Growth performance and meat quality of finishing pigs fed diets supplemented with antioxidants and organic acids in late summer
Hieu H. Le A B , Robert Hewitt
A B , Robert Hewitt  C , Sally Tritton C , Darryl Nicholas D’Souza C , Majid Shakeri A E , Yasir Iqbal A , Minh Ha A , Robyn D. Warner
C , Sally Tritton C , Darryl Nicholas D’Souza C , Majid Shakeri A E , Yasir Iqbal A , Minh Ha A , Robyn D. Warner  A , Frank R. Dunshea A D and Jeremy J. Cottrell
A , Frank R. Dunshea A D and Jeremy J. Cottrell  A *
A *
A
B
C
D
E Present address:
Abstract
Heat stress compromises growth performance and meat quality and results in economic losses in pork production.
We investigated the effects of supranutritional levels of selenium (Se) and vitamin E (VitE), along with organic acid blends, on the growth performance and meat quality of finishing pigs over a period of weeks during late summer to early autumn in Westbrook, Queensland, Australia.
A total of 264 crossbred pigs (25.8 ± 2.4 kg, mean ± s.d.) at 11 weeks of age were randomly assigned in a 2 × 2 × 2 factorial design with two aging times (2 or 5 days) nested within each pig. The factors included antioxidants (Se/E, with recommended or supranutritional doses of Se and VitE), an organic acids (OA) blend added to drinking water (control vs supplemented), and sex (female vs male).
Between 16 and 18 weeks of age, high Se/E decreased daily feed intake (P = 0.010) but had no effects on average daily gain or feed conversion efficiency (FCE). Male pigs grew faster (P = 0.040) and had a higher FCE than females (P = 0.050). Supplementation with OA increased FCE in males but not females (OA × Sex interaction, P = 0.035). Between Weeks 16 and 20, male pigs grew faster (P < 0.001), tended to eat more (P = 0.057), and had higher FCE (P = 0.002) than females (P < 0.001). There were no main effects of Se/E or OA on meat quality, except protein oxidation was reduced by high Se/E (P = 0.047). Sex impacted only Warner-Bratzler shear force (WBSF), with male pigs having lower WBSF than females (P = 0.053). Meat aging decreased WBSF (P < 0.001), but it increased cooking loss (P = 0.036), myofibrillar fragmentation index (P < 0.001), lipid oxidation (P < 0.001) and colour parameters (P < 0.001 for all).
Supplementation with Se/E for up to 10 weeks and OA for 5 weeks did not influence production parameters or pork quality in late summer, except that high Se/E decreased protein oxidation, and significant heat stress conditions were not experienced as expected.
Supplementation with Se/E and OA may be effective when environmental temperatures are higher.
Keywords: antioxidant, growth performance, heat stress, meat quality, organic acids, pigs, selenium, vitamin E.
Introduction
High environmental temperature is responsible for reducing growth performance, reproduction, meat quality, and productivity, resulting in economic loss in pig production (Montilla et al. 2014; Rauw et al. 2020). Global warming is increasing the frequency and severity of extreme heatwave events, and there is growing concern about the impacts of heat stress (HS) on pork production during summer (Renaudeau and Dourmad 2022). Heat stress is associated with respiratory alkalosis, oxidative stress and digestive dysfunction, and compromised productive parameters (Cui et al. 2016; Liu et al. 2016, 2022). Antioxidant enzymes and vitamins remove excess reactive oxygen species, and these pathways can be augmented by supplementation of selenium and vitamin E, respectively. Selenium (Se) is an integral element of glutathione peroxidase (GPx), an antioxidant enzyme that neutralises lipid peroxidation and protects the cell membrane damage (Hoekstra 1975). Previous studies reported that feeding a diet supplemented with Se improved antioxidant capacity in muscle tissue and positively affected the meat quality of finishing pigs (Chen et al. 2019; Bobček et al. 2004). Similarly, as a chain-breaking antioxidant, vitamin E (VitE) supplementation has been shown to reduce the occurrence of PSE meat (pale soft and exudative) and improve the oxidative stability of pork (Cheah et al. 1995; Hasty et al. 2002). The PSE pork is characterised by meat that has a pale colour and soft texture and is exudative, causing increased drip loss and cooking loss, reduced colour, and negatively affecting consumer acceptability (Lee and Choi 1999; Adzitey and Nurul 2011). Organic acids, alternative dietary supplements, are also widely used in pig production to enhance health, growth performance and meat quality (Chen et al. 2017; Morel et al. 2019). As an acidifier, organic acid could prevent respiratory alkalosis, which can happen in hot summer conditions (Nørgaard et al. 2010). Further, 2-hydroxy-4-(methylthio)butanoic acid (HMTBa), a unique source of methionine, has demonstrated the potential to alleviate stress in pigs by improving antioxidant and detoxification ability (Martín-Venegas et al. 2006; Wang et al. 2019). Dietary supplementation with HMTBa improves nutrient digestibility and intestinal microbial populations and protects against increased intestinal damage (Li et al. 2008; Martín-Venegas et al. 2013). In addition, feeding a diet with HMTBa above the recommendations over 14 days before slaughter improved pork quality by increasing glutathione content and decreasing thiobarbituric acid reactive substances (TBARS) levels in muscle (Lebret et al. 2018). The intrinsic differences between male and female pigs can independently influence (Xia et al. 2023) or interact with nutrition and the environment on productive traits, carcass characteristics and meat quality (Sundrum et al. 2011; Martins et al. 2023). However, immunocastration of male pigs is considered an alternative procedure performed during the finishing period to control boar taint in pork and improve the acceptability of customers (Marjeta et al. 2017; Werner et al. 2021). Ageing has improved pork quality by enhancing tenderness, flavour, juiciness and overall liking (Channon et al. 2004) but could also increase meat oxidation (Rant et al. 2019; Ribeiro et al. 2021). In Australia, pork raw materials are typically hung in a chilled room at an abattoir for 2 days before being delivered to supermarkets or butchers where pork can be available for sale, but its shelf life is no more than 5 days.
Therefore, the hypotheses being tested in this experiment were that antioxidants (Se and VitE) and blended organic mixture supplementation over late summer to early autumn, when the critical temperature for pigs is likely to be exceeded, would increase growth performance and improve the meat quality of growing-finishing pigs.
Materials and methods
The conduct of the project followed the Animal Care and Protection Act 2001, the Australian Code for the Care and Use of Animals for Scientific Purposes, 8th Edition 2013 (the Code) and all other relevant Commonwealth and State legislation and was approved by the Animal Ethics Committee of CHM Alliance Pty Ltd., Qld, Australia (Protocol no. CHM PP 116/18).
Environmental weather data
The experiment was conducted in a research facility located in Westbrook, Queensland, Australia. Daily weather observations during the experimental period were collected at Toowoomba Airport weather station (site number: 041529), located about 10 km from the experimental site. The meteorological data collected from the Australian Bureau of Meteorology included daily maximum temperature, temperature, and relative humidity at 9 am and 3 pm. The temperature–humidity index (THI) was calculated based on the equation described by Vashi et al. (2018) as follows:
where T is the environmental temperature (°C), and RH is the relative humidity (%).
Based on THI, the levels of heat stress were classified as follows: normal (THI < 74), mild heat stress (74 ≤ THI < 78), moderate or dangerous (78 ≤ THI < 82) and severe (THI > 82) (Mellado et al. 2018).
Growth performance trial
The experiment was conducted in a commercial research facility from February to May 2019 and consisted of three replicates with 24 pens (n = 11/pen) and 6 pens/treatment group. A total of 264 crossbred pigs at 11 weeks of age were randomly assigned in a 2 × 2 × 2 factorial design with the factors being antioxidants (Control vs supranutritionals levels of Se and VitE), organic acids (control vs supplemented) and sex (female vs male). In this study, pigs were fed a single diet through the entire experimental period (19.4% crude protein and 14 MJ digestible energy/kg, Table 1) containing the normal inclusion of Se and VitE for this farm (−Se/E, 0.1 g/tonne Se plus 40 g/tonne VitE) or high supranutritional levels of Se and VitE (+Se/E, 0.5 g/tonne Se plus 100 g/tonne VitE). In the last 5 weeks (from Week 16 to 20), pigs were fed the same diets as the previous stage, but half of the pigs from each diet were supplemented with organic acids (Activate®, Novus International, Inc. Mascot, NSW) in drinking water at 1 L/1000 L (+OA), whereas the other half were not supplemented (−OA). The bodyweight was recorded individually at the beginning of Week 11 and at the end of Weeks 15, 18 and 20. The average daily gain (ADG) was calculated from each pig, and daily feed intake (ADFI) and feed conversion efficiency (FCE) were calculated from each pen during experimental periods. The FCE was expressed as kg of ADG/kg of ADFI. As was normal practice on this farm, the male pigs were immunocastrated by vaccination with Improvac (Zoetis Australia Pty Ltd, Rhodes, NSW, Australia) at 13 and 17 weeks of age.
| Items | Amount | |
|---|---|---|
| Ingredient | % | |
| Wheat | 39.2 | |
| Sorghum | 25.0 | |
| Canola meal 37% | 11.8 | |
| Millrun 16% | 5.0 | |
| Soybean meal 46% | 7.75 | |
| Blood meal 90% | 1.0 | |
| Meat meal 51% | 5.0 | |
| Canola oil | 2.0 | |
| Molasses | 2.0 | |
| Limestone | 0.15 | |
| Salt | 0.25 | |
| Choline chloride 60% | 0.01 | |
| Lysine-HCl | 0.40 | |
| DL-Methionine | 0.045 | |
| L-Threonine | 0.03 | |
| L-Tryptophan | 0.005 | |
| Rovabio excel 10% | 0.05 | |
| Hi-Phos | 0.0075 | |
| Deodorase Farm pack | 0.1 | |
| Premix A | 0.2 | |
| Calculated values | ||
| Dry matter (%) | 90.0 | |
| Digestible energy (MJ/kg) | 14.0 | |
| Crude protein (%) | 19.4 | |
| Crude fibre (%) | 3.7 | |
| SID lysine/digestible energy (%/MJ) | 0.07 | |
SID lysine, standardised ileal digestible lysine.
Meat quality measurement
At the end of the experiment, 72 pigs (three pigs/pen of six pens/treatment) were randomly selected for meat quality measurement upon reaching market weight. These pigs were slaughtered at an abattoir, and longissimus dorsi muscles were collected and transported to the laboratory for meat quality analysis the following day (approximately 24 h postslaughter). Upon arrival at the laboratory, the meat samples were divided equally into two parts, and then randomly assigned to an aging period of either 2 or 5 days postslaughter at 4°C. All samples were frozen after aging at −20°C for further analysis.
The ultimate pH of the pork samples was measured after the pigs were slaughtered for about 24 h. The pH of the meat sample was measured using a spear-head electric pH probe (IJ44C, Ionode Pty. Ltd., Tennyson Queensland, Australia) attached to a waterproof pH-mV-temperature meter (WP80, TPS Pty. Ltd., Brisbane, Queensland, Australia) by inserting the pH probe approximately 2 cm inside meat for 30 s. The pH meter was calibrated using pH 4 and pH 7 buffer solutions (Hanna Instruments, Keysborough, Victoria, Australia) before the measurement. The pH value of each sample was the average result of five measurements (four around and one in the middle).
The surface colour of meat was measured on Day 2 and Day 5 postslaughter using a Hunterlab Miniscan EZ (Hunter Assoc. Labs Inc., VA, USA). The machine was calibrated against black and white tie references before measurement. Samples were allowed to bloom at 4°C for 20 min before measurement. Triplicates of surface colour were measured in the CIE system, and the value of L* (lightness), a* (redness) and b* (yellowness) was obtained from the average values of three readings.
The water-holding capacity (WHC) of meat samples was determined as expressed juice using the centrifugation method described by Laakkonen et al. (1970) and modified by Choi et al. (2014). Longissimus muscle samples (0.5 ± 0.05 g) were placed in a centrifugation tube (Centrifugal Filter Units, Merck Millipore, Tullagreen, Carrigtwohill, Co. Cork, Ireland) with filter units and then heated for 20 min at 80°C using a digital block heater (DBH40D, Ratek Instruments, Boronia, Victoria, Australia). After being cooled for 10 min at room temperature, samples were centrifuged at 2000g for 10 min at 4°C. The expressed juice was calculated as the difference of sample weight before and after heating and expressed as a percent of the original sample weight. A higher value of released juice will indicate a lower WHC.
The drip loss was assessed according to the EZ-DripLoss procedure described by Christensen (2003). Briefly, the longissimus dorsi muscles were cut into slices of 2.5 cm thickness. Three cylindrical muscle cores were taken in each slice by a fixed blade knife (25-mm diameter). Each core sample was placed into two special EZ-DripLoss containers with a funnel shape (Christensen Aps Industrivaengetand, Hilleroed, Denmark). All empty containers and samples were weighed using a precision laboratory scale (Model 1419 MP8-I; Sartorius, Gottingen, Germany). After storing at 4°C for 48 h, the container with meat and juice was weighed, then the meat was taken out to weigh the container with juice.
Cooking loss of pork samples was conducted as per the method of Channon et al. (2014) with some modifications. Pork samples were prepared in 3-cm thick slices with an approximate weight of 100 ± 5 g and placed into individual plastic bags. Then, the bags were suspended in a metal rack and cooked in a water bath (F38-ME, Julabo, Seelbach, Germany) preheated to 70°C. Samples were cooked to an internal temperature of 70°C (approximately 35 min). After cooking, samples were cooled in ice water for 30 min to prevent further cooking. Samples were gently dried with paper towels to remove the remaining moisture on the meat surface before they were reweighed to calculate cooking loss, presented as percentage weight loss resulting from cooking.
The Warner–Bratzler shear force (WBSF) was measured according to the method of Fang et al. (2018). After cooking loss measurement, the pork samples were wrapped in a plastic bag and chilled at 4°C overnight prior to texture analysis. Six rectangular strips of 1 cm2 × 4 cm were obtained from each sample by cutting parallel to the direction of muscle fibres. Warner–Bratzler Shear Force was measured using a shear blade (V-shaped) adapted to a texture analyser (Lloyd Instruments Ltd., Largo, FL, USA) with a 500N load cell, and the shearing speed was set at 300 mm/min. The peak of the shear force was recorded, and the mean WBSF (N) value for each sample was calculated from six strips.
Myofibrillar fragmentation index (MFI) was measured according to the method of Culler et al. (1978), as modified by Ha et al. (2019) with some modifications. Briefly, frozen meat samples (4 g) were homogenised in 7 mL of cold extraction buffer (50 mM Tris-HCl, 10 mM EDTA, pH 8.3) at 13,000 rpm for 10 s using a homogeniser (IKA Ultra Turrax ® T 25 digital, Staufen, Germany). Homogenates were centrifuged at 1500g for 10 min at 2°C, and the supernatants were discarded. The sample was resuspended in 25 mL of the extraction buffer, and this process was repeated twice. After the third wash, the pellet was resuspended in 5 mL of extraction buffer and vortexed to mix well. The suspension was filtered through a tea strainer to remove fat and connective tissue, and then another 5 mL of extraction buffer was added to wash the strainer. Protein concentration was determined using a Biuret assay with bovine serum albumin as standard. The myofibrillar protein suspension was diluted to a final protein concentration of 0.5 mg/mL with the extraction buffer, and 1 mL of the sample was transferred to a cuvette and then mixed well before reading the absorbance at 540 nm (A540) with a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) warmed for 20 min before assaying. The myofibrillar fragmentation index was calculated as A540 × 200.
Thiobarbituric acid reactive substances (TBARS) assay was used to quantify lipid oxidation for the 2 and 5-day aged samples following methods described by Ha et al. (2019) with minor modification. Briefly, 4 g of pork samples were homogenised in 7.5 mL of 10% (w/v) trichloroacetic acid (TCA) solution containing 0.1% ethylenediaminetetraacetic acid (EDTA) and 0.1% propyl gallate (PG) using a homogeniser (IKA Ultra Turrax ® T 25 digital, Staufen, Germany) at 19,000 rpm for 60 s. The homogenate was centrifuged at 2000g for 8 min at 4°C in a Rotina 380R Centrifuge (Hettich Instruments, Beverly, Massachusetts, United States). Afterwards, the supernatant was filtered through Grade 1 Whatman filter paper (Cat no. WHA1001-110, Whatman International Ltd, Maidstone, United Kingdom). An equal amount of filtrate (1 mL) and 0.02 M thiobarbituric acid solution (1 mL) were mixed in a screw-cap tube and incubated in the water bath at 95°C for 60 min before cooling in ice. The absorbance at 532 nm was measured with a spectrophotometer (Multiskan Spectrum, Thermofisher) and subtracted by the absorbance at 600 nm to correct nonspecific turbidity.
The standards were prepared from a 2 mM 1,1,3,3-tetraethoxypropane solution (TEP) with serial dilution using Milli-Q water. The final concentration of TEP ranges from 2 μM to 10 μM. Then 1 mL of standard solutions was mixed with 1 mL of 0.02 M TBA solution, the same as for the pork filtrate mentioned above. Results were expressed as mg malondialdehyde (MDA)/kg of meat using a calibration curve of TEP.
Protein carbonyl content was measured according to a method developed by Fagan et al. (1999) and with some modifications by Shakeri et al. (2020). Briefly, pork longissimus muscle (1 ± 0.01 g) was cut into small pieces and then homogenised in 5 mL of pyrophosphate buffer (2 mM sodium pyrophosphate, 10 mM tris maleate, 100 mM potassium chloride and 2 mM ethylene glycol tetraacetic acid, pH = 7.4) using an IKA homogeniser (T25 digital Ultra-Turrax®, Selangor, Malaysia) at 17,000 rpm for 40 s. The sample was then split into two equal aliquots of 1 mL. The aliquots were washed by adding 9 mL of HCl : acetone (3 : 100) (v/v) and centrifuged at 5000g for 5 min at 4°C. Then, samples were rewashed with 1 mL of HCl : acetone (3 : 100) (v/v) as mentioned above. The pellets were washed twice by adding 1 mL 10% (w/v) TCA, vortexed and centrifuged 5000g at 4°C for 5 min. One identical pellet was mixed with 0.5 mL of 10 mM 2,4-dinitrophenylhydrazine (DNPH) dissolved in 2 M HCl to determine carbonyl. Another pellet was mixed with 0.5 mL of 2 M HCl for protein measurement. Both samples were placed in the dark for 30 min with 10 s vortexes done every 10 min. Then, 0.5 mL of 20% TCA was added to samples, vortexed and left on ice for 10 min, then centrifuged at 5000g for 5 min at 4°C, and the supernatant was decanted. Samples were washed once more with 1 mL of 20% TCA before being washed three times with 5 mL of ethanol : ethyl acetate (1 : 1) (v/v). All tubes were centrifuged at 5000g for 5 min at 4°C to remove liquid in each wash. After that, tubes received 1 mL of 6 M guanidine hydrochloride solution (dissolved in 20 mM potassium dihydrogen phosphate, pH 2.3) and were shaken overnight at 4°C. The absorbance of supernatant from the tube containing DNPH was read at 370 nm to calculate carbonyl concentration. In comparison, that of the other tube was read at 280 nm to determine protein concentration. The protein content was calculated and expressed as nmol carbonyl/mg protein.
Statistical analyses
Since the hypothesis was that antioxidants would increase growth performance during the initial phase of the study, these data were analysed by t-test using Genstat Ver. 22. Data for growth performance for the main study was analysed by ANOVA with a fixed effect of antioxidant (Se/E), organic acids (OA) and sex, and replicate was included as a random effect. For meat quality analysis, the refrigerated storage time (T, days after postmortem) was an additional fixed effect in the model using Genstat 22nd version (VSN International, Hemel Hempstead, UK). It is important to note that the aging time for quality measurements was nested within each animal as a repeated measure. In the text, data were presented as the adjusted means of the main effects of antioxidants (−Se/E vs +Se/E), organic acids (−OA vs +OA), sex (female vs male) and meat aging (2 days vs 5 days). Data were expressed as adjusted means and standard error of the difference (sed) for the full interactions (Se/E × OA × Sex for production data and Se/E × OA × sex × T for meat quality). Fisher’s least significant difference test was applied to compare multiple means that were statistically significant. Differences were considered significant if the P-value ≤0.05, and a trend was considered if the P-value ≤0.10.
Results
Environmental conditions
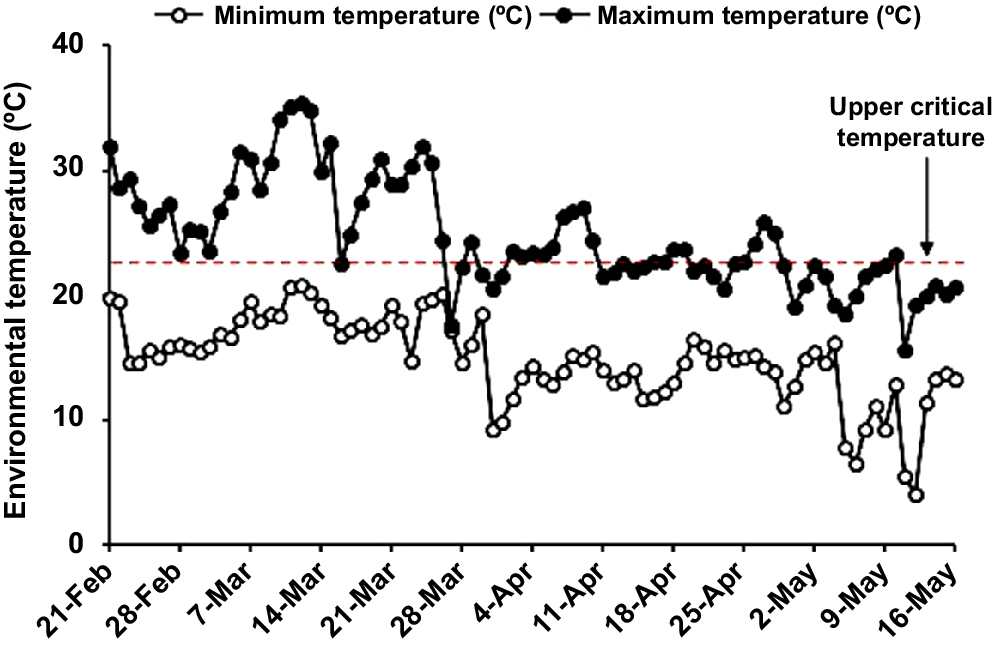
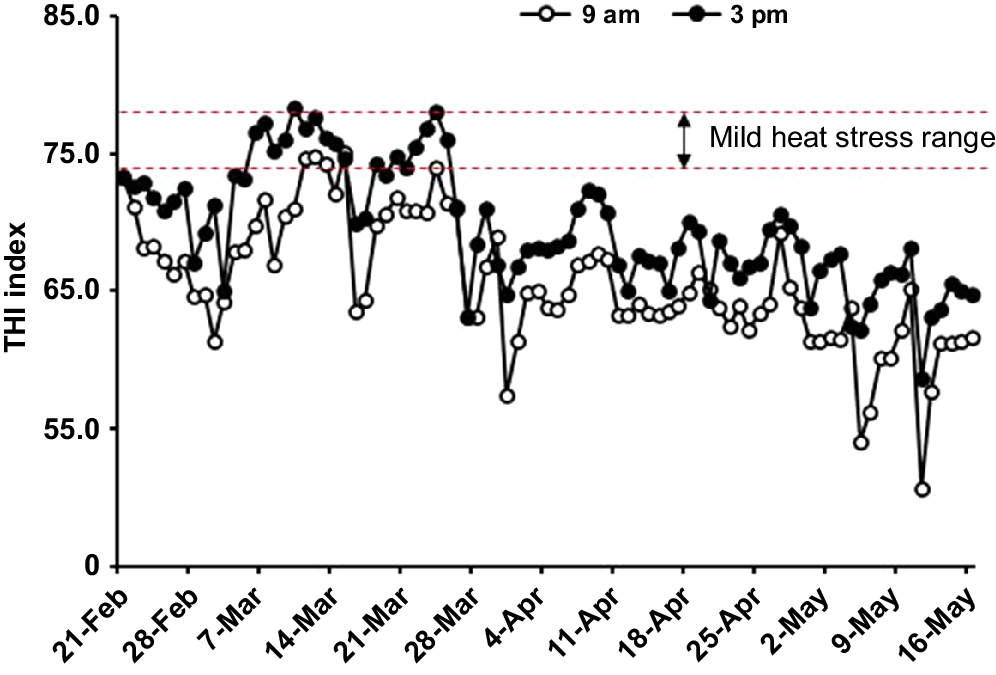
The daily temperatures and THI over the experimental period were expressed as the maximum and minimum values at 09:00 and 15:00 hours in Figs 1 and 2. According to Fig. 1, half time of the experiment had a maximum temperature higher than 23°C, known as the upper limit of the thermal neutral zone for growing-finishing pigs (Liu et al. 2022), and most of these days were distributed in late February and March. However, when it comes to the THI value, it illustrated that most of the experimental time, pigs were under suitable conditions because the THI values were below the heat stress threshold (THI < 74) (Fig. 2).
Daily maximum and minimum temperature during the experiment. The data were observed from Toowoomba airport weather station, Queensland, Australia (27.54°S, 151.91°E, site number: 041529), located approximately 9.8 km away from the experimental site. Replicate 1 lasted from 21 February to 2 May, Replicate 2 from 28 February to 9 May, and Replicate 3 from 7 March to 16 May.
The temperature–humidity index during the experiment. The data were observed from Toowoomba airport weather station, Queensland, Australia (27.54°S, 151.91°E, site number: 041529), located approximately 9.8 km away from the experimental site. Replicate 1 lasted from 21 February to 2 May, Replicate 2 from 28 February to 9 May, and Replicate 3 from 7 March to 16 May.
Growth performance
During the initial grower phase of the study before the introduction of the OA (Weeks 11–15), supplementation with Se/E tended to increase ADG (0.886 vs 0.923 kg/day, P = 0.063) but did not affect ADFI (P = 0.17) or FCE (P = 0.15) (data not shown). The effects of sex, Se/E and OA supplementation on the growth performance of finishing pigs during the finishing phase are presented in Table 2.
| Parameters | Sex, S | −Se/E | +Se/E | sed | P-value A | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −OA | +OA | −OA | +OA | S | Se/E | OA | S.OA | ||||
| Number of pigs B | 66 | 63 | 64 | 66 | |||||||
| Week 15–18 | 66 | 63 | 64 | 66 | |||||||
| ADG (kg/day) | F | 1.16 | 1.10 | 1.16 | 1.10 | 0.078 | 0.040 | 0.71 | 0.32 | 0.024 | |
| IM | 1.18 | 1.30 | 1.13 | 1.29 | |||||||
| ADFI (kg/day) | F | 2.62 | 2.56 | 2.56 | 2.46 | 0.094 | 0.52 | 0.010 | 0.32 | 0.51 | |
| IM | 2.72 | 2.64 | 2.46 | 2.52 | |||||||
| FCE | F | 0.442 | 0.433 | 0.456 | 0.447 | 0.028 | 0.050 | 0.17 | 0.11 | 0.035 | |
| IM | 0.433 | 0.493 | 0.464 | 0.513 | |||||||
| Week 18–20 | |||||||||||
| ADG (kg/day) | F | 1.04 | 1.02 | 0.88 | 1.08 | 0.123 | 0.042 | 0.30 | 0.45 | 0.46 | |
| IM | 1.19 | 1.17 | 1.09 | 1.10 | |||||||
| ADFI (kg/day) | F | 2.70 | 2.55 | 2.75 | 2.77 | 0.268 | 0.14 | 0.44 | 0.89 | 0.52 | |
| IM | 2.84 | 2.89 | 2.85 | 3.02 | |||||||
| FCE | F | 0.381 | 0.398 | 0.324 | 0.389 | 0.057 | 0.35 | 0.17 | 0.39 | 0.61 | |
| IM | 0.420 | 0.428 | 0.372 | 0.381 | |||||||
| Week 15–20 | |||||||||||
| ADG (kg/day) | F | 1.09 | 1.08 | 1.04 | 1.09 | 0.042 | 0.001 | 0.18 | 0.75 | 0.81 | |
| IM | 1.22 | 1.23 | 1.19 | 1.18 | |||||||
| ADFI (kg/day) | F | 2.60 | 2.58 | 2.57 | 2.63 | 0.118 | 0.057 | 0.45 | 0.96 | 0.77 | |
| IM | 2.78 | 2.75 | 2.67 | 2.67 | |||||||
| FCE | F | 0.418 | 0.412 | 0.409 | 0.405 | 0.017 | 0.002 | 0.63 | 1.00 | 0.58 | |
| IM | 0.444 | 0.453 | 0.446 | 0.448 | |||||||
| Final weight (kg) | F | 96.4 | 92.7 | 94.6 | 96.9 | 2.23 | 0.001 | 0.80 | 0.98 | 0.47 | |
| IM | 100 | 101 | 100 | 101 | |||||||
ADG, average daily gain; ADFI, average daily feed intake; FCE, feed conversion efficiency; F, female; IM, immunocastrated male; sed, standard error of the difference for the interaction between S, Se/E and OA.
Between 16 and 18 weeks of age, male pigs grew faster than female pigs (1.13 vs 1.22 kg/day, P = 0.040) (Table 2). Although there were no main effects of dietary supplements on ADG, there was a significant interaction (P = 0.024) such that OA supplementation increased ADG in males (1.16 vs 1.29 kg/day) but not in females (1.16 vs 1.0 kg/day). There was no effect of sex or OA on ADFI, whereas dietary Se/E supplementation decreased ADFI (2.64 vs 2.50 kg/day, P = 0.010) between 16 and 18 weeks. Between 16 and 18 weeks of age, male pigs had a higher FCE than female pigs (0.444 vs 0.476, P = 0.050) (Table 2). Although there were no main effects of dietary supplements on FCE, there was a significant interaction (P = 0.035) such that OA supplementation increased FCE in males (0.448 vs 0.503 kg/day) but not in females (0.449 vs 0.440). Between Weeks 18 and 20, the only significant effect was that male pigs grew more quickly than females (1.00 vs 1.14 kg/day, P = 0.042). Over the entire finisher phase between 16 and 20 weeks, male pigs grew faster (1.08 vs 1.20 kg/day, P = 0.001), tended to eat more (2.60 vs 2.72 kg/day, P = 0.057) had higher FCE (0.411 vs 0.447, P = 0.002) and were heavier at slaughter (95.2 vs 100.4 kg, P < 0.001) than females. There were no main or interactive effects of dietary supplementation over the full finishing period.
Meat quality
There were no effects of sex, Se/E or OA on pork loin pH, water holding capacity or drip loss (P > 0.05 for all, Table 3). WBSF was lower in males than in females (25.9 vs 28.1 N, P = 0.053), but there was no significant effect of Se/E (P = 0.13) or OA (P = 0.17). WBSF declined between Day 2 and Day 5 of aging (31.3 vs 22.7 N, P < 0.001). The cooking loss increased between Day 2 and Day 5 of aging (23.0 vs 23.9%, P = 0.036) but was not affected by sex (P = 0.53), Se/E (P = 0.82) or OA (P = 0.63) (Table 3).
| Parameters | Sex, S | −Se/E | +Se/E | sed | P-value A | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −OA | +OA | −OA | +OA | S | Se/E | OA | Day | ||||
| pH B | F | 5.66 | 5.65 | 5.64 | 5.65 | 0.029 | 0.50 | 0.65 | 0.72 | – | |
| IM | 5.68 | 5.64 | 5.65 | 5.66 | |||||||
| Expressed juice (%) C | F | 64.8 | 64.1 | 63.7 | 65.7 | 1.74 | 0.38 | 0.62 | 0.26 | – | |
| IM | 62.8 | 64.3 | 63.5 | 64.7 | |||||||
| Drip loss (%) C | F | 4.53 | 3.64 | 3.34 | 4.03 | 0.869 | 0.22 | 0.64 | 0.71 | – | |
| IM | 4.29 | 4.58 | 4.14 | 4.64 | |||||||
| WBSF (N) D | |||||||||||
| Day 2 | F | 1.429 (26.9) | 1.461 (28.9) | 1.493 (31.1) | 1.503 (31.8) | 0.052 | 0.053 | 0.13 | 0.17 | 0.001 | |
| IM | 1.494 (31.2) | 1.546 (35.2) | 1.509 (32.3) | 1.522 (33.3) | |||||||
| Day 5 | F | 1.361 (23.0) | 1.288 (19.4) | 1.296 (19.8) | 1.418 (26.2) | ||||||
| IM | 1.346 (22.2) | 1.36 (22.9) | 1.366 (23.2) | 1.393 (24.7) | |||||||
| Cooking loss (%) | |||||||||||
| Day 2 | F | 23.0 | 23.6 | 23.2 | 23.3 | 0.90 | 0.53 | 0.82 | 0.63 | 0.036 | |
| IM | 22.7 | 22.8 | 22.4 | 23.2 | |||||||
| Day 5 | F | 23.9 | 22.4 | 23.8 | 23.4 | ||||||
| IM | 24.1 | 26.1 | 23.8 | 24.0 | |||||||
Expressed juice was calculated as the difference of sample weight before and after heating and expressed as a percent of the original sample weight. A higher value of released juice will indicate a lower WHC.
WBSF, Warner–Bratzler Shear Force; F, female; IM, immunocastrated male; sed, standard error of the difference for the interaction between S, Se/E, OA and Day.
Protein oxidation, as measured by carbonyl units, was lower in pork from pigs that received the +Se/E diet during the finishing stage (2.60 vs 2.28 nmol/mg protein, P = 0.047), but there was no effect of sex, OA or day postslaughter (Table 4). There was no effect of sex (P = 0.22), AOX (P = 0.76) or OA (P = 0.51) on the myofibrillar fragmentation index. However, MFI increased between Day 2 and Day 5 postslaughter (45.2 vs 70.0, P < 0.001) (Table 4). There was no effect of sex, Se/E or OA on TBARS. However, TBARS increased between Day 2 and Day 5 postslaughter (0.099 vs 0.140 mg MDA/kg, P < 0.001) (Table 4). There were no effects of sex, Se/E or OA on any measures of colour (Table 4). However, L* (52.8 vs 54.7, P < 0.001), a* (7.34 vs 8.26, P < 0.001) and b* (14.7 vs 16.2, P < 0.001) all increased between 2 and 5 days of aging (Table 4).
| Parameters | Sex, S | −Se/E | +Se/E | sed | P-value A | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −OA | +OA | −OA | +OA | S | Se/E | OA | Day | ||||
| Carbonyl (nmol/mg protein) | |||||||||||
| Day 2 | F | 2.98 | 2.78 | 2.23 | 2.04 | 0.507 | 0.46 | 0.047 | 0.34 | 0.34 | |
| IM | 2.50 | 3.17 | 2.56 | 2.22 | |||||||
| Day 5 | F | 2.62 | 2.12 | 2.05 | 2.23 | ||||||
| IM | 2.45 | 2.14 | 2.86 | 2.10 | |||||||
| MFI | |||||||||||
| Day 2 | F | 40.1 | 49.9 | 47.6 | 52.1 | 11.63 | 0.22 | 0.76 | 0.51 | 0.001 | |
| IM | 49.1 | 41.5 | 40.9 | 40.2 | |||||||
| Day 5 | F | 57.2 | 80.8 | 81.4 | 71.8 | ||||||
| IM | 71.4 | 64.0 | 62.5 | 71.2 | |||||||
| TBARS (mg MDA/kg) | |||||||||||
| Day 2 | F | 0.135 | 0.115 | 0.110 | 0.082 | 0.0232 | 0.36 | 0.35 | 0.68 | 0.001 | |
| IM | 0.083 | 0.087 | 0.091 | 0.085 | |||||||
| Day 5 | F | 0.112 | 0.143 | 0.162 | 0.121 | ||||||
| IM | 0.150 | 0.161 | 0.127 | 0.143 | |||||||
| L* | |||||||||||
| Day 2 | F | 53.5 | 51.3 | 52.3 | 53.3 | 1.63 | 0.79 | 0.81 | 0.47 | 0.001 | |
| IM | 52.4 | 53.6 | 53.1 | 52.6 | |||||||
| Day 5 | F | 56.2 | 54.8 | 55.9 | 53.1 | ||||||
| IM | 53.5 | 54.8 | 54.4 | 54.6 | |||||||
| a* | |||||||||||
| Day 2 | F | 7.04 | 7.40 | 7.42 | 7.59 | 0.678 | 0.24 | 0.95 | 0.88 | 0.001 | |
| IM | 7.98 | 7.30 | 7.02 | 7.00 | |||||||
| Day 5 | F | 7.47 | 8.29 | 8.15 | 7.95 | ||||||
| IM | 8.55 | 8.50 | 8.91 | 8.27 | |||||||
| b* | |||||||||||
| Day 2 | F | 14.7 | 14.7 | 14.6 | 15.0 | 0.532 | 0.57 | 0.71 | 0.31 | 0.001 | |
| IM | 14.7 | 14.8 | 14.6 | 14.3 | |||||||
| Day 5 | F | 16.4 | 16.6 | 16.0 | 16.0 | ||||||
| IM | 15.6 | 16.3 | 16.1 | 16.6 | |||||||
MFI, myofibrillar fragmentation index; TBARS, thiobarbituric acid reactive substances; MDA, malondialdehyde; F, female; IM, immunocastrated male; sed, standard error of the difference for the interaction between S, Se/E, OA and Day.
Discussion
This experiment aimed to investigate whether supplementation with Se/E and OA can ameliorate symptoms associated with heat stress in finisher pigs during the late summer. The major findings of this study were that supplementation, either Se/E and OA or their combination above the amounts normally fed, had only subtle effects on feed intake, growth rate and feed efficiency of finishing pigs in the late summer. Furthermore, dietary supplements did not markedly improve objective meat quality and oxidative meat stability, although aging decreased shear force and increased cooking loss, L*, a*, b* values and lipid oxidation. This study could provide evidence that the recommended requirements for Se/E and OA are sufficient to avoid oxidative stress in pigs during cool weather, such as late summer. Therefore, these supranutritional supplements do not need to be added to grower-finisher pigs’ diets during periods where the THI is below the mild heat stress threshold. Nevertheless, there were some subtle positive effects of Se/E and OA supplementation that are worth exploring.
This experiment was originally planned to be conducted during summer conditions. However, for logistical reasons, the study was conducted later in the summer than anticipated. In the initial grower phase of the study, which was conducted earlier in the summer when the THI was close to the upper critical value, Se/E supplementation tended to increase ADG by approximately 4% with no change in ADFI or FCE. However, supplementation with Se/E and OA did not affect the growth performance of pigs in the finisher period. The lack of observed effects of dietary supplements on productive traits during the finisher phase may be attributed to the environmental conditions, as indicated by THI values, suggesting that pigs were mostly under normal conditions throughout the experimental duration. Interestingly, there was a positive effect of OA on ADG and FCE in the male pigs over the first 3 weeks of the finisher period. A previous study has demonstrated that male pigs can exhibit reduced performance in the transition period after dietary or housing changes (Dunshea 2001), so this finding suggests that OA supplementation may have assisted the male pigs with dealing with these stressors after this dietary change at 15 weeks of age. Additionally, it was also during this period when the immunocastration vaccine was administered to the male pigs, which can cause reduced ADFI and performance in the immediate period after the second vaccination (Dunshea et al. 2011). Biologically, male pigs are more vulnerable to stressors and diseases than females. For example, a greater mortality in barrows compared with gilts was observed on a commercial farm that had experienced porcine circovirus-associated disease in its history (Nevrkla et al. 2017). The improved growth rate and feed efficiency in male pigs fed OA suggest that the OA may have a more significant impact on the gut health of males by acidifying intestinal digesta (Tugnoli et al. 2020), reducing levels of pathogens such as pathogenic bacteria (De Busser et al. 2011; Roldan-Henao et al. 2023) and improving nutrient digestibility (Franco et al. 2005) compared with females. Environmental stressors, such as high summer temperatures, compromise pigs’ productive traits due to physiological responses (Oliveira et al. 2018; Poullet et al. 2022). Reduced feed intake is a common response to reduce heat production, and it is a major factor contributing to the poor growth rate of grower-finisher pigs in the hot season. It was estimated that the ADFI decreased by about 55 g for each degree above the upper limit of the thermoneutral zone (Le Bellego et al. 2002), and the reduction was greater at 100 g/degree when pigs were housed at 32.2°C (White et al. 2008). Dietary supplementation with AOX, such as Se and VitE could potentially improve growth performance by reducing the concentration of reactive oxygen species (ROS) and maintaining a balance of redox status (Gao et al. 2010; Liu et al. 2021; Sharaf et al. 2021). Similarly, organic acids, such as HMTBa, can be metabolised to become a source of methionine for animal requirements. In pigs, a diet supplemented with HMTBa improved growth performance, reduced pathogenic bacteria and increased the beneficial bacterial population in the gastrointestinal tract (Li et al. 2008; Wang et al. 2019; Qin et al. 2022). Although feeding Se and VitE has been shown to improve antioxidant capacity and reduce oxidative stress in heat-stressed pigs (Liu et al. 2016), the environmental conditions of this study might not have triggered oxidative stress; thus, dietary supplements might not exhibit their biological activities and affected production traits.
It has been reported that meat quality might be influenced by environmental factors, such as seasonal temperature. Under hot conditions, pigs might suffer the risk of oxidative stress in skeletal muscle (Montilla et al. 2014), which can impair meat quality (Pardo et al. 2021; Ponnampalam et al. 2022). For example, rearing pigs in summer conditions or high ambient temperature increases the occurrence of PSE meat (Gregory 2010; Čobanović et al. 2020), which is partly attributed to oxidative stress (Bernabucci et al. 2002; Ganesan et al. 2018). Reactive oxygen species can be scavenged by endogenous antioxidant enzymes or nutritional vitamins (Ponnampalam et al. 2022). By serving as a cofactor of glutathione peroxidase (an endogenous antioxidant enzyme), supplementation with Se reduced drip loss of broiler carcass (Downs et al. 2000) and improved the loin eye area in finishing pigs (Wolter et al. 1999). Similarly, feeding VitE (500 mg/kg) has been reported to reduce drip loss and PSE carcasses, coupled with inhibition of phospholipase in the longissimus thoracis of pigs (Cheah et al. 1995). Another feed additive, HMBTa, a source of methionine, has demonstrated a positive influence on meat quality by improving protein deposition and carcass composition (Lemme et al. 2020). However, the result of the present study shows that supplementation with either Se/E or OA or their combination did not impact meat quality parameters, such as pH, drip loss and WHC. Additionally, diets supplemented with Se/E and OA also did not exhibit improvements in the oxidative status of the meat, except that dietary Se/E reduced protein oxidation, as evidenced by reduced pork muscle carbonyl concentrations. As mentioned earlier, during only 4–6 weeks of the experimental duration, daily maximum temperatures were above the upper critical temperature threshold, and the THI showed mild heat stress. Exposure to such environments might not trigger oxidative stress, which could be the main reason for the lack of effects of Se/E and OA on meat quality. Similar to the effects observed for Se/E and OA, no significant differences were found in any measurements of longissimus muscle of finisher pigs between males and females, except for a tendency for WBSF to be higher in males than females.
Cooking loss is associated with water loss during the cooking process and represents the water-holding capacity of meat at high temperatures (Tang et al. 2013; Fan et al. 2020). Muscle water has three forms: bound, immobilised, and free water. Under high-temperature treatment (cooking), water can be converted from immobilised to free form, resulting in water loss (Tang et al. 2013). In the current study, the increase in cooking loss over the storage period illustrated that aging increased the immobilised and free water content and agreed with previous studies (Tang et al. 2013; Babür et al. 2019; Fan et al. 2020). The meat colour is an important parameter associated with the pH and water-holding capacity and might affect consumers’ acceptance (Tapp et al. 2011; Warner 2016). The form of myoglobin, the primary pigment in meat on the surface of the meat, is responsible for the colour of meat (Warner 2016). During the storage, meat colour changes from purple (reduced deoxymyoglobin) to bright red (oxymyoglobin) and brown (metmyoglobin). In this study, the higher L* values, a* values, and b* values on Day 5 indicated that pork samples became brighter and yellower with more intense redness. These results were consistent with the previous study, which reported that 7-day aging increased L* values, a* values and b* values of beef samples (Fan et al. 2020). Another study reported that storage time increased L* values and b* values but decreased a* values of dry-cured pork neck (Kim et al. 2014).
The reduced shear force on Day 5 compared to Day 2 of refrigerated storage samples indicates substantial myofibrillar proteolysis associated with aging. These results were supported by a nearly 30% reduction in WBSF, showing improvements in instrumental tenderness between Days 2 and 5 for stored pork. Postmortem changes occur in biochemistry and the activity of endogenous proteolytic enzymes such as calpain, lysosomal proteases, and cathepsins (Bhat et al. 2018). These proteases are responsible for the degradation of myofibrillar proteins and the tenderisation of meat during aging. These results were consistent with Kim et al. (2007) and Cho et al. (2016), who reported that aging time increased meat tenderness by increasing MFI and decreased WBSF. Recent studies have found little effect of aging on the WBSF of pork. For example, no improvement in WBSF and tenderness was observed in both the pork loin (M. longissimus thoracis) and silverside muscles (M. biceps femoris) between a 2-day and 7-day aging period (Channon et al. 2018a). Furthermore, there was no improvement in the WBSF of the pork loin between a 7-day and 28-day post-slaughter period, although a decreased WBSF was observed in silverside muscles (Channon et al. 2018b). In this study, the findings that a 5-day aging period resulted in reduced WBSF indicate that this may be the optimal time for obtaining the highest meat tenderness and consumer palatability. This result is particularly relevant in Australia, where pork carcasses are typically delivered to retail stores within 24–48 h after slaughter, allowing for an additional 3–5 days of retail sales. The higher concentration of TBARS observed on Day 5 compared to Day 2 of refrigerated storage indicated that meat aging was associated with increased lipid oxidation. This agreement with previous studies reported that lipid oxidation increased over storage time in broiler meat (Shakeri et al. 2019), pork (Kim et al. 2014) and lamb (Rant et al. 2019). The increased lipid oxidation coupled with the changes in colour values agrees with Akamittath et al. (1990), who reported that lipid oxidation had a close relationship with the discolouration of meat because the oxidation of muscle pigment catalysed this process. Furthermore, lipid oxidation can result in the development of rancid off-flavours, as well as the loss of the desirable characteristic flavour notes and palatability (Cheng and MacDonald 2019; Dhakal et al. 2022).
Conclusions
The results of this experiment showed immune-castrated male pigs had higher growth performance than females and only subtle beneficial effects of Se, VitE or OA on growth performance and meat quality under the experimental conditions. This study began during summer, but the pigs entered the finisher period in late summer and early autumn, when conditions had moderated, and significant heat stress conditions were not experienced as expected. Due to climatic conditions, the effectiveness of the treatments under heat stress could not be evaluated.
Data availability
The data presented in this study are available on request from the corresponding author.
Conflicts of interest
Robyn Warner and Frank Dunshea are Associate Editors of Animal Production Science. To mitigate this potential conflict of interest they had no editor-level access to this manuscript during peer review.
Declaration of funding
This study was funded by Australia Pork Limited (grant number 2016/081). Hieu Huu Le received a scholarship from the Vietnam International Education Cooperation Department (VIED) and The University of Melbourne. Australia Pork Limited approved the manuscript for publication but had no role in the design of the study, in the collection, analysis, or interpretation of data, or the writing of the manuscript.
Acknowledgements
The authors thank Ms Archana Abhijith and Meat Research Facility, School of Agriculture, Food and Ecosystem Sciences, Faculty of Science, The University of Melbourne, for their help and support of this study. We would also like to thank the Australian Bureau of Meteorology for their support with the meteorological data.
References
Adzitey F, Nurul H (2011) Pale soft exudative (PSE) and dark firm dry (DFD) meats: causes and measures to reduce these incidences – a mini review. International Food Research Journal 18, 11-20.
| Google Scholar |
Akamittath JG, Brekke CJ, Schanus EG (1990) Lipid oxidation and color stability in restructured meat systems during frozen storage. Journal of Food Science 55, 1513-1517.
| Crossref | Google Scholar |
Babür TE, Tekinşen KK, Gürbüz Ü (2019) Some quality qualifications of cooked meat sous vide in the storage process. MANAS Journal of Engineering 7, 34-41.
| Google Scholar |
Bernabucci U, Ronchi B, Lacetera N, Nardone A (2002) Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. Journal of Dairy Science 85, 2173-2179.
| Crossref | Google Scholar | PubMed |
Bhat ZF, Morton JD, Mason SL, Bekhit AE-DA (2018) Role of calpain system in meat tenderness: a review. Food Science and Human Wellness 7, 196-204.
| Crossref | Google Scholar |
Bobček B, Lahučký R, Mrázová J, Bobček R, Novotná K, Vašíček D (2004) Effects of dietary organic selenium supplementation on selenium content, antioxidative status of muscles and meat quality of pigs. Czech Journal of Animal Science 49, 411-417.
| Crossref | Google Scholar |
Channon HA, Kerr MG, Walker PJ (2004) Effect of Duroc content, sex and ageing period on meat and eating quality attributes of pork loin. Meat Science 66, 881-888.
| Crossref | Google Scholar | PubMed |
Channon HA, Taverner MR, D’Souza DN, Warner RD (2014) Aitchbone hanging and ageing period are additive factors influencing pork eating quality. Meat Science 96, 581-590.
| Crossref | Google Scholar | PubMed |
Channon HA, D’Souza DN, Dunshea FR (2018a) Eating quality traits of shoulder roast and stir fry cuts outperformed loin and silverside cuts sourced from entire and immunocastrated male pigs. Meat Science 136, 104-115.
| Crossref | Google Scholar | PubMed |
Channon HA, D’Souza DN, Dunshea FR (2018b) Diet composition and slaughter age up to 24weeks have minimal impact on pork eating quality of loin steaks and silverside roasts from female pigs. Meat Science 135, 94-101.
| Crossref | Google Scholar | PubMed |
Cheah KS, Cheah AM, Krausgrill DI (1995) Effect of dietary supplementation of vitamin E on pig meat quality. Meat Science 39, 255-264.
| Crossref | Google Scholar | PubMed |
Chen JL, Zheng P, Zhang C, Yu B, He J, Yu J, Luo JQ, Mao XB, Huang ZQ, Chen DW (2017) Benzoic acid beneficially affects growth performance of weaned pigs which was associated with changes in gut bacterial populations, morphology indices and growth factor gene expression. Journal of Animal Physiology and Animal Nutrition 101, 1137-1146.
| Crossref | Google Scholar | PubMed |
Chen J, Tian M, Guan W, Wen T, Yang F, Chen F, Zhang S, Song J, Ren C, Zhang Y, Song H (2019) Increasing selenium supplementation to a moderately-reduced energy and protein diet improves antioxidant status and meat quality without affecting growth performance in finishing pigs. Journal of Trace Elements in Medicine and Biology 56, 38-45.
| Crossref | Google Scholar | PubMed |
Cheng JL, MacDonald MJ (2019) Effect of heat stress on vascular outcomes in humans. Journal of Applied Physiology 126, 771-781.
| Crossref | Google Scholar | PubMed |
Cho S, Kang SM, Seong P, Kang G, Kim Y, Kim J, Lee S, Kim S (2016) Effect of aging time on physicochemical meat quality and sensory property of Hanwoo bull beef. Korean Journal for Food Science of Animal Resources 36, 68-76.
| Crossref | Google Scholar | PubMed |
Choi J-S, Lee H-J, Jin S-K, Choi Y-I, Lee J-J (2014) Comparison of carcass characteristics and meat quality between Duroc and crossbred pigs. Korean Journal for Food Science of Animal Resources 34, 238-244.
| Crossref | Google Scholar | PubMed |
Christensen LB (2003) Drip loss sampling in porcine m. longissimus dorsi. Meat Science 63, 469-477.
| Crossref | Google Scholar | PubMed |
Čobanović N, Stajković S, Blagojević B, Betić N, Dimitrijević M, Vasilev D, Karabasil N (2020) The effects of season on health, welfare, and carcass and meat quality of slaughter pigs. International Journal of Biometeorology 64, 1899-1909.
| Crossref | Google Scholar | PubMed |
Cui Y, Hao Y, Li J, Bao W, Li G, Gao Y, Gu X (2016) Chronic heat stress induces immune response, oxidative stress response, and apoptosis of finishing pig liver: a proteomic approach. International Journal of Molecular Sciences 17, 393.
| Crossref | Google Scholar | PubMed |
Culler RD, JR FCP, Smith GC, Cross HR (1978) Relationship of myofibril fragmentation index to certain chemical, physical and sensory characteristics of bovine longissimus muscle. Journal of Food Science 43, 1177-1180.
| Crossref | Google Scholar |
De Busser EV, Dewulf J, Zutter LD, Haesebrouck F, Callens J, Meyns T, Maes W, Maes D (2011) Effect of administration of organic acids in drinking water on faecal shedding of E. coli, performance parameters and health in nursery pigs. The Veterinary Journal 188, 184-188.
| Crossref | Google Scholar | PubMed |
Dhakal J, Holt D, Aldrich CG, Knueven C (2022) Effects of antimicrobial addition on lipid oxidation of rendered chicken fat. Translational Animal Science 6, txac011.
| Crossref | Google Scholar |
Downs KM, Hess JB, Bilgili SF (2000) Selenium source effect on broiler carcass characteristics, meat quality and drip loss. Journal of Applied Animal Research 18, 61-71.
| Crossref | Google Scholar |
Dunshea FR (2001) Sexual dimorphism in growth of sucking and growing pigs. Asian-Australasian Journal of Animal Sciences 14, 1610-1615.
| Crossref | Google Scholar |
Dunshea FR, Cronin GM, Barnett JL, Hemsworth PH, Hennessy DP, Campbell RG, Luxford B, Smits RJ, Tilbrook AJ, King RH, McCauley I (2011) Immunisation against gonadotrophin-releasing hormone (GnRH) increases growth and reduces variability in group-housed boars. Animal Production Science 51, 695-701.
| Crossref | Google Scholar |
Fagan JM, Sleczka BG, Sohar I (1999) Quantitation of oxidative damage to tissue proteins. The International Journal of Biochemistry & Cell Biology 31, 751-757.
| Crossref | Google Scholar | PubMed |
Fan Y, Han Z, Arbab AAI, Yang Y, Yang Z (2020) Effect of aging time on meat quality of Longissimus Dorsi from yunling cattle: a new hybrid beef cattle. Animals 10, 1897.
| Crossref | Google Scholar | PubMed |
Fang Z, Lin D, Warner RD, Ha M (2018) Effect of gallic acid/chitosan coating on fresh pork quality in modified atmosphere packaging. Food Chemistry 260, 90-96.
| Crossref | Google Scholar | PubMed |
Franco LD, Fondevila M, Lobera MB, Castrillo C (2005) Effect of combinations of organic acids in weaned pig diets on microbial species of digestive tract contents and their response on digestibility. Journal of Animal Physiology and Animal Nutrition 89, 88-93.
| Crossref | Google Scholar | PubMed |
Ganesan S, Pearce SC, Gabler NK, Baumgard LH, Rhoads RP, Selsby JT (2018) Short-term heat stress results in increased apoptotic signaling and autophagy in oxidative skeletal muscle in Sus scrofa. Journal of Thermal Biology 72, 73-80.
| Crossref | Google Scholar | PubMed |
Gao J, Lin H, Wang XJ, Song ZG, Jiao HC (2010) Vitamin E supplementation alleviates the oxidative stress induced by dexamethasone treatment and improves meat quality in broiler chickens. Poultry Science 89, 318-327.
| Crossref | Google Scholar | PubMed |
Gregory NG (2010) How climatic changes could affect meat quality. Food Research International 43, 1866-1873.
| Crossref | Google Scholar |
Ha M, McGilchrist P, Polkinghorne R, Huynh L, Galletly J, Kobayashi K, Nishimura T, Bonney S, Kelman KR, Warner RD (2019) Effects of different ageing methods on colour, yield, oxidation and sensory qualities of Australian beef loins consumed in Australia and Japan. Food Research International 125, 108528.
| Crossref | Google Scholar | PubMed |
Hasty JL, van Heugten E, See MT, Larick DK (2002) Effect of vitamin E on improving fresh pork quality in Berkshire- and Hampshire-sired pigs. Journal of Animal Science 80, 3230-3237.
| Crossref | Google Scholar | PubMed |
Hoekstra WG (1975) Biochemical function of selenium and its relation to vitamin E. Federation Proceedings 34, 2083-2089.
| Google Scholar | PubMed |
Kim J-H, Cho S-H, Seong P-N, Hah K-H, Kim H-K, Park B-Y, Lee J-M, Kim D-H, Ahn C-N (2007) Effect of ageing temperature and time on the meat quality of longissimus muscle from Hanwoo steer. Food Science of Animal Resources 27, 171-178.
| Crossref | Google Scholar |
Kim I-S, Jin S-K, Yang M-R, Ahn DU, Park J-H, Kang S-N (2014) Effect of packaging method and storage time on physicochemical characteristics of dry-cured pork neck products at 10°C. Asian-Australasian Journal of Animal Sciences 27, 1623-1629.
| Crossref | Google Scholar | PubMed |
Laakkonen E, Wellington GH, Sherbon JN (1970) Low-temperature, long-time heating of bovine muscle 1. Changes in tenderness, water-binding capacity, pH and amount of water-soluble components. Journal of Food Science 35, 175-177.
| Crossref | Google Scholar |
Le Bellego L, van Milgen J, Noblet J (2002) Effect of high temperature and low-protein diets on the performance of growing-finishing pigs. Journal of Animal Science 80, 691-701.
| Crossref | Google Scholar | PubMed |
Lebret B, Batonon-Alavo DI, Perruchot M-H, Mercier Y, Gondret F (2018) Improving pork quality traits by a short-term dietary hydroxy methionine supplementation at levels above growth requirements in finisher pigs. Meat Science 145, 230-237.
| Crossref | Google Scholar | PubMed |
Lee YB, Choi YI (1999) PSE (pale, soft, exudative) Pork: the causes and solutions – review. Asian-Australasian Journal of Animal Sciences 12, 244-252.
| Crossref | Google Scholar |
Lemme A, Naranjo V, de Paula Dorigam JC (2020) Utilization of methionine sources for growth and Met+Cys deposition in broilers. Animals 10, 2240.
| Crossref | Google Scholar | PubMed |
Li Z, Yi G, Yin J, Sun P, Li D, Knight C (2008) Effects of organic acids on growth performance, gastrointestinal pH, intestinal microbial populations and immune responses of weaned pigs. Asian-Australasian Journal of Animal Sciences 21, 252-261.
| Crossref | Google Scholar |
Liu F, Cottrell JJ, Furness JB, Rivera LR, Kelly FW, Wijesiriwardana U, Pustovit RV, Fothergill LJ, Bravo DM, Celi P, Leury BJ, Gabler NK, Dunshea FR (2016) Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Experimental Physiology 101, 801-810.
| Crossref | Google Scholar | PubMed |
Liu L, Chen D, Yu B, Luo Y, Huang Z, Zheng P, Mao X, Yu J, Luo J, Yan H, He J (2021) Influences of selenium-enriched yeast on growth performance, immune function, and antioxidant capacity in weaned pigs exposure to oxidative stress. BioMed Research International 2021, 5533210.
| Google Scholar | PubMed |
Liu F, Zhao W, Le HH, Cottrell JJ, Green MP, Leury BJ, Dunshea FR, Bell AW (2022) Review: What have we learned about the effects of heat stress on the pig industry? Animal 16, 100349.
| Crossref | Google Scholar | PubMed |
Marjeta Č-P, Martin Š, Galia Z (2017) Immunocastration as alternative to surgical castration in pigs. In ‘Theriogenology’. (Ed. C Rita Payan) pp. 109–126. (IntechOpen: Rijeka) doi:10.5772/intechopen.68650
Martins JM, Charneca R, Garrido N, Albuquerque A, Jerónimo E, Guerreiro O, Lage P, Marmelo C, Costa F, Ramos A, Martin L (2023) Influence of sex on meat and fat quality from heavy alentejano pigs finished outdoors on commercial and high fiber diets. Animals 13, 3099.
| Crossref | Google Scholar | PubMed |
Martín-Venegas R, Geraert PA, Ferrer R (2006) Conversion of the methionine hydroxy analogue DL-2-hydroxy-(4-methylthio) butanoic acid to sulfur-containing amino acids in the chicken small intestine. Poultry Science 85, 1932-1938.
| Crossref | Google Scholar | PubMed |
Martín-Venegas R, Brufau MT, Guerrero-Zamora AM, Mercier Y, Geraert P-A, Ferrer R (2013) The methionine precursor DL-2-hydroxy-(4-methylthio)butanoic acid protects intestinal epithelial barrier function. Food Chemistry 141, 1702-1709.
| Crossref | Google Scholar | PubMed |
Mellado M, Gaytán L, Macías-Cruz U, Avendaño L, Meza-Herrera C, Lozano EA, Rodríguez Á, Mellado J (2018) Effect of climate and insemination technique on reproductive performance of gilts and sows in a subtropical zone of Mexico. Austral Journal of Veterinary Sciences 50, 27-34.
| Crossref | Google Scholar |
Montilla SIR, Johnson TP, Pearce SC, Gardan-Salmon D, Gabler NK, Ross JW, Rhoads RP, Baumgard LH, Lonergan SM, Selsby JT (2014) Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature 1, 42-50.
| Crossref | Google Scholar |
Morel PCH, Chidgey KL, Jenkinson CMC, Lizarraga I, Schreurs NM (2019) Effect of benzoic acid, sodium butyrate and sodium butyrate coated with benzoic acid on growth performance, digestibility, intestinal morphology and meat quality in grower-finisher pigs. Livestock Science 226, 107-113.
| Crossref | Google Scholar |
Nevrkla P, Hadaš Z, Horký P, Kamanová V (2017) Effect of genotype and sex of piglets on their losses before weaning. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis 65, 893-897.
| Crossref | Google Scholar |
Nørgaard JV, Fernández JA, Sørensen KU, Wamberg S, Poulsen HD, Kristensen NB (2010) Effect of benzoic acid supplementation on acid–base status and mineral metabolism in catheterized growing pigs. Livestock Science 134, 116-118.
| Crossref | Google Scholar |
Oliveira RF, Moreira RHR, Abreu MLT, Gionbelli MP, Teixeira AO, Cantarelli VS, Ferreira RA (2018) Effects of air temperature on physiology and productive performance of pigs during growing and finishing phases. South African Journal of Animal Science 48, 627-635.
| Crossref | Google Scholar |
Pardo Z, Fernández-Fígares I, Lachica M, Lara L, Nieto R, Seiquer I (2021) Impact of heat stress on meat quality and antioxidant markers in Iberian pigs. Antioxidants 10, 1911.
| Crossref | Google Scholar |
Ponnampalam EN, Kiani A, Santhiravel S, Holman BWB, Lauridsen C, Dunshea FR (2022) The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals: antioxidant action, animal health, and product quality – invited review. Animals 12, 3279.
| Crossref | Google Scholar | PubMed |
Poullet N, Rauw WM, Renaudeau D, Riquet J, Giorgi M, Billon Y, Gilbert H, Gourdine J-L (2022) Plasticity of feeding behaviour traits in response to production environment (temperate vs. tropical) in group-housed growing pigs. Scientific Reports 12, 847.
| Crossref | Google Scholar | PubMed |
Qin X, Zhang D, Qiu X, Zhao K, Zhang S, Liu C, Lu L, Cui Y, Shi C, Chen Z, Hao R, Li Y, Yang S, Wang L, Wang H, Cao B, Su H (2022) 2-Hydroxy-4-(Methylthio) butanoic acid isopropyl ester supplementation altered ruminal and cecal bacterial composition and improved growth performance of finishing beef cattle. Frontiers in Nutrition 9, 833881.
| Crossref | Google Scholar |
Rant W, Radzik-Rant A, Świątek M, Niżnikowski R, Szymańska Ż, Bednarczyk M, Orłowski E, Morales-Villavicencio A, Ślęzak M (2019) The effect of aging and muscle type on the quality characteristics and lipid oxidation of lamb meat. Archives Animal Breeding 62, 383-391.
| Crossref | Google Scholar | PubMed |
Rauw WM, de Mercado de la Peña E, Gomez-Raya L, García Cortés LA, Ciruelos JJ, Gómez Izquierdo E (2020) Impact of environmental temperature on production traits in pigs. Scientific Reports 10, 2106.
| Crossref | Google Scholar | PubMed |
Renaudeau D, Dourmad JY (2022) Review: Future consequences of climate change for European Union pig production. Animal 16, 100372.
| Crossref | Google Scholar | PubMed |
Ribeiro FA, Lau SK, Pflanzer SB, Subbiah J, Calkins CR (2021) Color and lipid stability of dry aged beef during retail display. Meat Science 171, 108274.
| Crossref | Google Scholar | PubMed |
Roldan-Henao M, Dalsgaard A, Cardona-Castro N, Restrepo-Rivera L, Veloza-Angulo LC, Alban L (2023) Pilot study of the productivity and Salmonella seroprevalence in pigs administered organic acids. Frontiers in Veterinary Science 10, 1123137.
| Crossref | Google Scholar | PubMed |
Shakeri M, Cottrell JJ, Wilkinson S, Le HH, Suleria HAR, Warner RD, Dunshea FR (2019) Growth performance and characterization of meat quality of broiler chickens supplemented with betaine and antioxidants under cyclic heat stress. Antioxidants 8, 336.
| Crossref | Google Scholar | PubMed |
Shakeri M, Cottrell JJ, Wilkinson S, Le HH, Suleria HAR, Warner RD, Dunshea FR (2020) Dietary betaine reduces the negative effects of cyclic heat exposure on growth performance, blood gas status and meat quality in broiler chickens. Agriculture 10, 176.
| Crossref | Google Scholar |
Sharaf AK, El-Darawany AA, Nasr AS, Habeeb AAM (2021) Alleviation the negative effects of summer heat stress by adding selenium with vitamin E or AD3E vitamins mixture in drinking water of female rabbits. Biological Rhythm Research 52, 535-548.
| Crossref | Google Scholar |
Sundrum A, Aragon A, Schulze-Langenhorst C, Bütfering L, Henning M, Stalljohann G (2011) Effects of feeding strategies, genotypes, sex, and birth weight on carcass and meat quality traits under organic pig production conditions. NJAS: Wageningen Journal of Life Sciences 58, 163-172.
| Crossref | Google Scholar |
Tang S, Yu J, Zhang M, Bao E (2013) Effects of different heat stress periods on various blood and meat quality parameters in young Arbor Acer broiler chickens. Canadian Journal of Animal Science 93, 453-460.
| Crossref | Google Scholar |
Tapp WN, III, Yancey JWS, Apple JK (2011) How is the instrumental color of meat measured? Meat Science 89, 1-5.
| Crossref | Google Scholar | PubMed |
Tugnoli B, Giovagnoni G, Piva A, Grilli E (2020) From acidifiers to intestinal health enhancers: how organic acids can improve growth efficiency of pigs. Animals 10, 134.
| Crossref | Google Scholar | PubMed |
Vashi Y, Naskar S, Chutia T, Banik S, Singh AK, Goswami J, Sejian V (2018) Comparative assessment of native, crossbred and exotic pigs during different seasons (winter, spring and summer) based on rhythmic changes in the levels of serum cortisol, lactate dehydrogenase levels and PBMC HSP70 mRNA expression pattern. Biological Rhythm Research 49, 725-734.
| Crossref | Google Scholar |
Wang Y, Yin X, Yin D, Lei Z, Mahmood T, Yuan J (2019) Antioxidant response and bioavailability of methionine hydroxy analog relative to DL-methionine in broiler chickens. Animal Nutrition 5, 241-247.
| Crossref | Google Scholar | PubMed |
Werner D, Baldinger L, Bussemas R, Büttner S, Weißmann F, Ciulu M, Mörlein J, Mörlein D (2021) Early immunocastration of pigs: from farming to meat quality. Animals 11, 298.
| Crossref | Google Scholar | PubMed |
White HM, Richert BT, Schinckel AP, Burgess JR, Donkin SS, Latour MA (2008) Effects of temperature stress on growth performance and bacon quality in grow-finish pigs housed at two densities. Journal of Animal Science 86, 1789-1798.
| Crossref | Google Scholar | PubMed |
Wolter B, Ellis M, McKeith FK, Miller KD, Mahan DC (1999) Influence of dietary selenium source on growth performance, and carcass and meat quality characteristics in pigs. Canadian Journal of Animal Science 79, 119-121.
| Crossref | Google Scholar |
Xia JQ, Liu DY, Liu J, Jiang XP, Wang L, Yang S, Liu D (2023) Sex effects on carcass characteristics, meat quality traits and meat amino acid and fatty acid compositions in a novel Duroc line pig. Journal of Animal Physiology and Animal Nutrition 107, 129-135.
| Crossref | Google Scholar | PubMed |


