World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts
Magnus Unemo A K , Monica M. Lahra B , Michelle Cole C , Patricia Galarza D , Francis Ndowa E , Irene Martin F , Jo-Anne R. Dillon G , Pilar Ramon-Pardo H , Gail Bolan I and Teodora Wi JA World Health Organization Collaborating Centre for Gonorrhoea and Other STIs, Department of Laboratory Medicine, Faculty of Medicine and Health, Örebro University, SE-701 85 Örebro, Sweden.
B World Health Organization Collaborating Centre for Sexually Transmitted Infections and Antimicrobial Resistance, New South Wales Health Pathology, Microbiology, Randwick, NSW, Australia.
C National Infection Service, Public Health England, London, UK.
D National Reference Laboratory for STDs, National Institute of Infectious Diseases – ANLIS ‘Dr Carlos G. Malbrán’, Buenos Aires, Argentina.
E Skin and Genitourinary Medicine Clinic, Harare, Zimbabwe.
F Public Health Agency of Canada, National Microbiology Laboratory, Winnipeg, MB, Canada.
G University of Saskatchewan, Saskatoon, SK, Canada.
H Communicable Diseases and Environmental Determinants of Health Department Pan American Health Organization/World Health Organization, Washington, DC, USA.
I Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
J Department of Reproductive Health and Research, World Health Organization, Geneva, Switzerland.
K Corresponding author. Email: magnus.unemo@regionorebrolan.se
Sexual Health 16(5) 412-425 https://doi.org/10.1071/SH19023
Submitted: 7 February 2019 Accepted: 29 May 2019 Published: 23 August 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Antimicrobial resistance (AMR) in Neisseria gonorrhoeae is a serious public health problem, compromising the management and control of gonorrhoea globally. Resistance in N. gonorrhoeae to ceftriaxone, the last option for first-line empirical monotherapy of gonorrhoea, has been reported from many countries globally, and sporadic failures to cure especially pharyngeal gonorrhoea with ceftriaxone monotherapy and dual antimicrobial therapies (ceftriaxone plus azithromycin or doxycycline) have been confirmed in several countries. In 2018, the first gonococcal isolates with ceftriaxone resistance plus high-level azithromycin resistance were identified in England and Australia. The World Health Organization (WHO) Global Gonococcal Antimicrobial Surveillance Program (GASP) is essential to monitor AMR trends, identify emerging AMR and provide evidence for refinements of treatment guidelines and public health policy globally. Herein we describe the WHO GASP data from 67 countries in 2015–16, confirmed gonorrhoea treatment failures with ceftriaxone with or without azithromycin or doxycycline, and international collaborative actions and research efforts essential for the effective management and control of gonorrhoea. In most countries, resistance to ciprofloxacin is exceedingly high, azithromycin resistance is present and decreased susceptibility or resistance to ceftriaxone has emerged. Enhanced global collaborative actions are crucial for the control of gonorrhoea, including improved prevention, early diagnosis, treatment of index patient and partner (including test-of-cure), improved and expanded AMR surveillance (including surveillance of antimicrobial use and treatment failures), increased knowledge of correct antimicrobial use and the pharmacokinetics and pharmacodynamics of antimicrobials and effective drug regulations and prescription policies (including antimicrobial stewardship). Ultimately, rapid, accurate and affordable point-of-care diagnostic tests (ideally also predicting AMR and/or susceptibility), new therapeutic antimicrobials and, the only sustainable solution, gonococcal vaccine(s) are imperative.
Additional keywords: antimicrobial resistance, azithromycin, ceftriaxone, gonorrhoea, Neisseria gonorrhoeae, treatment.
Introduction
In 2016, the World Health Organization (WHO) estimated 86.9 million cases of gonorrhoea, which remains a global public health concern, among adults worldwide.1–5 Effective and accessible antimicrobial treatment is essential for gonorrhoea management, but antimicrobial resistance (AMR) in Neisseria gonorrhoeae has emerged to all previous first-line therapeutic drugs.3–6 The extended-spectrum cephalosporin (ESC) ceftriaxone is the last option for empirical first-line gonorrhoea monotherapy, but decreased susceptibility or resistance (DS/R) has been reported worldwide,3–24 and sporadic failures to cure especially pharyngeal gonorrhoea with ceftriaxone monotherapy have been confirmed in several countries.9,22,25–30 From 2011, dual antimicrobial therapy (ceftriaxone 250–500 mg plus azithromycin 1–2 g) was introduced for empirical first-line therapy in many countries.5,31–34 Worryingly, the first failure of treating pharyngeal gonorrhoea with dual therapy was reported in 2016,35 international spread of ceftriaxone-resistant gonococcal strains was confirmed in 2015–1915–21 and the first strain with resistance to ceftriaxone plus high-level azithromycin resistance was isolated in England and Australia in 2018.22–24
In 2012, the WHO published a global action plan to control the spread and impact of AMR in N. gonorrhoeae.3,36 This plan is linked to the WHO global action plan on AMR.37 The gonococcal global action plan stresses the key priorities, including: advocacy for increased awareness on correct antimicrobial use; effective drug regulations and prescription policies; effective early prevention, diagnosis, sexual partner notification, treatment, including test of cure (TOC), and control of gonorrhoea; research into alternative and especially novel treatment options and vaccine(s); and strengthened regular, quality-assured and comparable AMR surveillance nationally and internationally, including systematic monitoring of treatment failures and research into the use of molecular AMR prediction methods.3,36 Gonococcal AMR surveillance is essential to identify emerging AMR, monitor AMR trends and to provide evidence for revisions of global, regional and national gonorrhoea management guidelines and public health strategies and policies.
This paper describes WHO Global Gonococcal Antimicrobial Surveillance Program (GASP) data from 67 countries in 2015–16, reviews reported confirmed gonorrhoea treatment failures with ceftriaxone with or without azithromycin or doxycycline, highlights the crucial need for molecular AMR surveillance and describes international collaborative actions and research efforts essential for the effective management and control of gonorrhoea.
WHO GASP
Since 2009, WHO GASP,6 initially established in 1990,38 has been significantly expanded. WHO GASP consists of reference laboratories globally that are networked with other international and national programs, such as the European Gonococcal Antimicrobial Surveillance Program (Euro-GASP),7,8 the US Gonococcal Isolate Surveillance Project (GISP; https://www.cdc.gov/std/gisp/, accessed 28 June 2019),39,40 the Canadian GASP,41 the Gonococcal Antimicrobial Susceptibility Surveillance Program – Argentina (GASSP),42 the UK Gonococcal Resistance to Antimicrobials Surveillance Program (GRASP)43 and the Australian Gonococcal Surveillance Program (AGSP).44
The WHO recommends that gonorrhoea management guidelines are revised based on recent and quality-assured gonococcal AMR surveillance data and first-line empirical gonorrhoea treatment be discontinued when the level of treatment failures and/or in vitro AMR reach 5%.3–6 However, the evidence for the ≥5% AMR threshold is limited, and proportions of >1% and >3% resistance in high-frequency transmitting populations have also been suggested.45,46 It is important to consider whether dual therapy is used, making the threshold for single antimicrobial suboptimal, and the availability of new antimicrobials. Further, the prevalence and local epidemiology of gonorrhoea and AMR, aetiological diagnostics or syndromic management, transmission frequency, sexual partner tracing strategies and treatment strategies and cost are important.4,47,48
WHO GASP data in 2015–16
The structure, sampling and testing methodologies, quality assurance (QA) and quality control (QC) practices, including the well-characterised 2016 WHO gonococcal reference strains for intra- and interlaboratory comparisons,49–51 of WHO GASP have been described previously.6 Briefly, the aims of WHO GASP include collecting ≥100 representative gonococcal isolates per country and year, ideally using quantitative methods for determination of the minimum inhibitory concentration (MIC) of antimicrobials (agar dilution method or MIC gradient strip tests, such as Etest (bioMérieux, Marcy-l’Étoile, France)) and using validated and standardised interpretative criteria (i.e. most WHO GASP countries use the breakpoints stated by the European Committee on Antimicrobial Susceptibility Testing (EUCAST; www.eucast.org, accessed 28 June 2019) or the Clinical and Laboratory Standards Institute (CLSI; www.clsi.org, accessed 28 June 2019)).
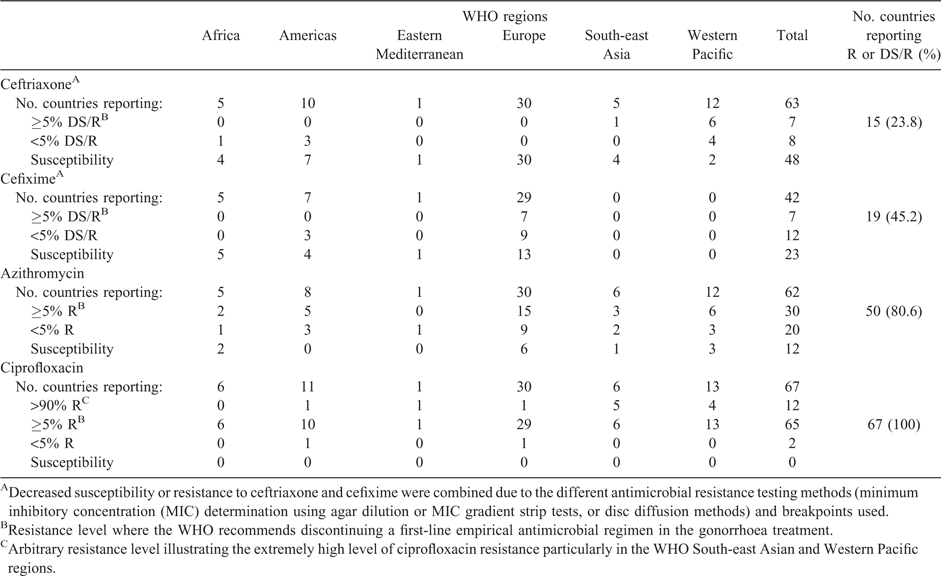
From 1 January 2015 to 31 December 2016, 67 countries reported gonococcal AMR data to the WHO (Geneva, Switzerland) for ceftriaxone (63 countries), cefixime (42 countries), azithromycin (62 countries) and/or ciprofloxacin (67 countries). Fifty-two (77.6%) countries reported data in both 2015 (56 countries; 83.6%) and 2016 (62 countries; 92.5%). The number of countries reporting data for at least one antimicrobial annually was the same as in 2014 (52 countries).
In 2015–16, as in 2014, the WHO European Region (EUR)52 had the highest number of reporting countries (30 countries; including 26 European Union (EU)/European Economic Area (EEA) countries), followed by the Western Pacific Region (WPR;53 13 countries), Region of Americas (including Latin America, Caribbean54,55 and North America with the US39,40 (www.cdc.gov/std/gisp/default.htm) and Canada;41 11 countries), South-east Asian Region (SEAR;56 6 countries), African Region (AFR;57 6 countries) and the Eastern Mediterranean Region (EMR; 1 reporting country).
Of countries monitoring susceptibility to ceftriaxone (63 countries), cefixime (42 countries), azithromycin (62 countries) and/or ciprofloxacin (67 countries), 23.8%, 45.2%, 80.6% and 100% of countries respectively reported isolates showing DS/R in 2015–16 (Table 1).
The most recent WHO GASP data are summarised in Figs 1–4.
Ceftriaxone and cefixime
In 2015–16, 64 countries reported on susceptibility to ceftriaxone and/or cefixime, which is significantly more countries compared with 2014 (45 countries; P < 0.05). Thirty-two (50%) countries reported DS/R to either of these ESCs, which is a significant decrease from the 66% of countries in 2009–146 (P < 0.05). In 2016, 58 countries reported data on susceptibility to ceftriaxone and 22.4% (13/58) reported isolates with DS/R (Fig. 1). Thirty-nine countries reported data on susceptibility to cefixime and 48.7% (19/39) reported isolates with DS/R (Fig. 2).
In the Euro-GASP,7,8,58 2660 isolates from 25 EU/EEA countries in WHO EUR were tested in 2016. No isolates were resistant to ceftriaxone, compared with one (0.05%) in 2015 and five (0.23%) in 2014.8 Furthermore, no resistance to ceftriaxone was found in 2015–16 in the non-EU/EEA WHO EUR countries of Belarus (36 isolates), Kyrgyzstan (n = 72), Russia (n = 293) and Ukraine (n = 58; Fig. 1). Cefixime-resistant isolates were detected in 56% (14/25) of the EU//EEA countries, but the overall resistance was stable at 2.1% (1.7% in 2015, 2.0% in 2014). Twenty-four per cent of countries (n = 6; Croatia (11.1%), Luxembourg (10.0%), Hungary (8.5%), Belgium (8.1%), Germany (6.4%) and Poland (5.2%)) reported ≥5% resistance. As in 2015, cefixime resistance was highest among heterosexual males (2.2%), followed by females (2%) and men who have sex with men (MSM; 0%).8 No cefixime resistance was detected in Ukraine, but cefixime resistance was detected in Belarus (22.2%) and Kyrgyzstan (1.4%; Fig. 2).
In the WHO WPR in 2016, DS/R to ceftriaxone was reported by 83.3% (10/12) of countries reporting on ceftriaxone susceptibility in 9763 isolates, an increase from 71% (5/7) in 2014. Six countries (China, Japan, Mongolia, Singapore, Republic of Korea and Vietnam) reported DS/R in ≥5% of isolates. Only two countries (New Caledonia and Cambodia) reported all isolates as susceptible to ceftriaxone in 2016 (Fig. 1).59 However, only two isolates were tested in Cambodia.
In the WHO SEAR, DS/R to ceftriaxone was reported in one (Myanmar) of the countries (n = 5) reporting ceftriaxone susceptibility data in 907 isolates in 2016. However, only five isolates were tested in Myanmar. Only susceptible isolates were found in Bhutan, India, Sri Lanka and Thailand (Fig. 1).59,60
In the WHO Region of Americas, DS/R to ceftriaxone in 2016 was reported in three (33.3%) of the nine countries reporting on ceftriaxone susceptibility in 12 887 isolates in 2016 (Peru (2.4%), Canada (1.8%; vs 2.7% in 201441) and the US (0.3%; vs 0.4% in 2014; Fig. 1). Notably, in 2014, DS/R to ceftriaxone was confirmed in Argentina and reported from Bolivia (disc diffusion results not confirmed by MIC determination).6,61 DS/R to cefixime in 2016 was documented in Argentina (1.4%), Canada (0.3%; vs 1.1% in 201441) and the US (0.3%; declined from 0.9% in 2012; www.cdc.gov/std/gisp/default.htm, accessed 28 June 2019). All isolates in Brazil,62 Chile and Paraguay (and Peru in 2015) were susceptible to cefixime (Fig. 2). In 2013–14, DS/R to cefixime was reported also in Chile and Uruguay.6
In 2016, only four of 47 WHO AFR countries (8.5%) provided ceftriaxone susceptibility data for 496 isolates and no DS/R was reported from Cote d’Ivoire,63 South Africa or Zimbabwe.64 However, one of 35 isolates (3.0%) from Madagascar showed DS/R to ceftriaxone, but this single isolate was not confirmed using MIC determination (Fig. 1). Notably, DS/R to ceftriaxone has been reported from South Africa and Uganda.6 In 2016, no DS/R to cefixime was detected in Cote d’Ivoire,63 Malawi, South Africa or Zimbabwe (Fig. 2).64
In the WHO EMR, only Pakistan (4.5%; 1/22) provided ceftriaxone and cefixime susceptibility data for 64 isolates in 2016 and no DS/R to these ESCs was found (Figs 1, 2).
Azithromycin
In 2016, 59 countries reported data on azithromycin susceptibility and 83.0% (49/59) of countries reported resistant isolates, which is similar (P > 0.05) to the 81% (47/58) and 78% (35/45) reported in 2009–14 and 2014 respectively. In 2016, 29 (49.1%) countries reported ≥5% azithromycin resistance (Fig. 3), which is significantly (P < 0.05) higher than in 2014 (28.9%; 13/45).6
In the WHO EUR, azithromycin-resistant isolates were detected in 84% (21/25) of Euro-GASP countries, but the overall resistance in the EU/EEA was stable at 7.5% (7.1% in 2015 and 7.9% in 2014).7,8 Fifty-two per cent (13/25) of Euro-GASP countries reported ≥5% resistance.8 As in 2015, azithromycin resistance was most common among heterosexual males (7.6%), followed by MSM (5.6%) and females (5.3%).8 The azithromycin resistance levels in Belarus, Russia and Ukraine were 13.9%, 6.5% and 4.0% respectively; however, no resistance was found in Kyrgyzstan (Fig. 3).
In the WHO WPR, 75.0% (9/12) of settings reported azithromycin-resistant isolates in 2016, compared with 78% (7/9) in 2014. Five settings reported ≥5% resistance (Australia, Cambodia (only two isolates tested), China, Hong Kong, Japan and New Zealand) and three countries reported <5% resistance (Brunei, Mongolia and Singapore; Fig. 3).59
In the WHO SEAR, azithromycin resistance was detected in all six countries reporting in 2016, compared with 83% (5/6) in 2014. Three (50%) countries (India, Myanmar and Indonesia) reported ≥5% resistance (Fig. 3), but only three and five isolates were tested with a disc diffusion method in Myanmar and Indonesia respectively.
In the WHO Region of Americas, azithromycin resistance was identified in all eight countries reporting in 2016. Five (62.5%) countries (Brazil,62 Canada, Columbia, Cuba and Peru) reported ≥5% resistance, whereas three (37.5%) reported <5% resistance (Argentina, Chile and the US; 3.6% vs 2.5% in 2014;6 Fig. 3).
In the WHO AFR, Cote d’Ivoire63 and Malawi reported azithromycin-resistant isolates (2.0% and 12.6% respectively) in 2016, whereas no azithromycin resistance was detected in Madagascar or South Africa. Notably, ≥5% azithromycin resistance was reported in Uganda in 2014, South Africa in 2013 and Kenya in 20146 and 2015 (Fig. 3).
In the WHO EMR, 4.7% of isolates in Pakistan were resistant to azithromycin in 2016 (Fig. 3).
Ciprofloxacin
In 2016, the highest ciprofloxacin resistance levels were recorded in the WHO SEAR (94.2%) and WPR (72.0%). In these WHO regions, nine of the 19 reporting countries (47.4%) reported >90% resistance and six (33.3%) countries reported >80% resistance. Also in the WHO AFR (69.1%), EMR (Pakistan: 96.9%) and EUR (45.4%), three countries reported >90% resistance, namely Madagascar, Pakistan and Kyrgyzstan respectively. Countries belonging to all the remaining WHO regions reported ≥70% ciprofloxacin resistance (Argentina, Cuba, Peru, Cote D’Ivoire,63 Malawi, South Africa, Iceland, Luxembourg;7,8 Fig. 4).
Confirmed gonorrhoea treatment failures with ceftriaxone, with or without azithromycin or doxycycline, and characterised ceftriaxone-resistant strains
Confirmed failures to cure gonorrhoea with ceftriaxone alone or combined with azithromycin or doxycycline are summarised in Table 2. Sporadic failures to cure gonorrhoea with ceftriaxone (250–1000 mg) have been confirmed during the past decade in Japan, Australia, Slovenia, Sweden and the UK.9,21,25–30 In 2016, the first global failure to cure pharyngeal gonorrhoea with dual therapy (ceftriaxone 500 mg plus azithromycin 1 g) was confirmed in England.35 An internationally spreading ceftriaxone-resistant gonococcal strain caused a failure to cure pharyngeal gonorrhoea with ceftriaxone 250 mg × 1 (plus doxycycline) in 2017 in France19 and with ceftriaxone 1 g × 1 in 2018 in England.21 In 2018, the first global gonococcal strain with ceftriaxone resistance plus high-level azithromycin resistance caused a treatment failure of pharyngeal gonorrhoea with ceftriaxone (1 g; plus doxycycline) in England.22 All confirmed gonorrhoea treatment failures, except one recent case in the UK,21 have been pharyngeal infection, a site where gonorrhoea is usually asymptomatic, tissue penetration of several antimicrobials is limited and new genetic AMR determinants can be acquired from coexisting non-gonococcal Neisseria species.65,66 Gonococcal infections acquired in the WHO WPR and SEAR represent most of the confirmed ceftriaxone treatment failures, but several have been acquired also in the EUR.9,19,21,22,25–30,35 The burden of ceftriaxone treatment failures is unknown, and it is essential to enhance gonococcal surveillance, including verification of treatment failures after treatment with recommended ceftriaxone and ESC plus azithromycin dual therapies. The WHO recommends, where feasible, examination of pre- and post-treatment isolates for MICs of relevant antimicrobials, molecular epidemiological genotype, molecular AMR determinants and documentation of all treatments administered and excluding reinfection.3,6,45,67
The ceftriaxone MICs of strains causing the confirmed ceftriaxone treatment failures ranged from 0.016 to 4 mg L–1. To cure gonorrhoea, 20–24 h of free ceftriaxone above MIC (fT>MIC) is required.68 According to Monte Carlo simulations, with doses of ceftriaxone 500 mg × 1 and ceftriaxone 1 g × 1, sufficient fT>MIC (≥20 h) will not be reached in up to 5% of individuals infected with gonococcal strains with ceftriaxone MICs of ≥0.06 and ≥0.125 mg L–1 respectively.68 This agrees with observations regarding the relatively low ceftriaxone MICs of strains causing several of the confirmed treatment failures (Table 2). The low ceftriaxone MICs (0.016–0.03 mg L–1) of strains causing some of these failures (Table 2) probably reflects a low bioavailability of ceftriaxone and the difficulties in curing pharyngeal gonorrhoea.4,9,25–30,65,67 Enhanced understanding of the pharmacokinetic and pharmacodynamic properties of current and novel antimicrobials in anogenital and pharyngeal gonorrhoea is critical. Promisingly, a murine genital tract infection model recently showed that the pharmacokinetic and pharmacodynamic relationships for ESCs in mice reflected those in humans with in vivo efficacy against an ESC-susceptible strain requiring an fT>MIC of >20–24 h.69 Furthermore, hollow-fibre bioreactor models70 are under development for gonococci.
The first extensively drug-resistant (XDR)67 gonococcal strains with high-level ceftriaxone resistance were reported from Japan, France and Spain in 2011–12.9–11 No additional cases caused by these strains were detected, and it was subsequently verified that their resistance-determining mosaic penicillin-binding protein 2 (PBP2) caused reduced fitness in vitro and in mice,71 which likely limited their spread. Worryingly, the failure to treat pharyngeal gonorrhoea with ceftriaxone in 2017 in France19 and in 2018 in England21 (Table 2) was caused by an internationally spreading ceftriaxone-resistant strain (FC428), initially identified in 2015 in Japan.15 FC428 subclones have been reported in Australia, Canada, Denmark, France, Ireland, England, Japan, China and Singapore in 2017–19.15–21,72 This is the first ceftriaxone-resistant gonococcal clone maintaining an adequate fitness and spreading internationally. FC428 and most of its subclones15–21,72 have originated from the WHO SEAR and WPR. The FC428 clade contains the ceftriaxone resistance-mediating mosaic penA-60.001 allele,73 which may have originated from Neisseria cinerea.74 The first gonococcal strain with ceftriaxone resistance plus high-level azithromycin resistance (WHO Q) identified in 2018 in England (infection acquired in Thailand) and Australia (one associated with travel to the SEAR) also harboured mosaic penA-60.001.22–24 This further indicates that the ceftriaxone resistance-mediating mosaic penA-60.001 does not substantially decrease the fitness of strains, enabling the international spread of ceftriaxone resistance.
Molecular surveillance of AMR in Neisseria gonorrhoeae
N. gonorrhoeae has used almost all known AMR mechanisms (enzymatic antimicrobial destruction or modification, modification or protection of antimicrobial targets, increased export (e.g. through MtrCDE efflux pump) and decreased uptake (e.g. through the porin PorB)).4 Some AMR determinants, particularly target alterations, directly cause AMR, whereas others require combination with other AMR determinants to cause AMR. The AMR determinants conferring resistance to currently and previously recommended treatment options have been detailed elsewhere.4,75,76
To enhance WHO GASP, it is essential to strengthen the gonococcal culture capacity internationally. It is also imperative to continue to develop and use appropriate molecular tests to detect gonococcal AMR determinants to predict AMR, particularly where gonococcal culture is unavailable. Many polymerase chain reaction (PCR) assays have been developed for this purpose.4,77–81 However, the prediction of susceptibility or resistance to antimicrobials other than ciprofloxacin and testing of clinical, especially extragenital, specimens have limitations, and few assays have been appropriately validated and quality assured.77 PCRs detecting specific ceftriaxone-resistant strains such as H041,9 F8910 and FC42815 have been developed.82–84 The N. gonorrhoeae Sequence Typing for Antimicrobial Resistance (NG-STAR; https://ngstar.canada.ca, accessed 28 June 2019), examining seven AMR determinants, has standardised the nomenclature of AMR determinants and can be used to track AMR strains and indirectly AMR prediction.73 Whole-genome sequencing (WGS) can elucidate the emergence, transmission and evolution of AMR and national and international spread of AMR gonococcal strains, but can also be used to predict AMR with relatively adequate accuracy.85,86 It was recently reported that rapid, real-time sequencing using the small hand-held MinION (Oxford Nanopore Technologies, Oxford, UK) can relatively accurately sequence genomes and predict resistance to ciprofloxacin and azithromycin, as well as DS/R to ESCs in gonococcal isolates.87 Unfortunately, genetic AMR prediction will never completely replace phenotypic AMR testing, which also detects AMR due to unknown AMR determinants.
In the future, molecular point-of-care (POC) tests detecting N. gonorrhoeae and AMR determinants for multiple antimicrobials will be available. These POC tests could be used for prompt diagnosis, AMR surveillance and to guide individualised treatments.77–80,88
Discussion
In 2015–16, WHO GASP documented sustained high levels of ciprofloxacin resistance, stable relatively high resistance to azithromycin and the emergence of DS/R to ESCs. Major concerns remain regarding the lack of AMR data in many countries worldwide, especially in the WHO AFR (12.8% of countries reporting any data in 2015–16) and EMR (4.5%), but also in the WHO Region of Americas (31.4%), WPR (44.8%), SEAR (54.5%) and EUR (55.6%; 83.9% in the EU/EEA7,8). Further, there are concerns regarding the representativeness of data due to low numbers of isolates (<100 isolates tested annually), selection of isolates, limited information about the total number of isolates and gonococcal diagnostic tests (culture and nucleic acid amplification tests (NAAT)), and the methodologies used in several countries. Critically, many countries with limited gonococcal AMR surveillance have high gonorrhoea incidences, suboptimal gonorrhoea diagnosis, over-the-counter access to antimicrobials, as well as limited availability of optimal antimicrobial treatment (e.g. high-quality ceftriaxone or dual therapy with ceftriaxone plus azithromycin). These factors combine to create the prerequisites for the emergence and spread of gonococcal AMR.4,67 To develop evidence-based treatment guidelines based on validated surveillance information, it is crucial to strengthen the WHO GASP and other GASPs (both more countries and more representative isolates), including the use of only MIC determination (agar dilution or MIC gradient strip tests) instead of different disc diffusion methods, and to monitor treatment failures. Strengthened AMR surveillance is especially urgent in the WHO AFR, where some progress has been made recently,63,64,89 and in the EMR. In the SEAR and WPR, there has been a substantial increase in the number of countries providing surveillance data for both ceftriaxone and azithromycin, and in ceftriaxone MIC testing across the Asia–Pacific;59 however, much more needs to be done in these regions because many ceftriaxone-resistant strains have originated from this region.15–23
To enhance WHO GASP, political engagement and financial commitment nationally and internationally is essential. An increased awareness among politicians (e.g. at national Ministries of Health) and healthcare professionals (at clinics, laboratories and public health organisations) that regular, representativeness and quality-assured gonococcal AMR surveillance should be the foundation of national AMR action plans to control gonorrhoea, part of routine diagnostics and/or surveillance and used to refine treatment recommendations is imperative. Additional gonococcal reference laboratories need to be established, and clinical and laboratory training must be provided regarding: (1) sample collection, transportation and preservation; (2) laboratory methodologies, including culture and AMR testing; and (3) appropriate QA, including use of internal QCs and external quality assessment (EQA).90–92 Appropriate research evaluating the precision, opportunities and cost-effectiveness of molecular AMR assays is important. These methods can supplement the phenotypic AMR surveillance and substantially increase the sample size in countries where gonococcal culture is limited. In remote Australia, molecular AMR assays have been used to enhance gonococcal AMR surveillance for several years.79,81–84 However, the limitations of molecular AMR prediction should be considered (e.g. cross-reactions with non-gonococcal species in clinical, particularly pharyngeal, specimens, suboptimal sensitivity and specificity in the AMR prediction for several key antimicrobials and that new AMR determinants will be undetected).4,77–80 Continued research to describe novel AMR determinants, and ideally their induction or selection, evolution and biological fitness, is imperative. In the future, new rapid molecular POC tests, including gonococcal detection and AMR prediction, will guide individualised treatment at the first healthcare visit.4,77–80 These rapid POC tests will improve the management and control of gonorrhoea and gonococcal AMR by directing appropriate treatment and sparing last-line antimicrobials, thus mitigating the emergence and spread of AMR. WGS is revolutionising knowledge of AMR, AMR prediction and the transmission of AMR gonococcal strains, and is informing the development of gonococcal diagnostics and a vaccine.51,85–87,93–100 It cannot be excluded that rapid, real-time sequencers, such as the hand-held MinION or similar sequencer, will be used for gonococcal detection and prediction of AMR in the future, including at POC.87 However, currently, the wider use of WGS is limited by its complexity and cost.
The main limitations of WHO GASP (and most other GASPs) include the limited number of countries, particularly in the WHO AFR and EMR, the low number and suboptimal representativeness (geographically, from all risk groups, sexes and anatomical sites) of isolates, the use of disc diffusion methods for AMR testing in some countries, the lack of standardised global QA including QCs and EQA, the lack of harmonised breakpoints for decreased susceptibility and/or resistance, the limited epidemiological data for gonorrhoea patients (except in Euro-GASP7,8,58 and US GISP39,40), the limited information on treatment and treatment failures and the delays in publication of AMR data, limiting the value of the data, particularly for early warning of AMR emergence. The WHO, together with the liaised GASPs, is continuously addressing these limitations. For example, training is regularly provided in GASP methodologies to culture more representative samples of gonococcal isolates. MIC determination (agar dilution or different MIC gradient strip tests, which also differ substantially in performance and quality101) is increasingly used and supported, and all ceftriaxone resistance identified by disc diffusion methods should be verified by MIC determination at national or international reference laboratories. Regional WHO reference centres assist with WHO reference strains49–51 for QA and ideally EQA, and the most frequently used CLSI (CLSI; www.clsi.org, accessed 28 June 2019) and EUCAST (EUCAST; www.eucast.org, accessed 28 June 2019) resistance breakpoints are increasingly harmonised in collaboration with GASPs. However, the suboptimal correlates between existing resistance breakpoints and treatment outcomes, particularly for azithromycin, need to be improved, making surveillance of treatment failures essential. Finally, in some sentinel countries (e.g. the Philippines and Thailand60), the WHO in collaboration with the US Centers for Disease Control and Prevention has initiated an enhanced GASP (EGASP) aiming to collect standardised and quality-assured epidemiological and clinical information, including treatment failures, linked to AMR data, as collected in Euro-GASP and GISP.7,8,39,40,58
In general, improved prevention, including primary prevention, testing (to also detect asymptomatic urogenital gonorrhoea in women and extragenital gonorrhoea in both sexes), management (including TOC) and control of gonorrhoea, ideally linked to general HIV and sexually transmissible infection (STI) prevention,3,36 and public health guidelines and policies are essential. Key prevention actions include: education regarding symptomatic and asymptomatic STIs; promotion of safe sexual behaviours, including increased condom use; behaviour change communication programs; enhanced sexual partner notification and treatment; expansion of targeted interventions, including screening in some settings for vulnerable populations (sex workers, MSM, adolescents and STI patients and their sexual partners); and think-out-of-box research, such as using antiseptic mouthwash to prevent pharyngeal gonorrhoea.102 However, the only sustainable solution for gonorrhoea control is adequate uptake of a sufficiently effective gonococcal vaccine. In the recent decade, significant progress in gonococcal vaccine development has been made, and increased research should be strongly promoted.103–109
Until a gonococcal vaccine is available, gonorrhoea control will need to rely on appropriate, timely and affordable antimicrobial treatment in combination with prevention strategies, diagnosis and surveillance. In 2016, the WHO published new guidelines for gonorrhoea treatment.5 In countries lacking recent, local and quality-assured gonococcal AMR data (and TOC), the WHO recommends dual therapy (ceftriaxone 250 mg plus azithromycin 1 g OR cefixime 400 mg plus azithromycin 1 g).5 Where feasible, the regimen with ceftriaxone plus azithromycin is preferable, and this regimen appears to be highly effective (very few ceftriaxone-resistant strains also show resistance to azithromycin). This recommendation is similar to the recommendations of dual antimicrobial therapy in many other regional or national gonorrhoea treatment guidelines.31–34 Notably, evidence for the selection of gonococcal resistance to azithromycin by the use of ceftriaxone plus azithromycin therapy for gonorrhoea is limited.110,111 Gonococcal azithromycin resistance is likely mostly a consequence of the widespread use of azithromycin monotherapy to treat respiratory tract infections, chlamydia and/or non-gonococcal urethritis.8,111 Nevertheless, new antimicrobials for monotherapy and/or inclusion in a dual therapy, which should be considered for all novel antimicrobials, for urogenital and extragenital gonorrhoea is essential. Antimicrobials such as lefamulin,112,113 SMT-571,114 gepotidacin115–117 and especially zoliflodacin118–122 must be studied further.123,124
In conclusion, gonococcal AMR remains a major global public health concern, which compromises treatment and control of gonorrhoea. WHO GASP, including its liaised GASPs, provides invaluable data regarding decreased susceptibility and resistance to therapeutically relevant antimicrobials. However, WHO GASP must continue to be significantly improved and sustainable, and national and international leadership and political and financial commitment are imperative. The inclusion of gonococcal AMR in the Global AMR Surveillance System (GLASS) (http://www.who.int/antimicrobial-resistance/global-action-plan/surveillance/glass/en/, accessed 28 June 2019) demonstrates the relevance of this public health threat and will also support the expansion of WHO GASP. Countries must strengthen their gonococcal AMR surveillance programs in the context of their national AMR programs, taking the opportunity of the investment in development of microbiology laboratory capacity. Work to improve the monitoring of antimicrobial use, the conservation and regulation of antimicrobials, monitoring prescription policies and enhancing awareness of the correct use of antimicrobials is also underway internationally. Research efforts should be of highest priority, including developing novel antimicrobials for gonorrhoea treatment (in conjunction with strategies to conserve these and other antimicrobials), gonococcal vaccine(s) and new diagnostic tests (including POC tests for simultaneous detection of gonococci and AMR) for diagnosis and surveillance.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors are very grateful to all the collaborators and contributors to WHO GASP globally. This research did not receive any specific funding.
References
[1] World Health Organization (WHO). Report on global sexually transmitted infection surveillance, 2018. Geneva: WHO; 2018. Available online at: https://www.who.int/reproductivehealth/publications/stis-surveillance-2018/en/ [verified February 2019].[2] Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 1997; 349 1868–73.
| Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1.Crossref | GoogleScholarGoogle Scholar | 9217758PubMed |
[3] World Health Organization (WHO). Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. Geneva: WHO; 2012. Available online at: http://www.who.int/reproductivehealth/publications/rtis/9789241503501/en/ [verified 28 June 2019].
[4] Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014; 27 587–613.
| Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future.Crossref | GoogleScholarGoogle Scholar | 24982323PubMed |
[5] World Health Organization (WHO). WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva: WHO; 2016. Available online at: http://www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/ [verified 28 June 2019].
[6] Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, et al Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 2017; 14 e1002344
| Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action.Crossref | GoogleScholarGoogle Scholar | 28746372PubMed |
[7] Cole MJ, Spiteri G, Jacobsson S, Woodford N, Tripodo F, Amato-Gauci AJ, et al Overall low extended-spectrum cephalosporin resistance but high azithromycin resistance in Neisseria gonorrhoeae in 24 European countries, 2015. BMC Infect Dis 2017; 17 617
| Overall low extended-spectrum cephalosporin resistance but high azithromycin resistance in Neisseria gonorrhoeae in 24 European countries, 2015.Crossref | GoogleScholarGoogle Scholar | 28893203PubMed |
[8] Day MJ, Spiteri G, Jacobsson S, Woodford N, Amato-Gauci AJ, Cole MJ, et al Stably high azithromycin resistance and decreasing ceftriaxone susceptibility in Neisseria gonorrhoeae in 25 European countries, 2016. BMC Infect Dis 2018; 18 609
| Stably high azithromycin resistance and decreasing ceftriaxone susceptibility in Neisseria gonorrhoeae in 25 European countries, 2016.Crossref | GoogleScholarGoogle Scholar | 30509194PubMed |
[9] Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, et al Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 2011; 55 3538–45.
| Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone.Crossref | GoogleScholarGoogle Scholar | 21576437PubMed |
[10] Cámara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, et al Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother 2012; 67 1858–60.
| Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain.Crossref | GoogleScholarGoogle Scholar | 22566592PubMed |
[11] Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant N. gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 2012; 56 1273–80.
| High-level cefixime- and ceftriaxone-resistant N. gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure.Crossref | GoogleScholarGoogle Scholar | 22155830PubMed |
[12] Gianecini R, Oviedo C, Stafforini G, Galarza P. Neisseria gonorrhoeae resistant to ceftriaxone and cefixime, Argentina. Emerg Infect Dis 2016; 22 1139–41.
| Neisseria gonorrhoeae resistant to ceftriaxone and cefixime, Argentina.Crossref | GoogleScholarGoogle Scholar | 27191699PubMed |
[13] Lahra MM, Ryder N, While DM. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Engl J Med 2014; 371 1850–1.
| A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia.Crossref | GoogleScholarGoogle Scholar | 25372111PubMed |
[14] Deguchi T, Yasuda M, Hatazaki K, Kameyama K, Horie K, Kato T, et al New clinical strain of Neisseria gonorrhoeae with decreased susceptibility to ceftriaxone in Japan. Emerg Infect Dis 2016; 22 142–4.
| New clinical strain of Neisseria gonorrhoeae with decreased susceptibility to ceftriaxone in Japan.Crossref | GoogleScholarGoogle Scholar | 26689442PubMed |
[15] Nakayama S, Shimuta K, Furubayashi K, Kawahata T, Unemo M, Ohnishi M. New ceftriaxone and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother 2016; 60 4339–41.
| New ceftriaxone and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan.Crossref | GoogleScholarGoogle Scholar | 27067334PubMed |
[16] Lahra MM, Martin I, Demczuk W, Jennison AV, Lee KI, Nakayama SI, et al Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis 2018; 24 735–40.
| Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain.Crossref | GoogleScholarGoogle Scholar |
[17] Lefebvre B, Martin I, Demczuk W, Deshaies L, Michaud S, Labbé AC, et al Ceftriaxone-resistant Neisseria gonorrhoeae, Canada, 2017. Emerg Infect Dis 2018; 24 381–3.
| Ceftriaxone-resistant Neisseria gonorrhoeae, Canada, 2017.Crossref | GoogleScholarGoogle Scholar |
[18] Terkelsen D, Tolstrup J, Johnsen CH, Lund O, Larsen HK, Worning P, et al Multidrug-resistant Neisseria gonorrhoeae infection with ceftriaxone resistance and intermediate resistance to azithromycin, Denmark, 2017. Euro Surveill 2017; 22.
| Multidrug-resistant Neisseria gonorrhoeae infection with ceftriaxone resistance and intermediate resistance to azithromycin, Denmark, 2017.Crossref | GoogleScholarGoogle Scholar | 29067905PubMed |
[19] Poncin T, Fouere S, Braille A, Camelena F, Agsous M, Bebear C, et al Multidrug-resistant Neisseria gonorrhoeae failing treatment with ceftriaxone and doxycycline in France, November 2017. Euro Surveill 2018; 23.
| Multidrug-resistant Neisseria gonorrhoeae failing treatment with ceftriaxone and doxycycline in France, November 2017.Crossref | GoogleScholarGoogle Scholar | 29845928PubMed |
[20] Golparian D, Rose L, Lynam A, Mohamed A, Bercot B, Ohnishi M, et al Multidrug-resistant Neisseria gonorrhoeae isolate, belonging to the internationally spreading Japanese FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin in Ireland, August 2018. Euro Surveill 2018; 23.
| Multidrug-resistant Neisseria gonorrhoeae isolate, belonging to the internationally spreading Japanese FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin in Ireland, August 2018.Crossref | GoogleScholarGoogle Scholar | 30482267PubMed |
[21] Eyre DW, Town K, Street T, Barker L, Sanderson N, Cole MJ, et al Detection in the United Kingdom of the Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, October to December 2018. Euro Surveill 2019; 24.
| Detection in the United Kingdom of the Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, October to December 2018.Crossref | GoogleScholarGoogle Scholar | 30862336PubMed |
[22] Eyre DW, Sanderson ND, Lord E, Regisford-Reimmer N, Chau K, Barker L, et al Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 2018; 23.
| Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018.Crossref | GoogleScholarGoogle Scholar | 29991383PubMed |
[23] Whiley DM, Jennison A, Pearson J, Lahra MM. Genetic characterization of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect Dis 2018; 18 717–18.
| Genetic characterization of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin.Crossref | GoogleScholarGoogle Scholar | 29976521PubMed |
[24] Jennison AV, Whiley D, Lahra MM, Graham RM, Cole MJ, Hughes G, et al Genetic relatedness of ceftriaxone-resistant and high-level azithromycin resistant Neisseria gonorrhoeae cases, United Kingdom and Australia, February to April 2018. Euro Surveill 2019; 24.
| Genetic relatedness of ceftriaxone-resistant and high-level azithromycin resistant Neisseria gonorrhoeae cases, United Kingdom and Australia, February to April 2018.Crossref | GoogleScholarGoogle Scholar | 30808445PubMed |
[25] Golparian D, Ohlsson A, Janson H, Lidbrink P, Richtner T, Ekelund O, et al Four treatment failures of pharyngeal gonorrhoea with ceftriaxone (500 mg) or cefotaxime (500 mg), Sweden, 2013 and 2014 [published erratum appears in Euro Surveill 2014; 19: pii/20874]. Euro Surveill 2014; 19.
| Four treatment failures of pharyngeal gonorrhoea with ceftriaxone (500 mg) or cefotaxime (500 mg), Sweden, 2013 and 2014 [published erratum appears in Euro Surveill 2014; 19: pii/20874].Crossref | GoogleScholarGoogle Scholar | 25108533PubMed |
[26] Unemo M, Golparian D, Potonik M, Jeverica S. Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011. Euro Surveill 2012; 17 pii:20200
| 23231859PubMed |
[27] Read PJ, Limnios EA, McNulty A, Whiley D, Lahra MM. One confirmed and one suspected case of pharyngeal gonorrhoea treatment failure following 500 mg ceftriaxone in Sydney, Australia. Sex Health 2013; 10 460–2.
| One confirmed and one suspected case of pharyngeal gonorrhoea treatment failure following 500 mg ceftriaxone in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 24028864PubMed |
[28] Tapsall J, Read P, Carmody C, Bourne C, Ray S, Limnios A, et al Two cases of failed ceftriaxone treatment in pharyngeal gonorrhoea verified by molecular microbiological methods. J Med Microbiol 2009; 58 683–7.
| Two cases of failed ceftriaxone treatment in pharyngeal gonorrhoea verified by molecular microbiological methods.Crossref | GoogleScholarGoogle Scholar | 19369534PubMed |
[29] Chen MY, Stevens K, Tideman R, Zaia A, Tomita T, Fairley CK, et al Failure of ceftriaxone 500 mg to eradicate pharyngeal gonorrhoea, Australia. J Antimicrob Chemother 2013; 68 1445–7.
| Failure of ceftriaxone 500 mg to eradicate pharyngeal gonorrhoea, Australia.Crossref | GoogleScholarGoogle Scholar |
[30] Unemo M, Golparian D, Hestner A. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill 2011; 16 pii:19792
| 22085601PubMed |
[31] Workowski KA, Bolan GA, Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64 1–137.
| 26042815PubMed |
[32] Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 2013; 24 85–92.
| 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults.Crossref | GoogleScholarGoogle Scholar | 24400344PubMed |
[33] Romanowski B, Robinson J, Wong T. Gonococcal infections chapter. In Canadian guidelines on sexually transmitted infections. Ottawa: Public Health Agency of Canada; 2013. Available online at: www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/assets/pdf/section-5-6-eng.pdf [verified 28 June 2019].
[34] Australasian Sexual Health Alliance (ASHA). Gonorrhoea. In Australian STI management guidelines for use in primary care. Sydney: ASHA; 2016. Available online at: www.sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea#management [verified 28 June 2019].
[35] Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, et al Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 2016; 374 2504–6.
| Failure of dual antimicrobial therapy in treatment of gonorrhea.Crossref | GoogleScholarGoogle Scholar | 27332921PubMed |
[36] Ndowa F, Lusti-Narasimhan M, Unemo M. The serious threat of multidrug-resistant and untreatable gonorrhoea: the pressing need for global action to control the spread of antimicrobial resistance, and mitigate the impact on sexual and reproductive health. Sex Transm Infect 2012; 88 317–8.
| The serious threat of multidrug-resistant and untreatable gonorrhoea: the pressing need for global action to control the spread of antimicrobial resistance, and mitigate the impact on sexual and reproductive health.Crossref | GoogleScholarGoogle Scholar | 22798629PubMed |
[37] World Health Organization (WHO). Global action plan on antimicrobial resistance. Geneva: WHO; 2015. Available online at: http://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ [verified 28 June 2019].
[38] World Health Organization (WHO). Global surveillance network for gonococcal antimicrobial susceptibility. WHO/VDT/90-452. Geneva: WHO; 1990. Available online at: http://apps.who.int/medicinedocs/documents/s16348e/s16348e.pdf [verified 28 June 2019].
[39] Kirkcaldy RD, Kidd S, Weinstock HS, Papp JR, Bolan GA. Trends in antimicrobial resistance in Neisseria gonorrhoeae in the USA: the Gonococcal Isolate Surveillance Project (GISP), January 2006–June 2012. Sex Transm Infect 2013; 89 iv5–10.
| Trends in antimicrobial resistance in Neisseria gonorrhoeae in the USA: the Gonococcal Isolate Surveillance Project (GISP), January 2006–June 2012.Crossref | GoogleScholarGoogle Scholar | 24243881PubMed |
[40] Kirkcaldy RD, Harvey A, Papp JR, Del Rio C, Soge OO, Holmes KK, et al Neisseria gonorrhoeae antimicrobial susceptibility surveillance – the Gonococcal Isolate Surveillance Project, 27 sites, United States, 2014. MMWR Surveill Summ 2016; 65 1–19.
| Neisseria gonorrhoeae antimicrobial susceptibility surveillance – the Gonococcal Isolate Surveillance Project, 27 sites, United States, 2014.Crossref | GoogleScholarGoogle Scholar | 27414503PubMed |
[41] Martin I, Sawatzky P, Liu G, Allen V, Lefebvre B, Hoang L, et al Decline in decreased cephalosporin susceptibility and increase in azithromycin resistance in Neisseria gonorrhoeae, Canada. Emerg Infect Dis 2016; 22 65–7.
| Decline in decreased cephalosporin susceptibility and increase in azithromycin resistance in Neisseria gonorrhoeae, Canada.Crossref | GoogleScholarGoogle Scholar | 26689114PubMed |
[42] Gianecini R, Romero MLM, Oviedo C, Vacchino M, Galarza P, Gonococcal Antimicrobial Susceptibility Surveillance Programme-Argentina (GASSP-AR) Working Group. Emergence and spread of Neisseria gonorrhoeae isolates with decreased susceptibility to extended-spectrum cephalosporins in Argentina, 2009 to 2013. Sex Transm Dis 2017; 44 351–5.
| Emergence and spread of Neisseria gonorrhoeae isolates with decreased susceptibility to extended-spectrum cephalosporins in Argentina, 2009 to 2013.Crossref | GoogleScholarGoogle Scholar | 28499284PubMed |
[43] Town K, Obi C, Quaye N, Chisholm S, Hughes G, GRASP Collaborative Group. Drifting towards ceftriaxone treatment failure in gonorrhoea: risk factor analysis of data from the Gonococcal Resistance to Antimicrobials Surveillance Programme in England and Wales. Sex Transm Infect 2017; 93 39–45.
| Drifting towards ceftriaxone treatment failure in gonorrhoea: risk factor analysis of data from the Gonococcal Resistance to Antimicrobials Surveillance Programme in England and Wales.Crossref | GoogleScholarGoogle Scholar | 27382010PubMed |
[44] Lahra MM, Enriquez RP, Prince of Wales Hospital, Randwick, for The National Neisseria Network. Australian Gonococcal Surveillance Programme, 1 July to 30 September 2016. Commun Dis Intell Q Rep 2017; 41 E109–10.
| 28385144PubMed |
[45] UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance. Strategies and laboratory methods for strengthening surveillance of sexually transmitted infections 2012. Geneva: World Health Organization; 2012. Available online at: http://apps.who.int/iris/bitstream/10665/75729/1/9789241504478_eng.pdf [verified 28 June 2019].
[46] Centers for Disease Control and Prevention (CDC) Antibiotic-resistant strains of Neisseria gonorrhoeae: policy guidelines for detection, management and control. MMWR 1987; 36 1S–18S.
[47] Ison CA, Deal C, Unemo M. Current and future treatment options for gonorrhoea. Sex Transm Infect 2013; 89 iv52–6.
| Current and future treatment options for gonorrhoea.Crossref | GoogleScholarGoogle Scholar | 24106339PubMed |
[48] Roy K, Wang SA, Meltzer MI. Optimizing treatment of antimicrobial-resistant Neisseria gonorrhoeae. Emerg Infect Dis 2005; 11 1265–73.
| Optimizing treatment of antimicrobial-resistant Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 16102317PubMed |
[49] El-Rami FE, Zielke RA, Wi T, Sikora AE, Unemo M. Quantitative proteomics of the 2016 WHO Neisseria gonorrhoeae reference strains surveys vaccine candidates and antimicrobial resistance determinants. Mol Cell Proteomics 2019; 18 127–50.
| Quantitative proteomics of the 2016 WHO Neisseria gonorrhoeae reference strains surveys vaccine candidates and antimicrobial resistance determinants.Crossref | GoogleScholarGoogle Scholar | 30352803PubMed |
[50] Unemo M, Fasth O, Fredlund H, Limnios A, Tapsall J. Phenotypic and genetic characterization of the 2008 WHO Neisseria gonorrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes. J Antimicrob Chemother 2009; 63 1142–51.
| Phenotypic and genetic characterization of the 2008 WHO Neisseria gonorrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes.Crossref | GoogleScholarGoogle Scholar | 19318360PubMed |
[51] Unemo M, Golparian D, Sánchez-Busó L, Grad Y, Jacobsson S, Ohnishi M, et al The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother 2016; 71 3096–108.
| The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization.Crossref | GoogleScholarGoogle Scholar | 27432602PubMed |
[52] Unemo M, Ison CA, Cole M, Spiteri G, van de Laar M, Khotenashvili L. Gonorrhoea and gonococcal antimicrobial resistance surveillance networks in the WHO European Region, including the independent countries of the former Soviet Union. Sex Transm Infect 2013; 89 iv42–6.
| Gonorrhoea and gonococcal antimicrobial resistance surveillance networks in the WHO European Region, including the independent countries of the former Soviet Union.Crossref | GoogleScholarGoogle Scholar | 24243879PubMed |
[53] Lahra MM, Lo YR, Whiley DM. Gonococcal antimicrobial resistance in the Western Pacific Region. Sex Transm Infect 2013; 89 iv19–23.
| Gonococcal antimicrobial resistance in the Western Pacific Region.Crossref | GoogleScholarGoogle Scholar | 24243875PubMed |
[54] Dillon JA, Trecker MA, Thakur SD, Gonococcal Antimicrobial Surveillance Program Network in Latin America and the Caribbean 1990–2011. Two decades of the gonococcal antimicrobial surveillance program in South America and the Caribbean: challenges and opportunities. Sex Transm Infect 2013; 89 iv36–41.
| Two decades of the gonococcal antimicrobial surveillance program in South America and the Caribbean: challenges and opportunities.Crossref | GoogleScholarGoogle Scholar | 24243878PubMed |
[55] Thakur SD, Araya P, Borthagaray G, Galarza P, Hernandez AL, Payares D, et al Resistance to ceftriaxone and azithromycin in Neisseria gonorrhoeae isolates from 7 countries of South America and the Caribbean: 2010–2011. Sex Transm Dis 2017; 44 157–60.
| 28178114PubMed |
[56] Bala M, Kakran M, Singh V, Sood S, Ramesh V, Members of WHO GASP SEAR Network. Monitoring antimicrobial resistance in Neisseria gonorrhoeae in selected countries of the WHO South-East Asia Region between 2009 and 2012: a retrospective analysis. Sex Transm Infect 2013; 89 iv28–35.
| Monitoring antimicrobial resistance in Neisseria gonorrhoeae in selected countries of the WHO South-East Asia Region between 2009 and 2012: a retrospective analysis.Crossref | GoogleScholarGoogle Scholar | 24243876PubMed |
[57] Ndowa FJ, Francis JM, Machiha A, Faye-Kette H, Fonkoua MC. Gonococcal antimicrobial resistance: perspectives from the African region. Sex Transm Infect 2013; 89 iv11–5.
| Gonococcal antimicrobial resistance: perspectives from the African region.Crossref | GoogleScholarGoogle Scholar | 24243873PubMed |
[58] Spiteri G, Cole M, Unemo M, Hoffmann S, Ison C, van de Laar M. The European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP) – a sentinel approach in the European Union (EU)/European Economic Area (EEA). Sex Transm Infect 2013; 89 iv16–18.
| The European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP) – a sentinel approach in the European Union (EU)/European Economic Area (EEA).Crossref | GoogleScholarGoogle Scholar | 24243874PubMed |
[59] George CRR, Enriquez RP, Gatus BJ, Whiley DM, Lo Y-R, Ishikawa N, et al Systematic review and survey of Neisseria gonorrhoeae antimicrobial resistance data in the Asia Pacific, 2011 to 2016. PLoS One 2019; 14 e0213312
| Systematic review and survey of Neisseria gonorrhoeae antimicrobial resistance data in the Asia Pacific, 2011 to 2016.Crossref | GoogleScholarGoogle Scholar | 30943199PubMed |
[60] Sirivongrangson P, Girdthep N, Sukwicha W, Buasakul P, Tongtoyai J, Weston E, et al The first year of the global Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP) in Bangkok, Thailand, 2015–2016. PLoS One 2018; 13 e0206419
| The first year of the global Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP) in Bangkok, Thailand, 2015–2016.Crossref | GoogleScholarGoogle Scholar | 30412586PubMed |
[61] Gianecini RA, Golparian D, Zittermann S, Litvik A, Gonzalez S, Oviedo C, et al Genome-based epidemiology and antimicrobial resistance determinants in Neisseria gonorrhoeae isolates with decreased susceptibility and resistance to extended-spectrum cephalosporins in Argentina, 2011–2016. J Antimicrob Chemother 2019; 74 1551–9.
| Genome-based epidemiology and antimicrobial resistance determinants in Neisseria gonorrhoeae isolates with decreased susceptibility and resistance to extended-spectrum cephalosporins in Argentina, 2011–2016.Crossref | GoogleScholarGoogle Scholar | 30820563PubMed |
[62] Bazzo ML, Golfetto L, Gaspar PC, Pires AF, Ramos MC, Franchini M, et al First nationwide antimicrobial susceptibility surveillance for Neisseria gonorrhoeae in Brazil, 2015–16. J Antimicrob Chemother 2018; 73 1854–61.
| First nationwide antimicrobial susceptibility surveillance for Neisseria gonorrhoeae in Brazil, 2015–16.Crossref | GoogleScholarGoogle Scholar | 29635367PubMed |
[63] Yéo A, Kouamé-Blavo B, Kouamé CE, Ouattara A, Yao AC, Gbedé BD, et al Establishment of a gonococcal antimicrobial surveillance programme (GASP), in accordance with WHO standards, in Côte d’Ivoire, Western Africa, 2014–2017. Sex Transm Dis 2019; 46 179–84.
| Establishment of a gonococcal antimicrobial surveillance programme (GASP), in accordance with WHO standards, in Côte d’Ivoire, Western Africa, 2014–2017.Crossref | GoogleScholarGoogle Scholar | 30461598PubMed |
[64] Latif AS, Gwanzura L, Machiha A, Ndowa F, Tarupiwa A, Gudza-Mugabe M, et al Antimicrobial susceptibility in Neisseria gonorrhoeae isolates from five sentinel surveillance sites in Zimbabwe, 2015–2016. Sex Transm Infect 2018; 94 62–6.
| Antimicrobial susceptibility in Neisseria gonorrhoeae isolates from five sentinel surveillance sites in Zimbabwe, 2015–2016.Crossref | GoogleScholarGoogle Scholar | 28476914PubMed |
[65] Lewis DA. Will targeting oropharyngeal gonorrhoea delay the further emergence of drug-resistant Neisseria gonorrhoeae strains? Sex Transm Infect 2015; 91 234–7.
| Will targeting oropharyngeal gonorrhoea delay the further emergence of drug-resistant Neisseria gonorrhoeae strains?Crossref | GoogleScholarGoogle Scholar | 25911525PubMed |
[66] Chow EPF, Maddaford K, Trumpour S, Fairley CK. Translating mouthwash use for gonorrhoea prevention into a public health campaign: identifying current knowledge and research gaps. Sex Health 2019;
| Translating mouthwash use for gonorrhoea prevention into a public health campaign: identifying current knowledge and research gaps.Crossref | GoogleScholarGoogle Scholar | 31203836PubMed |
[67] Tapsall JW, Ndowa F, Lewis DA, Unemo M. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther 2009; 7 821–34.
| Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 19735224PubMed |
[68] Chisholm SA, Mouton JW, Lewis DA, Nichols T, Ison CA, Livermore DM. Cephalosporin MIC creep among gonococci: time for a pharmacodynamics rethink? J Antimicrob Chemother 2010; 65 2141–8.
| Cephalosporin MIC creep among gonococci: time for a pharmacodynamics rethink?Crossref | GoogleScholarGoogle Scholar | 20693173PubMed |
[69] Connolly KL, Eakin AE, Gomez C, Osborn BL, Unemo M, Jerse AE. Pharmacokinetic data are predictive of in vivo efficacy for cefixime and ceftriaxone against susceptible and resistant Neisseria gonorrhoeae strains in the gonorrhea mouse model. Antimicrob Agents Chemother 2019; 63 e01644-18
| Pharmacokinetic data are predictive of in vivo efficacy for cefixime and ceftriaxone against susceptible and resistant Neisseria gonorrhoeae strains in the gonorrhea mouse model.Crossref | GoogleScholarGoogle Scholar | 30642924PubMed |
[70] Drusano GL. Pre-clinical in vitro infection models. Curr Opin Pharmacol 2017; 36 100–6.
| Pre-clinical in vitro infection models.Crossref | GoogleScholarGoogle Scholar | 29035729PubMed |
[71] Vincent LR, Kerr SR, Tan Y, Tomberg J, Raterman EL, Dunning Hotopp JC, et al In vivo-selected compensatory mutations restore the fitness cost of mosaic penA alleles that confer ceftriaxone resistance in Neisseria gonorrhoeae. MBio 2018; 9 e01905-17
| In vivo-selected compensatory mutations restore the fitness cost of mosaic penA alleles that confer ceftriaxone resistance in Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 29615507PubMed |
[72] Ko KKK, Chio MTW, Goh SS, Tan AL, Koh TH, Rahman NBA. First case of ceftriaxone-resistant multidrug-resistant Neisseria gonorrhoeae in Singapore. Antimicrob Agents Chemother 2019; 63 e02624-18
| First case of ceftriaxone-resistant multidrug-resistant Neisseria gonorrhoeae in Singapore.Crossref | GoogleScholarGoogle Scholar | 30858209PubMed |
[73] Demczuk W, Sidhu S, Unemo M, Whiley DM, Allen VG, Dillon JR, et al Neisseria gonorrhoeae sequence typing for antimicrobial resistance (NG-STAR): a novel antimicrobial resistance multilocus typing scheme for tracking the global dissemination of N. gonorrhoeae strains. J Clin Microbiol 2017; 55 1454–68.
| Neisseria gonorrhoeae sequence typing for antimicrobial resistance (NG-STAR): a novel antimicrobial resistance multilocus typing scheme for tracking the global dissemination of N. gonorrhoeae strains.Crossref | GoogleScholarGoogle Scholar | 28228492PubMed |
[74] Igawa G, Yamagishi Y, Lee KI, Dorin M, Shimuta K, Suematsu H, et al Neisseria cinerea with high ceftriaxone MIC is a source of ceftriaxone and cefixime resistance-mediating penA sequences in Neisseria gonorrhoeae. Antimicrob Agents Chemother 2018; 62 e02069-17
| Neisseria cinerea with high ceftriaxone MIC is a source of ceftriaxone and cefixime resistance-mediating penA sequences in Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 29311079PubMed |
[75] Wadsworth CB, Arnold BJ, Sater MRA, Grad YH. Azithromycin resistance through interspecific acquisition of an epistasis-dependent efflux pump component and transcriptional regulator in Neisseria gonorrhoeae. MBio 2018; 9 e01419-18
| Azithromycin resistance through interspecific acquisition of an epistasis-dependent efflux pump component and transcriptional regulator in Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 30087172PubMed |
[76] Rouquette-Loughlin CE, Reimche JL, Balthazar JT, Dhulipala V, Gernert KM, Kersh EN, et al Mechanistic basis for decreased antimicrobial susceptibility in a clinical isolate of Neisseria gonorrhoeae possessing a mosaic-like mtr efflux pump locus. MBio 2018; 9 e02281-18
| Mechanistic basis for decreased antimicrobial susceptibility in a clinical isolate of Neisseria gonorrhoeae possessing a mosaic-like mtr efflux pump locus.Crossref | GoogleScholarGoogle Scholar | 30482834PubMed |
[77] Donà V, Low N, Golparian D, Unemo M. Recent advances in the development and use of molecular tests to predict antimicrobial resistance in Neisseria gonorrhoeae. Expert Rev Mol Diagn 2017; 17 845–59.
| Recent advances in the development and use of molecular tests to predict antimicrobial resistance in Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 28741392PubMed |
[78] Low N, Unemo M. Molecular tests for the detection of antimicrobial resistant Neisseria gonorrhoeae: when, where, and how to use? Curr Opin Infect Dis 2016; 29 45–51.
| Molecular tests for the detection of antimicrobial resistant Neisseria gonorrhoeae: when, where, and how to use?Crossref | GoogleScholarGoogle Scholar | 26658656PubMed |
[79] Goire N, Lahra MM, Chen M, Donovan B, Fairley CK, Guy R, et al Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat Rev Microbiol 2014; 12 223–9.
| Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance.Crossref | GoogleScholarGoogle Scholar | 24509781PubMed |
[80] Sadiq ST, Mazzaferri F, Unemo M. Rapid accurate point-of-care tests combining diagnostics and antimicrobial resistance prediction for Neisseria gonorrhoeae and Mycoplasma genitalium. Sex Transm Infect 2017; 93 S65–8.
| Rapid accurate point-of-care tests combining diagnostics and antimicrobial resistance prediction for Neisseria gonorrhoeae and Mycoplasma genitalium.Crossref | GoogleScholarGoogle Scholar | 28684610PubMed |
[81] Whiley DM, Trembizki E, Buckley C, Freeman K, Baird RW, Beaman M, et al Molecular antimicrobial resistance surveillance for Neisseria gonorrhoeae, Northern Territory, Australia. Emerg Infect Dis 2017; 23 1478–85.
| Molecular antimicrobial resistance surveillance for Neisseria gonorrhoeae, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar | 28820128PubMed |
[82] Goire N, Ohnishi M, Limnios AE, Lahra MM, Lambert SB, Nimmo GR, et al Enhanced gonococcal antimicrobial surveillance in the era of ceftriaxone resistance: a real-time PCR assay for direct detection of the Neisseria gonorrhoeae H041 strain. J Antimicrob Chemother 2012; 67 902–5.
| Enhanced gonococcal antimicrobial surveillance in the era of ceftriaxone resistance: a real-time PCR assay for direct detection of the Neisseria gonorrhoeae H041 strain.Crossref | GoogleScholarGoogle Scholar | 22207596PubMed |
[83] Goire N, Lahra MM, Ohnishi M, Hogan T, Liminios AE, Nissen MD, et al Polymerase chain reaction-based screening for the ceftriaxone-resistant Neisseria gonorrhoeae F89 strain. Euro Surveill 2013; 18 20444
| Polymerase chain reaction-based screening for the ceftriaxone-resistant Neisseria gonorrhoeae F89 strain.Crossref | GoogleScholarGoogle Scholar | 23594520PubMed |
[84] Whiley DM, Mhango L, Jennison AV, Nimmo G, Lahra MM. Direct detection of penA gene associated with ceftriaxone-resistant Neisseria gonorrhoeae FC428 strain by using PCR. Emerg Infect Dis 2018; 24 1573–5.
| Direct detection of penA gene associated with ceftriaxone-resistant Neisseria gonorrhoeae FC428 strain by using PCR.Crossref | GoogleScholarGoogle Scholar | 30016236PubMed |
[85] Eyre DW, De Silva D, Cole K, Peters J, Cole MJ, Grad YH, et al WGS to predict antibiotic MICs for Neisseria gonorrhoeae. J Antimicrob Chemother 2017; 72 1937–47.
| WGS to predict antibiotic MICs for Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 28333355PubMed |
[86] Harris SR, Cole MJ, Spiteri G, Sánchez-Busó L, Golparian D, Jacobsson S, et al Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: a genomic survey. Lancet Infect Dis 2018; 18 758–68.
| Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: a genomic survey.Crossref | GoogleScholarGoogle Scholar | 29776807PubMed |
[87] Golparian D, Donà V, Sánchez-Busó L, Foerster S, Harris S, Endimiani A, et al Antimicrobial resistance prediction and phylogenetic analysis of Neisseria gonorrhoeae isolates using the Oxford Nanopore MinION sequencer. Sci Rep 2018; 8 17596
| Antimicrobial resistance prediction and phylogenetic analysis of Neisseria gonorrhoeae isolates using the Oxford Nanopore MinION sequencer.Crossref | GoogleScholarGoogle Scholar | 30514867PubMed |
[88] Hall CL, Harrison MA, Pond MJ, Chow C, Harding-Esch EM, Sadiq ST. Genotypic determinants of fluoroquinolone and macrolide resistance in Neisseria gonorrhoeae. Sex Health 2019;
| Genotypic determinants of fluoroquinolone and macrolide resistance in Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 31366421PubMed |
[89] Tayimetha CY, Unemo M. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates in Yaoundé, Cameroon from 2009 to 2014. Sex Transm Dis 2018; 45 e101–3.
| 30234796PubMed |
[90] Sawatzky P, Liu G, Dillon JA, Allen V, Lefebvre B, Hoang L, et al Quality assurance for antimicrobial susceptibility testing of Neisseria gonorrhoeae in Canada, 2003 to 2012. J Clin Microbiol 2015; 53 3646–9.
| Quality assurance for antimicrobial susceptibility testing of Neisseria gonorrhoeae in Canada, 2003 to 2012.Crossref | GoogleScholarGoogle Scholar | 26338862PubMed |
[91] Sawatzky P, Martin I, Galarza P, Carvallo MET, Araya Rodriguez P, Cruz OMS, et al Quality assurance for antimicrobial susceptibility testing of Neisseria gonorrhoeae in Latin American and Caribbean countries, 2013–2015. Sex Transm Infect 2018; 94 479–82.
| Quality assurance for antimicrobial susceptibility testing of Neisseria gonorrhoeae in Latin American and Caribbean countries, 2013–2015.Crossref | GoogleScholarGoogle Scholar | 29674407PubMed |
[92] Cole MJ, Quaye N, Jacobsson S, Day M, Fagan E, Ison C, et al Ten years of external quality assessment (EQA) of Neisseria gonorrhoeae antimicrobial susceptibility testing in Europe elucidate high reliability of data. BMC Infect Dis 2019; 19 281
| Ten years of external quality assessment (EQA) of Neisseria gonorrhoeae antimicrobial susceptibility testing in Europe elucidate high reliability of data.Crossref | GoogleScholarGoogle Scholar | 30909883PubMed |
[93] Demczuk W, Lynch T, Martin I, Van Domselaar G, Graham M, Bharat A, et al Whole-genome phylogenomic heterogeneity of Neisseria gonorrhoeae isolates with decreased cephalosporin susceptibility collected in Canada between 1989 and 2013. J Clin Microbiol 2015; 53 191–200.
| Whole-genome phylogenomic heterogeneity of Neisseria gonorrhoeae isolates with decreased cephalosporin susceptibility collected in Canada between 1989 and 2013.Crossref | GoogleScholarGoogle Scholar | 25378573PubMed |
[94] Demczuk W, Martin I, Peterson S, Bharat A, Van Domselaar G, Graham M, et al Genomic epidemiology and molecular resistance mechanisms of azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J Clin Microbiol 2016; 54 1304–13.
| Genomic epidemiology and molecular resistance mechanisms of azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014.Crossref | GoogleScholarGoogle Scholar | 26935729PubMed |
[95] Grad YH, Kirkcaldy RD, Trees D, Dordel J, Harris SR, Goldstein E, et al Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study. Lancet Infect Dis 2014; 14 220–6.
| Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study.Crossref | GoogleScholarGoogle Scholar | 24462211PubMed |
[96] Grad YH, Harris SR, Kirkcaldy RD, Green AG, Marks DS, Bentley SD, et al Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J Infect Dis 2016; 214 1579–87.
| Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013.Crossref | GoogleScholarGoogle Scholar | 27638945PubMed |
[97] Jacobsson S, Golparian D, Cole M, Spiteri G, Martin I, Bergheim T, et al WGS analysis and molecular resistance mechanisms of azithromycin-resistant (MIC >2 mg/L) Neisseria gonorrhoeae isolates in Europe from 2009 to 2014. J Antimicrob Chemother 2016; 71 3109–16.
| WGS analysis and molecular resistance mechanisms of azithromycin-resistant (MIC >2 mg/L) Neisseria gonorrhoeae isolates in Europe from 2009 to 2014.Crossref | GoogleScholarGoogle Scholar | 27432597PubMed |
[98] De Silva D, Peters J, Cole K, Cole MJ, Cresswell F, Dean G, et al Whole-genome sequencing to determine transmission of Neisseria gonorrhoeae: an observational study. Lancet Infect Dis 2016; 16 1295–303.
| Whole-genome sequencing to determine transmission of Neisseria gonorrhoeae: an observational study.Crossref | GoogleScholarGoogle Scholar | 27427203PubMed |
[99] Ezewudo MN, Joseph SJ, Castillo-Ramirez S, Dean D, Del Rio C, Didelot X, et al Population structure of Neisseria gonorrhoeae based on whole genome data and its relationship with antibiotic resistance. PeerJ 2015; 3 e806
| Population structure of Neisseria gonorrhoeae based on whole genome data and its relationship with antibiotic resistance.Crossref | GoogleScholarGoogle Scholar | 25780762PubMed |
[100] Ryan L, Golparian D, Fennelly N, Rose L, Walsh P, Lawlor B, et al Antimicrobial resistance and molecular epidemiology using whole-genome sequencing of Neisseria gonorrhoeae in Ireland, 2014–2016: focus on extended-spectrum cephalosporins and azithromycin. Eur J Clin Microbiol Infect Dis 2018; 37 1661–72.
| Antimicrobial resistance and molecular epidemiology using whole-genome sequencing of Neisseria gonorrhoeae in Ireland, 2014–2016: focus on extended-spectrum cephalosporins and azithromycin.Crossref | GoogleScholarGoogle Scholar | 29882175PubMed |
[101] Jönsson A, Jacobsson S, Foerster S, Cole MJ, Unemo M. Performance characteristics of newer MIC gradient strip tests compared with the Etest for antimicrobial susceptibility testing of Neisseria gonorrhoeae. APMIS 2018; 126 822–7.
| Performance characteristics of newer MIC gradient strip tests compared with the Etest for antimicrobial susceptibility testing of Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 30191618PubMed |
[102] Chow EP, Howden BP, Walker S, Lee D, Bradshaw CS, Chen MY, et al Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study. Sex Transm Infect 2017; 93 88–93.
| Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study.Crossref | GoogleScholarGoogle Scholar | 27998950PubMed |
[103] Jerse AE, Deal CD. Vaccine research for gonococcal infections: where are we? Sex Transm Infect 2013; 89 iv63–8.
| Vaccine research for gonococcal infections: where are we?Crossref | GoogleScholarGoogle Scholar | 24243883PubMed |
[104] Edwards JL, Jennings MP, Apicella MA, Seib KL. Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine development. Crit Rev Microbiol 2016; 42 928–41.
| Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine development.Crossref | GoogleScholarGoogle Scholar | 26805040PubMed |
[105] Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, et al Effectiveness of a Group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 2017; 390 1603–10.
| Effectiveness of a Group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study.Crossref | GoogleScholarGoogle Scholar | 28705462PubMed |
[106] Unemo M, Sikora AE. Infection: proof of principle for effectiveness of a gonorrhoea vaccine. Nat Rev Urol 2017; 14 643–4.
| Infection: proof of principle for effectiveness of a gonorrhoea vaccine.Crossref | GoogleScholarGoogle Scholar | 28858332PubMed |
[107] Gottlieb SL, Johnston C. Future prospects for new vaccines against sexually transmitted infections. Curr Opin Infect Dis 2017; 30 77–86.
| 27922851PubMed |
[108] Liu Y, Hammer LA, Liu W, Hobbs MM, Zielke RA, Sikora AE, et al Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol 2017; 10 1594–608.
| Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model.Crossref | GoogleScholarGoogle Scholar | 28272393PubMed |
[109] Gottlieb SL, Jerse AE, Delany-Moretlwe S, Deal C, Giersing BK. Advancing vaccine development for gonorrhoea and the Global STI Vaccine Roadmap. Sex Health 2019; In press.
[110] Clifton S, Town K, Furegato M, Cole M, Mohammed H, Woodhall SC, et al Is previous azithromycin treatment associated with azithromycin resistance in Neisseria gonorrhoeae? A cross-sectional study using national surveillance data in England. Sex Transm Infect 2018; 94 421–6.
| Is previous azithromycin treatment associated with azithromycin resistance in Neisseria gonorrhoeae? A cross-sectional study using national surveillance data in England.Crossref | GoogleScholarGoogle Scholar | 29511067PubMed |
[111] Unemo M, Workowski K. Dual antimicrobial therapy for gonorrhoea: what is the role of azithromycin? Lancet Infect Dis 2018; 18 486–8.
| Dual antimicrobial therapy for gonorrhoea: what is the role of azithromycin?Crossref | GoogleScholarGoogle Scholar | 29523498PubMed |
[112] Jacobsson S, Paukner S, Golparian D, Jensen JS, Unemo M. In vitro activity of the novel pleuromutilin lefamulin (BC-3781) and effect of efflux pump inactivation on multidrug-resistant and extensively-drug resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother 2017; 61 e01497-17
| In vitro activity of the novel pleuromutilin lefamulin (BC-3781) and effect of efflux pump inactivation on multidrug-resistant and extensively-drug resistant Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 28893785PubMed |
[113] Paukner S, Gruss A, Jensen JS. In vitro activity of lefamulin against sexually transmitted bacterial pathogens. Antimicrob Agents Chemother 2018; 62 e02380-17
| In vitro activity of lefamulin against sexually transmitted bacterial pathogens.Crossref | GoogleScholarGoogle Scholar | 29530863PubMed |
[114] Jacobsson S, Mason C, Khan N, Meo P, Unemo M. In vitro activity of the novel oral antimicrobial SMT-571, with a new mechanism of action, against MDR and XDR Neisseria gonorrhoeae – future treatment option for gonorrhoea? J Antimicrob Chemother 2019; 74 1591–4.
| In vitro activity of the novel oral antimicrobial SMT-571, with a new mechanism of action, against MDR and XDR Neisseria gonorrhoeae – future treatment option for gonorrhoea?Crossref | GoogleScholarGoogle Scholar | 30778550PubMed |
[115] Jacobsson S, Golparian D, Scangarella-Oman N, Unemo M. In vitro activity of the novel triazaacenaphthylene gepotidacin (GSK2140944) against MDR Neisseria gonorrhoeae. J Antimicrob Chemother 2018; 73 2072–7.
| In vitro activity of the novel triazaacenaphthylene gepotidacin (GSK2140944) against MDR Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 29796611PubMed |
[116] Scangarella-Oman NE, Hossain M, Dixon PB, Ingraham K, Min S, Tiffany CA, et al Microbiological analysis from a Phase 2 randomized study in adults evaluating single oral doses of gepotidacin in the treatment of uncomplicated urogenital gonorrhea caused by Neisseria gonorrhoeae. Antimicrob Agents Chemother 2018; 62 e01221-18
| Microbiological analysis from a Phase 2 randomized study in adults evaluating single oral doses of gepotidacin in the treatment of uncomplicated urogenital gonorrhea caused by Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 30249694PubMed |
[117] Taylor SN, Morris DH, Avery AK, Workowski KA, Batteiger BE, Tiffany CA, et al Gepotidacin for the treatment of uncomplicated urogenital gonorrhea: a Phase 2, randomized, dose-ranging, single-oral dose evaluation. Clin Infect Dis 2018; 67 504–12.
| Gepotidacin for the treatment of uncomplicated urogenital gonorrhea: a Phase 2, randomized, dose-ranging, single-oral dose evaluation.Crossref | GoogleScholarGoogle Scholar | 29617982PubMed |
[118] Taylor SN, Marrazzo J, Batteiger BE, Hook EW, Seña AC, Long J, et al Single-dose zoliflodacin (ETX0914) for treatment of urogenital gonorrhea. N Engl J Med 2018; 379 1835–45.
| Single-dose zoliflodacin (ETX0914) for treatment of urogenital gonorrhea.Crossref | GoogleScholarGoogle Scholar | 30403954PubMed |
[119] Basarab GS, Kern GH, McNulty J, Mueller JP, Lawrence K, Vishwanathan K, et al Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial Type II topoisomerases. Sci Rep 2015; 5 11827
| Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial Type II topoisomerases.Crossref | GoogleScholarGoogle Scholar | 26168713PubMed |
[120] Foerster S, Golparian D, Jacobsson S, Hathaway LJ, Low N, Shafer WM, et al Genetic resistance determinants, in vitro time–kill curve analysis and pharmacodynamic functions for the novel topoisomerase II inhibitor ETX0914 (AZD0914) in Neisseria gonorrhoeae. Front Microbiol 2015; 6 1377
| Genetic resistance determinants, in vitro time–kill curve analysis and pharmacodynamic functions for the novel topoisomerase II inhibitor ETX0914 (AZD0914) in Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 26696986PubMed |
[121] Jacobsson S, Golparian D, Alm RA, Huband M, Mueller J, Jensen JS, et al High in-vitro activity of the novel spiropyrimidinetrione AZD0914, a DNA gyrase inhibitor, against multidrug resistant Neisseria gonorrhoeae isolates suggests a new effective option for oral treatment of gonorrhea. Antimicrob Agents Chemother 2014; 58 5585–8.
| High in-vitro activity of the novel spiropyrimidinetrione AZD0914, a DNA gyrase inhibitor, against multidrug resistant Neisseria gonorrhoeae isolates suggests a new effective option for oral treatment of gonorrhea.Crossref | GoogleScholarGoogle Scholar | 24982070PubMed |
[122] Unemo M, Ringlander J, Wiggins C, Fredlund H, Jacobsson S, Cole M, European Collaborative Group. High in vitro susceptibility to the novel spiropyrimidinetrione ETX0914 (also known as AZD0914) among 873 contemporary clinical Neisseria gonorrhoeae isolates in 21 European countries during 2012–2014. Antimicrob Agents Chemother 2015; 59 5220–5.
| High in vitro susceptibility to the novel spiropyrimidinetrione ETX0914 (also known as AZD0914) among 873 contemporary clinical Neisseria gonorrhoeae isolates in 21 European countries during 2012–2014.Crossref | GoogleScholarGoogle Scholar | 26077246PubMed |
[123] Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, et al Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17 e235–79.
| Sexually transmitted infections: challenges ahead.Crossref | GoogleScholarGoogle Scholar | 28701272PubMed |
[124] Lewis D. New treatment options for Neisseria gonorrhoeae in the era of emerging antimicrobial resistance. Sex Health 2019;
| New treatment options for Neisseria gonorrhoeae in the era of emerging antimicrobial resistance.Crossref | GoogleScholarGoogle Scholar | 31292063PubMed |