Mature herbs as supplements to ruminant diets: effects on in vitro ruminal fermentation and ammonia production
Alexandra N. Kapp-Bitter A B , Uta Dickhoefer C , Michael Kreuzer B and Florian Leiber A D
A D
A Department of Livestock Sciences, Research Institute of Organic Agriculture (FiBL), Ackerstrasse 113, 5070 Frick, Switzerland.
B Institute of Agricultural Sciences, ETH Zurich, Universitätstrasse 2, 8092 Zurich, Switzerland.
C Animal Nutrition and Rangeland Management in the Tropics and Subtropics, University of Hohenheim, Schloß Hohenheim 1, 70599 Stuttgart, Germany.
D Corresponding author. Email: florian.leiber@fibl.org
Animal Production Science 61(5) 470-479 https://doi.org/10.1071/AN20323
Submitted: 28 May 2020 Accepted: 20 November 2020 Published: 14 December 2020
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Context: High concentrations of crude protein in ruminant diets may lead to excessive production of ruminal ammonia, which may stress the animal’s metabolism and impact nitrogen efficiency. This may become a problem in zero-concentrate feeding systems when pasture grass is rich in crude protein. Polyphenols such as tannins may protect part of dietary protein from ruminal degradation and thus inhibit ammonia formation.
Aims: The present study screened mature herbs for their potential to mitigate ruminal ammonia formation in cattle, when provided as a supplement to a forage diet.
Methods: Thirty-five temperate-climate, herbaceous meadow plant species (including three legumes) that appear in biodiverse natural and sown pastures were investigated for their effects on ruminal ammonia production. Aboveground material was harvested during ripening of the seeds and analysed for nutrient and phenol concentrations. Net energy and protein absorbable at the duodenum were calculated. Incubations (24 h) with cattle rumen fluid following the in vitro Hohenheim Gas Test protocol were performed to compare the effects of the test plants on ruminal gas and ammonia formation. Test plants replaced one-third of a basal mixture consisting of 57% Lolium perenne L. and 43% Medicago sativa L. (air-dry-matter basis). Results were compared with those obtained with the basal mixture alone.
Key results: According to regression analysis, ammonia concentration after incubation was negatively related to concentrations of total extractable phenols and total tannins in feed mixtures, whereas the relationship was weakly positive with dietary crude protein. In 23 and 19 of the test diets, respectively, in vitro gas production (indicating ruminal organic matter digestibility) and ammonia concentrations in the incubation medium after 24 h were significantly lower than with the basal mixture alone. Incubations containing Galium verum L., Leontodon hispidus L., Lotus corniculatus L., Onobrychis viciifolia Scop., Plantago lanceolata L., Sanguisorba minor Scop. and Scabiosa columbaria L. maintained gas production and estimated in vitro organic matter digestibility while at the same time lowering ammonia concentrations.
Conclusions: Seven mature herbs of a screening of 35 proved to have potential for positive effects on ruminal protein utilisation without impairing fermentation.
Implications: These herbs are of particular interest as dietary supplements for dairy cows grazing protein-rich pastures.
Keywords: condensed tannins, forbs, hydrolysable tannins, plant secondary compounds, protein efficiency.
Introduction
When cattle consume diets excessive in rumen-degradable crude protein (CP), the net ammonia production in the rumen may rise because the fermentable carbohydrate and thus energy supply from these diets limit rumen microbial protein synthesis. Excessive ammonia is absorbed from the rumen, leading to a burden for the ruminant’s metabolism as well as environmental pollution, with easily soluble nitrogen (N)-containing compounds excreted via their urine (Reynolds and Kristensen 2008; Sinz et al. 2019a). Excess dietary rumen-degradable CP supply occurs, for example, in low-input ruminant systems during autumn, when grasses with high CP concentrations but limited energy content are abundant (Pacheco and Waghorn 2008). An alternative approach to compensate for high-protein pasture and conserved forage builds on including plants with elevated concentrations of secondary compounds such as tannins in the diet of ruminants. Tannins bind to feed proteins, with bond formation depending on factors such as pH of the medium, type of protein and other plant compounds (Perez-Maldonado et al. 1995). Accordingly, tannin–protein complexes are formed under ruminal pH conditions. These complexes hamper rumen microbial CP degradation and thus decelerate ammonia formation. Part of these tannin-protected proteins may be released in the abomasum and digested there and in the small intestine (Piluzza et al. 2014). In this way, they can contribute to covering the ruminant’s amino acid requirements. Alternatively, the complexes remain, or are re-established under small intestinal pH conditions, and the protein is excreted with the faeces (Dschaak et al. 2011). Based on these mechanisms, cattle grazing on herb-rich pastures or receiving herbaceous supplements may increase their protein utilisation (Totty et al. 2013). Together with further implications for animal health and welfare (Leiber et al. 2020), this should encourage farmers trying to establish herbs as supplements to cows grazing young grass in spring or autumn. However, it is important to know first the extent to which suitable herb species would contribute to improving protein digestion in ruminants.
Several screenings of herbal species for their nutrient and phenolic contents have been made. Examples are the studies by Macheboeuf et al. (2014), who analysed a large collection of wild plants grown in the French Massif Central Area, Jayanegara et al. (2011), who investigated several alpine forages, and Terranova et al. (2018), who screened woody plants grown in temperate climatic conditions. Still, data on temperate-climate meadow herbs and herbaceous legumes are scarce. These species typically occur on swards established to generate a high biodiversity and are harvested late, often accomplished during seed ripening. In order to assess the suitability of mature, herb-rich swards as supplements that influence protein digestion, plants need to be investigated specifically at this mature stage, because concentrations of phenols can either increase or decrease during plant development, depending on species and other factors (Kälber et al. 2014; Stewart et al. 2019).
The present study was conducted to assess a large number of temperate climate herbs and legumes harvested during the seed-ripening stage. They were compared with respect to their nutrient and phenolic concentrations and their efficiency to reduce in vitro the ammonia concentration in rumen fluid while maintaining organic matter digestibility (OMD) when added to a standard diet. The overall aim was to identify plant species particularly promising at late harvest. Herbs with low rumen ammonia production and high ruminal fermentation rates could then be used as supplements for cattle in periods of dietary CP excess.
Materials and methods
Swiss ecotypes of 35 plant species from 13 plant families (as listed in Table 1) were used. They had been grown as pure cultures in Lenggenwil, Switzerland (47°28′31.34″N, 9°11′11.67″E; 580 m a.m.s.l.). Harvest took place from July to August 2016, when the seeds of the plants ripened. For each plant species, 5–10 kg wet weight was sampled by cutting 1 cm aboveground, divided into three batches, dried at 48°C for 24 h, and milled through a 0.5-mm sieve (SK100; Retsch, Haan, Germany). Pure stands of Lolium perenne L. and Medicago sativa L. were also harvested during the ripening period (first cut from seed-production fields) and treated the same way to form the basal mixture.
Sample analyses for nutrient and phenolic concentrations
All analyses were repeated three times per individual plant species by using the different batches of the harvested material (i.e. n = 3 per test plant species). In the dried material, concentrations of dry matter (DM), total ash, CP, crude fibre, and neutral (NDF) and acid (ADF) detergent fibre were determined with near-infrared reflectance (NIR) spectroscopy (NIRFlex N-500; Büchi, Flawil, Switzerland). This equipment had been previously calibrated with 180 samples from different mixed grass–herb swards analysed in parallel by wet chemistry. For each variable and plant species, the arithmetic mean of three NIR determinations was used. The OM was calculated as DM minus total ash. Using compositional data, net energy for lactation (NEL) and absorbable protein at the duodenum were estimated using regressions of Agroscope (2020). The absorbable protein at the duodenum is limited to either the sum of microbial protein from energy supply and rumen-undegradable protein (APDE) or the sum of microbial protein from rumen-degradable CP and rumen-undegradable protein (APDN) (Colin-Schoellen et al. 2000; Agroscope 2020).
For analysing total extractable phenols (TEP), 60 mg ground plant material was extracted with 6 mL 70% aqueous acetone (v/v). The supernatant was filtered (Cameo syringe filter, non-sterile, pore size 1.2 µm; GVS, Bologna, Italy). From this extract, 0.02 mL was taken to determine the amount of TEP and 1.0 mL the amount non-tannin phenols (NTP), using the Folin-Ciocâlteu method described in detail by Makkar (2003). Absorption was measured at 725 nm with a spectrophotometer (BioSpectrometer D30; Eppendorf, Hamburg, Germany). Total tannins (TT) were calculated as the difference between TEP and NTP, and given as equivalents of tannic acid. Condensed tannins (CT) were analysed following the protocol for the butanol–HCl assay (Makkar 2003). Hydrolysable tannins (HT) are equivalent to the difference between TT and CT. Absorption was measured at 550 nm and given as leucocyanidin equivalents.
In vitro study of ammonia production and organic matter digestibility
As a control, the basal diet, consisting of L. perenne and M. sativa, was incubated at a ratio of 0.57 : 0.43 (114 mg and 86 mg DM) according to the method of Menke and Steingass (1988). Each of the 35 test plants was incubated together with the basal diet at a ratio of 0.30 : 0.70 (test plant : basal diet). For each of the 36 combinations, 200 mg was incubated in a Hohenheim Gas Test apparatus on two different days (two runs) in three syringes each, giving six observations per plant. Along with the test mixtures, in each run, six blank syringes and three syringes each filled with either hay or concentrate as Hohenheim Gas Test standards were incubated for later adjustment of the different runs (Menke and Steingass 1988).
Rumen fluid was taken before the morning feeding from four cannulated lactating Jersey cows (mixture from two cows per run). These cows had been fed with a total mixed ration containing grass and maize silage, grass hay, barley straw and a concentrate mixture at a forage : concentrate ratio of 0.68 : 0.32 (on DM basis). Housing of, and rumen fluid collection from, the rumen-cannulated cows were approved by the Regierungspräsidium Stuttgart, Germany (licence no. A401/14 TE).
After filtration of the rumen fluid, a buffer solution was added to stabilise the pH. A 30-mL sample of this mixture was placed in the pre-warmed syringes and the air was removed. Subsequently, the syringes were placed in a water bath at 39°C for 24 h. The syringes were gently shaken hourly during the first 6 h of incubation. When the gas production exceeded 70 mL after 8 h, syringes were reset to 35 mL by releasing part of the gas produced. After 24 h, the total amount of gas produced was recorded and the incubation fluid was placed in sediment tubes for ammonia analysis by a pH meter (Model 713; Metrohm, Herisau, Switzerland) equipped with an ion-selective electrode. The ammonia value was corrected for the concentration in the blanks. To calculate the net gas production, the mean gas volume produced from the syringes containing only buffered rumen fluid (i.e. blanks) was subtracted from the volumes measured with the respective test diets. This result was adjusted by using a correction factor calculated from the observed and expected net gas productions from the standard hay and standard concentrate. In vitro OMD (IVOMD) was calculated based on the compositional data and the net gas production (Menke and Steingass 1988) as: IVOMD (mg/g OM) = 148.8 + 8.893 × gas production (mL/200 mg DM) + 0.448 × dietary CP (mg/g DM) + 0.651 × dietary total ash (mg/g DM).
Statistical analyses
SPSS Statistics version 24 (IBM, Armonk, NY, USA) was used for statistical analysis. For the in vitro data, the 35 diets containing the test plants were treated as fixed effects and the two incubation runs in the Hohenheim Gas Test as random effects (n = 6 per test-plant-based diet). For assessing differences between the basal mixture and the means obtained with the test plants, Tukey’s procedure was used, and P < 0.05 was considered to be significant. In addition, a principal component analysis (PCA) was performed including all available data from the 35 test diets (total n = 3 × 35 for nutrient and phenol concentrations and IVOMD, and n = 6 × 35 for gas production and ammonia concentration). For this purpose, the compositional data were calculated for the respective diets from composition and proportions of test plants and basal mixture. The first two principal components (PCs) were used to calculate the factor scores for each plant. Finally, based on the data averaged per test-plant-based diet, a regression analysis was performed with gas production and ammonia concentration of the inoculum after 24 h of incubation as dependent variables, and CP, TEP and TT as independent variables. The analyses were first performed with linear, quadratic, exponential and logarithmic terms. The best fitting model is presented, considering P < 0.05 as significant. If none of the models was significant, the linear regression is indicated.
Results
Chemical composition of the test plants
Concentrations of OM covered a range from 832 g/kg DM (Silene nutans) to 973 g/kg DM (Stachys officinalis) (Table 1). The median was 919 g/kg DM. The CP concentrations ranged from 72 g/kg DM for Leucanthemum vulgare to 204 g/kg DM for Campanula rapunculoides with its median was 120 g/kg DM. The concentration of crude fibre was highest in L. vulgare (422 g/kg DM) and lowest in C. rapunculoides (175 g/kg DM). The median was 304 g/kg DM. NDF content was very low in C. rapunculoides at 115 g/kg DM. The highest NDF content of 744 g/kg DM was found in Picris hieracioides, and the median was 452 g/kg DM. The median of ADF was 382 g/kg DM, with the lowest ADF content of 84 g/kg DM found in Knautia arvensis, and the highest (555 g/kg DM) in P. hieracioides. The highest calculated contents of NEL (5.10 MJ/kg DM), APDE (92 g/kg DM) and APDN (131 g/kg DM) were found in C. rapunculoides, and the lowest NEL (1.47 MJ/kg DM) and APDE values (35 g/kg DM) were calculated for L. vulgare. APDN was lowest in L. vulgare and Galium mollugo (both 45 g/kg DM). The medians for these variables were 3.45 MJ NEL/kg, 66 g APDE/kg DM and 76 g APDN/kg DM. A large number of individual plants differed in composition (P < 0.05) from the basal mixture (values in bold in Table 1).
The highest concentrations of TEP and NTP were detected in Origanum vulgare at 192 and 94 g/kg DM, respectively, and the lowest in Tragopogon pratensis subsp. orientalis at 6 and 6 g/kg DM, respectively, with medians at 48 and 33 g/kg DM (Table 2). The concentration of TT was highest in Sanguisorba minor at 135 g/kg DM and lowest in T. pratensis subsp. orientalis, for which no tannins were detected. The median for TT was 15 g/kg DM. Eight plants contained CT: Campanula rapunculus, L. corniculatus, O. viciifolia, Primula elatior, S. minor, Silene vulgaris, S. officinalis and Thymus pulegioides. Among these plants, O. viciifolia had the highest CT concentration at 38 g/kg DM. The median CT concentrations of these eight plants was 4 g/kg DM. The differences between TT and CT show that most test plants were mainly characterised by HT (data for HT not presented because not directly analysed). Except for Anthyllis vulneraria subsp. carpatica, Leucanthemum vulgare and Silene flos-cuculi, all test plants differed (P < 0.05) in at least one of the concentrations of TEP, NTP and TT from those of the basal mixture (values in bold in Table 2).
In vitro organic matter digestibility, gas production and ammonia concentration after incubation
The pH of the incubation fluid after 24 h of incubation was 6.8–6.9 (data not shown). The highest net gas production numerically during 24 h was realised with the basal mixture, at 57 mL/200 mg DM (Table 2). When one-third of the basal mixture was replaced by the test plants, the diet containing S. pratensis resulted in the highest gas production per 200 mg DM (53 mL/24 h), and the diet with O. vulgare the lowest (24 mL/24 h). The median was 43 mL/200 mg DM during 24 h. Compared with the basal mixture, gas production was decreased (P < 0.05) with 23 of the 35 test diets. The median of IVOMD was 541 g/kg OM, the lowest value was 336 g/kg OM (with O. vulgare) and the highest value 684 g/kg OM with P. lanceolata. Compared with the basal mixture, IVOMD was decreased (P < 0.05) with 18 of the 35 test diets. The median ruminal ammonia concentration of the incubation fluid after 24 h of incubation was 16 mmol/L. The lowest value was 11 mmol/L (with P. hieracioides) and the highest was 22 mmol/L (with S. flos-cuculi). Nineteen test diets led to lower (P < 0.05) ammonia concentrations after 24 h of incubation than the basal mixture alone.
Results of principal component and regression analyses
A close relationship among variables of plant composition and in vitro ruminal fermentation variables was shown by PCA, with the first PC explaining 38.8% and the second 21.3% of the variation, adding up to 60.1% (Fig. 1). Antagonistic relationships were observed for the concentrations of TEP, NTP or TT with net gas production or ammonia concentration, and for fibre contents (NDF and ADF) on the one side with CP, APDN, APDE and NEL on the other. Separation of ammonium formation from gas production and IVOMD by PCA was not possible.

|
The plot of the first two PC scores in Fig. 2 describes the association of each plant to the factor scores. In general, those test plants situated in the upper half of the plot were rich in phenols, and those in the lower half had high gas production and ammonia concentrations after 24 h when incubated in combination with the basal mixture. The test-plant species arranged at the right side of the plot had high CP concentration and high nutritive values concerning APDE, APDN and NEL, whereas the ones on the left side were characterised by high fibre concentrations. The test plants allocated to the upper right quadrant of the plot were relatively rich in phenols and had a high nutritional value (i.e. rich in CP, NEL, APDE and APDN). When incubated together with the basal mixture, they had the potential to reduce ammonia concentrations in vitro while improving the nutritional value of the mixed diet concerning NEL, APDE and APDN compared with the basal mixture alone. The plants found in this quadrant included C. rapunculoides, O. viciifolia, O. vulgare, P. atrata, P. lanceolata, P. elatior, P. vulgaris, S. pratensis and S. minor. However, no plant reached the centre or even the right upper corner of this quadrant, which would have meant a clear association of low ammonia production and high nutritional quality. On the other hand, in particular C. rapunculus, G. mollugo, G. verum, H. pilosella, L. hispidus, L. vulgare, P. hieracioides, S. columbaria, S. vulgaris and T. pratensis subsp. orientalis had low concentrations of TEP, similar to those of the basal diet, and resulted in high gas production and ammonia concentrations when incubated together with the basal mixture.

|
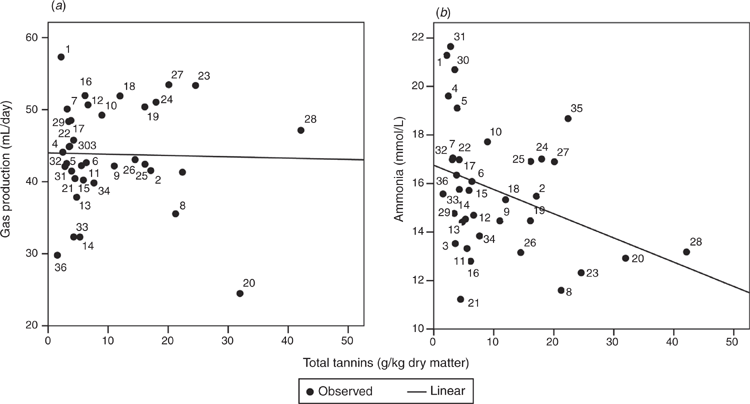
Regression analyses served to identify relationships of CP, TEP and TT concentrations of the test-plant based diets on the one hand with in vitro gas production and ammonia concentrations on the other hand. As expected, CP concentration was positively related (P < 0.05) to ammonia concentration after 24 h of incubation (Fig. 3b), whereas the relationship of CP and total gas production was not significant (Fig. 3a). There was a quadratic relationship (P < 0.05) between TEP and gas production, with an increase followed by a decrease at higher TEP concentrations of the diet (Fig. 4a). Incubating test plants with the basal mixture reduced ammonia concentrations in a linear relation (P < 0.05) with increasing contents of TEP (Fig. 4b) and TT (Fig. 5b). No relationship was found between TT and gas production (Fig. 5a).

|

|
|
Discussion
The goal of the present study was to compare different temperate herb species harvested at a late growth stage with respect to their nutrient and phenol concentrations and their influence on gas production and ammonia formation during 24 h of in vitro incubation in rumen fluid together with a basal diet. One objective was to identify herbs that have a high digestibility (estimated as IVOMD from nutrient composition and gas volume) and yet, at the same time, are able to lower ammonia formation in rumen. Such plants would be of value for further investigation into developing forage-based feed additives for improving protein utilisation by dairy cows. A second objective was to establish relationships among compositional and fermentation variables across a large number of plant species for this late harvest stage. All 35 plants tested were grown at the same time and at the same site, which enhanced comparability among plants but restricted the applicability of the results to these conditions. The dietary inclusion of 30% was high with respect to practical feasibility; however, levels of tannin supplementation that are too low may fail to achieve effects (Kapp-Bitter et al. 2020). The voluntary intake of plants containing high phenolic contents may be reduced or, in certain metabolic states of the animal, unchanged or even increased (Villalba et al. 2015). However, these effects should be the subject of future investigations and of targeted on-farm management.
Ammonia-mitigation potential of the test plants
Technically, ammonia could be measured only once, at the end-point of incubation after 24 h. Therefore, this approach does not reflect temporal dynamics of ruminal N metabolism, but it can serve as an indicator of ammonia formation in vivo. The in vitro ammonia concentration in the inoculum after 24 h of incubation increased with increasing CP concentration of the plants, as expected (Frank and Swensson 2002; Chanthakhoun et al. 2014). Ammonia production depends on the rumen-degradable CP concentration of the diet and, simultaneously, on the accessible energy available in the rumen (Reynolds and Kristensen 2008). Absorbed ammonia must be considered as a loss, and is even a metabolic burden for the liver (Parker et al. 1995), and it contributes significantly to blood and milk urea concentrations and thus urinary N excretion (Nousiainen et al. 2004). It is expected that tannins will protect dietary proteins from ruminal degradation by the formation of complexes (Perez-Maldonado et al. 1995), which could reduce the generation of ammonia and improve protein efficiency of dairy cows. There is in vitro (Jayanegara et al. 2011; Sinz et al. 2019b) and in vivo evidence (Kälber et al. 2012; Sinz et al. 2019a) that tannin-rich forage herbs may have this effect. It is important to identify forage combinations that simultaneously lower ruminal ammonia concentration and maintain fermentation efficiency, including microbial protein synthesis (Jayanegara et al. 2013; Sinz et al. 2019b).
Results of the present study confirm the expected negative relationship between phenol concentrations and ammonia formation during in vitro incubation. The reasons for this include especially the protein-binding effects of tannins (McSweeney et al. 2001) as discussed above. Although other factors cannot be excluded, the present results suggest that the ammonia production was negatively related with TT concentrations. Among the tannins, CT are likely most efficient for lowering ammonia production in rumen (Naumann et al. 2017; Koenig et al. 2018). However, because only a few of the test plants contained detectable levels of CT, the relationships with CT established in the present study should be considered with care. Accordingly, the position of CT in the PCA was not clear in one direction, as was also the case in the study by Jayanegara et al. (2011). Rather, it is likely that HT played a major role here. Additionally, of the 19 test-plant diets that lowered ammonia concentration, all except those with P. lanceolata, S. vulgaris and T. pratensis subsp. orientalis had greater NTP concentration than the basal mixture. Thus, effects of other phenols such as flavonoids (Waghorn and McNabb 2003; Sinz et al. 2018) or differences concerning other compounds (e.g. CP content) have to be considered. Further, there were incubation mixtures with greater TEP content in which the ammonia concentration in the inoculum after 24 h was not different from the basal mixture even if the TT content of the test plants was greater than that in the basal mixture. This is consistent with results of previous research, where effects on ammonia formation of phenol-rich plants were found to depend on the plant species, the extracts of which were investigated by Sinz et al. (2019b). Although not investigated in the present study, plant bioactive lipid compounds may also have affected ammonia formation (Khiaosa-ard and Zebeli 2013).
Relationships between ammonia mitigation and nutritive and digestion characteristics
It was not possible to separate effects on the different incubation variables, meaning that ammonia was mitigated together with gas and, consequently, also IVOMD in most cases. The desired candidate would be a herb that results in incubation variables that are displayed in the upper right corner of the PCA, indicating low ammonia formation at high dietary protein. No test plant completely fulfilled this criterion. However, some approached this goal, among them C. rapunculoides and S. pratensis, both of which had rather high protein content and still only average ammonia formation. Both were characterised by high proportions of NTP. On the other hand, O. vulgare and S. minor expressed low ammonia formation at average nutrient concentrations. These plants were both characterised by high TEP concentrations but almost an absence of CT, indicating that HT indeed had a major effect, which is in line with the literature (Jayanegara et al. 2011; Aboagye et al. 2019; Stewart et al. 2019). However, again, the NTP fraction was also clearly present in these plants and might have played a role (Sinz et al. 2018). The two herbs with high CT concentrations (L. corniculatus and O. viciifolia) also had quite clear mitigating effects on ammonia formation, as expected (Mueller Harvey 2006; Dschaak et al. 2011; Ghelichkhan et al. 2018), at fair dietary protein contents. Among the test plants of the present study, O. viciifolia is the most researched with respect to ammonia formation. It was found earlier to have clear rumen-ammonia-mitigating properties across various cultivars and harvest dates (Scharenberg et al. 2009; Azuhnwi et al. 2012; Grosse Brinkhaus et al. 2016).
In the PCA, negative relationships of concentrations of NEL, APDE and APDN with fibre (NDF and ADF) concentrations were expected because fibrous carbohydrates have a lower digestibility than other carbohydrates. There was a quadratic relationship between dietary TEP concentrations and gas production, indicating that at low and high TEP concentrations adverse effects will occur, whereas intermediate TEP concentrations may even promote ruminal nutrient degradation. This supports the observation that higher TEP concentrations impair certain rumen bacteria and thus, for instance, inhibit cellulolysis (McSweeney et al. 2001; Min et al. 2002) and, with this, nutrient degradation. It explains why apparent total tract digestibility declined with increasing dietary TEP concentrations in the in vivo studies of Yang et al. (2017) and Henke et al. (2017).
Conclusions
Among 35 mature herbs grown in a temperate climate, we did not find one that would decrease ammonia formation and at the same time improve IVOMD in in vitro rumen fermentation. We found indications of a correlation of HT and NTP with ammonia mitigation by this plant type. Galium verum, Leontodon hispidus, Lotus corniculatus, Onobrychis viciifolia, Plantago lanceolata, Sanguisorba minor and Scabiosa columbaria mitigated ammonia and moderately maintained IVOMD. These plants might be beneficial for lowering ammonia production when supplemented to grassland-based diets excessive in CP, and this without impairing nutritional quality. Implementation of such plants in farm practice appears realistic because all plants investigated were grown for seed production, thus making them available for cultivation. Further studies should investigate whether these plants will also exhibit the detected effects in vivo, and whether this is the case at lower dietary proportions.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This research was supported by the Swiss National Science Foundation (project no. 31003A_166425). The authors are grateful to the seed producer Johannes Burri from Fenaco, who helped with sampling of the plants, and to Herrmann Baumgärtner for his help with the laboratory analysis at the University of Hohenheim.
References
Aboagye IA, Oba M, Koenig KM, Zhao GY, Beauchemin KA (2019) Use of gallic acid and hydrolyzable tannins to reduce methane emission and nitrogen excretion in beef cattle fed a diet containing alfalfa silage. Journal of Animal Science 97, 2230–2244.| Use of gallic acid and hydrolyzable tannins to reduce methane emission and nitrogen excretion in beef cattle fed a diet containing alfalfa silage.Crossref | GoogleScholarGoogle Scholar | 30906949PubMed |
Agroscope (2020) Feeding recommendations for ruminants. [In German] Agroscope, Posieux FR, Switzerland. Available at https://www.agroscope.admin.ch/agroscope/de/home/services/dienste/futtermittel/fuetterungsempfehlungen-wiederkaeuer.html [Accessed 03 December 2020]
Azuhnwi BN, Thomann B, Arrigo Y, Boller B, Hess HD, Kreuzer M, Dohme-Meier F (2012) Ruminal dry matter and crude protein degradation kinetics of five sainfoin (Onobrychis viciifolia Scop) accessions differing in condensed tannin content and obtained from different harvests. Animal Feed Science and Technology 177, 135–143.
| Ruminal dry matter and crude protein degradation kinetics of five sainfoin (Onobrychis viciifolia Scop) accessions differing in condensed tannin content and obtained from different harvests.Crossref | GoogleScholarGoogle Scholar |
Chanthakhoun V, Wanapat M, Berg J, Kang S (2014) Influence of crude protein and energy level on feed intake, ruminal ammonia nitrogen, and methylglyoxal production in swamp buffaloes (Bubalus bubalis). Journal of Animal and Plant Sciences 24, 1716–1723.
Colin-Schoellen O, Jurjanz S, Laurent F (2000) Metabolizable protein supply (APDE) and restricted level of ruminally degradable nitrogen (APDN) in total mixed rations: effect on milk production and composition and on nitrogen utilization by dairy cows. Livestock Production Science 67, 41–53.
| Metabolizable protein supply (APDE) and restricted level of ruminally degradable nitrogen (APDN) in total mixed rations: effect on milk production and composition and on nitrogen utilization by dairy cows.Crossref | GoogleScholarGoogle Scholar |
Dschaak CM, Williams CM, Holt MS, Eun JS, Young AJ, Min BR (2011) Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. Journal of Dairy Science 94, 2508–2519.
| Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 21524543PubMed |
Frank B, Swensson C (2002) Relationship between content of crude protein in rations for dairy cows and milk yield, concentration of urea in milk and ammonia emissions. Journal of Dairy Science 85, 1829–1838.
| Relationship between content of crude protein in rations for dairy cows and milk yield, concentration of urea in milk and ammonia emissions.Crossref | GoogleScholarGoogle Scholar | 12201534PubMed |
Ghelichkhan M, Eun JS, Christensen RG, Stott RD, MacAdam JW (2018) Urine volume and nitrogen excretion are altered by feeding birdsfoot trefoil compared with alfalfa in lactating dairy cows. Journal of Animal Science 96, 3993–4001.
| Urine volume and nitrogen excretion are altered by feeding birdsfoot trefoil compared with alfalfa in lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 29982473PubMed |
Grosse Brinkhaus A, Bee G, Silacci P, Kreuzer M, Dohme-Meier F (2016) Effect of exchanging Onobrychis viciifolia and Lotus corniculatus for Medicago sativa on ruminal fermentation and nitrogen turnover in dairy cows. Journal of Dairy Science 99, 4384–4397.
| Effect of exchanging Onobrychis viciifolia and Lotus corniculatus for Medicago sativa on ruminal fermentation and nitrogen turnover in dairy cows.Crossref | GoogleScholarGoogle Scholar | 26995129PubMed |
Henke A, Dickhoefer U, Westreicher-Kristen E, Knappstein K, Molkentin J, Hasler M, Susenbeth A (2017) Effect of dietary Quebracho tannin extract on feed intake, digestibility, excretion of urinary purine derivatives and milk production in dairy cows. Archives of Animal Nutrition 71, 37–53.
| Effect of dietary Quebracho tannin extract on feed intake, digestibility, excretion of urinary purine derivatives and milk production in dairy cows.Crossref | GoogleScholarGoogle Scholar | 27830586PubMed |
Jayanegara A, Marquardt S, Kreuzer M, Leiber F (2011) Nutrient and energy content, in vitro ruminal fermentation characteristics and methanogenic potential of alpine forage plant species during early summer. Journal of the Science of Food and Agriculture 91, 1863–1870.
| Nutrient and energy content, in vitro ruminal fermentation characteristics and methanogenic potential of alpine forage plant species during early summer.Crossref | GoogleScholarGoogle Scholar | 21480269PubMed |
Jayanegara A, Marquardt S, Wina E, Kreuzer M, Leiber F (2013) In vitro indications for favourable non-additive effects on ruminal methane mitigation between high-phenolic and high-quality forages. British Journal of Nutrition 109, 615–622.
| In vitro indications for favourable non-additive effects on ruminal methane mitigation between high-phenolic and high-quality forages.Crossref | GoogleScholarGoogle Scholar |
Kälber T, Kreuzer M, Leiber F (2012) Silages containing buckwheat and chicory: quality, digestibility and nitrogen utilisation by lactating cows. Archives of Animal Nutrition 66, 50–65.
| Silages containing buckwheat and chicory: quality, digestibility and nitrogen utilisation by lactating cows.Crossref | GoogleScholarGoogle Scholar | 22397096PubMed |
Kälber T, Kreuzer M, Leiber F (2014) Milk fatty acid composition of dairy cows fed green whole-plant buckwheat, phacelia or chicory in their vegetative and reproductive stage. Animal Feed Science and Technology 193, 71–83.
| Milk fatty acid composition of dairy cows fed green whole-plant buckwheat, phacelia or chicory in their vegetative and reproductive stage.Crossref | GoogleScholarGoogle Scholar |
Kapp-Bitter AN, Dickhoefer U, Suglo E, Baumgartner L, Kreuzer M, Leiber F (2020) Graded supplementation of chestnut tannins to dairy cows fed protein-rich spring pasture: effects on indicators of protein utilization. Journal of Animal and Feed Sciences 29, 97–104.
| Graded supplementation of chestnut tannins to dairy cows fed protein-rich spring pasture: effects on indicators of protein utilization.Crossref | GoogleScholarGoogle Scholar |
Khiaosa-ard R, Zebeli Q (2013) Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants. Journal of Animal Science 91, 1819–1830.
| Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants.Crossref | GoogleScholarGoogle Scholar | 23345564PubMed |
Koenig KM, Beauchemin KA, McGinn SM (2018) Feeding condensed tannins to mitigate ammonia emissions from beef feedlot cattle fed high-protein finishing diets containing distillers grains. Journal of Animal Science 96, 4414–4430.
| Feeding condensed tannins to mitigate ammonia emissions from beef feedlot cattle fed high-protein finishing diets containing distillers grains.Crossref | GoogleScholarGoogle Scholar | 30032212PubMed |
Leiber F, Walkenhorst M, Holinger M (2020) The relevance of feed diversity and choice in nutrition of ruminant livestock. Landbauforschung 70, 35–38.
| The relevance of feed diversity and choice in nutrition of ruminant livestock.Crossref | GoogleScholarGoogle Scholar |
Macheboeuf D, Coudert L, Bergeault R, Lalière G, Niderkorn V (2014) Screening of plants from diversified natural grasslands for their potential to combine high digestibility, and low methane and ammonia production. Animal 8, 1797–1806.
| Screening of plants from diversified natural grasslands for their potential to combine high digestibility, and low methane and ammonia production.Crossref | GoogleScholarGoogle Scholar | 25046582PubMed |
Makkar HPS (2003) ‘Quantification of tannins in tree foliage: a laboratory manual.’ (Springer Verlag: Dordrecht, Netherlands)
McSweeney CS, Palmer B, McNeill DM, Krause DO (2001) Microbial interactions with tannins: nutritional consequences for ruminants. Animal Feed Science and Technology 91, 83–93.
| Microbial interactions with tannins: nutritional consequences for ruminants.Crossref | GoogleScholarGoogle Scholar |
Menke KH, Steingass H (1988) Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Animal Research and Development 28, 7–55.
Min BR, Attwood GT, Reilly K, Sun W, Peters JS, Barry TN, McNabb WC (2002) Lotus corniculatus condensed tannins decrease in vivo populations of proteolytic bacteria and affect nitrogen metabolism in the rumen of sheep. Canadian Journal of Microbiology 48, 911–921.
| Lotus corniculatus condensed tannins decrease in vivo populations of proteolytic bacteria and affect nitrogen metabolism in the rumen of sheep.Crossref | GoogleScholarGoogle Scholar | 12489781PubMed |
Mueller-Harvey I (2006) Unravelling the conundrum of tannins in animal nutrition and health. Journal of the Science of Food and Agriculture 86, 2010–2037.
| Unravelling the conundrum of tannins in animal nutrition and health.Crossref | GoogleScholarGoogle Scholar |
Naumann HD, Tedeschi LO, Zeller WE, Huntley NF (2017) The role of condensed tannins in ruminant animal production: advances, limitations and future directions. Revista Brasileira de Zootecnia 46, 929–949.
| The role of condensed tannins in ruminant animal production: advances, limitations and future directions.Crossref | GoogleScholarGoogle Scholar |
Nousiainen J, Shingfield KJ, Huhtanen P (2004) Evaluation of milk urea nitrogen as a diagnostic of protein feeding. Journal of Dairy Science 87, 386–398.
| Evaluation of milk urea nitrogen as a diagnostic of protein feeding.Crossref | GoogleScholarGoogle Scholar | 14762082PubMed |
Pacheco D, Waghorn GC (2008) Dietary nitrogen definitions, digestion, excretion and consequences of excess for grazing ruminants. Proceedings of the New Zealand Grassland Association 70, 107–116.
| Dietary nitrogen definitions, digestion, excretion and consequences of excess for grazing ruminants.Crossref | GoogleScholarGoogle Scholar |
Parker DS, Lomax MA, Seal CJ, Wilton JC (1995) Metabolic implications of ammonia production in the ruminant. The Proceedings of the Nutrition Society 54, 549–563.
| Metabolic implications of ammonia production in the ruminant.Crossref | GoogleScholarGoogle Scholar | 8524901PubMed |
Perez-Maldonado RA, Norton BW, Kerven GL (1995) Factors affecting in vitro formation of tannin-protein complexes. Journal of the Science of Food and Agriculture 69, 291–298.
| Factors affecting in vitro formation of tannin-protein complexes.Crossref | GoogleScholarGoogle Scholar |
Piluzza G, Sulas L, Bullitta S (2014) Tannins in forage plants and their role in animal husbandry and environmental sustainability: a review. Grass and Forage Science 69, 32–48.
| Tannins in forage plants and their role in animal husbandry and environmental sustainability: a review.Crossref | GoogleScholarGoogle Scholar |
Reynolds CK, Kristensen NB (2008) Nitrogen recycling through the gut and the nitrogen economy of ruminants: an asynchronous symbiosis. Journal of Animal Science 86, E293–E305.
| Nitrogen recycling through the gut and the nitrogen economy of ruminants: an asynchronous symbiosis.Crossref | GoogleScholarGoogle Scholar | 17940161PubMed |
Scharenberg A, Kreuzer M, Dohme F (2009) Suitability of sainfoin (Onobrychis viciifolia) hay as a supplement to fresh grass in dairy cows. Asian-Australasian Journal of Animal Sciences 22, 1005–1015.
| Suitability of sainfoin (Onobrychis viciifolia) hay as a supplement to fresh grass in dairy cows.Crossref | GoogleScholarGoogle Scholar |
Sinz S, Kunz C, Liesegang A, Braun U, Marquardt S, Soliva CR, Kreuzer M (2018) In vitro bioactivity of various pure flavonoids in ruminal fermentation, with special reference to methane formation. Czech Journal of Animal Science 63, 293–304.
| In vitro bioactivity of various pure flavonoids in ruminal fermentation, with special reference to methane formation.Crossref | GoogleScholarGoogle Scholar |
Sinz S, Liesegang A, Kreuzer M, Marquardt S (2019a) Do supplements of Acacia mearnsii and grapeseed extracts alone or in combination alleviate metabolic nitrogen load and manure nitrogen emissions of lambs fed a high crude protein diet? Archives of Animal Nutrition 73, 306–323.
| Do supplements of Acacia mearnsii and grapeseed extracts alone or in combination alleviate metabolic nitrogen load and manure nitrogen emissions of lambs fed a high crude protein diet?Crossref | GoogleScholarGoogle Scholar | 31164000PubMed |
Sinz S, Marquardt S, Soliva CR, Braun U, Liesegang A, Kreuzer M (2019b) Phenolic plant extracts are additive in their effects against in vitro ruminal methane and ammonia formation. Asian-Australasian Journal of Animal Sciences 32, 966–976.
| Phenolic plant extracts are additive in their effects against in vitro ruminal methane and ammonia formation.Crossref | GoogleScholarGoogle Scholar | 30744370PubMed |
Stewart EK, Beauchemin KA, Dai X, MacAdam JW, Christensen RG, Villalba JJ (2019) Effect of tannin-containing hays on enteric methane emissions and nitrogen partitioning in beef cattle. Journal of Animal Science 97, 3286–3299.
| Effect of tannin-containing hays on enteric methane emissions and nitrogen partitioning in beef cattle.Crossref | GoogleScholarGoogle Scholar | 31242504PubMed |
Terranova M, Kreuzer M, Braun U, Schwarm A (2018) In vitro screening of temperate climate forages from a variety of woody plants for their potential to mitigate ruminal methane and ammonia formation. The Journal of Agricultural Science 156, 929–941.
| In vitro screening of temperate climate forages from a variety of woody plants for their potential to mitigate ruminal methane and ammonia formation.Crossref | GoogleScholarGoogle Scholar |
Totty VK, Greenwood SL, Bryant RH, Edwards GR (2013) Nitrogen partitioning and milk production of dairy cows grazing simple and diverse pastures. Journal of Dairy Science 96, 141–149.
| Nitrogen partitioning and milk production of dairy cows grazing simple and diverse pastures.Crossref | GoogleScholarGoogle Scholar | 23164232PubMed |
Villalba JJ, Provenza FD, Catanese F, Distel RA (2015) Understanding and manipulating diet choice in grazing animals. Animal Production Science 55, 261–271.
| Understanding and manipulating diet choice in grazing animals.Crossref | GoogleScholarGoogle Scholar |
Waghorn GC, McNabb WC (2003) Consequences of plant phenolic compounds for productivity and health of ruminants. The Proceedings of the Nutrition Society 62, 383–392.
| Consequences of plant phenolic compounds for productivity and health of ruminants.Crossref | GoogleScholarGoogle Scholar | 14506885PubMed |
Yang K, Wei C, Zhao GY, Xu ZW, Lin SX (2017) Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility. Journal of Animal Physiology and Animal Nutrition 101, 302–310.
| Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility.Crossref | GoogleScholarGoogle Scholar | 27272696PubMed |




