Addition of sodium metabisulfite and microbial phytase, individually and in combination, to a sorghum-based diet for broiler chickens from 7 to 28 days post-hatch
H. H. Truong A B , D. J. Cadogan C , S. Y. Liu A and P. H. Selle A DA Poultry Research Foundation within The Faculty of Veterinary Science, The University of Sydney, 425 Werombi Road, Camden, NSW 2570, Australia.
B Poultry CRC, University of New England, Armidale, NSW 2351, Australia.
C Feedworks, Lancefield, Vic. 3435, Australia.
D Corresponding author. Email: peter.selle@sydney.edu.au
Animal Production Science 56(9) 1484-1491 https://doi.org/10.1071/AN14841
Submitted: 30 September 2014 Accepted: 20 January 2015 Published: 30 April 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Sodium metabisulfite (SMBS; 1.75 g/kg) and phytase (1000 FTU/kg), individually and in combination, were included in steam-pelleted, sorghum-based (580 g/kg) broiler diets from 7 to 28 days post-hatch. Rapid visco-analysis starch pasting properties of dietary treatments were monitored. Parameters of growth performance, nutrient utilisation, relative organ weights, toe ash, excreta moisture, apparent starch and nitrogen digestibility coefficients and disappearance rates in four small intestinal segments were determined. There were significant treatment interactions in the proximal jejunum (P < 0.01) and distal ileum (P < 0.05) for nitrogen digestibility coefficients. SMBS alone significantly increased jejunal nitrogen digestibility by 14.9% (0.634 vs 0.552) but the response to SMBS in combination with phytase was negligible (0.558 vs 0.552). SMBS alone significantly increased ileal nitrogen digestibility by 4.92% (0.786 vs 0.732) but the combination numerically improved digestibility by 0.96% (0.739 vs 0.732). SMBS alone tended to increase starch digestibility by 12.0% (0.691 vs 0.617; P = 0.064) in the proximal jejunum and increased rapidly digestible starch by 17.2% (116 vs 99 g/bird.day; P < 0.02). However, SMBS tended to depress apparent metabolisable energy by 0.33 MJ (P < 0.10). Therefore, consideration is given to the mechanisms influencing starch digestion rates, energy utilisation and nitrogen digestibility interactions between SMBS and phytase in this feeding study.
Additional keywords: dilated proventriculi, disulfide cross-linkages, kafirin, RVA starch pasting properties, slowly digestible starch.
Introduction
Graded inclusions of the sulfite-reducing agent sodium metabisulfite (SMBS) in sorghum-casein diets (Selle et al. 2013) and conventional sorghum-based broiler diets (Liu et al. 2014; Selle et al. 2014) displayed promise in two previous studies. The positive effects of SMBS on energy utilisation and efficiency of feed conversion appeared to stem from either the oxidative-reductive depolymerisation of starch polysaccharides or the reduction of disulfide cross-linkages in proteins. It was suggested that the oxidative-reductive depolymerisation reactions generated slowly digestible starch and this was the main factor which drove the observed improvements. Previously it was shown (Liu et al. 2014) that SMBS significantly influenced the rapid visco-analysis (RVA) pasting properties as seven graded SMBS inclusions (0–5.25 g/kg) linearly decreased final viscosity of starch (r = –0.925) in sorghum-based diets and starch extracted from those diets (r = –0.986). Presumably this was a consequence of SMBS-induced starch depolymerisation and for this reason RVA properties of the dietary treatments were determined in this study. Problems associated with ‘wet litter’ in broiler houses are a production and welfare issue of increasing importance (Collett 2012) and thus excreta moisture content was determined. The first objective of this feeding study was to confirm the effects of SMBS in conventional sorghum-based broiler diets at a specific inclusion rate of 1.75 g/kg, which was considered appropriate from the previous studies. However, the inclusion of phytate-degrading feed enzymes in broiler diets is now routinely practiced globally (Ravindran and Selle 2010). Thus, the second objective was to determine the effects of including 1.75 g/kg SMBS and 1000 FTU/kg phytase, individually and in tandem, in sorghum-based diets. The combined inclusions of these two dissimilar feed additives need to be compatible for this approach to be feasible. However, as responses to phytase in sorghum-based broiler diets appear comparatively modest (Selle et al. 2010) it is possible that the combined inclusion of phytase and SMBS may be advantageous.
Materials and methods
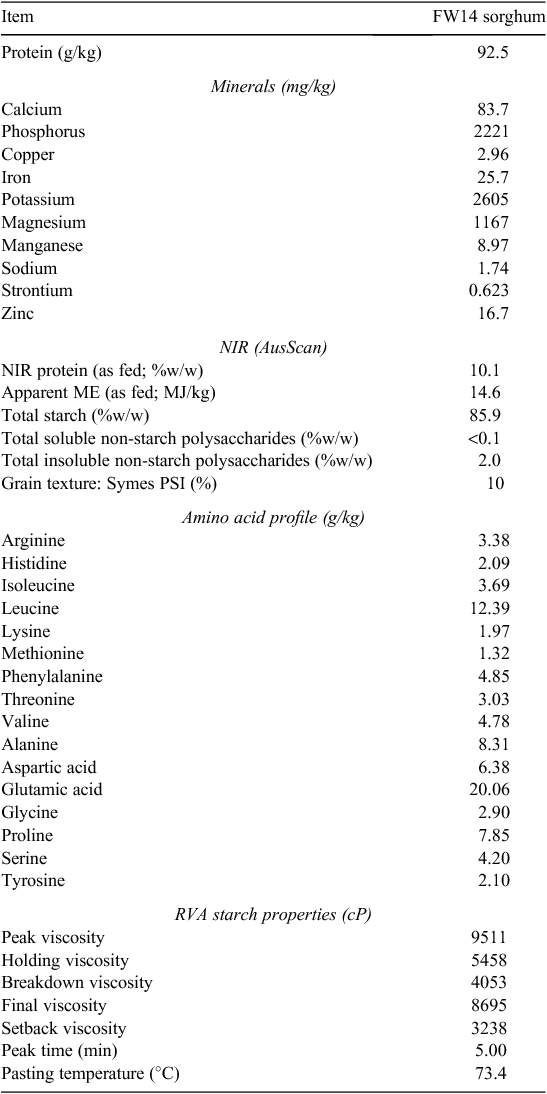
The 92.5 g/kg crude protein red grain sorghum FW14 used in this feeding study was characterised as shown in Table 1. The amino acid profile was determined by high performance liquid chromatography, mineral composition was obtained by inductively coupled plasma mass spectrometry, starch pasting properties by RVA as described below and grain texture by the particle size index method (Symes 1965). Sorghum FW14 was subjected to AusScan (Pork CRC Ltd, Willaston, SA, Australia) and had a predicted metabolisable energy density in broilers of 14.6 MJ/kg. The analysed phosphorus (P) content of sorghum FW14 was 2.22 g/kg and the estimated phytate-P content was 1.84 g/kg or 6.51 g/kg phytate. The grain had a PSI texture of 10, which is in the ‘very hard’ category and a kafirin index [g/kg leucine – (basic amino acids)] of 4.9.
|
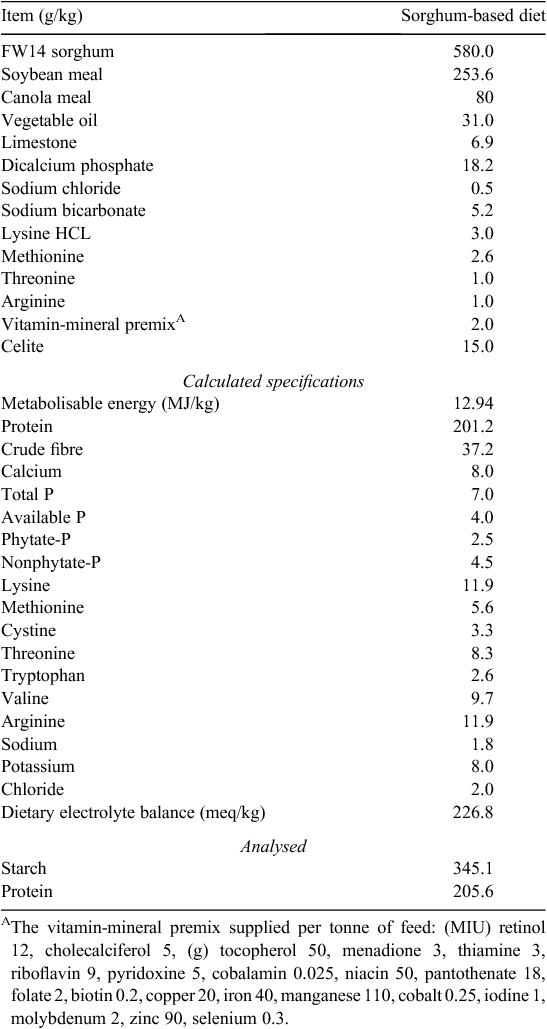
Sorghum FW14 was incorporated into a basal grower diet at 580 g/kg formulated to contain 201.2 g/kg of protein with an energy density of 12.94 MJ/kg as shown in Table 2. The basal diet was formulated to be P-adequate with a non-phytate-P content of 4.5 g/kg. SMBS and a Buttiauxella phytase (Axtra PHY, Danisco Animal Nutrition, Marlborough, Wiltshire, UK) were included in the basal diet at 1.75 g/kg and 1000 FTU/kg, respectively. With the addition of SMBS, dietary sodium (Na) levels and dietary electrolyte balances were maintained constant by manipulating Na bicarbonate levels. Levels of the dietary marker, Celite, were adjusted to accommodate inclusions of SMBS, Na bicarbonate and phytase. Sorghum FW14 was hammer-milled through a 3.2-mm screen before being mixed into complete diets, which were steam-pelleted at a conditioning temperature of 80°C.
|
Starch pasting properties of the four experimental diets were determined with a rapid visco-analyser (RVA-4, Newport Scientific Pty Ltd, Warriewood, NSW, Australia) as shown in Table 3. Procedures outlined by Hernandez et al. (2008) were followed. Within 13-min intervals, 28 g of mixtures of diets and water (15 : 85 w/w) on a dry basis were prepared and held at a temperature of 50°C for 1 min and then heated from 50°C to 95°C. After holding the hot paste at 95°C for 2.5 min, the slurry was again cooled to 50°C, and then held at that temperature for 2 min.
A total of 168 male broiler chicks (Ross 308) were offered a proprietary starter ration from 1 to 7 days post-hatch. The chicks were housed in an environmentally controlled facility under a ‘23 h-on’ lighting regime. On Day 7, birds were wing-tagged, weighed and assigned to 28 cages on the basis of bodyweight. Each of the four dietary treatments was offered to seven replicate cages (six birds per cage) from 7 to 28 days post-hatch. Feed intakes were monitored throughout the feeding period and, on Day 28, birds were weighed and euthanised (intravenous injection of sodium pentobarbitone). Weight gains of each bird were determined and feed conversion ratios calculated from feed intakes per cage. The incidence of dead or culled birds was recorded daily and their bodyweights were used to adjust feed conversion ratio calculations.
On Day 28, gizzard pH was obtained by inserting a pH meter into digesta within the gizzard and relative weights of emptied gizzards and pancreata were determined. During this procedure an unusually high incidence of grossly dilated proventriculi was noticed and therefore recorded. One toe was collected from each bird in a replicate and pooled to determine bone mineralisation and toe ash was expressed as a percentage of dry weight. Severed toes from each replicate were pooled and dried to a constant weight at 100°C and then ashed in a muffle furnace at 600°C for 6 h.
Total excreta were collected from 23 to 26 days post-hatch from each cage to determine nutrient utilisation. Feed intakes and excreta outputs were recorded for this 72-h period. Excreta were air-forced oven-dried for 24 h at 80°C and excreta moisture was determined. Gross energy (GE) of diets and excreta were determined using a Parr 1281 adiabatic bomb calorimeter (Parr Instrument Co., Moline, IL, USA). Apparent metabolisable energy (AME), ME : GE ratios, nitrogen (N) retention and N-corrected apparent metabolisable energy (AMEn) on a dry matter basis were calculated by the following formulae.
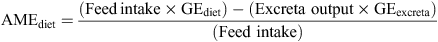
The ME : GE ratios were calculated by dividing AME values of diets recorded for each cage by GE of the relevant diets. N content of the diets and excreta were obtained using an FP-428 determinator (Leco Corporation, St Joseph, MI, USA). N retention was calculated using the following formula:

The AMEn (MJ/kg) values were calculated by correcting to zero N retention by applying the factor of 36.54 kJ/g N retained in the body (Hill and Anderson 1958).
Acid-insoluble ash (AIA, Celite World Minerals, Lompoc, CA, USA) was included in diets at ~15 g/kg as an inert marker to determine apparent digestibility coefficients of N and starch in the four small intestinal sites. The small intestines were removed from euthanised birds and digesta in their entirety were gently expressed from the proximal jejunum (PJ), distal jejunum, proximal ileum and distal ileum (DI) and pooled for each cage. Proximal jejunal samples were taken from the end of the duodenal loop to the midpoint with Meckel’s diverticulum and distal jejunal samples from the midpoint to the diverticulum. Proximal ileal samples were taken from Meckel’s diverticulum to the midpoint to the ileo-caecal junction and distal ileal samples were taken from below this midpoint. The digesta samples were freeze-dried and AIA concentrations were determined by the method of Siriwan et al. (1993). N concentrations were determined as outlined above and starch concentrations in diets and digesta were determined by a procedure based on dimethyl sulfoxide, α-amylase and amyloglucosidase, as described by Mahasukhonthachat et al. (2010). The apparent digestibility coefficients for N and starch at four small intestinal sites were calculated using the following equation:

Quantities of rapidly and slowly digestible starch were calculated from the following equations to determine starch disappearance rates (g/bird.day) from the defined region of the small intestine. Calculations were based on average daily feed intakes from 23 to 28 days post-hatch, analysed dietary nutrient concentrations and determined digestibility coefficients in the PJ and DI. Rapidly digestible starch is defined as starch digested in the PJ and slowly digestible starch is starch digested in the three posterior segments of the small intestine. N disappearance rates were similarly determined.
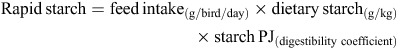
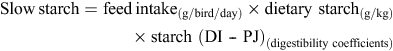
Experimental data was analysed as a 2 by 2 factorial arrangement of dietary treatments using the IBM SPSS Statistics 20 program (IBM Corporation, Somers, NY, USA). The experimental units were cage means and statistical procedures included univariate ANOVA using the general linear models procedure, linear regressions and Pearson correlations. When appropriate, the significance of pair-wise comparisons was taken into consideration. A probability level of less than 5% was considered to be statistically significant. The feeding study complied with specific guidelines approved by the Animal Ethics Committee of the University of Sydney.
Results
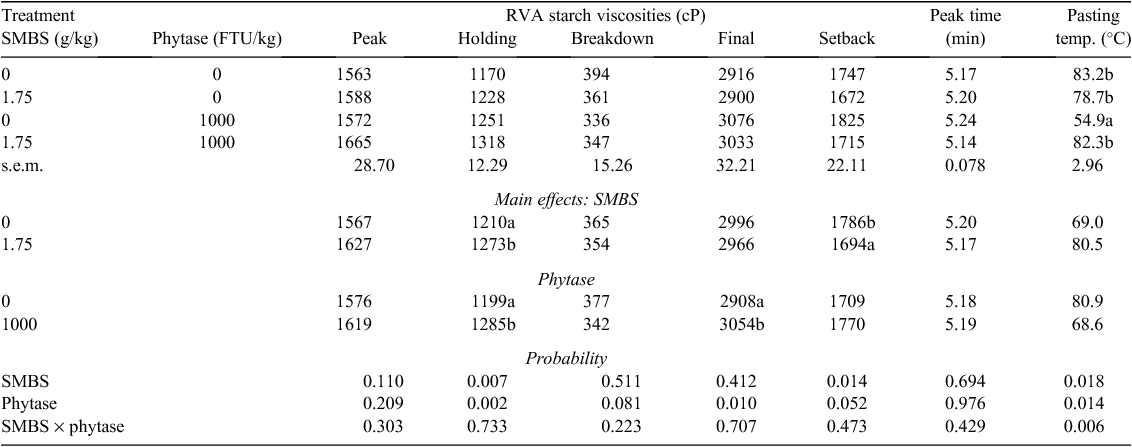
Effects of SMBS and phytase on starch RVA properties of sorghum-based diets steam-pelleted at a conditioning temperature of 80°C are shown in Table 3. SMBS increased holding viscosity by 5.21% (1273 vs 1210 cP; P < 0.01) and decreased setback viscosity by 5.15% (1694 vs 1786 cP; P < 0.015). Phytase increased holding (1285 vs 1199 cP; P < 0.01) and final (3054 vs 2908 cP; P < 0.025) starch viscosities by 7.17% and 5.02%, respectively. There was a significant interaction (P < 0.01) between SMBS and phytase for pasting temperature as values for the diet containing phytase alone was significantly less than the other three treatments.
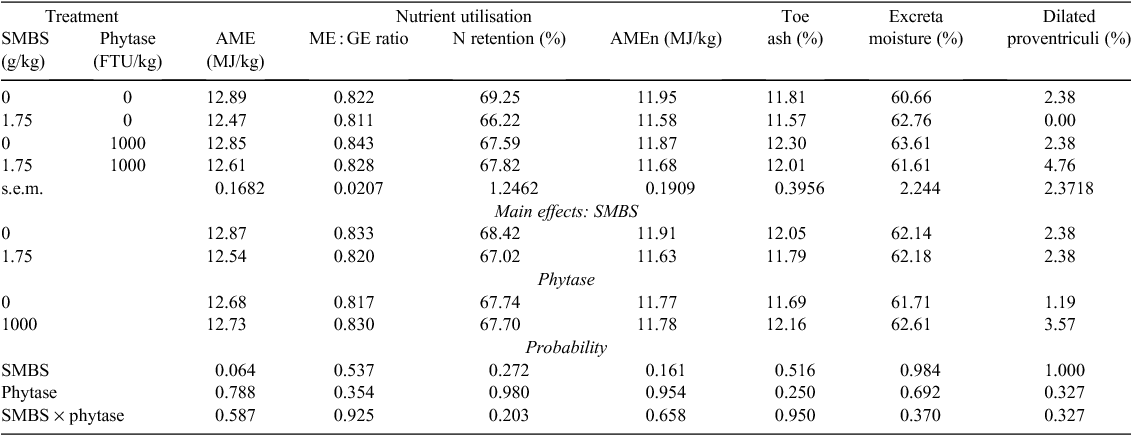
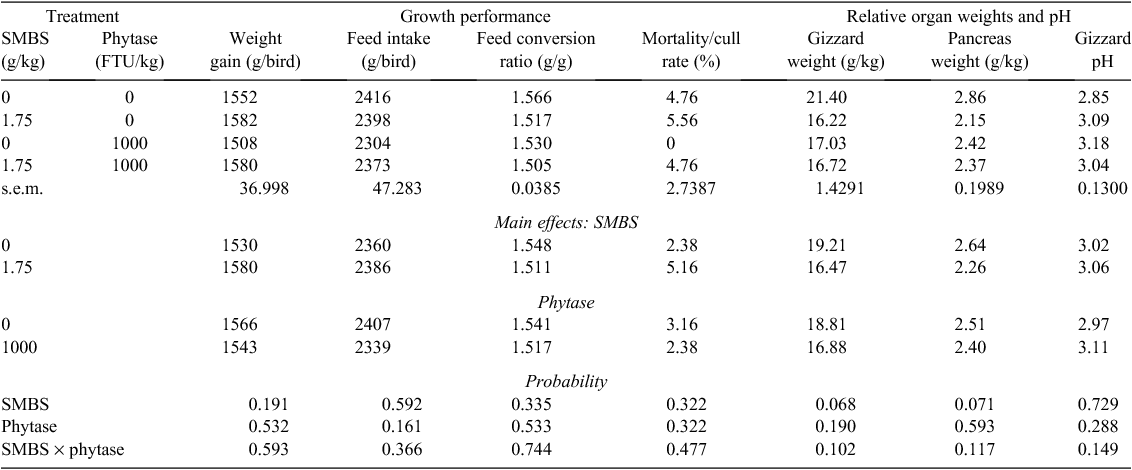
Effects of SMBS and phytase on growth performance, relative organ weights and gizzard pH are shown in Table 4 where there were no significant differences due to treatments. The overall mortality/cull rate was 3.77%, which is acceptable, and was unrelated to treatment (P > 0.30). SMBS numerically improved weight gain by 3.27% and tended to reduce relative gizzard and pancreas weights (P < 0.10). Effects of SMBS and phytase on nutrient utilisation, toe ash, excreta moisture and the incidence of dilated proventriculi are shown in Table 5. Significant differences due to treatment were not observed. However, SMBS tended to depress AME by 0.33 MJ (P < 0.10) and AMEn by 0.28 MJ (P < 0.20). Phytase did not influence toe ash or excreta moisture. The overall incidence of dilated proventriculi was 2.38% but this was not related to treatment.
|
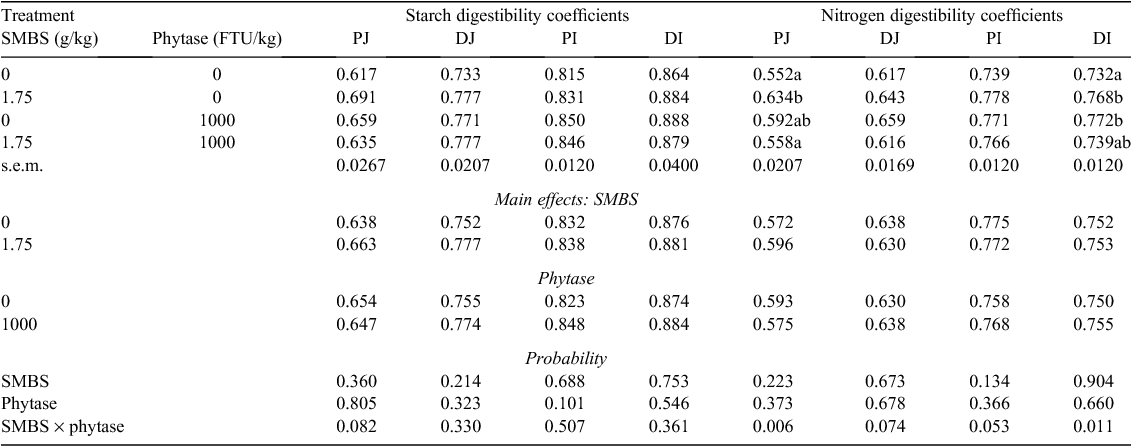
Effects of SMBS and phytase on apparent starch and N digestibility coefficients in the four small intestinal segments are shown in Table 6. The distal ileal starch digestibility coefficient in the control diet was 0.864, which is quite typical for sorghum-based diets. There are no significant differences for starch digestibility coefficients due to treatment but an interaction between main effects approached significance (P = 0.082) in the PJ. SMBS alone tended to increase starch digestibility by 12.0% (0.691 vs 0.617; P = 0.064), which closely approached significance on the basis of a pair-wise comparison. However, when SMBS was combined with phytase the numerical increase was 2.92%. SMBS alone numerically increased starch digestibility coefficients in descending small intestinal segments by 12.0%, 6.00%, 1.96% and 2.31%, respectively.
The overall distal ileal N digestibility coefficient in the sorghum-based diets was 0.753 and there were significant treatment interactions for N digestibility coefficients in the PJ (P < 0.01) and DI (P < 0.02) and interactions approached significance in the distal jejunum (P = 0.074) and proximal ileum (P = 0.053) for birds fed diets containing SMBS. For example, in the DI, SMBS alone significantly improved N digestibility by 4.92% (0.768 vs 0.732) and the significant improvement with phytase alone was 5.46% (0.772 vs 0.732). Individually, SMBS increased N digestibility coefficients in descending small intestinal segments by 14.9 (P = 0.006), 4.21 (P = 0.319), 5.28 (P = 0.018) and 4.92% (P = 0.053) where the probability of pair-wise comparisons are shown in parentheses. The corresponding figures for phytase alone were 7.25 (P = 0.149), 6.81 (P = 0.119), 4.33 (P = 0.047) and 5.46% (P = 0.033). In contrast, SMBS and phytase in combination did not tangibly influence N digestibility coefficients; for example in the DI the ‘response’ to the combination was 0.96% (0.739 vs 0.732).
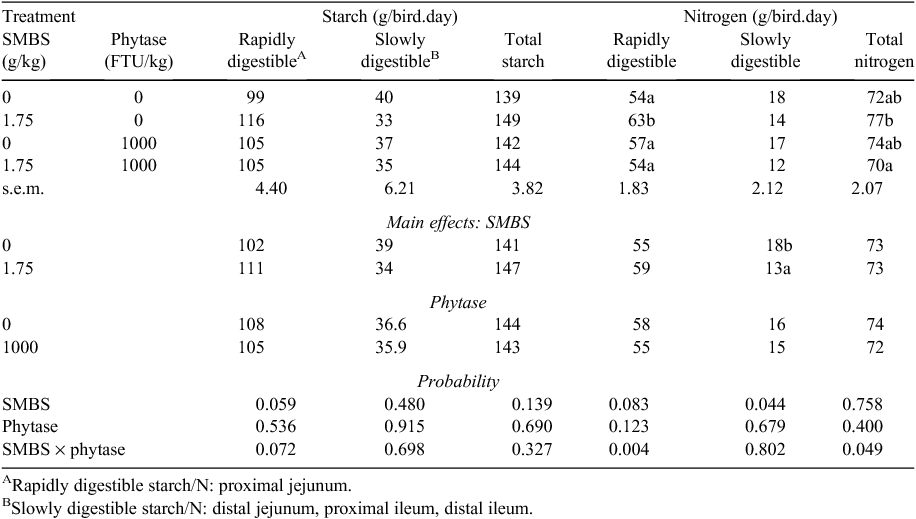
Effects of SMBS and phytase on the amounts of rapidly and slowly digestible starch and N and apparent total disappearance rates of starch and N in the jejunum and ileum are shown in Table 7. For rapidly digestible starch the SMBS × phytase interaction (P = 0.072) approached significance as did the main effect of SMBS (P = 0.059). SMBS alone increased rapidly digestible starch by 17.2% (116 vs 99 g/bird; P = 0.013) with a reciprocal tendency to decrease slowly digestible starch. There was a significant interaction (P < 0.05) for total N disappearance rates from the jejunum and ileum. N disappearance with SMBS alone was significantly greater by 10.0% (77 vs 70 g/bird) than SMBS in combination with phytase. There was also a significant SMBS × phytase interaction (P < 0.005) for rapid N disappearance rates from the PJ. SMBS generated significantly more rapidly digestible N than the other three treatments including an increase of 16.7% (63 vs 54 g/bird) relative to the control diet. Reciprocally, SMBS reduced slowly digestible N by 27.8% (13 vs 18 g/bird; P < 0.05) as a main effect.
Discussion
Dilatation of the proventriculus was recorded in 2.38% of birds at 28 days post-hatch but was unrelated to treatment. This condition, also called ‘Macaw disease’, is transmissible in broilers (Dormitorio et al. 2007). However, this unusually high figure represents only the grossly dilated proventriculi. The likelihood is the incidence of grossly and undetected dilated proventriculi was an unnecessary cause of variation in this study. There were not any significant treatment effects on toe ash, which confirms the P-adequacy of the diets. Also, phytase (and SMBS) had no effects on excreta moisture, which is noteworthy given the anecdotal association between phytase inclusions in broiler diets and wet litter.
Possible relationships between in vitro RVA starch properties and pig and poultry performance parameters have not been extensively investigated. However, Doucet et al. (2010) did find that final starch viscosity of diets in particular, was negatively correlated with starch digestibility coefficients at the small intestinal midpoint in weaner pigs. Interestingly, dietary final starch viscosity was found to be positively correlated with starch disappearance rates in the PJ (r = 0.385; P < 0.05) but negatively correlated with AME (r = –0.376; P < 0.05) in the present study. This supports the proposal that slowly digestible starch advantages energy utilisation, which is discussed below, and that RVA starch properties of grains and diets may be indicative of broiler performance.
The lack of robust responses following the inclusion of phytase in sorghum-based broiler diets is not without precedent. As reported by Selle at al. (1999), the addition of a fungal phytase at 600 FTU/kg to a P-adequate, sorghum-based diet with standard nutrient specifications did not improve weight gain, feed conversion efficiency and N retention in broilers from 7 to 25 days post-hatch. Thus the ‘extra-phosphoric’ effects of phytase on protein and energy utilisation (Ravindran and Selle 2010) were not in evidence in the Selle at al. (1999) study, as was the case in the present study. The likelihood is that the ‘extra-phosphoric’ effects of phytase stem in part from the prevention of de novo binary protein-phytate complex formation in the proventriculus and gizzard by the exogenous enzyme hydrolysing phytate before complex formation (Selle et al. 2000). However, kafirin, the major sorghum protein fraction, contains a paucity of basic amino acids and, as a consequence, may not be bound readily by phytate (Selle et al. 2010). This may be a straightforward explanation for the muted phytase responses observed in sorghum-based broiler diets.
The significant SMBS-phytase treatment interactions recorded for N digestibility coefficients in the PJ and DI in the present study are noteworthy. SMBS and phytase individually supported better N digestibility than their combined inclusions. SMBS alone significantly increased N digestibility by 14.9% and 4.92% in the PJ and DI, respectively. The genesis of these positive responses probably stem from reductions of disulfide cross-linkages in proteins, especially the β- and γ-kafirin fractions in sorghum (Selle et al. 2010). The reducing agent, SMBS, has been shown to decrease disulfide cross-linkages and increase free sulfydryl groups in sorghum-based broiler diets (Selle et al. 2014). Phytase alone significantly increased N digestibility in the DI by 5.46% and numerically increased N digestibility by 7.25% in the PJ. In contrast, SMBS and phytase in combination essentially did not alter N digestibility coefficients with notional increases of 1.09% and 0.96% in the PJ and DI, respectively. One possible explanation for these interactions is that SMBS-induced reductions of disulfide cross-linkages changed the structure of proteins and diminished their propensity to be complexed by phytate, which would attenuate phytase responses. Another possibility is that SMBS may have reduced disulfide cross-linkages in the exogenous phytase thereby altering its structure and stability and compromising the bioefficacy of the feed enzyme. However, given the data generated by Wyss et al. (1999), this appears to be unlikely as these researchers reported that several reducing agents, including dithiothreitol and mercaptoethanol, did not affect the enzymatic activity of four phytases by more than 11%. Alternatively, two reducing agents (dithiothreitol and sodium sulfite) stimulated in vitro degradation of feather meal by four proteases (Pedersen et al. 2012). Clearly, further research into the combined inclusions of SMBS and phytase is required in this respect.
However, a greater conundrum is the divergent impacts of SMBS observed in the present study in comparison to previous studies (Selle et al. 2013, 2014; Liu et al. 2014), especially in relation to energy utilisation. Initially, the prime reason for evaluating SMBS in sorghum-based broiler diets was because of its potential to reduce disulfide cross-linkages, especially in the β- and γ-kafirin fractions of sorghum protein and enhance protein digestibility. This was based on the premise that similar reducing agents had been shown to increase the in vitro protein digestibility of sorghum (Hamaker et al. 1987; Rom et al. 1992; Oria et al. 1995). However, graded inclusions of SMBS had negligible impacts on N digestibility in the initial studies (Selle et al. 2013, 2014), which was not anticipated. In contrast, 1.75 g/kg SMBS increased N digestibility coefficients in the PJ and DI and increased N disappearance rates in the PJ to significant extents in the present study. The sorghum used in the Selle et al. (2014) study contained (g/kg) 78.6 protein, 9.6 leucine, 3.5 arginine, 1.9 histidine and 2.1 lysine with a kafirin index of 2.1; whereas, in the present study, the corresponding concentrations were 92.5, 12.4, 3.4, 2.1, 2.0, respectively, with a kafirin index of 4.9. The higher protein and leucine contents, coupled with the higher kafirin index, suggest that sorghum FW14 contained more kafirin. Although speculative, the positive N digestibility responses observed in the present study may have been pursuant to SMBS reducing disulfide cross-linkages in kafirin, which was not declared in the previous study because of the lower sorghum kafirin content.
In the previous study SMBS linearly depressed starch digestibility coefficients in the PJ (r = –0.444; P = 0.001) and generated less rapidly, but more slowly, digestible starch to significant extents. Again in contrast, 1.75 g/kg SMBS alone increased starch digestibility coefficients in the PJ and generated 17.2% more rapidly digestible starch in the present study. This dichotomy in extents and sites of starch digestion may account for the marked discrepancies in energy utilisation responses to SMBS between this and previous studies. The benefits of the slowly digestible starch, or starch digested in the three posterior segments of the small intestine, in broiler diets in relation to feed conversion efficiency were initially demonstrated by Weurding and his colleagues (Weurding et al. 2001, 2003a, 2003b; Weurding 2002). Importantly, these benefits were shown to extend to energy utilisation (AME, AMEn) in the Liu et al. (2014) study where consideration was given to the probable underlying mechanisms.
In the previous study (Liu et al. 2014; Selle et al. 2014), slowly digestible starch was linked to unequivocal increases in energy utilisation (AME and AMEn) generated by SMBS. This sulfite-reducing agent has the capacity to depolymerise starch polysaccharides via oxidative-reductive reactions (Paterson et al. 1996, 1997) and enhanced energy utilisation was attributed to these oxidative-reductive reactions. It is generally held that kafirin negatively influences sorghum starch utilisation via biophysical and biochemical protein-starch interactions involving kafirin protein bodies and starch granules in sorghum endosperm (Taylor 2005). Thus it appears that disruption of disulfide cross-linkages in kafirin by SMBS, as evidenced by enhanced N digestibility, may have facilitated starch digestion and promoted more rapidly digestible starch in the present study and energy utilisation was depressed. The generation of slowly digestible starch was certainly not evident in the present study and this may have been a consequence of SMBS reducing disulfide cross-linkages in kafirin, thereby diminishing starch-protein interactions and accelerating starch digestion. That N proximal jejunal digestibility coefficients were positively correlated with starch disappearance rates (r = 0.568; P < 0.005) in the same site supports this proposal.
Graded SMBS inclusions linearly reduced peak, holding, final and setback RVA starch viscosities to significant extents in sorghum-based diets in the previous study, which was attributed to starch depolymerisation (Liu et al. 2014). However, the impact of SMBS on RVA starch viscosity parameters was less pronounced in the present study. Instructively, proteins may interact with starch and influence their RVA pasting properties (Zhang and Hamaker 1998; Ito et al. 2006) and the assumed higher kafirin concentration in sorghum FW14 may have influenced these outcomes. Therefore, it is evident that the influence of SMBS in sorghum-based diets is variable and appears dependent on the properties of the specific sorghum grain where the kafirin content may be a critical factor. Clearly, further research into the inclusion of SMBS in sorghum-based diets is required, which would be greatly facilitated by the quantification of kafirin concentrations in sorghum grains.
Acknowledgements
We acknowledge the financial contribution from Feedworks towards the funding of this feeding study. We also acknowledge the financial support for Ms Ha Truong provided by her postgraduate scholarship awarded by the Poultry CRC established by the Australian Government’s Cooperative Research Centres Program.
References
Collett SR (2012) Nutrition and wet litter problems in poultry. Animal Feed Science and Technology 173, 65–75.| Nutrition and wet litter problems in poultry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjvVenurw%3D&md5=1b3cdc07434280191bdbab52fd08cd15CAS |
Dormitorio TV, Giambrone JJ, Hoerr FJ (2007) Transmissible proventriculitis in broilers. Avian Pathology 36, 87–91.
| Transmissible proventriculitis in broilers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2s3ntValtg%3D%3D&md5=1b62c8da119a121d1e7e9d27562f7abaCAS | 17479367PubMed |
Doucet FJ, White GA, Wulfert F, Hill SE, Wiseman J (2010) Predicting in vivo starch digestibility coefficients in newly weaned pigs from in vitro assessment of diets using multivariate analysis. The British Journal of Nutrition 103, 1309–1318.
| Predicting in vivo starch digestibility coefficients in newly weaned pigs from in vitro assessment of diets using multivariate analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlvVensLs%3D&md5=4b3f6842a4ecacc32afaafc2925b4130CAS | 20021701PubMed |
Hamaker BR, Kirleis AW, Butler LG, Axtell JD, Mertz ET (1987) Improving the in vitro protein digestibility of sorghum with reducing agents. Proceedings of the National Academy of Sciences of the United States of America 84, 626–628.
| Improving the in vitro protein digestibility of sorghum with reducing agents.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXhtlOhsLs%3D&md5=ec544e646713cf3d2675c31025fdd52cCAS | 16593805PubMed |
Hernandez R, Capareda SC, Hays DB, Portillo OR, Rooney WL (2008) Effect of grain sorghum protein digestibility on starch gelatinization and enzymatic conversion to glucose. In ‘2008 ASABE annual international meeting, Providence, RI’. (American Society of Agricultural and Biological Engineers: St Joseph, MI)
Hill FW, Anderson DL (1958) Comparison of metabolizable energy and productive energy determinations with growing chicks. The Journal of Nutrition 64, 587–603.
Ito A, Harrori M, Yoshida T, Watanabe A, Sato R, Takahashi K (2006) Regulatory effect of amino acids on the pasting behaviour of potato starch is attributable to its binding to the starch chain. Journal of Agricultural and Food Chemistry 54, 10191–10196.
| Regulatory effect of amino acids on the pasting behaviour of potato starch is attributable to its binding to the starch chain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1GjsLfF&md5=6e0691fab1a0390f05c83e8f9709e430CAS | 17177559PubMed |
Liu SY, Selle PH, Khoddami A, Roberts TH, Cowieson AJ (2014) Graded inclusions of sodium metabiulphite in sorghum-based diets: II. Modification of starch pasting properties in vitro and beneficial impacts on strach digestion dynamics in broiler chickens. Animal Feed Science and Technology 190, 68–78.
| Graded inclusions of sodium metabiulphite in sorghum-based diets: II. Modification of starch pasting properties in vitro and beneficial impacts on strach digestion dynamics in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXisFegtL8%3D&md5=f9be5d0b1184f4c55edae8d4bae58ce5CAS |
Mahasukhonthachat K, Sopade PA, Gidley MJ (2010) Kinetics of starch digestion and functional properties of twin-screw extruded sorghum. Journal of Cereal Science 51, 392–401.
| Kinetics of starch digestion and functional properties of twin-screw extruded sorghum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVeksrg%3D&md5=6ec666c44c3b17de1d4a2c9e393291ffCAS |
Oria MP, Hamaker BR, Shull JM (1995) Resistance of sorghum α-, β-, and γ-kafirins to pepsin digestion. Journal of Agricultural and Food Chemistry 43, 2148–2153.
| Resistance of sorghum α-, β-, and γ-kafirins to pepsin digestion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXntFCis70%3D&md5=d4eb93f7e998a80e8e6a4ac3ba4e1424CAS |
Paterson LA, Mitchell JR, Hill SE, Blanshard JMV (1996) Evidence for sulfite induced oxidative reductive polymerisation of starch polysaccharides. Carbohydrate Research 292, 143–151.
| Evidence for sulfite induced oxidative reductive polymerisation of starch polysaccharides.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmtlSmtr0%3D&md5=001eb643c943257b63ed0ec735a65b7eCAS |
Paterson LA, Hill SE, Mitchell JR, Blanshard JMV (1997) Sulfite and oxidative-reductive depolymerization reactions. Food Chemistry 60, 143–147.
| Sulfite and oxidative-reductive depolymerization reactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXktFWitrY%3D&md5=2e8ca888506178a2f340b56114563bdfCAS |
Pedersen MB, Yu S, Plumstead P, Dalsgaard S (2012) Comparison of four feed proteases for improvement of poultry feather meal. Journal of Animal Science 90, 350–352.
| Comparison of four feed proteases for improvement of poultry feather meal.Crossref | GoogleScholarGoogle Scholar | 23365376PubMed |
Ravindran V, Selle PH (2010) Phytate: anti-nutritive effects on energy and amino acid utilization. In ‘Proceedings, 46th eastern nutrition conference’. pp. 1–12. (Animal Nutrition Association of Canada: Ottawa, Canada)
Rom DL, Shull JM, Chandrashekar A, Kirleis AW (1992) Effects of cooking and treatment with sodium bisulphite on in vitro protein digestibility and microstructure of sorghum flour. Cereal Chemistry 69, 178–181.
Selle PH, Ravindran V, Pittolo PH, Bryden WL (1999) An evaluation of microbial phytase in sorghum-based broiler diets. Proceedings, Australian Poultry Science Symposium 11, 97–100.
Selle PH, Ravindran V, Caldwell RA, Bryden WL (2000) Phytate and phytase: consequences for protein utilisation. Nutrition Research Reviews 13, 255–278.
| Phytate and phytase: consequences for protein utilisation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtlKmtr8%3D&md5=aa55b443e06ecb476f6f3251f9983a93CAS | 19087442PubMed |
Selle PH, Cadogan DJ, Li X, Bryden WL (2010) Implications of sorghum in broiler chicken nutrition. Animal Feed Science and Technology 156, 57–74.
| Implications of sorghum in broiler chicken nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtVCksr4%3D&md5=753b3b48a8171e3a0b51caa33f230a15CAS |
Selle PH, Liu SY, Cai J, Caldwell RA, Cowieson AJ (2013) Preliminary assessment of including a reducing agent (sodium metabisulphite) in ‘all-sorghum’ diets for broiler chickens. Animal Feed Science and Technology 186, 81–90.
| Preliminary assessment of including a reducing agent (sodium metabisulphite) in ‘all-sorghum’ diets for broiler chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFOnsrrL&md5=960324ac338c08e8d4b3c821753c5784CAS |
Selle PH, Liu SY, Cai J, Caldwell RA, Cowieson AJ (2014) Graded inclusions of sodium metabiulphite in sorghum-based diets: I. Reduction of disulphide cross-linkages in vitro and enhancement of energy utilisation and feed conversion efficiency in broiler chickens. Animal Feed Science and Technology 190, 59–67.
| Graded inclusions of sodium metabiulphite in sorghum-based diets: I. Reduction of disulphide cross-linkages in vitro and enhancement of energy utilisation and feed conversion efficiency in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1GrsLc%3D&md5=3c3e02122df062520434ca652a2b61e2CAS |
Siriwan P, Bryden WL, Mollah Y, Annison EF (1993) Measurement of endogenous amino-acid losses in poultry. British Poultry Science 34, 939–949.
| Measurement of endogenous amino-acid losses in poultry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXivFOgsLg%3D&md5=34b97d5206bd2efdd2a035e1a1918c15CAS | 8156432PubMed |
Symes KJ (1965) The inheritance of grain hardness in wheat as measured by the particle size index. Australian Journal of Agricultural Research 16, 113–123.
| The inheritance of grain hardness in wheat as measured by the particle size index.Crossref | GoogleScholarGoogle Scholar |
Taylor JRN (2005) Non-starch polysaccharides, protein and starch: form function and feed-highlights on sorghum. Proceedings, Australian Poultry Science Symposium 17, 9–16.
Weurding RE (2002) Kinetics of starch digestion and performance of broiler chickens. PhD Thesis, Wageningen University, The Netherlands.
Weurding RE, Veldman A, Veen WAG, van der Aar PJ, Verstegen MWA (2001) Starch digestion rate in the small intestine of broiler chickens differs amongst feedstuffs. The Journal of Nutrition 131, 2329–2335.
Wyss M, Brugger R, Kronenberger A, Remy R, Fimbel R, Oesterhelt G, Lehmann M, van Loon APGM (1999) Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): catalytic properties. Applied and Environmental Microbiology 65, 367–373.
Zhang G, Hamaker BR (1998) Low α-amylase starch digestibility of cooked sorghum flours and the effect of protein. Cereal Chemistry 75, 710–713.
| Low α-amylase starch digestibility of cooked sorghum flours and the effect of protein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmtlKnt7o%3D&md5=781ba1e4b5bff26e5b175dafd91b7ff4CAS |