Assessing factors affecting adult female white-tailed deer survival in the Northern Great Plains
Katherine L. Moratz A , Bailey S. Gullikson A , Eric S. Michel A C , Jonathan A. Jenks A , Daniel M. Grove B and William F. Jensen B
A C , Jonathan A. Jenks A , Daniel M. Grove B and William F. Jensen B
A Department of Natural Resource Management, South Dakota State University, Brookings, SD 57007, USA.
B North Dakota Game and Fish Department, Bismarck, ND 58501, USA.
C Corresponding author. Email: eric.michel@sdstate.edu
Wildlife Research 45(8) 679-684 https://doi.org/10.1071/WR18032
Submitted: 20 February 2018 Accepted: 16 September 2018 Published: 18 December 2018
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Context: Documenting cause-specific mortality and deriving survival estimates for a population are both vital to understanding potential restrictions to population growth. Survival varies among populations of the same species and depends on several factors, including climatic events, density-dependent and density-independent factors, observed predator composition and whether recreational hunting occurs. Therefore, understanding factors affecting adult survival and estimating survival rates at biologically important times will help refine management of these populations.
Aims: We aimed to assess cause-specific mortality, estimate survival rates, and determine at what part of the winter (January to April) most mortalities occurred for female white-tailed deer located in the Northern Great Plains region of the USA.
Methods: We captured 165 adult female white-tailed deer (Odocoileus virginianus) located in western North Dakota and north-western South Dakota, USA, during the winters of 2014 and 2015. We fitted individuals with Very High Frequency (VHF) radio-collars and located them 1–3 times per week to monitor survival. We investigated all mortalities to establish proximate cause of death.
Key Results: Survival was lowest during our Hunt time period (S = 0.93), although hunter harvest was not the leading cause of mortality. Predation was the greatest source of mortality, particularly during our Post-hunt time period. Additionally, almost 90% of mortalities occurring during the Post-hunt time period happened during late winter before spring green up.
Conclusions and Implications: Predation was the main source of mortality for adult females in our study, with coyotes (Canis latrans) being the sole predator capable of depredation in our study area. Predation by coyotes may indicate that potential factors, including winter severity and nutritional restrictions, have decreased female body condition, making individuals more susceptible to predation. Although we report relatively high survival, managers should consider the possibility that coyotes may impact adult populations, particularly in regions where other large-sized predators occur, or in regions where coyotes are newly established. Managers should also acknowledge that overwinter density estimates may need to be adjusted during severe winters to account for mortalities that occur after population surveys are conducted.
Additional keywords: grassland Ecosystems, mortality, Odocoileus virginianus, predation.
Introduction
Understanding what factors affect adult survival of long-lived ungulates is important because adult survival tends to be highly elastic (i.e. greatest ability to affect population dynamics), thus variation in adult survival will affect population growth (Gaillard et al. 1998; Gaillard et al. 2000). Juvenile survival is generally more variable than adult survival, and therefore seemingly has more influence on population dynamics (Gaillard et al. 1998; Chitwood et al. 2015). Consequently, establishing survival rates for each age class is important when considering various management actions such as recreational adult harvest (Robinson et al. 2014).
Survival rates for populations of the same species are affected by several factors, such as winter severity (roe deer, Capreolus capreolus, Gaillard et al. 1993; white-tailed deer, Odocoileus virginianus, DelGiudice et al. 2002; ring-necked pheasants, Phasianus colchicus, Gabbert et al. 1999), density-dependent and density-independent factors (white-tailed deer, Simard et al. 2010; caribou, Rangifer tarandus, Tyler 2010), predator composition (elk, Cervus canadensis, Brodie et al. 2013) and human harvest (white-tailed deer, Brinkman et al. 2004; wild boar, Sus scrofa, Toïgo et al. 2008). Predation and human harvest also can have additive mortality effects on populations (Sandercock et al. 2011; Brodie et al. 2013). Therefore, estimating survival and cause-specific mortality for a population allows for a better understanding of the viability of a population, and is essential for management.
Survival estimates and cause-specific sources of mortality vary among populations of the same species. For example, Bishop et al. (2005) found that survival and cause-specific sources of mortality varied for three adjacent populations of mule deer fawns (Odocoileus hemionus) in south-western Idaho, while Gingery et al. (2018) reported variation in white-tailed deer fawn survival across North America. Although white-tailed deer annual and seasonal mortality has been well documented in the Northern Great Plains region of North America (western Minnesota, USA, DelGiudice et al. 2002; Brinkman et al. 2004; eastern North Dakota, USA, Smith et al. 2007; Sternhagen 2015; and eastern South Dakota, USA, Grovenburg et al. 2011), there is no information regarding the impacts of hunter harvest, predation, and other mortality causes for white-tailed deer in the grassland ecosystem of the western Dakotas. Therefore, our goal was to estimate survival to evaluate the viability of a population of white-tailed deer captured from three study areas in North Dakota and South Dakota, USA. Specifically, our objectives were to assess if survival of adult female white-tailed deer varied throughout autumn (September to December) and winter, assess the impact of human harvest and document cause-specific sources of mortality.
Materials and methods
Study area
We investigated survival rates and cause-specific mortality of female white-tailed deer captured in Dunn and Grant counties, North Dakota and Perkins County, South Dakota (Fig. 1) during 2014 and 2015. Counties were located in the north-western Great Plains Level III Ecoregion (Bryce et al. 1998).
We focused deer capture in a 1492 km2 area in the south-western portion of Dunn County (47.2122° N, 102.7260° W), a 1865 km2 area in the south-western portion of Grant County (46.3951° N, 101.5536° W) and a 1492 km2 area in the central portion of Perkins County (45.3888° N, 102.3224° W). Grasslands, cropland and forested areas were the dominant cover types and ranged from 60 to 86%, 11 to 26% and 0.01 to 9%, respectively (Cropland Data Layer, U.S. Department of Agriculture 2015). Estimated minimum white-tailed deer density was lowest in Dunn County (1.0 deer per km2 in 2011; Stillings et al. 2016) and greatest in Grant County (1.8 deer per km2 in 2011; Stillings et al. 2016). Thirty-year mean annual precipitation ranged from 41.2 cm (Grant County) to 44.9 cm (Perkins County), and variation in thirty-year mean monthly temperature was greatest in Perkins County and ranged from –12.1°C to 30.3°C (North Dakota State Climate Office 2016).
Capture methods
We captured adult female (≥1.5-year-old) white-tailed deer via helicopter net guns (Native Range Capture Services, Elko, NV) in Dunn, Grant and Perkins counties from 24 February to 2 March 2014. Helicopter crew members hobbled, blindfolded and fitted individuals with Very High Frequency (VHF) radio-collars (model M2610B, Advanced Telemetry Systems, Isanti, MN) programmed with an 8-h mortality delay. We captured additional adult female white-tailed deer in Grant County via helicopter net guns (Quicksilver Air Inc., Peyton, CO) on 14 February 2015. In 2015, the helicopter capture crew transported captured individuals below the helicopter in canvas transport bags to a processing site where 1 mL Banamine and 3 mL BO-SE (Selenium and Vitamin E) were given to each individual (D. M. Grove, NDGF, pers. comm.), and age was estimated (yearling or adult) based on tooth wear and replacement before release (Severinghaus 1949). We censored all mortalities that occurred within 26 days post-capture, regardless of ultimate cause of death, to remove mortalities potentially caused by capture stress (Beringer et al. 1996). All handling methods followed the American Society of Mammalogists guidelines for mammal care and use (Sikes et al. 2016), and South Dakota State University Institutional Animal Care and Use Committee also approved all procedures under protocol 13-091A.
We located deer 1–3 times per week using aerial telemetry, omnidirectional whip antennas and handheld telemetry equipment. We investigated mortalities immediately after detecting a mortality signal and transported carcasses to the North Dakota Game and Fish Wildlife Laboratory in Bismarck, North Dakota, to confirm proximate cause of death. We determined a predation event occurred if there was evidence of haemorrhaging. We classified disease and predation mortalities as natural causes of mortality, and hunter harvest and vehicle collision mortalities (VCM) as human-related mortalities.
Statistical analysis
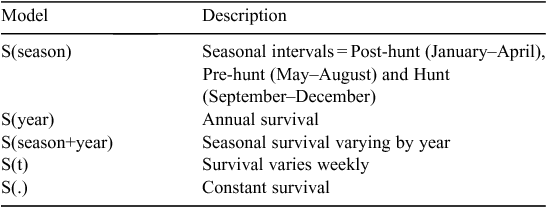
We summarised weekly female mortality using telemetry data and developed a candidate set of five models for comparison (Table 1). We estimated annual S(year) and seasonal S(season) survival rates using the Kaplan–Meier method (Kaplan and Meier 1958) adapted for staggered entry (Pollock et al. 1989) in Program MARK version 6.0 (White and Burnham 1999; Cooch and White 2016). Recreational hunting season dates were similar across study areas and occurred from 29 August 2014 to 4 January 2015 and 4 September 2015 to 3 January 2016 in North Dakota. Recreational hunting in South Dakota occurred from 27 September 2014 to 15 January 2015 and 26 September 2015 to 15 January 2016. We calculated seasonal survival rates for three time periods during 2014 and 2015: Post-hunt (January–April), Pre-hunt (May–August) and Hunt (September–December). We also assessed a model describing a combination of seasonal and annual survival S(season+year). We then compared annual and seasonal survival and their combination with models representing constant survival S(.) and weekly survival (S(t)). We used the logit link function to calculate all β parameter estimates. We ranked these five models using Akaike’s Information Criterion corrected for small sample size (AICc) and considered models within 2 ΔAICc units as competing (Burnham and Anderson 2002). We considered variables significant when 95% confidence intervals (CIs) of β estimates excluded zero. Although we included a model to describe the effects of female age (yearling or adult), only 14 of 165 (8.5%) females were classified as yearlings. Therefore, we did not incorporate an age model because of a lack of variation in the sample.
|
Results
We captured and radio-collared 50 adult female white-tailed deer via helicopter net gun in each study area during February and March 2014. We captured and radio-collared 15 additional female white-tailed deer in Grant County during 2015 due to high mortality in 2014. We observed seven total capture-related mortalities across 2014 and 2015 and removed those individuals from analyses. We were unable to locate one individual after December 2014 and therefore censored it from the 2015 analysis.
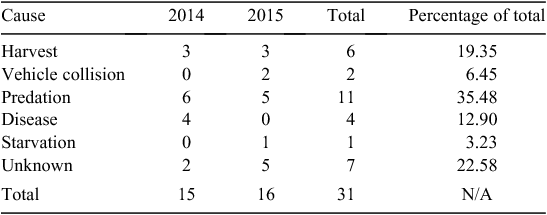
We documented 31 mortalities during the study (Table 2): 16 (52%) related to natural causes (predation, disease and starvation); and 8 (26%) human-related mortalities. Predation (n = 11, 35%) and hunter harvest (n = 6, 19%) were leading sources of mortalities; however, we were unable to assign cause-specific sources for ~22% (n = 7) of mortalities. Epizootic haemorrhagic disease and acidosis were the only two diseases responsible for mortalities in our study (n = 4).
|
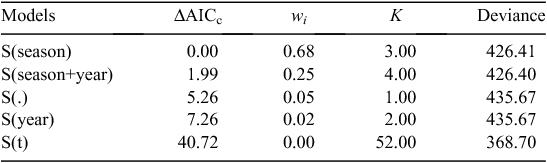
Model selection indicated that S(season) was the best approximating model (wi = 0.68, K = 3; Table 3), with survival varying by season (Post-hunt: β = 5.886, 95% CI = 5.265–6.506; Pre-hunt: β = 7.065, 95% CI = 6.084–8.04; Hunt: β = 5.591, 95% CI = 5.114–6.067). S(season+year) appeared to compete with our S(Season) models (ΔAICc = 1.99, wi = 0.25, K = 4). However, survival did not vary between years (β = –0.032, 95% CI = –0.747 – 0.682) and therefore, we only interpreted our S(season) model (Arnold 2010). Survival for the entirety of the study was 0.88 (95% CI = 0.833–0.913) and was similar between years (2014: 0.88, 95% CI = 0.805–0.925; 2015: 0.88 95% CI = 0.814–0.926). Survival was greatest in the Pre-hunt period (0.98, 95% CI = 0.962–0.995), and least during the Hunt period (0.93; 95% CI = 0.898–0.959); however, harvest only accounted for 35% (n = 6) of mortalities that occurred during this period.
|
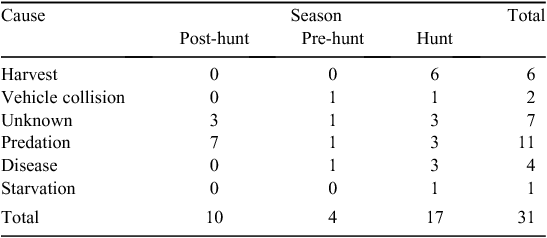
Mortalities during the Post-hunt period were attributed to predation (n = 7) and unknown causes (n = 3; Table 4). Across study areas, 90% of Post-hunt mortalities occurred after 15 March through 30 April (4 in 2014; 5 in 2015).
|
Discussion
We observed variation in seasonal and annual survival rates among study areas that were both consistent with and higher than previously published literature. Seasonal survival rates (Post-hunt, Pre-hunt and Hunt) mostly support previous research reported in eastern South Dakota (Post-hunt = 97%, Pre-hunt = 97%, Hunt = 80%, Grovenburg et al. 2011), south-western Minnesota (Post-hunt = 95%, Pre-hunt = 100%, Hunt = 80%, Brinkman et al. 2004) and eastern North Dakota (Post-hunt = 98%, Pre-hunt = 98%, Hunt = 97%, Sternhagen 2015). Survival during our Hunt time period varied from previous studies and was 13% greater in our study compared with studies conducted in eastern South Dakota (Grovenburg et al. 2011) and south-western Minnesota (Brinkman et al. 2004). Furthermore, our annual survival (88%) was slightly lower than an unhunted population (93% survival within the Kejimkujik National Park) in Nova Scotia, Canada (Patterson et al. 2002), but much higher than a hunted population in Illinois, USA (71%; Nixon et al. 1991). Relatively high survival in our study could be attributed to the decreased number of mortalities attributed to hunter harvest during the Hunt time period. For example, DelGiudice et al. (2002), Brinkman et al. (2004) and Grovenburg et al. (2011) all reported that hunter harvest was the leading cause of adult mortality during the hunting season. One explanation for this difference could be that antlerless deer tag availability decreased in western North and South Dakota during 2014 and 2015 (Huxoll 2015; Stillings et al. 2016). Additionally, many hunters chose to shoot males over females when given the opportunity (Jenks et al. 2002), and may also have selected against harvesting radio-collared deer, though hunters did not discriminate against radio-collared deer in Pennsylvania (Buderman et al. 2014). Regardless, our observations may not have captured the typical extent of hunting mortality for adult female white-tailed deer (Magle et al. 2012).
Although we report a relatively low predation rate by coyotes on adult female white-tailed deer (35% of all mortalities, 70% during Post-hunt, 25% during Pre-hunt and 18% during the Hunt time periods), there were discrepancies among the effects of coyotes on adult populations of white-tailed deer in the literature. For example, coyote predation of adult female white-tailed deer accounted for up to 64% (7 of 11) of mortalities in northern New Brunswick (Whitlaw et al. 1998), whereas coyotes only accounted for ~29% of mortalities in the central Appalachians of West Virginia (Campbell et al. 2005). No mortalities of adult female white-tailed deer could be attributed to coyote predation in a population in South Carolina (Kilgo et al. 2016). The overall impacts of coyote predation on adult populations is unclear. Nevertheless, coyotes are considered the leading predator of white-tailed deer neonates in the Northern Great Plains (Brinkman et al. 2004; Grovenburg et al. 2011, 2012), and are most likely capable of depredating adult white-tailed deer in the presence of inclement weather (deep snow), or when individuals have depleted body reserves, making them more susceptible to predation events (hypothesised by Demarais et al. (2000) and Ballard (2011) and displayed by white-tailed deer fawns (Mech 2007)). Therefore, although we report a relatively high annual survival rate (S = 0.88), we hypothesise that the increased number of predations that occurred during the Post-hunt time period (compared with other time periods in our study) indicate that this population of white-tailed deer was most susceptible to predation after the recreational hunting season occurred, potentially because of poor body condition related to increased winter severity. Nevertheless, continual monitoring should occur to assess if there are any effects of the predator population on population growth of white-tailed deer in the Northern Great Plains.
We report an overwhelming majority (90%) of adult female white-tailed deer mortalities occurred from 15 March through 30 April, indicating increased susceptibility to mortality in late winter (Mautz 1978). The high proportion of mortalities that occurred during late winter was likely influenced by winter severity. The Deer Winter Severity Index (an index that accounts for snow depth and temperature from November to April; Brinkman et al. 2005) was either above the threshold considered to be severe (2014) or slightly below the severe threshold (2015; Moratz 2016). Regardless, the high proportion of mortalities that occurred during late winter could be problematic for managers that derive density estimates based on techniques such as flying aerial surveys, particularly if those surveys are conducted before most mortalities occur. Managers should therefore be aware that up to 90% of overwinter mortalities may occur after 15 March and density estimates should be adjusted when winters are severe. If estimates are not adjusted, then inappropriate harvest recommendations for the following fall hunting season may be made.
Conclusion
Our results provide baseline information that will help improve white-tailed deer management in the western grasslands region of the Northern Great Plains, an area where baseline survival information has not been previously documented. Although adult female white-tailed deer survival was lowest during the Hunt time period, predation accounted for the largest proportion of mortality throughout our study, particularly during the Post-hunt time period. This result indicates that predation events (likely attributed to coyotes) may be compounded by winter severity (Van Deelen et al. 1997; DelGiudice et al. 2006) and a lack of nutritional resources (DePerno et al. 2000), leading to decreased body condition of adults, making them more susceptible to predation. However, more information is needed to assess if predation displays additive or compensatory effects on this population. If compensatory, then managers may consider increasing female harvest quotas to increase hunter participation. If predation displays an additive effect on population survival, potentially related to the relatively low densities reported for these study areas, then managers may consider reducing harvest quotas for females to alleviate potential negative effects, though the effect of predation may vary depending on age class (DelGiudice et al. 2002). Regardless, the survival estimates we report for adult female white-tailed deer in the Northern Great Plains region suggest that this is a viable population and neither hunting nor predation is likely having negative impacts on the population.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank M. Festa-Bianchet and two anonymous reviewers for the helpful comments on previous versions. We thank the North Dakota Game and Fish Department, South Dakota Department of Game, Fish and Parks, the Department of Natural Resource Management at South Dakota State University and numerous private landowners for their help and cooperation with this project. We thank field technicians C. Argabright, S. Bard, A. Dwire, S. Harrington, R. Johnson, A. Kauth and R. Kelble for their efforts tracking deer. Special thanks to J. Faught for his countless hours of telemetry flights. This work was supported by the Federal Aid to Wildlife Restoration and administered through North Dakota Game and Fish Department (Study No. W-67-R) and South Dakota Department of Game, Fish and Parks (Study No. 7555). Any mention of trade, product or firm names is for descriptive purposes only, and does not imply endorsement by the US Government.
References
Arnold, T. W. (2010). Uniformative parameters and model selection using Akaikie’s Information Criterion. The Journal of Wildlife Management 74, 1175–1178.Ballard, W. (2011). Population dynamics. In ‘Biology and Management of White-tailed Deer’. (Ed. D. G. Hewitt.) pp. 251–286. (CRC Press: Boca Raton, FL.)
Beringer, J., Hansen, L. P., Wilding, W., Fischer, J., and Sheriff, S. L. (1996). Factors affecting capture myopathy in white-tailed deer. The Journal of Wildlife Management 60, 373–380.
| Factors affecting capture myopathy in white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Bishop, C. J., Unsworth, J. W., and Garton, E. O. (2005). Mule deer survival among adjacent populations in southwest Idaho. The Journal of Wildlife Management 69, 311–321.
| Mule deer survival among adjacent populations in southwest Idaho.Crossref | GoogleScholarGoogle Scholar |
Brinkman, T. J., Jenks, J. A., DePerno, C. S., Haroldson, B., and Osborn, R. G. (2004). Survival of white-tailed deer in an intensively farmed region of Minnesota. Wildlife Society Bulletin 32, 726–731.
| Survival of white-tailed deer in an intensively farmed region of Minnesota.Crossref | GoogleScholarGoogle Scholar |
Brinkman, T. J., DePerno, C. S., Jenks, J. A., and Haroldson, B. S. (2005). Movement of female white-tailed deer: effects of climate and intensive row-crop agriculture. The Journal of Wildlife Management 69, 1099–1111.
| Movement of female white-tailed deer: effects of climate and intensive row-crop agriculture.Crossref | GoogleScholarGoogle Scholar |
Brodie, J., Johnson, H., Mitchell, M., Zager, P., Proffitt, K., Hebblewhite, M., Kauffman, M., Johnson, B., Bissonette, J., Bishop, C., Gude, J., Herbert, J., Hersey, K., Hurley, M., Lukacs, P. M., McCorquodale, S., McIntire, E., Nowak, J., Sawyer, H., Smith, D., and White, P. J (2013). Relative influence of human harvest, carnivores, and weather on adult female elk survival across western North America. Journal of Applied Ecology 50, 295–305.
| Relative influence of human harvest, carnivores, and weather on adult female elk survival across western North America.Crossref | GoogleScholarGoogle Scholar |
Bryce, S. A., Omernik, J. M., Pater, D. E., Ulmer, M., Scharr, J., Freeouf, J., Johnson, R., Kuck, P., and Azevedo, S. H. (1998). Ecoregions of North Dakota and South Dakota. (Map poster). US Geological Survey, Jamestown, ND.
Buderman, F. E., Diefenbach, D. R., Rosenberry, C. S., Wallingford, B. D., and Long, E. S. (2014). Effect of hunter selectivity on harvest rates of radio-collared white-tailed deer in Pennsylvania. The Journal of Wildlife Management 78, 1456–1465.
| Effect of hunter selectivity on harvest rates of radio-collared white-tailed deer in Pennsylvania.Crossref | GoogleScholarGoogle Scholar |
Burnham, K. P., and Anderson, D. R. (2002). ‘Model Selection and Multimodel Inference: a Practical Information-theoretic Approach.’ (Springer-Verlag: New York.)
Campbell, T. A., Laseter, B. R., Ford, W. M., and Miller, K. V. (2005). Characteristics of a central Appalachian white-tailed deer herd. Wildlife Society Bulletin 33, 212–221.
| Characteristics of a central Appalachian white-tailed deer herd.Crossref | GoogleScholarGoogle Scholar |
Chitwood, M. C., Lashley, M. A., Kilgo, J. C., Moorman, C. E., and DePerno, C. S. (2015). White-tailed deer population dynamics and adult female survival in the presence of a novel predator. The Journal of Wildlife Management 79, 211–219.
| White-tailed deer population dynamics and adult female survival in the presence of a novel predator.Crossref | GoogleScholarGoogle Scholar |
Cooch, E., and White, G. C. (2016). ‘Program Mark: a Gentle Introduction.’ (Cornell University: Ithaca, NY.)
DelGiudice, G. D., Riggs, M. R., Joly, P., and Pan, W. (2002). Winter severity, survival, and cause-specific mortality of female white-tailed deer in north-central Minnesota. The Journal of Wildlife Management 66, 698–717.
| Winter severity, survival, and cause-specific mortality of female white-tailed deer in north-central Minnesota.Crossref | GoogleScholarGoogle Scholar |
DelGiudice, G. D., Fieberg, J., Riggs, M. R., Powell, M. C., and Pan, W. (2006). A long-term age-specific survival analysis of female white-tailed deer. The Journal of Wildlife Management 70, 1556–1568.
| A long-term age-specific survival analysis of female white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Demarais, S., Miller, K. V., and Jacobson, H. A. (2000). White-tailed deer. In ‘Ecology and Management of Large Mammals in North America’. (Eds S. Demarais and P. R. Krausman.) pp. 601–619. (Prentice-Hall, Inc. Upper Saddle River, NJ.)
DePerno, C. S., Jenks, J. A., Griffin, S. L., and Rice, L. A. (2000). Female survival rates in a declining white-tailed deer population. Wildlife Society Bulletin 28, 1030–1037.
Gabbert, A. E., Leif, A. P., Purvis, J. R., and Flake, L. D. (1999). Survival and habitat use by ring-necked pheasants during two disparate winters in South Dakota. The Journal of Wildlife Management 63, 711–722.
| Survival and habitat use by ring-necked pheasants during two disparate winters in South Dakota.Crossref | GoogleScholarGoogle Scholar |
Gaillard, J. M., Delorme, D., Boutin, J. M., and Van Laere, G. V. (1993). Roe deer survival patterns: a comparative analysis of contrasting populations. Journal of Animal Ecology 62, 778–791.
| Roe deer survival patterns: a comparative analysis of contrasting populations.Crossref | GoogleScholarGoogle Scholar |
Gaillard, J. M., Festa-Bianchet, M., and Yoccoz, N. G. (1998). Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends in Ecology & Evolution 13, 58–63.
| Population dynamics of large herbivores: variable recruitment with constant adult survival.Crossref | GoogleScholarGoogle Scholar |
Gaillard, J. M., Festa-Bianchet, M., Yoccoz, N. G., Loison, A., and Toïgo, C (2000). Temporal variation in fitness components and population dynamics of large herbivores. Annual Review of Ecology and Systematics 31, 367–393.
| Temporal variation in fitness components and population dynamics of large herbivores.Crossref | GoogleScholarGoogle Scholar |
Gingery, T. M., Diefenbach, D. R., Wallingford, B. D., and Rosenberry, C. S. (2018). Landscape-level patterns in fawn survival across North America. The Journal of Wildlife Management 82, 1003–1013.
| Landscape-level patterns in fawn survival across North America.Crossref | GoogleScholarGoogle Scholar |
Grovenburg, T. W., Swanson, C. C., Jacques, C. N., DePerno, C. S., Klaver, R. W., and Jenks, J. A. (2011). Female white-tailed deer survival across ecoregions in Minnesota and South Dakota. American Midland Naturalist 165, 426–435.
| Female white-tailed deer survival across ecoregions in Minnesota and South Dakota.Crossref | GoogleScholarGoogle Scholar |
Grovenburg, T. W., Klaver, R. W., and Jenks, J. A. (2012). Survival of white-tailed deer fawns in the grasslands of the Northern Great Plains. The Journal of Wildlife Management 76, 944–956.
| Survival of white-tailed deer fawns in the grasslands of the Northern Great Plains.Crossref | GoogleScholarGoogle Scholar |
Huxoll, C. (2015). Big game harvest projections. Game Report No. 2016–02. South Dakota Game, Fish and Parks, Pierre, SD.
Jenks, J. A., Smith, W. P., and DePerno, C. S. (2002). Maximum sustained yield harvest versus trophy management. The Journal of Wildlife Management 66, 528–535.
| Maximum sustained yield harvest versus trophy management.Crossref | GoogleScholarGoogle Scholar |
Kaplan, E. L., and Meier, P. (1958). Nonparametric estimation from incomplete observations. Journal of the American Statistical Association 53, 457–481.
| Nonparametric estimation from incomplete observations.Crossref | GoogleScholarGoogle Scholar |
Kilgo, J. C., Vukovich, M., Conroy, M. J., Ray, H. S., and Ruth, C. (2016). Factors affecting survival of adult female white-tailed deer after coyote establishment in South Carolina. Wildlife Society Bulletin 40, 747–753.
| Factors affecting survival of adult female white-tailed deer after coyote establishment in South Carolina.Crossref | GoogleScholarGoogle Scholar |
Magle, S. B., Chamberlin, J. C., and Matthews, N. E. (2012). Survival of white-tailed deer in Wisconsin’s Chronic Wasting Disease zone. Northeastern Naturalist 19, 67–76.
| Survival of white-tailed deer in Wisconsin’s Chronic Wasting Disease zone.Crossref | GoogleScholarGoogle Scholar |
Mautz, W. W. (1978). Sledding on a bushy hillside: the fat cycle in deer. Wildlife Society Bulletin 6, 88–90.
Mech, L. D. (2007). Femur-marrow fat of white-tailed deer fawns killed by wolves. The Journal of Wildlife Management 71, 920–923.
| Femur-marrow fat of white-tailed deer fawns killed by wolves.Crossref | GoogleScholarGoogle Scholar |
Moratz, K. L. (2016). Effect of oil and gas development on survival and health of white-tailed deer in the Western Dakotas. PhD Thesis. South Dakota State University, Brookings, SD.
Nixon, C. M., Hansen, L. P., Brewer, P. A., and Chelsvig, J. E. (1991). Ecology of white-tailed deer in an intensively farmed region of Illinois. Wildlife Monographs 118, 3–77.
North Dakota State Climate Office (2016). Monthly station normal of temperature, precipitation, and heating and cooling degree days 1981–2010. Available at https://www.ndsu.edu/ndsco/data/ 30yearaverage/#c343059. [Accessed 12 July 2016]
Patterson, B. R., MacDonald, B. A., Lock, B. A., Anderson, D. G., and Benjamin, L. K. (2002). Proximate factors limiting population growth of white-tailed deer in Nova Scotia. The Journal of Wildlife Management 66, 511–521.
| Proximate factors limiting population growth of white-tailed deer in Nova Scotia.Crossref | GoogleScholarGoogle Scholar |
Pollock, K. H., Winterstein, S. R., Bunck, C. M., and Curtis, P. D. (1989). Survival analysis in telemetry studies: the staggered entry design. The Journal of Wildlife Management 53, 7–15.
| Survival analysis in telemetry studies: the staggered entry design.Crossref | GoogleScholarGoogle Scholar |
Robinson, K. F., Diefenbach, D. R., Fuller, A. K., Hurst, J. E., and Rosenberry, C. S. (2014). Can managers compensate for coyote predation of white-tailed deer? The Journal of Wildlife Management 78, 571–579.
| Can managers compensate for coyote predation of white-tailed deer?Crossref | GoogleScholarGoogle Scholar |
Sandercock, B. K., Nilsen, E. B., Brøseth, H., and Pedersen, H. C. (2011). Is hunting mortality additive or compensatory? Effects of experimental harvest on the survival and cause-specific mortality of willow ptarmigan. Journal of Animal Ecology 80, 244–258.
| Is hunting mortality additive or compensatory? Effects of experimental harvest on the survival and cause-specific mortality of willow ptarmigan.Crossref | GoogleScholarGoogle Scholar |
Severinghaus, C. W. (1949). Tooth development and wear as criteria of age in white-tailed deer. The Journal of Wildlife Management 13, 195–216.
| Tooth development and wear as criteria of age in white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Sikes, R. S., Animal Care and Use Committee of the American Society of Mammalogists (2016). 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy 97, 663–688.
| 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education.Crossref | GoogleScholarGoogle Scholar |
Simard, M. A., Coulson, T., Gingras, A., and Côté, S. D. (2010). Influence of density and climate on population dynamics of a large herbivore under harsh environmental conditions. The Journal of Wildlife Management 74, 1671–1685.
| Influence of density and climate on population dynamics of a large herbivore under harsh environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Smith, J. R., Sweitzer, R. A., and Jensen, W. F. (2007). Diets, movements, and consequences of providing wildlife food plots for white-tailed deer in central North Dakota. The Journal of Wildlife Management 71, 2719–2726.
| Diets, movements, and consequences of providing wildlife food plots for white-tailed deer in central North Dakota.Crossref | GoogleScholarGoogle Scholar |
Sternhagen, K. M. (2015). An evaluation of life history parameters and management of white-tailed deer (Odocoileus virginianus) in the Red River Valley of northeastern North Dakota. PhD Thesis, South Dakota State University, Brookings, SD.
Stillings, B., Jensen, W., and Smith, J. (2016). Study No. C–I: Deer population studies. Project No. W-67-R-56, Report No. A-232, North Dakota Game and Fish Department, Bismarck, ND.
Toïgo, C., Servanty, S., Gaillard, J. M., Brandt, S., and Baubet, E. (2008). Disentangling natural from hunting mortality in an intensively hunted wild boar population. The Journal of Wildlife Management 72, 1532–1539.
Tyler, N. J. C. (2010). Climate, snow, ice, crashes, and declines in populations of reindeer and caribou (Rangifer tarandus L.). Ecological Monographs 80, 197–219.
| Climate, snow, ice, crashes, and declines in populations of reindeer and caribou (Rangifer tarandus L.).Crossref | GoogleScholarGoogle Scholar |
US Department of Agriculture (USDA) (2015). USDA, National Agricultural Statistics Service (NASS), Cropland Data Layer. USDA, NASS Marketing and Information Services Office, Washington, DC.
Van Deelen, T. R., Campa, H., Haufler, J. B., and Thompson, P. D. (1997). Mortality patterns of white-tailed deer in Michigan’s Upper Peninsula. The Journal of Wildlife Management 61, 903–910.
| Mortality patterns of white-tailed deer in Michigan’s Upper Peninsula.Crossref | GoogleScholarGoogle Scholar |
White, G. C., and Burnham, K. P. (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139.
| Program MARK: survival estimation from populations of marked animals.Crossref | GoogleScholarGoogle Scholar |
Whitlaw, H. A., Ballard, W. B., Sabine, D. L., Young, S. J., Jenkins, R. A., and Forbes, G. J. (1998). Survival and cause-specific mortality rates of adult white-tailed deer in New Brunswick. The Journal of Wildlife Management 62, 1335–1341.
| Survival and cause-specific mortality rates of adult white-tailed deer in New Brunswick.Crossref | GoogleScholarGoogle Scholar |



