The mechanical properties of bettong and potoroo foods
D. Rex Mitchell A B * , Justin A. Ledogar
A B * , Justin A. Ledogar  C , Damien Andrew
C , Damien Andrew  D , Ian Mathewson E , Vera Weisbecker
D , Ian Mathewson E , Vera Weisbecker  A B and Karl Vernes
A B and Karl Vernes  F
F
A
B
C
D
E
F
Abstract
Potoroid marsupials (bettongs and potoroos of the family Potoroidae) are considered ecosystem engineers because of the roles they play in maintaining biodiversity. However, severe declines since European arrival have necessitated intense conservation efforts. Vital to these efforts is an understanding of the physical challenges that define their niches. The mechanical properties of their foods, such as toughness and stiffness, represent a physical interface with the environment that can contribute to quantitatively defining their niches. Here, we provide mechanical property data from wild bettong and potoroo foods, such as roots and tubers, fruit, fungi, invertebrates, seeds, and leaves. Toughness ranged from approximately 56.58 J/m2 (fungal sporocarp of Descolea sp.) to 2568.15 J/m2 (tubers of the blue yam, Brunoniella australis). Similarly, stiffness of the wild foods ranged from 1.15 MPa for Descolea sp. to 30.4 MPa for B. australis. However, the mechanical demands of accessing the kernels from within the shells (testae) of sandalwood and quandong (Santalum spp.) seeds far exceed measurements of any foods tested. We also tested some farmed foods, alongside inclusion of data from previous studies. Taken together, these data can also improve selection of comparable foods in designing diets for potoroids, and other species, in captivity.
Keywords: captive management, conservation, diet, elastic modulus, fracture toughness, habitat use, mastication, Potoroidae.
Introduction
Bettongs and potoroos are considered ecosystem engineers (Jones et al. 1994; Neilly and Schwarzkopf 2018; Davies et al. 2019; Decker et al. 2019; Ross et al. 2020) because their habitual digging and burrowing behaviours used in foraging (Garkaklis et al. 2004; Vernes and Jarman 2014), seed caching (Murphy et al. 2005; Chapman 2015), and refuge (Sander et al. 1997) are obvious examples of autogenic environmental change (Jones et al. 1994). These disturbances lead to bioturbation and aeration of soil, creating litter traps and facilitating incorporation of nutrients. This can in turn alter vegetation compositions and nutrient cycling (Neilly and Schwarzkopf 2018; Ross et al. 2019, 2020). Furthermore, potoroids are known dispersers of seeds and fungal spores through their specialised diets (Claridge et al. 1992; Vernes et al. 2002; Eldridge and James 2009; Palmer et al. 2021). Yet potoroid distribution and diversity has been greatly affected by European settlement (Short 1998; Westerman et al. 2004), with many species now extinct and others suffering niche contractions (Short 1998). Revitalising populations and distributions of remaining species is an essential consideration for maintaining biodiversity and ecosystem health in Australia. Resolution of this goal has been approached through translocations (Christensen and Burrows 1994; Short and Turner 2000; Priddel and Wheeler 2004) and the establishment of reserves (Bice and Moseby 2008).
Important to these conservation efforts is a clearer understanding of the niche parameters of potoroids because an awareness of abiotic and biotic limitations helps to inform decisions of locality and habitat choice for future populations. Different species of bettongs and potoroos have evolved from various environments with historic distributions across much of Australia; and from contrasting environments come contrasting resources. The mechanical properties of food are quantifiable metrics that can delineate fundamental niche limits. Studies involving potoroid feeding ecology mention contrasts in food hardness or resistance between species (e.g. Mitchell et al. 2018); however, these assumptions remain unqualified in the literature. Most research into the properties of different foods has been limited to primate diets (Agrawal et al. 1997; Williams et al. 2005; Coiner-Collier et al. 2016; Laird et al. 2020), or plant materials usually in the context of browsing and grazing herbivores (Sanson et al. 2001; Read and Sanson 2003; Caldwell et al. 2016). Here, we provide food mechanical property data for food groups known to be dominant components of potoroid diets.
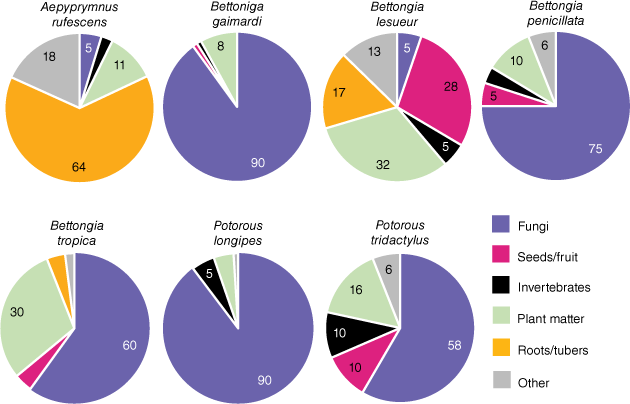
Potoroids are known specialists of nutrient-rich, highly digestible foods. The diets of the seven extant species of potoroids include high proportions of such disparate food groups as hypogeous fungal sporocarps (i.e. truffles), fibrous roots, tubers, bulbs, the stems and leaves of plants and grasses, fruits, flowers, and insects (Seebeck et al. 1989). Fig. 1 illustrates dietary variation across extant species of potoroos and bettongs. Some species, such as members of the genus Potorous, have diets dominated by truffles, while the rufous bettong (Aepyprymnus rufescens) has a diet dominated by fibrous roots and tubers. We predicted these food groups would be discrete in toughness and stiffness. However, of additional interest was the particularly durable shells (testae) of Santalum spp., which some bettongs are known to crack open to extract the nutritious kernels within (McNamara 2014; Chapman 2015). We expected these shells to represent a maximum extreme of biting capacity that these species would encounter, so the testae of these seeds were also tested.
Proportions of different food groups in the diets of all extant species of the Potoroidae. Proportions are averaged across seasons and publications when applicable (Guiler 1971; Bennett and Baxter 1989; Taylor 1992; Claridge et al. 1993; Green et al. 1999; McIlwee and Johnson 2002; Vernes et al. 2002; Bice and Moseby 2008; Robley et al. 2008; Zosky et al. 2017). Note: grass material identified in McIlwee and Johnson (2002) as the dominant component of the Aepyprymnus rufescens diet has been confirmed to be tuber and stem-base material (Christopher Johnson, pers. comm.). Numbers indicate rounded proportions greater than 5%.
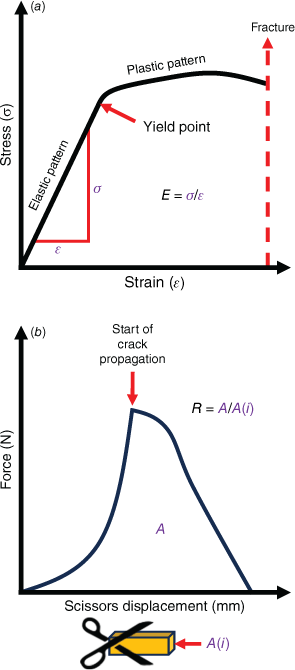
Foods consumed by animals in the wild are often described in terms of their puncture and crushing resistance, which have been collectively described as mechanical properties (Lambert et al. 2004; Wieczkowski 2009). Although informative, these terms do not fully describe the mechanical properties of food breakdown in the oral cavity. Measures that more meaningfully describe a food’s resistance to fragmentation include the Young’s modulus (E) – a measure of material stiffness or resistance to crack formation – and fracture toughness R – a measure of resistance to fracture propagation (Lucas 2004). When a force is applied to an object, some force is dispersed throughout the structure of the object. This force per unit of area is stress (σ), measured in newton per metre square (N/m2). When stress within the object reaches a magnitude greater than a critical yield point, it can cause deformation of the object’s structure (Fig. 2a). This deformation, calculated by dividing the change in length of the material by its initial length, is called strain (ε). Young’s modulus (E), calculated by dividing stress by strain, offers an estimation of how much force is required to instigate a degree of deformation in the material (Lucas et al. 2001; Lucas 2004). The greater the value of E, the stiffer the material, and the more force is needed to deform it. Fracture toughness (R) represents the energy required to propagate a crack in a material of a given area and is represented by joules per metre squared (J/m2) (Fig. 2b). These data obtained from potoroid food groups will not only benefit conservation initiatives of these important species through better understanding their physical limitations in their respective environments, but will also help identification of suitable dietary substitutes in captivity (Williams et al. 2005).
(a) During the application of forces to a material, stress and strain increase linearly in an elastic pattern until reaching a critical yield point. Stress then exhibits a plastic pattern, being unable to return to its original form, until reaching a final point of fracture. Young’s modulus (E) is calculated by dividing the stress (σ) by the strain (ε) at the initial, linear portion of the curve. (b) When commencing a cut through a piece of material with a cross-section of A(i), the force increases with the initial resistance until the point of crack propagation, beyond which force steadily decreases until the cut is complete. Toughness (R) is calculated by dividing the area (A) beneath the curve by A(i).
Methods
Field collections and the majority of mechanical property sampling took place in August 2023 in the New England bioregion (Table 1). Targeted species of introduced and native plants with roots and tubers consumed by A. rufescens (Schlager 1982) were carefully uprooted. Plant samples were identified using reproductive or vegetative morphological characteristics and compared to vouchers lodged in the N. C. W. Beadle herbarium. Six species of subterranean fungi were raked from the topsoil at the base of eucalypts (Eucalyptus spp.) and identified to either genus or species based on morphological characters of sporocarps, microscopic examination of spores, and comparisons with collections made during previous studies in this region (Danks et al. 2010, 2013; Nest et al. 2023). Samples of browse and fruit were also collected from the Simpson Desert and brought to Armidale at time of sampling. Sandalwood seeds and quandong seeds were bought online as dry samples but fresh quandongs were also later collected locally from South Australia to compare the properties of the two food conditions. Fresh samples were stored in zip-lock bags and refrigerated. Measurement of mechanical properties took place within 24 h, with the exception of fresh quandongs, which we obtained on a later date. These were flown to J. A. L’s lab in Tennessee and tested ~1 week after their collection. These were tested by the same researcher (J. A. L.) on the same equipment, but on a date following the sampling period for the other foods. Fungi and plant material in the New England bioregion were collected under Department of Planning and Environment Scientific Licence SL102681.
| Species | Latitude | Longitude | Locality | Food type | |
|---|---|---|---|---|---|
| Arthropodium milleflorum | −31.084 | 150.950 | Oxley Scenic Lookout, Tamworth, NSW | Roots/tubers | |
| Hypochaeris radicata | −30.417 | 151.632 | Newholme Field Station, Armidale, NSW | Roots/tubers | |
| Brunoniella australis | −31.084 | 150.950 | Oxley Scenic Lookout, Tamworth, NSW | Roots/tubers | |
| Hydrocotyle laxiflora | −30.418 | 151.637 | Newholme Field Station, Armidale, NSW | Roots/tubers | |
| Taraxacum officinale | −30.480 | 151.646 | University of New England, Armidale, NSW | Roots/tubers | |
| Hysterangium sp. | −30.483 | 151.640 | University of New England, Armidale, NSW | Fungi | |
| Scleroderma verrucosum | −30.483 | 151.640 | University of New England, Armidale, NSW | Fungi | |
| Cortinarius (‘Thaxterogaster’) sp. | −30.483 | 151.640 | University of New England, Armidale, NSW | Fungi | |
| Labyrinthomyces varius | −30.417 | 151.636 | Newholme Field Station, Armidale, NSW | Fungi | |
| Descolea sp. 1 | −30.417 | 151.637 | Newholme Field Station, Armidale, NSW | Fungi | |
| Descolea sp. 2 | −30.417 | 151.637 | Newholme Field Station, Armidale, NSW | Fungi | |
| Enchylaena tomentosa | −25.846 | 139.031 | Simpson Desert, Birdsville, Qld | Fruit | |
| Sclerolaena intricata | −25.846 | 140.031 | Simpson Desert, west of Birdsville, Qld | Fruit | |
| Salsola australis | −25.904 | 139.351 | Simpson Desert, Birdsville, Qld | Browse | |
| Maireana coronata | −25.904 | 140.351 | Simpson Desert, west of Birdsville, Qld | Browse | |
| Aristida holathera | −25.846 | 140.031 | Simpson Desert, west of Birdsville, Qld | Nuts/seeds | |
| Santalum spicatum | N/A | N/A | – | Nuts/seeds | |
| S. acuminatum dry | N/A | N/A | – | Nuts/seeds | |
| S. acuminatum fresh | −34.893 | 137.462 | Hardwicke Bay, SA | Nuts/seeds | |
| Panesthia cribrata | −30.417 | 151.632 | Newholme Field Station, Armidale, NSW | Invertebrates | |
| Acrossidius tasmaniae larvae | −30.417 | 151.632 | Newholme Field Station, Armidale, NSW | Invertebrates |
We also included data from farmed foods, sampled both by ourselves and by others for previous research, as a means of comparison. This information was included as familiar reference points to assist understanding of the dietary delimitations inherent in some potoroid diets, and to identify foods of similar properties for formulating diets of captive animals.
Some measurements were taken to compare the physical dimensions of wild food types. This included the maximum diameter of roots/tubers, and maximum diameters of fungi, fruits, and whole browse, as the most likely maximum axes bitten across by potoroids.
Mechanical tests used to quantify the E and R of foods were performed using a Lucas Scientific FLS-2 portable tester, an updated version of the FLS-1 tester (Darvell et al. 1996; Lucas et al. 2001), fitted with 50, 100, and 1000 N load cells. The tester is similar to Instron materials testing machines used in engineering and materials science laboratories, and has been used to collect the mechanical properties of mammalian foods (mainly primate) in the wild for over 20 years (Wright 2005; McGraw et al. 2016; Talebi et al. 2016; van Casteren et al. 2016, 2019; Paine et al. 2018; Chalk‐Wilayto et al. 2022; Laird et al. 2020, 2022). The tester consists of three central components: (1) a stainless-steel test stand that houses interchangeable load cells, (2) a data integration box which records compressive and tensile forces in real time, as well as the displacement of the tester’s crosshead, and (3) a computer with software that reads the data output from the integration box. Interchangeable parts allow researchers to perform a wide variety of mechanical tests (Lucas et al. 2001).
Food stiffness was quantified using a compression test. Our study limited stiffness tests to foods that could be cut into either a cube or cylindrical shape for the purposes of crushing. Food samples were crushed between the testing platen and compression jig, and Young’s modulus (E) was estimated by taking the slope of the force–displacement curve within its elastic range (i.e. before permanent shape change). The toughness of food tissues was quantified using a scissors-cutting test (Lucas 2004). Most samples were cut into thin matchstick-shaped cuboids or, in the case of leaves and insect cuticle, thin rectangular sheets. Values for fracture toughness (R) were estimated by quantifying the area under a force–displacement curve divided by crack area (Lucas et al. 2001; Lucas 2004). To account for anisotropic variation, at least two measurements of E and R per specimen were taken and averaged when possible. Additionally, we calculated the stress- and displacement-limited fragmentation indices (E.R0.5 and R/E0.5) when both metrics were quantified, as these measures best describe food fragmentation in the oral cavity (Lucas et al. 2001). These indices are effectively properties in themselves (Agrawal et al. 1997). Lastly, whole Santalum spp. (sandalwood and quandong) seeds were loaded until failure using the compression test in order to estimate the maximum force in newtons required to initiate a crack in the outer testae.
Results
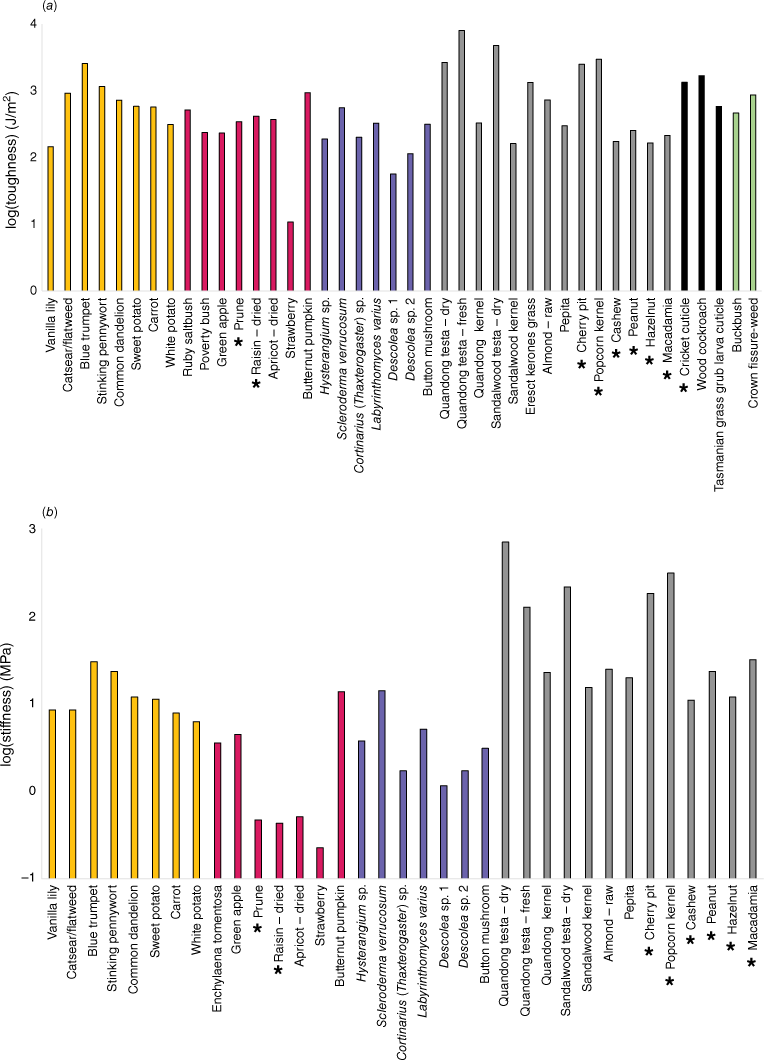
All samples were collected from a maximum soil depth of approximately 15 cm. The deepest materials were tuberous roots of the vanilla lily (Arthropodium milleflorum). The raw data are presented in Table 2. Some samples, such as pumpkin, which had much higher toughness and stiffness than other fruits, make analysis of food group averages problematic. However, in general, toughness values for edible materials were higher in seeds, roots/tubers, browse, and insect cuticle. Toughness values generally were lower in fruit, fungi, and some nuts. Similar results were found for stiffness; however, some materials, such as insect cuticle and browse, could not be tested for Young’s modulus (E) with the equipment used.
| Species | Common name | (R) | (E) | Max ⌀ (mm) | Group | |
|---|---|---|---|---|---|---|
| Arthropodium milleflorum | Vanilla lily | 145.85 | 8.66 | 15.4 | Roots/tubers | |
| Hypochaeris radicata | Catsear/flatweed | 925.55 | 8.52 | 6.2 | Roots/tubers | |
| Brunoniella australis | Blue trumpet | 2568.15 | 30.40 | 6.2 | Roots/tubers | |
| Hydrocotyle laxiflora | Stinking pennywort | 1166.55 | 23.89 | 2.3 | Roots/tubers | |
| Taraxacum officinale | Common dandelion | 727.10 | 12.00 | 8.5 | Roots/tubers | |
| Ipomoea batatas | Sweet potato | 586.80 | 11.40 | Roots/tubers | ||
| Daucus carota | Carrot | 572.70 | 7.83 | Roots/tubers | ||
| Solanum tuberosum | White potato | 313.30 | 6.34 | Roots/tubers | ||
| Enchylaena tomentosa | Ruby saltbush | 518.75 | 3.54 | 6.1 | Fruits | |
| Sclerolaena intricata | Poverty bush | 237.75 | NA | 1.8 | Fruits | |
| Malus domestica | Green apple | 233.10 | 4.56 | Fruits | ||
| Prunus armeniaca | Apricot – dried | 371.80 | 0.51 | Fruits | ||
| Fragaria ananassa | Strawberry | 10.80 | 0.23 | Fruits | ||
| Cucurbita moschata | Butternut pumpkin | 939.10 | 14.14 | Fruits | ||
| Hysterangium sp. | Native truffle | 189.80 | 3.76 | 18.1 | Fungi | |
| Scleroderma verrucosum | Earthball fungus | 557.15 | 14.18 | 15.9 | Fungi | |
| Cortinarius (‘Thaxterogaster’) sp. | Native fungus | 201.30 | 1.70 | 39.7 | Fungi | |
| Labyrinthomyces varius | Native truffle | 327.20 | 5.15 | 16.8 | Fungi | |
| Descolea sp. 1 | Native truffle | 56.575 | 1.15 | 14.6 | Fungi | |
| Descolea sp. 2 | Native truffle | 114.5 | 1.74 | 11.4 | Fungi | |
| Agaricus bisporus | Button mushroom | 316.1 | 3.15 | Fungi | ||
| Santalum acuminatum | Dry quandong testa | 2675.5 | 734.00 | 19.4 | Nuts/seeds | |
| Santalum acuminatum | Fresh quandong testa | 8050.2 | 130.67 | Nuts/seeds | ||
| Santalum acuminatum | Quandong kernel | 329.5 | 23.11 | Nuts/seeds | ||
| Santalum spicatum | Dry sandalwood testa | 4779.3 | 222.33 | 21.5 | Nuts/seeds | |
| Santalum spicatum | Sandalwood kernel | 162.15 | 15.63 | Nuts/seeds | ||
| Aristida holathera | Erect kerosene grass | 1342.10 | NA | 1.1 | Nuts/seeds | |
| Prunus amygdalus | Almond – raw | 733.6 | 25.19 | Nuts/seeds | ||
| Cucurbita pepo | Pepita | 299.4 | 20.39 | Nuts/seeds | ||
| Panesthia cribrata | Wood cockroach | 1693.23 | N/A | Invertebrates | ||
| Acrossidius tasmaniae larva | Tasmanian grass grub | 585.2 | N/A | Invertebrates | ||
| Salsola australis | Buckbush | 466.38 | N/A | <4.3 | Browse | |
| Maireana coronata | Crown fissure-weed | 868.28 | N/A | 2.1 | Browse |
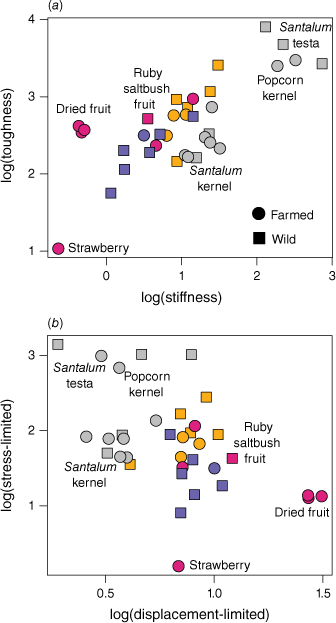
The data are presented graphically in Fig. 3, alongside additional data obtained from other studies of farmed foods. The lowest values of both toughness and stiffness belong to farmed fresh strawberries followed by wild fungal sporocarps of Descolea sp. The toughest and stiffest edible material belonged to the tuberous roots of the blue yam (Brunoniella australis). However, this species also had smaller tuber diameters as some other species tested (Table 2), which may reduce the challenge of processing them. The upper extremes of both metrics belong to the particularly resistant, inedible testae of sandalwood and quandong seeds (Santalum spp.). Interestingly, while we found the testa of quandong seeds (Santalum acuminatum) had the highest values for both toughness and stiffness, the testa of a dry quandong seed was lower in toughness and higher in stiffness than that of a fresh quandong seed, indicating a shift in relative properties with drying. Fig. 4a shows a plot of toughness–stiffness, with both values log-transformed. The pattern shows a general correlation between the two metrics, but there are some deviations. For example, nuts and seeds tend to be higher in stiffness and lower in toughness, while dried fruits and some tubers show the reverse pattern.
Food material property data: (a) fracture toughness, (b) material stiffness (Young’s modulus). *Additional data obtained from Agrawal et al. (1997) or Williams et al. (2005). Orange = roots/tubers, red = fruit, blue = fungi, grey = nuts/seeds, black = invertebrates, green = browse.
Plots of foods with measurements of both toughness and stiffness: (a) logarithmic conversions of toughness–stiffness, (b) fragmentation indices – stress-limited (R.E0.5) ~ displacement-limited (R/E0.5). Orange = roots/tubers, red = fruit, blue = fungi, grey = nuts/seeds, black = invertebrates, green = browse.
We additionally tested the ability of the FLS-2 tester to crush whole Santalum spp. seeds. The first sandalwood seed (Santalum spicatum) had a diameter of 20.4 mm and cracked open with 315 N of force. The second, with a diameter of 21.5 mm, was stopped due to slippage at 255 N. However, turning the seed 90° caused this seed to crack open on second attempt at 125 N. An attempt to crack open a dried quandong seed (Santalum acuminatum) nearly exceeded the limitations of a 1000 N load cell before being aborted. This suggests that >100 kg of direct force is required to crack open a quandong seed and indicates quandong testae are not only tougher and stiffer than all other materials tested, but the structure (thickness) of the testae offers additional resistance to cracking.
The fragmentation indices (Fig. 4b) identify a pattern of inverse correlation from high stress-limited foods to high displacement-limited foods. This roughly corresponds to a continuum of brittle foods to chewy foods, from stiff nuts and seeds to highly malleable dried fruits. The only wild fruit sample, from the ruby saltbush (Enchylaena tomentosa), represents the highest displacement index excluding farmed dried fruits. In addition, the only commercial foods with properties nearing the stress-limited index of Santalum testae were the popcorn kernel and cherry pit. With exception to the extremes of toughness, stiffness, and softness, there tends to be a high degree of overlap between farmed and wild samples from each food group.
Discussion
We have provided a collection of food mechanical property data from a range of food groups consumed by bettongs and potoroos. Our results are validated through similar findings for the same farmed foods measured in previous studies (Agrawal et al. 1997; Williams et al. 2005) and they detail a continuum of food resistance from softer fungi and fruit to more resistant roots and tubers, to the most resistant insect cuticle, browse, nuts, and seeds. These results can help identify some of the challenges species face in selecting food items in the wild, across both native and novel habitats. They can also help explain some of the dietary restructuring observed in potoroids with the arrival of alien resources. For example, the heavy consumption of the introduced flatweed, Hypochaeris radicata, by the rufous bettong (Schlager 1982) is aligned with this species’ dietary habits, because the mechanical properties of flatweed roots do not exceed the values of the roots from native species in its diet, such as Brunoniella australis (Schlager 1982). While there may certainly be chemical and nutritional concerns if potoroids feed on introduced species, having less challenging mechanical properties than typical foods likely eliminates any initial mechanical barrier to the consumption of novel resources. Despite a focus on potoroid diets, many of these food materials are also obviously consumed by other small mammals. Our results are therefore relevant in conservation and management considerations for other species as well.
The new knowledge of food mechanical properties we have presented alongside comparable farmed foods can be informative for developing captive diet regimes and feed formulations. Food mechanical properties can influence skull structure at both developmental and evolutionary scales in mammals (Weisbecker et al. 2019; Mitchell et al. 2020, 2021), such that softer, structurally weaker foods in captive diets might not lead to adequate skull development needed for tasks expected to be undertaken in the wild. This might affect the ability of captive-reared animals to establish and persist during rapid environmental change, such as brought about by human disturbance and translocations. Appropriate substitutions in captive diets should not only seek to emulate nutritional content of wild diets, but also mechanical properties (Hartstone-Rose et al. 2014). For example, our results show that the fruits of wild ruby saltbush are highest of all wild foods on the displacement-limited index. These properties might be best substituted with dried or semi-dried fruits to match this condition. However, some materials regularly interacted with, such as some native seeds with especially resistant casings, may have no obvious substitutes.
An important observation of some potoroid species, such as the woylie (Bettongia penicillata) and burrowing bettong (Bettongia lesueur), is their ability to crack open the exceedingly resistant shells of sandalwood and quandong seeds to access the kernel inside (Murphy et al. 2005, 2015; McNamara 2014; Chapman 2015). The toughness and stiffness of Santalum spp. testae are higher than the highest values found for other foods in previous studies (e.g. cherry pit; Williams et al. 2005) and far exceeded any edible materials measured in this study. An attempt to crack open a quandong seed with our tester failed upon approaching the maximum 1000 N limits of the machinery, suggesting at least ~100 kg of direct force in one place is needed to manage the task. The fact that woylies can regularly crack these open, alongside other examples of the hardest nuts known, including macadamias (Macadamia tetraphylla) and Brazil nuts (Bertholletia excelsa) (McNamara 2014), with skulls typically measuring less than 90 mm long (McDowell et al. 2015) is impressive. This is compounded by the fact that the mechanical properties are determined for the material itself, having been standardised for thickness. All else being equal, two foods might differ in accessibility based on thickness alone (Sanson et al. 2001), and quandongs have especially thick testae, measuring 2.2–2.4 mm across (Pardoe et al. 2019). In fact, the properties of quandong shells might even prevent germination of their own contents (Loveys and Jusaitis 1994), often only made possible with intervention either from cracking or passage through the digestive system of megafauna and resultant softening of the testa (Pardoe et al. 2019). Our results identify a potential behavioural attribute that might assist bettongs with the task of cracking open Santalum seeds. We found that a fresh quandong testa was extremely tough (8050.2 J/m2) but less stiff (130.67 MPa) compared to a dried testa (2675.5 J/m2, 734 MPa), which suggests that the regular, yet seemingly haphazard caching (‘scatter-hoarding’) of these seeds (Murphy et al. 2005; Chapman 2015), might serve to dry out the testae in order to crack them open more easily. Rotating a sandalwood seed 90° seemed to weaken the testa in our cracking tests as well, suggesting that multiple bites from different angles could incrementally weaken its structure.
Potoroids typically use their large sectorial premolars to cut through most foods and crack open hard nut casings (Schlager 1982; Sanson 1989; McNamara 2014). For cracking open nuts and seeds, bettongs tend to repeatedly bite hard with their premolars onto the testa, while manipulating its position in the oral cavity with their forelimbs (McNamara 2014). Considerable effort is reportedly applied to cracking the hardest nuts, however, the exact way this is ultimately achieved is not clear. The biomechanics of cracking open exceedingly resistant Santalum seeds by bettongs warrant more extensive study.
Declaration of funding
D. R. M. and V. W. were supported by the Australian Research Council Centre of Excellence for Australian Biodiversity and Heritage grant (CE170100015). J. A. L. was funded by Leakey Foundation grant (40463).
Acknowledgements
We thank Sofie Costin for access to fresh quandongs and Jen Silcock from the Queensland Herbarium for collecting and identifying plant material from the Simpson Desert. We also thank Andrew Harper and Max Tischler from Australian Desert Expeditions who organised and led the camel trek which allowed us to gather material from the Simpson Desert. This work was conducted on the unceded traditional lands of the Kaurna (Adelaide) and Anaiwan and Kamilaroi (New England Bioregion) people and the Wangkangurru and Yarluyandi people (Simpson Desert).
References
Agrawal, K. R., Lucas, P. W., Prinz, J. F., and Bruce, I. C. (1997). Mechanical properties of foods responsible for resisting food breakdown in the human mouth. Archives of Oral Biology 42(1), 1-9.
| Crossref | Google Scholar | PubMed |
Bennett, A. F., and Baxter, B. J. (1989). Diet of the long-nosed potoroo, Potorous tridactylus (Marsupialia: Potoroidae), in South-western Victoria. Australian Wildlife Research 16, 263-271.
| Crossref | Google Scholar |
Bice, J., and Moseby, K. (2008). Diets of the re-introduced greater bilby (Macrotis lagotis) and burrowing bettong (Bettongia lesueur) in the Arid Recovery Reserve, Northern South Australia. Australian Mammalogy 30(1), 1-12.
| Crossref | Google Scholar |
Caldwell, E., Read, J., and Sanson, G. D. (2016). Which leaf mechanical traits correlate with insect herbivory among feeding guilds? Annals of Botany 117(2), 349-361.
| Crossref | Google Scholar | PubMed |
Chalk‐Wilayto, J., Fogaça, M. D., Wright, B. W., van Casteren, A., Fragaszy, D. M., Izar, P., Visalberghi, E., Strait, D. S., Ross, C. F., and Wright, K. A (2022). Effects of food material properties and embedded status on food processing efficiency in bearded capuchins. American Journal of Biological Anthropology 178(4), 617-635.
| Crossref | Google Scholar |
Chapman, T. F. (2015). Reintroduced burrowing bettongs (Bettongia lesueur) scatter hoard sandalwood (Santalum spicatum) seed. Australian Journal of Zoology 63(1), 76-79.
| Crossref | Google Scholar |
Claridge, A. W., Tanton, M. T., Seebeck, J. H., Cork, S. J., and Cunningham, R. B. (1992). Establishment of ectomycorrhizae on the roots of two species of Eucalyptus from fungal spores contained in the faeces of the long‐nosed potoroo (Potorous tridactylus). Australian Journal of Ecology 17(2), 207-217.
| Crossref | Google Scholar |
Claridge, A. W., Tanton, M. T., and Cunningham, R. B. (1993). Hypogeal fungi in the diet of the long-nosed potoroo (Potorous tridactylus) in mixed-species and regrowth Eucalypt forest stands in South-eastern Australia. Wildlife Research 20, 321-338.
| Crossref | Google Scholar |
Coiner-Collier, S., Scott, R. S., Chalk-Wilayto, J., Cheyne, S. M., Constantino, P., Dominy, N. J., Elgart, A. A., Glowacka, H., Loyola, L. C., Ossi-Lupo, K., Raguet-Schofield, M., Talebi, M. G., Sala, E. A., Sieradzy, P., Taylor, A. B., Vinyard, C. J., Wright, B. W., Yamashita, N., Lucas, P. W., and Vogel, E. R. (2016). Primate dietary ecology in the context of food mechanical properties. Journal of Human Evolution 98, 103-118.
| Crossref | Google Scholar | PubMed |
Danks, M., Lebel, T., and Vernes, K. (2010). ‘Cort short on a mountaintop’ – Eight new species of sequestrate Cortinarius from sub-alpine Australia and affinities to sections within the genus. Persoonia 24, 106-126.
| Crossref | Google Scholar | PubMed |
Danks, M., Lebel, T., Vernes, K., and Andrew, N. (2013). Truffle-like fungi sporocarps in a eucalypt-dominated landscape: patterns in diversity and community structure. Fungal Diversity 58(1), 143-157.
| Crossref | Google Scholar |
Darvell, B. W., Lee, P. K. D., Yuen, T. D. B., and Lucas, P. W. (1996). A portable fracture toughness tester for biological materials. Measurement Science and Technology 7, 954-962.
| Crossref | Google Scholar |
Davies, G. T. O., Kirkpatrick, J. B., Cameron, E. Z., Carver, S., and Johnson, C. N. (2019). Ecosystem engineering by digging mammals: effects on soil fertility and condition in Tasmanian temperate woodland. Royal Society Open Science 6(1), 180621.
| Crossref | Google Scholar | PubMed |
Decker, O., Eldridge, D. J., and Gibb, H. (2019). Restoration potential of threatened ecosystem engineers increases with aridity: broad scale effects on soil nutrients and function. Ecography 42(8), 1370-1382.
| Crossref | Google Scholar |
Eldridge, D. J., and James, A. I. (2009). Soil‐disturbance by native animals plays a critical role in maintaining healthy Australian landscapes. Ecological Management & Restoration 10, S27-S34.
| Crossref | Google Scholar |
Garkaklis, M. J., Bradley, J. S., and Wooller, R. D. (2004). Digging and soil turnover by a mycophagous marsupial. Journal of Arid Environments 56(3), 569-578.
| Crossref | Google Scholar |
Green, K., Tory, M. K., Mitchell, A. T., Tennant, P., and May, T. W. (1999). The diet of the long‐footed potoroo (Potorous longipes). Australian Journal of Ecology 24(2), 151-156.
| Crossref | Google Scholar |
Guiler, E. R. (1971). Food of the potoroo (Marsupialia, Macropodidae). Journal of Mammalogy 52(1), 232-234.
| Crossref | Google Scholar |
Hartstone-Rose, A., Selvey, H., Villari, J. R., Atwell, M., and Schmidt, T. (2014). The three-dimensional morphological effects of captivity. PLoS One 9(11), e113437.
| Crossref | Google Scholar | PubMed |
Jones, C. G., Lawton, J. H., and Shachak, M. (1994). Organisms as Ecosystem Engineers. Oikos 69, 373-386.
| Crossref | Google Scholar |
Laird, M. F., Wright, B. W., Rivera, A. O., Fogaça, M. D., van Casteren, A., Fragaszy, D. M., Izar, P., Visalberghi, E., Scott, R. S., Strait, D. S., Ross, C. F., and Wright, K. A. (2020). Ingestive behaviors in bearded capuchins (Sapajus libidinosus). Scientific Reports 10(1), 20850.
| Crossref | Google Scholar | PubMed |
Laird, M. F., Punjani, Z., Oshay, R. R., Wright, B. W., Fogaça, M. D., van Casteren, A., Izar, P., Visalberghi, E., Fragazy, D., Strait, D. S., Ross, C. F., and Wright, K. A. (2022). Feeding postural behaviors and food geometric and material properties in bearded capuchin monkeys (Sapajus libidinosus). American Journal of Biological Anthropology 178(1), 3-16.
| Crossref | Google Scholar |
Lambert, J. E., Chapman, C. A., Wrangham, R. W., and Conklin-Brittain, N. L. (2004). Hardness of cercopithecine foods: implications for the critical function of enamel thickness in exploiting fallback foods. American Journal of Physical Anthropology 125(4), 363-368.
| Crossref | Google Scholar | PubMed |
Loveys, B. R., and Jusaitis, M. (1994). Stimulation of germination of quandong (Santalum acuminatum) and other Australian native plant seeds. Australian Journal of Botany 42(5), 565-574.
| Crossref | Google Scholar |
Lucas, P. W., Beta, T., Darvell, B. W., Dominy, N. J., Essackjee, H. C., Lee, P. K., Osorio, D., Ramsden, L., Yamashita, N., and Yuen, T. D. (2001). Field kit to characterize physical, chemical and spatial aspects of potential primate foods. Folia Primatol 72(1), 11-25.
| Crossref | Google Scholar | PubMed |
McDowell, M. C., Haouchar, D., Aplin, K. P., Bunce, M., Baynes, A., and Prideaux, G. J. (2015). Morphological and molecular evidence supports specific recognition of the recently extinct Bettongia anhydra (Marsupialia: Macropodidae). Journal of Mammalogy 96(2), 287-296.
| Crossref | Google Scholar |
McGraw, W. S., Van Casteren, A., Kane, E. E., Geissler, E., Burrows, B., and Daegling, D. J. (2016). Feeding and oral processing behaviors of two colobine monkeys in Tai Forest, Ivory Coast. Journal of Human Evolution 98, 90-102.
| Crossref | Google Scholar | PubMed |
McIlwee, A. P., and Johnson, C. N. (2002). The contribution of fungus to the diets of three mycophagous marsupials in Eucalyptus forests, revealed by stable isotope analysis. Functional Ecology 12(2), 223-231.
| Crossref | Google Scholar |
McNamara, J. A. (2014). Bettong diet and dentition. The South Australian Naturalist 88(2), 80-90.
| Crossref | Google Scholar |
Mitchell, D. R., Sherratt, E., Ledogar, J. A., and Wroe, S. (2018). The biomechanics of foraging determines face length among kangaroos and their relatives. Proceedings of the Royal Society B: Biological Sciences 285(1881), 20180845.
| Crossref | Google Scholar | PubMed |
Mitchell, D. R., Sherratt, E., Sansalone, G., Ledogar, J. A., Flavel, R. J., and Wroe, S. (2020). Feeding Biomechanics Influences Craniofacial Morphology at the Subspecies Scale among Australian Pademelons (Macropodidae: Thylogale). Journal of Mammalian Evolution 27(2), 199-209.
| Crossref | Google Scholar |
Mitchell, D. R., Wroe, S., Ravosa, M. J., and Menegaz, R. A. (2021). More challenging diets sustain feeding performance: applications toward the captive rearing of wildlife. Integrative Organismal Biology 3(1), obab030.
| Crossref | Google Scholar | PubMed |
Murphy, M. T., Garkaklis, M. J., and Hardy, G. E. S. J. (2005). Seed caching by woylies Bettongia penicillata can increase sandalwood Santalum spicatum regeneration in Western Australia. Austral Ecology 30, 747-755.
| Crossref | Google Scholar |
Murphy, M., Howard, K., Hardy, G. E. S. J., and Dell, B. (2015). When losing your nuts increases your reproductive success: sandalwood (Santalum spicatum) nut caching by the woylie (Bettongia penicillata). Pacific Conservation Biology 21(3), 243-252.
| Crossref | Google Scholar |
Neilly, H., and Schwarzkopf, L. (2018). Heavy livestock grazing negatively impacts a marsupial ecosystem engineer. Journal of Zoology 305(1), 35-42.
| Crossref | Google Scholar |
Nest, C., Elliott, T. F., Cooper, T., and Vernes, K. (2023). Seasonal consumption of mycorrhizal fungi by a marsupial-dominated mammal community. Fungal Ecology 64, 101247.
| Crossref | Google Scholar |
Paine, O. C. C., Koppa, A., Henry, A. G., Leichliter, J. N., Codron, D., Codron, J., Lambert, J. E., and Sponheimer, M. (2018). Grass leaves as potential hominin dietary resources. Journal of Human Evolution 117, 44-52.
| Crossref | Google Scholar | PubMed |
Palmer, B. J., Beca, G., Erickson, T. E., Hobbs, R. J., and Valentine, L. E. (2021). New evidence of seed dispersal identified in Australian mammals. Wildlife Research 48(7), 635-642.
| Crossref | Google Scholar |
Pardoe, C., Fullagar, R., and Hayes, E. (2019). Quandong stones: A specialised Australian nut-cracking tool. PLoS One 14(10), e0222680.
| Crossref | Google Scholar | PubMed |
Priddel, D., and Wheeler, R. (2004). An experimental translocation of brush-tailed bettongs (Bettongia penicillata) to western New South Wales. Wildlife Research 31, 421-432.
| Crossref | Google Scholar |
Read, J., and Sanson, G. D. (2003). Characterizing sclerophylly: the mechanical properties of a diverse range of leaf types. New Phytologist 160(1), 81-99.
| Crossref | Google Scholar | PubMed |
Robley, A. J., Short, J., and Bradley, S. (2008). Dietary overlap between the burrowing bettong (Bettongia lesueur) and the European rabbit (Oryctolagus cuniculus) in semi-arid coastal Western Australia. Wildlife Research 28, 341-349.
| Crossref | Google Scholar |
Ross, C. E., Munro, N. T., Barton, P. S., Evans, M. J., Gillen, J., Macdonald, B. C. T., McIntyre, S., Cunningham, S. A., and Manning, A. D. (2019). Effects of digging by a native and introduced ecosystem engineer on soil physical and chemical properties in temperate grassy woodland. PeerJ 7, e7506.
| Crossref | Google Scholar | PubMed |
Ross, C. E., McIntyre, S., Barton, P. S., Evans, M. J., Cunningham, S. A., and Manning, A. D. (2020). A reintroduced ecosystem engineer provides a germination niche for native plant species. Biodiversity and Conservation 29(3), 817-837.
| Crossref | Google Scholar |
Sander, U., Short, J., and Turner, B. (1997). Social organisation and warren use of the burrowing bettong, Bettongia lesueur (Macropodoidea: Potoroidae). Wildlife Research 24, 143-157.
| Crossref | Google Scholar |
Sanson, G., Read, J., Aranwela, N., Clissold, F., and Peeters, P. (2001). Measurement of leaf biomechanical properties in studies of herbivory: Opportunities, problems and procedures. Austral Ecology 26, 535-546.
| Crossref | Google Scholar |
Short, J. (1998). The extinction of rat-kangaroos (Marsupialia: Potoroidae) in New South Wales, Australia. Biological Conservation 86(3), 365-377.
| Crossref | Google Scholar |
Short, J., and Turner, B. (2000). Reintroduction of the burrowing bettong Bettongia lesueur (Marsupialia: Potoroidae) to mainland Australia. Biological Conservation 96, 185-196.
| Crossref | Google Scholar |
Talebi, M. G., Sala, E. A., Carvalho, B., Villani, G. M., Lucas, P. W., and van Casteren, A. (2016). Membrane-plate transition in leaves as an influence on dietary selectivity and tooth form. Journal of Human Evolution 98, 18-26.
| Crossref | Google Scholar | PubMed |
Taylor, R. J. (1992). Seasonal changes in the diet of the Tasmanian bettong (Bettongia gaimardi), a mycophagous marsupial. Journal of Mammalogy 73(2), 408-414.
| Crossref | Google Scholar |
van Casteren, A., Venkataraman, V., Ennos, A. R., and Lucas, P. W. (2016). Novel developments in field mechanics. Journal of Human Evolution 98, 5-17.
| Crossref | Google Scholar | PubMed |
van Casteren, A., Wright, E., Kupczik, K., and Robbins, M. M. (2019). Unexpected hard-object feeding in western lowland gorillas. American Journal of Physical Anthropology 170(3), 433-438.
| Crossref | Google Scholar | PubMed |
Vernes, K., and Jarman, P. (2014). Long-nosed potoroo (Potorous tridactylus) behaviour and handling times when foraging for buried truffles. Australian Mammalogy 36(1), 128-130.
| Crossref | Google Scholar |
Vernes, K., Castellano, M., and Johnson, C. N. (2002). Effects of season and fire on the diversity of hypogeous fungi consumed by a tropical mycophagous marsupial. Journal of Animal Ecology 70(6), 945-954.
| Crossref | Google Scholar |
Weisbecker, V., Guillerme, T., Speck, C., Sherratt, E., Abraha, H. M., Sharp, A. C., Terhune, C. E., Collins, S., Johnston, S., and Panagiotopoulou, O. (2019). Individual variation of the masticatory system dominates 3D skull shape in the herbivory-adapted marsupial wombats. Frontiers in Zoology 16, 41.
| Crossref | Google Scholar | PubMed |
Westerman, M., Loke, S., and Springer, M. S. (2004). Molecular phylogenetic relationships of two extinct potoroid marsupials, Potorous platyops and Caloprymnus campestris (Potoroinae: Marsupialia). Molecular Phylogenetics and Evolution 31(2), 476-485.
| Crossref | Google Scholar | PubMed |
Wieczkowski, J. (2009). Brief communication: Puncture and crushing resistance scores of Tana river mangabey (Cercocebus galeritus) diet items. American Journal of Physical Anthropology 140(3), 572-577.
| Crossref | Google Scholar | PubMed |
Williams, S. H., Wright, B. W., Truong, V., Daubert, C. R., and Vinyard, C. J. (2005). Mechanical properties of foods used in experimental studies of primate masticatory function. American Journal of Primatology 67(3), 329-346.
| Crossref | Google Scholar | PubMed |
Wright, B. W. (2005). Craniodental biomechanics and dietary toughness in the genus Cebus. Journal of Human Evolution 48(5), 473-492.
| Crossref | Google Scholar | PubMed |
Zosky, K. L., Wayne, A. F., Bryant, K. A., Calver, M. C., and Scarff, F. R. (2017). Diet of the critically endangered woylie (Bettongia penicillata ogilbyi) in south-western Australia. Australian Journal of Zoology 65(5), 302-312.
| Crossref | Google Scholar |


