Prevalence of five curable sexually transmitted infections and associated risk factors among tertiary student men who have sex with men in Nairobi, Kenya: a respondent-driven sampling survey†
Samuel Waweru Mwaniki A B * , Peter Mwenda Kaberia C , Peter Mwangi Mugo D and Thesla Palanee-Phillips E F
A B * , Peter Mwenda Kaberia C , Peter Mwangi Mugo D and Thesla Palanee-Phillips E F
A School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
B University Health Services, Administration and Campus Support Services, University of Nairobi, Nairobi, Kenya.
C Department of Mathematics, Faculty of Science and Technology, University of Nairobi, Nairobi, Kenya.
D Kenya Medical Research Institute – Wellcome Trust Research Programme, Nairobi, Kenya.
E Wits Reproductive Health and HIV Institute, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
F Department of Epidemiology, School of Public Health, University of Washington, Seattle, WA, USA.
Sexual Health 20(2) 105-117 https://doi.org/10.1071/SH22114
Submitted: 7 July 2022 Accepted: 21 December 2022 Published: 24 January 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Background: Young men who have sex with men (MSM) are a key population at high risk of sexually transmitted infections (STIs). We conducted a respondent-driven sampling (RDS) bio-behavioural survey to estimate the prevalence of five curable STIs: chlamydia, gonorrhoea, syphilis, trichomoniasis and Mycoplasma genitalium infection, and associated risk factors among tertiary student MSM (TSMSM) in Nairobi, Kenya.
Methods: Between February and March 2021, we recruited 248 TSMSM aged ≥18 years who self-reported engaging in anal and/or oral sex with another man in the past year. Samples collected included urine, anorectal and oropharyngeal swabs for pooled Chlamydia trachomatis, Mycoplasma genitalium, Neisseria gonorrhoeae and Trichomonas vaginalis testing using multiplex nucleic acid amplification tests, and venous blood for serological Treponema pallidum screening and confirmation of current infection. Participants self-completed a behavioural survey on a REDCap digital platform. Data analysis was done using RDS-Analyst (v0.72) and Stata (v15). Differences in proportions were examined using the chi-squared (χ2) test, and unweighted multivariate logistic regression was used to assess factors associated with STI prevalence.
Results: RDS-adjusted prevalence rates of at least one of the five STIs, chlamydia, gonorrhoea, Mycoplasma genitalium infection, trichomoniasis and latent syphilis were 58.8%, 51.0%, 11.3%, 6.0%, 1.5% and 0.7%, respectively. Factors independently associated with STI prevalence were inconsistent condom use (adjusted odds ratio (AOR) = 1.89, 95% confidence interval (CI): 1.03–3.47, P = 0.038) and the last sex partner being a regular partner (AOR = 2.35, 95% CI: 1.12–4.92, P = 0.023).
Conclusion: STI prevalence among TSMSM in Nairobi, Kenya, is disturbingly high, demonstrating urgent need for tailored testing, treatment and prevention interventions for this population.
Keywords: condom use, digital health, nucleic acid amplification tests (NAATs), risk reduction counselling, sex partner, sexual behaviour, sexual minorities, Sub-Saharan Africa, young key populations, young men who have sex with men (YMSM).
Introduction
Globally, compared to the general population, gay, bisexual and other men who have sex with men (collectively referred to as MSM) experience higher rates of sexually transmitted infections (STIs) such as chlamydia, gonorrhoea and syphilis.1 Data from sub-Saharan African (SSA) countries have shown high rates of these and other STIs among MSM, including chlamydia in Tanzania – 7.5%2 and Kenya – 26%3; gonorrhoea in Tanzania – 14.4%2 and Kenya – 26%3; syphilis in Malawi – 12.3%4 and Uganda – 9%5; Mycoplasma genitalium infection in Nigeria – 36.8%6; and trichomoniasis in South Africa – 9%.7
In terms of sexual behaviour, MSM may engage in both receptive and insertive anal and oral sex, making them susceptible to anorectal and oropharyngeal STIs besides the usual genital STIs. Unlike the genital STIs, extra-genital STIs are often asymptomatic and thus remain largely undiagnosed and untreated.8 The World Health Organization (WHO) recommends offering MSM periodic testing for asymptomatic STIs using serological tests for syphilis and nucleic acid amplification tests (NAATs) for chlamydia and gonorrhoea.9 Whereas serological tests are affordable and easy to use, NAATs require expensive technology and personnel with technical training, and are thus not routinely used in SSA countries. As a result, management of STIs in most SSA countries including Kenya is largely syndromic,10 leading to missed cases and inadequate management (because many STIs are asymptomatic in early infection), elevated risk of acquisition and transmission of human immunodeficiency virus (HIV),11 and future complications such as infertility.12 Simulation studies have shown that this sub-optimal treatment of STIs plays a significant role in HIV infection among young MSM (YMSM) aged 18–24 years.13 Besides, MSM infected with asymptomatic STIs may unknowingly infect sex partners, thus spreading the infections. Further, commensal oropharyngeal Neisseria spp. may transfer genetic material to untreated oropharyngeal Neisseria gonorrhoeae, leading to the development of resistance to extended spectrum cephalosporins.14 Taken together, these factors make surveillance of STIs critical, especially among key populations at heightened risk of infection.
Although more expensive than culturing of bacterial microorganisms, NAATs are currently the gold standard for detecting asymptomatic extra-genital bacterial STIs, given their high sensitivity and specificity.15 Pooling samples from different anatomical sites from an individual and testing them together can be used to lower the cost of NAATs,16 and has been shown to perform as well as single-anatomic site testing for Chlamydia trachomatis and Neisseria gonorrhoeae among MSM in various settings.17–22
At the onset of the developmental stage of emerging adulthood (18–25 years), in-school individuals transition from secondary to tertiary institutions.23 In tertiary institutions, most students find themselves in an environment with limited direct supervision of their behaviour, and increased freedom and opportunities to engage in behavioural risks such as casual sex.24 For tertiary student MSM (TSMSM), exposure to peers with similar sexual orientation and behaviour provides an environment with more socialising opportunities compared to their secondary school days.25 There is evidence to suggest that TSMSM may seek casual sex partners online, have sex more often, and engage in condomless sex, group sex and sex work.26 Moreover, TSMSM have been shown to have early sexual debuts, forced sex experiences, high sex partner turnover, concurrent sex partners, inconsistent condom use, and alcohol and drug use.27 As well, structural factors, such as criminalisation of homosexuality in Kenya, and societal stigma and discrimination towards individuals with same-sex behaviour, may further limit healthcare engagement for TSMSM,28 making it difficult to access services for prevention, screening and treatment of STIs. In sum, these behavioural and structural factors increase the risk of STIs among TSMSM, making them an important population to focus on for STIs research. In Kenya, little is known about the prevalence of STIs and associated risk factors among YMSM, including TSMSM. As part of the effort to bridge this knowledge gap, the current study examined the prevalence of five curable STIs (chlamydia, gonorrhoea, syphilis, trichomoniasis and Mycoplasma genitalium infection), and associated demographic, behavioural and contextual risk factors among TSMSM in Nairobi, Kenya.
Methods
The study methods are detailed in the published study protocol,29 and summarised below.
Study design and setting
A cross-sectional bio-behavioural survey was conducted between February and March 2021, just after stringent coronavirus disease 2019 (COVID-19) prevention and control restrictions were eased in Kenya, and tertiary institutions resumed in-person teaching. Nairobi, Kenya’s capital, was selected due to its large population of tertiary students, with approximately 150 campuses of various universities and colleges located in the city and within its metropolis.30
Participants, sampling, and recruitment
TSMSM were eligible to participate in the study if they were willing and able to provide written informed consent for study participation, were aged ≥18 years, provided proof of registration as a student in a university or college in Nairobi, were assigned male sex at birth, and reported consensual receptive or insertive anal and/or oral sexual intercourse with another man in the last 12 months. The sample size was primarily calculated to estimate the prevalence of HIV among TSMSM, based on WHO 2017 guidelines for bio-behavioural surveys among populations at higher risk of HIV infection.31 Using the Cochran formula,32 a design effect of three to account for clustering, estimated a HIV prevalence of 4.1% based on a previous study among TSMSM in South Africa,33 a level of precision of 0.05 and 10% non-response; we used this to determine that a minimum sample size of 200 TSMSM would be required. The results of the primary objective of the study are reported elsewhere.34 Respondent-driven sampling (RDS) was used following findings from formative qualitative research, which showed that this sampling method was appropriate and acceptable for recruiting TSMSM into HIV/STI research.35 From the TSMSM who took part in the formative research, six seeds were purposively selected based mainly on their self-reported personal network sizes and ability to recruit their peers. The seeds completed half a day training during which they were briefed on the purpose of the study, study procedures, eligibility criteria for prospective participants and types of incentives that would be offered. In addition, the seeds were offered a recruitment script, which if needed, would be used to inform their peers about the study. Upon completing the study procedures, each seed was issued three coupons to recruit their peers. A member of the study team used a prescribed form to screen prospective participants recruited by the seeds and subsequent participants. The screening involved checking the validity of the coupons (based on a unique code for each coupon linked to the seed starting off each recruitment chain), and fulfilment of the other set eligibility criteria. Upon completing the study procedures, each participant was briefed on how to recruit their peers into the study, issued three coupons and a recruitment script similar to the one issued to the seeds. Coupons were valid for a period of 2 weeks. Each seed and subsequent participant could recruit only a maximum of three peers into the study. Issuing of three coupons went on until the survey had recruited 120 participants (inclusive of seeds). Subsequently, two coupons were issued until the 150th participant, after which no more coupons were issued. The study remained open for recruitment for 2 weeks after the last coupons were issued, and closed with 248 TSMSM having participated. Each participant was reimbursed 1000 Kenyan shillings (equivalent to USD10 at that time) for their time and expenses related to transport to and from the study site, and 300 Kenyan shillings (equivalent to USD3) for every peer they recruited into the study. In addition, participants were offered condoms and water-based lubricants.
Data collection tools and procedures
The behavioural survey was conducted by self-administered interviews on tablets using a questionnaire set up on REDCap software (Vanderbilt University, TN, USA). The questionnaire was adapted from a validated questionnaire available from the integrated HIV bio-behavioural surveillance toolbox.36 The questionnaire contained questions on sociodemographic characteristics, sexual behaviour and other contextual characteristics (symptoms of STIs, use of geosocial networking applications and condom affordability and accessibility), and was administered in English, the language of instruction in Kenyan tertiary institutions.
Biological specimen collection and testing
After completing the behavioural survey, participants were offered testing for HIV34 and other STIs. Each participant provided approximately 3–4 mL of venous blood for serological syphilis testing using a rapid plasma reagin (RPR) test for screening and a Treponema pallidum haemagglutination assay (TPHA) test for confirmation of current syphilis infection. Additionally, each participant provided approximately 20–30 mL of first void urine, one pharyngeal, and one anorectal swab for testing of Chlamydia trachomatis, Mycoplasma genitalium, Neisseria gonorrhoeae and Trichomonas vaginalis. Participants self-collected the urine sample in a sterile jar after receiving instructions from the clinician. All pharyngeal swabs were collected by a clinician. A majority (approximately three-quarters) of the anorectal swabs were collected by a clinician, whereas one-quarter were self-collected by participants after receiving instructions from the clinician. Pharyngeal swabs were collected by swabbing the tonsils and the posterior pharynx using flocked swabs. Anorectal swabs were collected by inserting a flocked swab 3 cm into the anus and gently rotating for 5–10 s. Each swab was placed in a separate tube containing universal transport medium. All samples were transported to the laboratory within 24 h. Urine, pharyngeal and anorectal samples from each individual were pooled within 24 h. As per literature,21,37 the tubes containing the pharyngeal and anorectal swabs were robustly shaken for 15 s, the swabs pressed firmly on the sides of the tube to get rid of fluid, and then discarded. Subsequently, 1 mL of fluid from each of these tubes was mixed with 1 mL of urine in a sterile tube to make a volume of 3 mL. Samples were stored at 2–8°C as per the NAAT manufacturer’s instructions and tested within 72 h from collection. Deoxyribonucleic acid (DNA) extraction was done using the QIAamp® kit (Qiagen®, Germany) and DNA detection was done using a multiplex real-time polymerase chain reaction (PCR) test kit for Chlamydia trachomatis, Mycoplasma genitalium, Neisseria gonorrhoeae and Trichomonas vaginalis (Sacace Biotechnologies®, Italy) on a Rotor-Gene Q (Qiagen®, Germany) thermocycler.
Ethics approval
This study was approved by the University of the Witwatersrand Human Research Ethics Committee-Medical (Ref. No. M200215) and University of Nairobi-Kenyatta National Hospital Ethics and Research Committee (Ref. No. P990/12/2019). All participants provided written informed consent.
Data analysis
Data analysis excluded six seeds who were purposely selected to begin the RDS recruitment. Weighted STI prevalence with 95% confidence intervals (CI) were calculated using RDS Analyst (RDS-A) software (ver. 0.72).38 Weighting was done using Gile’s successive sampler estimator, which takes to account the self-reported participant network size, recruitment patterns, and estimated size of the study population.39 We asked the following question to estimate a participant’s network size: ‘How many TSMSM who study and live in Nairobi, who know you by name, and you know them by name, have you spoken to in the last 4 weeks/1 month?’. Due to the COVID-19 restrictions in place just before the survey, we added the following clarification: ‘By speaking, we mean either talking face-to-face or communicating on the phone whether through calling, texting or voice notes’. As per the Joint United Nations Programme on HIV/AIDS recommendations,40 an estimated population size of 8406 for TSMSM (1.45% of male tertiary students in the Nairobi metropolis) was used, with 95% CI and 1000 bootstraps.
Stata (ver. 15; StataCorp LLC, College Station, TX, USA) was used for further analysis of the unweighted data. Some continuous variables such as age and self-reported number of sex partners were converted into binary categories and analysed as such. Categorical variables were summarised using proportions, and differences in proportions examined using the chi-squared (χ2) test. Multivariate logistic regression models were used to measure associations between various factors and STI prevalence. Testing positive for at least one of the five STIs was used as the primary outcome variable. All exposure variables with a P < 0.1 in bivariate analysis and without missing values were included in the multivariate model. Unweighted regression models were used because they have been shown to perform better than weighted regression models for RDS data.41 Both adjusted odds ratio (AOR) and 95% confidence interval (CI) were calculated for each exposure variable in the final model. All statistical tests were two-sided. Variables with a P-value <0.05 were considered statistically significant.
Results
Characteristics of TSMSM
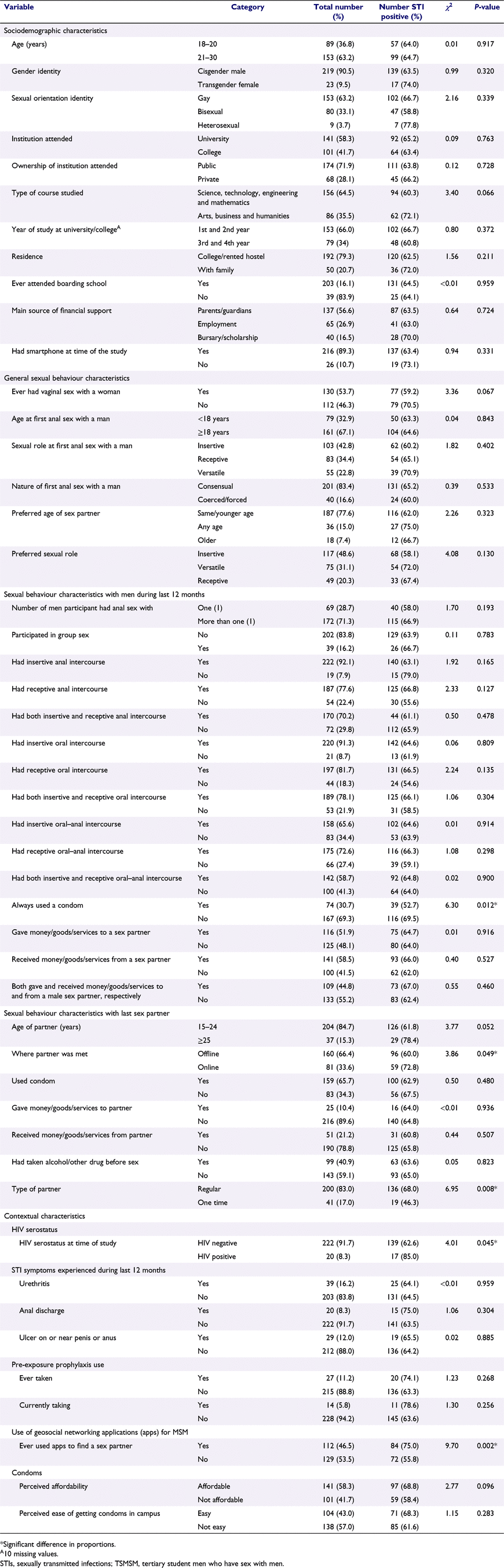
Six seeds recruited 242 participants in eight waves, resulting in a total of 248 TSMSM. The details of the recruitment networks are depicted in Fig. 1. The characteristics of participants in total and by STI status (proportion that tested positive for at least one of the five STIs) are shown in Table 1. Median age was 21 years (interquartile range, IQR, 20–22), with 96.3% of participants aged ≤24 years. More than half (58.3%) attended university, close to three-quarters (71.9%) were in public institutions, and four-fifths (79.3%) resided in hostels inside or outside their institutions, away from their families. A majority (89.3%) owned a smart phone at the time of the study, almost half (46.5%) had ever used a mobile phone geosocial networking application (app) for MSM to meet a sex partner, and one-third (33.6%) had met their last sex partner online. Sexual behaviours that may increase the risk of STIs at the genital, anorectal and oropharyngeal anatomic sites were also common among participants in the 12 months preceding the survey. Almost three-quarters (70.2%) had engaged in both receptive and insertive anal–penile intercourse, more than three-quarters (78.1%) had engaged in both receptive and insertive oral–penile intercourse and more than half (58.7%) had had engaged in both receptive and insertive oral–anal intercourse.
|
|
|
STIs prevalence, differences in proportions and associated factors
The unadjusted and RDS-adjusted prevalence of various STIs among study participants is shown in Table 2. In RDS-adjusted estimates, more than half (58.8%, 95% CI: 50.4–67.3%) of study participants tested positive for at least one of the five STIs, with chlamydia (51.0%, 95% CI: 42.3–59.8%) followed by gonorrhoea (11.3%, 95% CI: 6.1–16.5%), then Mycoplasma genitalium infection (6.0%, 95% CI: 2.6–9.4%) being the three most prevalent STIs. Co-infection with more than one STI was also observed with chlamydia/gonorrheoa (5.7%, 95% CI: 2.7–8.7%) being the most common type of co-infection, followed by chlamydia/Mycoplasma genitalium infection (4.5%, 95% CI: 2.3–6.7%), then gonorrhoea/Mycoplasma genitalium infection and chlamydia/gonorrhoea/Mycoplasma genitalium (both at 1.7%, 95% CI: 0.1–3.3%).

|
Table 1 also shows the results of bivariate analysis. These results revealed that STI prevalence (testing positive for at least one of the five STIs) was statistically significantly higher among participants who used condoms inconsistently when having sex with a man during the 12 months preceding the survey (χ2 = 6.30, P = 0.012), who met their last sex partner online (χ2 = 3.86, P = 0.049), whose last sex partner was a regular partner (χ2 = 6.95, P = 0.008), who tested positive for HIV at the time of the study (χ2 = 4.01, P = 0.045), and who had ever used a geosocial networking app for MSM to find a sex partner (χ2 = 9.70, P = 0.002).
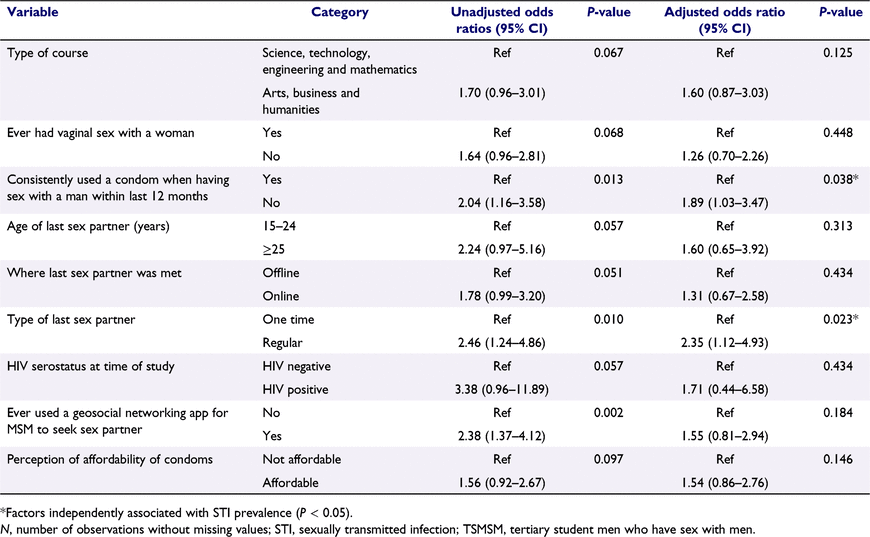
The results of multivariate logistic regression analysis are shown in Table 3. As shown by the AOR, independent factors associated with testing positive for at least one of the five STIs were: inconsistent condom use when having sex with a man within the 12 months preceding the survey (AOR 1.89, 95% CI: 1.03–3.47, P = 0.038) and the last sex partner being a regular partner (AOR = 2.35, 95% CI: 1.12–4.92, P = 0.023).
|
Discussion
Our study aimed to estimate the prevalence of five curable STIs and associated risk factors among TSMSM in Nairobi, Kenya. We found that more than half (58.8%) of study participants had at least one of the five STIs, with the descending order of prevalence being chlamydia, gonorrhoea, Mycoplasma genitalium infection, trichomoniasis and syphilis. In terms of co-infection, chlamydia/gonorrhoea was the most common followed by chlamydia/Mycoplasma genitalium infection, and gonorrhoea/Mycoplasma genitalium infection at the same rate as chlamydia/gonorrhoea/Mycoplasma genitalium infection. Co-infection with at least one of the five STIs and HIV was at the rate of 2.9% (95% CI: 0.8–4.9%).
The prevalence of chlamydia was almost double that observed among MSM in Coastal Kenya – 26%,3 and more than double that observed among YMSM in Vietnam – 22%.42 We found a prevalence of gonorrhoea (11%) less than half that observed among MSM in Coastal Kenya – 26%3 and similar to that seen among YMSM in Vietnam – 12%.42 Overall, the higher prevalence of chlamydia compared to gonorrhea in our study is consistent with evidence from systematic reviews that has shown that despite chlamydia and gonorrhoea being the most prevalent STIs among MSM, chlamydia is more prevalent than gonorrhoea,43 possibly due to chlamydia’s longer duration of infection and higher transmissibility as compared to gonorrhoea.44 For Mycoplasma genitalium infection and trichomoniasis, the observed prevalence was a sixth of that seen among MSM in Nigeria – 37%6 and in South Africa – 9%,7 respectively. The observed prevalence of latent syphilis was seven-fold less than that found among TSMSM in China – 4.7%, where evidence suggests that the syphilis epidemic among TSMSM has been growing, and is fuelled by a high prevalence of risky sexual behaviours.26
The overall prevalence of STIs among TSMSM in our study was higher than that in the general population in Kenya, as shown by a study that found the prevalence of chlamydia, Mycoplasma genitalium infection, gonorrhoea, trichomoniasis and at least one of the four aforementioned STIs was 16.8%, 28.6%, 7.1%, 7.1% and 49.5%, respectively.45 Therefore, while not neglecting the general population, there is need to focus more attention on the prevention and control of STIs among key populations such as MSM, including TSMSM. This approach may further foster benefits for the prevention and control of STIs in the general population, given that MSM who also have sex with women (36.8% in our study) form a bridge population that may transmit STIs to women, and these women in turn transmit to men in the general population, thus amplifying the STI epidemics.
In the present study, inconsistent condom use and having a regular sex partner were independently associated with STI prevalence. The inconsistent condom use observed in our study could have been due to problems with affordability and accessibility of condoms, with 41.7% and 57.0% of study participants finding condoms ‘not affordable’ and ‘not easy’ to access in campus, respectively. Inconsistent condom use has also been seen among TSMSM in China and YMSM in Sweden and New Zealand, and is attributed to having a regular sex partner and believing that condoms reduce sexual pleasure.46–48 Another study of YMSM in Hong Kong revealed that despite knowledge of the risk of condomless anal sex (CAS), socio-cultural norms and expectations relating to sexual positioning (insertive partner/top or receptive partner/bottom) contribute to inconsistent condom use, with CAS and condomless internal ejaculation considered a demonstration of intimacy and commitment.49 Interestingly, in our study, the last sex partner being a regular partner was a risk factor for STIs, and this could be linked to reduced condom use with regular partners, as revealed in the foregoing studies.46–48
This study has several implications for future research and development of interventions in the response to STIs among YMSM, including TSMSM. Since condoms remain the leading STI prevention tool, public health practitioners should ensure affordability, availability and accessibility of condoms for YMSM, including for those in tertiary learning institutions. Additionally, practitioners should aim to provide more compelling condom awareness, education and training so as to promote condom use among YMSM. Most importantly, to ensure condom use and effectiveness, interventions need to speak to perceptions of pleasure, relationships and sexual positioning issues that have been shown to have a negative impact on consistent condom use, despite YMSM being aware of the risk of CAS. In the case of Kenya, further research is needed to ascertain whether the studded/dotted condoms routinely distributed by the government and popularised under the tag ‘prevention with pleasure’,50 appeal to and meet the needs of YMSM, including TSMSM. Behaviours (such as CAS) that put MSM at risk of infection with STIs also put them at risk of HIV infection. Indeed, a 2019 modelling study among MSM in the USA showed that 10.4% of incident HIV infections were attributable to prevalent chlamydia and gonorrhoea infections.51 Given the observed high prevalence of STIs in our sample, it is important to reinforce the need for interventions such as pre-exposure prophylaxis (PrEP) to prevent new HIV infections among TSMSM. From our study sample, only a paltry 11.2% and 5.8% had ever taken and were currently taking PrEP, respectively. Further research is required to understand the low use of PrEP in this population, despite PrEP being freely available in Kenya. However, we note that although the use of PrEP decreases the risk of HIV infection, there is evidence to suggest that it may also lead to a high incidence of STIs among MSM, as a result of additional risk compensation – reduced sexual inhibition leading to increased risky sexual behaviour such as condomless sex.52 Accordingly, because PrEP does not prevent other STIs, we thus recommend its use as part of a comprehensive sexual health package, which should also include STI screening and treatment, condom use promotion and risk-reduction counselling.
Our findings revealed that 89.3% of our study participants owned a smart phone at the time of the survey. This finding is consistent with previous work that demonstrated that 95% of Kenyan tertiary students own a smart phone, and are regular and savvy users of the internet.53 In addition, almost half (46.5%) of our study participants had used a geosocial networking mobile phone application for MSM (such as Grindr) to find a sex partner, and one-third (33.6%) had met their last sex partner online. As such, online platforms could potentially be used for reaching TSMSM with interventions that promote and provide STI testing, treatment and prevention services. A systematic review conducted in 2017 showed that online interventions have the potential to address STIs among YMSM,54 and such interventions have also been recommended by others following a study that found that most MSM in Kenya and South Africa regularly socialise online.55 Online interventions are particularly recommended due to their private and discrete nature, which may enable TSMSM to bypass some of the barriers they encounter while seeking services in physical health facilities, especially the experiences of stigma and discrimination by healthcare providers.28,56 Indeed, a consortium of universities within the Nairobi metropolis has developed, and deployed for use is a mobile phone app called RADA (slang for ‘be alert’).57 Among other things, the app provides information on sexual and reproductive health matters to tertiary students, but its design and content is largely heteronormative. Considerations should therefore be made for the development of an app that suits and meets the needs of TSMSM. This could be achieved through collaboration with, and support from various stakeholders, including: researchers, policymakers, practitioners, funders, and TSMSM as the eventual app users.
To our knowledge, our study is the first one to investigate the prevalence and associated risk factors of five curable STIs among TSMSM in Kenya, and possibly in SSA countries, using the RDS method. Besides the traditional STIs (chlamydia, gonorrhoea and syphilis), we also investigated two emerging STIs (Mycoplasma genitalium infection and trichomoniasis) that are also prevalent among MSM. Pooling of samples from the three anatomical sites offered us a cost-effective way to detect extra-genital agents of STIs that would otherwise be undetected due to their usually asymptomatic nature.8 Nevertheless, even with the pooling of samples, detection of agents of STIs using NAATs is still costly and not routinely available in resource-limited settings such as Kenya. In the absence of NAATs, treatment of STIs in SSA countries is likely to continue being syndromic. For MSM, quarterly screening that includes physical examination for genital, anorectal and oropharyngeal STIs, and assessment of risky sexual behaviours, can complement the syndromic management of STIs. To further support early diagnosis and hence appropriate treatment of STIs, we suggest that policymakers and providers consider the use of accurate, affordable and timely point-of-care tests for STIs among MSM.58
Our findings should be viewed in light of some limitations. RDS is a peer-referral, probability-based sampling method that is susceptible to underestimation or overestimation of study outcomes. To offset this limitation, when calculating our sample size, we applied a design effect of three to account for the clustering that occurs due to homophily and minimise the traditional bias associated with snowball sampling, and used RDS-adjusted analysis to estimate the prevalence of various STIs. To minimise the effect of seed selection bias, we excluded from analysis the six seeds who were purposely selected to start off recruitment. Because we asked about past life events and experiences, there was a possibility of recall bias. To mitigate this, most of our questions focused on events and experiences that occurred within 12 months before the study, with only a few questions focusing on periods longer than 12 months. In addition, we asked questions about sexual behaviour, which may be affected by social desirability bias. To minimise this, participants self-administered the behavioural survey questionnaire on a tablet computer, as this has been shown to be more effective in offsetting this kind of bias, compared to face-to-face interviews.59 Approximately three-quarters of anorectal samples were collected by the clinician and the other quarter by participants after they received instructions from the clinician. This could potentially lead to variability in the test results of the various STIs. However, evidence suggests that when participants are given and are able to follow clear instructions on self-collection of anorectal samples, clinician-collected and self-collected samples yield concordant results.60 The cross-sectional nature of the study limits inferences about the direction of causality though the factors we identified to be associated with STIs prevalence, and are plausibly causal based on findings from previous studies.46–48 Finally, although we used a comprehensive set of variables in the logistic regression analysis, we cannot rule out the possibility of residual confounding from other variables that were not assessed, such as experiences of stigma, discrimination and violence. Despite these limitations, this survey offers valuable lessons on the burden of five curable STIs and associated risk factors among TSMSM, and hopefully will be useful in informing further research, and development of interventions for the prevention and control of STIs in this key but understudied population, both in Kenya and other similar settings.
Conclusion
We found a high prevalence of STIs among TSMSM that was associated with inconsistent condom use and regular sex partners. Accordingly, tailored interventions for testing, treatment and prevention of STIs are urgently called for in this population. Particularly, accurate, affordable and timely point-of-care tests for agents of STIs are required to optimise treatment and control. Given the high prevalence of STIs observed and their plausible association with incident HIV infection, there is need to implement modern HIV prevention interventions such as PrEP in this population. Further, interventions that increase condom use efficacy and offer risk-reduction counselling are requisite, with online platforms being a potential avenue for the delivery of such interventions.
Data availability
The data that support this study cannot be publicly shared due to ethical or privacy reasons, but may be shared upon reasonable request to the corresponding author, if appropriate.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
SWM was supported by the Consortium for Advanced Research Training in Africa (CARTA). CARTA is jointly led by the African Population and Health Research Center and the University of the Witwatersrand, and funded by the Carnegie Corporation of New York (Grant No. G-19-57145), SIDA (Grant No. 54100113), Uppsala Monitoring Center, Norwegian Agency for Development Cooperation (NORAD), and by the Wellcome Trust (reference no. 107768/Z/15/Z) and the UK Foreign, Commonwealth & Development Office, with support from the Developing Excellence in Leadership, Training and Science in Africa (DELTAS Africa) program. The statements made and views expressed are solely the responsibility of the Fellow.
Acknowledgements
The authors would like to thank the Partners for Health and Development in Africa (PHDA) team for hosting and supporting data collection at the TRANSFORM study site in Nairobi, Kenya. We are equally grateful to Babatunde Adedokun of the Department of Medicine and Center for Global Health, University of Chicago, Chicago, USA, for his guidance throughout the data analysis process. Lastly, we thank all the study participants without whom the study would not have been possible.
References
[1] World Health Organization (WHO). Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Geneva, Switzerland: WHO; 2021. Available at https://www.who.int/publications/i/item/9789240027077[2] Ross MW, Nyoni J, Ahaneku HO, Mbwambo J, McClelland RS, McCurdy SA. High HIV seroprevalence, rectal STIs and risky sexual behaviour in men who have sex with men in Dar es Salaam and Tanga, Tanzania. BMJ Open 2014; 4 e006175
| High HIV seroprevalence, rectal STIs and risky sexual behaviour in men who have sex with men in Dar es Salaam and Tanga, Tanzania.Crossref | GoogleScholarGoogle Scholar |
[3] Sanders EJ, Thiong’o AN, Okuku HS, Mwambi J, Priddy F, Shafi J, et al. High prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infections among HIV-1 negative men who have sex with men in coastal Kenya. Sex Transm Infect 2010; 86 440–1.
| High prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infections among HIV-1 negative men who have sex with men in coastal Kenya.Crossref | GoogleScholarGoogle Scholar |
[4] Wirtz AL, Trapence G, Kamba D, Gama V, Chalera R, Jumbe V, et al. Geographical disparities in HIV prevalence and care among men who have sex with men in Malawi: results from a multisite cross-sectional survey. Lancet HIV 2017; 4 e260–9.
| Geographical disparities in HIV prevalence and care among men who have sex with men in Malawi: results from a multisite cross-sectional survey.Crossref | GoogleScholarGoogle Scholar |
[5] Kim EJ, Hladik W, Barker J, Lubwama G, Sendagala S, Ssenkusu JM, et al. Sexually transmitted infections associated with alcohol use and HIV infection among men who have sex with men in Kampala, Uganda. Sex Transm Infect 2016; 92 240–5.
| Sexually transmitted infections associated with alcohol use and HIV infection among men who have sex with men in Kampala, Uganda.Crossref | GoogleScholarGoogle Scholar |
[6] Crowell TA, Lawlor J, Lombardi K, Nowak RG, Hardick J, Odeyemi S, et al. Anorectal and urogenital Mycoplasma genitalium in Nigerian men who have sex with men and transgender women: prevalence, incidence, and association with HIV. Sex Transm Dis 2020; 47 202–6.
| Anorectal and urogenital Mycoplasma genitalium in Nigerian men who have sex with men and transgender women: prevalence, incidence, and association with HIV.Crossref | GoogleScholarGoogle Scholar |
[7] Hoffman CM, Fritz L, Radebe O, Dubbink JH, McIntyre JA, Kock MM, et al. Rectal Trichomonas vaginalis infection in South African men who have sex with men. Int J STD AIDS 2018; 29 1444–7.
| Rectal Trichomonas vaginalis infection in South African men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[8] Dudareva-Vizule S, Haar K, Sailer A, Wisplinghoff H, Wisplinghoff F, Marcus U, et al. Prevalence of pharyngeal and rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among men who have sex with men in Germany. Sex Transm Infect 2014; 90 46–51.
| Prevalence of pharyngeal and rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among men who have sex with men in Germany.Crossref | GoogleScholarGoogle Scholar |
[9] World Health Organization. Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people. Geneva, Switzerland: World Health Organization; 2011.
[10] National AIDS and STI Control Programme (NASCOP). Kenya National Guidelines for prevention, management and control of sexually transmitted infections. Nairobi, Kenya: National AIDS and STI Control Programme (NASCOP); 2018.
[11] Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999; 75 3–17.
| From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection.Crossref | GoogleScholarGoogle Scholar |
[12] Aral SO. Sexually transmitted diseases: magnitude, determinants and consequences. Int J STD AIDS 2001; 12 211–5.
| Sexually transmitted diseases: magnitude, determinants and consequences.Crossref | GoogleScholarGoogle Scholar |
[13] Beck EC, Birkett M, Armbruster B, Mustanski B. A data-driven simulation of HIV spread among young men who have sex with men: role of age and race mixing and STIs. JAIDS J Acquir Immune Defic Syndr 2015; 70 186–94.
| A data-driven simulation of HIV spread among young men who have sex with men: role of age and race mixing and STIs.Crossref | GoogleScholarGoogle Scholar |
[14] Lewis DA. Will targeting oropharyngeal gonorrhoea delay the further emergence of drug-resistant Neisseria gonorrhoeae strains? Sex Transm Infect 2015; 91 234–7.
| Will targeting oropharyngeal gonorrhoea delay the further emergence of drug-resistant Neisseria gonorrhoeae strains?Crossref | GoogleScholarGoogle Scholar |
[15] Van Der Pol B, Chernesky M, Gaydos CA, Hook EW, Joseph AM, Christensen K, et al. Multicenter comparison of nucleic acid amplification tests for the diagnosis of rectal and oropharyngeal Chlamydia trachomatis and Neisseria gonorrhoeae infections. J Clin Microbiol 2022; 60 e01363-21
| Multicenter comparison of nucleic acid amplification tests for the diagnosis of rectal and oropharyngeal Chlamydia trachomatis and Neisseria gonorrhoeae infections.Crossref | GoogleScholarGoogle Scholar |
[16] Aboud L, Xu Y, Chow EPF, Wi T, Baggaley R, Mello MB, et al. Diagnostic accuracy of pooling urine, anorectal, and oropharyngeal specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae: a systematic review and meta-analysis. BMC Med 2021; 19 285
| Diagnostic accuracy of pooling urine, anorectal, and oropharyngeal specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[17] Sultan B, White JA, Fish R, Carrick G, Brima N, Copas A, et al. The “3 in 1” study: pooling self-taken pharyngeal, urethral, and rectal samples into a single sample for analysis for detection of Neisseria gonorrhoeae and Chlamydia trachomatis in men who have sex with men. J Clin Microbiol 2016; 54 650–6.
| The “3 in 1” study: pooling self-taken pharyngeal, urethral, and rectal samples into a single sample for analysis for detection of Neisseria gonorrhoeae and Chlamydia trachomatis in men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[18] Speers DJ, Chua I-LJ, Manuel J, Marshall L. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis from pooled rectal, pharyngeal and urine specimens in men who have sex with men. Sex Transm Infect 2018; 94 293–7.
| Detection of Neisseria gonorrhoeae and Chlamydia trachomatis from pooled rectal, pharyngeal and urine specimens in men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[19] Thielemans E, Wyndham-Thomas C, Henrard S, De Vleeschouwer A, Steensels D, Montesinos I, et al. Screening for Chlamydia trachomatis and Neisseria gonorrhoeae infections in men who have sex with men: diagnostic accuracy of nucleic acid amplification test on pooled urine, anorectal, and pharyngeal specimens. Sex Transm Dis 2018; 45 195–8.
| Screening for Chlamydia trachomatis and Neisseria gonorrhoeae infections in men who have sex with men: diagnostic accuracy of nucleic acid amplification test on pooled urine, anorectal, and pharyngeal specimens.Crossref | GoogleScholarGoogle Scholar |
[20] De Baetselier I, Osbak KK, Smet H, Kenyon CR, Crucitti T. Take three, test one: a cross-sectional study to evaluate the molecular detection of Chlamydia trachomatis and Neisseria gonorrhoeae in pooled pharyngeal, anorectal and urine samples versus single-site testing among men who have sex with men in Belgium. Acta Clin Belg 2020; 75 91–5.
| Take three, test one: a cross-sectional study to evaluate the molecular detection of Chlamydia trachomatis and Neisseria gonorrhoeae in pooled pharyngeal, anorectal and urine samples versus single-site testing among men who have sex with men in Belgium.Crossref | GoogleScholarGoogle Scholar |
[21] Durukan D, Read TRH, Bradshaw CS, Fairley CK, Williamson DA, De Petra V, et al. Pooling pharyngeal, anorectal, and urogenital samples for screening asymptomatic men who have sex with men for Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol 2020; 58 e01969-19
| Pooling pharyngeal, anorectal, and urogenital samples for screening asymptomatic men who have sex with men for Chlamydia trachomatis and Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar |
[22] Almeria J, Pham J, Paris KS, Heskett KM, Romyco I, Bristow CC. Pooled 3-anatomic-site testing for Chlamydia trachomatis and Neisseria gonorrhoeae: a systematic review and meta-analysis. Sex Transm Dis 2021; 48 e215–22.
| Pooled 3-anatomic-site testing for Chlamydia trachomatis and Neisseria gonorrhoeae: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[23] Arnett JJ. Emerging adulthood: a theory of development from the late teens through the twenties. Am Psychol 2000; 55 469–80.
| Emerging adulthood: a theory of development from the late teens through the twenties.Crossref | GoogleScholarGoogle Scholar |
[24] Fromme K, Corbin WR, Kruse MI. Behavioral risks during the transition from high school to college. Dev Psychol 2008; 44 1497–504.
| Behavioral risks during the transition from high school to college.Crossref | GoogleScholarGoogle Scholar |
[25] Renn K. LGBTQ students on campus: issues and opportunities for higher education leaders. Higher Education Today. 2017. Available at https://www.higheredtoday.org/2017/04/10/lgbtq-students-higher-education/ [Cited 10 June 2021].
[26] Fan S, Yang Z, Hou F, Yu M, Luo Z, Liao M, et al. HIV and syphilis and sexual risk behaviours among men who have sex with men attending university in China: a systematic review and meta-analysis. Sex Health 2019; 16 554–65.
| HIV and syphilis and sexual risk behaviours among men who have sex with men attending university in China: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[27] Brink JG. Considerations for South African higher education: a ‘national student men who have sex with men’ sexual behavior survey. S Afr J High Educ 2017; 31 184–207.
| Considerations for South African higher education: a ‘national student men who have sex with men’ sexual behavior survey.Crossref | GoogleScholarGoogle Scholar |
[28] Mwaniki SW, Kaberia PM, Mugo PM, Palanee-Phillips T. “What if I get sick, where shall I go?”: a qualitative investigation of healthcare engagement among young gay, bisexual and other men who have sex with men in Nairobi, Kenya. 2022;
| “What if I get sick, where shall I go?”: a qualitative investigation of healthcare engagement among young gay, bisexual and other men who have sex with men in Nairobi, Kenya.Crossref | GoogleScholarGoogle Scholar |
[29] Mwaniki SW, Mugo PM, Palanee-Phillips T. Project BESPOKE (integrated bio-behavioral assessment of HIV and STI among young tertiary student men who have sex with men in Nairobi, Kenya): a respondent-driven sampling survey protocol. Front Public Health 2021; 9 619694
| Project BESPOKE (integrated bio-behavioral assessment of HIV and STI among young tertiary student men who have sex with men in Nairobi, Kenya): a respondent-driven sampling survey protocol.Crossref | GoogleScholarGoogle Scholar |
[30] Study in Kenya. Universities and colleges in Nairobi County. 2021. Available at https://studyinkenya.co.ke/universities-and-colleges-in-nairobi-county?page=1 [Cited 24 June 2022].
[31] WHO, CDC, UNAIDS FHI 360. Biobehavioural survey guidelines for populations at risk for HIV. Geneva: World Health Organization; 2017.
[32] Cochran WG. Sampling techniques. 3rd edn. New York: John Wiley & Sons, Ltd; 1977.
[33] Higher Education HIV/AIDS Programme South Africa (HEAIDS). HIV prevalence and related factors: higher education sector study South Africa. Johannesburg, South Africa: HEAIDS; 2009.
[34] Mwaniki SW, Kaberia PM, Mugo PM, Palanee-Phillips T. HIV prevalence and associated risk factors among young tertiary student men who have sex with men (MSM) in Nairobi, Kenya: a respondent-driven sampling survey. 2022;
| HIV prevalence and associated risk factors among young tertiary student men who have sex with men (MSM) in Nairobi, Kenya: a respondent-driven sampling survey.Crossref | GoogleScholarGoogle Scholar |
[35] Mwaniki SW, Kaberia PM, Mugo PM, Palanee-Phillips T. “My friends would believe my word”: appropriateness and acceptability of respondent-driven sampling in recruiting young tertiary student men who have sex with men for HIV/STI research in Nairobi, Kenya. Int J Environ Res Public Health 2022; 19 7331
| “My friends would believe my word”: appropriateness and acceptability of respondent-driven sampling in recruiting young tertiary student men who have sex with men for HIV/STI research in Nairobi, Kenya.Crossref | GoogleScholarGoogle Scholar |
[36] University of California San Francisco (UCSF). Integrated HIV bio-behavioural surveillance toolbox. Available at https://globalhealthsciences.ucsf.edu/resources/integrated-hiv-bio-behavioral-surveillance-toolbox [Cited 24 September 2021].
[37] Dean JA, Bell SFE, Coffey L, Debattista J, Badman S, Redmond AM, et al. Improved sensitivity from pooled urine, pharyngeal and rectal specimens when using a molecular assay for the detection of chlamydia and gonorrhoea near point of care. Sex Transm Infect 2021; 97 471–2.
| Improved sensitivity from pooled urine, pharyngeal and rectal specimens when using a molecular assay for the detection of chlamydia and gonorrhoea near point of care.Crossref | GoogleScholarGoogle Scholar |
[38] Handcock M, Fellows I, Gile K. RDS analyst: software for the analysis of respondent-driven sampling data, version 0.72. Available at http://wiki.stat.ucla.edu/hpmrg/index.php/RDS_Analyst_Install [Cited 7 October 2021].
[39] Gile KJ. Improved inference for respondent-driven sampling data with application to HIV prevalence estimation. J Am Stat Assoc 2011; 106 135–46.
| Improved inference for respondent-driven sampling data with application to HIV prevalence estimation.Crossref | GoogleScholarGoogle Scholar |
[40] UNAIDS (Joint United Nations Programme on HIV/AIDS). Recommended population size estimates of men who have sex with men. 2020. Available at https://www.unaids.org/sites/default/files/media_asset/2020-recommended-population-size-estimates-of-men-who-have-sex-with-men_en.pdf [Cited 7 October 2021].
[41] Avery L, Rotondi N, McKnight C, Firestone M, Smylie J, Rotondi M. Unweighted regression models perform better than weighted regression techniques for respondent-driven sampling data: results from a simulation study. BMC Med Res Methodol 2019; 19 202
| Unweighted regression models perform better than weighted regression techniques for respondent-driven sampling data: results from a simulation study.Crossref | GoogleScholarGoogle Scholar |
[42] Adamson PC, Bhatia R, Tran KDC, Bui HTM, Vu D, Shiraishi RW, et al. Prevalence, anatomic distribution, and correlates of Chlamydia trachomatis and Neisseria gonorrhoeae infections among a cohort of men who have sex with men in Hanoi, Vietnam. Sex Transm Dis 2022; 49 504–10.
| Prevalence, anatomic distribution, and correlates of Chlamydia trachomatis and Neisseria gonorrhoeae infections among a cohort of men who have sex with men in Hanoi, Vietnam.Crossref | GoogleScholarGoogle Scholar |
[43] Dewart CM, Bernstein KT, DeGroote NP, Romaguera R, Turner AN. Prevalence of rectal chlamydial and gonococcal infections: a systematic review. Sex Transm Dis 2018; 45 287–93.
| Prevalence of rectal chlamydial and gonococcal infections: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[44] Chow EPF, Camilleri S, Ward C, Huffam S, Chen MY, Bradshaw CS, et al. Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: a systematic review. Sex Health 2016; 13 199–204.
| Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[45] Maina AN, Mureithi MW, Ndemi JK, Revathi G. Diagnostic accuracy of the syndromic management of four STIs among individuals seeking treatment at a health centre in Nairobi, Kenya: a cross-sectional study. Pan Afr Med J 2021; 40 138
| Diagnostic accuracy of the syndromic management of four STIs among individuals seeking treatment at a health centre in Nairobi, Kenya: a cross-sectional study.Crossref | GoogleScholarGoogle Scholar |
[46] Wang H, Yu S, Cross W, Lam L, Banik B, Zhang K. Condom use consistency and associated factors among college student men who have sex with men from seven colleges in Changsha city: a cross-sectional survey. HIV/AIDS - Res Palliat Care 2021; 13 557–69.
| Condom use consistency and associated factors among college student men who have sex with men from seven colleges in Changsha city: a cross-sectional survey.Crossref | GoogleScholarGoogle Scholar |
[47] Johansson K, Persson KI, Deogan C, El-Khatib Z. Factors associated with condom use and HIV testing among young men who have sex with men: a cross-sectional survey in a random online sample in Sweden. Sex Transm Infect 2018; 94 427–33.
| Factors associated with condom use and HIV testing among young men who have sex with men: a cross-sectional survey in a random online sample in Sweden.Crossref | GoogleScholarGoogle Scholar |
[48] Lachowsky NJ, Saxton PJW, Hughes AJ, Dickson NP, Summerlee AJS, Milhausen RR, et al. Younger gay and bisexual men’s condom use with main regular sexual partner in New Zealand. AIDS Educ Prev 2015; 27 257–74.
| Younger gay and bisexual men’s condom use with main regular sexual partner in New Zealand.Crossref | GoogleScholarGoogle Scholar |
[49] Yeo TED, Fung TH. Between ‘0’ and ‘1’: safer sex and condom use among young gay men in Hong Kong. Cult Health Sex 2016; 18 294–307.
| Between ‘0’ and ‘1’: safer sex and condom use among young gay men in Hong Kong.Crossref | GoogleScholarGoogle Scholar |
[50] Kilonzo E. Kenya set for free studded condoms ahead of Valentine’s Day. Nation Africa. 2017. Available at https://nation.africa/kenya/news/Kenya-free-studded-condoms-Valentine-s-Day-HII-Aids/1056-3810724-la49guz/index.html
[51] Jones J, Weiss K, Mermin J, Dietz P, Rosenberg ES, Gift TL, et al. Proportion of incident human immunodeficiency virus cases among men who have sex with men attributable to gonorrhea and chlamydia: a modeling analysis. Sex Transm Dis 2019; 46 357–63.
| Proportion of incident human immunodeficiency virus cases among men who have sex with men attributable to gonorrhea and chlamydia: a modeling analysis.Crossref | GoogleScholarGoogle Scholar |
[52] Scott HM, Klausner JD. Sexually transmitted infections and pre-exposure prophylaxis: challenges and opportunities among men who have sex with men in the US. AIDS Res Ther 2016; 13 5
| Sexually transmitted infections and pre-exposure prophylaxis: challenges and opportunities among men who have sex with men in the US.Crossref | GoogleScholarGoogle Scholar |
[53] Ojino R, Mich L. Mobile applications in university education: the case of Kenya. J E-Learn Knowl Soc 2018; 14 111–25.
| Mobile applications in university education: the case of Kenya.Crossref | GoogleScholarGoogle Scholar |
[54] Knight R, Karamouzian M, Salway T, Gilbert M, Shoveller J. Online interventions to address HIV and other sexually transmitted and blood-borne infections among young gay, bisexual and other men who have sex with men: a systematic review. J Int AIDS Soc 2017; 20 e25017
| Online interventions to address HIV and other sexually transmitted and blood-borne infections among young gay, bisexual and other men who have sex with men: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[55] Fearon E, Bourne A, Tenza S, Palanee-Phillips T, Kabuti R, Weatherburn P, et al. Online socializing among men who have sex with men and transgender people in Nairobi and Johannesburg and implications for public health-related research and health promotion: an analysis of qualitative and respondent-driven sampling survey data. J Int AIDS Soc 2020; 23 e25603
| Online socializing among men who have sex with men and transgender people in Nairobi and Johannesburg and implications for public health-related research and health promotion: an analysis of qualitative and respondent-driven sampling survey data.Crossref | GoogleScholarGoogle Scholar |
[56] Bowen D, Jabson J, Kamen C. mHealth: an avenue for promoting health among sexual and gender minority populations? mHealth 2016; 2 36
| mHealth: an avenue for promoting health among sexual and gender minority populations?Crossref | GoogleScholarGoogle Scholar |
[57] Google Play. Rada University of Nairobi. Available at https://play.google.com/store/apps/details?id=com.radauon.radauon2&hl=en&gl=US [Cited 11 May 2022].
[58] Unemo M. Accurate, rapid, point-of-care tests for sexually transmitted infections. Lancet Infect Dis 2021; 21 584–6.
| Accurate, rapid, point-of-care tests for sexually transmitted infections.Crossref | GoogleScholarGoogle Scholar |
[59] Adebajo S, Obianwu O, Eluwa G, Vu L, Oginni A, Tun W, et al. Comparison of audio computer assisted self-interview and face-to-face interview methods in eliciting HIV-related risks among men who have sex with men and men who inject drugs in Nigeria. PLoS ONE 2014; 9 e81981
| Comparison of audio computer assisted self-interview and face-to-face interview methods in eliciting HIV-related risks among men who have sex with men and men who inject drugs in Nigeria.Crossref | GoogleScholarGoogle Scholar |
[60] van der Helm JJ, Hoebe CJPA, van Rooijen MS, Brouwers EEHG, Fennema HSA, Thiesbrummel HFJ, et al. High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women. Sex Transm Dis 2009; 36 493–7.
| High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women.Crossref | GoogleScholarGoogle Scholar |
† A version of this manuscript was submitted and posted as a pre-print on the Research Square platform, and is available from the link below: https://www.researchsquare.com/article/rs-1828548/v1.


