Embryo development and survival in peripubertal ewe lambs
Jennifer L. Juengel A * , Laurel D. Quirke A , Jacqui Peers-Adams A , Peter D. Johnstone B and Peter Smith A
A * , Laurel D. Quirke A , Jacqui Peers-Adams A , Peter D. Johnstone B and Peter Smith A
A Agricultural Systems and Reproduction, Animal Science, Invermay Agricultural Centre, AgResearch Ltd, Puddle Alley, Mosgiel 9092, New Zealand.
B Bioinformatics & Statistics, Invermay Agricultural Centre, AgResearch Ltd, Puddle Alley, Mosgiel 9092, New Zealand.
Animal Production Science 63(12) 1177-1187 https://doi.org/10.1071/AN22417
Submitted: 9 November 2022 Accepted: 18 March 2023 Published: 13 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Mating of ewe lambs can increase farm profitability, although uptake is limited by low reproductive success. Reproductive loss to Day 35 of pregnancy in peripubertal ewe lambs is greater than in adult ewes; however, the precise timing of this loss is unknown.
Aim: We aimed to define more clearly early embryo development and key times of loss in peripubertal ewes.
Methods: Health and development of embryos from naturally cycling crossbred ewes ~8 months of age were recorded. Following mating across 2 years, ewes were assigned to three groups (n = 80–87 per group): assessed on Day 3 of pregnancy, on Day 14, or between Day 35 and birth. For Day 3 and Day 14 groups, ewes were humanely killed, and embryos were assessed by microscope following recovery from the reproductive tract. Ultrasonography at around Days 35 and 70, and lambing data, were used to assess loss in the third group.
Key results: By Day 3 of pregnancy, 22.9% of ova released were not present as healthy embryos (P < 0.05). Embryo survival on Day 14 tended (P < 0.10) to decrease further, with 34.0% (±4.5%) of ova released not present as healthy embryos. No decrease was detected between Days 14 and 35, whereas between Day 35 and birth, an additional 6.8% reproductive loss occurred (P < 0.05). Attainment of puberty prior to introduction of the fertile ram did not affect reproductive loss. Structures collected on Day 3 ranged from one cell to 12 cells: 11% (13/120) being one cell; 49% (59/120) two to four cells; and the remainder (48/120) at least five or six cells. Conceptus length at Day 14 ranged from 5.3 to 200.0 mm, with large variation between and within animals; within-ewe variation was 67% of between-ewe variation. Concentration of progesterone at time of collection was associated (P < 0.001) with developmental stage on Day 3, but not Day 14, of pregnancy.
Conclusions: Reproductive loss in peripubertal ewes primarily occurred prior to Day 14, with much of this loss before Day 3.
Implications: Improving oocyte and oviduct quality is important to supporting normal fertilisation and early embryo development in peripubertal ewe lambs, thus improving reproductive success.
Keywords: crossbred ewes, embryo development, embryo survival, ewe lambs, mating of ewe lambs, morphology of day 14 embryo, morphology of day 3 embryo, reproductive loss.
Introduction
Incorporation of mating of ewe lambs has the potential to increase farm productivity, both directly and by reducing the environmental impact per kg meat produced (Edwards and Juengel 2017; Rosales Nieto et al. 2018; Farrell et al. 2020). However, mating of ewe lambs has variable adoption rates around the world, with ≤30% of New Zealand, Australian and UK farms currently mating ewe lambs (Kenyon et al. 2014; Rees and Phillips 2016). One factor limiting uptake of mating of ewe lambs may be the reduced reproductive output of ewe lambs, which is ~60% of what can be achieved in mature ewes (Edwards and Juengel 2017). Development of methods to improve reproductive output of ewe lambs may support uptake of mating of ewe lambs, thus improving farm profitability while reducing environmental impacts.
In order to develop new methods to improve reproductive output of ewe lambs, key points of reproductive loss must be identified as the first step to establishing pathways to mitigate these losses. The majority of reproductive loss to birth in ewe lambs occurs in the first month of pregnancy (Paganoni et al. 2014; Edwards et al. 2016) and is approximately double that observed in mature ewes (Edwards et al. 2016). In adult ewes, the majority of embryo loss occurs during the first 2 weeks of pregnancy (O’Connell et al. 2016), likely prior to maternal recognition of pregnancy; most ewes that fail to become pregnant do not show a delay in expression of oestrus, indicating that the embryo was lost prior to pregnancy being recognised by the dam (Quinlivan et al. 1966). Approximately one-third of the loss during the first 2 weeks of pregnancy occurs by Day 4, with the rest occurring between Days 4 and 14 (O’Connell et al. 2016). Fertilisation failure is low, estimated at 1% (O’Connell et al. 2016). This identifies the need for increased understanding of oocyte quality, embryonic genome activation, and oviductal and uterine environment prior to implantation to enable the development of new methods to improve embryo survival in adult ewes (O’Connell et al. 2016).
In healthy ewe lambs, loss after the first month of pregnancy (i.e. late embryonic and fetal loss) is low (Paganoni et al. 2014; Edwards et al. 2016), whereas early embryo loss is often high, ranging from 30% to 60% (Paganoni et al. 2014; Edwards et al. 2016). However, when these losses occur in naturally mated ewe lambs has not been determined. Reciprocal embryo transfer studies between yearling and adult ewes highlighted the potential role of oocyte quality and oviduct environment in driving differences in embryo survival between the two age groups (Quirke and Hanrahan 1977, 1983). However, those studies used hormonal intervention for the synchronisation of cycles as well as embryo transfer. Hormonal treatment has the potential to mask individual variation in hormone levels and would induce puberty in prepubertal animals; thus, whether these are key factors underlying the reduced embryo survival observed in naturally cycling and naturally mated ewe lambs is unknown. The aims of the present study were to determine the rate of fertilisation failure and embryo loss, and to examine potential factors influencing embryo/fetal loss, in naturally mated ewe lambs. The natural variation present in embryo development within and between ewe lambs was also explored.
Materials and methods
Experimental design
All experiments were conducted in accordance with New Zealand animal welfare regulations with approval for the experiment given by the Invermay Animal Ethics Committee as application numbers 13979 and 14376. The experiment was conducted at the Invermay Agricultural Centre (45°51′S, 170°23′E) during 2017 and 2018. All lambs were crossbreds, with the dominant breeds being Coopworth, Romney and Texel. They were born in spring (September) and were bred in the following autumn (late April–early May) as ewe lambs at ~8 months of age.
They were drenched (1 mL/10 kg) at ~4-week intervals as needed to control internal parasites beginning in November (i.e. at ~2 months of age). The drench contained abamectin (2 g/L) and levamisole hydrochloride (80 g/L) with 5 mg cobalt and 1 mg selenium/mL (MSD Animal Health, Upper Hutt, New Zealand; or Nexan, Auckland, New Zealand). All ewe lambs were supplemented with vitamin B12 (Virbac New Zealand, Hamilton, New Zealand) at weaning and with iodine (Bayer Animal Health, Auckland, New Zealand) ~2 months prior to introduction to the fertile ram. They received vaccinations against leptospirosis and the major clostridial diseases with Ultravac 7in1 (Zoetis, Auckland, New Zealand), or Multine 5-in-1; (MSD Animal Health) and Leptoshield (Zoetis), in December and January. They were also vaccinated against Toxoplasma gondii and Campylobacter fetus fetus/Campylobacter jejuni (Toxovax and CampyVax4; MSD Animal Health) in February and in March (CampyVax4 only).
Following weaning in summer (December) at ~2–3 months of age, ewe lambs were placed on a ryegrass and white clover pasture with free access to water to reach a minimum liveweight of 39 kg by the beginning of mating at ~8 months of age. Lambs not reaching the minimum liveweight were removed from the trial. Approximately 1% of animals did not reach the liveweight minimum, with ~150 animals continuing in the trial each year. Liveweight was recorded at the time of introduction of the fertile rams. Ewes continued to be pastured with free access to water throughout the trial. If pasture was insufficient in winter, as assessed by experienced farm staff monitoring ewe growth and pasture coverage, supplemental ryegrass silage was provided to meet the animals’ nutritional needs.
In order to assess whether the ewe lambs had attained puberty prior to introduction of the fertile ram, they were first exposed to three vasectomised rams fitted with a marking harness for a minimum of 18 days (i.e. one reproductive cycle). The rump of each ewe was assessed for the presence/absence of a mating mark just prior to introduction of the fertile ram; those that had been marked were considered to have attained puberty. Following removal of the vasectomised rams, three adult intact crossbred rams (dominant breeds Coopworth, Romney and Texel) fitted with harnesses with crayons were placed with the ewes at a ewe:ram ratio of ~50:1. Ewes were observed each morning for mating marks. The first day that a mark was recorded was considered as Day 1 of pregnancy because the ewe would have been first mated in the previous 24 h. Rams were removed from the ewes after 17–18 days, when sufficient numbers of ewes had been bred.
Ewes were randomly assigned for tissue collection on the afternoon of Day 3 (Day 3 group) or the afternoon of Day 14 (Day 14 group), or to undergo ultrasound examination at around Days 35 and 70 and then complete pregnancy and a recorded lambing (Day 35 group). A single blood sample was collected into a vacutainer with lithium heparin additive (Becton Dickinson, Franklin Lakes, NJ, USA) at tissue collection for the Day 3 and Day 14 groups. Following centrifugation, plasma was collected and stored at −20°C for determination of progesterone concentrations.
Determination of embryo number and embryo measurements
For the Day 3 and Day 14 groups, ewes were humanely killed by captive bolt and exsanguination, and their reproductive tracts were collected post-mortem. The number of corpora lutea (CL) with a visible ovulation stigma on each ovary was used to determine the ovulation rate. For embryo collection on Day 3, the uterine horn and oviduct were flushed twice with 5 mL compound sodium lactate (Hartmann’s) solution (Baxter Healthcare, Sydney, NSW, Australia) with 1 mM 3-(N-morpholino)propanesulfonic acid (MOPS), or EMCARE Complete Ultra Flushing Solution (ICPbio Reproduction, Auckland, New Zealand), using a syringe with a blunt needle. The solution was collected via a polyethylene tube (OD 2.5 mm, ID 1.5 mm; TE Connectivity, Schaffhausen, Switzerland) inserted into the fimbria of the oviduct to recover embryos. Each side of the reproductive tract was flushed the same way twice.
In the Day 14 group, the uterine horns were partitioned from each other and then each was isolated from the uterine body. Hartmann’s solution with 1 mM MOPS or EMCARE Complete Ultra Flushing Solution (10 mL) was used to flush embryos from the horns. The uterine body was also flushed if the embryo was not recovered intact. This was done twice for each uterine horn if the number of embryos recovered was less than the number of CL present.
The embryos recovered were assessed and graded as previously described (O’Connell et al. 2016), using a Nikon SMZ800 microscope (maximum magnification 63×; Nikon, Tokyo, Japan), and photographed using a Nikon DS-Fi1 camera. For the Day 3 group, the number of cells present in each recovered structure was recorded. The photographs were used to measure the embryo diameter (Day 3 group), width of the zona pellucida (Day 3 group), and conceptus length (Day 14 group), using NIS Elements BR3.2.64-bit software (calibrated to measure pixels). The average of two measurements was used to obtain embryo diameter (measurements at right angles to each other and from the outer edge of the zona pellucida) and zona pellucida width (measurements at 180° angles to each other).
For the Day 35 group, the ovulation rate of the ewes was measured by laparoscopic assessment of CL with a visible ovulation stigma during the first oestrus cycle of the mated ewes after fertile rams were introduced (range Day 4–14 of pregnancy). The number of embryos was measured at around Day 35 of pregnancy (range Day 31–37) by ultrasonography with a transrectal B-mode ultrasound scanner (Aloka SSD-900 or SonoScape S2V; Euromed Electronics NZ, Auckland, New Zealand). One ewe was missed during this scanning; hence, the number of fetuses was not measured in this ewe until Day 46. She had not undergone any loss and thus we could be confident that the measurement obtained on Day 46 would be a true representation of her number of embryos present on Day 35. An abdominal ultrasound of the ewes was also performed at around Day 70 (range Day 55–83) by a commercial operator. These ewes were allowed to lamb, and they were closely monitored at parturition to allow assignment of lambs, born dead or alive, to each dam in order to determine the number of lambs born.
Measurement of progesterone concentrations
Progesterone was measured using the IBL Coat-a-Count RIA (IBL, Hamburg, Germany). This assay required an overnight incubation at 4°C, but all other steps followed the manufacturer’s instructions as previously validated for sheep plasma samples (Smith et al. 2019). The lower limit detected was 0.2 ng/mL (determined by linearity of serially diluted samples). Average concentrations of three quality-control standards included in each assay were 8.86 ng/mL (high standard), 4.56 ng/mL (medium standard) and 1.87 ng/mL (low standard). Inter-assay coefficients of variation (CVs) for the high, medium and low standards were 2.75%, 4.90% and 4.42%, respectively. Intra-assay CVs averaged 5.32%, 7.81% and 15.37% for the high, medium and low standards, respectively.
Data calculations
For each ewe, a survival score was calculated as the number of healthy embryos recovered divided by the number of CL. Health of the embryo was assessed by using the criteria outlined by O’Connell et al. (2016). For the Day 3 group, given that the embryos would still be within the zona pellucida, if an ovum/embryo was not recovered for each CL, it was considered a missing value. At least one ovum/embryo had to be recovered from a ewe in the Day 3 group for her to be retained in the dataset. The embryo health score was based on a single ovum/embryo for 22 twin-ovulating animals, and the average of two embryos/ova for four triplet-ovulating animals. For the Day 14 and Day 35 groups, if an embryo was not recovered/observed, it was considered to be unhealthy, because the healthy embryos on Day 14 were easy to identify in the uterine flush or on Day 35 by ultrasound examination. Survival scores were also calculated on Day 70 and 148 of pregnancy, respectively, by dividing the number of fetuses at mid-pregnancy (i.e. ~Day 70), and the number of lambs born, by the ovulation rate. In instances where the number of embryos/fetuses detected by ultrasonography was less than the number of lambs born, the number of embryos/fetuses was corrected to the number of lambs born to prevent overestimation of survival from an error in ultrasonography measurements (n = 2 animals on Day 35, and n = 5 animals on Day 70).
Statistical analyses
Data were analysed using GenStat Ver. 17 (VSN International 2014). Parsimonious modelling was used to determine which interactions between main effects to retain in the model, with only those that reach significance (P < 0.05) being retained.
The effects of year on differences in weight at introduction of the fertile ram, ovulation rate and percentage attaining puberty prior to introduction to the fertile ram were determined using restricted maximum likelihood (REML) with year being included as a main effect in the model.
Differences in survival scores among Days 3, 14 and 35 were assessed by using regression analysis with the year and day of pregnancy, and their interaction, included in the model. To determine whether significant embryo loss had occurred by Day 3, the standard errors of differences of predictions were used to determine whether survival score on Day 3 was different from 1. Differences in survival scores among Days 35, 70 and 148 (birth) were determined using REML for repeated measures (day of pregnancy), using an autoregressive (1) structure. Year and the interaction between year and day of pregnancy was also included in the model.
The effects of liveweight of the ewe and whether the ewes had attained puberty at the time of introduction of the fertile ram on survival scores were assessed with regression analysis and a generalised linear model with logit link and binomial distribution. Information from the survival scores on Days 3, 14 and 148 (birth) were used in this analysis. These data were limited to ewes ovulating two ova because there were too few observations in each year in animals ovulating one ovum or three ova to resolve adequately the interactions between year and number of ova released. There were 56–65 observations from ewes releasing two ova per pregnancy day, with a minimum of 24 observations for any pregnancy day in either year. The model included the main effects of year of trial, pregnancy stage, attainment of puberty and liveweight at fertile ram introduction. Interactions between main effects were examined but were excluded because they were not significant.
Animals were also classified into pregnant (at least one healthy embryo/fetus present at assessment) or non-pregnant (no healthy embryo/fetus present at assessment) for Days 3, 14 and 148 (birth). Analysis (REML) was undertaken to determine whether concentrations of progesterone (natural log transformed to normalise distribution; included Days 3 and 14 groups only), liveweight of the ewe at fertile ram introduction, and percentage that had attained puberty prior to fertile ram introduction differed between ewes that were pregnant and those that failed to become pregnant. The fixed effects included in the models were year, pregnancy day, pregnancy status (pregnant or not pregnant), and any significant interactions.
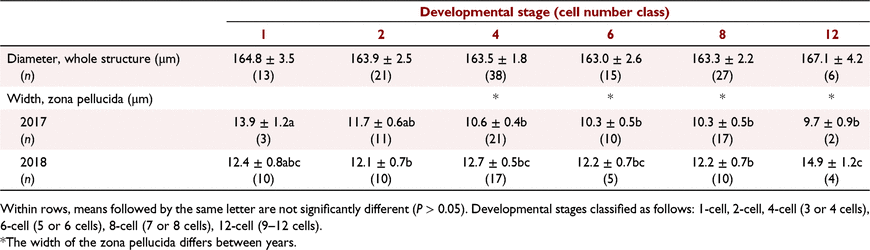
Factors affecting differences in embryo size and zona pellucida thickness on Day 3, and conceptus length on Day 14, were assessed using REML. The model included year and cell number (Day 3 only) as a main effect with animal as a random effect. Cell numbers were classified as follows: 1-cell, 2-cell, 4-cell (3 or 4 cells), 6-cell (5 or 6 cells), 8-cell (7 or 8 cells), or 12-cell (9–12 cells). All data are presented as mean ± s.e.m.
Additionally, maternal factors potentially associated with embryo development were assessed using the measurements of the most developed embryo for each pregnant ewe. The most developed embryo was chosen to represent the potential of the maternal environment for embryo development. Total number of cells was used for Day 3 and conceptus length was used for Day 14. For Day 3, the model included liveweight of the ewe at fertile ram introduction, whether the ewe had attained puberty prior to fertile ram introduction, year of the trial, and progesterone at time of tissue collection. A significant interaction between year of the trial and liveweight of the ewe was observed; thus, the interaction was also retained in the model. For Day 14, the main fixed effects were liveweight of the ewe at fertile ram introduction, whether the ewe had attained puberty prior to fertile ram introduction, year of the trial, and progesterone at time of tissue collection. Interactions between main effects were examined, and no significant interactions were observed; thus, the model fitted main effects only.
Results
Animal characteristics for each year of the trial
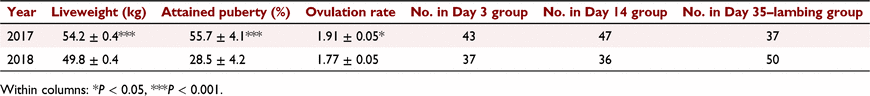
Average liveweight of the ewes, as well as the percentage of the ewe lambs that had attained puberty at the time of introduction of the fertile ram and average ovulation rate, differed between years (Table 1). The difference in average ovulation rate between years was related to the differences in liveweight because ovulation rates were very similar when liveweight was also included in the model (rates 1.81 ± 0.05 and 1.82 ± 0.05 for 2017 and 2018, respectively). The numbers of animals in each pregnancy day group for each year are given in Table 1.
Pattern of embryo loss
The pattern of embryo loss in ewe lambs is shown in Fig. 1. By Day 3 of pregnancy, 22.9% of the ova released were not present as a healthy embryo, representing significant loss of reproductive potential. This loss included total failure of fertilisation/first cleavage in 10 of the 80 animals, representing approximately half of the loss observed by Day 3 of pregnancy. Embryo survival scores tended (P < 0.10) to decrease further by Day 14 of pregnancy to 66.0% ± 4.5% (i.e. 34% of the ova released were not present as a healthy embryo). There were no differences between embryo survival scores from ewes collected on Day 35 of pregnancy and those collected on Day 14 of pregnancy. Similarly, no significant change in embryo survival score was observed between Day 35 and Day 70 of pregnancy, although a small loss (P < 0.05) was observed by birth, with loss between measurements around Day 35 and Day 70 being 1.8% and between around Day 70 and birth being 5.0%.
Factors associated with fertilisation and embryo/fetal survival
No differences (P = 0.67) were observed in the percentage of ewes attaining puberty prior to fertile ram introduction between pregnant (48.7% ± 3.2%) and non-pregnant (45.6% ± 6.3%) ewes. Similarly, reproductive loss did not differ (P = 0.68) between animals that had attained puberty prior to the fertile ram introduction (31.9% ± 4.6%) and those that had not (29.1% ± 4.6%). Liveweight at fertile ram introduction was not associated with survival score (P = 0.15). However, ewes that were pregnant at assessment for pregnancy status were heavier (P < 0.05) when the fertile ram was introduced than those that were not (52.2 ± 0.3 kg vs 50.8 ± 0.6 kg). When assessing progesterone concentrations, there was an interaction between the year of the trial and pregnancy stage and whether the ewe was pregnant, but no consistent pattern in concentrations of progesterone between pregnant and non-pregnant animals across years was observed (Table 2).

|
Characteristics of embryos on Days 3 and 14
Structures collected on Day 3 after marking by the fertile ram ranged from one cell to 12 cells (Fig. 2). There was no effect of year of collection on average size of the ovum/zygote/embryo on Day 3 (Table 3). Variation in size of the structures was small and relatively similar between and within animals. For zona pellucida width, a complex interaction (P < 0.05) between developmental stage and year was observed (Table 3). The zona was wider in the more developed embryos collected in the second year than in the first year. In the first year, the less developed embryos had a wider zona than the more developed embryos. In the second year, the most developed embryos (i.e. those with 12 cells) generally had a larger zona than the less developed embryo. Some variability in zona pellucida width was observed both between and within animals; the within-animal variation was 26% of the between-animal variation.
|
Conceptuses collected on Day 14 varied in size, ranging from 5.3 to 200.0 mm in length (Fig. 3). Even with this variability, conceptuses collected in the first year were ~60% (P < 0.001) of the size of those collected in the second year (49.93 ± 5.65 mm vs 78.49 ± 5.98 mm for 2017 and 2018, respectively; all healthy conceptuses included in analysis). Large variability in conceptus length was observed both between and within animals, with the within-animal variation being 67% of the between-animal variation.
Factors associated with embryo development
When examining the potential effects of maternal environment on development of the embryo, the classification of the most advanced embryo obtained from the ewe was utilised because that embryo represents the maternal potential to support embryo development. On Day 3 of pregnancy, no effect of year of the trial (P = 0.92) or whether the ewe lamb had attained puberty prior to introduction of the fertile ram (P = 0.82) was observed on developmental stage of the embryo. More advanced embryos were recovered from ewe lambs with higher concentrations of progesterone (P < 0.001), with an increase of 3.89 cells for every 1 ng increase in progesterone concentration. Additionally, a complex interaction was observed between liveweight at introduction of the fertile ram and year of the trial (P < 0.01). In the first year, liveweight did affect embryo development; embryos from heavier ewe lambs were more advanced (P < 0.001), with an additional 0.26 ± 0.07 cell for every 1 kg increase in liveweight. However, no such significant relationship was observed between liveweight and embryo development in the second year (P = 0.16); in fact, the relationship was negative (i.e. as liveweight increased the number of cells seemed to decrease), explaining the significant interaction. On Day 14 of pregnancy, no effect of liveweight of the ewe lamb (P = 0.70), concentrations of progesterone (P = 0.14), or whether the ewe lamb had attained puberty prior to mating to the fertile ram (P = 0.55) was observed on size of the conceptus (largest healthy conceptus included for each ewe). However, conceptuses collected in the second year were longer (P < 0.001) than those collected in the first year (52.04 ± 7.4 mm and 91.93 ± 7.63 mm for first and second year, respectively; largest healthy conceptus included for each ewe lamb).
Discussion
As has been observed previously in adult ewes (O’Connell et al. 2016), the present study found that the majority of embryo/fetal loss in ewe lambs occurs in the first 2 weeks of pregnancy. However, fertilisation failure was 11% in the ewe lambs in the present study, compared with a previously observed 1% failure in naturally mated adult ewes (O’Connell et al. 2016). Similarly, loss to Day 3 (which includes fertilisation/cleavage failure) represented approximately two-thirds of the embryo loss observed during the first 2 weeks in the ewe lamb (present study), with loss between Day 3 and 14 being approximately one-third. By contrast, in the adult ewe, approximately two-thirds of the embryo loss observed during the first 2 weeks of pregnancy occurred between Days 4 and 14 (O’Connell et al. 2016). This suggests that oocyte quality and oviductal environment may be under more stress in the ewe lamb than the adult ewe. This agrees with previous studies using a multiple ovulation program followed by embryo transfer to examine the differences in embryo survival between adult ewes and ewe lambs. Embryos from mature ewes had similar survival rates following transfer to ewe lambs or mature ewes (Quirke and Hanrahan 1983), whereas embryos from ewe lambs had lower survival than embryos from mature ewes when transferred into mature ewes (Quirke and Hanrahan 1977). Thus, the present results in naturally cycling ewe lambs, which is the physiological condition of most ewes mating as lambs, are consistent with previous results from hormonally treated animals.
Some studies have shown significant embryo/fetal loss (15–25%) after Day 30 of pregnancy in both adult ewes (Dixon et al. 2007; Ferreira-Silva et al. 2018) and ewe lambs (Clune et al. 2022). The reasons for the observed greater embryo fetal loss past Day 30 of pregnancy in those studies are unclear but could be linked to ewe breed, nutrition, presence of infectious disease, or other management (e.g. hormonal treatments) or environmental differences (e.g. temperature–humidity index) (Dixon et al. 2007; Clune et al. 2022). The ewe lambs in the present study were fully vaccinated against known infectious agents causing embryo/fetal loss. Additionally, they were managed to optimise growth prior to mating.
In our study, whether the ewe lamb attained puberty prior to introduction of the fertile ram did not affect fertilisation/cleavage/embryo failure. This seems at odds with studies that show positive effects of attaining puberty prior to introduction of the fertile ram on pregnancy rate or average number of lambs per ewe lamb exposed to the ram (Edwards et al. 2016). However, it is important to note that the present study examined only ewes that were mated by the fertile ram, in contrast to being exposed to the fertile ram. We have previously shown that the positive effect of attaining puberty prior to fertile ram introduction on ewe lamb fertility was related to an increased chance of the ewe being mated by the fertile ram rather than any change in overall fertilisation/cleavage/embryo failure (Edwards et al. 2016). Nonetheless, another study comparing ewe lambs mated on their first, second or third oestrus did detect a difference in fertilisation/cleavage/embryo failure, with ewes mated on their first oestrus having more than twice the loss of those mated on their second or third oestrus (Hare and Bryant 1985). There are several experimental differences between the two studies, with the previous study comparing the animals mated on the first oestrus, which is earlier in the breeding season with those mated on the second and third oestrus. which are later in the breeding season, the use of different breeds of sheep, as well as quite different fertilisation/cleavage/embryo failure rates, which were much lower in the present study than the previous study. Previous studies have shown a breed and sire effect on fertility in ewe lambs (Hare and Bryant 1985; Paganoni et al. 2014).
In our study, ewe lambs that became pregnant had greater liveweights at fertile ram introduction than those that failed to become pregnant. This is consistent with previous studies showing that the number of ewe lambs lambing was positively associated with liveweight of the ewe lamb at introduction of the fertile ram (Gaskins et al. 2005; Rosales Nieto et al. 2013; Paganoni et al. 2014; Corner-Thomas et al. 2015). However, in the present study, overall embryo loss was not strongly affected by liveweight. It is also important to note that the previous studies included influences of liveweight on the animal attaining puberty (Rosales Nieto et al. 2013; Paganoni et al. 2014; Edwards et al. 2015) and thus increased opportunity to become pregnant, whereas in our study, only animals that had been bred by the fertile ram were assigned to a pregnancy assessment group, and hence, those that were assessed for embryo loss had attained puberty. Therefore, liveweight may have some effect on pregnancy rate of the ewe lambs mated, but its strong effect on overall pregnancy rate in ewe lambs is likely primarily driven by increasing the number of ewe lambs that attain puberty and hence are mated during the breeding season.
Progesterone concentrations have also been shown to be linked to increased embryo survival in adult ewes (Parr 1992; O’Connell et al. 2013). However, no consistent relationship between higher progesterone concentrations and embryo survival were noted in our study. In one year, concentrations of progesterone collected on Day 14 were lower in the non-pregnant ewes than the pregnant ewes, but such differences were not observed in the other year or on Day 3. Failure to observe a relationship between progesterone concentrations and pregnancy rate may have been linked to the timing or frequency of sample collection. Typically, differences in progesterone concentrations related to embryo survival are observed only in the early stages of the cycle in animals sampled over multiple days (O’Connell et al. 2013). Thus, the single sample collected in the present study at the day of embryo collection may have been insufficient to detect a relationship between progesterone and embryo survival. Further studies, with more frequent collections of blood samples, are required to establish whether a positive relationship between progesterone concentrations and embryo survival exists in ewe lambs as it does in adult animals.
The embryo developmental stage was similar to that expected based on observations in adult ewes (Rowson and Moor 1966; O’Connell et al. 2016). The high variability in embryo developmental stage/size both between and within ewe lambs in the present study was similar to that observed in adult ewes (Rowson and Moor 1966; O’Connell et al. 2016). A potential cause of variation between ewe lambs is that detection of oestrus was undertaken only once per day, meaning that time of breeding could vary by as much as 24 h. Conceptus length approximately triples from Day 13 to Day 14 in sheep (Rowson and Moor 1966). Furthermore, in ewes with multiple ovulations, ovulation does not occur synchronously, and breeding can occur multiple times during oestrus; therefore, fertilisation and initiation of embryo development could be asynchronous. However, this timing difference is likely to be relatively small, because the median interval between ovulations in twin-ovulating ewes was <2 h (Whyman et al. 1979). However, variations in size do not appear linked to differences in embryo survival because embryos of varying size recovered from a single donor had similar developmental capabilities (Rowson and Moor 1966). Similarly, previous immunisation with an androstenedione-based vaccine that was associated with reduced embryo size did not affect embryo survival (O’Connell et al. 2016).
We observed a positive relationship between progesterone concentrations and conceptus size/development on Day 3, but not Day 14, of pregnancy. The effects of supplementary progesterone on increasing conceptus/fetal size is well documented in both sheep and cattle (Kleemann et al. 1994; Mann et al. 2006; Satterfield et al. 2006; Fermin et al. 2018a, 2018b). Additionally, Shorten et al. (2018a) showed a link between increased concentrations of progesterone in recipients and increased conceptus length when examining bovine embryos collected on Day 15 of pregnancy that had been transferred into recipients on Day 7 of pregnancy. However, the effects of progesterone concentrations on advancing embryo development are linked to increased progesterone early in the reproductive cycle. Ewes with shorter than average oestrous cycle durations had more-developed embryos on Day 13 of pregnancy than ewes with longer duration oestrous cycles (Nephew et al. 1991). Although increased progesterone concentrations were observed on Days 2–4 of pregnancy, progesterone concentrations were not different on other days of the cycle nor was embryo survival different between groups (Nephew et al. 1991). Therefore, the single sample collected at the time of embryo collection in the Day 14 group in the present study may not have been sufficient to observe relationships between progesterone concentrations and conceptus length. In addition, the relationship between progesterone concentrations and length of the conceptus on Day 15 of pregnancy was much weaker when examined in bovine embryos that were developed in vivo following artificial insemination (Shorten et al. 2018b) than in embryos produced in vitro and transferred to the uterus on Day 7; this is more consistent with our study, where no correlation was observed on Day 14 following natural mating. Changes in other steroids may also regulate conceptus length. Treatment with progesterone alone on Day 11.5 of pregnancy in sheep did not increase conceptus length on Day 13 (Nephew et al. 1994). However treatment with human chorionic gonadotropin (hCG), which resulted in an increase in both progesterone and oestrogen, on Day 11.5 of pregnancy did increase conceptus length on Day 13, providing evidence for a role of estrogen in regulating conceptus length during this stage of pregnancy (Nephew et al. 1994). Additionally, in adult ewes, previous immunisation against androstenedione retarded embryo development through unknown mechanisms (O’Connell et al. 2016). However, this was not linked with a decrease in embryo survival, which is consistent with considerable plasticity in embryo development being tolerated in the ewe.
In the present study, on Day 3, size of the unfertilised oocyte/embryo was not associated with changes in developmental stage. All structures collected at Day 3 were still enclosed within the zona pellucida and thus were similar in size to a full-grown oocyte. Some previous studies have linked increased oocyte volume to improved oocyte quality (Gandolfi et al. 1998; El Shourbagy et al. 2006; Murakoshi et al. 2013; Reader et al. 2015), whereas others have not (O’Brien et al. 1996, 2000). We saw no evidence that unfertilised oocytes collected on Day 3 were smaller than embryos that were developing normally at this time. Differences in the thickness of the zona pellucida have also been associated with differences in embryo quality grade in humans (Balakier et al. 2012), and oocytes that failed to fertilise had thicker zona pellucida than embryos collected 2 days post-insemination in rabbits (Marco-Jiménez et al. 2012). In our study, in the first year, unfertilised oocytes did have a thicker zona pellucida than the more advanced developing embryos, but no such relationship was observed in the second year. Thus, whether increased thickness of the zona inhibits fertilisation could not be clearly defined in the study. Previous studies comparing in vitro matured oocytes between adults and lambs did not observe differences in zona pellucida thickness, but cleavage rate of the prepubertal lambs was greater than that observed in adult oocytes (Reader et al. 2015). Overall, whether thickness of the zona pellucida is negatively associated with fertilisation success in yearling sheep warrants additional research.
We observed a positive relationship between liveweight of the ewe and embryo development on Day 3 in the first year of the trial, but this was not extended to the second year or Day 14. Previous studies have shown differences in embryo size following transfer of embryos into different sheep breeds with a small or large body size, with the larger ewes having longer conceptuses on Day 19 (Sharma et al. 2013; Fermin et al. 2018b). Thus, increased liveweight may advance embryo development. However, given the large variation in conceptus length on Day 14, with large variation both within and between years, large separation in liveweight, such as would be observed when using separate breeds of sheep, may be necessary to observe effects on Day 14.
Quite surprisingly, we observed major differences in average conceptus length on Day 14 between the two study years. The underlying factors causing this difference are unknown because the animals were of similar genetics, being sourced from the same flocks with some of the rams used in both years. As discussed previously, differences in progesterone or liveweight may be related to differences in conceptus length but could not explain the differences because the year in which the conceptuses were longer, the ewes were lower in liveweight, and progesterone concentrations were not different between years. Interestingly, the relationship between embryo developmental stage and liveweight of the ewe on Day 3 of pregnancy was not observed in the second year, when conceptuses were much longer by Day 14. It seems likely that some other unidentified environmental factor was affecting embryo development/growth differently between the two study years. We have previously observed unexplained environmental factors not related to overall nutrition (as measured by liveweight gain of the ewe lamb) that affect reproductive potential of ewe lambs. In the previous study, the percentage of ewes attaining puberty varied greatly from year to year, with the year-to-year variation unable to be explained by differences in average liveweight of the ewe lambs (Edwards et al. 2015).
Conclusions
Fertilisation/cleavage/embryo failure prior to Day 3 of pregnancy accounted for ~55% of the reproductive loss observed during pregnancy in ewe lambs. Additional loss occurred between Days 3 and 14 of pregnancy (accounting for ~28% of the loss during pregnancy) with the remainder occurring after Day 35. The pattern of loss during the first 2 weeks of pregnancy in the ewe lamb appeared to differ from that reported for the adult ewe (O’Connell et al. 2016), with more loss occurring prior to genome activation in the ewe lamb. This highlights the critical importance of research to understand and improve the quality of the oocyte and oviduct to increase reproductive rates in ewe lambs.
Data availability
Data used in the manuscript are available from the corresponding author upon reasonable request.
Conflicts of interest
The authors declare they have no known conflicts of interest.
Declaration of funding
This work was supported by AgResearch’s Strategic Science Investment Fund from NZ Ministry of Business, Innovation and Employment. Approval for publication was granted by AgResearch.
Acknowledgements
The authors acknowledge the contribution of Jo Stanton and Karen Reader at the University of Otago, Dunedin, New Zealand, for discussions regarding embryo loss in ewe lambs. The authors thank members of the Reproduction Team and farm staff at Invermay for help with data collection and animal management.
References
Balakier H, Sojecki A, Motamedi G, Bashar S, Mandel R, Librach C (2012) Is the zona pellucida thickness of human embryos influenced by women’s age and hormonal levels? Fertility and Sterility 98, 77–83.| Is the zona pellucida thickness of human embryos influenced by women’s age and hormonal levels?Crossref | GoogleScholarGoogle Scholar |
Clune T, Lockwood A, Hancock S, Thompson AN, Beetson S, Campbell AJD, Glanville E, Brookes D, Trengove C, O’Handley R, Kearney G, Jacobson C (2022) Abortion and lamb mortality between pregnancy scanning and lamb marking for maiden ewes in Southern Australia. Animals (Basel) 12, 10
| Abortion and lamb mortality between pregnancy scanning and lamb marking for maiden ewes in Southern Australia.Crossref | GoogleScholarGoogle Scholar |
Corner-Thomas RA, Ridler AL, Morris ST, Kenyon PR (2015) Ewe lamb live weight and body condition scores affect reproductive rates in commercial flocks. New Zealand Journal of Agricultural Research 58, 26–34.
| Ewe lamb live weight and body condition scores affect reproductive rates in commercial flocks.Crossref | GoogleScholarGoogle Scholar |
Dixon AB, Knights M, Winkler JL, Marsh DJ, Pate JL, Wilson ME, Dailey RA, Seidel G, Inskeep EK (2007) Patterns of late embryonic and fetal mortality and association with several factors in sheep. Journal of Animal Science 85, 1274–1284.
| Patterns of late embryonic and fetal mortality and association with several factors in sheep.Crossref | GoogleScholarGoogle Scholar |
Edwards SJ, Juengel JL (2017) Limits on hogget lambing: the fertility of the young ewe. New Zealand Journal of Agricultural Research 60, 1–22.
| Limits on hogget lambing: the fertility of the young ewe.Crossref | GoogleScholarGoogle Scholar |
Edwards SJ, Juengel JL, O’Connell AR, Johnstone PD, Farquhar PA, Davis GH (2015) Attainment of puberty by ewes in the first year of life is associated with improved reproductive performance at 2 years of age. Small Ruminant Research 123, 118–123.
| Attainment of puberty by ewes in the first year of life is associated with improved reproductive performance at 2 years of age.Crossref | GoogleScholarGoogle Scholar |
Edwards SJ, Smaill B, O’Connell AR, Johnstone PD, Stevens DR, Quirke LD, Farquhar PA, Juengel JL (2016) Reduced ovulation rate, failure to be mated and fertilization failure/embryo loss are the underlying causes of poor reproductive performance in juvenile ewes. Animal Reproduction Science 167, 125–132.
| Reduced ovulation rate, failure to be mated and fertilization failure/embryo loss are the underlying causes of poor reproductive performance in juvenile ewes.Crossref | GoogleScholarGoogle Scholar |
El Shourbagy SH, Spikings EC, Freitas M, St John JC (2006) Mitochondria directly influence fertilisation outcome in the pig. Reproduction 131, 233–245.
| Mitochondria directly influence fertilisation outcome in the pig.Crossref | GoogleScholarGoogle Scholar |
Farrell LJ, Kenyon PR, Morris ST, Tozer PR (2020) The impact of hogget and mature flock reproductive success on sheep farm productivity. Agriculture 10, 566
| The impact of hogget and mature flock reproductive success on sheep farm productivity.Crossref | GoogleScholarGoogle Scholar |
Fermin LM, Pain SJ, Gedye KR, Morel PCH, Kenyon PR, Blair HT (2018a) Timing of exogenous progesterone administration is critical for embryo development and uterine gene expression in an ovine model of maternal constraint. Reproduction, Fertility and Development 1699–1712.
| Timing of exogenous progesterone administration is critical for embryo development and uterine gene expression in an ovine model of maternal constraint.Crossref | GoogleScholarGoogle Scholar |
Fermin LM, Pain SJ, Morel PCH, Gedye KR, Kenyon PR, Blair HT (2018b) Effect of exogenous progesterone on embryo size and ewe uterine gene expression in an ovine ‘dam size’ model of maternal constraint. Reproduction, Fertility and Development 30, 766–778.
| Effect of exogenous progesterone on embryo size and ewe uterine gene expression in an ovine ‘dam size’ model of maternal constraint.Crossref | GoogleScholarGoogle Scholar |
Ferreira-Silva JC, Freitas Neto LM, Moura MT, Filho FT, Oliveira LRS, Bartolomeu CC, Oliveira MAL (2018) Conceptus loss in Santa Inês ewes carrying twin pregnancies by natural mating or embryo transfer. Theriogenology 115, 94–98.
| Conceptus loss in Santa Inês ewes carrying twin pregnancies by natural mating or embryo transfer.Crossref | GoogleScholarGoogle Scholar |
Gandolfi F, Milanesi E, Pocar P, Luciano AM, Brevini TAL, Acocella F, Lauria A, Armstrong DT (1998) Comparative analysis of calf and cow oocytes during in vitro maturation. Molecular Reproduction and Development 49, 168–175.
| Comparative analysis of calf and cow oocytes during in vitro maturation.Crossref | GoogleScholarGoogle Scholar |
Gaskins CT, Snowder GD, Westman MK, Evans M (2005) Influence of body weight, age, and weight gain on fertility and prolificacy in four breeds of ewe lambs. Journal of Animal Science 83, 1680–1689.
| Influence of body weight, age, and weight gain on fertility and prolificacy in four breeds of ewe lambs.Crossref | GoogleScholarGoogle Scholar |
Hare L, Bryant MJ (1985) Ovulation rate and embryo survival in young ewes mated either at puberty or at the second or third oestrus. Animal Reproduction Science 8, 41–52.
| Ovulation rate and embryo survival in young ewes mated either at puberty or at the second or third oestrus.Crossref | GoogleScholarGoogle Scholar |
Kenyon PR, Thompson AN, Morris ST (2014) Breeding ewe lambs successfully to improve lifetime performance. Small Ruminant Research 118, 2–15.
| Breeding ewe lambs successfully to improve lifetime performance.Crossref | GoogleScholarGoogle Scholar |
Kleemann DO, Walker SK, Seamark RF (1994) Enhanced fetal growth in sheep administered progesterone during the first three days of pregnancy. Journal of Reproduction and Fertility 102, 411–417.
| Enhanced fetal growth in sheep administered progesterone during the first three days of pregnancy.Crossref | GoogleScholarGoogle Scholar |
Mann GE, Fray MD, Lamming GE (2006) Effects of time of progesterone supplementation on embryo development and interferon-τ production in the cow. The Veterinary Journal 171, 500–503.
| Effects of time of progesterone supplementation on embryo development and interferon-τ production in the cow.Crossref | GoogleScholarGoogle Scholar |
Marco-Jiménez F, Naturil-Alfonso C, Jiménez-Trigos E, Lavara R, Vicente JS (2012) Influence of zona pellucida thickness on fertilization, embryo implantation and birth. Animal Reproduction Science 132, 96–100.
| Influence of zona pellucida thickness on fertilization, embryo implantation and birth.Crossref | GoogleScholarGoogle Scholar |
Murakoshi Y, Sueoka K, Takahashi K, Sato S, Sakurai T, Tajima H, Yoshimura Y (2013) Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. Journal of Assisted Reproduction and Genetics 30, 1367–1375.
| Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume.Crossref | GoogleScholarGoogle Scholar |
Nephew KP, McClure KE, Ott TL, Dubois DH, Bazer FW, Pope WF (1991) Relationship between variation in conceptus development and differences in estrous cycle duration in ewes. Biology of Reproduction 44, 536–539.
| Relationship between variation in conceptus development and differences in estrous cycle duration in ewes.Crossref | GoogleScholarGoogle Scholar |
Nephew KP, Cárdenas H, McClure KE, Ott TL, Bazer FW, Pope WF (1994) Effects of administration of human chorionic gonadotropin or progesterone before maternal recognition of pregnancy on blastocyst development and pregnancy in sheep. Journal of Animal Science 72, 453–458.
| Effects of administration of human chorionic gonadotropin or progesterone before maternal recognition of pregnancy on blastocyst development and pregnancy in sheep.Crossref | GoogleScholarGoogle Scholar |
O’Brien JK, Dwarte D, Ryan JP, Maxwell WM, Evans G (1996) Developmental capacity, energy metabolism and ultrastructure of mature oocytes from prepubertal and adult sheep. Reproduction, Fertility and Development 8, 1029–1037.
| Developmental capacity, energy metabolism and ultrastructure of mature oocytes from prepubertal and adult sheep.Crossref | GoogleScholarGoogle Scholar |
O’Brien JK, Dwarte D, Ryan JP, Maxwell WMC, Evans G (2000) Comparison of in vitro maturation, in vitro fertilization, metabolism and ultrastructure of oocytes from prepubertal and adult pigs. Reproduction in Domestic Animals 35, 101–107.
| Comparison of in vitro maturation, in vitro fertilization, metabolism and ultrastructure of oocytes from prepubertal and adult pigs.Crossref | GoogleScholarGoogle Scholar |
O’Connell AR, Hurst PR, Davis GH, McNatty KP, Taylor SL, Juengel JL (2013) An earlier rise in systemic progesterone and increased progesterone in the uterine vein during early pregnancy are associated with enhanced embryonic survival in the ewe. Theriogenology 80, 269–274.
| An earlier rise in systemic progesterone and increased progesterone in the uterine vein during early pregnancy are associated with enhanced embryonic survival in the ewe.Crossref | GoogleScholarGoogle Scholar |
O’Connell AR, Demmers KJ, Smaill B, Reader KL, Juengel JL (2016) Early embryo loss, morphology, and effect of previous immunization against androstenedione in the ewe. Theriogenology 86, 1285–1293.
| Early embryo loss, morphology, and effect of previous immunization against androstenedione in the ewe.Crossref | GoogleScholarGoogle Scholar |
Paganoni BL, Ferguson MB, Fierro S, Jones C, Kearney GA, Kenyon PR, Macleay C, Vinoles C, Thompson AN (2014) Early reproductive losses are a major factor contributing to the poor reproductive performance of Merino ewe lambs mated at 8-10 months of age. Animal Production Science 54, 762–772.
| Early reproductive losses are a major factor contributing to the poor reproductive performance of Merino ewe lambs mated at 8-10 months of age.Crossref | GoogleScholarGoogle Scholar |
Parr RA (1992) Nutrition-progesterone interactions during early pregnancy in sheep. Reproduction, Fertility and Development 4, 297–300.
| Nutrition-progesterone interactions during early pregnancy in sheep.Crossref | GoogleScholarGoogle Scholar |
Quinlivan TD, Martin CA, Taylor WB, Cairney IM (1966) Estimates of pre- and perinatal mortality in the New Zealand Romney Marsh ewe. I. Pre- and perinatal mortality in those ewes that conceived to one service. Journal of Reproduction and Fertility 11, 379–390.
| Estimates of pre- and perinatal mortality in the New Zealand Romney Marsh ewe. I. Pre- and perinatal mortality in those ewes that conceived to one service.Crossref | GoogleScholarGoogle Scholar |
Quirke JF, Hanrahan JP (1977) Comparison of the survival in the uteri of adult ewes of cleaved ova from adult ewes and ewe lambs. Journal of Reproduction and Fertility 51, 487–489.
| Comparison of the survival in the uteri of adult ewes of cleaved ova from adult ewes and ewe lambs.Crossref | GoogleScholarGoogle Scholar |
Quirke JF, Hanrahan JP (1983) Comparison of the survival of fertilized eggs from adult ewes in the uteri of adult ewes and ewe lambs. Journal of Reproduction and Fertility 68, 289–294.
| Comparison of the survival of fertilized eggs from adult ewes in the uteri of adult ewes and ewe lambs.Crossref | GoogleScholarGoogle Scholar |
Reader KL, Cox NR, Stanton J-AL, Juengel JL (2015) Mitochondria and vesicles differ between adult and prepubertal sheep oocytes during IVM. Reproduction, Fertility and Development 27, 513–522.
| Mitochondria and vesicles differ between adult and prepubertal sheep oocytes during IVM.Crossref | GoogleScholarGoogle Scholar |
Rees E, Phillips K (2016) Breeding from ewe lambs. Available at https://projectblue.blob.core.windows.net/media/Default/Research%20Papers/Beef%20&%20Lamb/ewelambblueprint_210710-final-report.pdf [Verified 4 November 2022]
Rosales Nieto CA, Ferguson MB, Macleay CA, Briegel JR, Martin GB, Thompson AN (2013) Selection for superior growth advances the onset of puberty and increases reproductive performance in ewe lambs. Animal 7, 990–997.
| Selection for superior growth advances the onset of puberty and increases reproductive performance in ewe lambs.Crossref | GoogleScholarGoogle Scholar |
Rosales Nieto CA, Thompson AN, Martin GB (2018) A new perspective on managing the onset of puberty and early reproductive performance in ewe lambs: a review. Animal Production Science 58, 1967–1975.
| A new perspective on managing the onset of puberty and early reproductive performance in ewe lambs: a review.Crossref | GoogleScholarGoogle Scholar |
Rowson LE, Moor RM (1966) Development of the sheep conceptus during the first fourteen days. Journal of Anatomy 100, 777–785.
Satterfield MC, Bazer FW, Spencer TE (2006) Progesterone regulation of preimplantation conceptus growth and galectin 15 (LGALS15) in the ovine uterus. Biology of Reproduction 75, 289–296.
| Progesterone regulation of preimplantation conceptus growth and galectin 15 (LGALS15) in the ovine uterus.Crossref | GoogleScholarGoogle Scholar |
Sharma RK, Parkinson TJ, Kenyon PR, Jenkinson CMC, Blair HT (2013) Uterine environment and early embryonic development in sheep. Small Ruminant Research 115, 67–70.
| Uterine environment and early embryonic development in sheep.Crossref | GoogleScholarGoogle Scholar |
Shorten PR, Ledgard AM, Donnison M, Pfeffer PL, McDonald RM, Berg DK (2018a) A mathematical model of the interaction between bovine blastocyst developmental stage and progesterone-stimulated uterine factors on differential embryonic development observed on Day 15 of gestation. Journal of Dairy Science 101, 736–751.
| A mathematical model of the interaction between bovine blastocyst developmental stage and progesterone-stimulated uterine factors on differential embryonic development observed on Day 15 of gestation.Crossref | GoogleScholarGoogle Scholar |
Shorten PR, Donnison M, McDonald RM, Meier S, Ledgard AM, Berg D (2018b) A mathematical model of in vivo bovine blastocyst developmental to gestational Day 15. Journal of Dairy Science 101, 8401–8416.
| A mathematical model of in vivo bovine blastocyst developmental to gestational Day 15.Crossref | GoogleScholarGoogle Scholar |
Smith P, Stanton J-AL, Quirke L, Juengel JL (2019) Gestational nutrition 1: alterations to gestational nutrition can increase indicators of fertility in sheep. Reproduction 157, 199–213.
| Gestational nutrition 1: alterations to gestational nutrition can increase indicators of fertility in sheep.Crossref | GoogleScholarGoogle Scholar |
VSN International (2014) ‘GenStat for windows.’ 17th edn. (VSN International: Hemel Hempstead, UK) Available at GenStat.co.uk
Whyman D, Johnson DL, Knight TW, Moore RW (1979) Intervals between multiple ovulations in PMSG-treated and untreated ewes and the relationship between ovulation and oestrus. Journal of Reproduction and Fertility 55, 481–488.
| Intervals between multiple ovulations in PMSG-treated and untreated ewes and the relationship between ovulation and oestrus.Crossref | GoogleScholarGoogle Scholar |