Dietary calcium supplementation affects nutrient digestibility and antler-production performance during the antler-velvet growth period of male sika deer
Weili Sun A * , Haiping Zhao A * , Kun Bao A , Chunyi Li A and Guangyu Li A BA Institute of Special Animal and Plant Sciences, Chinese Academy of Agriculture Sciences, Jilin Provincial Key Laboratory for Molecular Biology of Special Economic Animals, No. 4899, Juye Street, Jingyue District, Changchun City, Jilin Province 130112, China.
B Corresponding author. Email: tcslgy@126.com
Animal Production Science 59(9) 1689-1695 https://doi.org/10.1071/AN17862
Submitted: 11 December 2017 Accepted: 29 October 2018 Published: 25 January 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Effects of calcium (Ca) supplementation on nutrient digestibility, physiochemical characteristics and antler growth in farmed male sika deer were investigated. Eighteen sika deer (6 years old, 105.50 ± 5.05 kg) were assigned into the following three treatments where they had ad libitum access to water for 90 days: (1) control (C), basal diet containing 0.5% Ca; (2) Ca1.10, basal diet supplemented with 0.6% Ca; and (3) Ca1.70, basal diet supplemented with 1.2% Ca. The basal diet contained 0.50% Ca and 0.34% phosphorus (P). Each group consisted of the same ratio of Ca to P (provided as CaCO3 and CaHPO4). The results showed that the digestibility of dry matter (DM) and crude protein in the Ca1.70 group was lower than in the other two groups. The digestibilities of Ca, P and neutral detergent fibre in the Ca1.10 group were higher than those in the C group and Ca1.70 group (P < 0.05). Concentrations of Ca and P in faeces increased with an increasing supplementation level of Ca and the highest concentrations were observed in the Ca1.70 group (P < 0.05). There were no differences in the concentrations of parathyroid hormone, alkaline phosphatase and osteocalcin among the treatments. Testosterone and oestradiol concentrations of the Ca1.7 group were higher than those of the C and Ca1.10 groups (P < 0.05). Average daily gains of fresh antler weight and dry antler weight of the groups Ca1.10 and Ca1.70 were greater than those of the C (P < 0.05). Fresh and dry antler yields of the Ca1.10 group were higher than those of the other groups (P < 0.05). In conclusion, optimal level of Ca supplement was found to be total Ca concentration of 1.10–1.70%, on the basis of DM, which significantly increased feed digestibility and antler daily gain for the 6-year-old sika deer.
Additional keywords: antler growth, Ca, P.
Introduction
Velvet antler is a precious animal-based traditional Chinese medicine. Previous studies on humans and rats have convincingly shown the health benefits of deer velvet-antler supplements (Wu et al. 2013). Antlers of deer arguably are the fastest and most robust bone development organs in the animal kingdom. An antler is composed of ~50% minerals and of these, 45% is calcium (Ca), and 19% phosphorus (P) (Chapman 1975). Composition explained a mean variability of 77% in antler length and weight. Bodyweight and size, in turn, influenced mineral composition (Landete-Castillejos et al. 2007). Animal skeletons not only provide strong framework for supporting muscles and protect delicate organs and tissues, such as bone marrow, but also form joints to allow movement, and are malleable to allow growth. Furthermore, the skeletal reserve of Ca actively supports Ca homeostasis (Bain and Watkins 1993). Calcium metabolism must be described before the assessment of the Ca value of feeds can be addressed. Calcium is absorbed from the diet according to need by a hormonally regulated process in the small intestine (Schneider 1985; Bronner 1987), up to limits set by the diet and by the net movement of Ca into or out of the skeleton.
Growth of deer antlers requires large amounts of P and Ca in a short period (less than 90 days). It has been calculated out that a pair of red deer antlers weighing 13 kg and grown for 130 days corresponds to an average daily increase of 100 g of bone (Chapman 1975). The source of these large amounts of minerals used for antler growth has not clearly been determined; namely, whether they are obtained directly from food or, in part, at the expense of other parts of the body, or both. Indeed, a similar picture was found in the ribs, metacarpus, metatarsus and tibia of mule deer, where it was found that there was a cyclic mobilisation of cortical bone minerals during antler growth, despite high quantities of minerals in the food that could be accessed (Brown 1990; Grasman and Hellgren 1993). During antler growth, there was a marked increase in resorption foci and a decrease in bone density and the reverse occurred on cessation of antler growth, so that the cortical bone soon returned to a normal, stable configuration. Before the rutting season, during the fitness-recovery period in July and August, the process reverses and bone mineral density is restored. In mule deer, mineral resorption is highest in the ribs, reaching 23% during the middle period of antler growth, and falling to less than 3% by the time antler growth is completed (Chapman 1975). Resorption was greatest during the mid-period of antler growth, reaching 23% for ribs, 13% for the metacarpus and 10% for the metatarsus (Banks et al.1968b). After antler growth was completed, the resorption in the ribs fell to less than 3%. This cyclical, physiological osteoporosis was confirmed by measurement of the density of the bone, and contents of ash, Ca, magnesium (Mg) and P (Banks et al. 1968; Hillman et al. 1973). Recently, scientists have aimed to identify the genes and pathways that significantly alter their expression levels when osteoporosis develops as well as in the reverse phase in the deer skeleton during the antler growth cycle of deer (Borsy et al. 2009; Stéger et al. 2010). These genes include IGSF4, FABP3, FABP4, FKBP2, TIMP2, TMSB4X, TRIB, and members of the Wnt signalling.
An improvement in antler formation was noted in deer that were fed Ca and P supplements as compared with the deer that were on the Ca- and P-deficient diets (Magruder et al. 1957). The Ca-deficient diet was again observed to limit antler growth when compared with with Ca-supplemented diet, but the effect was not as marked as in the other deficiencies. Studies have reported the suitable concentration of P in white-tail deer and red deer (French et al. 1956; Muir et al. 1987). However, the effects of suitable concentration of Ca and P on the deer nutrient digestibility and the velvet antler production remain to be demonstrated in Chinese sika deer. The aim of the present study was to evaluate the effects of dietary Ca on nutrient digestibility, blood biochemical parameters and production performance of velvet antlers for the farmed male sika deer during velvet-antler growth period.
Materials and methods
The experiment was conducted under the animal care and user guidelines at the Antler Deer Farm of the Institute of Special Animal and Plant Research, Chinese Academy of Agricultural Science (Jilin, China), from 15 May 2015 to 15 August 2015.
Animals
Eighteen 6-year-old male sika deer with an average bodyweight of 105.50 ± 5.05 kg were randomly assigned into three groups (six deer/group) and offered the same level of nutrients except for Ca and P contents (the same ratio of Ca to P). All of deer were fed a totally mixed rations and supplemented with 0% (control), 0.6% (Ca1.10) and 1.2% (Ca1.70) Ca respectively. Calcium and P were added to the diets in the form of CaCO3 and CaHPO4 (Jilin Teyan Feed Co., Zuojia, Jilin, Jilin Province, China) during the feed processing. The ingredients and composition of the basal diet are listed in Table 1.

|
Experiment design
The experiments were started from the hard antler button casting and finished when antlers grew to the final stage (but still in velvet, ~90 days). Antler removal day was determined by the farm professional technicians. The digestion trials were conducted three times in the end of first month, second month and last month, and lasted for 4 days each time. Faecal samples were collected individually in the same time during the period of digestion trials. The total velvet-antler growth experiment lasted for 90 days. The digestibility (%) of dry matter (DM), crude protein (CP), neutral detergent fibre (NDF), acid detergent fibre (ADF), Ca and P were determined using the method of ash insoluble in 2 mol/L HCl. The apparent digestibilities of DM, CP, ADF, NDF, Ca and P were determined according to the following equation (Huang et al. 2015).

where D represents the nutrient apparent digestibility; A is the concentration of HCl insoluble ash in diet; A1 is the concentration of HCl-insoluble ash in faeces; B is the concentration of nutrients in diet; B1 is the concentration of nutrients in faeces.
Management
Each group of experimental deer was kept under uniform conditions by housing them in 10 m × 20 m enclosures. Deer in the same group were separated with fences and walls and feed was supplied separately, with equal shares of the ration every day. Animals had access to fresh and clean water ad libitum. Feed intake was recorded precisely. All training and blood sampling of the animals were performed by the same person. The experiments were conducted after 1-week adjustment period, during which the animals were accustomed to the experimental feed.
Blood sampling
Blood samples were collected at 65th days after the study started, through jugular venipuncture in the morning before feeding. Deer were anesthetised with xylazine hydrochloride (Qing dao Hanhe Animal and Plant Medicine Co., Qing dao, Shandong Province, China), which was administered by blow-gun dart syringe at a dosage of 0.5–3.0 mg/kg of bodyweight. Deer were recovered by tolazoline hydrochloride (Sigma Chemical Co., St Louis, MO, USA). The blood was collected into the separation gel coagulation promoting tubes. After centrifuging for 10 min at 3000g, serum was collected and stored in 2-mL plastic vials at −20°C to further analysis.
Faeces sampling
Faeces free of sand and other debris were carefully collected individually everywhere in the pen at 0600 hours every day during the digestion trial period, and then sprayed with diluted sulfuric acid to avoid the loss of nitrogen. For chemical analysis, faeces was dried at 65°C and ground to sieve through the 1-mm mesh and preserved in airtight plastic bags. Feeds was processed similarly to faeces before chemical analysis.
Antler sampling
Velvet antlers were removed by a professional technician using a saw, under the guidance of the institutional velveting regime. Antlers were removed at the same stage of their development. The procedures were as follows: removal of antlers was conducted under general anaesthesia (xylazine hydrochloride, 1.5–2.0 mL/100 kg bodyweight), and the anaesthesia was reversed using Nikethamide (1.5–2.0 mL/100 kg bodyweight) after antler harvesting. Fresh antler yield was measured. Antlers were stored at −80°C and then processed using a freeze drier at −20°C for 3 days. Dry antler yield was measured after being processed. The whole dried antlers were ground into powder, which was then sieved through a 30-mesh screen for further analysis. DM content and average daily gain of antlers were calculated using the following formula.



Chemical analysis
Dry matter, CP, ADF, NDF, P, Ca and ash insoluble in HCl for diet and faeces were analysed according to the methods from Association of Official Analytical Chemists (AOAC International 2005). Calcium contents in serum, faeces, antlers and diets were measured by the method of EDTA complexometric.
Statistical analyses
The data were presented as means ± s.d. Data were analysed using ANOVA as a completely randomised design using the procedure of SAS (SAS Institute Incorporated, Version 8). Analysis of variance and comparison of significance were performed by using Duncan’s multiple-range tests in SAS. Trends were significantly different if probability values of P < 0.05 were obtained.
Results
Nutrient apparent digestibility
Effects of diet Ca supplementation on the digestibility of nutrients during antler growing period in 6-year-old deer are shown in Table 2. The digestibilities of DM and CP in the control group and Ca1.10 group were higher than that of Ca1.70 group during the entire experimental period on the whole (P < 0.05). The digestibility of Ca in the control group was highly significantly (P < 0.01) lower than in the other treatments, and there was no difference between the groups Ca1.10 and Ca1.70. The results indicated the same trends in the 3 months of the experimental period. The digestibilities of P and NDF in the Ca1.10 group were significantly (P < 0.05) higher than those in the Ca1.70 group, and higher than or similar to those in the control group. No difference was found in the digestibility of ether extract and ADF between the control and the other treatment groups.
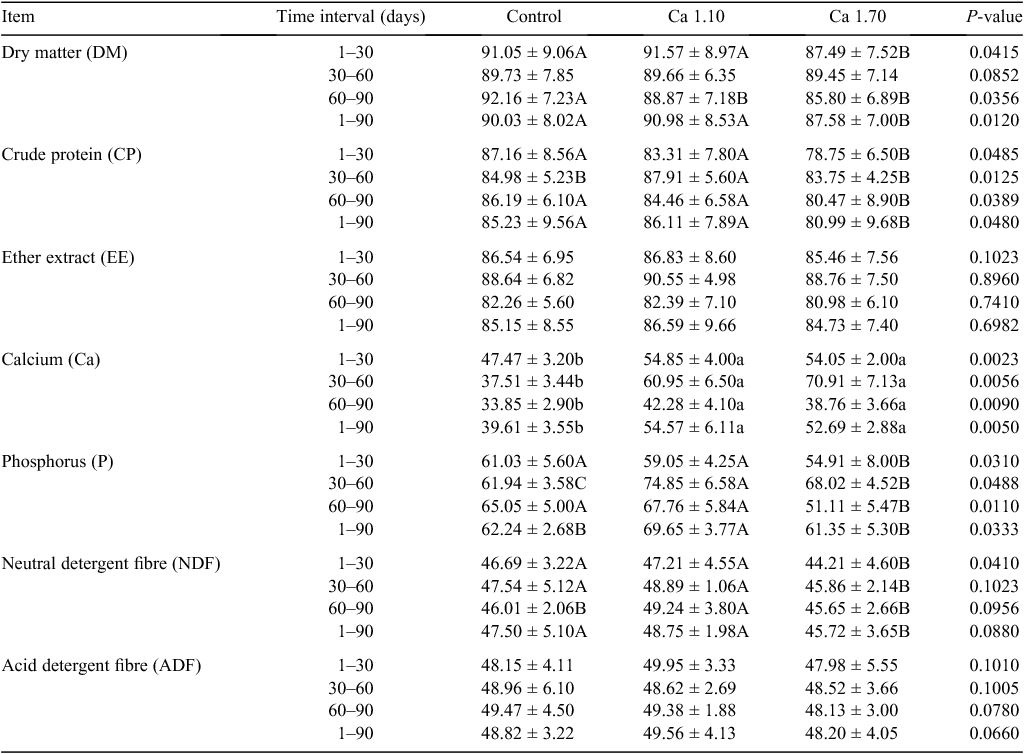
|
Calcium and P in serum, faeces and antlers
Calcium and P concentrations in serum, faeces and antlers and Mg concentration in serum are given in Table 3. Calcium, P and Mg concentrations in serum were very similar among the treatments. No significant differences in Ca, P and ash concentrations in the base of antlers were found among the dietary treatments. Calcium concentration in faeces was increased when treated with the Ca supplementation. Calcium concentrations in Groups Ca1.10 and Ca1.70 were significantly (P < 0.05) higher than in control, and there was no significant (P > 0.05) difference in P between Group Ca1.10 and Group Ca1.70. Phosphorus concentrations in the faeces were increased when animals were treated with Ca supplementation, and they were highly significantly (P < 0.01) lower in the control group than in the other treatments.
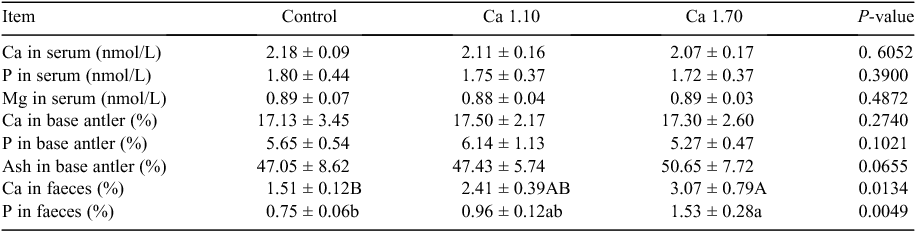
|
Parathyroid hormone (PTH), alkaline phosphatase (ALP), osteocalin, testosterone and oestradiol in serum
Concentrations of PTH, ALP and osteocalcin in serum were tested in the present study. No significant (P > 0.05) differences of PTH, ALP and osteocalcin were found among the treatments. Testosterone and oestradiol concentrations in serum are shown in Table 4. Testosterone and Oestradiol concentrations of Group Ca1.70 were significantly (P < 0.05) higher than those in the control and Ca1.10 groups.
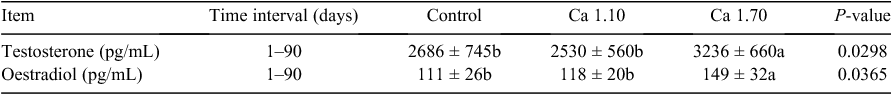
|
Production performance
Effects of dietary Ca and P supplementation on production performance of velvet antler are shown in Table 5. Fresh antler mass and dry antler mass were affected by dietary Ca application level. Antler masses of both fresh and dry velvet antler were significantly (P < 0.05) higher than those of the other two groups. No significant (P > 0.05) difference in the DM content of antler was found among the treatments. The number of days between antler initiation and harvesting in the Group Ca1.70 was decreased significantly (P < 0.05) compared with that of the control group and Ca1.10 group. Average daily gains of fresh antler and dry antler mass in the Groups Ca1.10 and Ca1.70 were significantly (P < 0.05) higher than those in the control group.
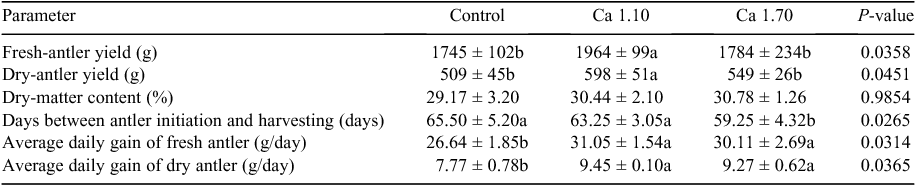
|
Discussion
Nutrient apparent digestibility
In the present study, the digestibility of Ca, P, DM, CP and NDF were affected by different dietary Ca supplementation levels. Our results suggested that the dietary supplementation level of Ca1.2% significantly decreased the digestibilities of CP and DM. The digestibilities of Ca and P increased in Group Ca1.10 and decreased in Group Ca1.70. Excess concentration of Ca in the diet decreased digestibility. Chu (2005) reported that diets with different Ca supplementation levels could influence the digestibility of Ca in lactating cows. Chu (2005) also reported that Ca concentration increased in the faeces and Ca digestibility decreased with the dairy Ca supplementation in lactating cows. On the basis of the above studies, there is sufficient evidence to state that Ca digestibility will be influenced by the Ca concentrations, and then the ADG changes as well, in some animals such as cow and sika deer. To have an adequate concentration of Ca in feed is important for growth performance and antler growth.
Dietary Ca nutrition is important to skeleton growth in poultry and, similarly, to antler growth and bone structure in deer. Sika deer produces as much as 10 kg of antler yearly and it could deposit as much as 100 g of mineral substance during the fastest antler-growth period each day (Chapman 1975; Li and Suttie 1996; Li et al. 1999). It has been reported that Cervidae (e.g. white-tailed deer, red deer, sika deer, among others) need a lot of minerals, especially Ca and P, to support this antler growth (Moen and Pastor 1996; Magruder et al. 1957). All the minerals are no doubt provided from the feed supply. During the rapid growth period of velvet antlers, minerals come not only from feed supply, but also from the skeleton, especially the ribs of deer, although the feed Ca concentration is enough for body growth needs. Our results showed that high dietary Ca supplementation level (total Ca 1.7%) reduced the digestibility of most of the nutrients, such as DM, CP, P and NDF. We infer that the absorption rate of Ca in sika deer has an upper limit.
Calcium and P in serum, faeces and antlers
Our research found that the percentage of ash in the base of antlers (2–3 cm from the base) ranged from 47.05% to 50.65%. Calcium and P concentrations in the faeces increased with dietary supplementation of Ca and P. The ratio of Ca to P of deer antlers ranged from 2.85% to 3.28% in sika deer in the present study. In a previous study, the following values were reported: Ca (18.0–22.7%) in white-tailed deer (French et al. 1956); Ca (18.8%) and P (8.9%) in fallow deer (Brown 1990) and Ca (22.0%, 24.9%) in red deer antlers and roe deer antlers respectively (Anke et al. 1973). However, the range of the ratio of Ca to P of 2.85–3.28% demonstrated that the ratio of Ca to P in basal part is much higher than that of in the upper part. Previous studies have shown that the chemical composition and physical properties of antlers depend on antler location, stage of antler development and deer species (French et al. 1956; Anke et al. 1973; Brown 1990; Wu et al. 2013). Most published references are based on hard and mature antlers. Calcium concentration of the antlers in the present study showed far less variation when expressed as a percentage of ash (37.2–39.2%) and this may imply the fact that the antlers from different species contain different amounts of organic matter. The lowest values for Ca and P are found in fallow deer and the highest values in Mexican deer (subspecies of white-tailed deer). These differences appear to be species specific.
Chen et al. (2014) reported that the Ca concentration of hard antlers is higher than that of other minerals in red deer, being 21.64%, and in sika deer it was 22.53%. Phosphorus concentration is 9.44% in red deer and 10.05% in sika deer. Our results demonstrated that the Ca range of the base of antlers was 17.13–17.50%, and the P range was 5.27–6.14%. Hard antlers are much more mineralised than are velvet antlers obviously.
In the present study, Ca and P concentrations in the faeces and antlers increased with the supplementation of Ca. Digestibilities of Ca and P were not significantly affected by the Ca supplementation level in the feed. We can conclude that the extremely high intake of Ca and P can cause high faeces Ca and P residues, which could lead to the danger of soil pollution. It is important to find out the suitable concentration of Ca and P in feed, so as to sustain maximal antler growth and, at the same time, decrease soil pollution. So, the balance between animal production and environment protection is our mission. We recommend that the appropriate Ca supplementation level is 1.10% in 6-year-old sika deer. Chu et al. (2010) reported that in the lactating cows with and increasing supplementation level of dietary Ca, serum ALP activity, and serum osteocalcin concentrations increased, and they were significantly (P < 0.05) higher in the Ca 0.96% treatment than in the Ca 0.50% treatment, but there was no significant (P > 0.05) difference between the 0.68% and 0.83% treatments; serum PTH concentration decreased, and was significantly (P < 0.05) higher in the Ca 0.50% treatment than in the other groups (Chu et al. 2010). So, the results demonstrated the sorption ability of Ca and P.
Hormone concentrations in serum
No significant (P > 0.05) differences of PTH, ALP and osteocalcin in serum were found among the treatments. Testosterone and oestradiol concentrations in serum were highest in the Group Ca1.70, and significantly higher than those of the control and Ca1.10 groups. Li et al. (1999) reported that testosterone concentration in serum increases with the growth of velvet antler, especially during the period of rapid growth. When the testosterone concentration in serum was the highest, the velvet-antler calcification also reached a plateau. The results of the present study agree with those reports, and we found that velvet antlers of the Group Ca1.70 grew fastest and ossified earlier than did those in the other groups (Table 5). Consequently, ossification process of velvet antlers was induced by the increase in testosterone concentration.
Antler production performance
Deer antlers are renewed in yearly cycles. Antlers are bony appendages developed from outgrowths of the frontal bone of the skull, referred to as pedicles, in most species of the deer family (Li et al. 2009). Moen and Pastor (1996) reported that Ca and P must be resorbed from bone during peak antler growth in males in reindeer, when >25 g/day of Ca and >12 g/day of P are being deposited in antlers. Females are capable of meeting Ca demands during the period of antler growth, with no bone resorption, but P was resorbed from bone during the final stages of antler mineralisation. Calcium and P are required for gestation, lactation and antler development. During the period of antler growth in males, the bone Ca : P ratio increased from a normal value ~2.0 to ~3.3 when Ca and P were uncoupled in bone resorption. This ratio may be higher than the physiologically acceptable level, suggesting that some of the bone were resorbed from the body bone. For resorption and excretion of Ca, results similar to male reindeer were obtained in the present study. An alternative mechanism to maintain a constant Ca : P ratio would be to increase the availability of P at a faster rate as the bone P is depleted (Grasman and Hellgren 1993). In the present study, fresh antler mass, dry antler mass, and antler DM content were not affected by dietary Ca supplementation level (P > 0.05). We inferred that high concentration of Ca and P can induce a rapid antler-velvet growth and calcification (P < 0.05).
Conclusions
Diet with different levels of Ca supplementation could influence the digestibility of main nutrients such as CP, Ca and P. When both speed and average daily gain of antler growth, also the less faecal excretion, are taken into consideration, the suitable level of Ca concentration is recommended to be from 1.10% to 1.70% during the antler growth period for 6-year-old sika deer.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work is supported by a Leading Talent and Creative Team project (20121810), Major Research Project of Science and Technology (20140203018NY), from Jilin province, and National Key R&D Program of China (No. 2018YFC1706600).
References
Anke M, Groppel B, Reissig W, Ludke H, Grun M, Dittrich G (1973) Manganese deficiency in ruminants. 3. Disorders of reproduction, skeleton and nerves caused by manganese deficiency in female ruminants and their progeny. Archiv fur Tierernahrung 23, 197–211.| Manganese deficiency in ruminants. 3. Disorders of reproduction, skeleton and nerves caused by manganese deficiency in female ruminants and their progeny.Crossref | GoogleScholarGoogle Scholar | 4731545PubMed |
AOAC International (2005) ‘Official methods of analysis of AOAC International.’ 18th edn. (AOAC International: Gaithersburg, MD)
Bain SD, Watkins BA (1993) Local modulation of skeletal growth and bone modeling in poultry. The Journal of Nutrition 123, 317–322.
| Local modulation of skeletal growth and bone modeling in poultry.Crossref | GoogleScholarGoogle Scholar | 8429381PubMed |
Banks WJ, Epling GP, Kainer RA, Davis RW (1968) Antler growth and osteoporosis. II. Gravimetric and chemical changes in the costal compacta during the antler growth cycle. The Anatomical Record 162, 399–405.
| Antler growth and osteoporosis. II. Gravimetric and chemical changes in the costal compacta during the antler growth cycle.Crossref | GoogleScholarGoogle Scholar | 5701620PubMed |
Borsy A, Podani J, Steger V, Balla B, Horvath A, Kosa JP, Gyurjan I, Molnar A, Szabolcsi Z, Szabo L, Jako E, Zomborszky Z, Nagy J, Semsey S, Vellai T, Lakatos P, Orosz L (2009) Identifying novel genes involved in both deer physiological and human pathological osteoporosis. Molecular Genetics and Genomics Mgg 281, 301–313.
| Identifying novel genes involved in both deer physiological and human pathological osteoporosis.Crossref | GoogleScholarGoogle Scholar | 19107525PubMed |
Bronner F (1987) Intestinal Ca absorption: mechanisms and applications. The Journal of Nutrition 117, 1347–1352.
| Intestinal Ca absorption: mechanisms and applications.Crossref | GoogleScholarGoogle Scholar | 3305814PubMed |
Brown RD (1990) Horns, pronghorns, and antler. In ‘Nutrition and antler development’. (Eds GA Bubenik, AB Bubenik) pp. 426–441. (Springer-Verlag: New York)
Chapman DI (1975) Antler-bone of contention. Mammal Review 5, 121–172.
| Antler-bone of contention.Crossref | GoogleScholarGoogle Scholar |
Chen Y, Li FT, Qian DW, Jiang Q, Duan JA (2014) Analysis and evaluation of red deer horn and the plum blossom antlers off dish in inorganic element. Chinese Traditional Patent Medicine 36, 2577–2582.
Chu HP (2005) ‘Study on optimum supply of calcium and phosphorus in dairy cows.’ Master’s Thesis. College of Animal Science and Technology, Shandong Agricultural University, Taian City.
Chu HP, Wang ZH, Li FC (2010) Effects of dietary Ca level on Ca metabolism in lactating dairy cows. Chinese Journal of Animal Nutrition 22, 1286–1292. [in Chinese]
French CE, Mcewen LC, Magruder ND, Ingram RH, Swift RW (1956) Nutrient requirements for growth and antler development in the white-tailed deer. The Journal of Wildlife Management 20, 221–232.
| Nutrient requirements for growth and antler development in the white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Grasman BT, Hellgren EC (1993) Phosophorus nutrition in white-tailed deer: nutrition balance, physiological responses, and antler growth. Ecology 74, 2279–2296.
| Phosophorus nutrition in white-tailed deer: nutrition balance, physiological responses, and antler growth.Crossref | GoogleScholarGoogle Scholar |
Hillman JR, Davis RW, Abdelbaki YZ (1973) Cyclic bone remodeling in deer. Calcified Tissue Research 12, 323–330.
| Cyclic bone remodeling in deer.Crossref | GoogleScholarGoogle Scholar | 4746698PubMed |
Huang J, Zhang TT, Bao K, Li GY, Wang KY (2015) Effect of supplementation of lysine and methionine on growth performance, nutrients digestibility and serum biochemical indices for growing sika deer (Cervus nippon) fed protein deficient diet. Italian Journal of Animal Science 14, 60–65.
| Effect of supplementation of lysine and methionine on growth performance, nutrients digestibility and serum biochemical indices for growing sika deer (Cervus nippon) fed protein deficient diet.Crossref | GoogleScholarGoogle Scholar |
Landete-Castillejos T, Garcia A, Gallego L (2007) Body weight, early growth and antler size influence antler bone mineral composition of Iberian red deer (Cervus elaphus hispanicus). Bone 40, 230–235.
| Body weight, early growth and antler size influence antler bone mineral composition of Iberian red deer (Cervus elaphus hispanicus).Crossref | GoogleScholarGoogle Scholar | 16949898PubMed |
Li C, Suttie JM (1996) Histological examination of the antlerogenic region of red deer (Cervus elaphus) hummels. New Zealand Veterinary Journal 44, 126–130.
| Histological examination of the antlerogenic region of red deer (Cervus elaphus) hummels.Crossref | GoogleScholarGoogle Scholar | 16031913PubMed |
Li CY, Littlejohn RP, Suttie JM (1999) Effects of insulin-like growth factor 1 and testosterone on the proliferation of antlerogenic cells in vitro. Journal of Experimental Zoology 284, 82–90.
| Effects of insulin-like growth factor 1 and testosterone on the proliferation of antlerogenic cells in vitro.Crossref | GoogleScholarGoogle Scholar |
Li CY, Yang FH, Sheppard A (2009) Adult stem cells and mammalian epimorphic regeneration-insights from studying annual renewal of deer antlers. Current Stem Cell Research & Therapy 4, 237–251.
| Adult stem cells and mammalian epimorphic regeneration-insights from studying annual renewal of deer antlers.Crossref | GoogleScholarGoogle Scholar |
Magruder ND, French CE, McEwen LC, Swift RW (1957) ‘Nutritional requirements of White-tailed deer for growth and antler development II.’ Bulletin 628. Pennsylvania State University, College of Agriculture, Agricultural Experiment Station.
Moen R, Pastor J (1996) Simulating antler growth and energy, nitrogen, calcium and phosphorus metabolism in caribou. In ‘The seventh North American caribou conference’, 19–21 August 1996, Thunder Bay, Ontario, Canada.
Muir PD, Sykes AR, Barrell GK (1987) Growth and mineralization of antlets in red deer (Cervus elaphbus). New Zealand Journal of Agricultural Research 30, 305–315.
| Growth and mineralization of antlets in red deer (Cervus elaphbus).Crossref | GoogleScholarGoogle Scholar |
Schneider A (1985) Eruptive processes, mineralization and isotopic evolution of the Los Frailes Karikari region/Bolivia. Chemistry (Weinheim an der Bergstrasse, Germany) 16, 13330–13334.
Stéger V, Molnar A, Borsy A, Gyurjan I, Szabolcsi Z, Dancs G, Molnar J, Papp P, Nagy J, Puskas L, Barta E, Zomborszky Z, Horn P, Podani J, Semsey S, Lakatos P, Orosz L (2010) Antler development and coupled osteoporosis in the skeleton of red deer Cervus elaphus: expression dynamics for regulatory and effector genes. Molecular Genetics and Genomics 284, 273–287.
| Antler development and coupled osteoporosis in the skeleton of red deer Cervus elaphus: expression dynamics for regulatory and effector genes.Crossref | GoogleScholarGoogle Scholar | 20697743PubMed |
Wu FF, Li HQ, Jin LJ, Li XY, Ma YS, You JS, Li SY, Xu YP (2013) Deer antler base as a traditional Chinese medicine: a review of its traditional uses, chemistry and pharmacology. Journal of Ethnopharmacology 145, 403–415.
| Deer antler base as a traditional Chinese medicine: a review of its traditional uses, chemistry and pharmacology.Crossref | GoogleScholarGoogle Scholar |
* These authors contributed equally to this work.


