Interactions between microbial consortia in biofilms: a paradigm shift in rumen microbial ecology and enteric methane mitigation
R. A. LengSchool of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia. Email: rleng@ozemail.com.au
Animal Production Science 54(5) 519-543 https://doi.org/10.1071/AN13381
Submitted: 14 September 2013 Accepted: 21 January 2014 Published: 28 February 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Minimising enteric CH4 emissions from ruminants is a current research priority because CH4 contributes to global warming. The most effective mitigation strategy is to adjust the animal’s diet to complement locally available feed resources so that optimal production is gained from a minimum of animals. This essay concentrates on a second strategy – the use of feed additives that are toxic to methanogens or that redirect H2 (and electrons) to inhibit enteric CH4 emissions from individual animals. Much of the published research in this area is contradictory and may be explained when the microbial ecology of the rumen is considered.
Rumen microbes mostly exist in organised consortia within biofilms composed of self-secreted extracellular polymeric substances attached to or within feed particles. In these biofilms, individual colonies are positioned to optimise their use of preferred intermediates from an overall process of organic matter fermentation that generates end-products the animal can utilise. Synthesis of CH4 within biofilms prevents a rise in the partial pressure of H2 (pH2) to levels that inhibit bacterial dehydrogenases, and so reduce fermentation rate, feed intake and digestibility. In this context, hypotheses are advanced to explain changes in hydrogen disposal from the biofilms in the rumen resulting from use of anti-methanogenic feed additives as follows.
Nitrate acts as an alternative electron sink when it is reduced via NO2– to NH3 and CH4 synthesis is reduced. However, efficiency of CH4 mitigation is always lower than that predicted and decreases as NO3– ingestion increases. Suggested reasons include (1) variable levels of absorption of NO3–or NO2– from the rumen and (2) increases in H2 production. One suggestion is that NO3– reduction may lower pH2 at the surface of biofilms, thereby creating an ecological niche for growth of syntrophic bacteria that oxidise propionate and/or butyrate to acetate with release of H2.
Chlorinated hydrocarbons also inhibit CH4 synthesis and increase H2 and formate production by some rumen methanogens. Formate diffuses from the biofilm and is converted to HCO3– and H2 in rumen fluid and is then excreted via the breath. Short-chain nitro-compounds inhibit both CH4 and formate synthesis when added to ruminal fluid but have little or no effect in redirecting H2 to other sinks, so the pH2 within biofilms may increase to levels that support reductive acetogenesis. Biochar or activated charcoal may also alter biofilm activity and reduce net CH4 synthesis; direct electron transfer between microbes within biofilms may also be involved. A final suggestion is that, during their sessile life stage, protozoa interact with biofilm communities and help maintain pH2 in the biofilm, supporting methanogenesis.
Additional keywords: chlorinated hydrocarbons, direct interspecies electron transport, electron acceptors, formate, inter-bacterial distance, motility symbiosis, partial pressure hydrogen, reductive acetogenesis, role of protozoa, short-chain nitro-compounds, syntrophism, transparent exopolymer particles, viscotactic spirochete.
Introduction
Methane (CH4) is a potent greenhouse gas that has a high priority for mitigation because of its detrimental global warming potential and because, in combination with tropospheric ozone and carbon (C) black, it is a health hazard that could reduce the life expectancy of 3.1 billion people worldwide (UNEP 2011). CH4 produced by ruminants is targeted as a significant and potentially mitigatable source of this greenhouse gas (Hristov et al. 2013).
Under normal feeding conditions, CH4 production is an inescapable consequence of the fermentation of organic matter (OM) in the digestive tract of ruminant animals. Theoretically, the generation of CH4 in the rumen can be decreased by the following factors (McAllister and Newbold 2008; Eckard et al. 2010; Morgavi et al. 2010; Cottle et al. 2011; Hristov et al. 2013):
-
by promoting a shift in fermentation toward production of the more reduced volatile fatty acids (VFA), e.g. propionate;
-
on nutrient deficient diets, which increase microbial growth efficiency (microbes are more reduced than the VFA end products and are therefore a sink for hydrogen (H2)) by providing supplements of minerals and, where the crude protein content of the diet is low, a non-protein nitrogen (N) source;
-
by addition of feed additives that inhibit methanogenesis (e.g. bromochloromethane, BCM) or have a high affinity for bioreduction (e.g. long-chain unsaturated fatty acids);
-
immunisation against methanogens;
-
defaunation of the rumen: because of e.g. physical association of methanogens in or on the surface of rumen protozoa;
-
stimulation of the growth of bacteriophages that infect and lyse methanogens;
-
supplementation of a diet with compounds that specifically promote the growth of bacteria and/or Archaea that use compounds such as nitrates and sulfates and have a higher affinity for H2 than do methanogens; and
-
by creating an environment in the rumen that encourages the growth of
-
-
reductive acetogenic microbes, and
-
methanotrophic microbes.
-
Recently, attempts were made to create a new microbial habitat in the rumen by including biochar in the diet, to increase the inert surface area for biofilm formation that may allow close association of both methanotrophs and methanogens and increase anaerobic CH4 oxidation (see Leng et al. 2012a) or improve overall microbial-growth efficiency (Leng et al. 2012b, 2012c; Liu et al. 2012). McAllister and Cheng (1996) proposed that methanogenesis cannot be eliminated without adverse effects on ruminant production, and there is a general view that the clearance of dissolved H2 from the rumen by methanogens is critical for maintenance of a low partial pressure of H2 at the sites of fermentation; this condition, in turn, is a pre-requisite for the regeneration of cofactors such as NADH, NADPH and reduced ferridoxins that are necessary for continuous glycolytic activity by the rumen microbial consortia. Syntrophic acetogenic bacteria grow in mixed culture with H2-consuming bacteria such as methanogens. A simultaneous electron transfer from an organism fermenting OM to a H2-consuming species (termed interspecies H2 or electron transfer) is putatively essential for growth and metabolism. More particularly, recent developments have indicated that the crucial factor in releasing electrons from reduced cofactors (allowing the glycolytic pathway to function) is the partial pressure of H2 in the biofilm matrix or in aggregate forms such as flocs in digesters (Thiele et al. 1988) associated with the plant particles (Wolin 1979; McAllister and Newbold 2008; Janssen 2010).
The production of CH4 in the rumen can be reduced by more than 90% by direct inhibition using chlorinated hydrocarbons or CH4 analogues added to feed (see e.g. McCrabb et al. 1997). Surprisingly, with this type of inhibition, there is no reduction in feed digestibility or production and the reduction in CH4 release is accompanied by a concomitant stoichiometric production of H2 (Mitsumori et al. 2012). If it is assumed that the H2 is produced in fermentative sites in the biofilm, it is reasonable to expect an increased partial pressure of H2 at these sites and, therefore, adverse effects on feed digestion and intake. The studies with the CH4 analogue, BCM, have created a conundrum. If rumen microbial ecology can change to produce H2, thereby maintaining fermentation efficiency and animal production, then the use of compounds toxic to methanogens or inhibitory for the pathways of CH4 synthesis (such as BCM, chloroform or tannins), or even immunisation against methanogens, are not rational ways of ameliorating greenhouse-gas emissions because these strategies are likely to release H2 in place of CH4. When H2 reaches the troposphere, it reacts with hydroxyl radicals and perturbs the distribution of CH4 and ozone; its effect is that of a greenhouse gas with a global-warming potential (GWP) of 5.8 over a 100-year time horizon (Derwent et al. 2006). Because it takes 4 mol of H2 to form 1 mol of CH4 that has a GWP of 23, little is gained from mitigating 1 mol enteric CH4 if the consequence is the release of 4 mol H2 to the atmosphere. (This situation could change in the future if the GWP of CH4 is assessed at a much higher level.)
In the following discussion, an attempt is made to rationalise the potential value of enteric CH4 mitigation by inhibiting methanogens, and to suggest research priorities. However, the arguments developed depend on an understanding of the roles of biofilms in anaerobic rumen digestion and their importance is explored in the initial section of this review.
Rumen microbial ecology
In the past, the rumen has been viewed as a milieu of microorganisms (a microbial ‘soup’) which, as a unit, is highly effective in degrading feed resources to VFA, with the ATP generated being used to synthesise the microbial polymers required for cell growth (Annison and Lewis 1959). There has been a gradual change in this perception as the concept of anaerobic, microbial communities in discrete, organised and structured systems has become recognised as essential for the control of the complex hydrolytic and enzymatic breakdown of feed in the rumen (Cheng and Costerton 1980; Costerton et al. 1987; McAllister et al. 1994; Cheng et al. 1995; McAllister and Cheng 1996; Costerton 2007; Edwards et al. 2007). Environmental microbiologists have long recognised that associated complex bacterial communities are responsible for driving all the major biogeochemical nutrient cycles within the earth’s biosphere (C, sulfur (S) and N cycles) that maintain relative stability in the biosphere (Davey and O’Toole 2000). Until recently, the lack of methods for exploring these microbial communities in situ has hampered detailed analyses in the rumen. Traditionally, studies of rumen microbes were performed using organisms isolated from rumen fluid and cultured in roll tubes (Hungate 1966). Application of technologies that were independent of cultured microbes has shown that species diversity in the rumen has been vastly underestimated (Rappé and Giovannoni 2003; Edwards et al. 2008). As this technology evolves, it is being adapted to examine microbial communities in their natural habitat and is playing a major role in describing the rumen biome (Bath et al. 2013).
The importance of rumen microbial ecology
Over the past 50 years, rumen microbiologists have emphasised the need for attachment of bacteria to feed materials, to enable them to efficiently digest OM in the rumen (Cheng and Costerton 1980; Cheng et al. 1980). Measurements of the sites of bacterial ATP formation and their location in the rumen indicated that the majority of ATP was associated with plant particles (Forsberg and Lam 1977; Craig et al. 1987) and isotope-dilution studies using organisms labelled with 15N suggested that 80–90% of the bacteria in the rumen are associated with particulate matter (Rodríguez et al. 2003). Krebs (1987) labelled bacteria with 35S-sulfate and examined the movement of 35S between the free-floating and particle-associated organisms and found that 80% of the microbes washed out of the rumen had been particle-associated and that bacteria moved between particles without entering the free-floating bacterial pool. It is now clear that particle-associated microbes play the most important role in rumen digestion (McAllister et al. 1994; Mayorga et al. 2007; Edwards et al. 2008). These microbes are found in associated consortia embedded in a biofilm matrix where end products produced by one colony are sequentially used by closely associated colonies.
Although compounds such as VFA, amino acids and ammonia (NH3), and gases such as CH4, H2 and carbon dioxide (CO2), diffuse into and out of the biofilm, these materials can be expected to be in higher concentrations within the biofilm matrix than in the external rumen fluid. Thus, the bicarbonate (HCO3–) that is reduced during methanogenesis will probably be mostly drawn from within the biofilm matrix (that has diffused in and/or been produced locally) rather than from a single homogenous HCO3– pool in rumen fluid. In this connection, Loughnan (1982) infused H14CO3– into the rumen of sheep and showed that the specific radioactivity of excreted CH4 was less than 50% of that of the HCO3–-C in the rumen fluid. This result would be expected if some of the C in CH4 were derived from unlabelled HCO3– produced close to methanogenic colonies in the biofilms and the remainder came from HCO3– that diffused from the external rumen fluid via water channels in the biofilm structure. An alternative possibility is that formate produced during fermentation of OM or synthesised in situ could have supplied unlabelled C for methanogenesis (see later in text); however, when Loughnan (1982) infused 14C-labelled formate into the rumen contents of the same sheep, formate-C made little contribution to CH4. Notably, some methanogens produce formate and its production is greatly enhanced when CH4 synthesis is inhibited by CH4 analogues (Bleicher and Winter 1994). In vitro, addition of anthroquinone to rumen fluid resulted in the accumulation of formate (Asanuma et al. 1998)
It is generally accepted that attachment of rumen microbes to feed particles is essential in maintaining a high rate of solubilisation of feed OM. In general, however, ruminant nutritionists have not connected the consequences to the actual mechanisms of fermentative digestion that require several different species of microbes to act in concert. Perhaps the term ‘interspecies electron transfer’ has not conveyed the concept of the organised biofilm mode of degradation. Recently, Wang and Chen (2009) and Weimer et al. (2009) highlighted the stark differences between the efficiency of fermentative production of bioethanol based on cellulosic feed stock (by planktonic yeast cells) and the efficiency of rumen organisms when converting cellulosic biomass to short-chain VFA. Clearly, the rumen has also evolved highly efficient mechanisms for these processes (Wang and Chen 2009) that depend on organised sequential breakdown of the cellulosic biomass and involve numerous species of organisms (in particular, bacteria, fungi and protozoa).
The modes of breakdown of complex plant OM in the rumen were thoroughly reviewed by McAllister and his colleagues (McAllister et al. 1994) and were reviewed more generally by Costerton (2007) and Leng (2011). In the current presentation, emphasis is placed on the interspecies transfer of electrons that facilitates, and perhaps integrates, the fermentative processes. McAllister et al. (1994) emphasised the need for microbes to attach to feed particles, to initiate the consortia that then enzymatically solubilise the complex components and circumvent barriers that restrict access to the more fermentable OM substrates within plant particles.
The microbial colonies, encased in self-produced polymeric substances, both grow inward to access the internal fermentable materials, as well as access those on the surfaces of feed particles. Anaerobic fungi, in contrast, grow within the plant structures (Gordon and Phillips 1998). They produce sporangia that release zoospores that actively invade plant materials, particularly in the areas where damage to the waxy surface has occurred. Penetration of fungal mycelia through plant particles weakens the structures and promotes more rapid reduction of particle size and greater access for other organisms. The fungi are in close contact with the biofilm consortia or can be considered as an extension of the biofilm into the solid plant particles. They actively provide hydrolytic breakdown products and H2 and/or formate that can be assimilated by the associated microbial colonies (Nagpal et al. 2009). For plant cell-wall degradation, such anaerobic fungi produce a wide range of hydrolytic enzymes such as cellulases, hemicellulases, proteases, amylases, feruloyl and p-coumaryl esterases, various disaccharidases, pectinases and exonucleases (Nagpal et al. 2009). Rumen fungi produce appreciable amounts of H2 and, therefore, are advantaged by being close to methanogens; they also produce a range of hydrolytic end products that provide substrates for other associated bacterial colonies.
The initial colonisers that adhere to the surface of plant materials are bacteria. Primary colonisers attach to newly ingested forage particles, exude extracellular polymeric substances and then develop into biofilm colonies on perennial ryegrass leaf, for instance, in less than 1 h (Huws et al. 2013). Bacteria have evolved signalling mechanisms that enable them to communicate and co-ordinate their activities so that they can respond quickly to environmental changes (such as establishment of nearby bacteria or the presence of nutrients or toxins); they exhibit a wide range of interactive, multicellular behaviors such as dispersal, nutrient acquisition, biofilm formation and quorum sensing (West et al. 2006).
The assembly of non-motile cellulolytic bacteria such as F. succinogenes on a feed particle, usually close to where the waxy cuticle has been damaged, may be facilitated by motility symbiosis between the bacteria and spirochetes (Stanton and Canale-Parola 1980). These workers studied Treponema bryantii, a highly motile, viscotactic spirochete that grows on soluble sugars but is unable to utilise cellulose and is representative of one of seven morphological types of spirochetes found in the rumen (Stanton and Canale-Parola 1979). T. bryantii was shown to migrate through culture media in vitro and position itself near cellulosic fibres being degraded by F. succinogenes bacteria. These workers suggest passive motility of bacteria occurs due to close proximity between the spirochetes and the F. succinogenes, rather than actual attachment. The incessant movement of the spirochetes towards potential substrate appears to propel the cellulolytic bacteria towards their non-diffusible substrate in the feed particle (Stanton and Canale-Parola 1980). At the same time, the T. bryantii positions itself to access the soluble sugars released by the hydrolytic breakdown of the complex polysaccharides in the feed particle by F.succinogenes (Stanton and Canale-Parola 1980).
There is evidence that most aquatic ecosystems contain planktonic transparent exopolymer particles (TEPs) which may also be present in the rumen. These particles are organic microgels that are partly composed of polymers of fucose and rhamnose that are highly surface-active (Bar-Zeevet al. 2012) TEPs may originate from dissolved polymeric organic matter or from preformed biofilms and display most of the characteristics of developing biofilms, except they are not attached to a surface. However, they are extremely ‘sticky’ and, within minutes of exposure of solid particles to an aqueous medium, can start to adhere to solid surfaces and begin the process of biofilm development. Although TEPs have not been identified in the rumen, there is a high probability that they exist as sloughed biofilm fragments released from the surface of feed particles during rumination (Leng 2011). These fragments would be analogous to ‘protobiofilms’, i.e. TEPs that are colonised with microbes and can quickly form ‘hot spots’ of biofilm microbes on the surface of inert particles (Bar-Zeev et al. 2012).
After biofilms are established as outlined in Fig. 1, the initial colonisers then begin the process of hydrolysing complex structural compounds in solid materials. Next, apparently attracted by the solubilised materials, and probably also by signalling molecules (or inducer molecules) produced by other bacteria (Williams 2007), secondary colonisers are attracted and establish colonies by embedding themselves in the extracellular polymeric substances around the initial colonisers and grow by assimilating some of the intermediate products of bacterial hydrolysis (mainly simple sugars and other compounds such as peptides and amino acids). Further microbes with specific substrate requirements become associated as the biofilm grows. These bacteria are users of end products released by the microbes that initially establish sessile colonies and by the fungal biomass within the plant particles, and the biofilms grow as they become associated. The consortia that develop progressively degrade both complex and simple carbohydrates via the glycolytic pathways to VFA; the H2 that is also produced is mostly incorporated into CH4 by methanogenic Archaea. The extent of degradation of true protein within the biofilm consortia is not understood, but McAllister et al. (1994) suggested that this was the mode of utilisation of feed proteins (i.e. attached colonies). In addition, the fungi have high protease activity that may have a role in protein degradation, because the plant structural proteins increase the integrity of plant cell wall (Wallace and Joblin 1985). Proteins are either degraded via peptides to amino acids that are utilised in cell growth, or degraded further to organic acids and NH3, presumably with interspecies transfer of N compounds playing a major role in microbial growth. Methanogenic colonies are always found in the biofilms attached to surfaces of solid substrates (Cheng et al. 1981); these methanogens are distributed within a cluster on the outer layers of the biofilm (Song et al. 2005). Biofilms with a high level of microbial digestive ability are always composed of complex multi-species layers of microbes or as separate but associated colonies (Stoodley et al. 2002).
A key feature of biofilm organisation is that the interspecies distances among colonies are small and metabolic end products of one species become substrates for nearby species until the final end products accumulate and diffuse into the external rumen fluid. Feedback inhibition by end products from one colony of organisms can affect other colonisers, so the ease of transfer of intermediates among colonies and the eventual diffusion to the bulk fluid regulates the breakdown of feed particles. The biofilm mode of fermentative degradation allows a greatly increased rate of OM breakdown as compared with that in planktonic communities that are not in organised consortia (de Bok et al. 2004; Wang and Chen 2009). Feedback from H2 is particularly important because, if it were not removed, it would inhibit the re-oxidation of reduced cofactors produced in the fermentation pathways and restrict glycolysis and feed-degradation rate.
Syntrophism in biofilm communities and inter-bacterial distance
Syntrophism is used to describe the cooperation of two or more metabolically different bacteria that depend on each other to be able to degrade particular substrates and share the energy released for their maintenance and growth. The term was coined to describe the close cooperation between VFA-oxidising, fermenting bacteria and H2-oxidising methanogens (McInerney et al. 1979). However, the benefits to colonies in close proximity to each other are not restricted to those involved in CH4 formation; they apply universally where the end products of one microbial species are the substrates for another species. The distance between bacterial syntrophs is critical in energy terms. Proximity can overcome energy barriers and make coupled growth possible. The relative proximity of organisms also allows reactions to proceed at rates unobtainable in mixed suspensions of planktonic cells (de Bok et al. 2004).
The transfer of a metabolite between microorganisms occurs by diffusion, as defined by Fick’s law, which is described by the equation:
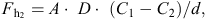
where Fh2 = flux of metabolite, D = diffusion coefficient in water, A = surface of producers, C = concentration of metabolite and d = distance between microorganisms (see Fig. 2 adapted from de Bok et al. 2004).

|
The effect of distance on the flux of interspecies electron carriers between producing and consuming organisms is shown in Fig. 2. Assuming a bacterium has a diameter of 2 µm, it can be calculated that, at densities 108, 109, 1010 and 1011 cells/mL, the inter-microbial distances in dispersed organisms are ~25, 10, 4 and 0.5 µm, respectively (Stams and Plugge 2009), as compared with distances in aggregates, flocs or biofilms of ~0.05 µm (de Bok et al. 2004). The interspecies distance may be even smaller in the case of Archaea that are appreciably smaller than bacteria. The need for consortia to bring microbes close to each other to facilitate H2 transfer applies in all anaerobic ecosystems. It also applies to species that use direct interspecies electron transfer via pila (Malvankar and Lovley 2012) or via solid conducting surfaces such as activated charcoal (Liu et al. 2012), or where microbial aggregates are electrically conductive, as has been demonstrated in waste water-treatment plants (Morita et al. 2011). In treatment plants, transfer of electrons occurs between fermenting bacteria and methanogens in biofilms containing colonies of Geobacter spp. (Reguera et al. 2005). Thus, it is possible that materials with charged particles such as biochar or activated charcoal or montmorilonite clay may facilitate both primary (hydrogenotrophic) and secondary (acetoclastic) fermentations by providing a large surface area as microbial habitat, and facilitating methanogenesis by electrical conductance between organisms (Leng et al. 2013).
Interspecies H2 transfer
The term ‘interspecies H2 transfer’ is often used to describe the transfer of H2 from fermenting organisms to methanogenic Archaea. A low partial pressure of H2 in the vicinity of actively fermenting organisms that contain hydrogenase enzymes enables the reduced cofactors to be re-oxidised. This releases electrons or H2 which are quickly taken up by methanogens that use the H2 to reduce HCO3– to CH4. This is efficient because the fermenting microbial consortia are organised in self-produced biofilms with optimal interspecies localisation (McAllister et al. 1994). The closer the microbes that use H2 are to those that produce it, the more rapid the rate of CH4 production (de Bok et al. 2004). It appears that interspecies electron transfer from primary fermenting organisms that produce H2 and VFA can be coupled to the reduction of several compounds including HCO3– (by methanogens), nitrate (by nitrate-reducing bacteria, NRB) and sulfate (by S-reducing bacteria, SRB).
Alternate electron acceptors include unsaturated fatty acids (Czerkawski et al. 1966), sulfate (Marty and Demeyer 1973) and nitrate (Allison and Macfarlane 1988). The presence of these acceptors can alter the microbial mix within the biofilm and divert electron flow away from methanogenesis. Hence, the inclusion of these salts in a diet seems to offer a logical means of lowering ruminal CH4 production (Leng 2008). The competition for H2 is affected by the Gibbs free-energy change of the reactions, so the reduction of nitrate (AGo = –163 KJ/mol) and sulfate (AGo = –152 KJ/mol) is thermodynamically more favourable than HCO3– reduction (AGo = –130 KJ/mol); their higher affinity for H2 gives NRB and SRB a further competitive advantage over methanogens (Oremland 1988), provided that the distances between colonies of SRB and NRB in the attached biofilm are similar to that of the competing methanogens. Essentially, the NRB and SRB must be concentrated in the biofilm matrix to outcompete the methanogens. The SRB and NRB occur naturally in the rumen (Coleman 1960; Hungate 1966; Howard and Hungate 1976; Cheng et al. 1988; Leng 2008) and experiments with sheep and cattle indicate that the population density of these species increases as the concentration of their respective electron acceptors in the ruminant diet increases (Alaboudi and Jones 1985; Hao et al. 2009). Which microbial species is the most successful in using the particular oxidised substrate (HCO3–, nitrate or sulfate) depends on the distance between the colonies of the organism involved in the interspecies transfer, the partial pressure of H2 and the Gibbs free-energy change of the reaction. There is a substantial suppression of methanogenesis when dietary nitrate is reduced to ammonia in rumen fluid (see later discussions) and NRB colonies must take up a position in the biofilm that favours nitrate reduction, rather than sulfate or HCO3– reduction. Similarly, SRB must be favourably distributed when competing with methanogens and NRB, although many species of NRB will assume the role of SRB when nitrate is not available (Moura et al. 2007). In summary, the affinity of various substrates for H2 varies directly with the Gibbs free-energy change of the relevant reaction. This suggests that interspecies distances among the NRB, SRB and methanogens in the rumen are similar and that these species are distributed within the biofilms associated with plant particles. In a planktonic cell culture and in the absence of a biofilm micro-environment, the H2 partial pressure can probably exert little effect on fermentation rate, as H2 is relatively insoluble and diffuses only slowly through water.
In waste-water treatment plants, the interspecies transfer of H2 is self-organised within microbial syntrophs by their aggregation into methanogenic granules and/or flocs, which brings together two or more cooperating species (de Bok et al. 2004). This appears to happen in the rumen at times when the animal is given liquid feeds, for example when molasses is the main energy source (Rowe et al. 1979; Leng 2011) and where the quantity of feed particles provides minimal surface area for biofilm formation. However, it is likely that the colonies of microbes in biofilms are spatially more dispersed than in flocs, granules or other aggregates. The biofilm communities that form on surfaces of feed particles grow inward as cellulose and other plant structural components are hydrolysed but also form mushroom-like ‘gels’ that hold sessile colonies of inter-associated microbes (McAllister et al. 1994).
The NRB and SRB in waste water are found together with methanogens in biofilms where both nitrate and sulfate are abundant (Martínez Amador et al. 2011), but these species have not been examined in biofilms in rumen digesta. Interspecies H2 transfer in microbial aggregates has been modelled and its relative importance is indicated by the calculated rates of reactions, which depend on the distance among the different species (Fig. 2 after de Bok et al. 2004).
Formate in interspecies H2 transfer
Syntrophic interactions (the combined effect of two organisms in completing a chemical reaction) usually involve interspecies H2 transfer, although formate may act as an alternative electron carrier. The H2 and formate concentrations in syntrophic cultures are usually extremely low, and it is therefore difficult to determine which is the more important electron carrier. Many of the syntrophs involved are able to produce both H2 and formate, and most of the methanogenic partners are able to oxidise both substrates. In addition, and of major importance, is that methanogens (which can metabolise both H2 and formate) are usually able to reversibly produce formate from H2 and HCO3–(Beaty and McInerney 1987). Rumen anaerobic fungi growing in vitro on cellulose (Bauchop and Montfort 1981) and wheat straw (Lowe et al. 1987) produced considerable amounts of formate, which would have no effect on the partial pressure of H2 at the site of fermentative activity within the feed particle. This formate may diffuse towards the bulk fluid and may be converted to H2 and CO2 or CH4 by feed-associated methanogens, or by planktonic microbes in the fluid phase, to these same end products.
The first evidence that formate could be used as an electron carrier was obtained with microbes from a whey-treating reactor when the rate of CH4 formation by syntrophic butyrate-degrading cultures could not be explained by interspecies H2 transfer alone (Thiele et al. 1988; Boone et al. 1989). Using diffusion models based mainly on Fick’s law, it was predicted that interspecies formate transfer could sustain an uptake of electrons for methanogenesis that was 100 times faster than was interspecies H2 transfer. A similar modelling approach demonstrated the importance of formate in propionate-degrading and butyrate-degrading co-cultures (Dong and Stams 1995). Further evidence for formate transfer came from growth and biochemical studies; Syntrophobacter fumaroxidans grew well on propionate in co-culture with methanogens that could use both H2 and formate, but no measurable growth was observed with methanogens that used only H2 (Dong et al. 1994; Stams and Plugge 2009).
Recently, Felchner-Zwirello et al. (2013) measured inter-bacterial distances between the propionate degraders and methanogens in syntrophic associations within granules that increased in size with time after inoculation of the growth media. According to these authors, the two microbial types find each other (probably by quorum sensing) and the aggregates increase in size over time, while the interspecies distances decrease from 5.30 to 0.29 μm. At the same time, the maximum possible H2 flux is increased from 1.1 to 10.3 nmol/mL.min. The results indicated that aggregation and reduction of the interspecies distance between inter-dependent microbes is highly advantageous in these complex ecosystems.
Recent studies have suggested that, in many anaerobic systems, formate is an important interspecies carrier of the H2 that is used to produce CH4 (Crable et al. 2011). In such systems, inhibition of methanogenesis with CH4 analogues has demonstrated that methanogens that produce small amounts of formate in flocs increase formate production by a factor of 10 in the presence of the CH4 inhibitor chloroform (Thiele and Zeikus 1988). In contrast, methanogens that used only HCO3– and H2 did not produce any formate when inhibited with the same CH4 analogue. More recent studies have shown that the synthesis of formate from H2 and HCO3– by pure cultures of methanogens or complex methanogenic consortia was much increased when H2 utilisation for CH4 synthesis was inhibited with chloroform, ethanol or bromoethanesulfonic acid (Bleicher and Winter 1994).
In summary, methanogenesis in biofilms or other microbial aggregates is dependent on interspecies transfer of electrons, either via H2 or formate. In both cases, only CH4 is released to the bulk medium. When CH4 analogues are used to inhibit methanogenesis, it is suggested that the inhibition results in the release of formate to the bulk fluid where it is converted to H2 and CO2. This would produce only a moderate increase in the partial pressure of H2 in rumen fluid due to its relatively large volume, and any increase in the biofilm would be small. H2, being relatively insoluble, would be excreted quickly via the rumen gas cap. The change of site of H2 production would thus maintain the partial pressure of H2 commensurate, with oxidation of NADH at the fermentation site on the feed particle. The interspecies transfer requires the methanogens to be closely associated with the fermentative organisms. In flocs, formate transfer appears to be 100 times more important than the transfer of H2 (Thiele and Zeikus 1988). Formate, being more soluble than H2, can produce higher concentrations of substrate at the surface of microbes but H2 diffuses 30 times faster than does formate. On the basis of calculations of diffusion kinetics (Boone et al. 1989), formate would be the preferred electron transfer system in planktonic cultures where the carrier molecule has to diffuse over a relatively long aqueous path; transfer of H2 would be more efficient in densely packed aggregates that dominate in anaerobic digesters. H2 would probably also be a more efficient carrier in sediments and in other microbial biofilms. It seems the relatively rapid turnover of rumen contents (2–8%/h) requires growth of methanogens to be more rapid than in some other fermentation systems; faster growth necessitates a close relationship between hydrogenotrophic organisms and primary fermentation organisms including bacteria, protozoa and fungi.
Little or no research has been undertaken to evaluate the ability of rumen biofilm microbial consortia to produce and use formate. Although formate is produced as an end product of primary fermentation of cellulose to VFA by F. succinogenes (Suen et al. 2011), the rate of conversion of formate to H2 in rumen biofilms is not known for any diet because methods have not been developed to measure formate fluxes within and from the biofilm matrix. If methanogenesis is inhibited, the fluxes will most probably be represented by the passage rates through the rumen-fluid pools (Hungate et al. 1970).
Aspects of rumen protozoan metabolism and potential for H2 transfer between methanogens and protozoa
Rumen protozoa are mainly holotrich and entodiniomorphid ciliates (Williams 1986). The type and biomass of protozoa present depends on diet, feed intake and the feeding patterns. The roles played by protozoa in ruminant nutrition are enigmatic. Ruminants are able to survive and grow without the presence of protozoa in the rumen and, following defaunation, appear to have more dietary and microbial protein available for digestion (for reviews, see Bird et al. 1979; Eugène et al. 2004). Fauna-free ruminants also have higher microbial-growth efficiencies due to the higher net passage of microbial N to the lower tract, which is usually attributed to the absence of protozoal predation on bacteria (Williams 1986). Fauna-free ruminants also produce a higher proportion of propionate in the total VFA. As a consequence of the higher ratio of propionate to acetate production and the higher microbial cell outflow from the rumen (both of which are electron sinks), defaunated ruminants therefore tend to emit less enteric CH4 (Morgavi et al. 2010).
The population density of protozoa in the bulk fluid of the rumen (the usual site of sampling), however, varies according to the daily feeding regimen. The entodiniomorphid population in rumen fluid decreases for up to 16 h after the animal ingests feed and then increases and returns to the pre-feeding level (Warner 1965). The holotrich population, consisting mainly of species of larger protozoa, e.g. Isotricha and Dasytricha that are 50–100 µm in diameter and predominant on diets high in soluble carbohydrates (Valdez et al. 1977), declines for a period of 12–20 h after feeding (Williams 1986) but the numbers in rumen fluid return to pre-feeding levels within 4–6 h. The numbers of Isotricha spp. and Dasytricha ruminantium begin to increase in the fluid phase before (0.5–2 h) feeding (Williams 1986), apparently because they sense the impending availability of substrate. An approximately three-fold increase in numbers of Dasytricha and Isotricha spp. was observed in the 2-h post-feeding period in cattle given red clover (Clarke 1977) or sugarcane (Valdez et al. 1977). In animals fed more often than once a day, a similar rise and fall in numbers in rumen fluid occurs in the shorter time between meals, indicating that protozoal sequestration and re-entry into rumen fluid are related to changes in the presence of feed (Michalowski and Muszynski 1978).
Various hypotheses have been proposed to explain the post-feeding decrease in holotrich numbers and their subsequent return to pre-feeding levels in the fluid phase. The apparent disappearance of the large ciliate protozoa has been attributed to several factors, as summarised by Williams (1986) and listed below.
-
Increased dilution rate in the rumen associated with feed intake.
-
Protozoal lysis as a consequence of an over-accumulation of storage polysaccharide. This suggestion is unlikely to be true because protozoal populations return to the same densities in rumen fluid before, or shortly after, the next meal.
-
Settlement of the protozoa in the rumen when their density increases with engulfment of feed particles or the storage of starch-like materials. This suggestion is not credible because protozoa have been found to attach to materials likely to be part of the mat that floats near the surface of the fluid of the rumen. The rumen is also continuously stirred by muscular contractions that ensure mixing. Nevertheless, large protozoa in rumen fluid do settle to the bottom of test tubes when left to stand.
-
Sequestration of the protozoa onto feed particles or onto the wall of the reticulo-rumen. Isotricha spp., for example, have a specialised attachment organelle (Orpin and Hail 1983) that allows them to adhere to plant particles after feeding (Orpin and Letcher 1978). This attachment to feed particles may have evolved as a mechanism that permits protozoa to associate with other microbial consortia that are also attached to surfaces of, or within, feed particles in biofilms. Such an association suggests that protozoa are interacting, and possibly cross-feeding with the biofilm microbial consortia.
The sequestration theory was extended by Abe et al. (1981) to explain a four-fold increase in holotrich numbers in rumen fluid in the hour after feeding and the ensuing abrupt decline in numbers. Large numbers of holotrichs were observed to associate with the reticulum wall after an overnight fast (Abe et al. 1981). These workers proposed that the holotrichs sequester on the wall of the reticulum and then migrate into the rumen after new feed arrives. The migration into the rumen may represent a response to a chemical stimulus, or the contraction of the reticulum during feed ingestion or in anticipation of feeding. Glucose entering the rumen shortly after feeding has been shown to increase the protozoal density in rumen fluid, probably because holotrich protozoa migrate into the rumen from sequestration sites in the reticulum (Murphy et al. 1985).
Protozoa in rumen contents quickly collect methanogens (by engulfment or attachment) after the animal ingests feed, going from virtually symbiants to 10−4 per protozoan within 1–2 h (Ushida and Jouany 1996; Tokura et al. 1997). This suggests that H2 produced by protozoa attracts methanogens (as the increase in numbers was too rapid to have been the result of cell division). An alternative possibility is that the protozoa sequester on to feed particle surfaces where they are close to, and can attract methanogens, probably from the outer layers of the biofilm matrix or the walls of the rumen and reticulum. These methanogens may be engulfed by the protozoa or voluntarily detach from the biofilm and re-attach to the protozoal hydrogenosomes where they form clusters. When soluble carbohydrate is freely available, it appears that protozoa quickly acquire enough methanogens to effectively maintain a low partial pressure of H2 at the sites of conversion of polysaccharides to organic acids. It is also possible that, when attached to particles, protozoa align their hydrogenosomes so that there is a shorter distance between the sites of protozoal H2 production and the organisms that can provide the most available, energetically favourable electron acceptor. This acceptor will usually be HCO3– or CO2, but other compounds with higher affinity for electrons could be used when available. A further possibility that is worthy of exploration is that direct transfer of electrons may result where two organisms are in physical contact (Morita et al. 2011).
Is the reason for the cyclic patterns of appearance of protozoa in rumen fluid related to their need to seek out soluble carbohydrates and/or to reduce cytoplasmic H2 partial pressure?
The attachment by protozoa to feed particles in the rumen is highly advantageous because this closely associates protozoa with both a continuous source of soluble substrates (largely sugars from hydrolysis of polysaccharides by fibrolytic bacteria) and the partial pressure of H2 is kept at a low level by associated methanogens. It seems likely that protozoa attach to the surface of feed particles at sites where bacterial and fungal activities are highest, e.g. the partially digested areas where the initial bacterial and fungal colonisers of the biofilm have stripped and solubilised complex plant components, with production of simple sugars which are not used in their own metabolism. For instance, the genome of F. succinogenes (one of the most prevalent cellulolytic bacteria) encodes for several enzymes capable of degrading an array of polysaccharides (Suen et al. 2011). This species appears to use these enzymes to gain access to cellulose in plant particles by solubilising the compounds surrounding cellulose fibres, but appears to utilise only glucose, cellobiose and cellodextrins to obtain energy for maintenance and growth. It has incomplete pathways (enzymes are not present) for the utilisation of galactose, mannose, fructose and pentose sugars and it makes these monosaccharides available for use by other fermenting bacteria or protozoa. Protozoa are able to take advantage of these monosaccharides and so are possibly attracted to the sites where fibrolytic activity is highest (Williams 1986), where they anchor themselves (Orpin and Letcher 1978). In addition, they would be close to the methanogenic colonies or have these methanogens attached to their external surface which would ensure they maintain partial pressures of H2 at the low levels required to enable them to ferment the sugars produced by the hydrolytic bacteria nearby. It is also possible that protozoa with their often large population of associated methanogens (up to 10−4/cell) provide a mobile depot for uptake of H2 that can quickly move to the sites where fermentation rate is the highest and most H2 is being produced; they would also be ideally placed to engulf and digest bacteria, obtaining amino acids which, in synchrony with the availability of ATP from fermentation of sugars, would enable them to grow and divide more quickly. By so doing, they may be expected to reduce the population densities of hydrolytic bacteria and perhaps even methanogenic Archaea at the sites of the highest cellulolytic activity. This hypothesis, however, is contrary to conclusions arising from the meta-analysis by Eugène et al. (2004) who concluded that fibre digestion is consistently lower in fauna-free than in faunated ruminants, while duodenal N flow rate, expressed as a ratio of N intake, is enhanced.
This apparent conflict may be resolved if protozoa associated with feed particles assist in supporting hydrolytic solubilisation of the complex polysaccharides by their high rate of removal of the soluble sugars. This would prevent feedback inhibition of hydrolysis of polysaccharides, in particular by F. succinogenes, but also possibly other dominant cellulolytic bacteria such as Ruminococcus flavefaciens and R. albus. In the absence of protozoa, the fibrolytic activity depends on hydrolysis and fermentation by bacterial and fungal populations and the removal of H2 by methanogens. In contrast, when protozoa are abundant, they could potentially take up the soluble sugars produced by F. succinogenes more rapidly; if allowed to accumulate, these sugars might limit overall fibrolytic activity. Protozoa store these sugars as amylopectin, thereby avoiding the immediate production of H2 from this source and reducing feedback inhibition of glycolysis by both monosaccharide accumulation and high rates of H2 production. Overall, this would improve cell-wall carbohydrate digestibility as compared with that in fauna-free animals. The lower fibrolytic activity in the fauna-free rumen is compensated for by a higher net microbial growth (a consequence of less protozoal predation) and a resulting increase in the ratio of protein to energy in the substrate absorbed.
In summary, it is suggested that protozoa spend a considerable proportion of the day as sessile organisms attached to feed particles or the rumen or reticulum wall. When attached to particles, they are closer to the biofilm matrices and microbial colonies where particulate OM is being fermented and, ultimately, H2, HCO3– and organic acids are produced. The protozoa scavenge some of the soluble intermediates and preferentially store them as glycogen-like materials, or utilise them for energy metabolism and growth. The protozoa may also benefit from a close association with the fermentative bacterial consortia where there is a high population of microbial cells facilitating predation. Additionally, they provide a dense mobile population of methanogens that can be attracted to sites where H2 is being rapidly produced and, when feed is high in soluble sugars, they are attracted into the fluid phase, still carrying adherent methanogenic symbionts; this again provides a more dense mobile concentration of methanogens in the bulk fluid where fermentation of sugar is rapid. Protozoa that detach from feed particles are likely to have more adherent methanogens when they have been associated for long periods with biofilm matrices on feed particles. This explains the observation that methanogens increase in numbers on the planktonic protozoa at too high a rate to be a result of growth. When the soluble sugars in rumen fluid are exhausted, the protozoa move to sites on particles where structural carbohydrates are being more slowly mobilised during the breakdown of plant OM and where the biofilm-embedded methanogens maintain a low partial pressure of H2. At the same time, they have a source of available protein from the particle-associated bacteria that are mostly hydrolytic microbes.
If the above hypotheses prove to be correct, the explanation for how the rumen adapts to the absence of protozoa and returns to pre-defaunation methanogenesis (Bird et al. 2008; Hegarty et al. 2008) resides in a simple increase in the hydrolytic bacteria and methanogenic communities in the biofilms attached to plant particles in the fauna-free rumen, with an increase in Ruminococcus species at the expense of F. succinogenes facilitated by potentially lower levels of those sugars that are not utilised by the latter organism. This argument is supported to some extent by the work of Mosoni et al.(2011) who showed that the abundance of cellulolytic bacteria and methanogens was higher in sheep during a long-term (2 years), fauna-free period than it was in faunated sheep. Recognition of (1) the potential roles of biofilm-associated microbes in facilitating fermentation (by maintaining partial pressures of H2 low in the immediate environment of the microbial consortia) and (2) the apparent plasticity in the end-product production by methanogens are discussed in the following sections, in relation to ongoing research aimed at enteric CH4 mitigation.
Methane-mitigation strategies and consequencies
Several reviewers have discussed potential mechanisms for reducing enteric CH4 emissions from ruminant animals (for a comprehensive coverage, see Hristov et al. (2013). In general, the most effective way to reduce CH4 release per unit of production is to reduce the amount of feed an animal used to produce a unit of product. This is best achieved by feeding highly digestible diets with no nutritional deficiencies. Ideally, the diets will be based on cereal grain, thereby allowing the animal to produce to its genetic potential. However, for economic reasons, feed ingredients generally need to be locally available and, in the majority of countries and, in particular, in those considered to be developing countries, the available ingredients will be by-products of plant production (Preston and Leng 1987). Under these conditions, the priority is to optimise production efficiency by management practices. For diets based on local biomass, this will generally be achieved by pre-treatment of feed staples to increase their digestibility and the provision of balanced supplementation (Preston and Leng 1987; Leng 1991, 2004), together with good management practices that minimise any ill-thrift syndromes (Leng 2005).
Mitigation of enteric CH4 per animal or per unit of digestible feed intake – the priorities
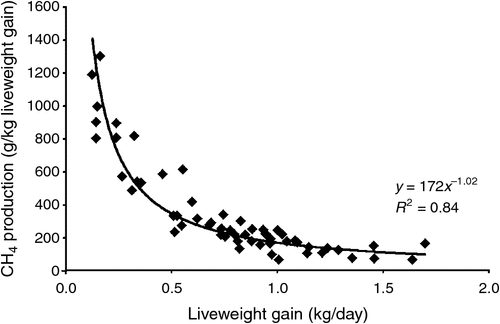
By applying these simple nutritional and management principles, the improvement in utilisation of cereal straw by ruminants that can be achieved has been well demonstrated. In India, milk production, which is largely from cows fed straw or other poor-quality forages, has been markedly improved by the application of good feed management (Banerjee 1994). In the northern wheatbelt of China, cattle growth rates on straw, treated to enhance digestibility and with strategic supplementation, approached 0.9 kg/day, which is 50–75% of the growth rate that could be achieved with similar animals fed grain-based feedlot diets (Dolberg and Finlayson 1995; Cungen et al. 1999). At these growth rates, the numbers of animals that can be fattened on the same quantity of untreated straw is increased by 10–13-fold, with a concomitant large decrease in the amount of CH4 per kg of liveweight or per kg of animal protein produced (see Fig. 3 from Klieve and Ouwerkerk 2007).
|
Optimising productivity per unit of feed intake is by far the most important approach to lowering the world’s enteric CH4 production from ruminants because most of ruminants exist under poor nutritional conditions (Steinfeld et al. 2006). The primary strategies are relatively well established (Preston and Leng 1987) but their implementation is limited by logistic problems. The next priority is to incorporate more ‘sophisticated’ approaches that may reduce CH4 production from individual animals with no detriment to production levels. The direct approach to mitigating rumen CH4 production has been referred to above and will not be discussed further here; the following discussion will focus on manipulation of rumen function to mitigate CH4 production. However, as the majority of recent research in this area has been undertaken on animals given diets based on high-quality feed resources that will become more expensive in the future, the practicality of rumen-centred approaches should always be critically assessed. Grains, for example, may be more efficiently used in the future for human consumption, or to produce pig and poultry meats. As the demand for animal proteins increases with increasing wealth and population, the demand for ruminant feed has to be met by using locally produced biomass and locally available supplements (Leng 2004; Devendra and Leng 2011).
The effects of methanogen inhibitors on fermentative metabolism of OM
Methane analogues, including BCM, are potent inhibitors of methanogenesis (Bauchop 1967). Addition of BCM to rumen contents in vitro, or to the rumen directly, strongly inhibited CH4 production (McCrabb et al. 1997; Goel et al. 2009; Mitsumori et al. 2012). The most successful compounds tested in vivo have been the chlorinated hydrocarbons, including BCM, 2-bromoethane sulfonate (BES), chloroform and cyclodextrin. Inclusion of any of these compounds in the diets of sheep, goats and cattle has reduced CH4 production by 50–100% (Immig et al. 1996; Lila et al. 2004; Knight et al. 2011; Mitsumori et al. 2012). Notably, when Sawyer et al. (1974) added increasing concentrations of BCM to the diet of lambs for 105 days, CH4 excretion was substantially reduced, with no effects on feed intake, digestibility, molar proportions of VFA in ruminal fluid, or on animal growth and production. In a series of experiments with Brahman-cross steers, Tomkins et al. (2009) reported a 93% reduction in CH4 production when BCM was included in the diet at 0.3 g/100 kg BW. There were no treatment differences in daily liveweight gain, feed intake, feed efficiency and carcass quality. In a study in which goats were given 0.3 g BCM/100 kg BW for 10 weeks, Abecia et al. (2012) reported a 33% reduction in CH4 production per unit of DM intake and an increase in the molar proportion of rumen propionate in the total VFA of nearly 40%. These workers observed a highly significant 36% increase in milk yield, with no difference in DM intake, which was attributed to a higher proportion of propionate in the ruminal VFA production. The increase in propionate proportions in the VFA was largely due to a reduction in branched-chain fatty-acid production. An alternative explanation for the increase in milk yield is that there was a concomitant increase in escape protein from the rumen. This suggestion stems from the fact that branched-chain VFA are formed largely from the fermentation of feed protein, but it would be necessary for the goats to be given a diet such that the protein to energy ratio in the substrates absorbed limited the efficiency of milk formation.
Adaptation to the chemicals occurred in some studies and the CH4 mitigation effect became lower with time (Johnson et al. 1972; Immig et al. 1996). However, the effect of BCM appeared to be persistent (Sawyer et al. 1974; Tomkins et al. 2009; Abecia et al. 2012). Recently, Knight et al. (2011) found that there was an immediate lowering of rumen CH4 production in dry cows given chloroform in their diet and the effect persisted for up to 42 days. However, CH4 production gradually increased to 62% of the pre-treatment levels over this period, indicating that there was some adaptation to chloroform by the rumen ecosystem. When dietary BCM concentration was increased stepwise every 8 days, from zero to 5 g/100 kg liveweight in the diet of sheep, CH4 production was almost completely inhibited (by 91%), with no effect on diet digestibility (Mitsumori et al. 2012). A concomitant decrease in the population of H2-sensitive cellulose-digesting bacteria (Ruminococcus spp.) was observed with an increase in numbers of F. succinogenes, a cellulolytic bacterium that produces formate. The alteration to the microbial population occurred both in vivo (Mitsumori et al. 2012) and in vitro (Goel et al. 2009).
Mitsumori et al. (2012) concluded that the inhibition of methanogenesis was accounted for by the release of H2, which they predicted but not actually measured (being outside the limits of detection, given the relatively high gas-flow rate through their calorimeters). It seems reasonable, however, in the light of the research discussed above, to accept that methanogenesis was almost completely suppressed, with H2 gas being released in its place. This observation appears to be contrary to the often-repeated concept that, at high partial pressures of H2 in the rumen, the oxidation of reduced cofactors generated in the fermentative pathways will inhibit the rates of fermentation of feed materials. This enigma may be resolved if formate is produced by the biofilm consortia, and by fungi in particular (Lowe et al. 1987), and is then released into the external fluid and converted to H2 and CO2 by enzymes produced by the planktonic bacteria (see Formate in interspecies H2 transfer, earlier in the paper). This concept is further discussed in the next section, in relation to the apparent differences in action on rumen gas production when CH4 analogues or short-chain nitro-compounds are used to mitigate CH4 production.
Inhibition of methanogenesis with short-chain nitro-compounds as compared with CH4 analogues
Studies by Anderson and colleagues have shown that short-chain nitro-compounds such as nitroethane, 2 nitroethanol, 2-nitro-1-propanol and 3-nitro-1-propionic acid, dimethyl-2-nitroglutarate and 2-nitro-methyl-propionate inhibit ruminal CH4 production in vitro (for references, see Anderson et al. 2010) and nitroethane and 2-nitro-1-propanol have been shown to reduce CH4-producing activity in vivo (Anderson et al. 2006; Gutierrez-Bañuelos et al. 2007; Brown et al. 2011). These nitro-compounds inhibit both CH4 and formate synthesis (Anderson et al. 2008). Inhibition of methanogenesis is accompanied by increased formate production when BCM (a coenzyme M inhibitor) and other CH4 analogues are incubated with methanogens or complex consortia of methanogens (Thiele and Zeikus 1988; Bleicher and Winter 1994). In contrast to the majority of inhibitors, these nitro-compounds do not bring about marked changes in the molar proportions of VFA produced by the mixed microbial population (Bleicher and Winter 1994; Anderson et al. 2003; Brown et al. 2011). Any H2 released by reversal of cofactor reduction does not yet have an identified sink other than that attributable to reduction of the nitro-compound itself, and on the basis of stoichiometry, this could account for only a small fraction of the H2 removed (Božic et al. 2009). It appears that some other unidentified H2 sink is being utilised, because feed intake and digestibility are relatively unaffected. Anderson et al. (2010) discussed the possibility that the H2 is used by Denitrobacterium detoxificans, an obligate, non-fermentative, anaerobic bacterium that conserves energy via respiration. Alternatively, reductive acetogens (that are present in measurable numbers in the rumen) may be responsible for the H2 removal. However, at the partial pressures of H2 maintained in biofilm matrices, these bacteria would probably be outcompeted by methanogens because they have a 10- to 40-fold lower H2 threshold than do acetogens (Greening and Leedle 1989; Breznak and Blum 1991). Nevertheless, even under ‘normal’ feeding conditions, acetogens appear to be present in the rumen in significant numbers and this suggests that they can obtain energy without reducing HCO3– (Le Van et al. 1998). Balautia producta, Eubacterium limosum and Acetitomaculum ruminis are chemolithoautotrophic acetogenic bacteria that have been isolated from the bovine rumen (Boccazzi and Patterson 2013) but are not considered to be the primary H2-consuming organisms because their numbers are consistently lower than those of methanogens. Many organisms that oxidise H2 to acetate can also use sugars, and this may be the principal role of these organisms in the rumen.
The factors dictating whether reductive acetogenesis or methanogenesis will predominate in anaerobic environments are not yet fully understood. It appears possible that when nitro-compounds are incubated with rumen fluid, H2 not accounted for may enter reductive acetogenesis. For example, Pinder and Patterson (2012) showed that an acetogen isolated from rumen contents displayed cellular growth in two phases (diauxie) when incubated with glucose under a gas phase of H2/CO2 (80 : 20). Acetate, formate and H2 were detected during growth on glucose, but only acetate was detected during later growth on H2 and HCO3–. This acetogen would be well suited to a medium receiving intermittent inputs, where sugars that become available after each input are rapidly removed by fermentation and then the organisms switch to utilising H2 as an alternative source of energy.
The effects of short-chain nitro-compounds on CH4 production were evaluated in vitro in batch cultures of rumen fluid (Anderson et al. 2010). After incubation at 39°C for 24 h under 100% CO2 in ruminal-fluid cultures containing nitro-compounds, the CH4 production was reduced to 8% of that in control cultures, whereas total VFA production was greater than in the control incubations. Addition of nitroethane appeared to be particularly active in this respect, because acetate production rate was markedly increased (from 41 to 87 mol/L over 24 h) and, theoretically, only 50% of the increase (21 mol/L) was attributable to possible degradation of the nitroethane. The simultaneous increase in propionate and butyrate production in batch cultures containing nitroethane relative to controls also accounted for some of the decrease in CH4 production. The authors cautiously stated that it was ‘attractive’ to speculate that, in these cultures and particularly within the nitroethane incubation, conditions may have been conducive to reduction of HCO3– to acetate via acetogenesis. An alternative explanation is that the results were an artefact of the incubation technique such that control fermentations were inhibited. For example, CH4 and small amounts of H2 would have accumulated in the head space in the control cultures and increased the partial pressure of CH4 and H2 in solution. This could inhibit the removal of CH4 and H2 from biofilms and so inhibit the amounts of OM fermented, whereas much less gases would have accumulated in the cultures containing nitro-compounds. Head space gas pressures could also lower the amounts of VFA produced by the control incubations. Further research is needed in this area.
It seems possible that, when CH4 and formate synthesis are both inhibited, acetogenesis rapidly takes over the role of maintaining H2 partial pressure below the level that would inhibit the regeneration of cofactors needed to maintain the glycolytic breakdown of both simple and complex carbohydrates in the rumen. This is consistent with the property of self-generation in biofilms of anaerobic ecosystems that have evolved to adapt to changing conditions and to protect the intrinsic conditions needed for bacterial growth and survival and to maintain custom-made communities that can respond to nutrient opportunities (Costerton 2007). As Costerton (2007) observed ‘if the particles in an anaerobic digester consolidate their structure, so that a methanogenic core is surrounded by concentric layers of heterotrophs with graded H2 tolerance’, we must be open to the notion that other microbial communities undergo dynamic restructuring.
Nitro-compounds and chlorinated hydrocarbons reduce CH4 emissions in different ways. As discussed above, it appears likely that CH4 analogues cause methanogens in the rumen biofilm to switch to formate synthesis (Bleicher and Winter 1994) and this provides a potential explanation for the results obtained in the studies of Mitsumori et al. (2012). Production of formate and its diffusion from the biofilm would reduce the H2 concentration in those organisms that have hydrogenase activity; the formate would leave the biofilm and be then diluted in the large volume of rumen fluid where it can be converted to H2 and CO2 by dispersed bacteria. As formate breakdown occurs at a significant distance from the sites of fermentative activity, the H2 partial pressure in the biofilm would be kept below a level that would inhibit oxidation of NADH, and H2 rather than CH4 would be excreted in the eructated gases, cf. studies of the effects of feeding BCM to sheep (Mitsumori et al. 2012). Nitro-compounds, however, appear to block both methanogenesis and formate synthesis, thus allowing the partial pressure of H2 in colonies in the biofilm matrix to initially increase to levels sufficient to promote the growth of acetogens. Further H2 accumulation in the biofilm would then be prevented by reductive acetogenesis. Supporting the above concept, Anderson et al. (2010) showed there was only a small increase in H2 production in rumen fluid incubated with nitro-compounds as compared with control incubations. When CH4 inhibition was in response to the presence of BCM, H2 production appeared stochiometrically to match the decrease in CH4 production (Mitsumori et al. 2012).
Bleicher and Winter (1994) showed that most methanogens were able to produce formate, particularly when fermentative activity and H2 production were high, but if the partial pressure of H2 declined, they produced H2 and CO2 so as to maintain formate for CH4 production. In the light of results on formate synthesis from H2 and HCO3– and its re-utilisation by all formate-utilising methanogens, Bleicher and Winter (1994) argued that the concept of interspecies formate transfer proposed by Thiele and Zeikus (1988) should be reconsidered. An alternative explanation as to why formate is produced is that its synthesis pathway has evolved to enable methanogens to outcompete acetogens for H2. That is, in complex ecosystems with excess H2, formate synthesis by methanogens may serve as a means of disposing of surplus reducing power that would, if allowed to increase at the site of fermentative activity, promote reductive acetogenesis at the expense of methanogenesis.
Some of the differences in the literature concerning the relative reduction of methanogenesis and H2 production almost certainly arise from comparisons of in vitro and in vivo gaseous exchanges. In the intact animal, the rumen gaseous environment is controlled by the production rates of H2 and CH4 and their solubilities. In vivo, both are removed rapidly from the gas space by eructation and so they do not accumulate in rumen fluid to any extent. The partial pressure of H2 in the extracellular polymeric substances in the biofilm will be elevated as compared to that in the rumen fluid. In incubations in vitro, if the gases are allowed to accumulate in the head space, the partial pressure of H2 in the incubation fluid may in turn affect the partial pressure of H2 in the biofilm; for this reason, in vitro experiments may not replicate in vivo conditions where H2 is removed by eructation. This effect may be particularly significant where the pressure in the gas space in the incubation vessels is allowed to increase throughout the incubation period. As Bleicher and Winter (1994) pointed out, it is not the H2 concentration in the gas phase, but the H2 concentration in the vicinity of the microorganisms that is crucial for formate and CH4 generation; the same reasoning must apply to reductive acetogenesis.
The potentially high formate concentration in the biofilm relative to rumen fluid was demonstrated by Hungate et al. (1970). A dialysis bag containing a buffered salt mix was placed in the rumen for 1 h and the fluid was analysed for formate and compared with the formate concentration in whole-rumen contents. The concentration in the whole-rumen contents was 1000 times that of the dialysate, whereas the concentrations of the acetate, propionate and butyrate were the same at both sites. The formate in the non-fluid contents was attributed to the formate contained in microorganisms, but it is possible that it more accurately represented both intracellular formate and formate in the biofilm matrices. It is potentially possible that formate is retained in microbes, or its diffusion through the biofilm to the external medium is slowed by either chemical or physical binding to the extracellular polymeric substances. Such a mechanism would be advantageous in the potential control of partial pressure of H2 by methanogens, especially if they can switch on formate production to lower the H2 pressure at the surface of the biofilm matrix, or increase it by activating formate dehydrogenase. Bleicher and Winter (1994) also argued that formate generation by methanogens is a means for disposal of surplus H2, which can be reversed when the reducing power is lowered. Stewart (2003) summarised diffusion in biofilms in the following four points:
-
diffusion is the predominant solute transport process within cell clusters,
-
the time scale for diffusive equilibration of a non-reacting solute will range from a fraction of a second to tens of minutes in most biofilm systems,
-
diffusion limitation readily leads to gradients in the concentration of reacting solutes and, hence, to gradients in physiology, and
-
water channels can carry solutes into or out of the interior of a biofilm, but they do not guarantee access to the middle of cell clusters.
Nitro-compounds such as nitroethane inhibit ruminal methanogenesis by as much as 90% in vitro (Anderson et al. 2003) and by more than 43% in vivo (Anderson et al. 2006), via inhibition of formate and H2 oxidation (Anderson et al. 2008). If rumen protozoa depend on associated methanogens to enable reduced cofactors to be re-oxidised, then protozoal populations should be reduced in the presence of short chain nitro-compounds. In spite of this, there appear to be no reports of the effects of these compounds on ruminal protozoa.
The ruminal effects of promoting NRB and/or SRB by supplementation with alternative electron acceptors (nitrate or sulfate) on methanogenesis, and potential consequences for the microbial ecology
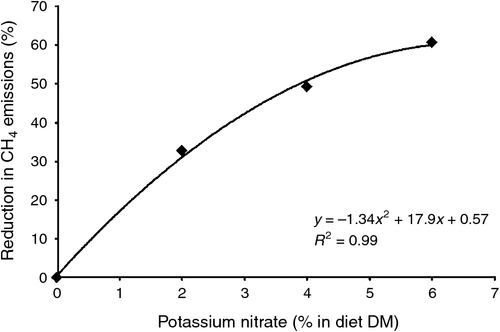
Ruminants given low-protein diets are able to use non-protein N in the rumen, usually in the form of urea or NH3, to stimulate fermentative digestion and feed intake (see Preston and Leng1987). It has been recognised for some time that nitrate salts can be used to replace urea because both are degraded to NH3, which is a principal source of N for microbial protein synthesis and growth. In addition, the chemical reduction of nitrate and/or sulfate to NH3 and/or H2S in the rumen provides alternative electron sinks and animals given nitrate in the diet have lower CH4 emissions. Depending on the basal diet, there will always be a balance between the amount of nitrate required to satisfy the fermentable-N requirements of the ruminal biota and the potential reduction in CH4 production that can be achieved. In addition, the response in CH4 emission to increases in ingested nitrate is curvilinear. One of the first reports of this effect was that of Sophea and Preston (2011); when nitrate progressively replaced urea in a diet given to goats, the apparent effectiveness of the nitrate in reducing CH4 decreased (Fig. 4). The reduction in CH4 production was assessed by the method of Madsen et al. (2010), which uses the lowering in the ratio of CH4 to CO2 in breath as an index of the proportional reduction in CH4 release. The response to increased nitrate intake was curvilinear, reaching 60% reduction when all the urea-N was replaced by nitrate-N in the diet, i.e. CH4 mitigation per unit of nitrate decreased with increasing nitrate supply. Without knowing the CH4 production rate, however, the absolute reduction in CH4 production rate cannot be calculated.
|
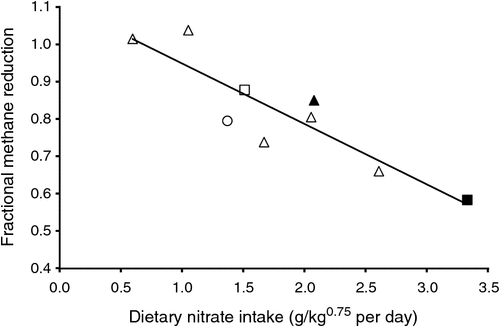
Studies by Hulshof et al. (2012) in cattle in which nitrate progressively replaced dietary urea also showed a progressive decline in the actual CH4 mitigation compared with the theoretical reduction; the latter was based on the stoichiometric prediction that 100 g of dietary nitrate reduced to NH3 should lower CH4 emissions by 25.8 g (assuming that all of the added nitrate was converted to ammonia in the rumen and the resulting reducing equivalents were used solely for CH4 mitigation). The available data on this ratio (termed here ‘fractional CH4 reduction’) from reported studies where nitrate has replaced urea in ruminant diets have been summarised by (van Zijderveld 2011; see Fig. 5).
|
There was a negative correlation between the amount of nitrate given per kilogram of metabolic bodyweight and the fractional CH4 reduction. The apparently inefficient use of nitrate has been suggested to originate from differences among animal species or incomplete nitrate reduction in the rumen (van Zijderveld 2011) or from re-direction of VFA production toward propionate production in the rumen. This difference in CH4 mitigation by dietary nitrate was attributed to the production of more reduced end products (e.g. propionate or microbial cells) in the rumen in dairy cows fed high-concentrate diets (van Zijderveld et al. 2011) than in sheep and beef cattle on much more fibrous feeds (Nolan et al. 2010; van Zijderveld et al. 2010). However, in the studies reported by van Zijderveld (2011) in cattle ingesting the same basal ration, the apparent fractional CH4 reduction decreased with increasing dietary nitrate supply, confirming the results of Sophea and Preston (2011).
The potential explanation for the apparently low efficiency of CH4 mitigation by dietary nitrate based on changes in metabolism of VFA in the rumen (syntrophic butyrate and propionate oxidation to acetate) is not straight forward. There appear to be at least three major (or combination) reasons that bring about the apparently inefficient fractional CH4 reduction as nitrate concentrations in a diet are increased, as follows:
-
nitrate or nitrite produced in the rumen is absorbed and excreted in the urine,
-
nitrate alters the microbial ecology of the rumen and stimulates additional H2 production when compared with urea as the fermentable N source, and
-
nitrate stimulates formate production by methanogens which diffuses into the bulk fluid and is converted to H2 that is removed by eructation.
If the first is correct, then nitrate and/or nitrite excretion in the urine could result in the release of nitrous oxides to the atmosphere, which carries high greenhouse-gas implications that could offset the benefits of the reduction in CH4 production. This would be a major impediment to the acceptance of nitrate supplementation of ruminants as a means of mitigating greenhouse-gas production. At the highest reported level of intake of nitrate, which was by dairy cows, the fractional CH4 reduction from added nitrate was 59%. If 41% of the nitrate had not been reduced to NH3, a deficiency of rumen-degradable N could have been expected to reduce feed intake and digestibility and lower milk yield. However, this did not occur. An alternative explanation is that replacing urea with nitrate resulted in a change in microbial communities in the rumen, leading to changes in the production of acetate relative to propionate and butyrate, and an increase in H2 production. When nitrate replaced urea in the rumen, there was also a tendency for an increased microbial cell yield (cells also represent an electron sink) (Nolan et al. 2010). Increased acetate relative to propionate production may result from a channelling of more carbohydrate through pyruvate and acetyl CoA in the fermentative pathways, or it could be a result of acetogenic oxidation of butyrate and propionate by the boosted nitrate-reducing capacity of rumen contents and increased populations of NRB (Alaboudi and Jones 1985) and SRB. The latter, from animal and human large intestine, have been shown to oxidise propionate and butyrate to acetate (Gibson 1990).
In anaerobic environments rich in OM, nitrate stimulates the growth of syntrophic organisms through changes in the partial pressures of H2 within the particle-associated biofilm consortia. It is argued above that the reduction of nitrate to NH3 occurs in the biofilm communities associated with feed OM present in the rumen. As shown by Bleicher and Winter (1994), for many pure cultures of methanogens and for a complex sewage-sludge culture, some formate was formed as an intermediate during growth on H2 and CO2. High concentrations of formate were formed from H2 and HCO3– when conditions for methanogenesis were impaired by the presence of CH4 analogues such as bromoethanesulfonic acid or chloroform, or an elevated redox potential in co-cultures with nitrate reducers. The formation of formate rather than H2 under such circumstances would allow the production of small amounts of H2, e.g. when inclusion of nitrate in the diet of sheep lowered CH4 production substantially (van Zijderveld et al. 2010).
The potential for syntrophic oxidation of butyrate and propionate in the rumen in nitrate/sulfate-supplemented ruminants
Syntrophism is essential in CH4 production, which involves an interaction between H2 and formate-producing microbes with H2 and formate-using partners. The Gibbs free-energy changes involved in syntrophic metabolism are very low, i.e. close to the minimum free-energy change needed to sustain microbial growth. In single cultures, the oxidation of butyrate to acetate and H2 is energetically unfavourable. However, when methanogens are co-cultured with bacteria capable of butyrate oxidation, methanogenesis significantly lowers the concentration of H2 (down to 10−5 atm or ~1 Pa) and thereby shifts the equilibrium of the butyrate oxidation reaction to non-standard conditions. The concentration of one product is lowered and the reaction is shifted towards net energetically favourable conditions (for butyrate oxidation: ΔG°’ = +48.2 kJ/mol, but ΔG’ = –8.9 kJ/mol at 10−5 atm H2). The higher affinity of SRB or NRB for H2 can lower the partial pressure of H2 at the site of butyrate oxidation more rapidly, and to a greater extent in the biofilm matrix (Lovley and Godwin 1988), potentially increasing the growth rates of butyrate-oxidising bacteria. Methanogens appear to be unable to use H2 below partial pressures of 6.5 Pa (Lovley 1985). However, the threshold partial pressures of several methanogens have recently been shown to vary from 1 to 4.7 Pa and the most dominant methanogen in the rumen (Methanobrevibacter spp.) was reported to have a H2 threshold of 4.7 Pa (Kim 2012).
Butyrate-oxidising organisms similar to Syntrophomonas wolfei have been isolated from the rumen (McInerney et al. 1981) and have been shown to oxidise butyrate to acetate when H2 partial pressures are maintained at extremely low levels by co-culture with methanogens (Lovley and Godwin 1988). The same species is also known to oxidise propionate to acetate. The situation is complicated further by the fact that syntrophic propionate-oxidising bacteria also appear to be able to reduce sulfate (Schink 1997) and perhaps nitrate (Moura et al. 2007). Nitrate, having a much higher affinity than CO2 for electron capture, may maintain the H2 partial pressure low enough to enable these organisms to proliferate and this could explain the lowered net production of butyrate when nitrate replaces urea in diets (Farra and Satter 1971) (see later in the text). These organisms also obtain ATP for growth from these reactions (Schink 1997), which would increase the net microbial growth in the rumen. It has been calculated that the rate of OM degradation in H2-syntrophic co-cultures is dependent on the efficiency with which H2-consuming organisms can grow when concentrations of H2 are low (Cord-Ruwisch et al. 1988). In the light of these observations, the terminal electron acceptor in anaerobic systems may be the limiting factor for the rate of substrate oxidation. This fits with the observation that syntrophic co-cultures grow more rapidly with sulfate reducers than with methanogens as H2 scavengers (McInerney et al. 1981), and therefore growth of S. wolfei with nitrate should be higher than that with sulfate or HCO3– as terminal H2 acceptors. In support of this concept, Lovley and Godwin (1988) showed that the H2 concentrations associated with the specified predominant terminal electron-accepting reactions in bottom sediments of a variety of surface-water environments were as follows: methanogenesis 7–10 nM; sulfate reduction, 1.5 nM and nitrate reduction, <0.05 nM.
The most successful hydrogenotrophic species in any anaerobic system is the one that keeps the H2 partial pressure below the level that is necessary to allow H2 uptake by competitors and so SRB should have lower H2 threshold levels than do methanogenic bacteria. In fact, it has been demonstrated that thresholds of H2 oxidation were about one order of magnitude lower in sediments that contained sulfate as well as bicarbonate as electron acceptors (Lovley et al. 1982). In addition, the threshold levels of H2 for nitrate reduction are extremely low (Cord-Ruwisch et al. 1988; Kim 2012), indicating that nitrate would establish the most favourable growth conditions for syntrophic metabolism of butyrate or propionate to acetate.
Calculating the potential additional H2 production as a result of syntrophic metabolism in the rumen
The NRB (which are probably also capable of reducing S) are able to utilise several organic compounds and produce H2 and C intermediates for the synthesis of cells. Two electron donating reactions are shown below. These reactions could explain the changes in VFA production patterns in rumen fluid that occur when nitrate is introduced into the diet (Farra and Satter 1971). Stimulation of the growth of the syntrophic microbes, with concomitant increase in H2 could also explain a lower apparent efficiency of CH4 mitigation when dietary nitrate is reduced to NH3.
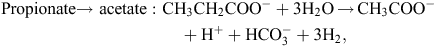
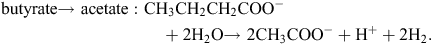
On the basis of stoichiometry, each 100 g of nitrate reduced to ammonia in the rumen would lower CH4 production by 25.8 g and, if ~40% of the nitrate escaped reduction in the rumen (van Zijderveld et al. 2011), then CH4 production would be lowered by 10.3 g CH4 or 0.645 mol CH4. Four mol of H2 are required to produce 1 mol of CH4, so an additional H2 production of 2.58 mol would account for the apparent inefficiency in CH4 mitigation. The 2.58 mol of H2 unaccounted for could be produced by the conversion of 0.86 mol of propionate, or 1.29 mol of butyrate to acetate, occurring in response to changes in the microbial ecology as a result of the presence of nitrate.
From measurement of VFA production rates in the rumen of beef cattle fed diets based on ground corn, Sharp et al. (1982) found that the net production of acetate was 34 mol/day, of propionate 16 mol/day and of butyrate 6.3 mol/day. In the CH4-mitigation studies discussed above, the intake of of DM from the total mixed ration by dairy cows was three times the feed intake of the beef cattle (19 kg/day versus 6.2 kg/day). Assuming a roughly three-fold VFA production in the rumen of dairy cows as compared with the beef animals and an intake of 400 g nitrate/day by the dairy cows, then the amount of propionate needed to be converted to acetate to account for the actual reduction in CH4 production relative to that calculated is ~6–7% of production. If, however, butyrate oxidation was the only pathway affected by nitrate, then 29% of the butyrate would need to be converted to acetate. These calculations are being made here only so as to gauge the magnitude of the changes that may occur to the production of rumen metabolites and, therefore, the feasibility for this being a result of the change in the microbial ecology discussed above when switching from urea to nitrate as the fermentable N source in a diet.
From a review of seven publications, where inter-conversions of VFA in the rumen have been calculated from isotope-dilution studies, 0.45–15.4% of acetate was produced directly from propionate and 1–21% of the acetate was directly produced from butyrate in the rumen (Sharp et al. 1982). These data provide evidence for syntrophic oxidation of propionate and butyrate (Schink 1997) to occur to a limited extent under a variety of feeding conditions. None of these studies included nitrate as a component of the diet; however, the direct utilisation of these VFA could have been attributed to the activity of SRB/NRB that can utilise a diverse range of substrates (Muyzer and Stams 2008).
Farra and Satter (1971) showed that the ratio of acetate in total VFA in rumen fluid increased after adaptation of dairy cows to a nitrate-based diet. Following adaptation to nitrate in a feed (4% of DM intake), the percentages (mol/100 mol total VFA) of these VFA changed as follows: for acetate from 62.3% to 80.2% (increased by 22.3%); propionate from 19.6% to 14.7% (decreased by 25%); and butyrate from 16.1% to 5.0% (decreased by 69%). A lower level of nitrate (2% of DM intake, which was similar to that fed by van Zijderveld et al. 2011) increased acetate (mol %) from 51.5% to 65.7% and decreased propionate (mol %) from 33.6% to 20.9% without altering butyrate proportions. Nolan et al. (2010) reported a similar response in VFA proportions in rumen fluid when sheep fed oaten hay in equal meals each hour were given nitrate to replace urea in the diet, i.e. the acetate to propionate ratio increased markedly (from 3.22 to 4.28) and butyrate proportions were reduced. It appears that the effect of nitrate on VFA production increases with increasing feed intake. There would therefore be markedly different responses to dietary nitrate depending on the experimental feeding strategies; in addition, the effects in animals fed at frequent intervals could be quite different from those fed once daily. In the research of van Zijderveld et al. (2010), the VFA concentrations and proportions were not altered when urea N was quantitatively replaced by nitrate N but the samples analysed were collected 24 h after the meal was offered. The results may be misleading because Farra and Satter (1971) observed an increase in the acetate to propionate ratio in total VFA and a fairly rapid fall in butyrate proportions in the rumen fluid of cows, following ingestion of feed containing nitrate, but both measures returned to ‘normal’ after the nitrate was apparently fully reduced to NH3. Hulshof et al. (2012) studied cattle fed nitrate in a sugarcane–maize silage diet and found that dietary nitrate lowered CH4 production by 32% at an apparent mitigation efficiency of 87%. These workers reported a small increase in the proportion of acetate relative to propionate but no effect on the butyrate percentage (mol/100 mol) in the total VFA in rumen fluid.
Clearly, the diet must have considerable influence on the microbial ecology of the rumen and on the response when urea is replaced with nitrate as a fermentable N source. However, it does appear that any change in the microbial ecology leads to higher levels of H2 production, and this, in turn, increases the requirement for electron acceptors. In the past, a change in the proportions of VFA has been attributed to whether carbohydrate fermentation is directed into the more or less reduced VFA. Generally, metabolism of VFA is associated with a relatively low energy yield (ATP) and slow growth of organisms, which is more similar to sludge fermentations. The relatively high liquid turnover rate in the rumen (2–8%/h) often prevents their establishment in the rumen of most secondary fermentative (acetoclastic) organisms because of their slow growth rate. Nevertheless, it is emphasised here that NRB appear to have growth rates high enough to enable them to maintain high population densities in the rumen. Dwyer et al. (1988) described an organism termed NASF-2 which is a strictly anaerobic, non-spore-forming, acetogenic, H2-reducing, butyrate-oxidising bacterium that resembles S. wolfei. When given optimum conditions (i.e. a low partial pressure of H2), this organism grew exponentially and had a doubling time of 10 h, which would be compatible with substantial growth in the rumen.
Potential mechanism to overcome H2 accumulation and inhibition of hydrogenase activity
Formate production as a potential mechanism to overcome H2 inhibition of hydrogenase activity
Formate synthesis may play an important role in the control of H2 partial pressure in rumen digesta. In most studies in which attempts have been made to mitigate enteric CH4, formate production has not been monitored. Normally, the partial pressure of H2 in methanogenic biofilms is relatively low because the H2 is used immediately for CH4 production and so there is minimal production of formate. However, when the methanogens are partially inhibited, formate may be produced and released into the bulk rumen fluid as reported for flocs in biodigesters (Thiele et al. 1988). Planktonic organisms that have a formate dehydrogenase would then rapidly convert the formate to H2 and CO2 (Doetsch et al. 1953). Hungate et al. (1970) demonstrated that the amount of formate in rumen contents was 1000-fold higher than that in particle-free rumen fluid (a dialysate of rumen digesta), even though the total amount present was still extremely small. This could indicate that most of the formate is present in biofilms. When methanogenesis is inhibited, formate may be produced in the rumen microbial consortia associated with digesta particles, thereby ensuring a low H2 concentration that would not inhibit bacterial hydrogenase activity; accordingly, renewal of functional coenzymes in the fermentative organisms closely attached to and within the biofilm matrix would still be possible. This hypothesis is supported by research with R. flavefaciens (Shi et al. 1997), indicating that, at high growth rates, this species produces formate by reducing HCO3–; at lower growth rates, H2 is formed via hydrogenase. Similarly, when reducing equivalents are in higher concentration, the other dominant cellulolytic species (R. albus) also reduces HCO3– to formate (Miller and Wolin 1973; Asanuma et al. 1990). Formate production by anaerobic fungi could also be involved (Lowe et al. 1987). In fungi, a shift in fermentation toward formate production seemingly maintains low H2 partial pressures. The available evidence suggests that reducing equivalents may be balanced through formate or H2 production, without affecting the yields of the major C-containing fermentation end products. In the rumen digesta, methanogenesis or HCO3– reduction to formate are both systems that compete for available H2. A small proportion of both formate and H2 will normally diffuse into the external rumen fluid where formate is converted back to CO2 and H2. Both gases may be excreted together with CH4 via the gas cap in the rumen.
A similar scenario occurred in flocs from a whey-processing digester (Thiele and Zeikus 1988). Formate production by digester contents or purified digester flocs was dependent on HCO3– and either ethanol or lactate, but not H2, as an electron donor. During syntrophic methanogenesis, flocs were the primary site for formate production via ethanol-dependent HCO3– reduction. Floc preparations accumulated formate, reaching concentrations four-fold higher than digester contents. The formate was generated from reduction of HCO3–, as the formate production continued when methanogenesis was inhibited by chloroform and the primary site for formate cleavage to CO2 and H2 was the dispersed flora. More than 90% of the syntrophic conversion of ethanol to CH4 by mixed cultures containing mainly Desulfovibrio vulgaris and Methanobacterium formicicum was mediated via interspecies formate transfer and less than10% was mediated via interspecies H2 transfer. Mixed consortia of sewage sludge or pure cultures of methanogens (both H2 and formate utilising) generated some formate, even at high partial pressures of H2 (Bleicher and Winter 1994). When partial pressures of H2 decreased, the formate was taken up again and converted to CH4. If methanogenesis was inhibited by BES, methanogens with the ability to use formate for methanogenesis produced formate from H2 and HCO3–. No formate was excreted by methanogens that could use only H2 and HCO3–.
The conundrum presented by the lack of effect of high H2 production rates in the rumen on feed utilisation when CH4 analogues are administered can be explained if the methanogenic Archaea are capable of synthesising formate and using it as the carrier for interspecies transfer of H2 when methanogenesis is inhibited. When H2 partial pressures are relatively high, formate-producing Archaea may become more dominant and prevent further increases in the partial pressure of H2. In support of this hypothesis, methanogenic bacteria with coccobacillus morphology – similar to Methanobrevibacter ruminantium isolated from bovine rumen fluid – grew rapidly and metabolised formate extremely quickly (Lovley et al. 1984). However, recent research has indicated that, even though the short-chain nitro-compounds are inhibitory to methanogens, formate is not always produced (Anderson et al. 2008).
A curious effect when feeding BCM was that, even when CH4 was reduced by 91%, protozoal numbers in the rumen were not affected (Mitsumori et al. 2012). As noted previously, rumen protozoa share a symbiotic relationship with methanogens and participate in interspecies H2 transfer; this transfer provides methanogens with the H2 they need to reduce HCO3– to CH4 and thereby continue to function. It has been estimated that the methanogens associated, both intracellularly and extracellularly, with the ciliate protozoa are responsible for 9–37% of the CH4 production in the rumen. However, the dependency of protozoa on methanogens to re-oxidise NADH and allow glycolysis to continue must be questionable, unless the methanogenic symbionts also produce formate (Hook et al. 2010). If rumen methanogens produce formate that is subsequently converted to H2 and CO2 that are eructated by the animal, then direct inhibition of CH4 is an inappropriate way to mitigate unwanted gas emissions from ruminants because H2 is just as potent a green-house gas as CH4.
Reductive acetogenesis
Creating conditions to support the growth of reductive acetogens is a further strategy for maintaining a low partial pressure of H2 at the fermentative sites in the rumen digesta (Joblin 1999). However, in the rumen, methanogens usually outcompete acetogens for H2. The usual reason put forward for this is that the reduction of CO2 to acetate is thermodynamically less favourable than the reduction of HCO3– to CH4 (McAllister and Newbold 2008). In this connection, however, it is interesting to note that CH4 is not produced in the fermentative areas in the gut of macropods (Kempton et al. 1976) where methanogenesis is apparently replaced by reductive acetogenesis (Ouwerkerk et al. 2009).
Acetogens appear to be present in the rumen in numbers similar to or slightly lower than those of methanogens (Leedle and Greening 1988) and are present in higher numbers in gnobiotic lambs (Le Van et al. 1998; Fonty et al. 2007). Also, acetogens are capable of interspecies H2 transfer when in co-culture with R. albus (Miller 1995). However, the system seems not to support acetogens in the presence of methanogens (Fonty et al. 2007). Growth of acetogens in the rumen is probably limited by their inability to establish in the biofilm consortia close enough to the site of H2 production, to enable them to compete effectively for H2 at the partial pressure maintained by the methanogens. However, in the presence of chlorobromoethane, H2 is produced in amounts that should induce an increase in the partial pressure of H2, without stimulation of reductive acetogenesis. In all probability, acetogens are unable to replace methanogens, numbers being kept low by a combination of Gibbs free-energy change (leaving them relatively less competitive) and their need to associate more closely with the acetogenic fermenting microbes to have any chance of competing. Close proximity to acetate-producing organisms would induce a higher acetate concentration at the site in the biofilm and this would result in feedback inhibition when acetate is produced by HCO3– reduction.
A detailed understanding of how kangaroos support acetate synthesis from H2 and HCO3– in their forestomach would enhance the possibility that this pathway might be introduced effectively in the rumen. There appears to be little information as to the extent of biofilm formation in the tubiform foregut of kangaroos but the uni-directional propulsion of feed through the forestomach may restrict the potential for inoculation of feed particles with hydrolytic and syntrophic organisms. In ruminants, inoculation of feed particles may be largely through rumination (Leng 2011). Although contraction and some regurgitation (termed merycism) occurs in the forestomach of macropods, this is a sporadic event (Hume 1982) and inoculation of feed with microbes may be facilitated by the blind sac – a pouch formed at the junction of the oesophagus and the forestomach. This pouch may provide a reservoir of microbes similar to that in the vermiform appendix of the large bowl of humans. This appendix is now believed to be a reservoir of microbes that form colonies with adherent biofilms in the bowel following recovery after their collapse under, for instance, antibiotic treatment (Bollinger et al. 2007).
It seems possible that acetogenesis rather than methanogenesis is promoted when the establishment of biofilms or the inoculation of digesta is slow and new feed is poorly mixed with residual contents. There is also the possibility that stomach anatomy is important in determining the microbial ecology of the forestomach of ruminants compared with kangaroos. The rumen has evolved to quickly expel gases produced in fermentation. Gases produced collect rapidly in the gas cap or dome and the strong mixing contractions of the rumen result in frequent eructation with gases either entering the mouth directly, or being drawn into the lungs before being expelled in breath (Dougherty 1968).
The solubilities of CH4 and H2 in water at 37°C are ~0.016 g/kg and 0.0014 g/kg, respectively. Thus, if methanogenesis occurred in the kangaroo forestomach, in the absence of a mechanism such as eructation, it might quickly increase the CH4 concentration in the biofilm consortia and the bulk fluid. This in turn could initially stimulate H2 production, with a concomitant increase in partial pressures to a level compatible with establishment of reductive acetogenesis. Such interplay between the concentrations of gases could be responsible for maintaining a reductive acetogenic population that generates less net-gas production. When methanogenic Archaea are inhibited by CH4 analogues, formate is produced (Bleicher and Winter 1994) but is quickly converted to CO2 and H2 by fermenting organisms. Thus, if formate were produced in the kangaroo forestomach, it would not necessarily reduce the partial pressure of gases. It, therefore, seems that acetate synthesis may offer the only means of relieving the partial pressure of H2 within the biofilm. The biofilm mode of fermentation of OM appears to be very delicately balanced by the partial pressures of gases capable of end-product inhibition, but this interplay appears to be crucial for the mitigation of CH4. Further discussion of the potential for reductive acetogenesis to be bolstered in the presence of nitro-compounds is provided in Inhibition of methanogenesis with short-chain nitro-compounds as compared with CH4 analogues, earlier in the paper.
Methanotrophic activities
Methane emissions from biological systems represent a balance between production by methanogenic Archaea and oxidation by methanotrophic microorganisms. CH4 oxidation has been reported in both aerobic and anaerobic environments (Hanson and Hanson 1996). Stocks and McCleskey (1964) isolated CH4-oxidising bacteria from the rumen of steers that were similar to methanotrophic anaerobes isolated from soil and water and Mitsumori et al. (2002) demonstrated that methanotrophs were present in both rumen fluid and in biofilm attached to the rumen wall. However, studies using an artificial rumen indicated that an insignificant amount of the CH4 flux was anaerobically oxidised by a reversal of methanogenesis, with sulfate as the terminal electron acceptor (Kajikawa and Newbold 2003; Kajikawa et al. 2003).
Recent studies have demonstrated that the application of biochar to soils supporting rice production lowered CH4 release (Liu et al. 2011) and this was a result of increased numbers of methanotrophic proteobacteria. Biochar amendment greatly increased the ratio of methanotrophic to methanogenic abundances in paddy soils (Feng et al. 2012). The possibility of increasing methanotrophic activity in the rumen in a similar manner led to a hypothesis that increasing microbial habit with material such as biochar, which has a large surface area to weight ratio, might reduce the net rate of CH4 production (Leng et al. 2012a). To test this hypothesis, biochar was added to an in vitro incubation of rumen fluid; the presence of biochar resulted in a 15% reduction in CH4 release (Hansen et al. 2012; Leng et al. 2012a, 2012b, 2012c). Biochar added to diets of cattle also decreased their CH4 emissions and, at the same time, increased the efficiency of liveweight gain (Leng et al. 2012b; Sophal et al. 2013). The following question is raised by the research with biochar: does the relatively large surface area and highly porous structure of biochar provide a favourable habitat for the organisms involved in a methanogenic–methanotrophic interaction, increasing the potential for anaerobic CH4 oxidation? Recently, research revealed a further property of biochar that may be important in this context; it seems that electrical conductivity of biochar surfaces may facilitate direct electron transfer among syntrophic organisms (S. Cheng, A. E. Rotaru, N. S. Malvankar, F. Liu, K. Nevin, D. R. Lovley, pers. comm.).
Brunauer–Emmett–Teller (BET) surface area is a measure of the ability of a material to absorb gases and also of its accessible surface area for microbial attachment. Biochars often have BET surface areas of 2–40 m2/g but biochars with much greater surface areas may be produced by particular production technologies. The use of biochar and/or activated charcoal in ruminant diets has been shown to mitigate enteric CH4 (Leng et al. 2012a, 2012b, 2012c). A concentrated research effort is needed to refine the mode of action.
Direct interspecies electron transfer
Direct interspecies electron transfer (DIET) may be a more effective mechanism for interspecies electron exchange under anaerobic conditions than is indirect transfer via reduced molecules such as H2 and formate. Improved rates of CH4 production in biodigesters after inoculation with activated charcoal have been shown to result from a more rapid exchange of electrons between bacteria and methanogenic Archaea attached to the surface of charcoal by conduction across its surface (Liu et al. 2012). The demonstration that charcoals enable direct electron transfer suggests that stimulation of metabolism in methanogenic digesters may be attributed, at least in part, to better interspecies electrical connections than those forged biologically. Biochar appears to have similar properties in promoting direct electron transfer (S. Cheng, A. E. Rotaru, N. S. Malvankar, F. Liu, K. Nevin, D. R. Lovley, pers. comm.), but in the rumen it is suspected that biochar surfaces provide habitat for more efficient and rapid microbial growth that may also favour a closer relationship between methanotrophs and methanogens. DIET was found to be an important process for interspecies electron exchange in multi-species aggregates from a methanogenic digester in which Geobacter and Methanosaeta spp. predominated (Morita et al. 2011).
Conclusions
The solubilisation of plant OM within biofilms in the rumen has bestowed numerous advantages on the ruminant animal; in particular, it provides a highly efficient mode of digestion of structural components of plants, yielding nutrients the animal can absorb (organic acids) or digest (microbial cells) to meet their nutrient requirements.
That biofilms provide a micro-environment with opportunities for important interactions between microbes is apparently often not taken into account in studies aimed at mitigating enteric CH4. An in-depth understanding of microbial ecology is a valuable asset when attempting to manipulate anaerobic microbial ecosystems, and ongoing research of biofilms in the ruminant digestive system is a high-priority research area. Biofilm communities in the rumen appear to be self-organising and they adapt to changes in the animal’s diet or other perturbations. The resilience of these microbial structures and their ability to elicit changes in either the composition of colonies, or the end products excreted, suggests that simply surveying for substances with anti-methanogenic properties may not be rewarding. The accidental discovery that chlorinated hydrocarbons inhibit methanogenesis made by Bauchop (1967) has led to many subsequent studies aimed at inhibiting methanogenesis in the rumen by feeding CH4 analogues. Other workers have surveyed a large number of CH4 inhibitors from natural sources.
The direct inhibition of methanogenesis by blocking metabolic pathways (e.g. using CH4 analogues or nitro-compounds) is probably impractical. Research, particularly into the biochemistry of waste-water treatment, has shown that when the methanogenic pathway of H2 uptake is inhibited, the Archaea switch to produce formate from H2 and CO2. Mitsumori et al. (2012) fed BCM to sheep and this inhibited CH4 production by over 90%, but with a concomitant increase in the production of H2. Unfortunately, the H2 emissions from the animals would have the same greenhouse consequences as do CH4 emissions. The consequences of inhibiting methanogenesis with BCM and perhaps many other natural feed ingredients can be explained as follows: the rumen Archaea adjust by producing formate, thereby maintaining the required low partial pressure of H2 within the biofilm matrix. The formate diffuses to the external rumen fluid where it is reconverted to H2 and CO2 by the planktonic microbes. Because H2 solubility in water is low, H2 is quickly released from the rumen fluid into the rumen gas cap. The best evidence for this hypothesis was provided by Bleicher and Winter (1994) who demonstrated that methanogenic consortia from sludge, and also those methanogens in culture that utilise formate as a means of interspecies H2 transfer, become net producers of formate from H2 and CO2 when methanogenic pathways are blocked. Importantly, the conversion of formate to H2 and CO2 is a reversible process in methanogens that possess enzymes capable of both HCO3– reduction and oxidation (Crable et al. 2011).
Nitro-compounds have been shown to inhibit CH4 and formate synthesis and yet have only minor effects on other aspects of digestion. It is argued above that the biofilm consortia have evolved several survival and growth strategies, including the ability to recruit different microbial species to adjust to changing nutrient availabilities, threats from toxic compounds and other changes in the local environment. From the above discussion, it appears that the partial pressure of H2 is a component of mechanisms that control the microbial diversity within the biofilm and therefore the extent and composition of fermentative end products. In particular, maintaining an appropriately low H2 partial pressure at the site of fermentative activity is a critical strategy; depending on the H2 partial pressure, the electron acceptors may be CH4, formate or acetate or, in the presence of nitrate or sulfate, NH3 and H2S, respectively.
If the direct chemical inhibition of CH4 production results in the methanogens using formate as an electron sink, which is then released and metabolised to H2 and CO2 in ruminal fluid, there is little to be gained by survey research for natural or synthetic CH4 inhibitors unless their effects on formate or H2 production are also tested. The ability of methanogens to produce formate provides greater justification for investigations into the potential role of dietary substances that act as alternative electron acceptors, such as nitrate and sulfate. As well as acting as electron acceptors, the presence of these substances may also be responsible for major changes to the microbial ecosystem and so further research is required to optimise their benefits. Concepts such as improving the microbial habitat or stimulating direct electron transfer through electrical conductance, e.g. by including biochars in the diet, may be applicable and may increase the efficiency of microbial growth. A focussed research effort to better understand the role of rumen protozoa and ways by which they interact with the methanogens is also a priority.
Acknowledgements
I thank Professor John Nolan for his many contributions to this essay in both discussions and assistance with editing. I also acknowledge the late Professor J. William Costerton, the father of biofilm research, who through his book ‘The Biofilm Primer’ stimulated my interest in the biofilm mode of life and led me to spend many hours researching the literature in this fascinating emerging science.
References
Abe M, Iriki T, Tobe N, Shibui H (1981) Sequestration of holotrich protozoa in the reticulo-rumen of cattle. Applied and Environmental Microbiology 41, 758–765.Abecia L, Toral PG, Martín-García AI, Martínez G, Tomkins NW, Molina-Alcaide E, Newbold CJ, Yaňez-Ruiz DR (2012) Effect of bromochloromethane on methane emission, rumen fermentation pattern, milk yield, and fatty acid profile in lactating dairy goats. Journal of Dairy Science 95, 2027–2036.
| Effect of bromochloromethane on methane emission, rumen fermentation pattern, milk yield, and fatty acid profile in lactating dairy goats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xksleitrw%3D&md5=47e56251124ff85a8c2d69c66d89bc49CAS | 1:CAS:528:DC%2BC38Xksleitrw%3D&md5=47e56251124ff85a8c2d69c66d89bc49CAS | 22459848PubMed |
Alaboudi AR, Jones GA (1985) Effect of acclimation to high nitrate intakes on some rumen fermentation parameters in sheep. Canadian Journal of Animal Science 65, 841–849.
| Effect of acclimation to high nitrate intakes on some rumen fermentation parameters in sheep.Crossref | GoogleScholarGoogle Scholar |
Allison C, Macfarlane GT (1988) Effect of nitrate on methane production and fermentation by slunies of human faecal bacteria. Journal of General Microbiology 134, 1397–1405.
Anderson RC, Callaway TR, Van Kessel JA, Jung YS, Edrington TS, Nisbet DJ (2003) Effect of select nitrocompounds on ruminal fermentation, an initial look at their potential to reduce economic and environmental costs associated with ruminal methanogenesis. Bioresource Technology 90, 59–63.
| Effect of select nitrocompounds on ruminal fermentation, an initial look at their potential to reduce economic and environmental costs associated with ruminal methanogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvFGlsrY%3D&md5=cd4045a1faa4b842e7718d0bb332bc0aCAS | 12835058PubMed |
Anderson RC, Carstens GE, Miller RK, Callaway TR, Schultz CL, Edrington TS, Harvey RB, Nisbet DJ (2006) Effect of oral nitroethane and 2 nitropropanol administration on methane-producing activity and volatile fatty acid production in the ovine rumen. Bioresource Technology 97, 2421–2426.
| Effect of oral nitroethane and 2 nitropropanol administration on methane-producing activity and volatile fatty acid production in the ovine rumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnsFOlsLg%3D&md5=350fefbeb50bc402f3a04c37ad7a5ae0CAS | 1:CAS:528:DC%2BD28XnsFOlsLg%3D&md5=350fefbeb50bc402f3a04c37ad7a5ae0CAS | 16303299PubMed |
Anderson RC, Krueger NA, Stanton TB, Callaway TR, Edrington TS, Harvey RB, Jung YS, Nisbet DJ (2008) Effects of select nitrocompounds on in vitro ruminal fermentation during conditions of limiting or excess added reductant. Bioresource Technology 99, 8655–8661.
| Effects of select nitrocompounds on in vitro ruminal fermentation during conditions of limiting or excess added reductant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVOmtbfE&md5=5a2dc44a563e52213463d5eb83e906d0CAS | 18538564PubMed |
Anderson RC, Huwe JK, Smith DJ, Stanton TB, Krueger NA, Callaway TR, Edrington TS, Harvey RB, Nisbet DJ (2010) Effect of nitroethane, dimethyl-2-nitroglutarate and 2-nitro-methyl-propionate on ruminal methane production and hydrogen balance in vitro. Bioresource Technology 101, 5345–5349.
| Effect of nitroethane, dimethyl-2-nitroglutarate and 2-nitro-methyl-propionate on ruminal methane production and hydrogen balance in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXks1GltLg%3D&md5=955ca968cbe2d2c452424f1cdc60a923CAS | 20194018PubMed |
Annison EF, Lewis D (1959) ‘Metabolism in the rumen.’ (John Wiley and Sons: New York)
Asanuma N, Wamoto M, Hino T (1990) The production of formate, a substrate for methanogenesis, from compounds related with the glyoxalate cycle by mixed ruminal microbes. Journal of Animal Science 70, 67–73.
Asanuma N, Iwamoto M, Hino T (1998) Formate metabolism by ruminal microorganisms in relation to methanogenesis. Animal Science and Technology 69, 576–584.
Banerjee A (1994) ‘Dairying systems in India. World animal review 79(2).’ (FAO: Rome)
Bar-Zeev E, Berman-Frank I, Girshevitz O, Berman T (2012) Revised paradigm of aquatic biofilm formation facilitated by microgel transparent exopolymer particles. Proceedings of the National Academy of Sciences of the United States of America 109, 9119–9124.
| Revised paradigm of aquatic biofilm formation facilitated by microgel transparent exopolymer particles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XovF2gu70%3D&md5=2729c04ee672e116f6817599882dc2e5CAS | 1:CAS:528:DC%2BC38XovF2gu70%3D&md5=2729c04ee672e116f6817599882dc2e5CAS | 22615362PubMed |
Bath C, Morrison M, Ross EM, Hayes BJ, Cocks BG (2013) The symbiotic rumen microbiome and cattle performance: a brief review. Animal Production Science 53, 876–881.
Bauchop T (1967) Inhibition of rumen methanogens by methane analogues. Journal of Bacteriology 94, 171–175.
Bauchop T, Montfort D (1981) Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Applied and Environmental Microbiology 42, 1103–1110.
Beaty PS, McInerney MJ (1987) Growth of Syntrophomonas wolfei in pure cultures on crotonate. Archives of Microbiology 147, 389–393.
| Growth of Syntrophomonas wolfei in pure cultures on crotonate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXktlKns7c%3D&md5=bb16e5f40a806450802bcfe854d847e3CAS |
Bird SH, Hill MK, Leng RA (1979) The effects of defaunation of the rumen on the growth of lambs on low-protein-high-energy diets. The British Journal of Nutrition 42, 81–87.
| The effects of defaunation of the rumen on the growth of lambs on low-protein-high-energy diets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXmtV2rtb8%3D&md5=5e13e1d08f2671c5101facf42d15849fCAS | 486396PubMed |
Bird SH, Hegarty RS, Woodgate R (2008) Persistence of defaunation effects on digestion and methane production in ewes. Australian Journal of Experimental Agriculture 48, 152–155.
| Persistence of defaunation effects on digestion and methane production in ewes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovFSg&md5=79256be09a520d3d99fc925cfb8923eaCAS | 1:CAS:528:DC%2BD1cXovFSg&md5=79256be09a520d3d99fc925cfb8923eaCAS |
Bleicher K, Winter J (1994) Formate production and utilisation by methanogens and by sludge consortia: interference with the concept of interspecies formate transfer. Applied Microbiology and Biotechnology 40, 910–915.
| Formate production and utilisation by methanogens and by sludge consortia: interference with the concept of interspecies formate transfer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXksVWrtbc%3D&md5=8b2c24b7d2f4d7e7838f50b5c833221fCAS | 1:CAS:528:DyaK2cXksVWrtbc%3D&md5=8b2c24b7d2f4d7e7838f50b5c833221fCAS |
Boccazzi P, Patterson JA (2013) Isolation and initial characterization of acetogenic ruminal bacteria resistant to acidic conditions. Agricultural Food and Analytical Bacteriology 3, 129–144.
Bollinger RR, Barbas AS, Bush EL, Lin SS, Parkera W (2007) Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. Journal of Theoretical Biology 249, 826–831.
| Biofilms in the large bowel suggest an apparent function of the human vermiform appendix.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlGhtrfF&md5=7ba24b65f6fe92e5073ef0a0a5c499f4CAS | 1:CAS:528:DC%2BD2sXhtlGhtrfF&md5=7ba24b65f6fe92e5073ef0a0a5c499f4CAS |
Boone DR, Johnson RL, Liu Y (1989) Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems, and implications in the measurement of KM for H2 or formate uptake. Applied and Environmental Microbiology 55, 1735–1741.
Božic AK, Anderson RC, Carstens GE, Ricke SC, Callaway TR, Yokoyama MT, Wang JK, Nisbet D (2009) Effects of the methane-inhibitors nitrate, nitroethane, lauric acid, Lauricidin and the Hawaiian marine algae Chaetoceros on ruminal fermentation in vitro. Bioresource Technology 100, 4017–4025.
| Effects of the methane-inhibitors nitrate, nitroethane, lauric acid, Lauricidin and the Hawaiian marine algae Chaetoceros on ruminal fermentation in vitro.Crossref | GoogleScholarGoogle Scholar | 19362827PubMed |
Breznak JA, Blum JS (1991) Mixotrophy in the termite gut acetogen, Sporomusa termitida. Archives of Microbiology 156, 105–110.
| Mixotrophy in the termite gut acetogen, Sporomusa termitida.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXlsFCktLY%3D&md5=268dd2b40b5b2648cc675a2a1e360620CAS | 1:CAS:528:DyaK3MXlsFCktLY%3D&md5=268dd2b40b5b2648cc675a2a1e360620CAS |
Brown EG, Anderson RC, Carstensc GC, Gutierrez-Banuelos H, McReynolds JL, Slay LJ, Callaway TR, Nisbet DJ (2011) Effects of oral nitroethane administration on enteric methane emissions and ruminal fermentation in cattle. Animal Feed Science and Technology 166–167, 275–281.
| Effects of oral nitroethane administration on enteric methane emissions and ruminal fermentation in cattle.Crossref | GoogleScholarGoogle Scholar |
Cheng K-J, Costerton JW (1980) The formation of microcolonies by rumen bacteria. Canadian Journal of Microbiology 26, 1104–1113.
| The formation of microcolonies by rumen bacteria.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M7htFKhtg%3D%3D&md5=378e178ff87b133c97f63d70b312a211CAS | 1:STN:280:DyaL3M7htFKhtg%3D%3D&md5=378e178ff87b133c97f63d70b312a211CAS | 7459724PubMed |
Cheng KJ, Fay JP, Howarth RE, Costerton JW (1980) Sequence of events in the digestion of fresh legume leaves by rumen bacteria. Applied and Environmental Microbiology 40, 613–625.
Cheng K-J, Fay JP, Coleman RN, Milligan LP, Costerton JW (1981) Formation of bacterial microcolonies on feed particles in the rumen. Applied and Environmental Microbiology 41, 298–305.
Cheng K-J, Phillippe RC, Majak W (1988) Identification of rumen bacteria that anaerobically degrade nitrate. Applied and Environmental Microbiology 34, 1099–1102.
Cheng K-J, McAllister TA, Costerton JW (1995) Biofilm of the ruminant digestive tract. In ‘Microbial biofilms’. (Eds H Lappin-Scott, JM Costerton) pp. 221–232. (Cambridge University Press: Cambridge, UK)
Clarke RTJ (1977) Protozoa in the rumen ecosystem. In ‘Microbial ecology of the gut’. (Eds RTJ Clarke, T Bauchop) pp. 251–275. (Academic Press: New York)
Coleman RN (1960) A sulphate-reducing bacterium from the sheep rumen. Journal of General Microbiology 22, 423–436.
| A sulphate-reducing bacterium from the sheep rumen.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF3c7jtFGkuw%3D%3D&md5=8750c4248b457cbbc759f79e846d55bcCAS | 1:STN:280:DyaF3c7jtFGkuw%3D%3D&md5=8750c4248b457cbbc759f79e846d55bcCAS |
Cord-Ruwisch R, Seitz HJ, Conrad R (1988) The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Archives of Microbiology 149, 350–357.
| The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXht1yhurc%3D&md5=fed3a93e7f7a674725da036e9f935bc8CAS |
Costerton JW (2007) The biofilm primer 1. In ‘Springer series on biofilms’. (Ed. C Eckey) p. 165. (Springer-Verlag: Berlin)
Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JG, Dasgupta M, Marrie TJ (1987) Bacterial biofilms in nature and disease. Annual Review of Microbiology 41, 435–464.
| Bacterial biofilms in nature and disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXmtlalu70%3D&md5=1e115d9b35eac9bac68129c8c0a349e9CAS | 3318676PubMed |
Cottle DJ, Nolan JV, Wiedemann SG (2011) Ruminant enteric methane mitigation: a review. Animal Production Science 51, 491–514.
| Ruminant enteric methane mitigation: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntVGisLY%3D&md5=5199c518bcbf607cd2261a9a90b2975eCAS | 1:CAS:528:DC%2BC3MXntVGisLY%3D&md5=5199c518bcbf607cd2261a9a90b2975eCAS |
Crable BR, Plugge CML, McInerney MJ, Stams AJM (2011) Formate formation and formate conversion in biological fuels production. Enzyme Research 2011, 532536
| Formate formation and formate conversion in biological fuels production.Crossref | GoogleScholarGoogle Scholar | 21687599PubMed |
Craig WM, Broderick GA, Ricker DB (1987) Quantitation of microorganisms associated with the particulate phase of ruminal ingesta. The Journal of Nutrition 117, 56–62.
Cungen L, Tingshuang SZG, Waldron S (1999) The ‘Straw for Ruminants’ project in China. Agricultural and natural resource economics discussion paper 4/99. School of Natural and Rural Systems Management, University of Queensland, Brisbane.
Czerkawski JW, Blaxter KL, Wainman FW (1966) The metabolism of oleic, linoleic and linolenic acids by sheep with reference to their effects on methane production. The British Journal of Nutrition 20, 349–362.
| The metabolism of oleic, linoleic and linolenic acids by sheep with reference to their effects on methane production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF28Xkt1ensrw%3D&md5=d6592ba772ab7650934abac3f5321f4dCAS | 5938713PubMed |
Davey ME, O’Toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiology and Molecular Biology Reviews 64, 847–867.
| Microbial biofilms: from ecology to molecular genetics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltFWjtrc%3D&md5=c5c84bf3120d0f92d529383bd90e15daCAS | 11104821PubMed |
de Bok FAM, Plugge CM, Stams AJM (2004) Interspecies electron transfer in methanogenic propionate degrading consortia. Water Research 38, 1368–1375.
| Interspecies electron transfer in methanogenic propionate degrading consortia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhvVGgs78%3D&md5=780b1de1b9f0348158ac332396ccbf03CAS | 1:CAS:528:DC%2BD2cXhvVGgs78%3D&md5=780b1de1b9f0348158ac332396ccbf03CAS |
Derwent R, Simmonds P, O’Doherty S, Manning A, Collins W, Stevenson D (2006) Global environmental impacts of the hydrogen economy. International Journal of Nuclear Hydrogen Production and Application 1, 57–67.
| Global environmental impacts of the hydrogen economy.Crossref | GoogleScholarGoogle Scholar |
Devendra C, Leng RA (2011) Feed resources for animals in Asia: issues, strategies for use, intensification and integration for increased productivity. Asian–Australasian Journal of Animal Sciences 24, 303–321.
| Feed resources for animals in Asia: issues, strategies for use, intensification and integration for increased productivity.Crossref | GoogleScholarGoogle Scholar |
Doetsch RN, Robinson RQ, Brown RE, Shaw JC (1953) Catabolic reactions of mixed suspensions of bovine rumen bacteria. Journal of Dairy Science 36, 825–831.
| Catabolic reactions of mixed suspensions of bovine rumen bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG3sXms1KnsA%3D%3D&md5=93249f38dcea1bf4631831bf74e074ceCAS | 1:CAS:528:DyaG3sXms1KnsA%3D%3D&md5=93249f38dcea1bf4631831bf74e074ceCAS |
Dolberg F, Finlayson P (1995) Treated straw for beef production in China. World Animal Review 82, 14–21.
Dong X, Stams AJM (1995) Evidence for H2 and formate formation during syntrophic butyrate and propionate degradation. Anaerobe 1, 35–39.
| Evidence for H2 and formate formation during syntrophic butyrate and propionate degradation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XkvV2ltLY%3D&md5=4690bf58ae040a799f7922b334f10cdbCAS | 1:CAS:528:DyaK28XkvV2ltLY%3D&md5=4690bf58ae040a799f7922b334f10cdbCAS | 16887505PubMed |
Dong X, Plugge CM, Stams AJM (1994) Anaerobic degradation of propionate by a mesophilic acetogenic bacterium in coculture and triculture with different methanogens. Applied and Environmental Microbiology 60, 2834–2838.
Dougherty RW (1968) Section 6. Physiology of eructation in ruminants. In ‘Handbook of physiology. Volume V’. (Ed. CF Code) pp. 2695–2698. (American Physiological Society: Washington, DC)
Dwyer DF, Weeg-Aerssens E, Shelton DR, Tiedje JM (1988) Bioenergetic conditions of butyrate metabolism by a syntrophic, anaerobic bacterium in co-culture with hydrogen-oxidizing methanogenic and sulfidogenic bacteria. Applied and Environmental Microbiology 54, 1354–1359.
Eckard RJ, Grainger C, de Klein CAM (2010) Options for the abatement of methane and nitrous oxide from ruminant production: a review. Livestock Science 130, 47–56.
| Options for the abatement of methane and nitrous oxide from ruminant production: a review.Crossref | GoogleScholarGoogle Scholar |
Edwards JE, Huws SA, Kim EJ, Kingston-Smith AH (2007) Characterization of the dynamics of initial bacterial colonisation of non-conserved forage in the bovine rumen. FEMS Microbiology Ecology 62, 323–335.
| Characterization of the dynamics of initial bacterial colonisation of non-conserved forage in the bovine rumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsValtr7F&md5=ce91c9b458b2348f5ade81eac700e848CAS | 1:CAS:528:DC%2BD2sXhsValtr7F&md5=ce91c9b458b2348f5ade81eac700e848CAS | 17941835PubMed |
Edwards JE, Huws SA, Kim EJ, Lee MRF, Kingston-Smith AH, Scollan ND (2008) Advances in microbial ecosystem concepts and their consequences for ruminant agriculture. Animal Production Science 2, 653–660.
Eugène M, Archimede H, Sauvant D (2004) Quantitative meta-analysis on the effects of defaunation of the rumen on growth, intake and digestion in ruminants. Livestock Production Science 85, 81–97.
| Quantitative meta-analysis on the effects of defaunation of the rumen on growth, intake and digestion in ruminants.Crossref | GoogleScholarGoogle Scholar |
Farra PA, Satter LD (1971) Manipulation of the ruminal fermentation III. Effect of nitrate on ruminal volatile fatty acid production and milk composition. Journal of Dairy Science 54, 1018–1024.
| Manipulation of the ruminal fermentation III. Effect of nitrate on ruminal volatile fatty acid production and milk composition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3MXksVKgur4%3D&md5=00e76480de3774c07a3e1460b1d96dbaCAS | 1:CAS:528:DyaE3MXksVKgur4%3D&md5=00e76480de3774c07a3e1460b1d96dbaCAS |
Felchner-Zwirello M, Winter J, Gallert J (2013) Interspecies distances between propionic acid degraders and methanogens in syntrophic consortia for optimal hydrogen transfer. Applied Microbiology and Biotechnology 97, 9193–9205.
| Interspecies distances between propionic acid degraders and methanogens in syntrophic consortia for optimal hydrogen transfer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFarurrN&md5=8ec6ad9e242299802fc8e24b8308bb7fCAS | 23233207PubMed |
Feng Y, Xu Y, Yu Y, Xie Z, Lin X (2012) Mechanisms of biochar decreasing methane emission from Chinese paddy soils. Journal of Soils Biology and Biochemistry 46, 80–88.
| Mechanisms of biochar decreasing methane emission from Chinese paddy soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XosFCltw%3D%3D&md5=ffd9a46d144634f0a3635df757d551e4CAS | 1:CAS:528:DC%2BC38XosFCltw%3D%3D&md5=ffd9a46d144634f0a3635df757d551e4CAS |
Fonty G, Joblin K, Chavarot M, Roux R, Naylor G, Michallon F (2007) Establishment and development of ruminal hydrogenotrophs in methanogen free lambs. Applied and Environmental Microbiology 73, 6391–6403.
| Establishment and development of ruminal hydrogenotrophs in methanogen free lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1ektLrK&md5=1a516268787d3743a46b5481d9b924fdCAS | 17675444PubMed |
Forsberg CW, Lam K (1977) Use of adenosine-5′-triphosphate as an indicator of the microbiota biomass in rumen contents. Applied and Environmental Microbiology 33, 528
Gibson GR (1990) Physiology and ecology of the sulphate-reducing bacteria. The Journal of Applied Bacteriology 69, 769–797.
| Physiology and ecology of the sulphate-reducing bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhtl2nsb4%3D&md5=7a6b324afb14d25124947de3369f0041CAS | 1:CAS:528:DyaK3MXhtl2nsb4%3D&md5=7a6b324afb14d25124947de3369f0041CAS | 2286579PubMed |
Goel G, Makkar HPS, Becker K (2009) Inhibition of methanogens by bromochloromethane: effects on microbial communities and rumen fermentation using batch and continuous fermentations. The British Journal of Nutrition 101, 1484–1492.
| Inhibition of methanogens by bromochloromethane: effects on microbial communities and rumen fermentation using batch and continuous fermentations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnslSlsLo%3D&md5=f3b4f426f264761183d0503c752d24e1CAS | 1:CAS:528:DC%2BD1MXnslSlsLo%3D&md5=f3b4f426f264761183d0503c752d24e1CAS | 19243639PubMed |
Gordon GLR, Phillips MW (1998) The role of anaerobic gut fungi in ruminants. Nutrition Research Reviews 11, 133–168.
| The role of anaerobic gut fungi in ruminants.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M%2FjsF2rsQ%3D%3D&md5=b5eafa448ec37555c97b8cd4c49414a2CAS |
Greening RC, Leedle JA (1989) Enrichment and isolation of Acetitomaculum ruminis gen. nov., sp. nov.: acetogenic bacteria from the bovine rumen. Archives of Microbiology 151, 399–406.
| Enrichment and isolation of Acetitomaculum ruminis gen. nov., sp. nov.: acetogenic bacteria from the bovine rumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXkt1SlsL0%3D&md5=62388b17475fa6b85b8714553147f391CAS | 2500921PubMed |
Gutierrez-Bañuelos H, Anderson RC, Carstens GE, Slay LJ, Ramlachan N, Horrocks SM, Callaway TR, Edrington TS, Nisbet DJ (2007) Zoonotic bacterial populations, gut fermentation characteristics and methane production in feedlot steers during oral nitroethane treatment and after the feeding of an experimental chlorate product. Anaerobe 13, 21–31.
| Zoonotic bacterial populations, gut fermentation characteristics and methane production in feedlot steers during oral nitroethane treatment and after the feeding of an experimental chlorate product.Crossref | GoogleScholarGoogle Scholar | 17208022PubMed |
Hansen HH, Storm IMLD, Sell AM (2012) Effect of biochar on in vitro rumen methane production. Acta Agriculturae Scandinavica. Section A – Animal Science 62, 305–309.
| Effect of biochar on in vitro rumen methane production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXntVGjuro%3D&md5=060638e3c5a804a0c0e6697b005d58bbCAS | 1:CAS:528:DC%2BC3sXntVGjuro%3D&md5=060638e3c5a804a0c0e6697b005d58bbCAS |
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiological Reviews 60, 439–471.
Hao TP, Do HQ, Preston TR, Leng RA (2009) Nitrate as a fermentable nitrogen supplement for goats fed forage based diets low in true protein. Livestock Research for Rural Development 21, Article #10. Available at http://www.lrrd.org/lrrd21/1/trin21010.htm. [Verified 11 February 2014]
Hegarty RS, Bird SH, Vanselow BA, Woodgate R (2008) Effects of the absence of protozoa from birth or from weaning on the growth and methane production of lambs. The British Journal of Nutrition 100, 1220–1227.
| Effects of the absence of protozoa from birth or from weaning on the growth and methane production of lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsV2lsr3L&md5=be5929bfcaeffc9783c14e0a60b69b8cCAS | 1:CAS:528:DC%2BD1cXhsV2lsr3L&md5=be5929bfcaeffc9783c14e0a60b69b8cCAS | 18479584PubMed |
Hook SE, Wright NDG, McBride BW (2010) Methanogens: methane producers of the rumen and mitigation strategies. Archaea 2010, 945785
| Methanogens: methane producers of the rumen and mitigation strategies.Crossref | GoogleScholarGoogle Scholar | 21253540PubMed |
Howard BH, Hungate RE (1976) Desulfovibrio of the sheep rumen. Applied and Environmental Microbiology 32, 592–602.
Hristov AN, Oh J, Firkins JL, Dijkstra J, Kebreab E, Waghorn G, Makkar HPS, Adesogan AT, Yang W, Lee C, Gerber PJ, Henderson B, Tricarico JM (2013) Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. Journal of Animal Science 91, 5045–5069.
| Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslKktrrL&md5=4f67430a31a04d014f12be800c812ad3CAS | 24045497PubMed |
Hulshof RBA, Berndt A, Gerrits WJJ, Dijkstra J, van Zijderveld SM, Newbold JR, Perdok HB (2012) Dietary nitrate supplementation reduces methane emission in beef cattle fed sugarcane based diets. Journal of Animal Science 90, 2317–2323.
| Dietary nitrate supplementation reduces methane emission in beef cattle fed sugarcane based diets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFehsbjN&md5=96a48ee0214f4fa24c2e164cb62708e8CAS | 1:CAS:528:DC%2BC38XhtFehsbjN&md5=96a48ee0214f4fa24c2e164cb62708e8CAS |
Hume ID (1982) ‘Digestive physiology and nutrition of marsupials.’ (Cambridge University Press: Cambridge, UK)
Hungate RE (1966) ‘The rumen and its microbes.’ (Academic Press: New York)
Hungate RE, Smith W, Bauchop T, Yu I, Rabinowitz JC (1970) Formate as an intermediate in the bovine rumen fermentation. Journal of Bacteriology 102, 389–397.
Huws SA, Mayorga OL, Theodorou MK, Onime LA, Kim EJ, Cookson AH, Newbold CJ, Kingston-Smith AH (2013) Successional colonization of perennial ryegrass by rumen bacteria. Letters in Applied Microbiology 56, 186–196.
| Successional colonization of perennial ryegrass by rumen bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjtVakur8%3D&md5=395d09e022630a07d55d78273a4e0c68CAS | 1:CAS:528:DC%2BC3sXjtVakur8%3D&md5=395d09e022630a07d55d78273a4e0c68CAS | 23206248PubMed |
Immig I, Demeyer D, Fiedler D, Van Nevel C, Mbanzamihigo L (1996) Attempts to induce reductive acetogenesis into a sheep rumen. Archives of Animal Nutrition 49, 363–370.
Janssen PH (2010) Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Animal Feed Science and Technology 160, 1–22.
| Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtV2itLvF&md5=f711073648fa8a61705a2f9dffb04381CAS |
Joblin KN (1999) Ruminal acetogens and their potential to lower ruminant methane emissions. Australian Journal of Agricultural Research 50, 1307–1313.
| Ruminal acetogens and their potential to lower ruminant methane emissions.Crossref | GoogleScholarGoogle Scholar |
Johnson ED, Wood AS, Stone JB, Moran ET (1972) Some effects of methane inhibition in ruminants (steers). Canadian Journal of Animal Science 52, 703–712.
| Some effects of methane inhibition in ruminants (steers).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXkt1SitQ%3D%3D&md5=29dd570c274b0779f9d5c4b53452c6a0CAS |
Kajikawa H, Newbold CJ (2003) Methane oxidation in the rumen. Journal of Animal Science 78, 291
Kajikawa H, Valdes C, Hillman K, Wallace RJ, Newbold CJ (2003) Methane oxidation and its coupled electron-sink reactions in ruminal fluid. Letters in Applied Microbiology 36, 354–357.
| Methane oxidation and its coupled electron-sink reactions in ruminal fluid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlsFeltLc%3D&md5=d47819bea58b8bd4caf7026e99bb211bCAS | 1:CAS:528:DC%2BD3sXlsFeltLc%3D&md5=d47819bea58b8bd4caf7026e99bb211bCAS | 12753241PubMed |
Kempton TJ, Murray RM, Leng RA (1976) Methane production and digestibility measurements in grey kangaroo and sheep. Australian Journal of Biological Sciences 29, 209–214.
Kim CC (2012) Identification of rumen methanogens, characterization of substrate requirements and measurement of hydrogen thresholds. MSc Thesis, Massey University, Palmerston North, New Zealand.
Klieve AV, Ouwerkerk D (2007) Comparative greenhouse gas emissions from herbivores. In ‘7th international symposium on the nutrition of herbivores’. (Eds QX Meng, LP Ren, ZJ Cao) pp. 487–500. (China Agricultural University Press: Beijing, China)
Knight T, Ronimus RS, Dey D, Tootill C, Naylor G, Evans P, Molano G, Smith A, Tavendale M, Pinares-Patino CS, Clark H (2011) Chloroform decreases rumen methanogenesis and methanogen populations without altering rumen function in cattle. Animal Feed Science and Technology 166–167, 101–112.
| Chloroform decreases rumen methanogenesis and methanogen populations without altering rumen function in cattle.Crossref | GoogleScholarGoogle Scholar |
Krebs GL (1987) The rumen ecosystem – kinetics of microbial pools and effects on microbial protein yield. PhD Thesis, University of New England, Armidale, NSW.
Le Van TD, Robinson JA, Ralph J, Greening RC, Smolenski WJ, Leedle JAZ, Schaefer DM (1998) Assessment of reductive acetogenesis with indigenous ruminal bacterium populations and Acetitomaculum ruminis. Applied and Environmental Microbiology 64, 3429–3436.
Leedle JAZ, Greening RC (1988) Methanogenic and acidogenic bacteria in the bovine rumen: postprandial changes after feeding high- or low-forage diets once daily. Applied and Environmental Microbiology 54, 505–506.
Leng RA (1991) Improving ruminant production and reducing methane emissions from ruminants by strategic supplementation. A report prepared for the Environmental Protection Agency of the USA. EPA/400/1-91/004. Available at http://nepis.epa.gov/ [Verified 3 August 2013]
Leng RA (2004) Requirements for protein meals for ruminant meat production in developing countries. In ‘Protein sources for the animal feed industry. Expert consultation and workshop, Bangkok’. pp. 225–254. (FAO: Rome) Available at http://www.fao.org/docrep/007/y5019e/y5019e0f.htm [ Verified 18 February 2014]
Leng RA (2005) Metabolisable protein requirements of ruminants fed roughage based diets. In ‘International AHAT/BSAT conference on livestock–crop systems to meet the challenges of globalisation. Vol. 1’. (Eds P Rawlinson, C Wachirapakorn, P Pakdee, M Wanapat) pp. 330–347. (British Society of Animal Science: Penicuik, UK)
Leng RA (2008) ‘The potential of feeding nitrate to reduce enteric methane production in ruminants.’ (Department of Climate Change, Commonwealth Government of Australia: Canberra) Available at http://www.penambulbooks.com [Verified 11 February 2014]
Leng RA (2011) The rumen – a fermentation vat or a series of organized structured microbial consortia: implications for the mitigation of enteric methane production by feed additives. Livestock Research for Rural Development 23, Article #258. Available at http://www.lrrd.org/lrrd23/12/leng23258.htm. [Verified 3 August 2013]
Leng RA, Inthapanya S, Preston TR (2012a) Biochar lowers net methane production from rumen fluid in vitro. Livestock Research for Rural Development 24, Article #103. Available at http://www.lrrd.org/lrrd24/6/sang24103.htm. [Verified 3 August 2013]
Leng RA, Preston TR, Inthapanya S (2012b) Biochar reduces enteric methane and improves growth and feed conversion in local ‘Yellow’ cattle fed cassava root chips and fresh cassava foliage. Livestock Research for Rural Development 24, Article #199. Available at http://www.lrrd.org/lrrd24/11/leng24199.htm. [Verified 3 August 2013]
Leng RA, Preston TR, Inthapanya S (2012c) Methane production is reduced in an in vitro incubation when the rumen fluid is taken from cattle that previously received biochar in their diet. Livestock Research for Rural Development 24, Article #211. Available at http://www.lrrd.org/lrrd24/11/sang24211.htm. [Verified 3 August 2013]
Leng RA, Inthapanya S, Preston TR (2013) All biochars are not equal in lowering methane production in in vitro rumen incubations. Livestock Research for Rural Development 25, Article #106. Available at http://www.lrrd.org/lrrd25/6/leng25106.htm [Verified 9 August 2013]
Lila ZA, Mohammed N, Tatsuoka N, Kanda S, Kurokawa Y, Itabashi H (2004) Effect of cyclodextrin diallyl maleate on methane production, ruminal fermentation andmicrobes in vitro and in vivo. Animal Science Journal 75, 15–22.
| Effect of cyclodextrin diallyl maleate on methane production, ruminal fermentation andmicrobes in vitro and in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXis1Shu7Y%3D&md5=aa168d5b55ce6e809aaa86f8ad47f421CAS | 1:CAS:528:DC%2BD2cXis1Shu7Y%3D&md5=aa168d5b55ce6e809aaa86f8ad47f421CAS |
Liu YX, Yang M, Wu YM, Wang HL, Chen YX, Wu WX (2011) Reducing CH4 and CO2 emissions from water-logged paddy soil with biochar. Journal of Soils and Sediments 11, 930–939.
| Reducing CH4 and CO2 emissions from water-logged paddy soil with biochar.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVegtLnK&md5=b840aca65b5b0d2bd7b3707da16198d7CAS |
Liu F, Rotaru A-E, Shrestha PM, Malvankar NS, Nevin K, Lovley DR (2012) Promoting direct interspecies electron transfer with activated carbon. Energy and Environmental Science 5, 8982–8989. Available at http://works.bepress.com/kelly_nevin/59 [Verified 3 August 2013]
Loughnan M (1982) Methane synthesis in the rumen in the presence and absence of chemical manipulators of fermentation. MRurSci Thesis, University of New England, Armidale, NSW.
Lovley DR (1985) Minimum threshold for hydrogen metabolism in methanogenic bacteria. Applied and Environmental Microbiology 49, 1530–1531.
Lovley D, Godwin S (1988) Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments. Geochimica et Cosmochimica Acta 52, 2993–3003.
| Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXot1SltQ%3D%3D&md5=1a265a3bf2c07b03a25876cd0ae685f7CAS | 1:CAS:528:DyaL1MXot1SltQ%3D%3D&md5=1a265a3bf2c07b03a25876cd0ae685f7CAS |
Lovley DR, Dwyer DF, Klug MJ (1982) Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Applied and Environmental Microbiology 43, 1373–1379.
Lovley DR, Greening RC, Ferry JG (1984) Rapidly growing rumen methanogenic organism that synthesizes Coenzyme M and has a high affinity for formate. Applied and Environmental Microbiology 48, 81–87.
Lowe SE, Theodorou MK, Trinci APJ (1987) Cellulases and xylanase of an anaerobic rumen fungus grown on wheat straw, wheat straw holocellulose, cellulose, and xylan. Applied and Environmental Microbiology 53, 1216–1223.
Madsen J, Bjerg BS, Hvelplund T, Weisbjerg MR, Lund P (2010) Methane and carbon dioxide ratio in excreted air for quantification of methane production in ruminants. Livestock Science 129, 223–227.
| Methane and carbon dioxide ratio in excreted air for quantification of methane production in ruminants.Crossref | GoogleScholarGoogle Scholar |
Malvankar NS, Lovley DR (2012) Microbial nanowires: a new paradigm for biological electron transfer and bioelectronics. ChemSusChem 5, 1039–1046.
| Microbial nanowires: a new paradigm for biological electron transfer and bioelectronics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XntlSksbs%3D&md5=33ff093950ec7f43411ec61c88fe7b6bCAS | 1:CAS:528:DC%2BC38XntlSksbs%3D&md5=33ff093950ec7f43411ec61c88fe7b6bCAS | 22614997PubMed |
Martínez Amador SY, Garza-García Y, Rodríguez de la Garza JA, Rodríguez Martínez J (2011) Nitrate reduction, sulfate reduction and methanogenesis interrelation in fixed and suspended bed batch reactors. Current Research Journal of Biological Sciences 3, 591–596.
Marty RJ, Demeyer DI (1973) The effect of inhibitors of methane production on fermentation pattem and stoichiometry in vitro using rumen contents from sheep given molasses. The British Journal of Nutrition 30, 369–376.
| The effect of inhibitors of methane production on fermentation pattem and stoichiometry in vitro using rumen contents from sheep given molasses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXjsFCgsw%3D%3D&md5=2f7f24041100c150c79ee67f85a12305CAS | 1:CAS:528:DyaE2cXjsFCgsw%3D%3D&md5=2f7f24041100c150c79ee67f85a12305CAS | 4746693PubMed |
Mayorga OL, Huws SA, Kim EJ, Kingston-Smith AH, Newbold CJ, Theodorou MK (2007) Biofilm formation by rumen microbes on fresh perennial ryegrass under in vitro anaerobic conditions. In ‘UK Molecular Microbial Ecology Group 13th Meeting, University of Liverpool, 16-17 July 2007’.
McAllister TA, Cheng KJ (1996) Microbial strategies in the ruminal digestion of cereal grains. Animal Feed Science and Technology 62, 29–36.
| Microbial strategies in the ruminal digestion of cereal grains.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xns1Sht7o%3D&md5=244ca478d4051e812766d514aeabe91eCAS | 1:CAS:528:DyaK28Xns1Sht7o%3D&md5=244ca478d4051e812766d514aeabe91eCAS |
McAllister TA, Newbold CJ (2008) Redirecting rumen fermentation to reduce methanogenesis. Australian Journal of Experimental Agriculture 48, 7–13.
| Redirecting rumen fermentation to reduce methanogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovVKh&md5=2cf3f257293eca4b5ea313d16a0d7e84CAS | 1:CAS:528:DC%2BD1cXovVKh&md5=2cf3f257293eca4b5ea313d16a0d7e84CAS |
McAllister TA, Bae HD, Jones GA, Cheng KJ (1994) Microbial attachment and feed digestion in the rumen. Journal of Animal Science 72, 3004–3018.
McCrabb GJ, Berger KT, Magner T, May C, Hunter RA (1997) Inhibiting methane production in Brahman cattle with a novel compound and the effects on growth. Australian Journal of Agricultural Research 48, 323–329.
| Inhibiting methane production in Brahman cattle with a novel compound and the effects on growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXislals7Y%3D&md5=3438173e8aa95aad13fc44ada2af82e0CAS |
McInerney MJ, Bryant MP, Pfennig N (1979) Anaerobic bacterium that degrades fatty acids in syntrophic association with methanogens. Archives of Microbiology 122, 129–135.
| Anaerobic bacterium that degrades fatty acids in syntrophic association with methanogens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXltlKrt7Y%3D&md5=54eeb28941d83cf4d7cc062233661b06CAS | 1:CAS:528:DyaE1MXltlKrt7Y%3D&md5=54eeb28941d83cf4d7cc062233661b06CAS |
McInerney MJ, Bryant MR, Hespell B, Costerton W (1981) Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic, syntrophic, fatty acid oxidising bacterium. Applied and Environmental Microbiology 41, 1029–1039.
Michalowski T, Muszynski P (1978) Diurnal variations in number of ciliate protozoa in the rumen of sheep fed once and twice daily. The Journal of Agricultural Science 90, 1–5.
| Diurnal variations in number of ciliate protozoa in the rumen of sheep fed once and twice daily.Crossref | GoogleScholarGoogle Scholar |
Miller TL (1995) Ecology of methane production and hydrogen sinks in the rumen. In ‘Ruminant physiology: digestion, metabolism, growth and reproduction’. (Eds W von Engelhardt, S Leonhard-Marek, G Breves, D Giesecke) pp. 317–331. (Ferdinand Enke Verlag: Stuttgart, Germany)
Miller TL, Wolin MJ (1973) Formation of hydrogen and formate by Ruminococcus albus. Journal of Bacteriology 116, 836–846.
Mitsumori M, Ajisaka N, Tajima K, Kajikawa H, Kurihara M (2002) Detection of Proteobacteria from the rumen by PCR using methanotroph-specific primers. Letters in Applied Microbiology 35, 251–255.
| Detection of Proteobacteria from the rumen by PCR using methanotroph-specific primers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xntl2lsL8%3D&md5=370d5313ac7143631ad26b3f6ce5a395CAS | 1:CAS:528:DC%2BD38Xntl2lsL8%3D&md5=370d5313ac7143631ad26b3f6ce5a395CAS | 12180951PubMed |
Mitsumori M, Shinkai T, Takenaka A, Enishi O, Higuchi K, Kobayashi Y, Nonaka I, Asanuma N, Denman SE, McSweeney CS (2012) Responses in digestion, rumen fermentation and microbial populations to inhibition of methane formation by a halogenated methane analogue. The British Journal of Nutrition 108, 482–491.
| Responses in digestion, rumen fermentation and microbial populations to inhibition of methane formation by a halogenated methane analogue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFClu7fK&md5=da64721d0aa62073419fee9d4be3adfeCAS | 1:CAS:528:DC%2BC38XhtFClu7fK&md5=da64721d0aa62073419fee9d4be3adfeCAS | 22059589PubMed |
Morgavi DP, Forano E, Martin C, Newbold CJ (2010) Microbial ecosystem and methanogenesis in ruminants. Animal Feed Science and Technology 4, 1024–1036.
Morita I, Malvankar NS, Franks AE, Summers ZM, Giloteaux L, Rotaru AE, Rotaru C, Lovley DR (2011) Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. MBio 2, e00159-11
| Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates.Crossref | GoogleScholarGoogle Scholar |
Mosoni P, Martin C, Forano E, Morgavi DP (2011) Long-term defaunation increases the abundance of cellulolytic ruminococci and methanogens but does not affect the bacterial and methanogen diversity in the rumen. Journal of Animal Science 89, 783–791.
| Long-term defaunation increases the abundance of cellulolytic ruminococci and methanogens but does not affect the bacterial and methanogen diversity in the rumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjtlSmurk%3D&md5=05d9766eb07f9f359ec589cf915f086eCAS | 1:CAS:528:DC%2BC3MXjtlSmurk%3D&md5=05d9766eb07f9f359ec589cf915f086eCAS | 21346137PubMed |
Moura JG, Gonzales P, Moura I, Fauque G (2007) Dissimilatory nitrate and nitrite ammonification by sulphate-reducing eubacteria. In ‘Sulphur-reducing bacteria environmental and engineering systems’. (Eds LL Barton, WA Hamilton) pp. 241–264. (Cambridge University Press: Cambridge, UK)
Murphy MR, Drone PE, Woodford ST (1985) Factors stimulating migration of holotrich protozoa into the rumen. Applied and Environmental Microbiology 49, 1329–1331.
Muyzer G, Stams AJM (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nature Reviews. Microbiology 6, 441–454.
Nagpal R, Puniya AK, Griffith GW, Goel G, Puniya M, Sehgal JP, Singh K (2009) Chapter 17. Anaerobic rumen fungi: potential and applications. In ‘Agriculturally important micro-organisms. Vol. 1’. (Eds GG Khachatourians, DK Arora, TP Rajendran, AK Srivastava) pp. 375–393. (Academic World International: Kamal, India)
Nolan JV, Hegarty RS, Hegarty J, Godwin IR, Woodgate R (2010) Effects of dietary nitrate on rumen fermentation, methane production and digesta kinetics in sheep. Animal Production Science 50, 801–806.
| Effects of dietary nitrate on rumen fermentation, methane production and digesta kinetics in sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVyrtbzP&md5=fec0fdf6d058f63b6cb019493a242d75CAS | 1:CAS:528:DC%2BC3cXhtVyrtbzP&md5=fec0fdf6d058f63b6cb019493a242d75CAS |
Oremland RS (1988) Biochemistry of methanogenic bacteria. In ‘Biology of anaerobic microorganisms’. (Ed. AJB Zehnder) pp. 641–705. (John Wiley & Sons: New York)
Orpin CG, Hail FJ (1983) Surface structures of the rumen holotrich protozoon Isotricha intestinalis with particular reference to the attachment zone. Current Microbiology 8, 321–325.
| Surface structures of the rumen holotrich protozoon Isotricha intestinalis with particular reference to the attachment zone.Crossref | GoogleScholarGoogle Scholar |
Orpin CG, Letcher AJ (1978) Some factors controlling the attachment of the rumen holotrich protozoa Isotricha intestinalis and I. prostoma to plant particles in vitro. Journal of General Microbiology 106, 33–40.
| Some factors controlling the attachment of the rumen holotrich protozoa Isotricha intestinalis and I. prostoma to plant particles in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXlsVehsr0%3D&md5=1b4b8e5a15b262b8168ddce0d9b02401CAS | 1:CAS:528:DyaE1cXlsVehsr0%3D&md5=1b4b8e5a15b262b8168ddce0d9b02401CAS | 418148PubMed |
Ouwerkerk D, Maguire AJ, McMillen L, Klieve AV (2009) Hydrogen utilising bacteria from the forestomach of eastern grey (Macropus giganteus) and red (Macropus rufus) kangaroos. Animal Production Science 49, 1043–1051.
| Hydrogen utilising bacteria from the forestomach of eastern grey (Macropus giganteus) and red (Macropus rufus) kangaroos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1Gmtb%2FK&md5=b12f958798ab2b3d539622083865d3b6CAS |
Pinder RS, Patterson JA (2012) Glucose and hydrogen utilization by an acetogenic bacterium isolated from ruminal contents. Agricultural Food and Analytical Bacteriology 2, 253–274.
Preston TR, Leng RA (1987) ‘Matching livestock systems to available resources in the tropics and subtropics.’ (Penambul Books: Armidale, NSW)
Rappé MS, Giovannoni SJ (2003) The uncultured microbial majority. Annual Review of Microbiology 57, 369–394.
| The uncultured microbial majority.Crossref | GoogleScholarGoogle Scholar | 14527284PubMed |
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435, 1098–1101.
| Extracellular electron transfer via microbial nanowires.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltl2ntrc%3D&md5=55e5fef787bed89538fa5336cdf62480CAS | 1:CAS:528:DC%2BD2MXltl2ntrc%3D&md5=55e5fef787bed89538fa5336cdf62480CAS | 15973408PubMed |
Rodríguez CJ, González J, Alvir MR, Redondo R, Cajarville C (2003) Effects of feed intake on composition of sheep rumen contents and their microbial population size. The British Journal of Nutrition 89, 97–103.
| Effects of feed intake on composition of sheep rumen contents and their microbial population size.Crossref | GoogleScholarGoogle Scholar |
Rowe JB, Loughnan ML, Nolan JV, Leng RA (1979) Secondary fermentation in the rumen of a sheep given a diet based on molasses. The British Journal of Nutrition 41, 393–397.
| Secondary fermentation in the rumen of a sheep given a diet based on molasses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXktFyktLo%3D&md5=d7415b58639b208edaae2dab4c7a221eCAS | 1:CAS:528:DyaE1MXktFyktLo%3D&md5=d7415b58639b208edaae2dab4c7a221eCAS | 427091PubMed |
Sawyer MS, Hoover WJ, Sniffen CJ (1974) Effects of a ruminal methane inhibitor on growth and energy metabolism in the ovine. Journal of Animal Science 38, 908–914.
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiology and Molecular Biology Reviews 61, 262–280.
Schink B, Thauer RK (1988) Energetics of syntrophic methane formation and the influence of aggregation. In ‘Granular anaerobic sludge: microbiology and technology’. (Eds G Lettinga, AJB Zehnder, JTC Grotenhuis, LW Hulshoff-Pol) pp. 5–17. (Pudoc: Wageningen, The Netherlands)
Sharp WM, Johnson RR, Owens FN (1982) Ruminal VFA production with steers fed whole or ground corn grain. Journal of Animal Science 55, 1505–1514.
Shi Y, Weimer P, Ralph J (1997) Formation of formate and hydrogen, and flux of reducing equivalents and carbon in Ruminococcus flavefaciens FD-1. Antonie van Leeuwenhoek 72, 101–109.
| Formation of formate and hydrogen, and flux of reducing equivalents and carbon in Ruminococcus flavefaciens FD-1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmsVamtrg%3D&md5=91c3cb5edc6e50f0bd0e884e520907b5CAS | 1:CAS:528:DyaK2sXmsVamtrg%3D&md5=91c3cb5edc6e50f0bd0e884e520907b5CAS | 9298188PubMed |
Song H, Clarke WP, Blackall LL (2005) Concurrent microscopic observations and activity measurements of cellulose hydrolyzing and methanogenic populations during the batch anaerobic digestion of crystalline cellulose. Biotechnology and Bioengineering 91, 369–378.
| Concurrent microscopic observations and activity measurements of cellulose hydrolyzing and methanogenic populations during the batch anaerobic digestion of crystalline cellulose.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXntlCnsL8%3D&md5=8505201f521de59b26ae753fcc683ee9CAS | 1:CAS:528:DC%2BD2MXntlCnsL8%3D&md5=8505201f521de59b26ae753fcc683ee9CAS | 15991234PubMed |
Sophal C, Khang DN, Preston TR, Leng RA (2013) Nitrate replacing urea as a fermentable N source decreases enteric methane production and increases the efficiency of feed utilization in Yellow cattle. Livestock Research for Rural Development 25, Article #113. http://www.lrrd.org/lrrd25/7/soph25113.htm [Verified 14 July 2013]
Sophea IV, Preston TR (2011) Effect of different levels of supplementary potassium nitrate replacing urea on growth rates and methane production in goats fed rice straw, mimosa foliage and water spinach. Livestock Research for Rural Development 23, Article #71. http://www.lrrd.org/lrrd23/4/soph23071.htm [Verified 5 August 2013]
Stams AJM, Plugge CM (2009) Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nature Reviews. Microbiology 7, 568–577.
| Electron transfer in syntrophic communities of anaerobic bacteria and archaea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXos1Wrsb0%3D&md5=246d2da71fa7a68ef5e39dedbb1e2cc4CAS | 1:CAS:528:DC%2BD1MXos1Wrsb0%3D&md5=246d2da71fa7a68ef5e39dedbb1e2cc4CAS |
Stanton TB, Canale-Parola E (1979) Enumeration and selective isolation of rumen spirochetes. Applied and Environmental Microbiology 38, 965–973.
Stanton TB, Canale-Parola E (1980) Treponema bryantii sp. nov. a rumen spirochete that interacts with cellulolytic bacteria. Archives of Microbiology 127, 145–156.
| Treponema bryantii sp. nov. a rumen spirochete that interacts with cellulolytic bacteria.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M%2FjvVehsg%3D%3D&md5=529c7ddca1c5f48eb6508a9cb2dd6535CAS | 7425785PubMed |
Steinfeld H, Gerber P, Wassenaar T, Castel V, Rosales M, de Haan C (2006) ‘Livestock’s long shadow: environmental issues and options.’ (Food and Agriculture Organisation: Rome)
Stewart PS (2003) Diffusion in biofilms. Journal of Bacteriology 185, 1485–1491.
| Diffusion in biofilms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhs1Sisrs%3D&md5=3d996cbc368acebc7b611d26edaf286aCAS | 1:CAS:528:DC%2BD3sXhs1Sisrs%3D&md5=3d996cbc368acebc7b611d26edaf286aCAS | 12591863PubMed |
Stocks PK, McCleskey CS (1964) Morphology and physiology of Methanomonas methanooxidans. Journal of Bacteriology 88, 1071–1077.
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annual Review of Microbiology 56, 187–209.
| Biofilms as complex differentiated communities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xos1Gis7o%3D&md5=5d7bc8bd8a0e227221038b29a0a3a33cCAS | 1:CAS:528:DC%2BD38Xos1Gis7o%3D&md5=5d7bc8bd8a0e227221038b29a0a3a33cCAS | 12142477PubMed |
Suen G, Weimer PJ, Stevenson D, Aylward FO, Boyum J, Deneke J, Drinkwater C, Ivanova NN, Mikhailova N, Chertkov O, Goodwin LA, Currie CR, Mead D, Brumm PJ (2011) The complete genome sequence of Fibrobacter succinogenes S85 reveals a cellulolytic and metabolic specialist. PLoS ONE 6, e18814
| The complete genome sequence of Fibrobacter succinogenes S85 reveals a cellulolytic and metabolic specialist.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlt1ais7o%3D&md5=bff765ddbcbd19327e2cee86762fbc57CAS | 1:CAS:528:DC%2BC3MXlt1ais7o%3D&md5=bff765ddbcbd19327e2cee86762fbc57CAS | 21526192PubMed |
Thiele JH, Zeikus JG (1988) Control of interspecies electron flow during anaerobic digestion: significance of formate transfer versus hydrogen transfer during syntrophic methanogenesis in flocs. Applied and Environmental Microbiology 54, 20–29.
Thiele JH, Chartrain M, Zeikus JG (1988) Control of interspecies electron flow during anaerobic-digestion – role of floc formation in syntrophic methanogenesis. Applied and Environmental Microbiology 54, 10–19.
Tokura M, Ushida K, Miyazaki K, Kojima Y (1997) Methanogens associated with rumen ciliates. FEMS Microbiology Ecology 22, 137–143.
| Methanogens associated with rumen ciliates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXptlSluw%3D%3D&md5=647d8a800c03b956b828665ccbf0d544CAS | 1:CAS:528:DyaK2sXptlSluw%3D%3D&md5=647d8a800c03b956b828665ccbf0d544CAS |
Tomkins NW, Colegate SM, Hunter RA (2009) A bromochloromethane formulation reduces enteric methanogenesis in cattle fed grain-based diets. Animal Production Science 49, 1053–1058.
| A bromochloromethane formulation reduces enteric methanogenesis in cattle fed grain-based diets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVWqtLnE&md5=9d7f6949e23b7bfc802fdd20e21a4eeeCAS | 1:CAS:528:DC%2BD1MXhsVWqtLnE&md5=9d7f6949e23b7bfc802fdd20e21a4eeeCAS |
UNEP (2011) ‘Near-term climate protection and clean air benefits: actions for controlling short-lived climate forcers.’ (United Nations Environment Programme (UNEP): Nairobi, Kenya). Available at http://www.unep.org/publications/ebooks/SLCF/UNEP. [Verified 3 August 2013]
Ushida K, Jouany JP (1996) Methane production associated with rumen ciliated protozoa and its effect on protozoan activity. Letters in Applied Microbiology 23, 129–132.
| Methane production associated with rumen ciliated protozoa and its effect on protozoan activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlsFSks7s%3D&md5=d27ae9750b3b0406640025a7a8d29491CAS | 1:CAS:528:DyaK28XlsFSks7s%3D&md5=d27ae9750b3b0406640025a7a8d29491CAS | 8987455PubMed |
Valdez RE, Alvarez FJ, Ferreiro HM, Guerra F, Lopez J, Priego A, Blackburn TH, Leng RA, Preston TR (1977) Rumen function in cattle given sugar cane. Tropical Animal Production 2, 260–272.
van Zijderveld SM (2011) Dietary strategies to reduce methane emissions from ruminants. PhD Thesis, Wageningen University, The Netherlands.
van Zijderveld SM, Gerrits WJJ, Apajalahti JA, Newbold JR, Dijkstra J, Leng RA, Perdok HB (2010) Nitrate and sulfate: effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. Journal of Dairy Science 93, 5856–5866.
| Nitrate and sulfate: effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjs1Kis7Y%3D&md5=2c90a9348249f958588d724bd6912c2aCAS | 1:CAS:528:DC%2BC3MXjs1Kis7Y%3D&md5=2c90a9348249f958588d724bd6912c2aCAS | 21094759PubMed |
van Zijderveld SM, Gerrits WJJ, Dijkstra J, Newbold JR, Hulshof RBA, Perdok HB (2011) Persistency of methane mitigation by dietary nitrate supplementation in dairy cows. Journal of Dairy Science 94, 4028–4038.
| Persistency of methane mitigation by dietary nitrate supplementation in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsVylur4%3D&md5=b403e5eac8ed8ca1f5a91dddfa81918fCAS | 21787938PubMed |
Wallace RJ, Joblin KN (1985) Proteolytic activity of a rumen anaerobic fungus. FEMS Microbiology Letters 29, 19–25.
| Proteolytic activity of a rumen anaerobic fungus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXlsFKqu7c%3D&md5=1e19ab578c500486e51ead15b0f3fadeCAS |
Wang ZW, Chen S (2009) Potential of biofilm-based biofuel production. Applied and Environmental Microbiology 83, 1–18.
Warner ACI (1965) Factors influencing numbers and kinds of microorganisms in the rumen. In ‘Physiology of digestion in the ruminant’. (Eds RW Dougherty, RS Allen, W Burroughs, NL Jacobson, AD McGilliard) pp. 346–359. (Butterworths: Washington, DC)
Weimer PJ, Russell JB, Muck RE (2009) Lessons from the cow: what the ruminant animal can teach us about consolidated bioprocessing of cellulosic biomass. Bioresource Technology 100, 5323–5331.
| Lessons from the cow: what the ruminant animal can teach us about consolidated bioprocessing of cellulosic biomass.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXoslGgsrY%3D&md5=afc370d2ca2a1e0cdf0e94d93412285cCAS | 19560344PubMed |
West SA, Griffin AS, Gardner A, Diggle SP (2006) Social evolution theory for microbes. Nature Reviews. Microbiology 4, 597–607.
| Social evolution theory for microbes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmvFamtbk%3D&md5=e3e7260fcf3525f077f68fecc0f8143aCAS | 16845430PubMed |
Williams AG (1986) Rumen holotrich ciliate protozoa. Microbiological Reviews 50, 25–49.
Williams P (2007) Quorum sensing, communication and cross-kingdom signaling in the bacterial world. Microbiology 153, 3923–3938.
| Quorum sensing, communication and cross-kingdom signaling in the bacterial world.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlvVw%3D&md5=75b54779306bd8d5936d7527226dd5a2CAS | 1:CAS:528:DC%2BD1cXlvVw%3D&md5=75b54779306bd8d5936d7527226dd5a2CAS | 18048907PubMed |
Wolin MJ (1979) The rumen fermentation: a model for microbial interactions in anaerobic ecosystems. Advances in Microbial Ecology 3, 49–77.
 Ronald Alfred Leng is Professor Emeritus of Nutritional Biochemistry at the University of New England (UNE). Ron was born in England and studied Agriculture at the University of Nottingham before coming to Australia in 1959 to pursue a PhD in the Faculty of Rural Science at UNE. On successful completion of his PhD he was appointed Lecturer in Nutrition in 1963 and he was progressively promoted over the next 10 years to a Personal Chair in Nutritional Biochemistry. Ron was the first to be awarded the degree of DRurSc in 1972. He was a member of the Faculty of Rural Science for 37 years. It was as a member of the Faculty that he developed his fascination for and began his research into rumen microbial ecology and the utilisation of poor quality forages by ruminants. Ron was made an Officer of the Order of Australia in 1991 for his contribution to development of systems of using poor quality feeds for ruminant meat and milk production in Australia and in developing countries. Ron has been a Distinguished Visiting Professor at Iowa State University (USA) and Nihon University (Japan).The Australian Society of Animal Production made him a Fellow in 1996. In 2002, he received the Han Award from the Asian-Australasian Association of Animal Production Societies ‘in recognition of his outstanding contribution to animal production which is of international significance’. He has been a consultant to the governments of more than 30 countries through United Nations Development Programs. In 1990, he helped to establish The University of Tropical Agriculture in Asia and travels annually to lecture and examine students enrolled in higher degree courses at the campuses in Cambodia, Vietnam and Laos. Ron has published in excess of 400 papers in peer reviewed journals and has published 8 books in areas related to animal production science.
Ronald Alfred Leng is Professor Emeritus of Nutritional Biochemistry at the University of New England (UNE). Ron was born in England and studied Agriculture at the University of Nottingham before coming to Australia in 1959 to pursue a PhD in the Faculty of Rural Science at UNE. On successful completion of his PhD he was appointed Lecturer in Nutrition in 1963 and he was progressively promoted over the next 10 years to a Personal Chair in Nutritional Biochemistry. Ron was the first to be awarded the degree of DRurSc in 1972. He was a member of the Faculty of Rural Science for 37 years. It was as a member of the Faculty that he developed his fascination for and began his research into rumen microbial ecology and the utilisation of poor quality forages by ruminants. Ron was made an Officer of the Order of Australia in 1991 for his contribution to development of systems of using poor quality feeds for ruminant meat and milk production in Australia and in developing countries. Ron has been a Distinguished Visiting Professor at Iowa State University (USA) and Nihon University (Japan).The Australian Society of Animal Production made him a Fellow in 1996. In 2002, he received the Han Award from the Asian-Australasian Association of Animal Production Societies ‘in recognition of his outstanding contribution to animal production which is of international significance’. He has been a consultant to the governments of more than 30 countries through United Nations Development Programs. In 1990, he helped to establish The University of Tropical Agriculture in Asia and travels annually to lecture and examine students enrolled in higher degree courses at the campuses in Cambodia, Vietnam and Laos. Ron has published in excess of 400 papers in peer reviewed journals and has published 8 books in areas related to animal production science.



