Carbon (δ13C) dynamics in agroecosystems under traditional and minimum tillage systems: a review
C. J. Smith A C and P. M. Chalk B C
A C and P. M. Chalk B C
A CSIRO Agriculture, GPO Box 1700, Canberra, ACT 2601, Australia.
B Faculty of Veterinary and Agricultural Sciences, University of Melbourne, Parkville, Vic. 3010, Australia.
C Corresponding authors. Email: Chris.J.Smith@csiro.au; chalkphillip@gmail.com
Soil Research 59(7) 661-672 https://doi.org/10.1071/SR21056
Submitted: 2 March 2021 Accepted: 28 April 2021 Published: 30 June 2021
Journal compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Following cultivation, substantial loss of soil organic matter occurs in surface soil layers. No-till is an agronomic practice to reverse or slow the loss of soil organic matter. We reviewed 95 research papers that used 13C natural abundance of soils to quantify the impact of tillage on the C dynamics of cropping systems. New C (from current cropping systems) accumulated in the surface soil under no-till, whereas the most extreme cultivation (mouldboard ploughing) mixed new C throughout the soil. There was a decline in soil C with years of cultivation. Compared with land that had been tilled, no-till generally had little impact on the accumulation on soil organic C. Tillage and residue retention caused stratification in C stocks that depended on tillage depth, with the highest C concentrations and stocks found in the surface under no-till. Shifts in the δ13C signature indicated significant exchange of ‘new’ C for the original (old) C. Tillage methods had no impact on the size and δ13C signature of the microbial biomass pool. Change in δ13C indicates that microbial biomass rapidly incorporates new carbon. The largest change in the δ13C values (Δ13C) was observed in the coarse sand fraction, whereas the smallest change occurred in the clay fraction. Comparison of conventional vs no-till showed inconsistent results on the effect of tillage on C in the different particle size fractions. Natural 13C abundance data show that no-till cropping systems do not result in increases in soil organic C in the top 0.30 m of soil.
Keywords: tillage, no-till, zero till, C3, C4, soil microbial biomass, δ13C, C sequestration.
Introduction
The preferential fixation of 12C over 13C during photosynthesis results in the δ13C signature of C3 plants (Calvin cycle) being lower (more negative) than that of C4 plants (Hatch-Slack pathway) (Smith and Epstein 1971; Farquhar et al. 1982, 1989; Lloyd and Farquhar 1994). In undisturbed systems, the soil mirrors the δ13C signature of the dominant vegetation. When a C3 forest is clear-felled for continuous cultivation of C4 maize (Zea mays), for example, the soil δ13C signature gradually changes towards that of the replacement C4 vegetation.
The proportion of soil C derived from C inputs (crop or residue) can be calculated using an isotope end-member mixing model (Balesdent et al. 1987), with the assumptions of similar isotopic fractionation during humification of C3 and C4 plant residues (Dorodnikov et al. 2007), and a constant temporal 13C abundance of the input C. Growing C4 plants on a soil that has previously been under C3 vegetation, or vice versa, can be considered as an in-situ labelling of the organic matter incorporated into the soil and is best used in the case of several successive cultivations (Balesdent et al. 1987). Based on measured changes in C stocks and δ13C signatures, the turnover times of the original and replacement soil organic matter fractions can be estimated (Zach et al. 2006; Chalk et al. 2021).
Smith and Chalk (2020) reviewed the effect of tillage systems on nitrogen cycling by using 15N tracing techniques and concluded that no-till had little impact on 15N fertiliser recovery, or the accumulation of N released from the mineralisation of ‘native’ soil organic matter. However, the influence of tillage systems on the stability and turnover of soil organic matter in the soil profile was not considered. Additionally, Smith and Chalk (2020) found the effect of no-till was restricted to the surface soil layers (<0.05 m), but the 15N studies could not confirm or refute the widely-held view that soil organic N increased under no-till, and by inference, soil organic C sequestration. New insights with respect to the influence of tillage on the stability and turnover of soil organic C may be obtained by measuring changes in the soil δ13C signatures in systems that have undergone a C3 to C4 vegetation shift, where original vs replaced C pools can be identified in situ. We reviewed studies of the effects of tillage on shifts in 13C natural abundance (δ13C) implemented for varying lengths of time following the clearing of native vegetation.
In several of the papers reviewed, C in a soil layer was reported as a concentration rather than an amount, and the key soil property, bulk density, was not reported. Consequently, it was not possible to convert C concentrations into C stocks in the various soil layers. Furthermore, nominal sampling depths vary among studies, because of choice, as well as disturbance caused by tillage, and comparisons of tillage effects using mass-based concentrations and a material coordinate (defined by integrating the bulk density profile down from the soil surface; Smiles 2009) cannot be made. That is, comparisons should be made on values adjusted to an equivalent depth based on the weight of soil being the same in the comparisons (Resh et al. 2002; Zach et al. 2006). The objective of the review is to examine C dynamics under traditional and minimal tillage systems using the in situ natural 13C abundance technique, taking into account C stocks rather than C concentrations whenever possible. We do not review 13C enrichment studies because pulse labelling of plants with 13CO2 results in a non-uniform label (Sangster et al. 2010). Furthermore, such studies are short-term by nature and consequently do not provide information on long-term soil C dynamics.
δ13C signatures of CO2 and selected organic materials
Plants preferentially fix 12C over 13C and accordingly have a lower δ13C value compared with atmospheric CO2 (Farquhar et al. 1989; Lloyd and Farquhar 1994). The C3 photosynthetic pathway (Calvin cycle) results in stored C depleted in δ13C of ~18‰ relative to the atmosphere (Farquhar et al. 1982). Since 1975, δ13C in the atmosphere is not constant and has steadily decreased (Francey et al. 1999; Keeling et al. 2017). Ice core records of atmospheric CO2 show that it had a δ13C value of approximately –6.5‰ (1387–1900 years; Francey et al. 1999), which has declined to around –8‰ (Keeling et al. 2005, 2017; Dean et al. 2014). The decline in δ13CO2 is linked to the burning of fossil fuels derived from the organic remains of organisms (mainly plants) that lived millions of years ago. The δ13C of oil or coal is like modern C3 plants with values ranging from −32 to −21‰ for oil and −28 to −23‰ for coal relative to the Vienna Pee Dee Belemnite (VPDB) standard (Given 1984; Warwick and Ruppert 2016; Xu et al. 2017; Winiger et al. 2019). The annual trend in the decrease in atmospheric δ13C is explained by the addition of CO2 to the atmosphere that must come from the terrestrial biosphere and/or fossil fuels. As a result of the change in the atmospheric δ13C, carbon (C) inputs from plants are likely to be more depleted than in the past, thus increasing the uncertainty in subsequent C cycling calculations.
In general, the average δ13C abundance for a range of organic materials of C3 origin under a range of climates was –28.0 ± 1.9‰ (Balesdent et al. 1987, 1988, 1990, 1993; Natelhoffer and Fry 1988; Vitorello et al. 1989; Wanniarachchi et al. 1999; Desjardins et al. 1994; Cadisch et al. 1996; Andriulo et al. 1999; Gerzabek et al. 2001; Kaler at al. 2018), whereas the average value from C4-derived organic sources was –12.5 ± 1.5‰ (Balesdent et al. 1987, 1990; Desjardins et al. 1994; Puget et al. 1995; Cadisch et al. 1996; Andriulo et al. 1999; Wanniarachchi et al. 1999; Hansen et al. 2004; Kristiansen et al. 2005; Fuentes et al. 2010). There are small differences in the isotopic composition of plants in different land use systems. For example, forest, –29.0 ± 1.8‰ (Natelhoffer and Fry 1988; Desjardins et al. 1994; Cadisch et al. 1996); grass and cereal, –28.4 ± 2.3‰ (Balesdent et al. 1990; Andriulo et al. 1999; Fuentes et al. 2010); organic wastes from animal housing such as manure, straw and slurries, –27.6 ± 1.5 (Gerzabek et al. 2001); and peat, –25.6‰ (Gerzabek et al. 2001).
Soil C stocks under tillage systems
Definitions of tillage systems are somewhat variable and were described by Smith and Chalk (2020). The systems and the degree of soil disturbance and mixing needs to be understood in order to make valid comparisons between different experiments. This is not the case in much of the literature. There is a need to use standard terminology in tillage experiments to quantify the impact of the tillage on incorporation of residues, tillage depth and soil bulk density. This will enable C and isotope stocks to be correctly determined on an area basis. Furthermore, soil C and 13C stocks of different treatments should be compared on an equivalent mass of soil, not on a fixed depth basis (Smiles 2009; Lam et al. 2013).
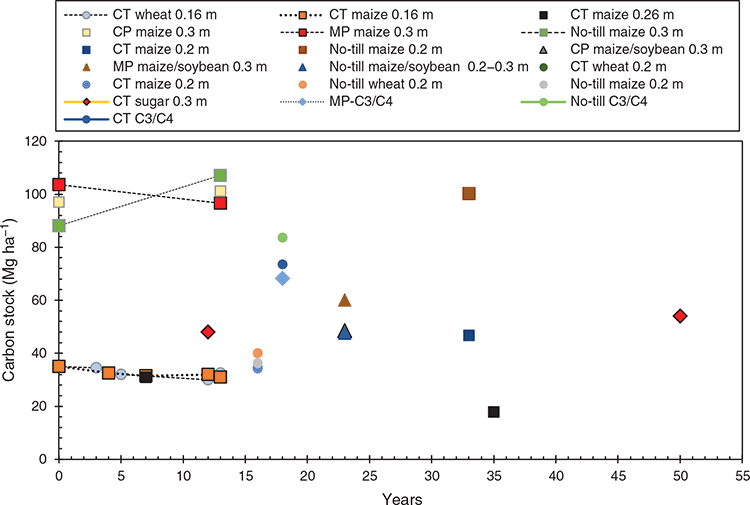
The influence of mouldboard ploughing (MP), chisel ploughing (CP), conventional tillage (CT) and no-tillage (NT), except for soil disturbance during the seeding operation are in Fig. 1. Where possible, C stocks in the surface 0.3 m are presented because the cultivation methods disturbed the soil to different depths. For example, mouldboard ploughing inverts the soil to a depth of 0.2 m and moves organic material from the surface to depth.
|
There was a general decline in soil C concentrations and stocks with years of cultivation of virgin land (Lefroy et al. 1993; Jolivet et al. 1997; Andriulo et al. 1999), which can either be described by a single or double exponential decay model. Of the 10 studies that report C stocks (Vitorello et al. 1989; Desjardins et al. 1994; Balesdent et al. 1998; Andriulo et al. 1999; Collins et al. 1999; Clapp et al. 2000; Dolan et al. 2006; Jantalia et al. 2007; Fuentes et al. 2010; de Sant-Anna et al. 2017), time series data are lacking, especially for long-term cultivation studies. Carbon stocks showed a reduction in C when comparisons were made on the same mass of soil equal to that under 0.3 m of native vegetation (Jantalia et al. 2007). Whilst no-till systems reduced the C loss compared to conventional tillage, the largest losses occurred with mouldboard ploughing and/or twice-yearly tillage (Jantalia et al. 2007). Differences in the C stocks because of land-use change and no-tillage were predominately restricted to the surface soil, whereas mouldboard ploughing resulted in decreases in the C stock in the surface 0.25 m (Clapp et al. 2000; Layese et al. 2002; Huggins et al. 2007; Loss et al. 2012).
Ploughing and harrowing in annual conventional tillage systems promoted greater loss of soil C compared to no-till (Loss et al. 2016). In a 13-year continuous maize study, soil C stocks were found to be sensitive to tillage and harvest of the aboveground plant material (Clapp et al. 2000). Continuous maize grown after a low input mixture of lucerne (Medicago sativa) showed a small decrease in soil C in the annually tilled (MP, CP) treatments. In the no-till treatment, C stocks in the surface plough layer (0.15 m) remained unchanged when the stover was harvested but increased when the stover was returned (Clapp et al. 2000). In contrast, C stocks were unaffected by tillage (non-till compared to MP and CP) when summed to 0.5 m (Dolan et al. 2006), nor did tillage system affect the mineralisation of soil organic C (Liu et al. 2015).
Tillage and residue retention cause stratification in C stocks that are dependent on tillage type (depth) with the highest C concentrations and stocks found in the surface (<0.05 m) under NT (Balesdent et al. 1990; Andriulo et al. 1999; Machado et al. 2003; Allmaras et al. 2004; Dolan et al. 2006; Huggins et al. 2007). For comparison of the effect of tillage on soil C stocks, comparisons need to be made by sampling the tilled layer and ensuring that the cumulative mass of soil per unit area of cross-section is the same between the different treatments. Furthermore, soil C is responsive to the quantity of organic C applied, including the recycling of animal manures (Gerzabek et al. 2001), which in turn is influenced by climate, crop selection, agronomic management, fertilisation and residue management.
Soil δ13C signatures under tillage systems
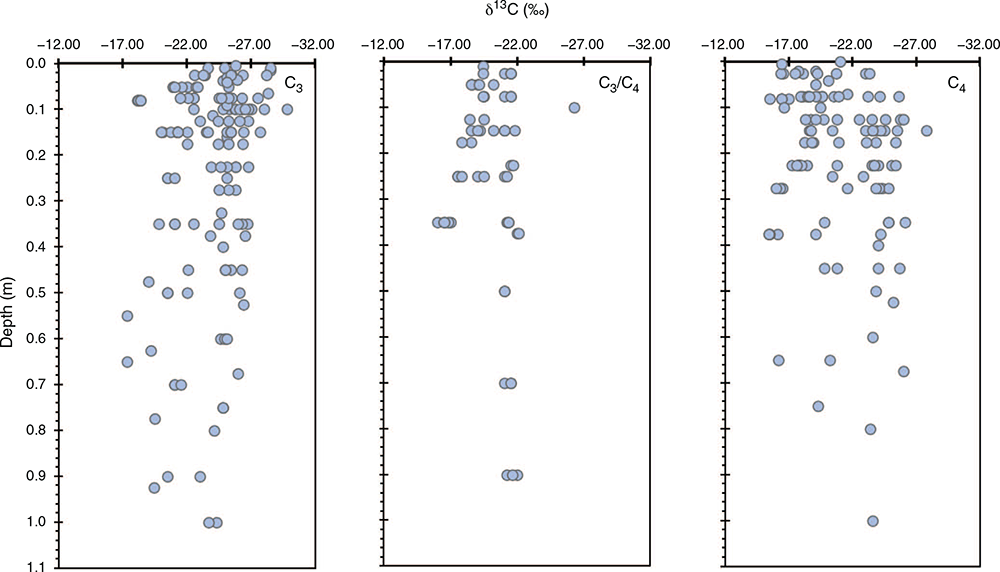
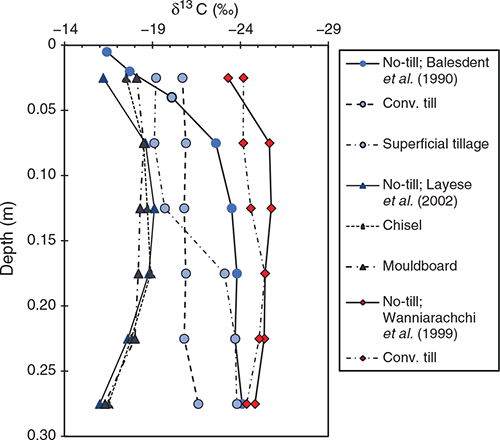
The δ13C signatures of soil organic C under C3, C4 and mixed plant systems show end members ranging from –28‰ under C3 vegetation to –15‰ from C4 vegetation (Fig. 2). Mixed plant communities have δ13C values that lie between these two end member signatures. In general, the δ13C composition of the soil C tended to decrease with soil depth suggesting that the soil C at depth was predominately derived from C3 plant material. The δ13C values were lower than –12‰ in land use systems that supported C4 plants, which is the value one would anticipate if the soil C was derived solely from C4 plants. It should be noted that in all the papers reviewed, the C was determined on soil samples that had carbonates (CO3) removed, thereby ensuring the δ13C signature is that of organic C. Systems with a mixture of C3 and C4 plants have intermediate δ13C values ranging from –17‰ to –22‰. The δ13C values decreased for higher proportions of C4 crops in the rotation, and for the length of time the C4 crops were grown.
|
Although not shown in Fig. 2, the soil organic C declined from the surface to 1.0 m depth, while the turnover period of soil C below the cultivation depth increased with tillage. The δ13C signature integrated for the profile reflects more closely the current vegetation under CT compared to NT (Machado et al. 2003), with significantly ‘newer’ C under CT compared to NT, which in CT extended below the cultivation depth. The difference between the two tillage systems reflects the retention of the fresh C in the surface layer (0.05 m), which declines rapidly with depth under NT. Under CT, the fresh C is distributed through the plough layer and declined below the plough layer. Tillage had no significant effect on the total amount of C present to 0.4m, but the effect was significant only when the surface soil (defined as 0.2 m) was considered (Machado et al. 2003). Furthermore, the result remained to be corrected after adjusting for different bulk densities and using the same mass of soil.
Stratification in soil C under the different tillage regimes is highlighted by δ13C values, with the least negative values in the very surface soil. δ13C values decreased under CP or MP, which mixes plant residues throughout the depth of cultivation (Table 1). The stratification was most pronounced in the studies of Balesdent et al. (1990) and Layese et al. (2002) and to a lesser extent in Wanniarachchi et al. (1999) as shown in Fig. 3. The highest δ13C values were reported by Balesdent et al. (1990) and the value (–16.4‰) was closer to that of the maize residue compared to the values under CT or superficial tillage. These results emphasise the need to sample to depth and make comparisons on adjusted soil C values based on the same mass of soil, if the effect of tillage practices on soil C stocks is to be accurately assessed, as discussed by Jantalia et al. (2007).
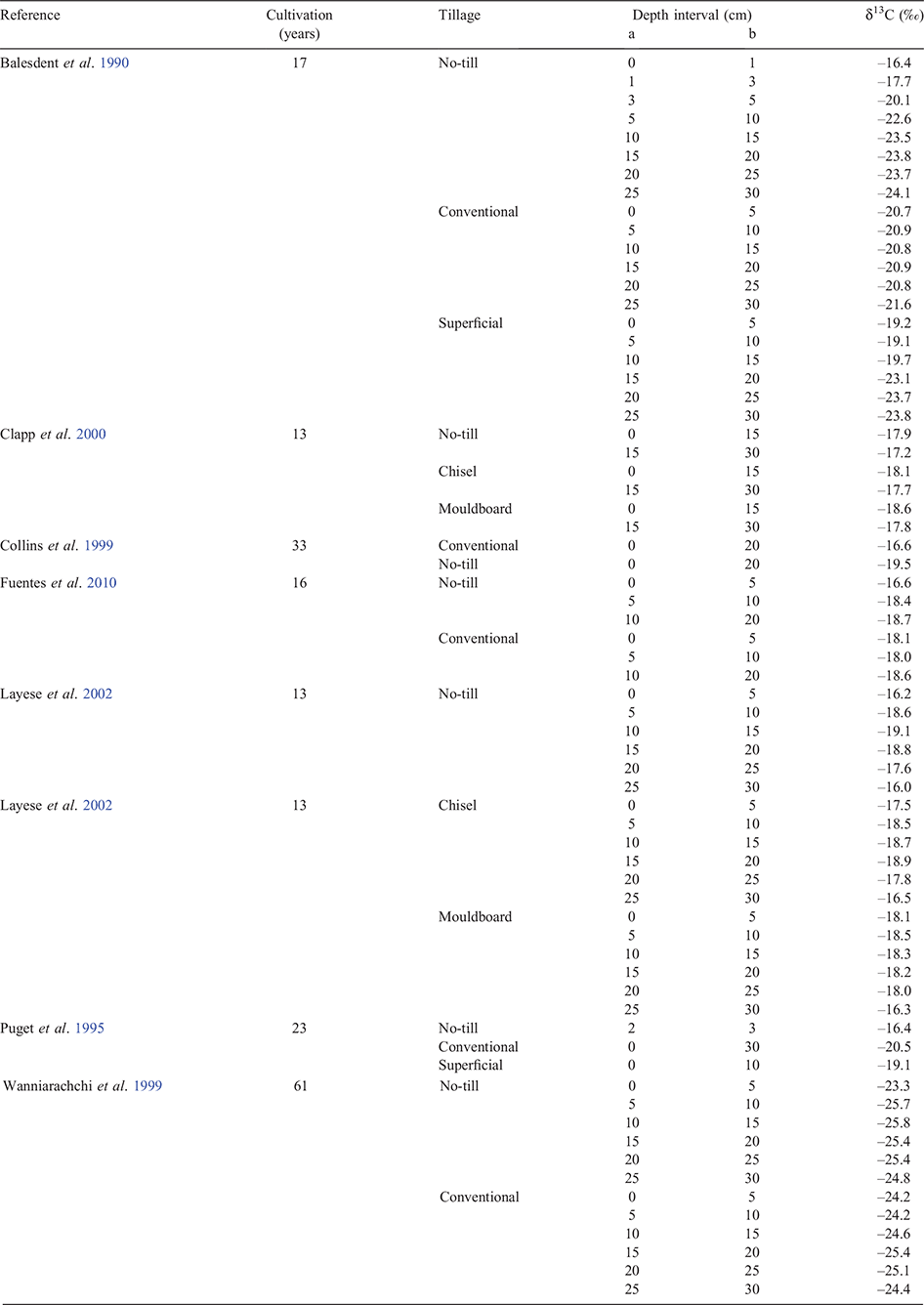
|
|
Whilst there was no large change in the C stocks when summed to a constant mass of soil (Balesdent et al. 1990; Andriulo et al. 1999; Clapp et al. 2000; Allmaras et al. 2004; Dolan et al. 2006), the shifts in the δ13C signature indicated significant exchange of ‘new’ C for the original (old) C (Fig. 3; Zang et al. 2018). The data highlight the dynamic nature of the soil C and enable the contribution of the recently derived C to the total C stock in a soil layer and profile to be estimated. Furthermore, assuming soil organic C decomposition follows first order kinetics, the mean residence time of the soil C can be estimated from the mass balance and isotopic composition data (Balesdent and Mariotti 1996; Zang et al. 2018; Chalk et al. 2021).
Mean residence times under tillage systems
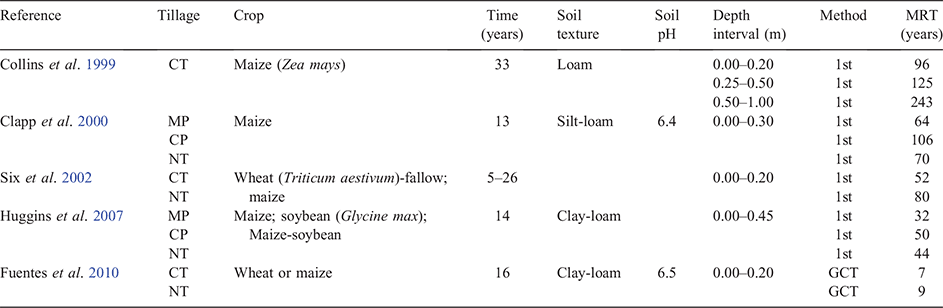
Mean residence time (MRT) is the average length of time that an element such as C spends in a given pool that is not uniform in composition. Each component or fraction of the pool changes at a different rate. The turnover of C is calculated using either a first order decay model (Six et al. 1998; Six and Jastrow 2002) or a double exponential decay model representing a rapid and then a slow change in the δ13C signature of soil organic matter pools (Arrouays et al. 1995; Jolivet et al. 1997). The MRT is calculated as the reciprocal of the decomposition rate (Six and Jastrow 2002).
The effect of tillage on the MRT of C at soil depth intervals is summarised in Table 2. In general, MRTs increase with depth. In the surface soil, MRT values are quite variable, ranging from <10 to 100 years under cultivation systems. Conventional tillage and MP tended to have shorter MRT values compared to CP. NT systems had a similar spread in MRT values with the average value of 53 years in the surface 0.3 m of soil. The MRTs under NT were larger than those under MP and CT. Apparent difference in MRT among tillage systems integrate several variables, including intrinsic soil properties (e.g. pH and texture), the amounts of residues produced and incorporated into the soil and the method of calculation (Table 2).
Distribution of 13C among soil constituents
Microbial biomass
Change in microbial biomass is regarded as an early indicator of tillage and crop rotation effects on soil organic matter compared with total organic C or N measurements and is a potential index of C sequestration (Powlson and Jenkinson 1981; Carter 1986; Powlson et al. 1987; Balota et al. 1998; Sá et al. 2001; Potthoff et al. 2003). Furthermore, microbial biomass can be a valuable tool for understanding changes in soil carbon, nutrient availability, aggregate stability, and quality (Smith and Paul 1990; Doran and Parkin 1994; Brookes 1995; Sparling 1997; Dalal 1998).
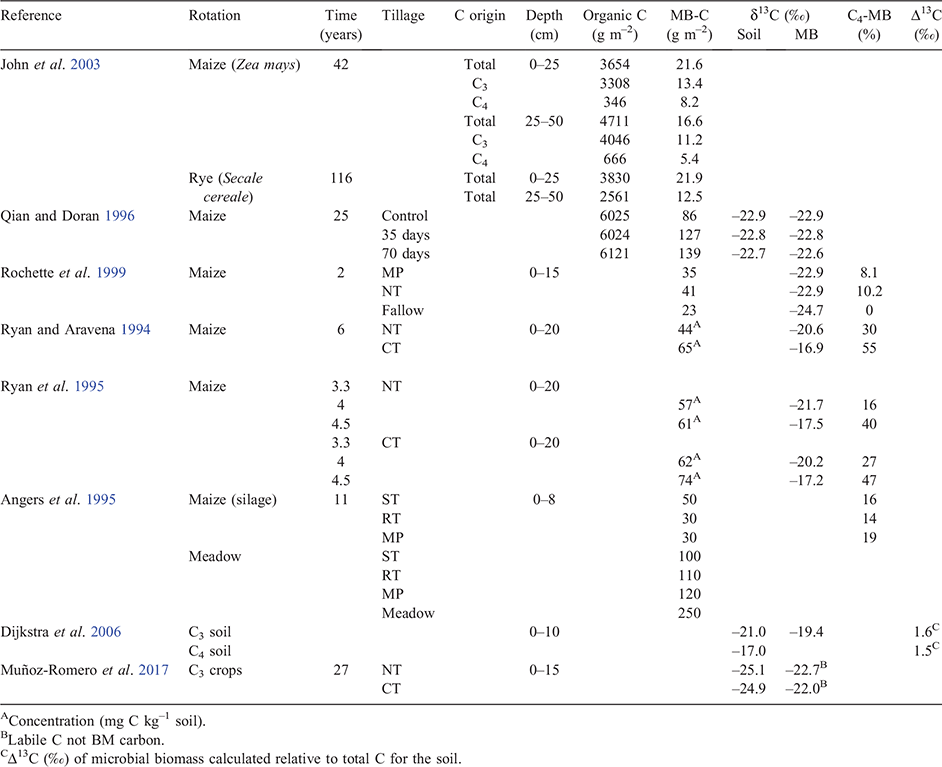
It might be anticipated that tillage treatments such as MP or CT that incorporate residues may have a greater effect on MB than NT, where residues are largely left on the soil surface. However, the limited data available allow few definitive comparisons (Table 3). Generally, there were only small differences in MB-C under maize rotation between NT and MP treatments (Rochette et al. 1999), between NT and CT (Ryan and Aravena 1994, Ryan et al. 1995) and between ST, RT and MP (Angers et al. 1995).
However, after 4 to 6 years of continuous maize, a larger percentage of the total microbial biomass was derived from C4-C under CT compared to NT management (Ryan and Aravena 1994; Ryan et al. 1995) (Table 3), which may reflect the degree of mixing of residues and soil under different tillage regimes. Microbial biomass varies seasonally according to crop growth, with an annual minimum before planting and a maximum during the growing season (Ryan et al. 1995). We therefore conclude that MB-C per se is a poor indicator of long-term changes in soil C under different tillage regimes, representing only 1–2% of soil organic C.
Soil organic C fractions
Tillage intensity generally increase the turnover rate of soil organic matter and decreases soil aggregate stability. The reduced aggregate stability and increased soil organic C decomposition is a function of the physical disturbance (Six et al. 1998). The turnover rate of the soil organic matter in the different particle size fractions has the potential to provide a basis for the partitioning the larger soil organic C pool into smaller functional pools. The changes in the soil δ13C signature before and after cultivation of either C4 or C3 plants (Δ13C) for different particle size fractions are in Fig. 4, where plant sequences of C4 to C3 or C3 to C4 are shown in the upper and lower panels, respectively. Changing from C3 to C4 vegetation cover, in general resulted in a decrease δ13C values (positive shift in Δ13C) and vice versa for a C4 to C3 vegetation change. In most studies, the largest change in the Δ13C values was observed in the coarse sand fraction (200–2000 µm), whereas the smallest change occurred in the clay fraction. Collectively, these data confirm the slower turnover rate of soil organic C in clay (as evident by the lower replacement of the original C), the fastest turnover associated with coarse and fine sand, and intermediate values for silt fractions. Carbon in macroaggregates in general had a mean residence time 42 ± 18 years, whereas microaggregate-associated C has a mean residence time of 206 ± 95 years (Six and Jastrow 2002).
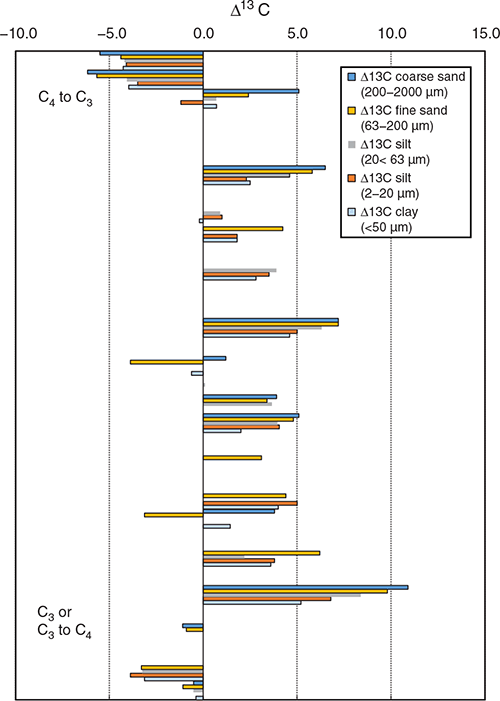
|
Comparison of CT (including mouldboard ploughing) to NT showed inconsistent results of the effect of tillage on C in the different particle size fractions (Fig. 5). In contrast, Six et al. (1999) reported greater C accumulation in NT compared to CT because of the slower turnover of C in macroaggregates under NT. According to Denef et al. (2007), the formation of macroaggregates is important to the long-term C stabilisation in NT systems. Formation of macroaggregates serves as an early indicator for potential total SOC sequestration caused by the greater stability and slowed turnover of C in macroaggregates. More frequent tillage may lead to the formation of small aggregates rich in labile C (Urbanek et al. 2011). The δ13C signature in the surface soil (<0.01 m) suggests that C4-C derived from maize is the dominant fraction of C in agroecosystems, accounting for up to 80% of the total soil organic C concentration (Jha et al. 2017).
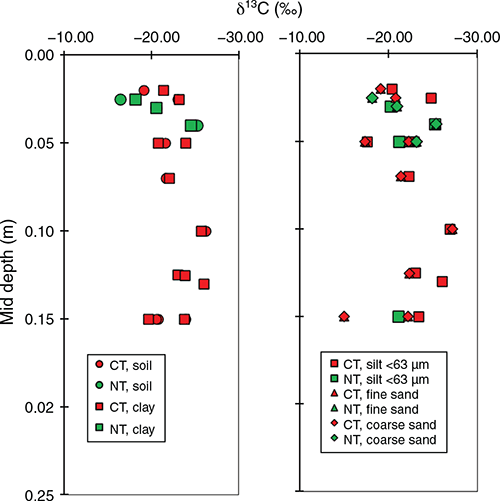
|
The light fraction of soil organic C is dynamic and responds to short-term shifts in organic C storage. This pool has been shown to contain charcoal or a char component formed by the incomplete burning of organic residues (Skjemstad et al. 1994; Murage et al. 2007). Jantalia et al. (2007) estimated that 40% of soil C was charcoal in a soybean (Glycine max) rotation on an Oxisol in the Cerrado region of Brazil. The char or charcoal is generally composed of C from the original vegetation, and consequently may lead to misinterpretation of C turnover. For example, Murage et al. (2007) showed that the turnover of the light fraction of organic matter appears to be 2.5 times slower compared to the same pool without charcoal. Given that most of the cropping systems involve burning of residues, the char component should be removed from the light fraction when determining the changes to this pool. In a comparison of a soil under forest and under maize cultivation, the light fraction of soil organic matter had a shift in the δ13C value (Δ13C) of +10‰, indicating rapid mineralisation of C in the light fraction compared to the other particle size fractions (Gregorich et al. 1995). However, sample char or charcoal was not determined, and thus the turnover of the light fraction may have been overestimated.
The soil particulate organic matter fraction (POM) has been reported to be affected by cultivation of a forest soil (Balesdent et al. 1998), with the change being reached within the first few years after forest clearing. Six et al. (1999) suggest that free POM is mostly affected by plant C input rates. Generally, coarse and fine POM increased under NT, with C derived from C3 plants being mainly associated with the finer compared to the coarse particles (Liang et al. 2014). The turnover time of POM increased with NT compared to CT, especially in the finer POM (53–250 µm) fraction, which largely controls aggregate stability (Six et al. 1998, 2002).
Role of carbon sequestration in climate change mitigation
The potential of C sequestration in soil to off-set the anthropogenic increase in atmospheric CO2 has been discussed by several authors (e.g. Luo et al. 2011; Lam et al. 2013; Amundson and Biardeau 2018; Sun et al. 2020). The studies reported the impact of different tillage practices and rotations on changes in soil C stocks in different soil layers. Generally, the accumulation of C is the largest in the surface layer and declines exponentially with depth. Whilst the studies all use total C stocks, where possible, there is an opportunity to use knowledge of changes in the δ13C isotopic signatures of soil C to confirm estimates of net and gross C changes that are likely to occur over longer times scales. The shifts in δ13C signatures provide valuable information on the residence times of C in soil and assist in confirming that predictions of tillage and rotations on C storage are reasonable. Acton et al. (2013) reported a framework for soil C and isotopic mass balance modelling for forests that includes multiple C pools, including the vertical distribution and turnover of soil C. There is a need to include similar routines in agricultural system models used to evaluate the long-term impact of reduced tillage on soil C sequestration.
Conclusions
Generally, the literature shows that tillage has little impact on the accumulation on soil organic C when the comparisons are made on the same volume of soil that the different tillage systems influence. Ideally the comparisons should be made using mass of soil as the x coordinate to allow for differences in bulk density. Tillage, however, does cause stratification in soil C with the accumulation of the largest amounts of C in the surface layers under no-till systems. In contrast, the use of mouldboard ploughing that inverts the soil to depths of 20–30 cm distributes the new C throughout this layer. The relative 13C signature of the carbon is an ideal natural in situ labelling technique that confirms the long-term fate of the C when it is incorporated into the soil. The technique is limited to systems where there is a change in the δ13C signature of the newly applied land use system from that developed under the previous system. The shift in the δ 13C signature is a powerful tool for quantifying the fate and turnover times of the different sources of C in the soil. These findings are consistent with the previous review by Smith and Chalk (2020) that showed tillage having little effect on soil N dynamics.
Soil C stocks are the balance between new C inputs in the form of crop residues and root biomass and the decomposition of the new and older soil C. Net primary productivity of the plant system regulates potential C input which in turn is constrained by climate, nutrients and agronomic management.
Conflicts of interest
The authors declare no conflict of interest.
Declaration of funding
This research did not receive any specific funding.
References
Acton P, Fox J, Campbell E, Rowe H, Wilkinson M (2013) Carbon isotopes for estimating soil decomposition and physical mixing in well-drained forest soils. Journal of Geophysical Research. Biogeosciences 118, 1532–1545.| Carbon isotopes for estimating soil decomposition and physical mixing in well-drained forest soils.Crossref | GoogleScholarGoogle Scholar |
Allmaras RR, Linden DR, Clapp CE (2004) Corn-residue transformations into root and soil carbon as related to nitrogen, tillage, and stover management. Soil Science Society of America Journal 68, 1366–1375.
| Corn-residue transformations into root and soil carbon as related to nitrogen, tillage, and stover management.Crossref | GoogleScholarGoogle Scholar |
Amundson R, Biardeau L (2018) Soil carbon sequestration is an elusive climate mitigation tool. Proceedings of the National Academy of Sciences of the United States of America 115, 11652–11656.
| Soil carbon sequestration is an elusive climate mitigation tool.Crossref | GoogleScholarGoogle Scholar | 30425181PubMed |
Andriulo A, Guérif J, Mary B (1999) Evolution of soil carbon with various cropping sequences on the rolling pampas. Determination of carbon origin using variations in natural 13C abundance. Agronomie 19, 349–364.
| Evolution of soil carbon with various cropping sequences on the rolling pampas. Determination of carbon origin using variations in natural 13C abundance.Crossref | GoogleScholarGoogle Scholar |
Angers DA, Voroney RP, Côté D (1995) Dynamics of soil organic matter and corn residues affected by tillage practices. Soil Science Society of America Journal 59, 1311–1315.
| Dynamics of soil organic matter and corn residues affected by tillage practices.Crossref | GoogleScholarGoogle Scholar |
Antil RS, Gerzabek MH, Haberhauer G, Eder G (2005) Long-term effects of cropped vs. fallow and fertilizer amendments on soil organic matter I. Organic carbon. Journal of Plant Nutrition and Soil Science 168, 108–116.
| Long-term effects of cropped vs. fallow and fertilizer amendments on soil organic matter I. Organic carbon.Crossref | GoogleScholarGoogle Scholar |
Arrouays D, Balesdent J, Mariotti A, Girardin C (1995) Modelling organic carbon turnover in cleared temperate forest soils converted to maize cropping by using 13C natural abundance measurements. Plant and Soil 173, 191–196.
| Modelling organic carbon turnover in cleared temperate forest soils converted to maize cropping by using 13C natural abundance measurements.Crossref | GoogleScholarGoogle Scholar |
Balesdent J, Mariotti A (1996) Measurement of soil organic matter turnover using 13C natural abundance. In ‘Mass Spectrometry of Soils.’ (Eds TW Boutton, S-I Yamasaki), pp. 83−111. (Marcel Dekker, New York)
Balesdent J, Mariotti A, Guillet B (1987) Natural 13C abundance as a tracer for studies of soil organic matter dynamics. Soil Biology & Biochemistry 19, 25–30.
| Natural 13C abundance as a tracer for studies of soil organic matter dynamics.Crossref | GoogleScholarGoogle Scholar |
Balesdent J, Wagner GH, Mariotti A (1988) Soil organic matter turnover in long-term field experiments as revealed by carbon-13 natural abundance. Soil Science Society of America Journal 52, 118–124.
| Soil organic matter turnover in long-term field experiments as revealed by carbon-13 natural abundance.Crossref | GoogleScholarGoogle Scholar |
Balesdent J, Mariotti A, Boisgontier D (1990) Effect of tillage on soil organic carbon mineralization estimated from 13C abundance in maize fields. European Journal of Soil Science 41, 587–596.
| Effect of tillage on soil organic carbon mineralization estimated from 13C abundance in maize fields.Crossref | GoogleScholarGoogle Scholar |
Balesdent J, Girardin C, Mariotti A (1993) Site-related δ13C of tree leaves and soil organic matter in a temperate forest. Ecology 74, 1713–1721.
| Site-related δ13C of tree leaves and soil organic matter in a temperate forest.Crossref | GoogleScholarGoogle Scholar |
Balesdent J, Besnard E, Arrouays D, Chenu C (1998) The dynamics of carbon in particle-size fractions of soil in a forest-cultivation sequence. Plant and Soil 201, 49–57.
| The dynamics of carbon in particle-size fractions of soil in a forest-cultivation sequence.Crossref | GoogleScholarGoogle Scholar |
Balota EL, Colozzi-Filho A, Andrade DS, Hungria M (1998) Microbial biomass and its activity in soils under different tillage and crop rotation systems. Revista Brasileira de Ciência do Solo 22, 641–649.
| Microbial biomass and its activity in soils under different tillage and crop rotation systems.Crossref | GoogleScholarGoogle Scholar |
Bonde TA, Christensen BT, Cerri CC (1992) Dynamics of soil organic matter as reflected by natural 13C abundance in particle size fractions of forested and cultivated oxisols. Soil Biology & Biochemistry 24, 275–277.
| Dynamics of soil organic matter as reflected by natural 13C abundance in particle size fractions of forested and cultivated oxisols.Crossref | GoogleScholarGoogle Scholar |
Brookes PC (1995) The use of microbial parameters in monitoring soil pollution by heavy metals. Biology and Fertility of Soils 19, 269–279.
| The use of microbial parameters in monitoring soil pollution by heavy metals.Crossref | GoogleScholarGoogle Scholar |
Cadisch G, Imhof H, Urquiaga S, Boddey RM, Giller KE (1996) Carbon turnover (δ13C) and nitrogen mineralization potential of particulate light soil organic matter after rainforest clearing. Soil Biology & Biochemistry 28, 1555–1567.
| Carbon turnover (δ13C) and nitrogen mineralization potential of particulate light soil organic matter after rainforest clearing.Crossref | GoogleScholarGoogle Scholar |
Carter MR (1986) Microbial biomass and mineralizable nitrogen in solonetzic soils: Influence of gypsum and lime amendments. Soil Biology & Biochemistry 18, 531–537.
| Microbial biomass and mineralizable nitrogen in solonetzic soils: Influence of gypsum and lime amendments.Crossref | GoogleScholarGoogle Scholar |
Chalk PM, Balieiro FC, Chen D (2021) Chapter Two - Quantitative estimation of carbon dynamics in terrestrial ecosystems using natural variations in the δ13C abundance of soils and biota: A review. Advances in Agronomy 167, 63–104.
| Chapter Two - Quantitative estimation of carbon dynamics in terrestrial ecosystems using natural variations in the δ13C abundance of soils and biota: A review.Crossref | GoogleScholarGoogle Scholar |
Clapp CE, Allmaras RR, Layese MF, Linden DR, Dowdy RH (2000) Soil organic carbon and 13C abundance as related to tillage, crop residue, and nitrogen fertilization under continuous corn management in Minnesota. Soil & Tillage Research 55, 127–142.
| Soil organic carbon and 13C abundance as related to tillage, crop residue, and nitrogen fertilization under continuous corn management in Minnesota.Crossref | GoogleScholarGoogle Scholar |
Collins HP, Christenson DR, Blevins RL, Bundy LG, Dick WA, Huggins DR, Paul EA (1999) Soil carbon dynamics in corn-based agroecosystems: Results from carbon-13 natural abundance. Soil Science Society of America Journal 63, 584–591.
| Soil carbon dynamics in corn-based agroecosystems: Results from carbon-13 natural abundance.Crossref | GoogleScholarGoogle Scholar |
Dalal RC (1998) Soil microbial biomass – what do the numbers really mean? Australian Journal of Experimental Agriculture 38, 649–665.
| Soil microbial biomass – what do the numbers really mean?Crossref | GoogleScholarGoogle Scholar |
de Sant-Anna SA, Jantalia CP, Sá JM, Vilela L, Marchão RL, Alves BJ, Urquiaga S, Boddey RM (2017) Changes in soil organic carbon during 22 years of pastures, cropping or integrated crop/livestock systems in the Brazilian Cerrado. Nutrient Cycling in Agroecosystems 108, 101–120.
| Changes in soil organic carbon during 22 years of pastures, cropping or integrated crop/livestock systems in the Brazilian Cerrado.Crossref | GoogleScholarGoogle Scholar |
Dean JR, Leng MJ, Mackay AW (2014) Is there an isotopic signature of the Anthropocene? The Anthropocene Review 1, 276–287.
| Is there an isotopic signature of the Anthropocene?Crossref | GoogleScholarGoogle Scholar |
Denef K, Zotarelli L, Boddey RM, Six J (2007) Microaggregate-associated carbon as a diagnostic fraction for management-induced changes in soil organic carbon in two Oxisols. Soil Biology & Biochemistry 39, 1165–1172.
| Microaggregate-associated carbon as a diagnostic fraction for management-induced changes in soil organic carbon in two Oxisols.Crossref | GoogleScholarGoogle Scholar |
Desjardins T, Andreux F, Volkoff B, Cerri CC (1994) Organic carbon and 13C contents in soils and soil size-fractions, and their changes due to deforestation and pasture installation in eastern Amazonia. Geoderma 61, 103–118.
| Organic carbon and 13C contents in soils and soil size-fractions, and their changes due to deforestation and pasture installation in eastern Amazonia.Crossref | GoogleScholarGoogle Scholar |
Dijkstra P, Ishizu A, Doucett R, Hart SC, Schwartz E, Menyailo OV, Hungate BA (2006) 13C and 15N natural abundance of the soil microbial biomass. Soil Biology & Biochemistry 38, 3257–3266.
| 13C and 15N natural abundance of the soil microbial biomass.Crossref | GoogleScholarGoogle Scholar |
Dolan MS, Clapp CE, Allmaras RR, Baker JM, Molina JAE (2006) Soil organic carbon and nitrogen in a Minnesota soil as related to tillage, residue and nitrogen management. Soil & Tillage Research 89, 221–231.
| Soil organic carbon and nitrogen in a Minnesota soil as related to tillage, residue and nitrogen management.Crossref | GoogleScholarGoogle Scholar |
Doran JW, Parkin TB (1994) Defining and assessing soil quality, In ‘Defining Soil Quality for a Sustainable Environment’ (Eds JW Doran et al.). pp.3−21. (Soil Science Society America: Madison, WI)
Dorodnikov M, Fangmeier A, Kuzyakov Y (2007) Thermal stability of soil organic matter pools and their δ13C values after C3–C4 vegetation change. Soil Biology & Biochemistry 39, 1173–1180.
| Thermal stability of soil organic matter pools and their δ13C values after C3–C4 vegetation change.Crossref | GoogleScholarGoogle Scholar |
Farquhar GD, O’Leary MHO, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Australian Journal of Plant Physiology 9, 121–137.
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination during photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40, 503–537.
| Carbon isotope discrimination during photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Francey RJ, Allison CE, Etheridge DM, Trudinger CM, Enting IG, Leuenberger M, Leuenberger RL, Michel E, Steele LP (1999) A 1000-year high precision record of δ13C in atmospheric CO2. Tellus 51, 170–193.
| A 1000-year high precision record of δ13C in atmospheric CO2.Crossref | GoogleScholarGoogle Scholar |
Fuentes M, Govaerts B, Hidalgo C, Etchevers J, Gonzáles-Martin I, Hernández-Hierro JM, Sayre KD, Dendooven L (2010) Organic carbon and stable 13C isotope in conservation agriculture and conventional systems. Soil Biology & Biochemistry 42, 551–557.
| Organic carbon and stable 13C isotope in conservation agriculture and conventional systems.Crossref | GoogleScholarGoogle Scholar |
Gerzabek MH, Haberhauer G, Kirchmann H (2001) Soil organic matter pools and carbon-13 natural abundances in particle-size fractions of a long-term agricultural field experiment. Soil Science Society of America Journal 65, 352–358.
| Soil organic matter pools and carbon-13 natural abundances in particle-size fractions of a long-term agricultural field experiment.Crossref | GoogleScholarGoogle Scholar |
Given PH (1984) An essay on the organic geochemistry of coal. Coal Science 3, 63–252.
| An essay on the organic geochemistry of coal.Crossref | GoogleScholarGoogle Scholar |
Gregorich EG, Monreal CM, Ellert BH (1995) Turnover of soil organic matter and storage of corn residue carbon estimated from natural 13C abundance. Canadian Journal of Soil Science 75, 161–167.
| Turnover of soil organic matter and storage of corn residue carbon estimated from natural 13C abundance.Crossref | GoogleScholarGoogle Scholar |
Hansen EM, Christensen BT, Jensen LS, Kristensen K (2004) Carbon sequestration in soil beneath long-term Miscanthus plantations as determined by 13C abundance. Biomass and Bioenergy 26, 97–105.
| Carbon sequestration in soil beneath long-term Miscanthus plantations as determined by 13C abundance.Crossref | GoogleScholarGoogle Scholar |
Huggins DR, Allmaras RR, Clapp CE, Lamb JA, Randall GW (2007) Corn-soybean sequence and tillage effects on soil carbon dynamics and storage. Soil Science Society of America Journal 71, 145–154.
| Corn-soybean sequence and tillage effects on soil carbon dynamics and storage.Crossref | GoogleScholarGoogle Scholar |
Jantalia CP, Resck DVS, Alves BJR, Zotarelli L, Urquiaga S, Boddey RM (2007) Tillage effect on C stocks of a clayey Oxisol under a soybean-based crop rotation in the Brazilian Cerrado region. Soil & Tillage Research 95, 97–109.
| Tillage effect on C stocks of a clayey Oxisol under a soybean-based crop rotation in the Brazilian Cerrado region.Crossref | GoogleScholarGoogle Scholar |
Jastrow JD, Miller RM, Boutton TW (1996) Carbon dynamics of aggregate-associated organic matter estimated by carbon-13 natural abundance. Soil Science Society of America Journal 60, 801–807.
| Carbon dynamics of aggregate-associated organic matter estimated by carbon-13 natural abundance.Crossref | GoogleScholarGoogle Scholar |
Jha P, Verma S, Lal R, Eidson C, Dheri GS (2017) Natural 13C abundance and soil carbon dynamics under long-term residue retention in a no-till maize system. Soil Use and Management 33, 90–97.
| Natural 13C abundance and soil carbon dynamics under long-term residue retention in a no-till maize system.Crossref | GoogleScholarGoogle Scholar |
John B, Ludwig B, Flessa H (2003) Carbon dynamics determined by natural 13C abundance in microcosm experiments with soils from long-term maize and rye monocultures. Soil Biology & Biochemistry 35, 1193–1202.
| Carbon dynamics determined by natural 13C abundance in microcosm experiments with soils from long-term maize and rye monocultures.Crossref | GoogleScholarGoogle Scholar |
Jolivet C, Arrouays D, Andreux F, Lévèque J (1997) Soil organic carbon dynamics in cleared temperate forest spodosols converted to maize cropping. Plant and Soil 191, 225–231.
| Soil organic carbon dynamics in cleared temperate forest spodosols converted to maize cropping.Crossref | GoogleScholarGoogle Scholar |
Kaler AS, Bazzer SK, San-Saez A, Ray JD, Fritschi FB, Purcell LC (2018) Carbon isotope ratio fractionation among plant tissue of Soybean. Plant Phenome Journal 1, 180002
| Carbon isotope ratio fractionation among plant tissue of Soybean.Crossref | GoogleScholarGoogle Scholar |
Keeling CD, Piper SC, Bacastow RB, Wahlen M, Whorf TP, Heimann M, Meijer HA (2005) Atmospheric CO2 and 13CO2 exchange with the terrestrial biosphere and oceans from 1978 to 2000: Observations and carbon cycle implications. In ‘A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems. Ecological Studies (Analysis and Synthesis)’ (Eds IT Baldwin et al.) Vol. 177, pp. 83–113. (Springer: New York, NY)
Keeling RF, Graven HD, Welp LR, Resplandy L, Bi J, Piper SC, Sun Y, Bollendacher A, Meijer AJ (2017) Atmospheric evidence for a global secular increase in carbon isotopic discrimination of land photosynthesis. Proceedings of the National Academy of Sciences of the United States of America 114, 10361–10366.
| Atmospheric evidence for a global secular increase in carbon isotopic discrimination of land photosynthesis.Crossref | GoogleScholarGoogle Scholar | 28893986PubMed |
Kristiansen SM, Hansen EM, Jensen LS, Christensen BT (2005) Natural 13C abundance and carbon storage in Danish soils under continuous silage maize. European Journal of Agronomy 22, 107–117.
| Natural 13C abundance and carbon storage in Danish soils under continuous silage maize.Crossref | GoogleScholarGoogle Scholar |
Lam SK, Chen D, Mosier AR, Roush R (2013) The potential for carbon sequestration in Australian agricultural soils is technically and economically limited. Scientific Reports 3, 2179
| The potential for carbon sequestration in Australian agricultural soils is technically and economically limited.Crossref | GoogleScholarGoogle Scholar | 23846398PubMed |
Layese MF, Clapp CE, Allmaras RR, Linden DR, Copeland SM, Molina JAE, Dowdy RH (2002) Current and relic carbon using natural abundance of carbon-13. Soil Science 167, 315–326.
| Current and relic carbon using natural abundance of carbon-13.Crossref | GoogleScholarGoogle Scholar |
Lefroy RD, Blair GJ, Strong WM (1993) Changes in soil organic matter with cropping as measured by organic carbon fractions and 13C natural isotope abundance. Plant and Soil 155–156, 399–402.
| Changes in soil organic matter with cropping as measured by organic carbon fractions and 13C natural isotope abundance.Crossref | GoogleScholarGoogle Scholar |
Liang A, Chen S, Zhang X, Chen X (2014) Short-term effects of tillage practices on soil organic carbon turnover assessed by δ13C abundance in particle-size fractions of black soils from Northeast China. TheScientificWorldJournal 2014, 514183
| Short-term effects of tillage practices on soil organic carbon turnover assessed by δ13C abundance in particle-size fractions of black soils from Northeast China.Crossref | GoogleScholarGoogle Scholar | 25162052PubMed |
Liu E, Wang J, Zhang Y, Angers DA, Yan C, Oweis T, He W, Liu Q, Chen B (2015) Priming effect of 13C-labelled wheat straw in no-tillage soil under drying and wetting cycles in the Loess Plateau of China. Scientific Reports 5, 13826
| Priming effect of 13C-labelled wheat straw in no-tillage soil under drying and wetting cycles in the Loess Plateau of China.Crossref | GoogleScholarGoogle Scholar | 26345303PubMed |
Lloyd J, Farquhar GD (1994) 13C discrimination during CO2 assimilation by the terrestrial biosphere. Oecologia 99, 201–215.
| 13C discrimination during CO2 assimilation by the terrestrial biosphere.Crossref | GoogleScholarGoogle Scholar | 28313874PubMed |
Loss A, Pereira MG, Perin A, Beutler SJ, Anjos LHCD (2012) Carbon, nitrogen and natural abundance of δ13C e δ15N of light-fraction organic matter under no-tillage and crop-livestock integration systems. Acta Scientiarum. Agronomy 34, 465–472.
| Carbon, nitrogen and natural abundance of δ13C e δ15N of light-fraction organic matter under no-tillage and crop-livestock integration systems.Crossref | GoogleScholarGoogle Scholar |
Loss A, Pereira MG, Costa EM, Beutler SJ, Piccolo MC (2016) Soil fertility, humic fractions and natural abundance of 13C and 15N in soil under different land use in Paraná State, Southern Brazil. Idesia 34, 27–38.
| Soil fertility, humic fractions and natural abundance of 13C and 15N in soil under different land use in Paraná State, Southern Brazil.Crossref | GoogleScholarGoogle Scholar |
Luo Z, Wang E, Sun OJ, Smith CJ, Probert ME (2011) Modeling long-term soil carbon dynamics and sequestration potential in semi-arid agro-ecosystems. Agricultural and Forest Meteorology 151, 1529–1544.
| Modeling long-term soil carbon dynamics and sequestration potential in semi-arid agro-ecosystems.Crossref | GoogleScholarGoogle Scholar |
Machado PLOA, Sohi SP, Gaunt JL (2003) Effect of no‐tillage on turnover of organic matter in a Rhodic Ferralsol. Soil Use and Management 19, 250–256.
| Effect of no‐tillage on turnover of organic matter in a Rhodic Ferralsol.Crossref | GoogleScholarGoogle Scholar |
Muñoz-Romero V, Lopez-Bellido RJ, Fernandez-Garcia P, Redondo R, Murillo S, Lopez-Bellido L (2017) Effects of tillage, crop rotation and N application rate on labile and recalcitrant soil carbon in a Mediterranean Vertisol. Soil & Tillage Research 169, 118–123.
| Effects of tillage, crop rotation and N application rate on labile and recalcitrant soil carbon in a Mediterranean Vertisol.Crossref | GoogleScholarGoogle Scholar |
Murage EW, Voroney P, Beyaert RP (2007) Turnover of carbon in the free light fraction with and without charcoal as determined using the 13C natural abundance method. Geoderma 138, 133–143.
| Turnover of carbon in the free light fraction with and without charcoal as determined using the 13C natural abundance method.Crossref | GoogleScholarGoogle Scholar |
Natelhoffer KJ, Fry B (1988) Controls on natural nitrogen-15 and carbon-13 abundance in forest soil organic matter. Soil Science Society of America Journal 52, 1633–1640.
| Controls on natural nitrogen-15 and carbon-13 abundance in forest soil organic matter.Crossref | GoogleScholarGoogle Scholar |
Potthoff M, Loftield N, Buegger F, Wick B, John B, Joergensen RG, Flessa H (2003) The determination of δ13C in soil microbial biomass using fumigation-extraction. Soil Biology & Biochemistry 35, 947–954.
| The determination of δ13C in soil microbial biomass using fumigation-extraction.Crossref | GoogleScholarGoogle Scholar |
Powlson DS, Jenkinson DS (1981) A comparison of the organic matter, biomass, adenosine triphosphate and mineralizable nitrogen contents of ploughed and direct drilled soils. Journal of Agricultural Science 97, 713–721.
| A comparison of the organic matter, biomass, adenosine triphosphate and mineralizable nitrogen contents of ploughed and direct drilled soils.Crossref | GoogleScholarGoogle Scholar |
Powlson DS, Brookes PC, Christensen BT (1987) Measurement of soil microbial biomass provides an early indication of changers in total soil organic matter due to straw incorporation. Soil Biology & Biochemistry 19, 159–164.
| Measurement of soil microbial biomass provides an early indication of changers in total soil organic matter due to straw incorporation.Crossref | GoogleScholarGoogle Scholar |
Puget P, Chenu C, Balesdent J (1995) Total and young organic matter distributions in aggregates of silty cultivated soils. European Journal of Soil Science 46, 449–459.
| Total and young organic matter distributions in aggregates of silty cultivated soils.Crossref | GoogleScholarGoogle Scholar |
Qian JH, Doran JW (1996) Available carbon released from crop roots during growth as determined by carbon-13 natural abundance. Soil Science Society of America Journal 60, 828–831.
| Available carbon released from crop roots during growth as determined by carbon-13 natural abundance.Crossref | GoogleScholarGoogle Scholar |
Resh S, Binkley D, Parrotta JA (2002) Greater soil carbon sequestration under nitrogen-fixing trees comparted with Eucalyptus species. Ecosystems 5, 217–231.
| Greater soil carbon sequestration under nitrogen-fixing trees comparted with Eucalyptus species.Crossref | GoogleScholarGoogle Scholar |
Rochette P, Angers DA, Flanagan LB (1999) Maize residue decomposition measurement using soil surface carbon dioxide fluxes and natural abundance of carbon-13. Soil Science Society of America Journal 63, 1385–1396.
| Maize residue decomposition measurement using soil surface carbon dioxide fluxes and natural abundance of carbon-13.Crossref | GoogleScholarGoogle Scholar |
Roscoe R, Burrman P (2003) Tillage effects on soil organic matter in density fractions of a Cerrado Oxisol. Soil & Tillage Research 70, 107–119.
| Tillage effects on soil organic matter in density fractions of a Cerrado Oxisol.Crossref | GoogleScholarGoogle Scholar |
Roscoe R, Burrman P, Velthorst EJ, Vasconcellos CA (2001) Soil organic matter dynamics in density and particle size fractions as revealed by the 13C/12C isotopic ratio in a Cerrado’s oxisol. Geoderma 104, 185–202.
| Soil organic matter dynamics in density and particle size fractions as revealed by the 13C/12C isotopic ratio in a Cerrado’s oxisol.Crossref | GoogleScholarGoogle Scholar |
Ryan MC, Aravena R (1994) Combining 13C natural abundance and fumigation-extraction methods to investigate soil microbial biomass turnover. Soil Biology & Biochemistry 26, 1583–1585.
| Combining 13C natural abundance and fumigation-extraction methods to investigate soil microbial biomass turnover.Crossref | GoogleScholarGoogle Scholar |
Ryan MC, Aravena R, Gillham RW (1995) The use of 13C natural abundance to investigate the turnover of the microbial biomass and active fractions of soil organic matter under two tillage treatments. In: ‘Soils and Global Change’ (Eds R Lal, J Kimble, E Levine, BA Stewart). pp. 351−360. (CRC Press, Boca Raton)
Sá JC de M, Cerri CC, Dick WA, Lal R, Filho SPV, Picollo MC, Feigl BE (2001) Organic matter dynamics and carbon sequestration rates for a tillage chronosequence in a Brazilian Oxisol. Soil Science Society of America Journal 65, 1486–1499.
| Organic matter dynamics and carbon sequestration rates for a tillage chronosequence in a Brazilian Oxisol.Crossref | GoogleScholarGoogle Scholar |
Sangster A, Knight D, Farrell R, Bedard-Haughn A (2010) Repeat−pulse 13CO2 labeling of canola and field pea: implications for soil organic matter studies. Rapid Communications in Mass Spectrometry 24, 2791–2798.
| Repeat−pulse 13CO2 labeling of canola and field pea: implications for soil organic matter studies.Crossref | GoogleScholarGoogle Scholar | 20857436PubMed |
Shang C, Tiessen H (2000) Carbon turnover and carbon-13 natural abundance in organo-mineral fractions of a tropical dry forest soil under cultivation. Soil Science Society of America Journal 64, 2149–2155.
| Carbon turnover and carbon-13 natural abundance in organo-mineral fractions of a tropical dry forest soil under cultivation.Crossref | GoogleScholarGoogle Scholar |
Sisti CPJ, dos Santos HP, Kohhann R, Alves BJR, Urquiaga S, Boddey RM (2004) Change in carbon and nitrogen stocks in soil under 13 years of conventional or zero tillage in southern Brazil. Soil & Tillage Research 76, 39–58.
| Change in carbon and nitrogen stocks in soil under 13 years of conventional or zero tillage in southern Brazil.Crossref | GoogleScholarGoogle Scholar |
Six J, Jastrow JD (2002) Organic matter turnover. In ‘Encyclopedia of Soil Science’ (Ed. R Lal). pp. 936−942. (Marcel Dekker, New York)
Six J, Elliott ET, Paustian K, Doran JW (1998) Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Science Society of America Journal 62, 1367–1377.
| Aggregation and soil organic matter accumulation in cultivated and native grassland soils.Crossref | GoogleScholarGoogle Scholar |
Six J, Elliott ET, Paustian K (1999) Aggregate and soil organic matter dynamics under conventional and no-tillage systems. Soil Science Society of America Journal 63, 1350–1358.
| Aggregate and soil organic matter dynamics under conventional and no-tillage systems.Crossref | GoogleScholarGoogle Scholar |
Six J, Feller C, Denef K, Ogle SM, Sa JC de M, Albrecht A (2002) Soil organic matter, biota and aggregation in temperate and tropical soils – Effects of no-tillage. Agronomie 22, 755–775.
| Soil organic matter, biota and aggregation in temperate and tropical soils – Effects of no-tillage.Crossref | GoogleScholarGoogle Scholar |
Skjemstad JO, Catchpoole VR, Lefeuvre RP (1994) Carbon dynamics in Vertisols under several crops as assessed by natural abundance 13C. Soil Research 32, 311–321.
| Carbon dynamics in Vertisols under several crops as assessed by natural abundance 13C.Crossref | GoogleScholarGoogle Scholar |
Smiles DE (2009) Quantifying carbon and sulphate loss in drained acid sulphate soils. European Journal of Soil Science 60, 64–70.
| Quantifying carbon and sulphate loss in drained acid sulphate soils.Crossref | GoogleScholarGoogle Scholar |
Smith CJ, Chalk PM (2020) A review of the role of 15N in tracing N dynamics in agro-ecosystems under alternative systems of tillage management. Soil & Tillage Research 197, 104496
| A review of the role of 15N in tracing N dynamics in agro-ecosystems under alternative systems of tillage management.Crossref | GoogleScholarGoogle Scholar |
Smith BN, Epstein S (1971) Two categories of 13C/12C ratios for higher plants. Plant Physiology 47, 380–384.
| Two categories of 13C/12C ratios for higher plants.Crossref | GoogleScholarGoogle Scholar | 16657626PubMed |
Smith JL, Paul EA (1990) The significance of soil microbial biomass estimations. In ‘Soil Biochemistry’ (Eds JM Bollag, G Stotzkv). Vol. 6, pp. 357−396. (Marcel Dekker, New York)
Sparling GP (1997) Soil microbial biomass, activity and nutrient cycling as indicators of soil health, In ‘Biological Indicators of Soil Health’ (Eds CE Pankhurst, BM Doube, VVSR Gupta), pp. 97–119 (CAB International, Wallingford)
Sun W, Canadell JG, Yu L, Yu L, Zhang W, Smith P, Fischer T, Haung Y (2020) Climate drives global soil carbon sequestration and crop yield changes under conservation agriculture. Global Change Biology 26, 3325–3335.
| Climate drives global soil carbon sequestration and crop yield changes under conservation agriculture.Crossref | GoogleScholarGoogle Scholar | 31953897PubMed |
Urbanek E, Smucker AJ, Horn R (2011) Total and fresh organic carbon distribution in aggregate size classes and single aggregate regions using natural 13C/12C tracer. Geoderma 164, 164–171.
| Total and fresh organic carbon distribution in aggregate size classes and single aggregate regions using natural 13C/12C tracer.Crossref | GoogleScholarGoogle Scholar |
Vitorello YA, Cerri CC, Andreux F, Feller C, Victoria RL (1989) Organic matter and natural carbon 13C distribution in forested and cultivated Oxisols. Soil Science Society of America Journal 53, 773–778.
| Organic matter and natural carbon 13C distribution in forested and cultivated Oxisols.Crossref | GoogleScholarGoogle Scholar |
Wanniarachchi SD, Voroney RP, Vyn TJ, Beyaert RP, MacKenzie AF (1999) Tillage effects on the dynamics of total and corn-residue-derived soil organic matter in two southern Ontario soils. Canadian Journal of Soil Science 79, 473–480.
| Tillage effects on the dynamics of total and corn-residue-derived soil organic matter in two southern Ontario soils.Crossref | GoogleScholarGoogle Scholar |
Warwick PD, Ruppert LF (2016) Carbon and oxygen isotopic composition of coal and carbon dioxide derived from laboratory coal combustion: A preliminary study. International Journal of Coal Geology 166, 128–135.
| Carbon and oxygen isotopic composition of coal and carbon dioxide derived from laboratory coal combustion: A preliminary study.Crossref | GoogleScholarGoogle Scholar |
Winiger P, Barrett TE, Sheesley RJ, Huang L, Sharma S, Barrie LA, Yttri KE, Evangeliou N, Eckhardt S, Stohl A, Klimont Z, Heyes C, Semiletov IP, Dudarev OV, Charkin A, Shakhova N, Holmstrand H, Andersson A, Gustafsson Ö (2019) Source apportionment of circum-Arctic atmospheric black carbon from isotopes and modelling. Science Advances 5, eaau8052
| Source apportionment of circum-Arctic atmospheric black carbon from isotopes and modelling.Crossref | GoogleScholarGoogle Scholar | 30788434PubMed |
Xu J, Lee X, Xio W, Cao C, Liu S, Wen X, Xu J, Zhang Z, Zhao J (2017) Interpreting the 13C/12C ratio of carbon dioxide in an urban airshed in the Yangtze River Delta, China. Atmospheric Chemistry and Physics 17, 3385–3399.
| Interpreting the 13C/12C ratio of carbon dioxide in an urban airshed in the Yangtze River Delta, China.Crossref | GoogleScholarGoogle Scholar |
Zach A, Tiessen H, Noellemeyer E (2006) Carbon turnover and carbon‐13 natural abundance under land use change in semiarid savanna soils of La Pampa, Argentina. Soil Science Society of America Journal 70, 1541–1546.
| Carbon turnover and carbon‐13 natural abundance under land use change in semiarid savanna soils of La Pampa, Argentina.Crossref | GoogleScholarGoogle Scholar |
Zang H, Blagodatskaya E, Wen Y, Xu X, Dyckmans J, Kuzyakov Y (2018) Carbon sequestration and turnover in soil under the energy crop Miscanthus: Repeated 13C natural abundance approach and literature synthesis. Global Change Biology. Bioenergy 10, 262–271.
| Carbon sequestration and turnover in soil under the energy crop Miscanthus: Repeated 13C natural abundance approach and literature synthesis.Crossref | GoogleScholarGoogle Scholar |