A trial reintroduction of the western quoll to a fenced conservation reserve: implications of returning native predators
R. S. West A D , L. Tilley B and K. E. Moseby A CA Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia.
B School of Biological Sciences, The University of Adelaide, Adelaide, SA 5005, Australia.
C Arid Recovery Ltd, PO Box 147, Roxby Downs, SA 5725, Australia.
D Corresponding author: Email: rebecca.west@unsw.edu.au
Australian Mammalogy 42(3) 257-265 https://doi.org/10.1071/AM19041
Submitted: 16 June 2019 Accepted: 29 September 2019 Published: 16 October 2019
Journal Compilation © Australian Mammal Society 2020 Open Access CC BY-NC-ND
Abstract
While fenced reserves provide sanctuary for many threatened prey species, few projects have reintroduced native threatened predators, despite their potential role in regulating prey, addressing prey naivety, trophic regulation, and predator conservation. We aimed to investigate a set of issues unique to predator reintroduction into fenced reserves: how to contain predators that are naturally wide roaming, how to estimate carrying capacity, and will native predators impact resident threatened species? We conducted a trial reintroduction of four western quolls (Dasyurus geoffroii) (two males, two females) into a fenced reserve where four threatened prey species had been reintroduced and feral cats and foxes excluded. We monitored quoll survival, diet, movement and reproduction. Nocturnal foraging ranges measured over a fortnight were 3–17 km2, favouring burrows in dune habitat for shelter. Dietary analysis indicated a preference for reintroduced bettongs and western barred bandicoots, and resident hopping mice. Successful breeding was recorded but the two male quolls eventually escaped the reserve by climbing over the external fence and did not return. Results suggest that quoll reintroductions to fenced reserves will require fence designs that enable quolls to climb back into the reserve, threat management outside the reserve, and close monitoring of prey species.
Additional keywords: chuditch, fenced reserve, predator, quoll, reintroduction, translocation.
Introduction
Many threatened species are conserved on islands and fenced conservation reserves where they are protected from introduced species, human interference and habitat alteration. Usually, these conservation areas protect populations of herbivorous or granivorous mammals, reptiles and birds (Slotow et al. 2005; Burns et al. 2012; Legge et al. 2018) that can subsequently undergo spectacular increases in abundance (Moseby et al. 2018). Globally, reintroductions of predators into fenced reserves are less common except in Africa (Hayward 2009). Translocating predators to fenced conservation reserves poses a series of unique questions and issues, such as: Will the predators endanger the resident prey species? Are fenced reserves large enough to sustain viable populations of predators? And how are wide roaming predator species contained and monitored? Effects of reintroducing or removing predators from ecological systems can be difficult to predict due to the complex nature of predator–prey interactions (Glen and Dickman 2005; Gervasi et al. 2012).
The potential benefits of predator reintroduction include conservation of the predators themselves, regulating prey species, addressing issues of prey naivety and recreating natural ecosystems. The return of native predators to degraded ecosystems, such as wolves to Yellowstone National Park, has successfully reduced herbivore overabundance, leading to a reduction in browsing pressure on vegetation (Ripple and Beschta 2004). Furthermore, studies in Africa (Slotow et al. 2005) and Australia (Moseby et al. 2018) have highlighted the issues of overpopulation of herbivores when predators are excluded. In Australia, research on trophic cascades has been used to support arguments for the reintroduction of dingoes (Canis lupus dingo) and Tasmanian devils (Sarcophilus harrisii) to mainland ecosystems, where they have been excluded or driven to extinction (Glen et al. 2007; Hunter et al. 2015). Prey species isolated from predators can become naïve, losing antipredator behaviour and increasing their susceptibility to predation (Blumstein and Daniel 2005). Introducing predators can help reverse this behaviour (Moseby et al. 2016; West et al. 2018). Finally, many native predators are themselves threatened species and require protection (Ginsberg 2001). For example, large predators such as the Iberian lynx (Lynx pardinus) and tiger (Panthera tigris) are threatened due to human persecution, loss of habitat and declines in prey species (Vargas et al. 2008; Sanderson et al. 2010).
In Australia, native predators have suffered decline and extinction due to human persecution, introduction of feral predators, habitat clearance and disease. The recent increase in fenced reserves in Australia (Legge et al. 2018) presents an opportunity to re-establish predators and their ecosystem services and learn more about the challenges of reintroducing predators to fenced reserves. We trialled releasing the western quoll (Dasyurus geoffroii), a nationally threatened native marsupial predator, into the Arid Recovery Reserve (herein Reserve), a large 123-km2 fenced conservation reserve in arid South Australia (SA). Western quolls once occupied more than 70% of Australia’s mainland but their range declined by 95% after the introduction of feral cats and foxes (DEC 2012). The Reserve excludes introduced cats (Felis catus), foxes (Vulpes vulpes) and rabbits (Oryctolagus cuniculus) from 6000 ha, and four nationally threatened mammal species have been reintroduced: greater stick-nest rat (Leporillus conditor) in 1998, burrowing bettong (Bettongia lesueur) in 1999, greater bilby (Macrotis lagotis) in 2000 and western barred bandicoot (Perameles bougainville) in 2001 (Moseby et al. 2011). Burrowing bettongs have increased to high levels and in 2016 averaged 1.3 bettongs per hectare (Moseby et al. 2018). In situ populations of native rodents have also increased compared with those in outside areas (Moseby et al. 2009). These increases in mammal abundance have been attributed to the absence of mammalian predators within the Reserve (Moseby et al. 2009) and a trial reintroduction of a native predator was proposed to restore ecosystem balance (Moseby et al. 2018).
We aimed to examine three key issues related to the reintroduction of a native predator: (1) ease of containment within a fenced reserve; (2) potential carrying capacity; and (3) the impact on resident prey species.
Materials and methods
Study area
The Reserve is fenced with a 1.8-m-high wire netting fence (Moseby and Read 2006) to exclude introduced rabbits, cats and foxes. A range of habitats are present including longitudinal sand dunes separated by clay interdunal swales, small swamps and stony rises. Vegetation includes chenopod (saltbush/bluebush) and Acacia shrublands. Rainfall is erratic, failing to reach the long-term average of 160 mm in 60% of years. The Reserve is divided into six paddocks, from which feral species are removed from four sections totalling 60 km2 (Fig. 1). Three different fence designs are used in the Reserve. The external fence and some internal fences are 1.8 m high and made from 50-mm-diameter wire netting on the top 90 cm and 30-mm-diameter wire netting below 90 cm (high fence, Fig. 1). It has an outward-facing wire netting floppy top that extends out for 60 cm and a 30-cm foot netting (Moseby and Read 2006) extending out from the base of the fence on both sides. The second design (low fence) is used on the internal fences separating three of the paddocks and is 90 cm high and made from 30-mm wire mesh (Fig. 1). The original Main Exclosure is surrounded by the same 1.8-m-high fence design but with the addition of two electric wires at heights of 120 and 150 cm (electrified high fence, Fig. 1). Electric wires are offset from the fence by 60–80 mm. Quolls were reintroduced into the Northern Expansion of the reserve (quoll treatment). The Main Exclosure was used as a comparative control treatment as quolls were excluded from this area of the reserve by the electric fence.

|
Quoll releases
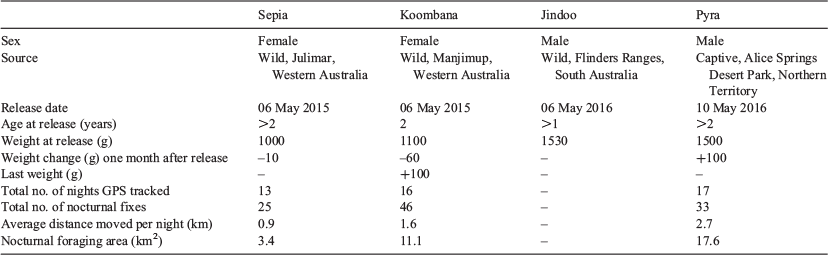
In May 2015 two female quolls were released into the Northern Expansion (Fig. 1). A single-sex release was conducted initially to allow the option of reversing the reintroduction if significant unexpected impacts on resident species were detected. Females were captured at wild sites in Western Australia (Table 1), fitted with VHF collars (25 g, Sirtrack Ltd, New Zealand) and held in captivity at Native Animal Rescue, Malaga, Western Australia, for several days before release. On the day of release, quolls were transported by aeroplane to Wilpena airstrip, Flinders Ranges, SA, and then by car to the Reserve, ~420 km away. They were released on the night of capture into abandoned bettong warrens within a 0.6-ha release pen where they were kept for seven days before being allowed access to the rest of the Reserve.
|
In May 2016, two male quolls fitted with VHF radio-collars (25 g, Sirtrack Ltd) were released into the Northern Expansion (Table 1). One male was sourced from the reintroduced population in the Flinders Ranges, SA, and the other from a captive population at the Alice Springs Desert Park, Northern Territory. Both males were released at dusk close to the female quolls.
Ease of containment
Ease of containment was determined using radio-tracking and camera trap data to investigate whether the internal and external reserve fence designs could contain quolls. We analysed radio-tracking data to examine the number of Reserve sections visited by each quoll, the number of times quolls left the Reserve, and their total retention time within the Reserve. Consecutive radio-tracking fixes were examined to determine how many times quolls traversed each of the different fence types. If quolls left the Reserve, attempts were made to return them to the Reserve.
Carrying capacity
We used radio-tracking and trapping data to investigate the habitat selection, movement, and reproductive output of reintroduced quolls to estimate carrying capacity. Due to the wide roaming behaviour of quolls and the undulating habitat, reliably locating quolls on a daily basis was difficult, and so home ranges were not estimated. Instead, we estimated a short-term nocturnal foraging area, by swapping the VHF collar to a GPS Pinpoint collar (30 g, Sirtrack Ltd) for a period of 13–17 days for three of the four quolls (2 females, 1 male; Table 1). Pinpoint collars were not fitted to quolls until at least two months after release to exclude any panic dispersal and settling movements. Pinpoint collars collected three fixes per night (at 2100, 0000 and 0300 hours) and also emitted a VHF signal. The collars stored a maximum of 50 fixes. Nocturnal foraging area for each individual was calculated using a convex hull (the smallest polygon enclosing the GPS fixes) in ArcGIS (ESRI 2014).
All quolls were radio-tracked daily for the first month after release and subsequently at weekly intervals to identify diurnal shelter sites. Diurnal shelter sites were scored by type (warren, single-entrance burrow, stick-nest rat nest, other), species resident (determined by tracks) and by habitat (dune or swale). Selection of habitat (dune/swale) was tested using Chi-square tests (IBM SPSS Statistics 23) by comparing the observed habitat use (shelter or nocturnal fixes) with 100 random habitat fixes generated in ArcGIS (ESRI 2014). Random points were generated across the quoll-accessible areas of the Reserve (First, Second and Northern Expansions). High-resolution aerial imagery was used to define each random point and each diurnal or nocturnal fix as dune or swale.
Quolls were trapped, weighed and checked for reproductive status approximately one month after release and then at 2–3-month intervals. As female quolls have exclusive home ranges (Serena and Soderquist 1989), we used the total reserve area divided by the nocturnal range of each female quoll to provide an estimate of carrying capacity.
Impacts on resident prey species
We assessed dietary selectivity and changes in prey abundance to investigate the potential impacts on resident prey species. Changes in activity of mammalian prey species within the Reserve were monitored using track counts conducted two weeks before quoll release, then at 4, 8 and 14 months after reintroduction within the Northern Expansion (quolls present) and Main Exclosure (control area, quolls absent). Track counts involved walking a 1-km transect along a sand dune that was cleared of tracks (see Moseby et al. (2018) for detailed method). The following morning tracks of bettongs, bilbies, bandicoots and stick-nest rats that entered the transect were counted. In addition, tracks of small mammals (spinifex hopping mice (Notomys alexis) and plains mice (Pseudomys australis)) were also counted for the first 10 m of every 100 m along the transect and multiplied by 10 to provide an estimate of track count per kilometre. Significant positive relationships between track counts and population size have been identified in the burrowing bettong (Moseby et al. 2018), but track counts were predominantly used as a measure of activity and thus a proxy for possible encounter rates between quolls and prey species.
The size of reintroduced stick-nest rats, their threatened status, and the conspicuousness of their nests suggested that they may be vulnerable to quoll predation. However, they are difficult to monitor using track counts so instead we fitted radio-collars (9 g, Sirtrack Ltd) to 21 stick-nest rats (8 males, 13 females) before the quoll release and conducted weekly radio-tracking for the first 10 months after quoll release to determine survival. Rats were trapped at their nests and fitted with collars in both the quoll treatment (n = 11) and control treatment (n = 10). Multiple nests were trapped to ensure that radio-collared rats were spread throughout both treatment areas to maximise the chances of detecting predation events.
Quoll scats were collected by searching the vicinity of diurnal shelter sites, searching opportunistically and during trapping events. Mammalian prey species were identified by Desert Wildlife Services using microscopic analysis of hair samples extracted from the scats (after Brunner and Coman 1974). The frequency of occurrence of dietary items was expressed as a percentage in all scat samples with items classified as native rodent (Notomys or Pseudomys), bettong, bandicoot, stick-nest rat, invertebrate, reptile, bird, dasyurid, and introduced rodent (Mus).
To determine the proportion of mammal species in the quoll diet relative to availability, the three track counts collected after quoll release were averaged and used to calculate a proportional availability for each prey species (number of each prey track per total track). This analysis examined only the three reintroduced species for which track counts are reliable (bilby, bettong, western barred bandicoot) and native rodents.
We also examined selectivity by converting track counts to a comparative biomass availability based on the body mass of each species. The mass of an average bettong is 1600 g while the native rodent Notomys alexis weighs, on average, 35 g. Thus, higher track numbers of N. alexis does not reflect a larger availability of rodent biomass compared to that of bettongs. We therefore calculated a scaling factor relative to bettong mass for the other species (bettong mass/species mass), using the following average weights: bettong and bilby = 1600 g, bandicoot = 200 g, native rodents = 35 g. The average track count was multiplied by the scaling factor to convert track counts per kilometre to biomass available.
To calculate the degree of quoll selectivity on prey species, we used Jacob’s preference index (Jacobs 1974).

where r is the proportion of each species within scats and p is the proportional availability of each species within the reserve. The index (D) ranges from –1 (complete avoidance) to 1 (exclusive predation), with 0 showing no selection. This index was calculated for (a) the proportion of individual tracks per kilometre in relation to the proportion of scats containing each species, and (b) the proportion of available biomass in relation to the proportion of scats containing each species.
Results
Containment
Both female quolls remained in the Reserve for the entire study (18 months after release). Both male quolls, Jindoo and Pyra, left the Reserve 4 and 64 days after release respectively. The wild-caught male Jindoo travelled 6 km in the first night and roamed across the 60 km2 feral-free area (Fig. S1, available as supplementary material). Jindoo was captured at a rabbit warren 310 m outside the Reserve 5 days after release and returned to a female quoll’s warren within the Reserve. However, this did not assist with retention within the Reserve and the male was last captured on a camera trap north of the Northern Expansion, 15 days after first release. Despite two radio-tracking flights covering a 30-km radius of the Reserve and numerous searches on foot and trapping events, this male was not found. The other male, Pyra, was also captured and returned to the reserve after being located at a rabbit warren 150 m outside the reserve on Night 65 after release. After being captured and returned, Pyra remained within the reserve for another 35 days before the radio-tracking signal was lost.
The nocturnal fixes demonstrated that both male and female quolls could easily traverse the low internal fences dividing some of the paddocks and suggested that one female breached the high electrified fence 4 times over a 16-night period (Fig. 2). However, checks of the fence revealed holes under the foot netting from digging bettongs, so it is not known if she climbed over or under the electrified fence. Of the shelter sites collected for male Jindoo, three were inside the Main Exclosure, suggesting that he was also able to enter and exit this section of the reserve (Fig. S1).

|
Carrying capacity
The nocturnal foraging areas were between 3 and 18 km2 across an average 14-day period (Table 1, Fig. 2). The male Pyra used the largest area (17.6 km2); however, there were also large differences in the area used by the two females (3.4 versus 11.1 km2).
In total, 177 quoll shelter site records were obtained (137 female, 40 male). Active bettong warrens were predominantly used as shelter sites (71%, n = 126), with single-entrance bettong burrows (28%, n = 50) also utilised and one recorded within an abandoned stick-nest rat nest. In all, 99% of shelter sites were located within dune habitat (175 of 177), which was strongly selected for (χ2 = 151.3, d.f. = 1, P < 0.001). A total of 104 GPS habitat fixes were obtained using the pinpoint collars. These active nocturnal fixes were also predominately in dune habitat (86%, n = 89), compared to swale (n = 15), suggesting a preference for dune habitat (χ2 = 60.6, d.f. = 1, P < 0.001).
On all trapping occasions, quolls were in good condition and maintained their body weight (Table 1). In July 2016, the female quoll Sepia was captured with four pouch young (date of birth estimated at 16 July 2016 according to head length of young: Serena and Soderquist 1988). This female was found dead above ground three months later but at least three of her young survived to weaning as they were later captured. The other female was still alive at the conclusion of this study (November 2016) but is not known to have produced young. Both males were lost from the reserve before completion of this study.
Impacts on resident prey species
Track counts of threatened species in the quoll treatment area and control area displayed the same fluctuations over the three counts following quoll release (Fig. 3). Despite the GPS fixes suggesting that some quolls gained access, the small proportion of the control area used and the fact that no quoll tracks were recorded during track transects suggests that the control area had a very low visitation rate by quolls and can still be considered a control. Track counts suggest that the quolls did not decrease threatened prey species abundance in the short term (18 months after release) (Fig. 3).
Seventy-four scats, including three latrines of 6, 6 and 30 scats, were analysed. Latrines are often used by multiple quoll individuals (Kruuk and Jarman 1995) so each scat was considered independent. Of the four threatened prey species, bettong, western barred bandicoot and stick-nest rat remains were found in quoll scats, but not bilby (Table 2, Fig. 4). Some scats contained traces of more than one prey item (total 85 prey items across the 74 scats). The frequency of occurrence (frequency of prey item/all prey items) for prey was 52% native rodent (Notomys or Pseudomys) (n = 44), 25% western barred bandicoot (n = 21), 22% bettong (n = 19), 2% house mouse (n = 2), 2% reptile (n = 2), 0.01% for stick-nest rat (n = 1), dasyurid (n = 1), invertebrate (n = 1), bird (n = 1) and unknown mammal (n = 1) (Fig. 4). Of the 19 detections of bettong remains within the scats, four of these were identified as juvenile bettongs by the size of the claws present.

|
When examining dietary selection using proportional track abundance, quolls selected for bettongs and bandicoots and against native rodents and bilbies (Table 2). However, when using proportional biomass of prey species rather than proportional track abundance, quolls were selecting for native rodents and bandicoots (Table 2). None of the 21 radio-collared stick-nest rats were killed by quolls in the first 10 months after quoll release before the collars were removed. One rat died from predation by a raptor.
Discussion
The ability of conservation managers to make informed decisions regarding predator reintroductions can be hindered by inadequate knowledge of the responses of resident species, and the predators themselves, to reintroduction (Hayward et al. 2007c). The consequences of predator reintroduction can be further compounded within closed systems where limitations on natural dispersal lead to issues associated with impacts to native prey and maintenance of genetic diversity (Hayward et al. 2007b; Miller et al. 2013). However, the challenges and possible negative effects of predator reintroductions need to be understood due to the difficulty in reversing these decisions once the ‘cat is out of the bag’. This study used a small-scale trial to examined the potential issues of releasing an Australian native predator to a fenced reserve. The results provide important insights into the possible consequences and key considerations when attempting to establish native predators to restricted areas.
Ease of containment
The 1.8-m-high Reserve fence was unable to contain reintroduced quolls, which climbed out over the fence. Quolls also entered the exclusion area of the reserve, which was electrified; however, it is likely that this was through holes under the fence. The Reserve fence is designed to prevent entry from the outside, so these findings are not unexpected. Semiarboreal predators such as quolls (Serena et al. 1991) and leopards (Panthera pardus) (see Hayward et al. 2007a) are likely to present additional containment issues compared with exclusively terrestrial larger predators such as lions (Panthera leo) and dingoes, where fence height can be used to eliminate the chances of escape.
Male quolls escaped more times than females, likely a result of larger home ranges and wider roaming behaviour (Serena and Soderquist 1989; Rayner et al. 2012), and their polygynous mating strategy driving them to seek out multiple females (Soderquist and Serena 1990). Although both males were returned to the Reserve they eventually escaped. Male mammals usually roam further than females (Greenwood 1980) and therefore male predators may be more likely to leave protected areas (Pimm et al. 2006). The broad-ranging movement patterns of predators will have implications for the successful establishment of predators within fenced reserves and the maintenance of genetic diversity. Future studies should focus on the development of measures to enable quolls to re-enter fenced reserves if they exit. Such measures could include one-way gates, ramps or gates operated by shape recognition software to enable re-entry, although these designs will require research and development to ensure they do not simultaneously enable entry of feral species. The long-term genetic consequences of losing a proportion of males before or after each breeding season will also need examination. If this is likely to significantly influence long-term genetic persistence, and a suitable internal barrier to prevent climbing out is not developed, then regular genetic audits (White et al. 2018) and supplementation of additional males before the breeding season in some years may need to be considered to reduce inbreeding. It may be possible to increase the area of occupancy for reintroduced predators by including areas adjacent to the reserve if threats outside the reserve can be suitably managed. This may assist in the maintenance of genetic diversity by supporting a population of the reintroduced predator both within and adjacent to the reserve if gene flow between the two areas can be secured.
Carrying capacity
A key consideration when reintroducing predators into closed systems is the number of individuals that the area can sustain (Hayward 2009). Sustainable carrying capacity depends on the provision of suitable habitat for the predator, predation rates on resident prey, the population growth rate of both the predator and resident prey species, and the home-range requirements and social structuring of the reintroduced predator. Due to the small sample size and short study period, the current study is limited in its ability to estimate predation and population growth rates. However, the movement and habitat selection results can assist in making carrying capacity predictions to test in future releases. The current study found that quolls were able to survive and maintain body condition, and reproduce, suggesting that the environment was suitable in terms of the provision of habitat, shelters and prey. Accurate home-range requirements, including seasonal variation, could not be determined due to the limited number of animals and the short study duration (Goldingay 2015); however, the nocturnal ranges provide an estimate of potential home-range requirements. The 3.4-km2 nocturnal range area observed for one of the females is equivalent to that found in wild quoll populations (female territories 3–4 km2) (Serena and Soderquist 1989), while the 11.1 km2 observed for the other female is much larger than previously observed. As female quolls are known to occupy exclusive home ranges, the range of these two females would therefore suggest that the 123 km2 Reserve could support between 11 and 36 female quolls with non-overlapping home ranges. This would equate to a total population size of between 22 and 72 quolls, given that the sex ratio of male and female quoll populations is close to parity (DEC 2012). Carrying capacity of African predators in conservation areas has been shown to be significantly related to the abundance of their preferred prey (Hayward et al. 2007c), suggesting that these carrying capacity estimates are likely to fluctuate according to prey densities, which vary widely in arid areas in response to seasonal conditions (Letnic et al. 2005). Further studies to calculate predation and population growth rates in addition to habitat requirements will be required to further examine carrying capacity.
Impact on prey species
The potential impact on resident prey species is a key concern for reintroduction and management of predator populations (Tambling and Du Toit 2005; Hayward et al. 2007b). Dietary analysis revealed that quolls did eat bettongs and exhibited strong selection for rodents but also showed selection for another reintroduced species, the western barred bandicoot. Unlike previous quoll diet studies, the scats contained few invertebrates (Serena et al. 1991). Population monitoring did not detect any significant decline in any mammal species, although our study was limited to only four individual predators in the first 18 months after release, and populations of these prey species had grown significantly in the 18 years since reintroduction. For this study, only one transect was established in the control and quoll treatments for prey activity comparisons. Future studies would benefit from multiple shorter transects or repeated visits within a short period to allow for the inclusion of standard errors within estimates. Larger-scale releases will need to conduct intensive monitoring of the reintroduced prey species populations in conjunction with quoll diet analysis to determine the long-term impacts of quoll predation, including potential sex-specific effects. The ability of quolls to breach fences may prevent excessive predation pressure, unlike predator translocations to island populations where restriction of dispersal has led to high population increases in quolls (Griffiths et al. 2017) and overabundance of other predator species within fenced reserves (Hayward et al. 2007b). Reintroduced quolls selected dune habitat over swale, evident from shelter site selection and nocturnal habitat fixes, and predominantly sheltered in bettong warrens. Thus prey species using dune habitat and sheltering in bettong warrens may be more likely to be susceptible to population decline from quoll predation and require closer monitoring. Concerns about significant impacts to previously reintroduced species by the reintroduction of a predator could be allayed by attempting to exclude predators from an area of the fenced reserve after release.
Implications
The reintroduction of native predators may assist with rebalancing trophic systems within fenced reserves and tackling issues of prey naïveté and overpopulation. However, the restricted size of most fenced reserves suggests that issues with small population size, genetic diversity and impacts to coexisting threatened species must be considered before release. Predator species that can escape reserves create issues for genetic diversity but may be less risky in terms of impacts to resident species. Practitioners should carefully consider the costs and benefits of full versus partial containment of predators reintroduced to fenced reserves. The results of this study suggest that appropriate trial releases can assist with assessing the benefits and costs of predator reintroductions before proceeding to full-scale releases. Trials could include releasing animals of a single sex only and implementing exclusion zones to quantify and identify any possible effects.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Arid Recovery is an independent conservation initiative supported by BHP, the South Australian Department for Environment and Water (DEW), the University of Adelaide and Bush Heritage Australia. Funding was provided by Arid Recovery, The Nature Foundation of South Australia, the Australian Wildlife Society, the University of Adelaide (L. Tilley, Honours project support). This research was conducted under approval from the South Australian Wildlife Ethics Committee (1/2014 – M2). We thank the WA Department for Biodiversity, Conservation and Attractions, DEW and the Alice Springs Desert Park for the provision of quolls for the reintroduction. The Foundation for Australia’s Most Endangered species (FAME) and David Peacock were instrumental in returning quolls to SA and enabling us to add value to the SA DEW’s Flinders Ranges reintroduction. Thank you to Desert Wildlife Services for scat analyses. We also thank Arid Recovery staff for assistance with monitoring, in particular Kimberley Solly, and research interns Kaarissa Harring-Harris, Ruth Shepherd, Evan Griffith and Sam Fischer. Thanks to Kath Tuft for assistance with the project and David Paton for cosupervision of students.
References
Blumstein, D. T., and Daniel, J. C. (2005). The loss of anti-predator behaviour following isolation on islands. Proceedings of the Royal Society of London. Series B, Biological Sciences 272, 1663–1668.| The loss of anti-predator behaviour following isolation on islands.Crossref | GoogleScholarGoogle Scholar |
Brunner, H., and Coman, B. J. (1974). ‘The Identification of Mammalian Hair.’ (Inkata Press: Melbourne.)
Burns, B., Innes, J., and Day, T. (2012). The use and potential of pest-proof fencing for ecosystem restoration and fauna conservation in New Zealand. In ‘Fencing for Conservation’. (Eds M. Somers, and M. Hayward.) pp. 65–90. (Springer: New York.)
DEC (2012). Chuditch (Dasyurus geoffroii) recovery plan. Department of Environment and Conservation, Perth.
ESRI (2014). ArcGIS Desktop: Release 10.3.
Gervasi, V., Nilsen, E. B., Sand, H., Panzacchi, M., Rauset, G. R., Pedersen, H. C., Kindberg, J., Wabakken, P., Zimmermann, B., and Odden, J. (2012). Predicting the potential demographic impact of predators on their prey: a comparative analysis of two carnivore–ungulate systems in Scandinavia. Journal of Animal Ecology 81, 443–454.
| Predicting the potential demographic impact of predators on their prey: a comparative analysis of two carnivore–ungulate systems in Scandinavia.Crossref | GoogleScholarGoogle Scholar | 22077484PubMed |
Ginsberg, J. R. (2001). Setting priorities for carnivore conservation: what makes carnivores different? In ‘Carnivore Conservation’. (Eds J. L. Gittleman, S. M. Funk, D. Macdonald, and R. K. Wayne.) pp. 498–523. (Cambridge University Press: Cambridge.)
Glen, A. S., and Dickman, C. R. (2005). Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biological Reviews of the Cambridge Philosophical Society 80, 387–401.
| Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management.Crossref | GoogleScholarGoogle Scholar | 16094805PubMed |
Glen, A. S., Dickman, C. R., Soule, M. E., and Mackey, B. (2007). Evaluating the role of the dingo as a trophic regulator in Australian ecosystems. Austral Ecology 32, 492–501.
| Evaluating the role of the dingo as a trophic regulator in Australian ecosystems.Crossref | GoogleScholarGoogle Scholar |
Goldingay, R. L. (2015). A review of home-range studies on Australian terrestrial vertebrates: adequacy of studies, testing of hypotheses, and relevance to conservation and international studies. Australian Journal of Zoology 63, 136–146.
| A review of home-range studies on Australian terrestrial vertebrates: adequacy of studies, testing of hypotheses, and relevance to conservation and international studies.Crossref | GoogleScholarGoogle Scholar |
Greenwood, P. J. (1980). Mating systems, philopatry and dispersal in birds and mammals. Animal Behaviour 28, 1140–1162.
| Mating systems, philopatry and dispersal in birds and mammals.Crossref | GoogleScholarGoogle Scholar |
Griffiths, A. D., Rankmore, B., Brennan, K., and Woinarski, J. C. Z. (2017). Demographic evaluation of translocating the threatened northern quoll to two Australian islands. Wildlife Research 44, 238–247.
| Demographic evaluation of translocating the threatened northern quoll to two Australian islands.Crossref | GoogleScholarGoogle Scholar |
Hayward, M. W. (2009). Moving beyond the descriptive: predicting the responses of top-order predators to reintroduction. In ‘Reintroduction of Top-order Predators’. (Eds M. W. Hayward, and M. Somers.) pp. 345–370. (Blackwell Publishing: Oxford.)
Hayward, M. W., Adendorff, J., Moolman, L., Hayward, G. J., and Kerley, G. (2007a). The successful reintroduction of leopard Panthera pardus to the Addo Elephant National Park. African Journal of Ecology 45, 103–104.
| The successful reintroduction of leopard Panthera pardus to the Addo Elephant National Park.Crossref | GoogleScholarGoogle Scholar |
Hayward, M. W., Kerley, G. I., Adendorff, J., Moolman, L. C., O’brien, J., Sholto-Douglas, A., Bissett, C., Bean, P., Fogarty, A., and Howarth, D. (2007b). The reintroduction of large carnivores to the Eastern Cape, South Africa: an assessment. Oryx 41, 205–214.
| The reintroduction of large carnivores to the Eastern Cape, South Africa: an assessment.Crossref | GoogleScholarGoogle Scholar |
Hayward, M. W., O’Brien, J., and Kerley, G. I. (2007c). Carrying capacity of large African predators: predictions and tests. Biological Conservation 139, 219–229.
| Carrying capacity of large African predators: predictions and tests.Crossref | GoogleScholarGoogle Scholar |
Hunter, D. O., Britz, T., Jones, M., and Letnic, M. (2015). Reintroduction of Tasmanian devils to mainland Australia can restore top-down control in ecosystems where dingoes have been extirpated. Biological Conservation 191, 428–435.
| Reintroduction of Tasmanian devils to mainland Australia can restore top-down control in ecosystems where dingoes have been extirpated.Crossref | GoogleScholarGoogle Scholar |
Jacobs, J. (1974). Quantitative measurement of food selection. Oecologia 14, 413–417.
| Quantitative measurement of food selection.Crossref | GoogleScholarGoogle Scholar | 28308662PubMed |
Kruuk, H., and Jarman, P. (1995). Latrine use by the spotted-tailed quoll (Dasyurus maculatus: Dasyuridae, Marsupialia) in its natural habitat. Journal of Zoology (London, England) 236, 345–349.
| Latrine use by the spotted-tailed quoll (Dasyurus maculatus: Dasyuridae, Marsupialia) in its natural habitat.Crossref | GoogleScholarGoogle Scholar |
Legge, S., Woinarski, J. C., Burbidge, A. A., Palmer, R., Ringma, J., Radford, J. Q., Mitchell, N., Bode, M., Wintle, B., and Baseler, M. (2018). Havens for threatened Australian mammals: the contributions of fenced areas and offshore islands to the protection of mammal species susceptible to introduced predators. Wildlife Research 45, 627–644.
| Havens for threatened Australian mammals: the contributions of fenced areas and offshore islands to the protection of mammal species susceptible to introduced predators.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., Tamayo, B., and Dickman, C. R. (2005). The responses of mammals to La Nina (El Nino Southern Oscillation)–associated rainfall, predation, and wildfire in central Australia. Journal of Mammalogy 86, 689–703.
| The responses of mammals to La Nina (El Nino Southern Oscillation)–associated rainfall, predation, and wildfire in central Australia.Crossref | GoogleScholarGoogle Scholar |
Miller, S., Bissett, C., Parker, D., Burger, A., Courtenay, B., Dickerson, T., Naylor, S., Druce, D., Ferreira, S., and Slotow, R. (2013). Management of reintroduced lions in small, fenced reserves in South Africa: an assessment and guidelines. South African Journal of Wildlife Research 43, 138–154.
| Management of reintroduced lions in small, fenced reserves in South Africa: an assessment and guidelines.Crossref | GoogleScholarGoogle Scholar |
Moseby, K., and Read, J. (2006). The efficacy of feral cat, fox and rabbit exclusion fence designs for threatened species protection. Biological Conservation 127, 429–437.
| The efficacy of feral cat, fox and rabbit exclusion fence designs for threatened species protection.Crossref | GoogleScholarGoogle Scholar |
Moseby, K. E., Hill, B. M., and Read, J. L. (2009). Arid recovery – a comparison of reptile and small mammal populations inside and outside a large rabbit, cat and fox‐proof exclosure in arid South Australia. Austral Ecology 34, 156–169.
| Arid recovery – a comparison of reptile and small mammal populations inside and outside a large rabbit, cat and fox‐proof exclosure in arid South Australia.Crossref | GoogleScholarGoogle Scholar |
Moseby, K., Read, J., Paton, D., Copley, P., Hill, B., and Crisp, H. (2011). Predation determines the outcome of 10 reintroduction attempts in arid South Australia. Biological Conservation 144, 2863–2872.
| Predation determines the outcome of 10 reintroduction attempts in arid South Australia.Crossref | GoogleScholarGoogle Scholar |
Moseby, K. E., Blumstein, D. T., and Letnic, M. (2016). Harnessing natural selection to tackle the problem of prey naïveté. Evolutionary Applications 9, 334–343.
| Harnessing natural selection to tackle the problem of prey naïveté.Crossref | GoogleScholarGoogle Scholar | 26834826PubMed |
Moseby, K., Lollback, G., and Lynch, C. (2018). Too much of a good thing; successful reintroduction leads to overpopulation in a threatened mammal. Biological Conservation 219, 78–88.
| Too much of a good thing; successful reintroduction leads to overpopulation in a threatened mammal.Crossref | GoogleScholarGoogle Scholar |
Pimm, S. L., Dollar, L., and Bass, O. L. (2006). The genetic rescue of the Florida panther. Animal Conservation 9, 115–122.
| The genetic rescue of the Florida panther.Crossref | GoogleScholarGoogle Scholar |
Rayner, K., Chambers, B., Johnson, B., Morris, K. D., and Mills, H. R. (2012). Spatial and dietary requirements of the chuditch (Dasyurus geoffroii) in a semiarid climatic zone. Australian Mammalogy 34, 59–67.
| Spatial and dietary requirements of the chuditch (Dasyurus geoffroii) in a semiarid climatic zone.Crossref | GoogleScholarGoogle Scholar |
Ripple, W. J., and Beschta, R. L. (2004). Wolves and the ecology of fear: can predation risk structure ecosystems? A.I.B.S. Bulletin 54, 755–766.
Sanderson, E. W., Forrest, J., Loucks, C., Ginsberg, J., Dinerstein, E., Seidensticker, J., Leimgruber, P., Songer, M., Heydlauff, A., and O’Brien, T. (2010). Setting priorities for tiger conservation: 2005–2015. In ‘Tigers of the World’. 2nd edn. (Eds R. Tilson and P. Nyhus.) pp. 143–161. (Elsevier.)
Serena, M., and Soderquist, T. (1988). Growth and development of pouch young of wild and captive Dasyurus geoffroii (Marsupialia, Dasyuridae). Australian Journal of Zoology 36, 533–543.
| Growth and development of pouch young of wild and captive Dasyurus geoffroii (Marsupialia, Dasyuridae).Crossref | GoogleScholarGoogle Scholar |
Serena, M., and Soderquist, T. (1989). Spatial organization of a riparian population of the carnivorous marsupial Dasyurus geoffroii. Journal of Zoology 219, 373–383.
| Spatial organization of a riparian population of the carnivorous marsupial Dasyurus geoffroii.Crossref | GoogleScholarGoogle Scholar |
Serena, M., Soderquist, T. R., and Morris, K. (1991). The chuditch (Dasyurus geoffroii). Department of Conservation and Land Management, Perth.
Slotow, R., Garaï, M. E., Reilly, B., Page, B., and Carr, R. D. (2005). Population dynamics of elephants re-introduced to small fenced reserves in South Africa. South African Journal of Wildlife Research 35, 23–32.
Soderquist, T., and Serena, M. (1990). Occurrence and outcome of polyoestry in wild western quolls, Dasyurus geoffroii (Marsupialia: Dasyuridae). Australian Mammalogy 13, 205–208.
Tambling, C. J., and Du Toit, J. T. (2005). Modelling wildebeest population dynamics: implications of predation and harvesting in a closed system. Journal of Applied Ecology 42, 431–441.
| Modelling wildebeest population dynamics: implications of predation and harvesting in a closed system.Crossref | GoogleScholarGoogle Scholar |
Vargas, A., Sánchez, I., Martínez, F., Rivas, A., Godoy, J. A., Roldan, E., Simón, M., Serra, R., Perez, M., and Ensenat, C. (2008). The Iberian lynx Lynx pardinus conservation breeding program. International Zoo Yearbook 42, 190–198.
| The Iberian lynx Lynx pardinus conservation breeding program.Crossref | GoogleScholarGoogle Scholar |
West, R., Letnic, M., Blumstein, D. T., and Moseby, K. E. (2018). Predator exposure improves anti‐predator responses in a threatened mammal. Journal of Applied Ecology 55, 147–156.
| Predator exposure improves anti‐predator responses in a threatened mammal.Crossref | GoogleScholarGoogle Scholar |
White, L. C., Moseby, K. E., Thomson, V. A., Donnellan, S. C., and Austin, J. J. (2018). Long-term genetic consequences of mammal reintroductions into an Australian conservation reserve. Biological Conservation 219, 1–11.
| Long-term genetic consequences of mammal reintroductions into an Australian conservation reserve.Crossref | GoogleScholarGoogle Scholar |




