Sign surveys can be more efficient and cost effective than driven transects and camera trapping: a comparison of detection methods for a small elusive mammal, the numbat (Myrmecobius fasciatus)
Anke Seidlitz A E , Kate A. Bryant
A E , Kate A. Bryant  B , Nicola J. Armstrong
B , Nicola J. Armstrong  C , Michael C. Calver
C , Michael C. Calver  A and Adrian F. Wayne
A and Adrian F. Wayne  D
D
A Environmental and Conservation Sciences, Murdoch University, Murdoch, WA 6150, Australia.
B Centre for Climate-Impacted Terrestrial Ecosystems, Harry Butler Institute, Murdoch University, Murdoch, WA 6150, Australia.
C Mathematics and Statistics, Murdoch University, Murdoch, WA 6150, Australia.
D Department of Biodiversity, Conservation and Attractions, Locked Bag 2, Manjimup, WA 6258, Australia.
E Corresponding author. Email: anke.seidlitz@gmx.net
Wildlife Research - https://doi.org/10.1071/WR20020
Submitted: 3 February 2020 Accepted: 25 January 2021 Published online: 10 March 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Context: Determining the most efficient detection method for a target species is key for successful wildlife monitoring and management. Driven transects and sign surveys are commonly used to monitor populations of the endangered numbat (Myrmecobius fasciatus). Camera trapping is being explored as a new method. These methods were unevaluated for efficacy and cost for numbat detection.
Aims: To compare efficacy and costing of driven transects, sign surveys and camera trapping for detecting numbats in the Upper Warren region, Western Australia.
Methods: Seven repeat sign surveys and driven transects, as well as 4 months of camera trapping, were conducted concurrently at 50 sites along three transects. Numbat detection rates and costing of the three techniques were compared, and detection probabilities were compared between sign surveys and camera trapping.
Key results: Numbat signs were detected during 88 surveys at 39 sites, exceeding camera trapping (26 detections at 13 sites) and driven transects (seven detections near five sites). The estimated probability for detecting a numbat or a sign thereof (at a site where numbats were present) ranged from 0.21 to 0.35 for a sign survey, and 0.02 to 0.06 for 7 days of camera trapping. Total survey costs were lowest for driven transects, followed by camera trapping and sign surveys. When expressed as cost per numbat detection, sign surveys were cheapest.
Conclusions: Comparative studies of survey methods are essential for optimal, cost-effective wildlife monitoring. Sign surveys were more successful and cost effective than camera trapping or driven transects for detecting numbats in the Upper Warren region. Together with occupancy modelling, sign surveys are appropriate to investigate changes in occupancy rates over time, which could serve as a metric for long-term numbat monitoring.
Implications: There is no ‘best’ method for wildlife surveys. Case-specific comparison of animal detection methods is recommended to ensure optimal methods. For the numbat population in the Upper Warren region, further studies are needed to improve numbat detection rates from camera trapping, and to test sign surveys in autumn (March to May), when surviving juvenile numbats have established their own territory and assumptions regarding population closure are less likely to be violated.
Keywords: Animal signs, diggings, non-invasive techniques, numbat, sampling efficiency, scat, wildlife management, wildlife monitoring.
Introduction
Determining efficient detection methods for a target animal is key for successful monitoring and management. This is because management goals are commonly stated in terms of population size or distribution, such as increases for threatened species, reductions for pest species or stable numbers for harvested species (Lancia et al. 2005). Given that few animal species are sufficiently visible and concentrated for accurate, complete counts, population parameters are commonly estimated using a range of methods (Boyce 1995). Finding the most appropriate detection method for a target animal is important because detection success can differ among methods (Silveira et al. 2003; Wayne et al. 2005; Vine et al. 2009; Croose et al. 2019). Inappropriate detection methods may cause inaccurate estimates of species distribution and abundance, inefficient use of resources, incorrect management decisions and possibly legal challenges (Caughley and Gunn 1996; Thompson et al. 1998).
One species that is difficult to detect is the endangered numbat (Myrmecobius fasciatus), a small (500–700 g), obligate termitophagous, diurnal, Australian endemic marsupial (Cooper 2011; Hayward et al. 2015). Since European settlement, habitat loss, exotic predators and inappropriate fire regimes have contributed to the numbat’s decline (Friend and Page 2017). It now occurs in only 1% of its former range, with an estimated <1000 mature animals in ongoing decline (Woinarski et al. 2014; Friend and Page 2017). Conservation efforts successfully reintroduced numbats in several areas (Western Australia: Batalling forest block, Boyagin Nature Reserve, Dragon Rocks Nature Reserve, Mount Gibson, Tutanning Nature Reserve; New South Wales: Scotia Sanctuary; South Australia: Yookamurra Sanctuary (Friend and Page 2017; Australian Wildlife Conservancy, https://www.australianwildlife.org/where-we-work/mt-gibson, accessed 20 December 2019)). The only two remaining natural populations are in south-west Western Australia, in the Dryandra Woodland and the Upper Warren region (UWR) (Woinarski et al. 2014). The present study focuses on the UWR numbat population, where adequate monitoring information is lacking due to the absence of effective detection methods to assess population trends, size or distribution.
Obtaining reliable population estimates for numbats is challenging (Hayward et al. 2015). Conventional mammal trapping is unsuitable because no known lures or baits attract numbats (Burrows and Christensen 2002). In limited soil types tracking may be possible (Hayward et al. 2015), but it lacks wide potential. Instead, numbats are commonly monitored directly by sightings from driven transects (Calaby 1960; Friend 1990), with results reported as relative abundance indices (sightings per 100 km) (Friend 1990; Friend and Thomas 2003; Hayward et al. 2015). However, comparisons of relative abundance indices to monitor population trends may be problematic because the assumption of constant detectability – the probability of actually detecting an animal when present – is often violated (Gese 2001). Animal detectability can be affected by factors such as weather, time of day, vegetation density and observer ability (Gese 2001). More robust numbat population estimates from driven transects were achieved by Berry et al. (2019) within the fenced Scotia Sanctuary by applying distance sampling methods accounting for detection probabilities. Because distance sampling techniques require relatively high animal sighting rates (60–80 animals) (Buckland et al. 2001), they are impractical for monitoring numbats in the UWR where sighting rates from driven transects were very low, with 0.3 and 1.45 sightings per 100 km in 1995 and 1996 respectively (Friend and Page 2017).
Alternatively to direct sightings, animals can be detected indirectly from feeding signs and scats (Gese 2001; Sutherland 2006). Numbats leave typical signs (diggings and scats), suitable for indirect detection (Calaby 1960; Friend and Thomas 2003), and results of numbat sign surveys have previously been presented as presence–absence indices (percentage of sites occupied) (Friend 1990; Friend and Thomas 2003). Because abundance-occupancy relationships are commonly positively related (Gaston et al. 2000), these indices have been used to monitor numbat population trends in some subpopulations (Friend and Thomas 2003). However, the indices used were the naïve count of sites occupied, ignoring detection probability. Using raw counts without accounting for detection probability biases population estimates (Tyre et al. 2003; Gu and Swihart 2004; Guillera-Arroita et al. 2014). More reliable estimates of numbat occupancy can be derived from repeat sign surveys in conjunction with occupancy modelling developed by MacKenzie et al. (2002). Occupancy modelling accounts for imperfect detection of a species, avoiding the negative bias of naïve presence–absence indices (MacKenzie et al. 2018).
More recently, technology has provided another option in the form of camera traps (Swann and Perkins 2014), used to monitor a range of fauna in varied settings (see examples in Meek et al. (2014)). Although camera traps were originally deemed unsuccessful for numbats because the species occurs at low densities (Hayward et al. 2015), they have successfully detected other elusive mammals that occur at low densities (Gompper et al. 2006; Vine et al. 2009; Paull et al. 2012), and unpublished pilot studies in the UWR confirm that numbats can be detected by camera traps (Julia Wayne, unpubl. data). Although camera trap data may be used in various ways to estimate animal population parameters (O’Brien 2011), they were used in our study to derive numbat detection probabilities from occupancy modelling to allow direct comparison with the results from sign surveys. If numbat detections by camera traps prove sufficiently high for capture–recapture methods, camera-trapping techniques could be developed further to allow individual numbat identification using dorsal pelage patterns.
There are no published assessments of the relative effectiveness of driven transects, sign searches and camera trapping for numbat detection. We therefore aimed to compare the efficacy and costing of the three different methods for detecting numbats within the UWR. Specifically, we compared the overall number of detections, and the cumulative increase of sites with detections as a function of effort of the three methods. Furthermore, we compared numbat detection probabilities from sign searches and camera trapping. Finally, we compared the costing of the three methods to evaluate method feasibility. Determining the most efficient detection method for numbats in the UWR will help develop appropriate survey methods to robustly assess population parameters, and therefore improve monitoring and management of the species. The findings may be relevant for monitoring other small mammals elsewhere.
Materials and methods
Study area, transects and sites
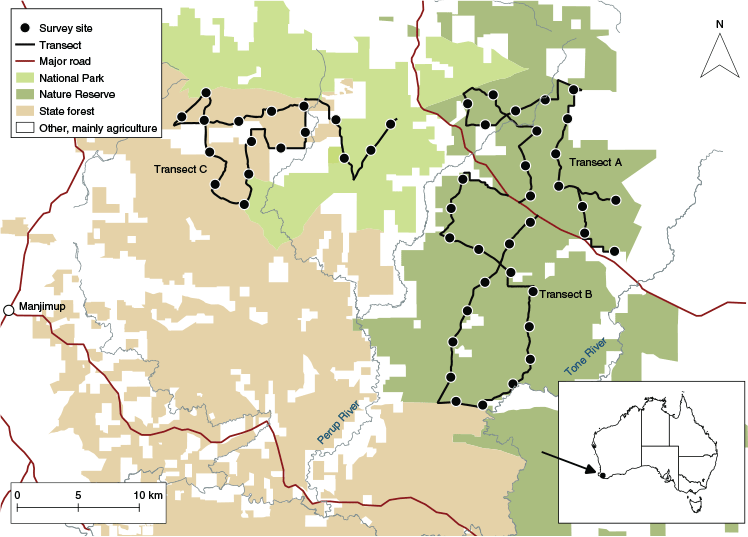
The present study was conducted in areas of national park, nature reserve and state forest within the UWR, ~300 km south of Perth, Western Australia (Fig. 1). The UWR is part of the South‐western Australian Global Biodiversity Hotspot (Myers et al. 2000), and has a high conservation value because it supports many rare and endangered species (Burrows and Christensen 2002). Within the UWR, open sclerophyll forests and woodlands cover more than 140 000 ha of publicly managed land, with jarrah (Eucalyptus marginata), marri (Corymbia calophylla) and in some places wandoo (Eucalyptus wandoo) as dominant tree species (Yeatman et al. 2016). Forest management activities conducted by state authorities include prescribed fuel-reduction burns, timber harvesting and feral predator control using 1080-poisoned bait (Wayne et al. 2013). Forested areas are surrounded by privately owned land predominantly used for agriculture and forestry (Fig. 1). The region experiences a temperate mediterranean climate, with an annual average rainfall of ~650–900 mm (Zosky et al. 2017).
Three existing transects, consisting of transect A (~54 km), B (~56 km) and C (~61 km), and 50 existing survey sites were utilised (Fig. 1). Transects were established in 1992 to monitor numbats (Julia Wayne pers. comm.) and followed unsealed double-lane roads (~34 km) and single-lane tracks (~138 km). Survey sites (16–17 sites per transect) were established in 2015 for numbat camera-trapping trials (Julia Wayne pers. comm.). Survey sites were, on average, 2.38 km apart (min 1.88 km, max 2.88 km; Fig. 1). We deemed distances between sites sufficiently large to avoid redetection of numbats at different sites (the largest home-range size of a numbat in the UWR was measured to be 123.51 ha (Christensen et al. 1984)).
At each survey site, 10 plots (40 × 100 m) were established for this study, with five plots located on each side of the transect. When the transect was bordered by private property all plots were situated on the same side (n = 6 sites). Plots were placed adjacent to each other with the long edge perpendicular to the transect. A central plot was reserved for camera trapping at each survey site. The remaining nine plots were used for sign surveys.
Survey period and team
Camera trapping was conducted from September to December 2017 (4 months), and sign surveys and driven transects from mid-September to mid-December (3 months). This coincides with the Australian spring and early summer, and is immediately before and during juvenile numbat dispersal (Friend 1989), and therefore the time of maximum numbat abundance when detection is most likely. The three survey types (driven transects, sign surveys and camera trapping) were tested simultaneously to avoid seasonal or yearly differences in numbat abundance. All surveys were conducted by the first author, and 23 volunteers helped with driven transects and sign surveys. Volunteers received training before surveys to become familiar with required tasks. Nineteen volunteers participated in one, three volunteers in two, and one in three driven transects and sign surveys.
Driven transects
Driven transects were repeated seven times along the entire length of transect A, B and C. Periods between repeat driven transects were weather dependent, and on average were 7 days (min. 6 days, max. 10 days). The driven transect procedure was adapted from Calaby (1960) and Friend (1990). A car was driven at ~15 km h−1 on warm, calm days. Numbats rest during midday hours on hot days (Christensen et al. 1984), so we interrupted driven transects when temperatures exceeded 28°C (thermometer used: Kestrel 3000 Pocket Weather Meter). During driven transects, one driver (first author) and three additional observers (volunteers) scanned the areas in front and on either side for numbats. A numbat detection from driven transects is here defined as a sighting by any observer. When a numbat was detected, date, time, GPS location and the numbat’s distance to the road were recorded. Distance to road was estimated by counting steps. We did not use a more exact measurement because the main aim was to assess driven transects as a detection method, not to calculate population estimates by, for example, distance sampling techniques. For logistical reasons, driven transects were usually interrupted for sign searches when a survey site was reached.
Sign surveys
Seven repeat 5-day sign surveys were conducted during the same time as driven transects. They consisted of searches for numbat signs (fresh diggings and scats as described by Calaby (1960), Christensen et al. (1984) and Connell and Friend (1985)). During each sign survey, one plot was searched at each of the 50 survey sites. Within each site, plots were chosen randomly with no plot searched twice. Searches were conducted by the first author and three volunteers. To maximise search area within each plot, team members walked ~5 m apart in a straight line, searching for numbat signs 2.5 m left and right. The search team walked up one side of the plot (covering half of the plot width) and down the other side (covering the other half of the plot width). When numbat diggings and/or scats were found, the signs were recorded and searching stopped. The finding of one or more numbat signs (i.e. an indirect detection of a numbat/s) on a plot during a survey was defined as a single numbat detection at that survey site. All numbat scats found were inspected by the first author using a 20× magnifying glass in the field, and later verified under a dissecting microscope. When no signs were found, searches ended after the team searched the entire plot (~20 min).
Camera trapping
We used Reconyx PC900 camera traps because they are highly regarded (Glover-Kapfer et al. 2019), and commonly used by the Department of Biodiversity, Conservation and Attractions (DBCA) for camera trap studies in the UWR. At each of the 50 sites, one camera trap was attached to a tree ~25 cm above ground. This camera height was found to be most suitable for numbats (Seidlitz et al. 2020). Camera traps faced southwards to avoid direct sun glare, and towards forest clearings to minimise vegetation obstructing the field of view within the first ~5 m from the camera. A 5-m detection distance and a detection angle of 40° (see manufacturer’s specifications: Reconyx Inc. 2017) result in a detection area of ~8.7 m2 (calculated as a circle sector). Vegetation near cameras was minimally pruned to reduce false-trigger events. Camera traps were located centrally on a plot with a minimum distance of 30 m to the transect. Cameras were set to take 10 images when triggered using the ‘rapid fire’ function, with no delay between triggers. The camera’s passive infrared sensor sensitivity was set to high. Batteries and SD cards were changed monthly. Images from camera trapping were scanned for numbats using FastStone Image Viewer version 6.2 (FastStone Soft 2019). A numbat detection was defined as a camera trap trigger resulting in one or more images depicting a numbat partially or wholly. To take into account that a numbat may stay in front of a camera trap for an extended period, detections were only counted when there was a minimum of 60 min between subsequent detections (Tobler et al. 2008; Rovero and Marshall 2009).
Cost analysis
Costs for each survey method were calculated independently. We considered travel, personnel, volunteer and material costs as main expense categories. Travel cost was based on the DBCA vehicle hire charge of AU$1.00 per km, and covered distances travelled to, from and between sites. Personnel cost was determined at an hourly rate of AU$69.17, the 2020 rate of a well qualified fauna conservation officer at DBCA (including 45% payroll overheads). Personnel cost included time for equipment preparation, installation, maintenance and post-survey care, for survey planning, volunteer-related communication and preparation, scat validation under the microscope, data digitalisation and time in the field. Volunteer cost covered a daily food and accommodation allowance of AU$30.00. Material cost included AU$10.00 for stationery for all survey methods, and AU$20.00 for scat collection and storage materials for sign surveys. For camera trap related material costs, we divided the 2017 purchase price of camera traps, rechargeable batteries, and SD cards by 5, assuming that these materials can be used for further numbat surveys over 5 years (20% depreciation rate).
Evaluating method effectiveness and data analysis
To compare method efficacy, several performance metrics were evaluated, including raw numbat detections (e.g. total number of detections as defined above for each method), and the accumulation of sites with numbat detections over time using the Vegan Community Ecology package version 2.5–5 (Oksanen et al. 2019) in R version 3.5.0 (R Core Team 2018). Site accumulation was estimated from indirect numbat detections from sign surveys (scats and diggings), and from direct detections from camera trapping (numbat images) and driven transects (sightings). Sightings of numbats from driven transects were assigned to the nearest survey site to allow site accumulation for this transect-based method. To compare sighting rates from driven transects with historic sighting rates (sightings per 100 km) in the UWR, the number of sightings was divided by the number of kilometres driven and multiplied by 100. Additionally, efficacy of sign surveys and camera trapping were compared by determining the probability of numbat detection for each method. Probability of detection (the probability that a species is detected at a site given its presence) was calculated using the single season occupancy modelling framework (MacKenzie et al. 2018). A matrix with detections (1) and non-detections (0) was established from spatial replicates (50 sites) and temporal repeats (repeat surveys). Similar to other studies, temporal repeats for camera trapping were established by dividing camera trap data into 7-day periods (Gálvez et al. 2016). Models to estimate detection probabilities were fitted using the RPresence package version 2.12.33 (MacKenzie and Hines 2018) in R. We acknowledge that the assumption of population closure may have been violated by conducting surveys during juvenile numbat dispersal. This may have affected occupancy estimates (the probability that a species is present at a site), which were not the aim for the present study. Because we aimed to compare the efficiency of detection methods, we consider the survey timing reasonable, and because occupancy was not estimated, the occupancy component of models was kept constant (psi ~1) and habitat covariates were not used. Models were fitted with combinations of the covariates ‘method’, ‘transect’ and ‘site’ before comparison using the Akaike Information Criterion (Akaike 1974; Burnham and Anderson 2002). The model with the lowest value was chosen to determine numbat detection probabilities for the different methods. Model fit was examined by computing the c-hat value using the goodness of fit test in the RPresence package on the global model (the most complex model with the greatest number of parameters), as described in MacKenzie et al. (2018), Chapter 4. Further software used were Microsoft Excel for data digitalisation and survey cost calculations, and QGIS 3.2 Bonn for computing maps and distance travelled (QGIS Development Team 2019).
Results
During seven repeat surveys, we drove ~1198 km along transects A, B and C, and searched 350 plots at 50 survey sites for sign searches. Camera trapping at 50 survey sites resulted in 156 966 images. Forty-seven camera traps operated between 124 and 127 days (mean 126.5 days). Three camera traps had reduced periods (92, 95 and 103 days) due to either unknown or operator errors (code lock not entered correctly). Days on which camera traps failed to operate were included in the occupancy modelling data matrix as missing observations. In total, camera traps operated on 6235 full days.
Numbat detections were highest from sign surveys and lowest from driven transects (Fig. 2). The first author (skilled observer) found 63% of numbat signs detected during sign surveys. Numbats were detected on transects A, B and C with the use of sign surveys and camera trapping. No numbats were detected on transect B during driven transects (Fig. 2). Seven numbat detections were recorded from driven transects, giving a relative abundance index of 0.584 detections per 100 km.

|
The increase in the number of survey sites with numbat detections as a function of effort was greatest from sign surveys (Fig. 3). After seven repeat surveys, numbats were detected at 39 (78%) and 13 (26%) survey sites from sign surveys and camera trapping respectively. During driven transects, numbats were detected near five (10%) survey sites. Numbats were detected at 40 sites (80%) from all three detection methods combined.

|
Using occupancy modelling, the initial model (including the explanatory variable of interest ‘method’ only) had an AIC of 624.21. The AIC did not improve when ‘method + site’ (AIC = 624.78) or ‘method * site’ (AIC = 655.46) were included. The AIC did improve by adding ‘method + transect’ (AIC = 623.62) and ‘method * transect’ (AIC = 622.98). Expanding the model further with ‘method + transect + site’ or ‘method * transect + site’ did not improve it (AIC = 628.78 and 628.11 respectively). Therefore, the final model included ‘method’ and ‘transect’, and the interaction thereof as covariates to estimate detection probabilities. The estimated c-hat for the global model, after 3000 bootstrap iterations, was 1.93, noting that values greater than 1 suggest there is more variation in the observed data than expected by the model (MacKenzie and Bailey 2004). Detection probability estimates for finding a numbat or a sign thereof (at a site where numbats were present) ranged from 0.02 to 0.06 for camera trapping (per 7-day period), and from 0.21 to 0.35 for sign surveys (per survey; Table 1).

|
Total survey cost was lowest for driven transects and highest for sign surveys (Table 2). Sign surveys were 13% (AU$3780) more expensive than camera trapping and 44% (AU$8896) more expensive than driven transects. The lowest cost per numbat detection was achieved by sign surveys, with driven transects being 8.7 times, and camera trapping 2.9 times more expensive (Table 2).

|
Discussion
The present study broadens the understanding of the usefulness and cost efficiency of three survey methods for detecting elusive species occurring at low densities. Under the conditions reported here, sign surveys were considerably more successful and cost effective at detecting numbats in the UWR than driven transects or camera trapping. Even though the true numbat occupancy rate is unknown, we conclude here that sign surveys were more accurate than camera trapping or driven transects because the number of sites with numbat detections from sign surveys (39) was closest to the total number of sites with evidence of numbat presence from all three methods combined (40). Several studies found camera trapping to be more successful for animal detection when compared with other methods including sign surveys (see Wearn and Glover-Kapfer (2019) for an analysis of 104 method-comparing studies). Even though some of these studies compared methods similar to the ones compared here, results are not directly comparable because bait was used to attract target species (Di Cerbo and Biancardi 2013; Greene et al. 2016), target species were considerably larger than numbats (Bartolommei et al. 2012; Anile et al. 2014) or camera traps were set at burrow entrances (Ellis et al. 2017).
The success of sign surveys for detecting numbats during the present study may be attributed to several factors. Animal signs persist for a long time (Heinemeyer et al. 2008). Depending on weather, we assume that numbat diggings last for several days, and scats possibly for several weeks. Therefore, evidence of numbats can be detected long after the animal has left. Although advantageous for sign detection, the long persistence of animal signs also poses a disadvantage: it remains unknown when exactly the animal was present in the area (contrasting direct sightings or captures on camera, which provide information on the time of capture). To resolve this, it needs to be determined how long numbat scats persist in the natural environment – a subject for future studies. Numbat diggings are typically shallow, numerous and spread over several square meters, making them conspicuous and characteristic. Even though numbat scats are small, they are easily recognisable (Connell and Friend 1985), are often found with diggings or on logs and termite mounds (Calaby 1960), and therefore can be detected by skilful observers. Finally, the search area was large (40 × 100 m) and searched by four observers (the lead author and three volunteers), resulting in a detection probability of 21–35%. As the lead author found most numbat signs (63%), the efficacy and precision of sign surveys could be improved by using fewer but more skilled observers. This might allow a reduction of observers as well as repeat surveys needed to achieve the same numbat detection probability, reducing survey cost.
Sign surveys could be applied for future numbat studies in the UWR in various ways. Sign surveys in conjunction with occupancy modelling could investigate changes in occupancy over time. Additionally, differences in occupancy rates among study sites with varying habitat types or management activities could be explored by including the relevant covariates in model design (Okes and O’Riain 2017; Romano et al. 2018; Silveira et al. 2018). Furthermore, DNA analysis from scat samples could open new avenues, including numbat population abundance estimates from capture–recapture analysis (Piggott et al. 2006; Mondol et al. 2009; Kindberg et al. 2011).
Camera trapping detected fewer numbats than sign surveys. Even though camera traps operated continuously (contrasting the shorter duration of sign surveys), and over an extended survey period (4 instead of 3 months for sign surveys), their numbat detection probability was low (2–6%). Multiple factors may have contributed. Camera traps sample a small detection zone (Apps and McNutt 2018b), so a numbat could pass near a camera trap and stay undetected (Gillespie et al. 2015; Pease et al. 2016). Some animals may even avoid camera traps (Meek et al. 2016). Additionally, camera traps may not always trigger when an animal moves within the detection zone (Jumeau et al. 2017; Urbanek et al. 2019; Seidlitz et al. 2020), especially when the animal is small (Gompper et al. 2006; Damm et al. 2010; Rowcliffe et al. 2011; Urbanek et al. 2019).
However, camera traps have many advantages warranting further trials to improve numbat detection probabilities and reduce costs. Evaluation of camera trap images does not need highly skilled personnel, so cost could be reduced by using volunteers. Furthermore, camera traps take images of multiple species. Therefore, cameras could collect data for multiple projects, allowing cost sharing. Whereas sign surveys result in presence–absence data (unless scat DNA analysis is possible), images from camera traps may identify individuals, breeding status, age and other demographic attributes (Jędrzejewski et al. 2017). Numbat detection rates from camera traps could be improved by, for example: targeted camera placement in preferred habitat areas (Harris et al. 2013), increasing the number of camera traps per site (Pease et al. 2016; O’Connor et al. 2017); or by using different camera trap models (e.g. Fancourt et al. (2018), who increased rabbit detection rates by using camera traps with wider detection angles). Some of these options may not be cost effective because they may increase survey cost. However, significant cost savings can be achieved by using a cheaper, potentially more effective camera trap model (Driessen et al. 2017; Apps and McNutt 2018a; Fancourt et al. 2018). To be able to use camera trapping as a survey method for numbat monitoring in the UWR in conjunction with occupancy modelling, the numbat detection probabilities would need to be increased. If detection probabilities are as low as 10% (2–4% in this study), more than 26 repeat surveys (here a repeat survey was defined as a 7-day camera trap interval) are necessary to allow reliable estimates of occupancy (MacKenzie et al. 2018, p. 461).
The least successful method was driven transects. Similar to results from driven transects conducted in the UWR during the mid-1990s, few numbats were sighted (0.584 detections per 100 km). This low detection rate is unsuitable for population estimates from distance sampling. Furthermore, relative abundance indices from driven transects with such low detection rates are unlikely to facilitate confident detection of real temporal changes in the numbat population. Numbats are small and well camouflaged, and therefore difficult to spot. Additionally, numbats are likely to hide when a car is approaching, and the typically dense vegetation of the UWR restricts observations. It is thus unlikely that driven transects can be improved to become a successful numbat detection method for long-term monitoring in the UWR.
Sign surveys were more successful and cost effective than camera trapping or driven transects for detecting numbats in the UWR. During sign surveys, special attention must be paid to ensure that animal signs are correctly identified to avoid false negative or false positive identifications. We are confident here that signs were identified correctly because diggings were only counted when numerous fresh diggings were found, and scats were validated by microscopy. We recommend sign surveys for numbat monitoring in the UWR, with the use of occupancy modelling. Until reliable numbat abundance estimates can be developed, for example, from capture–recapture methods using numbat scat DNA analysis or camera trapping, it remains unknown how occupancy estimates relate to true numbat abundance. However, they do provide information on areas occupied by numbats. Such knowledge can help identify habitat preferences, informing management of the species’ responses to timber harvesting, prescribed fuel reduction burns and introduced predator control. To improve occupancy estimates we recommend investigating factors affecting the production, persistence and detection of numbat signs, because these factors influence detection probabilities. Furthermore, although the occupancy models used in this study were adequate for comparing survey methods, they could be improved by including additional covariates (e.g. habitat and environmental variables) that may better account for the variation in observed numbat detections. We further recommend exploring the improvement of numbat detection rates from camera trapping, and to test sign surveys in autumn when surviving juvenile numbats have established their own territory, and assumptions regarding closure (e.g. occupancy status at each site does not change over the survey season) are less likely to be violated. Even though occupancy model extensions are available to assess the robustness of results when closure assumption violations are suspected (MacKenzie et al. 2018, Chapter 6), it is best to sample populations when closure assumptions can be met (MacKenzie et al. 2018, p. 149). The present study highlights the importance of finding the most appropriate detection method for a target animal, but it needs to be acknowledged that a method’s success depends on many factors, including species’ characteristics, population density, habitat type, personnel skills, type of equipment used and survey timing. We recommend case-specific comparison of animal detection methods to ensure optimal methods are used for successful and cost-effective monitoring.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was conducted under the permit O2968/17 of the Murdoch University Animal Ethics Committee. We thank all volunteers and co-workers for their invaluable help with this project. Special thanks to Tony Friend who showed the fieldwork team how to find and identify numbat signs. We are also grateful to the Department of Biodiversity, Conservation and Attractions for providing camera trapping equipment and other in-kind contributions. Furthermore, we thank WWF-Australia for obtaining and managing project funds. This project was supported by funding from the Western Australian Government’s State NRM Program (A16002), Murdoch University and Coles Supermarkets Australia Pty Ltd. Many thanks also to Blackwood Basin Group for providing reduced-rate field accommodation at Perup Nature’s Guesthouse.
References
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control 19, 716–723.| A new look at the statistical model identification.Crossref | GoogleScholarGoogle Scholar |
Anile, S., Ragni, B., Randi, E., Mattucci, F., and Rovero, F. (2014). Wildcat population density on the Etna volcano, Italy: a comparison of density estimation methods. Journal of Zoology 293, 252–261.
| Wildcat population density on the Etna volcano, Italy: a comparison of density estimation methods.Crossref | GoogleScholarGoogle Scholar |
Apps, P., and McNutt, J. W. (2018a). Are camera traps fit for purpose? A rigorous, reproducible and realistic test of camera trap performance. African Journal of Ecology 56, 710–720.
| Are camera traps fit for purpose? A rigorous, reproducible and realistic test of camera trap performance.Crossref | GoogleScholarGoogle Scholar |
Apps, P. J., and McNutt, J. W. (2018b). How camera traps work and how to work them. African Journal of Ecology 56, 702–709.
| How camera traps work and how to work them.Crossref | GoogleScholarGoogle Scholar |
Bartolommei, P., Manzo, E., and Cozzolino, R. (2012). Evaluation of three indirect methods for surveying European pine marten in a forested area of central Italy. Hystrix, the Italian Journal of Mammalogy 23, 91–94.
| Evaluation of three indirect methods for surveying European pine marten in a forested area of central Italy.Crossref | GoogleScholarGoogle Scholar |
Berry, L. E., L’ Hotellier, F. A., Carter, A., Kemp, L., Kavanagh, R. P., and Roshier, D. A. (2019). Patterns of habitat use by three threatened mammals 10 years after reintroduction into a fenced reserve free of introduced predators. Biological Conservation 230, 1–9.
| Patterns of habitat use by three threatened mammals 10 years after reintroduction into a fenced reserve free of introduced predators.Crossref | GoogleScholarGoogle Scholar |
Boyce, M. S. (1995). Anticipating consequences of wolves in Yellowstone: model validation. In ‘Ecology and Conservation of Wolves in a Changing World’. (Eds L. N. Carbyn, S. H. Fritts and D. R. Seip.) pp. 199–209. (Canadian Circumpolar Institute: Edmonton, Alberta, Canada.)
Buckland, S. T., Anderson, D. R., Burnham, K. P., Laake, J. L., Borchers, D. L., and Thomas, L. N. (2001). ‘Introduction to Distance Sampling: Estimating Abundance of Biological Populations.’ (Oxford University Press: Oxford.)
Burnham, K. P., and Anderson, D. R. (2002). ‘Model Selection and Multimodel Inference: A Practical Information–Theoretic Approach.’ 2nd edn. (Springer: New York.)
Burrows, N. D., and Christensen, P. E. S. (2002). Long-term trends in native mammal capture rates in a jarrah forest in south-western Australia. Australian Forestry 65, 211–219.
| Long-term trends in native mammal capture rates in a jarrah forest in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Calaby, J. H. (1960). Observations on the banded ant-eater Myrmecobius f. fasciatus Waterhouse (marsupialia), with particular reference to its food habits. Proceedings of the Zoological Society of London 135, 183–207.
| Observations on the banded ant-eater Myrmecobius f. fasciatus Waterhouse (marsupialia), with particular reference to its food habits.Crossref | GoogleScholarGoogle Scholar |
Caughley, G., and Gunn, A. (1996) ‘Conservation Biology in Theory and Practice.’ (Blackwell Science: Cambridge, MA, USA.)
Christensen, P., Maisey, K., and Perry, D. (1984). Radiotracking the numbat, Myrmecobius fasciatus, in the Perup Forest of Western Australia. Wildlife Research 11, 275–288.
| Radiotracking the numbat, Myrmecobius fasciatus, in the Perup Forest of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Connell, G. W., and Friend, A. J. (1985). Searching for numbats. Landscope 1, 21–26.
Cooper, C. E. (2011). Myrmecobius fasciatus (Dasyuromorphia: Myrmecobiidae). Mammalian Species 43, 129–140.
| Myrmecobius fasciatus (Dasyuromorphia: Myrmecobiidae).Crossref | GoogleScholarGoogle Scholar |
Croose, E., Birks, J. D. S., Martin, J., Ventress, G., MacPherson, J., and O’Reilly, C. (2019). Comparing the efficacy and cost-effectiveness of sampling methods for estimating population abundance and density of a recovering carnivore: the European pine marten (Martes martes). European Journal of Wildlife Research 65, 37.
| Comparing the efficacy and cost-effectiveness of sampling methods for estimating population abundance and density of a recovering carnivore: the European pine marten (Martes martes).Crossref | GoogleScholarGoogle Scholar |
Damm, P. E., Grand, J. B., and Barnett, S. W. (2010). Variation in detection among passive infrared triggered-cameras used in wildlife research. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 64, 125–130.
Di Cerbo, A. R., and Biancardi, C. M. (2013). Monitoring small and arboreal mammals by camera traps: effectiveness and applications. Acta Theriologica 58, 279–283.
| Monitoring small and arboreal mammals by camera traps: effectiveness and applications.Crossref | GoogleScholarGoogle Scholar |
Driessen, M. M., Jarman, P. J., Troy, S., and Callander, S. (2017). Animal detections vary among commonly used camera trap models. Wildlife Research 44, 291–297.
| Animal detections vary among commonly used camera trap models.Crossref | GoogleScholarGoogle Scholar |
Ellis, K. S., Larsen, R. T., Whiting, J. C., Wilson, T. L., and McMillan, B. R. (2017). Assessing indirect measures of abundance and distribution with remote cameras: simplifying indices of activity at pygmy rabbit burrows. Ecological Indicators 77, 23–30.
| Assessing indirect measures of abundance and distribution with remote cameras: simplifying indices of activity at pygmy rabbit burrows.Crossref | GoogleScholarGoogle Scholar |
Fancourt, B. A., Sweaney, M., and Fletcher, D. B. (2018). More haste, less speed: pilot study suggests camera trap detection zone could be more important than trigger speed to maximise species detections. Australian Mammalogy 40, 118–121.
| More haste, less speed: pilot study suggests camera trap detection zone could be more important than trigger speed to maximise species detections.Crossref | GoogleScholarGoogle Scholar |
FastStone Soft (2019). FastStone Image Viewer for Windows. Available at www.faststone.org [verified 20 February 2019].
Friend, J. A. (1989). 22. Myrmecobiidae. In ‘Fauna of Australia’. (Eds D. W. Walton and B. J. Richardson.) pp. 1–18. (AGPS Canberra: Canberra, ACT, Australia.)
Friend, J. A. (1990). The numbat Myrmecobius fasciatus (Myrmecobiidae): history of decline and potential for recovery. Proceedings of the Ecological Society of Australia 16, 369–377.
Friend, J. A., and Page, M. J. (2017). Numbat (Myrmecobius fasciatus) Recovery Plan. Wildlife Management Program No. 60. WA Department of Parks and Wildlife, Perth, WA.
Friend, J. A., and Thomas, N. D. (2003). Conservation of the numbat (Myrmecobius fasciatus). In ‘Predators with Pouches: The Biology of Carnivorous Marsupials’. (Eds M. Jones, C. Dickman and M. Archer.) pp. 452–463. (CSIRO Publishing: Melbourne, Vic., Australia.)
Gálvez, N., Guillera-Arroita, G., Morgan, B. J. T., and Davies, Z. G. (2016). Cost-efficient effort allocation for camera-trap occupancy surveys of mammals. Biological Conservation 204, 350–359.
| Cost-efficient effort allocation for camera-trap occupancy surveys of mammals.Crossref | GoogleScholarGoogle Scholar |
Gaston, K. J., Blackburn, T. M., Greenwood, J. J. D., Gregory, R. D., Quinn, R. M., and Lawton, J. H. (2000). Abundance–occupancy relationships. Journal of Applied Ecology 37, 39–59.
| Abundance–occupancy relationships.Crossref | GoogleScholarGoogle Scholar |
Gese, E. M. (2001). Monitoring of terrestrial carnivore populations. In ‘Carnivore Conservation’. (Eds J. L. Gittleman, S. M. Funk, D. W. MacDonald and R. K. Wayne.) pp. 372–396. (Cambridge University Press: Cambridge.)
Gillespie, G. R., Brennan, K., Gentles, T., Hill, B., Low Choy, J., Mahney, T., Stevens, A., and Stokeld, D. (2015). A guide for the use of remote cameras for wildlife survey in northern Australia. National Environmental Research Program, Northern Australia Hub. (Charles Darwin University: Casuarina, NT, Australia.)
Glover-Kapfer, P., Soto-Navarro, C. A., and Wearn, O. R. (2019). Camera-trapping version 3.0: current constraints and future priorities for development. Remote Sensing in Ecology and Conservation 5, 209–223.
| Camera-trapping version 3.0: current constraints and future priorities for development.Crossref | GoogleScholarGoogle Scholar |
Gompper, M. E., Kays, R. W., Ray, J. C., Lapoint, S. D., Bogan, D. A., and Cryan, J. R. (2006). A comparison of noninvasive techniques to survey carnivore communities in northeastern North America. Wildlife Society Bulletin 34, 1142–1151.
| A comparison of noninvasive techniques to survey carnivore communities in northeastern North America.Crossref | GoogleScholarGoogle Scholar |
Greene, D. U., McCleery, R. A., Wagner, L. M., and Garrison, E. P. (2016). A comparison of four survey methods for detecting fox squirrels in the southeastern United States. Journal of Fish and Wildlife Management 7, 99–106.
| A comparison of four survey methods for detecting fox squirrels in the southeastern United States.Crossref | GoogleScholarGoogle Scholar |
Gu, W., and Swihart, R. K. (2004). Absent or undetected? Effects of non-detection of species occurrence on wildlife–habitat models. Biological Conservation 116, 195–203.
| Absent or undetected? Effects of non-detection of species occurrence on wildlife–habitat models.Crossref | GoogleScholarGoogle Scholar |
Guillera-Arroita, G., Lahoz-Monfort, J. J., MacKenzie, D. I., Wintle, B. A., and McCarthy, M. A. (2014). Ignoring imperfect detection in biological surveys is dangerous: a response to ‘Fitting and Interpreting Occupancy Models’. PLoS One 9, e99571.
| Ignoring imperfect detection in biological surveys is dangerous: a response to ‘Fitting and Interpreting Occupancy Models’.Crossref | GoogleScholarGoogle Scholar | 25075615PubMed |
Harris, G., Farley, S., Russell, G. J., Butler, M. J., and Selinger, J. (2013). Sampling designs matching species biology produce accurate and affordable abundance indices. PeerJ 1, e227.
| Sampling designs matching species biology produce accurate and affordable abundance indices.Crossref | GoogleScholarGoogle Scholar | 24392290PubMed |
Hayward, M. W., Poh, A. S. L., Cathcart, J., Churcher, C., Bentley, J., Herman, K., Kemp, L., Riessen, N., Scully, P., Diong, C. H., Legge, S., Carter, A., Gibb, H., and Friend, J. A. (2015). Numbat nirvana: conservation ecology of the endangered numbat (Myrmecobius fasciatus) (Marsupialia: Myrmecobiidae) reintroduced to Scotia and Yookamurra Sanctuaries, Australia. Australian Journal of Zoology 63, 258–269.
| Numbat nirvana: conservation ecology of the endangered numbat (Myrmecobius fasciatus) (Marsupialia: Myrmecobiidae) reintroduced to Scotia and Yookamurra Sanctuaries, Australia.Crossref | GoogleScholarGoogle Scholar |
Heinemeyer, K. S., Ulizio, T. J., and Harrison, R. L. (2008). Natural sign: tracks and scats. In ‘Noninvasive Survey Methods for Carnivores’. (Eds R. A. Long, P. MacKay, W. J. Zielinski and J. C. Ray.) pp. 45–74. (Island Press: Washington, DC, USA.)
Jędrzejewski, W., Puerto, M. F., Goldberg, J. F., Hebblewhite, M., Abarca, M., Gamarra, G., Calderón, L. E., Romero, J. F., Viloria, Á. L., Carreño, R., Robinson, H. S., Lampo, M., Boede, E. O., Biganzoli, A., Stachowicz, I., Velásquez, G., and Schmidt, K. (2017). Density and population structure of the jaguar (Panthera onca) in a protected area of Los Llanos, Venezuela, from 1 year of camera trap monitoring. Mammal Research 62, 9–19.
| Density and population structure of the jaguar (Panthera onca) in a protected area of Los Llanos, Venezuela, from 1 year of camera trap monitoring.Crossref | GoogleScholarGoogle Scholar |
Jumeau, J., Petrod, L., and Handrich, Y. (2017). A comparison of camera trap and permanent recording video camera efficiency in wildlife underpasses. Ecology and Evolution 7, 7399–7407.
| A comparison of camera trap and permanent recording video camera efficiency in wildlife underpasses.Crossref | GoogleScholarGoogle Scholar | 28944025PubMed |
Kindberg, J., Swenson, J. E., Ericsson, G., Bellemain, E., Miquel, C., and Taberlet, P. (2011). Estimating population size and trends of the Swedish brown bear Ursus arctos population. Wildlife Biology 17, 114–123.
| Estimating population size and trends of the Swedish brown bear Ursus arctos population.Crossref | GoogleScholarGoogle Scholar |
Lancia, R. A., Kendall, W. L., Pollock, K. H., and Nichols, J. D. (2005). Estimating the number of animals in wildlife populations. In ‘Techniques for Wildlife Investigations and Management’. (Ed. E. B. Clait.) pp. 106–153. (Wildlife Society: Bethesda, MD, USA.)
MacKenzie, D. I., and Bailey, L. L. (2004). Assessing the fit of site-occupancy models. Journal of Agricultural, Biological, and Environmental Statistics 9, 300–318.
| Assessing the fit of site-occupancy models.Crossref | GoogleScholarGoogle Scholar |
MacKenzie, D., and Hines, J. (2018). RPresence: R Interface for Program PRESENCE. R package version 2.12.33. Available at https://www.usgs.gov/software/presence [verified 5 April 2018].
MacKenzie, D. I., Nichols, J. D., Lachman, G. B., Droege, S., Royle, J. A., and Langtimm, C. A. (2002). Estimating site occupancy rates when detection probabilities are less than one. Ecology 83, 2248–2255.
| Estimating site occupancy rates when detection probabilities are less than one.Crossref | GoogleScholarGoogle Scholar |
MacKenzie, D. I., Nichols, J. D., Royle, J. A., Pollock, K. H., Bailey, L. L., and Hines, J. E. (2018). ‘Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence.’ 2nd edn. (Academic Press: London, UK.)
Meek, P., Fleming, P., Ballard, G., Banks, P., Claridge, A., Sanderson, J., and Swann, D. (Eds) (2014). ‘Camera Trapping: Wildlife Management and Research.’ (CSIRO Publishing: Melbourne, Vic., Australia.)
Meek, P., Ballard, G., Fleming, P., and Falzon, G. (2016). Are we getting the full picture? Animal responses to camera traps and implications for predator studies. Ecology and Evolution 6, 3216–3225.
| Are we getting the full picture? Animal responses to camera traps and implications for predator studies.Crossref | GoogleScholarGoogle Scholar | 27096080PubMed |
Mondol, S., Karanth, K. U., Samba Kumar, N., Gopalaswamy, A. M., Andheria, A., and Ramakrishnan, U. (2009). Evaluation of non-invasive genetic sampling methods for estimating tiger population size. Biological Conservation 142, 2350–2360.
| Evaluation of non-invasive genetic sampling methods for estimating tiger population size.Crossref | GoogleScholarGoogle Scholar |
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
| Biodiversity hotspots for conservation priorities.Crossref | GoogleScholarGoogle Scholar | 10706275PubMed |
O’Brien, T. G. (2011). Abundance, density and relative abundance: a conceptual framework. In ‘Camera Traps in Animal Ecology: Methods and Analyses’. (Eds A. F. O’Connell, J. D. Nichols and K. U. Karanth.) pp. 71–96. (Springer: New York, NY, USA.)
O’Connor, K. M., Nathan, L. R., Liberati, M. R., Tingley, M. W., Vokoun, J. C., and Rittenhouse, T. A. G. (2017). Camera trap arrays improve detection probability of wildlife: investigating study design considerations using an empirical dataset. PLoS One 12, .
| Camera trap arrays improve detection probability of wildlife: investigating study design considerations using an empirical dataset.Crossref | GoogleScholarGoogle Scholar | 28422973PubMed |
Okes, N. C., and O’Riain, M. J. (2017). Otter occupancy in the Cape Peninsula: estimating the probability of river habitat use by Cape clawless otters, Aonyx capensis, across a gradient of human influence. Aquatic Conservation 27, 706–716.
| Otter occupancy in the Cape Peninsula: estimating the probability of river habitat use by Cape clawless otters, Aonyx capensis, across a gradient of human influence.Crossref | GoogleScholarGoogle Scholar |
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P. R., O’Hara, R. B., Simpson, G. L., Solymos, P., Stevens, M. H. H., Szoecs, E., and Wagner, H. (2019). vegan: Community Ecology Package, R package version 2.5–5. Available at https://CRAN.R-project.org/package=vegan [verified 20 December 2019].
Paull, D. J., Claridge, A. W., and Cunningham, R. B. (2012). Effective detection methods for medium-sized ground-dwelling mammals: a comparison between infrared digital cameras and hair tunnels. Wildlife Research 39, 546–553.
| Effective detection methods for medium-sized ground-dwelling mammals: a comparison between infrared digital cameras and hair tunnels.Crossref | GoogleScholarGoogle Scholar |
Pease, B. S., Nielsen, C. K., and Holzmueller, E. J. (2016). Single-camera trap survey designs miss detections: impacts on estimates of occupancy and community metrics. PLoS One 11, .
| Single-camera trap survey designs miss detections: impacts on estimates of occupancy and community metrics.Crossref | GoogleScholarGoogle Scholar | 27902733PubMed |
Piggott, M. P., Banks, S. C., Stone, N., Banffy, C., and Taylor, A. C. (2006). Estimating population size of endangered brush-tailed rock-wallaby (Petrogale penicillata) colonies using faecal DNA. Molecular Ecology 15, 81–91.
| Estimating population size of endangered brush-tailed rock-wallaby (Petrogale penicillata) colonies using faecal DNA.Crossref | GoogleScholarGoogle Scholar | 16367832PubMed |
QGIS Development Team (2019). QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available at http://qgis.osgeo.org [verified 15 February 2019].
R Core Team (2018). R: A language and environment for statistical computing. Available at http://www.r-project.org [verified 20 December 2019].
Reconyx Inc. (2017). Reconyx Hyperfire Instruction Manual Version 20151130v1. Available at https://www.reconyx.com/img/file/HyperFireManual.pdf [verified 10 July 2020].
Romano, A., Costa, A., Salvidio, S., Menegon, M., Garollo, E., Tabarelli de Fatis, K., Miserocchi, D., Matteucci, G., and Pedrini, P. (2018). Forest management and conservation of an elusive amphibian in the Alps: habitat selection by the golden alpine salamander reveals the importance of fine woody debris. Forest Ecology and Management 424, 338–344.
| Forest management and conservation of an elusive amphibian in the Alps: habitat selection by the golden alpine salamander reveals the importance of fine woody debris.Crossref | GoogleScholarGoogle Scholar |
Rovero, F., and Marshall, A. R. (2009). Camera trapping photographic rate as an index of density in forest ungulates. Journal of Applied Ecology 46, 1011–1017.
| Camera trapping photographic rate as an index of density in forest ungulates.Crossref | GoogleScholarGoogle Scholar |
Rowcliffe, J. M., Carbone, C., Jansen, P. A., Kays, R., and Kranstauber, B. (2011). Quantifying the sensitivity of camera traps: an adapted distance sampling approach. Methods in Ecology and Evolution 2, 464–476.
| Quantifying the sensitivity of camera traps: an adapted distance sampling approach.Crossref | GoogleScholarGoogle Scholar |
Seidlitz, A., Bryant, K. A., Armstrong, N., Calver, M., and Wayne, A. (2020). Optimising camera trap height and model increases detection and individual identification rates for a small mammal, the numbat (Myrmecobius fasciatus). Australian Mammalogy , .
| Optimising camera trap height and model increases detection and individual identification rates for a small mammal, the numbat (Myrmecobius fasciatus).Crossref | GoogleScholarGoogle Scholar |
Silveira, L., Jácomo, A. T. A., and Diniz-Filho, J. A. F. (2003). Camera trap, line transect census and track surveys: a comparative evaluation. Biological Conservation 114, 351–355.
| Camera trap, line transect census and track surveys: a comparative evaluation.Crossref | GoogleScholarGoogle Scholar |
Silveira, M., Tomas, W. M., Fischer, E., and Bordignon, M. O. (2018). Habitat occupancy by Artibeus planirostris bats in the Pantanal wetland, Brazil. Mammalian Biology 91, 1–6.
| Habitat occupancy by Artibeus planirostris bats in the Pantanal wetland, Brazil.Crossref | GoogleScholarGoogle Scholar |
Sutherland, W. J. (Ed.) (2006). ‘Ecological Census Techniques: a Handbook.’ 2nd edn. (Cambridge University Press: Cambridge.)
Swann, D. E., and Perkins, N. (2014). Camera trapping for animal monitoring and management: a review of applications. In ‘Camera Trapping: Wildlife Management and Research’. (Eds P. D. Meek, P. J. S. Fleming, G. Ballard, P. Banks, A. W. Claridge, J. Sanderson and D. Swann.) pp. 3–12. (CSIRO Publishing: Melbourne, Vic., Australia.)
Thompson, W. L., White, G. C., and Gowan, C. (1998) ‘Monitoring Vertebrate Populations.’ (Academic Press: San Diego, CA, USA.)
Tobler, M. W., Carrillo‐Percastegui, S. E., Pitman, R. L., Mares, R., and Powell, G. (2008). An evaluation of camera traps for inventorying large‐ and medium‐sized terrestrial rainforest mammals. Animal Conservation 11, 169–178.
| An evaluation of camera traps for inventorying large‐ and medium‐sized terrestrial rainforest mammals.Crossref | GoogleScholarGoogle Scholar |
Tyre, A. J., Tenhumberg, B., Field, S. A., Darren, N., Kirsten, P., and Possingham, H. P. (2003). Improving precision and reducing bias in biological surveys: estimating false-negative error rates. Ecological Applications 13, 1790–1801.
| Improving precision and reducing bias in biological surveys: estimating false-negative error rates.Crossref | GoogleScholarGoogle Scholar |
Urbanek, R. E., Ferreira, H. J., Olfenbuttel, C., Dukes, C. G., and Albers, G. (2019). See what you’ve been missing: an assessment of Reconyx® PC900 hyperfire cameras. Wildlife Society Bulletin , .
| See what you’ve been missing: an assessment of Reconyx® PC900 hyperfire cameras.Crossref | GoogleScholarGoogle Scholar |
Vine, S. J., Crowther, M. S., Lapidge, S. J., Dickman, C. R., Mooney, N., Piggott, M. P., and English, A. W. (2009). Comparison of methods to detect rare and cryptic species: a case study using the red fox (Vulpes vulpes). Wildlife Research 36, 436–446.
| Comparison of methods to detect rare and cryptic species: a case study using the red fox (Vulpes vulpes).Crossref | GoogleScholarGoogle Scholar |
Wayne, A. F., Cowling, A., Ward, C. G., Rooney, J. F., Vellios, C. V., Lindenmayer, D. B., and Donnelly, C. F. (2005). A comparison of survey methods for arboreal possums in jarrah forest, Western Australia. Wildlife Research 32, 701–714.
| A comparison of survey methods for arboreal possums in jarrah forest, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Wayne, A. F., Maxwell, M. A., Ward, C. G., Vellios, C. V., Ward, B. G., Liddelow, G. L., Wilson, I., Wayne, J. C., and Williams, M. R. (2013). Importance of getting the numbers right: quantifying the rapid and substantial decline of an abundant marsupial, Bettongia penicillata. Wildlife Research 40, 169–183.
| Importance of getting the numbers right: quantifying the rapid and substantial decline of an abundant marsupial, Bettongia penicillata.Crossref | GoogleScholarGoogle Scholar |
Wearn, O. R., and Glover-Kapfer, P. (2019). Snap happy: camera traps are an effective sampling tool when compared with alternative methods. Royal Society Open Science 6, 181748.
| Snap happy: camera traps are an effective sampling tool when compared with alternative methods.Crossref | GoogleScholarGoogle Scholar | 31032031PubMed |
Woinarski, J., Harrison, P., and Burbidge, A. (2014) ‘The Action Plan for Australian Mammals 2012.’ (CSIRO Publishing: Melbourne, Vic., Australia.)
Yeatman, G. J., Wayne, A. F., Mills, H. R., and Prince, J. (2016). Temporal patterns in the abundance of a critically endangered marsupial relates to disturbance by roads and agriculture. PLoS One 11, e0160790.
| Temporal patterns in the abundance of a critically endangered marsupial relates to disturbance by roads and agriculture.Crossref | GoogleScholarGoogle Scholar | 27501320PubMed |
Zosky, K. L., Wayne, A. F., Bryant, K. A., Calver, M. C., and Scarff, F. R. (2017). Diet of the critically endangered woylie (Bettongia penicillata ogilbyi) in south-western Australia. Australian Journal of Zoology 65, 302–312.
| Diet of the critically endangered woylie (Bettongia penicillata ogilbyi) in south-western Australia.Crossref | GoogleScholarGoogle Scholar |