Multi-century times-since-fire and prior fire interval determine biomass carbon stocks in obligate-seeder eucalypt woodlands
Carl R. Gosper A B * , Colin J. Yates A , Georg Wiehl B , Alison O’Donnell B and Suzanne M. Prober C
A B * , Colin J. Yates A , Georg Wiehl B , Alison O’Donnell B and Suzanne M. Prober C
A
B
C
Abstract
Understanding the influence of fires on terrestrial carbon stocks is important for informing global climate models and underpinning land management-based carbon markets.
To quantify biomass carbon in south-western Australia’s Great Western Woodlands – the world’s largest extant Mediterranean-climate woodland – with time-since-fire and prior fire interval.
Plot-based measurement of live and dead tree and shrub size, woody debris volume and litter mass across a ~400-year chronosequence to calculate biomass carbon.
Biomass carbon increased with time-since-fire, reaching >65 Mg C ha−1, although the rate of increase declined in mature woodlands. Biomass carbon decreased after fire in these obligate-seeder woodlands, while a longer prior fire interval buffered carbon fluxes through retained large standing dead trees and fallen woody debris.
The current age class distribution of the ~95,000 km2 of eucalypt woodlands in the region may support ~0.453 Pg C. Further refinement of carbon estimates explicitly considering variation in woodland type and climate, a continuous woodland age distribution and soil carbon are required to underpin a carbon methodology.
Biomass carbon would be maximised by reducing the extent of bushfires impacting woodlands, focussing on existing mature stands that support the greatest carbon stocks.
Keywords: aboveground carbon, ecological fire management, Eucalyptus salubris, fire regime, Great Western Woodlands, multi-century chronosequence, stand-replacement, succession.
Introduction
Fluxes of carbon into and out of terrestrial habitats, resulting from photosynthesis, ecosystem respiration, disturbances such as fire and climatic events, can be of significant magnitude. Hence, their inclusion as core components of global carbon models and carbon accounting methodologies (Intergovernmental Panel on Climate Change 2006; Bloom et al. 2016). As concerns have increased over anthropogenic alterations to the global carbon cycle, specifically increasing concentrations of carbon dioxide (CO2) and other gases in the atmosphere driving global heating, the potential for terrestrial habitats to be a source or sink of carbon has received increasing attention. In some situations, where changes in land management demonstrably increase carbon stocks and their persistence in terrestrial habitats, land managers, including Indigenous peoples, have been able to access carbon markets as an income stream (Russell-Smith et al. 2013; Murphy et al. 2015). However, baseline carbon stocks and the potential impacts of disturbances and land management on ecosystem carbon storage capacity are poorly understood in many regions of the world, limiting our ability to quantify ecosystem contributions to the global carbon cycle or the effectiveness of carbon mitigation strategies.
Fire regimes are a strong influence on the carbon stocks of terrestrial habitats (Liao et al. 2020). Fires consume biomass, particularly finer fuels such as leaves and litter but also larger pieces such as coarse woody debris, transitioning carbon from vegetation biomass to the atmosphere and soil (Aponte et al. 2014; Loehman et al. 2014). Fires also generate coarse woody debris, locking in future releases of carbon though subsequent fires and/or decomposition (Gosper et al. 2019a). The amount of carbon held in soil organic matter is affected by fire through the balance of changes in organic matter consumption, biomass input and rates of decomposition (Agbeshie et al. 2022; Pellegrini et al. 2022; Cheng et al. 2023).
Various aspects of the fire regime, such as fire intensity, extent, season and interval, can have a large bearing on the amount of carbon released from biomass during and after fires (Aponte et al. 2014; Gosper et al. 2019a; Liao et al. 2020). Fires interact with vegetation structure and function to mediate changes in biomass carbon, through patterns of mortality of pre-fire individuals and stimulation of post-fire resprouting or recruitment (Clarke et al. 2015; Nolan et al. 2018). Vegetation communities dominated by obligate-seeder species may exhibit particularly pronounced fluxes in biomass carbon in response to fire. Individuals of obligate-seeder species are, by definition, killed by fires of intensities typical of the habitat in which they occur; hence, fires result in an immediate transition from stands comprised mostly of live plants to stands comprised mostly of dead plants. Thus, fires trigger initial carbon release through combustion and set in train future carbon releases to the atmosphere through decomposition (Loehman et al. 2014; Gosper et al. 2018, 2019a). In contrast, in vegetation dominated by resprouting species, protected buds allow individuals to resprout after fire from retained live biomass (Clarke et al. 2015; Nolan et al. 2018), buffering fluxes in carbon stocks after most fire events.
The Great Western Woodlands (GWW) of south-western Australia comprise the world’s largest extant Mediterranean-climate woodland, with eucalypt woodlands, eucalypt shrublands, scrub and salt lakes extending over an area of ~160,000 km2 (Watson et al. 2008). In addition to their extent, the eucalypt woodlands of the GWW and adjoining regions are unique in several respects. First, the dominant eucalypt trees are mostly obligate-seeders, where fires and other disturbances are typically stand-replacing, in contrast to most other eucalypt-dominated communities (Yates et al. 1994; Gosper et al. 2018). Second, the eucalypt woodland trees are remarkably large (to >20 m in height, and >1 m diameter) considering the dry to semi-arid climate in which they occur (Milewski 1981; Gosper et al. 2018). Third, the spatial extent of woodlands in the GWW relative to non-treed vegetation types is much higher than comparable Mediterranean-climate regions (Prober et al. 2012). This combination of factors suggests that biomass carbon stocks in eucalypt woodlands of the GWW are likely to be significant, and that these stocks are likely to be highly contingent on the fire regime.
Berry et al. (2010) estimated mean biomass carbon stocks of GWW vegetation (woodlands and shrublands, including live and dead biomass, above and below ground) as 21 Mg C ha−1, with some woodland areas supporting up to 100 Mg C ha−1. Although eucalypt woodlands of the GWW undergo multi-century succession in vegetation composition and structure, and woody debris biomass and structure is affected by time-since-fire and prior fire interval (Gosper et al. 2013a, 2013b, 2019a), there are currently no published data on how time-since-fire and fire interval influence biomass carbon stocks at the stand level.
Fires comprise the most widespread stand-replacing disturbance affecting obligate-seeder eucalypt woodlands of the GWW (Yates et al. 1994; Gosper et al. 2018). Nearly 40% of total woodland area has burnt over recent decades (post ~1969) in a number of often very large (>100,000 ha) bushfires (McCaw et al. 2014; Gosper et al. 2019b; Jucker et al. 2023) possibly burning at an unprecedented frequency and extent (Berry et al. 2010). Although mature eucalypt woodlands have relatively low flammability under moderate fire weather conditions because of their discontinuous fuel structure, their probability of burning increases under extreme fire weather and climate conditions (O’Donnell et al. 2014). Dense sapling stands regenerating after fire exhibit higher flammability than mature woodlands and may instigate a positive flammability-time-since-fire feedback (Gosper et al. 2013b, 2014). For these reasons, some form of fire management intervention is likely necessary to maintain or increase biomass carbon stocks in GWW eucalypt woodlands. As a basis for establishing the potential for fire management practices to maintain or increase carbon stocks in obligate-seeder eucalypt woodlands, we addressed the following research questions:
How does woody biomass and carbon stock change with time-since-fire?
Is post-fire woody biomass and carbon stock affected by the length of the prior fire interval? and
How does the contribution of different components (trees, shrubs, coarse woody debris, litter) to total stand biomass vary with aspects of the fire regime?
Materials and methods
Study sites, their time-since-fire and prior fire interval
A multi-century chronosequence of sites in Eucalyptus salubris F.Muell. (gimlet) dominated woodlands was established in 2010–12 (Gosper et al. 2013a, 2013b). All plots had a dominant crown layer of E. salubris, sometimes in association with other eucalypts. E. salubris is a non-lignotuberous tree widespread across the GWW that is killed by fire (Gosper et al. 2018). In this study, we sampled in 2016 a subset of 30 plots spanning the entire range of times since fire represented in the chronosequence for more detailed estimates of biomass carbon stocks, with the 30 plots falling into two geographic clusters: (1) ‘northern’; and (2) ‘southern’ sites (Fig. 1, see Supplementary Table S1). Vegetation biomass was sampled within relatively uniform stands of equal-aged woodland approximately 2 ha in area (typically 100 × 200 m but some were 400 × 50 m if required by site factors such as fire boundaries or vegetation types; Fig. S1). Plots with substantial recent or historical disturbance other than fire were avoided.
(a) Location of study sites and their time-since-fire (divided into time-since-fire classes for ease of visual interpretation) in relation to the extent of eucalypt woodlands (major vegetation types 4 Woodland and 5 Medium-low woodland of Beard et al. (2013)) and whether woodlands have burnt since ~1969 in bushfires recorded in Department of Biodiversity, Conservation and Attractions (2022) in the Great Western Woodlands (GWW). Insert shows the location of the GWW in the context of the state of Western Australia. Example photographs (photos C. Gosper) of the time-since-fire classes are used to illustrate the most frequently encountered cohorts of the tree component of stand biomass. (b) young class, in this case with a long prior fire interval; (c) intermediate class; and (d) mature class.
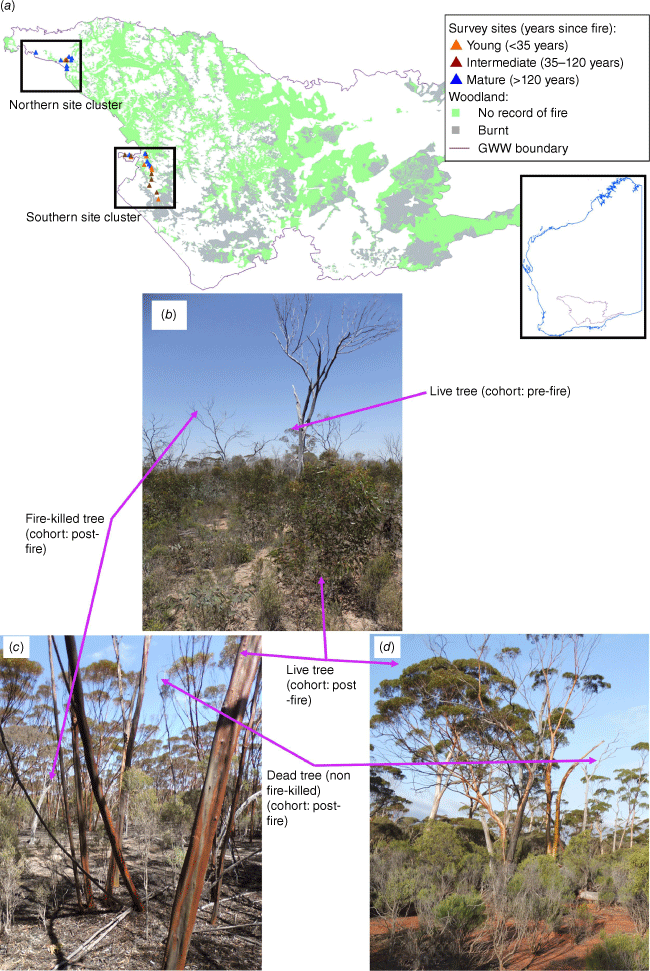
Time-since-fire of each plot was estimated from a combination of: (1) fire history data, primarily derived from remote sensing (Department of Biodiversity, Conservation and Attractions 2022) for areas burnt since ~1972; and (2) stand time-since-fire estimates derived from a size-age model in E. salubris calibrated by growth ring counts and remotely-sensed imagery (using the methods of Gosper et al. 2013c and their more conservative Model 2). For all sites that had not burnt since ~1972, stand age was estimated based on the larger sample of trees measured in this study (n = 52 c.f. n = 4–16 in previously published estimates in Gosper et al. 2013a, 2013b). In a handful of cases for sites clearly last burnt shortly prior to 1972, the size-age model underestimated stand age compared to the likely minimum age based on the fire history dataset (Department of Biodiversity, Conservation and Attractions 2022); in these cases, a fire date of 1972 (minimum age of 44 years) was set. Plot stand ages were estimated to range from 9 to ~369 years at the time of sampling.
As E. salubris tree size was used to estimate the time-since-fire of longer-unburnt sites, analyses of mean tree size and mean biomass against site time-since-fire are not independent, noting that the tree size and biomass data does include measurements from species other than E. salubris. However, due to ubiquitous hollow formation in older trees, there is no method available to estimate the time-since-fire of sites independently of tree size.
Prior fire interval was determined for sites burnt <25 years previously by estimating the age of fire-killed standing dead trees from their stem diameters. The age of fire-killed trees was estimated using the same size-age model of Gosper et al. (2013c) described above for live trees; however, 6 mm was added to the measured stem diameter of dead trees to account for the thickness of bark (Gosper et al. 2018) assumed to have been combusted or shed prior to measurement. Due to a lack of data on rates of persistence of standing dead trees of different sizes, and difficulty in identification of long-dead trees and hence the applicability of the size-age model which was developed on a single species, these estimates of prior fire intervals are of lower certainty than the estimates of ages of live trees. Consequently, prior fire intervals were grouped into the age classes of Gosper et al. (2013a, 2013b): short (young fire-killed tree stands <~35 years at the time of burning), intermediate (~35–120 years); and long (mature stands >~120 years). The prior intervals of sites burnt >25 years ago were unable to be calculated due to the absence of sufficient standing fire-killed trees.
Vegetation measurements
We estimated biomass carbon stocks through measurement of live and dead standing trees, live and dead standing shrubs, coarse woody debris (CWD), and litter on the ground surface. Measurements did not include shrubs <2.5 cm stem diameter at 10 cm from the base (D10), nor grasses or annual forbs, assuming their contribution to overall stand biomass to be insignificant and/or subject to wide inter-annual or seasonal variability.
In standing trees and shrubs, individuals of live, fire-killed, and dead from causes other than fire were identified and separately sampled as distinct cohorts of the tree and shrub biomass components. Cohorts were based on different temporal periods of origin, reflecting the obligate-seeder nature of E. salubris and most other GWW eucalypt and shrub species (Yates et al. 1994; Gosper et al. 2016, 2018). Times of stand origin refer to the fire that led to the establishment of a particular cohort, which we refer to as (from youngest to oldest): ‘post-fire’, ‘pre-fire’, and in some cases, with an origin from the fire(s) before the most recent two fires, as ‘older than pre-fire’. Fig. 1 gives examples for trees.
Due to the highly variable density of tree and shrub cohort and component combinations with variation in the fire regime (Gosper et al. 2013a, 2013b, 2019a), a uniform sampling approach could not be applied. For the post-fire cohorts of live and dead from causes other than fire, the plotless sampling point-centred quarter method (Cottam and Curtis 1956) was used with 13 sampling points for trees (total individual tree n = 52) or seven sampling points for shrubs (n = 28), distributed at 50-m intervals on transects across the site (Fig. S1). At each sampling point, the distance to the closest individual in each quadrant was used to derive an estimate of density for each cohort and component combination (e.g. number of individuals ha−1 of live post-fire trees). For the pre-fire and older than pre-fire cohorts of live and fire-killed trees and shrubs which invariably occurred at low density, all individuals within cumulative 0.125 ha fixed-area subplots were sampled until a minimum of 50 individuals were encountered or a maximum sample area of 1 ha searched, with density estimates converted to a per ha basis. For the post-fire cohort of fire-killed trees and fire-killed shrubs, a visual field assessment of the density of individuals was used to determine which of the plotless or fixed-area sampling methods would most appropriately measure stand characteristics. For higher density cohorts (>~50 individuals ha−1) the plotless method was used (site n = 3, noting that most sites >~40 years post-fire lacked a fire-killed cohort), while the fixed-area method was used for lower density stands (n = 9).
Using a combination of plotless and fixed-area sampling methods potentially introduces bias into density estimates; however, we note that variation in methodology was primarily between rather than within cohort and component combinations. Further, applying a single method across all cohorts and components would introduce errors, such as inaccuracy in plot layout and counting errors contributing to density estimate errors using fixed-area approaches in high density stands, while lack of independence of samples of point-centred quarters potentially violated an assumption of that method in low density stands.
For each individual standing plant sampled, its identity (species – if alive) and whether it was alive or dead was recorded, along with its stem diameter at 10 and 130 cm above the base (D10 and D130) for eucalypts (trees), and D10 for shrubs. In the case of individuals with broken trunks and a large part of the canopy lost (estimated as >50%), trunk length and smallest, largest and midpoint diameter and the proportion of the trunk that was not hollow was measured. In multi-stemmed individuals, the quadratic mean of the multiple stems (Dind) was calculated to represent total trunk size (diameter; D) in a single value (Dequiv):
Dead standing plants were defined as having complete canopy death. For dominant obligate-seeder eucalypts such as E. salubris, this equated to complete individual death, but for infrequent co-occurring lignotuber-resprouter eucalypts, the post-fire top-killed portion was sampled as a standing dead tree even if basal resprouting had occurred. Dead individuals were classified into one of five decay classes as described by Woldendorp et al. (2002): (1) dead leaves and bark firmly attached; (2) leaves, bark and/or small branches lost but little apparent decay in wood; (3) leaves, bark and most branches lost and moderately decayed wood; (4) decayed (soft, crumbly) wood but in original shape; and (5) highly decayed (soft, crumbly) wood usually with loss of original shape.
All dead plant material on the ground was considered either CWD or litter. CWD was dead wood >2.5 cm in diameter lying on the ground surface for at least part of its length. Litter was all remaining dead plant material on the soil surface. It was not possible to allocate CWD and litter to cohorts of origin.
CWD was sampled along transects with the intercept method of Van Wagner (1968), as described by Gosper et al. (2019a), including measures of piece diameter, the proportion of the piece’s diameter that was intact (not hollow or completely decomposed) and total transect length, along with visual assessment of decay class. Total wood volume of CWD (CWDWVol) was calculated with a modified version of Van Wagner’s (1968) formula, accounting for the absence of wood in hollow centres using the estimate of the proportion of the piece that was intact. In this calculation, the hollow portion was assumed to be the inner part of a cylindrical piece.
where D is piece diameter (cm); Dh is diameter of hollow portion (cm); and L is transect length (m)
A litter sample collection point was placed at a random distance along each of four 50 m transects systematically placed through the site. Each litter sample consisted of four bulked 0.35 × 0.35 cm (0.125 m2) quadrat subsamples placed at systematic locations around the central point (point sample area 0.5 m2; total area sampled per site 2 m2; Fig. S1), with all litter collected down to the mineral soil. Litter samples were sieved to 0.5 mm and sorted to remove soil and rocks, air-dried, then oven-dried (65°C for 48 h) to constant mass and weighed.
Vegetation biomass
For live trees and shrubs, aboveground biomass (AGB) and belowground biomass (BGB) were estimated separately on an individual tree and shrub basis using published allometric equations used in carbon accounting in Australia (Table 1). Generic plant growth form allometric equations have produced robust estimates of stand biomass across a wide span of geographic regions and climates in multi-species ecosystems, negating the need to develop multiple local or species-specific relationships or the inclusion of additional predictive variables beyond trunk diameter (Paul et al. 2016, 2019). Paul et al. (2013, 2016, 2019) found that the key differences in allometry are between, rather than within, growth forms, likely attributable to differences in biomass allocation and stem geometry related to differences in disturbance response type, lifespan, wood density and environmental conditions, which determine the proportion of branches and biomass allocation to lignotubers. Consequently, we follow the growth forms of Paul et al. (2016, 2019), with the following forms represented in our samples: eucalypt trees (Eucalyptus spp. including obligate-seeders, and stem- and lignotuber-resprouters that typically develop a monopodial growth form); multi-stemmed eucalypt trees (lignotuber-resprouter mallees) and shrubs to small trees (many non-Eucalyptus genera). Allometric models were all of the power-law form, which have proven effective over a wide range of species, sites and conditions (Paul et al. 2013, 2016, 2019), with the Baskerville correction factor applied when back-transforming to natural scales (Table 1):
where a and b are constants; and D is trunk diameter.
| Plant growth form | Biomass (kg) | Correction factor | Source | Source species: sample diameter range (cm) | Diameter range this study (cm) | |
|---|---|---|---|---|---|---|
| Eucalyptus trees | ln(AGB) = –2.016 + 2.375 × ln(D130) | 1.0668 | Paul et al. (2016) | Generic tree eucalypts: 0.5–169 | 0.1–100 | |
| ln(BGB) = –2.682 + 2.212 × ln(D130) | 1.0958 | Paul et al. (2019) | Generic tree eucalypts: 1.1–139 | |||
| Eucalyptus mallees | ln(AGB) = –2.757 + 2.474 × ln(D10) | 1.0787 | Paul et al. (2016) | Generic mallees: 0.5–61.5 | 0.1–60 | |
| ln(BGB) = –2.946 + 2.302 × ln(D10) | 1.1160 | Paul et al. (2019) | Generic mallees: 1.0–81.1 | |||
| Shrubs | ln(AGB) = –3.007 + 2.428 × ln(D10) | 1.1281 | Paul et al. (2016) | Generic shrubs: 0.3–50.0 | 2.5–40.5 | |
| ln(BGB) = –3.553 + 2.185 × ln(D10) | 1.1601 | Paul et al. (2019) | Generic shrubs: 0.6–98.4 |
AGB, aboveground biomass; BGB, belowground biomass; D10/D130, trunk diameter (cm) at 10/130 cm above the ground.
Eucalyptus trees included E. salubris, E. ravida, E. salmonophloia, E. urna, E. moderata, E. capillosa, E. longicornis, E. yilgarnensis, E. celastroides, and E. transcontinentalis. Eucalyptus mallees included E. sheathiana, E. loxophleba subsp. lissophloia, E. phenax, E. cylindrocarpa, and E. calycogona. Shrubs include Acacia, Melaleuca, Hakea, Eremophila, Senna, Dodonaea, Alyxia, Santalum, Exocarpos, Grevillea, Pittosporum, Daviesia, and Scaevola.
AGB in broken trunks of live trees was calculated based on the product of trunk volume (V), wood density and the proportion of the trunk not lost to hollow formation:
V was calculated using Newton’s formula where all the remaining trunk could be accessed or Huber’s formula where taking all measurements was not possible, as follows:
where L is length (m); Ab is cross-sectional area (CSA) at large end (m2); Am is CSA at midpoint; and As is CSA at small end [CSA = πr2 where r = radius]
As wood density values were not available for all sampled species and dead individuals were often not identifiable, we used a fixed wood density value of the dominant tree and a common shrub (E. salubris, Acacia acuminata; 940 kg m−3; Forest Products Commission (2022)) in all calculations.
BGB of live trees with broken trunks was assumed to be the same as for undamaged trees of equal trunk diameter.
The biomass in standing dead trees and shrubs (stags/snags, including suspended stems) varies with the time since plant death because of changes in canopy, bark and wood condition. The equivalent live biomass of standing dead trees and shrubs was calculated using the same methods as for intact or broken live trees or shrubs as appropriate. Equivalent live biomass was then multiplied by (i) a fraction of 0.766 accounting for the loss of biomass held in combustible, rapidly shed or decomposed components (estimated combustible fraction of total biomass = 0.234; Jonson and Freudenberger 2011); and (ii) a decay fraction based on assessed decay class to account for the loss of wood density with time (Woldendorp et al. 2002):
As we lack data on relationships between above- and belowground biomass in standing dead plants, we assumed that belowground biomass had the same loss of rapidly decomposed components, same decay class and (if applicable) percentage trunk remaining as assigned to the aboveground portion. An exception was for stumps (dead broken trunks <1.5 m in height), for which no belowground biomass was allocated.
Coarse woody debris (CWD) biomass was calculated through the product of wood volume, wood density and decay fraction:
For litter, sample mass was divided by quadrat area to calculate biomass loading (kg m−2), and then averaged across all samples per site.
Stand biomass
For each biomass component and cohort combination, results were then expressed per ha, summing above- and belowground components:
e.g. Total post-fire fire-killed tree biomass (FKTBio):
Stand biomass components and cohorts were then aggregated for totals of all trees, all shrubs, and overall (trees + shrubs + CWD + litter).
Carbon content
Biomass (Mg ha−1) was converted to carbon (Mg C ha−1) using an established conversion factor of 0.50 (Gifford 2000).
Statistical analysis
We used regression models to examine the relationships between the density, individual biomass and stand biomass of components and cohorts with time-since-fire, fitted using the polynomial standard curves regression module of Sigmaplot 10.0 (Systat Software Inc., https://systatsoftware.com/sigmaplot/). The regression forms tested represented a range of ecologically plausible scenarios of temporal changes in density and biomass based on previous studies of woodland dynamics (Gosper et al. 2013b, 2018, 2019a): a consistent rate of increase/decrease over time (linear); an accelerating rate of change over time (exponential); and a rapid increase or decrease with the rate of change declining over time to approach an asymptote (power or inverse). In cases where there was both an ecologically plausible mechanism and evidence in the data of non-monotonic relationships, a quadratic polynomial model was also tested representing a change in the direction of the relationship at an intermediate time-since-fire. Model selection was based upon minimising Akaike Information Criterion corrected for small sample sizes (AICc). An exception was for individual biomass of fire-killed trees and shrubs, which were not statistically analysed as these biomass components exclusively occurred shortly after fire. We applied square-root transformation to time-since-fire to reduce the leverage of the few longest-unburnt plots, and log10 transformations of density and biomass to meet analysis assumptions. Alternative regression model forms and summary statistics are included in Table S2.
To explore the possibility of spatial differences in density and biomass, we used a t-test comparing ‘northern’ and ‘southern’ (Fig. 1) clusters of sites where there was no overall significant regression relationship with time-since-fire. To explore the role of prior fire interval, we used a one-way ANOVA to test whether density or biomass differed between short, intermediate and long prior intervals solely among recently-burnt (<25 years post-fire) plots, and where there was no overall significant regression relationship with time-since-fire, testing a regression only including recently-burnt sites with either a long or short prior fire interval (i.e. excluding intermediate and short, and intermediate and long prior intervals respectively). These analyses of fire interval were not conduced for shrubs as individuals in recently-burnt sites largely were of a size below the measurement threshold.
The relative contribution of trees, shrubs, CWD and litter to total biomass was calculated per site. Linear regression was used to investigate if the relative contribution of these major biomass pools varied with time-since-fire.
Results
Tree biomass
Stand live tree biomass (above- and belowground) from the post-fire cohort increased across the span of the chronosequence, ranging from less than 10 Mg ha−1 in recently-burnt stands (>9 years post-fire) through to over 100 Mg ha−1 in long-unburnt stands (>~80 Mg ha−1 in aboveground biomass; Fig. 2c). The rate of increase in biomass slowed in older stands, represented by a power model. As live tree density declined sharply with time-since-fire (Fig. 2a), increasing mean tree size (Fig. 2b) more than offset the decline in density over the chronosequence such that while stand biomass approached a plateau, the woodlands nevertheless continued to accumulate tree biomass many centuries after fire. Prior fire interval had no influence on individual or stand biomass or density of the post-fire cohort of live trees shortly after fire.
Contributions of tree density (a, d, g) and mean tree biomass(b, e, h) to stand biomass (c, f, i) for live trees (a–c), fire-killed trees (d–f) and dead trees from inter-fire mortality (g–i), in the context of time-since-fire (TSF) and prior fire interval (Interval) for recently-burnt sites. Regression relationships for TSF are based on the post-fire cohort, and the contribution from any cohort arising from previous fire events are shown separately (×). Note non-linear scales of both axes and that the units of stand biomass regression models (c, f, i) are in kg ha-1 but are ploted here in Mg ha-1. Alternative models considered are listed in Table S2. Interval was tested by ANOVA across three classes (long, intermediate, short), except for fire-killed tree mean biomass as tree size was used to estimate prior interval (e), and dead trees from causes other than fire (g–i) as these were rare in recently-burnt sites. ***P < 0.001; **P < 0.01; *P < 0.05.
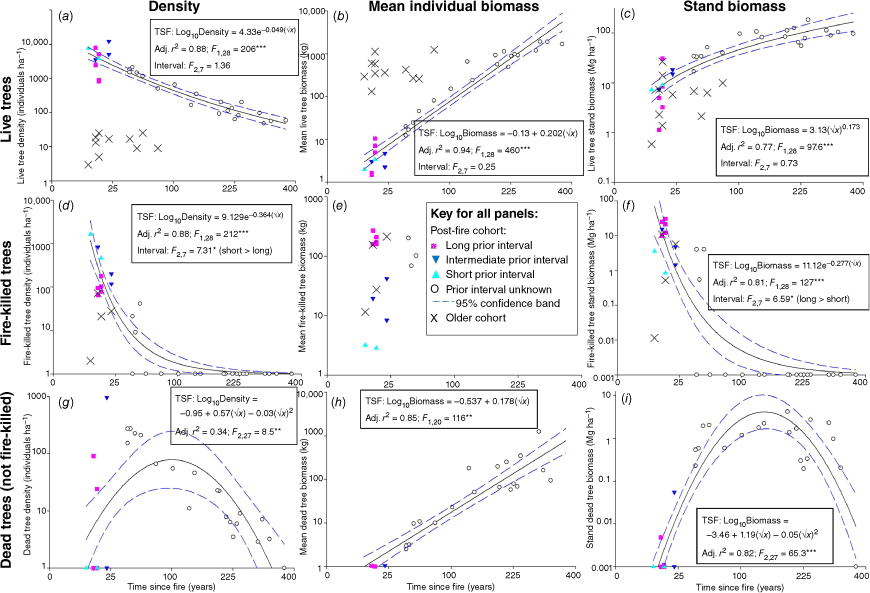
Approximately 60% of recently-burnt sites (<25 years post-fire) had either live trees surviving from the pre-fire cohort, and/or fire-killed trees that had an origin older than the pre-fire cohort, especially when E. salmonophloia F.Muell. formed a component. The density of individuals from the pre-fire cohort was invariably low, but as these trees were often of significant size, their contribution to total stand biomass was within the range of the post-fire cohort through to ~25 years after fire (Fig. 2a–f).
Standing fire-killed trees of the post-fire cohort contributed a substantial quantity of biomass shortly after fire (often exceeding 20 Mg ha−1; Fig. 2f). Prior fire interval had a significant effect on density and stand biomass in recently-burnt sites. Although pairwise comparisons were inconsistent, the density of the post-fire cohort of standing fire-killed trees was greater after shorter prior intervals, while stand biomass was greater after longer prior fire intervals (Fig. 2d, f). Attrition of standing fire-killed trees was complete in sampled stands by approximately 50 years post-fire.
Few standing trees died over the inter-fire period from causes other than fire in both recently-burnt and long-unburnt sites; density of standing dead trees from causes other than fire instead peaked in the middle of the chronosequence (Fig. 2g). Although mean dead tree size increased with time-since-fire (Fig. 2h), because the density of dead trees from causes other than fire peaked at an intermediate time-since-fire, stand dead tree biomass also showed a peaked response (Fig. 2i). The contribution of dead trees from causes other than fire to overall site biomass was substantially lower than that of live trees throughout the chronosequence, and fire-killed trees at recently-burnt sites. There were insufficient records of trees killed by causes other than fire to test whether there were differences in recently-burnt sites associated with prior fire interval.
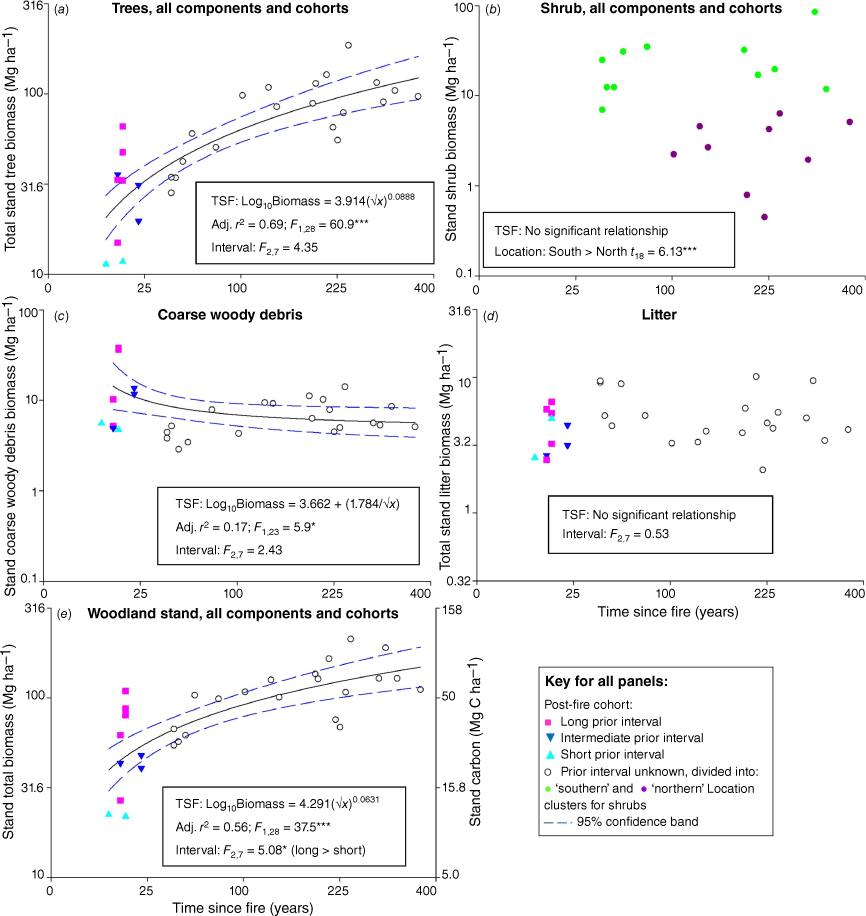
Total standing tree biomass (live and dead of all cohorts) increased with time-since-fire, and as for the post-fire live cohort, the rate of increase slowed in older stands (Fig. 3a). However, there is a strong indication that a monotonic increase in total tree biomass may only be typical of sites with a short prior interval. The legacy of standing fire-killed trees in the decades following fires after long, and to a lesser extent intermediate, prior fire intervals, results in many of these sites having higher stand tree biomass shortly after fire than once these fire-killed trees have fallen into the CWD pool by ~50 years post-fire. While there is substantial site-to-site variation, older stands mostly had a mean above- and belowground biomass of standing trees exceeding 100 Mg ha−1. There was no significant effect of prior fire interval on total tree biomass in recently-burnt sites.
Total stand biomass in Eucalyptus salubris woodlands in the context of time-since-fire (TSF) and prior fire interval (Interval) for recently-burnt sites, of (a) standing trees (all cohorts of live, fire-killed and dead from causes other than fire); (b) standing shrubs (all cohorts of live, fire-killed and dead from causes other than fire), excluding recently-burnt sites where shrubs were smaller than the measurement threshold, separated into those distributed in northern and southern locations; (c) coarse woody debris, with regression relationship shown based on recently-burnt sites with a long prior interval only (i.e. excluding short and intermediate prior intervals); (d) litter; and (e) all cohorts of all biomass components. Note non-linear scales of both axes and that the units of stand biomass regression models (a–e) are in kg ha-1 but are ploted here in Mg ha-1. Alternative models considered are listed in Table S2. Interval was tested by ANOVA across three classes (long, intermediate, short). ***P < 0.001; **P < 0.01; *P < 0.05.
Shrub biomass
There was no evidence that stand live shrub biomass of the post-fire cohort changed in a consistent manner with time-since-fire in sites greater than ~25 years since fire (Fig. 4c). Live shrub biomass in the post-fire cohort in sites <~25 years since fire was not measured as recruiting plants were smaller than the 2.5-cm minimum diameter size threshold we applied, but is assumed to be low. Decreasing density of the post-fire cohort of live shrubs (Fig. 4a) offset increasing mean live biomass (Fig. 4b) with time-since-fire such that no pattern in stand shrub biomass was apparent. As for live trees, some recently-burnt sites supported live shrubs that survived the recent fire. However, unlike for live trees, there was evidence of spatial differences in stand live shrub biomass, with southern sites consistently supporting higher biomass (>~10 Mg ha−1) than northern sites (<~10 Mg ha−1).
Contributions of shrub density (a, d, g) and mean shrub biomass (b, e, h) to stand biomass (c, f, i) for live shrubs (a–c), fire-killed shrubs (d–f) and dead shrubs from inter-fire mortality (g–i) greater than 2.5 cm diameter at the base, in the context of time-since-fire (TSF) and prior fire interval for recently-burnt sites. Regression relationships for TSF are based on the post-fire cohort, and the contribution from any cohort arising from previous fire events are shown separately (×). Note non-linear scales of both axes and that the units of stand biomass (c, f, i) regression models are in kg ha-1 but are ploted here in Mg ha-1. Alternative models considered are listed in Table S2. Sites are separated into those distributed in the northern and southern portions of the study area (Fig. 1), with differences between these Locations assessed by t-test when there was no relationship with TSF. ***P < 0.001; **P < 0.01; *P < 0.05.
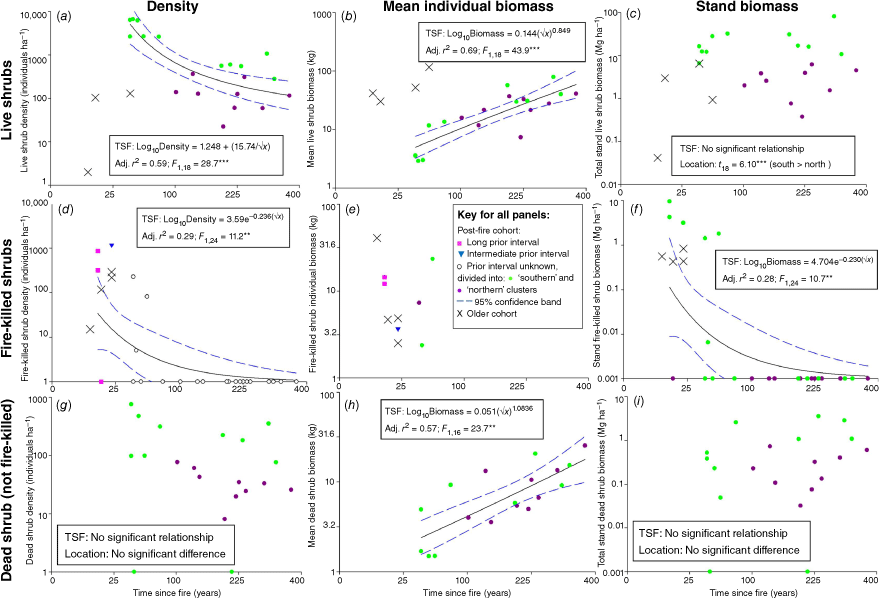
Fire-killed shrub density and stand biomass of the post-fire cohort rapidly declined to zero by ~50 years post-fire (at those sites where fire-killed shrubs exceeded the size threshold; Fig. 4d–f), with some sites having fire-killed shrubs of a pre-fire cohort. The post-fire cohort of shrubs dead from causes other than fire at sites >~25 years since fire showed no relationship with time-since-fire, nor with location, for density (Fig. 4g). Dead shrub individual biomass increased with time-since-fire (Fig. 4h), yet this did not result in any consistent pattern in stand biomass (Fig. 4i).
Total standing shrub biomass (live and dead of all cohorts) showed no consistent pattern with time-since-fire, although the difference between northern and southern sites persisted (Fig. 3b). Post-fire live shrub biomass was the overwhelming contributor to stand total shrub biomass.
Coarse woody debris (CWD) and litter biomass
There was no significant relationship between CWD biomass and time-since-fire when recently-burnt sites of all prior fire intervals were included in the analysis (Table S2). However, there was a relationship if only recently-burnt sites with a long prior interval were included, showing that if preceded by a long fire interval, CWD biomass was greatest shortly after fire (often >10 Mg ha−1), then declined (Fig. 3c). However, prior fire interval did not have a significant effect on CWD biomass in recently-burnt sites.
There was no evidence that litter biomass (~5 Mg ha−1) changed systematically with time-since-fire, in response to prior fire interval, or with location (Fig. 3d).
All biomass and carbon content
Total biomass across all components and cohorts was driven by the contribution of trees, and thus increased over the span of the chronosequence from ~40 Mg ha−1 (20 Mg C ha−1) when recently-burnt (>9 years post-fire) through to >130 Mg ha−1 (65 Mg C ha−1) when long-unburnt. The rate of increase declined over time (Fig. 3e). Prior fire interval had a significant effect on total biomass. Some plots 15 years post-fire with a long prior fire interval had equivalent biomass as plots ~100 years post-fire, noting that biomass in the recently-burnt with long prior interval plots would be expected to decline over the intermediate period with decomposition of fire-killed trees before beginning to increase. Even in the longest-unburnt plots, woodlands continued to sequester C.
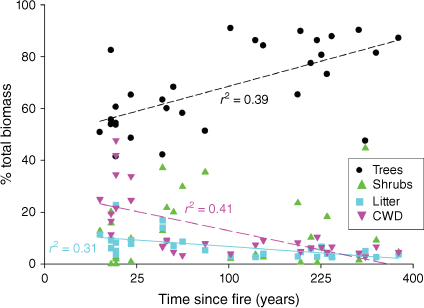
Across all stand ages, trees were the dominant contributors to biomass. While trees always formed >50% of biomass, their relative importance increased with time-since-fire until contributing ~80% of biomass in long-unburnt sites (Fig. 5). The relative contribution of CWD to biomass was greatest in recently-burnt sites (>25%), but then rapidly declined. Litter showed a similar pattern of change across the chronosequence to CWD, but with a lower peak post-fire and a shallower decline in contribution to total biomass with time.
Relative contribution of trees, shrubs, leaf litter and coarse woody debris (CWD) to total stand biomass in Eucalyptus salubris woodlands with times since fire. Linear regression relationships (with adjusted r2) are shown, except for shrubs where there was no significant relationship between percent shrub biomass and time-since-fire. Note non-linear scale of x axis.
Discussion
Fire management opportunities to maximise carbon stocks
We demonstrated that obligate-seeder eucalypt woodlands in the GWW store and sequester significant quantities of biomass carbon, beyond ~ 65 Mg C ha−1 in mature woodlands (~54 Mg C ha−1 in live vegetation, ~52 Mg C ha−1 in above-ground biomass), excluding soil carbon. These estimates are somewhat higher than the mean of ~44 Mg C ha−1 in GWW woodland sites in Berry et al. (2010; based on their table 3.3), as may be expected through Berry et al. (2010) likely sampling some non-mature stands by chance as they were not able to account for time-since-fire. Nevertheless, we confirm their argument that this ~160,000-km2 area has an important role to play in national and global carbon management. In comparison to broader-scale estimates of biomass carbon, stocks in mature GWW eucalypt woodlands are high compared to other parts of the globe with similar semi-arid climate (average for live woody biomass in the tropical dry non-forest biome is 24.4 Mg C ha−1; Xu et al. 2021), reflecting the exceptional stature of GWW eucalypt trees relative to the semi-arid environment in which they occur (Milewski 1981; Gosper et al. 2018). Indeed, our measurements suggest that the global spatial model of live biomass stocks of Xu et al. (2021) may underestimate live biomass carbon in obligate-seeder eucalypt woodlands, as Xu et al. (2021) have this region supporting less than 20 Mg C ha−1, despite ~40% of woodlands in the region being mature (Jucker et al. 2023) and likely supporting close to 50 Mg C ha−1. At a national scale, mature GWW eucalypt woodland aboveground biomass of ~80 Mg ha−1 or more falls at the upper end of estimates of aboveground biomass in the region in Liao et al. (2020) and Williams et al. (2021), but does exceed the average modelled aboveground biomass for Australian woodlands of ~50 Mg ha−1 in Roxburgh et al. (2019). Using the area of ~95,000 km2 of obligate-seeder eucalypt woodland in the GWW, a current age class distribution estimated at 22% young, 36% intermediate and 42% mature (Jucker et al. 2023), median age class values of 17.5, 77.5 and 260 years respectively, and an assumption that the carbon stocks of the E. salubris woodlands sampled here are representative of woodlands across the region, allows a rough estimate of woodland carbon stock. The woodland may thus support regional biomass carbon stocks of ~0.453 Pg C (0.362 Pg C aboveground). With Liao et al. (2020) estimating total current aboveground carbon in Australian terrestrial vegetation of 6.6 Pg C and Williams et al. (2021) estimating total potential aboveground carbon of 16.1 Pg C, GWW eucalypt woodlands may comprise ~5.5% of Australia’s current total or 2.2% of the potential total, in 1.2% of Australia’s land area.
Time-since-fire and prior fire interval had a strong bearing on vegetation biomass and carbon stocks in obligate-seeder eucalypt woodlands. As would be expected in a community dominated by obligate-seeder trees, fires resulted in substantial loss of carbon formerly held in live trees, although net loss shortly post-fire was highly dependent on the prior fire interval. Biomass carbon stocks shortly after fire in previosuly mature woodlands (long prior interval) were greater than after fire in previously young woodlands (short prior interval), but these differences largely dissipated by ~50 years after fire. Carbon transitioned to fire-killed standing dead trees and CWD, and the remainder presumably to the atmosphere and soil. As standing dead trees and CWD represent medium-term but transient reservoirs of carbon (mostly lost by ~50 years after fire), fires in mature woodlands ultimately lead to the net loss (i.e. partly offset by gains sequestered in regrowth) ~50 years post-fire of about half pre-fire biomass carbon (~ 33 Mg C ha−1).
The greatest potential carbon benefit at the plot scale from management aimed at altering fire regimes would be if the extent of bushfires in mature woodlands can be reduced leading to avoided carbon losses. However, sequestration of carbon in woodlands recovering post-fire is also significant, occurring most rapidly in the early years post-fire. The slope of the relationship between time-since-fire and biomass carbon stocks was not linear, with the annual quantity of carbon sequestered per unit area declining over time. Eucalyptus salubris woodlands act as a carbon sink to the value of ~0.25 Mg C ha−1 year−1 at 50 years post-fire, ~0.16 Mg C ha−1 year−1 at 100 years post-fire, decreasing to ~0.07 Mg C ha−1 year−1 in 400-year-old woodlands. The spatial context of the extent of woodland of different fire ages also needs to be considered. Recent decades have seen multiple large bushfires >100,000 ha in extent impacting mature obligate-seeder woodlands (McCaw et al. 2014; Gosper et al. 2019b; Jucker et al. 2023), instigating large carbon losses and producing large extents of recovering woodlands.
While this study provides strong evidence of the value of biomass carbon stocks in GWW obligate-seeder woodlands and that these stocks respond strongly to fire regimes, further research is required to provide the data to underpin emissions reductions methodologies (e.g. Australian Emissions Reduction Fund https://www.cleanenergyregulator.gov.au/ERF) and thereby provide a financial mechanism to support improved fire management. Obligate-seeder woodlands in the GWW span a climate gradient of ~400–200 mm mean annual rainfall. As rainfall may affect biomass allocation and biomass carbon stocks (Zerihun et al. 2006), and with our samples distributed towards the wetter end of the GWW rainfall gradient (Fig. 1), we recommend further sampling of stand structure over a greater diversity of local conditions and species composition. It is likely that, for a given age, woodlands in wetter environments support greater carbon stocks. Yet, much of the mature woodland occurs in drier parts of the GWW (Fig. 1), suggesting greater likelihood of avoiding fire and attaining greater age in these areas. Furthermore, adjacent lignotuber-resprouter dominated mallee eucalypt communities are likely to provide significant additional carbon stocks (Paul et al. 2016). As aboveground biomass in mallee eucalypts is killed in most fires (Noble 2001), the carbon stocks of GWW mallee communities are also in need of quantification in a fire regime context. Further, soil carbon was not measured in this study. As carbon in soil organic matter can form a substantial proportion of ecosystem carbon stocks, and as the stability and responses to fire of soil organic matter can vary between ecosystems and with fire characteristics (Agbeshie et al. 2022; Pellegrini et al. 2022; Cheng et al. 2023), we recommend investigation of soil carbon in GWW eucalypt woodlands explicitly considering time-since-fire, fire interval and fire intensity.
Even when sufficient data across conditions and species are available to parameterise robust carbon models with variation in fire regime parameters across GWW eucalypt woodlands, the feasibility of fire management approaches to alter recent fire regimes in a direction conferring increased carbon sequestration and/or avoided loss is yet to be determined. Specifically, the optimal balance of investment into various approaches and the spatial distribution of actions to change fire patterns, while uncertain, is currently being planned and delivered by state agency (Department of Biodiversity, Conservation and Attractions) and other land managers in an adaptive management framework. Current fire management actions targeted at woodland communities and/or adjoining mallee and shrublands include prescribed fire and other pre-fire mitigation works including fuel modification and fuel breaks, and there is the possibility of integrating emerging rapid fire detection and suppression approaches (Burrows and McCaw 2013; Shinneman et al. 2019; Yebra et al. 2021). Broad-scale prescribed burning, an approach used widely in managing flammable fuel and carbon in other ecosystems (Burrows and McCaw 2013; Russell-Smith et al. 2013), appears to have poor prospects for application in obligate-seeder eucalypt woodlands themselves. Even low intensity surface fires kill the majority of E. salubris trees (Gosper et al. 2018), indicating that such prescribed fires would instigate carbon losses that would not be recoverable for decades. Avoiding broad-scale prescribed fire in obligate-seeder woodlands is also consistent with documented practices of Indigenous communities, whose use of fire in these woodlands was highly targeted, selective and limited in extent (Prober et al. 2016), and contemporary operations. Alternatively, prescribed fire in more flammable (O’Donnell et al. 2011) adjoining vegetation which occurs in a mosaic with woodlands offers promise, is consistent with past Indigenous practices (Prober et al. 2016), aligns with existing fire management directions and plans (Department of Environment and Conservation 2008), though requires an increased and ongoing commitment of resources.
Technical constraints
Our study area lies towards the arid end of the range of samples upon which the allometric relationships in Paul et al. (2016, 2019) were generated. However, given the lack of substantial improvement in model fit with inclusion of climatic parameters in Paul et al. (2016, 2019), we have no reason to think that the allometric equations used may not be robust in E. salubris woodlands. However, as pointed out by Paul et al. (2016), larger trees (D130 > 50 cm) and mature trees with extensive hollow trunk development (both of which are common in long-unburnt E. salubris woodlands and other semi-arid woodlands; Zerihun et al. 2006; Gosper et al. 2013b, 2013,c) are poorly represented in studies in which allometric equations are derived, potentially introducing bias into biomass estimates and offering opportunities for improving model precision. Fatemi et al. (2011) similarly concluded that stand age may be an important factor influencing aboveground allometry and the accuracy of biomass estimation.
Implications for conservation management
Shrub biomass, both in absolute quantity and as a proportion of site biomass, was substantially greater in southern parts of the study area. The ecological mechanism underlying this difference is unknown, although it may be related to the lower aridity of southern sites. The differences in shrub biomass across GWW eucalypt woodlands may have important ecological ramifications beyond their contribution to biomass carbon. Bushfires in southern parts of the GWW have burnt a greater proportion of woodland area over recent decades than bushfires in the north (Fig. 1; Gosper et al. 2018, 2019b; Jucker et al. 2023). Greater shrub cover is a plausible explanation, with shrubs contributing to greater fuel connectivity both vertically (ladder fuels) and horizontally and contributing to increased fuel hazard (Gould et al. 2011).
The large individual biomass of fire-killed dead trees in woodlands with a long prior fire interval, which was derived from large live individual size before fire, indicates an important conservation resource in addition to buffering carbon fluxes. These large dead trees and pieces of coarse woody debris are likely to provide significant hollow resources for fauna for decades after fire (Lindenmayer et al. 1990; Gosper et al. 2019a). As development of new hollows in regenerating vegetation will take centuries (Gosper et al. 2018), careful management of large dead trees and large coarse woody debris to minimise impacts from timber cutting and mining disturbance will have conservation benefits.
Supplementary material
On plot layout, site details and statistical models are available as Supplementary material (available online).
Acknowledgements
We acknowledge and pay our respects to the Traditional Owners in the Great Western Woodlands on whose land this work was conducted. We thank Maxime Zahedi (CSIRO) and Cameron McArtney (Curtin University, CSIRO) for field assistance. The E. salubris chronosequence was established with the support of the Department of Biodiversity, Conservation and Attractions, CSIRO Environment, and the Australian Government through its Terrestrial Ecosystem Research Network (TERN) Great Western Woodlands SuperSite.
Author contributions
C.G.: conceptualisation, data curation, formal analysis, investigation, methodology, validation, visualisation, writing – original draft. C.Y.: conceptualisation, funding acquisition, supervision, writing – review and editing. G.W.: investigation, methodology, resources. A.O.: validation, writing – review and editing. S.P.: conceptualisation, funding acquisition, supervision, writing – review and editing.
References
Agbeshie AA, Abugre S, Atta-Darkwa T, Awuah R (2022) A review of the effects of forest fire on soil properties. Journal of Forestry Research 33, 1419-1441.
| Crossref | Google Scholar |
Aponte C, Tolhurst KG, Bennett LT (2014) Repeated prescribed fires decrease stocks and change attributes of coarse woody debris in a temperate eucalypt forest. Ecological Applications 24, 976-989.
| Crossref | Google Scholar | PubMed |
Beard JS, Beeston GR, Harvey JM, Hopkins AJM, Shepherd DP (2013) The vegetation of Western Australia at the 1:3,000,000 scale. Explanatory memoir, 2nd edition. Conservation Science Western Australia 9, 1-252.
| Google Scholar |
Bloom AA, Exbrayat J-F, van der Velde IR, Feng L, Williams M (2016) The decadal state of the terrestrial carbon cycle: Global retrievals of terrestrial carbon allocation, pools, and residence times. Proceedings of the National Academy of Sciences 113, 1285-1290.
| Crossref | Google Scholar | PubMed |
Burrows N, McCaw L (2013) Prescribed burning in southwestern Australian forests. Frontiers in Ecology and the Environment 11, e25-e34.
| Crossref | Google Scholar |
Cheng Y, Luo P, Yang H, Li H, Luo C, Jia H, Huang Y (2023) Fire effects on soil carbon cycling pools in forest ecosystems: a global meta-analysis. Science of The Total Environment 895, 165001.
| Crossref | Google Scholar | PubMed |
Clarke PJ, Lawes MJ, Murphy BP, Russell-Smith J, Nano CEM, Bradstock R, Enright NJ, Fontaine JB, Gosper CR, Radford I, Midgley JJ, Gunton RM (2015) A synthesis of postfire recovery traits of woody plants in Australian ecosystems. Science of The Total Environment 534, 31-42.
| Crossref | Google Scholar | PubMed |
Cottam G, Curtis JT (1956) The use of distance measures in phytosociological sampling. Ecology 37, 451-460.
| Crossref | Google Scholar |
Department of Biodiversity, Conservation and Attractions (DBCA) (2022) DBCA Fire History (DBCA-060). Available at https://catalogue.data.wa.gov.au/dataset/dbca-fire-history [verified 26 September 2023]
Department of Environment and Conservation (2008) Goldfields Regional Fire Management Plan 2008–2013. (Department of Environment and Conservation: Kensington) Available at https://library.dbca.wa.gov.au/#record/123236 [verified 25 May 2024]
Fatemi FR, Yanai RD, Hamburg SP, Vadeboncoeur HA, Arthur MA, Briggs RD, Levine CR (2011) Allometric equations for young northern hardwoods: the importance of age-specific equations for estimating aboveground biomass. Canadian Journal of Forest Research 41, 881-891.
| Crossref | Google Scholar |
Forest Products Commission (FPC) (2022) FPC: Inland forests, woodlands and desert timber species of Western Australia poster. Available at https://www.wa.gov.au/government/publications/fpc-inland-forests-woodlands-and-desert-timber-species-of-western-australia-poster [verified 18 September 2023]
Gosper CR, Yates CJ, Prober SM (2013a) Floristic diversity in fire-sensitive eucalypt woodlands shows a ‘U’-shaped relationship with time-since-fire. Journal of Applied Ecology 50, 1187-1196.
| Crossref | Google Scholar |
Gosper CR, Prober SM, Yates CJ (2013b) Multi-century changes in vegetation structure and fuel availability in fire-sensitive eucalypt woodlands. Forest Ecology and Management 310, 102-109.
| Crossref | Google Scholar |
Gosper CR, Prober SM, Yates CJ, Wiehl G (2013c) Estimating the time since fire of long-unburnt Eucalyptus salubris (Myrtaceae) stands in the Great Western Woodlands. Australian Journal of Botany 61, 11-21.
| Crossref | Google Scholar |
Gosper CR, Yates CJ, Prober SM, Wiehl G (2014) Application and validation of visual fuel hazard assessments in dry Mediterranean-climate woodlands. International Journal of Wildland Fire 23, 385-393.
| Crossref | Google Scholar |
Gosper CR, Prober SM, Yates CJ (2016) Continental-scale syntheses of Australian pyromes – misclassification of south-western eucalypt woodlands misinforms management. Journal of Biogeography 43, 858-861.
| Crossref | Google Scholar |
Gosper CR, Yates CJ, Cook GD, Harvey JM, Liedloff AC, McCaw WL, Thiele KR, Prober SM (2018) A conceptual model of vegetation dynamics for the unique obligate-seeder eucalypt woodlands of south-western Australia. Austral Ecology 43, 681-695.
| Crossref | Google Scholar |
Gosper CR, Yates CJ, Fox E, Prober SM (2019a) Time since fire and prior fire interval shape woody debris dynamics in obligate-seeder woodlands. Ecosphere 10(12), e02927.
| Crossref | Google Scholar |
Gosper CR, Fox E, Burbidge AH, Craig MD, Douglas TK, Fitzsimons JA, McNee S, Nicholls AO, O’Connor J, Prober SM, Watson DM, Watson SJ, Yates CJ (2019b) Multi-century periods since fire in an intact woodland landscape favour bird species declining in an adjacent agricultural region. Biological Conservation 230, 82-90.
| Crossref | Google Scholar |
Gould JS, McCaw WL, Cheney NP (2011) Quantifying fine fuel dynamics and structure in dry eucalypt forest (Eucalyptus marginata) in Western Australia for fire management. Forest Ecology and Management 262, 531-546.
| Crossref | Google Scholar |
Jonson JH, Freudenberger D (2011) Restore and sequester: estimating biomass in native Australian woodland ecosystems for their carbon-funded restoration. Australian Journal of Botany 59, 640-653.
| Crossref | Google Scholar |
Jucker T, Gosper CR, Wiehl G, Yeoh PB, Raisbeck-Brown N, Fischer FJ, Graham J, Langley H, Newchurch W, O’Donnell AJ, Page GFM, Zdunic K, Prober SM (2023) Using multi-platform LiDAR to guide the conservation of the world’s largest temperate woodland. Remote Sensing of Environment 296, 113745.
| Crossref | Google Scholar |
Liao Z, Van Dijk AIJM, He B, Larraondo PR, Scarth PF (2020) Woody vegetation cover, height and biomass at 25-m resolution across Australia derived from multiple site, airborne and satellite observations. International Journal of Applied Earth Observation and Geoinformation 93, 102209.
| Crossref | Google Scholar |
Lindenmayer DB, Norton TW, Tanton MT (1990) Differences between wildfire and clearfelling on the structure of montane ash forests of Victoria and their implications for fauna dependent on tree hollows. Australian Forestry 53, 61-68.
| Crossref | Google Scholar |
Loehman RA, Reinhardt E, Riley KL (2014) Wildland fire emissions, carbon, and climate: Seeing the forest and the trees – A cross-scale assessment of wildfire and carbon dynamics in fire-prone, forested ecosystems. Forest Ecology and Management 317, 9-19.
| Crossref | Google Scholar |
Milewski AV (1981) A comparison of vegetation height in relation to the effectiveness of rainfall in the Mediterranean and adjacent arid parts of Australia and South Africa. Journal of Biogeography 8, 107-116.
| Crossref | Google Scholar |
Noble JC (2001) Lignotubers and meristem dependence in mallee (Eucalyptus spp.) coppicing after fire. Australian Journal of Botany 49, 31-41.
| Crossref | Google Scholar |
Nolan RH, Sinclair J, Eldridge DJ, Ramp D (2018) Biophysical risks to carbon sequestration and storage in Australian drylands. Journal of Environmental Management 208, 102-111.
| Crossref | Google Scholar | PubMed |
O’Donnell AJ, Boer MM, McCaw WL, Grierson PF (2011) Vegetation and landscape connectivity control wildfire intervals in unmanaged semi-arid shrublands and woodlands in Australia. Journal of Biogeography 38, 112-124.
| Crossref | Google Scholar |
O’Donnell AJ, Boer MM, McCaw WL, Grierson PF (2014) Scale-dependent thresholds in the dominant controls of wildfire size in semi-arid southwest Australia. Ecosphere 5(7), art93.
| Crossref | Google Scholar |
Paul KI, Roxburgh SH, England JR, Ritson P, Hobbs T, Brooksbank K, John Raison R, Larmour JS, Murphy S, Norris J, Neumann C, Lewis T, Jonson J, Carter JL, McArthur G, Barton C, Rose B (2013) Development and testing of allometric equations for estimating above-ground biomass of mixed-species environmental plantings. Forest Ecology and Management 310, 483-494.
| Crossref | Google Scholar |
Paul KI, Roxburgh SH, Chave J, England JR, Zerihun A, Specht A, Lewis T, Bennett LT, Baker TG, Adams MA, Huxtable D, Montagu KD, Falster DS, Feller M, Sochacki S, Ritson P, Bastin G, Bartle J, Wildy D, Hobbs T, Larmour J, Waterworth R, Stewart HTL, Jonson J, Forrester DI, Applegate G, Mendham D, Bradford M, O’Grady A, Green D, Sudmeyer R, Rance SJ, Turner J, Barton C, Wenk EH, Grove T, Attiwill PM, Pinkard E, Butler D, Brooksbank K, Spencer B, Snowdon P, O’Brien N, Battaglia M, Cameron DM, Hamilton S, McAuthur G, Sinclair J (2016) Testing the generality of above-ground biomass allometry across plant functional types at the continent scale. Global Change Biology 22, 2106-2124.
| Crossref | Google Scholar | PubMed |
Paul KI, Larmour J, Specht A, Zerihun A, Ritson P, Roxburgh SH, Sochacki S, Lewis T, Barton CVM, England JR, Battaglia M, O’Grady A, Pinkard E, Applegate G, Jonson J, Brooksbank K, Sudmeyer R, Wildy D, Montagu KD, Bradford M, Butler D, Hobbs T (2019) Testing the generality of below-ground biomass allometry across plant functional types. Forest Ecology and Management 432, 102-114.
| Crossref | Google Scholar |
Pellegrini AFA, Harden J, Georgiou K, Hemes KS, Malhotra A, Nolan CJ, Jackson RB (2022) Fire effects on the persistence of soil organic matter and long-term carbon storage. Nature Geoscience 15, 5-13.
| Crossref | Google Scholar |
Prober SM, Thiele KR, Rundel PW, Yates CJ, Berry SL, Byrne M, Christidis L, Gosper CR, Grierson PF, Lemson K, Lyons T, Macfarlane C, O’Connor MH, Scott JK, Standish RJ, Stock WD, van Etten EJB, Wardell-Johnson GW, Watson A (2012) Facilitating adaptation of biodiversity to climate change: a conceptual framework applied to the world’s largest Mediterranean-climate woodland. Climatic Change 110, 227-248.
| Crossref | Google Scholar |
Prober SM, Yuen E, O’Connor MH, Schultz L (2016) Ngadju kala: Australian Aboriginal fire knowledge in the Great Western Woodlands. Austral Ecology 41, 716-732.
| Crossref | Google Scholar |
Roxburgh SH, Karunaratne SB, Paul KI, Lucas RM, Armston JD, Sun J (2019) A revised above-ground maximum biomass layer for the Australian continent. Forest Ecology and Management 432, 264-275.
| Crossref | Google Scholar |
Russell-Smith J, Cook GD, Cooke PM, Edwards AC, Lendrum M, Meyer C, Whitehead PJ (2013) Managing fire regimes in north Australian savannas: applying Aboriginal approaches to contemporary global problems. Frontiers in Ecology and the Environment 11, e55-e63.
| Crossref | Google Scholar |
Shinneman DJ, Germino MJ, Pilliod DS, Aldridge CL, Vaillant NM, Coates PS (2019) The ecological uncertainty of wildfire fuel breaks: examples from the sagebrush steppe. Frontiers in Ecology and the Environment 17, 279-288.
| Crossref | Google Scholar |
Van Wagner CE (1968) The line intercept method of forest fuel sampling. Forest Science 14, 20-26.
| Crossref | Google Scholar |
Williams K, Hunter B, Schmidt B, Woodward E, Cresswell I (2021) Australia state of the environment 2021: land. Independent report to the Australian Government Minister for the Environment. (Commonwealth of Australia: Canberra, ACT) 10.26194/6EAM-6G50
Xu L, Saatchi SS, Yang Y, Yu Y, Pongratz J, Bloom AA, Bowman K, Worden J, Liu J, Yin Y, Domke G, McRoberts RE, Woodall C, Nabuurs G-J, de-Miguel S, Keller M, Harris N, Maxwell S, Schimel D (2021) Changes in global terrestrial live biomass over the 21st century. Science Advances 7, eabe9829.
| Crossref | Google Scholar | PubMed |
Yates CJ, Hobbs RJ, Bell RW (1994) Landscape-scale disturbances and regeneration in semi-arid woodlands of southwestern Australia. Pacific Conservation Biology 1, 214-221.
| Crossref | Google Scholar |
Yebra M, Barnes N, Bryant C, Cary GJ, Durrani S, Lee J-U, Lindenmayer D, Mahony R, Prinsley R, Ryan P, Sharp R, Stocks M, TridFgell A, Zhou X (2021) An integrated system to protect Australia from catastrophic bushfires. Australian Journal of Emergency Management 36, 20-22.
| Crossref | Google Scholar |
Zerihun A, Montagu KD, Hoffmann MB, Bray SG (2006) Patterns of below- and above-ground biomass in Eucalyptus populnea woodland communities of northeast Australia along a rainfall gradient. Ecosystems 9, 501-515.
| Crossref | Google Scholar |


