Supplementation of reduced protein diets with l-arginine and l-citrulline for broilers challenged with subclinical necrotic enteritis. 2. Intestinal permeability, microbiota, and short-chain fatty acid production
Hiep Thi Dao A B , Nishchal K. Sharma A , Reza Barekatain C D , Sarbast K. Kheravii A , Emma J. Bradbury E , Shu-Biao Wu
A B , Nishchal K. Sharma A , Reza Barekatain C D , Sarbast K. Kheravii A , Emma J. Bradbury E , Shu-Biao Wu  A and Robert A. Swick
A and Robert A. Swick  A *
A *
A School of Environmental and Rural Science, Faculty of Science, Agriculture, Business and Law, University of New England, Armidale, NSW 2351, Australia.
B Faculty of Animal Science, Vietnam National University of Agriculture, Trau Quy Town, Gia Lam District, Hanoi, 100000, Vietnam.
C South Australian Research and Development Institute, Roseworthy Campus, Roseworthy, SA 5371, Australia.
D School of Animal and Veterinary Sciences, University of Adelaide, Roseworthy, SA 5371, Australia.
E Ridley AgriProducts, Melbourne, Vic. 3000, Australia.
Animal Production Science 62(13) 1250-1265 https://doi.org/10.1071/AN21394
Submitted: 28 July 2021 Accepted: 21 March 2022 Published: 2 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Necrotic enteritis (NE) has been considered a major threat to broiler gut health and growth performance.
Aims: This study aimed at investigating the effects of l-arginine (Arg) or l-citrulline (Cit) supplementation on intestinal morphology, short-chain fatty acid (SCFA), microbiota count, gut permeability, and pH in broilers fed reduced-protein diets during subclinical NE challenge.
Methods: Ross 308 cockerels (n = 720) were randomly assigned to six experimental treatments with eight replicates of 15 birds per pen. The treatments were standard protein without NE challenge (SP−), or with NE challenge (SP+); reduced protein (two percentage points lower crude protein) without NE challenge (RP−), or with NE challenge (RP+); RP+ plus added Arg (103% of Ross 308 requirement, RPA+) and RPC+ where supplemental Arg in RPA+ was replaced with Cit. A 2 × 2 factorial arrangement was employed for the first four treatments. Factors were NE (− or +) and protein concentration (SP or RP). Treatments SP+, RP+, RPA+, and RPC+ were analysed by one-way ANOVA.
Key results: Necrotic enteritis × protein interactions were detected for serum fluorescein isothiocyanate dextran (FITC-d) level, C. perfringens (P < 0.05) count in the caeca (P < 0.01), and acetic acid (P < 0.01) and total SCFA concentrations in the ileum on Day 16 (P < 0.001). Feeding the RP diet reduced serum FITC-d concentration, number of C. perfringens in the caeca, and increased acetic acid and total SCFA concentrations in the ileum compared with the SP group only in birds challenged with NE. Birds in the RPC+ treatment had greater jejunal villus height (P < 0.001), and lower caecal C. perfringens and Enterobacteriaceae count than did those in the SP+ treatment (P ≤ 0.001).
Conclusions: The results indicated a benefit to gut health of broilers during NE challenge when replacing crystalline Arg with Cit in RP diets.
Implications: In part, replacement of Arg by Cit in the RP diets is of great potential to increase gut health, reduce growth loss, thus, minimising negative effects of NE in broilers.
Keywords: arginine, citrulline, gut health, low protein, meat chicken, microbiota, morphology, necrotic enteritis.
Introduction
The gastrointestinal tract (GIT) plays a crucial role in the growth performance and health of chickens. An optimal morphological architecture is crucial for the digestion, absorption, and transportation of nutrients in birds (Swatson et al. 2002; Zanu et al. 2020a). Inside the GIT, the microbiota are known to regulate the rates of mucin production, epithelial cell proliferation, and short-chain fatty acid (SCFA) composition (Deplancke and Gaskins 2001; Hörmann et al. 2014) that are important to fight against negative effects from the environment (Den Besten et al. 2013; Apajalahti and Vienola 2016). Necrotic enteritis (NE), a disease caused by Clostridium perfringens, has been considered a major threat to broiler gut health as gut integrity is impaired with major shifts in the microbiota observed (Wilson et al. 2005; Stanley et al. 2012, 2014; Latorre et al. 2018). While the inclusion of antibiotics in broiler diets can effectively control NE (Widyaratne 2012), a ban on in-feed antibiotic growth promoters in several countries has intensified the search for alternatives to control NE.
Reduction in dietary protein level has been reported to increase intestinal morphology and SCFA level (Gu and Li 2004; Opapeju et al. 2008; Apajalahti and Vienola 2016). This effect is probably associated with the decrease in the population of pathogenic bacteria as a result of there being less undigested protein in the hindgut (Belloir et al. 2017; Hilliar et al. 2019, 2020a). Reducing the dietary protein level may decrease ammonia production in the hindgut as there are lower concentrations of fermentable nitrogenous substrates (He et al. 2015; Sharma et al. 2017). Ammonia production has been reported to increase intestinal pH and facilitate the growth of C. perfringens (Paiva and McElroy 2014; Qaisrani et al. 2015). Hence, reduced dietary protein may benefit the gut by reducing C. perfringens counts and, in turn, help alleviate the adverse effects of subclinical NE on growth performance.
As a precursor of nitric oxide and polyamines, arginine (Arg) plays an important role in mucosal development, villus physiology (Murakami et al. 2012), and proliferation and migration of intestinal epithelial cells in the gut (Rhoads and Wu 2009). Dietary Arg supplementation has been reported to preserve gut morphology in Eimeria- and/or C. perfringens-challenged birds by decreasing intestinal lesion scores, increasing villus height, villus height to crypt depth ratio, and villus surface area (Tan et al. 2014; Laika and Jahanian 2017; Zhang et al. 2018). Citrulline (Cit), a metabolite of Arg, has been demonstrated to have Arg-sparing effects in chickens (Tamir and Ratner 1963; Su and Austic 1999). The effects of Arg and Cit supplementation in reduced protein diets on growth performance, carcass traits, internal organ weights, intestinal lesion score, and serum uric acid concentration during NE were reported in the first part of this series (Dao et al. 2022). The findings of that report showed that supplementation of Cit to the reduced-protein diets promoted growth performance and recovery in NE-challenged birds compared with standard protein diets or reduced-protein diets supplemented with Arg. The objective of the present work was to provide further insight into the effects of Arg and Cit supplementation in reduced-protein diets on intestinal morphology, microbiota composition, SCFA, gut permeability, and pH in broilers subjected to the NE challenge.
Materials and methods
Experimental design and diets
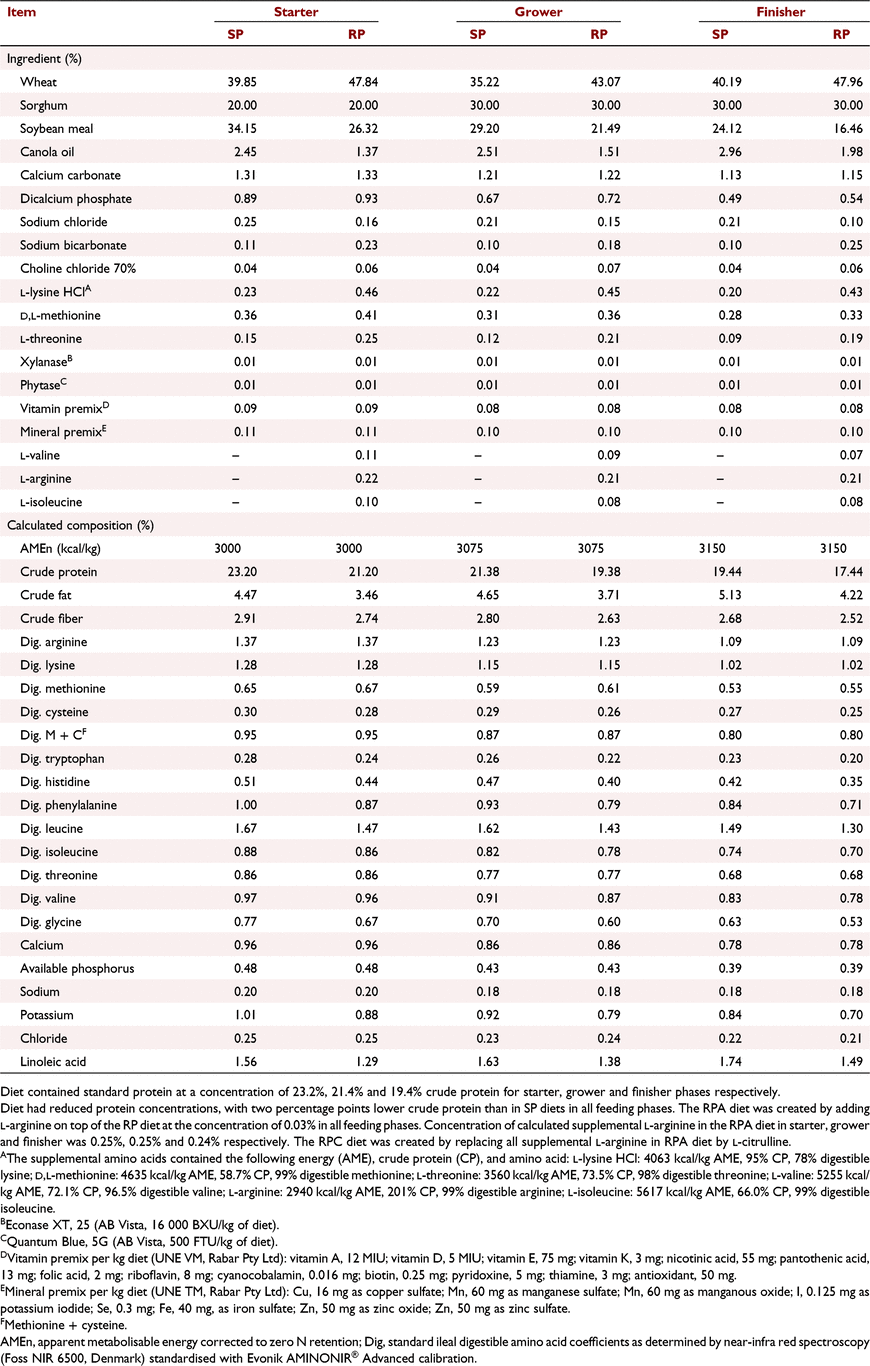
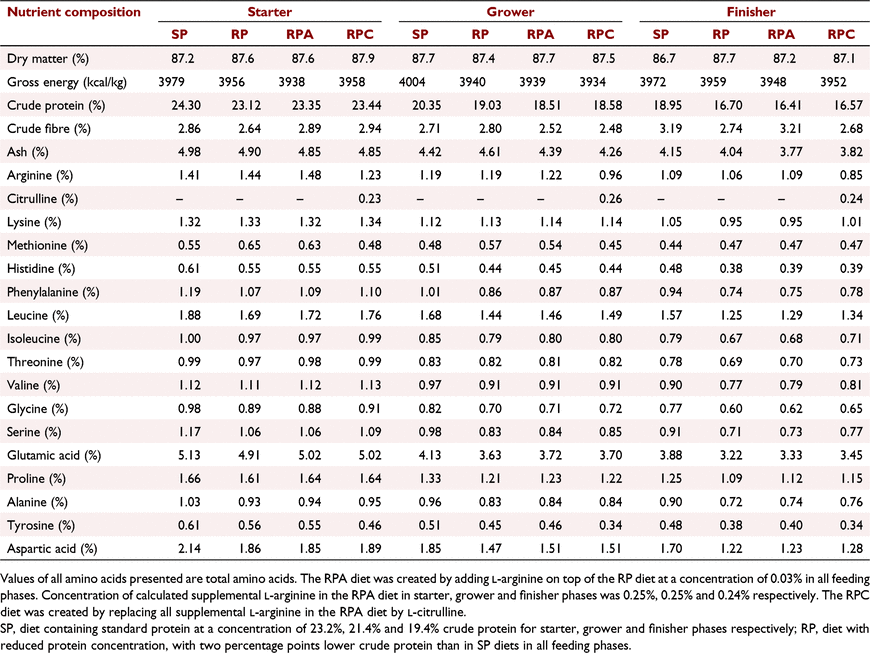
The study was implemented at the Centre of Animal Research and Teaching at the University of New England, Armidale, New South Wales, Australia, approved by its Animal Ethics Committee (Approval number: AEC19-119), and met the requirements of the Australian code of practice to care and use of animals for scientific purposes (NHMRC 2013). Day-old Ross 308 cockerels (n = 720) were allocated to 48 equal-sized floor pens (120 × 80 cm), with 15 birds per pens. Starting pen weights were similar across treatments. Birds were grown to mimic commercial conditions with hardwood shavings as bedding material in environmentally controlled rooms. Feed and water were provided ad libitum using nipple drinkers and tube feeders throughout the 35-day feeding study. The temperature, lighting, and ventilation conditions followed Ross 308 recommendations (Aviagen 2014a). Six treatments were used in this study, with eight replicate pens per treatment. The treatments were as follows: standard protein diet without NE challenge (SP−), or with NE challenge (SP+); reduced-protein diet balanced with crystalline amino acids without NE challenge (RP−), or with NE challenge (RP+); RP diet supplemented with additional Arg to 103% of requirement (equal to 15% additional supplemental crystalline Arg) with NE challenge (RPA+); and RP with Cit replacing all supplemental Arg in previous treatment with NE challenge (RPC+). A 2 × 2 factorial arrangement was employed for the first four treatments. Factors were NE (− or +) and protein level (SP or RP). In addition, all six treatments were analysed by one-way ANOVA. There was a two percentage point difference in crude protein level between SP and RP diets for all feeding phases. The concentrations of essential amino acids in the RP diet were equivalent to those in the SP diet and in accordance with Ross 308 broiler nutrition specifications (Aviagen 2014b). Concentrations of added crystalline Arg in the RP treatments in starter, grower, and finisher phases were 0.217%, 0.213%, and 0.212% respectively. Concentrations of added crystalline Arg in the RPA+ treatment in starter, grower, and finisher phases were 0.249%, 0.245%, and 0.244% respectively. Concentrations of Cit in the RPC+ treatment were equivalent to the Arg concentration in the RPA+ treatment. Details on diet composition and nutrient contents are presented in Tables 1 and 2. Arginine and Cit were supplemented to the RP diet at the expense of wheat. Feeds were provided as crumbles for starter (Days 0–10), and pellets for grower (Days 10–24), and finisher (Days 24–35) phases. Details on feed analysis were provided in the first part of this series (Dao et al. 2022).
|
|
Necrotic enteritis challenge
Subclinical NE was established following procedures previously described by Rodgers et al. (2015). Birds in the challenged groups (SP+, RP+, RPA+, and RPC+) were orally inoculated with 1 mL of sterile phosphate-buffered solution (PBS) containing a vaccine strain of Eimeria with 5000 sporulated oocysts of Eimeria acervulina, 5000 sporulated oocysts of Eimeria maxima, and 2500 sporulated oocysts of Eimeria brunetti (Eimeria Pty Ltd, Melbourne, Vic., Australia) on Day 9 and 1 mL of C. perfringens with an approximate concentration of 108 CFU (EHE-NE18 strain, Commonwealth Scientific and Industrial Research Organization, Geelong, Australia) in a starch thioglycollate broth on Day 14. Birds in the unchanged control groups (SP− and RP−) were given 1 mL of sterile PBS on Day 9 and 1 mL of sterile thioglycollate broth media as a sham treatment on Day 14. The results of the first part of this series showed that subclinical NE was successfully established in the current study as shown by increased intestinal lesion scores on Day 16, decreased feed intake and bodyweight gain and increased feed conversion ratio in the grower, finisher, and overall growth periods in NE-challenged birds.
Morphometric measurements of jejunum
On Day 16, three birds per pen were randomly selected, weighed, electrically stunned (MEFE CAT 44N, Mitchell Engineering Food Equipment, Clontarf, Qld, Australia), and euthanised by decapitation for sample collection. Jejunal samples were obtained and flushed with PBS and approximately 2 cm of jejunal tissue (mid-point between the end of duodenum and the Meckel’s diverticulum) was collected for morphometric measurements. The samples were fixed with 10% buffered formalin in 50-mL containers until processing. The jejunal samples were sectioned and processed using standard haematoxylin and eosin assay as described by Golder et al. (2011). The histological slides were scanned by NanoZoomer 2.0-RS (Model C10730-12, Hamamatsu Photonics K.K., Hamamatsu, Japan) and then readings were performed on the scanned slides using NDP.scan 2.5.8 software supplied by the same company. Five villi per section were randomly selected for measurements of villus height, crypt depth, basal width, middle width, and apical width, resulting in 10 measurements per sample and 30 measurements per pen. The apparent villus surface area was calculated as (basal width + apical width)/2 × villus height, following Zanu et al. (2020b).
Caecal DNA extraction and quantification of bacteria
To quantify microbiota populations, caecal digesta from three birds per pen on Day 16 were pooled in 50-mL containers. Then, subsamples of the pooled caecal digesta were aliquoted into 2-mL Eppendorf tubes and snap-frozen with liquid nitrogen during the sampling. All the samples were stored at −20°C until analysis. The DNA of caecal content was extracted, then the relative amounts of Bacillus spp., Bacteroides spp., Bifidobacterium spp., C. perfringens, Enterobacteriaceae, Lactobacillus spp., Ruminococcus spp., and total bacteria, expressed as log10 genomic DNA copies per gram of caecal digesta, were quantified as described by Kheravii et al. (2017). The quantitative real-time PCR (Rotorgene 6000 real-time PCR machine, Corbett, Sydney, NSW, Australia) was employed to determine the bacterial populations. The specific 16S rRNA primers used for the quantification of different bacterial populations are presented in Table 3.

|
Determination of succinic acid, lactic acid, and SCFA
Pooled ileal and caecal digesta from three birds per pen on Day 16 were used for the analysis of succinic acid, lactic acid, and SCFA. The samples were stored at −20°C until analysis. Concentrations of succinic acid, lactic acid, and SCFA in the samples were determined in duplicate, following procedures described by Wu et al. (2010).
Gut permeability with fluorescein isothiocyanate dextran in blood serum
The fluorescein isothiocyanate dextran (FITC-d, Sigma Aldrich, NSW, Australia; average mole weight of 4000 and FITC-d:glucose of 1:250) was used as a marker to measure the gut permeability on Day 16. The FITC-d solution was prepared, kept at 4°C, and wrapped in aluminium foil to avoid light exposure. Three birds per pen were selected, marked, and inoculated with a 1 mL dose of FITC-d (4.17 mg/kg body weight dissolved in water). Vacutainers (Becton, Dickinson UK Ltd, Plymouth, UK) that contained spray-coated silica and a polymer gel were used to collect the blood from sampled birds between 2 and 2.5 h after the inoculation. Then, blood samples were centrifuged at 3000g at 4°C for 10 min to separate the serum. Serum samples were stored at −20°C until further analysis. To determine FITC-d concentration, a standard curve was prepared following the method previously described by Prado-Rebolledo et al. (2017). Concentrations of FITC-d in diluted serum (1:1 PBS), blank, and standard samples were determined at an excitation wavelength of 480 nm and an emission wavelength of 520 nm by using a Synergy MX plate reader (Biotek Instruments, Bedfordshire, UK).
Measurements of ileal and caecal pH
Immediately after dissecting, the intact ileum and caeca were removed from three birds per pen on Day 16. The pH was measured using a digital pH metre (Mettler-Toledo, UK) with a spear tip piercing pH electrode (Sensorex, Garden Grove, CA, USA) by directly inserting the pH metre probe into the digesta of the caeca and distal ileum. Three readings were taken for each sample. The probe was washed with ultra-pure water (ICW 3000 water purifier for ion chromatography; Millipore, Burlington, MA, USA) between the samples to avoid cross-contamination.
Data analyses
R Commander (version 3.3.1, R Foundation for Statistical Computing, Vienna, Austria) was used to analyse data. All data were tested for normality and variance homogeneity before analysis. First, a two-way ANOVA was used to test the interaction between NE challenge (no or yes) and protein level (SP or RP) excluding RPA+ and RPC+ treatments (2 × 2 factorial arrangement of treatments). Then, one-way ANOVA was used to test statistical differences among the four NE-challenged treatments (SP+, RP+, RPA+, and RPC+). Tukey’s posthoc test was used to identify pairwise differences between the treatments from significant ANOVA results. The P-value of <0.05 was considered significant.
Results
Jejunal morphology
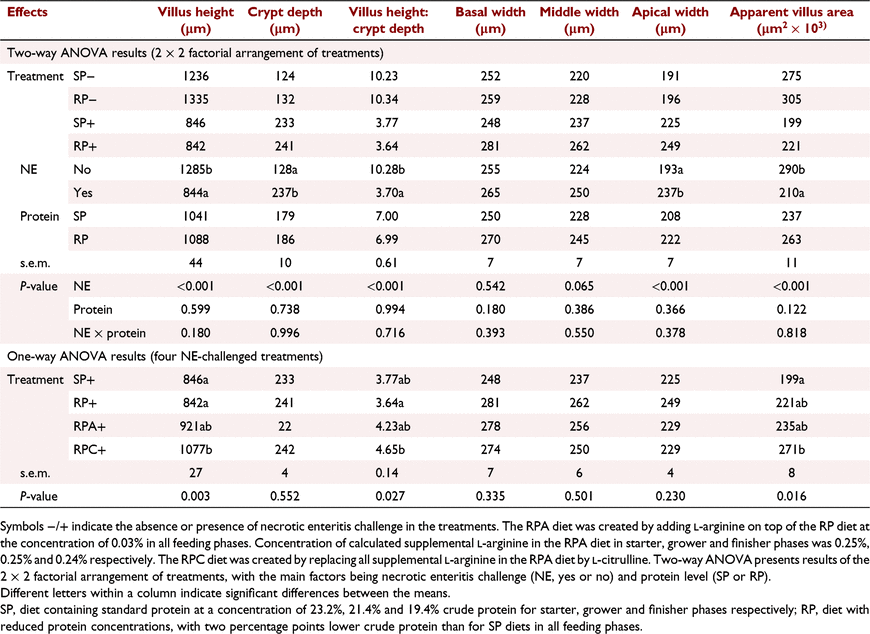
Results on jejunal morphology on Day 16 are presented in Table 4 and Fig. 1. No NE × protein interaction was detected for jejunal morphology (Table 4). Necrotic enteritis challenge as the main effect reduced villus height (P < 0.001), villus height to crypt depth ratio (P < 0.001), and apparent villus area (P < 0.001), while increasing crypt depth (P < 0.001), and apical width (P < 0.001), and tended to increase middle width (P = 0.065) on Day 16. Protein level as the main effect did not affect morphological parameters in the jejunum on Day 16. The addition of Cit to the RP+ treatment increased villus height (P < 0.01) and villus height to crypt depth ratio (P < 0.05) compared with the RP+ treatment, and increased apparent villus area (P < 0.05) compared with the SP+ treatment on Day 16 (Table 4).
|
Caecal microbiota
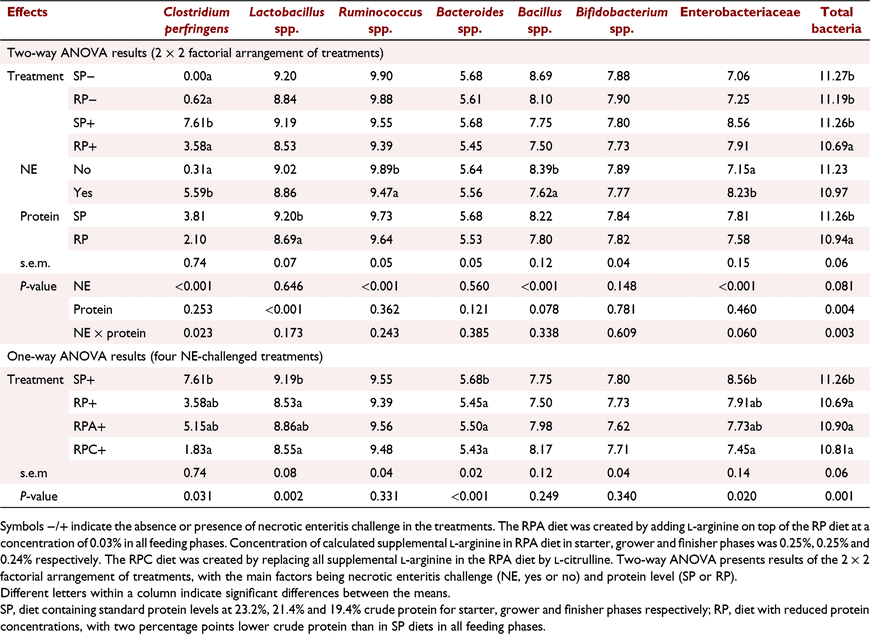
Necrotic enteritis × protein interactions were detected for caecal C. perfringens (P < 0.05) and total bacteria counts (P < 0.01). Feeding the RP diet reduced C. perfringens and total bacteria counts in the caeca compared with the SP group only in birds challenged with NE (Table 5). Similarly, a tendency for a NE × protein interaction was observed for caecal Enterobacteriaceae count (P = 0.06). The number of Enterobacteriaceae tended to decrease in birds fed the RP diet compared with the SP diet only when challenged with NE. Necrotic enteritis challenge as the main effect decreased numbers of Ruminococcus spp. (P < 0.001) and Bacillus spp. (P < 0.001) in the caeca on Day 16 (Table 5). Numbers of caecal Lactobacillus spp., Bacteroides spp., and Bifidobacterium spp. were not influenced by the NE challenge. Feeding the RP diet reduced the number of Lactobacillus spp. (P < 0.001) and tended to reduce the number of Bacillus spp. in the caeca on Day 16 (P = 0.078) compared with those of the SP fed birds as shown by the main effect of protein level in Table 5. Supplementation of Cit to the RP+ treatment reduced C. perfringens and Enterobacteriaceae counts in the caeca compared with those of the SP+ treatment on Day 16 (P < 0.05; Table 5).
|
Ileal and caecal succinic acid, lactic acid, and SCFA
The results on the concentrations of ileal and caecal succinic acid, lactic acid, and SCFA on Day 16 are shown in Tables 6 and 7. Interactions for NE × protein were observed for acetic acid (P < 0.01), succinic acid (P < 0.01), and total SCFA (P < 0.001) concentrations in the ileum on Day 16. Concentrations of ileal acetic acid and total SCFA were increased in birds fed the RP diets compared with the SP-fed birds only when challenged with NE (P < 0.01), whereas the concentration of ileal succinic acid was increased in birds fed the SP diets only when without NE challenge (P < 0.01, Table 6). A tendency for interaction between NE and protein level was detected for caecal butyric acid concentration on Day 16 (P = 0.059). The butyric acid concentration was increased in caecal contents in birds fed the SP diet compared with the RP diet only when challenged with NE (Table 7).

|

|
Necrotic enteritis challenge increased ileal lactic acid (P < 0.001) but decreased propionic acid (P < 0.001) concentrations compared with unchallenged birds, as shown in Table 6. In the caeca, NE challenge increased butyric (P < 0.01), isobutyric (P < 0.05), and isovaleric acid concentrations (P < 0.05) on Day 16 compared with unchallenged birds. Feeding the RP diets increased acetic acid (P < 0.001), lactic acid (P < 0.05) and total SCFA (P < 0.05) but decreased propionic acid (P < 0.05) and butyric acid (P < 0.05) in the caeca on Day 16, when compared with SP diets, irrespective of the NE challenge (Table 7).
Supplementation of Arg and Cit to the RP+ treatment did not affect concentrations of succinic acid, lactic acid, and SCFA in the ileum on Day 16 (P > 0.05, Table 6). Supplementation of Cit to the RP+ treatment decreased concentration of butyric acid (P < 0.001) compared with the RP+ treatment and decreased concentration of valeric acid (P < 0.05) compared with the SP+ treatment in the caeca on Day 16 (Table 7).
Serum FITC-d concentration, ileal and caecal pH
A NE × protein interaction was observed for gut leakage as evidenced by Day 16 serum FITC-d concentrations (P < 0.001, Table 8). Serum FITC-d concentation was decreased in birds fed the RP diet compared with those offered the SP diet only when challenged with NE. The one-way ANOVA results showed that supplementation of either Arg or Cit to the RP+ treatment did not affect serum FITC-d concentration on Day 16 when compared with the RP control treatment (P > 0.05), as shown in Table 8.

|
No NE by protein interactions were detected for ileal or caecal pH on Day 16 (P > 0.05), as shown in Table 8. Necrotic enteritis challenge decreased ileal pH (P < 0.001) but did not affect caecal pH on Day 16. Birds fed the RP diet had a higher caecal pH (P < 0.01) than, but a similar ileal pH to those offered the SP diet on Day 16, as shown by the main effect of protein level in Table 8. Supplementation of either Arg or Cit to the RP+ treatment did not affect ileal pH. Birds in the RPC+ treatment had a higher caecal pH than did those in the SP+ treatment (P < 0.05), whereas the caecal pH values of RP+, RPA+, and SP+ treatments were not different (Table 8).
Discussion
Necrotic enteritis challenge decreased villus height, villus height to crypt depth ratio and apparent villus area, and increased crypt depth, villus apical width, and middle width on Day 16 in the current study. Similar findings have been previously reported in broilers challenged with Eimeria spp. and/or C. perfringens (Onrust et al. 2018; Wu et al. 2018; Xue et al. 2018). The decreased villus height and thereby the smaller absorptive area reduce digestion and absorption capacity (Gao et al. 2008). Also, a deeper crypt may not be preferred as it indicates increased tissue turnover, and thus a higher maintenance requirement that might consequently reduce feed conversion efficiency and growth (Gao et al. 2008; Zanu et al. 2020c). This was illustrated by the reduced bodyweight gain and feed efficiency in NE-challenged birds, as reported in the first part of this series (Dao et al. 2022). The increased crypt depth may also suggest inflammation and toxin secretions by enteric pathogens (Gao et al. 2008), and the wider villus may indicate a reduction in nutrient absorptive area and possibly an increase of gut-associated immune tissues (Chen et al. 2015). Furthermore, alterations in the GIT microbiota population following the NE challenge have been reported in birds (Stanley et al. 2012, 2014; Latorre et al. 2018). The increase in the numbers of C. perfringens in NE-challenged birds was expected and may confirm the success of the NE challenge model established in the current study. The NE challenge increased the number of Enterobacteriaceae, and decreased numbers of Bacillus spp. and Ruminococcus spp. in the caeca, which is similar to the results of other reports (Keerqin et al. 2017; Gharib-Naseri et al. 2019; Zanu et al. 2020d). Bacillus spp. are Gram-positive, aerobic bacteria, that have been illustrated to have beneficial effects on gut health through the production of extracellular enzymes, and thus have been used as a source of industrial enzymes (Hong et al. 2009; Latorre et al. 2015, 2016). Whereas Ruminococcus spp. plays various important roles in digestion, and production of bacteriocins and butyric acid that might influence the proliferation of intestinal epithelial cells and the development of C. perfringens in the GIT (Crost et al. 2011; Rinttilä and Apajalahti 2013). The decrease of Bacillus spp. and Ruminococcus spp. may indicate digestion disruption and impaired immune functions leading to reduced performance in NE challenged birds in the current study.
Short-chain fatty acids are required to maintain redox equivalent production within the anaerobic microbial community in the gut (Den Besten et al. 2013). As neutral or slightly alkaline is a favourable environment for the majority of gut pathogenic bacteria (Rodrigues and Choct 2018), the high level of SCFA can lower gut pH and inhibit the development of many pathogens (Ricke 2003; Huyghebaert et al. 2011). The results of the current study showed that the NE challenge increased lactic acid concentration, decreased propionic acid concentration in the ileum, and increased the concentrations of butyric, isobutyric, and isovaleric acids in the caeca of respective groups. The alterations in the ileal and caecal SCFA profile might indicate the changes in the gut microbiome in NE-challenged birds. Similar results were reported by Hilliar et al. (2020b). Meanwhile, no difference in the concentrations of caecal propionic, butyric, isobutyric, valeric, isovaleric, and lactic acids between NE-challenged and unchallenged birds were observed by Kheravii et al. (2018). It is generally accepted that higher concentrations of lactic acid, succinic acid, and SCFA in the gut indicate a healthier gut (Wu et al. 2016). However, NE disease is typically associated with the small intestine rather than the caeca per se (Timbermont et al. 2011; Shojadoost et al. 2012). Hence, higher concentrations of lactic acid, succinic acid, and SCFA in the caeca do not mean that birds will be protected from NE infection (Wu et al. 2016). Besides, increases in butyric acid concentration in the caeca might indicate the response of birds to the increase of C. perfringens as a result of NE challenge that can occur via the influence of the immune system (Meijer et al. 2010) or stimulation of intestinal mucin gene expression (Gaudier et al. 2004).
The intestinal pH is crucial for nutrient solubility and the microorganism population as the microbes rely on the pH of the environment where they reside (Zanu et al. 2020c). In the current study, the NE challenge decreased distal ileal pH but did not affect caecal pH on Day 16. These findings were consistent with the results on concentrations of succinic acid, lactic acid, and SCFA in the ileum and caeca on Day 16 in the current study. In particular, the NE challenge resulted in a marked increase in the concentration of lactic acid (2.4 times), which was the most abundant acid in the ileum, accounting for 94% and 88% of total examined ileal acids in NE-challenged and unchallenged birds respectively. Meanwhile, the total SCFA that accounted for 91–92% of examined acids in the caeca were not different between the challenged and unchallenged groups. The decreased ileal pH in NE-challenged birds has been previously reported and is believed to be a result of the increase in the numbers of lactic- and butyric acid-producing bacteria (Hilliar et al. 2020b).
Dietary protein concentration did not influence jejunal morphology in the current study. Similar results were found by Buwjoom et al. (2010) and Laudadio et al. (2012). Conversely, decreased jejunal villus height and villus height to crypt depth ratio in birds fed RP diets compared with those offered SP diets have been reported by others (Dehghani and Jahanian 2012; Houshmand et al. 2012). The differences in dietary protein level, diet composition, and bird age might be the reasons for discrepancies among the studies. Meanwhile, the decrease in gut permeability as shown by the serum FITC-d concentration on Day 16 as a result of reducing dietary protein compared with the SP diet when challenged with NE might be associated with less undigested feed materials and lower NE incidence than for the SP diet, as reported in the first part of this series (Dao et al. 2022). Noticeably, feeding the RP diets reduced numbers of caecal Lactobacillus spp. and total bacteria compared with those of the SP diets in the current study. Lactobacilli have been generally known as beneficial bacteria for the host due to their ability to use complex plant-derived carbohydrates, including non-starch polysaccharides, and produce SCFA (Biddle et al. 2013). The higher inclusion level of starch from wheat and less soy-derived non-starch polysaccharides in the RP diets than in the SP diets in the current study may reduce the substrates that are essential for the growth of Lactobacillus spp. and anaerobic bacteria, and thus reduce the abundance of these groups. In addition, it is possible that the increased nitrogen digestibility in the upper GIT in birds fed RP diets compared with those fed the SP diets (Hilliar et al. 2019; Dao et al. 2021a, 2021b) might reduce nutrient bypass to the caeca and, consequently, limit microbial populations reliant on nitrogenous substrates. The NE × protein interactions obtained in the current study indicate that feeding the RP diets might help reduce disease severity and promote recovery in NE-challenged birds by lowering C. perfringens and Enterobacteriaceae counts compared with the SP diets. However, it is noteworthy that feeding an RP diet may also reduce beneficial bacteria such as Lactobacillus spp. in the caeca.
Dietary protein concentration is associated with the development of the gut microbial population and SCFA profile (Apajalahti and Vienola 2016). The results of the current study showed a beneficial effect of the RP over the SP diet on some aspects of the gut health when birds were challenged with NE that were indicated by the increases of total SCFA and acetic acid concentrations in the ileum and the increases of acetic acid, lactic acid, and total SCFA in the caeca in the respective group. This effect of the RP diets may be related to the reduction of harmful bacteria, including C. perfringens and Enterobacteriaceae, in the caeca, as observed in the current study. Decreased concentrations of caecal propionic acid and butyric acid were also observed in the RP-fed birds compared with the SP-fed birds in the current study. The decreased caecal butyric acid concentration in birds fed the RP compared with the SP has been previously reported and is believed to attribute to lower non-starch polysaccharide content in the RP diets (Hilliar et al. 2020b). Studies on humans have also shown that the serum butyrate level is increased in protein-rich diets, whereas propionate is decreased in carbohydrate-rich diets (Mueller et al. 2020). The results of the current study reflect differential effects of the RP diets on individual SCFA in different segments of the gut, which are most likely attributed to the diet composition.
Arginine is associated with proliferation and migration of intestinal epithelial cells, intestinal mucosa development, and villus morphology (Rhoads and Wu 2009; Murakami et al. 2012). Dietary Arg supplementation has been shown to increase villus height, villus height to crypt depth ratio, villus surface area, and gut integrity in birds subjected to the Eimeria and/or C. perfringens challenge (Tan et al. 2014; Laika and Jahanian 2017; Zhang et al. 2018). Although Cit effects are rarely being reported in birds, studies on mice and rats have shown that Cit supplementation was effective in increasing intestinal barrier functions (Jegatheesan et al. 2016; Sellmann et al. 2017). Furthermore, dietary Arg supplementation can deplete Arg degradation pathways caused by pathogenic bacteria, including C. perfringens, and enhance T-cell proliferation and function, thus inhibiting the development of these bacteria (Stadelmann et al. 2013; Tan et al. 2015). In addition, combined supplementation of Arg, threonine, and glutamine has been reported to benefit gut microbiota and promote an immune response in birds subjected to Eimeria and E. coli challenges (Gottardo et al. 2017; Bortoluzzi et al. 2018). In the current study, the addition of Cit to the RP diet increased villus height, villus height to crypt depth ratio, and apparent villus area, whereas Arg supplementation did not affect jejunal morphology in NE-challenged birds on Day 16. Similarly, supplementation of Arg to the RP diet did not affect caecal microbiota counts, whereas Cit supplementation reduced the numbers of caecal C. perfringens and Enterobacteriaceae in the NE-challenged birds in the current study. The advantages of Cit as a delivery form of Arg are that it is less toxic to the intestine (Grimble 2007) and can escape the degradation of arginase activity in the GIT compared with Arg (McCarty 2010). This may explain more beneficial effects of Cit than Arg in enhancing gut morphology and reducing numbers of caecal pathogenic bacteria in birds suffering from NE challenge in the current study. However, no beneficial effects of either Arg or Cit supplementation on gut permeability were observed in the current study. Supplementation of Arg and/or Cit to RP diets at higher levels than the levels used in the current study is worthwhile for further studies.
Conclusions
In conclusion, the NE challenge caused gut damage by impairing jejunal morphological architecture, increasing the number of C. perfringens, and decreasing numbers of Bacillus spp. and Ruminococcus spp. in the caeca. Feeding RP diets with approximately two percentage points lower crude protein supported gut health of NE-challenged birds by reducing C. perfringens in the caeca compared with the SP diets, as shown by the result of NE × protein interactions. Increased ileal acetic acid and total SCFA concentrations were also observed in birds fed the RP diets compared with those fed the SP diets. Supplementation of Cit to the RP diets further increased the beneficial effects of RP diets by increasing jejunal villus height, villus height to crypt depth ratio, and apparent villus area, and by reducing C. perfringens and Enterobacteriaceae counts in the caeca. Hence, it can be concluded that compared with RP or RPA diet, in part replacement of Arg by Cit in the RPC diet may have beneficial effects on gut health of broiler chickens during the NE challenge.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Declaration of funding
The authors thank Poultry Hub Australia for their financial support for this study (grant number: 18-414).
Acknowledgements
We thank Australian Proteome Analysis Facility, University of Macquarie, Australia for feed analysis. We thank Evonik (South East Asia) Pte. Ltd. (Singapore) for NIRS feed analysis. Also, the authors thank Mr Jonathon Clay studying at School of Environmental and Rural Science and the Poultry Research and Teaching Unit, the University of New England, Australia, for their help during the experiment and laboratory analysis.
References
Apajalahti J, Vienola K (2016) Interaction between chicken intestinal microbiota and protein digestion. Animal Feed Science and Technology 221, 323–330.| Interaction between chicken intestinal microbiota and protein digestion.Crossref | GoogleScholarGoogle Scholar |
Aviagen (2014a) ‘Ross 308 broiler management handbook.’ (Ross Breeders Limited: Newbridge, Midlothian, Scotland)
Aviagen (2014b) ‘Ross 308 broiler nutrition specification.’ (Ross Breeders Limited: Newbridge, Midlothian, Scotland)
Bartosch S, Fite A, Macfarlane GT, McMurdo MET (2004) Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Applied and Environmental Microbiology 70, 3575–3581.
| Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota.Crossref | GoogleScholarGoogle Scholar | 15184159PubMed |
Belloir P, Méda B, Lambert W, Corrent E, Juin H, Lessire M, Tesseraud S (2017) Reducing the CP content in broiler feeds: impact on animal performance, meat quality and nitrogen utilization. Animal 11, 1881–1889.
| Reducing the CP content in broiler feeds: impact on animal performance, meat quality and nitrogen utilization.Crossref | GoogleScholarGoogle Scholar | 28462773PubMed |
Biddle A, Stewart L, Blanchard J, Leschine S (2013) Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 5, 627–640.
| Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities.Crossref | GoogleScholarGoogle Scholar |
Bortoluzzi C, Rochell SJ, Applegate TJ (2018) Threonine, arginine, and glutamine: influences on intestinal physiology, immunology, and microbiology in broilers. Poultry Science 97, 937–945.
| Threonine, arginine, and glutamine: influences on intestinal physiology, immunology, and microbiology in broilers.Crossref | GoogleScholarGoogle Scholar | 29294123PubMed |
Buwjoom T, Yamauchi K, Erikawa T, Goto H (2010) Histological intestinal alterations in chickens fed low protein diet. Journal of Animal Physiology and Animal Nutrition 94, 354–361.
| Histological intestinal alterations in chickens fed low protein diet.Crossref | GoogleScholarGoogle Scholar | 19906144PubMed |
Chen J, Tellez G, Richards JD, Escobar J (2015) Identification of potential biomarkers for gut barrier failure in broiler chickens. Frontiers in Veterinary Science 2, 14
| Identification of potential biomarkers for gut barrier failure in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 26664943PubMed |
Crost EH, Ajandouz EH, Villard C, Geraert PA, Puigserver A, Fons M (2011) Ruminococcin C, a new anti-Clostridium perfringens bacteriocin produced in the gut by the commensal bacterium Ruminococcus gnavus E1. Biochimie 93, 1487–1494.
| Ruminococcin C, a new anti-Clostridium perfringens bacteriocin produced in the gut by the commensal bacterium Ruminococcus gnavus E1.Crossref | GoogleScholarGoogle Scholar | 21586310PubMed |
Dao HT, Sharma NK, Bradbury EJ, Swick RA (2021a) Response of meat chickens to different sources of arginine in low-protein diets. Journal of Animal Physiology and Animal Nutrition 105, 731–746.
| Response of meat chickens to different sources of arginine in low-protein diets.Crossref | GoogleScholarGoogle Scholar | 33410556PubMed |
Dao HT, Sharma NK, Bradbury EJ, Swick RA (2021b) Effects of l-arginine and l-citrulline supplementation in reduced protein diets for broilers under normal and cyclic warm temperature. Animal Nutrition 7, 927–938.
| Effects of l-arginine and l-citrulline supplementation in reduced protein diets for broilers under normal and cyclic warm temperature.Crossref | GoogleScholarGoogle Scholar | 34703910PubMed |
Dao HT, Sharma NK, Daneshmand A, Kumar A, Bradbury EJ, Wu S-B, Swick RA (2022) Supplementation of reduced protein diets with L-arginine and L-citrulline for broilers challenged with subclinical necrotic enteritis. 1. Growth, carcass yield, and intestinal lesion scores. Animal Production Science
| Supplementation of reduced protein diets with L-arginine and L-citrulline for broilers challenged with subclinical necrotic enteritis. 1. Growth, carcass yield, and intestinal lesion scores.Crossref | GoogleScholarGoogle Scholar |
Dehghani N, Jahanian R (2012) Interactive impacts of dietary organic acids and crude protein levels on performance and gut morphology of broiler chick. Worlds Poultry Science Journal 68, 1–4.
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research 54, 2325–2340.
| The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism.Crossref | GoogleScholarGoogle Scholar | 23821742PubMed |
Deplancke B, Gaskins HR (2001) Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. The American Journal of Clinical Nutrition 73, 1131S–1141S.
| Microbial modulation of innate defense: goblet cells and the intestinal mucus layer.Crossref | GoogleScholarGoogle Scholar | 11393191PubMed |
Gao J, Zhang HJ, Yu SH, Wu SG, Yoon I, Quigley J, Gao YP, Qi GH (2008) Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poultry Science 87, 1377–1384.
| Effects of yeast culture in broiler diets on performance and immunomodulatory functions.Crossref | GoogleScholarGoogle Scholar | 18577619PubMed |
Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C (2004) Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. American Journal of Physiology-Gastrointestinal and Liver Physiology 287, G1168–G1174.
| Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose.Crossref | GoogleScholarGoogle Scholar | 15308471PubMed |
Gharib-Naseri K, Kheravii SK, Keerqin C, Morgan N, Swick RA, Choct M, Wu S-B (2019) Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens. Poultry Science 98, 6422–6432.
| Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 31424518PubMed |
Golder HM, Geier MS, Forder REA, Hynd PI, Hughes RJ (2011) Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile. British Poultry Science 52, 500–506.
| Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile.Crossref | GoogleScholarGoogle Scholar | 21919578PubMed |
Gottardo ET, Burin ÁM, Lemke BV, Silva AM, Pasa CLB, Fernandes JIM (2017) Immune response in Eimerria sp. and E. Coli chalenged birds. Austral Journal of Veterinary Sciences 49, 175–184.
| Immune response in Eimerria sp. and E. Coli chalenged birds.Crossref | GoogleScholarGoogle Scholar |
Grimble GK (2007) Adverse gastrointestinal effects of arginine and related amino acids. The Journal of Nutrition 137, 1693S–1701S.
| Adverse gastrointestinal effects of arginine and related amino acids.Crossref | GoogleScholarGoogle Scholar | 17513449PubMed |
Gu X, Li D (2004) Effect of dietary crude protein level on villous morphology, immune status and histochemistry parameters of digestive tract in weaning piglets. Animal Feed Science and Technology 114, 113–126.
| Effect of dietary crude protein level on villous morphology, immune status and histochemistry parameters of digestive tract in weaning piglets.Crossref | GoogleScholarGoogle Scholar |
He LW, Meng QX, Li DY, Zhang YW, Ren LP (2015) Influence of feeding alternative fiber sources on the gastrointestinal fermentation, digestive enzyme activities and mucosa morphology of growing Greylag geese. Poultry Science 94, 2464–2471.
| Influence of feeding alternative fiber sources on the gastrointestinal fermentation, digestive enzyme activities and mucosa morphology of growing Greylag geese.Crossref | GoogleScholarGoogle Scholar | 26316338PubMed |
Hilliar M, Huyen N, Girish CK, Barekatain R, Wu S, Swick RA (2019) Supplementing glycine, serine, and threonine in low protein diets for meat type chickens. Poultry Science 98, 6857–6865.
| Supplementing glycine, serine, and threonine in low protein diets for meat type chickens.Crossref | GoogleScholarGoogle Scholar | 31433853PubMed |
Hilliar M, Hargreave G, Girish CK, Barekatain R, Wu S-B, Swick RA (2020a) Using crystalline amino acids to supplement broiler chicken requirements in reduced protein diets. Poultry Science 99, 1551–1563.
| Using crystalline amino acids to supplement broiler chicken requirements in reduced protein diets.Crossref | GoogleScholarGoogle Scholar | 32111322PubMed |
Hilliar M, Keerqin C, Girish CK, Barekatain R, Wu S-B, Swick RA (2020b) Reducing protein and supplementing crystalline amino acids, to alter dietary amino acid profiles in birds challenged for subclinical necrotic enteritis. Poultry Science 99, 2048–2060.
| Reducing protein and supplementing crystalline amino acids, to alter dietary amino acid profiles in birds challenged for subclinical necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 32241490PubMed |
Hong HA, Khaneja R, Tam NMK, Cazzato A, Tan S, Urdaci M, Brisson A, Antonio Gasbarrini A, Barnes I, Cutting SM (2009) Bacillus subtilis isolated from the human gastrointestinal tract. Research in Microbiology 160, 134–143.
| Bacillus subtilis isolated from the human gastrointestinal tract.Crossref | GoogleScholarGoogle Scholar | 19068230PubMed |
Hörmann N, Brandão I, Jäckel S, Ens N, Lillich M, Walter U, Reinhardt C (2014) Gut microbial colonization orchestrates TLR2 expression, signaling and epithelial proliferation in the small intestinal mucosa. PLoS ONE 9, e113080
| Gut microbial colonization orchestrates TLR2 expression, signaling and epithelial proliferation in the small intestinal mucosa.Crossref | GoogleScholarGoogle Scholar | 25396415PubMed |
Houshmand M, Azhar K, Zulkifli I, Bejo MH, Kamyab A (2012) Effects of non-antibiotic feed additives on performance, immunity and intestinal morphology of broilers fed different levels of protein. South African Journal of Animal Science 42, 23–32.
| Effects of non-antibiotic feed additives on performance, immunity and intestinal morphology of broilers fed different levels of protein.Crossref | GoogleScholarGoogle Scholar |
Huyghebaert G, Ducatelle R, Van Immerseel F (2011) An update on alternatives to antimicrobial growth promoters for broilers. The Veterinary Journal 187, 182–188.
| An update on alternatives to antimicrobial growth promoters for broilers.Crossref | GoogleScholarGoogle Scholar | 20382054PubMed |
Jegatheesan P, Beutheu S, Freese K, Waligora-Dupriet A-J, Nubret E, Butel M-J, Bergheim I, De Bandt J-P (2016) Preventive effects of citrulline on Western diet-induced non-alcoholic fatty liver disease in rats. British Journal of Nutrition 116, 191–203.
| Preventive effects of citrulline on Western diet-induced non-alcoholic fatty liver disease in rats.Crossref | GoogleScholarGoogle Scholar |
Keerqin C, Wu S-B, Svihus B, Swick R, Morgan N, Choct M (2017) An early feeding regime and a high-density amino acid diet on growth performance of broilers under subclinical necrotic enteritis challenge. Animal Nutrition 3, 25–32.
| An early feeding regime and a high-density amino acid diet on growth performance of broilers under subclinical necrotic enteritis challenge.Crossref | GoogleScholarGoogle Scholar | 29767124PubMed |
Kheravii SK, Swick RA, Choct M, Wu S-B (2017) Coarse particle inclusion and lignocellulose-rich fiber addition in feed benefit performance and health of broiler chickens. Poultry Science 96, 3272–3281.
| Coarse particle inclusion and lignocellulose-rich fiber addition in feed benefit performance and health of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 28637332PubMed |
Kheravii SK, Swick RA, Choct M, Wu S-B (2018) Effect of oat hulls as a free choice feeding on broiler performance, short chain fatty acids and microflora under a mild necrotic enteritis challenge. Animal Nutrition 4, 65–72.
| Effect of oat hulls as a free choice feeding on broiler performance, short chain fatty acids and microflora under a mild necrotic enteritis challenge.Crossref | GoogleScholarGoogle Scholar | 30167486PubMed |
Laika M, Jahanian R (2017) Increase in dietary arginine level could ameliorate detrimental impacts of coccidial infection in broiler chickens. Livestock Science 195, 38–44.
| Increase in dietary arginine level could ameliorate detrimental impacts of coccidial infection in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Latorre JD, Hernandez-Velasco X, Kuttappan VA, Wolfenden RE, Vicente JL, Wolfenden AD, Bielke LR, Prado-Rebolledo OF, Morales E, Hargis BM, Tellez G (2015) Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and Clostridium perfringens proliferation using an in vitro digestive model in different poultry diets. Frontiers in Veterinary Science 2, 25
| Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and Clostridium perfringens proliferation using an in vitro digestive model in different poultry diets.Crossref | GoogleScholarGoogle Scholar | 26664954PubMed |
Latorre JD, Hernandez-Velasco X, Wolfenden RE, Vicente JL, Wolfenden AD, Menconi A, Bielke LR, Hargis BM, Tellez G (2016) Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Frontiers in Veterinary Science 3, 95
| Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry.Crossref | GoogleScholarGoogle Scholar | 27812526PubMed |
Latorre JD, Adhikari B, Park SH, Teague KD, Graham LE, Mahaffey BD, Baxter MFA, Hernandez-Velasco X, Kwon YM, Ricke SC, Bielke LR, Hargis BM, Tellez G (2018) Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Frontiers in Veterinary Science 5, 199
| Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 30186844PubMed |
Laudadio V, Passantino L, Perillo A, Lopresti G, Passantino A, Khan RU, Tufarelli V (2012) Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poultry Science 91, 265–270.
| Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels.Crossref | GoogleScholarGoogle Scholar | 22184453PubMed |
Layton A, McKay L, Williams D, Garrett V, Gentry R, Sayler G (2006) Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Applied and Environmental Microbiology 72, 4214–4224.
| Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water.Crossref | GoogleScholarGoogle Scholar | 16751534PubMed |
Lee DH, Zo YG, Kim SJ (1996) Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Applied and Environmental Microbiology 62, 3112–3120.
| Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism.Crossref | GoogleScholarGoogle Scholar | 8795197PubMed |
McCarty MF (2010) Potential utility of full-spectrum antioxidant therapy, citrulline, and dietary nitrate in the management of sickle cell disease. Medical Hypotheses 74, 1055–1058.
| Potential utility of full-spectrum antioxidant therapy, citrulline, and dietary nitrate in the management of sickle cell disease.Crossref | GoogleScholarGoogle Scholar | 20089363PubMed |
Meijer K, de Vos P, Priebe MG (2010) Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Current Opinion in Clinical Nutrition and Metabolic Care 13, 715–721.
| Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health?Crossref | GoogleScholarGoogle Scholar | 20823773PubMed |
Mueller NT, Zhang M, Juraschek SP, Miller ER, Appel LJ (2020) Effects of high-fiber diets enriched with carbohydrate, protein, or unsaturated fat on circulating short chain fatty acids: results from the OmniHeart randomized trial. The American Journal of Clinical Nutrition 111, 545–554.
| Effects of high-fiber diets enriched with carbohydrate, protein, or unsaturated fat on circulating short chain fatty acids: results from the OmniHeart randomized trial.Crossref | GoogleScholarGoogle Scholar | 31927581PubMed |
Murakami AE, Fernandes JIM, Hernandes L, Santos TC (2012) Effects of starter diet supplementation with arginine on broiler production performance and on small intestine morphometry. Pesquisa Veterinária Brasileira 32, 259–266.
| Effects of starter diet supplementation with arginine on broiler production performance and on small intestine morphometry.Crossref | GoogleScholarGoogle Scholar |
NHMRC (2013) ‘Australian code of practice for the care and use of animals for scientific purposes.’ 8th edn. (The National Health and Medical Research Council: Australia)
Onrust L, Van Driessche K, Ducatelle R, Schwarzer K, Haesebrouck F, Van Immerseel F (2018) Valeric acid glyceride esters in feed promote broiler performance and reduce the incidence of necrotic enteritis. Poultry Science 97, 2303–2311.
| Valeric acid glyceride esters in feed promote broiler performance and reduce the incidence of necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 29562369PubMed |
Opapeju FO, Rademacher M, Blank G, Nyachoti CM (2008) Effect of low-protein amino acid-supplemented diets on the growth performance, gut morphology, organ weights and digesta characteristics of weaned pigs. Animal 2, 1457–1464.
| Effect of low-protein amino acid-supplemented diets on the growth performance, gut morphology, organ weights and digesta characteristics of weaned pigs.Crossref | GoogleScholarGoogle Scholar | 22443903PubMed |
Paiva D, McElroy A (2014) Necrotic enteritis: applications for the poultry industry. Journal of Applied Poultry Research 23, 557–566.
| Necrotic enteritis: applications for the poultry industry.Crossref | GoogleScholarGoogle Scholar |
Prado-Rebolledo OF, Delgado-Machuca JJ, Macedo-Barragan RJ, Garcia-Márquez LJ, Morales-Barrera JE, Latorre JD, Hernandez-Velasco X, Tellez G (2017) Evaluation of a selected lactic acid bacteria-based probiotic on Salmonella enterica serovar Enteritidis colonization and intestinal permeability in broiler chickens. Avian Pathology 46, 90–94.
| Evaluation of a selected lactic acid bacteria-based probiotic on Salmonella enterica serovar Enteritidis colonization and intestinal permeability in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 27545145PubMed |
Qaisrani SN, Van Krimpen MM, Kwakkel RP, Verstegen MWA, Hendriks WH (2015) Dietary factors affecting hindgut protein fermentation in broilers: a review. World’s Poultry Science Journal 71, 139–160.
| Dietary factors affecting hindgut protein fermentation in broilers: a review.Crossref | GoogleScholarGoogle Scholar |
Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P (2009) Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. British Journal of Nutrition 101, 541–550.
| Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii.Crossref | GoogleScholarGoogle Scholar |
Requena T, Burton J, Matsuki T, Munro K, Simon MA, Tanaka R, Watanabe K, Tannock GW (2002) Identification, detection, and enumeration of human Bifidobacterium species by PCR targeting the transaldolase gene. Applied and Environmental Microbiology 68, 2420–2427.
| Identification, detection, and enumeration of human Bifidobacterium species by PCR targeting the transaldolase gene.Crossref | GoogleScholarGoogle Scholar | 11976117PubMed |
Rhoads JM, Wu G (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37, 111–122.
| Glutamine, arginine, and leucine signaling in the intestine.Crossref | GoogleScholarGoogle Scholar |
Ricke SC (2003) Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poultry Science 82, 632–639.
| Perspectives on the use of organic acids and short chain fatty acids as antimicrobials.Crossref | GoogleScholarGoogle Scholar | 12710485PubMed |
Rinttilä T, Apajalahti J (2013) Intestinal microbiota and metabolites – implications for broiler chicken health and performance. Journal of Applied Poultry Research 22, 647–658.
| Intestinal microbiota and metabolites – implications for broiler chicken health and performance.Crossref | GoogleScholarGoogle Scholar |
Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A (2004) Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. Journal of Applied Microbiology 97, 1166–1177.
| Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR.Crossref | GoogleScholarGoogle Scholar | 15546407PubMed |
Rodgers NJ, Swick RA, Geier MS, Moore RJ, Choct M, Wu S-B (2015) A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Diseases 59, 38–45.
| A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 26292532PubMed |
Rodrigues I, Choct M (2018) The foregut and its manipulation via feeding practices in the chicken. Poultry Science 97, 3188–3206.
| The foregut and its manipulation via feeding practices in the chicken.Crossref | GoogleScholarGoogle Scholar | 29893913PubMed |
Sellmann C, Jin CJ, Engstler AJ, De Bandt J-P, Bergheim I (2017) Oral citrulline supplementation protects female mice from the development of non-alcoholic fatty liver disease (NAFLD). European Journal of Nutrition 56, 2519–2527.
| Oral citrulline supplementation protects female mice from the development of non-alcoholic fatty liver disease (NAFLD).Crossref | GoogleScholarGoogle Scholar | 27496089PubMed |
Sharma NK, Keerqin C, Wu S-B, Choct M, Swick RA (2017) Emissions of volatile odorous metabolites by Clostridium perfringens – in vitro study using two broth cultures. Poultry Science 96, 3291–3297.
| Emissions of volatile odorous metabolites by Clostridium perfringens – in vitro study using two broth cultures.Crossref | GoogleScholarGoogle Scholar | 28651342PubMed |
Shojadoost B, Vince AR, Prescott JF (2012) The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Veterinary Research 43, 74
| The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review.Crossref | GoogleScholarGoogle Scholar | 23101966PubMed |
Stadelmann B, Hanevik K, Andersson MK, Bruserud O, Svärd SG (2013) The role of arginine and arginine-metabolizing enzymes during Giardia – host cell interactions in vitro. BMC Microbiology 13, 256
| The role of arginine and arginine-metabolizing enzymes during Giardia – host cell interactions in vitro.Crossref | GoogleScholarGoogle Scholar | 24228819PubMed |
Stanley D, Keyburn AL, Denman SE, Moore RJ (2012) Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Veterinary Microbiology 159, 155–162.
| Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 22487456PubMed |
Stanley D, Wu S-B, Rodgers N, Swick RA, Moore RJ (2014) Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS ONE 9, 104739
| Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens.Crossref | GoogleScholarGoogle Scholar |
Su CL, Austic RE (1999) The recycling of l-citrulline to l-arginine in a chicken macrophage cell line. Poultry Science 78, 353–355.
| The recycling of l-citrulline to l-arginine in a chicken macrophage cell line.Crossref | GoogleScholarGoogle Scholar | 10090261PubMed |
Swatson HK, Gous R, Iji PA, Zarrinkalam R (2002) Effect of dietary protein level, amino acid balance and feeding level on growth, gastrointestinal tract, and mucosal structure of the small intestine in broiler chickens. Animal Research 51, 501–515.
| Effect of dietary protein level, amino acid balance and feeding level on growth, gastrointestinal tract, and mucosal structure of the small intestine in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Tamir H, Ratner S (1963) A study of ornithine, citrulline and arginine synthesis in growing chicks. Archives of Biochemistry and Biophysics 102, 259–269.
| A study of ornithine, citrulline and arginine synthesis in growing chicks.Crossref | GoogleScholarGoogle Scholar | 14061730PubMed |
Tan J, Applegate TJ, Liu S, Guo Y, Eicher SD (2014) Supplemental dietary l-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens. British Journal of Nutrition 112, 1098–1109.
| Supplemental dietary l-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Tan J-Z, Guo Y-M, Applegate TJ, Du E-C, Zhao X (2015) Dietary l-arginine modulates immunosuppression in broilers inoculated with an intermediate strain of infectious bursa disease virus. Journal of the Science of Food and Agriculture 95, 126–135.
| Dietary l-arginine modulates immunosuppression in broilers inoculated with an intermediate strain of infectious bursa disease virus.Crossref | GoogleScholarGoogle Scholar | 24728981PubMed |
Timbermont L, Haesebrouck F, Ducatelle R, Van Immerseel F (2011) Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathology 40, 341–347.
| Necrotic enteritis in broilers: an updated review on the pathogenesis.Crossref | GoogleScholarGoogle Scholar | 21812711PubMed |
Widyaratne GP (2012) The role of protein and amino acid nutrition in controlling Clostridium perfringens in the gastrointestinal tract of broiler chickens. PhD thesis, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
Wilson J, Tice G, Brash ML, Hilaire SS (2005) Manifestations of Clostridium perfringens and related bacterial enteritides in broiler chickens. World’s Poultry Science Journal 61, 435–449.
| Manifestations of Clostridium perfringens and related bacterial enteritides in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Wise MG, Siragusa GR (2007) Quantitative analysis of the intestinal bacterial community in one-to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. Journal of Applied Microbiology 102, 1138–1149.
| Quantitative analysis of the intestinal bacterial community in one-to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets.Crossref | GoogleScholarGoogle Scholar | 17381758PubMed |
Wu S-B, Rodgers N, Choct M (2010) Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens. Avian Diseases 54, 1058–1065.
| Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 20945788PubMed |
Wu S-B, Rodgers NJ, Cui G, Sun Y, Choct M (2016) Dynamics of intestinal metabolites and morphology in response to necrotic enteritis challenge in broiler chickens. Avian Pathology 45, 346–356.
| Dynamics of intestinal metabolites and morphology in response to necrotic enteritis challenge in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 27245303PubMed |
Wu Y, Shao Y, Song B, Zhen W, Wang Z, Guo Y, Shahid MS, Nie W (2018) Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. Journal of Animal Science and Biotechnology 9, 9
| Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 29416856PubMed |
Xue GD, Barekatain R, Wu SB, Choct M, Swick RA (2018) Dietary l-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poultry Science 97, 1334–1341.
| Dietary l-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge.Crossref | GoogleScholarGoogle Scholar | 29452407PubMed |
Zanu HK, Kheravii SK, Morgan NK, Bedford MR, Swick RA (2020a) Over-processed meat and bone meal and phytase effects on broilers challenged with subclinical necrotic enteritis: part 2. Inositol phosphate esters hydrolysis, intestinal permeability, hematology, jejunal gene expression and intestinal morphology. Animal Nutrition 6, 488–498.
| Over-processed meat and bone meal and phytase effects on broilers challenged with subclinical necrotic enteritis: part 2. Inositol phosphate esters hydrolysis, intestinal permeability, hematology, jejunal gene expression and intestinal morphology.Crossref | GoogleScholarGoogle Scholar | 33364465PubMed |
Zanu HK, Kheravii SK, Morgan NK, Bedford MR, Swick RA (2020b) Interactive effect of dietary calcium and phytase on broilers challenged with subclinical necrotic enteritis: part 2. Gut permeability, phytate ester concentrations, jejunal gene expression, and intestinal morphology. Poultry Science 99, 4914–4928.
| Interactive effect of dietary calcium and phytase on broilers challenged with subclinical necrotic enteritis: part 2. Gut permeability, phytate ester concentrations, jejunal gene expression, and intestinal morphology.Crossref | GoogleScholarGoogle Scholar | 32988528PubMed |
Zanu HK, Keerqin C, Kheravii SK, Morgan NK, Wu S-B, Bedford MR, Swick RA (2020c) Influence of meat and bone meal, phytase, and antibiotics on broiler chickens challenged with subclinical necrotic enteritis: 1. growth performance, intestinal pH, apparent ileal digestibility, cecal microbiota, and tibial mineralization. Poultry Science 99, 1540–1550.
| Influence of meat and bone meal, phytase, and antibiotics on broiler chickens challenged with subclinical necrotic enteritis: 1. growth performance, intestinal pH, apparent ileal digestibility, cecal microbiota, and tibial mineralization.Crossref | GoogleScholarGoogle Scholar | 32111321PubMed |
Zanu HK, Kheravii SK, Morgan NK, Bedford MR, Swick RA (2020d) Over-processed meat and bone meal and phytase effects on broilers challenged with subclinical necrotic enteritis: part 1. Performance, intestinal lesions and pH, bacterial counts and apparent ileal digestibility. Animal Nutrition 6, 313–324.
| Over-processed meat and bone meal and phytase effects on broilers challenged with subclinical necrotic enteritis: part 1. Performance, intestinal lesions and pH, bacterial counts and apparent ileal digestibility.Crossref | GoogleScholarGoogle Scholar | 33005765PubMed |
Zhang Y, Chen DW, Yu B, He J, Yu J, Mao XB, Wang JX, Luo JQ, Huang ZQ, Cheng GX, Zheng P (2015) Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets. Journal of Animal Science 93, 2967–2976.
| Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets.Crossref | GoogleScholarGoogle Scholar | 26115283PubMed |
Zhang B, Lv Z, Li Z, Wang W, Li G, Guo Y (2018) Dietary l-arginine supplementation alleviates the intestinal injury and modulates the gut microbiota in broiler chickens challenged by Clostridium perfringens. Frontiers in Microbiology 9, 1716
| Dietary l-arginine supplementation alleviates the intestinal injury and modulates the gut microbiota in broiler chickens challenged by Clostridium perfringens.Crossref | GoogleScholarGoogle Scholar | 30108569PubMed |


