mouth disease) in Southeast Asia: Lao PDR and Cambodia in focus and the importance of international partnerships. Microbiology Australia 42, 156-160.
| Crossref | Google Scholar |
Siengsanan-Lamont J, Theppangna W, Phommachanh P, Khounsy S, Selleck PW, Matsumoto N, Gleeson LJ, Blacksell SD (2022) Abattoir-based serological surveillance and spatial risk analysis of foot-and-mouth disease, brucellosis, and Q fever in Lao PDR large ruminants. Tropical Medicine and Infectious Disease 7, 78.
| Crossref | Google Scholar | PubMed |
Singanallur NB, Nampanya S, MacPhillamy I, Soukvilay V, Keokhamphet C, Bush RD, Khounsy S, Dhand NK, Windsor P, Vosloo W (2020) Serological evidence of foot-and-mouth disease infection in goats in Lao PDR. Frontiers in Veterinary Science 7, 544.
| Crossref | Google Scholar | PubMed |
Srikawkheaw N, Lawhavinit O-A (2007) Detection of antibodies against melioidosis from animal sera in Thailand by indirect haemagglutination test. Kasetsart Journal – Natural Science 41, 81-85.
| Google Scholar |
Su W, Xing C, Wu Y, Wang Y, Ding H, He H (2015) Complete genome sequence of a novel mutant of Peste des Petits Ruminants virus obtained from China. Genome Announcements 3, e01504–14.
| Google Scholar | PubMed |
Subharat S, Meunsene D, Putthana V, Tiwari H, Firestone SM (2023) Field epidemiology capacity of the national veterinary services of Lao PDR: an online survey. Frontiers in Veterinary Science 10, 1096554.
| Crossref | Google Scholar | PubMed |
Sun M-J, Di D-D, Li Y, Zhang Z-C, Yan H, Tian L-L, Jing Z-G, Li J-P, Jiang H, Fan W-X (2016) Genotyping of Brucella melitensis and Brucella abortus strains currently circulating in Xinjiang, China. Infection, Genetics and Evolution 44, 522-529.
| Crossref | Google Scholar | PubMed |
Techapornroong M, Ekgatat M (2018) Brucellosis, four case series in Prapokklao hospital. The Journal of Prapokklao Hospital Clinical Medical Education Center 35, 214-221.
| Google Scholar |
Tian G-Z, Cui B-Y, Piao D-R, Zhao H-Y, Li L-Y, Liu X, Xiao P, Zhao Z-Z, Xu L-Q, Jiang H, Li Z-J (2017) Multi-locus variable-number tandem repeat analysis of Chinese Brucella strains isolated from 1953 to 2013. Infectious Diseases of Poverty 6, 89.
| Crossref | Google Scholar | PubMed |
Viengvilay P, Phengvilaysouk A, Phengsvanh P, Walkden-Brown S, Gray D (2017) Smallholder goat rearing systems in Lao PDR. The Lao Journal of Agriculture and Forestry 37, 149.
| Google Scholar |
Walkden-Brown S, Colvin A, Millar J, Hoang N, Phengsavanh P, Phengvilaysouk A, Olmo L, Xaikhue T, Van NH, Ba NX, Cuc NTK, Nga BT, Nguyen DV, Le SV, Jayasekara P, Liehr E, Nguyen CV, Werf Jvd, Heras-Saldana Sdl, McNeill D, Kongmanila D, Porsavathdy P (2023) Final report: goat production systems and marketing in Lao PDR and Vietnam. ACIAR Project No: LS-2017-034. Australian Centre for International Agricultural Research, Canberra, ACT, Australia.
Wang Z, Bao J, Wu X, Liu Y, Li L, Liu C, Suo L, Xie Z, Zhao W, Zhang W, Yang N, Li J, Wang S, Wang J (2009) Peste des Petits Ruminants virus in Tibet, China. Emerging Infectious Diseases 15, 299-301.
| Crossref | Google Scholar | PubMed |
Wang D-F, Yang X-Y, Wei Y-R, Li J-J, Bolati H, Meng X-X, Tuerxun G, Nuerdan N, Wu J-Y (2023) Genome characterization of the Caprine arthritis-encephalitis virus in China: a retrospective genomic analysis of the earliest Chinese isolates. Journal of Integrative Agriculture 22, 872-880.
| Crossref | Google Scholar |
Whitley NC, Oh S-H, Lee SJ, Schoenian S, Kaplan RM, Storey B, Terrill TH, Mobini S, Burke JM, Miller JE, Perdue MA (2014) Impact of integrated gastrointestinal nematode management training for US goat and sheep producers. Veterinary Parasitology 200, 271-275.
| Crossref | Google Scholar | PubMed |
WHO (2017) Joint external evaluation of IHR core capacities of the Lao People’s Democratic Republic: Mission report 17-24 February 2017. (World Health Organization) Available at &https://apps.who.int/iris/bitstream/handle/10665/258554/WHO-WHE-CPI-REP-2017.35-eng.pdf?sequence=1&isAllowed=y [Accessed 28 March 2023]
Wilson RT (2007) Status and prospects for livestock production in the Lao People’s Democratic Republic. Tropical Animal Health and Production 39, 443-452.
| Crossref | Google Scholar | PubMed |
Windsor PA, Nampanya S, Tagger A, Keonam K, Gerasimova M, Putthana V, Bush RD, Khounsy S (2017) Is orf infection a risk to expanding goat production in developing countries? A study from Lao PDR. Small Ruminant Research 154, 123-128.
| Crossref | Google Scholar |
Windsor PA, Nampanya S, Putthana V, Keonam K, Johnson K, Bush RD, Khounsy S (2018) The endoparasitism challenge in developing countries as goat raising develops from smallholder to commercial production systems: a study from Laos. Veterinary Parasitology 251, 95-100.
| Crossref | Google Scholar | PubMed |
World Bank (2022) DataBank. Available at https://databank.worldbank.org/ [Verified 21 February 2022]
World Bank (2023) World development indicators. Available at https://databank.worldbank.org/reports.aspx?source=2&country=LAO# [Verified 9 September 2023]
Wu X, Li L, Li J, Liu C, Wang Q, Bao JY, Zou Y, Ren W, Wang H, Zhang Y, Lv Y, Liu F, Wang S, Ma H, Wang Z (2016) Peste des Petits Ruminants viruses re-emerging in China, 2013-2014. Transboundary and Emerging Diseases 63, e441-e446.
| Crossref | Google Scholar | PubMed |
Xaikhue T, Olmo L, Colin AF, Theppangna W, Vannivong T, Phommasone P, Walkden-Brown SW (2023) Evaluation of anthelmintic efficacy in goats in Savannakhet province, Lao PDR. In ‘9th International Conference on Sustainable Animal Agriculture for Developing Countries’, Vientiane, Lao PDR, 21–24 November. (SAADC)
Xayalath S, Mujitaba MA, Ortega ADSV, Rátky J (2021) A review on the trend of livestock breeds in Laos. Acta Agraria Debreceniensis 1, 227-237.
| Crossref | Google Scholar |
Xaydalasouk K, Innoula N, Putthana V, Chanthavongsa K, Snoeck CJ, Hubschen JM, Oudomphone P, Chan B, Muller CP, Black AP, Pommasichan S, Pauly M (2021) High seroprevalence of foot and mouth disease in Laos: call for nationwide vaccination campaigns and disease surveillance. Transboundary and Emerging Diseases 68, 2345-2352.
| Crossref | Google Scholar | PubMed |
Yang H, Gu W, Li Z, Zhang L, Liao D, Song J, Shi B, Hasimu J, Li Z, Yang Z, Zhong Q, Li H (2021) Novel putative bluetongue virus serotype 29 isolated from inapparently infected goat in Xinjiang of China. Transboundary and Emerging Diseases 68, 2543-2555.
| Crossref | Google Scholar | PubMed |
Yu Y, Duan X, Liu Y, Ma J, Song B, Lian Z, Cui Y (2020) Laboratory diagnosis of a NZ7-like Orf virus infection and pathogen genetic characterization, particularly in the VEGF gene. Frontiers in Veterinary Science 7, 538.
| Crossref | Google Scholar | PubMed |
Zhang K, Liu Y, Kong H, Shang Y, Liu X (2014) Comparison and phylogenetic analysis based on the B2L gene of orf virus from goats and sheep in China during 2009-2011. Archives of Virology 159, 1475-1479.
| Crossref | Google Scholar | PubMed |
Zheng X, Xia Q, Xia L, Li W (2019) Review: endemic melioidosis in Southern China: past and present. Tropical Medicine and Infectious Disease 4, 244-258.
| Crossref | Google Scholar | PubMed |