Regional patterns of continuing decline of the eastern quoll†
Calum X. Cunningham A B * , Zach Aandahl A , Menna E. Jones A , Rowena Hamer A C and Christopher N. Johnson A DA School of Natural Sciences, University of Tasmania, Private Bag 55, Hobart, Tas. 7001, Australia.
B School of Environmental and Forest Sciences, University of Washington, Seattle, WA 98195, USA.
C Tasmanian Land Conservancy, 827 Sandy Bay Road, Sandy Bay, Tas. 7005, Australia.
D Australian Research Council Centre of Excellence for Australian Biodiversity and Heritage, School of Natural Sciences, University of Tasmania, Private Bag 55, Hobart, Tas. 7001, Australia.
Australian Mammalogy 45(2) 151-159 https://doi.org/10.1071/AM22010
Submitted: 12 March 2022 Accepted: 5 September 2022 Published: 20 September 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the Australian Mammal Society. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Like many other Australian mammals, eastern quolls (Dasyurus viverrinus) were widespread in the south-east of mainland Australia but went extinct there during the 20th century. The species remained abundant in Tasmania until it rapidly declined from 2001 to 2003, coinciding with a period of unsuitable weather. We provide an updated analysis of eastern quoll population trends in Tasmania using a time series of annual spotlight counts (1985–2019) collected across most of the species’ range. Eastern quolls were widespread and abundant in Tasmania until the early 2000s. In addition to the previously documented severe decline in the early 2000s in the east and northeast, we present new evidence of an earlier decline in the north (mid-1990s) and a more recent decline in the south (~2009). Declines have continued unabated during the last decade, resulting in a ~67% decline since the late 1990s in the area with high quoll abundance. Although the major decline in the early 2000s coincided with unfavourable weather, the continuing and more recent declines suggest other undetermined causes are also involved. We can no longer assume the presence of eastern quolls in Tasmania ensures the species’ long-term survival, highlighting the urgent need to conserve the remaining populations in Tasmania.
Keywords: climate change, change point regression, critical weight range, Dasyurus viverrinus, integrated nested laplace approximation, population decline, random field, range decline, species distribution.
Introduction
Like many small and medium-sized Australian mammal species (Woinarski et al. 2015), the eastern quoll (Dasyurus viverrinus) was once widespread across the south-eastern Australian mainland (Peacock and Abbott 2014) but has declined severely since European colonisation. Eastern quolls have been assumed extinct on the Australian mainland since the mid-to-late 20th century (Burbidge and Woinarski 2016), with the last confirmed sighting in 1963 (Frankham et al. 2017). However recent reports suggest the species possibly survived at low abundance for some time after, with an eastern quoll specimen obtained in 1989 in NSW (Frankham et al. 2017), and several reported sightings in the 2000s from members of the public (Hope et al. 2020). In 2018 and 2019, 60 quolls were reintroduced to Booderee National Park, NSW, as a first step in re-establishing wild eastern quoll populations on the Australian mainland, however the reintroduction ultimately failed, with all animals now assumed deceased (Robinson et al. 2020; Glenday and Kennedy 2021).
Standardised spotlight surveys (1990–2009) and non-systematic data (1967–1989) indicate eastern quolls were abundant and widespread in lutruwita/Tasmania until the early 2000s when densities declined sharply (Rounsevell et al. 1991; Fancourt et al. 2013, 2015a). While these population declines were initially attributed to the mesopredator release of feral cats, stemming from the disease-induced decline of the Tasmanian devil (Hollings et al. 2014), later research suggested they were associated with a period of unsuitable weather over much of the species’ distribution from 2001 to 2003 (Fancourt et al. 2015a). Following a return to suitable weather, the population did not recover, suggesting other factors might be preventing recovery, such as a ‘predator pit’ caused by feral cats (Fancourt et al. 2015a). Based on a 52% decline of spotlighting sightings in the decade to 2009, eastern quolls were listed as Endangered under the IUCN Red List (Burbidge and Woinarski 2016). The species is listed as Endangered under the federal Environment Protection and Biodiversity Conservation Act 1999 but is not listed under the Tasmanian Threatened Species Protection Act 1995.
The most recent published assessment of eastern quoll population trends used survey data from 1990 to 2009 (Fancourt et al. 2013, 2015a). The aim of this paper is to provide an updated analysis of eastern quoll population trends across much of Tasmania, review possible causes of decline, and suggest actions for recovery. We use a 35-year (1985–2019) standardised spotlighting dataset from across Tasmania to characterise changes in the distribution and relative abundance of eastern quolls. We apply recent advances in species distribution modelling to fit spatiotemporal models of relative abundance through time. We also use change-point models to identify the timing of change in regional population trajectories and evaluate whether the onset of decline is consistent with the weather-induced decline hypothesis.
Materials and methods
Data
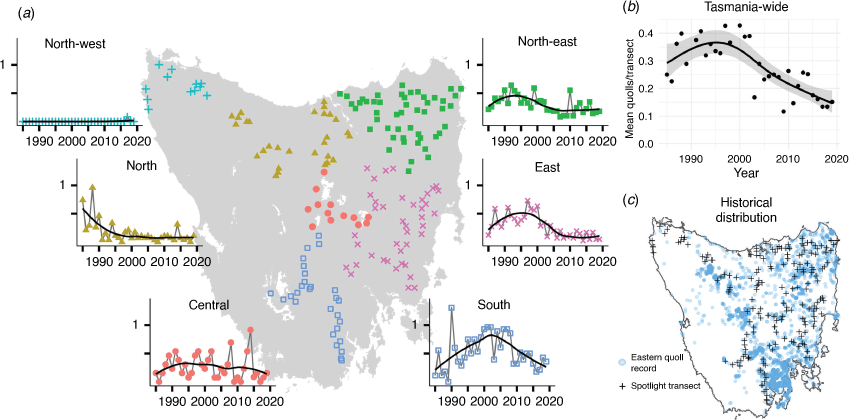
The Tasmanian State Government initiated annual spotlight surveys of wildlife in 1975. The methodology was further standardised and the number of transects increased from 50 to 132 in 1985, and then to ~170 in the 1990s (Supplementary Table S1). Here, we analysed the standardised annual spotlighting surveys across Tasmania from 1985 to 2019, totalling 5761 transect counts (Fig. 1).
|
The spotlight surveys were initially established to monitor populations of herbivores subject to harvest, while recording all sightings of non-domestic mammals, including eastern quolls (Hocking and Driessen 1992). The same transects are surveyed each year, however the number of transects has increased over time (Supplementary Table S1). We visually evaluated the effect of survey additions in the 1990s, which generally did not appear to contribute to the observed trends (Supplementary Fig S1). The most notable difference occurred in the ‘South’ region, where the number of transects increased from 9 in the 1980s to 28 in 1992 (Supplementary Table S1). In this region, analyses of the full dataset resulted in more uniform average annual spotlight counts and weaker inter-annual trends. We therefore opted to analyse the full dataset as the more conservative option and because it comprised a more comprehensive sample of the region.
Each transect follows a 10-km section of hardened or gravel road. Transects are driven once per year at a speed of 20 km/h, with a handheld spotlight used to observe animals on both sides of the road (for details, see Hocking and Driessen 1992; Hollings et al. 2014). Transects are surveyed during the summer months (1985 refers to the summer of 1985/1986 etc.) to ensure comparability across years. However, because transects are only surveyed once per year, this prevents the use of statistical techniques that separate the state and detection processes. While we consider the count of eastern quolls per transect as an index of relative abundance (see Fig. 1 for trends), we acknowledge that the spotlight counts are a combined measure of the detection and abundance processes (MacKenzie and Kendall 2002). We note that year-to-year changes in environmental conditions (e.g. grass heights) could also alter detection probability, but it is unlikely that vegetation-induced changes in detection probability have changed systematically through time. Nevertheless, this long-term dataset is of rare spatial and temporal scope, and has accurately identified population changes in other species including Tasmanian devils (Sarcophilus harrisii; Hawkins et al. 2006; Hollings et al. 2014; Lazenby et al. 2018; Cunningham et al. 2021), bare-nosed wombats (Vombatus ursinus; Carver et al. 2021), and fallow deer (Dama dama; Cunningham et al. 2022). Importantly, the changes in the trajectories of other species have occurred at different points in time and have been verified by additional field methods, providing confidence that the spotlight dataset is capable of accurately measuring population changes despite an inability to separate the abundance and detection processes.
We grouped transects into six regions (Fig. 1) based on proximity. We attempted to align our regions with the IBRA bioregions, with some aggregation necessary in areas where few transects fell in an IBRA bioregion. Our regions align with IBRA bioregions as follows: ‘North-west’ fits within the King bioregion; ‘North’ comprises the Northern Slopes bioregion as well as three southernmost transects that fall just over the boundary of the Central Highlands bioregion; ‘Central’ falls within the eastern half of the Central Highlands bioregion; ‘Northeast’ comprises both the Ben Lomond and Furneaux bioregions; ‘East’ comprises the Northern Midlands and South East bioregions; ‘South’ fits entirely within the Southern Ranges bioregion (DSEWPC 2012).
To visualise the area over which quolls have been historically recorded and approximate their historical geographic range, we downloaded 10 118 presence-only records of eastern quolls from the Tasmanian Natural Values Atlas (Department of Natural Resources and Environment Tasmania 2022) between 1975 and 2022 (downloaded 15 June 2022). This atlas hosts presence-only data originating from a range of sources, and as such, should not be interpreted as systematic survey.
Statistical analysis
Spatial changes in the distribution of eastern quolls
We modelled changes in the distribution of eastern quolls using a spatiotemporal autoregressive model. This approach aims to characterise the realised distribution of eastern quolls through time. We fitted the models using integrated nested Laplace approximation (INLA), an accurate and fast option for Bayesian inference from spatial data. To fit the models, we used the ‘inlabru’ R package (Bachl et al. 2019; R Core Team 2019), which is an extension of the R-INLA package (Rue et al. 2009; Bakka et al. 2018).
INLA is useful for modelling spatial data because spatial dependence between observations can be modelled using a continuous correlation process known as a Gaussian random field. A Gaussian random field is a spatially continuous process where random variables at any point in space are normally distributed and are spatially correlated with other random points via a continuous correlation process (Bachl et al. 2019). R-INLA approximates the continuous correlation process using a stochastic partial differential equation (SPDE). The SPDE requires discretising the study domain into a series of abutting triangles, known as a mesh. We discretised Tasmania into a mesh with internal edge lengths of 10 km, corresponding to the scale of the spotlight transects (inla.mesh.2d function of the R-INLA package).
Using the mesh, a Gaussian random field is then approximated by an SPDE. We constructed a spatiotemporal SPDE to allow the spatial correlation between observations to change through time. To model the temporal dependence in eastern quoll observations between consecutive years, we began by fitting first- and second-order autoregressive models (Gómez-Rubio 2020). These models, however, had convergence problems, probably because of relatively sparse sightings and moderate year-to-year variability. To smooth some of the year-to-year variability, we grouped surveys into 5-year periods, which solved the convergence problems. We fitted the spatio-temporal random field using a first-order autoregressive model (Gómez-Rubio 2020), which in effect correlated observations in consecutive 5-year periods. We used a Matérn correlation structure for the SPDE, and specified a prior probability of 0.1 that the range was less than 10 km and a 0.5 probability that the standard deviation was greater than 1 km. For further details about SPDEs, see Lindgren et al. (2011).
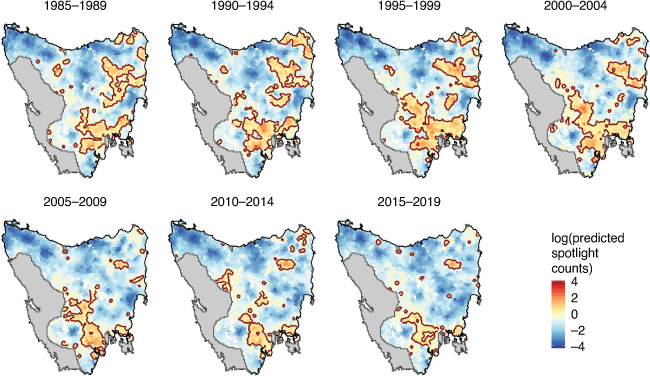
We used the count of eastern quolls per transect as the response variable and fitted the model using the negative binomial distribution to account for overdispersion (Ver Hoef and Boveng 2007). We modelled counts in response to the spatio-temporal Gaussian random field described above. From this model, we produced maps of the relative abundance of eastern quolls in 5-year periods from 1985 to 2019. We visualised areas with high relative abundance using a contour around the areas in which the model-estimated relative abundance was at least one quoll per transect.
Change-point modelling
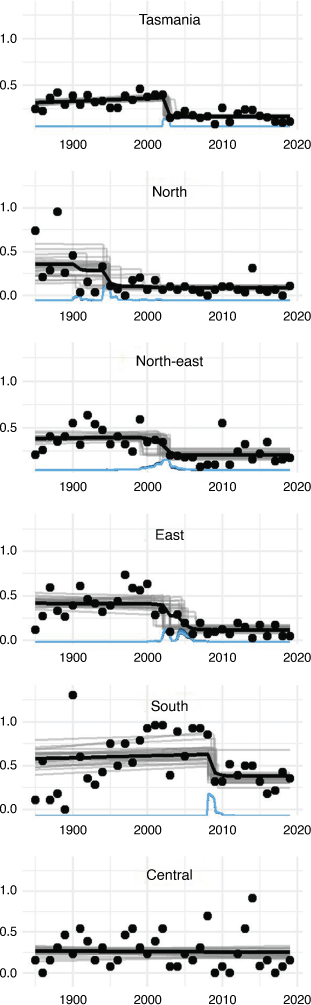
We used change point models to infer if, and when, changes in transect counts of eastern quolls occurred in the different regions of Tasmania. We modelled change points by performing segmented regression with the R package ‘mcp’ (Lindeløv 2020). Because the literature contains strong evidence for a step-change in quoll abundance (Fancourt et al. 2013, 2015a), we considered three-simple models: (1) a one-segment null model with no change point, (2) a two-segment model that included a single disjoined change point (a step change), and (3) a two-segment model with a disjoined change point with differing variance between the segments. We fitted these models for each region and selected the best-performing model using the Estimated Log Predictive Density (ELPD) from approximate leave-one-out cross-validation, in which larger values represent better predictive accuracy.
Results
Prior to the early 2000s, spotlight counts of eastern quolls were increasing in the Northeast, East and South (Fig. 1) and so too was the proportion of transects on which quolls were observed (Fig. 2b). Spatio-temporal distribution models of spotlight data between 1985 and 2019 show that eastern quolls occupied much of eastern Tasmania at high densities until the early 2000s (Fig. 3), when the model-estimated area with at least one quoll per spotlight transect peaked at 11 500 km2 (Fig. 2a). The population then went into a decline that has continued for almost two decades (Fig. 2). Eastern quolls are now regionally abundant only in southern Tasmania, along with small and isolated pockets in other areas (Fig. 3). Since the population peak in the late 1990s, relative abundance measured across most of the species’ range has declined by 60% (Fig. 1b) and the model-estimated area with at least one quoll per transect has declined by 67%, now at ~3750 km2 (Fig. 2a). This declining trend has continued in the most recent decade (Figs. 1, 2). Note that these estimates of percentage change are relative to the population peaks in the late 1990s rather than commencement of monitoring in 1985. We chose this reference point because there is a suggestion that carnivore populations in the 1980s were still recovering from widespread use of strychnine poisoning (McCallum and Jones 2006), with additional hypotheses that an increase in forest-pasture mosaics (Carver et al. 2021) and pasture improvement (Kirkpatrick and Bridle 2007) could also contribute to the increasing trajectories. In either case, the population immediately prior to the severe decline provides the most useful reference point for the population size that could be supported by the current environment.

|
There was strong support for the occurrence of change points that differed among regions (Table 1). The best-performing model for the North, where peak densities were lower than in more southerly populations, indicates a change-point in the early-to-mid 1990s (Fig. 4). In the East and Northeast regions, where initial abundances were among the highest, a distinct step-change occurred in the early 2000s (Fig. 4). In the South region, the population underwent a more recent step-change around 2009. There was no evidence of population change in the Central region.
|

|
Discussion
This study adds to understanding of recent changes in the distribution and abundance of eastern quolls in Tasmania. Using an updated analysis of long-term monitoring data, we confirm earlier conclusions that the Tasmanian population of eastern quolls declined severely during the early 2000s (Fancourt et al. 2013, 2015a). We further show that this decline was composed of regional declines that occurred at different times, occurring first in the North region and most recently in the South (with no decline observed in the Central region). The general decline of the overall population has continued into recent years with little or no change in trend. The cumulative effect of ongoing declines has been very significant: the relative abundance and distribution of eastern quolls in Tasmania has evidently been reduced by more than 60% over the last two decades. The species is likely extinct on mainland Australia, but until recently it was widespread and abundant in Tasmania and was therefore considered to be safe from complete extinction. We can no longer assume that the existence of eastern quolls in Tasmania ensures its long-term survival.
As well as confirming the general decline of eastern quolls in Tasmania, our results provide new understanding of the pattern of decline. Perhaps most concerning is new evidence of a more recent step-change around 2009 in the South region, where the species was thought to be most secure. We also present new evidence that populations in the North region began declining up to 10 years before the onset of decline in the South and East. Pre-decline abundances were lower in the North than in the South and East regions, and the northern parts of Tasmania have generally lower climatic suitability for the species (Fancourt et al. 2015a). Therefore, this geographic pattern of decline could suggest that low-density populations at the periphery of the species’ environmental niche are more susceptible to decline than populations in the core of the niche (Lomolino and Channell 1995). Nonetheless, the ultimate magnitude of reduction in distribution and abundance has been similar in the northern and southern regions. In the Central region, which lies on the cool western margin of the species’ distribution in Tasmania, abundance has been consistently low and there were no indications of a recent change in abundance. A caveat for the Central region is that there are fewer spotlight transects, so our power to detect change is lower. Eastern quolls are present at high density on Bruny Island in the far southeast (Driessen et al. 2011). Bruny Island is not included in the spotlight monitoring program that produced the data analysed here, but anecdotal evidence and unpublished field studies (Cyril Scomparin pers. comm.) suggest the species remains abundant there.
It is not clear what has caused the reduction of abundance and distribution of eastern quolls in Tasmania. Fancourt et al.’s (2015a) study, which revealed a fall in abundance in the early 2000s, attributed this change to a period of unsuitable weather. The climatic niche of the eastern quoll in Tasmania is characterised by cool dry winters (Fancourt et al. 2015a). Between 2000 and 2003, a series of unusually warm and wet winters caused a large temporary contraction in the total area of Tasmania providing that climatic niche. The sharp decline in abundance detected by Fancourt et al. (2015a), and confirmed by our analysis, coincided with this event. Why these changed conditions reduced the abundance of eastern quolls is unknown, but Fancourt et al. (2018) suggested that effects on the phenology of key invertebrate or vertebrate prey might have been involved. Favourable weather conditions had returned to most of the species’ range by 2005, but abundance did not recover, presumably because some other factor prevented this.
Our results suggest that the causes of decline may be more complex than proposed by Fancourt et al. (2015a), for two reasons. First, we show that in the North of Tasmania, eastern quoll relative abundance began falling in the early 1990s, when Fancourt et al.’s (2015a) analysis suggests weather conditions were generally favourable for the species throughout its range. It remains plausible that changed weather conditions triggered later declines in the East and Northeast, but it is difficult to make that case for the earlier declines in the North. Possibly, some localised weather event triggered those early declines, during a period when eastern quoll populations in the South and East were increasing (as also shown by our analysis), but at present it is not clear what that event might have been. Second, while the weather anomaly of the early 2000s may be able to account for the onset of decline in the core of the species’ range in the Northeast and East, it cannot explain why declines have continued throughout Tasmania since that time. Other causes are likely responsible for this, but at present it is not clear what those factors might be, or which of several plausible factors is most important.
A leading candidate is predation by the feral cat (Felis catus). The eastern quoll is among the Australian native mammal species with the highest susceptibility to invasive predators, including the feral cat (Radford et al. 2018). Feral cats are widespread in Tasmania, and their densities are highest in the agricultural regions that form a substantial part of the former distribution of eastern quolls (Hollings et al. 2014; Hamer et al. 2021). Predation by cats, particularly of juvenile quolls, may hold low-density quoll populations in a ‘predator pit’, and explain why populations that declined during temporary periods of unfavourable weather did not subsequently recover (Fancourt et al. 2015b). Elsewhere in Australia, the northern quoll has suffered substantial range contractions to topographically complex environments, presumably where predation from introduced predators poses a lower threat (Moore et al. 2019).
Densities of feral cats have increased across much of eastern Tasmania following declines of the Tasmanian devil due to disease (Hollings et al. 2014, 2016; Cunningham et al. 2020). This disease emerged in northeast Tasmania in the mid-1980s (Patton et al. 2020) with substantial population declines commencing in the mid-1990s (Hawkins et al. 2006; Cunningham et al. 2021). The increasing abundance of feral cats could help to explain the continuing declines of eastern quolls across most of their distributional range. Furthermore, the effects of cats may interact with changes to the structure of low, dense vegetation that provides refuge and escape possibilities (as shown for small mammals in northern Australia; McGregor et al. 2014, 2015; Leahy et al. 2016). The role of feral cats and vegetation structure in suppressing eastern quolls could be experimentally tested at large scale using translocations of quolls into areas where quolls persist at low densities or have recently disappeared, combined with supplementation of vegetative cover, artificial refuges and short to medium-term control of feral cats. Any investigation of the causes of continuing decline of eastern quolls should also consider possible effects of food availability due to changes in land use and climate.
When the eastern quoll was listed as Endangered under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999, the Threatened Species Scientific Committee (2015) prepared conservation advice and recommended recovery actions. Since publication of this advice, most effort has been directed towards ex-situ management, including the establishment of a captive breeding program under the Tasmanian Quoll Conservation Program, establishment of insurance populations in fenced sanctuaries on the mainland (e.g. Wilson et al. 2020), and reintroduction to the wild on the Australian mainland (Robinson et al. 2020). Some consideration has also been given to establishing the species on offshore islands (Barlow et al. 2021). However, there has been limited work towards halting and reversing declines within their extant Tasmanian distribution, except for plans to manage feral cats on Bruny Island.
In light of the continuing decline of wild populations of eastern quolls, we recommend additional focus on safeguarding populations in Tasmania. First, a critical review of the state-wide monitoring of the species is required. We suggest that it would valuable to expand the spotlight surveys into areas that are currently unmonitored (e.g. western Tasmania), and use other monitoring techniques (e.g. cameras) in areas of the Central Plateau that are within the historical range of eastern quolls but where an absence of roads makes spotlight surveys unfeasible. The spotlighting transects provide an invaluable long-term dataset but were designed to monitor harvested species (Hocking and Driessen 1992). Because each transect is surveyed only once per year, there is no obvious way to use this dataset alone to disentangle changes in abundance versus changes in detection probability, or the potential for changes in spotlight counts to arise from changes in the observers. Thus, it is possible the observed trends are driven by a change in either detection probability or abundance, or both. To combat this shortcoming, future research may benefit from recent advances in integrated modelling, which can facilitate the simultaneous analysis of multiple datasets while leveraging the strengths of each (Miller et al. 2019). Jointly analysing the spotlight dataset in conjunction with an occupancy model of camera data may allow future researchers to separate the detection and abundance processes, while continuing to leverage the long-term value of the spotlight dataset. Importantly, this may help correct for likely biases in sightability in different vegetation classes. Second, preliminary results from a recent pilot study in the Tasmanian central highlands support supplementing wild populations with captive-bred animals (Hamer et al. 2022). If this produces a sustained increase in the abundance of wild populations, extending these trials to lowland regions where declines have been most severe could be valuable. Although there is urgency to act while quolls still occupy some parts of Tasmania in moderate abundance, recovery actions need to fit within an experimental and adaptive management framework. Such a framework that monitors the fates of released individuals can provide valuable information on the threats and major causes of mortality at release sites (e.g. Robinson et al. 2020) and therefore help to test hypotheses about the factors responsible for the original and ongoing population declines.
In conclusion, declines in eastern quoll populations in Tasmania have continued over the last two decades. The extinction of this species in the wild is a possibility requiring urgent investment and attention. Ensuring that wild quolls persist in the wild should be given high priority, and conservation actions should be embedded within an experimental framework to diagnose current drivers of population decline.
Data availability
The spotlight data used in this paper were used under agreement with the Department of Natural Resources and Environment Tasmania (formerly Department of Primary Industries, Parks, Water and Environment). Please contact them to obtain the data.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Supplementary material
Supplementary material is available online.
Acknowledgements
We would like to acknowledge the palawa peoples of lutruwita, the traditional custodians of the lands on which this work was completed. We thank Michael Driessen, Greg Hocking and the Department of Natural Resources and Environment Tasmania for providing the spotlight dataset.
References
Bachl, F. E., Lindgren, F., Borchers, D. L., and Illian, J. B. (2019). inlabru: an R package for Bayesian spatial modelling from ecological survey data. Methods in Ecology and Evolution 10, 760–766.| inlabru: an R package for Bayesian spatial modelling from ecological survey data.Crossref | GoogleScholarGoogle Scholar |
Bakka, H., Rue, H., Fuglstad, G.-A., Riebler, A., Bolin, D., Illian, J., Krainski, E., Simpson, D., and Lindgren, F. (2018). Spatial modeling with R-INLA: A review. WIREs Computational Statistics 10, e1443.
| Spatial modeling with R-INLA: A review.Crossref | GoogleScholarGoogle Scholar |
Barlow, M. M., Johnson, C. N., McDowell, M. C., Fielding, M. W., Amin, R. J., and Brewster, R. (2021). Species distribution models for conservation: identifying translocation sites for eastern quolls under climate change. Global Ecology and Conservation 29, e01735.
| Species distribution models for conservation: identifying translocation sites for eastern quolls under climate change.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A., and Woinarski, J. (2016). Dasyurus viverrinus. The IUCN Red List of Threatened Species. Available at
| Crossref | [downloaded on 13 October 2020]
Carver, S., Charleston, M., Hocking, G., Gales, R., and Driessen, M. M. (2021). Long-Term Spatiotemporal Dynamics and Factors Associated with Trends in Bare-Nosed Wombats. The Journal of Wildlife Management 85, 449–461.
| Long-Term Spatiotemporal Dynamics and Factors Associated with Trends in Bare-Nosed Wombats.Crossref | GoogleScholarGoogle Scholar |
Cunningham, C. X., Johnson, C. N., and Jones, M. E. (2020). A native apex predator limits an invasive mesopredator and protects native prey: Tasmanian devils protecting bandicoots from cats. Ecology Letters 23, 711–721.
| A native apex predator limits an invasive mesopredator and protects native prey: Tasmanian devils protecting bandicoots from cats.Crossref | GoogleScholarGoogle Scholar |
Cunningham, C. X., Comte, S., McCallum, H., Hamilton, D. G., Hamede, R., Storfer, A., Hollings, T., Ruiz-Aravena, M., Kerlin, D. H., Brook, B. W., Hocking, G., and Jones, M. E. (2021). Quantifying 25 years of disease-caused declines in Tasmanian devil populations: host density drives spatial pathogen spread. Ecology Letters 24, 958–969.
| Quantifying 25 years of disease-caused declines in Tasmanian devil populations: host density drives spatial pathogen spread.Crossref | GoogleScholarGoogle Scholar |
Cunningham, C. X., Perry, G. L. W., Bowman, D. M. J. S., Forsyth, D. M., Driessen, M. M., Appleby, M., Brook, B. W., Hocking, G., Buettel, J. C., French, B. J., Hamer, R., Bryant, S. L., Taylor, M., Gardiner, R., Proft, K., Scoleri, V. P., Chiu-Werner, A., Travers, T., Thompson, L., Guy, T., and Johnson, C. N. (2022). Dynamics and predicted distribution of an irrupting ‘sleeper’ population: fallow deer in Tasmania. Biological Invasions 24, 1131–1147.
| Dynamics and predicted distribution of an irrupting ‘sleeper’ population: fallow deer in Tasmania.Crossref | GoogleScholarGoogle Scholar |
Department of Natural Resources and Environment Tasmania (2022). Natural Values Atlas v.3.11.1. Dataset downloaded June 15 2022. Available at https://www.naturalvaluesatlas.tas.gov.au/
Driessen, M., Carlyon, K., Gales, R., Mooney, N., Pauza, M., Thurstans, S., Visoiu, M., and Wise, P. (2011). Terrestrial mammals of a sheep-grazing property on Bruny Island, Tasmania. Papers and Proceedings of the Royal Society of Tasmania 145, 51–64.
| Terrestrial mammals of a sheep-grazing property on Bruny Island, Tasmania.Crossref | GoogleScholarGoogle Scholar |
DSEWPC (Department of Sustainability, Environment, Water, Population, and Communities) (2012). Interim Biogeographic Regionalisation for Australia (IBRA), Version 7. URL: https://www.environment.gov.au/fed/catalog/search/resource/details.page?uuid=%7B1273FBE2-F266-4F3F-895D-C1E45D77CAF5%7D
Fancourt, B. A., Hawkins, C. E., and Nicol, S. C. (2013). Evidence of rapid population decline of the eastern quoll (Dasyurus viverrinus) in Tasmania. Australian Mammalogy 35, 195–205.
| Evidence of rapid population decline of the eastern quoll (Dasyurus viverrinus) in Tasmania.Crossref | GoogleScholarGoogle Scholar |
Fancourt, B. A., Bateman, B. L., VanDerWal, J., Nicol, S. C., Hawkins, C. E., Jones, M. E., and Johnson, C. N. (2015a). Testing the Role of Climate Change in Species Decline: Is the Eastern Quoll a Victim of a Change in the Weather? PLoS One 10, e0129420.
| Testing the Role of Climate Change in Species Decline: Is the Eastern Quoll a Victim of a Change in the Weather?Crossref | GoogleScholarGoogle Scholar |
Fancourt, B. A., Hawkins, C. E., Cameron, E. Z., Jones, M. E., and Nicol, S. C. (2015b). Devil declines and catastrophic cascades: is mesopredator release of feral cats inhibiting recovery of the eastern quoll? PLoS One 10, e0119303.
| Devil declines and catastrophic cascades: is mesopredator release of feral cats inhibiting recovery of the eastern quoll?Crossref | GoogleScholarGoogle Scholar |
Fancourt, B. A., Hawkins, C. E., and Nicol, S. C. (2018). Mechanisms of climate-change-induced species decline: spatial, temporal and long-term variation in the diet of an endangered marsupial carnivore, the eastern quoll. Wildlife Research 45, 737–750.
| Mechanisms of climate-change-induced species decline: spatial, temporal and long-term variation in the diet of an endangered marsupial carnivore, the eastern quoll.Crossref | GoogleScholarGoogle Scholar |
Frankham, G. J., Thompson, S., Ingleby, S., Soderquist, T., and Eldridge, M. D. B. (2017). Does the ‘extinct’ eastern quoll (Dasyurus viverrinus) persist in Barrington Tops, New South Wales?. Australian Mammalogy 39, 243–247.
| Does the ‘extinct’ eastern quoll (Dasyurus viverrinus) persist in Barrington Tops, New South Wales?.Crossref | GoogleScholarGoogle Scholar |
Glenday, J., and Kennedy, A. (2021). Can extinct eastern quolls really return to the wild? ABC News, 21 May 2021. Available at https://www.abc.net.au/news/2021-05-22/can-extinct-australian-eastern-quolls-return-to-wild/100140048?utm_medium=social&utm_content=sf246101740&utm_campaign=abc_illawarra&utm_source=m.facebook.com&sf246101740=1
Gómez-Rubio, V. (2020). Chapter 8 Temporal Models. In ‘Bayesian inference with INLA’. (CRC Press.) Available at https://becarioprecario.bitbucket.io/inla-gitbook/ch-temporal.html
Hamer, R. P., Gardiner, R. Z., Proft, K. M., Johnson, C. N., and Jones, M. E. (2021). A triple threat: high population density, high foraging intensity and flexible habitat preferences explain high impact of feral cats on prey. Proceedings of the Royal Society B: Biological Sciences 288, 20201194.
| A triple threat: high population density, high foraging intensity and flexible habitat preferences explain high impact of feral cats on prey.Crossref | GoogleScholarGoogle Scholar |
Hamer, R. P., Robinson, N., Brewster, R., Barlow, M., Guinane, M., Humphrey, M., Mifsud, A., and Kutt, A. S. (2022). Not waiting for the death knell. A pilot study to examine supplementation and survivorship in a declining population of Tasmanian eastern quoll (Dasyurus viverrinus). Research Square , .
| Not waiting for the death knell. A pilot study to examine supplementation and survivorship in a declining population of Tasmanian eastern quoll (Dasyurus viverrinus).Crossref | GoogleScholarGoogle Scholar |
Hawkins, C. E., Baars, C., Hesterman, H., Hocking, G. J., Jones, M. E., Lazenby, B., Mann, D., Mooney, N., Pemberton, D., Pyecroft, S., Restani, M., and Wiersma, J. (2006). Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biological Conservation 131, 307–324.
| Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii.Crossref | GoogleScholarGoogle Scholar |
Hocking, G. J., and Driessen, M. M. (1992). ‘Tasmanian spotlight survey manual: a set of instructions and maps for conducting spotlight surveys in Tasmania.’ (Department of Parks Wildlife and Heritage.)
Hollings, T., Jones, M., Mooney, N., and McCallum, H. (2014). Trophic cascades following the disease-induced decline of an apex predator, the Tasmanian devil. Conservation Biology 28, 63–75.
| Trophic cascades following the disease-induced decline of an apex predator, the Tasmanian devil.Crossref | GoogleScholarGoogle Scholar |
Hollings, T., Jones, M., Mooney, N., and McCallum, H. (2016). Disease-induced decline of an apex predator drives invasive dominated states and threatens biodiversity. Ecology 97, 394–405.
| Disease-induced decline of an apex predator drives invasive dominated states and threatens biodiversity.Crossref | GoogleScholarGoogle Scholar |
Hope, B., Soderquist, T., and Eldridge, M. D. B. (2020). Eastern quoll (Dasyurus viverrinus Shaw, 1800): a review of recent sightings on mainland Australia. Australian Mammalogy 42, 144–151.
| Eastern quoll (Dasyurus viverrinus Shaw, 1800): a review of recent sightings on mainland Australia.Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick, J., and Bridle, K. (2007). ‘People, sheep and nature conservation: the Tasmanian experience.’ (CSIRO PUBLISHING.)
Lazenby, B. T., Tobler, M. W., Brown, W. E., Hawkins, C. E., Hocking, G. J., Hume, F., Huxtable, S., Iles, P., Jones, M. E., Lawrence, C., Thalmann, S., Wise, P., Williams, H., Fox, S., and Pemberton, D. (2018). Density trends and demographic signals uncover the long-term impact of transmissible cancer in Tasmanian devils. Journal of Applied Ecology 55, 1368–1379.
| Density trends and demographic signals uncover the long-term impact of transmissible cancer in Tasmanian devils.Crossref | GoogleScholarGoogle Scholar |
Leahy, L., Legge, S. M., Tuft, K., McGregor, H. W., Barmuta, L. A., Jones, M. E., and Johnson, C. N. (2016). Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildlife Research 42, 705–716.
| Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas.Crossref | GoogleScholarGoogle Scholar |
Lindeløv, J. K. (2020). mcp: An R package for regression with multiple change points. OSF Preprints [Preprint]
| Crossref |
Lindgren, F., Rue, H., and Lindström, J. (2011). An explicit link between Gaussian fields and Gaussian Markov random fields: the stochastic partial differential equation approach. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 73, 423–498.
| An explicit link between Gaussian fields and Gaussian Markov random fields: the stochastic partial differential equation approach.Crossref | GoogleScholarGoogle Scholar |
Lomolino, M. V., and Channell, R. (1995). Splendid Isolation: Patterns of Geographic Range Collapse in Endangered Mammals. Journal of Mammalogy 76, 335–347.
| Splendid Isolation: Patterns of Geographic Range Collapse in Endangered Mammals.Crossref | GoogleScholarGoogle Scholar |
MacKenzie, D. I., and Kendall, W. L. (2002). How should detection probability be incorporated into estimates of relative abundance? Ecology 83, 2387–2393.
| How should detection probability be incorporated into estimates of relative abundance?Crossref | GoogleScholarGoogle Scholar |
McCallum, H., and Jones, M. (2006). To Lose Both Would Look Like Carelessness: Tasmanian Devil Facial Tumour Disease. PLOS Biology 4, e342.
| To Lose Both Would Look Like Carelessness: Tasmanian Devil Facial Tumour Disease.Crossref | GoogleScholarGoogle Scholar |
McGregor, H. W., Legge, S., Jones, M. E., and Johnson, C. N. (2014). Landscape Management of Fire and Grazing Regimes Alters the Fine-Scale Habitat Utilisation by Feral Cats. PLoS One 9, e109097.
| Landscape Management of Fire and Grazing Regimes Alters the Fine-Scale Habitat Utilisation by Feral Cats.Crossref | GoogleScholarGoogle Scholar |
McGregor, H., Legge, S., Jones, M. E., and Johnson, C. N. (2015). Feral cats are better killers in open habitats, revealed by animal-borne video. PLoS One 10, e0133915.
| Feral cats are better killers in open habitats, revealed by animal-borne video.Crossref | GoogleScholarGoogle Scholar |
Miller, D. A. W., Pacifici, K., Sanderlin, J. S., and Reich, B. J. (2019). The recent past and promising future for data integration methods to estimate species’ distributions. Methods in Ecology and Evolution 10, 22–37.
| The recent past and promising future for data integration methods to estimate species’ distributions.Crossref | GoogleScholarGoogle Scholar |
Moore, H. A., Dunlop, J. A., Valentine, L. E., Woinarski, J. C. Z., Ritchie, E. G., Watson, D. M., and Nimmo, D. G. (2019). Topographic ruggedness and rainfall mediate geographic range contraction of a threatened marsupial predator. Diversity and Distributions 25, 1818–1831.
| Topographic ruggedness and rainfall mediate geographic range contraction of a threatened marsupial predator.Crossref | GoogleScholarGoogle Scholar |
Patton, A. H., Lawrance, M. F., Margres, M. J., Kozakiewicz, C. P., Hamede, R., Ruiz-Aravena, M., Hamilton, D. G., Comte, S., Ricci, L. E., Taylor, R. L., Stadler, T., Leaché, A., McCallum, H., Jones, M. E., Hohenlohe, P. A., and Storfer, A. (2020). A transmissible cancer shifts from emergence to endemism in Tasmanian devils. Science 370, eabb9772.
| A transmissible cancer shifts from emergence to endemism in Tasmanian devils.Crossref | GoogleScholarGoogle Scholar |
Peacock, D., and Abbott, I. (2014). When the ‘native cat’ would ‘plague’: historical hyperabundance in the quoll (Marsupialia : Dasyuridae) and an assessment of the role of disease, cats and foxes in its curtailment. Australian Journal of Zoology 62, 294–344.
| When the ‘native cat’ would ‘plague’: historical hyperabundance in the quoll (Marsupialia : Dasyuridae) and an assessment of the role of disease, cats and foxes in its curtailment.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2019). ‘R: A Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna, Austria.)
Radford, J. Q., Woinarski, J. C. Z., Legge, S., Baseler, M., Bentley, J., Burbidge, A. A., Bode, M., Copley, P., Dexter, N., Dickman, C. R., Gillespie, G., Hill, B., Johnson, C. N., Kanowski, J., Latch, P., Letnic, M., Manning, A., Menkhorst, P., Mitchell, N., Morris, K., Moseby, K., Page, M., and Ringma, J. (2018). Degrees of population-level susceptibility of Australian terrestrial non-volant mammal species to predation by the introduced red fox (Vulpes vulpes) and feral cat (Felis catus). Wildlife Research 45, 645–657.
| Degrees of population-level susceptibility of Australian terrestrial non-volant mammal species to predation by the introduced red fox (Vulpes vulpes) and feral cat (Felis catus).Crossref | GoogleScholarGoogle Scholar |
Robinson, N. M., Dexter, N., Brewster, R., Maple, D., MacGregor, C., Rose, K., Hall, J., and Lindenmayer, D. B. (2020). Be nimble with threat mitigation: lessons learned from the reintroduction of an endangered species. Restoration Ecology 28, 29–38.
| Be nimble with threat mitigation: lessons learned from the reintroduction of an endangered species.Crossref | GoogleScholarGoogle Scholar |
Rounsevell, D., Taylor, R., and Hocking, G. (1991). Distribution records of native terrestrial mammals in Tasmania. Wildlife Research 18, 699–717.
| Distribution records of native terrestrial mammals in Tasmania.Crossref | GoogleScholarGoogle Scholar |
Rue, H., Martino, S., and Chopin, N. (2009). Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 71, 319–392.
| Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations.Crossref | GoogleScholarGoogle Scholar |
Threatened Species Scientific Committee (2015). ‘Conservation Advice Dasyurus viverrinus Eastern Quoll.’ (Department of the Environment: Canberra.)
Ver Hoef, J. M., and Boveng, P. L. (2007). Quasi‐Poisson vs. negative binomial regression: how should we model overdispersed count data? Ecology 88, 2766–2772.
| Quasi‐Poisson vs. negative binomial regression: how should we model overdispersed count data?Crossref | GoogleScholarGoogle Scholar |
Wilson, B. A., Evans, M. J., Batson, W. G., Banks, S. C., Gordon, I. J., Fletcher, D. B., Wimpenny, C., Newport, J., Belton, E., Rypalski, A., Portas, T., and Manning, A. D. (2020). Adapting reintroduction tactics in successive trials increases the likelihood of establishment for an endangered carnivore in a fenced sanctuary. PLoS One 15, e0234455.
| Adapting reintroduction tactics in successive trials increases the likelihood of establishment for an endangered carnivore in a fenced sanctuary.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Burbidge, A. A., and Harrison, P. L. (2015). Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences 112, 4531–4540.
| Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement.Crossref | GoogleScholarGoogle Scholar |
† A preprint of an earlier version of this manuscript can be found at https://doi.org/10.1101/2022.03.10.483855.