Overcoming barriers to HIV pre-exposure prophylaxis (PrEP) coverage in Australia among Medicare-ineligible people at risk of HIV: results from the MI-EPIC clinical trial
Curtis Chan A # * , Doug Fraser
A # * , Doug Fraser  A # , Stefanie Vaccher
A # , Stefanie Vaccher  A , Barbara Yeung A , Fengyi Jin
A , Barbara Yeung A , Fengyi Jin  A , Janaki Amin
A , Janaki Amin  A B , Nila J. Dharan
A B , Nila J. Dharan  A , Andrew Carr
A , Andrew Carr  C , Catriona Ooi
C , Catriona Ooi  D , Matthew Vaughan E , Jo Holden F , Cherie Power F , Andrew E. Grulich
D , Matthew Vaughan E , Jo Holden F , Cherie Power F , Andrew E. Grulich  A , Benjamin R. Bavinton
A , Benjamin R. Bavinton  A and for the MI-EPIC Research Group
A and for the MI-EPIC Research Group
A The Kirby Institute, UNSW Sydney, Sydney, NSW, Australia.
B Department of Health Systems and Populations, Macquarie University, North Ryde, NSW, Australia.
C St Vincent’s Hospital, Darlinghurst, Sydney, NSW, Australia.
D Clinic 16, Sydney, NSW, Australia.
E ACON, Sydney, NSW, Australia.
F NSW Ministry of Health, Sydney, NSW, Australia.
Handling Editor: Nittaya Phanuphak
Sexual Health 18(6) 453-459 https://doi.org/10.1071/SH21096
Submitted: 5 May 2021 Accepted: 26 July 2021 Published: 13 December 2021
© 2021 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Background: Overseas-born people who are ineligible for government-subsidised health care experience barriers to accessing HIV pre-exposure prophylaxis (PrEP) in Australia. This study aimed to assess a program providing free PrEP to overseas-born adults at risk of acquiring HIV.
Methods: Medicare-Ineligible Expanded Implementation in Communities (MI-EPIC) was a single-arm, open-label trial of daily tenofovir disoproxil fumarate/emtricitabine as PrEP. Six clinics recruited Medicare-ineligible adults who met HIV risk criteria in New South Wales, Australia. We recorded data on HIV and sexually transmitted infection (STI) diagnoses, and PrEP dispensing from July 2019 to June 2020. PrEP adherence as a medication possession ratio (MPR) was calculated as pills dispensed divided by days. We administered an optional survey on behaviours and attitudes to PrEP and sexual health.
Results: The 221 participants (206 men; 93.2%) had a median age of 29 years (IQR 26–34). Participants were mostly born in Asia (53.4%), Latin America or the Caribbean (25.3%), or Europe (10.9%). Adherence was high; 190 participants (86.0%) had an MPR of >60%. Of 121 survey participants, 42 (34.7%) completed the survey in a language other than English. Of participants who had not used PrEP in the 6 months before enrolment (n = 45, 37.2%), the most common reasons were cost (n = 22, 48.9%), and lack of knowledge about accessing PrEP (n = 20, 44.4%).
Conclusions: Medicare-ineligible people at risk of HIV demonstrate high adherence when given access to free PrEP and translated information. Increasing PrEP awareness and reducing barriers to accessing PrEP in this high-risk population should be priorities in HIV prevention.
Keywords: social determinants, gay men, HIV prevention, men who have sex with men, public health, pre-exposure prophylaxis, HIV/AIDS, migrants.
Introduction
HIV pre-exposure prophylaxis (PrEP) is highly effective at preventing HIV,1 and the scale-up of PrEP has become a priority in HIV prevention strategies globally.2 In Australia and internationally, HIV continues to disproportionately affect priority populations including gay, bisexual, and other men who have sex with men (GBM).2,3
In Australia, PrEP uptake has been high4 and has led to a substantial decrease in new HIV diagnoses.3,5 This decrease was driven by declines in HIV diagnoses among GBM born in Australia from 445 new HIV diagnoses in 2015 to 299 diagnoses in 2019.6 However, there was no decrease in national HIV diagnoses among GBM born overseas, from 296 (39.5% of all HIV diagnoses) in 2015 to 291 (48.7%) in 2019.6 Internationally, migrants face a higher HIV burden due to social, structural, and political barriers including language, social exclusion, and the fear that seeking HIV care or testing positive for HIV could adversely affect their visa status.7 Previous research has also found poor knowledge of how to navigate the healthcare system in a new country is a barrier for newly arrived residents.7,8
An important reason for the disparity in HIV trends between overseas-born and Australian-born GBM might be the inequity of access to the Australian universal healthcare system, Medicare. For people with access to Medicare, the cost to the patient for 30 pills of PrEP is a maximum of AUD41.30 for general customers and AUD6.60 for concession card holders (e.g. students, pensioners).9 The total cost of subsidised prescription medicines to individual patients is capped each year (AUD1497.20 and AUD316.80 for general and concession populations respectively) and, after reaching that cap, general patients pay AUD6.60 for 30 pills and concession card holders pay nothing. The unsubsidised cost of generic PrEP in Australia varies between pharmacies and over time; at the time of writing, PrEP was being sold for approximately AUD160 for 30 pills by national discount chemists.10,11 Medicare also subsidises general practice visit fees, and usually completely covers the cost of required laboratory testing for those eligible.
Overseas-born individuals who are not eligible for Medicare in Australia are systematically disadvantaged in accessing publicly subsidised PrEP.12 To access affordable PrEP, individuals without Medicare may rely on personal importation from international pharmacies, charitable compassionate access schemes, and publicly funded sexual health services. These alternative avenues still require clinic visits for sexual health screening and a prescription, which can incur additional costs except at a publicly funded sexual health clinic that provides free testing for at-risk populations without a Medicare card.13 Although publicly funded sexual health clinics may cover the cost of clinic visits and lab testing, they may not be readily accessible to all. Previous research in Australia has found that not having Medicare was associated with lower rates of PrEP use.14
Addressing structural barriers to PrEP use in overseas-born people at risk of HIV could lead to higher rates of use and HIV prevention coverage. We report results from the Medicare Ineligible Expanded Implementation in Communities (MI-EPIC) study, designed to provide free PrEP to Medicare-ineligible people in Australia at high risk of HIV infection. The MI-EPIC study used the existing clinical trial infrastructure of a previous PrEP implementation trial15 to provide a pathway for Medicare-ineligible people to access free PrEP within the Australian health care system. The aim of this study was to assess this PrEP access program for overseas-born adults at a high risk of acquiring HIV who were not otherwise eligible for government-subsidised PrEP.
Methods
Study design and participants
The MI-EPIC study was an open-label, single-arm trial of daily, oral, generic, co-formulated tenofovir disoproxil fumarate (300 mg) and emtricitabine (200 mg) as HIV PrEP, aimed at Medicare-ineligible adults. The protocol-defined procedures of the MI-EPIC study were identical to that of the large-scale PrEP trial, EPIC-NSW, which has been reported previously.15,16
Participants were able to enrol in the study if they were ineligible for Medicare (i.e. not an Australian citizen or permanent resident and unable to access Australia’s universal healthcare coverage), aged ≥18 years, resided in New South Wales (NSW) or visited regularly enough to attend clinic follow-up visits, were confirmed HIV negative by a fourth-generation HIV antibody–antigen test within 7 days of commencing PrEP, and had a high and ongoing risk of HIV infection through sexual exposure, as defined by the NSW PrEP prescribing guidelines.17 These criteria included any of the following in the past 3 months: receptive condomless anal intercourse with any casual male partner; at least one episode of rectal gonorrhoea, rectal chlamydia, or infectious syphilis; engaging in ‘chemsex’ (in Australia, this sexualised drug use usually involves the use of crystal methamphetamine); or at least one episode of condomless anal intercourse with an HIV-positive partner who was either not on treatment or who was on treatment but had a detectable HIV viral load.17 Previous EPIC-NSW participants were eligible to participate in the MI-EPIC study provided they were ineligible for Medicare. The MI-EPIC study was approved by the Human Research Ethics Committee of St Vincent’s Hospital (Sydney, NSW, Australia).
Procedures
The study was promoted online by community organisations through social and digital media. It was also promoted in sexual health clinic waiting rooms, and patients were approached directly by clinicians to participate. Study materials, including online promotions, posters, flyers, as well as the participant information sheet and consent form and survey were developed in seven community languages commonly spoken in Australia (Arabic, simplified Chinese, English, Portuguese, Spanish, Thai, and Vietnamese). The first versions of study materials were developed in English, and then translated into the other languages by certified translators. Translations were checked by community members prior to study rollout.
The procedures for screening, enrolment, and follow up were identical to those of EPIC-NSW15 and were managed by the clinical sites: five publicly funded sexual health clinics and one publicly funded, hospital-based HIV clinic. The clinics were selected based on their willingness to be involved in the study and their ability to recruit Medicare-ineligible participants who qualified for PrEP. Potential participants received information about the study and underwent a behavioural risk assessment via a brief clinician-administered questionnaire. Participants were enrolled between 9 July 2019 and 31 March 2020 and were followed up until 30 June 2020.
Study visits were conducted according to the New South Wales PrEP guidelines.18 At baseline, participants were dispensed PrEP and had tests for sexually transmissible infections (STIs; gonorrhoea, chlamydia, infectious syphilis) and HIV. This included throat swab, anal swab, and urine sample for chlamydia and gonorrhoea, and blood tests for syphilis and HIV. Participants attended follow-up visits 1 month and 3 months after baseline, then every 3 months thereafter. At the 1-month visit, participants received an HIV test whereas the 3-monthly visits included both HIV and STI testing. PrEP was dispensed at each visit if participants still met the PrEP guidelines. Paper dispensing logs that recorded the date and dose of PrEP dispensed at each visit were kept at each site and participants were dispensed sufficient PrEP to allow daily dosing until their next scheduled visit.
Measures and data extraction
Demographic characteristics of participants including gender, age, sexual identity, country of birth, year of arrival in Australia, and postcode of residence were recorded by the study nurse or doctor at the baseline visit in an electronic database. Using a published method,19 we grouped participants’ postcode of residence into four categories based on the proportion of adult males estimated to be gay within each postcode (<5%, 5–9.9%, 10–19.9%, and ≥20%).
Adherence was estimated using a medication possession ratio (MPR), reported as the proportion of days during the study period that a participant had study medication in possession, which was calculated as the number of pills dispensed divided by the number of days between study visits. These data were based on medication dispensing records at each site and were truncated to a maximum of 1.
Three of the six participating study sites (accounting for 26.7% of the enrolled participants) were part of the Australian Collaboration for Coordinated Enhanced Sentinel Surveillance of Sexually Transmissible Infections and Blood Borne Viruses (ACCESS) network, a national sexual health surveillance network that collects and collates de-identified clinical data from sexual health centres and other health services across Australia.20 HIV and STI testing data were collected by the ACCESS network for these sites and were entered manually by remaining sites in a secure online database designed for this study. STI positivity was calculated at baseline as the number of individuals who had a positive result for gonorrhoea, chlamydia, or infectious syphilis divided by the number who were tested for each. Baseline STI tests were included in the analysis if they were conducted within 21 days before or after the enrolment date.
Survey
At enrolment, participants were invited by email to complete an optional online survey using SurveyGizmo (Boulder, CO, USA). Clinic staff had no access to the survey or responses. The survey collected demographic, behavioural, and attitudinal data. To identify structural barriers to PrEP access, participants were asked about PrEP knowledge, English-language proficiency, visa status, and health insurance. Participation in the survey was completely voluntary and there were no additional incentives to complete the survey.
Analysis
Analyses were conducted using Stata ver. 14.2 (StataCorp, College Station, TX, USA). Frequencies and percentages of demographic characteristics, survey responses and adherence measures are reported. Comparison of demographic characteristics between participants who completed the survey and those who did not complete the survey were conducted using chi-squared tests of homogeneity.
Results
A total of 222 participants consented to participate. One participant was excluded during screening due to low estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2. Of the 221 included in the study, one-quarter (n = 56, 25.3%) of participants had previously been enrolled in the EPIC-NSW study. Two individuals formally withdrew during study follow up; one due to side-effects from the medication, and the other due to relocation to another country and inability to attend study follow-up visits. There was one serious adverse event in a participant who reported suicidal ideation; this was classified by the study medical officer as potentially related to the study drug. There was one participant who had a confirmed HIV diagnosis during the study. This participant was not taking PrEP daily at the time of likely HIV exposure.
Participant characteristics
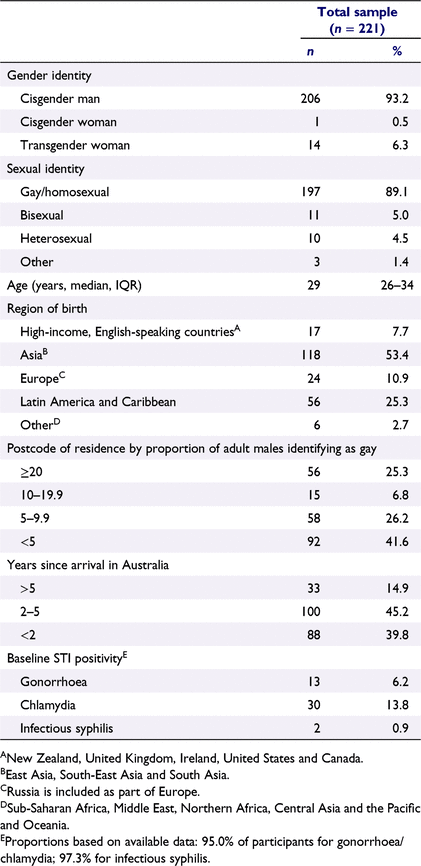
Most participants were male (n = 206, 93.2%) and identified as gay (89.1%; Table 1). The median age at enrolment was 29 years (IQR 26–34). Half (53.4%) were born in Asia, one-quarter (25.3%) in Latin America or the Caribbean, and 10.9% in Europe. Most participants (85.1%) had been living in Australia for ≤5 years, including 39.8% for <2 years. One-quarter (25.3%) reported living in a suburb where the estimated proportion of gay men in that postcode is ≥20%, 6.8% live in a suburb with 10–19.9% of gay men, 26.8% live in a suburb with 5–9.9% of gay men, and 41.6% live in a suburb with <5% of gay men. Of the 221 participants, 210 (95.0%) had gonorrhoea and chlamydia test results at any anatomical site available at baseline, of which 6.2% (n = 13) were diagnosed with gonorrhoea and 13.8% (n = 20) were diagnosed with chlamydia. Of the 215 (97.3%) participants with baseline data available for syphilis, 0.9% (n = 2) had infectious syphilis.
|
In the 3 months before enrolment, 90.1% (n = 199) of participants reported receptive condomless anal intercourse with a casual partner. More than one-fifth (n = 50, 22.6%) reported a diagnosis of infectious syphilis, rectal chlamydia, or rectal gonorrhoea. Almost one in seven (n = 32, 14.5%) reported recent methamphetamine use. Condomless anal intercourse with an HIV-positive partner who did not have undetectable viral load was reported by only three individuals (1.4%). Two-thirds (n = 150, 67.9%) of participants met only one behavioural eligibility criterion, 57 (25.8%) met two, and 14 (6.4%) met three or more criteria.
Just over half of participants (n = 121, 54.8%) completed the baseline survey. Participants who completed the survey were more likely to be male (97.5% vs 88.0%, P = 0.018) and to identify as gay (94.2% vs 83.0%, P = 0.020). Among survey respondents, 71 (58.7%) were on a student or training visa, 21 (17.4%) were on skilled migration visa, 12 (9.9%) were on a working holiday and the remaining 17 (14.0%) held another type of visa (e.g. family/partner, bridging, refugee). Half of survey participants (n = 67, 55.4%) reported that English was the main language they used outside of work or study, and 70.2% (n = 85) reported speaking and understanding English either fluently or very well. One-third (n = 42, 34.7%) completed the survey in a language other than English. When restricted to those who reported that their main language was not English (n = 54), almost half (48.2%) completed the survey in a language other than English. Of those individuals, 15 (35.7%) completed the survey in Spanish, nine (21.4%) in Chinese and eight (19.1%) in Thai.
Approximately two-thirds (66.1%, n = 80) of survey respondents had private health insurance, but only a small minority (n = 3, 3.8%) reported that the cost of PrEP was covered by their private health insurance. There were 76 (62.8%) participants who had taken PrEP in the 6 months prior to the study enrolment. When asked about where they most recently obtained PrEP, 36 (47.4%) had received it through EPIC-NSW or another trial, 25 (32.9%) reported obtaining PrEP online, six (5.0%) received it as post-exposure prophylaxis (PEP) through a hospital or sexual health centre, six (5.0%) purchased PrEP from a chemist of pharmacy in another country, and three (2.5%) purchased PrEP in an Australian chemist or pharmacy. Participants who had not taken PrEP in the 6 months prior to enrolment (n = 45, 37.2%) were asked about the reasons. The most common reasons were that they could not afford PrEP (n = 22, 48.9%), did not know how to access it (n = 20, 44.4%), or could not get a prescription for it (n = 10, 22.2%); three (6.7%) had not heard of PrEP before enrolling.
PrEP adherence
All 221 participants were dispensed PrEP. Over the study period, the mean MPR was 87.0% (SD: 22.3), with a median of 100.0% (IQR: 83.3–100.0%). Overall, 31 participants (14.0%) had an average MPR of <60%, which is indicative of taking fewer than four pills per week on average. There were 66 (29.9%) participants who had an average MPR between 60.0 and 99.9%, and 124 participants (56.1%) had an average MPR of 100% (indicating near daily adherence).
Discussion
The MI-EPIC study demonstrated that a PrEP access program for overseas-born adults with a high risk of HIV who were not eligible for government-subsidised PrEP could be delivered in the Australian context. Adherence to PrEP was high; more than half of participants (56.1%) had an MPR consistent with taking a pill every day, and 86% of participants had an MPR that suggested they were taking at least four pills per week, consistent with high levels of protection against HIV infection.21 The mean MPR of 87% is comparable to adherence reported in EPIC-NSW, the largest PrEP implementation study conducted in Australia, which ranged from 64 to 93% throughout the study.22
Almost half (48.9%) of participants who had not used PrEP in the 6 months before study commencement reported PrEP affordability had contributed to this, in line with previous Australian evidence indicating the cost of accessing healthcare services can deter migrant populations from seeking services like HIV testing and PrEP.8,13,23 There is no adequate system covering costs of PrEP in Australia for Medicare-ineligible individuals. Private health insurance is required for temporary visitors on certain visas, but of the two-thirds of the survey participants who reported having private health insurance, almost all (96.2%) believed PrEP was not included in their policy. This is likely a correct belief as most mandatory private health insurance policies for working and student visa requirements do not cover the cost of PrEP.24 For example, minimum requirements for an Overseas Student Health Cover policy necessary for a student visa in Australia includes AUD50 to be paid by the insurer for drugs on the Pharmaceutical Benefits Scheme (PBS) on each purchase, and up to AUD300 of insurer contributions in total per year, across all medicines.25 If the unsubsidised cost of a month’s supply of PrEP is approximately AUD160, an individual on Overseas Student Health Cover would have to pay AUD110 for each of the first six purchases in a year, and then pay full price for the remainder of the year. This would be on top of the cost of paying for the health insurance and would also exhaust their contributions for other non-PrEP medications.
Because private health insurance does not provide the required support for PrEP, other avenues should be explored. In late 2020, the Australian Government indicated a commitment to work with State and Territory Governments to secure an agreement to provide subsidised access to HIV treatment regardless of Medicare status.26 If the proportion of Medicare-ineligible GBM, who are PrEP eligible, is similar to that of Australian-born GBM,4 then approximately 5000 Medicare-ineligible people would take PrEP in Australia, and a similar scheme to HIV treatment for PrEP could be initiated if determined to be cost effective.
One alternative to Medicare-ineligible individuals purchasing government-subsidised PrEP is personal importation from an overseas pharmacy, although this remains relatively uncommon.4 To purchase pharmaceuticals online, a valid Australian prescription is required, and the cost is approximately AUD60 for 90 pills. There are no specific data on Medicare-ineligible men, but among community-attached gay men in Australia, only 10.3% report purchasing PrEP online from overseas4 despite it being cheaper than through the PBS. This suggests that people are not aware of this system or that it is difficult to use, and these issues could be exacerbated in overseas-born individuals for whom English-language ability could be a limiting factor.
Publicly funded sexual health clinics provide partial financial assistance by offering clinical consultation and free HIV/STI testing regardless of Medicare status, but do not fund the cost of PrEP itself. There are potential barriers to visiting these free services that also apply to initiating PrEP. Permanent residency applications are affected by HIV status, and although approved student visas cannot be revoked after a positive test, overseas-born GBM may not want to test for HIV because of their beliefs and fears of social and legal repercussions of an HIV diagnosis, as well as because of low self-perceived risk.27 Poor knowledge of how to navigate the healthcare system in a new country is an ongoing issue for migrants, both in Australia and internationally.7,8,27 These issues are further compounded by HIV-related stigma and homophobia,7,8,27 as well as gaps in knowledge by healthcare service providers when dealing with HIV and sexual health-related issues.27–30 There have been recent initiatives to address these issues, such as a website launched by the NSW Government that compiles information for international students about navigating the NSW Health system (https://internationalstudents.health.nsw.gov.au/). More work will be needed to assess the impacts of these initiatives in the future.
Being unaware of PrEP and having challenges to navigate the Australian healthcare system were identified as barriers to access in this study. Almost half (44.4%) of participants who had not used PrEP in the 6 months preceding enrolment said it was because they did not know how to access it. Gaps in knowledge may be exacerbated by language and other cultural factors. Although 70.2% of survey participants self-reported good English proficiency, only half said their main language outside the workplace was English. Language barriers are also a commonly reported issue in accessing health care, particularly with HIV testing and treatment,7,8,31 and this is likely to extend to PrEP. In this study, the materials, including recruitment information and the survey, were translated into seven languages and half of the participants whose main language was not English completed the survey in a language other than English. This high uptake of an optional survey when provided in a language other than English supports existing recommendations that research and healthcare information that targets migrant populations should provide culturally appropriate materials.32,33
New strategies need to be considered to reach Medicare-ineligible populations who would benefit from taking PrEP. As reflected by our study, these populations live in a range of areas and more investment into publicly funded sexual health clinics outside of inner-city ‘gay’ areas should be considered to facilitate linkage to healthcare services and clinicians who can initiate conversations about PrEP. Existing services can also be adapted to suit the needs of Medicare-ineligible populations, particularly where language is a barrier. For example, in Sydney, a community-based peer-led HIV/STI testing service for GBM was adapted to be administered entirely in Chinese led by Chinese-speaking GBM peers.34 The distribution of culturally appropriate materials in online spaces, such as Facebook or WeChat groups, has the potential to reach people who would not engage with generic sexual health information. Such materials should acknowledge different levels of HIV knowledge, and also would need to address HIV-related stigma affecting this population, which could be a barrier to accessing HIV testing and PrEP initiation.27 Reaching this population continues to be a challenge in HIV prevention, and innovative approaches are needed to engage with this group that are not reached by current methods.
There were several limitations to this study. The generalisability of data from the optional survey are limited because only half the study participants completed the survey. MPR is an imperfect measure of adherence and does not provide information about how participants took PrEP; even with estimates of medication in possession, study participants may have used a non-daily dosing strategy. As participants were often recruited at publicly funded sexual health clinics, these Medicare-ineligible GBM were connected to clinical care and may not be representative of other Medicare-ineligible GBM. Long-term PrEP use and access is difficult to assess due to the short follow-up period.
Conclusion
Australia’s national HIV strategy goal of the virtual elimination of HIV transmission will not be achieved if overseas-born populations are systematically excluded from accessing effective HIV prevention strategies such as PrEP. A continuing challenge for HIV prevention in Australia and internationally is identifying the groups who experience barriers to HIV prevention strategies and mitigating those barriers where possible. Medicare-ineligible populations at risk of HIV, particularly overseas-born GBM, face unique difficulties regarding healthcare access that are often reinforced by social and cultural factors. This study demonstrated that when access to HIV prevention medication was provided and language barriers were reduced, interest in PrEP and adherence to medication was high. If Australia is to successfully eliminate HIV transmission, then access to affordable HIV prevention services for all Australians will also be required.
Data availability
The data that support this study cannot be publicly shared due to ethical and privacy reasons.
Conflicts of interest
Barbara Yeung had received a travel grant from Mylan Australia to present at the HIV Clinical Care meeting in the Australian Capital Territory (ACT). Andrew Carr has received research funding from Gilead Sciences, MSD and ViiV Healthcare; lecture and travel sponsorships from Gilead Sciences and ViiV Healthcare; and has served on advisory boards for Gilead Sciences, MSD and ViiV Healthcare. Andrew E. Grulich reports a grant from Seqirus Australia, personal fees from MSD, and in-kind support from GSK outside of the submitted work, and is a Joint Editor of Sexual Health, but was blinded from the peer-review process for this paper.
Declaration of funding
The MI-EPIC study was funded by the New South Wales Ministry of Health.
Acknowledgements
The MI-EPIC research group includes Rebecca Guy, Fengyi Jin, Nila Dharan, Stefanie Vaccher, Tobias Vickers, and Barbara Yeung from the Kirby Institute; Janaki Amin from Macquarie University; Andrew Carr from St Vincent’s Hospital; Katherine Brown from Illawara Sexual Health Clinic; Christopher Carmody from Liverpool Sexual Health Clinic; Catriona Ooi from Clinic 16; Nathan Ryder from Hunter New England Sexual Health; David Smith from Lismore Sexual Health Centre; Kerry Chant, Jo Holden, Christine Selvey, Cherie Power, and Bill Whittaker from the NSW Ministry of Health; Nicolas Parkhill, Karen Price, and Matthew Vaughan from ACON.
References
[1] Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 2016; 30 1973–83.| Effectiveness and safety of oral HIV preexposure prophylaxis for all populations.Crossref | GoogleScholarGoogle Scholar | 27149090PubMed |
[2] World Health Organization. Consolidated HIV strategic information guidelines: driving impact through programme monitoring and management. Geneva: WHO; 2020.
[3] Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2018. Sydney: Kirby Institute; 2018.
[4] Chan C, Broady T, Bavinton BR, Mao L, Bear B, Mackie B, et al. Gay Community Periodic Survey: Sydney 2020. Sydney: Centre for Social Research in Health, UNSW Sydney; 2020.
[5] NSW Health. NSW HIV strategy 2016–2020: quarter 4 & annual 2018 data report. Sydney: Centre for Population Health; 2019.
[6] Kirby Institute. National HIV notification Q1 2015–Q4 2019. Sydney: Kirby Institute; 2020.
[7] UNAIDS. The Gap Report. Geneva: UNAIDS; 2014.
[8] Gray C, Lobo R, Narciso L, Oudih E, Gunaratnam P, Thorpe R, et al. Why I can’t, won’t or don’t test for HIV: insights from Australian Migrants born in Sub-Saharan Africa, Southeast Asia and Northeast Asia. Int J Environ Res Public Health 2019; 16 1034
| Why I can’t, won’t or don’t test for HIV: insights from Australian Migrants born in Sub-Saharan Africa, Southeast Asia and Northeast Asia.Crossref | GoogleScholarGoogle Scholar |
[9] Pharmaceutical Benefits Scheme. Schedule of pharmaceutical benefits: Section 100 – items available under special arrangement – volume 2 – effective 1 November 2020. Canberra: Department of Health; 2020.
[10] Pharmacy Online. Tenofovir/Emtricitabine Tab 300mg/200mg X 30 (generic for Truvada). 2021. Available at https://www.pharmacyonline.com.au/tenofovir-emtricitabine-tab-300mg-200mg-x-30-auth [verified 9 April 2021].
[11] Chemist Direct. Tenofovir/Emtricitabine Tab 300mg/200mg X 30 (generic for Truvada). 2021. Available at https://www.chemistdirect.com.au/tenofovir-emtricitabine-tab-300mg-200mg-x-30-auth [verified 9 April 2021].
[12] Medland NA, Chow EPF, Read THR, Ong JJ, Chen M, Denham I, et al. Incident HIV infection has fallen rapidly in men who have sex with men in Melbourne, Australia (2013–2017) but not in the newly-arrived Asian-born. BMC Infect Dis 2018; 18 410
| Incident HIV infection has fallen rapidly in men who have sex with men in Melbourne, Australia (2013–2017) but not in the newly-arrived Asian-born.Crossref | GoogleScholarGoogle Scholar | 30126355PubMed |
[13] Walia AM, Fairley CK, Bradshaw CS, Chen MY, Chow EPF. Disparities in characteristics in accessing public Australian sexual health services between Medicare-eligible and Medicare-ineligible men who have sex with men. Aust NZ J Public Health 2020; 44 363–8.
| Disparities in characteristics in accessing public Australian sexual health services between Medicare-eligible and Medicare-ineligible men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[14] MacGibbon J, Lea T, Ellard J, Murphy D, Kolstee J, Power C. Access to subsidised healthcare affects HIV pre-exposure prophylaxis (PrEP) uptake among gay and bisexual men in Australia: results of national surveys 2013–19. J Acquir Immune Defic Syndr 2021; 86 430–5.
| 33230031PubMed |
[15] Zablotska IB, Selvey C, Guy R, Price K, Holden J, Schmidt H-M. Expanded HIV pre-exposure prophylaxis (PrEP) implementation in communities in New South Wales, Australia (EPIC-NSW): design of an open label, single arm implementation trial. BMC Public Health 2018; 18 210.
| 29394918PubMed |
[16] Grulich AE, Guy R, Amin J, Jin F, Selvey C, Holden J, et al. Population-level effectiveness of rapid, targeted, high-coverage roll-out of HIV pre-exposure prophylaxis in men who have sex with men: the EPIC-NSW prospective cohort study. The Lancet HIV 2018; 5 e629–37.
| Population-level effectiveness of rapid, targeted, high-coverage roll-out of HIV pre-exposure prophylaxis in men who have sex with men: the EPIC-NSW prospective cohort study.Crossref | GoogleScholarGoogle Scholar | 30343026PubMed |
[17] Centre for Population Health. Pre-exposure prophylaxis of HIV with antiretroviral medications. Sydney: New South Wales Ministry of Health; 2016.
[18] Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM). The ASHM PrEP guidelines: Sept 2019 update. Sydney: ASHM; 2019.
[19] Callander D, Mooney-Somers J, Keen P, Guy R, Duck T, Bavinton BR, et al. Australian ‘gayborhoods’ and ‘lesborhoods’: a new method for estimating the number and prevalence of adult gay men and lesbian women living in each Australian postcode. Int J Geogr Info Sci 2020; 34 2160–76.
| Australian ‘gayborhoods’ and ‘lesborhoods’: a new method for estimating the number and prevalence of adult gay men and lesbian women living in each Australian postcode.Crossref | GoogleScholarGoogle Scholar |
[20] Callander D, Moreira C, El-Hayek C, Asselin J, van Gemert C, Watchirs Smith L, et al. Monitoring the control of sexually transmissible infections and blood-borne viruses: protocol for the Australian Collaboration for Coordinated Enhanced Sentinel Surveillance (ACCESS). JMIR Res Protoc 2018; 7 e11028
| Monitoring the control of sexually transmissible infections and blood-borne viruses: protocol for the Australian Collaboration for Coordinated Enhanced Sentinel Surveillance (ACCESS).Crossref | GoogleScholarGoogle Scholar | 30459142PubMed |
[21] Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4 151ra125
| 22972843PubMed |
[22] Grulich AE, Jin F, Bavinton BR, Yeung B, Hammoud MA, Amin J. Long-term protection from HIV infection in the expanded and extended EPIC-NSW study of oral HIV pre-exposure prophylaxis in gay and bisexual men. The Lancet HIV 2021; 8 E486–E494.
| Long-term protection from HIV infection in the expanded and extended EPIC-NSW study of oral HIV pre-exposure prophylaxis in gay and bisexual men.Crossref | GoogleScholarGoogle Scholar | 34217426PubMed |
[23] Blackshaw LCD, Chow EPF, Varma R, Healey L, Templeton DJ, Basu A, et al. Characteristics of recently arrived Asian men who have sex with men diagnosed with HIV through sexual health services in Melbourne and Sydney. Aust NZ J Public Health 2019; 43 424–8.
| Characteristics of recently arrived Asian men who have sex with men diagnosed with HIV through sexual health services in Melbourne and Sydney.Crossref | GoogleScholarGoogle Scholar |
[24] Private Health Insurance Ombudsman. Overseas visitors & overseas students. 2021. Available at https://www.privatehealth.gov.au/health_insurance/overseas/index.htm
[25] Department of Health. Deed for the provision of overseas student health cover. Canberra: Department of Health; 2017.
[26] National Association of People with HIV Australia (NAPWHA), Australian Federation of AIDS Organisations (AFAO). People who are ineligible for Medicare to gain access to HIV treatment and care [sector release]. 2020. Available at https://napwha.org.au/medicare-ineligibles-gain-access-to-subsidised-hiv-treatment-and-care/ [verified 1 December 2020].
[27] Philpot SP, Aung E, Prestage G, Mao L, Chen T, Varma R, et al. Qualitative interviews with overseas-born gay and bisexual men recently diagnosed with HIV from non-English speaking countries: report of results. Sydney: Kirby Institute; 2021.
[28] Skolnik AA, Bokhour BG, Gifford AL, Wilson BM, Van Epps P. Roadblocks to PrEP: what medical records reveal about access to HIV pre-exposure prophylaxis. J Gen Intern Med 2020; 35 832–8.
| Roadblocks to PrEP: what medical records reveal about access to HIV pre-exposure prophylaxis.Crossref | GoogleScholarGoogle Scholar | 31705471PubMed |
[29] Lane W, Heal C, Banks J. HIV pre-exposure prophylaxis: knowledge and attitudes among general practitioners. Aust J Gen Pract 2019; 48 722–7.
| HIV pre-exposure prophylaxis: knowledge and attitudes among general practitioners.Crossref | GoogleScholarGoogle Scholar | 31569318PubMed |
[30] Zablotska IB, O’Connor CC. Preexposure prophylaxis of HIV infection: the role of clinical practices in ending the HIV epidemic. Curr HIV/AIDS Rep 2017; 14 201–10.
| Preexposure prophylaxis of HIV infection: the role of clinical practices in ending the HIV epidemic.Crossref | GoogleScholarGoogle Scholar | 29071519PubMed |
[31] Körner H. Late HIV diagnosis of people from culturally and linguistically diverse backgrounds in Sydney: the role of culture and community. AIDS Care 2007; 19 168–78.
| Late HIV diagnosis of people from culturally and linguistically diverse backgrounds in Sydney: the role of culture and community.Crossref | GoogleScholarGoogle Scholar | 17364395PubMed |
[32] Ethnic Communities’ Council of Victoria (ECCV). An investment not an expense: enhancing health literacy in culturally and linguistically diverse communities: ECCV policy paper. Melbourne: ECCV; 2012.
[33] Andrulis DP, Brach C. Integrating literacy, culture, and language to improve health care quality for diverse populations. Am J Health Behav 2007; 31 122–33.
| Integrating literacy, culture, and language to improve health care quality for diverse populations.Crossref | GoogleScholarGoogle Scholar |
[34] Chan C, Patel P, Johnson K, Vaughan V, Price K, McNulty A, et al. Evaluation of ACON’s community-based a[TEST] HIV and STI testing services, 2015–2019. Sydney: Kirby Institute; 2020.


