Implications of elevated threonine plasma concentrations in the development of reduced-crude protein diets for broiler chickens
Shemil P. Macelline A B , Peter V. Chrystal A C , Sonia Yun Liu A B and Peter H. Selle A D E
A D E
A Poultry Research Foundation within The University of Sydney, Camden Campus, 425 Werombi Road, NSW 2570, Australia.
B School of Life and Environmental Sciences, Faculty of Science, The University of Sydney, NSW 2006, Australia.
C Baiada Poultry Pty Limited, Pendle Hill, NSW 2145, Australia.
D Sydney School of Veterinary Science, The University of Sydney, Sydney, NSW 2006, Australia.
E Corresponding author. Email: peter.selle@sydney.edu.au
Animal Production Science 61(14) 1442-1448 https://doi.org/10.1071/AN20554
Submitted: 2 October 2020 Accepted: 22 April 2021 Published: 17 May 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
There is a real quest to develop reduced-crude protein diets to facilitate sustainable chicken-meat production. However, pronounced elevations in threonine plasma concentrations in systemic plasma have consistently been observed pursuant to crude protein reductions in diets for broiler chickens. The aim of the present Perspective was to consider the genesis and consequences of these elevated threonine concentrations. A series of five reduced-crude protein feeding studies with maize-based diets completed on the Camden Campus of Sydney University was the basis of the present Perspective. Collectively, an average reduction in dietary crude protein from 212 to 167 g/kg generated a mean increase of 64.8% (867 versus 526 μmol/L) in threonine plasma concentrations. This was attributed to the downregulation of hepatic threonine dehydrogenase activity, which catalyses threonine to acetyl-CoA and glycine and a mechanism for this inhibition is proposed. Tangible reductions in dietary crude protein usually impair feed conversion efficiency and increase fat deposition. Threonine plasma concentrations are elevated by these reductions and the likelihood is that threonine concentrations may be an indicative biomarker of the precision with which efficient reduced-CP broiler diets are formulated and, if so, would facilitate their successful development.
Keywords: amino acids, broiler chickens, glucose, protein, starch, threonine.
Introduction
There is considerable interest in the development of reduced-crude protein (CP) diets for broiler chickens for which there is a compelling justification as outlined by Greenhalgh et al. (2020a). The successful development of reduced-CP diets will attenuate emissions of nitrogen and ammonia, which is environmentally advantageous. Reduced-CP diets will enhance litter quality and reduce the incidence of foot-pad lesions. Poor litter quality is a complex problem and is detrimental to bird welfare, which is an issue of increasing importance (Dunlop et al. 2016). Furthermore, reduced-CP diets have the potential to reduce the dependence of chicken-meat industry on soybean meal (Selle et al. 2020). This would be a real advantage in countries that rely on importations of soybean meal. Nevertheless, the successful development of reduced-CP diets is fraught with challenges, as considered by Chrystal et al. (2020a), because dietary CP reductions usually compromise feed conversion ratios (FCR), with associated increases in fat deposition.
Threonine is the third limiting amino acid in diets for broiler chickens after methionine and lysine (Kidd and Kerr 1996) and constitutes 36.3 g/kg of whole-body protein in broiler chickens (Wu 2014). Additionally, threonine is the dominant amino acid in avian mucin (Fang et al. 1993). The importance of threonine was demonstrated by free threonine concentrations in portal plasma taken from the anterior mesenteric vein (Selle et al. 2016). Increasing portal threonine plasma concentrations were linearly associated with both enhanced weight gain (r = 0.915, P < 0.0001) and feed conversion efficiency (r = –0.773, P < 0.01) in broilers from 7 to 28 days post-hatch in this study.
Free amino acid concentrations in systemic plasma are indicative of protein or amino acid utilisation in broiler chickens (Dean and Scott 1966; Fernández-Fígares et al. 1997). However, an intriguing outcome was reported by Fancher and Jensen (1989) in which female broilers were offered maize-based diets containing either 183 or 159 g/kg CP. Three 159 g/kg CP diets containing 1.3 g/kg non-bound threonine and different inclusions of other non-bound (synthetic, crystalline) amino acids, were compared with one 183 g/kg CP diet. The transition from 183 to 159 g/kg CP diets generated an average increase in systemic threonine plasma concentration of 124% (1959 versus 876 mmol/L), whereas plasma concentrations of the balance of amino acids assessed essentially remained static. Thus, this outcome raises the obvious question as to the genesis of these elevated threonine plasma concentrations pursuant to reductions in dietary CP. Moreover, this conundrum became increasingly more relevant when similar elevations in threonine plasma concentrations were repeatedly observed in broiler chickens offered maize-based diets on the Camden Campus of Sydney University. Therefore, the purpose of the present paper was to review these outcomes and to consider their genesis and implications of elevated threonine plasma concentrations in the context of the development of reduced-CP diets for sustainable chicken-meat production.
Impact of dietary CP reductions on threonine plasma concentrations
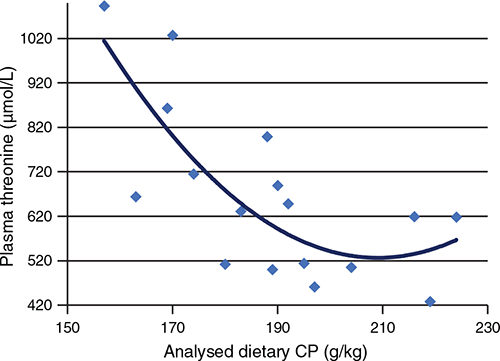
In a series of five feeding studies, off-sex, male Ross 308 chickens were offered maize-based diets from 7 to 35 days post-hatch, with dietary CP concentrations ranging from 222 to 156 g/kg (Moss et al. 2018; Chrystal et al. 2020b; 2020c, 2020d, 2021). The relevant data are presented in Table 1 where the dietary mean molar concentration of threonine was 68.3 mmolar, with a standard deviation of ±4.87. In contrast, free threonine plasma concentrations ranged from 428 to 1093 μmol/L around a mean value of 664 μmol/L ± 189.9 in systemic plasma. Instructively, there is a quadratic relationship (r = 0.797, P < 0.001) between analysed dietary CP concentrations and free threonine plasma concentrations, as shown in Fig. 1. The regression equation predicts that elevations of threonine plasma concentrations will be observed once dietary CP concentrations are reduced below 206 g/kg. When the highest- and lowest-CP diets in each experiment were compared, analysed CP concentrations decreased by an average of 42 g/kg from 212 to 170 g/kg and, in response, free threonine plasma concentrations increased by an average of 64.8% (867 versus 526 µmol/L). Across the individual experiments, elevations in threonine plasma concentrations ranged from 39.6% (863 versus 396 µmol/L) in Chrystal et al. (2021) to 116.4% (1027 versus 619 µmol/L) in Chrystal et al. (2020c). Clearly, the elevations in threonine plasma concentrations are variable; however, they are related to both FCR and relative fat-pad weights. From the same lowest to highest CP comparison, FCR were compromised by 4.29% (1.545 versus 1.481) and fat–pad weights were 77.6% heavier in the lowest CP diet (13.28 versus 7.48 g/kg). Compromised efficiency of feed conversion and heavier relative fat-pad weights are typical responses to reductions in dietary CP, so it is noteworthy that threonine plasma concentrations are quadratically related to heavier relative fat-pad weights (r = 0.680, P = 0.024) and compromised FCR (r = 0.569, P = 0.065), when the tabulated data are considered in their entirety. This is not to suggest that elevated threonine plasma concentrations are generating these critical negative responses, but they may be indicative of compromised performance when birds are offered reduced-CP diets.
|
|
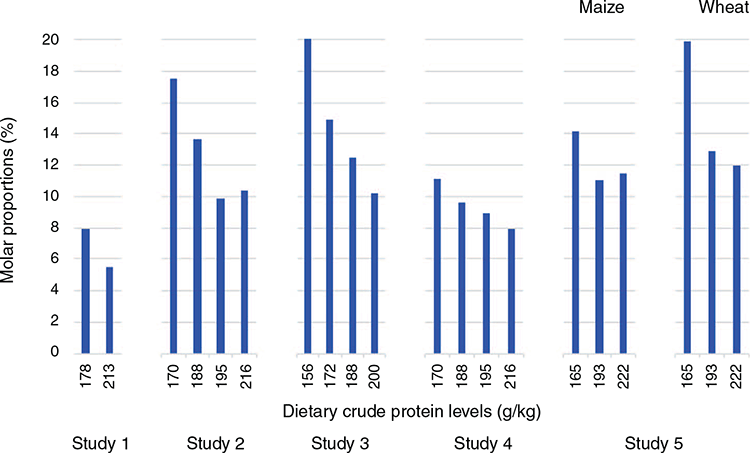
The molar proportions of free threonine relative to total amino acid concentrations in systemic plasma are illustrated in Fig. 2. In Studies 1–4, molar proportions of plasma threonine increased in an essentially linear fashion in response to declining dietary CP from an average of 8.48% to 14.16%, which is an increase of 67.0%, when the highest- and lowest-CP diets in each experiment were considered. In Study 5 (Chrystal et al. 2021), both maize and wheat-based diets were investigated where the 66.1% (19.91 versus 11.99%) increase in threonine molar proportions with wheat was more pronounced than the 24.1% (14.18 versus 11.43%) increase with maize-based diets. This difference between wheat and maize is discussed later.
|
A rationale for elevated threonine plasma concentrations
Threonine is an essential amino acid (Malinovsky 2018); therefore, the elevations in plasma threonine concentrations illustrated in Fig. 1 cannot stem from the biosynthesis of threonine in poultry. Alternatively, the elevations could be the result of decreased threonine catabolism where potentially three enzymes have the capacity to catabolise threonine in the liver. These include threonine dehydratase, which degrades threonine to α-ketobutyrate; threonine aldolase, which degrades threonine to acetaldehyde and glycine; and threonine dehydrogenase (TDH), which degrades threonine to acetyl-CoA and glycine (Davis and Austic 1982). Threonine is principally degraded by TDH in poultry; instructively, Akagi et al. (2004) found hepatic TDH activity (88%) to be dominant in avian species (Japanese quail). In contrast, threonine dehydratase (93%) was the dominant enzyme in rats. Akagi et al. (2004) classified threonine as a ketogenic amino acid in avian species because TDH metabolises to acetyl-CoA, whereas threonine is usually regarded as a glucogenic amino acid in mammals (D’Andrea 2000). Thus, it is plausible that elevated free threonine plasma concentrations in broiler chickens are essentially generated by the downregulation of hepatic TDH activity.
Several investigations into TDH activity in poultry have been completed (Davis and Austic 1997; Yuan et al. 2000; Yuan and Austic 2001; Lee et al. 2011, 2014, 2016) without reaching finite conclusions. The activity of TDH in hepatic mitochondria was specifically examined in Davis and Austic (1997) and it was suggested that hepatic TDH activity is influenced more so by concentrations of dietary protein or other amino acids than by threonine per se. Yuan and Austic (2001) reported that lowering dietary CP from 320 to 230 g/kg reduced total TDH activity in hepatic mitochondria of chickens by 48.3% (18.3 versus 35.4 units). This would be expected to elevate free threonine plasma concentrations, but a dietary CP of 320 g/kg is very much higher than standard. However, Lee et al. (2014) found that TDH activity in chicks was stimulated by additional dietary threonine and protein and, presumably, lowering dietary concentrations of threonine and/or protein would have the reverse effect. The mean analysed dietary threonine concentration was 8.16 g/kg and ranged from 7.4 to 9.1 g/kg in the five nominated experiments; therefore, there were no gross variations in dietary threonine concentrations in association with dietary CP reductions. However, the above findings suggest that reductions in dietary protein may depress TDH activity.
Of relevance is that Guerranti et al. (2001) investigated the inhibition of hepatic TDH activity in rats in a study where the focus was on fatty acids. These researchers concluded that acetyl-CoA and its derivatives depressed TDH activity by selective feedback inhibition as acetyl-CoA is a major end product of threonine catabolism. However, the catabolism of ketogenic amino acids, and specifically threonine by TDH, generates acetyl-CoA, which is a central metabolic intermediate capable of influencing the activity of numerous enzymes (Pietrocola et al. 2015). Pivotally, glucose may be metabolised to acetyl-CoA (Shi and Tu 2015), and Kaempfer et al. (1991) investigated the fraction of hepatic acetyl-CoA that is derived from glucose in rats. These researchers found that the quantity of acetyl-CoA derived from glucose fluctuates with the availability of carbohydrate. Typically, dietary starch:protein ratios, starch, and potentially glucose, concentrations are increased in reduced-CP diets, while fat concentrations are decreased. From the data in Table 1, analysed dietary starch concentrations increase by 33.0% (416 versus 313 g/kg) and analysed dietary starch:protein ratios increase from 1.49 to 2.45 when the lowest- to highest-CP diets are compared. Therefore, high starch/glucose concentrations in birds offered reduced-CP diets could be expected to increase acetyl-CoA concentrations which, in turn, could inhibit or downregulate hepatic TDH activity by selective feedback inhibition, resulting in elevated free threonine plasma concentrations. It is conceded that the proposed rationale is speculative. It is possible that lower dietary protein concentrations are contributing to elevated threonine plasma concentrations by depressing TDH activity; however, this mechanism may be subordinate to the proposed rationale. Nevertheless, it should be possible to assess the validity of the rationale by analysing hepatic acetyl-CoA concentrations (Shurubor et al. 2017) and TDH activity (Aoyama and Motokawa 1981) allied to threonine plasma concentrations in birds offered reduced-CP diets; certainly, this is our intention.
Insulin and glucagon
A mechanism whereby threonine plasma concentrations in birds offered reduced-CP diets has been tentatively identified. Nevertheless, this mechanism is not operating in a ‘metabolic vacuum’ and the conversion of glucose to acetyl-CoA is pivotal to the proposed mechanism. Plasma glucose concentrations are higher in avian than in mammalian species (15.3 versus 7.6 mM/L) by a two-fold factor (Braun and Sweazea 2008), but the biological basis for this difference has not been defined (Scanes 2009). Broiler chickens offered reduced-CP diets are effectively subject to a ‘starch overload’ and the resultant increased intestinal uptakes of glucose have an impact on the pancreatic secretion of insulin and glucagon, powerful hormones that have either anabolic or catabolic functions respectively, to the extent that molar ratios of insulin to glucagon have been described as determinants of avian carbohydrate metabolism (Hazelwood 1984). In addition, insulin has been shown to increase leptin expression in liver, but not in adipose tissue, of chickens and, reciprocally, glucagon has been shown to decrease hepatic expression of leptin but not in adipose tissue (Taouis et al. 2001). However, avian species appear to be sensitive to glucagon but resistant to insulin (Braun and Sweazea 2008). The likelihood is that both insulin and glucagon affect the performance of birds offered reduced-CP diets, including elevated threonine plasma concentrations, compromised FCR and heavier relative fat-pad weights. Of relevance is that excessive fat deposition in rapidly growing broilers has been attributed to increased plasma concentrations of insulin and glucagon and, possibly, insulin resistance (Sinsigalli et al. 1987). In relation to starch and protein digestive dynamics, Selle and Liu (2019) concluded that a better delineation of the starch–glucose–insulin axis in avian physiology is necessary and this caveat equally applies to the reciprocal glucose homeostatic roles of insulin and glucagon in broiler chickens offered reduced-CP diets.
Threonine, glycine and serine
In theory, threonine is a precursor of glycine (Baker et al. 1972) and both TDH and threonine aldolase catalyse the enzymatic conversion of threonine to glycine and glycine and serine are inter-convertible in poultry (Sugahara and Kandatsu 1976). Although classified as non-essential amino acids, glycine and serine, or glycine equivalents, have been shown to be of critical importance in reduced-CP broiler diets, and Siegert and Rodehutscord (2019) recommended that glycine equivalent requirements fall within a broad range from 11 to 20 g/kg. However, the notable increases in plasma threonine concentrations do not indicate that threonine is being converted to glycine and, in turn, serine. Indeed, increasing threonine plasma concentrations quadratically depressed glycine plasma concentrations to significant extents in both Chrystal et al. (2020c), where r = 0.486 and P = 0.036, and Chrystal et al. (2020d), where r = 0.632 and P = 0.002. Increasing threonine plasma concentrations tended to depress glycine concentrations quadratically (r = 0.514, P = 0.117) when consideration was given to all the Table 1 data. This agrees with the proposal that conversion of threonine to glycine and acetyl-CoA by TDH is being downregulated and, as a consequence, threonine is not serving as a glycine precursor. In more general terms, this is consistent with the conclusion of D’Mello (1973) that threonine is not readily degraded to glycine and does not act as a glycine precursor.
Dietary starch:protein ratios
Typically, soybean meal inclusions are decreased, and maize increased, in the formulation of reduced-CP diets. This substitution achieves reductions in CP and then targeted amino acid requirements are met by increasing inclusions of an expanding array of non-bound amino acids. For example, soybean meal inclusions were decreased by 52.7% (157 versus 331 g/kg) and maize inclusions increased by 31.6% (732 versus 556 g/kg) collectively, with the transition from the highest- to lowest-CP diets in Chrystal et al. (2020b, 2020c, 2020d, 2021). Also, inclusions of non-bound amino acids increased from 5.8 to 26.7 g/kg to meet requirements. This resulted in an expansion of analysed dietary starch:protein ratios from 1.55 to 2.50 in these four studies. There is a quadratic relationship between analysed dietary starch:protein ratios with relative fat-pad weights (r = 0.754, P = 0.006) and a linear relationship with FCR (r = 0.417, P = 0.096) across the Table 1 studies; thus, expanding starch:protein ratios may have deleterious impacts on these critical parameters.
The biological validity of these statistically significant outcomes is problematic; nevertheless, they prompted an initial evaluation of the strategy of limiting or ‘capping’ expansions of starch:protein ratios in reduced-CP diets. Some promise was displayed in this evaluation by Greenhalgh et al. (2020b). Broiler chicks were offered 197.5 g/kg CP wheat-based diets with dietary starch:protein ratios of either 1.97 or 1.63 from 7 to 35 days post-hatch. Diets with the narrower dietary starch:protein ratio supported better growth performance with a significant improvement in weight gain of 10.4% (2161 versus 1958 g/bird) and a numerical improvement in FCR of 4.04% (1.616 versus 1.684). The partial or total substitution of soybean meal (475 g/kg CP) with full-fat soy (360 g/kg CP) is one approach where dietary CP could be reduced to some extent without an increase in dietary starch concentrations. The proposed rationale that glucose from dietary starch is downregulating TDH activity because it is a source of acetyl-CoA suggests that the strategy of capping expansions in dietary starch:protein ratios merits further investigations. The total replacement of soybean meal with full-fat soy in broiler diets would lower dietary CP by ~30 g/kg without increasing dietary starch concentrations. More tangible CP reductions would necessitate some increases in dietary feed grain inclusions and starch concentrations, but modest increases may be accommodated. If the strategy of capping dietary starch:protein ratios proves to be a viable approach, it is quite possible that elevations in threonine plasma concentrations would be moderated.
Maize versus wheat as a feed grain in reduced-CP diets
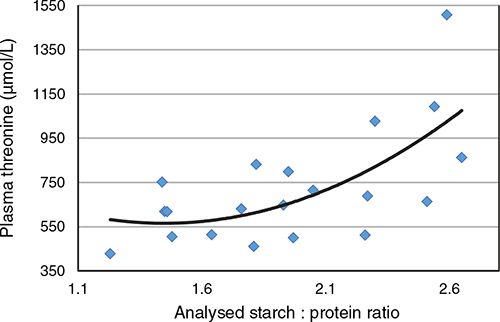
The feed grain component, usually maize or wheat, is increased at the expense of protein-rich feedstuffs, usually soybean meal, in reduced-CP diets. Axiomatically, this increases quantities of dietary starch and expands dietary starch:protein ratios. Moreover, as shown in Fig. 3, there is a quadratic increase (r = 0.664, P = 0.007) in free threonine plasma concentrations in response to expanding dietary starch:protein ratios from the Table 1 data. Given these increases in maize or wheat inclusions, the starch and protein concentrations of the feed grain assume more importance. Moreover, it does appear that the birds are better able to accommodate CP reductions of maize-based diets than of wheat-based diets (Chrystal et al. 2021). In this study, birds offered 165 g/kg CP maize-based diets significantly outperformed birds offered wheat based by 53.0% (2370 versus 1549 g/bird) in weight gain, 22.4% (3481 versus 2843 g/bird) in feed intake and by 19.9% (1.473 versus 1.840) in FCR. Interestingly, systemic plasma threonine concentrations were 75% (180 versus 103 µg/mL) higher in birds offered the wheat-based diet. Again, this is not to suggest that the difference in plasma threonine concentrations was causative, but quite possibly indicative, of the inferior growth performance supported by the wheat-based diet. The factors contributing to the relative inferiority of wheat need to be identified. The higher protein concentration of wheat results in more non-bound amino acids and less ‘intact’ soy protein in reduced-CP diets than is the case with maize. Also, there is the likelihood that wheat starch may be digested more rapidly than is maize starch, which is the case under in vitro conditions (Giuberti et al. 2012). These three factors are possibly involved in the inferiority of wheat in this context, which appears to be reflected in elevated threonine plasma concentrations.
|
Conclusions
In conclusion, tangible reductions in dietary CP usually compromise FCR, increase fat deposition and trigger elevations in free threonine systemic plasma concentrations. The elevated threonine concentrations are not thought to be causative; nevertheless, the likelihood is that they are indicative of a compromised performance. The strategy of limiting increases in starch concentrations in reduced-CP broiler diets may enhance performance. However, if the proposed mechanism whereby threonine dehydrogenase is downregulated is valid, elevations in free threonine plasma concentrations may be attenuated as a secondary effect. Finally, the likelihood is that free threonine plasma concentrations may be an indicative biomarker of the precision with which efficient reduced-CP broiler diets are formulated and, if so, would facilitate their successful development.
Conflicts of interest
The authors declare that there not any conflicts of interest.
Acknowledgements
First, we recognise the financial support provided by Australian Government Research and Training Program International Scholarship awarded to Shemil Macelline by Department of Education, Skills and Employment. Second, the authors acknowledge Evonik Nutrition & Care GmbH for their generous support and guidance. Third, we thank Dr Leon McQuade and Mr Bernard McInerney of Australian Proteome Analysis Facility (Macquarie University) for their amino acid analyses of plasma samples.
References
Akagi S, Sato K, Ohmori S (2004) Threonine metabolism in Japanese quail liver. Amino Acids 26, 235–242.| Threonine metabolism in Japanese quail liver.Crossref | GoogleScholarGoogle Scholar | 15221503PubMed |
Aoyama Y, Motokawa Y (1981) L-Threonine dehydrogenase of chicken liver. The Journal of Biological Chemistry 256, 12367–12373.
| L-Threonine dehydrogenase of chicken liver.Crossref | GoogleScholarGoogle Scholar | 7028754PubMed |
Baker DH, Hill TM, Kleiss AJ (1972) Nutritional evidence concerning formation of glycine from threonine in the chick. Journal of Animal Science 34, 582–586.
| Nutritional evidence concerning formation of glycine from threonine in the chick.Crossref | GoogleScholarGoogle Scholar | 5018009PubMed |
Braun EJ, Sweazea KL (2008) Glucose regulation in birds. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology 151, 1–9.
| Glucose regulation in birds.Crossref | GoogleScholarGoogle Scholar |
Chrystal PV, Selle PH, Liu SY (2020a) Facilitating the acceptance of tangibly reduced-crude protein diets for chicken-meat production. Animal Nutrition 6, 247–257.
| Facilitating the acceptance of tangibly reduced-crude protein diets for chicken-meat production.Crossref | GoogleScholarGoogle Scholar | 33005758PubMed |
Chrystal PV, Moss AF, Khoddami A, Naranjo VD, Selle PH, Liu SY (2020b) Impacts of reduced-crude protein diets on key parameters in male broiler chickens offered maize-based diets. Poultry Science 99, 505–516.
| Impacts of reduced-crude protein diets on key parameters in male broiler chickens offered maize-based diets.Crossref | GoogleScholarGoogle Scholar | 32416837PubMed |
Chrystal PV, Moss AF, Khoddami A, Naranjo VD, Selle PH, Liu SY (2020c) Effects of reduced crude protein levels, dietary electrolyte balance and energy density on the performance of broiler chickens offered maize-based diets with evaluations of starch, protein and amino acid metabolism. Poultry Science 99, 1421–1431.
| Effects of reduced crude protein levels, dietary electrolyte balance and energy density on the performance of broiler chickens offered maize-based diets with evaluations of starch, protein and amino acid metabolism.Crossref | GoogleScholarGoogle Scholar | 32115029PubMed |
Chrystal PV, Moss AF, Yin D, Khoddami A, Naranjo VD, Selle PH, Liu SY (2020d) Glycine equivalent and threonine inclusions in reduced-crude protein, maize-based diets impact on growth performance, fat deposition starch-protein digestive dynamics and amino acid metabolism in broiler chickens. Animal Feed Science and Technology 261, 114387
| Glycine equivalent and threonine inclusions in reduced-crude protein, maize-based diets impact on growth performance, fat deposition starch-protein digestive dynamics and amino acid metabolism in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Chrystal PV, Greenhalgh S, McInerney BV, McQuade LR, Selle PH, Liu SY (2021) Maize-based diets are more conducive to crude protein reductions than wheat-based diets for broiler chickens. Animal Feed Science and Technology 275, 114867
| Maize-based diets are more conducive to crude protein reductions than wheat-based diets for broiler chickens.Crossref | GoogleScholarGoogle Scholar |
D’Andrea G (2000) Classifying amino acids as gluco(glyco)genic, ketogenic, or both. Biochemical Education 28, 27–28.
| Classifying amino acids as gluco(glyco)genic, ketogenic, or both.Crossref | GoogleScholarGoogle Scholar | 10717451PubMed |
D’Mello JPF (1973) Aspects of threonine and glycine metabolism in the chick (Gallus domesticus). Nutrition and Metabolism 15, 357–363.
| Aspects of threonine and glycine metabolism in the chick (Gallus domesticus).Crossref | GoogleScholarGoogle Scholar |
Davis AT, Austic RE (1982) Threonine-degrading enzymes in the chicken. Poultry Science 61, 2107–2111.
| Threonine-degrading enzymes in the chicken.Crossref | GoogleScholarGoogle Scholar | 6817321PubMed |
Davis AT, Austic RE (1997) Dietary protein and amino acid levels alter threonine dehydrogenase activity in hepatic mitochondria of Gallus domesticus. The Journal of Nutrition 127, 738–744.
| Dietary protein and amino acid levels alter threonine dehydrogenase activity in hepatic mitochondria of Gallus domesticus.Crossref | GoogleScholarGoogle Scholar |
Dean WF, Scott HM (1966) Use of free amino acid concentrations in blood plasma of chicks to detect deficiencies and excesses of dietary amino acids. The Journal of Nutrition 88, 75–83.
| Use of free amino acid concentrations in blood plasma of chicks to detect deficiencies and excesses of dietary amino acids.Crossref | GoogleScholarGoogle Scholar | 5900635PubMed |
Dunlop MW, Moss AF, Groves PJ, Wilkinson SJ, Stuetz RM, Selle PH (2016) The multidimensional causes of ‘wet litter’ in chicken-meat production. The Science of the Total Environment 562, 766–776.
| The multidimensional causes of ‘wet litter’ in chicken-meat production.Crossref | GoogleScholarGoogle Scholar | 27110988PubMed |
Fancher BI, Jensen LS (1989) Dietary protein levels and essential amino acid content: influence upon female broiler performance during the growing period. Poultry Science 68, 897–908.
| Dietary protein levels and essential amino acid content: influence upon female broiler performance during the growing period.Crossref | GoogleScholarGoogle Scholar | 2780478PubMed |
Fang R, Mantle M, Ceri H (1993) Characterization of quail intestinal mucin as a ligand for endogenous quail lectin. The Biochemical Journal 293, 867–872.
| Characterization of quail intestinal mucin as a ligand for endogenous quail lectin.Crossref | GoogleScholarGoogle Scholar | 8352754PubMed |
Fernández-Fígares I, Prieto C, Nieto R, Aguilera JF (1997) Free amino acid concentrations in plasma, muscle and liver as indirect measures of protein adequacy in growing chickens. Animal Science 64, 529–539.
| Free amino acid concentrations in plasma, muscle and liver as indirect measures of protein adequacy in growing chickens.Crossref | GoogleScholarGoogle Scholar |
Giuberti G, Gallo A, Cerioli C, Masoero F (2012) In vitro starch digestion and predicted glycemic index of cereal grains commonly utilized in pig nutrition. Animal Feed Science and Technology 174, 163–173.
| In vitro starch digestion and predicted glycemic index of cereal grains commonly utilized in pig nutrition.Crossref | GoogleScholarGoogle Scholar |
Greenhalgh S, Chrystal PV, Selle PH, Liu SY (2020a) Reduced-crude protein diets in chicken-meat production: justification for an imperative. World’s Poultry Science Journal 76, 537–548.
| Reduced-crude protein diets in chicken-meat production: justification for an imperative.Crossref | GoogleScholarGoogle Scholar |
Greenhalgh S, McInerney BV, McQuade LR, Chrystal PV, Khoddami A, Zhuang MAM, Liu SY, Selle PH (2020b) Capping dietary starch:protein ratios in moderately reduced crude protein, wheat-based diets showed promise but further reductions generated inferior growth performance in broiler chickens from 7 to 35 days post-hatch. Animal Nutrition 6, 168–178.
| Capping dietary starch:protein ratios in moderately reduced crude protein, wheat-based diets showed promise but further reductions generated inferior growth performance in broiler chickens from 7 to 35 days post-hatch.Crossref | GoogleScholarGoogle Scholar | 32542197PubMed |
Guerranti R, Pagani R, Neri S, Errico SV, Leoncini RE, Marinello E (2001) Inhibition and regulation of rat liver L-threonine dehydrogenase by different fatty acids and their derivatives. Biochimica et Biophysica Acta (BBA) - General Subjects 1568, 45–52.
| Inhibition and regulation of rat liver L-threonine dehydrogenase by different fatty acids and their derivatives.Crossref | GoogleScholarGoogle Scholar |
Hazelwood RL (1984) Pancreatic hormones, insulin/glucagon molar ratios, and somatostatin determinants of avian carbohydrate metabolism. The Journal of Experimental Zoology 232, 647–652.
| Pancreatic hormones, insulin/glucagon molar ratios, and somatostatin determinants of avian carbohydrate metabolism.Crossref | GoogleScholarGoogle Scholar | 6151582PubMed |
Kaempfer S, Blackham M, Christiansen M, Wu K, Cesar D, Vary T, Hellerstein MK (1991) Fraction of hepatic cytosolic acetyl-CoA derived from glucose in vivo: relation to PDH phosphorylation state. American Journal of Physiology (Endocrinology and Metabolism 23) 260, E865–E875.
| Fraction of hepatic cytosolic acetyl-CoA derived from glucose in vivo: relation to PDH phosphorylation state.Crossref | GoogleScholarGoogle Scholar |
Kidd MT, Kerr BJ (1996) L-Threonine for poultry: a review. Journal of Applied Poultry Research 5, 358–367.
| L-Threonine for poultry: a review.Crossref | GoogleScholarGoogle Scholar |
Lee CW, Oh YJ, Son YS, An WG (2011) Effects of dietary protein and threonine supply on in vitro liver threonine dehydrogenase activity and threonine efficiency in rat and chicken. Asian-Australasian Journal of Animal Sciences 24, 1417–1424.
| Effects of dietary protein and threonine supply on in vitro liver threonine dehydrogenase activity and threonine efficiency in rat and chicken.Crossref | GoogleScholarGoogle Scholar |
Lee CW, Cho IJ, Lee YJ, Son YS, Kwak I, Ahn YT, Kim SC, An WG (2014) Effects of dietary levels of glycine, threonine and protein on threonine efficiency and threonine dehydrogenase activity in hepatic mitochondria of chicks. Asian-Australasian Journal of Animal Sciences 27, 69–76.
| Effects of dietary levels of glycine, threonine and protein on threonine efficiency and threonine dehydrogenase activity in hepatic mitochondria of chicks.Crossref | GoogleScholarGoogle Scholar | 25049928PubMed |
Lee CW, Zhao R, Cho IJ, Byun SH, Kim YW, Kim YS, Kim YS, Kim SC, An WG (2016) Influence of protein supply on threonine efficiency and threonine catabolism in hepatic mitochondria of chicks and rats. Annals of Animal Science 16, 223–234.
| Influence of protein supply on threonine efficiency and threonine catabolism in hepatic mitochondria of chicks and rats.Crossref | GoogleScholarGoogle Scholar |
Malinovsky AV (2018) Why threonine is an essential amino acid in mammals and birds: studies at the enzyme level. Biochemistry (Moscow) 83, 795–799.
| Why threonine is an essential amino acid in mammals and birds: studies at the enzyme level.Crossref | GoogleScholarGoogle Scholar |
Moss AF, Sydenham CJ, Khoddami A, Naranjo VD, Liu SY, Selle PH (2018) Dietary starch influences growth performance, nutrient utilisation and digestive dynamics of protein and amino acids in broiler chickens offered low-protein diets. Animal Feed Science and Technology 237, 55–67.
| Dietary starch influences growth performance, nutrient utilisation and digestive dynamics of protein and amino acids in broiler chickens offered low-protein diets.Crossref | GoogleScholarGoogle Scholar |
Pietrocola F, Galluzzi L, Pedro JMB-S, Madeo F, Kroemer G (2015) Acetyl coenzyme A: a central metabolite and second messenger. Cell Metabolism 21, 805–821.
| Acetyl coenzyme A: a central metabolite and second messenger.Crossref | GoogleScholarGoogle Scholar | 26039447PubMed |
Scanes CG (2009) Perspectives on the endocrinology of poultry growth and metabolism. General and Comparative Endocrinology 163, 24–32.
| Perspectives on the endocrinology of poultry growth and metabolism.Crossref | GoogleScholarGoogle Scholar | 19393657PubMed |
Selle PH, Liu SY (2019) The relevance of starch and protein digestive dynamics in poultry. Journal of Applied Poultry Research 28, 531–545.
| The relevance of starch and protein digestive dynamics in poultry.Crossref | GoogleScholarGoogle Scholar |
Selle PH, Dorigam JCdeP, Chrystal PV, Liu SY (2020) Synthetic and crystalline amino acids: alternatives to soybean meal in chicken-meat production. Animals 10, 1079
Selle PH, Truong HH, McQuade LR, Moss AF, Liu SY (2016) Reducing agent and exogenous protease additions, individually and in combination, to wheat- and sorghum-based diets interactively influence parameters of nutrient utilisation and digestive dynamics in broiler chickens. Animal Nutrition 2, 303–311.
| Reducing agent and exogenous protease additions, individually and in combination, to wheat- and sorghum-based diets interactively influence parameters of nutrient utilisation and digestive dynamics in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 29767134PubMed |
Shi L, Tu BP (2015) Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Current Opinion in Cell Biology 33, 125–131.
| Acetyl-CoA and the regulation of metabolism: mechanisms and consequences.Crossref | GoogleScholarGoogle Scholar | 25703630PubMed |
Shurubor YI, D’Aurelio M, Clark-Matott J, Isakova EP, Deryabina YI, Beal MF, Cooper AJL, Krasnikov BF (2017) Determination of Coenzyme A and acetyl-coenzyme A in biological samples using HPLC with UV detection. Molecules 22, 1388
| Determination of Coenzyme A and acetyl-coenzyme A in biological samples using HPLC with UV detection.Crossref | GoogleScholarGoogle Scholar |
Siegert W, Rodehutscord M (2019) The relevance of glycine and serine in poultry nutrition: a review. British Poultry Science 60, 579–588.
| The relevance of glycine and serine in poultry nutrition: a review.Crossref | GoogleScholarGoogle Scholar | 31116025PubMed |
Sinsigalli NA, McMurty JP, Cherry JA, Siegel PB (1987) Glucose tolerance, plasma insulin and immunoreactive glucagon in chickens selected for high and low body weight. The Journal of Nutrition 117, 941–947.
| Glucose tolerance, plasma insulin and immunoreactive glucagon in chickens selected for high and low body weight.Crossref | GoogleScholarGoogle Scholar | 3295145PubMed |
Sugahara M, Kandatsu M (1976) Glycine serine interconversion in the rooster. Agricultural and Biological Chemistry 40, 833–837.
Taouis M, Dridi S, Cassy S, Benomar Y, Raver N, Rideau N, Picard M, Williams J, Gertler A (2001) Chicken leptin: properties and actions. Domestic Animal Endocrinology 21, 319–327.
| Chicken leptin: properties and actions.Crossref | GoogleScholarGoogle Scholar | 11872323PubMed |
Wu G (2014) Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. Journal of Animal Science and Biotechnology 5, 34
| Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition.Crossref | GoogleScholarGoogle Scholar | 24999386PubMed |
Yuan J-H, Austic RE (2001) The effect of dietary protein level on threonine dehydrogenase activity in chickens. Poultry Science 80, 1353–1356.
| The effect of dietary protein level on threonine dehydrogenase activity in chickens.Crossref | GoogleScholarGoogle Scholar | 11558922PubMed |
Yuan J-H, Davis AJ, Austic RE (2000) Temporal response of hepatic threonine dehydrogenase in chickens to the initial consumption of a threonine-imbalanced diet. The Journal of Nutrition 130, 2746–2752.
| Temporal response of hepatic threonine dehydrogenase in chickens to the initial consumption of a threonine-imbalanced diet.Crossref | GoogleScholarGoogle Scholar | 11053516PubMed |


