Long-term post-fire succession of reptiles in an urban remnant in south-western Australia
Robert A. Davis A * and Michael D. Craig
A * and Michael D. Craig  B
B
A
B
Abstract
Reptile responses to fire may differ between remnants and contiguous vegetation but this is poorly understood.
We aimed to explore long-term (≤15 years) post-fire responses of reptiles in an urban Banksia woodland remnant.
We trapped reptiles for 10 nights in November and December each year between 2009 and 2023 inclusive (except 2014 and 2019) to estimate relative abundance. We used mixed models to explore differences between unburnt and burnt sites and changes in both over time.
The reptile community showed short-term negative responses to fire, but communities had returned to their pre-fire state within 3 years. Two species showed short-term (3 and 5 years respectively) negative responses to fire while two species showed positive responses; one in the first year post-fire only, and the other in sites >8 years post-fire. There did not appear to be consistent differences in fire responses between this study and studies conducted in contiguous Banksia woodlands, although differences in study designs renders this conclusion equivocal.
Reptile communities in Banksia woodland remnants, and the species they contain appear to be relatively robust to a wide range of fire regimes.
Keywords: fire, fragmentation, Kings Park, monitoring, Perth, reptile, urban, Western Australia.
Introduction
Many reptile species have limited dispersal capabilities (Munguia-Vega et al. 2013; François et al. 2021; Mulhall et al. 2022), which when combined with their often small home ranges and sensitivity to changes in microhabitats and vegetation structure (Craig et al. 2014; Bradley et al. 2022), can render them highly sensitive to disturbance events such as fire (Nimmo et al. 2013; Smith et al. 2013). As a result, fire has been shown to be a significant factor influencing the composition of reptile communities (Clarke et al. 2021; Partridge et al. 2023). Several species have been shown to be most abundant in long unburnt vegetation (Nimmo et al. 2012; Doherty et al. 2015) and recolonisation of burnt areas can be a relatively slow process leading to a source-sink population dynamic between habitat patches of differing post-fire ages (Driscoll 2004; Driscoll et al. 2012; Simms et al. 2019). Hence, understanding long-term successional responses of reptiles to fire is critical for their effective conservation and management (Driscoll et al. 2010b; Clarke et al. 2021).
The conservation and management of reptiles in urban remnants is challenging with reptile communities in even large remnants being relatively depauperate compared to contiguous habitats (How and Dell 2000; Keinath et al. 2017). Adding to the challenge is the fact that reptiles in urban remnants may display different post-fire responses to populations in contiguous habitat, due to the disruption of source-sink dynamics (Driscoll et al. 2010a, 2012). In addition, remnants are often subject to altered fire regimes due to increased fire frequency due to arson (Stenhouse 2001; Hero et al. 2013) and increased fire frequency and severity due to invasion by fire-promoting weeds (Crosti et al. 2007; Fisher et al. 2009; Ramalho et al. 2014). These altered fire regimes combine with other disturbances, such as edge effects (Urbina-Cardona et al. 2006; Garden et al. 2007; McAlpine et al. 2015), to further alter vegetation structure (Ibanez et al. 2017; Gomes et al. 2022). In combination, these pressures, along with a changing climate (How et al. 2022), have the potential to strongly influence post-fire responses of reptiles in urban remnants. However, whether post-fire responses differ between remnants and contiguous vegetation remains poorly understood and the ecological mechanisms behind any potential differences, even less well understood.
Banksia woodlands are a threatened ecological community confined to sandy soils on the Swan Coastal Plain of south-western Australia where the canopy is dominated by species of Banksia (Ritchie et al. 2021). Banksia woodlands support a very rich herpetofauna of over 60 species and woodlands in and close to the major city of Perth have been extensively cleared and fragmented. Furthermore, fire is a natural disturbance in Banksia woodlands although the frequency of fire has increased over recent decades, particularly in urban remnants, so understanding post-fire responses in remnants is critical for the conservation of these reptile communities in remnants. While post-fire responses of reptiles in contiguous Banksia woodlands have been previously studied (Bamford 1995; Valentine et al. 2012), responses in remnants remain less well understood (see Davis and Doherty 2015). This study intended to address this knowledge gap by continuing the study of Davis and Doherty (2015) to explore longer-term (≤15 years) post-fire responses of reptiles in Kings Park, a large Banksia woodland remnant located within the Perth Metropolitan Area and compare the results with studies from contiguous Banksia woodlands. The questions asked were: (1) does the reptile community change over 15 years post-fire; (2) does the abundance of individual species change over 15 years post-fire; and (3) how do any patterns compare to post-fire responses in contiguous Banksia woodlands.
Materials and methods
Study site
Kings Park is located approximately 1.5 km from the centre of Perth, Western Australia (31°57′39″S, 115°49′56″E) and contains 267 ha of mixed Banksia, Allocasuarina, and Eucalyptus woodland alongside a smaller section of botanic gardens (Davis and Wilcox 2013). This woodland portion of the park is remnant native vegetation and constitutes a large urban remnant bounded to the south-east by a steep limestone escarpment leading down to the Swan River and by high-density urban development on the other three sides (Davis and Wilcox 2013). The climate is Mediterranean with mild, wet winters and hot, dry summers. Mean annual rainfall at the Subiaco Wastewater Treatment Plant, 3.6 km west of the study site, is 716.3 mm, with >75% falling between May and September inclusive. Maximum temperatures at Perth Metro, 6.0 km NE of the study area, average 31.6°C in the hottest month (February) and 18.5°C in the coolest month (July). The park has a long history of arson, wildfire and prescribed burning and, between 1962 and 2011, various areas of Kings Park’s 267 ha of remnant woodland burnt between 0 and 11 times. However, most burns were small (<2% of fires are >25 ha) and 11% (29 ha) of the woodland has remained unburnt over this period, 39% (104 ha) has had only a single fire and <1 ha has had six or more fires (Miller 2012). In January 2009, a wildfire burnt 40 ha of bushland on the scarp and plateau at Kings Park which provided an opportunity to initiate a study of the long-term post-fire dynamics of the reptile community. None of this area had been subsequently burnt up until February 2024.
Reptile sampling
To sample the reptile community, we established 10 sampling grids, five that were in the area of the 2009 fire, and five that were in older unburnt vegetation (two were last burnt in 1978 and three in 1998). All sites were placed as far apart as possible (50–500 m) within the constraints of the fire scar and other logistical constraints within the park (see fig. 1 in Davis and Doherty 2015). Each sampling grid consisted of nine pitfall traps set up in a 3 × 3 grid, with each trap 10 m apart. The pitfall traps were white PVC buckets 40 cm deep and 30 cm in diameter located in the centre of a 7-m long and 20-cm tall drift fence running in a random direction. All sites were monitored for 10 nights in the late austral spring and early summer (November and December) each year between 2009 and 2023 inclusive, except for 2014 and 2019 (see Supplementary Table S1). Traps were checked early each morning and cleared of all captured animals, which were measured and released at the point of capture. Conditions during trapping were generally warm and sunny. For all species, we calculated the minimum number of individuals captured at each site (MKTBA) in each year by removing from the dataset individuals that were recaptured at the same site within the same year. Research was approved by the ECU Animal Ethics Committee under project approval number 4064. Trapping was undertaken under a fauna licence granted by the Department of Biodiversity Conservation and Attractions and permission to work in Kings Park was provided by Mr Steve Easton and Mr Ryan Glowacki (Botanic Gardens and Parks Authority).
Statistical analyses
To analyse the effect of fire on reptile community composition we first constructed a between site-year resemblance matrix using the Bray–Curtis similarity measure. For this analysis, we included reptile community composition for each year at each site. We then visually represented community composition using a Principal Coordinates Analysis (PCO) and analysed the influence of fire with a Permutational Analysis of Variance (PERMANOVA) with Treatment (Unburnt or Burnt) and Year as fixed factors and Site as a random factor. For these analyses, we used Primer 7.0 (Anderson et al. 2008). To analyse the effect of fire on reptile community metrics (overall reptile abundance – sum of MKTBA for all species and species richness – number of species captured) and the relative abundance of individual species (MKTBA), we used a general linear mixed model with Treatment and Year as fixed factors and Site as a random factor. For these analyses we used the nlme package (Pinheiro et al. 2023) in R4.2.0 (R Development Core Team 2022) and restricted analyses on individual species to those where more than 10 individuals were captured. For all analyses, the results we were interested in were a treatment effect, showing consistent differences between unburnt and burnt sites and a treatment by year interaction, indicating that differences between unburnt and burnt sites changed across years due to post-fire succession. We did not analyse any fire variables, including historical variables, because the limited spatial spread of our sites, with all sites burnt in a single fire, meant there was negligible variation in fire variables and they failed to add significant explanatory significance beyond whether sites were unburnt or burnt.
Results
Reptile community
Across all 13 years, we captured 1701 individuals of 19 species from seven families, which included 11 species of Scincidae, two species of Pygopodidae, one species of Gekkonidae, one species of Agamidae, one species of Varanidae, one species of Typhlopidae, and two species of Elapidae. The most commonly captured species were Ctenotus fallens (489 individuals), Hemiergis quadrilineata (318), Lerista elegans (167), Cryptoblepharus buchananii (149), Morethia obscura (108), Pogona minor (108), Menetia greyii (71), Lerista praepedita (70), Lerista lineopunctulata (52), Christinus marmoratus (49), Lialis burtonis (33) and Cyclodomorphus celatus (30). For other less common captures, see Supplementary Table S2.
Effects of fire
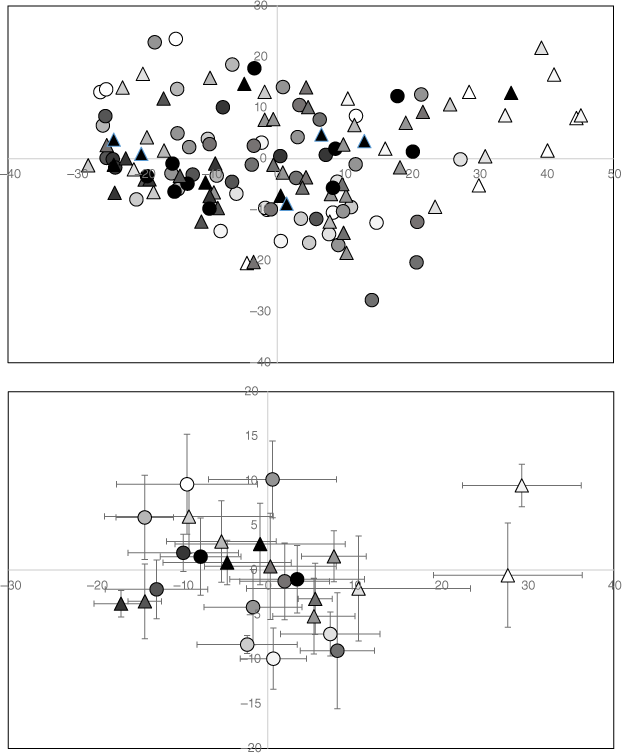
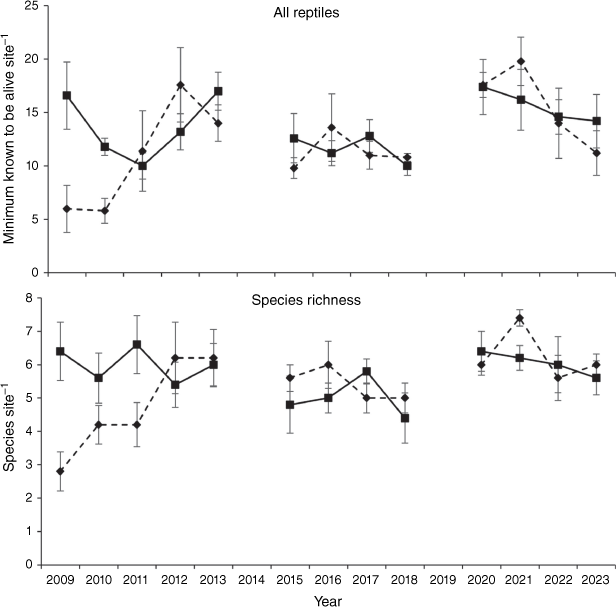
Community composition did not differ between unburnt and burnt sites (Pseudo-F1,96 = 1.58, P = 0.223) but there was a significant treatment by year interaction (Pseudo-F12,96 = 2.08, P = 0.001) with composition in the first 2 years post-fire being significantly different from sites with the longest time since fire and unburnt sites (Fig. 1). Overall reptile relative abundance showed no difference between unburnt and burnt sites (t8 = 1.78, P = 0.113) and there was no significant treatment by time interaction (t118 = −1.78, P = 0.078), although overall reptile relative abundance was lower in burnt sites in the first 2 years post-fire. Reptile species richness did show a difference between unburnt and burnt sites (t8 = 3.15, P = 0.014) but this was driven by a significant treatment by time interaction (t118 = −3.14, P = 0.002) with species richness lower in the first 3 years post-fire but the difference disappearing after that time (Fig. 2).
Results of the Principle Coordinates Analysis of reptile community composition in the first 15 years post-fire showing both individual sites (top) and the mean (±s.e.) of unburnt and burnt sites for each year (bottom). Unburnt sites are shown as circles and burnt sites as triangles with the colour of the shapes becoming progressively darker with increasing post-fire age. Note that community composition differs greatly between unburnt and burnt sites in the first 2 years post-fire but the difference disappears after this time.
Overall reptile abundance and species richness for unburnt (solid lines) and burnt (broken lines) sites for the first 15 years post-fire. All values are shown as mean ± s.e.
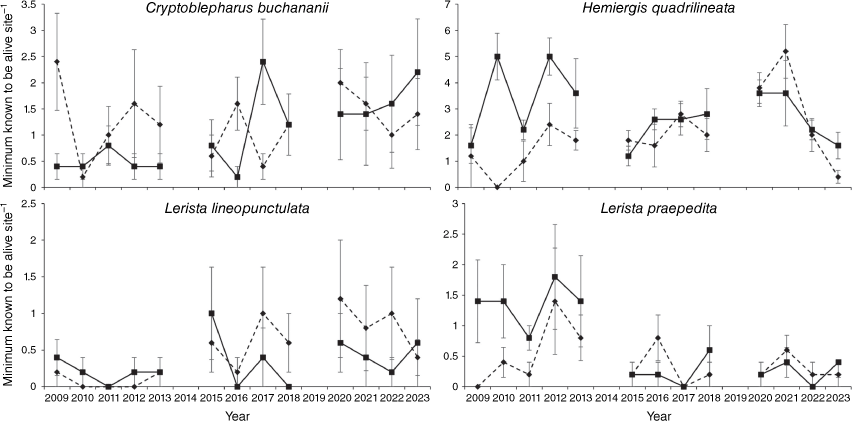
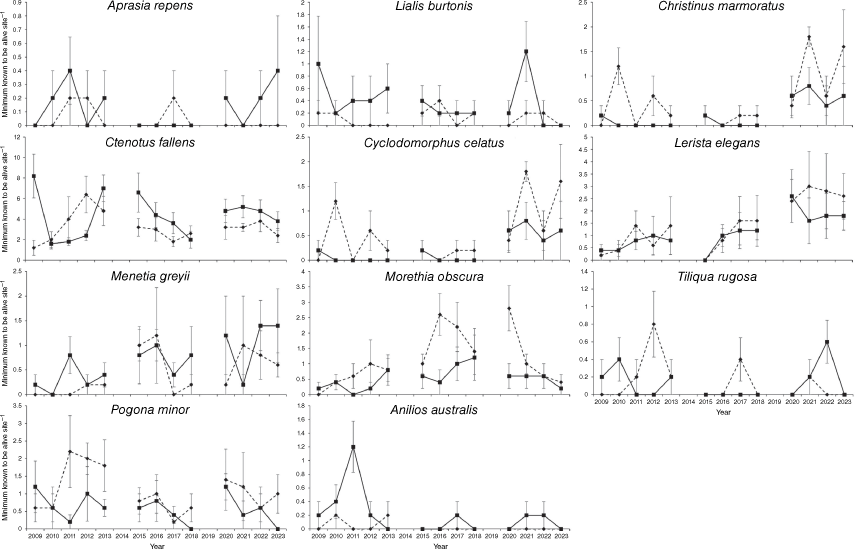
Among individual species, H. quadrilineata relative abundance showed a significant difference between unburnt and burnt sites (t8 = 2.66, P = 0.029) but this was due to a significant treatment by time interaction (t118 = −2.65, P = 0.009) with relative abundance being generally lower in the first 5 years post-fire, except for the first year (Fig. 3). L. praepedita also differed in relative abundance between unburnt and burnt sites (t8 = 2.62, P = 0.031) and this was driven by a significant treatment by time interaction (t118 = −2.61, P = 0.010) with relative abundance lower in the first 3 years post-fire but the difference disappearing after that time (Fig. 3). L. lineopunctulata relative abundance did not differ significantly between unburnt and burnt sites (t8 = 2.04, P = 0.075) but there was a significant treatment by time interaction (t18 = −2.05, P = 0.043) with relative abundance being higher in burnt sites more than 8 years post-fire. C. buchananii relative abundance also did not differ significantly between unburnt and burnt sites (t8 = −2.23, P = 0.057) but there was a significant treatment by time interaction (t118 = 2.22, P = 0.028) with relative abundance being higher in burnt sites in the first 12 months post-fire (Fig. 3). No other species showed any difference in relative abundance between unburnt and burnt sites nor a significant treatment by time interaction (Fig. 4, Table S2).
Discussion
Over the first 15-years post-fire, we found that most post-fire responses of reptiles were relatively short-lived, with no noticeable negative effects of fire on the reptile community for more than 5 years post-fire. Community metrics showed no noticeable response to fire after more than 3 years post-fire and only four species showed significant responses to fire. L. praepedita showed a reduced abundance for 3 years post-fire while H. quadrilineata was the species that showed the longest negative response to fire, with relative abundance reaching pre-fire levels 6 years post-fire. The positive responses to fire were that C. buchananii was more abundant in burnt areas in the first year post-fire and the relative abundance of L. lineopunctulata increased above pre-fire levels after 8 years post-fire. There was also no evidence of negative fire effects on rare species (≤6 individuals captured) that we did not analyse statistically. Furthermore, three of those rare species (Varanus tristis, C. australis, Psuedonaja affinis) have been recorded in a wide range of fire ages in other studies (Bamford 1995; Valentine et al. 2009) and persist in numerous urban remnants (How and Dell 1994, 2000) so they do not appear to be negatively affected by fire. Furthermore, P. affinis is often more abundant in disturbed habitats and feeds extensively on Mus musculus (Maryan and Bush 1996), which often responds positively to fire (e.g. Recher et al. 2009; Kelly et al. 2011). Therefore, the patterns we observed in these rare species are almost certainly due to stochasticity rather than reflecting an actual fire response. As expected, our results are very similar to the earlier study (Davis and Doherty 2015). The only additional information was that our longer-term study identified the post-fire age when H. quadrilineata recovered its post-fire abundance, that Lerista lineopunctulata abundance increased in burnt area in the longer term, and increased our confidence that rare species are not negatively affected by fire.
In general, our study mirrored responses from two other studies conducted in contiguous Banksia woodlands (Bamford 1995; Valentine et al. 2012). Neither of those studies found that overall reptile abundance or species richness showed a significant response to fire; however, Valentine et al. (2012) only examined sites 4 years post-fire or older so their difference from our study was likely due to their different study design. The fact that we recorded initial decreases in overall abundance and species richness could indicate that several, less common species that are resilient to fire are absent from our remnant or that there are fewer source populations to recolonise after fire. Individual species’ responses were also generally similar. P. minor, C. buchananii, Ctenotus fallens and Lerista elegans all showed no response to fire in either study (Bamford 1995; Valentine et al. 2012). One of these species, C. fallens, potentially disperses widely between bushland remnants as evidenced by a low Fst in a previous population genetics study that included Kings Park and this may enable it to recolonise after fire (Virens et al. 2015). In the case of C. buchananii, the lack of observed response was probably because its abundance was mainly related to canopy cover, which regrew rapidly post-fire (Valentine et al. 2012). Our study only found a response to fire by C. buchananii in the first year post-fire and the other studies only looked at sites 2 years or more post-fire so, again, this difference was due to study design. Hemiergis initialis abundance was lower post-fire in contiguous woodlands (Valentine et al. 2012), similar to this study, which was likely due to reduced litter cover in the first few years post-fire (Valentine et al. 2012). This species was not captured by Bamford (1995). Three species’ responses showed small variations between studies. We observed the abundance of L. praepedita was lower in the first 3 years post-fire whereas both studies in contiguous woodlands found no relationship with time since fire (Bamford 1995; Valentine et al. 2012). However, Valentine et al. (2012) only examined sites more than 4 years post-fire, so any difference was most likely due to the different study design, and the species was rarely captured by Bamford (1995). M. greyii and M. obscura were both found to increase in abundance with increasing post-fire age by Valentine et al. (2012) but both Bamford (1995) and this study found no relationship with fire. The relationship for M. obscura in Valentine et al. (2012), however, was driven by a high abundance of this species in sites more than 20 years post-fire, which is older than any burnt sites in this study. It is unclear whether that was also the case for M. greyii but Valentine et al. (2012) commented that this species’ relationship with post-fire age was weak.
Overall, all three studies exploring reptile responses to fire in Banksia woodlands found similar patterns. All differences between species were minor and inconsistent between remnant and contiguous woodlands with many differences appearing to result from different study designs. All three studies found that reptile recovery post-fire was relatively rapid and, although this recovery extended over decades for a few species, these species recovered rapidly and did not appear to be highly vulnerable to short inter-fire intervals. We also found no evidence that individual species’ responses differed consistently between remnants and contiguous woodlands, at least over the post-fire period explored in this study (i.e. <15 years). This suggests that differences in habitat structure (e.g. increased weed cover) between remnants and contiguous habitat (Ramalho et al. 2014) did not result in significantly different post-fire responses. There was limited evidence that the reptile community in remnants displayed short-term (≤3 years) negative responses that were not evident in contiguous woodlands but again, differences in study design render this conclusion speculative. Given that remnants display lower species richness, losing primarily rare and specialised species, it is possible that reptile communities in remnants are less resilient to disturbances like fire, than those in contiguous habitats, but this requires further research. The impacts of climate change on reptiles and interactions with fire, are poorly understood and require future study. How et al. (2022) analysed a 35-year dataset of reptiles in Banksia woodlands near to our study site and found that abundance was significantly correlated with preceding rainfall. They found only limited community compositional change due to annual rainfall variation.
In conclusion, our study found that post-fire responses in a Banksia woodland remnant were generally fairly minor with the few negative responses lasting no more than 5 years and a short-term (1 year) and longer-term (>8 years) positive effect. This suggests the reptile community should be fairly resilient to a wide range of fire regimes including those with short inter-fire intervals down to 3 years. The fact that reptiles occurred, in reduced numbers for two species, in areas immediately post-fire indicated that population connectivity will be maintained even under short-fire intervals. We found no consistent differences in post-fire responses between remnants and contiguous woodlands, although our results suggested the overall reptile community may show a greater negative response to fire in the first 3 years post-fire. Given fragmentation of many habitats is increasing, we encourage future fire research to explored whether differences in post-fire responses exist between contiguous and fragmented habitats. Understanding whether differences exist is likely to be important for the effective conservation of reptile populations in remnants, particularly as climate change is likely to change fire regimes to more frequent, intense fires in many ecosystems. Our study also further emphasises the importance of long-term monitoring and time series data such as this study (Dornelas et al. 2018) in addressing ecological and management questions.
Data availability
Data for this project is available from the BioTime database (https://biotime.st-andrews.ac.uk/download.php) and detailed data may be requested from the authors.
Declaration of funding
Some funding for some years of the project was provided by the Botanic Gardens and Parks Authority.
Acknowledgements
The authors acknowledge the Botanic Gardens and Parks Authority (BGPA) at Kings Park, for their ongoing support and encouragement of this project. C. McChesney, provided vegetation data used in analyses, S. Easton and R. Glowacki assisted with site selection, project ideas and resources, and the BGPA bushland crew assisted with installing and checking pitfall traps. M. Cosentino and E. Virens from Edith Cowan University and C. Irvine from the University of Western Australia all contributed to fauna trapping. The work was inspired and guided by the insights of Prof. R. How and J. Dell from their previous work in the park.
References
Bamford MJ (1995) Responses of reptiles to fire and increasing time after fire in Banksia woodland. CALMScience Supplement 4, 175-186.
| Google Scholar |
Bradley HS, Craig MD, Cross AT, Tomlinson S, Bamford MJ, Bateman PW (2022) Revealing microhabitat requirements of an endangered specialist lizard with LiDAR. Scientific Reports 12(1), 5193 PMCID: 35338156.
| Crossref | Google Scholar | PubMed |
Clarke MF, Kelly LT, Avitabile SC, Benshemesh J, Callister KE, Driscoll DA, Ewin P, Giljohann K, Haslem A, Kenny SA, Leonard S, Ritchie EG, Nimmo DG, Schedvin N, Schneider K, Watson SJ, Westbrooke M, White M, Wouters MA, Bennett AF (2021) Fire and its interactions with other drivers shape a distinctive, semi-arid ‘Mallee’ ecosystem. Frontiers in Ecology and Evolution 9, 647557.
| Crossref | Google Scholar |
Craig MD, Grigg AH, Hobbs RJ, Hardy GESJ (2014) Does coarse woody debris density and volume influence the terrestrial vertebrate community in restored bauxite mines? Forest Ecology and Management 318, 142-150.
| Crossref | Google Scholar |
Crosti R, Dixon KW, Ladd PG, Yates CJ (2007) Changes in the structure and species dominance in vegetation over 60 years in an urban bushland remnant. Pacific Conservation Biology 13(3), 158-170.
| Crossref | Google Scholar |
Davis RA, Doherty TS (2015) Rapid recovery of an urban remnant reptile community following summer wildfire. PLoS One 10(5), e0127925.
| Crossref | Google Scholar | PubMed |
Davis RA, Wilcox JA (2013) Adapting to suburbia: bird ecology on an urbanbushland interface in Perth, Western Australia. Pacific Conservation Biology 19(2), 110-120.
| Crossref | Google Scholar |
Doherty TS, Davis RA, Van Etten EJB, Collier N, Krawiec J (2015) Response of a shrubland mammal and reptile community to a history of landscape-scale wildfire. International Journal of Wildland Fire 24(4), 534-543.
| Crossref | Google Scholar |
Dornelas M, Antão LH, Moyes F, et al. (2018) BioTIME: a database of biodiversity time series for the Anthropocene. Global Ecology and Biogeography 27(7), 760-786.
| Crossref | Google Scholar | PubMed |
Driscoll DA (2004) Extinction and outbreaks accompany fragmentation of a reptile community. Ecological Applications 14(1), 220-240.
| Crossref | Google Scholar |
Driscoll DA, Kirkpatrick JB, McQuillan PB, Bonham KJ (2010a) Classic metapopulations are rare among common beetle species from a naturally fragmented landscape. Journal of Animal Ecology 79(1), 294-303 PMCID: 19694875.
| Crossref | Google Scholar |
Driscoll DA, Lindenmayer DB, Bennett AF, Bode M, Bradstock RA, Cary GJ, Clarke MF, Dexter N, Fensham R, Friend G, Gill M, James S, Kay G, Keith DA, MacGregor C, Russell-Smith J, Salt D, Watson James JEM, Richard RJ, York A (2010b) Fire management for biodiversity conservation: Key research questions and our capacity to answer them. Biological Conservation 143(9), 1928-1939.
| Crossref | Google Scholar |
Driscoll DA, Whitehead CA, Lazzari J (2012) Spatial dynamics of the knob-tailed gecko Nephrurus stellatus in a fragmented agricultural landscape. Landscape Ecology 27(6), 829-841.
| Crossref | Google Scholar |
Fisher JL, Loneragan WA, Dixon K, Delaney J, Veneklaas EJ (2009) Altered vegetation structure and composition linked to fire frequency and plant invasion in a biodiverse woodland. Biological Conservation 142(10), 2270-2281.
| Crossref | Google Scholar |
François D, Ursenbacher S, Boissinot A, Ysnel F, Lourdais O (2021) Isolation-by-distance and male-biased dispersal at a fine spatial scale: a study of the common European adder (Vipera berus) in a rural landscape. Conservation Genetics 22(5), 823-837.
| Crossref | Google Scholar |
Garden JG, McAlpine CA, Possingham HP, Jones DN (2007) Habitat structure is more important than vegetation composition for local-level management of native terrestrial reptile and small mammal species living in urban remnants: a case study from Brisbane, Australia. Austral Ecology 32(6), 669-685.
| Crossref | Google Scholar | PubMed |
Gomes LP, Dias PB, Dias HM, Kunz SH (2022) Growing at the forest edges: how natural regeneration develops under fragmentation. IForest 15(4), 240-247.
| Crossref | Google Scholar |
Hero JM, Castley JG, Butler SA, Lollback GW (2013) Biomass estimation within an Australian eucalypt forest: Meso-scale spatial arrangement and the influence of sampling intensity. Forest Ecology and Management 310, 547-554.
| Crossref | Google Scholar |
How RA, Dell J (1994) The zoogeographic significance of urban bushland remnants to reptiles in the Perth region, Western Australia. Pacific Conservation Biology 1(2), 132-140.
| Crossref | Google Scholar |
How RA, Dell J (2000) Ground vertebrate fauna of Perth’s vegetation remnants: impact of 170 years of urbanization. Pacific Conservation Biology 6(3), 198-217.
| Crossref | Google Scholar |
How RA, Cowan MA, How JR (2022) Decadal abundance patterns in an isolated urban reptile assemblage: monitoring under a changing climate. Ecology and Evolution 12(7), e9081.
| Crossref | Google Scholar | PubMed |
Ibanez T, Hequet V, Chambrey C, Jaffré T, Birnbaum P (2017) How does forest fragmentation affect tree communities? A critical case study in the biodiversity hotspot of New Caledonia. Landscape Ecology 32(8), 1671-1687.
| Crossref | Google Scholar |
Keinath DA, Doak DF, Hodges KE, Prugh LR, Fagan W, Sekercioglu CH, Buchart SHM, Kauffman M (2017) A global analysis of traits predicting species sensitivity to habitat fragmentation. Global Ecology and Biogeography 26(1), 115-127.
| Crossref | Google Scholar |
Kelly LT, Nimmo DG, Spence‐Bailey LM, Haslem A, Watson SJ, Clarke MF, Bennett AF (2011) Influence of fire history on small mammal distributions: insights from a 100-year post-fire chronosequence. Diversity and Distributions 17(3), 462-473.
| Crossref | Google Scholar |
Maryan B, Bush B (1996) The dugite or spotted brown snake Pseudonaja affinis. Herpetofauna 26(2), 22-34.
| Google Scholar |
McAlpine CA, Bowen ME, Smith GC, Gramotnev G, Smith AG, Lo Cascio A, Goulding W, Maron M (2015) Reptile abundance, but not species richness, increases with regrowth age and spatial extent in fragmented agricultural landscapes of eastern Australia. Biological Conservation 184, 174-181.
| Crossref | Google Scholar |
Mulhall SJ, Sitters H, Di Stefano J (2022) Vegetation cover and configuration drive reptile species distributions in a fragmented landscape. Wildlife Research 50, 792-806.
| Crossref | Google Scholar |
Munguia-Vega A, Rodriguez-Estrella R, Shaw WW, Culver M (2013) Localized extinction of an arboreal desert lizard caused by habitat fragmentation. Biological Conservation 157, 11-20.
| Crossref | Google Scholar |
Nimmo DG, Kelly LT, Spence‐Bailey LM, Watson SJ, Haslem A, White JG, Clarke MF, Bennett AF (2012) Predicting the century-long post-fire responses of reptiles. Global Ecology and Biogeography 21(11), 1062-1073.
| Crossref | Google Scholar |
Nimmo DG, Kelly LT, Spence-Bailey LM, Watson SJ, Taylor RS, Clarke MF, Bennett AF (2013) Fire mosaics and reptile conservation in a fire-prone region. Conservation Biology 27(2), 345-353 PMCID: 23163245.
| Crossref | Google Scholar | PubMed |
Partridge DA, Lewis T, Tran CT, Castley JG (2023) Fire and habitat variables explain reptile community abundance and richness in subtropical open eucalypt forests. International Journal of Wildland Fire 32, 1089-1108.
| Crossref | Google Scholar |
Pinheiro J, Bates D, DebRoy S, et al. (2023) nlme: Linear and Nonlinear Mixed Effects Models. https://cran.r-project.org/web/packages/nlme/nlme.pdf
Ramalho CE, Laliberté E, Poot P, Hobbs RJ (2014) Complex effects of fragmentation on remnant woodland plant communities of a rapidly urbanizing biodiversity hotspot. Ecology 95(9), 2466-2478.
| Crossref | Google Scholar |
Recher HF, Lunney D, Matthews A (2009) Small mammal populations in a eucalypt forest affected by fire and drought. I. Long-term patterns in an era of climate change. Wildlife Research 36(2), 143-158.
| Crossref | Google Scholar |
Ritchie AL, Svejcar LN, Ayre BM, Bolleter J, Brace A, Craig MD, Davis B, Davis RA, Van Etten EJB, Fontaine JB, Fowler WM, Froend RH, Groom C, Hardy GESJ, Hooper P, Hopkins AJM, Hughes M, Krauss SL, Leopold M, Miller BP, Miller RG, Ramalho CE, Ruthrof KX, Shaw C, Stevens JC, Tangney R, Valentine LE, Veneklaas EJ, Hobbs RJ (2021) A threatened ecological community: Research advances and priorities for Banksia woodlands. Australian Journal of Botany 69(2), 53-84.
| Crossref | Google Scholar |
Simms A, Scott M, Watson S, Leonard S (2019) Attenuated post-fire fauna succession: the effects of surrounding landscape context on post-fire colonisation of fauna. Wildlife Research 46(3), 247-255.
| Crossref | Google Scholar |
Smith AL, Michael Bull C, Driscoll DA (2013) Successional specialization in a reptile community cautions against widespread planned burning and complete fire suppression. Journal of Applied Ecology 50(5), 1178-1186.
| Crossref | Google Scholar |
Stenhouse R (2001) Management of urban remnant bushlands by the community and local government. Australian Journal of Environmental Management 8(1), 37-47.
| Crossref | Google Scholar |
Urbina-Cardona JN, Olivares-Pérez M, Reynoso VH (2006) Herpetofauna diversity and microenvironment correlates across a pasture-edge-interior ecotone in tropical rainforest fragments in the Los Tuxtlas Biosphere Reserve of Veracruz, Mexico. Biological Conservation 132(1), 61-75.
| Crossref | Google Scholar |
Valentine LE, Reaveley A, Johnson B, Fisher R, Wilson BA (2012) Burning in banksia woodlands: how does the fire-free period influence reptile communities? PLoS One 7(4), e34448 PMCID: 22496806.
| Crossref | Google Scholar | PubMed |
Virens E, Krauss SL, Davis RA, Spencer PBS (2015) Weak genetic structuring suggests historically high genetic connectivity among recently fragmented urban populations of the scincid lizard, Ctenotus fallens. Australian Journal of Zoology 63(4), 279-286.
| Crossref | Google Scholar |