Field Anaesthesia for Platypuses: a proven method and the case for non-veterinarian accreditation pathways
Gilad Bino A * and Tahneal Hawke A BA
B
Abstract
This communication details the implementation of field-based anaesthesia for platypuses, applied by non-veterinarians to over 500 individuals across 9 years, with a focus on enhancing animal welfare while enabling the collection of critical scientific data. By utilising portable anaesthetic machines and tailored protocols, safe handling and minimising stress during procedures such as tagging, sample collection, and minor surgeries are ensured. Given the impracticality and high costs of having veterinarians in the field along with researchers, we advocate for accreditation pathways to empower ecologists to independently perform these important procedures, thereby improving the efficiency and sustainability of wildlife research.
Keywords: animal welfare, capture techniques, conservation research, field techniques, isoflurane, mammal, monitoring vital signs, monotreme, non-invasive sampling, portable anaesthetic machines, wildlife handling.
Introduction
Recent advancements in field-based anaesthesia have underscored its essential role in wildlife research, particularly for non-invasive and minimally invasive procedures. Such techniques are increasingly employed to ensure the safe handling of animals during complex tasks, including tagging, sample collection, and minor surgeries (Chinnadurai et al. 2016; Fiorello et al. 2016; Kreeger et al. 2023). Innovations such as portable anaesthetic machines and remote drug delivery systems have revolutionised the ability to safely anaesthetise animals in challenging field environments (Deem et al. 2001; Fahlman 2008). These advancements are vital for minimising stress and reducing the risk of injury during capture and handling (Soulsbury et al. 2020). In wildlife conservation, these developments have been significant, enabling researchers to gather high-quality data on the health, genetics, and behaviour of wild animals, which is crucial for the development of effective conservation strategies (Karesh and Cook 1995; Deem et al. 2001; Junge and Louis 2005).
The use of anaesthesia in fieldwork with platypuses has a well-documented history, with isoflurane commonly employed because of its effectiveness in induction and maintenance. Early applications, such as those outlined by Booth and Connolly (2008), established foundational protocols now standard in Australian wildlife hospitals. Macgregor et al. (2015) significantly advanced these methods by describing comprehensive field-based anaesthetic protocols and monitoring procedures for platypuses. Macgregor’s studies were also the first to document large-scale field anaesthesia, reporting its use in over 150 platypuses and providing detailed guidelines on anaesthetic techniques, including isoflurane delivery, patient monitoring, and thermoregulation measures works laid the groundwork for subsequent adaptations in platypus anaesthesia, including modifications to enhance practicality in remote settings (Macgregor et al. 2014). Since 2015, we have been applying field-based anaesthesia in a range of environmental conditions to over 500 platypuses, refining techniques to improve efficiency, safety, and adaptability in remote settings. With this paper, we aim to formalise these refinements, advocate for structured accreditation pathways, and highlight the potential for trained non-veterinarians to safely and effectively administer anaesthesia, ensuring broader accessibility and consistency in field-based research.
Capture of platypuses is achieved using either unweighted mesh nets or fyke nets, depending on the specific river morphology and flow conditions (Bino et al. 2018). Once captured, platypuses are held in pillowcases in a cool, quiet, and dark place until they are ready for processing. We also use towels, which are lightly wrapped around the platypus or are simply used to cushion the surface. Trapping and handling were performed in accordance with guidelines of University of New South Wales Animal Care and Ethics Committee (e.g. 21/70B), New South Wales Fisheries (P15/0096-2.0 & OUT20/15426), and New South Wales Department of Primary Industries (SL101655). Given that platypuses have a typical body temperature range of 30–34°C, with the majority of recordings between 31°C and 33°C (Grigg et al. 1992; Macgregor 2015), it is critical to prevent overheating, particularly when ambient temperatures are high. Body temperature can be reduced by wetting the fur or lowering room temperature if indoors. To enable safe field anaesthesia in our work, we received specialised training from Dr Larry Vogelnest, senior veterinarian at Taronga Zoo, and were granted approval by the UNSW Animal Care and Ethics Committee (ACEC) to use these protocols under field conditions. Building on these foundational protocols, we incorporated specific adjustments for extended field use, such as temperature regulation, with warm water bottles positioned adjacent to the vaporiser to maintain efficient evaporation of isoflurane, particularly in colder environments. These modifications ensure safe, effective anaesthesia that is tailored to the variable conditions encountered in field research with platypuses.
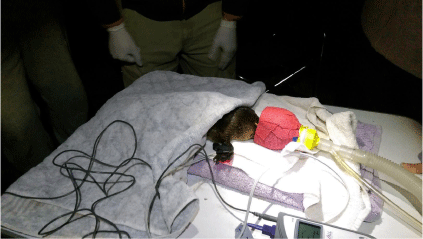
Prior to administering general anaesthesia, a visual examination of the platypus general body condition is performed for any signs that might be a concern about the platypus’s condition and could increase anaesthetic risk. To minimise handling and stress, while still in the pillowcase, platypuses are placed in an induction chamber (small Buster ICU), where they are exposed to 5% isoflurane in oxygen at a flow rate of 3 L/min for 5 min. Anaesthesia is administered using an anaesthetic machine equipped with a vaporiser (Darvall DVM ISO (CM) Low Flow) connected to an oxygen tank. It is important to prevent the vaporiser from reaching very cold temperatures (<5°C) to maintain efficient evaporation of the isoflurane, which is achieved by placing a warm hot water bottle next to the vaporiser (Vogelnest and Portas 2019). Isoflurane is the drug of choice for platypus anaesthesia, a practice commonly used at Taronga Zoo and other wildlife hospitals across Australia (Booth and Connolly 2008; Vogelnest and Portas 2019). We typically use a C-size medical-grade oxygen cylinder, sufficient for safely processing up to six platypuses. The animal is then removed and maintained under anaesthesia using a specialised T-piece face mask, which we fashioned from a 1 L milk bottle, moulded and padded to fit the bill of the platypus using GMV Medi-Vet Wrap Cohesive Elastic Bandage (Figs 1, 2). Anaesthesia is maintained with 1.5–3% isoflurane in oxygen at a reduced flow rate of 1.5 L/min (Bino et al. 2018; Vogelnest and Portas 2019).
During anaesthesia, vital signs, including pulse, oxygen saturation (SpO2), and temperature, are continuously monitored by using a Nellcor Oximax Technology Pulse Oximeter with Temperature Measurement (H100N). This ensures appropriate anaesthesia depth and stability throughout the procedure (Grigg and Roesler 2018). In low-temperature conditions (<10°C for males and potentially <15°C for females) (Macgregor 2015; Vogelnest and Portas 2019), or if the platypus’s body temperature drops below 30°C during induction and maintenance of anaesthesia, platypuses are placed on a warm water (approximately 30°C) bottle beneath a towel to prevent hypothermia, while avoiding overheating. This method, which does not require an external power source, has been effective in sub-zero ambient temperatures, ensuring nominal body temperature and successful recovery (Hawke et al. 2021a). Alternatively, Macgregor (2015) used a thermostatically controlled heat pad for all anaesthetics, requiring an external power source, and a warm water bottle at a set temperature for platypuses in holding sacks during cold conditions.
During maintenance of anaesthesia, platypus heart rates can vary widely. Typical parameters of 180–220 beats/min (Booth and Connolly 2008) and 114–162 beats/min (Macgregor et al. 2014) have been reported. In our studies, heart rates ranged from 66 to 220 beats/min in adult males and 83 to 242 beats/min in adult females, with juveniles exhibiting somewhat higher ranges (females: 130–255 beats/min; males: 118–207 beats/min, Table 1). Respiratory rates during maintenance of stable anaesthesia have been reported to range from 20–50 breaths/min (Booth and Connolly 2008) to 6–24 breaths/min (Macgregor et al. 2014). However, sudden-onset apnoeic/bradycardic events (SOABEs) may occur (Macgregor et al. 2014). These events, characterised by bradycardia (<30 beats/min) and apnoea (>1 min without breaths), require immediate intervention.
| Sex | Age | Heart rate (min–max) | |
|---|---|---|---|
| Female | Adult (n = 102) | 166.8 ± 35.4 (83–242) | |
| Juvenile (n = 18) | 188.7 ± 32.0 (130–255) | ||
| Male | Adult (n = 131) | 151.6 ± 29.0 (66–220) | |
| Juvenile (n = 14) | 168.5 ± 30.8 (118–207) |
Minimising stress and maintaining body temperature are essential, as SOABEs have been linked to factors such as low temperatures, dorsal recumbency, and extended pre-anaesthetic holding times (Macgregor et al. 2014; Macgregor 2015). Isoflurane-induced irritation of nasal chemoreceptors can trigger apnoea and bradycardia through a nasopharyngeal response akin to a dive reflex (Macgregor et al. 2014), These physiological responses highlight the importance of careful anaesthetic management, particularly during induction and recovery. Platypuses have a high blood oxygen affinity, with over 90% saturation at a PO₂ of approximately 50 mmHg (Frappell 2003). Maintaining SpO2 concentrations above 90% ensures adequate oxygenation and tissue perfusion under anaesthesia.
Threshold values were set at SpO2 <90%, heart rate <30 beats/min, respiratory rate <1 breaths/min, and body temperature <28°C as emergency indicators for immediate intervention. If any of these thresholds are crossed, the procedure is halted, and corrective measures implemented. To improve oxygen saturation, isoflurane flow can be reduced or stopped while administering supplemental oxygen. Isoflurane-induced respiratory depression reduces breathing depth and rate, impairing oxygen uptake. Ceasing isoflurane flow allows the anaesthetic to metabolise and the respiratory drive to recover, although this process is not instantaneous. Supplemental oxygen remains critical during this transition. When oxygen saturation is compromised due to ventilation-perfusion mismatch, airway obstruction, or pre-existing pulmonary or cardiovascular issues, stopping isoflurane alone may be insufficient. Additional measures include clearing airway obstructions, optimising ventilator settings, or employing positive end-expiratory pressure to improve alveolar recruitment. A gentle hand motion along the sides of the platypus from back to front can help stimulate a deep breath. Continuous monitoring of SpO₂, respiratory rate, heart rate, and body temperature is essential to ensure effective stability and recovery throughout the procedure.
Under anaesthesia, platypuses can be safely weighed, measured, and assessed for sex and age on the basis of spur morphology without the risk of envenomation (Williams et al. 2012). Anaesthesia also enables the collection of samples and attachment of tracking devices with minimal stress and discomfort, including passive integrated transponder (PIT) tag insertion (Grant and Whittington 1991; Macgregor et al. 2015), toe-web biopsies (Gongora et al. 2012; Mijangos et al. 2022), and prey material collected from the cheek pouch (McLachlan-Troup et al. 2010; Hawke et al. 2022). Collection of ~2 mL of blood from the venous sinus in the dorsal bill, typically required for biochemical and hormonal analysis (Stewart et al. 2021), would be extremely challenging and painful to the animal without anaesthesia. Field surgery to implant transmitters (Bino et al. 2018) and the precise collection of biometric data necessitate anaesthesia (Grigg et al. 1992).
The platypuses are typically anaesthetised for approximately 30 min when only sample collection is involved, and approximately 40 min when radio transmitters are being externally attached. For some studies, acoustic transmitters have been surgically implanted into the abdominal cavity (Bino et al. 2018; Hawke et al. 2021b), where animals remain anaesthetised for approximately 1 h. Following the procedures, animals are monitored until regaining consciousness, then left to recover in a quiet, dark chamber for at least 30 min, ensuring they regain full consciousness before release. This process is designed to minimise stress and reduce the likelihood of adverse effects, with recovery times generally being swift.
Discussion
Field-based anaesthesia of platypuses is a well-established and refined procedure that prioritises animal welfare, while enabling the collection of essential scientific data. As reported here and by others (Macgregor et al. 2014; Macgregor 2015), the aim of this protocol – whether applied directly or through an accredited pathway – is to ensure that platypus anaesthesia is as safe and effective in the hands of a trained practitioner as it is in those of an experienced researcher, even in the absence of direct veterinary oversight. To achieve this, the protocol includes detailed guidelines for recognising and responding to physiological changes during anaesthesia, clear thresholds for intervention, and step-by-step instructions for monitoring and maintaining stability. This approach minimises reliance on subjective judgment and instead focuses on objective, reproducible measures, such as real-time monitoring of oxygen saturation, heart rate, respiratory rate, and body temperature. By prioritising safety and establishing standardised practices, the protocol supports non-experienced practitioners in achieving consistent outcomes while maintaining the highest animal welfare standards.
Over the past 9 years (2016–2024), we have successfully implemented this protocol during platypus surveys conducted under various conditions, including temperatures as low as −5°C. Previous applications of field-based anaesthesia by others (Macgregor et al. 2014; Macgregor 2015) have similarly successfully been implemented. Our findings highlight the feasibility and safety of conducting field-based anaesthesia in situations where veterinary supervision is impractical, provided that researchers receive comprehensive and appropriate training. Although veterinarians were integral to our initial training, the routine administration of anaesthesia on 514 animals was conducted without direct veterinary oversight.
To formalise and expand this approach across Australia, we advocate for the development of a structured accreditation pathway, enabling qualified ecologists to independently perform anaesthesia on platypuses in field settings. Accreditation pathways would address the lack of consistent training opportunities, ensuring that practitioners have the necessary skills and knowledge to safely and effectively administer anaesthesia. By incorporating both foundational veterinary instruction and practical field training under experienced mentors, this model would create a robust and reliable framework for training ecologists and researchers.
Standardising training and accreditation require the establishment of an authoritative overseeing body to ensure consistency and competency in administering field-based anaesthesia. This body could operate under the guidance of existing organisations, such as the Australian Veterinary Association (AVA, https://www.ava.com.au/) or the Australian and New Zealand College of Veterinary Scientists (ANZCVS, https://anzcvs.org.au/), while integrating input from ecologists, wildlife researchers, and ethical review panels. Veterinary input would be critical in the foundational stages of training to provide participants with a robust understanding of anaesthetic principles, whereas experienced researchers would deliver practical, field-based mentorship. By formalising this dual-training model, the overseeing body would ensure that training programs meet high standards of animal welfare and procedural safety, addressing the logistical and financial constraints of field research.
While there is a trend in clinical veterinary work towards the use of veterinary technicians with undergraduate degrees, we propose a more targeted, competency-based training model for wildlife researchers. This approach focuses on developing specific skills relevant to platypus anaesthesia, minimising the time and financial barriers associated with broader veterinary training programs. Accreditation could require researchers to complete at least 20 supervised anaesthetic procedures under the mentorship of wildlife veterinarians or experienced researchers, ensuring that trainees meet rigorous standards before being qualified to work independently. It is anticipated that establishing such accreditation pathways would streamline Animal Ethics Committee (AEC) approvals by providing clear and consistent criteria for non-veterinarian qualifications. Although the ultimate decision rests with AECs, a formalised pathway offers a transparent framework for assessing competency, thereby enhancing the accessibility and scalability of field-based research.
The advantages of this accreditation model extend beyond platypus research. Remote or cryptic species, including small mammals, amphibians, and aquatic taxa, often encounter similar logistical challenges in field research. Although programs such as Safe Capture International (https://sdzwaacademy.org/safecapture/) already offer training for a variety of species, our proposed pathway aims to address gaps for taxa with unique physiological or ecological requirements that may not be fully covered by existing models. By enabling trained ecologists to safely and effectively perform anaesthesia, this framework could complement existing programs and promote the broader adoption of standardised practices in wildlife research.
We do not advocate for the complete removal of veterinary involvement but rather for reducing reliance on continuous veterinary oversight during fieldwork. Veterinary expertise remains crucial in the design and delivery of initial training programs, providing the foundational knowledge and ethical grounding necessary for safe anaesthetic practices. However, the practical realities of field-based research often render on-site veterinary supervision unfeasible. By formalising a dual-training model that incorporates veterinary input alongside mentorship from experienced researchers, we aim to uphold high standards of animal welfare and procedural safety while addressing logistical constraints.
This approach aligns with global trends in research ethics, emphasising the need for standardisation, competency-based training, and a steadfast commitment to animal welfare. Accreditation pathways not only ensure that researchers are technically proficient but also instil an understanding of ethical considerations, such as minimising stress, avoiding unnecessary interventions, and prioritising the well-being of the animal. These measures are vital for maintaining the trust of both the scientific community and the public in wildlife research.
In conclusion, field-based anaesthesia of platypuses is a proven method for minimising stress during complex procedures and enabling the collection of high-quality data critical to conservation efforts. A structured accreditation pathway would render this tool more accessible, consistent, and scalable, benefiting not only platypus research but also wildlife research more broadly. By empowering trained ecologists to perform anaesthesia independently, we can advance both animal welfare and conservation science, addressing a critical need in managing and protecting biodiversity amid ongoing environmental challenges.
Data availability
The data that support this study will be shared upon request to the corresponding author.
Acknowledgements
The authors extend their gratitude to the reviewer for their constructive feedback, which significantly improved the clarity and quality of this paper.
References
Bino, G., Kingsford, R. T., Grant, T., Taylor, M. D., and Vogelnest, L. (2018). Use of implanted acoustic tags to assess platypus movement behaviour across spatial and temporal scales. Scientific Reports 8(1), 5117.
| Crossref | Google Scholar | PubMed |
Chinnadurai, S. K., Strahl-Heldreth, D., Fiorello, C. V., and Harms, C. A. (2016). Best-practice guidelines for field-based surgery and anesthesia of free-ranging wildlife. I. Anesthesia and analgesia. Journal of Wildlife Diseases 52(2s), S14-S27.
| Crossref | Google Scholar | PubMed |
Deem, S. L., Karesh, W. B., and Weisman, W. (2001). Putting theory into practice: wildlife health in conservation. Conservation Biology 15(5), 1224-1233.
| Crossref | Google Scholar |
Fahlman, Å. (2008) Advances in wildlife immobilisation and anaesthesia. Doctoral dissertation, Swedish University of Agricultural Sciences, Uppsala, Sweden. Available at http://www.rhinoresourcecenter.com/pdf_files/124/1245946418.pdf
Fiorello, C. V., Harms, C. A., Chinnadurai, S. K., and Strahl-Heldreth, D. (2016). Best-practice guidelines for field-based surgery and anaesthesia of free-ranging wildlife. II. Surgery. Journal of Wildlife Diseases 52(2s), S28-S39.
| Crossref | Google Scholar | PubMed |
Frappell, P. B. (2003). Ventilation and metabolic rate in the platypus: insights into the evolution of the mammalian breathing pattern. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 136(4), 943-955.
| Crossref | Google Scholar |
Gongora, J., Swan, A. B., Chong, A. Y., Ho, S. Y., Damayanti, C. S., Kolomyjec, S., Grant, T., Miller, E., Blair, D., Furlan, E., and Gust, N. (2012). Genetic structure and phylogeography of platypuses revealed by mitochondrial DNA. Journal of Zoology 286(2), 110-119.
| Crossref | Google Scholar |
Grant, T. R., and Whittington, R. J. (1991). The use of freeze-branding and implanted transponder tags as a permanent marking method for platypuses, Ornithorhynchus anatinus (Monotremata: Ornithorhynchidae). Australian Mammalogy 14, 147-150.
| Crossref | Google Scholar |
Grigg, E. B., and Roesler, A. (2018). Anesthesia medication handling needs a new vision. Anesthesia & Analgesia 126(1), 346-350.
| Crossref | Google Scholar | PubMed |
Grigg, G., Beard, L., Grant, T., and Augee, M. (1992). Body-temperature and diurnal activity patterns in the Platypus (Ornithorhynchus-Anatinus) during winter. Australian Journal of Zoology 40(2), 135-142.
| Crossref | Google Scholar |
Hawke, T., Bino, G., and Kingsford, R. T. (2021a). Damming insights: variable impacts and implications of river regulation on platypus populations. Aquatic Conservation: Marine and Freshwater Ecosystems 31, 504-519.
| Crossref | Google Scholar |
Hawke, T., Bino, G., Kingsford, R. T., Iervasi, D., Iervasi, K., and Taylor, M. D. (2021b). Long-term movements and activity patterns of platypus on regulated rivers. Scientific Reports 11(1), 3590.
| Crossref | Google Scholar |
Hawke, T., Bino, G., Shackleton, M. E., Ross, A. K., and Kingsford, R. T. (2022). Using DNA metabarcoding as a novel approach for analysis of platypus diet. Scientific Reports 12(1), 2247.
| Crossref | Google Scholar | PubMed |
Junge, R. E., and Louis, E. E. (2005). Preliminary biomedical evaluation of wild ruffed lemurs (Varecia variegata and V. rubra). American Journal of Primatology 66(1), 85-94.
| Crossref | Google Scholar | PubMed |
Karesh, W. B., and Cook, R. A. (1995). Applications of veterinary medicine to in situ conservation efforts. Oryx 29(4), 244-252.
| Crossref | Google Scholar |
Macgregor, J. W., Holyoake, C., Fleming, P., Robertson, I. D., Connolly, J., and Warren, K. S. (2014). Investigation into the characteristics, triggers and mechanism of apnoea and bradycardia in the anaesthetized platypus (Ornithorhynchus anatinus). Conservation Physiology 2(1), cou053.
| Crossref | Google Scholar | PubMed |
Macgregor, J., Holyoake, C., Munks, S., Connolly, J. H., Robertson, I. D., Fleming, P. A., and Warren, K. (2015). Novel use of in-stream microchip readers to monitor wild platypuses. Pacific Conservation Biology 20(4), 376-384.
| Crossref | Google Scholar |
McLachlan-Troup, T. A., Dickman, C. R., and Grant, T. R. (2010). Diet and dietary selectivity of the platypus in relation to season, sex and macroinvertebrate assemblages. Journal of Zoology 280(3), 237-246.
| Crossref | Google Scholar |
Mijangos, J. L., Bino, G., Hawke, T., Kolomyjec, S. H., Kingsford, R. T., Sidhu, H., Grant, T., Day, J., Dias, K. N., and Gongora, J. (2022). Fragmentation by major dams and implications for the future viability of platypus populations. Communications Biology 5(1), 1127.
| Crossref | Google Scholar | PubMed |
Soulsbury, C., Gray, H., Smith, L., Braithwaite, V., Cotter, S., Elwood, R. W., Wilkinson, A., and Collins, L. M. (2020). The welfare and ethics of research involving wild animals: a primer. Methods in Ecology and Evolution 11(10), 1164-1181.
| Crossref | Google Scholar |
Stewart, J., Bino, G., Hawke, T., and Kingsford, R. T. (2021). Seasonal and geographic variation in packed cell volume and selected serum chemistry of platypuses. Scientific Reports 11(1), 15932.
| Crossref | Google Scholar |
Williams, G., Serena, M., and Grant, T. (2012). Age-related change in spurs and spur sheaths of the platypus (Ornithorhynchus anatinus). Australian Mammalogy 35(1), 107-114.
| Crossref | Google Scholar |