Disentangling the effect of sheep urine patch size and nitrogen loading rate on cumulative N2O emissions
Karina A. Marsden A B , Davey L. Jones A and David R. Chadwick AA School of Environment, Natural Resources and Geography, Bangor University, Bangor, Gwynedd, LL57 2UW, UK.
B Corresponding author. Email: k.marsden@bangor.ac.uk
Animal Production Science 56(3) 265-275 https://doi.org/10.1071/AN15613
Submitted: 15 September 2015 Accepted: 30 November 2015 Published: 9 February 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Ruminant urine nitrogen (N) concentration and volume are important parameters influencing the size and N loading rate of urine patches deposited to soil. Such parameters can influence N cycling and emissions of the greenhouse gas, nitrous oxide (N2O) from grazed grassland, yet, there is limited information on the effect of these parameters within typical ranges reported for sheep. We used an automated, high-frequency gas monitoring system to investigate N2O emissions from varying urine N application rates and patch sizes under field conditions. Using artificial sheep urine, we manipulated urine N concentration to provide two urine N application rates (4 and 16 g N/L; equivalent to 200 and 800 kg N/ha). We investigated the effect of urine patch size with equal N application rates (4 × 125 cm2 vs 500 cm2, at 200 and 800 kg N/ha) and the effect of patch size with unequal N application rates, but the same total amount of N applied (62.5 mL over 125 cm2 at 800 kg N/ha and 250 mL over 500 cm2 at 200 kg N/ha). Cumulative emissions of N2O generally increased with N loading rate, whether applied as one large urine patch or four smaller ones. Cumulative N2O emissions increased when the N was applied in four smaller urine patches compared with one large patch; this difference was significant at 800 kg N/ha, but not at 200 kg N/ha. When the total amount of N applied was held constant (1 g of N), the amount of N2O released was similar when urine was applied as a high N concentration small patch (800 kg N/ha) compared with a low N concentration large patch (200 kg N/ha). Urine N2O emission factors in this study were, on average, 10 times lower than the IPCC default of 1% for sheep excreta. This research clearly demonstrates that the chemical and physical nature of the urine patch influences N2O emissions, yet further research is required to gather more data on typical sheep urine volumes (individual and daily), urination frequency, urine N concentrations and the typical volumes of soil influenced by urine deposition, to provide more accurate estimates of emissions from sheep grazed pastures.
Additional keywords: agricultural systems, global climate change, microbial processes, ruminants.
Introduction
Ruminant urine N concentration, volume and frequency vary widely, influencing the size and nitrogen (N) loading rate of urine patches deposited to pasture soils. These parameters can influence the fate of urinary N in grazed pastures, including emissions of the powerful greenhouse gas nitrous oxide (N2O) and leaching of NO3– (Li et al. 2012). The default IPCC N2O emission factor for both cattle and sheep was 2% of applied N; however, due to the lower volume and higher frequency of sheep urine events, and more moderate levels of soil compaction under sheep-grazed pastures, the default emission factor has been lowered for sheep from 2% to 1% of the applied N (IPCC 2006). The range in urine patch sizes deposited by sheep and cattle (0.13–2 L) has been shown to influence N transformations and processes (e.g. longer retention of mineral N, greater plant growth and N uptake in larger patches) even when the total N applied is constant (Orwin et al. 2009). While these results display a clear difference between sheep and cattle urine patches, where large differences in urinary volume are produced between the two species, less work has been conducted on N cycling and N2O emissions within the typical ranges of urine patch sizes reported for sheep.
The concentration of N excreted in urine is a function of the amount of surplus metabolised N to be excreted, the volume of urine produced and the frequency of urine events (Hoogendoorn et al. 2010) and can range from 1 to 18 g N/L (Bristow et al. 1992; Oenema et al. 1997; Hoogendoorn et al. 2010). Urine volume is mainly influenced by water intake and the mineral load ingested by the animal (Selbie et al. 2015), and can be high when the moisture content of the diet is high, or when the herbage leaves are wet with rain water or dew (Doak 1952). Urine volume can also vary as a response to coping with changes in ambient temperatures (Betteridge et al. 2010a). Data for sheep urine volume and frequency are scarce, but typically individual sheep urine events range between 100 and 200 mL (Doak 1952; Haynes and Williams 1993). However, variation within individual sheep urine volumes can be high; for example, the mean and range of 40 individual urine events among six Welsh mountain ewes fed (ad libitum) a Lolium perenne L. dominated sward was 104 (18–397) mL (K. A. Marsden, unpubl. data). Daily sheep urine volume ranged from 0.5 to 3 L in Ledgard et al. (2008) and the frequency of urination events has been reported to range between 13 and 20 times per day (Doak 1952; Betteridge et al. 2010a, 2010b).
Urine volume, soil moisture status, soil type and topography can all influence the urine patch-wetted area and volume of soil influenced by a urine patch. The wetted area of a urine patch has been defined as the surface area covered by urine following deposition to soil, as opposed to the pasture response area, which can extend beyond the wetted area (Lantinga et al. 1987; Li et al. 2012). A typical wetted area for a sheep urine event with a volume of 150 mL is reported to be 300 cm2 (Doak 1952) and for 200 mL it is reported to be 430–550 cm2 (Williams and Haynes 1994). Typical urine applications of 4 L/m2 were reported to be utilised in Kelliher et al. (2014).
Due to the wide range in the volume and frequency of sheep urine, and the interactive effects of dietary N content, energy use and ambient temperature fluctuations, it is evident that sheep urine patches vary widely in N concentration and volume, resulting in patches of different sizes and of different N loading rates. It can be envisaged that at times where sheep may be fed a diet low in moisture content, or when ambient temperatures are high, their urine will be more concentrated, having a higher N concentration deposited over a small patch size. However, when the diet is high in moisture and the sheep is fully hydrated, urine may be deposited in larger-sized urine patches with a lower N concentration. This experiment was designed to test these two extremes, to determine how differences in sheep urine patch size and N concentration may influence N cycling and cumulative N2O emissions arising from such urine patches.
Using a high-frequency automated greenhouse gas monitoring system we assessed whether (1) cumulative N2O emissions will increase with increasing urine N loading rate, when keeping the urine patch size constant, (2) cumulative emissions will be higher from four small urine patches than from one large urine patch, when keeping the total N loading rate constant, and (3) cumulative emissions will be higher from a low N-concentration large urine patch than from a high N-concentration small urine patch, where N loading rate differs, but the total amount of N applied remains constant. The total amount of applied N equates to the total amount of N in the volume of applied urine. The N loading rate is a function of the urine N concentration and the surface area the urine is applied to (Selbie et al. 2015).
Materials and methods
Field site
The field site was established at the Henfaes Research Station, Abergwyngregyn, North Wales (53°14ʹN, 4°01ʹW) in March 2015. The soil at the site is classified as a Eutric Cambisol and is of mixed glacial till in origin, deposited ~10 000 years ago. The field was re-seeded in 1990 with a Lolium perenne L. and Trifolium repens L. mix. The field has since received moderate fertiliser applications (~120 kg N/ha.year in years 1990–2002 and ~60–80 kg N/ha.year in subsequent years) and is regularly grazed by Welsh Mountain ewes at a moderate stocking density (2 or 3 livestock units/ha). The area was fenced off just over 3 months before treatment application, to prevent the effect of recent livestock urine patches on monitored gas fluxes from the soil.
Soil sampling and analysis
Soil (0–10 cm) was sampled in triplicate at block level (n = 3) to determine soil characteristics (Table 1); this was conducted 4 days before treatment application. Soil moisture content was determined by oven drying (105°C, 24 h) and organic matter was determined by the loss-on-ignition (450°C, 16 h; Ball 1964). Soil pH and electrical conductivity were determined using standard electrodes in 1 : 2.5 (w/v) soil–distilled water suspensions. Total carbon (C) and N were determined on oven-dried and ground samples in a TruSpec® Analyzer (Leco Corporation, St Joseph, MI, USA). Dissolved C and dissolved N were determined in 1 : 5 (w/v) soil–0.5 M K2SO4 extracts, according to Jones and Willett (2006). Microbial biomass C and N were determined via the chloroform fumigation–extraction method of Voroney et al. (2008), using KEC and KEN correction factors of 0.35 and 0.5, respectively. Total extractable P, NO3– and NH4+ were determined in 0.5 M K2SO4 extracts via the methods of Murphy and Riley (1962), Miranda et al. (2001) and Mulvaney (1996), respectively. Exchangeable cations (sodium, potassium, calcium) within 1 : 5 (w/v) soil–1 M NH4Cl extracts were measured using a model 410 flame photometer (Sherwood Scientific, Cambridge, UK).

|
Experimental design and treatment application
Artificial sheep urine was made up according to Lucas and Jones (2006), the composition of which is in line with the suggestion of Kool et al. (2006) in containing at least urea and hippuric acid for a realistic simulation of N2O emissions from artificial urine patches. The N concentration of the artificial urine was modified by adjusting the proportion of urea to obtain a total of 4 g N/L and 16 g N/L, where all other urine constituents were held constant. The two N concentrations were chosen to reflect low and high N concentrations in the urine, within typical reported ranges for N concentration within sheep urine. The effect of urine patch size was investigated by comparing a single large urine patch with four smaller patches, while holding total N loading rate constant. The effect of patch size with unequal N loading rates, but the same total amount of applied N, was also assessed, by comparing a low N-concentration large urine patch with a high N-concentration small urine patch.
The study was set up in a randomised block design, with five treatments (n = 3), as follows: (1) control (no urine application), (2) large urine patch with a low N concentration (4 g N/L; 1 g of N applied in 250 mL covering 500 cm2; N loading rate = 200 kg N/ha), (3) large urine patch with a high N concentration (16 g N/L; 4 g of N applied in 250 mL, covering 500 cm2; N-loading rate = 800 kg N/ha), (4) four small urine patches with a low N concentration (4 g N/L; 4 × 0.25 g of N applied in 4 × 62.5 mL, covering 125 cm2 each; N loading rate = 200 kg N/ha) and (5) four small urine patches with a high N concentration (16 g N/L; 4 × 1 g of N applied in 4 × 62.5 mL, covering 125 cm2 each; N loading rate = 800 kg N/ha).
Duplicate plots were established, one to allow for chamber gas flux measurements and the other for soil sampling during the study. Artificial urine was applied by evenly pouring onto the soil, using a fixed template as an area guide. All urine patches were smaller than the chamber basal area (2500 cm2), ensuring additional room inside the chamber for the urine-patch diffusional area, which is important for accurately monitoring urine patch N2O emissions (Marsden et al. 2016). Soil cores (0–5 cm) were taken from the area of immediate urine application, during the course of the study. Soil sampling was conducted 4 days before treatment application (for measurement of background soil characteristics), on the day of a urine application, and 2, 4, 7, 10, 14, 17, 21, 28 and 35 days following treatment application. The soil sampling was conducted more frequently at the beginning of the study (three times in the first week) so as to provide information on soil N-transformation processes, which occur relatively quickly following organic N additions to soil (e.g. urea hydrolysis and nitrification). Soil sampling reduced in frequency throughout the remainder of the study (twice per week in the second and third week, and once per week, thereafter). When soil cores were taken, they were timed to match manual flux measurements taken from the control plots. Soil cores were returned immediately to the laboratory, where post-processing took place within 24 h of sampling. Prior to extraction, soils were homogenised by gently mixing within the sample bags.
Soil moisture was monitored within each individual chamber by using Acclima SDI-12 digital TDT® sensors (Acclima Inc., Meridian, ID, USA), monitoring at a 0.5-h measurement frequency. The sensors were inserted diagonally through the urine patch, and were in situ 3 weeks before urine application. Rhizon suction samplers (2.5-mm diameter, 5-cm porous tube length, 12-cm tubing length; Rhizosphere Research Products, Wageningen, The Netherlands) were also inserted at an angle of 45° into the centre of individual urine patches, 3 weeks before urine application. This allowed sampling of soil solution within the chamber in a non-destructive manner when the soil was wet enough for sample collection. Successful soil solution samples were collected 4 and 3 days before urine application, on the day of urine application and 2, 4, 7, 10, 14 and 17 days following urine application. Beyond this point, soil conditions were too dry, so samples were not collected. Additionally, air temperature was monitored both inside and outside chambers using Thermochron iButtons® (iButtonLink, Whitewater, WI, USA) logging temperature every 1 h. A weather station was located near the field site, where rainfall, soil (0–10 cm) and air temperature were monitored hourly.
Pasture biomass and N content
The pasture within chambers was cut 2 and 4 weeks following urine application. Samples were oven-dried (80°C; 24 h), weighed for biomass, and ground before analysis of N content, as described previously.
Automated and manual greenhouse gas measurements
Greenhouse gas fluxes were measured from 12 non-steady-state, non-through flow chambers connected to a mobile, automated high-frequency measurement system (Queensland University of Technology, Institute for Future Environments, Brisbane, Australia) as detailed in Scheer et al. (2014). Stainless-steel chamber bases were inserted into the ground (10 cm depth) for 4 weeks before treatment application, to ensure no effects from soil disturbance were observed during the study. Chambers (50 cm × 50 cm × 15 cm) were fixed to the bases, which opened and closed during sampling via pneumatic actuators. Chamber headspace samples were automatically pumped (~200 mL/min) from the chambers, through Teflon tubing to the sampling unit, which housed a LI-COR LI-820 non-dispersive infrared gas analyser (LI-COR, St Joseph, MI, USA) to measure the CO2 concentration, and a gas chromatograph (SRI 8610C, SRI International, Torrance, USA), equipped with a 63Ni electron capture detector and flame ionisation detector to measure N2O and CH4 concentrations in the chamber headspace, respectively. The samples passed through an Ascarite (sodium hydroxide-coated silica) column before entering the gas chromatograph, so as to remove moisture and CO2, which was changed periodically so as to protect the electron capture detector cell.
The sampling routine consisted of three blocks of four chambers, where each block of chambers close sequentially for a period of 1 h, while the other two sets are open, allowing restoration of ambient conditions. During the 1 h closure period, each chamber is sampled for 3 min, followed by a calibration standard (0.97 mg/m3 N2O; 1.71 g/m3 CO2; 2.12 mg/m3 CH4; ± 2% of the certified value, BOC Gases, Liverpool, UK). The 15 min cycle repeats four times, before the next set of chambers are sampled. One entire cycle takes 3 h to complete, allowing up to eight flux measurements per 24 h period.
The automated system consisted of 12 chambers only; therefore, control treatments were sampled manually from static chambers (n = 3), as N2O fluxes were expected to be minimal from this treatment. Manual chamber flux measurements were taken once per day, between 1000 hours and 1200 hours (de Klein and Harvey 2012) as close to daily as possible, for the duration of the experiment. The gas samples were taken by placing polypropylene upturned buckets (~26 cm in height) onto collars (26-cm diameter), inserted to a depth of 10 cm. The chambers were fitted with a re-sealable vent, to allow pressure equalisation when placing chambers onto bases, and were fitted with Suba-Seals ® (Sigma, Gillingham, UK) to allow sampling of the headspace. Samples were taken with a syringe every 15 min, over the period of 1 h, to match the automated system. Samples were stored in pre-evacuated 20 mL glass vials, before being analysed on a Clarus 500 gas chromatograph with a TurboMatrix headspace autoanalyser (Perkin Elmer, CT, USA).
Statistical analyses
Cumulative N2O emissions were determined by integration using the trapezoidal rule. The resulting cumulative emissions were corrected for the chamber area assumed to be unaffected by urine application (i.e. total chamber area = 0.25 m2, urine patch area = 0.05 m2 and control area unaffected by urine = 0.2 m2), by deducting the cumulative emissions arising from the control treatment over an area of 0.2 m2. Further calculations, e.g. emission factors, were based on the control-corrected values for cumulative emissions. To compare whether cumulative emissions or emission factors increased with increasing N loading rate, Student’s t-tests were conducted, comparing the results from Treatment 2 with those from Treatment 3 and results from Treatment 4 with those from Treatment 5. So as to determine whether emissions are greater from small or large urine patches (with the same N loading rate), the same procedure was used, comparing Treatment 2 with Treatment 4 and Treatment 3 with Treatment 5. To test the third hypothesis that cumulative emissions would be higher from a low N-concentration large urine patch (200 kg N/ha) than from a high N-concentration small urine patch (800 kg N/ha) with the same total amount of N applied, the results from Treatment 5 were divided by 4 (assuming that cumulative emissions from each individual urine patch within the chamber were equal) and compared with the results from Treatment 2. Differences in pasture biomass and foliar N content at 2 and 4 weeks following urine application were assessed via ANOVA with Tukey’s post hoc test, after assessing normality and homogeneity of variance. All statistical analyses were conducted in Minitab 17.0 (Minitab Inc., State College, PA, USA).
Results
Effect of urine N content and patch size on soil C and N dynamics
The dynamics of total (free and exchangeable) NH4+ and NO3– concentrations in soil can be seen in Fig. 1a, b, and the concentration of NH4+ and NO3– in soil solution (extracted with Rhizon samplers within the chambers) is presented in Fig. 1c, d. During the second half of the field trial, the lack of rainfall prevented the acquisition of soil water with the Rhizon samplers and, thus, there are no results to present. Extractable NH4+ increased up to 4 days following urine application in the treatments receiving a high N loading rate, and then followed a declining trend. As expected, extractable NH4+ peaked at a lower concentration in the lower N-containing treatments, and began to decline 2 days following urine application. There were no apparent differences in the urine-induced increase of soil NH4+ concentration due to patch size. The NO3– concentration steadily increased in the soil extracts and soil solution, with lower amounts of NO3– produced in the low N-containing treatments. Differences in NO3– concentration between patch sizes were evident, with less accumulation of NO3– in smaller patches, and with concentrations returning to background values faster than in large urine patches. In the high N-containing large urine patch, the NO3– concentration was still higher than the control at the end of the experiment, and it cannot be ruled out that further N2O emissions would have occurred after this time.
The total extractable dissolved N and organic C present in soil during the course of the study can be seen in Fig. 2a, b, and the soil-solution total dissolved N and C can be seen in Fig. 2c, d. Concentrations of extractable and soil-solution N were high at the beginning of the study, due to the large amounts of dissolved organic N deposited into the soil within the urine. The N persisted in the soil for longer periods in the high N-containing urine patches. The soil solution C was immediately high following urine application, and rapidly declined to control values. Dissolved organic C may have been higher in the high N-containing treatments, due to the increased proportion of urea in these treatments, and/or it may have been caused by a priming effect, which has been demonstrated for ruminant urine-influenced soils for both C (Lambie et al. 2013) and N (Di and Cameron 2008).
Pasture biomass and foliar N content
The pasture biomass and N content after 2 and 4 weeks following urine application can be seen in Table 2. As expected, the pasture biomass and foliar N content were generally larger in the urine treatments than in the control (except pasture biomass 4 weeks after urine application). Increasing the N loading rate in a single large urine patch did not increase (P > 0.05) pasture biomass 2 or 4 weeks after urine application, nor did it increase foliage N content in the first 2 weeks after urine application; however, foliage N did increase after 4 weeks (P < 0.05). Increasing the N loading rate in four smaller urine patches increased pasture biomass (P < 0.01) 2 weeks after urine application and increased foliar N content (P < 0.01) on both sampling occasions. Patch size had no effect (P > 0.05) on pasture biomass or foliar N content when applied at 200 kg N/ha, either 2 or 4 weeks following urine deposition. At 800 kg N/ha, the biomass was greater (P < 0.05) in four small urine patches than in one large urine patch after 2 weeks; however, no differences (P > 0.05) were observed after 4 weeks. No differences (P > 0.05) were observed between the foliar N content of four small patches and that of one large urine patch, when urine was applied at 800 kg N/ha.
N2O fluxes from artificial urine-influenced soil
The weather station data can be seen in Fig. 3a and the results for the N2O emissions over the course of the experiment can be seen in Fig. 3b, alongside the soil water-filled pore space (WFPS; calculated from the SDI-12 soil moisture sensors) in Fig. 3c. The initial peaks in N2O emissions were similar across all treatments where urine was applied, indicating that, initially, the N content was not limiting N2O production. Following the maxima of this initial peak, the two high N treatments begin to branch off from the low N treatments, and generally remained higher than the low N-containing treatments for ~2 weeks. The major peaks in N2O emissions occurred just after rainfall events (Fig. 3a, b). The largest N2O peaks were observed for the high N-containing small urine patches, which peaked at ~150 µg N2O-N/m2.h.
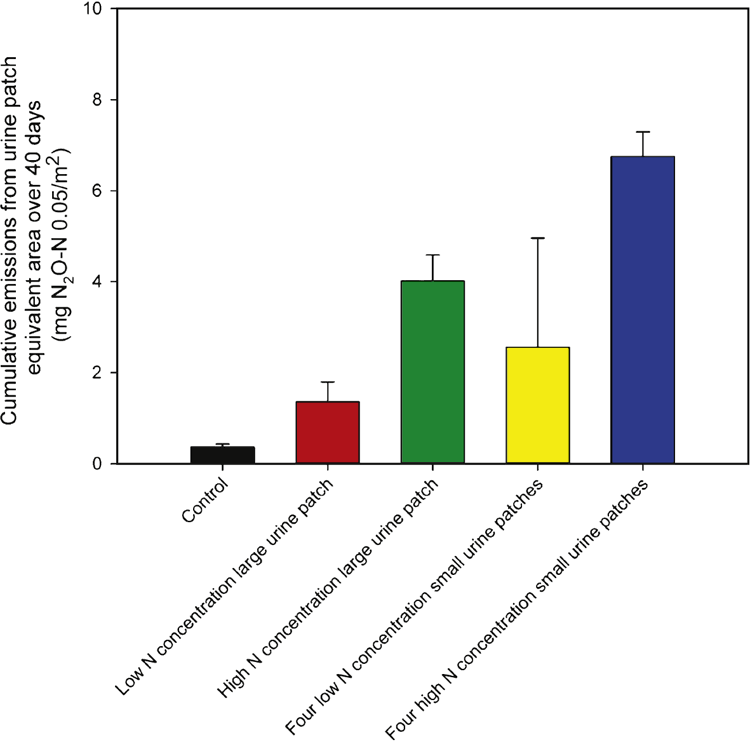
Cumulative N2O emissions
Cumulative N2O emissions (mg N2O-N/0.05 m2) over the 39-day measuring period (Fig. 4) followed the numerical trend of high-N small urine patches (6.75 ± 0.54) > high-N large patch (4.01 ± 0.57) > low-N small patches (2.56 ± 2.40) > low-N large patch (1.36 ± 0.44) > control (0.36 ± 0.07). The variability in cumulative N2O emissions was greater in the low-N small urine-patch treatment, which arose from one ‘high-emitting’ replicate.
Increasing the N loading rate from 200 to 800 kg N/ha within a large urine patch increased (P < 0.05) cumulative N2O emissions by a factor of 3, from 1.36 ± 0.44 to 4.01 ± 0.57 mg N2O-N/0.05 m2 over 39 days. No difference (P > 0.05) was observed between cumulative N2O emissions when increasing N concentration in the four small-sized urine patches. The non-significant effect of increasing N concentration on cumulative N2O emissions in the small urine patches is likely to be the result of the high N2O-emitting replicate; if this replicate is removed from the analysis, the difference then becomes significant (P < 0.01), although the potential for such high emissions cannot be ruled out from smaller urine patches. Increasing the number of replicates per treatment is recommended to capture spatial variability of N2O emissions under field conditions.
The second hypothesis was to determine whether cumulative N2O emissions were higher from four small than from one large urine patch (with the same N loading rate). No effect of patch size (P > 0.05) was observed between the cumulative emissions at the lower N loading rate (200 kg N/ha), regardless of whether the high-emitting replicate was included or removed. However, under a higher N loading rate (800 kg N/ha), cumulative N2O emissions were greater (P < 0.05) from the four small patches (6.75 ± 0.54 mg N2O-N/0.05 m2 over 40 days) than from a single large urine patch (4.01 ± 0.57 mg N2O-N/0.05 m2 over 40 days).
The third hypothesis was that cumulative emissions would be higher from a large urine patch with a low N concentration, than from a small urine patch with a high N concentration (manipulating N loading rate, but keeping total N applied constant). The cumulative emissions from a high N-concentration single small urine patch was 1.69 ± 0.14 mg N2O-N/0.05 m2 over the duration of the study, where no differences were found (P > 0.05) compared with the low N-concentration large urine patch, at 1.36 ± 0.44 mg N2O-N/0.05 m2.
Emission factors
The N2O emission factor for the low N-concentration large urine patch over 39 days was 0.10 ± 0.05% of the N applied and 0.09 ± 0.02% from the high N-concentration large urine patch. The emission factors from the four low N-concentration small urine patches were 0.22 ± 0.24% of the applied N, where one replicate had the highest emission factor of 0.69%, highlighting the large variability observed in this treatment. The N2O emission factor from the four high N-concentration small urine patches was 0.16 ± 0.01% of the total N applied and the emission factor from the high N-concentration large urine patch was 0.09 ± 0.02% of the N applied. No effect of patch size on N2O emission factors was observed in the low N-concentration treatments; however, the emission factor was significantly greater (P < 0.05) from the four small urine patches than from a single large urine patch, at 800 kg N/ha. No significant differences in N2O emission factors were observed when increasing N concentration from 4 to 16 g N L, either from four small patches, or from a single large patch.
Discussion
When urine was applied at the same N loading rate, NO3– concentrations did not peak as high, and returned to control values faster in four smaller urine patches than in a single large patch. The observed effect could have been due to the larger wetted perimeter created by the four small urine patches (179 cm) than by one large urine patch (89 cm). This may have allowed increased plant access to the nutrient-rich patch via roots and stolons, which may explain the greater plant biomass and foliar N content when increasing urine N loading rate in smaller urine patches. Diffusion away from the urine patch edge has been identified as an important mechanism for N processing and transformations in urine patches of different sizes (Orwin et al. 2009). The difference in patch sizes may have allowed a greater lateral, as opposed to vertical, diffusion of soluble N and C into surrounding soil, potentially influencing differing proportions of nitrifying and denitrifying microorganisms. A greater diffusion of NO3– beyond the area of immediate urine application may have also resulted in the lower peak of NO3– found in smaller urine patches, as soil cores were only taken where urine had been directly applied, and Rhizon samples were taken at the centre of the urine patch. The soil mineral-N concentration remained higher for a longer period with increasing urine N concentration, which means N losses can occur under partially differing environmental conditions from the high N treatment compared with the lower N treatment (Dijkstra et al. 2013).
Concentrations of NO3–, NH4+, total N and dissolved organic C in the urine patches (measured in both the soil extracts and the soil solution) tended to vary widely, having large standard errors. This large spatial variability in factors that can drive N2O emissions is likely to have contributed to the large observed variation in N2O emissions in the present study This could also be problematic when comparing urine-patch soil properties from duplicated plots with N2O emissions from chambers, as processes may be occurring at different rates in the different locations. We suggest the development of non-destructive sampling techniques for monitoring soil conditions within chambers to ensure monitored soil properties accurately reflect conditions within chambers.
In the present study, increasing urine N loading rate from 200 kg N/ha to 800 kg N/ha generally increased cumulative N2O emissions, but the emission factors were not significantly different. This is similar to the finding of Selbie et al. (2014), who found a curvilinear increase in cumulative N2O emissions on increasing N loading rate. However, increasing urine N loading rate had no effect on the fraction of N2O produced under laboratory (van Groenigen et al. 2005a) and field conditions (van Groenigen 2005b; Dai et al. 2013; Luo et al. 2013). However, urine-derived N2O emission factors have also been shown to decrease (Selbie et al. 2014) or increase alongside N loading rate (Singh et al. 2009). Results for the response of urine patch N2O emission factors to increasing N loading rates are contradictory and uncertain (Luo et al. 2013; Selbie et al. 2014).
When manipulating urine patch size and N loading rate, but maintaining the same total amount of N applied, no differences were found in cumulative N2O emissions or emission factors, which is similar to the findings of van Groenigen et al. (2005a). However, cumulative N2O emissions and emission factors were significantly greater from four small urine patches than from one large urine patch, when applied at 800 kg N/ha. It is difficult to determine a management strategy that would reduce N2O emissions on the basis of the results of this treatment, as it assumes a difference in the frequency of urine events, but with the same N concentration and total volume of urine excreted. In reality, ruminant urine N concentration and volume varies widely among and within days, and among individuals of the same species (Betteridge et al. 1986, 2013; Hoogendoorn et al. 2010). Nevertheless, on the basis of the results of the present study and the sheep urine patch sizes used here, it would seem that sheep that urinate more frequently in smaller volumes would emit more N2O than sheep urinating less frequently in larger volumes, given the same high urinary N concentration. Further work is required to determine how this effects other N-loss processes such as NO3– leaching and NH3 volatilisation, as, given the range of patch sizes excreted by cattle, increasing patch size has been shown to logarithmically increase NO3– leaching (Li et al. 2012).
It has been recognised that more information is required for values of ruminant urine volume, urine N concentration and urination frequency, where the development of sensor-based technology that can measure all three of these values (e.g. Betteridge et al. 2013) will be important for improving models to predict N losses from grazed grassland. An increase in our knowledge of how ruminant urine N concentration is affected by dietary factors will also be important; for example, Pacheco et al. (2010) found urinary N concentration of cattle urine to be related to the moisture content, dietary cation–anion difference and soluble sugar content of the forage. Increasing knowledge in these areas will also assist in a better understanding on the effect of differing management and mitigation strategies to reduce N losses (e.g. feed type, grazing intensity and inclusion of diuretics in the diet).
Increasing urine volume, for example, by the inclusion of NaCl in the diet, has been suggested as a way to reduce N losses as it would reduce N loading rate per urination, potentially promoting a better dispersion of N across the pasture (Costall and Betteridge 2010; Pacheco et al. 2010), although this will depend on livestock movement and behaviour. However, the results of our study have shown that N2O emissions were no different in patches of differing N loading rates, for the same amount of N applied. Therefore, for a reduction in N2O emissions from sheep urine patches, it is suggested that a reduction in dietary N would need to occur concurrently with any mitigation strategies based on increasing total urine volume (de Klein et al. 2014).
Both Ledgard et al. (2007) and Liu and Zhou (2014) showed that increasing NaCl in the diet of cattle and sheep, respectively, results in increased urine volume and frequency, but the average volume of individual urine events remained similar. Both Li et al. (2012) and Betteridge et al. (2013) showed that considering mean values (rather than varied values) for cattle urine volume, frequency and N concentration causes differences of ~5–10% in modelled NO3– leaching losses. The mean values for urine volume and N concentration are also often used in plot-based studies of urine-patch N2O emissions, which may also cause inaccuracies when upscaling such emissions.
Across all urine treatments, N2O emission factors were on average 10 times lower than the 1% default IPCC emission factor for sheep excreta (IPCC 2006). In a large study based on numerous field trials conducted across New Zealand, the mean sheep urine-patch emission factors from lowland pasture soils were also found to be lower than the default IPCC emission factor for excretal N deposited to pasture, at 0.55% of the applied N (Kelliher et al. 2014). The low emission factors found in the present study may have been caused by the relatively dry soil conditions that prevailed during the study. Low emission factors have been previously observed above sheep-grazed pastures under dry summer months, with maximum emissions occurring under cooler and wetter soil conditions (Allen et al. 1996; Saggar et al. 2007). Rainfall and soil drainage class have been identified as key variables influencing N2O emission factors from urine returns (de Klein et al. 2003), and a consideration of environmental and management factors (which are not considered in the IPCC Tier 1 approach) will undoubtedly be important for better constraining N2O emission estimates from grazed soils.
The values for emission factors in the present study should be considered with care, as they were derived from artificial urine and overall emissions were fairly low and variable, which may have masked some effects. It is suggested that similar studies should be conducted under differing environmental conditions to determine whether the effects hold true. Real urine is generally advocated for the calculation of emission factors (de Klein et al. 2003); however, the use of artificial urine was beneficial in the present study as it allowed the manipulation of N concentration. The artificial urine contained the recommended constituents for adequate representations of urine for N2O emissions (Kool et al. 2006) and the proportion of urea was increased to manipulate the N content, which is consistent with an increase in the proportion of urea excreted by ruminants occurring as a result of increased dietary crude protein (Dijkstra et al. 2013).
Conclusions
Cumulative N2O emissions generally increased, but emission factors remained similar, with increasing N loading rate (200–800 kg N/ha). Emission factors from a low N-concentration large urine patch (200 kg N/ha) were similar to those from a high N-concentration small urine patch (800 kg N/ha), when applying the same total amount of N. However, emissions were larger from four smaller patches than from one large urine patch under the same N loading rate (800 kg N/ha). These conclusions suggest that a reduction in overall dietary N would need to occur, alongside any mitigation strategies which manipulate urine volume, so as to be effective at reducing N2O emissions from sheep urine patches. For the same total volume and concentration of N excreted in the urine, sheep that urinate little and often may be causing greater emissions than sheep that urinate less frequently with a larger volume and patch size. With this in mind, further research is required to gather more data on typical sheep urine volume (both individual urine events and daily volumes), frequency and N concentrations. Such parameters may vary due to sheep breed, edaphic and climatic conditions, diet and management or mitigation practices (e.g. inclusion of salt in diet). Improving our knowledge on the links within the plant–animal–soil–atmosphere system will be important for determining more accurate emission estimates from sheep-grazed grasslands, and for determining the efficacy of N2O mitigation strategies.
Acknowledgements
We thank Christian Brunk at QUT for his assistance and support during the set up phase of the automated GHG system, Lara Jane Pritchard for assistance with field sampling and John Evans, Mark Hughes and Llinos Hughes for their assistance setting up the field trial and the two anonymous reviewers for their suggestions for improvements to the manuscript. KAM also thanks the School of Environment, Natural Resources and Geography, Bangor University, for sponsoring this study. This work was supported by the Natural Environment Research Council under Grant award NE/MO15351/1.
References
Allen AG, Jarvis SC, Headon DM (1996) Nitrous oxide emissions from soils due to inputs of nitrogen from excreta return by livestock on grazed grassland in the UK. Soil Biology & Biochemistry 28, 597–607.| Nitrous oxide emissions from soils due to inputs of nitrogen from excreta return by livestock on grazed grassland in the UK.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XjvF2itrY%3D&md5=43f99fa7076c870333b5da4c52334156CAS |
Ball DF (1964) Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. Journal of Soil Science 15, 84–92.
| Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXkt1Whtrg%3D&md5=3493cdbd6ed17859eb3b95da1e42a4afCAS |
Betteridge K, Andrews WGK, Sedcole JR (1986) Intake and excretion of nitrogen, potassium and phosphorus by grazing steers. The Journal of Agricultural Science 106, 393–404.
| Intake and excretion of nitrogen, potassium and phosphorus by grazing steers.Crossref | GoogleScholarGoogle Scholar |
Betteridge K, Costall D, Balladur S, Upsdell M, Umemura K (2010a) Urine distribution and grazing behaviour of female sheep and cattle grazing a sheep New Zealand hill pasture. Animal Production Science 50, 624–629.
| Urine distribution and grazing behaviour of female sheep and cattle grazing a sheep New Zealand hill pasture.Crossref | GoogleScholarGoogle Scholar |
Betteridge K, Hoogendoorn C, Costall D, Carter M, Griffiths W (2010b) Sensors for detecting and logging spatial distribution of urine patches of grazing female sheep and cattle. Computers and Electronics in Agriculture 73, 66–73.
| Sensors for detecting and logging spatial distribution of urine patches of grazing female sheep and cattle.Crossref | GoogleScholarGoogle Scholar |
Betteridge K, Costall DA, Li FY, Luo D, Ganesh S (2013) Why we need to know what and where cows are urinating – a urine sensor to improve nitrogen models. Proceedings of the New Zealand Grassland Association 75, 119–124.
Bristow A, Whitehead DC, Cockburn JE (1992) Nitrogenous constituents in the urine of cattle, sheep and goats. Journal of the Science of Food and Agriculture 59, 387–394.
| Nitrogenous constituents in the urine of cattle, sheep and goats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XmsVyqt7Y%3D&md5=ac08e01e85e3934a453614e867e5e52eCAS |
Costall K, Betteridge K (2010) Methods of delivering salt to cattle. Proceedings of the New Zealand Society of Animal Production 70, 296–298.
Dai Y, Di HJ, Cameron KC, He J-Z (2013) Effects of nitrogen application rate and a nitrification inhibitor dicyandiamide on ammonia oxidizers and N2O emissions in a grazed pasture soil. The Science of the Total Environment 465, 125–135.
| Effects of nitrogen application rate and a nitrification inhibitor dicyandiamide on ammonia oxidizers and N2O emissions in a grazed pasture soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWmtL3I&md5=9ed0f0d204b6f7c69dfda04834d233b6CAS | 23021462PubMed |
de Klein CAM, Harvey MJ (2012) ‘Nitrous oxide chamber methodology guidelines.’ (Global Research Alliance on Agricultural Greenhouse Gases, Ministry for Primary Industries: Wellington, New Zealand)
de Klein CAM, Barton L, Sherlock RR, Li Z, Littlejohn RP (2003) Estimating a nitrous oxide emission factor for animal urine from some New Zealand pasture soils. Australian Journal of Soil Research 41, 381–399.
| Estimating a nitrous oxide emission factor for animal urine from some New Zealand pasture soils.Crossref | GoogleScholarGoogle Scholar |
de Klein CAM, Luo J, Woodward KB, Styles T, Wise B, Lindsey S, Cox N (2014) The effect of nitrogen concentration in synthetic cattle urine on nitrous oxide emissions. Agriculture, Ecosystems & Environment 188, 85–92.
| The effect of nitrogen concentration in synthetic cattle urine on nitrous oxide emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXnslGhsr4%3D&md5=6992e906d5ca98e4dc938c91f285a91fCAS |
Di HJ, Cameron KC (2008) Sources of nitrous oxide from 15N-labelled animal urine and urea fertiliser with and without a nitrification inhibitor, dicyandiamide (DCD). Australian Journal of Soil Research 46, 76–82.
| Sources of nitrous oxide from 15N-labelled animal urine and urea fertiliser with and without a nitrification inhibitor, dicyandiamide (DCD).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhs1WmsLY%3D&md5=36a706ce984a27c9c1a12c36a5041ef7CAS |
Dijkstra J, Oenema O, van Groenigen JW, Spek JW, van Vuuren AM, Bannink A (2013) Diet effects on urine composition of cattle and N2O emissions. Animal 7, 292–302.
| Diet effects on urine composition of cattle and N2O emissions.Crossref | GoogleScholarGoogle Scholar | 23739471PubMed |
Doak BW (1952) Some chemical changes in the nitrogenous constituents of urine when voided on pasture. The Journal of Agricultural Science 42, 162–171.
| Some chemical changes in the nitrogenous constituents of urine when voided on pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG2cXjtlyitw%3D%3D&md5=f5511f44da4a382734f5170849faaad2CAS |
Haynes RJ, Williams PH (1993) Nutrient cycling and soil fertility in the grazed pasture ecosystem. Advances in Agronomy 49, 119–199.
| Nutrient cycling and soil fertility in the grazed pasture ecosystem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXltlygs7Y%3D&md5=7e66c458181ce52451e838bd2edf3b5eCAS |
Hoogendoorn CJ, Betteridge K, Costall DA, Ledgard SF (2010) Nitrogen concentration in the urine of cattle, sheep and deer grazing a common ryegrass/cocksfoot/white clover pasture. New Zealand Journal of Agricultural Research 53, 235–243.
| Nitrogen concentration in the urine of cattle, sheep and deer grazing a common ryegrass/cocksfoot/white clover pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFWnsLfI&md5=ef3ff16c6f84b77489cb1d759309cc5bCAS |
IPCC (2006) ‘IPCC guidelines for national greenhouse gas inventories. Vol. 4. Agriculture, forestry and other land use.’ Available at http://www.ipcc-nggip.iges.or.jp/public/2006gl/vol4.html [Verified 11 August 2015]
Jones DL, Willett VB (2006) Experimental evaluation methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biology & Biochemistry 38, 991–999.
| Experimental evaluation methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjvFSitr4%3D&md5=db935e16acd4399641c98f3e8382109cCAS |
Kelliher FM, Cox N, van der Weerden TJ, de Klein CAM, Luo J, Cameron KC, Di HJ, Giltrap D, Rys G (2014) Statistical analysis of nitrous oxide emission factors from pastoral agriculture field trials conducted in New Zealand. Environmental Pollution 186, 63–66.
| Statistical analysis of nitrous oxide emission factors from pastoral agriculture field trials conducted in New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvVajsrc%3D&md5=d09e39fad30fef5d7354f1d4d01e1a93CAS | 24361566PubMed |
Kool DM, Hoffland E, Abrahamse S(PA), van Groenigen JW (2006) What artificial urine composition is adequate for simulating soil N2O fluxes and mineral N dynamics? Soil Biology & Biochemistry 38, 1757–1763.
| What artificial urine composition is adequate for simulating soil N2O fluxes and mineral N dynamics?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xnt1yltbs%3D&md5=206d115195b4bdd9b88fe929fdeddf4fCAS |
Lambie SM, Schipper LA, Balks MR, Baisden WT (2013) Priming of soil decomposition leads to losses of carbon in soil treated with cow urine. Soil Research 51, 513–520.
| Priming of soil decomposition leads to losses of carbon in soil treated with cow urine.Crossref | GoogleScholarGoogle Scholar |
Lantinga EA, Keuning JA, Groenwold J, Deenen PJAG (1987) Distribution of excreted nitrogen by grazing cattle and its effects on sward quality, herbage production and utilization. In ‘Animal manure on grassland and fodder crops, fertilizer or waste?’. (Eds HG van der Meer, RJ Unwin, TA van Dijk, GC Ennik) pp. 103–117. (Kluwer Academic Publishers: Lancaster, UK)
Ledgard SF, Welten BG, Menneer JC, Betteridge K, Crush JR, Barton MD (2007) New nitrogen mitigation technologies for evaluation in the Lake Taupo catchment. Proceedings of the New Zealand Grassland Association 69, 117–121.
Ledgard SF, Menneer JC, Dexter MM, Kear MJ, Lindsey S, Peters JS, Pacheco DS (2008) A novel concept to reduce nitrogen losses from grazed pastures by administering soil nitrogen process inhibitors to ruminant animals: a study with sheep. Agriculture, Ecosystems & Environment 125, 148–158.
| A novel concept to reduce nitrogen losses from grazed pastures by administering soil nitrogen process inhibitors to ruminant animals: a study with sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjtlSku7Y%3D&md5=44754fcb0339fa1c406e33b401099b10CAS |
Li FY, Batteridge K, Cichota R, Hoogendoorn CJ, Jolly BH (2012) Effects of nitrogen load variation in animal urination events on nitrogen leaching from grazed pastures. Agriculture, Ecosystems & Environment 159, 81–89.
| Effects of nitrogen load variation in animal urination events on nitrogen leaching from grazed pastures.Crossref | GoogleScholarGoogle Scholar |
Liu H, Zhou D (2014) Mitigation of ammonia and nitrous oxide emissions from pasture treated with urine of sheep fed diets supplemented with NaCl. Animal Feed Science and Technology 192, 39–47.
| Mitigation of ammonia and nitrous oxide emissions from pasture treated with urine of sheep fed diets supplemented with NaCl.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmslamt7k%3D&md5=06fc1bbbc37446cf1ae6700c50b56856CAS |
Lucas SD, Jones DL (2006) Biodegradation of estrone and 17 β-estradiol in grassland soils amended with animal wastes. Soil Biology & Biochemistry 38, 2803–2815.
| Biodegradation of estrone and 17 β-estradiol in grassland soils amended with animal wastes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XotFKjurw%3D&md5=a4d9b44a94be534388460957bf20ad91CAS |
Luo J, Hoogendoorn C, van der Weerden T, Saggar S, de Klein C, Giltrap D, Rollo M, Rys G (2013) Nitrous oxide emissions from grazed hill land in New Zealand. Agriculture, Ecosystems & Environment 181, 58–68.
| Nitrous oxide emissions from grazed hill land in New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitVShtrjM&md5=e6114c80e715310d14d4e6ea6cd4a324CAS |
Marsden KA, Jones DL, Chadwick DR (2016) The urine patch diffusional area: an important N2O source? Soil Biology & Biochemistry 92, 161–170.
| The urine patch diffusional area: an important N2O source?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhslGltb7N&md5=38509adb78387794324da74fd751be8dCAS |
Miranda KM, Epsey MG, Wink DA (2001) A rapid, simple, spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5, 62–71.
| A rapid, simple, spectrophotometric method for simultaneous detection of nitrate and nitrite.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtFemsrs%3D&md5=ee61dbd83cbdc16152e15b25dc28ebf5CAS | 11178938PubMed |
Mulvaney RL (1996) Nitrogen – inorganic forms. In ‘Methods of soil analysis part 3’. (Ed. DL Sparks) pp. 1123–1184. (Soil Science Society of America Inc.: Madison, WI)
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27, 31–36.
| A modified single solution method for the determination of phosphate in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF38XksVyntr8%3D&md5=402b1fd8521d55dfa2240a31023d85f1CAS |
Oenema O, Velthof GL, Yamulki S, Jarvis SC (1997) Nitrous oxide emissions from grazed grassland. Soil Use and Management 13, 288–295.
| Nitrous oxide emissions from grazed grassland.Crossref | GoogleScholarGoogle Scholar |
Orwin KH, Bertram JE, Clough TJ, Condron LM, Sherlock RR, O’Callaghan M (2009) Short-term consequences of spatial heterogeneity in soil nitrogen concentrations caused by urine patches of different sizes. Applied Soil Ecology 42, 271–278.
| Short-term consequences of spatial heterogeneity in soil nitrogen concentrations caused by urine patches of different sizes.Crossref | GoogleScholarGoogle Scholar |
Pacheco D, Lowe K, Hickey M, Burke JL, Cosgrove GP (2010) Seasonal and dietary effects on the concentration of urinary N from grazing cows. In ‘Meeting the challenges of pasture-based dairying: proceedings of the 4th Australasian dairy science symposium’. (Eds GR Edwards, RH Bryant) pp. 68–73. (Lincoln University: Christchurch, New Zealand)
Saggar S, Hedley CB, Giltrap DL, Lambie SM (2007) Measured and modelled estimates of nitrous oxide emission and methane consumption from a sheep-grazed pasture. Agriculture, Ecosystems & Environment 122, 357–365.
| Measured and modelled estimates of nitrous oxide emission and methane consumption from a sheep-grazed pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmsFKhtro%3D&md5=a07a3f65fc6071880f3f7e03485df858CAS |
Scheer C, Rowlings DW, Firrel M, Deuter P, Morris S, Grace PR (2014) Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia. Soil Biology & Biochemistry 77, 243–251.
| Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht12ntL3M&md5=829f5c647177157a5492d65872a4ca04CAS |
Selbie DR, Cameron KC, Di HJ, Moir JL, Lanigan JG, Richards KG (2014) The effect of urinary nitrogen loading rate and a nitrification inhibitor on nitrous oxide emissions from a temperate grassland soil. The Journal of Agricultural Science 152, S159–S171.
| The effect of urinary nitrogen loading rate and a nitrification inhibitor on nitrous oxide emissions from a temperate grassland soil.Crossref | GoogleScholarGoogle Scholar |
Selbie DR, Buckthought LE, Shepherd MA (2015) The challenge of the urine patch for managing nitrogen in grazed pasture systems. Advances in Agronomy 129, 229–292.
| The challenge of the urine patch for managing nitrogen in grazed pasture systems.Crossref | GoogleScholarGoogle Scholar |
Singh J, Saggar S, Bolan NS (2009) Influence of dicyandiamide on nitrogen transformation and losses in cow-urine-amended soil cores from grazed pasture. Animal Production Science 49, 253–261.
| Influence of dicyandiamide on nitrogen transformation and losses in cow-urine-amended soil cores from grazed pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisFSnt78%3D&md5=41e1b473ec37ffa2dab2bf9999ccc4b1CAS |
van Groenigen JW, Kuikman PJ, de Groot WJM, Velthof GL (2005a) Nitrous oxide emission from urine-treated soil as influenced by urine composition and soil physical conditions. Soil Biology & Biochemistry 37, 463–473.
| Nitrous oxide emission from urine-treated soil as influenced by urine composition and soil physical conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFGgsrnF&md5=12b09cbcf935fa1988658ba13238db2aCAS |
van Groenigen JW, Velthof GL, van der Bolt FJE, Vos A, Kuikman PJ (2005b) Seasonal variation in N2O emissions from urine patches: effects of urine concentration, compaction and dung. Plant and Soil 273, 15–27.
| Seasonal variation in N2O emissions from urine patches: effects of urine concentration, compaction and dung.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXks1Ojs7w%3D&md5=5a6e32ffda81adb75dc76ab7e711fe01CAS |
Voroney RP, Brookes PC, Beyaert RP (2008) Soil microbial biomass C, N, P and S. In ‘Soil sampling and methods of analysis’. 2nd edn. (Eds MR Carter, EG Gregorich) pp. 637–651. (CRC Press: Boca Raton, FL)
Williams PH, Haynes RJ (1994) Comparison of initial wetting pattern, nutrient concentrations in soil solution and the fate of 15N-labelled urine in sheep and cattle urine patch areas of pasture soil. Plant and Soil 162, 49–59.
| Comparison of initial wetting pattern, nutrient concentrations in soil solution and the fate of 15N-labelled urine in sheep and cattle urine patch areas of pasture soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXlslyksrY%3D&md5=e973b19b1e44605b8f44d3976c16a0c4CAS |