Prevalence of bacterial vaginosis in postmenopausal women: a systematic review and meta-analysis
Linde L. Stewart A * , Lenka A. Vodstrcil A B C , Jacqueline Coombe
A * , Lenka A. Vodstrcil A B C , Jacqueline Coombe  A , Catriona S. Bradshaw A B C and Jane S. Hocking
A , Catriona S. Bradshaw A B C and Jane S. Hocking  A B
A B
A Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Vic., Australia.
B Melbourne Sexual Health Centre, Alfred Health, Carlton, Vic., Australia.
C Central Clinical School, Monash University, The Alfred Centre, Melbourne, Vic., Australia.
Sexual Health 19(1) 17-26 https://doi.org/10.1071/SH21083
Submitted: 3 May 2021 Accepted: 5 August 2021 Published: 23 February 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Bacterial vaginosis (BV), the most common cause of vaginal discharge in women of reproductive age, is associated with considerable reproductive and gynaecological sequelae and increases the risk of acquiring sexually transmissible infections including HIV. Although we understand the burden of BV in women of reproductive age, much less is known about the burden of BV in postmenopausal women. We undertook this systematic review and meta-analysis to estimate the prevalence of BV in postmenopausal women. The electronic databases PubMed, EMBASE, Web of Science, and The Cochrane Library were searched for English-language papers reporting on the prevalence of BV in postmenopausal women and published up until the end of July 2020. Search terms included: (prevalence OR survey OR proportion) AND ‘bacterial vaginosis’. Meta-analysis was used to calculate pooled estimates of prevalence. We identified 2461 unique references and assessed 328 full-text articles for eligibility, with 13 studies included in the meta-analysis. The prevalence of BV ranged from 2.0 to 57.1%, with a summary estimate of 16.93% (95% CI: 8.5–27.4; I2 = 97.9). There was considerable heterogeneity between studies and quality varied considerably. Further research is needed to provide a better understanding of the condition in postmenopausal women and understand its effect on their lives.
Keywords: bacterial vaginosis (BV), menopause, postmenopausal, prevalence, sexually transmissible, symptoms, vaginal discharge, vaginosis, women.
Introduction
Bacterial vaginosis (BV) is the most common cause of abnormal vaginal discharge in women of reproductive age. Characterised by a disturbance of vaginal microbiota, there is a marked depletion of Lactobacillus species and an increase in diversity of organisms including Gardnerella vaginalis, Atopobium vaginae, and genital mycoplasmas.1–6 The symptoms can include a thin homogeneous vaginal discharge and fishy odour and are accompanied by an increase in vaginal pH. Symptoms can be very distressing for some women, and recurrent BV can have a significant effect on a woman’s self-esteem and intimate relations, substantially affecting quality of life.7 However, a significant number of cases are asymptomatic.8
BV has been associated with poor obstetric and gynaecological sequelae in premenopausal women including preterm birth,9–15 spontaneous abortion,10,15 postpartum endometritis,16 and pelvic inflammatory disease (PID).16 There is considerable observational evidence that BV is sexually transmissible;8,17 therefore, sexually active postmenopausal women continue to be at risk of acquiring BV. Furthermore, women diagnosed with BV are at an increased risk of acquiring sexually transmissible infections (STIs) including HIV.8,18 Recurrence is common after treatment.19
BV is commonly diagnosed by one of two gold standard diagnostic methods: the Nugent score,20 or Amsel criteria.21 The Nugent score is based on counting bacterial morphotypes identified under a microscope. Amsel criteria are based on the presence of at least three of the four following signs and symptoms: a thin homogenous discharge, raised pH, detections of amines, and presence of Clue cells seen in a wet preparation viewed under a microscope. These two methods were developed in women of reproductive age and there is ongoing debate about whether these are appropriate for diagnosing BV in postmenopausal women where the absence of oestrogen effect the vaginal epithelium and microbiome.22
The global burden of BV in women of reproductive age is high. General population prevalence estimates range from 23 to 29% globally,23 with a higher burden in women who have sex with women,24–27 women of African ethnicity28–33 and women who engage in intravaginal practices (e.g. douching or insertion into the vagina of items or products for hygiene, enhancement of sex or treatment of symptoms).8,34 In contrast, there is very little prevalence data and no real understanding of the effect of BV in postmenopausal women. Women continue to be sexually active after menopause and are at risk of acquiring BV, so it is important to understand the burden of BV among these women. We undertook this systematic review and meta-analysis to determine the prevalence of BV in postmenopausal women.
Methods
Protocol and registration
The PRISMA statement was used to guide the reporting of results in this review and meta-analysis.35 Our review protocol is registered on the International Prospective Register of Systematic Reviews (PROSPERO), registration no.: CRD42019146598 (https://www.crd.york.ac.uk/PROSPERO/).
Search strategy
The electronic databases PubMed, EMBASE, Web of Science, and The Cochrane Library were searched for English-language studies that provided BV prevalence data, published before 20 July 2020. The search terms included (prevalence OR survey OR proportion) AND ‘bacterial vaginosis’ (Supplementary Table S1). Citation lists were examined for additional references.
Eligibility criteria
Studies were eligible for inclusion if they included BV prevalence data for postmenopausal women and the study was published in English. Women were defined as postmenopausal if they had not had a menstrual period for ≥12 months or were aged >50 years if menopausal status was not specified. Studies had to report on the prevalence of BV using an established, diagnostic method such as the Nugent score,20 Amsel criteria,21 or Hay/Ison criteria.36 Studies reporting on BV exclusively in women of reproductive age were excluded. Letters to the editor, reviews, editorial and discussion articles, conference abstracts without subsequent publication, or studies where the target population (postmenopausal women) sample size was <10 were excluded. Studies were reviewed by two authors (LLS and JC). Differences in opinion were resolved by consensus with other authors (JSH, LAV, CSB).
Authors were contacted if it was unclear whether the study included BV prevalence data for postmenopausal women. Given the timeframe of the search and that email addresses of authors were not available for several older publications, an a priori decision was made to only contact authors of papers published since 2009, focusing on the most recent decade of publications.
In addition, if the paper reported prevalence data on a sample of women across a broad age range, authors were only contacted if the study included either >100 total participants across all ages or if the sample size of postmenopausal women was ≥10. Fourteen authors were contacted, six responded and three were able to provide additional data eligible for inclusion (see Supplementary Table S3).
Data extraction
The data extracted included: (1) study details (author and year of publication reported); (2) country of study; (3) study population and setting (e.g. clinic or hospital or general population); (4) ethnicity; (5) study design (cross-sectional, cohort, case–control, randomised controlled trial); (6) age range of participants; (7) total sample size across all ages; (8) number of postmenopausal women; (9) number of postmenopausal women who tested positive for BV; (10) diagnostic method used; (11) BV prevalence in postmenopausal women; and (12) reason for visit. For the two community-based cohort studies and the randomised controlled trial, baseline data were extracted. For the population-based cohort study, the most recent data were used. One author (LLS) extracted the data, and a second author (JC) checked the extracted data. Discrepancies were resolved by consultation with a third author (JSH).
Outcome
The primary outcome was BV prevalence among postmenopausal women. This was measured as a proportion, with the numerator being the number of women diagnosed with BV and the denominator being the number of women tested for BV.
Analysis
Meta-analysis was used to calculate summary estimates of BV prevalence; random effects models were used. Where studies did not report 95% confidence intervals (CIs), these were calculated using available data. The I2 test was used to calculate the proportion of total variability in prevalence estimates that could be attributed to underlying study heterogeneity alone and not simply due to chance. An I2 value was considered moderate if >50% and high if >75%.37 Subgroup analyses were undertaken to investigate factors associated with heterogeneity. Subgroup analyses included assessing BV prevalence by diagnostic method (Nugent score or Amsel criteria) and population type (classified as gynaecology populations, general population-based or screening populations [e.g. cervical cancer or employment health screening] or HIV-positive population). To investigate the effect of individual studies on the overall summary estimate, a sensitivity analysis was undertaken in which the effect of removing each study on the summary estimate was explored. Data were analysed using Stata 16 (Version 16; StataCorp) applying the Metaprop syntax using the Freeman–Tukey double arcsine transformation.
Assessment of study quality
We assessed within-study bias using a combination of the following: evaluation criteria suggested by Sanderson et al.38 and AXIS, the critical appraisal tool for cross-sectional studies developed by Downes et al.39 We assessed the following parameters: (1) selection bias: setting and source population, the reporting of inclusion/exclusion criteria, sample representation of the source population/the use of consecutive sampling, reporting of response fraction, missing data; (2) measurement bias: appropriate diagnostic method, experience with diagnostic method; and (3) statistical bias: sample size and reporting of sample size calculations. Publication bias, assessed by funnel plot analysis, was not performed because of the low number of eligible studies.
Results
Study selection and characteristics
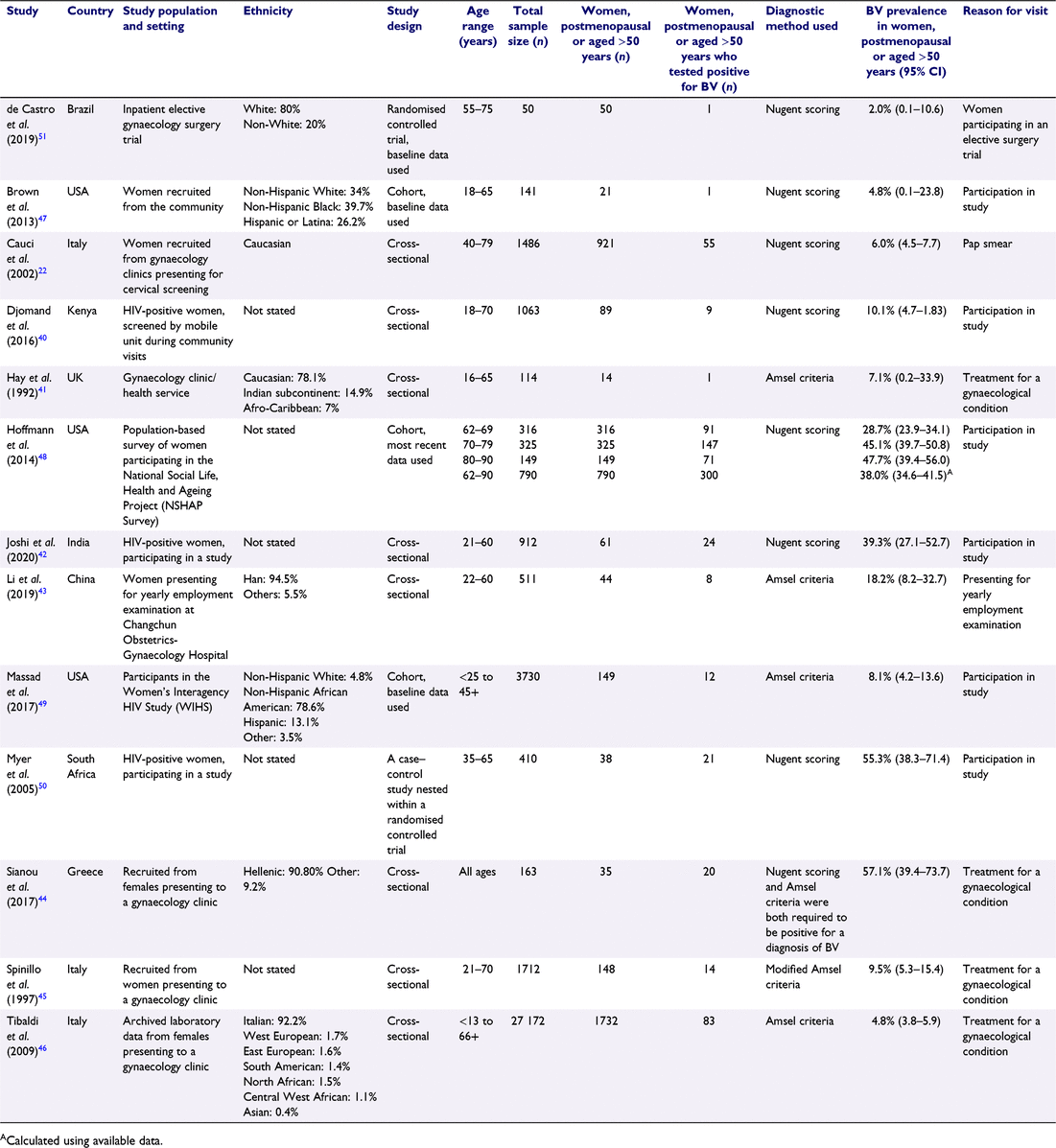
Overall, 5693 articles were identified, of which 2461 were unique studies. A total of 328 full-text articles were assessed for eligibility, with 13 studies eligible for inclusion in the review (see Fig. 1). Table 1 shows that of 13 eligible studies, eight were cross-sectional studies,22,40–46 three were cohort studies,47–49 one was a nested case–control study50 and one was a randomised controlled trial.51 Three studies were from the USA,47–49 three were from Italy,22,45,46 and one study from each of the UK,41 Kenya,40 South Africa,50 Brazil,51 Greece,44 China43 and India.42 The populations included were ethnically diverse, including Afro-Caribbean,41 Asian46, Caucasian,22,41,51 Central West African46, East European46, Han Chinese,43 Hellenic,44 Hispanic or Latina,47,49 Indian subcontinent,41 Italian,46 Non-Hispanic African American,49 Non-Hispanic Black,47 Non-Hispanic White,47,49 North African,46 South American46 and West European.46 Four studies recruited participants from gynaecological clinics or inpatient gynaecological wards,41,44,45,51 two were from community screening programs (cervical screening, employment screening),22,43 three were from HIV-positive populations,40,42,50 one was a population cohort,48 two were community-based cohorts47,49 and one used archived laboratory data.46 Seven studies used the Nugent score as the diagnostic method,22,40,42,47,48,50,51 four used Amsel criteria,41,43,46,49 one required both the Nugent score and Amsel criteria to be positive to make a diagnosis of BV,44 one used modified Amsel criteria,45 and none used Hay/Ison criteria. Sample sizes of postmenopausal women varied from 14 to 1732 with an overall total of 4092 women.
|
BV prevalence
Prevalence estimates ranged from 2.0 to 57.1%. The overall summary prevalence estimate for BV among postmenopausal women was 16.93% (95% CI 8.45–27.4%; I2 = 97.9%; P < 0.01) with marked heterogeneity (Fig. 2).
|
|
Subgroup analyses found that BV prevalence was higher in studies that used the Nugent score to diagnose BV (22.90%; 95% CI 8.64–41.18; I2 = 98.15; P < 0.01) than in those that used Amsel criteria (7.92%; 95% CI 4.05–12.76; I2 = 74.64; P < 0.01). BV prevalence was highest among HIV-positive women (25.06%; 95% CI 7.49–48.27; I2 = 94.6; P < 0.01) (Table 2).

|
The sensitivity analysis found that removing each study had little effect on the summary estimates, with the overall summary BV prevalence estimates for the remaining studies ranging from 14.50 to 18.64% with marked heterogeneity for each estimate (I2 > 93%).
Assessment of within-study bias
Risk of bias is summarised in Table 3 and Supplementary Table S2. Overall, the included studies were at very high risk of selection bias with all but one48 set in specialist clinic settings or high-risk populations (e.g. HIV-positive patients). Even the one population-based study had oversampled women of African American or Hispanic ethnicity.48,52 Only two (15.4%) studies reported consecutive recruitment of patients22,41 and none reported the retention of all data. Inclusion and exclusion criteria were described in four (30.8%) studies22,43,45,51 and a further four (30.8%) studies42,48–50 reported inclusion criteria alone. Overall, measurement bias was considered low because all studies used gold standard diagnostic methods. However, the risk of measurement bias was considered moderate in one study, which required that the Nugent score and Amsel criteria were both positive.44 Only three studies focused on postmenopausal women,45,48,51 with all other studies including adult women of all ages, reducing the available sample size for our target population.22,40–44,46,47,49,50 None of the studies reported any sample size calculations.

|
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis of BV prevalence specifically among postmenopausal women. Our review included a total of 4092 women and found an overall summary BV prevalence of 16.93%. However, the included studies were at considerable risk of bias; only one population-based study was identified,48 and our pooled estimate had marked heterogeneity, highlighting the scarcity of robust BV prevalence data for postmenopausal women.
There are several limitations present in this review. First, by including studies with women who were aged >50 years and not just those who were postmenopausal, our review will have included some women who are still premenopausal or perimenopausal. However, this highlights the lack of studies that focus specifically on postmenopausal women. Second, by limiting our search to studies published in English, some important studies may have been omitted. Third, there were several studies that we were unable to extract data specifically for postmenopausal women, even after contact with authors, so some important data may have been excluded from our analysis. However, this highlights the need for further studies with a focus on postmenopausal women. Furthermore, our search only identified one population-based study and only three studies specifically focusing on postmenopausal women.45,48,51 Finally, we chose to only include studies that used either the Nugent score,20 Amsel criteria21 or Hay/Ison criteria36 to diagnose BV.
Our subgroup analysis found that BV prevalence was higher in studies using the Nugent score than in studies that used Amsel criteria as the diagnostic method. It has been previously shown that the Amsel criteria has a lower sensitivity for BV than the Nugent score, and this may have contributed to the observed difference.53–55 Furthermore, BV prevalence was highest among HIV-positive women. However, there are concerns about how well these diagnostic methods work after menopause22,48 when the decline in oestrogen alters the vaginal epithelium and leads to a significant reduction or absence of lactobacilli.22,56 Currently, there is no standardised recommendation for the diagnosis of BV in postmenopausal women. According to a recent study that compared the relative abundances of vaginal microbiota with Nugent score,57 classic BV-associated organisms such as Gardnerella, Atopobium, Sneathia, and Megasphaera were strongly associated with an elevated Nugent score in premenopausal women, but in postmenopausal women, these same organisms were not. The authors concluded that a high Nugent score should not be used to infer a diagnosis of BV in postmenopausal women.57 This highlights the challenges with understanding BV in postmenopausal women and may contribute to the heterogeneity in prevalence estimates.
The use of hormone replacement therapy (HRT) is relatively common among postmenopausal women to alleviate the symptoms of menopause.56 Physiological changes in response to HRT can include significant increases in the relative abundance of Lactobacillus spp., a decrease in vaginal pH, as well as increased maturation of the vaginal epithelium.58 However, it remains unclear how the use of HRT could affect the pathogenesis, symptoms, or diagnosis of BV in postmenopausal women when there are already ongoing concerns about how well diagnostic methods work after menopause22,48 and, currently, there is no standardised recommendation for the diagnosis of BV in postmenopausal women. Only one study in our review compared the prevalence of BV in postmenopausal women who use HRT with those who did not, with the authors observing that the difference was not statistically significant.22
BV is associated with poor reproductive and obstetric outcomes, significant gynaecological sequelae,9–14,16,41,59,60 and a greater risk of STI acquisition, including HIV18,61–63 in women of reproductive age, but there is sparse discussion about its impact in postmenopausal women. However, women continue to be sexually active after menopause, often lacking knowledge of STIs and safer sexual practices,64 and are less likely to use condoms.65 A recent study found that STIs are increasing at a faster rate among older women than among younger women.66 Given the considerable evidence suggesting that BV is sexually transmissible,8,17 and the knowledge that women continue to be sexually active after menopause, postmenopausal women continue to be at risk of acquiring BV, which can increase their risk of other STIs including HIV.8,18,32,67–70
This review highlights that there are few quality studies on BV prevalence in postmenopausal women. Most studies were subject to considerable selection bias and lacked sample size calculations. Even the single population-based study had some selection bias with women of African American or Hispanic ethnicity being oversampled.48,52 A recent systematic review and meta-analysis by Peebles et al.,23 of global BV prevalence and costs among women of reproductive age in the general population, found similar marked heterogeneity. Nevertheless, our review suggests that BV may be highly prevalent among postmenopausal women.
Conclusion
BV is a condition we take seriously in younger women, but know very little about in postmenopausal women. Our review highlights how under-researched and poorly understood this important area of public health is. Quality research is needed to further our understanding of BV in postmenopausal women and understand its effect on their lives.
Supplementary material
Supplementary material is available online.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
Jane Hocking is a Joint Editor of Sexual Health, but was blinded from the peer-review process for this paper.
Declaration of funding
JSH is supported by a National Health and Medical Research Council (NHMRC) Senior Research Fellowship (1136117) and CSB is supported by a NHMRC Investigator Grant (1173361). No other funds were used for this work.
Author contributions
Designed the search strategy: LLS, JSH, CSB and LAV. Analysed the data: LLS, JC and JSH. Wrote the paper: LLS and JSH. Contributed to drafting/review of final manuscript: all authors.
References
[1] Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 2005; 353 1899–911.| Molecular identification of bacteria associated with bacterial vaginosis.Crossref | GoogleScholarGoogle Scholar | 16267321PubMed |
[2] Ferris MJ, Masztal A, Aldridge KE, Fortenberry JD, Fidel PL, Martin DH. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect Dis 2004; 4 5
| Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis.Crossref | GoogleScholarGoogle Scholar | 15018635PubMed |
[3] Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE 2012; 7 e37818
| Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria.Crossref | GoogleScholarGoogle Scholar | 22719852PubMed |
[4] Cox C, Watt AP, McKenna JP, Coyle PV. Mycoplasma hominis and Gardnerella vaginalis display a significant synergistic relationship in bacterial vaginosis. Eur J Clin Microbiol Infect Dis 2016; 35 481–7.
| Mycoplasma hominis and Gardnerella vaginalis display a significant synergistic relationship in bacterial vaginosis.Crossref | GoogleScholarGoogle Scholar | 26796553PubMed |
[5] Spiegel CA, Amsel R, Eschenbach D, Schoenknecht F, Holmes KK. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med 1980; 303 601–7.
| Anaerobic bacteria in nonspecific vaginitis.Crossref | GoogleScholarGoogle Scholar | 6967562PubMed |
[6] Plummer EL, Vodstrcil LA, Bodiyabadu K, et al. Are Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum associated with specific genital symptoms and clinical signs in nonpregnant women? Clin Infect Dis 2021; 73 659–68.
| Are Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum associated with specific genital symptoms and clinical signs in nonpregnant women?Crossref | GoogleScholarGoogle Scholar | 33502501PubMed |
[7] Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS ONE 2013; 8 e74378
| The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis.Crossref | GoogleScholarGoogle Scholar | 24040236PubMed |
[8] Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 2007; 34 864–9.
| The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health.Crossref | GoogleScholarGoogle Scholar | 17621244PubMed |
[9] Krohn MA, Hillier SL, Kiviat NB, Eschenbach DA. The severity of fetal membrane infection and pregnancy complications. Ann Epidemiol 1993; 3 78–85.
| The severity of fetal membrane infection and pregnancy complications.Crossref | GoogleScholarGoogle Scholar | 8287160PubMed |
[10] Donders GG, Van Calsteren K, Bellen G, et al. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG 2009; 116 1315–24.
| Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy.Crossref | GoogleScholarGoogle Scholar | 19538417PubMed |
[11] Hocevar K, Maver A, Vidmar Simic M, et al. Vaginal microbiome signature is associated with spontaneous preterm delivery. Front Med (Lausanne) 2019; 6 201
| Vaginal microbiome signature is associated with spontaneous preterm delivery.Crossref | GoogleScholarGoogle Scholar | 31552254PubMed |
[12] Svare JA, Schmidt H, Hansen BB, Lose G. Bacterial vaginosis in a cohort of Danish pregnant women: prevalence and relationship with preterm delivery, low birthweight and perinatal infections. BJOG 2006; 113 1419–25.
| 17010117PubMed |
[13] Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med 1995; 333 1737–42.
| Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant.Crossref | GoogleScholarGoogle Scholar | 7491137PubMed |
[14] Gravett MG, Nelson HP, DeRouen T, Critchlow C, Eschenbach DA, Holmes KK. Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome. JAMA 1986; 256 1899–903.
| Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome.Crossref | GoogleScholarGoogle Scholar | 3761496PubMed |
[15] Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ 1994; 308 295–8.
| Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage.Crossref | GoogleScholarGoogle Scholar | 8124116PubMed |
[16] Haggerty CL, Hillier SL, Bass DC, Ness RB. Bacterial vaginosis and anaerobic bacteria are associated with endometritis. Clin Infect Dis 2004; 39 990–5.
| Bacterial vaginosis and anaerobic bacteria are associated with endometritis.Crossref | GoogleScholarGoogle Scholar | 15472851PubMed |
[17] Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 2007; 109 114–20.
| Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data.Crossref | GoogleScholarGoogle Scholar | 17197596PubMed |
[18] Allsworth JE, Peipert JF. Severity of bacterial vaginosis and the risk of sexually transmitted infection. Am J Obstet Gynecol 2011; 205 113.e1–6.
| Severity of bacterial vaginosis and the risk of sexually transmitted infection.Crossref | GoogleScholarGoogle Scholar |
[19] Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193 1478–86.
| High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence.Crossref | GoogleScholarGoogle Scholar | 16652274PubMed |
[20] Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991; 29 297–301.
| Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation.Crossref | GoogleScholarGoogle Scholar | 1706728PubMed |
[21] Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 74 14–22.
| Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations.Crossref | GoogleScholarGoogle Scholar | 6600371PubMed |
[22] Cauci S, Driussi S, De Santo D, et al. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J Clin Microbiol 2002; 40 2147–52.
| Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women.Crossref | GoogleScholarGoogle Scholar | 12037079PubMed |
[23] Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transmit Dis 2019; 46 304–11.
| High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[24] Vodstrcil LA, Walker SM, Hocking JS, et al. Incident bacterial svaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis 2015; 60 1042–53.
| Incident bacterial svaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV.Crossref | GoogleScholarGoogle Scholar | 25516188PubMed |
[25] Evans AL, Scally AJ, Wellard SJ, Wilson JD. Prevalence of bacterial vaginosis in lesbians and heterosexual women in a community setting. Sex Transm Infect 2007; 83 470–5.
| Prevalence of bacterial vaginosis in lesbians and heterosexual women in a community setting.Crossref | GoogleScholarGoogle Scholar | 17611235PubMed |
[26] Bradshaw C, Bilardi J, Walker S, et al. Behavioural factors associated with bacterial vaginosis (BV) in women who have sex with women (WSW): the women on women’s (WOW) health study. Sex Transmit Infect 2011; 87 A32–3.
[27] Marrazzo JM, Koutsky LA, Eschenbach DA, Agnew K, Stine K, Hillier SL. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J Infect Dis 2002; 185 1307–13.
| Characterization of vaginal flora and bacterial vaginosis in women who have sex with women.Crossref | GoogleScholarGoogle Scholar | 12001048PubMed |
[28] Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol 2013; 209 505–23.
| The global epidemiology of bacterial vaginosis: a systematic review.Crossref | GoogleScholarGoogle Scholar | 23659989PubMed |
[29] Bukusi EA, Cohen CR, Meier AS, et al. Bacterial vaginosis: risk factors among Kenyan women and their male partners. Sex Transm Dis 2006; 33 361–7.
| Bacterial vaginosis: risk factors among Kenyan women and their male partners.Crossref | GoogleScholarGoogle Scholar | 16547451PubMed |
[30] Chico RM, Mayaud P, Ariti C, Mabey D, Ronsmans C, Chandramohan D. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: a systematic review. JAMA 2012; 307 2079–86.
| Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: a systematic review.Crossref | GoogleScholarGoogle Scholar | 22665107PubMed |
[31] Torrone EA, Morrison CS, Chen PL, et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med 2018; 15 e1002511
| Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies.Crossref | GoogleScholarGoogle Scholar | 29485986PubMed |
[32] Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis 2008; 35 78–83.
| A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology.Crossref | GoogleScholarGoogle Scholar | 17989585PubMed |
[33] Ness RB, Hillier S, Richter HE, et al. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc 2003; 95 201–12.
| 12749680PubMed |
[34] Brown J, Hess K, Brown S, Murphy C, Waldman A, Hezareh M. P1-S1.27 Intravaginal practices, lubrication, and bacterial vaginosis among women in Los Angeles. Sex Transm Infect 2011; 87 A110
| P1-S1.27 Intravaginal practices, lubrication, and bacterial vaginosis among women in Los Angeles.Crossref | GoogleScholarGoogle Scholar |
[35] Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6 e1000097
| Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.Crossref | GoogleScholarGoogle Scholar | 19621072PubMed |
[36] Ison CA, Hay PE. Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics. Sex Transm Infect 2002; 78 413–5.
| Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics.Crossref | GoogleScholarGoogle Scholar | 12473800PubMed |
[37] Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ ( 2003; 327 557–60.
| Measuring inconsistency in meta-analyses.Crossref | GoogleScholarGoogle Scholar |
[38] Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 2007; 36 666–6.
| Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography.Crossref | GoogleScholarGoogle Scholar | 17470488PubMed |
[39] Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016; 6 e011458
| Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS).Crossref | GoogleScholarGoogle Scholar | 27932337PubMed |
[40] Djomand G, Gao H, Singa B, et al. Genital infections and syndromic diagnosis among HIV-infected women in HIV care programmes in Kenya. Int J STD AIDS 2016; 27 19–24.
| Genital infections and syndromic diagnosis among HIV-infected women in HIV care programmes in Kenya.Crossref | GoogleScholarGoogle Scholar | 25614522PubMed |
[41] Hay PE, Taylorrobinson D, Lamont RF. Diagnosis of bacterial vaginosis in a gynecology clinic. Br J Obstet Gynaecol 1992; 99 63–6.
| Diagnosis of bacterial vaginosis in a gynecology clinic.Crossref | GoogleScholarGoogle Scholar | 1547176PubMed |
[42] Joshi S, Mane A, Muwonge R, et al. Prevalence and predictors of bacterial vaginosis in HIV-infected women in Maharashtra, India. Int J STD AIDS 2020; 31 541–52.
| Prevalence and predictors of bacterial vaginosis in HIV-infected women in Maharashtra, India.Crossref | GoogleScholarGoogle Scholar | 32233718PubMed |
[43] Li M, Li L, Wang R, et al. Prevalence and risk factors for bacterial vaginosis and cervicitis among 511 female workers attending gynecological examination in Changchun, China. Taiwan J Obstet Gynecol 2019; 58 385–89.
| Prevalence and risk factors for bacterial vaginosis and cervicitis among 511 female workers attending gynecological examination in Changchun, China.Crossref | GoogleScholarGoogle Scholar | 31122530PubMed |
[44] Sianou A, Galyfos G, Moragianni D, Baka S. Prevalence of vaginitis in different age groups among females in Greece. J Obstet Gynaecol 2017; 37 790–4.
| Prevalence of vaginitis in different age groups among females in Greece.Crossref | GoogleScholarGoogle Scholar | 28468531PubMed |
[45] Spinillo A, Bernuzzi AM, Cevini C, Gulminetti R, Luzi S, De Santolo A. The relationship of bacterial vaginosis, candida and trichomonas infection to symptomatic vaginitis in postmenopausal women attending a vaginitis clinic. Maturitas 1997; 27 253–60.
| The relationship of bacterial vaginosis, candida and trichomonas infection to symptomatic vaginitis in postmenopausal women attending a vaginitis clinic.Crossref | GoogleScholarGoogle Scholar | 9288698PubMed |
[46] Tibaldi C, Cappello N, Latino MA, Masuelli G, Marini S, Benedetto C. Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: risk factors and rates of occurrence. Clin Microbiol Infect 2009; 15 670–9.
| Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: risk factors and rates of occurrence.Crossref | GoogleScholarGoogle Scholar | 19558525PubMed |
[47] Brown JM, Hess KL, Brown S, Murphy C, Waldman AL, Hezareh M. Intravaginal practices and risk of bacterial vaginosis and candidiasis infection among a cohort of women in the United States. Obstet Gynecol 2013; 121 773–80.
| Intravaginal practices and risk of bacterial vaginosis and candidiasis infection among a cohort of women in the United States.Crossref | GoogleScholarGoogle Scholar | 23635677PubMed |
[48] Hoffmann JN, You HM, Hedberg EC, Jordan JA, McClintock MK. Prevalence of bacterial vaginosis and Candida among postmenopausal women in the United States. J Gerontol B Psychol Sci Soc Sci 2014; 69 S205–14.
| Prevalence of bacterial vaginosis and Candida among postmenopausal women in the United States.Crossref | GoogleScholarGoogle Scholar | 25360022PubMed |
[49] Massad LS, Evans CT, Kang R, et al. Correlates of bacterial vaginosis over long-term follow-up: impact of HIV infection. AIDS Res Hum Retroviruses 2017; 33 432–39.
| Correlates of bacterial vaginosis over long-term follow-up: impact of HIV infection.Crossref | GoogleScholarGoogle Scholar | 27841674PubMed |
[50] Myer L, Denny L, Telerant R, Souza M, Wright TC, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case–control study. J Infect Dis 2005; 192 1372–80.
| Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case–control study.Crossref | GoogleScholarGoogle Scholar | 16170754PubMed |
[51] de Castro EB, Brito LGO, Giraldo PC, Juliato CRT. Does the vaginal flora modify when a synthetic mesh is used for genital prolapse repair in postmenopausal women? A pilot, randomized controlled study. Female Pelvic Med Reconstr Surg 2019; 25 284–8.
| Does the vaginal flora modify when a synthetic mesh is used for genital prolapse repair in postmenopausal women? A pilot, randomized controlled study.Crossref | GoogleScholarGoogle Scholar | 29324569PubMed |
[52] O’Muircheartaigh C, Eckman S, Smith S. Statistical design and estimation for the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci 2009; 64 i12–19.
| Statistical design and estimation for the national social life, health, and aging project.Crossref | GoogleScholarGoogle Scholar | 19567827PubMed |
[53] Taj N, Ul Alam MS. Taj N, Ul Alam MS. Bacterial vaginosis in pregnant women and its diagnosis using Amsel’s clinical criteria and Nugent’s method. Pak J Med Health Sci 2014; 8 133–5.
[54] Sapra KJ, Randis TM, Whittier S, Gelber SE, Ratner AJ. Characteristics of women with bacterial vaginosis undetected by Amsel criteria. Reprod Sci 2013; 20 192A
[55] Sha BE, Chen HY, Wang QJ, Zariffard MR, Cohen MH, Spear GT. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J Clin Microbiol 2005; 43 4607–12.
| Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women.Crossref | GoogleScholarGoogle Scholar | 16145114PubMed |
[56] Sturdee DW, Panay N. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric 2010; 13 509–22.
| Recommendations for the management of postmenopausal vaginal atrophy.Crossref | GoogleScholarGoogle Scholar | 20883118PubMed |
[57] Mitchell CM, Srinivasan S, Ma N, et al. Bacterial communities associated with abnormal Nugent score in postmenopausal versus premenopausal women. J Infect Dis 2021; 223 2048–52.
| Bacterial communities associated with abnormal Nugent score in postmenopausal versus premenopausal women.Crossref | GoogleScholarGoogle Scholar | 33107562PubMed |
[58] Shen J, Song N, Williams CJ, et al. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci Rep 2016; 6 24380
| Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis.Crossref | GoogleScholarGoogle Scholar | 27103314PubMed |
[59] Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol 2005; 162 585–90.
| A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease.Crossref | GoogleScholarGoogle Scholar | 16093289PubMed |
[60] Haggerty CL, Ness RB, Totten PA, et al. Presence and concentrations of select bacterial vaginosis-associated bacteria are associated with increased risk of pelvic inflammatory disease. Sex Transm Dis 2020; 47 344–6.
| Presence and concentrations of select bacterial vaginosis-associated bacteria are associated with increased risk of pelvic inflammatory disease.Crossref | GoogleScholarGoogle Scholar | 32149953PubMed |
[61] Gallo MF, Macaluso M, Warner L, et al. Bacterial vaginosis, gonorrhea, and chlamydial infection among women attending a sexually transmitted disease clinic: a longitudinal analysis of possible causal links. Ann Epidemiol 2012; 22 213–20.
| Bacterial vaginosis, gonorrhea, and chlamydial infection among women attending a sexually transmitted disease clinic: a longitudinal analysis of possible causal links.Crossref | GoogleScholarGoogle Scholar | 22192490PubMed |
[62] Baisley K, Changalucha J, Weiss HA, et al. Bacterial vaginosis in female facility workers in north-western Tanzania: prevalence and risk factors. Sex Transm Infect 2009; 85 370–5.
| Bacterial vaginosis in female facility workers in north-western Tanzania: prevalence and risk factors.Crossref | GoogleScholarGoogle Scholar | 19473997PubMed |
[63] Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. Aids 2008; 22 1493–501.
| Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies.Crossref | GoogleScholarGoogle Scholar | 18614873PubMed |
[64] Lyons A, Heywood W, Fileborn B, et al. Sexually active older Australian’s knowledge of sexually transmitted infections and safer sexual practices. Aust NZ J Public Health 2017; 41 259–61.
| Sexually active older Australian’s knowledge of sexually transmitted infections and safer sexual practices.Crossref | GoogleScholarGoogle Scholar |
[65] Lindau ST, Leitsch SA, Lundberg KL, Jerome J. Older women’s attitudes, behavior, and communication about sex and HIV: a community-based study. J Womens Health (Larchmt) 2006; 15 747–53.
| Older women’s attitudes, behavior, and communication about sex and HIV: a community-based study.Crossref | GoogleScholarGoogle Scholar |
[66] Bourchier L, Malta S, Temple-Smith M, Hocking J. Do we need to worry about sexually transmissible infections (STIs) in older women in Australia? An investigation of STI trends between 2000 and 2018. Sexual Health 2020; 17 517–24.
| Do we need to worry about sexually transmissible infections (STIs) in older women in Australia? An investigation of STI trends between 2000 and 2018.Crossref | GoogleScholarGoogle Scholar | 33334416PubMed |
[67] Brotman RM, Klebanoff MA, Nansel TR, et al. Bacterial vaginosis assessed by Gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis 2010; 202 1907–15.
| Bacterial vaginosis assessed by Gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection.Crossref | GoogleScholarGoogle Scholar | 21067371PubMed |
[68] Bautista CT, Wurapa EK, Sateren WB, Hollingsworth BP, Sanchez JL. Longitudinal association of gonorrhea and bacterial vaginosis with repeat chlamydia diagnoses among U.S. Army women: a retrospective cohort analysis. Mil Med Res 2018; 5 37
| Longitudinal association of gonorrhea and bacterial vaginosis with repeat chlamydia diagnoses among U.S. Army women: a retrospective cohort analysis.Crossref | GoogleScholarGoogle Scholar | 30373657PubMed |
[69] Lv P, Zhao F, Xu X, Xu J, Wang Q, Zhao Z. Correlation between common lower genital tract microbes and high-risk human papillomavirus infection. Can J Infect Dis Med Microbiol 2019; 2019 9678104
| Correlation between common lower genital tract microbes and high-risk human papillomavirus infection.Crossref | GoogleScholarGoogle Scholar | 31885754PubMed |
[70] Wang W, Zhang XH, Li M, Hao CH, Liang HP. Association between vaginal infections and the types and viral loads of human papillomavirus: a clinical study based on 4,449 cases of gynecologic outpatients. Can J Infect Dis Med Microbiol 2020; 2020 9172908
| Association between vaginal infections and the types and viral loads of human papillomavirus: a clinical study based on 4,449 cases of gynecologic outpatients.Crossref | GoogleScholarGoogle Scholar | 32273935PubMed |


