Time trends in positive gonorrhoea diagnoses at the Christchurch Sexual Health Service (2012–2022): a data audit study
Hayley J. Denison A * , Julie Creighton B , Jeroen Douwes A , Maureen Coshall C and Heather Young C
A * , Julie Creighton B , Jeroen Douwes A , Maureen Coshall C and Heather Young C
A
B
C
Abstract
Gonorrhoea infections and antimicrobial resistance are rising in many countries, particularly among men who have sex with men, and an increasing proportion of infection is detected at extragenital sites. This study assessed trends in gonorrhoea diagnoses and antibiotic resistance at a sexual health service in New Zealand that followed national guidelines for specimen collection.
Routinely-collected data from Canterbury Health Laboratories of specimens taken at the Christchurch Sexual Health Service 2012–2022 were audited. Descriptive results included the number of patient testing events positive for gonorrhoea per year and site of infection (extragenital/urogenital). Annual test-positivity was calculated (number of positive patient testing events divided by total number of testing events) and the Cochran-Armitage Test for Trend was used to assess whether there was an association between test-positivity and year.
Of 52,789 patient testing events, 1467 (2.8%) were positive for gonorrhoea (81% male). Half (49.3%) of people (57.9% of males, 12.2% of females) with a gonorrhoea infection had an extragenital infection in the absence of a urogenital infection. The number of extragenital infections increased at a faster rate than urogenital among males. Test-positivity increased from 1.3% in 2012 to 5.8% in 2022 (P < 0.001). Antimicrobial resistance was identified in many isolates. Ciprofloxacin resistance was high, but there were no cases of ceftriaxone resistance.
This study highlights the importance of extragenital sampling and maintaining bacterial culture methods for accurate diagnosis and treatment. The observation that gonorrhoea positivity rate and antimicrobial resistance rates are rising in New Zealand calls for urgent action.
Keywords: antibiotic susceptibility testing, antimicrobial resistance, diagnosed infection, extragenital infection, gonorrhoea, impacts of covid, Neisseria gonorrhoeae, sexual health service use, test positivity, trends over time.
Background
The sexually transmitted infection (STI) gonorrhoea is caused by Neisseria gonorrhoeae, a Gram-negative, diplococcus shaped bacterium, which only colonises humans.1 Gonorrhoea is the second most common bacterial STI after chlamydia and is a major cause of cervicitis or urethritis.2N. gonorrhoeae can sometimes ascend to the upper genital tract in females, where it may cause complications such as pelvic inflammatory disease and ectopic pregnancy.3 There is also some evidence that N. gonorrhoeae can cause infertility in men.4
N. gonorrhoeae has an extraordinary capacity to alter its genetic material and is a highly adapted pathogen, having acquired or developed nearly all known physiological mechanisms of antimicrobial resistance.5 Over time, N. gonorrhoeae has developed resistance to almost all antimicrobials introduced for treatment of gonorrhoea, including penicillin, tetracycline, macrolides and fluroquinolones including ciprofloxacin. The extended-spectrum cephalosporin, ceftriaxone, is the last option for empirical first-line monotherapy in most countries.6 Worryingly, isolates with decreased susceptibility to ceftriaxone have now been reported in several countries.7 The World Health Organization has classified N. gonorrhoeae as a high-priority pathogen threatening human health.8
National-level surveillance data show that the rate of gonorrhoea is increasing in developed countries. In the US, the rate of reported gonorrhoea had increased 118% from 2009 to 2021.9 A similar rise in the rate of gonorrhoea has been seen in the UK and in Australia.10,11 The latest figures from New Zealand show that the 2022 national rate among males is double what it was in 2015 (81 per 100,000 population in 2015; 161 per 100,000 population in 2022) and almost double among females (62 per 100,000 population in 2015; 110 per 100,000 population in 2022).12 Although these figures are indicative of a rise in gonorrhoea infection, the real incidence rate is likely to be much higher, as rates are calculated from diagnosed cases and many infections go undetected. In addition, rates for 2020 and 2021 are affected by the impact of the COVID-19 pandemic, when sexual health services moved to urgent services only in compliance with national lockdowns and social distancing rules. Estimates of incidence rates over time in developing countries are lacking, as producing time-trend data requires STI surveillance systems which low-income countries generally do not have the resources to implement.
A disproportionately high percentage of gonorrhoea infections occur among men who have sex with men (MSM),13 and the incidence of infection among this group appears to be increasing.14 Overseas studies have found that extragenital gonorrhoea positivity has increased at a much faster rate than urogenital positivity among MSM.15 The Aotearoa New Zealand STI Guidelines recommend MSM (cisgender) have both an anorectal and a pharyngeal swab regardless of reported sexual practices or condom use as asymptomatic infection is common, and that females (cisgender) have an anorectal swab if they report anal sex in their sexual history.16 Selection of appropriate testing therefore requires knowledge of clients’ sexual orientation/behaviour.
New Zealand surveillance data compiled by the Institute of Environmental Science and Research shows that the number of urogenital infections is around three times the number of extragenital (anorectal and pharyngeal) infections.17 However, data are influenced by swabbing practices, so if extragenital testing does not take place, these infections will be missed and will be absent from surveillance data. The likelihood of missed infections is compounded by extragenital infections often being asymptomatic.18
The aims of this study were to assess time-trends of gonorrhoea diagnoses, including extragenital infection, and antibiotic resistance at a sexual health clinic in New Zealand where all clients are asked about their sexual orientation/behaviour and all MSM are offered anorectal and pharyngeal testing as well as urogenital testing.
Materials and methods
Study design
This study is an audit of routinely collected data from Canterbury Health Laboratories in Christchurch, New Zealand. Data relating to positive gonorrhoea diagnoses were extracted from laboratory computer records and permanently de-identified. All positive gonorrhoea diagnoses of specimens taken at the Christchurch Sexual Health Service (SHS) between January 2012 and December 2022 were included. The Christchurch SHS provides a specialist sexual health service for the Canterbury population (~660,000 people) and tertiary support to the wider South Island health regions. Access is via self-referral, primary care or secondary care referral. While the Christchurch SHS is the only specialist hospital sexual health service in the Canterbury region, a large amount of sexual health testing is done in general practice. In addition, there are smaller STI testing providers in the region including Burnett Foundation Aotearoa (formerly New Zealand AIDS Foundation), Sexual Wellbeing Aotearoa (formerly Family Planning), and Te Tahi Youth (a Youth Health ‘one stop shop’ for medical, sexual and mental health services). Therefore, this study only includes a portion of STI testing from the region.
Measures
The main outcome measures were specimen site and antimicrobial resistance. Specimen site was coded into either urogenital (vagina; cervix; first catch urine; urethra) or extragenital (pharynx; rectum). Susceptibility testing for each of the antibiotics ciprofloxacin, penicillin, tetracycline, azithromycin, and ceftriaxone was categorised as susceptible, intermediate, or resistant, according to current breakpoints. Sex, age, and date of specimen were also included in the dataset. Canterbury Health Laboratories provided a yearly denominator of the number of patient testing events each year (2012–2022) (each testing event could involve testing of multiple sites).
The total number of patient testing events per year excluded testing events that were for the purpose of confirming a positive or obtaining a culture after a positive nucleic acid amplification test (NAAT) test. These were identified by matching patient details for people with two positive test results within 30 days and checking the reason for the repeat on the test referral form. Tests of cure, recommended in certain circumstances, were also excluded when identified during this process.
Specimen collection methods
NAAT specimens were collected according to the Aotearoa New Zealand STI Guidelines.16 For females, a vulvovaginal swab is recommended. An anorectal swab is also recommended if the sexual health history includes anal sex. For men who have sex with women, a first-pass urine test is recommended. For MSM, a first-pass urine test plus pharyngeal and anorectal swabs are recommended. Swabs for NAAT testing were collected into multi-collect specimen collection kits, as recommended by the manufacturer (Abbott, IL, USA).
At the Christchurch SHS, N. gonorrhoeae is cultured from: body sites where people have a positive screening gonorrhoea NAAT test (prior to being treated); females who present with vaginal discharge; males with purulent penile discharge; people known to be a contact of a person with gonorrhoea infection. Cultures were taken by directly inoculating Thayer Martin plates (Fort Richard Laboratories, Auckland, New Zealand) with sample from cervix, vagina, pharynx, rectum or urethra.
Laboratory methods
Molecular detection of Neisseria gonorrhoeae DNA was performed by polymerase chain reaction (Abbott). For cultures taken as per the criteria outlined above, the Thayer Martin plates were incubated at 36°C in 5% CO2 and examined for growth for 3 days. Identification of N. gonorrhoeae consisted of typical colony morphology, a positive oxidase test and confirmation by matrix-assisted laser desorption/ionisation time-of-flight (Bruker Daltonics, USA). Antibiotic susceptibility testing against ceftriaxone and azithromycin (since 2013) was determined by minimum inhibitory concentration (MIC) gradient strips, and susceptibility against ciprofloxacin, penicillin and tetracycline were determined by the disc diffusion method, using CLSI (Clinical & Laboratory Standards Institute) and EUCAST (European Committee on Antimicrobial Susceptibility Testing) breakpoints. In addition, isolates were tested for β-lactamase production (conferring high-level resistance to penicillin) using the chromogenic BBL cefinase discs (BD, Sparks, USA).
Statistical analysis
Data were stratified by year (2012–2022) using recorded specimen date. Descriptive statistics were generated by year to assess time trends. Test-positivity was calculated by dividing the number of positive patient testing events (could include multiple sites positive) by the total number of testing events each year (after removal of confirmatory/culture swab secondary tests). The Cochran-Armitage Test for Trend was used to assess whether there was an association between test-positivity and year. All analyses were conducted using SAS ver. 9.4.
Results
Between January 2012 and December 2022, there were 52,789 patient testing events (each event could involve testing of multiple sites) at the Christchurch SHS that underwent gonorrhoea testing at Canterbury Health Laboratories. Of these, 1467 (2.8%) tested positive for gonorrhoea. Positive samples were more likely to be from male clients (81.0%) and from those in the 20–29 years age-group (41.0%) (Table 1). The median age of people with positive gonorrhoea diagnoses was 30 years (IQR 24–40). The median age among males (31 years; IQR 25–42) was higher than among females (25 years; IQR 20–33). More people tested positive for gonorrhoea at extragenital sites only (n = 723, 49.3%) than from urogenital sites only (n = 535, 36.5%). The remaining 209 people (14.3%) tested positive at both extragenital and urogenital sites. The majority of people (82.9%) had one positive gonorrhoea diagnosis during the period 2012–2022; 11.4% of people had two positive diagnoses and 5.7% of people had three or more positive diagnoses. Although identifying re-infection rate was not an a priori objective of this research, we conducted some exploratory analyses where we identified all of the people who had a positive diagnosis in 2019 and assessed whether they had another infection within 12 months. We found that 8.4% had a repeat infection diagnosed at the SHS within 12 months.
| Characteristic | Number of peopleA | Percentage of people | |
|---|---|---|---|
| Total | 1467 | 100.0 | |
| Sex | |||
| Female | 278 | 19.0 | |
| Male | 1189 | 81.0 | |
| Age (years) | |||
| 14–19 | 115 | 7.8 | |
| 20–29 | 602 | 41.0 | |
| 30–39 | 379 | 25.8 | |
| 40–49 | 190 | 13.0 | |
| 50+ | 181 | 12.3 | |
| Number of sites with a positive sample per person per visit | |||
| 1 | 1034 | 70.5 | |
| 2 | 329 | 22.4 | |
| 3 | 96 | 6.5 | |
| 4 | 8 | 0.6 | |
| Types of site where infection was identified | |||
| Genital only | 535 | 36.5 | |
| Extragenital only | 723 | 49.3 | |
| Both genital and extragenital | 209 | 14.3 | |
| Number of positive diagnoses per person from 2012 to 2022B | |||
| 1 | 961 | 82.9 | |
| 2 | 132 | 11.4 | |
| 3 | 40 | 3.5 | |
| 4 | 16 | 1.4 | |
| 5 | 5 | 0.4 | |
| 6 | 2 | 0.2 | |
| 7 | 3 | 0.3 | |
Trends in gonorrhoea diagnoses over time
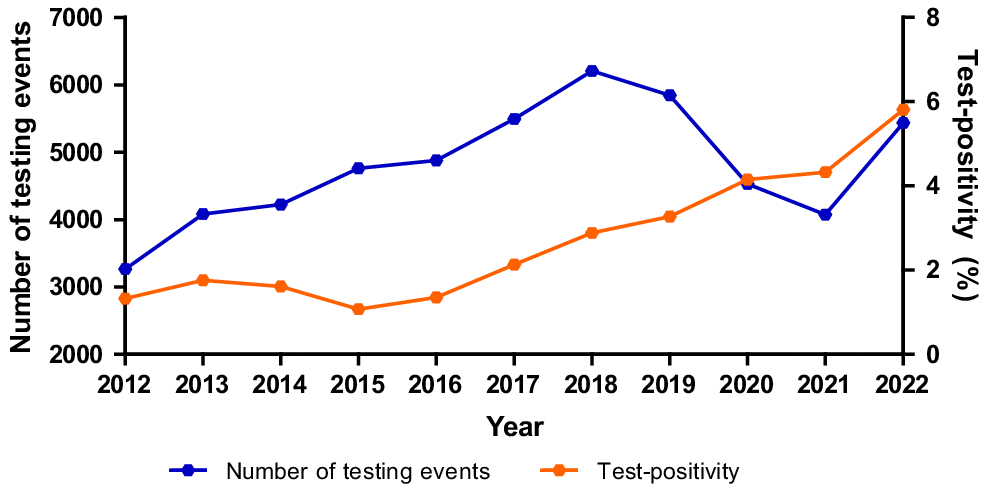
The number of positive diagnoses increased across the 11 years from 43 in 2012 to 316 in 2022, with the increase being much steeper among males, especially since 2016 (Table 2). These results are influenced by testing numbers, as the more people that test, the more infections that will be identified. The number of patient testing events at the sexual health service increased year-on-year from 2012 to 2018, almost doubling across that period (Fig. 1) before dipping slightly in 2019. It then reduced by around a third in 2020 and 2021 during the COVID-19 pandemic and then increased markedly in 2022, to almost pre-COVID levels. To assess whether the overall increase in testing was responsible for the changes observed in positive diagnoses over time, we also analysed test-positivity, which increased four-fold (1.3–5.8%) from 2012 to 2022 (Fig. 1). It increased year-on-year, even during 2020 and 2021 when both test numbers and positive gonorrhoea diagnoses dropped. A Cochran-Armitage test for trend showed a statistically significant increasing trend (P < 0.001), suggesting that gonorrhoea infection has increased over time independent of the increase in testing rates.
The number of testing events at the Christchurch Sexual Health Service processed by Canterbury Health Laboratories and the percentage of specimens that were positive, 2012–2022.
| Year | Female | Male | All | |||||
|---|---|---|---|---|---|---|---|---|
| Urogenital infection only | Extragenital infection (with or without urogenital infection) | Total | Urogenital infection only | Extragenital infection (with or without urogenital infection) | Total | Total | ||
| 2012 | 14 | 0 | 14 | 21 | 8 | 29 | 43 | |
| 2013 | 23 | 0 | 23 | 25 | 24 | 49 | 72 | |
| 2014 | 3 | 2 | 5 | 14 | 49 | 63 | 68 | |
| 2015 | 3 | 1 | 4 | 14 | 33 | 47 | 51 | |
| 2016 | 8 | 2 | 10 | 16 | 40 | 56 | 66 | |
| 2017 | 17 | 4 | 21 | 31 | 65 | 96 | 117 | |
| 2018 | 20 | 14 | 34 | 30 | 115 | 145 | 179 | |
| 2019 | 20 | 12 | 32 | 40 | 119 | 159 | 191 | |
| 2020 | 23 | 18 | 41 | 45 | 102 | 147 | 188 | |
| 2021 | 19 | 9 | 28 | 34 | 114 | 148 | 176 | |
| 2022 | 44 | 22 | 66 | 71 | 179 | 250 | 316 | |
When comparing results by site of infection, the number of extragenital (pharyngeal and anorectal) infections has increased at a faster rate than urogenital infections among males (Table 2). In 2022, the number of males with extragenital gonorrhoea was 2.5-times that of males with urogenital infection alone. Among females, the number of both extragenital and urogenital infections has increased, but to a much lesser extent and lower number overall (Table 2). In 2022, the number of extragenital infections was half the number of urogenital only infections among females. In total across the 11 years, 57.9% of males who had a gonorrhoea infection had an extragenital infection in the absence of a urogenital infection; among females, this was 12.2%.
Trends in antimicrobial resistance
The rate of resistance of N. gonorrhoeae isolates (2012–2022) to the antimicrobials ceftriaxone, ciprofloxacin, penicillin, tetracycline, and azithromycin and the presence of β-lactamase enzyme are in Table 3. In total across the 11 years, 37% of isolates tested were found to be resistant to ciprofloxacin, 11% to penicillin (6% of isolates were β-lactamase positive, conferring high-level resistance to penicillin), 18% to tetracycline, and 7% to azithromycin. Rates of resistance to ciprofloxacin remain high, with fluctuating resistance prevalence over the study period. The first azithromycin low-level resistant isolates (based on the epidemiological cut-off value of 1 mg/L) were detected in 2018, with the highest level of resistance (30%) found in 2020, likely due to the circulation of resistant clones. All azithromycin resistant isolates had MICs in the 2–4 mg/L range. No isolates were found to be resistant to ceftriaxone, instead there was a slight shift of MIC distribution towards higher susceptibility (MIC ≤ 0.016 mg/L) (MIC data not shown).
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | Total | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Number of N. gonorrhoeae isolates tested (n) | 38 | 50 | 46 | 29 | 42 | 63 | 90 | 97 | 100 | 87 | 158 | 801 | |
| β-lactamase positive | 4 (10.5) | 3 (6.0) | 2 (4.4) | 4 (13.8) | 6 (14.3) | 5 (7.9) | 9 (10.0) | 5 (5.2) | 1 (1.0) | 5 (5.8) | 5 (3.2) | 49 (6.1) | |
| Ceftriaxone | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Ciprofloxacin | 15 (39.5) | 12 (24.0) | 25 (54.4) | 18 (62.1) | 14 (33.3) | 17 (27.0) | 14 (15.6) | 41 (42.3) | 50 (50.0) | 28 (32.2) | 58 (36.7) | 292 (36.5) | |
| Penicillin | 4 (10.5) | 3 (6.0) | 5 (10.9) | 5 (17.2) | 11 (26.2) | 10 (15.9) | 9 (10.0) | 12 (12.4) | 2 (2.0) | 15 (17.2) | 11 (7.0) | 87 (10.9) | |
| Tetracycline | 7 (18.4) | 9 (18.0) | 7 (15.2) | 4 (13.8) | 7 (16.7) | 9 (14.3) | 10 (11.1) | 21 (21.7) | 29 (29.0) | 23 (26.4) | 19 (12.0) | 145 (18.1) | |
| Number of N. gonorrhoeae isolates tested (n) | 0 | 45 | 46 | 29 | 42 | 63 | 90 | 97 | 100 | 87 | 158 | 757 | |
| Azithromycin A | n/a | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (6.7) | 3 (3.1) | 30 (30.0) | 4 (4.6) | 5 (3.2) | 48 (6.3) |
Discussion
This study found that the number of gonorrhoea infections increased across the 11-year period, and that the increase has been steeper among males than females. There was a significant increasing trend in test-positivity rates, suggesting that along with a general increase in testing, there has also been an increase in community prevalence of gonorrhoea. Among males, the number of extragenital infections has increased at a faster rate than urogenital infections.
There are a few factors that would have contributed to the fluctuations in testing numbers observed in the study. HIV pre-exposure prophylaxis (PrEP) became publicly funded in 2018 which may have increased testing volumes, as the recommended testing regimen for renewal of PrEP includes NAAT swabs for chlamydia and gonorrhoea. Locally within the SHS, workforce capacity as well as self-testing and outreach initiatives to manage demand may have also impacted on testing volumes. The COVID-19 pandemic impacted testing numbers as asymptomatic testing was paused at the Christchurch SHS during Alert Levels 3 and 4 of the New Zealand COVID-19 Alert System (the highest two Alert Levels); this can be clearly seen in the data for 2020 and 2021. It is important to note that there have been no major changes to testing recommendations over the study period, including no changes to which sites are tested. While testing numbers rose and fell over the time period of the study, the test-positivity rate (proportion of tests that are positive for gonorrhoea) steadily increased year-on-year since 2015, indicating that the rise in gonorrhoea is independent of changes in testing rates.
The high number of people in this study with a gonorrhoea infection at an extragenital site only (49.3%) suggests that the number of undetected infections if only urogenital sites were screened would be high. A US study examining the records of MSM from 42 sexual health clinics found that 71.8% of rectal gonorrhoea infections and 73.8% of pharyngeal infections were associated with negative urethral tests at the same visit and would not have been detected with urethral screening alone.19 The possibility of missing extragenital infections among females is also a concern. In the current study, 12.2% of females with a gonorrhoea infection had an extragenital infection in the absence of a urogenital infection. However, universal extragenital sampling is not currently recommended by the Aotearoa New Zealand STI Guidelines for women,16 so it is likely these women had extragenital testing because they were at increased risk. In a study of female attendees at an STI clinic in Italy where vaginal, pharyngeal and rectal swabs were collected from every participant to test for Chlamydia trachomatis and N. gonorrhoeae, 5.2% of cases would have been missed if extragenital sites had not been tested. In addition, a meta-analysis of 14 studies conducted in the UK, USA, Canada, Australia and Europe found that rectal infection was independent of a history of anal sex.20 This suggests that universal extragenital sampling for females may be warranted, although more research into the prevalence of extragenital infections among females in New Zealand would be needed to inform this decision.
The data in this study are from an SHS; it is not known how much extragenital testing is being done by other providers. A study from The Netherlands found that more testing for C. trachomatis is done by general practitioners (GPs) and gynaecologists than STI clinics; however, anorectal or pharyngeal swabs are taken less often (<1%) in these settings than in STI clinics (14.4%).21 This suggests that awareness of the importance of extragenital testing guidelines could be increased among GPs and gynaecologists. Similar data is not currently available In New Zealand; national surveillance statistics are based on laboratory notification data that does not include health provider information. Clinician notification data could be used to identify rates of extragenital testing by provider types; gonorrhoea is a notifiable disease under the Health (Protection) Amendment Act 2016 (NZ), meaning there is a legal requirement for the diagnosing clinician to complete an anonymous case report for all gonorrhoea cases.22 However, currently this is not well completed.23
Antimicrobial resistance was identified in many N. gonorrhoeae isolates. Ciprofloxacin resistance is particularly high, as is observed globally.6 There is concerning evidence that resistance to azithromycin is increasing, although a downward trend has been observed for the past 2 years. There were no cases of ceftriaxone resistance in this study, and there have not been any reports of ceftriaxone resistance in New Zealand to date.24 The introduction of NAATs has resulted in a reduction of N. gonorrhoeae isolates available for susceptibility testing in many countries.25 It is important to maintain bacterial culture methods as results from susceptibility testing are critical to the formation of treatment guidelines. The Christchurch Sexual Health Service has maintained a positive culture rate of over 50% since the introduction of molecular testing.
These results are evidence that current policies and funding decisions are not reducing gonorrhoea infection rates. The rise in infection rates, along with the rise in antimicrobial resistance, is a serious concern for health. Manatū Hauora (NZ Ministry of Health) recently published the Sexually Transmitted and Blood Borne Infections (STBBI) Strategy that aims to provide a framework for delivering effective action on STBBI.26 However, the current action plans that fall under the strategic direction of the STBBI strategy do not include gonorrhoea, meaning that no new funding has been allocated to reducing the incidence and impacts of gonorrhoea in New Zealand.
There are several limitations of this study. First, to maintain anonymity, sexual behaviour data from the clinic could not be linked to the laboratory diagnosis data. Therefore, we could not determine the proportion of people at risk of extragenital infection who were tested at extragenital sites. Second, testing numbers by sex and age were not available, meaning that we could not assess changes in testers’ characteristics over time or calculate test-positivity and carry out tests for trend separately for these groups. This prevented interpretation of whether changes in testing demographics are influencing changes in positivity rates. Third, this study only includes gonorrhoea diagnoses from tests done at the Christchurch SHS, limiting the ability to assess reinfection rate due to infections diagnosed by another provider not being captured in the data. Lastly, the high rate of extragenital infection identified in this study could be because people at higher risk of infection at these sites may be more likely to attend sexual health clinics than primary care. While this could mean that the high rates are a finding relevant only to sexual health clinics, it does underscore the importance of having easily accessible and free sexual health services that reach at-risk populations. Differences in demographics and sexual behaviour between sexual health service clients and the general population also limit the generalisability of the antibiotic resistance results presented. A strength of this study is that it uses routinely-collected laboratory data for gonorrhoea diagnosis rather than self-report data from questionnaire or interview, eliminating social desirability and recall bias.
In conclusion, this study found that in a setting that has a testing protocol based on Aotearoa New Zealand STI testing recommendations for extragenital sites, the proportion of extragenital infections is high and appears to be increasing. This suggests that not offering extragenital testing could lead to missed infection and increased disease burden in the population. Therefore, sexual health training of healthcare providers to increase awareness of the importance of extragenital testing guidelines may be warranted.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
HD was supported by a Massey University Research Funding grant (RM21507) and a Lottery Health Research Postdoctoral Fellowship (LHR-2020-129824).
Acknowledgements
This study used de-identified data from specimens taken at the Christchurch SHS and tested at Canterbury Health Laboratories. We thank clinic and laboratory staff for their support of this work and their contribution to data collection.
References
1 Quillin SJ, Seifert HS. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol 2018; 16(4): 226-240.
| Crossref | Google Scholar | PubMed |
2 Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE 2015; 10(12): e0143304.
| Crossref | Google Scholar | PubMed |
3 Skerlev M, Culav-Koscak I. Gonorrhea: new challenges. Clin Dermatol 2014; 32(2): 275-281.
| Crossref | Google Scholar | PubMed |
4 Gimenes F, Souza RP, Bento JC, Teixeira JJV, Maria-Engler SS, Bonini MG, et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol 2014; 11(12): 672-687.
| Crossref | Google Scholar | PubMed |
5 Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014; 27(3): 587-613.
| Crossref | Google Scholar | PubMed |
6 Unemo M, Lahra MM, Escher M, Eremin S, Cole MJ, Galarza P, et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017-18: a retrospective observational study. Lancet Microbe 2021; 2(11): e627-e636.
| Crossref | Google Scholar | PubMed |
7 Unemo M, Lahra MM, Cole M, Galarza P, Ndowa F, Martin I, et al. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health 2019; 16(5): 412-425.
| Crossref | Google Scholar | PubMed |
8 Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18(3): 318-327.
| Crossref | Google Scholar | PubMed |
9 Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2021. Available at https://www.cdc.gov/std/statistics/2022/2021-STD-Surveillance-Report-PDF_ARCHIVED-2-16-24.pdf [Accessed 20 June 2024]
11 UK Health Security Agency. Official Statistics. Sexually transmitted infections (STIs): annual data tables. Table 1a: new STI diagnosis numbers and rates in England by gender, 2013 to 2022. 2024. Available at https://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables [Accessed 5 July 2023]
12 Institute of Environmental Science and Research. New Zealand Sexually Transmitted Infection (STI) Surveillance Dashboard. 2024. Available at https://www.esr.cri.nz/our-research/nga-kete/infectious-disease-intelligence/sexually-transmitted-infection-sti-surveillance/ [Accessed 5 July 2023]
13 Saxton PJW, McAllister SM, Thirkell CE, Ludlam AH, Bateman JP, Anglemyer AT, et al. Population rates of HIV, gonorrhoea and syphilis diagnoses by sexual orientation in New Zealand. Sex Transm Infect 2022; 98(5): 376-379.
| Crossref | Google Scholar | PubMed |
14 Kirkcaldy RD, Weston E, Segurado AC, Hughes G. Epidemiology of gonorrhoea: a global perspective. Sex Health 2019; 16(5): 401-411.
| Crossref | Google Scholar | PubMed |
15 Comninos NB, Garton L, Guy R, Callander D, Fairley CK, Grulich AE, et al. Increases in pharyngeal Neisseria gonorrhoeae positivity in men who have sex with men, 2011-2015: observational study. Sex Transm Infect 2020; 96(6): 432-435.
| Crossref | Google Scholar |
16 Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM) and New Zealand Sexual Health Society (NZSHS). Aotearoa New Zealand, STI management guidelines for use in primary care. 2024. Available at https://sti.guidelines.org.nz/ [Accessed 5 July 2023]
18 Chan PA, Robinette A, Montgomery M, Almonte A, Cu-Uvin S, Lonks JR, et al. Extragenital infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae: a review of the literature. Infect Dis Obstet Gynecol 2016; 2016: 5758387.
| Crossref | Google Scholar |
19 Patton ME, Kidd S, Llata E, Stenger M, Braxton J, Asbel L, et al. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men--STD Surveillance Network, United States, 2010-2012. Clin Infect Dis 2014; 58(11): 1564-1570.
| Crossref | Google Scholar | PubMed |
20 Chandra NL, Broad C, Folkard K, Town K, Harding-Esch EM, Woodhall SC, et al. Detection of Chlamydia trachomatis in rectal specimens in women and its association with anal intercourse: a systematic review and meta-analysis. Sex Transm Infect 2018; 94(5): 320-326.
| Crossref | Google Scholar | PubMed |
21 den Heijer CDJ, van Liere GAFS, Hoebe CJPA, van Bergen JEAM, Cals JWL, Stals FS, et al. Who tests whom? A comprehensive overview of Chlamydia trachomatis test practices in a Dutch region among different STI care providers for urogenital, anorectal and oropharyngeal sites in young people: a cross-sectional study. Sex Transm Infect 2016; 92(3): 211-217.
| Crossref | Google Scholar | PubMed |
22 New Zealand Parliament. Health (Protection) Amendment Act 2016, Schedule 1, Part 1, Section C—infectious diseases notifiable to medical officer of health without identifying information of patient or deceased person. 2016. Available at https://www.legislation.govt.nz/act/public/2016/0035/latest/DLM6223006.html [Accessed 14 June 2023]
23 Murray C, Rose SB, Kvalsvig A, Baker MG. Contact tracing for sexually transmitted infections in Aotearoa New Zealand: a review of clinician-notified gonorrhoea and syphilis data. J Prim Health Care 2023; 15(2): 167-171.
| Crossref | Google Scholar | PubMed |
25 World Health Organization. Regional Office for the Western Pacific. Review of national treatment guidelines for sexually transmitted infections in the Western Pacific Region [fact sheet]. License: CC BY-NC-SA 3.0 IGO. WHO Regional Office for the Western Pacific; 2018. Available at https://apps.who.int/iris/handle/10665/279732 [Accessed 14 June 2023]


