Observations on the behaviour of the northern hairy-nosed wombat (Lasiorhinus krefftii) in a translocated population
Kristina Sand Jørgensen A E , Alistair Melzer A , Dave Harper B and Owen T. Nevin A C DA School of Health, Medical and Applied Sciences, Central Queensland University, CQIRP, Ibis Avenue, Rockhampton, Qld 4702, Australia.
B Threatened Species Program, Department of Environment and Science, 55 Priors Pocket Road, Moggill, Qld 4070, Australia.
C University of Cumbria, Rydal Road, Ambleside, LA22 9BB, United Kingdom.
D Western Australian Biodiversity Science Institute, 133 St Georges Terrace, Perth, WA 6000, Australia.
E Corresponding author. Email: kristina.jorgensen@cqumail.com
Australian Mammalogy 43(1) 132-136 https://doi.org/10.1071/AM20010
Submitted: 1 February 2020 Accepted: 13 December 2020 Published: 20 January 2021
Journal Compilation © Australian Mammal Society 2021 Open Access CC BY-NC-ND
Abstract
The natural distribution of the critically endangered northern hairy-nosed wombat (Lasiorhinus krefftii) is confined to Epping Forest National Park, Queensland; however, a small number of animals have been translocated to establish an insurance population at Richard Underwood Nature Refuge (RUNR), Queensland. Northern hairy-nosed wombat behaviour is poorly understood, mostly due to its cryptic behaviour. Thirty-two wildlife cameras set up at burrow mouths at RUNR were used to capture social and solitary behaviour. Over a six month period between December 2016 and May 2017, 0.3% (21 videos of 6607) of recordings captured social behaviour, suggesting that the northern hairy-nosed wombat actively avoids social interactions at the burrow mouth. Vocalisation was only observed during social interaction. The results were similar to data from Epping Forest National Park and studies on other wombat species. In this respect the translocated population appeared to behave in a manner typical of the wild population.
Keywords: burrowing behaviour, mammal, marsupial, semi-arid, social interactions, translocation, wombats.
Introduction
Understanding the behaviour of endangered species can assist their conservation. The critically endangered northern hairy-nosed wombat (Lasiorhinus krefftii) is a large, herbivorous marsupial that displays nocturnal behaviour and a semi-fossorial nature (Shimmin and White 2002; Horsup 2004; Hogan et al. 2009). The species’ only natural population is confined to Epping Forest National Park, Queensland (22°21′S, 146°42′E) (Johnson 1991b); however, a small translocated population has been established within the species’ former range at St. George, Queensland (Johnson 1991b; Dinwoodie 2012) within the Richard Underwood Nature Refuge (RUNR; 27°40′3.62′′S, 148°42′14.27′′E). Between July 2009 and September 2010 five male and ten female wombats were brought to the site. Taking into account births and mortalities the population during this study was 10 animals.
The behaviour of the northern hairy-nosed wombat has been little studied due to the species’ nocturnal activity patterns and semi-fossorial behaviour (Johnson and Crossman 1990; Hogan et al. 2009). It is generally agreed, however, that the northern hairy-nosed wombat displays solitary behaviour (Johnson 1991a). Stenke (2000) directly observed social interactions between individuals at Epping Forest National Park; however, from 1300 observation hours, only 12 social interaction observations (wombat–wombat) were made.
The objectives of this study were to: (a) describe elements of solitary and social behavioural patterns of wild northern hairy-nosed wombats in the vicinity of burrow entrances; and (b) determine the effectiveness of using remote camera traps for behavioural studies of wombats.
Methods
Field site
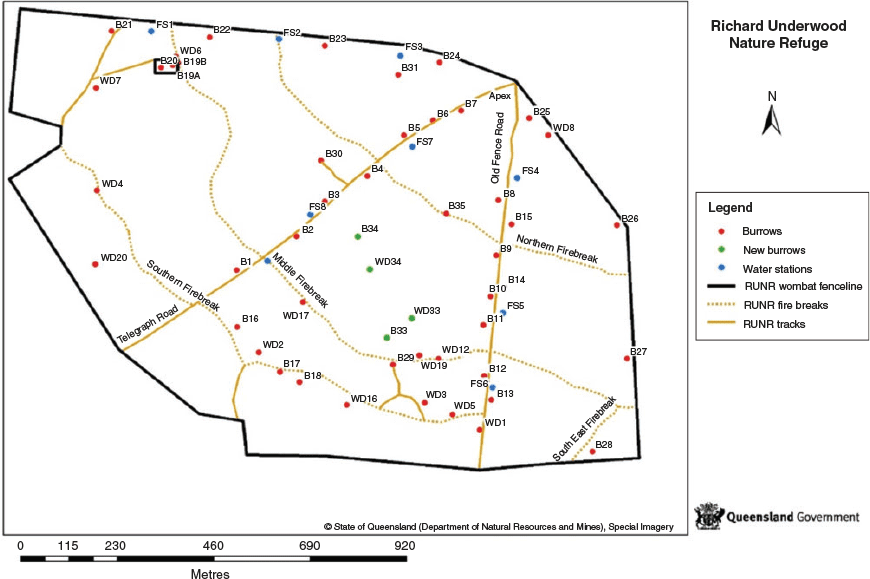
The RUNR is a 130 ha property surrounded by agricultural land, near St. George, Queensland, and is managed by the Queensland Department of Environment and Science (DES). The major vegetation communities found are tussock grassland, mainly dominated by the introduced buffel grass (Cenchrus ciliaris), open woodland with species including poplar box (Eucalyptus populnea), and dense woodland especially dominated by cypress pine (Callitris preissii). There are 61 burrows within the RUNR site (Fig. 1), occupied by 10 translocated northern hairy-nosed wombats, with 20 burrows recognised as ‘primary’ burrows (DES, unpubl. data) –currently known to be frequently occupied by wombats.
|
Remote camera monitoring
Eighteen burrows are equipped with one or more cameras in an ongoing monitoring program using 32 camera traps (Trophy Cam HD 119466/119467 and Trail Aggressor Cam HD 119776C, Bushnell). Cameras were only set at burrows known to be active (determined by presence of fresh tracks and scats) and were situated at the end of the burrow ‘runway’, at a distance of 3–5 m, to minimise disturbance.
Camera SD cards were retrieved every two days. All cameras were set to record 30 s videos when triggered, with 10 s delay between triggers, and were mounted on solar panel tripods (SLIK F153) to maintain battery charge. As the cameras had been deployed for approximately five years, wombats are assumed to be habituated to the cameras, and not to display any altered behaviour on recordings. Data were collected for a six month period (December 2016–May 2017).
Wombat behaviour
Following Stenke (2000), solitary behaviour was defined as any activity by a single wombat recorded in the field of view of a camera placed at the mouth of the burrow. Social behaviour was defined as any activity by two or more wombats recorded in the same field of view. However, these definitions are only based on the focal area of the behavioural observations, at the burrow mouth. Behavioural interactions outside and away from the burrow mouth were not investigated in this study. All video captures which included more than one wombat were identified within the dataset and extracted for analysis. Due to the highly unbalanced nature of the observations (0.3% social, 99.7% solitary) a randomised subset of solitary observations was selected to create numerical balance using a random number generator; this sample was stratified by date to ensure seasonal effects were captured in the analysis.
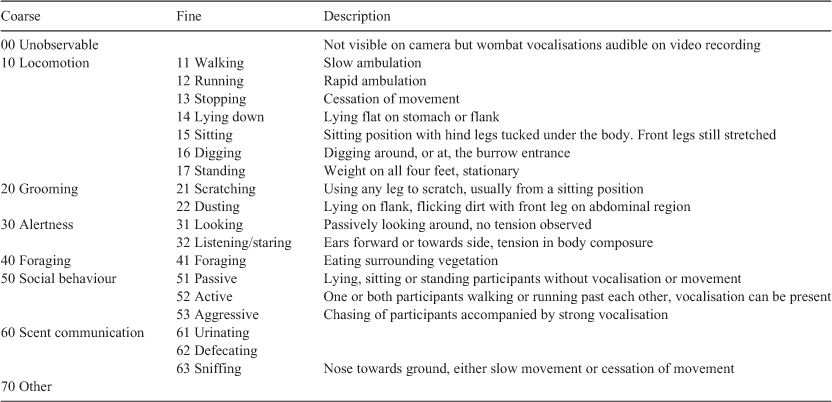
Videos were treated as focal animal samples (Altman 1974) and scored using an ethogram developed specifically for this study with more detailed levels of behavioural definition (Fine) nested within broader behavioural categories (Coarse) to enable post-hoc clustering of behaviours if required for analysis (Table 1). Behavioural descriptions are modified from those defined in previous wombat studies, and other fauna studies (Stenke 2000; Hogan 2004; Nevin and Gilbert 2005; Hogan et al. 2009).
|
Statistics
A contingency table analysis was used to test the difference between time in coarse behavioural categories for solitary and social wombats. A time budget was developed across the six month observation period for both solitary wombats and social interactions, outlining the basic behaviour of vocalisation, compared to the corresponding solitary behaviour. Behaviour was classified in three ways; (1) coarse behaviour, (2) fine behaviour and (3) ‘social’ behaviour.
Results
A total of 6607 video captures were collected between December 2016 and May 2017. Of these, just 21 (0.3%) represent social events, with two wombats present; the remainder were solitary individuals. There were no video captures with more than two wombats present. No wombats could be individually identified. Social events are therefore described between individuals as a broad description independent of sex or age.
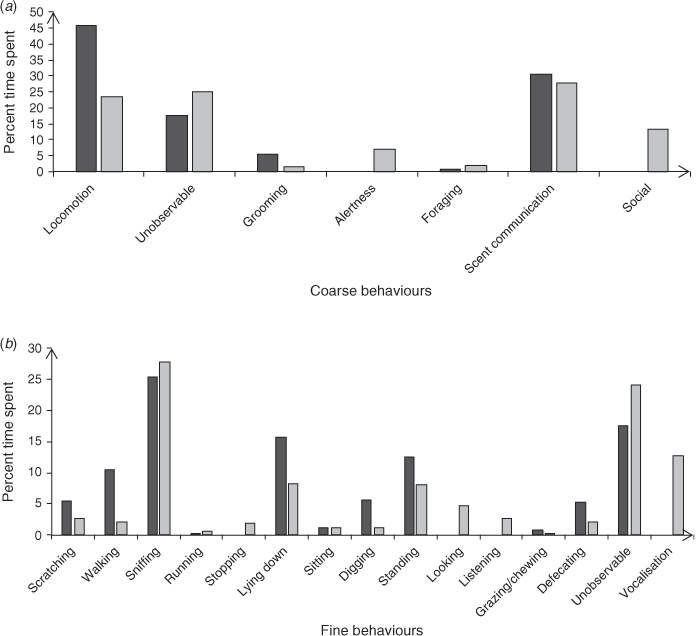
In cases where two animals appeared together, interaction occurred. This included chasing (in aggressive instances), vocalising and alertness. In the few instances where two animals were captured on camera vocalising, it appeared to be the approaching animal which started calling. Behaviours observed during social events included running, alertness, sitting and standing (Fig. 2). It was found that the coarse behaviour locomotion decreased during social interactions by approximately 50%.
The main behaviours of solitary animals were locomotion (walking and lying down), grooming (scratching) and scent marking (defecating) (Fig. 2). Additional behaviours observed in solitary events included lying down, sitting, and grooming.
There was a significant difference (χ2 = 12.59, d.f. = 6, P ≤ 0.01) in the frequencies of coarse behavioural categories between solitary and social individuals.
Discussion
This is the first study to examine the behaviour of a translocated population of the northern hairy-nosed wombat, and only the second behavioural study with this species overall. Out of the 6607 videos recorded, only 0.3% showed social interaction. Similar results were found by Stenke (2000), where out of 1300 observation hours, only 12 incidents of social behaviour were recorded. This suggests that the northern hairy-nosed wombat may avoid social interaction, presumably to facilitate maintenance of low metabolic activity, and digestion of low quality food with no excess energy expenditure (Woolnough 1998). However social interactions were occasionally recorded at the burrow entrances, and usually with apparent agonistic behaviour displays including alertness, vocalisation, and avoidance or chasing.
The use of digital video recording systems for monitoring the behaviour of the northern hairy-nosed wombat was shown to be successful but was limited to the immediate vicinity of the burrow entrances. Johnson and Crossman (1991) found similar results, where both solitary and communal behaviour were recorded in the species. Communal behaviour was referred to as an overlap of burrow use. In this study the frequency of solitary behaviour and social behavioural events were significantly different.
Vocalisations were only recorded at burrow entrance when two wombats were present. A significant increase in energy intensive behaviours, including walking and running, during social interactions was also seen. It may be that this increased energy cost is potentially driving the low occurrence of social interaction at burrow entrances. If this high frequency of solitary behaviour is a consequence of active avoidance, the mechanism for such avoidance remains unclear.
This study has demonstrated that cameras are a suitable method for monitoring and assessing the behaviour of northern hairy-nosed wombats at occupied burrows. Further investigation of behaviour can be accomplished by GPS-collaring, to explore ranging behaviour and provide more detailed data on burrow sharing in the northern hairy-nosed wombat. Social behaviour can furthermore be explored by adding proximity loggers to collars. This has been used in cattle (Patison et al. 2010) and koalas (Ellis et al. 2015), to determine interaction between individuals, and would potentially give an additional perspective in social behaviour of the northern hairy-nosed wombat.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
A special thanks to the Department of Environment and Heritage Protection for their help and contribution with fieldwork and technical advice for this project, and Central Queensland University for equipment used during the project. Funding was provided from the Wombat Foundation through the Department of Environment and Heritage Protection to assist with the project. Post Graduate Support Funding was received from the Central Queensland University to assist with the project, and a stipend provided from the Danish Government to KSJ was allocated throughout the duration of the project.
References
Altmann, J. (1974). Observational study of Behavior: sampling methods. Behaviour 49, 227–266.| Observational study of Behavior: sampling methods.Crossref | GoogleScholarGoogle Scholar | 4597405PubMed |
Dinwoodie, A. (2012). Northern hairy-nosed wombat, Lasiorhinus krefftii. In ‘Queensland’s threatened animals’. (Eds; L. K. Curtis, A. J. Dennis, K. R. McDonald, P. M. Kyne and S. J. S. Debus). pp. 354–355. (CSIRO Publishing: Melbourne.)
Ellis, W., Fitzgibbon, S., Pye, G., Whipple, B., Barth, B., Johnston, S., Seddon, J., Melzer, A., Higgins, D., and Bercovitch, F. (2015). The Role of Bioacoustic Signals in Koala Sexual Selection: Insights from Seasonal Patterns of Associations Revealed with GPS-Proximity Units. Plos One 10, e0130657.
| The Role of Bioacoustic Signals in Koala Sexual Selection: Insights from Seasonal Patterns of Associations Revealed with GPS-Proximity Units.Crossref | GoogleScholarGoogle Scholar | 26154295PubMed |
Hogan, L. A. (2004) The behaviour of captive common wombats. Honours thesis, University of Queensland.
Hogan, L. A., Phillips, C. J. C., Lisle, A., Horsup, A. B., Janssen, T., and Johnston, S. D. (2009). Remote monitoring of the behaviour and activity of captive southern hairy-nosed wombats (Lasiorhinus latifrons. Australian Mammalogy 31, 123–135.
| Remote monitoring of the behaviour and activity of captive southern hairy-nosed wombats (Lasiorhinus latifrons.Crossref | GoogleScholarGoogle Scholar |
Horsup, A. (2004). Recovery plan for the northern hairy-nosed wombat Lasiorhinus krefftii 2004-2008. Queensland Parks and Wildlife Service, Queensland Government Environmental Protection Agency, The State of Queensland, Environmental Protection Agency.
Johnson, C. N. (1991a). Behaviour and ecology of the northern hairy-nosed wombat Lasiohinus krefftii. Internal Report, Queensland National Parks and Wildlife Service.
Johnson, C. N. (1991b). Utilization of habitat by the northern hairy-nosed wombat Lasiorhinus krefftii. The Zoological Society of London 225, 495–507.
| Utilization of habitat by the northern hairy-nosed wombat Lasiorhinus krefftii.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. N., and Crossman, D. G. (1990). Sexual dimorphism in the northern hairy-nosed wombat, Lasiorhinus krefftii (Marsupialia: Vombatidae). Australian Mammalogy 14, 145–146.
Johnson, C. N., and Crossman, D. G. (1991). Dispersal and social organization of the northern hairy-nosed wombat Lasiorhinus krefftii. The Zoological Society of London 225, 605–613.
| Dispersal and social organization of the northern hairy-nosed wombat Lasiorhinus krefftii.Crossref | GoogleScholarGoogle Scholar |
Nevin, O. T., and Gilbert, B. K. (2005). Perceived risk, displacement and refuging in brown bears: positive impacts of ecotourism? Biological conservation 121, 611–622.
| Perceived risk, displacement and refuging in brown bears: positive impacts of ecotourism?Crossref | GoogleScholarGoogle Scholar |
Patison, K. P., Swain, D. L., Bishop-Hurley, G. J., Robins, G., Pattison, P., and Reid, D. J. (2010). Changes in temporal and spatial associations between pairs of cattle during the process of familiarisation. Applied Animal Behaviour Science 128, 10–17.
| Changes in temporal and spatial associations between pairs of cattle during the process of familiarisation.Crossref | GoogleScholarGoogle Scholar |
Shimmin, G., and White, C. (2002). Northern hairy-nosed wombat warrens: architecture, environment and gas exchange properties. Report of findings. Department of Environmental Biology, University of Adelaide.
Stenke, R. (2000). Study of the behaviour and the ecology of the northern hairy-nosed wombat (Lasiohinus krefftii) at Epping Forest National Park. A project report.
Woolnough, A. P. (1998). The feeding ecology of the northern hairy-nosed wombat, Lasiorhinus krefftii (Marsupialia: Vombatidae). PhD Thesis. Zoology and Tropical Ecology, James Cook University of North Queensland.