Effects of germination on the energy value of cereal grains for livestock
J. L. Black A B * , A. M. Tredrea C , S. H. Bird D , R. J. Hughes E and S. G. Nielsen F
A B * , A. M. Tredrea C , S. H. Bird D , R. J. Hughes E and S. G. Nielsen F
A John L Black Consulting, Warrimoo, NSW 2774, Australia.
B Centre for Rock Art Research + Management, University of Western Australia, Perth, WA 6009, Australia.
C The University of Sydney, IA Watson Plant Breeding Institute, Narrabri, NSW 2390, Australia.
D Previously with Department of Animal Science, University of New England, Armidale, NSW 2351, Australia.
E Previously with Livestock Sciences, South Australian Research and Development Institute, Roseworthy, SA 5371, Australia.
F Sharon Nielsen Statistical Consulting and Training, Kooringal, NSW 2650, Australia.
Animal Production Science 63(3) 256-268 https://doi.org/10.1071/AN22183
Submitted: 6 May 2022 Accepted: 13 September 2022 Published: 28 October 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Unusually wet weather in eastern Australia during the 2021 spring resulted in prolonged preharvest germination of a large proportion of cereal crops. An estimated 40–45% of wheat grown in New South Wales was downgraded from human consumption to feed-wheat. Similarly, preharvest germination of barley renders it unsuitable for malting or brewing.
Aims: To investigate the impact of wheat grown in 2021 and of various germination times on the energy value of cereal grain species for different livestock types.
Methods: Twenty-three samples of wheat harvested in 2021 were evaluated. Three experiments were also conducted with increasing germination times, as follows: (1) grain from wheat, barley and sorghum cultivars were germinated for 0–100 h; (2) sorghum grain was steeped in water and germinated for 5 or 10 days, with different periods of ensiling to simulate grain ‘reconstitution’ as practiced in cattle feedlots; (3) barley, wheat, sorghum and triticale grain was germinated for 0, 20 or 48 h and fed to meat chickens. Experiments 1 and 2 were conducted in vitro, with starch disappearance, starch digestion by animal-derived enzymes and starch fermentation by rumen microbes measured.
Key results: Short-term germination did not affect grain starch content within a cultivar, increased animal enzyme digestion of starch for barley, but not for wheat or sorghum. Longer germination of sorghum grain for 5–10 days substantially increased starch fermentability, which was further enhanced by anaerobic ensiling. Germination significantly increased the available-energy content of barley grain, but not wheat, sorghum or triticale for meat chickens.
Conclusions: The energy content of preharvest germinated grains for animals was not decreased, but increased for barley fed to chickens and for sorghum fed to ruminants after 5 days of germination.
Implications: Feeding preharvest germinated grains to livestock does not reduce energy availability, but may lead to fungal growth and mycotoxin formation.
Keywords: barley, energy availability, meat chickens, mycotoxin, preharvest sprouting, ruminants, sorghum, wheat.
Introduction
The 2021 spring and early summer in the cereal grain-growing areas of eastern Australia was unusually wet. Large areas of cereal grains were subjected to days of rainfall, low temperatures and high humidity shortly before harvest, which resulted in germination of the standing crops. The majority of wheat grown in Australia is used for making bread and similar products. Preharvest sprouting releases grain amylases, which hydrolyse the long-chain starch molecules, reduce gelling capacity of the starch and result in poor-quality bread (Newberry et al. 2018). Similarly, preharvest germination renders barley grain unsuitable for malting (Brookes 1980). The down-graded grain is frequently bought by livestock producers, but at a reduced price of ∼AUD20–50 per Mg compared with the price of grain used for human consumption (Newberry et al. 2018). An estimated 40–45% of wheat grown in New South Wales (NSW) in the 2021–22 season was downgraded to feed-wheat (Grain Central 2022). With such a large quantity of germinated grain available in Australia in this season, it is important to know the effect of preharvest sprouting on the nutritional quality of the grain for animals.
Commonly, preharvest germination of grains caused by increased amylase activity occurs after a brief event of rain and high humidity for ∼3 days and is rapidly ceased with hot, dry weather. Under many circumstances, only a small proportion of the grain starch is hydrolysed with the increased amylase activity. However, in the 2021–22 season, and particularly in areas of north-eastern NSW, the wet, cold and humid weather remained for weeks. Germination conditions and, hence, the amylase activity continued for an extended period, with a large proportion of the grain in cereal crops experiencing preharvest sprouting. Cereal grains are fed to livestock primarily as a source of energy because of their high starch content. Consequently, understanding the impact of various periods of germination on the energy value of various cereal grain species for different livestock types would help in assessing the value of available grains for the livestock industries. Samples of wheat grain were collected at harvest from the University of Sydney, Plant Breeding Institute at Narrabri in northern NSW and examined for germination and fungal development. Results from several experiments from the Premium Grains for Livestock Program (Black 2008) are presented to help clarify the usefulness of preharvest sprouted gains for livestock.
Materials and methods
Grain collected in 2021
Twenty-three samples of wheat heads were collected from the University of Sydney, IA Watson Plant Breeding Institute, Narrabri, New South Wales, during the 2021 harvest season. Harvesting was disrupted due to the weather being wet, cold and humid relative to normal seasons and the delay was exacerbated because the soil was frequently too wet for the harvest machinery to operate. Ripe grain remained unharvested for many days longer than normal under conditions ideal for fungal growth.
Records of rainfall and daily temperatures form Narrabri Airport for the months from September to December 2021 were compared with long-term averages. Relative humidity values from the Plant Breeding Institute from September to December 2021 were compared with average values from 2015 to 2019. Narrabri Airport is 4.7 km from the Plant Breeding Institute.
The collected grain heads were assessed for the extent of germination by using the Hagberg falling-number test (Hagberg 1960). Relative density of the grains, commonly termed ‘test weight’ (kg/hL), was determined for each sample. Starch content and the available-energy content (MJ/kg) of the grains for different livestock species was predicted using a near-infrared (NIR) system (van Barneveld et al. 2018).
Individual grains were photographed to illustrate the extent of germination and the development of red and black fungal growth. Selected samples of grain showing substantial discolouration through fungal growth were used to identify fungal species. Two 100-grain subsamples from each seed lot were surface-sterilised in 0.2% w/v sodium hypochlorite in 45% v/v ethanol for 1 min, then washed in sterile distilled water, and dried. Individual grains were placed onto 0.25 strength potato dextrose agar supplemented with 100 mg/L novobiocin to determine the incidence of Fusarium pseudograminearum (Fp; formerly F. graminearum Group 1), F. graminearum (Fg; formerly F. graminearum Group 2), Bipolaris sorokiniana (Bs) and Eutiarosporella spp. (Eut) infections. Cultures were incubated at 25°C under a combination of white and near-ultraviolet lights with a 12 h photoperiod for 7 days, before assessing the number of colonies of Fp/Fg, Bs and Eut that emerged from inside infected grains.
Effect of sprouted grain on energy availability and animal performance
Results from the Premium Grains for Livestock Program (PGLP) were used to assess the impact of grain germination times on the availability of energy to animals and on their performance. PGLP was established in 1996 and jointly funded by the Australian grains and animal industries. The project arose because of the rapidly increasing demand for grain by the intensive livestock industries in Australia and concern about a reliable supply of grain meeting quality specifications for their industries. Much of the ‘feed-grain’ available was of insufficient quality for animal industries to meet production specifications and deadlines. Grain growers had traditionally seen ‘feed-grains’ as down-graded grains unsuitable for human consumption and were not encouraged to produce quality grains for livestock because of the perceived lower price received. Consequently, the grains and animal industries recognised the opportunity to develop a research program to understand and measure grain characteristics determining their nutritional value for various animal types (Black 2008). As part of PGLP, in vitro and in vivo experiments were undertaken to determine the value of germinated grains for animals.
Germination experiments
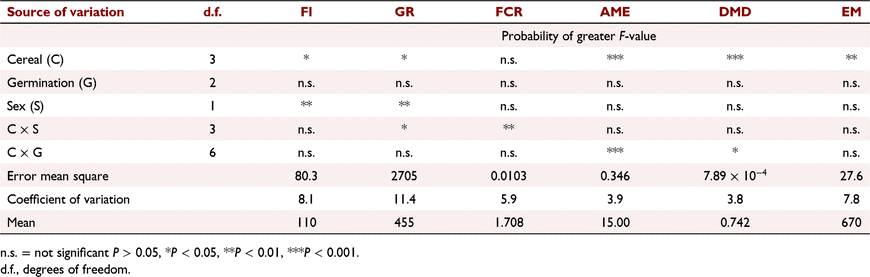
Three experiments were conducted in PGLP to assess the impact of germination on the energy value of cereal grains, with one experiment examining the impact on meat chicken productivity.
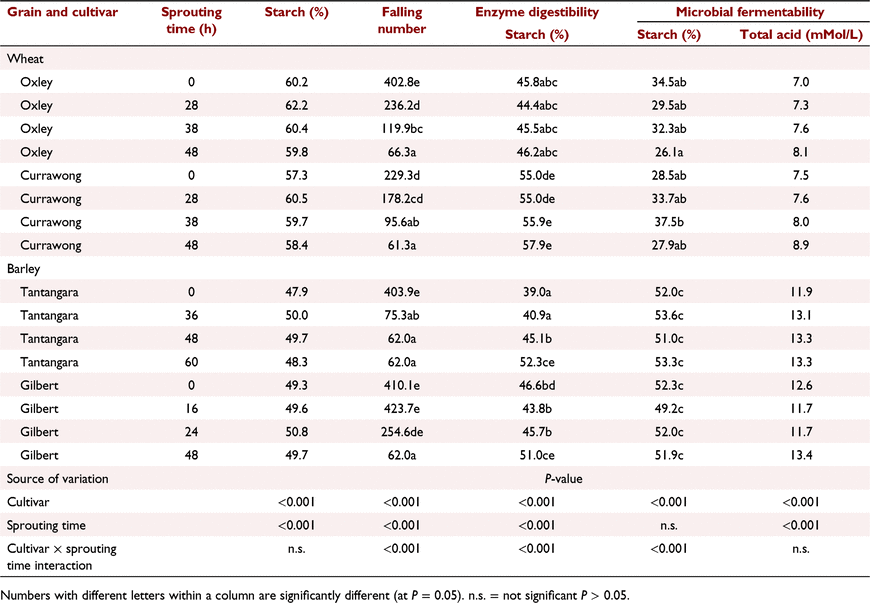
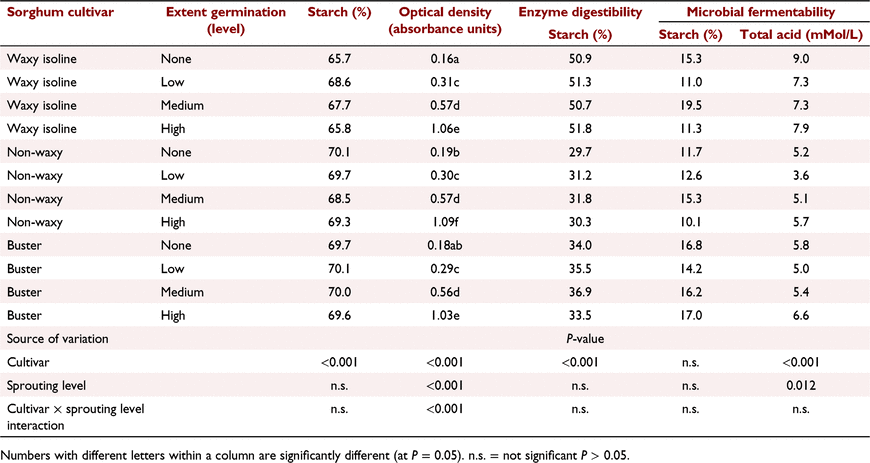
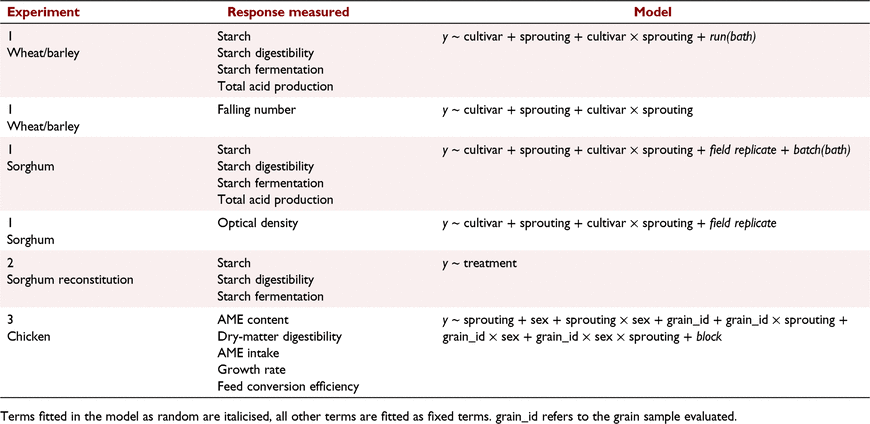
The first experiment simulated natural sprouting. The effects of germination time for two cultivars of wheat (Oxley and Currawong), two cultivars of barley (Tantangara and Gilbert) and three cultivars of sorghum (Buster, waxy and non-waxy isoline cultivars from plant breeder experimental lines numbered 7631 and 7632 respectively, within PGLP). The grains were grown at the University of Sydney, Plant Breeding Institute in Narrabri, NSW, Australia. Numerous grain heads from each cultivar were harvested when mature. The grain heads from a single cultivar were spaced vertically apart in perforated trays and placed on a carousel, within a rain-simulator cabinet. The carousel rotated the trays of heads, which were uniformly sprayed with water for 15 min every 2 h. High humidity within the cabinet was greater than 90% and temperature was maintained at 22°C to promote optimal sprouting conditions. Germination within the grain heads was ceased by placing the germinating sample in an aerated dehydrator at 40°C until the moisture content was approximately 12%. Three replicates of each grain cultivar for wheat and barley were either not germinated or germinated for three time periods, which varied among cultivars, as indicated in Table 1. The germination times were selected to provide differences in estimated α-amylase activity for wheat and barley by using the Hagberg falling-number test (Hagberg 1960). The α-amylase activity of each sorghum sample was estimated using the 620 nm absorbance colorimetric, optical density method of Barnes and Blakeney (1974). Following preliminary assessments of the impact of germination time of sorghum on optical density, three replicates of the cultivars Buster and non-waxy were germinated for 0 h, 40 h, 50 h, 60 h or 92 h, and cultivar waxy for 0 h, 50 h, 75 h, 95 h or 100 h. Within each sorghum cultivar, three individual replicates with varying germination times but similar optical density measurements were chosen for low, medium and high germination levels shown in Table 2. Measurements on each sample included (1) total starch, (2) the Hagberg falling-number test for wheat and barley and the optical density test for sorghum, (3) in vitro starch digestibility simulating animal digestive enzymes, and (4) in vitro starch fermentability and total acid formation simulating rumen microbial fermentation.
In the second experiment, sorghum was processed to simulate the germination and reconstitution process practiced in some Australian cattle feedlots. The process involved two steps. Sorghum grain was soaked in water to allow its moisture content to increase to approximately 30%. The moist grain was then placed under anaerobic storage in a silo for 2–3 weeks. There were six grain treatments, as follows:
-
Unprocessed: grain dry-rolled, but otherwise untreated;
-
Steeped: grain soaked in water until moisture content reached 30% and the grain then rolled;
-
Steeped and anaerobically ensiled for 21 days: wet grain (30%) was stored anaerobically for 21 days and then rolled;
-
Germinated 5 days: wet grain (30%) was allowed to germinate in air for 5 days and then rolled;
-
Germinated 10 days: wet grain (30%) was allowed to germinate in air for 10 days and then rolled;
-
Germinated 5 days and ensiled 16 days: wet grain (30%) was allowed to germinate in air for 5 days, stored anaerobically for 16 days and then rolled.
Measurements on each rolled sample included (1) total starch, (2) in vitro starch digestibility simulating animal digestive enzymes, and (3) in vitro starch fermentability simulating rumen microbial fermentation.
In the third experiment, previously presented as a preliminary conference report by Hughes and van Barneveld (2004), sufficient wheat, barley, sorghum and triticale for a meat chicken experiment was germinated for either 20 h or 48 h. Grain samples were germinated by placing batches in separate containers, covering with water at 20°C for 4 h, then draining the water and maintaining the damp grain at 20°C and 80% relative humidity for the specified times before being dried in an aerated dehydrator at 40°C. The germinated and unprocessed grains were included in test diets for meat chickens to measure grain apparent metabolisable energy (AME) and dry-matter digestibility, voluntary intake, growth rate and feed conversion efficiency, as described by Hughes (2008). Day-old chickens were feather-sexed at hatch and reared in floor pens in single-sex groups for 20 days by using commercial diets, then transferred in single-sex pairs to 72 metabolism cages in a controlled-temperature room to allow chickens to adapt to the cages. At 22 days of age, one bird was removed from each cage. Air temperature was maintained at 26°C at the start of the 7-day experiment and lowered daily until it was 23°C at the end. Birds were randomly allocated to test diets on Day 22 and given a 3-day period to adapt to diets. Each kilogram of pelleted test diet contained 800 g of the selected treatment grain, 155 g casein, 20 g dicalcium phosphate, 11 g limestone, 7 g dl-methionine, 2 g vitamin mix, 3 g sodium chloride and 2 g choline chloride (60%). Feed intake, liveweight gain and feed conversion were measured over 7 days, and excreta were collected for 4 days following the adaptation period. Gross-energy content of feed and excreta was measured by bomb calorimetry.
The experiment was approved by the Animal Ethics Committees of the University of Adelaide (W/32/01) and the Department of Primary Industries and Regions, South Australia (29/01).
Grain analyses
Falling-number starch-viscosity test
The Hagberg falling-number method (Hagberg 1960) provided an indirect measure of α-amylase activity in cereal grains. Whole grain samples of wheat and barley were ground in a laboratory mill fitted with a 0.5 mm screen, placed in a tube with distilled water and shaken to thoroughly homogenise. The tube was placed in a boiling water bath within a Perten Instruments falling-number machine. The falling number is defined as ‘total time required to activate a viscometer stirrer and allow it to fall a predetermined distance through an aqueous gel prepared from heating a mixture of flour or semolina, and water in a viscometer tube, and which is undergoing liquefaction due to attack by the enzyme α-amylase’ (American Association of Cereal Chemists International (AACC) 2010, International Method 56-81.03).
Optical density
The optical density method developed by Barnes and Blakeney (1974) was more suitable for estimating the α-amylase activity in sorghum grain than was the falling-number test. The sorghum grain samples were ground in a laboratory mill with a 0.5 mm screen and placed in a tube with a dye, as described by Barnes and Blakeney (1974). The dye cross-links with amylose to produce a complex that hydrates in water, but is insoluble in water. Hydrolysis of the starch by α-amylase during germination produces more amylose molecules and increases the intensity of the dyed-coloured fragments. Optical density of the solution was measured at an absorbance of 620 nm by using a spectrophotometer.
Test weight
Bulk density of grain samples in kg/hL was determined by the International Organisation of Standards method ISO 7971-1:2009 for cereal grains.
Total starch
Grain starch content was measured using the amyloglucosidase-α-amylase method of McCleary et al. (1997) following grinding of the whole grain sample in a laboratory mill with a 0.5 mm screen.
In vitro starch digestibility
In vitro starch digestibility simulating animal digestive enzymes was determined using the method of Bird et al. (1999). A sample of 0.1 g of the whole grain was milled with a 0.5 mm screen and incubated in a culture tube with amyloglucosidase and α-amylase enzymes and a buffer at 30°C for 60 min. The amount of starch digested was determined by measuring the difference between starch content in the original grain and starch remaining in the tube after 1 h incubation. Starch digestion was incomplete after 1 h, so the results represent a rate of digestion expressed as the percentage of initial starch that disappeared in 1 h.
In vitro starch digestibility and total acid formation simulating rumen microbial fermentation
A 10–30 g sample of grain milled through a 0.5 mm screen was placed in a flask containing rumen fluid and buffer, and incubated at 39°C for 5 h (Bird et al. 1999). Rumen fluid was collected from rumen-fistulated steers that were fed a diet containing 50% wheat grain, cereal straw and minerals. The amount of starch digested, total acid production and the production of acetic, propionic, butyric and lactic acids were measured at the end of the incubation. Acids within the incubated solution were measured using the method described by Erwin et al. (1961). The amount of starch fermented was determined by measuring the difference between starch content in the original grain and starch remaining in the flask after 5 h incubation. Acid production was estimated from the difference between the initial and final acid concentrations in the incubation flask. Starch fermentation was incomplete after 5 h, so the results represent a rate of rumen fermentation expressed as the percentage of initial starch that disappeared in 5 h.
NIR analyses
The NIR system described by van Barneveld et al. (2018) was used to predict the starch and available energy content (MJ/kg) of 2021-collected grains for meat chickens and pigs.
Statistical analyses
Standard regression analyses were used for analyses of the 2021 grain collection. The statistical models used for the PGLP observations to determine the significance of treatments on the experimental responses measured are given in Table 3. Experiment 1 was analysed statistically for wheat and barley cultivars together and separately for sorghum cultivars because of large differences between the groups in measured variables.
|
Results
Grains collected in 2021
Weather conditions
Records from Narrabri airport show that rainfall over the grain harvest period in 2021 was approximately 60% greater in September, 20% greater in October and 250% greater in November than the long-term average (Fig. 1). The period was considerably cooler than the long-term average for the months from September to December, with the maximum temperature for November being 3.5°C lower than average (Fig. 1). Similarly, the period was more humid than normal, particularly the minimum values in the middle of the day. The average minimum humidity at the Plant Breeding Institute over the years 2015–20 for the months from September to December was 13.6%, but during 2021 it was up to 10% higher (Fig. 1). The maximum humidity was also 1.3–2.7% higher than the average of 94.7% over the same months from 2015 to 2022.
Germination, test weight and NIR predictions
In total, 17 of the 23 opportunistically collected grains from 2021 had falling-number values of 62 or 63, indicating high levels of amylase activity and major changes to starch molecules associated with germination. Four samples had falling-number values between 100 and 200, indicating that a substantial number of individual grains were germinating and two samples had values between 200 and 300, suggesting that only a few grains had germinated. Many grains showed clear signs of sprouting with cotyledon formation.
Grain sample test weight ranged from 72.6 to 80.4 kg/hL. The lower test weight values were associated with low falling-number values, but the correlation between test weight and falling-number value was weak, with an R2 of 0.36. There was no relationship between test weight and NIR-predicted grain starch content (R2 = 0.007), but a weak negative relationship between falling-number and starch content (R2 = 0.36). There was no relationship between either falling-number (R2 = 0.003) or test weight (R2 = 0.03) and NIR-predicted AME for meat chickens. There was a weak positive relationship between test weight and NIR-predicted pig digestible energy content (DE), with an increase from 13.50 to 13.85 MJ/kg as test weight increased from 72.6 to 80.4 kg/hL. Similarly, there was a positive predicted relationship between falling-number and pig DE, with an increase from 13.60 to 14.06 MJ/kg as falling-number increased from 62 to 389.
Fungal growth
An examination of the 2021-collected grain samples showed ∼1% to be dark or reddish in colour (Fig. 2), which indicates fungal growth. Samples of isolated reddish- and black- coloured grains are illustrated in Fig. 3, with several grains showing clear signs of sprouting and cotyledon emergence. The fungus Fusarium pseudograminearum was recovered from all 10 externally sterilised reddish grains placed on potato agar, whereas Eutiarosporella spp., known as white grain disorder, was recovered from all 10 dark-coloured grains placed on the agar (Fig. 4).
|
|
|
|
Effect of sprouted grain on energy availability and animal performance – PGLP results
Germination of barley and wheat cultivars
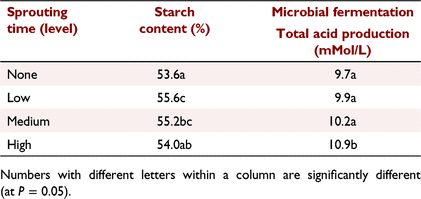
Although there were significant differences between wheat and barley cultivars for all variables measured in Experiment 1 from PGLP, the interaction between cultivar and germination time was significant only for falling-number values, starch digestibility and starch fermentability (Table 1). Increasing germination time significantly (P < 0.001) reduced the falling-number values for all cultivars. The disappearance of starch using in vitro animal enzyme digestion increased significantly (P < 0.05) with germination time for both cultivars of barley, but not for the wheat cultivars. Germination did not significantly affect the microbial fermentation of starch for any cultivars. However, when results across all cultivars were combined, there was a significant (P < 0.05) increase in total acid produced for the longest germination time (Table 4), suggesting a small increase in microbial fermentability with germination time. There was also a significant increase in measured grain starch content across cultivars following low and medium germination times, but not for the longest germination time (Table 4).
|
Germination of sorghum cultivars
Germination level, measured by optical density as an indicator of α-amylase activity, showed significant increases for all cultivars (Table 2). However, germination level did not significantly affect grain starch content, starch animal enzyme digestibility or starch fermentability. Germination level had a minor effect on total acid production following fermentation (P = 0.012), but the effect was not consistent across germination level, being 6.7, 5.3, 5.9 and 6.7 respectively, for germination levels none, low, medium and high. These results indicated that germination had minimal impacts on starch content, starch animal enzyme digestibility or starch fermentability by microbial enzymes. However, there were significant differences among sorghum cultivars, with starch content being lower and starch animal enzyme digestibility higher for the waxy cultivar.
Germination and reconstitution of sorghum
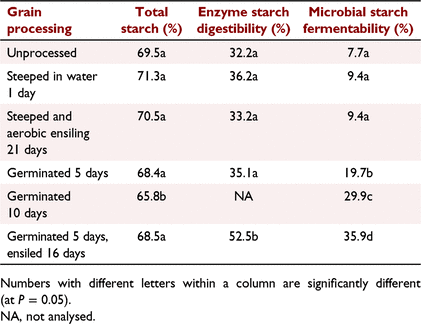
The relative effects of steeping in water followed by germination, ensiling or both germination and ensiling of sorghum grain on grain starch characteristics are shown in Table 5. Steeping sorghum grain in water until it reached 30% moisture or steeping and ensiling for 21 days did not significantly affect grain starch content, in vitro animal enzyme digestion or in vitro microbial fermentability of sorghum starch. Germinating sorghum grain for 5 days did not significantly change in vitro animal enzyme digestibility of sorghum starch, but significantly (P < 0.05) increased microbial fermentability of the starch. Germination of sorghum for 10 days significantly (P < 0.05) reduced total starch in the grain and greatly increased microbial fermentability of the starch (P < 0.05). Although a 5-day germination period followed by a 16-day ensiling period did not change total starch content of the sorghum, the treatment showed the greatest change in both in vitro animal enzyme digestion and in vitro fermentation of sorghum starch.
|
Meat chicken experiment
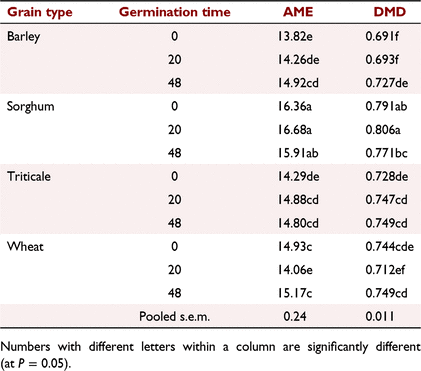
The results previously presented at a conference by Hughes and van Barneveld (2004) are given in Tables 6, 7, 8. Germination significantly (P < 0.05) increased the AME content of the barley sample by over 1 MJ/kg DM. Germination did not alter the AME content for either the sorghum or triticale sample. Germination of wheat for 20 h significantly reduced the AME content of wheat, but the energy value of wheat was recovered with 48 h germination (Table 6).
|
|
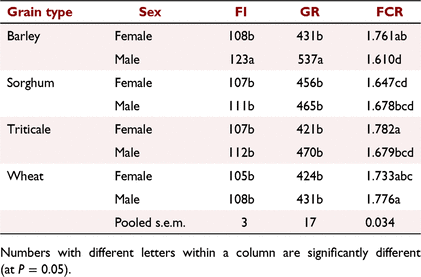
The effects of germination on feed intake, growth rate and feed conversion ratio (Table 7) confirmed that there was a positive impact for the sample of barley, but not for sorghum, triticale or wheat. The results showed a reduced intake, growth rate and poorer feed efficiency for wheat germinated for 20 h than for either ungerminated wheat or wheat germinated for 48 h. These results on the effects of germination on the energy value of cereal grains for meat chickens again indicated that there are likely to be different characteristics within the grain samples examined that result in an inconsistent response. Male birds tended to have higher feed intakes, growth rates and efficiency of feed use than did female birds (Table 8), as found in earlier experiments (Hughes 2003).
Discussion
The normal grain harvest period towards the end of the 2021 in north-eastern NSW was considerably wetter, colder and more humid than normal, which delayed harvesting and left grains within the inflorescences exposed to ideal conditions for germination and fungal development. Twenty-three opportunistically collected wheat samples from the University of Sydney Plant Breeding Institute all showed advanced germination as determined by falling-number values. Many grains were also discoloured by fungal growth. The fungi had penetrated into the endosperm of the grains because the fungi grew on agar plates despite being sterilised on the exterior. The widespread unusually wet and cold weather resulted in ∼45% of wheat grain harvested being downgraded to feed-wheat (Grain Central 2022). The large proportion of partially germinated grain raised concern about whether there were differences in the nutritional value of grains for livestock depending on the length of germination time, and whether the effects were similar for different grain species and animal types. Results obtained from the research conducted in PGLP allowed answers to some of these concerns. The predominance of fungal growth also raised queries about the possible impact of mycotoxins on animal health and performance when the grains were fed to livestock.
Reduced falling-number values for wheat and barley cultivars collected in 2021 and from the PGLP experiments confirmed that these grains would be unsuitable for bread-making (Newberry et al. 2018) or brewing (Brookes 1980). Short-term germination did not affect the starch content of cultivars of sorghum, but low and medium levels of germination apparently increased starch content for wheat and barley. This observation is difficult to explain and may be an artefact of the colorimetric method for measuring starch content. Optical density values for sorghum increased significantly with an increasing germination time, indicating an increase in α-amylase activity with longer germination times.
Germination times of 24–60 h significantly increased in vitro animal enzyme digestibility of starch for both cultivars of barley, but not that of starch from the wheat cultivars. The improvement in in vitro animal enzyme digestibility for barley from short-term germination was confirmed in the meat chicken experiment by the significant increase in AME and DMD for birds offered barley diets, but not for wheat, triticale or sorghum diets. Similarly, early research reported by Derera (2018) suggested that preharvest sprouting reduces the yield of barley but increases its digestibility in pigs. Although the changes from short-term germination of wheat in the in vitro animal enzyme starch digestion were not significant for the cultivars examined in this paper, previous research by Johnson and Taverner (1986) showed that feeding sprouted wheat improved the AME content of the grain for meat chickens and growth rate in young pigs. Lardy (2017) also showed that feeding 20% sprouted wheat to finisher pigs significantly increased growth rate.
Results showing that the in vitro fermentability of starch from the finely ground barley cultivars was significantly greater than that from the wheat cultivars confirmed earlier observations by Bird et al. (1999), where the in vitro starch fermentation rate of 20 Australian barley cultivars was 67% compared with 48% for seven wheat cultivars. However, this difference in in vitro starch fermentability between barley and wheat does not appear to be translated to differences in performance of feedlot cattle when fed as rolled grains. Lardy (2017) reported no difference in liveweight gain, feed intake or efficiency of feed use when wheat and barley were compared in traditional feedlot diets.
The NIR predictions suggested that there was no relationship between the falling-number value and AME content for meat chickens of wheat harvested during the 2021 season at the Narrabri Plant Breeding Institute. However, the NIR system predicted a decrease of 0.4 MJ/kg in the DE of the 2021-harvested grains for pigs as falling number declined from 389 to 62. The accuracy of the NIR calibrations for predicting the energy content of grain samples containing substantial proportions of fungal-affected red and black grains is unknown, but may be less accurate.
Short-term germination did not affect in vitro animal enzyme digestibility or fermentability of starch in the sorghum cultivars. This in vitro result is supported by the chicken experiment where germination of sorghum for 20 h or 48 h did not alter its AME content of the grain or bird performance. Similarly, earlier experiments with preharvest sprouted sorghum suggested that germination did not affect the growth rate or feed conversion efficiency of meat chickens or turkey poults (Rowland et al. 1978).
Although 48 h germination of wheat and barley cultivars increased the concentration of total acids in the in vitro fermentation assays, there appears to be no effect of preharvest sprouting of wheat or barley on the performance of feedlot cattle (Lardy 2017). Short-term germination of sorghum did not affect total acid production during the in vitro fermentation assay. However, the length of the germination period had a major impact on the fermentability of sorghum grain. Results from the germination and ensiling experiment showed that in vitro fermentation of sorghum starch approximately doubled after 5 days of germination and quadrupled after 10 days of germination (Table 5). Five days of germination followed by 16 days of ensiling further increased in vitro fermentation of sorghum starch by 4.7-fold compared with the unprocessed grain. This result supports the practice used by some feedlots in Australia.
Germination was identified as a key factor in the value of reconstitution of sorghum. The improved fermentability of reconstituted sorghum is thought to be partially due to the initiation of germination that occurs during the reconstitution process (Sullins et al. 1971). Lardy (2017) reported results from an experiment at Kansas State University, where growth rate of feedlot cattle was significantly improved by feeding heavily sprouted sorghum grain compared with unsprouted grain. However, the improved fermentability following 10 days of germination was associated with significantly reduced starch content of the grain (Table 5), which supports the lower test weights (kg/hL) reported for commercial germinated sorghum products (Fromme 2016).
The rate of digestion of starch in cereal grains is determined predominantly by the rate of diffusion of amylase into the grain (Ratanpaul et al. 2018). Amylase diffusion rate is affected by the thickness and integrity of the endosperm cell walls, the extent the starch granules are embedded within high-sulfur-containing kafirin prolamins in sorghum, the tightness the starch granules are packed together, the ratio of open structured amylopectin to more tightly formed amylose starch molecules in waxy grains, and grain processing such as particle size or application of moist heat (Black 2016). Although highly dependent on cultivar and growing conditions, amylase diffusion rates for normally grown cultivars were fastest for wheat, being approximately 20% faster than for barley and 100% faster than for normal sorghum (Ratanpaul et al. 2018). Amylase diffusion rate for waxy sorghum was 50% faster than for the non-waxy isoline. Endosperm cell walls are particularly thick in barley grains, thin in sorghum and of intermediate thickness for wheat and triticale (Black 2016). The slower amylase diffusion rates for normal sorghum than for wheat or barley are due to the high concentrations of low-solubility kafirin prolamins and tightly packed endosperm cells. Wu and Wall (1980) reported that sorghum prolamins, which protect the starch granules within the grain from enzyme attack, decline from 48% to 16% of total grain protein over 10 days following germination. Endosperm cell walls are rapidly degraded during germination (Andriotis et al. 2016), which increases amylase diffusion rate (Black 2016) and may explain why there was a greater response following short-term germination in starch digestibility and fermentability and for chicken performance for barley than for wheat, sorghum or triticale.
The PGLP research suggested, with confirmation from published sources, that the energy content of preharvest sprouted grains for animals was not decreased and, in some circumstances, was increased when compared with non-sprouted grain. The effects of germination were particularly favourable for a barley sample fed to meat chickens and sorghum fed to cattle after a germination period of at least 5 days.
The wheat samples collected in 2021 had a longer period of germination, with grains showing signs of cotyledon development, due to prolonged wet and cool weather, than the wheat samples used in the PGLP experiments. Despite the longer germination time, the NIR-predicted starch content or available energy for meat chickens or cattle was not affected by falling-number value. However, the NIR system predicted a small fall in the DE content of grain for pigs as falling number decreased. This predicted result for pigs would need confirmation with in vivo pig experiments. Overall, there is little evidence that the nutritional value of the 2021 grains was adversely affected by the preharvest sprouting.
Field-sprouted cereal grains are particularly susceptible to fungal growth during storage, with an associated mycotoxins development and negative impacts when fed to animals (Kochiieru et al. 2021). Fusarium pseudograminearum and Eutiarosporella species were identified in the 2021-harvested grains. The major Fusarium mycotoxins, deoxynivalenol, fumonisins, zearalenone, and T-2 toxin, have particularly negative effects on the health and performance of livestock (Holanda and Kim 2021). These Fusarium mycotoxins reduce feed intake of animals, can cause nausea, reduce feed digestibility, alter intestinal and pancreatic morphology and nutrient utilisation (Xu et al. 2022), as demonstrated in Australia by Blaney and Williams (1991). However, Eutiarosporella species, reclassified from Botryosphaeria zeae, do not appear to have an adverse effect when fed to animals (Kopinski and Blaney 2010).
Conclusions
There was no detrimental effect of sprouting per se on the energy value of grains for animals when germination is ceased within 48–60 h through drying under normal summer conditions. However, prolonged germination under continuing moist and low-temperature conditions may have a negative impact on animal performance because of loss of starch in the grain and increased susceptibility to mycotoxin development.
Data availability
Data from the 2021-collected wheat samples are available upon request to the Australasian Pork Research Institute Limited via the first Author. Data from PGLP Experiments 1 and 3 are also available from the first author. Data for Experiment 2 were collected and analysed at the University of New England in 2004 and all original data may not still be available.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Funds for analysis of the 2021-collected wheat samples were provided by Australian Pork Research Institute Limited. Funds for the research were through the Premium Grains for Livestock Program with contributions from the Grains R&D Corporation, Meat & Livestock Australia, Australian Pork Limited and Rural Industries R&D Corporation – Chicken Meat and Eggs.
Acknowledgements
The authors thank the Australian Pork Research Institute Limited for providing funds for analysis of the 2021-collected wheat samples. The authors acknowledge the contribution of Dr Steven Simpfendorfer from NSW Department of Primary Industries for his analysis of the fungal species on the 2021 wheat grains and for his identification of the fungal species. The authors also thank Robert van Barneveld for his contribution to the meat chicken experiment.
References
American Association of Cereal Chemists International (AACC) (2010) ‘Approved methods of analysis.’ 11th edn. (AACC International: St Paul, MN, USA)Andriotis VME, Rejzek M, Barclay E, Rugen MD, Field RA, Smith AM (2016) Cell wall degradation is required for normal starch mobilisation in barley endosperm. Scientific Reports 6, 33215
| Cell wall degradation is required for normal starch mobilisation in barley endosperm.Crossref | GoogleScholarGoogle Scholar |
Barnes WC, Blakeney AB (1974) Determination of cereal alpha-amylase using a commercially available dye-labelled substrate. Starch 26, 193–197.
| Determination of cereal alpha-amylase using a commercially available dye-labelled substrate.Crossref | GoogleScholarGoogle Scholar |
Bird SH, Rowe JB, Choct M, Stachiw S, Tyler P, Thompson RD (1999) In vitro fermentation of grain and enzymatic digestion of cereal starch. Recent Advances in Animal Nutrition in Australia 12, 53–61.
Black JL (2008) Premium grains for livestock program: component 1 – coordination. Final report. Grains R&D Corporation, Canberra, ACT, Australia.
Black JL (2016) Cereal grains as animal feed. In ‘Encyclopedia of food grains. Vol. 3’. 2nd edn. (Eds C Wrigley, H Corke, K Seetharaman, J Faubion) pp. 215–222. (Academic Press: Oxford, UK)
Blaney BJ, Williams KC (1991) Effective use in livestock feeds of mouldy and weather-damaged grain containing mycotoxins: case histories and economic assessments pertaining to pig and poultry industries of Queensland. Australian Journal of Agricultural Research 44, 993––1012.
Brookes PA (1980) The significance of pre-harvest sprouting of barley on malting and brewing. Creal Research Communications 8, 29–38.
Derera NF (2018) ‘Preharvest field sprouting in cereals.’ (CRC Press: Boca Raton, FL, USA) Available at https://books.google.com.au/books?id=7rxHDwAAQBAJ&dq=feeding+preharves+sprouted+grains+to+Pigs&source=gbs_navlinks_s
Erwin ES, Marco GJ, Emery EM (1961) Volatile fatty acid analysis of blood and rumen fluid by liquid gas chromatography. Journal of Dairy Science 44, 1768–1771.
| Volatile fatty acid analysis of blood and rumen fluid by liquid gas chromatography.Crossref | GoogleScholarGoogle Scholar |
Fromme D (2016) Pre-harvest sprouting and weathering of sorghum grain. Louisiana State University. Available at https://www.lsuagcenter.com/profiles/aiverson/articles/page1472760731224#:∼:text=The%20most%20obvious%20damaging%20effects,of%20mycotoxins%20(photo%203)
Grain Central (2022) Australia’s big wheat year big on low protein: AGIC Asia. Market report 4 March 2022. Available at https://www.graincentral.com/markets/australias-big-wheat-year-big-on-low-protein-agic-asia/
Hagberg S (1960) A rapid method for determining alpha-amylase activity. Cereal Chemistry 37, 218–222.
Holanda DM, Kim SW (2021) Mycotoxin occurrence, toxicity, and detoxifying agents in pig production with an emphasis on deoxynivalenol. Toxins 13, 171
| Mycotoxin occurrence, toxicity, and detoxifying agents in pig production with an emphasis on deoxynivalenol.Crossref | GoogleScholarGoogle Scholar |
Hughes RJ (2003) Sex and the single chicken. In ‘Proceedings of the Australian poultry science symposium’. Vol. 15, pp. 172–176. (University of Sydney)
Hughes RJ (2008) Relationship between digesta transit time and apparent metabolisable energy value of wheat in chickens. British Poultry Science 49, 716–720.
| Relationship between digesta transit time and apparent metabolisable energy value of wheat in chickens.Crossref | GoogleScholarGoogle Scholar |
Hughes RJ, van Barneveld RJ (2004) Effects of germination of grains on apparent metabolisable energy values and performance of broiler chickens. In ‘Proceedings of the Australian poultry science symposium’. Vol. 16, pp. 47–50. (University of Sydney)
Johnson RJ, Taverner MR (1986) The feeding quality of sprouted wheat for poultry and pigs. In ‘Proceedings of the 4th international symposium on pre-harvest sprouting in cereals’. pp. 222–227. (CRC Press: Port Macquarie, NSW, Australia)
Kochiieru Y, Mankevičienė A, Cesevičienė J, Semaškienė R, Ramanauskienė J, Gorash A, Janavičienė S, Venslovas E (2021) The impact of harvesting time on Fusarium mycotoxins in spring wheat grain and their interaction with grain quality. Agronomy 11, 642
| The impact of harvesting time on Fusarium mycotoxins in spring wheat grain and their interaction with grain quality.Crossref | GoogleScholarGoogle Scholar |
Kopinski JS, Blaney BJ (2010) Nutritive value and non-toxicity of Botryosphaeria zeae-infected wheat for weaner pigs. Journal of Animal Physiology and Animal Nutrition 94, 44–54.
| Nutritive value and non-toxicity of Botryosphaeria zeae-infected wheat for weaner pigs.Crossref | GoogleScholarGoogle Scholar |
Lardy G (2017) ‘Feeding value of sprouted grains.’ (North Dakota State University: Fargo, ND, USA) Available at https://www.ag.ndsu.edu/publications/livestock/feeding-value-of-sprouted-grains
McCleary BV, Gibson TS, Mugford DC, Collaborators (1997) Measurement of total starch in cereal products by amyloglucosidase-α-amylase method: collaborative study. Journal of AOAC International 80, 571–579.
| Measurement of total starch in cereal products by amyloglucosidase-α-amylase method: collaborative study.Crossref | GoogleScholarGoogle Scholar |
Newberry M, Zwart AB, Whan A, Mieog JC, Sun M, Leyne E, Pritchard J, Daneri-Castro SN, Ibrahim K, Diepeveen D, Howitt CA, Ral J-PF (2018) Does late maturing alpha-amylase impact wheat baking quality? Frontiers in Plant Science 9, 1356
| Does late maturing alpha-amylase impact wheat baking quality?Crossref | GoogleScholarGoogle Scholar |
Ratanpaul V, Williams BA, Black JL, Gidley MJ (2018) Apparent amylase diffusion rates in milled cereal grains determined in vitro: potential relevance to digestion in the small intestine of pigs. Journal of Cereal Science 82, 42–48.
| Apparent amylase diffusion rates in milled cereal grains determined in vitro: potential relevance to digestion in the small intestine of pigs.Crossref | GoogleScholarGoogle Scholar |
Rowland LO, Plyler JE, Bradley JW (1978) The feeding value of weather-damaged grain sorghum for poultry. Poultry Science 57, 180–185.
| The feeding value of weather-damaged grain sorghum for poultry.Crossref | GoogleScholarGoogle Scholar |
Sullins RD, Rooney LW, Riggs JK (1971) Physical changes in the kernel during reconstitution of sorghum grain. Cereal Chemistry 48, 567–575.
van Barneveld RJ, Graham H, Diffey S (2018) Predicting the nutritional quality of feed ingredients for pigs using near-infrared spectroscopy (NIRS) and chemical analysis. Animal Production Science 58, 709–718.
| Predicting the nutritional quality of feed ingredients for pigs using near-infrared spectroscopy (NIRS) and chemical analysis.Crossref | GoogleScholarGoogle Scholar |
Wu YV, Wall JS (1980) Lysine content of protein increased by germination of normal and high-lysine sorghums. Journal of Agricultural and Food Chemistry 28, 455–458.
| Lysine content of protein increased by germination of normal and high-lysine sorghums.Crossref | GoogleScholarGoogle Scholar |
Xu R, Elijah G, Kiarie EG, Yiannikouris A, Sun L, Niel A, Karrow NA (2022) Nutritional impact of mycotoxins in food animal production and strategies for mitigation. Journal of Animal Science and Biotechnology 13, 69
| Nutritional impact of mycotoxins in food animal production and strategies for mitigation.Crossref | GoogleScholarGoogle Scholar |